Abstract
This cohort study examines triple-negative breast cancer detection via mammography screening, treatment, and survival rates in African American and white American women.
Population-based breast cancer mortality is 40% higher among African American women compared with white American women,1 a disparity partly explained by 2-fold higher incidence of biologically aggressive triple-negative breast cancer (TNBC) in African American women.2 Screening mammography improves breast cancer survival through early detection, but TNBC is more challenging to detect mammographically compared with non-TNBC.3,4,5,6 The value of screening mammography in reducing disparities through early detection of the disproportionately high TNBC burden observed in African American women is therefore uncertain.
Methods
We used the Henry Ford Health System prospectively maintained database to evaluate patients who self-reported as African American or white American and had nonmetastatic TNBC (defined by American Society of Clinical Oncology/College of American Pathologists guidelines) diagnosed from January 2011 to December 2015. All were followed up until death, loss to follow-up, or study termination (September 14, 2018), whichever occurred first. Mammography screening–detected cancers were found on routinely scheduled mammography in the absence of clinical symptoms. This retrospective analysis of data from a prospectively maintained database accrued through standard-of-care cancer treatment was approved by the Henry Ford Health System institutional review board; for this type of study, formal consent was not required. This database and its analysis is in accordance with the ethical standards of the institutional research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The statistical programming language R version 3.3.1 (R Foundation for Statistical Computing) was used. Fisher exact and χ2 tests compared categorical variables; t tests were used for continuous variables. Overall, all-cause survival was compared using log-rank test and P values. Multivariate Cox proportional hazards regression was used. Two-sided P values <.05 were considered significant. Data analysis occurred from January 2011 to August 2018.
Results
Of 243 TNBC cases identified, 106 African American and 87 white American patients had adequate data for comparison. There were no significant differences between African American and white American patients by mean (range) age at diagnosis (61.3 [29-90] years vs 61.0 [27-90] years); mean (range) tumor size (2.2 [0.3-10.0] cm vs 2.7 [0.1-27.0] cm); or nodal status (node negative: 80 African American patients [75.5%] and 68 white American patients [78.2%]). Frequency of screening-detected disease was similar (62 African American patients [58.5%] and 39 white American patients [44.8%]). Most tumors in African American and white American patients were invasive ductal tumors (89 [84.0%] and 67 [77.0%], respectively); distribution of E3 ubiquitin-protein ligase MIB1/KI-67 scores (with scores ≥30%: 56 African American patients [52.8%] and 42 white American patients [48.3%]), frequency of lymphovascular invasion (11 African American patients [10.4%] and 15 white American patients [17.2%]; P = .30), and frequency of high-grade pathology (84 African American patients [79.2%] and 63 white American patients [72.4%]) were similar. African American patients had higher mean (SD) body mass index scores (calculated as weight in kilograms divided by height in meters squared; 32.2 vs 28.6; P = .001). Family histories were similar for African American and white American patients. About 90% of each group (91 African American patients [95.8%] and 77 white American patients [88.6%]) had some form of nonpublic insurance (including Medicare). Fewer African American patients than white American patients were referred for genetic testing or counseling (23 [21.7%] vs 33 [37.9%]; P = .03).
Lumpectomies were performed in 59 African American patients (55.7%) and 52 white American patients (59.8%). Among African American patients, 61 (57.5%) received postoperative or adjuvant chemotherapy and 24 (22.6%) neoadjuvant chemotherapy, compared with 53 (60.9%) and 17 (19.5%), respectively, in the white American group. African American patients were less likely to undergo contralateral prophylactic mastectomy (2 [1.9%] vs 10 [11.5%]; P = .01).
Compared with non–screening-detected cases, more mammography screening–detected TNBC cases were T1 (80 of 101 [79.2%] vs 33 of 89 [37.1%]; P < .001) and node negative (92 of 101 [91.1%] vs 53 of 89 [59.6%]; P < .001), and more resulted in a lumpectomy (69 of 101 [68.3%] vs 41 of 89 [46.1%]; P = .002). These patterns were identical within African American and white American subsets. There were no differences in body mass index or high-grade pathology prevalence between patients with screening-detected and non–screening-detected cases.
