Key Points
Question
Are racial/ethnic disparities in childhood and adolescent cancer survival associated with treatment amenability?
Finding
In this cohort study of 67 061 US children and adolescents, children and adolescents with racial/ethnic minority status had worse cancer survival compared with non-Hispanic white children and adolescents. Among non-Hispanic black and Hispanic (all races) children and adolescents, the disparity was generally greater for cancer types with higher vs lower relative survival rates.
Meaning
Survival disparities among racial/ethnic minority children and adolescents appear to be greater for cancer types that are generally more amenable to medical intervention.
Abstract
Importance
Although US cancer survival rates have increased over time, disparities by race/ethnicity remain, including for children and adolescents.
Objective
To examine whether racial/ethnic disparities in childhood and adolescent cancer survival vary by cancer type according to relative survival rates (RSRs), a marker for amenability to medical intervention.
Design, Setting, and Participants
In a retrospective cohort study using US Surveillance, Epidemiology, and End Results data, 67 061 children and adolescents diagnosed at ages 0 to 19 years with a first primary malignant cancer from January 1, 2000, to December 31, 2016, were evaluated. Data analysis was performed from June 19 to November 3, 2019. Participants were followed up from the dates of diagnosis to cancer death or the end of the follow-up period, whichever came first.
Exposures
Race/ethnicity defined as non-Hispanic white, non-Hispanic black, non-Hispanic American Indian/Alaskan Native, non-Hispanic Asian or Pacific Islander, or Hispanic (any race).
Main Outcomes and Measures
Cancer amenability was defined using 5-year RSRs for 103 cancer types. Cox proportional hazards regression was used to compute adjusted hazard ratios (aHRs) and 95% CIs for the association between race/ethnicity and cancer survival for high (>85% RSR), medium (70%-85% RSR), and low (<70% RSR) amenability categories.
Results
Among 67 061 cancer cases, 36 064 were male (53.8%); most individuals were non-Hispanic white (35 186 [52.5%]) followed by Hispanic of any race (19 220 [28.7%]), non-Hispanic black (7100 [10.6%]), non-Hispanic Asian or Pacific Islander (4981 [7.4%]), and non-Hispanic American Indian/Alaskan Native (574 [0.9%]). Mean (SD) age at diagnosis was 9.66 (6.41) years. Compared with non-Hispanic white children and adolescents, a higher aHR of death was observed for high- than low-amenability cancers for non-Hispanic black patients (high: aHR, 1.59; 95% CI, 1.41-1.80 vs low: aHR, 1.35; 95% CI, 1.24-1.47; P = .002 for interaction) and Hispanic (any race) patients (high: aHR, 1.63; 95% CI, 1.50-1.78 vs low: aHR, 1.16; 95% CI, 1.08-1.25; P < .001 for interaction). Results for other race/ethnicities showed similar patterns but were not statistically significant.
Conclusions and Relevance
Racial/ethnic minority children and adolescents were observed to have a higher risk of death than non-Hispanic white children and adolescents, with more amenable cancers having larger relative survival differences. This disparity may be associated with a combination of factors, including differences in access to health care resources.
This cohort study examines the association between race/ethnicity and cancer outcomes in children and adolescents of differing race/ethnicity.
Introduction
In 2019, an estimated 10 060 cancers were diagnosed among children aged 0 to 14 years in the United States, and 1190 were estimated to have died from the disease.1 Leukemia is the most prevalent cancer among children in this age group, accounting for 28% of all cases, followed by central nervous system cancers (26%).1 Among adolescents aged 15 to 19 years, the most common cancers are central nervous system cancers (21%) and lymphoma (20%). Five-year survival rates vary widely by cancer type, ranging from 66.4% to 99.7% for children and 46.2% to 99.2% for adolescents.1
Although childhood and adolescent cancer incidence rates have increased slightly from 1975 to 2006, mortality rates have dropped by more than 50%.2 The greatest mortality reductions have occurred for leukemia, gonadal cancer, and lymphoma, which have helped to avert an estimated 38 032 childhood and adolescent cancer deaths from 1975 to 2006.2 Improvements in childhood cancer care over the past several decades have led to substantial increases in 5-year relative survival rates (RSRs) from 58% in the mid-1970s to 83% in 2014.1 Investments in basic and clinical research have led to multimodal treatment strategies associated with this marked increase in survival among children and adolescents with cancer.3,4
Despite improvements in cancer survival for all racial/ethnic groups, there is evidence that survival improvements have benefited some groups more than others,5 particularly non-Hispanic white individuals and those with higher educational attainment.6 In a study of racial disparities in childhood and adolescent cancer survival over time, Pui et al7 reported a survival disparity between black and white patients for acute myeloid leukemia and neuroblastoma, which worsened from the earlier (1992-2000) to the later (2001-2007) cohort. This difference may indicate a disproportionate benefit from medical advances for certain races, which may manifest through factors associated with race, such as socioeconomic status, health care access, health literacy, treatment quality, and enrollment in clinical trials.8,9
Among children and adolescents, racial/ethnic disparities in cancer survival are well documented, particularly for those of African American and Hispanic descent.10,11,12,13 However, to our knowledge, no previous study has examined how these disparities compare for cancer types of varying survivability. Survival probability is an indicator of how amenable a cancer is to medical intervention.14,15 Studies in adults have shown greater racial/ethnic survival disparities for cancers with higher amenability than those with lower amenability.14,16 Cancers that are more amenable to medical intervention may provide greater opportunities for disparities to manifest, as those with fewer resources may have greater challenges obtaining a timely diagnosis and optimal treatment course.17 Using data from the US Surveillance, Epidemiology, and End Results (SEER) 18 registries18—a large, population-based database—we examined the association between race/ethnicity and childhood and adolescent cancer survival by varying levels of amenability as defined by RSRs, hypothesizing that disparities will decrease with decreasing amenability.
