This case series describes the venous vasculature in the hyaloid, retinal, and choroidal layers in patients with the EPAS1 gain-of-function mutation syndrome.
Key Points
Question
What is the pathogenesis of ocular abnormalities in patients with EPAS1 gain-of-function mutation syndrome?
Findings
In this case series of 3 patients with EPAS1 gain-of-function mutation syndrome, magnetic resonance imaging scans of the brain and orbit showed increased lateral extent of contrast at the posterior aspect of the globe. Ophthalmoscopy, fluorescein angiography, and optical coherence tomography images revealed disorganized and tortuous vasculature throughout the retina and choroid as well as substantial choroidal thickening, and a transgenic murine model confirmed these findings.
Meaning
Findings from this study suggested an association between gain-of-function mutation in the EPAS1 gene, along with the subsequent stabilization of HIF-2α, and the abnormal development of hypoxia-dependent tissues.
Abstract
Importance
Patients with the EPAS1 gain-of-function mutation syndrome (or Pacak-Zhuang syndrome) present with multiple paragangliomas or pheochromocytomas, duodenal somatostatinoma, polycythemia, headaches, and sometimes diminished visual acuity at an early age. The characteristic phenotype and known genetic cause of the syndrome provide an opportunity to study the role of hypoxia-inducible factor 2α (HIF-2α) in oxygen sensing, development in regions of physiologic hypoxia, and other pathological processes.
Objectives
To describe the ocular lesions in EPAS1 gain-of-function mutation syndrome and to establish whether early-onset diminished visual acuity is developmental or associated with long-term physiologic sequelae of the syndrome.
Design, Setting, and Participants
This clinical case series with a transgenic murine model study was conducted from July 2013 to June 2019. Participants were 3 patients referred by their primary care physicians to the National Institutes of Health for evaluation of recurrent and metastatic paragangliomas or pheochromocytomas accompanied by polycythemia. The syndrome and somatic mosaicism in patients were confirmed by the identification of gain-of-function mutations in the EPAS1 gene in resected tumors and other tissues.
Main Outcomes and Measures
Ocular findings in patients with EPAS1 gain-of-function mutation syndrome.
Results
A total of 3 patients (mean [SD] age, 29 [6.2] years) with confirmed ocular abnormalities were included in the study. Increased contrast accumulation at the posterior aspect of the globe was seen bilaterally on magnetic resonance imaging scans in all patients. Ophthalmoscopy images demonstrated fibrosis overlying the optic disc, tortuous and dilated retinal vessels, and retinal pigment epithelium changes. Optic disc edema and retinal exudates were also seen. Fluorescein angiography images showed leakage of dye from postcapillary venules surrounding the optic disc and highlighted aberrant retinal vascular patterns. Enhanced-depth imaging optical coherence tomography images showed substantial thickening of the choroid and dilation of choroidal vessels. The ocular features of the syndrome were confirmed with a transgenic model of mice with gain-of-function Epas1A529V mutation.
Conclusions and Relevance
In this case series, HIF-2α and hypoxia signaling was found to have a role in vessel development within the choroid and retina, indicating that the marked permanent choroidal thickening and tortuous and dilated veins seen in the choroid and retina in patients with EPAS1 gain-of-function mutation syndrome were suggestive of the persistence of venous elements within the developing mesenchyme. These findings may explain other eye and vascular abnormalities whose pathogenesis remains unclear.
Introduction
The EPAS1 gain-of-function mutation syndrome, or Pacak-Zhuang syndrome, is characterized by the constellation of multiple paragangliomas or pheochromocytomas, duodenal somatostatinoma, and polycythemia caused by postzygotic gain-of-function mutations in the EPAS1 gene (GeneID 2034), encoding for hypoxia-inducible factor 2α (HIF-2α).1,2 Mutations in EPAS1 alter the recognition of the hydroxylation domain of HIF-2α by prolyl hydroxylase domain-2–containing protein.3 This alteration results in the stabilization and reduced degradation of HIF-2α, leading to a pseudohypoxic state marked by the activation of downstream hypoxia-related genes and impaired response to changes in oxygen tension.3,4,5,6,7
The development of ocular structures requires proper coordination of hypoxia signaling.8,9,10,11 During development, the mesenchyme of the choroid condenses, and the endothelium within it organizes into vessels.12 The hyaloid artery, which has lesser caliber and lower systemic pressures, is replaced by the larger central retinal artery. The central retinal artery then goes on to branch into the radial plane, thus increasing oxygen tension. As the veins regress to the periphery, they migrate with the mesenchyme, dive into the choroid, and exit the globe. Subsequently, they form the vortex veins, which drain to the superior ophthalmic vein and the intracranial veins.13 Disruption of hypoxic signaling has been found to interfere with proper development of the retina and retinal vessels.14 This interference suggests that gain-of-function EPAS1 mutations, which lead to a persistent pseudohypoxic state, may have a substantial association with primary vascular and ocular development.
