This cross-sectional study identifies clinical, socioeconomic, personality, and mental health factors associated with health-related quality of life in patients with facial palsy.
Key Points
Question
What is the association of socioeconomic, personality, and mental health factors with health-related quality of life in patients with facial palsy?
Findings
This cross-sectional study found that health-related quality of life among 121 patients with facial palsy at a tertiary referral center for facial reanimation surgery appeared to be associated with age, bilateral facial palsy, severity of facial palsy, mental distress, and the personality traits of extraversion, conscientiousness, and emotional stability.
Meaning
It is important to assess socioeconomic, personality, and mental health factors when investigating health-related quality of life before and after facial palsy intervention to better interpret the results and evaluate treatment.
Abstract
Importance
Knowledge of factors associated with health-related quality of life in patients with facial palsy may aid in better interpreting outcomes of research and treatment.
Objective
To identify factors associated with health-related quality of life in patients with facial palsy.
Design, Setting, and Participants
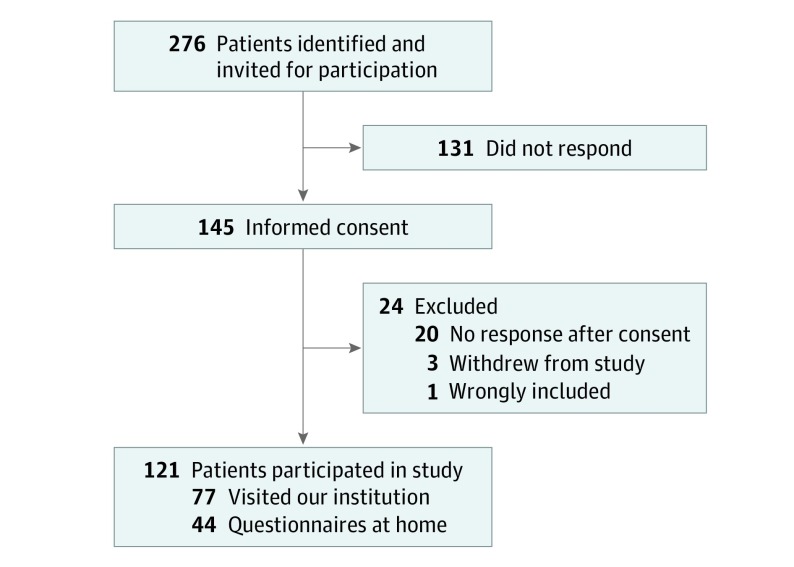
The inclusion period for participants in this cross-sectional study at the University Medical Center Groningen, a tertiary referral center for facial reanimation surgery, was March 1 to June 1, 2019. Patients aged at least 18 years with facial palsy who had undergone surgery for facial palsy between January 1, 2007, and January 1, 2018, and patients visiting the outpatient clinic of the University of Groningen Department of Plastic Surgery for their facial palsy between March 1 and June 1, 2019, were also asked to participate. Of 276 patients invited, 145 gave informed consent. Twenty patients did not respond after consent, 3 patients withdrew from the study, and 1 patient was wrongly included.
Main Outcomes and Measures
Health-related quality of life was measured using the Facial Clinimetric Evaluation Scale and the Facial Disability Index (physical score and social score). Facial function was assessed with the Sunnybrook Facial Grading System. Other variables were investigated using validated questionnaires, including the Duke University Religion Index, Ten-Item Personality Inventory, and Hospital Anxiety and Depression Scale. Multivariable linear regression analyses with stepwise backward selection were performed to identify associations with health-related quality of life. Because 44 Sunnybrook composite scores were missing, a sensitivity analysis was performed that excluded the Sunnybrook composite scores from the multivariable analysis.
Results
In total, 121 patients with facial palsy were included; their median age was 62 years (interquartile range, 48-71 years), and 63 (52%) were women. Sunnybrook composite score (β = 0.4; 95% CI, 0.2-0.5), extraversion (β = 2.6; 95% CI, 0.4-4.8), and anxiety (β = −2.4; 95% CI, −4.1 to −0.8) were associated with the Facial Clinimetric Evaluation Scale total score (R2 = 0.380; 95% CI, 0.212-0.548). The Sunnybrook composite score was associated with the Facial Disability Index physical score (β = 0.2; 95% CI, 0.0-0.4) (R2 = 0.084; 95% CI, −0.037 to 0.205). Bilateral facial palsy (β = −21.2; 95% CI, −32.3 to −10.1), extraversion (β = 2.7; 95% CI, 1.3-4.1), conscientiousness (β = 2.7; 95% CI, 0.2-5.2), emotional stability (β = 3.3; 95% CI, 1.7-4.8), and depression (β = −1.3; 95% CI, −2.5 to −0.1) were associated with the Facial Disability Index social score (R2 = 0.400; 95% CI, 0.262-0.538). In the sensitivity analysis, the Sunnybrook composite score was associated with age (Spearman ρ = −0.252).