Methods
Study Population
We obtained data for this retrospective cohort study from the SEER 18 registries database18 on individuals diagnosed with cancer between birth and 19 years. We restricted the study population to individuals diagnosed with a first primary cancer from January 1, 2000, through December 31, 2016. Data analysis was performed from June 19 to November 3, 2019. We excluded individuals diagnosed with other and unspecified cancers according to the International Classification of Childhood Cancer (ICCC).19 Participants were followed up from the date of diagnosis to the date of cancer death or the end of the follow-up period, whichever came first. This study was exempt from institutional review board approval and informed patient consent as publicly available data per the general policy of the Washington University Institutional Review Board. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Variables
A list of all SEER variables used in the analysis can be found in eTable 1 in the Supplement. Cancer types were based on ICCC definitions.19 Race/ethnicity was defined as non-Hispanic white, non-Hispanic black, non-Hispanic American Indian/Alaskan Native, non-Hispanic Asian or Pacific Islander, and Hispanic (any race). As proxies for socioeconomic status, we used SEER variables indicating percentage of high school education in 2000 and median family income (in tens) in 2000 in the county of residence, with both categorized into 5 quintiles (0%-20%, >20%-40%, >40%-60%, >60%-80%, and >80%). Insurance status was available for cases diagnosed from 2007 onward and was classified using the variable insurance recode (2007+) as uninsured, any Medicaid, or other insurance at the time of diagnosis. Observations with unknown survival time, missing/unspecified values for survival status, race/ethnicity, cancer type, educational level, or income were excluded from the analysis (<3%).
Main Outcomes and Measures
Cancer amenability to medical intervention was defined using 5-year RSRs, which are the ratio of survival rates of those with a particular cancer type relative to a comparable set of cancer-free individuals.20 Using the ICCC extended classification,19 we identified 103 cancer types in this data set. Relative survival rates were calculated for each cancer type among patients aged 0 to 19 years with a first primary malignant cancer diagnosed from 2000 to 2016 using SEER 18 data and SEER*Stat software, version 8.3.6.21 A list of all cancer types represented, RSRs, and counts can be found in eTable 2 in the Supplement. Ten individuals diagnosed with cancer types with RSRs equal to 0% were excluded. The interquartile range for cancer type RSRs ranged from 77.2% for neuroblastoma and ganglioneuroblastoma to 92.0% for nephroblastoma, with a median of 87.1% for all cases. To ensure sufficient case numbers in the low-amenability category, RSRs were initially grouped into low- (<70.0%), medium- (70.0%-85.0%), and high- (>85.0%) amenability categories. However, because cancers with RSRs lower than 70% may still be amenable, we also conducted analyses with a more stringent cutoff using categories of less than or equal to 50% and greater than 50% RSR. We hypothesized that we should see less disparities in survival for malignancies with RSRs less than or equal to 50% between racial/ethnic groups than those with RSRs greater than 50%.
Statistical Analysis
To evaluate whether childhood and adolescent cancer death rates varied by race/ethnicity, we used Cox proportional hazards regression to compute unadjusted and adjusted hazard ratios (aHRs) and 95% CIs for the association between race/ethnicity and cancer death, setting non-Hispanic white as the reference group. To examine disparities in survival at each amenability category level by race/ethnicity, we included an interaction term (race/ethnicity × amenability) in the model and covariates described below for adjusted models. Unadjusted and adjusted HRs for specific comparisons reported herein were computed using contrasts.22 We used Wald χ2 tests to assess whether HRs for childhood and adolescent cancer death for each race/ethnicity relative to non-Hispanic white patients were modified by amenability category.
Potential covariates were identified using a directed acyclic graph23 of known and suspected confounders for the association between race/ethnicity and child and adolescent cancer survival. Age, sex, parent race/ethnicity, parent income, parent educational level, cancer type, cancer stage at diagnosis, and insurance status were considered. Paths between the exposure and outcome were identified using the back-door criterion.24 Parent income and educational level provided a minimal set of covariates necessary for control of confounding bias (eFigure 1 in the Supplement). To adjust for parent income and educational level, proxies for socioeconomic status were included in adjusted models as described above. Health insurance provided another sufficient adjustment for this association. However, because SEER 18 registries only provide insurance status from 2007 onward, we performed subanalyses including insurance status for individuals diagnosed in the years 2007-2016. To account for potential confounding by age at diagnosis, we used age as the time scale instead of follow-up time according to the methods described by Cologne et al.25 Age at cancer death, last contact, or end of follow-up was calculated as the sum of the age at diagnosis in years, reported cancer survival time in years, and 0.041 years. We added 0.041 years to reported cancer survival time in years to account for cancer death or censorship occurring between integer months.26
The proportionality assumption of the Cox proportional hazards regression models was visually verified using scaled Schoenfeld residuals.27 Outliers were assessed using dfbeta, and removal did not significantly influence the effect estimates. All analyses and figures were generated with R software, version 3.6.1 (R Foundation). Statistical tests were 2-sided; HRs are presented with 95% CIs, and tests of interaction were performed with a type I error rate of 5% (P < .05). Code for analyses can be found at GitHub.28
Results
Among 68 684 cases initially identified, 1623 cases (2.4%) were excluded, leaving 67 061 cases in our analytic data set (eFigure 2 in the Supplement). Patients with cancers classified as low (n = 12 937), medium (n = 16 464), and high (n = 37 660) amenability had median ages at diagnosis of 10, 7, and 12 years; follow-up times of 40, 63, and 79 months; and RSRs of 62.8, 80.0, and 90.8, respectively. The sample included 36 064 males (53.8%); race/ethnicity categorization was 35 186 non-Hispanic white (52.5%), 7100 non-Hispanic black (10.6%), 574 non-Hispanic American Indian/Alaskan Native (0.9%), 4981 non-Hispanic Asian or Pacific Islander (7.4%), and 19 220 Hispanic (any race) (28.7%), with some variation across amenability categories. Mean (SD) age at diagnosis was 9.66 (6.41) years. The most common cancer types were precursor cell leukemias (13 478 [20.1%]), astrocytomas (5781 [8.6%]), and Hodgkin lymphomas (4706 [7.0%]) (Table 1).
