Key Points
Question
Is a 7-fold increased intake of vitamin D in pregnancy related to improved offspring bone health compared with standard dose?
Findings
In this prespecified analysis of a large-scale double-blinded randomized clinical trial, a high dose vs standard dose of vitamin D supplementation from pregnancy week 24 to 1 week post partum resulted in an overall higher bone mineralization status in the offspring during the first 6 years of life.
Meaning
This trial suggests that an increased vitamin D intake during pregnancy has the potential to promote greater bone health in the offspring, which could have a protective effect on risk of fractures.
Abstract
Importance
Studies suggest an association between maternal vitamin D status during pregnancy and offspring anthropometry and bone mineralization, but investigations are few and with mixed results.
Objective
To investigate the effect of a high dose vs standard dose of vitamin D supplementation in pregnancy on anthropometric and bone outcomes until age 6 years in the offspring.
Design, Setting, and Participants
A prespecified analysis of a double-blinded, randomized clinical trial in the Copenhagen Prospective Studies on Asthma in Childhood 2010 mother-child cohort that included 623 pregnant mothers and their 584 children. Data were analyzed between January 2019 and September 2019.
Interventions
Vitamin D supplementation of 2800 IU/d (high-dose) vs 400 IU/d (standard-dose) from pregnancy week 24 until 1 week after birth.
Main Outcomes and Measures
Longitudinal anthropometry assessments including length/height, weight, and body mass index until age 6 years and bone mineral content (BMC) and bone mineral density (BMD) at age 3 years and 6 years from dual-energy radiography absorptiometry scans.
Results
At age 6 years, 517 children (89%) completed the clinical follow-up. All participants were Danish and white; 261 were boys and 256 were girls. A mixed-effects model analysis of dual-energy radiography absorptiometry scan outcomes from ages 3 years and 6 years showed that children in the vitamin D vs placebo group had higher whole-body BMC: mean difference adjusted (aMD) for age, sex, height, and weight was 11.5 g (95% CI, 2.3-20.7; P = .01); higher whole-body-less-head BMC aMD was 7.5 g (95% CI, 1.5-13.5; P = .01); and higher head BMD aMD was 0.023 g/cm2 (95% CI, 0.003-0.004; P = .03). The largest effect was in children from vitamin D–insufficient mothers (<30 ng/mL; to convert to nanomoles per liter, multiply by 2.496) and among winter births. In a post hoc analysis, we found borderline lower incidence of fractures in the vitamin D group (n = 23 vs n = 36; incidence rate ratio, 0.62 [95% CI, 0.37-1.05]; P = .08), but no differences in any anthropometric outcomes. Adjustment for a concomitant ω-3 polyunsaturated fatty acids intervention did not change the results.
Conclusions and Relevance
High-dose vitamin D supplementation in pregnancy improved offspring bone mineralization through age 6 years compared with the standard dose, suggesting an increased recommended gestational intake, which may influence peak bone mass, fracture risk, and risk of osteoporosis later in life. We found no supplementation effect on anthropometric outcomes.
Trial Registration
ClinicalTrials.gov Identifier: NCT00856947
This prespecified secondary analysis of a randomized clinical trial investigates the effect of a high dose vs standard dose of vitamin D supplementation in pregnancy on anthropometric and bone outcomes until age 6 years in the offspring.
Introduction
Vitamin D deficiency has increased in the last few decades owing to changing lifestyles and dietary habits.1 Vitamin D is an essential hormone for bone mineralization2 that begins in utero and continues throughout childhood until early adulthood, when peak bone mass is reached, which is an important determinant for osteoporosis later in life.3,4 A decreased bone mineral status has been associated with risk of fractures already in childhood.5 Furthermore, severe vitamin D deficiency in childhood can lead to craniotabes and rickets,6 but studies examining the effect of maternal gestational vitamin D status on offspring anthropometrics and bone mineralization have shown contradictory results.
For anthropometry, observational studies have shown lower birth length and increased risk of small-for-gestational-age (SGA) birth in children born to mothers with vitamin D deficiency,7,8 while others did not confirm this.9,10 Two randomized clinical trials (RCTs) of prenatal vitamin D supplementation showed no effect on birth anthropometrics but reported accelerated growth until age one year in 134 and 117 children, respectively.11,12 In contrast, another larger RCT (n = 1164) from Bangladesh13 found no effect on infant growth at age 1 year. A 2018 meta-analysis14 of available RCTs reported reduced risk of SGA and greater length at age 1 year in the vitamin D group; however, the study from Bangladesh was not published when the meta-analysis was conducted and neither of the included RCTs evaluated the effect on anthropometrics later in childhood.
For bone mineralization, observational studies have shown association between maternal gestational vitamin D status and offspring bone mineral content (BMC) at age 9 years10 and peak bone mass at age 20 years,15 whereas others did not confirm such an association.16 To our knowledge, causality of these observations has only been tested in 1 large-scale RCT (n = 737), showing that supplementation with 1000 IU/d of vitamin D vs placebo in pregnancy did not affect offspring neonatal whole-body BMC.17 The dose used in this study may be considered relatively low.
We conducted a double-blinded RCT investigating the effect of a high dose of 2800 IU/d vs a standard dose of 400 IU/d vitamin D supplementation during pregnancy on offspring health outcomes in the Copenhagen Prospective Studies on Asthma in Childhood 2010 (COPSAC2010) cohort.18 In this report, we examined the effect on bone mineralization assessed by dual-energy radiograph absorptiometry (DXA) scans at age 3 and 6 years and the effect on birth weight and childhood growth until age 6 years, which were prespecified secondary outcomes.