In addition to presenting with endocrine tumors and polycythemia, some patients may show early-onset diminished visual acuity and headaches. The reason for this ocular involvement is poorly understood. The index patient in this case series (patient 1) had a particularly severe condition. Patient 1 was found to have a morning glory anomaly, a developmental anomaly of the choroid, retina, and head of the optic nerve. This finding prompted an ophthalmic investigation into the potential cause or changes associated with the visual acuity deterioration in other patients with the syndrome, with the aim of delineating the primary developmental implications of the syndrome from secondary hemodynamic, long-term visual acuity sequelae. In this study, we developed and evaluated a transgenic murine model with a somatic heterozygous Epas1A529V mutation (corresponding to the human EPAS1A530V mutation). Our goal was to establish the role of HIF-2α in the development of the pathological conditions of the eye observed in the patients.15
Methods
This case series was conducted from July 2013 to June 2019. It was approved by the institutional review board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH). All patients provided written informed consent.
Patient Selection and Imaging
Patients were referred for evaluation by their primary care physician to the NIH. Findings of paraganglioma, pheochromocytoma, polycythemia, and duodenal somatostatinoma were confirmed in these patients (Table). For EPAS1 mutation analysis, we obtained genomic DNA from tumor and circulating leukocyte with a DNA kit (NucleoSpin Tissue Kit; Macherey-Nagel). EPAS1 somatic mutations were confirmed with a digital polymerase chain reaction system (ddPCR; Bio-Rad Laboratories Inc). The original studies by Zhuang et al1 in 2012 and Pacak et al4 in 2013, which first described the syndrome, reported neuroendocrine and hematologic findings in patients with somatic gain-of-function mutations in EPAS1; however, ocular manifestations were undetected. Subsequent reports (Pacak et al17 in 2014 and Därr et al2 in 2016) built on these original clinical findings.
Table. Ocular Anomalies in Patients With EPAS1 Gain-of-Function Mutation Syndrome.
| Variable | Patient | ||
|---|---|---|---|
| 1a,b | 2a,b | 3a | |
| Age at onset of diagnosed condition, y | |||
| Polycythemia | 2 | Birth | Birth |
| PGL/PHEO | 15 | 14 | 18 |
| Ampullary somatostatinoma | 17 | 29 | 22 |
| First evidence of eye disease | 3 | 5 | 18 |
| Mutation analysis: tumors and circulating leukocytes | |||
| EPAS1 gain-of-function mutation | P531S | A530T | A530V |
| EPOR, HIF-1α, JAK2, PHD1/2, SDHB/C/D, VHL | Negative | Negative | Negative |
| Clinical characteristics, Snellen | |||
| Visual acuity at presentation to the NIH | |||
| OD | 20/400 | 20/20 | 20/16 |
| OS | 20/20 | 20/25 | 20/16 |
| Pertinent family history | None | None | None |
| Ophthalmic evaluation | |||
| Age at time of ophthalmic evaluation, y | 15 | 32 | 24 |
| Ophthalmoscopic imaging | |||
| Fibrosis over the optic disc | |||
| OD | Negative | Positive | Positive |
| OS | Positive | Positive | Positive |
| Optic disc edema | |||
| OD | Positive | Negative | Negative |
| OS | Negative | Negative | Negative |
| Retinal vessel tortuosity | |||
| OD | Positive | Positive | Positive |
| OS | Positive | Negative | Positive |
| Dilated veins | |||
| OD | Positive | Positive | Positive |
| OS | Negative | Positive | Positive |
| Morning glory anomaly | |||
| OD | Positive | Negative | Negative |
| OS | Negative | Negative | Negative |
| Retinal exudate | |||
| OD | Positive | Negative | Negative |
| OS | Negative | Negative | Negative |
| RPE changes | |||
| OD | Positive | Negative | Positive |
| OS | Negative | Negative | Positive |
| Fluorescein angiography | |||
| Leakage from veins | |||
| OD | Positive | Positive | NA |
| OS | Positive | Positive | NA |
| Peripheral retinal neovascularization | |||
| OD | Negative | Positive | NA |
| OS | Negative | Positive | NA |
| Hemangiomatous lesions | |||
| OD | Negative | Negative | NA |
| OS | Negative | Positive | NA |
| Optical coherence tomography | |||
| Choroid thickness (reference range: 274 μm16), μm | |||
| OD | 415 | 396 | 504 |
| OS | 307 | 447 | 623 |
Abbreviations: EPOR, erythropoietin receptor; HIF-1α, hypoxia-inducible factor 1a; JAK2, janus kinase 2; NA, not applicable; NIH, National Institutes of Health; OD, right eye; OS, left eye; PGL/PHEO, paraganglioma or pheochromocytoma; PHD1/2, prolyl hydroxylase 1 and 2; RPE, retinal pigment epithelium; SDHB/C/D, succinate dehydrogenase B, C, and D; VHL, von Hippel-Lindau syndrome.
