Key Points
Question
In adults with methicillin-resistant Staphylococcus aureus (MRSA) bacteremia, does the addition of 7 days of an antistaphylococcal β-lactam (flucloxacillin, cloxacillin, or cefazolin) to standard antibiotic therapy (vancomycin or daptomycin) lead to improved clinical outcomes at 90 days?
Findings
In this randomized clinical trial that included 352 patients and was stopped early because of increased risk of acute kidney injury in the intervention group, the addition of an antistaphylococcal β-lactam to standard therapy, compared with standard therapy alone, resulted in no significant difference in the primary composite end point of mortality, bacteremia, relapse, or treatment failure (35% vs 39%, respectively).
Meaning
Among patients with MRSA bacteremia, the addition of an antistaphylococcal β-lactam to standard antibiotic therapy did not significantly reduce the primary composite end point.
Abstract
Importance
Methicillin-resistant Staphylococcus aureus (MRSA) bacteremia is associated with mortality of more than 20%. Combining standard therapy with a β-lactam antibiotic has been associated with reduced mortality, although adequately powered randomized clinical trials of this intervention have not been conducted.
Objective
To determine whether combining an antistaphylococcal β-lactam with standard therapy is more effective than standard therapy alone in patients with MRSA bacteremia.
Design, Setting, and Participants
Open-label, randomized clinical trial conducted at 27 hospital sites in 4 countries from August 2015 to July 2018 among 352 hospitalized adults with MRSA bacteremia. Follow-up was complete on October 23, 2018.
Interventions
Participants were randomized to standard therapy (intravenous vancomycin or daptomycin) plus an antistaphylococcal β-lactam (intravenous flucloxacillin, cloxacillin, or cefazolin) (n = 174) or standard therapy alone (n = 178). Total duration of therapy was determined by treating clinicians and the β-lactam was administered for 7 days.
Main Outcomes and Measures
The primary end point was a 90-day composite of mortality, persistent bacteremia at day 5, microbiological relapse, and microbiological treatment failure. Secondary outcomes included mortality at days 14, 42, and 90; persistent bacteremia at days 2 and 5; acute kidney injury (AKI); microbiological relapse; microbiological treatment failure; and duration of intravenous antibiotics.
Results
The data and safety monitoring board recommended early termination of the study prior to enrollment of 440 patients because of safety. Among 352 patients randomized (mean age, 62.2 [SD, 17.7] years; 121 women [34.4%]), 345 (98%) completed the trial. The primary end point was met by 59 (35%) with combination therapy and 68 (39%) with standard therapy (absolute difference, −4.2%; 95% CI, −14.3% to 6.0%). Seven of 9 prespecified secondary end points showed no significant difference. For the combination therapy vs standard therapy groups, all-cause 90-day mortality occurred in 35 (21%) vs 28 (16%) (difference, 4.5%; 95% CI, −3.7% to 12.7%); persistent bacteremia at day 5 was observed in 19 of 166 (11%) vs 35 of 172 (20%) (difference, −8.9%; 95% CI, −16.6% to −1.2%); and, excluding patients receiving dialysis at baseline, AKI occurred in 34 of 145 (23%) vs 9 of 145 (6%) (difference, 17.2%; 95% CI, 9.3%-25.2%).
Conclusions and Relevance
Among patients with MRSA bacteremia, addition of an antistaphylococcal β-lactam to standard antibiotic therapy with vancomycin or daptomycin did not result in significant improvement in the primary composite end point of mortality, persistent bacteremia, relapse, or treatment failure. Early trial termination for safety concerns and the possibility that the study was underpowered to detect clinically important differences in favor of the intervention should be considered when interpreting the findings.
Trial Registration
ClinicalTrials.gov Identifier: NCT02365493
This randomized trial compares the effect of combining standard antibiotic therapy (intravenous vancomycin or daptomycin) with an antistaphylococcal β-lactam (intravenous flucloxacillin, cloxacillin, or cefazolin) vs standard therapy alone on a composite outcome of mortality, bacteremia, relapse, or treatment failure in adults with methicillin-resistant Staphylococcus aureus (MRSA) bacteremia.
Introduction
In 2017 in the United States, there were an estimated 120 000 cases of Staphylococcus aureus bacteremia resulting in 20 000 deaths.1 The mortality from S aureus bacteremia is higher for methicillin-resistant S aureus (MRSA) than for methicillin-susceptible S aureus (MSSA), typically at 20% to 25%.1,2 Despite the heavy burden of S aureus bacteremia, there is a paucity of evidence to guide treatment. Overall, there have been fewer than 2500 patients enrolled in published randomized clinical trials for S aureus bacteremia in the past 20 years, and fewer than 450 for MRSA bacteremia.3
The current standard therapy for MRSA bacteremia is vancomycin or daptomycin.4 Vancomycin has many shortcomings, including poor tissue penetration and slow killing time. Vancomycin has reduced efficacy against MSSA compared with antistaphylococcal β-lactams.5
A growing body of evidence suggests that adding a β-lactam to standard therapy for MRSA bacteremia may improve patient outcomes. In vitro laboratory data consistently demonstrate synergy of vancomycin or daptomycin with a β-lactam against MRSA strains, with an increase in the speed of bacterial killing.5 In vivo animal models of MRSA infection demonstrate improved survival with combination therapy.5 Ex vivo human studies highlight β-lactam–mediated potentiation of host antimicrobial peptides in killing MRSA.6 Retrospective studies have reported improved outcomes when β-lactams have been included during a treatment course for MRSA bacteremia.7,8 Results from 2 small clinical trials suggest that the combination of an antistaphylococcal β-lactam with vancomycin9 or daptomycin10 may reduce the duration of bacteremia9 or mortality.10
The CAMERA2 trial (Combination Antibiotics for Methicillin Resistant Staphylococcus aureus) tested the hypothesis that combination therapy with an antistaphylococcal β-lactam with either vancomycin or daptomycin would improve clinical outcomes in hospitalized adults with MRSA bacteremia as measured by a composite primary end point of mortality, microbiological persistence, relapse, or treatment failure.
Methods
Study Design and Setting
This study was an investigator-initiated, multicenter, open-label, parallel group, randomized clinical trial powered for superiority. Participants were recruited between August 2015 and July 2018 at 27 hospitals in Australia, Singapore, Israel, and New Zealand. Institutional ethics approval was obtained at each site and written informed consent was obtained from each participant or surrogate decision maker. The study protocol11 and the statistical analysis plan are available in Supplement 1 and Supplement 2.
