Key Points
Question
Do decisions about trading effort for reward differentiate patients whose depression is in remission and who are still taking medication from controls, and are these decisions associated with relapse after stopping medication?
Findings
This prognostic study found that patients whose depression was in remission but who still took medication were more sensitive to effort. These patients took longer to make these decisions, which was predictive of the risk of relapse after stopping antidepressants.
Meaning
Even when their depression is in remission, patients taking antidepressant medications show characteristic differences in how they trade rewards for effort, and these differences might be a clinically useful predictor of relapse if medication is discontinued.
Abstract
Importance
Nearly 1 in 3 patients with major depressive disorder who respond to antidepressants relapse within 6 months of treatment discontinuation. No predictors of relapse exist to guide clinical decision-making in this scenario.
Objectives
To establish whether the decision to invest effort for rewards represents a persistent depression process after remission, predicts relapse after remission, and is affected by antidepressant discontinuation.
Design, Setting, and Participants
This longitudinal randomized observational prognostic study in a Swiss and German university setting collected data from July 1, 2015, to January 31, 2019, from 66 healthy controls and 123 patients in remission from major depressive disorder in response to antidepressants prior to and after discontinuation. Study recruitment took place until January 2018.
Exposure
Discontinuation of antidepressants.
Main Outcomes and Measures
Relapse during the 6 months after discontinuation. Choice and decision times on a task requiring participants to choose how much effort to exert for various amounts of reward and the mechanisms identified through parameters of a computational model.
Results
A total of 123 patients (mean [SD] age, 34.5 [11.2] years; 94 women [76%]) and 66 healthy controls (mean [SD] age, 34.6 [11.0] years; 49 women [74%]) were recruited. In the main subsample, mean (SD) decision times were slower for patients (n = 74) compared with controls (n = 34) (1.77 [0.38] seconds vs 1.61 [0.37] seconds; Cohen d = 0.52; P = .02), particularly for those who later relapsed after discontinuation of antidepressants (n = 21) compared with those who did not relapse (n = 39) (1.95 [0.40] seconds vs 1.67 [0.34] seconds; Cohen d = 0.77; P < .001). This slower decision time predicted relapse (accuracy = 0.66; P = .007). Patients invested less effort than healthy controls for rewards (F1,98 = 33.970; P < .001). Computational modeling identified a mean (SD) deviation from standard drift-diffusion models that was more prominent for patients than controls (patients, 0.67 [1.56]; controls, –0.71 [1.93]; Cohen d = 0.82; P < .001). Patients also showed higher mean (SD) effort sensitivity than controls (patients, 0.31 [0.92]; controls, –0.08 [1.03]; Cohen d = 0.51; P = .05). Relapsers differed from nonrelapsers in terms of the evidence required to make a decision for the low-effort choice (mean [SD]: relapsers, 1.36 [0.35]; nonrelapsers, 1.17 [0.26]; Cohen d = 0.65; P = .02). Group differences generally did not reach significance in the smaller replication sample (27 patients and 21 controls), but decision time prediction models from the main sample generalized to the replication sample (validation accuracy = 0.71; P = .03).
Conclusions and Relevance
This study found that the decision to invest effort was associated with prospective relapse risk after antidepressant discontinuation and may represent a persistent disease process in asymptomatic remitted major depressive disorder. Markers based on effort-related decision-making could potentially inform clinical decisions associated with antidepressant discontinuation.
This prognostic study examines whether the decision to invest effort for rewards represents a persistent depression process after remission, predicts relapse after remission, or is affected by antidepressant discontinuation.
Introduction
Achieving remission is only the first step in the treatment of major depressive disorder; too often, an initially successful treatment is followed by a relapse. Major depressive disorder often takes a chronic relapsing-remitting course; hence, maintenance of remission requires dedicated attention. Relapse rates after antidepressant discontinuation are very high, with approximately 1 in 3 patients experiencing another depressive episode within 6 months.1 Current guidelines make explicit recommendations to continue treatment after the initial response,2,3 but, to our knowledge, no established predictors of relapse after antidepressant discontinuation exist,4 nor are the mechanisms underlying discontinuation known. Furthermore, recommendations about the length of maintenance treatment are based on assumptions about the natural course of depressive episodes5 and studies investigating the risk of relapse irrespective of treatment.6 The evidence about whether a longer treatment course reduces the risk of relapse after antidepressant discontinuation and whether patients with more depressive episodes benefit from longer treatment is ambiguous.7,8,9
A perception of decreased energy or increased fatigue is a core symptom of depression in the World Health Organization International Classification of Diseases,10,11 and its absence has a high negative predictive value for a diagnosis of depression.12 The decision to expend effort to obtain rewards also appears to be a robust feature of depression; patients with current depression, with subsyndromal symptoms, in their first episode of depression, and in remission all show a reduced willingness to expend effort for reward.13,14,15,16 Expending effort to obtain rewards is also central to psychotherapeutic interventions such as behavioral activation17; decisions about effort are known to be associated with neurotransmitters such as serotonin and dopamine18,19 and with antidepressants.20 As such, decisions about effort may well play an important role in the long-term course of depression and particularly in relapses after antidepressant discontinuation. Neurobiological and psychological theories, as well as diagnostic systems, have emphasized the role of reward processing, suggesting that effort investment is reduced in individuals with depression because the resulting reward experience is impaired.21,22,23,24 However, although self-reports of anhedonia can reliably be elicited in several ways, human studies have shown equivocal impairments in actual reward experience,25,26,27 and it remains unclear whether alterations in the tradeoffs between reward and effort arise from a change in the experience of rewards or efforts or from changes in how they are anticipated.