See also Därr et al.2
See also Pacak et al.17
This present series identified novel ocular findings in patients and further elucidated the role of HIF-2α in the development of ocular lesions. Patients were linked to previous studies as follows: patient 1 was equivalent to patient 3 in Pacak et al17 and patient 7 in Därr et al2; patient 2 was equivalent to patient 1 in Zhuang et al,1 patient 1 in Pacak et al,4 patient 2 in Pacak et al,17 and patient 3 in Därr et al2; and patient 3 was equivalent to patient 2 in Zhuang et al,1 patient 3 in Pacak et al,4 and patient 1 in Därr et al.2
Patients underwent magnetic resonance imaging (MRI) of the head and/or orbits with contrast for evaluation of headaches and diminished visual acuity. Ophthalmic evaluation included ophthalmoscopy and fluorescein angiography (FA) to assess the retina and retinal blood vessels. Enhanced-depth imaging optical coherence tomography (EDI-OCT) was used to evaluate the choroid.18,19
Transgenic Murine Model
Animal studies were approved by the Animal Care and Use Committee of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and were conducted in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Visual Research. The Epas1A529V mutation in mice (corresponding to the EPAS1A530V mutation in humans) was established by TALEN (transcription activator-like effector nucleases)-mediated homologous recombination.15 Laboratory and imaging evaluations were performed on 3-month-old mice.
In vivo MRI of the brain and orbits with contrast through tail vein injection was performed on mice to evaluate orbital vascular anomalies using a horizontal bore 9.4-T scanner (Bruker Avance platform; Bruker Biospin Inc). Ophthalmoscopic examinations and photography were performed with a custom-made endoscope (Xenon Nova; Karl Storz SE & Co. KG) attached to a camera (Nikon D90; Nikon Corp). Fluorescein angiography of the retina was performed using a retinal imaging system (Phoenix Micron III; Phoenix Technology Corp). On FA, the arterial, capillary filling, and venous phases were determined qualitatively by following the dye through the capillary bed. The EDI-OCT was performed with an OCT machine (Bioptigen; Leica Microsystems).
Enucleated eyes were fixed in 10% buffered formalin and processed for routine histological analysis at the Histology Core of the National Eye Institute. Hematoxylin-eosin staining (original magnification ×200) was performed on paraffin-embedded sections.
Results
Medical History
The clinical presentations of 3 patients (mean [SD] age, 29 [6.2] years) are summarized in the Table. All patients were found to have EPAS1 gain-of-function mutation syndrome through Sanger sequencing of resected tumors. The same mutations were also found in the circulating leukocytes of 2 of the 3 patients.
Patient 1
A man in his 20s with a medical history of polycythemia diagnosis at age 2 years and right adrenalectomy for pheochromocytoma was referred to the NIH for evaluation of multiple paragangliomas (Table). His ocular history was notable for persistent diminished visual acuity since age 3 years and weekly headaches since childhood. At 15 years of age, the patient underwent an MRI of the head and orbits for diminished visual acuity and headaches, and the MRI scan showed contrast leakage from the posterior aspect of the globe into the vitreous humor in the right eye. An eye examination at the NIH when he was aged 17 years revealed visual acuity of 20/400 OD and 20/20 OS. The right optic nerve had a morning glory disc abnormality with fibrous proliferation and marked disc edema. The macular edema was present with evidence of retinal hard exudate. Fibrous proliferation was found over the nasal aspect of the left optic disc with no evidence of macular edema.
Patient 2
A woman in her 30s with a medical history of polycythemia at birth, bilateral adrenalectomy for pheochromocytomas, and multiple paragangliomas was referred to the NIH for recurrent disease. She was found to have a duodenal ampullary somatostatinoma, which was later resected (Table). The patient first noticed eye problems as a young child owing to exotropia of her left eye. She was referred for ophthalmic evaluation at age 32 years. Her ocular examination, conducted at the NIH, showed that her vision was 20/20 OD and 20/15 OS. She had bilateral fibrous tissue on the optic nerves with evidence of peripheral retinal lesions. There was no macular edema.