Participants
Participants were hospitalized patients who were eligible if they met all inclusion criteria: (1) having a positive blood culture for MRSA; (2) able to be randomized within 72 hours of the first positive blood culture; (3) aged 18 years or older; and (4) likely to remain hospitalized for at least 7 days following randomization. Patients were excluded if they met any of the exclusion criteria: (1) history of type 1 hypersensitivity reaction to β-lactams; (2) polymicrobial bacteremia (excluding isolates judged by the site investigator to be contaminants); (3) previous participation in the trial; (4) known pregnancy; (5) treating clinician unwilling to allow patient to be enrolled; (6) patient currently receiving β-lactam therapy that could not be ceased or substituted for a non–β-lactam antibiotic; (7) patient expected to die in the next 48 hours; and (8) treatment limitations precluding use of antibiotics.
Randomization
Participants were randomized in a 1:1 ratio to the standard or combination therapy group using a web-based interactive randomization system (Spiral Software). Randomization was stratified by site and receipt of dialysis in permuted blocks of size 2, 4, or 6. Randomization codes were computer generated by a statistician not involved in the conduct of the trial. The day of randomization was considered study day 1.
Interventions
Participants randomized to standard therapy received either vancomycin or daptomycin according to treating clinician preference. Vancomycin was dosed in accordance with Australian12 or US4 guidelines with subsequent adjustment to maintain trough levels of 15 to 20 μg/mL. Daptomycin was dosed at 6 to 10 mg/kg per day. Doses were adjusted according to kidney function.11 Nonantibiotic management and duration of vancomycin or daptomycin administration were at clinician discretion, but the protocol recommended 14 to 42 days of intravenous treatment guided by the result of blood culture at 2 to 4 days, echocardiography, and management of infection foci. Those randomized to combination therapy received standard therapy plus an intravenous β-lactam (flucloxacillin, 2 g every 6 hours in Australia and New Zealand; cloxacillin, 2 g every 6 hours in Singapore and Israel) for the first 7 calendar days following randomization (including the day of randomization as day 1). Those with a history of non–type 1 hypersensitivity allergy to any penicillin received cefazolin, 2 g every 8 hours. Patients undergoing hemodialysis received cefazolin, 2 g 3 times per week after dialysis.
Outcomes
The primary outcome was a composite measure assessed 90 days after randomization with 4 components: (1) all-cause mortality; (2) persistent bacteremia at study day 5; (3) microbiological relapse defined as a positive blood culture for MRSA at least 72 hours after a preceding negative culture; and (4) microbiological treatment failure defined as a positive sterile site culture for MRSA at least 14 days after randomization. Secondary outcomes were (1) all-cause mortality at 14, 42, and 90 days; (2) persistent bacteremia at day 2; (3) persistent bacteremia at day 5; (4) acute kidney injury (AKI), defined as stage 1 or higher using modified RIFLE criteria13 (≥1.5-fold increase in serum creatinine; the criterion of urine output <0.5 mL/kg per hour was not included) at any time within the first 7 days or new need for renal replacement therapy (RRT) between day 1 and day 90 (participants already undergoing hemodialysis or peritoneal dialysis at randomization were excluded from this AKI end point); (5) microbiological relapse; (6) microbiological treatment failure; and (7) duration of intravenous antibiotic treatment. Serum creatinine measurements were included as part of the initial protocol at baseline and study days 2, 5, and 7, and at days 14 and 28 in an amended protocol.
The composite primary end point was assessed by a blinded end-point adjudication committee of 3 infectious disease physicians who were not involved in study design or patient recruitment.
Adverse Events
Because all drugs used were registered with established safety profiles, the adverse event reporting protocol was abbreviated. Site investigators were asked to record all adverse events (regardless of seriousness) that were thought to be related to vancomycin, daptomycin, or a study β-lactam. Expedited reporting of serious adverse events was required only for the combination therapy group and only if assessed to be at least possibly related to the study β-lactam.
Laboratory Methods
Oxacillin and vancomycin minimum inhibitory concentrations for each available index bacterial isolate were determined in a central laboratory by Sensititre broth microdilution (Thermo Fisher Scientific). Isolates underwent whole genome sequencing on the Illumina NextSeq platform and multilocus sequence type determined in silico using mlst version 2.16.4 (https://github.com/tseemann/mlst).
Statistical Methods
Sample Size
We estimated that the primary outcome would occur in 30% of participants in the control group based on a previous pilot trial.9 We aimed to detect a clinically meaningful absolute reduction in the primary end point of 12.5%. Opinions on a clinically significant margin were sought from members of the trial study group; estimates ranged from 10% to 15%. At a significance level of α = .05 and with a power of 80%, this resulted in a sample size of 440 (accounting for 10% dropout).
Study Populations
Participants were analyzed according to treatment randomization regardless of the treatment they actually received. The primary analysis population included all participants with data available for the primary end point. The per-protocol population was defined as (1) for the combination group, those who received at least 75% of study β-lactam doses; (2) for the standard treatment group, those who received no more than 1 defined daily dose of a study β-lactam after enrollment; and (3) for both groups, those with data available for the primary end point.
Analyses
For the primary end point and other categorical measures, the absolute difference in proportions was reported with corresponding 95% confidence intervals. The Fisher exact test was used for statistical comparisons. Continuous measures were summarized with medians or means as appropriate and compared using the Mann-Whitney U test or the t test. Post hoc analyses adjusting for randomization variables (study site and hemodialysis) via mixed-effects models were conducted for each of the primary and secondary outcomes.
Given only sparse missing data, complete case analyses were performed and reported throughout, with no assumptions made about missing data. Post hoc sensitivity analyses for the primary outcome were conducted as follows: (1) participants with missing end-point data treated as having treatment failure; (2) participants with missing data in the standard treatment group treated as having treatment success and those with missing data in the combination group treated as having treatment failure (worst-case scenario); (3) including only participants with an associated bacterial isolate and end-point data available; (4) including only participants with an associated S aureus isolate (excluding those with Staphylococcus argenteus) and end-point data; 5) including only participants with an associated MRSA isolate (excluding S argenteus and MSSA) and end-point data. A priori subgroups are listed in the full protocol, and subgroup analyses for the primary outcome were conducted. Wald tests were used to test for subgroup interactions. P values were 2-sided and all hypothesis tests were conducted at the α = .05 significance level. No adjustment was made for multiple comparisons, so findings for secondary outcomes and analyses should be interpreted as exploratory.