The AIDA study (Antidepressiva Absetzstudie), a longitudinal observational study, was designed to identify predictors of relapse after antidepressant discontinuation, investigate the mechanisms underlying relapse after antidepressant discontinuation, and cross-sectionally compare healthy controls with patients whose depression was in remission but who were still taking antidepressants. We report findings using an effort task in which participants had to choose how much effort in terms of repeated button presses to invest for varying levels of reward. We used detailed generative computational models of choice and decision time, with extensive validation to disentangle the cognitive processes associated with depression, with discontinuing antidepressant medication, and with the risk of a subsequent relapse. We hypothesized a priori that patients—in particular, those who would go on to experience relapse—would choose high-effort options less, would press buttons more slowly, and would take longer to reach decisions.
Methods
Study Design
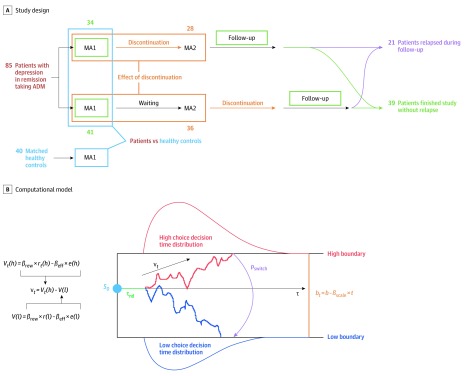
After a telephone screening, suitable participants were invited to an in-person baseline assessment to determine inclusion based on clinical interviews with trained staff (including I.M.B., J.G.W, and Q.J.M.H.). Participants were then randomized (Figure 1A). In group 1W2, the first main assessment (MA1) preceded gradual discontinuation of antidepressants during a period of up to 18 weeks and was followed by a second main assessment (MA2). In group 12W, both main assessments occurred before discontinuation of antidepressants. Participants were followed up for 6 months to assess relapse. Healthy controls matched for age, sex, and educational level underwent a telephone screening, a baseline assessment, and the MA1. Participants performed the physical effort task (eFigure 1 in the Supplement)28 and underwent self-rated and observer-rated reports (eAppendix 1 in the Supplement) during each main assessment. Study data were collected between July 1, 2015, and January 31, 2019. Study recruitment took place until January 2018. All participants provided informed written consent and received monetary compensation for their participation. Ethical approval for the study was obtained from the cantonal ethics commission Zurich and the ethics commission at the Campus Charité-Mitte, and procedures were in accordance with the Declaration of Helsinki.29
Figure 1. Study Design, Task, and Computational Model.
Study design. Patients with depression in remission taking antidepressant medication (ADM) and matched healthy controls were included in the study and assessed during main assessment 1 (MA1). Group differences between patients and healthy controls at MA1 indicate an association of disease persisting into medicated remission. Patients were randomized to either discontinue their medication prior to the second main assessment (MA2; group 1W2 [where W represents withdrawal]) or wait for a period of similar length and discontinue after MA2 (group 12W). Differences in changes between MA1 and MA2 in the 2 separate groups were investigated to gain an understanding of the consequences of discontinuation. After discontinuation, all patients entered the follow-up period of 6 months; some patients had a relapse during this period, while other patients finished this period without relapse. Comparing performance at MA1 between patients who relapsed and patients who did not relapse during follow-up provides information on differences between these patient subgroups and allowed for the identification of predictors of relapse after antidepressant discontinuation. The numbers indicate how many participants in the main sample were assessed at each stage. B, Computational model. The drift rate (νt) of the drift-diffusion model (DDM) depended on a weighted sum of effort (number of button presses) and reward for each of the 2 presented options. Parameters were individually fitted to provide measures of individual reward sensitivity (βrew), effort sensitivity (βeff), and a deviation from standard DDMs, implemented as a probability (pswitch) that allowed participants to choose the low-effort choice for small high-reward option (ie, 3 and 4) through an additional process. Further individually adjusted parameters were starting point (S0), nondecision time (τnd), starting boundary (b), and linear boundary scaling over trials (βscale).
Participants
We recruited participants who had experienced 1 severe depressive episode30 or multiple depressive episodes, had initiated antidepressant treatment during the last depressive episode and now achieved stable remission, and had reached the decision to discontinue their medication independently from and prior to study participation. See eAppendix 1 in the Supplement for detailed inclusion and exclusion criteria. In the present analyses, the sample from Zurich, Switzerland, is the main sample, and the sample from Berlin, Germany, is the replication sample.
Follow-up Procedure
After antidepressant discontinuation was completed, patients were contacted for telephone assessments at weeks 1, 2, 4, 6, 8, 12, 16, and 21 to assess relapse status. If core symptoms of depression were identified in the telephone interview, participants were invited to an in-person interview and relapse was assessed using the Structured Clinical Interview for DSM-IV-TR.31 If patients fulfilled the criteria for a major depressive episode, they were categorized as having relapsed, underwent the final assessment, exited the study, and re-referred to their treating physician. If no relapse occurred during the 6-month follow-up period, the final assessment took place in week 26.
Statistical Analysis
Data Analyses
The fraction of high-effort choices, the mean effort execution time (the mean time between button presses), and the mean decision time (time to first button press) were subjected to mixed-design analyses of variance. Main effects were 1-sided in accordance with the directions stated in the hypotheses in our a priori analysis plan (available at https://gitlab.ethz.ch/tnu/analysis-plans/aidaz_analysis_plan_effort_task). We corrected for the 3 main outcome measures using Bonferroni correction and, accordingly, set the required significance level to α = .017. See eAppendix 1 in the Supplement for more details.