Methods
Study Population
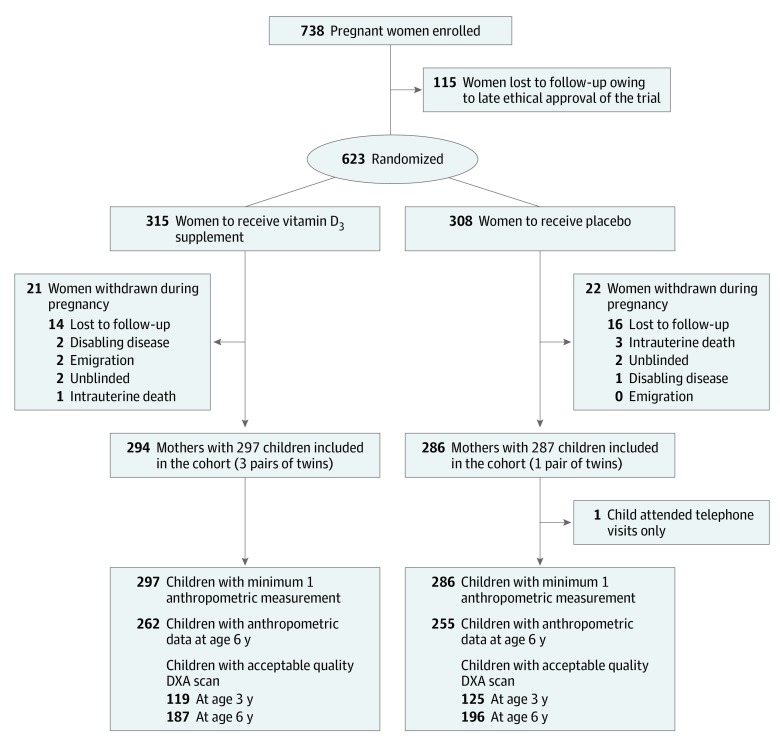
Participants were part of the population-based COPSAC2010 clinical mother-child cohort study including 738 pregnant women at 24 weeks of gestation and following up their 700 children with deep clinical phenotyping through childhood. Of the 738 recruited women, 623 participated in the vitamin D3 trial. The reduced number was owing to delayed ethical approval of the vitamin D study protocol. The cohort, baseline characteristics, and the enrollment process have previously been described in detail.18,19,20 The trial protocol and statistical analysis plan for phase 1 (0-3 years) and phase 2 (3-6 years) are available in Supplement 1, and CONSORT guidelines were followed.
The Vitamin D RCT
The pregnant women were randomly assigned (1:1) in a double-blinded manner to receive a daily dose of 2400 IU vitamin D3 (cholecalciferol) or matching placebo capsules from 24 weeks’ gestation until 1 week post partum. All women were encouraged to continue a daily intake of 400 IU of vitamin D3 as advised by the Danish Health Authority, making the study a dose comparison of 2800 IU/d (7-fold dose) vs 400 IU/d (standard dose). Serum 25-hydroxyvitamin D (25[OH]D) levels were measured at trial entry in week 24 and 1 week post partum (eMethods in Supplement 2) and counting returned capsules complemented adherence to intervention. Additionally, participants were enrolled in a 2 × 2 factorial designed trial of 2.4 g/d of long-chain ω-3 polyunsaturated fatty acids (LCPUFA).18 The vitamin D trial was registered on ClinicalTrials.gov and approved by the Danish Data Protection Agency and the local ethics committee. Both parents gave written informed consent before enrollment. The predefined primary study outcome was asthma/persistent wheeze in the first 3 years of life.18,20 Bone mineralization assessment with DXA scans and longitudinal anthropometric measurements were prespecified registered secondary outcomes.
Birth Anthropometrics
Information on birth weight and length were obtained from the parents and validated against the Danish Medical Birth Registry.21 The standardized fetal ultrasonography-based growth curves from Marsál et al22,23 were used to calculate each child’s birth weight as a percentile of their expected birth weight given their gestational age (GA) as a measure for size for GA. Prevalence of SGA was defined as being less than 2 SD on the fetal growth curves.22
Childhood Growth Assessments at Age 0 to 6 Years
Anthropometric measurements were obtained at every visit to the COPSAC research unit corresponding to age 1 week, 1 month, 3 months, 6 months, half-yearly until age 3 years, and thereafter yearly until age 6 years (eMethods in Supplement 2). All measurements were done 3 times using the mean values for analyses. z Scores for body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), height, and weight were calculated from the World Health Organization reference charts from age 1 week to 6 years and thereby adjusted for sex and age.24
DXA Scans at Age 3 and 6 Years
Whole-body DXA scans were performed with Lunar iDXA densitometer (GE Healthcare) with Encore analysis software with children lying on their back and were performed from head to toes in 1 movement lasting approximately 3 minutes. Thereafter, an experienced specialist examined all scan data and validated the quality of each image. The DXA scan provided measures of bone, lean soft tissue mass, and fat mass of the whole body including the head region.
Fractures
History of fractures was obtained through interviews with parents at the clinic visits and medical record checks until January 31, 2019. Fractures were defined by radiologically verified fractures of the larger long bones (clavicle, radius, ulna, tibia, fibula, femur, and humerus), excluding fissures (ie, minor cracks). Risk of fractures was added as a post hoc analysis.