Conclusions and Relevance
Bilateral facial palsy, age, severity of facial palsy, mental distress, and personality traits should be taken into account in future research and treatment of patients with facial palsy.
Introduction
Facial palsy alters the movement of mimic muscles and impedes expression of emotion (eg, smiling and being surprised).1 Eating and speaking may also be impeded because of facial palsy.2 Facial function can be assessed using facial grading systems, such as the Sunnybrook Facial Grading System (hereafter referred to as the Sunnybrook composite score).3 However, facial function alone does not represent all of the consequences of facial palsy on a patient’s health-related quality of life (HRQOL). In addition to its physical repercussions, facial palsy alters mental and social well-being.2,4,5 Validated instruments assessing HRQOL in patients with facial palsy include the Facial Clinimetric Evaluation (FaCE) Scale6 and the Facial Disability Index (FDI).7 Most patients with facial palsy and poor facial function experience lower HRQOL,2,4 although that association was not observed in studies8,9,10 examining facial palsy after acoustic neuroma surgery. This outcome suggests that some factors associated with HRQOL may be related to the cause of facial palsy. A study11 examining the role of religiosity in the quality of life of patients with facial palsy found that religious attendance was associated with HRQOL. Regarding mental well-being, depression and anxiety rates were found to be statistically significantly higher in patients with facial palsy than in control groups.5,12,13 Although anxiety has been associated with total HRQOL scores in patients with facial palsy, depression has not.2 In the literature,2,4 several factors explain 26% to 43% of the variance of HRQOL scores in patients with facial palsy, including sex, duration of facial palsy, facial function, anxiety, and oral commissure movement with smiling.2,14,15
It is unknown whether socioeconomic and personality factors alter HRQOL in patients with facial palsy, although such associations have been observed in patients with heart conditions.16,17 Those factors include marital status, educational level, occupational status, monthly income, and personality. Identifying factors associated with quality of life in patients with facial palsy may facilitate interpretation of outcomes of treatment in individual patients and outcomes of research in patients with facial palsy. Therefore, this study aimed to identify factors associated with HRQOL in patients with facial palsy, focusing on socioeconomic and personality factors. We hypothesized that socioeconomic and personality factors would be associated with HRQOL in patients with facial palsy. Furthermore, we expected to find associations with HRQOL that have been reported in the literature, such as severity of facial palsy and mental well-being.
Methods
Formal medical ethics review of this study was waived by the Institutional Review Board of the University Medical Center Groningen (UMCG), Groningen, the Netherlands, because it did not fall within the scope of the Medical Research Involving Human Subjects Act. Before participating in the study, written informed consent was obtained from all patients.
Data Collection
This cross-sectional study was conducted at the UMCG Department of Plastic Surgery, a tertiary referral center for facial reanimation surgery. Patients who had undergone surgery for facial palsy between January 1, 2007, and January 1, 2018, were eligible and received an invitation letter for participation. Because the inclusion period for participants was March 1 to June 1, 2019, patients visiting the outpatient clinic of the University of Groningen’s Department of Plastic Surgery for facial palsy in the same period were also asked to participate. Patients younger than 18 years were excluded because the HRQOL questionnaires used are only validated for adults. Participants were asked to provide medical photographs, have a video taken of the movement of their face, and complete questionnaires. When patients were not able to visit the UMCG, they completed questionnaires at home, and no photographs or videos were obtained.
The HRQOL was assessed using the Dutch versions of the FaCE Scale and the FDI. The FaCE Scale includes 15 questions with 5-point Likert-type responses (with 1 being the worst facial function and 5 being the best facial function).6 The FaCE Scale total score ranges from 0 (worst) to 100 (best). The FDI consists of 2 scales (FDI physical score and FDI social score), with each including 5 questions with 5-point Likert-type responses.7 Both scales range from 0 (worst) to 100 (best). The FDI has been translated according to a forward-backward method by 2 independent translators and validated for the Dutch population (B. ten Hoope, MD, and N. Talukder, BSc, unpublished data, 2019).
Five personality traits were assessed using the Dutch version of the Ten-Item Personality Inventory (TIPI).18 In this questionnaire, the participant judges in 10 pairs of traits to what extent each pair is applicable to his or her own personality by scoring the pairs from 1 (disagree strongly) to 7 (agree strongly). A score ranging from 1 to 7 is then calculated for extraversion, agreeableness, conscientiousness, emotional stability, and openness to experiences, with higher scores indicating the stronger presence of a personality trait.