Patient 3
A woman in her 20s with a history of polycythemia at birth, right adrenalectomy for pheochromocytoma, multiple paragangliomas, and duodenal ampullary somatostatinoma was referred to the NIH for elevated plasma catecholamines (Table). Her ocular history was notable for persistently red conjunctiva in both eyes since childhood. On ocular examination, her visual acuity was 20/16 in both eyes. She had evidence of fibrotic tissue on both optic nerves. No other ocular lesions were seen.
Ocular Anomalies in Syndromic Patients
Magnetic resonance imaging of the brain and orbit was performed to evaluate headaches and diminished visual acuity and showed bilateral increase in the lateral extent of contrast enhancement along the posterior aspect of the globe in all patients (eFigure 1A-D in the Supplement). All patients had radiographic signs of chronic raised intracranial pressure.20 Serial MRI scans of the brain and orbit of patient 1 at ages 15 years (eFigure 1A in the Supplement), 17 years (eFigure 1B in the Supplement), and 19 years (eFigure 1C in the Supplement) were suspicious for the presence of long-standing raised intracranial pressure as evidenced by tortuosity of the optic nerves and increased optic nerve sheath diameter. In addition, the MRI scan showed leakage of contrast throughout the entire thickness of the posterior globe, signifying the presence of a morning glory anomaly of the right eye. In patient 3, a similar pattern of contrast leakage overlying the optic nerve head was found, possibly suggesting the existence of a subtle variant of a morning glory anomaly not penetrating the retina and therefore not detectable on ophthalmoscopy.
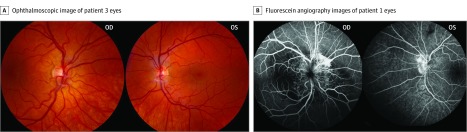
Ophthalmoscopic examination in all patients demonstrated abnormal vascular patterns at the optic nerve head, fibrosis overlying the optic disc, and retinal vascular tortuosity and dilation. Ophthalmoscopic images of the right eye of patient 1 showed retinal pigment epithelium changes and confirmed the morning glory anomaly. In addition, the right eye of patient 1 demonstrated evidence of macular edema emanating from the optic disc, leading to visual acuity loss. A fibrovascular membrane overlying the optic nerve, persistent vasculature over the optic nerve head, and pallor of the optic disc were seen in the left eye. In patient 2, peripheral retinal neovascularization and peripheral hemangiomatous lesions were found. In addition to having these conditions that were common to all patients in this case series, patient 3 also exhibited mild retinal pigment epithelium changes in both eyes (Figure 1A and Table).
Figure 1. Patient Retinal Findings.
A, Ophthalmoscopic image of patient 3′s right eye (left image) demonstrates vessel dilation and tortuosity and pallor of the optic disc. The patient’s left eye (right image) shows fibrosis, dilated and tortuous retinal vessels, pallor of the optic disc, and mild changes to the retinal pigment epithelium. B, Morning glory anomaly shown is specific to patient 1. The postcapillary venous-phase fluorescein angiography images demonstrate substantial leakage of dye in venous distribution. The patient’s left eye demonstrates abnormal vasculature and leakage of dye from vessels surrounding the optic disc.
Time-lapsed FA images revealed aberrant vascular patterns, postcapillary venule leakage of dye over the optic disc and surrounding retina, and vessel dilation and tortuosity in the left eye of patient 1 and bilaterally in patient 2. Abnormal contrast filling was present centrally in the right eye of patient 1, consistent with the known morning glory anomaly (Figure 1B; eFigure 2A in the Supplement). Peripheral neovascularization and leakage of dye in the periphery in patient 2 mimicked the appearance of a retinal hemangioblastoma. Patient 3 did not undergo FA at the NIH.
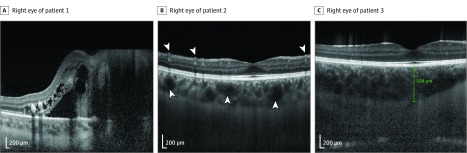
Analysis of OCT images with measurements taken at the fovea demonstrated substantial choroidal thickening and dilation of choroidal vessels in all patients as well as increased numbers of retinal vessels (Figure 2; eFigure 2B in the Supplement). Choroid measurements for each of the patients were as follows: patient 1, 415 μm OD and 307 μm OS; patient 2, 396 μm OD and 447 μm OS; and patient 3, 504 μm OD and 623 μm OS. Normal subfoveal choroidal thickness was 275 μm.