Table 1. Distribution of Selected Characteristics for Children and Adolescents Diagnosed With Cancer From 2000 to 2016 by Cancer Type Amenability.
| Characteristic | Amenability | |||
|---|---|---|---|---|
| Low (n = 12 937) | Medium (n = 16 464) | High (n = 37 660) | Overall (n = 67 061) | |
| 5-y Relative survival rate | ||||
| Mean (SD) | 0.61 (0.08) | 0.79 (0.04) | 0.92 (0.05) | 0.83 (0.13) |
| Median (range) | 0.63 (0.20-0.70) | 0.80 (0.71-0.84) | 0.91 (0.85-1.00) | 0.87 (0.20-1.00) |
| Survival months | ||||
| Mean (SD) | 62.5 (58.9) | 76.0 (60.3) | 85.9 (60.1) | 78.9 (60.6) |
| Median (range) | 40.0 (0-203) | 63.0 (0-203) | 79.0 (0-203) | 68.0 (0-203) |
| Sex, No. (%) | ||||
| Male | 7175 (55.5) | 8790 (53.4) | 20 099 (53.4) | 36 064 (53.8) |
| Female | 5762 (44.5) | 7674 (46.6) | 17 561 (46.6) | 30 997 (46.2) |
| Age at diagnosis, y | ||||
| Mean (SD) | 9.55 (6.12) | 7.84 (6.21) | 10.50 (6.42) | 9.66 (6.41) |
| Median (range) | 10.0 (0-19.0) | 7.0 (0-19.0) | 12.0 (0-19.0) | 10.0 (0-19.0) |
| Age, No. (%), y | ||||
| 0-4 | 3660 (28.3) | 6631 (40.3) | 10 473 (27.8) | 20 764 (31.0) |
| 5-9 | 2413 (18.7) | 3331 (20.2) | 5874 (15.6) | 11 618 (17.3) |
| 10-14 | 3271 (25.3) | 3062 (18.6) | 6940 (18.4) | 13 273 (19.8) |
| 15-19 | 3593 (27.8) | 3440 (20.9) | 14 373 (38.2) | 21 406 (31.9) |
| Race/ethnicity, No. (%) | ||||
| Non-Hispanic white | 6439 (49.8) | 9230 (56.1) | 19 517 (51.8) | 35 186 (52.5) |
| Non-Hispanic black | 1718 (13.3) | 1876 (11.4) | 3506 (9.3) | 7100 (10.6) |
| Non-Hispanic American Indian/Alaskan Native | 126 (1.0) | 128 (0.8) | 320 (0.8) | 574 (0.9) |
| Non-Hispanic Asian or Pacific Islander | 1017 (7.9) | 1161 (7.1) | 2803 (7.4) | 4981 (7.4) |
| Hispanic (any race) | 3637 (28.1) | 4069 (24.7) | 11 514 (30.6) | 19 220 (28.7) |
| Cancer type, No. (%)a | ||||
| Precursor cell leukemia | 0 | 0 | 13 478 (35.8) | 13 478 (20.1) |
| Astrocytoma | 0 | 5781 (35.1) | 0 | 5781 (8.6) |
| Hodgkin lymphoma | 0 | 0 | 4706 (12.5) | 4706 (7.0) |
| Thyroid carcinoma | 0 | 0 | 3141 (8.3) | 3141 (4.7) |
| Acute myeloid leukemia | 3089 (23.9) | 0 | 0 | 3089 (4.6) |
| Neuroblastoma and ganglioneuroblastoma | 0 | 3036 (18.4) | 0 | 3036 (4.5) |
| Nephroblastoma | 0 | 0 | 2204 (5.9) | 2204 (3.3) |
| Osteosarcoma | 1981 (15.3) | 0 | 0 | 1981 (3.0) |
| Malignant melanoma | 0 | 0 | 1865 (5.0) | 1865 (2.8) |
| Rhabdomyosarcoma | 1843 (14.2) | 0 | 0 | 1843 (2.7) |
The 10 most common cancer types in this data set are included. A complete list of cancer types with relative survival rates is available in eTable 2 in the Supplement.