Computational Modeling
To quantitatively evaluate the mechanisms associated with participants’ behavior in the task, we built and fitted a range of generative computational models representing putative computations during the task. Building such models allows for the identification of the computations required to produce the observed behavioral patterns in a holistic manner. The parameters in such models have an explicit mechanistic meaning in terms of their role in the model. The final model is depicted and described in Figure 1B. This is an augmented version of analytical drift-diffusion models (DDMs)32 in which the drift rate is determined by the difference between the high-value and the low-value option. Parameters were fitted with an empirical hierarchical bayesian procedure based on an expectation-maximization algorithm.33 We further ensured that all components of the model were necessary by quantitatively comparing it with reduced model versions.34 See eAppendix 1 in the Supplement for detailed model formulation, fitting, model recovery, parameter recovery, and model comparison methods. Parameters were compared between groups using 2-sample t tests.
Prediction Analyses
We fitted logistic regressions nested in a leave-one-out cross-validation for each variable that differed significantly between relapsers and nonrelapsers. Group membership of the left-out participant was predicted using parameter estimates (regression weights and optimized threshold) obtained from the participants included in the fit. The significance of the balanced accuracy was assessed using a binomial test comparing with 0.5.
Replication Analyses
Replication findings were considered significant at α = .05 in the same direction as the effect in the main sample. To validate the predictive accuracy obtained in the main sample, the replication sample was used as a left-out validation sample, and the parameters from the main sample were applied to it. To verify the nature of the mechanisms at play, the complete model validation procedure was independently repeated in the replication sample. For parameter estimation in the replication sample, we applied maximum a posteriori estimation using the hyperparameters estimated in the main sample.
Missing Data
Complete-case analyses were supplemented by Cox proportional hazards intention-to-treat regressions including patients who dropped out after MA1 (eAppendix 1 in the Supplement).
Results
Participants
The main Zurich sample contained 74 patients and 34 matched healthy controls. A total of 63 patients (85%) participated in MA2, including 28 (44%) who were not taking medication after discontinuation in group 1W2 and 35 (56%) who were taking medication prior to discontinuation in group 12W (eFigure 2 in the Supplement). The Berlin replication sample contained 27 patients and 21 healthy controls. A total of 25 patients (93%) were reassessed at MA2. Of these, 14 (56%) were reassessed after discontinuation and 11 (44%) were reassessed prior to discontinuation (eFigure 3 in the Supplement). There were no significant differences in terms of demographic, neuropsychological, or clinical measures between patients who did and patients who did not relapse during the follow-up period in either the complete-case or intention-to-treat analyses (Table 126,35,36,37,38,39; eTable 2 in the Supplement). Demographic, neuropsychological, and clinical measures of the replication sample are shown in eTable 1 in the Supplement and discussed in eAppendix 2 in the Supplement.
Table 1. Participant Characteristics for the Main Sample From Zurich.
| Variable | Patients vs HC | Relapsers vs Nonrelapsers | ||||
|---|---|---|---|---|---|---|
| Patients (n = 74) | HC (n = 34) | P Value | Relapsers (n = 21) | Nonrelapsers (n = 39) | P Value | |
| Demographics | ||||||
| Age, mean (SD), y | 34.1 (10.7) | 32.3 (10.2) | .40 | 35.2 (9.5) | 32.9 (11.1) | .42 |
| Male sex, No. (%) | 17 (23) | 12 (35) | .18 | 5 (24) | 9 (23) | .95 |
| Neuropsychology scores, mean (SD) | ||||||
| Intelligencea | 27.6 (4.6) | 27.7 (4.4) | .91 | 28.7 (3.7) | 27.5 (4.9) | .33 |
| Working memorya | 6.9 (2.1) | 8.0 (3.8) | .06 | 6.6 (1.6) | 7.0 (2.2) | .46 |
| Cognitive processing speeda | 25.1 (10.3) | 23.8 (5.6) | .49 | 23.8 (8.7) | 24.0 (6.8) | .92 |
| Executive functiona | 58.9 (16.2) | 58.3 (21.6) | .87 | 56.4 (16.0) | 60.3 (17.7) | .40 |
| Clinical measures, mean (SD) | ||||||
| No. of prior episodes | NA | NA | NA | 2.9 (2.0) | 2.6 (1.6) | .61 |
| Residual depressiona | 3.15 (2.9) | 0.65 (1.2) | <.001 | 2.0 (2.5) | 3.4 (2.7) | .06 |
| Disease severityb | NA | NA | NA | 0.06 (0.38) | −0.01 (0.36) | .47 |
| Medication loadb | NA | NA | NA | 0.007 (0.004) | 0.008 (0.004) | .34 |
| Covariates of interest, mean (SD)a | ||||||
| Anticipatory pleasure | 4.3 (0.6) | 4.4 (0.6) | .68 | 4.2 (0.58) | 4.3 (0.59) | .78 |
| Consummatory pleasure | 4.9 (0.6) | 4.9 (0.6) | .82 | 4.9 (0.56) | 5.0 (0.62) | .30 |
| Brooding | 10.0 (2.8) | 8.1 (2.3) | <.001 | 11.2 (2.9) | 9.6 (2.8) | .06 |
Abbreviations: HC, healthy controls; NA, not applicable.