Additional post hoc descriptions included charting the fold change in creatinine levels from baseline, reporting on day 90 outcomes for patients experiencing AKI, reporting on AKI stages using modified Kidney Disease: Improving Global Outcomes (KDIGO) criteria,14 and reporting the occurrence of AKI in participants receiving only flucloxacillin or cloxacillin or only cefazolin. Analyses were performed using Stata version 15 (StataCorp LP) and R version 3.6.0 (R Foundation for Statistical Computing).
Study Oversight
An independent study monitor visited each study site at least once per year and undertook source data verification for key data points on all study participants. The study was overseen by an independent data and safety monitoring board (DSMB) comprising 2 infectious disease physicians, a nephrologist, and an independent statistician. There was a planned interim analysis after 220 patients had been enrolled and followed up for 90 days.
In March 2018, at the planned interim analysis, with data from 220 participants, the DSMB raised concerns about emerging differences between the treatment groups with regard to AKI. The DSMB recommended ongoing recruitment but requested enhanced data collection of creatinine levels. In July 2018, the DSMB completed a further analysis, with data from 343 participants, showing a significantly higher rate of AKI in one group that could not be explained by small baseline differences and no signal of a decrease in mortality at 90 days in that group. Given that recruitment was close to 80% of the planned total of 440 and that rates of AKI and mortality were statistically unlikely to change in a clinically meaningful manner during the final phase of planned recruitment, the DSMB recommended ceasing patient recruitment on July 23, 2018. The trial management committee closed trial recruitment on July 26, 2018.
Results
Study Population
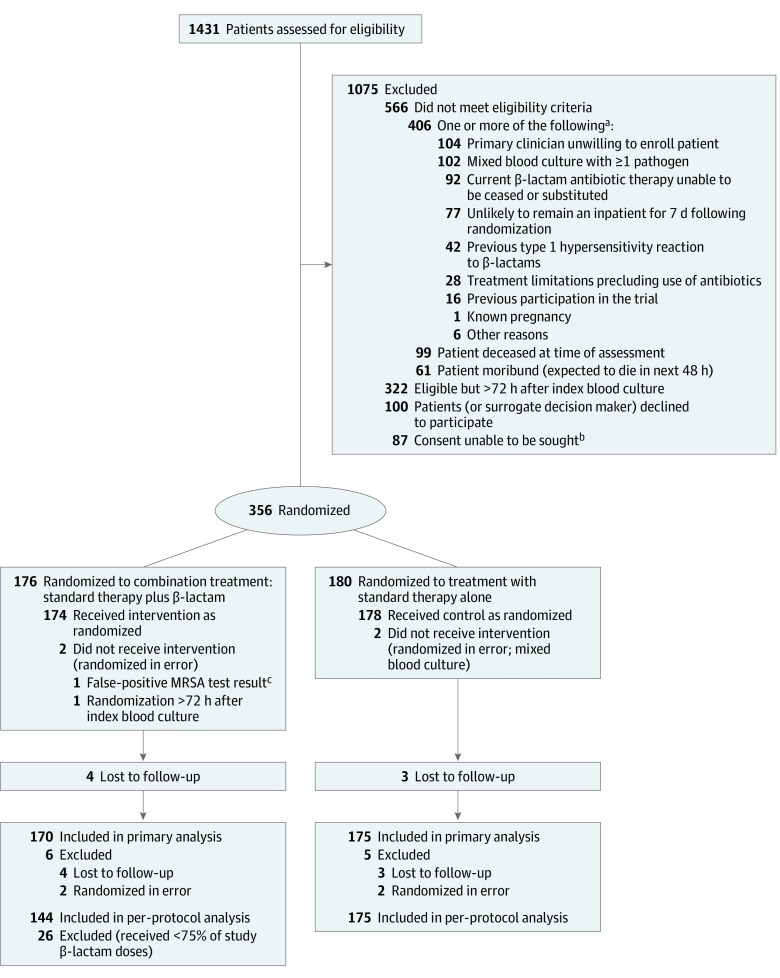
Of 1431 patients screened, 356 were randomized, of whom 4 were subsequently found to be ineligible (Figure 1). Of the remaining 352 patients, 174 were randomized to combination therapy and 178 to standard therapy. Seven patients were lost to follow-up, leaving 345 patients in the primary analysis population. An additional 26 patients in the combination therapy group did not receive at least 75% of study β-lactam doses and were excluded from the per-protocol population (Figure 1).
Figure 1. Patient Recruitment, Randomization, and Flow Through the CAMERA2 Trial.
aPatients could have more than 1 reason for exclusion.
bIncludes when a patient’s surrogate decision maker was unavailable, site was not approved for surrogate consent, or interpreters and/or investigators were unavailable.
cInitial rapid test result was called methicillin-resistant Staphylococcus aureus (MRSA) but final laboratory antimicrobial susceptibility test result was methicillin-susceptible S aureus.
The median age was 64 years (interquartile range, 49-77 years). Baseline characteristics were similar by treatment group (Table 1). Three hundred forty-nine patients (99%) received vancomycin, with day 1 to day 3 trough levels indicating appropriate dosing, and 13 (4%) received at least 1 dose of daptomycin during the study (Table 2). Fifty-five patients were undergoing dialysis at baseline. Genotypes and oxacillin and vancomycin minimum inhibitory concentrations of isolates were similar by treatment group (Table 1; eFigure 1 in Supplement 3).