All racial/ethnic minority groups in our sample showed worse cancer survival relative to the non-Hispanic white group (Table 2). Compared with non-Hispanic white children and adolescents, a higher hazard of death was observed for high- vs low-amenability cancers for black patients (high: aHR, 1.59; 95% CI, 1.41-1.80 vs low: aHR, 1.35; 95% CI, 1.24-1.47), non-Hispanic American Indian/Alaskan Native patients (high: aHR, 1.60; 95% CI, 1.12-2.28 vs low: aHR, 1.38; 95% CI, 1.05-1.82), non-Hispanic Asian or Pacific Islander patients (high: aHR, 1.46; 95% CI, 1.26-1.68 vs low: aHR, 1.33; 95% CI, 1.19-1.49), and Hispanic (any race) patients (high: aHR, 1.63; 95% CI, 1.50-1.78 vs low: aHR, 1.16; 95% CI, 1.08-1.25). Statistically significant effect modification by amenability category was observed for non-Hispanic black patients (P = .002 for interaction) and Hispanic (any race) patients (P < .001 for interaction) (Table 2).
Table 2. Risk of Cancer Death for Children and Adolescents Diagnosed With Cancer From 2000 to 2016 by Race/Ethnicity and Initial Cancer Amenability Categorya.
| Race/Ethnicity | Total, No. | Died, No. (%) | HR (95% CI) | |
|---|---|---|---|---|
| Unadjustedb | Adjustedc | |||
| Overall | ||||
| Non-Hispanic white | 35 186 | 4790 (13.6) | 1 [Reference] | 1 [Reference] |
| Non-Hispanic black | 7100 | 1477 (20.8) | 1.65 (1.56-1.75) | 1.51 (1.42-1.60) |
| Non-Hispanic American Indian/Alaskan Native | 574 | 113 (19.7) | 1.51 (1.25-1.82) | 1.53 (1.27-1.84) |
| Non-Hispanic Asian or Pacific Islander | 4981 | 859 (17.2) | 1.40 (1.30-1.51) | 1.45 (1.35-1.56) |
| Hispanic (any race) | 19 220 | 3211 (16.7) | 1.33 (1.28-1.40) | 1.34 (1.28-1.40) |
| Race/Ethnicity by Amenability | ||||
| Non-Hispanic white | ||||
| Low | 6439 | 2097 (32.6) | 1 [Reference] | 1 [Reference] |
| Medium | 9230 | 1542 (16.7) | 1 [Reference] | 1 [Reference] |
| High | 19 517 | 1151 (5.9) | 1 [Reference] | 1 [Reference] |
| Non-Hispanic black | ||||
| Low | 1718 | 678 (39.5) | 1.37 (1.26-1.50) | 1.35 (1.24-1.47) |
| Medium | 1876 | 469 (25.0) | 1.73 (1.56-1.92) | 1.70 (1.53-1.88) |
| High | 3506 | 330 (9.4) | 1.62 (1.43-1.83) | 1.59 (1.41-1.80) |
| P value for interaction | .002 | .002 | ||
| Non-Hispanic American Indian/Alaskan Native | ||||
| Low | 126 | 52 (41.3) | 1.36 (1.04-1.80) | 1.38 (1.05-1.82) |
| Medium | 128 | 30 (23.4) | 1.67 (1.16-2.40) | 1.73 (1.20-2.48) |
| High | 320 | 31 (9.7) | 1.60 (1.12-2.29) | 1.60 (1.12-2.28) |
| P value for interaction | .62 | .61 | ||
| Non-Hispanic Asian or Pacific Islander | ||||
| Low | 1017 | 371 (36.5) | 1.32 (1.18-1.47) | 1.33 (1.19-1.49) |
| Medium | 1161 | 265 (22.8) | 1.62 (1.42-1.84) | 1.64 (1.44-1.87) |
| High | 2803 | 223 (8.0) | 1.43 (1.24-1.65) | 1.46 (1.26-1.68) |
| P value for interaction | .06 | .05 | ||
| Hispanic (any race) | ||||
| Low | 3637 | 1290 (35.5) | 1.22 (1.14-1.31) | 1.16 (1.08-1.25) |
| Medium | 4069 | 838 (20.6) | 1.39 (1.28-1.51) | 1.33 (1.22-1.45) |
| High | 11 514 | 1083 (9.4) | 1.71 (1.57-1.85) | 1.63 (1.50-1.78) |
| P value for interaction | <.001 | <.001 | ||
Abbreviation: HR, hazard ratio.
The relative survival rates were categorized as low, less than 70%; medium, 70% to 85%; and high, greater than 85%. Non-Hispanic white children and adolescents were the reference group.
Unadjusted model using age as the time scale. The race/ethnicity-by-amenability models also included the amenability category and an interaction term between race/ethnicity and amenability category.
Adjusted model using age as the time scale. The model included percentage below high school educational level in county quintile and median family income in county quintile. The race/ethnicity-by-amenability models also included amenability category and an interaction term between race/ethnicity and amenability category.