Determined as follows: intelligence, Mehrfachwahl Wortschatz Test35; working memory, digit span backward test from the Wechsler Adult Intelligence Scale36; cognitive processing speed, Trail Making Test A37; executive processing speed, Trail Making Test B37; residual depression, Inventory of Depressive Symptomatology–Clinician Rated38; anticipatory pleasure, subscale of anticipatory pleasure of the Temporal Experience of Pleasure Scale26; consummatory pleasure, subscale of consummatory pleasure of the Temporal Experience of Pleasure Scale26; brooding, brooding subscale of the German version of the Response Style Questionnaire.39
Computation of the variables is described in eAppendix 1 in the Supplement.
Behavioral Results
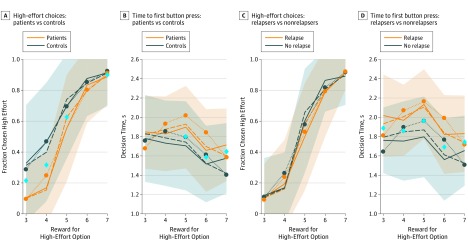
The decision to invest effort for reward distinguished patients with depression in remission who were still taking antidepressants from healthy controls and distinguished prospective relapsers from nonrelapsers. Patients chose the high-effort option less often than did controls (F1,98 = 33.970; P < .001), particularly when it yielded low rewards (interaction: F4,392 = 9.867; P < .001). Patients were also slower than healthy controls in reaching a decision (F1,103 = 6.128; P = .02), particularly when choosing the high-effort option (F1,45 = 3.875; P = .06; low effort, F1,27 = 0.325; P = .57). Patients who relapsed were slower to decide than those who did not relapse (F1,57 = 13.079; P < .001; see Figure 2 and Table 2 for results of choices and decision times in all groups).
Figure 2. Raw Behavioral Data and Model Fits in the Main Sample.
A, Fraction of high-effort choices as a function of reward offered for the high-effort choice comparing patients vs controls. B, Time to first button press as a function of reward offered for the high-effort choice comparing patients vs controls. C, Fraction of high-effort choices as a function of reward offered for the high-effort choice comparing relapsers vs nonrelapsers. D, Time to first button press as a function of reward offered for the high-effort choice comparing relapsers vs nonrelapsers. Solid lines indicate group mean values in the raw data and the surrounding shaded areas indicate the SDs of the raw data. The blue diamonds indicate significant post hoc tests corrected for the false-discovery rate for the individual reward levels. Dotted and dashed lines indicate the mean values of the surrogate data generated from models in all panels. The standard drift-diffusion model (DDM; dotted lines) forces fast decisions to accompany deterministic behavior and hence a prominent inverted U-shape dependence of decision times on reward levels (panels B and D). Inclusion of the deviation process allows the deterministic decisions to be accompanied by longer decision times (dashed lines).
Table 2. Behavioral Associations for Fraction of Effortful Choices and Decision Time in the Main Sample.
| Variable | Mean (SD) Probability | |||||
|---|---|---|---|---|---|---|
| Patients vs HC | Relapsers vs Nonrelapsers | |||||
| Patients (n = 74) | HC (n = 34) | P Valuea | Relapsers (n = 21) | Nonrelapsers (n = 39) | P Valuea | |
| Probability of high choice | 0.501 (0.408) | 0.693 (0.373) | <.001/<.001b | 0.472 (0.420) | 0.519 (0.408) | .30/.16b |
| Separate for reward levels | ||||||
| 3 | 0.070 (0.127) | 0.345 (0.375) | <.001 | 0.071 (0.140) | 0.074 (0.129) | .94 |
| 4 | 0.124 (0.143) | 0.494 (0.372) | <.001 | 0.153 (0.218) | 0.123 (0.137) | .90 |
| 5 | 0.549 (0.332) | 0.734 (0.314) | .02 | 0.479 (0.335) | 0.598 (0.317) | .51 |
| 6 | 0.855 (0.217) | 0.926 (0.141) | .10 | 0.788 (0.288) | 0.887 (0.158) | .51 |
| 7 | 0.908 (0.127) | 0.964 (0.062) | .03 | 0.924 (0.103) | 0.913 (0.117) | .90 |
| Decision time | 1.771 (0.382) | 1.608 (0.370) | .02/.35b | 1.954 (0.403) | 1.671 (0.337) | <.001/.43b |
| Separate for reward levels | ||||||
| 3 | 1.835 (0.365) | 1.771 (0.370) | .14 | 2.016 (0.415) | 1.737 (0.304) | .009 |
| 4 | 1.806 (0.338) | 1.689 (0.412) | .14 | 1.966 (0.360) | 1.710 (0.307) | .009 |
| 5 | 1.881 (0.401) | 1.643 (0.383) | .03 | 2.135 (0.355) | 1.761 (0.354) | .002 |
| 6 | 1.638 (0.401) | 1.462 (0.305) | .05 | 1.822 (0.398) | 1.524 (0.359) | .009 |
| 7 | 1.697 (0.346) | 1.528 (0.292) | .05 | 1.831 (0.387) | 1.620 (0.292) | .02 |
Abbreviation: HC, healthy control.
Unless stated otherwise, P values are determined from false discovery rate–corrected post hoc tests.
Of main effect/of interaction effect.
The groups did not differ in the vigor with which they executed the effortful behavior (the button press rate). Among those who went on to relapse, however, discontinuation of antidepressants reduced vigor numerically only (F1,25 = 3.914; P = .06; see eFigure 4A in the Supplement). Linear mixed-effects analyses replicated this pattern of findings (see eTable 3 and eAppendix 2 in the Supplement). Split-half reliability and test-retest reliability for all behavioral variables were high and moderate, respectively (see eTable 6 and eAppendix 2 in the Supplement).