The Dutch version of the Duke University Religion Index (DUREL) was used to measure the degree of religiosity.19 The questionnaire includes 5 questions assessing the following 3 aspects of religiosity: organizational religious activity (1 question), nonorganizational religious activity (1 question), and intrinsic religiosity (3 questions). Higher scores indicate that a person is more religious.
Anxiety and depression were assessed using the Dutch version of the Hospital Anxiety and Depression Scale (HADS).20 The HADS evaluates psychological distress with 14 questions divided into an anxiety scale and a depression scale. Each scale ranges from 0 to 21, with scores of 7 or lower indicating no anxiety or depression, 8 to 10 indicating moderate anxiety or depression, and 11 or higher indicating severe anxiety or depression.
With the Sunnybrook composite score, standardized photographs and videos were used to grade facial function.3 This scoring system has 3 components, including resting symmetry, voluntary movement, and synkinesis. The scores of the 3 components are converted into a total composite score, which ranges from 0 (complete paralysis) to 100 (normal function).
Statistical Analysis
The number of patients needed in our study was based on the following rule for multiple linear regression: for every variable appearing to be statistically significant in univariate analysis, 10 to 15 persons should be included in the study. We planned to explore 11 variables; therefore, a maximum of 110 to 165 persons was needed.
All statistical analyses were performed using SPSS Statistics for Windows, version 23.0 (IBM Corp). All statistical tests were 2 sided, and α = .05 was considered statistically significant.
Simple linear regression analyses were performed between the potential factors and the outcomes of the FaCE Scale total score, FDI physical score, and FDI social score separately. For categorical variables, dummy variables were constructed. All factors univariately associated with the outcome variable at P < .20 were entered into multiple linear regression analyses. For each outcome variable, a separate analysis was performed with a manual backward selection process until all P values were less than or equal to .05. The R2 values were calculated as a measure of model fit (explained variance). We calculated 95% CIs around the R2 values by bootstrapping.
We expected that some participants would not be willing or able to visit the UMCG (owing to distance from the institution); therefore, a recent Sunnybrook composite score could not be obtained. Because 44 Sunnybrook composite scores were missing, a sensitivity analysis was thus performed that excluded these scores from the multivariable analysis if the Sunnybrook composite scores were associated with the outcome measured in simple linear regression analyses. This consisted of one analysis that included the Sunnybrook composite score and another analysis of the total population that excluded this score. The differences between the analyses were then compared. In all multiple linear regression analyses, assumptions were checked and fulfilled. If the results needed clarification, post hoc analyses were conducted.
Results
In total, 276 patients with facial palsy were identified and invited for participation (Figure). Written informed consent was provided by 145 patients. Twenty patients did not respond after consent, 3 patients withdrew from the study, and 1 patient was wrongly included, leaving 121 patients (44%) to be included in this study. Their median age was 62 years (interquartile range, 48-71 years), and 63 (52%) were women (Table 1). Seventy-seven patients visited the UMCG, and the remaining 44 patients completed questionnaires at home. All patients had undergone surgery for their facial palsy. The most common cause was benign acoustic neuroma (31 [26%]), followed by Bell palsy (11 [9%]), benign parotid tumor (10 [8%]), trauma of soft tissue and temporal bone fracture (10 [8%]), and congenital (10 [8%]) (eTable 1 in the Supplement).
Figure. Flowchart of Patient Inclusion.
All patients had undergone surgery for their facial palsy.
Table 1. Patient Characteristics.