Figure 2. Patient Choroidal Findings.
The enhanced-depth imaging optical coherence tomography (EDI-OCT) images of the right eyes of 3 patients with measurements of choroid thickness taken at the fovea show thickening of the choroid layer and dilation of the choroidal vessels (arrowheads). The EDI-OCT image of patient 1’s right eye (A) demonstrates changes consistent with morning glory anomaly. Increased numbers of retinal vessels are also seen in patient 2’s right eye (arrowheads, B). Choroidal measurements are as follows: OD 415 μm (A), OD 396 μm (B), and OD 504 μm (C).
Confirmation of Ocular Anomalies in the Murine Model
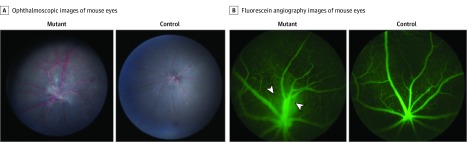
The characteristic ocular features of the syndrome were confirmed in transgenic mice with Epas1A529V mutation.15 In this study, the MRI scan of mouse brain and orbit showed increased contrast accumulation in the posterior aspect of the eye with dilation of the choroidal vessels and vortex veins bilaterally in mutant mice when compared with littermate control mice (n = 4) (eFigure 3A in the Supplement). Evaluation of the ophthalmoscopic images of 3-month-old mutant mice (n = 7) showed dilated and tortuous vessels, fibrosis overlying the optic disc, and abnormal patterning of retinal vasculature when compared with the littermate control mice (Figure 3A; eFigure 3B and eTable in the Supplement). The arterial phase, capillary filling phase, and venous phase images are shown in eFigure 3C in the Supplement.
Figure 3. Transgenic Murine Model Ocular Findings.
A, Ophthalmoscopic images of mutant mouse eye (left) show dilated vessels, vessel tortuosity, fibrosis over the optic disc, and disorganized retinal vasculature. Ophthalmoscopic image of control mouse eye is on the right. B, Fluorescein angiography images of mutant mouse eye (left) 10 minutes after injection highlights aberrant retinal vessel patterning and demonstrates persistence of hyaloid vessels (arrowheads) not found in the littermate control mouse eye (right).
Evaluation of the FA images of 3-month-old mice (n = 7) found dilated and tortuous vessels and evidence of venous persistence of varying severity. In a mouse with less severe changes (mutant 3), the venous plexus was found further out toward the periphery with plexiform arteriovenous shunting present (Video). A mouse with more severe changes (mutant 2), in contrast, had veins throughout the field coalescing at the optic nerve, as evidenced by postcapillary venule leakage over the optic disc (eTable in the Supplement). In addition, FA demonstrated evidence of persistence of hyaloid vessels 10 minutes after fluorescein injection (Figure 3B; eFigure 3E in the Supplement).
Video. Evidence of Arteriovenous Shunt in Epas1A529V Mutant Mouse.
Time-lapse fluorescein angiography demonstrates an arteriovenous shunt (arrow) in a mutant mouse. No shunts were found in control mice.
Evaluation of the EDI-OCT images of 3-month-old mice (n = 7) revealed thickening of the choroid and demonstrated dilated vessels within the choroid in the mutant mice when compared with littermate control mice, recapitulating the finding in the syndromic patients (eFigure 3F in the Supplement). Repeated ophthalmic evaluation of mutant mice 3 months after initial imaging showed no progression of eye disease (eFigure 4 in the Supplement). Histologic analysis of 6-month-old mutant mouse eyes demonstrated dilated, congested vessels in the retina, iris, and ciliary body. A neovascular membrane and persistent hyaloid vasculature were also seen (eFigure 5 in the Supplement). These findings were not seen in littermate control mice.
Discussion
The EPAS1 gain-of-function mutation syndrome was initially characterized by paraganglioma or pheochromocytoma, polycythemia, and somatostatinoma. One patient also presented with diminished visual acuity. Previous studies reported on ophthalmic findings of optic disc fibrosis and neovascularization, retinal hard exudate, and peripheral retinal neovascularization in patient 2 and attributed these findings to dysregulation of hypoxia signaling and subsequent increase in expression of hypoxia-related genes such as EPO (GeneID 2056), EDN1 (GeneID 1906), GLUT1 (Gene ID 6513), and VEGFA (GeneID 7422).2,17 In the present study, we identified developmental anomalies of venous vasculature in the hyaloid, retinal, and choroidal layers and proposed a mechanism by which these venous structures persist.