Statistical Analysis
The effects of high-dose vitamin D supplementation on birth weight, size for gestational age, and z scores of BMI, height, and weight at age 6 years (±6 months) were analyzed using t test. The effects on the longitudinal assessments of z-scored length/height, weight, and BMI and waist, thorax circumference, and head circumference from age 1 week to 6 years (up to 12 assessments per child) were analyzed in a random intercept mixed-effects model. Nonnormalized data were adjusted for age and sex.
The DXA scan data on BMC, lean soft tissue mass, and fat mass at age 3 and 6 years were analyzed using a multivariable linear regression model adjusted for age, sex, height, and weight.25 The effect of the vitamin D intervention on BMC and bone mineral density (BMD) outcomes combined for both ages was analyzed in a random intercept mixed-effects model.
The effects on childhood growth and bone mineralization were also analyzed stratified by maternal presupplementation 25(OH)D levels (insufficient [<30 ng/mL; to convert to nanomoles per liter, multiply by 2.496] vs sufficient ≥30 ng/mL]), season of birth (winter [December to February], spring [March to May], summer [June to August], and fall [September to November]), and as low season (November 8 to May 7) vs high season (May 8 to November 7) based on 25(OH)D levels.
The effect on the post hoc–defined end point of fractures was analyzed by a Poisson regression model accounting for repeated measurements. Statistical analyses were performed with R, version 3.4.1 (the R Foundation), with a 2-sided P value less than .05 considered indicative of significance. The trial was powered for persistent wheeze as primary outcome. No imputation was performed for missing data.
Results
We randomized 623 pregnant women from March 4, 2009, to November 17, 2010, living in Zealand, Denmark (55.4633° N; 11.7215° E); 43 women were withdrawn before childbirth, leaving 584 children, including 4 pairs of twins, eligible for analysis (Figure 1). There were no differences in baseline characteristics between the groups (eTable 1 in Supplement 2). The intervention resulted in increased maternal 25(OH)D level 1 week post partum (vitamin D vs placebo: mean [SD], 42.59 [14.30] ng/mL vs 29.29 [12.66] ng/mL; mean difference [MD], 13.30 ng/mL [95% CI, 11.10-15.5]; P < .001).
Figure 1. CONSORT Flowchart.
Birth Anthropometrics
There were no significant differences between the high-dose vitamin D and placebo group in GA, size for GA, prevalence of SGA cases, birth weight, or birth length (eTable 1 in Supplement 2).
Childhood Anthropometrics Through Age 6 Years
At age 6 years, 517 of 584 children (89%) had available anthropometric data: 262 in the vitamin D and 255 in the placebo group. There were no significant differences among children in the 2 groups regarding BMI, height, weight, waist, head circumference, and thorax circumference at age 6 years (Table 1) and no differences in a mixed-effects model of the longitudinal measurements from age 1 week to 6 years (eTable 2 in Supplement 2). Separate analyses of each point showed no differences between the groups on any of the anthropometric outcomes through age 6 years except for a marginally decreased z score BMI in the high-dose vitamin D group at age 3 years (MD, −0.16; P = .02) (eFigure 1 in Supplement 2).
Table 1. Anthropometric Outcomes at 6 Years of Age.
| Measurement | Mean (SD) | Estimate, Mean Difference (95% CI) | P Value | |
|---|---|---|---|---|
| Vitamin D (n = 262) | Placebo (n = 255) | |||
| z Scorea | ||||
| BMI | −0.03 (0.9) | 0.06 (0.8) | −0.10 (−0.24 to 0.05) | .19 |
| Height | 0.49 (0.9) | 0.53 (0.99) | −0.04 (−0.21 to 0.13) | .64 |
| Weight | 0.29 (0.90) | 0.37 (0.90) | −0.08 (−0.24 to 0.07) | .30 |
| Waist, cmb | 54.9 (3.87) | 55.2 (3.48) | −0.34 (−0.98 to 0.31) | .30 |
| Head, cmb | 52.1 (1.43) | 52 (1.45) | 0.06 (−0.19 to 0.30) | .65 |
| Thorax circumference, cmb | 56.21 (2.98) | 56.47 (3.07) | −0.36 (−0.87 to 0.15) | .17 |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
World Health Organization age- and sex-specific z scores for BMI, height and weight.
Adjusted for age and sex.
There was no evidence of interaction between child sex, mother’s preintervention 25(OH)D level, season of birth, or the concomitant ω-3 LCPUFA RCT and the vitamin D supplementation on any of the anthropometrics outcomes (all interaction P values >.05), and adjusting the models for these covariates did not change our findings (eTable 2 in Supplement 2).
DXA Scans at Age 3 Years
At age 3 years, 244 of 584 children (42%) had technically approved DXA scans, including a scan of the head (n = 218), total body less head (TBLH; n = 199), or total body (n = 177) after excluding scans owing to movement artifacts (Figure 1). There were no differences in baseline characteristics or intervention randomization among children with vs without available DXA data except for a higher rate of maternal smoking during pregnancy (n = 18 [5%] vs n = 5 [2%]; P = .05) (eTable 3 in Supplement 2).