| Variable | No. (%) or Median (IQR)a |
|---|---|
| General | |
| Sex | |
| Female | 63 (52) |
| Age, y | 62 (48-71) |
| Marital status (n = 120) | |
| No relationship | 30 (25) |
| Inhabitants residing per municipality | 55 939 (33 634-127 492) |
| DUREL subscale 1 (n = 120) | |
| Organizational religious activity never | 87 (72) |
| DUREL subscale 2 | |
| Nonorganizational religious activity never | 76 (63) |
| DUREL subscale 3 (n = 118) | 5.0 (3.0-11.0) |
| Intrinsic religiosity, 3-15 scale | |
| Socioeconomic Status | |
| Educational level (n = 120) | |
| Lower | 79 (65) |
| Occupational status (n = 119) | |
| Working | 67 (55) |
| Net income per month (n = 111) | |
| Low, <€1400 (<US $1562) | 37 (31) |
| Middle, €1400-€2400 (US $1562-$2678) | 41 (34) |
| High, >€2400 (>US $2678) | 33 (27) |
| Facial Palsy Related | |
| Duration of facial palsy, y | 12 (7-27) |
| Laterality of facial palsy | |
| Left | 57 (47) |
| Right | 59 (49) |
| Bilateral | 5 (4) |
| Cause | |
| Benign tumor | 44 (36) |
| Infection | 22 (18 |
| Trauma | 18 (15) |
| Head and neck cancer | 13 (11) |
| Congenital | 10 (8) |
| Other | 14 (12) |
| Sunnybrook composite score, 0-100 scale (n = 77) | 30 (25-42) |
| Personality | |
| Ten-Item Personality Inventory, 1-7 scale (n = 117) | |
| Extraversion | 4.5 (3.0-6.0) |
| Agreeableness | 4.0 (3.5-4.5) |
| Conscientiousness | 6.5 (5.5-7.0) |
| Emotional stability | 5.5 (4.0-6.5) |
| Openness to experiences | 5.0 (4.5-6.5) |
| Mental | |
| HADS anxiety, 0-21 scale (n = 120) | |
| No anxiety, ≤7 points | 45 (37) |
| Moderate anxiety, 8-10 points | 55 (45) |
| Severe anxiety, ≥11 points | 20 (17) |
| HADS depression, 0-21 scale (n = 117) | |
| No depression, ≤7 points | 11 (9) |
| Moderate depression, 8-10 points | 71 (59) |
| Severe depression, ≥11 points | 35 (29) |
| Health-Related Quality of Life, 0-100 Scale | |
| FaCE Scale total score (n = 111) | 52 (5.0-93.3) |
| FDI physical score (n = 109) | 70 (63-80) |
| FDI social score (n = 111) | 76 (64-84) |
Abbreviations: DUREL, Duke University Religion Index; FaCE, Facial Clinimetric Evaluation; FDI, Facial Disability Index; HADS, Hospital Anxiety and Depression Scale; IQR, interquartile range; Sunnybrook, Sunnybrook Facial Grading System.
Percentages are based on the total number of included patients (n = 121) unless otherwise indicated.
Overall results showed that the Sunnybrook composite score (β = 0.4; 95% CI, 0.2-0.5), extraversion (β = 2.6; 95% CI, 0.4-4.8), and anxiety (β = −2.4; 95% CI, −4.1 to −0.8) were associated with the FaCE Scale total score (R2 = 0.380; 95% CI, 0.212-0.548). The Sunnybrook composite score was associated with the FDI physical score (β = 0.2; 95% CI, 0.0-0.4) (R2 = 0.084; 95% CI, −0.037 to 0.205). Bilateral facial palsy (β = −21.2; 95% CI, −32.3 to −10.1), extraversion (β = 2.7; 95% CI, 1.3-4.1), conscientiousness (β = 2.7; 95% CI, 0.2-5.2), emotional stability (β = 3.3; 95% CI, 1.7-4.8), and depression (β = −1.3; 95% CI, −2.5 to −0.1) were associated with the FDI social score (R2 = 0.400; 95% CI, 0.262-0.538).
Specifically, simple linear regression analyses identified 11 factors associated with the FaCE Scale total score, including sex, age, nonorganizational religious activity, occupational status, duration of facial palsy, bilateral facial palsy, Sunnybrook composite score, extraversion, emotional stability, openness to experiences, and anxiety (eTable 2 in the Supplement). In multiple linear regression analyses with stepwise backward selection, the Sunnybrook composite score and extraversion were positively associated with the FaCE Scale total score, and anxiety was negatively associated with the FaCE Scale total score (with positively and negatively indicating the direction of β of the variable) (Table 2). The model fit (R2) was 0.380 (95% CI, 0.212-0.548), meaning that these 3 factors combined explained 38% of the variance of the FaCE Scale total score. After removing the Sunnybrook composite score from the analysis, age, bilateral facial palsy, and anxiety were negatively associated with the FaCE Scale total score, and extraversion was positively associated with the FaCE Scale total score (R2 = 0.205; 95% CI, 0.076-0.334) (Table 2).
Table 2. Multiple Linear Regression Analysis of the Facial Clinimetric Evaluation Scale Total Score.
| Variable | Including Sunnybrook Composite Score (n = 70)a | Excluding Sunnybrook Composite Score (n = 108)b | ||
|---|---|---|---|---|
| β (95% CI) | P Value | β (95% CI) | P Value | |
| Intercept | 50.2 (31.2 to 69.2) | >.001 | 74.7 (52.5 to 96.9) | >.001 |
| Age | NA | NA | −0.2 (−0.4 to 0.0) | .02 |
| Laterality of facial palsyc | NA | NA | −19.0 (−33.8 to −4.2) | .01 |
| Ten-Item Personality Inventory | ||||
| Extraversion | 2.6 (0.4 to 4.8) | .02 | 1.9 (0.3 to 3.8) | .047 |
| Anxiety | −2.4 (−4.1 to −0.8) | .005 | −2.1 (−3.7 to −0.5) | .009 |
| Sunnybrook composite score | 0.4 (0.2 to 0.5) | >.001 | NA | NA |
Abbreviations: NA, not applicable.