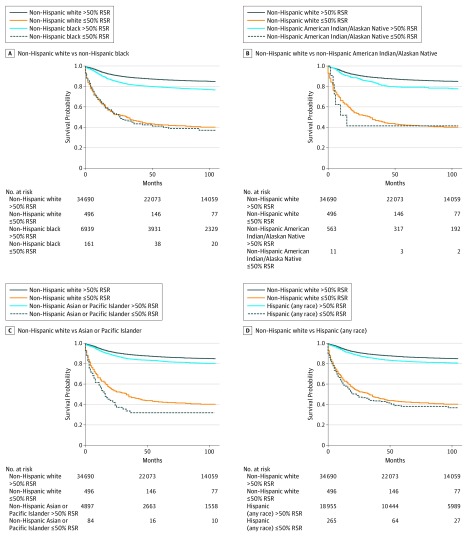
In our analyses categorizing amenability at less than or equal to 50% vs greater than 50% RSR, Kaplan-Meier survival curves showed clear differences in survival probabilities over the follow-up period for cancers with amenability less than or equal to 50% vs greater than 50% for both non-Hispanic black and non-Hispanic white patients. For Hispanic (any race) patients, the disparity differences for cancers with amenability less than or equal to 50% vs greater than 50% became more apparent after 50 months (Figure). In Cox proportional hazards regression models, non-Hispanic black patients had a higher hazard of death compared with non-Hispanic white patients for greater than 50% RSR cancers (aHR, 1.63; 95% CI, 1.53-1.73), while disparities were smaller for cancers with a less than or equal to 50% RSR (aHR, 1.17; 95% CI, 0.91-1.49). A similar pattern was observed for Hispanic (any race) patients, with a 30% greater hazard of death for greater than 50% RSR cancers (aHR, 1.30; 95% CI, 1.23-1.36) and a somewhat smaller hazard of death for less than or equal to 50% RSR cancers (aHR, 1.13; 95% CI, 0.92-1.39). Statistically significant effect modification of amenability by less than or equal to 50% (aHR, 1.17; 95% CI, 0.91-1.49) vs greater than 50% (aHR, 1.63; 95% CI, 1.53-1.73) RSR cancers was observed for non-Hispanic black patients (P = .009 for interaction) (Table 3). A subanalysis performed for the years 2007 to 2016 including insurance status as a covariate showed a similar general pattern with lower aHRs for low-amenability vs medium- and high-amenability cancers, indicating that racial/ethnic disparities are greater for more amenable childhood and adolescent cancers for these groups (eTable 3 in the Supplement). The pattern was consistent for non-Hispanic black but not Hispanic (any race) patients in models including the binary amenability variable (eTable 4 in the Supplement). We also include an analysis of survival disparities by major cancer types, with 5-year survival rates ranging from 63% to 96% in eTable 5 in the Supplement.
Figure. Survival Disparities Among Racial/Ethnic Groups .
Relative survival rates (RSRs) shown for non-Hispanic black (A), non-Hispanic American Indian/Alaskan Native (B), non-Hispanic Asian or Pacific Islander (C), and Hispanic (any race) children and adolescents relative to non-Hispanic white children and adolescents for cancer types with less than or equal to 50% and greater than 50% 5-year RSRs over time in survival months. Significant disparities were observed for greater than 50% compared with less than or equal to 50% 5-year RSR cancers for both non-Hispanic black and Hispanic (any race) patients relative to non-Hispanic white patients.
Table 3. Risk of Cancer Death for Children and Adolescents Diagnosed With Cancer From 2000 to 2016 by Race/Ethnicity and Cancer Amenability Categorya.
| Race/Ethnicity by Amenability | Total No. | Died, No. (%) | HR (95% CI) | |
|---|---|---|---|---|
| Unadjustedb | Adjustedc | |||
| Non-Hispanic white | ||||
| ≤50% | 496 | 265 (53.4) | 1 [Reference] | 1 [Reference] |
| >50% | 34 690 | 4525 (13.0) | 1 [Reference] | 1 [Reference] |
| Non-Hispanic black | ||||
| ≤50% | 161 | 87 (54.0) | 1.16 (0.91-1.48) | 1.17 (0.91-1.49) |
| >50% | 6939 | 1390 (20.0) | 1.66 (1.56-1.76) | 1.63 (1.53-1.73) |
| P value for interaction | .005 | .009 | ||
| Non-Hispanic American Indian/Alaskan Native | ||||
| ≤50% | 11 | 6 (54.5) | 1.16 (0.52-2.61) | 1.16 (0.52-2.62) |
| >50% | 563 | 107 (19.0) | 1.52 (1.25-1.84) | 1.52 (1.26-1.85) |
| P value for interaction | .53 | .53 | ||
| Non-Hispanic Asian or Pacific Islander | ||||
| ≤50% | 84 | 51 (60.7) | 1.32 (0.98-1.79) | 1.34 (0.99-1.81) |
| >50% | 4897 | 808 (16.5) | 1.40 (1.30-1.51) | 1.42 (1.32-1.54) |
| P value for interaction | .72 | .69 | ||
| Hispanic (any race) | ||||
| ≤50% | 265 | 143 (54.0) | 1.17 (0.95-1.43) | 1.13 (0.92-1.39) |
| >50% | 18 955 | 3068 (16.2) | 1.35 (1.29-1.41) | 1.30 (1.23-1.36) |
| P value for interaction | .17 | .21 | ||
Abbreviation: HR, hazard ratio.
Less than or equal to 50% and greater than 50% relative survival rates. Non-Hispanic white children and adolescents were the reference group.
Unadjusted model using age as the time scale. The race/ethnicity-by-amenability models also included the amenability category and an interaction term between race/ethnicity and amenability category.
Adjusted model using age as the time scale. The model included percentage below high school educational level in county quintile and median family income in county quintile, amenability category, and an interaction term between race/ethnicity and amenability category.
Discussion
To our knowledge, this is the first study to assess racial/ethnic disparities in childhood or adolescent cancer survival by cancer type RSRs. We report evidence that suggests that relative cancer survival disparities exist among all racial/ethnic minority children and adolescents in this data set and that these disparities are generally greater for cancers that are more amenable to medical intervention. We observed significant effect modification in our main analysis of relative survival disparities by cancer amenability for non-Hispanic black and Hispanic (any race) children and adolescents.