Computational Modeling
Model Building and Selection
To identify the cognitive mechanisms underlying the choice differences, we used computational modeling, fitting DDMs in which the drift rates depended on both the effort and the reward for each option such that increased difficulty with the choice led to smaller drift rates. In combination with a stringent model validation procedure (eAppendix 1 in the Supplement), this modeling allowed us to parametrically measure the processes whereby effort was traded off against reward and the resulting valuations were turned into choices and decision times. In standard DDMs, fast mean decision times are associated with a large drift rate and/or a strong bias and are therefore associated with more deterministic choice patterns. The standard DDM predicts a strong inverted U shape in the reaction times (Figure 2B and D; significant quadratic association of reward with decision times in the data generated from the DDM; t2 = –9.924; P = .01) to match the deterministic choice behavior at extreme reward levels (Figure 2A and C). However, deterministic choices in the data were not actually accompanied by faster reaction times (Figure 2B and D; quadratic association of reward with decision times in data; t2 = –0.276; P = .81). This discrepancy was particularly seen in patients during trials in which the high effort option yielded low rewards. To capture the slower decision times, the model would have had to produce a less deterministic rejection of the extreme reward options, which in turn would have led to a poorer fit to the choice data.
We therefore altered the standard DDM model to parametrically capture the deviation in the patients by including a pswitch parameter. This pswitch parameter shifted some of the probability mass from the high-effort choices to the low-effort choices in trials with low rewards. Including this process led to a good model fit (Figure 2A-D), and bayesian model comparison and model validation showed this model to be robust and parsimonious (eFigure 5, eFigure 6, and eFigure 7 and eTable 4 and eTable 5 in the Supplement).
Parameter Comparisons
Having identified a valid and parsimonious mechanism through the computational modeling procedure, we examined the parameter estimates of the model to gain mechanistic insight into the processes differentiating the groups.34
Comparing patients whose depression is in remission with healthy controls, we found that patients deviated more from the standard DDM than did controls (pswitch: patients, 0.67 [1.56]; controls, –0.71 [1.93]; t106 = 3.943; d = 0.82; 95% CI, 0.684-2.067; P < .001). Patients were also more sensitive to the effort (β for effort: patients, 0.31 [0.92]; controls, –0.08 [1.03]; t106 = 1.984; d = 0.51; 95% CI, 0.0003-0.786; P = .05) and had longer nondecision times (τ nondecision: patients, 3.35 [0.55]; controls, 3.04 [0.50]; t106 = 2.805; d = 0.58; 95% CI, 0.0912-0.531; P = .006).
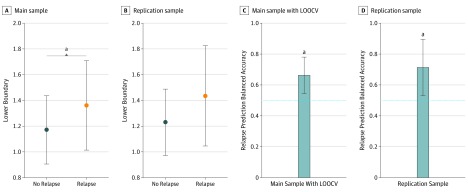
Patients who went on to relapse had a higher mean (SD) boundary compared with nonrelapsers (2.69 [0.44] vs 2.44 [0.46]; t58 = 2.027; d = 0.55; 95% CI, 0.0033-0.495; P = .05). This association seemed to be due to the boundary to the low-effort choice being higher (mean [SD]: relapsers, 1.36 [0.35]; nonrelapsers, 1.17 [0.26]; t58 = 2.366; Cohen d = 0.65; 95% CI, 0.029-0.349; P = .02), as depicted in Figure 3A. We confirmed this in exploratory analyses, finding that it was present across different models. Hence, relapsers needed more evidence before they could commit to a decision. See eFigure 10 in the Supplement for all parameter comparisons in the winning model.
Figure 3. Mean Lower Boundary and Relapse Prediction.
A, Mean lower boundary for relapse and no-relapse groups in the main sample. B, Mean lower boundary for relapse and no-relapse groups in the replication sample. Error bars indicate SDs. C, Balanced accuracy of relapse prediction using decision times for the main sample. D, Balanced accuracy of relapse prediction using decision times for the replication sample. Error bars indicate 95% bayesian credible intervals. Dashed blue lines indicate the chance level. LOOCV indicates leave-one-out cross-validation.
aP < .05 from chance level.
Prediction
Decision time predicted relapse (main sample balanced leave-one-out cross-validation accuracy = 0.66; P = .007; Figure 3C). The lower boundary did not improve accuracy further.
Covariates
Relapsers and nonrelapsers did not differ on any of the clinical variables (Table 1).26,33,34,35,36,37 Rumination did differ between patients and healthy controls (t106 = 3.417; 95% CI, 0.790-2.975; P < .001) but was not associated with decision time (r = –0.01; P = .88).
Replication Sample Results
The behavioral differences between patients and controls did not replicate. This was due to a difference between the healthy control samples between sites (F1,51 = 23.22; P < .001), whereas no difference between the patient samples was found (F1,91 = 3.219; P = .08). Despite these differences between the samples, computational model comparison and validation identified the same mechanisms in the replication sample (eFigure 8 in the Supplement).
The findings associated with relapse were qualitatively replicated but generally failed to reach statistical significance in the smaller replication sample. Patients who went on to relapse were also slower in deciding between the low-effort option and the high-effort option, which was also captured by an increase in the low boundary (eTable 7 and eFigure 9 in the Supplement; Figure 3B).
When applied to the replication sample, the prediction weights and thresholds from the main sample predicted relapse on the replication sample above chance, with a balanced accuracy of 0.71 (P = .03; Figure 3D). Patients who discontinued antidepressants and relapsed showed the same numerically reduced vigor during effort execution after discontinuation (F1,11 = 0.1.918; P = .19; eFigure 4B in the Supplement).