R2 = 0.380; 95% CI, 0.212 to 0.548.
R2 = 0.205; 95% CI, 0.076 to 0.334.
Unilateral is 0 and bilateral is 1.
Simple linear regression analyses identified 8 factors associated with the FDI physical score, including age, marital status, organizational religious activity, educational level, occupational status, cause, Sunnybrook composite score, and emotional stability (eTable 3 in the Supplement). In multiple linear regression analyses, the Sunnybrook composite score was positively associated with the FDI physical score (R2 = 0.084; 95% CI, −0.037 to 0.205) (Table 3). After removing the Sunnybrook composite score from the analysis, age was negatively associated with the FDI physical score (R2 = 0.040; 95% CI, −0.031 to 0.111), explaining 8% of the variance in the FDI physical score (Table 3).
Table 3. Multiple Linear Regression Analysis of the Facial Disability Index Physical Score.
| Variable | Including Sunnybrook Composite Score (n = 69)a | Excluding Sunnybrook Composite Score (n = 109)b | ||
|---|---|---|---|---|
| β (95% CI) | P Value | β (95% CI) | P Value | |
| Intercept | 64.2 (57.3 to 71.0) | >.001 | 80.1 (69.6 to 90.6) | >.001 |
| Age | NA | NA | −0.2 −(0.4 to 0.0) | .04 |
| Sunnybrook composite score | 0.2 (0.0 to 0.4) | .02 | NA | NA |
Abbreviations: NA, not applicable.
R2 = 0.084; 95% CI, −0.037 to 0.205.
R2 = 0.040; 95% CI, −0.031 to 0.111.
Simple linear regression analyses identified 9 factors associated with the FDI social score, including marital status, bilateral facial palsy, cause, extraversion, conscientiousness, emotional stability, openness to experiences, anxiety, and depression (eTable 4 in the Supplement). In multiple linear regression analyses, bilateral facial palsy and depression were negatively associated with the FDI social score, and extraversion, conscientiousness, and emotional stability were positively associated with the FDI social score (R2 = 0.400; 95% CI, 0.262-0.538), explaining 40% of the variance in the FDI social score (Table 4).
Table 4. Multiple Linear Regression Analysis of the Facial Disability Index Social Score Among 103 Patientsa.
| Variable | β (95% CI) | P Value |
|---|---|---|
| Intercept | 42.2 (21.1 to 63.3) | >.001 |
| Laterality of facial palsyb | −21.2 (−32.3 to −10.1) | <.001 |
| Ten-Item Personality Inventory | ||
| Extraversion | 2.7 (1.3 to 4.1) | .001 |
| Conscientiousness | 2.7 (0.2 to 5.2) | .03 |
| Emotional stability | 3.3 (1.7 to 4.8) | <.001 |
| Depression | −1.3 (−2.5 to −0.1) | .03 |
R2 = 0.400; 95% CI, 0.262 to 0.538.
Unilateral is 0 and bilateral is 1.
Two post hoc analyses were conducted. In the first analysis, we examined the association between age and the Sunnybrook composite score because age appeared to be associated with the FaCE Scale total score and with the FDI physical score after the Sunnybrook composite score was excluded from the analyses (Spearman ρ = −0.252; P = .03). In the second analysis, an independent t test was used to test the difference in the means of FaCE Scale total scores of 71 patients who visited the UMCG (mean [SD], 54.7 [16.4]) and 40 patients who did not visit the UMCG (mean [SD], 46.1 [18.5]). Ten patients (6 who visited the UMCG and 4 who did not visit the UMCG) had a missing FaCE Scale total score. The difference in means was 8.6 (95% CI, 1.9-15.3; P = .01).
Discussion
The Sunnybrook composite score, extraversion, and anxiety appeared to be associated with the FaCE Scale total score. Removing the Sunnybrook composite score from the analysis resulted in associations with age and bilateral facial palsy. The Sunnybrook composite score was the only variable associated with the FDI physical score. When removing the Sunnybrook composite score from the multivariable analysis, age became the only factor associated with the FDI physical score. In the final multivariable analysis, bilateral facial palsy, extraversion, conscientiousness, emotional stability, and depression were associated with the FDI social score.