Childhood cancer treatment in the United States is generally comprehensive. More than 90% of children and adolescents diagnosed with cancer are treated through a Children’s Oncology Group–affiliated cancer center,29 which is a program of the National Cancer Institute that provides standards and guidelines for treatment. Patients and families often receive support from a multidisciplinary team of experts, including physicians, psychologists, and social workers.30 Despite efforts to offer state-of-the-art therapy and enrollment into clinical trials to most children and adolescents with cancer in the United States31 as well as policies that lead to better health care access among children than adults,32 racial/ethnic survival disparities remain. These disparities likely result from several factors, including differences in clinical trial enrollment, adherence to therapy, disease biological characteristics, and pharmacogenetics.8 Beyond these factors, there is strong evidence that socioeconomic status mediates the association between race/ethnicity and childhood and adolescent cancer survival.8,33
We have shown that racial/ethnic disparities are greater overall for more amenable childhood and adolescent cancers as indicated by SEER RSRs.21 Tehranifar et al14 investigated this phenomenon for adults with cancer in the United States, proposing that disparities may emerge in situations in which social and economic capital can provide an advantage, including in obtaining an effective treatment for managing a disease. To this end, cancer types that have a high RSR, and therefore are generally more amenable to medical intervention, may be more dependent on resources related to social and economic capital for survival than cancers with lower RSRs, in which medical intervention may have less influence on survival. Using race and ethnicity as a model, the investigators observed disparities in cancer survival for African American, American Indian/Native Alaskan, and Hispanic patients relative to white patients, which were greater for amenable than for nonamenable cancers. In a subsequent study, this effect was observed to be pronounced for the youngest adult age group (20-34 years).15
In another study, Mahal et al16 investigated whether racial disparities in prostate cancer survival differed by Gleason score—a measure of prostate cancer staging. Disparities between black and white men were significant for low-grade Gleason 6 disease, while no disparity was observed for intermediate- to high-grade Gleason 7 to 10 disease, which is associated with a poorer prognosis. There has been debate as to how much of these reported disparities may be attributed to socioeconomic vs biological differences.34 To investigate this issue, Dess et al34 compared racial disparities in prostate cancer survival for different populations, including a cohort with very homogeneous care (ie, those enrolled in a randomized clinical trial) and what would be more typically seen with real-world care (ie, a population-based cohort, such as SEER). Disparities for black race were observed in the SEER cohort for low- and intermediate-risk disease but not for high-risk disease or for the clinical trial group who had similar access to care and standardized treatment between races. An implication of this study is that nonbiological factors are associated with these disparities—an implication that may also apply to childhood and adolescent cancer in light of the findings of our study.
Link and Phelan35 provided an explanation for this phenomenon with their social theory of fundamental causes, which states that socioeconomic status and the factors associated with it, such as wealth, health care access, knowledge, and social capital, are fundamental causes of disease—not merely proxies—because they allow individuals to avoid and better manage their illness. Despite improving medical knowledge about diseases and how to manage them, socioeconomic disparities have persisted throughout time.17,35 The ability to successfully manage many childhood cancers exists and is continuously improving. However, the manner in which this ability is distributed among groups may vary. Because more survivable cancers are highly amenable owing to their strong dependency on health care access and use, disparities may persist despite the fact that survival overall may be improving. These disparities are less pronounced for cancers with lower survival rates, as they are less dependent on health care access and use owing to fewer viable treatment options.
The experience of childhood cancer is fundamentally different than adult cancer. Children have the added benefit of higher rates of health insurance,32 as well as parental/guardian surveillance of health. Despite these potential differences, we have observed racial and ethnic disparities by cancer type amenability similar to what has been reported for adults. It is possible that the same forces creating this disparity for adults—whether socioeconomic or otherwise—act on children as well.
Limitations
Our results must be interpreted within the context of the study’s limitations. First, RSRs are a proxy for cancer amenability to medical intervention. Although we were able to demonstrate that relative differences in the hazard of cancer death decreases with decreasing cancer amenability in our main analysis using 3 amenability categories (<70%, 70%-85%, and >85% RSR), survival disparities by 5-year RSRs were not as clearly apparent for specific cancer types (eTable 5 in the Supplement). We could not produce meaningful statistics for specific cancer subtypes at the lower end of the 5-year RSR range owing to limitations in sample size. In our analyses using a more stringent amenability cutoff of 50% (≤50% and >50%) that grouped cancers broadly into the 2 RSR categories, the relative differences in the hazard of cancer death were more apparent, especially for non-Hispanic black patients, as would be expected if disparities result largely from socioeconomic factors.
Second, there is evidence of some race/ethnicity misclassification in SEER cohorts, particularly among non-Hispanic American Indian/Alaskan Native individuals and, to a lesser extent, Hispanic (any race) and non-Hispanic Asian or Pacific Islander individuals.36 Third, we did not explore cancer stage, which may be an important mediator of the observed disparities in cancer survival by race/ethnicity. Childhood cancer types are not consistently coded with respect to stage, and data are often missing.37 The SEER Historic Stage A variable is the most consistently recorded across cancer subtypes and is coded as local, regional, and distant, with all leukemia cases coded as distant. However, approximately one-third of the cases in our cohort were missing this variable. Understanding whether stage at diagnosis explains some of the racial/ethnic disparities in childhood cancer survival is an important area of future investigation. Fourth, our variables for income and educational level were obtained at the county level vs the individual level, which could result in misclassification36 and incomplete adjustment for socioeconomic status. Models adjusting for insurance status—a variable obtained at the individual level—may provide better adjustment for socioeconomic status.