Children in the vitamin D vs placebo group had significantly higher TBLH BMC (mean [SD] 293.8 [44.3] g vs 288.8 [41.7] g, mean difference adjusted [aMD] for age, sex, height, and weight: 6.1 g [95% CI, 0.1-12; P = .05]) and total body BMC (526.2 [60.3] g vs 513.5 [58.9] g; aMD: 9.9 g [95% CI, 0.3-19.6; P = .04]) (Table 2). The largest difference was seen among children born to mothers with insufficient vs sufficient preintervention 25(OH)D levels (total BMC, 537.5 [38.5] g vs 513.6 [66.2] g; aMD, 14.0 g [95% CI, 1.3-26.8; P = .03]) (eTable 4 in Supplement 2), although there was no statistically significant interaction with maternal preintervention 25(OH)D levels. There were no significant differences for head BMC; total body, head, or TBLH BMD; lean soft tissue; or fat mass (Table 2); however, head BMD values were higher in the vitamin D group among mothers with insufficient preintervention 25(OH)D level (eTable 4 in Supplement 2).
Table 2. DXA Scan Results at Age 3 and 6 Years and Longitudinal Combined Mixed-Effects Model Analyses in the High-Dose Vitamin D vs Placebo Groups.
| Measurement | Age | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 y | 6 y | 3 and 6 y Combined | ||||||||||||
| Vitamin D | Placebo | Adjusted Estimate (95% CI)a | P Value | Vitamin D | Placebo | Adjusted Estimate (95% CI)a | P Value | Adjusted Estimate (95% CI)a | P Value | |||||
| No. | Mean (SD) | No. | Mean (SD) | No. | Mean (SD) | No. | Mean (SD) | |||||||
| TBLH | ||||||||||||||
| BMD, g/cm2 | 94 | 0.44 (0.03) | 105 | 0.44 (0.03) | 0.005 (−0.002 to 0.012) | .14 | 187 | 0.56 (0.05) | 196 | 0.55 (0.04) | 0.005 (−0.002 to 0.011) | .15 | 0.005 (−0.001 to 0.005) | .11 |
| BMC, g | 94 | 293.8 (44.3) | 105 | 288.8 (41.7) | 6.1 (0.1 to 12.1) | .05 | 187 | 532.3 (86.6) | 196 | 523.9 (82.4) | 7.8 (0.6 to 15.1) | .03 | 7.5 (1.5 to 13.5) | .01 |
| Head | ||||||||||||||
| BMD, g/cm2 | 105 | 1.16 (0.1) | 113 | 1.14 (0.1) | 0.012 (−0.012 to 0.035) | .34 | 187 | 1.43 (0.13) | 196 | 1.40 (0.11) | 0.033 (0.010 to 0.057) | .01 | 0.023 (0.003 to 0.042) | .03 |
| BMC, g | 105 | 227.9 (25) | 113 | 225.4 (24.5) | −0.6 (−5.9 to 4.7) | .82 | 187 | 300.1 (34.2) | 196 | 293.8 (30.2) | 6.1 (0.4 to 11.7) | .03 | 3.0 (−1.8 to 7.9) | .22 |
| Total | ||||||||||||||
| BMD, g/cm2 | 82 | 0.61 (0.04) | 95 | 0.60 (0.04) | 0.007 (−0.003 to 0.017) | .16 | 187 | 0.72 (0.05) | 196 | 0.71 (0.05) | 0.009 (0.001 to 0.017) | .04 | 0.007 (−0.001 to 0.014) | .08 |
| BMC, g | 82 | 526.2 (60.3) | 95 | 513.5 (58.9) | 9.9 (0.3 to 19.6) | .04 | 187 | 833.2 (109.6) | 196 | 817.8 (101) | 13.9 (3.2 to 24.7) | .01 | 11.5 (2.3 to 20.7) | .01 |
| Lean mass, g | 82 | 10 816 (1125) | 95 | 10 712 (1285) | 76.7 (−177.3 to 330.8)b | .55 | 187 | 16 033 (1951) | 196 | 15 923 (2075) | −10.7 (−228.3 to 206.8)b | .92 | −6.4 (−198.6 to 185.2)b | .95 |
| Fat mass, g | 82 | 4276 (814) | 95 | 4316 (743) | 71.2 (−138.2 to 280.6)b | .50 | 187 | 5174 (1454) | 196 | 5349 (1413) | −105.2 (−349.4 to 139.1)b | .40 | −38.9 (−243.7 to 165.9)b | .71 |
Abbreviations: BMC, bone mineral content; BMD, bone mineral density; DXA, dual-energy radiograph absorptiometry; TBLH, total body less head.
Adjusted for age, sex, height, and weight.
Adjusted for age, sex, height, and height.2
DXA Scans at Age 6 Years
At age 6 years, 383 of 584 children (66%) had a technically approved DXA scan of the total body including the head (Figure 1). Children without available DXA data were taller at age 6 years (MD, 0.97 cm [95% CI, 0.02-1.9; P = .03]), but there were no other differences between children with vs without available DXA scan (eTable 3 in Supplement 2).
Children in the vitamin D vs placebo group had significantly higher TBLH BMC (aMD, 7.8 g; 95% CI, 0.6-15.1; P = .03); higher head BMD (0.033 g/cm2; 95% CI, 0.010-0.057; P = .01); higher head BMC (6.1 g; 95% CI, 0.4-11.7; P = .03); higher total BMD (0.009 g/cm2; 95% CI, 0.001-0.017; P = .04); and higher total BMC (13.9 g; 95% CI, 3.2-24.7; P = .01) (Table 2). The effect was most pronounced among children born to mothers with insufficient preintervention 25(OH)D levels (total BMC, 834.0 g vs 817.3 g [aMD, 17.9 g; 95% CI, 2.6-33.1; P = .02] and total BMD, aMD, 0.0125 g/cm2 [95% CI, 0.001-0.024; P = .04]) (eTable 4 in Supplement 2), but there was no significant interaction (P interaction for total BMC = .94 and P interaction for total BMD = .82). There were no significant differences in TBLH BMD, lean soft tissue, or fat mass (Table 2).