Discussion
The choice to exert effort to gain rewards differentiated patients whose depression was in remission and who were still taking antidepressants from healthy, never-depressed controls, and the time taken to make that decision was predictive of subsequent relapse. In addition, the choice process differentiated among patients who would go on to relapse after discontinuing their antidepressant medication. However, the choice process showed only marginal signatures of the medication discontinuation itself. Computational modeling identified some mechanisms underlying these differences and suggested novel mechanisms that were robust in the face of variations between sites. Several aspects of the findings deserve discussion.
First, the differentiation between the patient and control group was entirely located within the decision-making process itself and not in the actual execution of the effortful behavior, even though the computational model identified effort sensitivity as being one of the parameters differentiating the groups. Hence, even though patients whose depression was in remission weighted a prospective effort more negatively in their choices, they went on to perform it with equal vigor.
Second, reward sensitivity did not differentiate patients whose depression was in remission and who were still taking antidepressants from healthy controls. This finding is in keeping with a lack of group differences in self-reported anticipatory and consummatory pleasure in our study. However, it contrasts with results on reward and effort decisions in the depressed state itself, which appears to be associated with changes in both sensitivity to rewards and effort.14,15 Hence, effort anticipation may be more persistently altered than reward sensitivity.
Third, the duration of the decision process overall was predictive of subsequent relapse, and this predictive power generalized without decrement to the replication sample, which was treated as a left-out validation sample for all analyses presented. In the model, the effect was partially captured by the boundary to the low-effort choice, which was more pronounced in subsequent relapsers. The boundary effect is mechanistically distinct, uniquely associated with relapse, and suggests a particular struggle to accumulate evidence toward the low-effort choices among those who go on to relapse. It did not improve prediction accuracy beyond the decision times alone, suggesting that some of the predictive power of long decision times was taken up by other decision time–associated parameters or was reduced by the choices.
Fourth, decision times and choices were dissociated in patients; when having to expend large effort for relatively little reward, patients were highly deterministic in their choices but took longer to decide. The standard account for choices and reaction times, the DDM,40 strongly links determinism with speed and was unable to capture this pattern. Adding a mechanism, pswitch, that increased the probability of low-effort choices in trials with low rewards on offer enabled the model to capture the deviation from the standard DDM. This pswitch parameter distinguished the patients whose depression was in remission and who were still taking antidepressants from the controls and, hence, captured the selective deviation of the patient group from the standard DDM. It is possible that this pswitch deviation in the patients is indicative of a change-of-mind process.41,42,43 Clinically, this could relate to observations in behavioral activation psychotherapy17 whereby depression is thought to be characterized by a tendency to make plans that involve more effort and less reward than tolerable to the individual. Such plans result in last-minute changes of mind when the effort execution is imminent, and the repeated abandonment of plans is thought to be associated with self-efficacy. Behavioral activation treatment focuses on providing clients with tools to make realistic plans.
Fifth, the discontinuation of antidepressant medications did not have an effect on choice behavior or the exertion of the effortful behavior. This finding may be due to the limited statistical power of the study, but in the presence of a strong group difference, it suggests that antidepressants do not normalize decision-making abnormalities directly. The lack of effect on the exertion of the effortful behavior is surprising given that escitalopram, a highly selective serotonin reuptake inhibitor, reduces the process by which holding a grip becomes more effortful over time.20 The rate of button presses here showed no effect of antidepressant discontinuation. The fact that the effect of escitalopram was seen very rapidly20 but that no effect is seen after 5 half-times suggests either a slowing down of the recovery or a lack of sensitivity in our task. The design of the study allowed the association between discontinuation and relapse to be examined, albeit in a smaller sample. This nevertheless showed a consistent effect, with a slowing of button pressing after antidepressant discontinuation among those who went on to relapse, suggesting that the absence of an effect in the group as a whole might be due to differential effects in individuals, which in turn might be associated with relapse likelihood.
Limitations
The study has strengths, but also important limitations. First, while the patient groups were comparable, the healthy control behavior in the 2 sites differed. Hence, although the results pertaining to variation within the patient group was replicated qualitatively, our interpretations of the differences between controls and patients rely on the results in the main sample but clearly require examination of external motivating factors associated with task behavior in control participants. Second, test-retest reliability was at most moderate, suggesting one reason for the partial replication in the validation sample. Third, the sample was small, reflecting recruitment challenges for such studies. Fourth, the study used a purely observational design. Hence, any longitudinal associations could arise not from the discontinuation of the medication itself but from a nocebo-like response to the discontinuation. The absence of a robust association between discontinuation and vigor could be in keeping with such an interpretation.
Conclusions
In this study, choices about effortful behavior remained altered even after near-complete remission of depression, predisposed patients toward relapse, and predicted relapse, but the decision findings are dissociated from the actual execution of the effortful behaviors. This study exemplifies the possibility of applying computational techniques44,45,46 to a problem of eminent clinical importance, namely the management of antidepressant discontinuation.
eAppendix 1. Methods
eAppendix 2. Results
eFigure 1. Physical Effort Task
eFigure 2. CONSORT Diagrams of the Main Sample
eFigure 3. CONSORT Diagrams of the Replication Sample
eFigure 4. Relapse Discontinuation Interaction Effect for Effort Execution Time
eFigure 5. Parameter Recovery
eFigure 6. Model Fits
eFigure 7. Model Comparison in the Main Sample
eFigure 8. Model Comparison in the Replication Sample
eFigure 9. Raw Behavioral Data and Model Fits in the Replication Sample
eFigure 10. Parameter Comparisons
eTable 1. Participant Characteristics for the Replication Sample from Berlin
eTable 2. Dropout Comparisons and Cox Regressions
eTable 3. ANOVA and LMEM Comparisons
eTable 4. Model Recovery
eTable 5. Winning Models
eTable 6. Split-Half and Test-Retest Reliability
eTable 7. Behavioral Effects for Fraction of Effortful Choices and Decision Time in the Replication Sample
eReferences.