Conclusions
We found racial/ethnic disparities in childhood and adolescent cancer survival for non-Hispanic black, non-Hispanic American Indian/Alaskan Native, non-Hispanic Asian or Pacific Islander, and Hispanic (any race) patients. These disparities were larger overall for more survivable cancer types, which are generally more amenable to medical intervention. As childhood and adolescent cancer treatment continues to advance, the risk of leaving disadvantaged groups behind grows. Efforts should be made to promote health equity by race/ethnicity among all children and adolescents with cancer in the United States.
eFigure 1. Directed Acyclic Graph
eFigure 2. Flow Chart of Subject Exclusions
eTable 1. SEER Variables Used in the Analysis
eTable 2. Cancer Types Represented in the Analysis
eTable 3. Risk of Cancer Death for Children and Adolescents Diagnosed With Cancer From 2007-2016 by Race/Ethnicity and Cancer Amenability Category (Low: <70% RSR, Medium: 70%-85% RSR, High: >85% RSR) With Non-Hispanic Whites as the Reference Group and Adjusting for Insurance Status
eTable 4. Risk of Cancer Death for Children and Adolescents Diagnosed With Cancer From 2000-2016 by Race/Ethnicity and Cancer Amenability Category (≤50% and >50% RSR) With Non-Hispanic White as the Reference Group and Adjusting for Insurance Status
eTable 5. Risk of Cancer Death for Children and Adolescents Diagnosed With Cancer From 2000-2016 by Race/Ethnicity for Common Cancer Types
eReferences
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Smith MA, Seibel NL, Altekruse SF, et al. . Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28(15):2625-2634. doi: 10.1200/JCO.2009.27.0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robison LL, Armstrong GT, Boice JD, et al. . The Childhood Cancer Survivor Study: a National Cancer Institute–supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308-2318. doi: 10.1200/JCO.2009.22.3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barr RD, Ferrari A, Ries L, Whelan J, Bleyer WA. Cancer in adolescents and young adults: a narrative review of the current status and a view of the future. JAMA Pediatr. 2016;170(5):495-501. doi: 10.1001/jamapediatrics.2015.4689 [DOI] [PubMed] [Google Scholar]
- 5.Zeng C, Wen W, Morgans AK, Pao W, Shu XO, Zheng W. Disparities by race, age, and sex in the improvement of survival for major cancers: results from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program in the United States, 1990 to 2010. JAMA Oncol. 2015;1(1):88-96. doi: 10.1001/jamaoncol.2014.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212-236. doi: 10.3322/caac.20121 [DOI] [PubMed] [Google Scholar]
- 7.Pui CH, Pei D, Pappo AS, et al. . Treatment outcomes in black and white children with cancer: results from the SEER database and St Jude Children’s Research Hospital, 1992 through 2007. J Clin Oncol. 2012;30(16):2005-2012. doi: 10.1200/JCO.2011.40.8617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia S. Disparities in cancer outcomes: lessons learned from children with cancer. Pediatr Blood Cancer. 2011;56(6):994-1002. doi: 10.1002/pbc.23078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):83-103. doi: 10.3322/caac.21219 [DOI] [PubMed] [Google Scholar]
- 10.Truong B, Green AL, Friedrich P, Ribeiro KB, Rodriguez-Galindo C. Ethnic, racial, and socioeconomic disparities in retinoblastoma. JAMA Pediatr. 2015;169(12):1096-1104. doi: 10.1001/jamapediatrics.2015.2360 [DOI] [PubMed] [Google Scholar]
- 11.Linabery AM, Ross JA. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975-1999. Cancer. 2008;113(9):2575-2596. doi: 10.1002/cncr.23866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadan-Lottick NS, Ness KK, Bhatia S, Gurney JG. Survival variability by race and ethnicity in childhood acute lymphoblastic leukemia. JAMA. 2003;290(15):2008-2014. doi: 10.1001/jama.290.15.2008 [DOI] [PubMed] [Google Scholar]
- 13.Kahn JM, Keegan TH, Tao L, Abrahão R, Bleyer A, Viny AD. Racial disparities in the survival of American children, adolescents, and young adults with acute lymphoblastic leukemia, acute myelogenous leukemia, and Hodgkin lymphoma. Cancer. 2016;122(17):2723-2730. doi: 10.1002/cncr.30089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tehranifar P, Neugut AI, Phelan JC, et al. . Medical advances and racial/ethnic disparities in cancer survival. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2701-2708. doi: 10.1158/1055-9965.EPI-09-0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tehranifar P, Goyal A, Phelan JC, et al. . Age at cancer diagnosis, amenability to medical interventions, and racial/ethnic disparities in cancer mortality. Cancer Causes Control. 2016;27(4):553-560. doi: 10.1007/s10552-016-0729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahal BA, Berman RA, Taplin ME, Huang FW. Prostate cancer–specific mortality across Gleason scores in black vs nonblack men. JAMA. 2018;320(23):2479-2481. doi: 10.1001/jama.2018.11716 [DOI] [PubMed] [Google Scholar]
- 17.Phelan JC, Link BG, Tehranifar P. Social conditions as fundamental causes of health inequalities: theory, evidence, and policy implications. J Health Soc Behav. 2010;51(suppl):S28-S40. doi: 10.1177/0022146510383498 [DOI] [PubMed] [Google Scholar]
- 18.Surveillance, Epidemiology, and End Results Program Database. Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2018 Sub (1975-2016 varying)—Linked To County Attributes—Total US, 1969-2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission. http://www.seer.cancer.gov. Accessed November 30, 2019.