Findings in Relation to the Literature
A systematic review21 examining the association between personality characteristics and HRQOL in persons with different health states (eg, Parkinson disease, total hip replacement, and breast cancer) showed that traits characterized by positive affectivity, such as extraversion and agreeableness, were associated with higher HRQOL, whereas traits characterized by a negative affectivity, such as neuroticism, were associated with lower HRQOL. These outcomes support the findings in our study that extraversion was associated with a higher FaCE Scale total score and that extraversion, conscientiousness, and emotional stability were associated with a higher FDI social score. Personality traits were not associated with the FDI physical score, suggesting that personality does not alter a patient’s perception of his or her facial function, but it does alter the social consequences of facial palsy. Previous research suggests that the association between personality traits and well-being is a result of more effective coping strategies in people with personality traits characterized by positive affectivity.22
Another study2 examining factors that predict HRQOL in patients with facial palsy found an association between high anxiety levels and low HRQOL, similar to the results of the present study. Anxiety seemed to alter overall HRQOL and not specifically physical function or social function. Although depression has been associated with facial palsy, no association between depression and HRQOL in patients with facial palsy has been described in the literature.2,5,13 In the present study, greater depression was associated with lower FDI social scores. An explanation for these different findings may be the duration of facial palsy. Patients manifesting facial palsy for a longer period may have become adjusted to their condition and thus report fewer depressive symptoms. Patients included in this study were in that long-standing facial palsy category, with a median duration of facial palsy of 12 years (interquartile range, 7-27 years) (Table 1).
The Sunnybrook composite score was associated with the FDI physical score. In univariate analysis, R2 was 0.24; after removing the Sunnybrook composite score from the multivariable analysis, R2 decreased from 0.380 to 0.205. Because the Sunnybrook composite score and the FDI physical score both measure facial function of the affected side but from a different point of view (clinician vs patient), this association was expected. The Sunnybrook composite score was not associated with the FDI social score, suggesting that distortion of the face as objectively graded by a clinician is less important than personality traits and unilateral or bilateral facial palsy when it comes to social function. As stated earlier, 26% to 43% of the variance of HRQOL scores in patients with facial palsy was explained in the literature.2,14,15 In our study, 38% of the variance of the FaCE Scale total score was explained, and 40% of the FDI social score was explained (Table 4), which is within the range found in previous studies.2,14,15 Only 8% of the FDI physical score could be explained in the present study (Table 3), suggesting a large discrepancy between facial function graded by a clinician and facial function assessed by the patient.
In the literature,4,23 age has been associated with the FaCE Scale total score, FaCE subdomain scores, and both FDI physical score and FDI social score. In these previous studies, age and facial function appear together as HRQOL score predictors. In this study, age was associated with the FaCE Scale total score and with lower FDI physical scores after excluding the Sunnybrook composite score from the multivariable analysis. This finding can be explained by the results of our post hoc analysis, which showed a statistically significant negative association between age and the Sunnybrook composite score. If it is assumed that older people do not have worse facial function, the association found in the present study could be the result of sample variation. Another explanation might be that older individuals have less elastic skin, which causes asymmetry at rest to be more pronounced. Hypothetically, outcomes regarding recovery from facial palsy may be worse in older age groups than in younger age groups (eg, after Bell palsy or nerve reinnervating surgery). In the multivariable analysis, post hoc analysis showed that study sample age in our study was associated with the Sunnybrook composite score.
We found that bilateral facial palsy was associated with the FDI social score, which can be explained by the greater social disability experienced by patients with bilateral facial palsy due to their inability to express emotion by means of facial movement.24 Although only 5 patients in the present study had bilateral facial palsy, a statistically significant association was found between bilateral facial palsy and the FDI social score (Table 4).
Clinical Relevance
This study provides insight into factors that are important when measuring HRQOL in patients with facial palsy, including age, bilateral facial palsy, severity of facial palsy, mental distress, and the personality traits of extraversion, conscientiousness, and emotional stability. Observational studies in facial palsy research are the norm, and these factors should be taken into account. Furthermore, it may be beneficial for patients when the clinician considers these factors in discussing facial palsy treatment because mental distress and personality traits are often overlooked.
Strengths and Limitations
A strength of this study is that patients were assessed with validated instruments. Furthermore, measuring HRQOL with 2 instruments (the FDI physical score and the FDI social score) enabled us to examine the association of factors not only with overall HRQOL but also with physical function and social function separately.