Ophthalmic evaluation of the patients revealed primary ocular pathological processes, including substantial thickening of the choroidal layer and dilation and tortuosity of retinal and choroidal veins in all patients. Persistence of the fibrovascular membrane in all patients and morning glory anomaly in the eye of 1 patient suggested a failure of the mesenchyme to respond to neural tube induction. Anatomically, this response corresponds to the response of the choroid to developing optic nerve. The patients had minimal changes in eye condition and visual acuity on subsequent clinical examinations, suggesting the discontinuation of the ongoing, progressive process leading to the eye findings. Classically, retinal and choroidal development has been attributed to the proper coordination of the lens placode, which arises from neuroectoderm and induces the formation of retinal and optic nerve vasculature from the mesenchyme beneath it.9,10 Our finding of a primarily venous persistence and abnormal vasculature within the choroid, hyaloid membrane, and retina suggests that the inductive potential of the lens placode is not the only factor in ocular development.
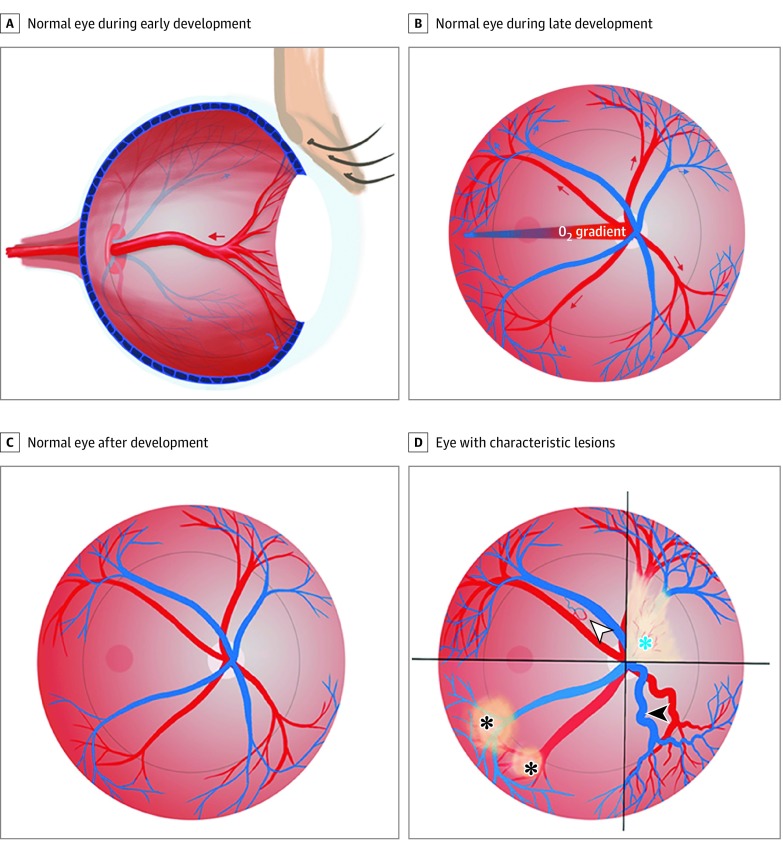
Connolly et al11 showed that the retinal and hyaloid vascular system begins as a polygonal network of capillary-like vessels that develop into arteries and veins from the remodeling of this primordial network. The distance by which the capillary bed extends from the center of the retina is dependent on a hypoxic gradient in development.11,21 Normally, as the arterial field penetrates centrally and advances to meet the hypoxic field in the periphery, the increasing oxygen tension leads to the regression of the capillary bed and veins to the periphery (Figure 4B).11 We believe that the ocular venous abnormalities and abnormal vascular patterning in patients with the EPAS1 gain-of-function mutation syndrome—findings that were also demonstrated in the corresponding murine model—are associated with aberrant hypoxia signaling and loss of response to the developing arterial field.
Figure 4. Schematic Illustrations.
A, The hyaloid artery supplying the lens is shown in a normal eye during development. As development continues, the hyaloid artery regresses (red arrow). B, A pattern of vessel growth is shown in a normal eye during development. As the arteries grow out from the optic disc and branch in the radial plane (red arrows), the increasing oxygen tension and decreased hypoxia signaling, mediated by hypoxia-inducible factor 2α (HIF-2α), causes the plexiform venous bed to regress to the periphery (blue arrows). C, When development is complete, the normal retinal vessel pattern is characterized by vessels that have emerged from the optic nerve head and spread, in a radial fashion, to meet the ora serrata in the periphery. D, The 4 characteristic lesions associated with a common HIF-2α gain-of-function-mediated disturbance of development are shown: fibrovascular membrane overlying the optic disc (blue asterisk), dilated and tortuous retinal vessels (white arrowhead), retinal pigment epithelium changes (black asterisks), and arteriovenous shunt (black arrowhead).