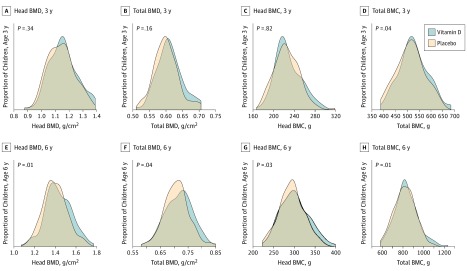
A random intercept mixed-effects model of the DXA outcomes from age 3 and 6 years combined (n = 427) revealed a significant effect of the high-dose vitamin D supplementation on TBLH BMC (aMD, 7.5 g; 95% CI, 1.5-13.5; P = .01); head BMD (0.023 g/cm2; 95% CI, 0.003-0.0042; P = .03); and total BMC (11.5 g; 95% CI, 2.3-20.7; P = .01) (Table 2). Figure 2 shows the proportional increase in head and total BMC and BMD at age 3 and 6 years stratified by treatment groups. Adjusting the analyses for the LCPUFA intervention did not change the results, and there was no evidence of interaction between the vitamin D and LCPUFA interventions except for head BMD (eTable 5 in Supplement 2), where stratified analyses by treatment groups demonstrated an effect in the group receiving high-dose vitamin D and olive oil compared with the group receiving standard-dose vitamin D and olive oil. We found no additive effect on head BMD of receiving both high-dose vitamin D and LCPUFA treatment (eTables 5 and 6 and eFigure 2 in Supplement 2).
Figure 2. Density Plots of Head and Total Body Bone Mineral Content (BMC) and Bone Mineral Density (BMD) at Age 3 and 6 Years in Children Born to Mothers Receiving High-Dose Vitamin D or Placebo During Pregnancy.
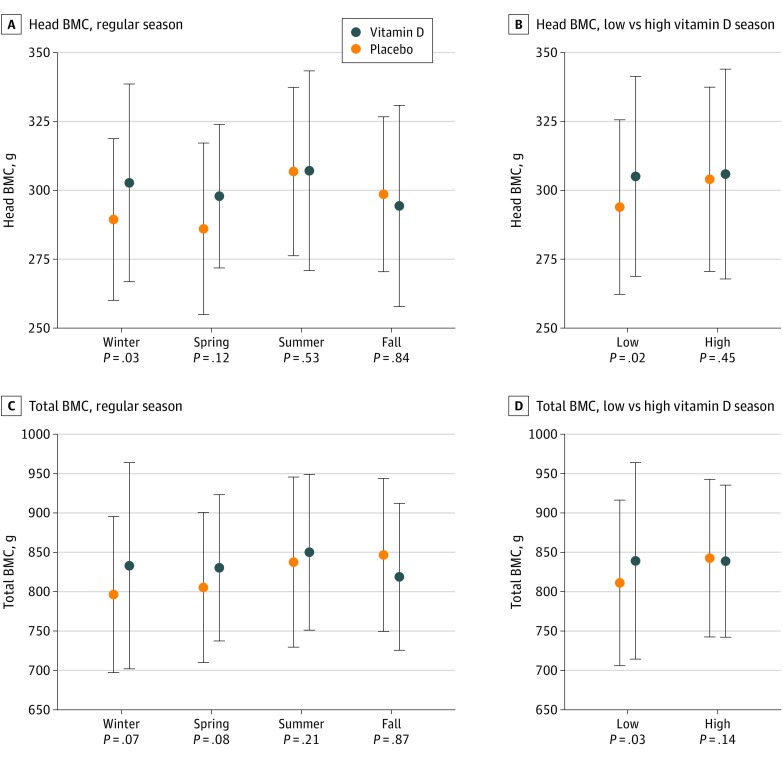
We investigated possible effect modification by season of birth and found that giving birth during winter was associated with the highest effect on head BMC. Furthermore, we stratified the analysis according to mothers’ preintervention 25(OH)D levels in a low vs high season (eFigure 3 in Supplement 2), which confirmed that giving birth in low season (November 8 to May 7) was associated with a significant supplementation effect on head BMC and total BMC, whereas no effects were observed in children born in high season (May 8 to November 7) (Figure 3) (eTable 7 in Supplement 2).
Figure 3. Effect of High-Dose Vitamin D vs Placebo on Head and Total Body Bone Mineral Content (BMC) Stratified by Birth Season at Age 6 Years.
A and C, Regular season. B and D, Low vs high vitamin D season.
Fractures
Among the 584 children, 59 fractures (vitamin D vs placebo: 23 vs 36) were registered in 55 children during the follow-up period and showed a trend toward a lower incidence in the vitamin D vs placebo group: n = 22 (7%) vs n = 33 (11%); incidence rate ratio, 0.62; 95% CI, 0.37-1.05; P = .08. The distribution of fracture types was 32 forearm (54%), 14 humerus (24%), 8 crus (14%), and 5 clavicle (8%); 4 children had 2 fractures. Adjusting for sex, birth season, preintervention 25(OH)D levels, and LCPUFA intervention did not change the results (incidence rate ratio, 0.64; 95% CI, 0.37-1.06; P = .09). There was no significant interaction with maternal preintervention 25(OH)D levels.