Table 1. Baseline Characteristics of Patients in the Primary Analysis Population.
| Characteristics | Combination Therapy (n = 174) | Standard Therapy (n = 178) |
|---|---|---|
| Age, median (IQR), y | 65 (51-76) | 63 (47-79) |
| Sex, No. (%) | ||
| Male | 121 (70) | 110 (62) |
| Female | 53 (30) | 68 (38) |
| Maintenance dialysis before study enrollment, No. (%) | 25 (14) | 30 (17) |
| Country, No. (%) | ||
| Australia/New Zealand | 124 (71) | 128 (72) |
| Singapore | 28 (16) | 28 (16) |
| Israel | 22 (13) | 22 (12) |
| Acquisition, No. (%)a | ||
| Nosocomial acquisition | 56 (32) | 48 (27) |
| Health care–associated infection | 105 (60) | 120 (67) |
| Time from index blood culture to randomization, median (IQR), d | 2 (1-2) | 2 (1-2) |
| Charlson Comorbidity Index, median (IQR)b | 5 (2-7) | 5 (2-7) |
| Pitt bacteremia score, median (IQR)c | 2 (2-3) | 2 (2-3) |
| SOFA score, median (IQR)d | 2 (1-4) | 1 (0-4) |
| Indwelling vascular device, No. (%)e | 95 (55) | 97 (54) |
| Indwelling prosthetic valve or cardiac device, No. (%) | 20 (11) | 14 (8) |
| Other intravascular foreign material, No. (%)f | 7 (4) | 5 (3) |
| Injecting drug use in the last 30 d, No. (%) | 14 (8) | 16 (9) |
| Recognized infection foci at time of index blood culture, No. (%) | ||
| Skin and soft tissue infection | 40 (23) | 50 (28) |
| Primary blood stream infection | 34 (20) | 35 (20) |
| Native osteoarticular | 31 (18) | 27 (15) |
| Intravenous line related | 25 (14) | 22 (12) |
| Pleuropulmonary infection | 13 (7) | 11 (6) |
| Device related | 9 (5) | 9 (5) |
| Infective endocarditis | 9 (5) | 6 (3) |
| Other | 13 (7) | 18 (10) |
| Any antibiotic in 72 h preceding randomization, No. (%) | 170 (98) | 174 (98) |
| Any β-lactam in 72 h preceding randomization, No. (%) | 111 (64) | 104 (58) |
| Drugs affecting kidney function in 48 h preceding randomization, No. (%)g | 98 (56) | 108 (61) |
| Baseline creatinine level, median (IQR), mg/dLh | 1.13 (0.8-2.5) | 1.22 (0.8-2.7) |
| Baseline C-reactive protein level, median (IQR), mg/L | 174 (92-269) | 161 (77-248) |
| Multilocus ST, No./total (%)i | ||
| ST22 | 33/160 (21) | 34/161 (21) |
| ST93 | 23/160 (14) | 28/161 (17) |
| ST45 | 21/160 (13) | 26/161 (16) |
| ST5 | 24/160 (15) | 15/161 (9) |
| ST239 | 7/160 (4) | 10/161 (6) |
| ST1 | 7/160 (4) | 9/161 (6) |
| ST30 | 5/160 (3) | 8/161 (5) |
| Otherj | 40/160 (25) | 31/161 (19) |
| Vancomycin MIC, No./total (%)i,k | ||
| ≤1 μg/mL | 152/160 (95) | 153/161 (95) |
| 2 μg/mL | 8/160 (5) | 8/161 (5) |
Abbreviations: IQR, interquartile range; ST, sequence type.
Nosocomial acquisition was indicated if patients were inpatients for more than 48 hours at the time of index blood culture collection. A health care–associated infection was indicated if patients had any of the following: outpatient parenteral antibiotic therapy service in the past 30 days, more than 48 hours in the hospital in the past 90 days, outpatient chemotherapy in the past 30 days, or living in a residential care facility.
The Charlson Comorbidity Index provides a 10-year mortality risk based on weighted comorbid conditions, ranging from 0 (no comorbid conditions) to 29, with a score of 4 associated with an estimated 10-year survival of 53%.15
The Pitt bacteremia score provides a measure of in-hospital mortality risk in patients with bloodstream infections based on clinical variables, ranging from 0 to 14, with a Pitt score of 4 or greater associated with a risk of mortality of approximately 40%.16
The Sequential Organ Failure Assessment (SOFA) score provides a mortality prediction score based on the degree of dysfunction of 6 organ systems, ranging from 0 to 24, with a SOFA score of 6 to 7 associated with a risk of mortality of approximately 20%.17 The SOFA score was based on the worst recorded parameters in the 24 hours preceding randomization.
Indwelling vascular devices included peripheral intravenous cannulas, hemodialysis synthetic arteriovenous grafts, vascaths, peripherally inserted central catheters, central venous catheters, tunneled lines, portacaths, and arterial lines. The presence of any of these was noted without making a judgment as to whether methicillin-resistant Staphylococcus aureus bacteremia was attributable to the vascular device.
Data collected indicated the presence of “other intravascular foreign material” without further information (it was a tick-box only, without a further text field).
Drugs affecting kidney function included radiocontrast dye, amphotericin B, loop diuretics, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, nonsteroidal anti-inflammatory drugs, aminoglycosides, and calcineurin inhibitors.
To convert creatinine to μmol/L, multiply by 88.4. Baseline creatinine was defined as the highest creatinine measurement in the 24 hours preceding randomization.
There were 321 isolates recovered for in silico genotyping by whole genome sequencing and determination of vancomycin minimum inhibitory concentration (MIC) by broth microdilution.
Three of these had genotypes consistent with Staphylococcus argenteus, which is recommended to be clinically managed as for S aureus.
Vancomycin MIC was tested by broth microdilution that uses a vancomycin range of 1 to 128 μg/mL (in 2-fold increments). No isolates had an MIC greater than 2 μg/mL.
Table 2. Characteristics of Patients During the Trial in the Primary Analysis Population.
| Characteristics | Combination Therapy (n = 174) | Standard Therapy (n = 178) |
|---|---|---|
| Final diagnosis of infective endocarditis, No. (%)a | 26 (15) | 16 (9) |
| Received vancomycin, No. (%)b | 171 (98) | 178 (100) |
| Received daptomycin, No. (%)b | 7 (4) | 6 (3) |
| Trough vancomycin level, mean (SD), μg/mL | ||
| Day 1 | 15.1 (8.1) | 14.7 (7.3) |
| Day 2 | 17.9 (9.1) | 17.2 (8.0) |
| Day 3 | 20.1 (7.6) | 19.2 (7.5) |
| Received any nonstudy antibiotic during days 1-7, No. (%)c | 53 (30) | 48 (27) |
| Infectious diseases consultation, No. (%) | 168 (97) | 171 (96) |
| Presumed infected source removed, No. (%) | 77/106 (73) | 84/105 (80) |
| Time to removal of infected source, median (IQR), dd | 0.0 (−1.0 to 2.0) | 0.0 (−1.0 to 2.0) |
| Echocardiogram performed, No. (%) | 161 (93) | 168 (94) |
| Transthoracic | 151 (87) | 151 (85) |
| Transesophageal | 61 (35) | 68 (38) |
Abbreviation: IQR, interquartile range.