In the EPAS1 gain-of-function mutation syndrome, oxygen sensing and the hypoxic gradient are perturbed by a persistent pseudohypoxic state. This state is associated with the failure of the venous field to regress from the center to the periphery, persistence of nascent venous elements, and shorter distance by which the artery can grow to meet a vein. Accompanying this situation is the failure of regression of the hyaloid vessels in the perpendicular axis, which may account for the presence of a fibrovascular membrane in all patients (Figure 4). EPAS1 deletion has been shown to cause increased regression of these vessels to the periphery, resulting in increased retinal arterial density and abnormal patterning of retinal vasculature.11,14 Thus, constitutive activation of HIF-2α and the subsequent pseudohypoxic state appear to be associated with the failure of plexiform venous connections to regress within the migrating mesenchyme of the choroid. The outcome of this failure is the aberrant venous configurations at varying distances from the midpoint of the radial axis of the retina, the development of which is dependent on a hypoxic gradient.22
The transgenic murine model we used provided the opportunity to evaluate the association between the gene mutation and the ocular anomalies and to establish whether the aberrant vessel configuration observed was associated with venous field persistence. Given that the ocular vasculature in mice paralleled the vascular pattern seen in humans throughout development, the mouse system appears to be suitable for modeling normal and pathological vascular processes in humans.11 The murine model recapitulated the findings of aberrant retinal vasculature, increased choroidal thickness, and persistent hyaloid membrane and vessels seen in the patients. This finding raised the question of whether the fibrosis present in the patients was secondary to postcapillary leakage, as previously reported,17 or the result of a persistent fibrovascular membrane. Further emulating the human syndrome, postcapillary venule leakage was seen on FA images of the mice as well. The contrast leakage occurred at different distances from the physiologic cup in different mutants, suggesting additional levels of regulation for severity of the syndrome.
Findings of anomalies in choroid and retinal vasculature in patients with the syndrome and in transgenic mice implicate HIF-2α as a critical component of the development of the choroid and retinal vasculature. Traumatic and hemodynamic pachychoroidopathies associated with impaired venous drainage and vortex system have been described in a previous study.16 To our knowledge, however, the present case series is the first to describe choroidal thickening associated with a developmental persistence of an enlarged venous system. Although some of the ophthalmic findings may be secondary sequelae of the syndrome, this study provided evidence that the choroidal abnormalities, abnormal vascular patterns, and optic nerve head pathological conditions are developmental in origin. Furthermore, when the mutant mice were reevaluated at later points, their eye findings were found to be unchanged, suggesting that the observed ocular abnormalities were largely developmental (eFigure 4 in the Supplement).
Patients with conditions suggestive of the syndrome should be screened with EDI-OCT for choroidal abnormalities and given a retinal examination with ophthalmoscopy and FA. Conversely, long-standing choroidal thickening and aberrant vascular patterning of the retina should raise clinical suspicion for this rare syndrome. The ocular findings in this syndrome, related to aberrant hypoxic signaling in development, may provide insight into other vascular anomalies of the eye and mesenchyme-derived tissue.
Limitations
A limitation of this study is that it was based on a small series of 3 patients with relatively short follow-up time. This small size may limit the ability to draw definitive conclusions regarding the spectrum of clinical phenotypes. The murine model can help future investigations into the mechanism of EPAS1 in vascular development.
Conclusions
This case series further characterized the EPAS1 gain-of-function mutation syndrome. It also described the development of vascular phenomena that was supported by a transgenic murine model. Findings of this study may provide insight into other vascular anomalies of the eye.