- 19.Surveillance, Epidemiology, and End Results Program ICCC Recode ICD-O-3/WHO 2008. https://seer.cancer.gov/iccc/iccc-who2008.html. Accessed November 25, 2018.
- 20.Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat. Relative survival. https://seer.cancer.gov/seerstat/WebHelp/Relative_Survival.htm. Accessed June 21, 2019.
- 21.Surveillance Research Program; National Cancer Institute. SEER*Stat software, version 8.3.6. https://seer.cancer.gov/seerstat. Updated August 8, 2019. Accessed August 10, 2019.
- 22.UCLA Statistical Consulting Group Statistical consulting. How can I test contrasts in R? R FAQ. https://stats.idre.ucla.edu/r/faq/how-can-i-test-contrasts-in-r/. Accessed November 1, 2019.
- 23.Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol. 2016;45(6):1887-1894. doi: 10.1093/ije/dyw341 [DOI] [PubMed] [Google Scholar]
- 24.Pearl J. Causality: Models, Reasoning, and Inference. 2nd ed New York: Cambridge University Press; 2009. doi: 10.1017/CBO9780511803161 [DOI] [Google Scholar]
- 25.Cologne J, Hsu WL, Abbott RD, et al. . Proportional hazards regression in epidemiologic follow-up studies: an intuitive consideration of primary time scale. Epidemiology. 2012;23(4):565-573. doi: 10.1097/EDE.0b013e318253e418 [DOI] [PubMed] [Google Scholar]
- 26.Surveillance, Epidemiology, and End Results Program. Calculation of survival time fields. https://seer.cancer.gov/survivaltime/SurvivalTimeCalculation.pdf. Updated September 11, 2013. Accessed November 3, 2019.
- 27.Bellera CA, MacGrogan G, Debled M, de Lara CT, Brouste V, Mathoulin-Pélissier S. Variables with time-varying effects and the Cox model: some statistical concepts illustrated with a prognostic factor study in breast cancer. BMC Med Res Methodol. 2010;10:20. doi: 10.1186/1471-2288-10-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.GitHub. kiJohnson/amenability-paper. https://github.com/kijohnson/Amenability-paper. Accessed November 6, 2019.
- 29.Children’s Oncology Group About us. https://www.childrensoncologygroup.org/index.php/aboutus. Accessed April 10, 2019.
- 30.Miller KD, Siegel RL, Lin CC, et al. . Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271-289. doi: 10.3322/caac.21349 [DOI] [PubMed] [Google Scholar]
- 31.O’Leary M, Krailo M, Anderson JR, Reaman GH; Children’s Oncology Group . Progress in childhood cancer: 50 years of research collaboration, a report from the Children’s Oncology Group. Semin Oncol. 2008;35(5):484-493. doi: 10.1053/j.seminoncol.2008.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Artiga S, Ubri P Key issues in children's health coverage. Kaiser Family Foundation website. https://www.kff.org/medicaid/issue-brief/key-issues-in-childrens-health-coverage/. Published February 15, 2017. Accessed April 15, 2019.
- 33.Kehm RD, Spector LG, Poynter JN, Vock DM, Altekruse SF, Osypuk TL. Does socioeconomic status account for racial and ethnic disparities in childhood cancer survival? Cancer. 2018;124(20):4090-4097. doi: 10.1002/cncr.31560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dess RT, Hartman HE, Mahal BA, et al. . Association of black race with prostate cancer–specific and other-cause mortality. JAMA Oncol. 2019;5(7):975-983. doi: 10.1001/jamaoncol.2019.0826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Link BG, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav. 1995;(spec No):80-94. doi: 10.2307/2626958 [DOI] [PubMed] [Google Scholar]
- 36.Colton MD, Hawkins M, Goulding D, Cockburn M, Green AL. Socioeconomics, race, and ethnicity in childhood cancer survival: accessing and addressing root causes of disparities. Cancer. 2018;124(20):3975-3978. doi: 10.1002/cncr.31558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta S, Aitken JF, Bartels U, et al. . Paediatric cancer stage in population-based cancer registries: the Toronto consensus principles and guidelines. Lancet Oncol. 2016;17(4):e163-e172. doi: 10.1016/S1470-2045(15)00539-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Directed Acyclic Graph
eFigure 2. Flow Chart of Subject Exclusions
eTable 1. SEER Variables Used in the Analysis
eTable 2. Cancer Types Represented in the Analysis
eTable 3. Risk of Cancer Death for Children and Adolescents Diagnosed With Cancer From 2007-2016 by Race/Ethnicity and Cancer Amenability Category (Low: <70% RSR, Medium: 70%-85% RSR, High: >85% RSR) With Non-Hispanic Whites as the Reference Group and Adjusting for Insurance Status
eTable 4. Risk of Cancer Death for Children and Adolescents Diagnosed With Cancer From 2000-2016 by Race/Ethnicity and Cancer Amenability Category (≤50% and >50% RSR) With Non-Hispanic White as the Reference Group and Adjusting for Insurance Status
eTable 5. Risk of Cancer Death for Children and Adolescents Diagnosed With Cancer From 2000-2016 by Race/Ethnicity for Common Cancer Types
eReferences