This study has limitations. When interpreting the results, one must keep in mind that this study calculates the HRQOL of a population. Population-based HRQOL is suitable for guiding clinical work but might not be a correct reflection of the needs of individual patients. Furthermore, the causes of facial palsy varied among the patients participating in this study. There were few patients with Bell palsy in the sample and more patients with acoustic neuroma, which is consistent with the UMCG Department of Plastic Surgery being a tertiary referral center that mainly performs facial reanimation surgery. Different causes of facial palsy could be associated with a discrepancy in the measured severity of facial palsy and in patients’ perceived HRQOL. The findings of a study25 comparing self-reported disability between patients with Bell palsy and those with acoustic neuroma suggest that perceived disability mainly depends on patients’ expectations and perceived control regarding the course of their condition. Patients with Bell palsy or other nonsurgical causes of facial palsy are acutely confronted with facial dysfunction, whereas patients with surgical causes of facial palsy have had the time to learn about this condition and make an informed decision regarding surgery based on the risks. Performing the present study at a plastic surgery outpatient clinic over a time span of more than 10 years implies that almost all patients included in this study had surgery for their facial palsy and are no longer in the acute phase of this condition. Therefore, the results of this study cannot be generalized to all patients with facial palsy, and interpretation of the findings may be particularly challenging for patients with less severe or short-term facial dysfunction.
Another limitation of this study is that many patients did not visit the UMCG and instead completed questionnaires at home. Post hoc analysis showed a statistically significantly lower FaCE Scale total score among patients who did not visit the UMCG compared with patients who did, indicating selection bias. Patients who completed questionnaires at home possibly found visiting the hospital too difficult, too far away, or the last appointment too long ago.
Conclusions
Age, bilateral facial palsy, severity of facial palsy, mental distress, and personality traits are associated with HRQOL in patients with long-standing facial palsy. It is important to take these and other factors identified in this study into account in future research and treatment of patients with facial palsy.
eTable 1. Detailed Patient Characteristics
eTable 2. Univariate Analysis FaCE Scale Total Score
eTable 3. Univariate Analysis FDI Physical Score
eTable 4. Univariate Analysis FDI Social Score
References
- 1.Gordin E, Lee TS, Ducic Y, Arnaoutakis D. Facial nerve trauma: evaluation and considerations in management. Craniomaxillofac Trauma Reconstr. 2015;8(1):1-13. doi: 10.1055/s-0034-1372522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tavares-Brito J, van Veen MM, Dusseldorp JR, Bahmad F Jr, Hadlock TA. Facial palsy–specific quality of life in 920 patients: correlation with clinician-graded severity and predicting factors. Laryngoscope. 2019;129(1):100-104. doi: 10.1002/lary.27481 [DOI] [PubMed] [Google Scholar]
- 3.Fattah AY, Gurusinghe ADR, Gavilan J, et al. ; Sir Charles Bell Society . Facial nerve grading instruments: systematic review of the literature and suggestion for uniformity. Plast Reconstr Surg. 2015;135(2):569-579. doi: 10.1097/PRS.0000000000000905 [DOI] [PubMed] [Google Scholar]
- 4.Kleiss IJ, Hohman MH, Susarla SM, Marres HA, Hadlock TA. Health-related quality of life in 794 patients with a peripheral facial palsy using the FaCE Scale: a retrospective cohort study. Clin Otolaryngol. 2015;40(6):651-656. doi: 10.1111/coa.12434 [DOI] [PubMed] [Google Scholar]
- 5.Nellis JC, Ishii M, Byrne PJ, Boahene KDO, Dey JK, Ishii LE. Association among facial paralysis, depression, and quality of life in facial plastic surgery patients. JAMA Facial Plast Surg. 2017;19(3):190-196. doi: 10.1001/jamafacial.2016.1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahn JB, Gliklich RE, Boyev KP, Stewart MG, Metson RB, McKenna MJ. Validation of a patient-graded instrument for facial nerve paralysis: the FaCE Scale. Laryngoscope. 2001;111(3):387-398. doi: 10.1097/00005537-200103000-00005 [DOI] [PubMed] [Google Scholar]
- 7.VanSwearingen JM, Brach JS. The Facial Disability Index: reliability and validity of a disability assessment instrument for disorders of the facial neuromuscular system. Phys Ther. 1996;76(12):1288-1298. doi: 10.1093/ptj/76.12.1288 [DOI] [PubMed] [Google Scholar]