Discussion
High-dose vitamin D supplementation with 2800 IU/d vs a standard dose of 400 IU/d from pregnancy week 24 to 1 week post partum led to a higher bone mineralization in the offspring’s first 6 years of life. The intervention effect was significant for total body BMC, TBLH BMC, and head BMD in a combined analysis of age 3 and 6 years, with the most pronounced effects observed in children born to mothers with low preintervention serum 25(OH)D level and in children born during winter season.
The strength of the study is the randomized, double-blinded, placebo-controlled design, with frequent clinical scheduled visits resulting in a high follow-up rate of 89% at age 6 years. The study is a population-based mother-child cohort representing the general Danish population, which allows for a generalization of the results. Furthermore, the observed effect on bone mineralization is biologically plausible.
Limitations
The main limitation of our study is that the trial was designed with asthma/persistent wheeze through age 3 years as the primary end point and not powered for bone or anthropometric outcomes. However, this is mitigated by the fact that an effect was found. Another limitation is the number of available DXA scans and that 157 children did not have an available DXA scan, which was primarily owing to unacceptable quality. We observed no interaction between the vitamin D and LCPUFA interventions on the anthropometric and DXA outcomes except for head BMD, where stratified analyses showed the largest effect of vitamin D in combination with olive oil and no additive effect in the vitamin D and LCPUFA group combined. Additionally, adjusting the analyses for LCPUFA did not change the results supporting an independent vitamin D effect on bone mineralization.
Our findings on DXA outcomes are consistent with previous observational studies linking low 25(OH)D levels in mid or late pregnancy to reduced total BMC outcomes in childhood10 and the age around peak bone mass, where it has been predicted that each 4.01-ng/mL increase in maternal serum 25(OH)D during pregnancy results in a total amount of up to 37.6 g more hydroxyapatite (BMC) and up to 0.007 g/cm2 higher BMD in the offspring at age 20 years.15
The well-established tracking of bone mineralization from early life throughout childhood and early adulthood is a key factor for the final peak bone mass gained and the subsequent risk of fractures5 and osteoporosis later in life.26 The clinical importance of peak bone mass has been evaluated by mathematical models predicting that a 10% increase would result in 13 years of delayed onset of osteoporosis, suggesting that factors contributing to even small increases in peak bone mass in early life could be an effective preventive strategy for fractures and osteoporosis4 later in life. In our study, we found an effect of the high-dose vitamin D intervention in the combined analysis of age 3 and 6 years DXA outcomes not only on total BMC but also on head BMD, which may represent the most sensitive compartment of intervention because 80% of the mineralization of the skull is achieved by age 3 years.27 We speculate that these intervention effects could be of importance for bone health and osteoporosis risk in adult life, which is supported by our likely underpowered post hoc analysis on fracture risk, suggesting an almost 40% reduced incidence of fractures of the larger bones in the high-dose vitamin D group.
We only identified 1 other pregnancy vitamin D RCT (the Maternal Vitamin D Osteoporosis Study [MAVIDOS])17 with child DXA outcomes, which did not show an effect of supplementation with 1000 IU/d plus self-administration of up to 400 IU/d on neonatal BMC measurements at 2 weeks post partum, but showed higher total BMD and BMC values among children born during the winter season in the intervention group.17 This overall negative trial result is in contrast to our findings and could be owing to the relative low supplementation dose,28 as it has been reported elsewhere that even small supplementation increases from 400 to 2000 IU/d resulted in a 52% higher chance of reaching vitamin D sufficiency at time of delivery in mothers.29 The MAVIDOS negative trial result could also be owing to the DXA scans in neonates only and not later in childhood.
Our trial showed that high-dose vitamin D supplementation during pregnancy had the largest effect on bone mineralization outcomes when the child was born during winter or spring. This could be a result of supplying the fetus with sufficient vitamin D in the crucial third trimester, where 80% of mineral content is accrued, most of the bone mineralization occurs, and secondary ossification centers take form.30 Vitamin D is a main regulator of calcium balance in the body through its actions on the intestine, kidneys, and role in bone metabolism,2 and a significant supplementation effect on cord blood and neonatal calcium levels has previously been described.31 Further, a longitudinal study demonstrated a positive association between placental calcium levels and BMC in offspring, suggesting that vitamin D insufficiency in the last trimester resulted in an impaired placental calcium transfer leading to reduced mineral content in childhood,10 which could be a mechanism underlying our findings. In accordance with the results from the MAVIDOS trial, the only other pregnancy vitamin D RCT with child DXA outcomes,17 we observed no effect on lean mass or fat mass.
Our null finding on anthropometrics in childhood are in line with a 2018 larger RCT13 reporting no differences in anthropometric outcomes at birth or age 1 year between vitamin D groups of up to 4000 IU/d vs placebo. In contrast, an earlier published meta-analysis14 reported accelerated growth in the first year of life and a reduced risk of SGA in children from mothers receiving prenatal high-dose vitamin D; however, there are several issues to consider in the meta-analysis: a high heterogeneity of up to 89% in the anthropometric findings, number of participants (lower than 200) per measuring point, and that the risk reduction of SGA was only seen in supplementation groups of 2000 IU/d or lower.