eTable. Ophthalmic Features of Three-Month-Old Epas1A529V Mutant Mice
eFigure 1. Patient Orbital and Intracranial MRI
eFigure 2. Additional Patient Ocular Findings
eFigure 3. Additional Epas1A529V Transgenic Mouse Model Ocular Findings
eFigure 4. Repeat Fundoscopic Evaluation of Epas1A529V Mutant Mice
eFigure 5. Histologic Analysis of Epas1A529V Mutant Mice
References
- 1.Zhuang Z, Yang C, Lorenzo F, et al. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N Engl J Med. 2012;367(10):922-930. doi: 10.1056/NEJMoa1205119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Därr R, Nambuba J, Del Rivero J, et al. Novel insights into the polycythemia-paraganglioma-somatostatinoma syndrome. Endocr Relat Cancer. 2016;23(12):899-908. doi: 10.1530/ERC-16-0231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang C, Sun MG, Matro J, et al. Novel HIF2A mutations disrupt oxygen sensing, leading to polycythemia, paragangliomas, and somatostatinomas. Blood. 2013;121(13):2563-2566. doi: 10.1182/blood-2012-10-460972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pacak K, Jochmanova I, Prodanov T, et al. New syndrome of paraganglioma and somatostatinoma associated with polycythemia. J Clin Oncol. 2013;31(13):1690-1698. doi: 10.1200/JCO.2012.47.1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5(5):343-354. doi: 10.1038/nrm1366 [DOI] [PubMed] [Google Scholar]
- 6.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399-408. doi: 10.1016/j.cell.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30(4):393-402. doi: 10.1016/j.molcel.2008.04.009 [DOI] [PubMed] [Google Scholar]
- 8.Dunwoodie SL. The role of hypoxia in development of the Mammalian embryo. Dev Cell. 2009;17(6):755-773. doi: 10.1016/j.devcel.2009.11.008 [DOI] [PubMed] [Google Scholar]
- 9.Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255-296. doi: 10.1146/annurev.cellbio.17.1.255 [DOI] [PubMed] [Google Scholar]
- 10.Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29(2):144-168. doi: 10.1016/j.preteyeres.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connolly SE, Hores TA, Smith LE, D’Amore PA. Characterization of vascular development in the mouse retina. Microvasc Res. 1988;36(3):275-290. doi: 10.1016/0026-2862(88)90028-3 [DOI] [PubMed] [Google Scholar]
- 12.Thompson H, Griffiths JS, Jeffery G, McGonnell IM. The retinal pigment epithelium of the eye regulates the development of scleral cartilage. Dev Biol. 2010;347(1):40-52. doi: 10.1016/j.ydbio.2010.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiel JW. The Ocular Circulation. San Rafael, CA: Morgan & Claypool Life Sciences; 2010. [PubMed] [Google Scholar]
- 14.Ding K, Scortegagna M, Seaman R, Birch DG, Garcia JA. Retinal disease in mice lacking hypoxia-inducible transcription factor-2alpha. Invest Ophthalmol Vis Sci. 2005;46(3):1010-1016. doi: 10.1167/iovs.04-0788 [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Cui J, Yang C, et al. A transgenic mouse model of Pacak-Zhuang syndrome with an Epas1 gain-of-function mutation. Cancers (Basel). 2019;11(5):667. doi: 10.3390/cancers11050667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012;31(5):377-406. doi: 10.1016/j.preteyeres.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pacak K, Chew EY, Pappo AS, et al. Ocular manifestations of hypoxia-inducible factor-2α paraganglioma-somatostatinoma-polycythemia syndrome. Ophthalmology. 2014;121(11):2291-2293. doi: 10.1016/j.ophtha.2014.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong IY, Koizumi H, Lai WW. Enhanced depth imaging optical coherence tomography. Ophthalmic Surg Lasers Imaging. 2011;42(suppl):S75-S84. doi: 10.3928/15428877-20110627-07 [DOI] [PubMed] [Google Scholar]
- 19.Koay CL, Quo MJ, Subrayan V. Reproducibility of choroidal thickness measurements in subjects on 3 spectral domain optical coherence tomography machines. Int Ophthalmol. 2017;37(3):655-671. doi: 10.1007/s10792-016-0306-4 [DOI] [PubMed] [Google Scholar]
- 20.Mollan SP, Davies B, Silver NC, et al. Idiopathic intracranial hypertension: consensus guidelines on management. J Neurol Neurosurg Psychiatry. 2018;89(10):1088-1100. doi: 10.1136/jnnp-2017-317440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutty GA, McLeod DS. Development of the hyaloid, choroidal and retinal vasculatures in the fetal human eye. Prog Retin Eye Res. 2018;62:58-76. doi: 10.1016/j.preteyeres.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashton N. Oxygen and the growth and development of retinal vessels: In vivo and in vitro studies: the XX Francis I. Proctor Lecture. Am J Ophthalmol. 1966;62(3):412-435. doi: 10.1016/0002-9394(66)91322-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Ophthalmic Features of Three-Month-Old Epas1A529V Mutant Mice
eFigure 1. Patient Orbital and Intracranial MRI
eFigure 2. Additional Patient Ocular Findings
eFigure 3. Additional Epas1A529V Transgenic Mouse Model Ocular Findings
eFigure 4. Repeat Fundoscopic Evaluation of Epas1A529V Mutant Mice
eFigure 5. Histologic Analysis of Epas1A529V Mutant Mice