The final diagnosis of infective endocarditis was defined by modified Duke criteria. Numbers differ from those recognized with infective endocarditis at baseline because further investigations were performed.
Some patients may have received both vancomycin and daptomycin during their time in the study.
The 5 most common nonstudy antibiotics were piperacillin-tazobactam (combination: n = 18; standard: n = 15), ceftriaxone (combination: n = 15; standard: n = 11), gentamicin (combination: n = 9; standard: n = 5), azithromycin (combination: n = 9; standard: n = 4), and metronidazole (combination: n = 5; standard: n = 5). Participants may have received more than 1 nonstudy antibiotic.
The source may have been removed prior to randomization, with days prior to randomization counted as a negative number of days. Patients may have had multiple infected sources. Removal of a presumed infected source included removal of vascular lines and foreign devices as well as procedures such as drainage of skin abscesses, drainage of deep or visceral abscesses, debridement of infected tissue, and operative joint irrigation and drainage.
Primary Outcome
In the primary analysis population, 59 of 170 patients (35%) in the combination therapy group and 68 of 175 (39%) in the standard treatment group met the primary outcome at day 90 (difference, −4.2%; 95% CI, −14.3% to 6.0%; P = .42). Results were consistent when the analysis was adjusted for the baseline stratification variables of study site and hemodialysis, for the per-protocol population (Table 3; eTable 1 in Supplement 3), and in post hoc sensitivity analyses, including when any losses to follow-up were counted as treatment failures (eTable 2 in Supplement 3).
Table 3. Primary and Secondary Outcomes.
| Outcomes | No./Total No. (%) | Risk Difference, % (95% CI) | P Value | |
|---|---|---|---|---|
| Combination Therapy | Standard Therapy | |||
| Primary Outcomea,b | ||||
| Primary analysis population | 59/170 (35) | 68/175 (39) | −4.2 (−14.3 to 6.0) | .42 |
| Per protocol | 47/144 (33) | 68/175 (39) | −6.2 (−16.7 to 4.3) | .25 |
| Secondary Outcomesc | ||||
| All-cause mortalityd | ||||
| Day 14 | 13/170 (8) | 13/174 (7) | 0.2 (−5.4 to 5.8) | .95 |
| Day 42 | 25/170 (15) | 19/174 (11) | 3.8 (−3.3 to 10.8) | .29 |
| Day 90 | 35/170 (21) | 28/174 (16) | 4.5 (−3.7 to 12.7) | .28 |
| Persistent bacteremiae | ||||
| Day 2 | 50/167 (30) | 61/173 (35) | −5.3 (−15.3 to 4.6) | .29 |
| Day 5 | 19/166 (11) | 35/172 (20) | −8.9 (−16.6 to −1.2) | .02 |
| Microbiological relapsea | 14/169 (8) | 18/175 (10) | −2.0 (−8.1 to 4.1) | .52 |
| Microbiological treatment failurea | 16/170 (9) | 17/175 (10) | −0.3 (−6.5 to 5.9) | .92 |
| Acute kidney injuryf | 34/145 (23) | 9/145 (6) | 17.2 (9.3 to 25.2) | <.001 |
| Duration of intravenous antibiotics, mean (SD), d | 29.3 (19.5) | 28.1 (17.4) | .72 | |
The primary outcome was a composite of mortality at day 90, persistent bacteremia at day 5, microbiological relapse (a positive blood culture for methicillin-resistant Staphylococcus aureus [MRSA] at least 72 hours after a preceding negative culture), and microbiological treatment failure (a positive sterile-site culture for MRSA at least 14 days after randomization).
The primary analysis population consisted of all participants with data available for the primary end point, who were analyzed according to treatment randomization, regardless of treatment received. The per-protocol population was defined as (1) for the combination group, those who received at least 75% of study β-lactam doses; (2) for the standard treatment group, those who received no more than 1 defined daily dose of study β-lactam; and (3) for both groups, those with data available for the primary end point.
Results for secondary outcomes are reported for the primary analysis population. Results for secondary outcomes for the per-protocol population are found in eTables 1 and 3 in Supplement 3.
One patient did not have mortality data available but did meet the criteria for persistent bacteremia and so met the primary composite end point.
The median time from the date of first positive blood culture to study day 2 was 4 days and from date of first positive blood culture to study day 5 was 7 days.
Participants undergoing dialysis at randomization were excluded from the acute kidney injury (AKI) outcome. Acute kidney injury was defined as at least stage 1 modified RIFLE criteria (1.5-fold increase in serum creatinine) at any time within the first 7 days or new need for renal replacement at any time between day 1 and day 90. There were 5 participants in the combination therapy group and 11 in the standard therapy group who did not have baseline creatinine measurement data but could still qualify for AKI if they required renal replacement therapy. When these participants with missing baseline creatinine measurement data were excluded from the analysis, AKI occurred in 34 of 140 (24%) in the combination therapy group and 9 of 134 (7%) in the standard therapy group (risk difference, 18%; 95% CI, 9.3%-26%; P < .001).
Secondary Outcomes
Prespecified secondary outcomes for the primary analysis population are presented in Table 3 and eFigure 2 in Supplement 3. Although mortality did not significantly differ between treatment groups at any time point, persistent bacteremia at study day 5 was significantly less common with combination therapy (19/166 [11%]) than with standard therapy (35/172 [20%]) (difference, −8.9%; 95% CI, −16.6 to −1.2%). Acute kidney injury (patients undergoing dialysis at baseline were excluded from this analysis) was significantly more common with combination therapy (34/145 [23%]) than with standard therapy (9/145 [6%]) (difference, 17.2%; 95% CI, 9.3%-25.2%). The secondary outcomes for the per-protocol population are presented in eTable 3 in Supplement 3. Because there was no difference in the primary outcome between treatment groups, a prespecified health economic analysis was not performed.
Prespecified Subgroup Analyses
Prespecified subgroup analyses showed no significant effect of the treatment on the composite primary outcome in any subgroup (eTable 4 and eFigure 3 in Supplement 3).
Reported Adverse Events
Adverse events were recorded by site investigators for 23 participants in the combination therapy group and 7 in the standard therapy group. The most commonly recorded adverse event was AKI (13/174 with combination therapy and 1/178 with standard therapy) (eTable 5 in Supplement 3). There were 5 reported serious adverse events: 4 episodes of AKI and 1 seizure (eTable 6 in Supplement 3).