Considering the beginning of the mineralization process and the forming of primary ossification centers in the first trimester,30 an earlier start of the intervention might have resulted in more pronounced treatment effects. Our placebo group had higher BMC values at age 6 years compared with other age-specific BMC references32; therefore, the supplementation effect may be even larger in other populations with high skin pigmentation, limited exposure to sunlight, and extensive use of skin protection. Lastly, future studies could possibly benefit from a higher supplementation dose than 2800 IU/d, which is considered safe in a 2018 meta-analysis14 including 24 clinical trials using as much as 5000 IU/d.
Conclusions
In conclusion, supplementation of 2800 IU/d of vitamin D from gestational week 24 resulted in an overall higher mineralization status in the offspring compared with standard dose, with the most pronounced effects among children born to mothers with insufficient 25(OH)D levels or giving birth during the months with least exposure to sunlight. These findings suggest that a 7-fold increased intake of vitamin D in pregnancy improves the child’s bone health through age 6 years, which is likely to influence peak bone mass, fracture risk, and risk of osteoporosis later in life.
Trial Protocol.
eMethods.
eReferences.
eTable 1. Baseline characteristics of the mother-child pairs in the vitamin D trial.
eTable 2. Repeated measurements mixed effect model analyses of anthropometrics from age 1 week to 6 years and analyses adjusted for birth season, LCPUFA and mothers baseline 25(OH)D levels at age 6 years in the high-dose vitamin D vs. placebo group.
eTable 3. Characteristics of children with available DXA scan compared with children with no available DXA scan at age 3 and 6 years.
eTable 4. Analyses stratified by maternal vitamin D pre-intervention levels (nmol/L: <75 insufficient, ≥75 sufficient).
eTable 5. Combined mixed effects model analyses additionally adjusted for LCPUFA intervention and p-values of interaction between vitamin D and LCPUFA.
eTable 6. Head BMD analyses of the high-dose vitamin D and LCPUFA treatment groups combined in a mixed effects model and differences between groups at age 3 and 6 years.
eTable 7. Age 6 years BMC values (g) stratified by birth seasons.
eFigure 1. Effect of high-dose vitamin D supplementation on anthropometric measurements from age 1 week to 6 years.
eFigure 2. Boxplots showing head BMD outcomes at age 3 and 6 years stratified by the high-dose vs. LCPUFA intervention groups.
eFigure 3. Illustration of vitamin D levels measured at pregnancy week 24. Highest level measured at 8th of August, lowest level at 7th of February, P<0.001.
Data Sharing Statement.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281. doi: 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- 2.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6)(suppl):1689S-1696S. doi: 10.1093/ajcn/80.6.1689S [DOI] [PubMed] [Google Scholar]
- 3.Heaney RP, Abrams S, Dawson-Hughes B, et al. Peak bone mass. Osteoporos Int. 2000;11(12):985-1009. doi: 10.1007/s001980070020 [DOI] [PubMed] [Google Scholar]
- 4.Hernandez CJ, Beaupré GS, Carter DR. A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos Int. 2003;14(10):843-847. doi: 10.1007/s00198-003-1454-8 [DOI] [PubMed] [Google Scholar]
- 5.Clark EM, Tobias JH, Ness AR. Association between bone density and fractures in children: a systematic review and meta-analysis. Pediatrics. 2006;117(2):e291-e297. doi: 10.1542/peds.2005-1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116(8):2062-2072. doi: 10.1172/JCI29449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodnar LM, Catov JM, Zmuda JM, et al. Maternal serum 25-hydroxyvitamin D concentrations are associated with small-for-gestational age births in white women. J Nutr. 2010;140(5):999-1006. doi: 10.3945/jn.109.119636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leffelaar ER, Vrijkotte TGM, van Eijsden M. Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: results of the multi-ethnic Amsterdam Born Children and their Development cohort. Br J Nutr. 2010;104(1):108-117. doi: 10.1017/S000711451000022X [DOI] [PubMed] [Google Scholar]
- 9.Gale CR, Robinson SM, Harvey NC, et al. ; Princess Anne Hospital Study Group . Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62(1):68-77. doi: 10.1038/sj.ejcn.1602680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Javaid MK, Crozier SR, Harvey NC, et al. ; Princess Anne Hospital Study Group . Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367(9504):36-43. doi: 10.1016/S0140-6736(06)67922-1 [DOI] [PubMed] [Google Scholar]
- 11.Roth DE, Perumal N, Al Mahmud A, Baqui AH. Maternal vitamin D3 supplementation during the third trimester of pregnancy: effects on infant growth in a longitudinal follow-up study in Bangladesh. J Pediatr. 2013;163(6):1605-1611. doi: 10.1016/j.jpeds.2013.07.030 [DOI] [PubMed] [Google Scholar]
- 12.Brooke OG, Butters F, Wood C. Intrauterine vitamin D nutrition and postnatal growth in Asian infants. Br Med J (Clin Res Ed). 1981;283(6298):1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roth DE, Morris SK, Zlotkin S, et al. Vitamin D supplementation in pregnancy and lactation and infant growth. N Engl J Med. 2018;379(6):535-546. doi: 10.1056/NEJMoa1800927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bi WG, Nuyt AM, Weiler H, Leduc L, Santamaria C, Wei SQ. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr. 2018;172(7):635-645. doi: 10.1001/jamapediatrics.2018.0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu K, Whitehouse AJ, Hart PH, et al. Maternal vitamin D status during pregnancy and bone mass in offspring at 20 years of age: a prospective cohort study. J Bone Miner Res. 2014;29(5):1088-1095. doi: 10.1002/jbmr.2138 [DOI] [PubMed] [Google Scholar]