- 8.Baumann I, Polligkeit J, Blumenstock G, Mauz PS, Zalaman IM, Maassen MM. Quality of life after unilateral acoustic neuroma surgery via middle cranial fossa approach. Acta Otolaryngol. 2005;125(6):585-591. doi: 10.1080/00016480510026935 [DOI] [PubMed] [Google Scholar]
- 9.Cross T, Sheard CE, Garrud P, Nikolopoulos TP, O’Donoghue GM. Impact of facial paralysis on patients with acoustic neuroma. Laryngoscope. 2000;110(9):1539-1542. doi: 10.1097/00005537-200009000-00024 [DOI] [PubMed] [Google Scholar]
- 10.Lassaletta L, Alfonso C, Del Rio L, Roda JM, Gavilan J. Impact of facial dysfunction on quality of life after vestibular schwannoma surgery. Ann Otol Rhinol Laryngol. 2006;115(9):694-698. doi: 10.1177/000348940611500908 [DOI] [PubMed] [Google Scholar]
- 11.Lucchetti G, De Rossi J, Gonçalves JPB, Lucchetti ALG. Peripheral facial palsy: does patients’ religiousness matter for the otorhinolaryngologist? J Relig Health. 2016;55(3):856-861. doi: 10.1007/s10943-015-0062-1 [DOI] [PubMed] [Google Scholar]
- 12.Fu L, Bundy C, Sadiq SA. Psychological distress in people with disfigurement from facial palsy. Eye (Lond). 2011;25(10):1322-1326. doi: 10.1038/eye.2011.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saadi R, Shokri T, Schaefer E, Hollenbeak C, Lighthall JG. Depression rates after facial paralysis. Ann Plast Surg. 2019;83(2):190-194. doi: 10.1097/SAP.0000000000001908 [DOI] [PubMed] [Google Scholar]
- 14.van Veen MM, Quatela O, Tavares-Brito J, et al. . Patient-perceived severity of synkinesis reduces quality of life in facial palsy: a cross-sectional analysis in 92 patients. Clin Otolaryngol. 2019;44(3):483-486. doi: 10.1111/coa.13322 [DOI] [PubMed] [Google Scholar]
- 15.van Veen MM, Tavares-Brito J, van Veen BM, et al. . Association of regional facial dysfunction with facial palsy–related quality of life. JAMA Facial Plast Surg. 2019;21(1):32-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Israelsson J, Thylén I, Strömberg A, Bremer A, Årestedt K. Factors associated with health-related quality of life among cardiac arrest survivors treated with an implantable cardioverter-defibrillator. Resuscitation. 2018;132:78-84. doi: 10.1016/j.resuscitation.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 17.Luttik ML, Jaarsma T, Veeger N, van Veldhuisen DJ. Marital status, quality of life, and clinical outcome in patients with heart failure. Heart Lung. 2006;35(1):3-8. doi: 10.1016/j.hrtlng.2005.08.001 [DOI] [PubMed] [Google Scholar]
- 18.Gosling SD, Rentfrow PJ, Swann WB Jr. A very brief measure of the Big-Five personality domains. J Res Pers. 2003;37(6):504-528. doi: 10.1016/S0092-6566(03)00046-1 [DOI] [Google Scholar]
- 19.Koenig H, Parkerson GR Jr, Meador KG. Religion index for psychiatric research. Am J Psychiatry. 1997;154(6):885-886. doi: 10.1176/ajp.154.6.885b [DOI] [PubMed] [Google Scholar]
- 20.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361-370. doi: 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 21.Huang IC, Lee JL, Ketheeswaran P, Jones CM, Revicki DA, Wu AW. Does personality affect health-related quality of life? a systematic review. PLoS One. 2017;12(3):e0173806. doi: 10.1371/journal.pone.0173806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Afshar H, Roohafza HR, Keshteli AH, Mazaheri M, Feizi A, Adibi P. The association of personality traits and coping styles according to stress level. J Res Med Sci. 2015;20(4):353-358. [PMC free article] [PubMed] [Google Scholar]
- 23.Volk GF, Granitzka T, Kreysa H, Klingner CM, Guntinas-Lichius O. Initial severity of motor and non-motor disabilities in patients with facial palsy: an assessment using patient-reported outcome measures. Eur Arch Otorhinolaryngol. 2017;274(1):45-52. doi: 10.1007/s00405-016-4018-1 [DOI] [PubMed] [Google Scholar]
- 24.Strobel L, Renner G. Quality of life and adjustment in children and adolescents with Moebius syndrome: evidence for specific impairments in social functioning. Res Dev Disabil. 2016;53-54:178-188. doi: 10.1016/j.ridd.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 25.Saito DM, Cheung SW. A comparison of facial nerve disability between patients with Bell’s palsy and vestibular schwannoma. J Clin Neurosci. 2010;17(9):1122-1125. doi: 10.1016/j.jocn.2010.01.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Detailed Patient Characteristics
eTable 2. Univariate Analysis FaCE Scale Total Score
eTable 3. Univariate Analysis FDI Physical Score
eTable 4. Univariate Analysis FDI Social Score