Post Hoc Analyses
In light of the increased AKI in the combination therapy group, the following post hoc exploratory analyses were conducted after excluding patients undergoing dialysis at baseline. P values were not calculated for these post hoc analyses.
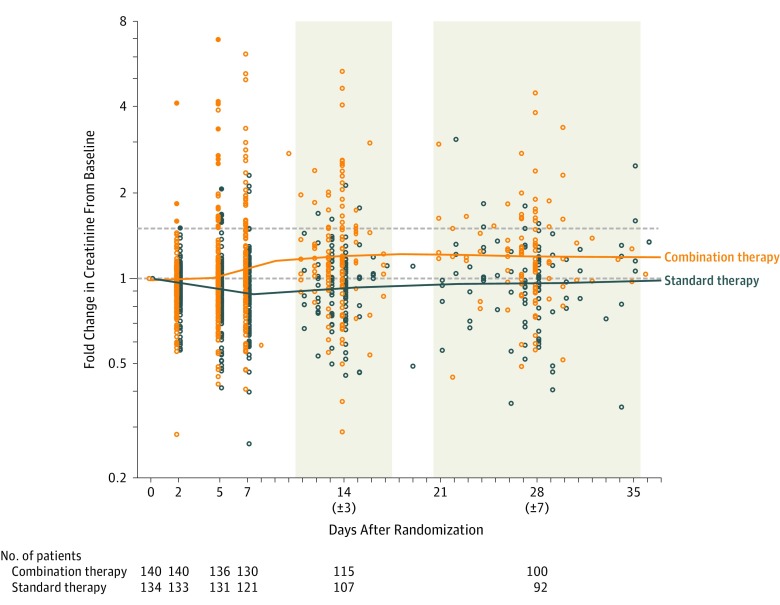
The fold change in serum creatinine levels from baseline was increased in the combination therapy group compared with the standard therapy group from study day 5 through day 30 (Figure 2). Of the 34 of 145 patients (23%) experiencing AKI with combination therapy, 6 required new RRT, and by day 90, 2 were still receiving RRT and 7 had died. In contrast, of the 9 of 145 patients (6%) experiencing AKI with standard therapy, 2 required new RRT; by day 90 none were still receiving RRT and 3 had died. When AKI was defined using modified KDIGO criteria, 36 of 145 (25%) in the combination therapy group experienced AKI compared with 13 of 145 (9%) in the standard therapy group, and a greater proportion of the AKI in the combination therapy group was of a higher severity (stage 2 or 3) (eTable 7 in Supplement 3).
Figure 2. Fold Change in Creatinine Levels vs Baseline Measurements.
Fold change in creatinine levels up to postrandomization day 30 for all patients except those undergoing dialysis or with missing creatinine at baseline with a log2 scale for the y-axis. The horizontal lines depict a fold change of 1 (solid) and 1.5 (dashed). Acute kidney injury was defined as a 1.5-fold or greater increase in serum creatinine any time in the first 7 days. Participants contributing data at baseline, day 2, 5, 7, 14 (±3) and 28 (±7) in each treatment group are shown. After excluding patients undergoing dialysis at baseline, there were 5 participants in the combination therapy and 11 participants in the standard therapy group with missing baseline creatinine measurements. Each individual contributed only 1 measurement to each of the time points (days 2, 5, and 7) or intervals (days 14 [±3] and 28 [±7]). If individuals had multiple measurements in either of the last 2 intervals, the measurements closest to day 14 and day 28 were used. Solid lines for combination and standard therapy are the loess-smoothed mean creatinine in each group over time.
Within the combination therapy group, 111 patients received only flucloxacillin or cloxacillin and 27 received only cefazolin. The characteristics of these 2 groups are presented in eTable 8 in Supplement 3. Thirty (27%) of the 111 who received only flucloxacillin (25/90 [28%]) or cloxacillin (5/21 [24%]) developed AKI (using modified RIFLE criteria) compared with 1 (4%) of the 27 who received only cefazolin.
Discussion
In patients with MRSA bacteremia, the addition of 7 days of an antistaphylococcal β-lactam to standard therapy did not statistically significantly reduce the occurrence of a composite of 90-day mortality, microbiological persistence, relapse, or treatment failure. The trial was stopped early because of an excess of AKI in the combination therapy group.
Methicillin-resistant S aureus bacteremia is difficult to treat and associated with high mortality.2 Novel treatment regimens need to balance potential improvements in efficacy with additional toxicities. The promise of efficacy of combination therapy for S aureus bacteremia demonstrated in in vitro and animal models has not been borne out in prospective studies measuring clinically relevant outcomes. In trials spanning 35 years, neither the addition of an aminoglycoside for S aureus endocarditis18 nor of rifampicin for S aureus bacteremia19 resulted in improved clinical outcomes; both agents were associated with increased toxicity. In the current trial, the signal of improved efficacy in the combination treatment group of a reduction in persistent bacteremia was counterbalanced by higher rates of AKI. Given the early termination, the trial may have been underpowered to demonstrate an improvement in the composite primary end point; however, it is likely that any potential gains in efficacy with combination therapy would be offset by the increased toxicity. These clinical trials demonstrating a lack of benefit for combination therapy across a broad range of patients with S aureus bacteremia should give pause to enthusiasm for combination therapy outside of clinical trials.
Duration of bacteremia is often considered a clinically useful surrogate end point for S aureus bacteremia. However, a reduction in duration of bacteremia with either a β-lactam or gentamicin18 has not translated to improved clinical outcomes in prospective trials. Therefore, the limitations of duration of bacteremia as a surrogate end point should be recognized in the design of future studies.
Cefazolin has been associated with less AKI than antistaphylococcal penicillins in retrospective data of patients with MSSA bacteremia20 and also when combined with vancomycin in the post hoc findings from this trial. Combining cefazolin with vancomycin may be less (or even not) nephrotoxic compared with flucloxacillin or cloxacillin. Cefazolin may be an agent that achieves improved efficacy while minimizing toxicity, and further trials of this combination are warranted.
Acute kidney injury is increasingly recognized as a serious complication regardless of the underlying cause. A recent systematic review involving more than 2 million participants found that individuals with AKI were at increased long-term risk for chronic kidney disease, end-stage kidney disease, and death.21 Even within the short follow-up in this study, there were more instances of requiring RRT in the combination group. Longer-term follow-up is planned.