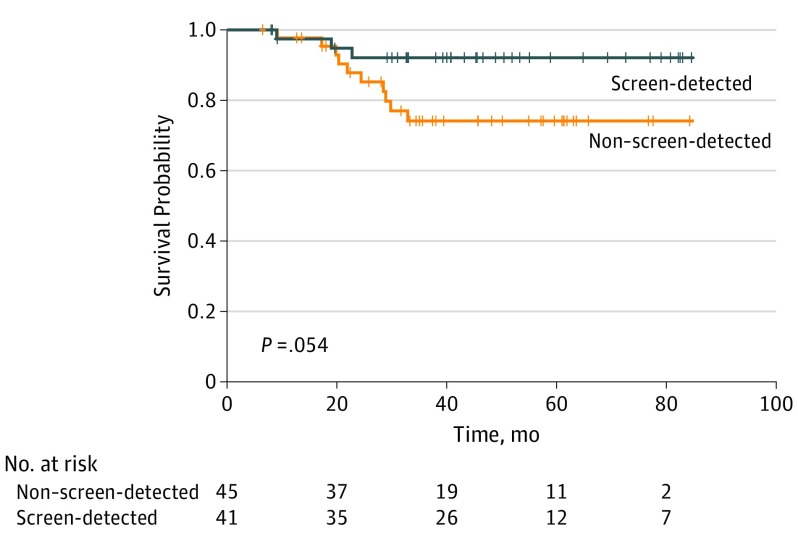
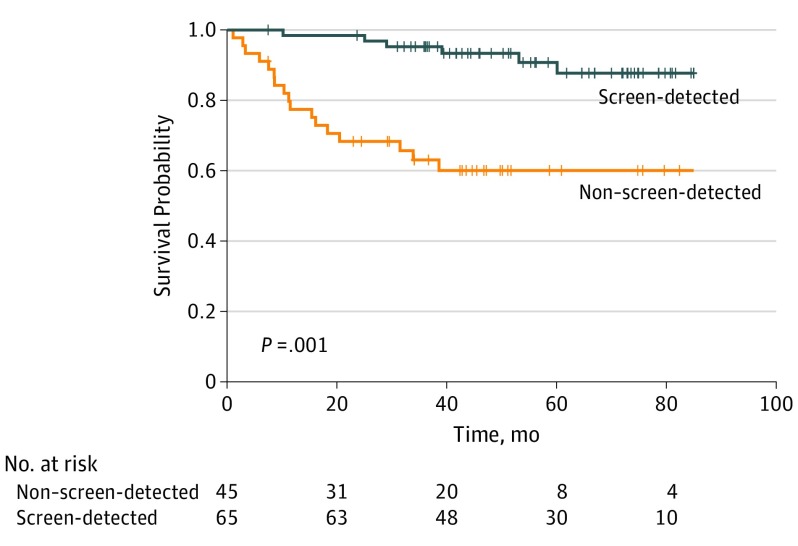
The median (range) follow-up was 50.3 (1-91) months for African American patients and 47.5 (6-91) months for white American patients. The frequency of local recurrence and distant relapse were not significantly different between the groups. Detection of TNBC through screening was associated with improved 4-year overall survival in African American patients (screening-detected cases: 93.2% [95% CI, 87.0%-99.9%]; non–screening detected cases, 59.1% [95% CI, 45.8%-76.2%]; P < .001) but not significantly improved in white American patients (screening-detected cases: 87.5% [95% CI, 76.5%-100%]; non–screening detected cases: 74.8% [95% CI, 62.3%-89.7%]).
Univariate factors associated with better survival included screening-detected disease (vs non–screening-detected disease; hazard ratio [HR], 0.3 [95% CI, 0.1-0.5]; P < .001), non-T1 disease (vs T1 disease; HR, 3.5 [95% CI, 1.8-6.9]; P < .001), and node positivity (vs node negativity; HR, 3.4 [95% CI, 1.8-6.7]; P < .001). High-grade disease (vs non–high grade disease; HR, 2.7 [95% CI, 0.96-7.7]; P = .049), lymphovascular invasion (present vs absent; HR, 3.2 [95% CI, 1.5-7.2]; P = .002), and receiving adjuvant/neoadjuvant chemotherapy (vs not receiving it; HR, 0.4 [95% CI, 0.2-0.8]; P = .006) were associated with worse prognosis (survival). Race/ethnicity, age, histology, MIB1 status, body mass index, insurance status, and parity were not associated with survival. On multivariate analysis, screen detection and high-grade pathology remained significant. The all-cause mortality hazard ratio for screening-detected vs non–screening-detected disease was 0.21 (95% CI, 0.10-0.45; P < .001); the hazard ratio for high-grade vs non–high-grade pathology was 3.37 (95% CI, 1.17-9.66). Figure 1 and Figure 2 show the improved survival for white American and African American patients, based on screening.
Figure 1. Overall Survival Probability for White American Patients With Triple-Negative Breast Cancer, Comparing Mammography Screening–Detected and Non–Screening-Detected Cases.
Generated by the ggsurvplot function in the R version 0.4.2 (R Foundation for Statistical Computing) package survminer.
Figure 2. Overall Survival Probability for African American Patients With Triple-Negative Breast Cancer, Comparing Mammography Screening–Detected and Non–Screening-Detected Cases.
Generated by the ggsurvplot function in the R version 0.4.2 (R Foundation for Statistical Computing) package survminer.
Discussion
We report equal outcomes among equitably treated African American patients and white American patients with TNBC. Screening mammography successfully detected early-stage TNBC, improving outcomes for both African American and white American patients. Screening mammography is therefore an important strategy for reducing race/ethnicity–associated breast cancer disparities by optimizing overall survival for both population subsets.
References
- 1.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015. CA Cancer J Clin. 2016;66(1):31-42. doi: 10.3322/caac.21320 [DOI] [PubMed] [Google Scholar]
- 2.Kohler BA, Sherman RL, Howlader N, et al. . Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107(6):djv048. doi: 10.1093/jnci/djv048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman LA, Reis-Filho JS, Morrow M, Carey LA, King TA. The 2014 Society of Surgical Oncology Susan G. Komen for the Cure symposium. Ann Surg Oncol. 2015;22(3):874-882. doi: 10.1245/s10434-014-4279-0 [DOI] [PubMed] [Google Scholar]
- 4.Bellio G, Marion R, Giudici F, et al. . Interval breast cancer versus screen-detected cancer. Clin Breast Cancer. 2017;17(7):564-571. doi: 10.1016/j.clbc.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 5.O’Brien KM, Mooney T, Fitzpatrick P, Sharp L. Screening status, tumour subtype, and breast cancer survival. Breast Cancer Res Treat. 2018;172(1):133-142. doi: 10.1007/s10549-018-4877-9 [DOI] [PubMed] [Google Scholar]
- 6.Puvanesarajah S, Nyante SJ, Kuzmiak CM, et al. . PAM50 and risk of recurrence scores for interval breast cancers. Cancer Prev Res (Phila). 2018;11(6):327-336. doi: 10.1158/1940-6207.CAPR-17-0368 [DOI] [PMC free article] [PubMed] [Google Scholar]