References
- 1.Geddes JR, Carney SM, Davies C, et al. . Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361(9358):653-661. doi: 10.1016/S0140-6736(03)12599-8 [DOI] [PubMed] [Google Scholar]
- 2.Bauer M, Pfennig A, Severus E, Whybrow PC, Angst J, Möller HJ; World Federation of Societies of Biological Psychiatry. Task Force on Unipolar Depressive Disorders . World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry. 2013;14(5):334-385. doi: 10.3109/15622975.2013.804195 [DOI] [PubMed] [Google Scholar]
- 3.National Institute for Health and Clinical Excellence Guidance: Depression: The Treatment and Management of Depression in Adults (Updated Edition). Leicester, UK: British Psychological Society; 2010. [PubMed] [Google Scholar]
- 4.Berwian IM, Walter H, Seifritz E, Huys QJM. Predicting relapse after antidepressant withdrawal—a systematic review. Psychol Med. 2017;47(3):426-437. doi: 10.1017/S0033291716002580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank E, Prien RF, Jarrett RB, et al. . Conceptualization and rationale for consensus definitions of terms in major depressive disorder: remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48(9):851-855. doi: 10.1001/archpsyc.1991.01810330075011 [DOI] [PubMed] [Google Scholar]
- 6.Keller MB, Shapiro RW, Lavori PW, Wolfe N. Relapse in major depressive disorder: analysis with the life table. Arch Gen Psychiatry. 1982;39(8):911-915. doi: 10.1001/archpsyc.1982.04290080031005 [DOI] [PubMed] [Google Scholar]
- 7.Andrews PW, Kornstein SG, Halberstadt LJ, Gardner CO, Neale MC. Blue again: perturbational effects of antidepressants suggest monoaminergic homeostasis in major depression. Front Psychol. 2011;2:159. doi: 10.3389/fpsyg.2011.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaymaz N, van Os J, Loonen AJM, Nolen WA. Evidence that patients with single versus recurrent depressive episodes are differentially sensitive to treatment discontinuation: a meta-analysis of placebo-controlled randomized trials. J Clin Psychiatry. 2008;69(9):1423-1436. doi: 10.4088/JCP.v69n0910 [DOI] [PubMed] [Google Scholar]
- 9.Viguera AC, Baldessarini RJ, Friedberg J. Discontinuing antidepressant treatment in major depression. Harv Rev Psychiatry. 1998;5(6):293-306. doi: 10.3109/10673229809003578 [DOI] [PubMed] [Google Scholar]
- 10.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders 3rd ed. Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- 11.World Health Organization International Classification of Diseases and Related Health Problems, 10th Revision. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 12.McGlinchey JB, Zimmerman M, Young D, Chelminski I. Diagnosing major depressive disorder VIII: are some symptoms better than others? J Nerv Ment Dis. 2006;194(10):785-790. doi: 10.1097/01.nmd.0000240222.75201.aa [DOI] [PubMed] [Google Scholar]
- 13.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35(3):537-555. doi: 10.1016/j.neubiorev.2010.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Treadway MT, Bossaller NA, Shelton RC, Zald DH. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol. 2012;121(3):553-558. doi: 10.1037/a0028813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang XH, Huang J, Zhu CY, et al. . Motivational deficits in effort-based decision making in individuals with subsyndromal depression, first-episode and remitted depression patients. Psychiatry Res. 2014;220(3):874-882. doi: 10.1016/j.psychres.2014.08.056 [DOI] [PubMed] [Google Scholar]
- 16.Cooper JA, Arulpragasam AR, Treadway MT. Anhedonia in depression: biological mechanisms and computational models. Curr Opin Behav Sci. 2018;22:128-135. doi: 10.1016/j.cobeha.2018.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimidjian S, Hollon SD, Dobson KS, et al. . Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol. 2006;74(4):658-670. doi: 10.1037/0022-006X.74.4.658 [DOI] [PubMed] [Google Scholar]
- 18.Salamone JD, Correa M, Farrar AM, Nunes EJ, Pardo M. Dopamine, behavioral economics, and effort. Front Behav Neurosci. 2009;3:13. doi: 10.3389/neuro.08.013.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Husain M, Roiser JP. Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nat Rev Neurosci. 2018;19(8):470-484. doi: 10.1038/s41583-018-0029-9 [DOI] [PubMed] [Google Scholar]
- 20.Meyniel F, Goodwin GM, Deakin JW, et al. . A specific role for serotonin in overcoming effort cost. Elife. 2016;5:e17282. doi: 10.7554/eLife.17282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewinsohn P, Youngren M, Grosscup S. Reinforcement and depression In: Depue RA, ed. The Psychobiology of Depressive Disorders: Implications for the Effects of Stress. New York, NY: Academic Press; 1979:291-316. [Google Scholar]
- 22.Jacobson NS, Dobson KS, Truax PA, et al. . A component analysis of cognitive-behavioral treatment for depression. J Consult Clin Psychol. 1996;64(2):295-304. doi: 10.1037/0022-006X.64.2.295 [DOI] [PubMed] [Google Scholar]