The association of penicillins with AKI, either alone or in combination with other agents, has been observed in retrospective studies. A systematic review including 15 studies concluded that kidney toxicity was greater for vancomycin plus piperacillin-tazobactam than for either agent alone or for vancomycin plus meropenem or cefepime.22 A systematic review of 6 studies including more than 1000 patients with MSSA bacteremia found higher rates of AKI for patients receiving monotherapy with antistaphylococcal penicillins (12%) than with cefazolin (3.4%).20 A key limitation of these studies is their retrospective nature. The present trial showed that combining flucloxacillin or cloxacillin with vancomycin resulted in a higher incidence of nephrotoxicity than vancomycin monotherapy.
Limitations
This study has several limitations. First, the findings may not be generalizable to regions outside of study sites where S aureus strains and the distribution of vancomycin minimum inhibitory concentrations may differ or where resources are more limited, and the findings may not hold true for other antistaphylococcal penicillins, such as nafcillin. However, the biochemical structures, antistaphylococcal activity and adverse effects are comparable among these antistaphylococcal penicillins. At least within the regions in which this trial was conducted, the dominant S aureus genotypes reflected the typical circulating clones.23,24
Second, the results are largely limited to vancomycin plus flucloxacillin or cloxacillin. Few patients were treated with daptomycin or cefazolin. Extrapolation to other antibiotics and β-lactams cannot be made. (Flu)cloxacillin or cefazolin were chosen as the adjunctive agents rather than ceftaroline (which has direct MRSA activity) because these agents are substantially cheaper in the participating countries and thus the trial results would be applicable in lower- and middle-income countries. The vancomycin dosing was based on maintaining trough levels of 15 to 20 μg/mL. Updated draft guidelines recommend using area-under-the-curve (AUC)–guided dosing rather than trough levels because AUC-guided dosing has been associated with a reduction in the risk of nephrotoxicity.25
Third, randomization occurred within 72 hours of index blood culture, and 98% of patients had received antibiotics in the preceding 72 hours, with 61% having received a β-lactam antibiotic. The study was not designed to test empirical therapy prior to identification of MRSA but reflects clinical practice at the point when definitive antibiotic choices are made.
Fourth, the study was open label because blinding of treating clinicians and patients would have been prohibitively expensive. However, the elements of the primary outcome were objective measures and determined by an adjudication committee blinded to treatment allocation.
Fifth, there was a low number of investigator-reported adverse events. Only adverse events thought by site investigators to be attributable to 1 or more study drugs were recorded. Most instances of AKI (indicated in routinely collected serum creatinine concentrations) were not reported as adverse events, perhaps because treating clinicians did not recognize a creatinine increase as an adverse event or did not attribute it to a β-lactam. This underlines the importance of collecting the relevant parameters for potential adverse events of interest as part of the main data collection rather than relying on recording by site investigators.
Conclusions
Among patients with MRSA bacteremia, addition of an antistaphylococcal β-lactam to standard antibiotic therapy with vancomycin or daptomycin did not result in significant improvement in the primary composite end point of mortality, persistent bacteremia, relapse, or treatment failure. Early trial termination for safety concerns and the possibility that the study was underpowered to detect clinically important differences in favor of the intervention should be considered when interpreting the findings.
Trial Protocol
Statistical Analysis Plan
eTable 1. Primary and Secondary Outcomes Adjusted for Baseline Stratification Variables of Study Site and Hemodialysis
eTable 2. Sensitivity Analyses for the Primary Outcomes, Unadjusted and Adjusted for Baseline Stratification Variables of Study Site and Hemodialysis
eTable 3. Secondary Outcomes in the Per Protocol Population, Unadjusted and Adjusted for Baseline Stratification Variables of Study Site and Hemodialysis
eTable 4. Subgroup Analyses for the Primary Outcome
eTable 5. Reported Adverse Events Thought to Be Related to Study Drugs (Vancomycin, Daptomycin or β-Lactam)
eTable 6. Reported Serious Adverse Events in the Combination Therapy Group
eTable 7. Occurrence of Acute Kidney Injury as Defined Using Modified Kidney Disease Improving Global Outcomes (KDIGO) Criteria
eTable 8. Baseline Characteristics of Patients in the Combination Treatment Group Who Received Only Flucloxacillin or Cloxacillin and Who Only Received Cefazolin, Excluding Those on Dialysis at Baseline
eFigure 1. Distribution of Oxacillin Minimum Inhibitory Concentrations (MIC, μg/mL) by Treatment Allocation as Determined by a Central Laboratory Using Broth Microdilution
eFigure 2. Primary and Secondary Outcomes for the Primary Analysis Population, as Percentages of Participants Meeting Those Outcomes by Allocated Treatment Group
eFigure 3. Forest Plot of Absolute Difference in Treatment Effect by Pre-specified Subgroups for the Primary Outcome
Data Sharing Statement
References
- 1.Kourtis AP, Hatfield K, Baggs J, et al. ; Emerging Infections Program MRSA Author Group . Vital signs: epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections—United States. MMWR Morb Mortal Wkly Rep. 2019;68(9):214-219. doi: 10.15585/mmwr.mm6809e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28(3):603-661. doi: 10.1128/CMR.00134-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holland TL, Chambers HF, Boucher HW, et al. . Considerations for clinical trials of Staphylococcus aureus bloodstream infection in adults. Clin Infect Dis. 2019;68(5):865-872. doi: 10.1093/cid/ciy774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu C, Bayer A, Cosgrove SE, et al. ; Infectious Diseases Society of America . Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-e55. doi: 10.1093/cid/ciq146 [DOI] [PubMed] [Google Scholar]