- 16.Lawlor DA, Wills AK, Fraser A, Sayers A, Fraser WD, Tobias JH. Association of maternal vitamin D status during pregnancy with bone-mineral content in offspring: a prospective cohort study. Lancet. 2013;381(9884):2176-2183. doi: 10.1016/S0140-6736(12)62203-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper C, Harvey NC, Bishop NJ, et al. ; MAVIDOS Study Group . Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): a multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2016;4(5):393-402. doi: 10.1016/S2213-8587(16)00044-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chawes BL, Bønnelykke K, Stokholm J, et al. Effect of vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: a randomized clinical trial. JAMA. 2016;315(4):353-361. doi: 10.1001/jama.2015.18318 [DOI] [PubMed] [Google Scholar]
- 19.Bisgaard H, Vissing NH, Carson CG, et al. Deep phenotyping of the unselected COPSAC2010 birth cohort study. Clin Exp Allergy. 2013;43(12):1384-1394. doi: 10.1111/cea.12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bisgaard H, Stokholm J, Chawes BL, et al. Fish Oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375(26):2530-2539. doi: 10.1056/NEJMoa1503734 [DOI] [PubMed] [Google Scholar]
- 21.Vinding RK, Stokholm J, Sevelsted A, et al. Effect of fish oil supplementation in pregnancy on bone, lean, and fat mass at six years: randomised clinical trial. BMJ. 2018;362:k3312. doi: 10.1136/bmj.k3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85(7):843-848. doi: 10.1111/j.1651-2227.1996.tb14164.x [DOI] [PubMed] [Google Scholar]
- 23.Vinding RK, Stokholm J, Sevelsted A, et al. Fish oil supplementation in pregnancy increases gestational age, size for gestational age, and birth weight in infants: a randomized controlled trial. J Nutr. 2019;149(4):628-634. doi: 10.1093/jn/nxy204 [DOI] [PubMed] [Google Scholar]
- 24.WHO Multicentre Growth Reference Study Group WHO Child Growth Standards: Growth velocity based on weight, length and head circumference: methods and development. Geneva, Switzerland: World Health Organization; 2009.
- 25.Prentice A, Parsons TJ, Cole TJ. Uncritical use of bone mineral density in absorptiometry may lead to size-related artifacts in the identification of bone mineral determinants. Am J Clin Nutr. 1994;60(6):837-842. doi: 10.1093/ajcn/60.6.837 [DOI] [PubMed] [Google Scholar]
- 26.Kalkwarf HJ, Gilsanz V, Lappe JM, et al. Tracking of bone mass and density during childhood and adolescence. J Clin Endocrinol Metab. 2010;95(4):1690-1698. doi: 10.1210/jc.2009-2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor A, Konrad PT, Norman ME, Harcke HT. Total body bone mineral density in young children: influence of head bone mineral density. J Bone Miner Res. 1997;12(4):652-655. doi: 10.1359/jbmr.1997.12.4.652 [DOI] [PubMed] [Google Scholar]
- 28.Moon RJ, Harvey NC, Cooper C, et al. Determinants of the maternal 25-hydroxyvitamin D response to Vitamin D supplementation during pregnancy. J Clin Endocrinol Metab. 2016;101(12):5012-5020. doi: 10.1210/jc.2016-2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26(10):2341-2357. doi: 10.1002/jbmr.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovacs CS. Bone metabolism in the fetus and neonate. Pediatr Nephrol. 2014;29(5):793-803. doi: 10.1007/s00467-013-2461-4 [DOI] [PubMed] [Google Scholar]
- 31.Brooke OG, Brown IR, Bone CD, et al. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. Br Med J. 1980;280(6216):751-754. doi: 10.1136/bmj.280.6216.751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zemel BS, Kalkwarf HJ, Gilsanz V, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96(10):3160-3169. doi: 10.1210/jc.2011-1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eMethods.
eReferences.
eTable 1. Baseline characteristics of the mother-child pairs in the vitamin D trial.
eTable 2. Repeated measurements mixed effect model analyses of anthropometrics from age 1 week to 6 years and analyses adjusted for birth season, LCPUFA and mothers baseline 25(OH)D levels at age 6 years in the high-dose vitamin D vs. placebo group.
eTable 3. Characteristics of children with available DXA scan compared with children with no available DXA scan at age 3 and 6 years.
eTable 4. Analyses stratified by maternal vitamin D pre-intervention levels (nmol/L: <75 insufficient, ≥75 sufficient).
eTable 5. Combined mixed effects model analyses additionally adjusted for LCPUFA intervention and p-values of interaction between vitamin D and LCPUFA.
eTable 6. Head BMD analyses of the high-dose vitamin D and LCPUFA treatment groups combined in a mixed effects model and differences between groups at age 3 and 6 years.
eTable 7. Age 6 years BMC values (g) stratified by birth seasons.
eFigure 1. Effect of high-dose vitamin D supplementation on anthropometric measurements from age 1 week to 6 years.
eFigure 2. Boxplots showing head BMD outcomes at age 3 and 6 years stratified by the high-dose vs. LCPUFA intervention groups.
eFigure 3. Illustration of vitamin D levels measured at pregnancy week 24. Highest level measured at 8th of August, lowest level at 7th of February, P<0.001.
Data Sharing Statement.