- 23.Huys QJM, Pizzagalli DA, Bogdan R, Dayan P. Mapping anhedonia onto reinforcement learning: a behavioural meta-analysis. Biol Mood Anxiety Disord. 2013;3(1):12. doi: 10.1186/2045-5380-3-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 25.Dichter GS, Smoski MJ, Kampov-Polevoy AB, Gallop R, Garbutt JC. Unipolar depression does not moderate responses to the Sweet Taste Test. Depress Anxiety. 2010;27(9):859-863. doi: 10.1002/da.20690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gard DE, Germans Gard M, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: a scale development study. J Res Pers. 2006;40:1086-1102. doi: 10.1016/j.jrp.2005.11.001 [DOI] [Google Scholar]
- 27.Huys QJM, Daw ND, Dayan P. Depression: a decision-theoretic analysis. Annu Rev Neurosci. 2015;38:1-23. doi: 10.1146/annurev-neuro-071714-033928 [DOI] [PubMed] [Google Scholar]
- 28.Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol Psychiatry. 2013;74(2):130-136. doi: 10.1016/j.biopsych.2012.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 30.Wakefield JC, Schmitz MF. When does depression become a disorder? using recurrence rates to evaluate the validity of proposed changes in major depression diagnostic thresholds. World Psychiatry. 2013;12(1):44-52. doi: 10.1002/wps.20015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wittchen HU, Fydrich T. Strukturiertes klinisches Interview für DSM-IV: Manual zum SKID-I und SKID-II. Göttingen, DE: Hofgrefe; 1997. [Google Scholar]
- 32.Navarro DJ, Fuss IG. Fast and accurate calculations for first-passage times in Wiener diffusion models. J Math Psychol. 2009;53:222-230. doi: 10.1016/j.jmp.2009.02.003 [DOI] [Google Scholar]
- 33.Huys QJM, Cools R, Gölzer M, et al. . Disentangling the roles of approach, activation and valence in instrumental and pavlovian responding. PLoS Comput Biol. 2011;7(4):e1002028. doi: 10.1371/journal.pcbi.1002028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huys QJM. Bayesian approaches to learning and decision-making In: Anticevic A, Murray J, eds. Computational Psychiatry: Mathematical Modelling of Mental Illness. Cambridge, MA: Academic Press; 2017:247-271. [Google Scholar]
- 35.Lehr S. Mehrfachwahl-Wortschatz-Intelligenztest MWT-B. Balingen, DE: Spitta; 2005. [Google Scholar]
- 36.Wechsler D. Wechsler Adult Intelligence Scale—Fourth edition (WAIS-IV). San Antonio, TX: Psychological Corporation; 2014. [Google Scholar]
- 37.Reitan RM. Validity of the Trial Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271-276. doi: 10.2466/pms.1958.8.3.271 [DOI] [Google Scholar]
- 38.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26(3):477-486. doi: 10.1017/S0033291700035558 [DOI] [PubMed] [Google Scholar]
- 39.Huffziger S, Kühner C. Die Ruminationsfacetten Brooding und Reflection: Eine psychometrische Evaluation der deutschsprachigen Version RSQ-10D. Z Klin Psychol Psychother (Gott). 2012;41:38-46. doi: 10.1026/1616-3443/a000118 [DOI] [Google Scholar]
- 40.Ratcliff R, Smith PL. A comparison of sequential sampling models for two-choice reaction time. Psychol Rev. 2004;111(2):333-367. doi: 10.1037/0033-295X.111.2.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Resulaj A, Kiani R, Wolpert DM, Shadlen MN. Changes of mind in decision-making. Nature. 2009;461(7261):263-266. doi: 10.1038/nature08275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci. 2014;18(8):414-421. doi: 10.1016/j.tics.2014.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy PR, Robertson IH, Harty S, O’Connell RG. Neural evidence accumulation persists after choice to inform metacognitive judgments. Elife. 2015;4:e11946. doi: 10.7554/eLife.11946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stephan KE, Mathys C. Computational approaches to psychiatry. Curr Opin Neurobiol. 2014;25:85-92. doi: 10.1016/j.conb.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 45.Wang XJ, Krystal JH. Computational psychiatry. Neuron. 2014;84(3):638-654. doi: 10.1016/j.neuron.2014.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huys QJM, Maia TV, Frank MJ. Computational psychiatry as a bridge from neuroscience to clinical applications. Nat Neurosci. 2016;19(3):404-413. doi: 10.1038/nn.4238 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Methods
eAppendix 2. Results
eFigure 1. Physical Effort Task
eFigure 2. CONSORT Diagrams of the Main Sample
eFigure 3. CONSORT Diagrams of the Replication Sample
eFigure 4. Relapse Discontinuation Interaction Effect for Effort Execution Time
eFigure 5. Parameter Recovery
eFigure 6. Model Fits
eFigure 7. Model Comparison in the Main Sample
eFigure 8. Model Comparison in the Replication Sample
eFigure 9. Raw Behavioral Data and Model Fits in the Replication Sample
eFigure 10. Parameter Comparisons
eTable 1. Participant Characteristics for the Replication Sample from Berlin
eTable 2. Dropout Comparisons and Cox Regressions
eTable 3. ANOVA and LMEM Comparisons
eTable 4. Model Recovery
eTable 5. Winning Models
eTable 6. Split-Half and Test-Retest Reliability
eTable 7. Behavioral Effects for Fraction of Effortful Choices and Decision Time in the Replication Sample
eReferences.