- 5.Davis JS, Van Hal S, Tong SY. Combination antibiotic treatment of serious methicillin-resistant Staphylococcus aureus infections. Semin Respir Crit Care Med. 2015;36(1):3-16. doi: 10.1055/s-0034-1396906 [DOI] [PubMed] [Google Scholar]
- 6.Sakoulas G, Okumura CY, Thienphrapa W, et al. . Nafcillin enhances innate immune-mediated killing of methicillin-resistant Staphylococcus aureus. J Mol Med (Berl). 2014;92(2):139-149. doi: 10.1007/s00109-013-1100-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casapao AM, Jacobs DM, Bowers DR, Beyda ND, Dilworth TJ; REACH-ID Study Group . Early administration of adjuvant β-lactam therapy in combination with vancomycin among patients with methicillin-resistant Staphylococcus aureus bloodstream infection: a retrospective, multicenter analysis. Pharmacotherapy. 2017;37(11):1347-1356. doi: 10.1002/phar.2034 [DOI] [PubMed] [Google Scholar]
- 8.Jorgensen SCJ, Zasowski EJ, Trinh TD, et al. . Daptomycin plus β-lactam combination therapy for methicillin-resistant Staphylococcus aureus bloodstream infections: a retrospective, comparative cohort study [published online August 12, 2019]. Clin Infect Dis. doi: 10.1093/cid/ciz746 [DOI] [PubMed] [Google Scholar]
- 9.Davis JS, Sud A, O’Sullivan MVN, et al. ; Combination Antibiotics for Methicillin Resistant Staphylococcus aureus (CAMERA) Study Group . Combination of vancomycin and β-lactam therapy for methicillin-resistant Staphylococcus aureus bacteremia: a pilot multicenter randomized controlled trial. Clin Infect Dis. 2016;62(2):173-180. doi: 10.1093/cid/civ808 [DOI] [PubMed] [Google Scholar]
- 10.Geriak M, Haddad F, Rizvi K, et al. . Clinical data on daptomycin plus ceftaroline versus standard of care monotherapy in the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2019;63(5):e02483-18. doi: 10.1128/AAC.02483-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tong SY, Nelson J, Paterson DL, et al. ; CAMERA2 Study Group and the Australasian Society for Infectious Diseases Clinical Research Network . CAMERA2—combination antibiotic therapy for methicillin-resistant Staphylococcus aureus infection: study protocol for a randomised controlled trial. Trials. 2016;17(1):170. doi: 10.1186/s13063-016-1295-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antibiotic Expert Group Therapeutic Guidelines: Antibiotic Version 15. Melbourne, Australia: Therapeutic Guidelines Ltd; 2014. [Google Scholar]
- 13.Mehta RL, Kellum JA, Shah SV, et al. ; Acute Kidney Injury Network . Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1-138. [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 16.Chow JW, Yu VL. Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: a commentary. Int J Antimicrob Agents. 1999;11(1):7-12. doi: 10.1016/S0924-8579(98)00060-0 [DOI] [PubMed] [Google Scholar]
- 17.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754-1758. doi: 10.1001/jama.286.14.1754 [DOI] [PubMed] [Google Scholar]
- 18.Korzeniowski O, Sande MA. Combination antimicrobial therapy for Staphylococcus aureus endocarditis in patients addicted to parenteral drugs and in nonaddicts: a prospective study. Ann Intern Med. 1982;97(4):496-503. doi: 10.7326/0003-4819-97-4-496 [DOI] [PubMed] [Google Scholar]
- 19.Thwaites GE, Scarborough M, Szubert A, et al. ; United Kingdom Clinical Infection Research Group (UKCIRG) . Adjunctive Rifampicin for Staphylococcus aureus Bacteraemia (ARREST): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391(10121):668-678. doi: 10.1016/S0140-6736(17)32456-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weis S, Kesselmeier M, Davis JS, et al. . Cefazolin versus anti-staphylococcal penicillins for the treatment of patients with Staphylococcus aureus bacteremia. Clin Microbiol Infect. 2019;25(7):818-827. doi: 10.1016/j.cmi.2019.03.010 [DOI] [PubMed] [Google Scholar]
- 21.See EJ, Jayasinghe K, Glassford N, et al. . Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019;95(1):160-172. doi: 10.1016/j.kint.2018.08.036 [DOI] [PubMed] [Google Scholar]
- 22.Luther MK, Timbrook TT, Caffrey AR, Dosa D, Lodise TP, LaPlante KL. Vancomycin plus piperacillin-tazobactam and acute kidney injury in adults: a systematic review and meta-analysis. Crit Care Med. 2018;46(1):12-20. doi: 10.1097/CCM.0000000000002769 [DOI] [PubMed] [Google Scholar]
- 23.Coombs GW, Daley DA, Lee YT, Pang S; Australian Group on Antimicrobial Resistance . Australian Group on Antimicrobial Resistance (AGAR) Australian Staphylococcus aureus Sepsis Outcome Programme (ASSOP) annual report 2016. Commun Dis Intell (2018). 2018;42:42. [PubMed] [Google Scholar]
- 24.Htun HL, Kyaw WM, de Sessions PF, et al. . Methicillin-resistant Staphylococcus aureus colonisation: epidemiological and molecular characteristics in an acute-care tertiary hospital in Singapore. Epidemiol Infect. 2018;146(14):1785-1792. doi: 10.1017/S0950268818001966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neely MN, Kato L, Youn G, et al. . Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-e02017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eTable 1. Primary and Secondary Outcomes Adjusted for Baseline Stratification Variables of Study Site and Hemodialysis
eTable 2. Sensitivity Analyses for the Primary Outcomes, Unadjusted and Adjusted for Baseline Stratification Variables of Study Site and Hemodialysis
eTable 3. Secondary Outcomes in the Per Protocol Population, Unadjusted and Adjusted for Baseline Stratification Variables of Study Site and Hemodialysis
eTable 4. Subgroup Analyses for the Primary Outcome
eTable 5. Reported Adverse Events Thought to Be Related to Study Drugs (Vancomycin, Daptomycin or β-Lactam)
eTable 6. Reported Serious Adverse Events in the Combination Therapy Group
eTable 7. Occurrence of Acute Kidney Injury as Defined Using Modified Kidney Disease Improving Global Outcomes (KDIGO) Criteria
eTable 8. Baseline Characteristics of Patients in the Combination Treatment Group Who Received Only Flucloxacillin or Cloxacillin and Who Only Received Cefazolin, Excluding Those on Dialysis at Baseline
eFigure 1. Distribution of Oxacillin Minimum Inhibitory Concentrations (MIC, μg/mL) by Treatment Allocation as Determined by a Central Laboratory Using Broth Microdilution
eFigure 2. Primary and Secondary Outcomes for the Primary Analysis Population, as Percentages of Participants Meeting Those Outcomes by Allocated Treatment Group
eFigure 3. Forest Plot of Absolute Difference in Treatment Effect by Pre-specified Subgroups for the Primary Outcome
Data Sharing Statement