This secondary analysis of a randomized clinical trial assesses the DRCR Retina Network protocol–defined approach and outcomes of patients with center-involved diabetic macular edema and good vision after initial observation and receiving aflibercept injections for 2 years.
Key Points
Question
For eyes with center-involved diabetic macular edema and good vision, what was the DRCR Retina Network Protocol V protocol–defined approach for initial observation plus aflibercept only if visual acuity worsened, and which characteristics were associated with receiving aflibercept injections?
Findings
In this secondary analysis of a randomized clinical trial, during 2 years, 80 of 236 eyes (34%) assigned to initial observation received aflibercept. Participants who had thicker retinas, more severe diabetic retinopathy, or a nonstudy eye receiving diabetic macular edema treatment within 4 months of baseline were more likely to receive aflibercept.
Meaning
The findings suggest that understanding this approach to initial observation is important for clinicians who manage eyes with center-involved diabetic macular edema and good visual acuity.
Abstract
Importance
Among eyes with center-involved diabetic macular edema (CI-DME) and good visual acuity (VA), randomized clinical trial results showed no difference in VA loss between initial observation plus aflibercept only if VA decreased, initial focal/grid laser plus aflibercept only if VA decreased, or prompt aflibercept. Understanding the initial observation approach is relevant to patient management.
Objective
To assess the DRCR Retina Network protocol-defined approach and outcomes of initial observation with aflibercept only if VA worsened.
Design, Setting, and Participants
This was a post hoc secondary analyses of a randomized clinical trial of the DRCR Retina Network Protocol V that included 91 US and Canadian sites from November 2013 to September 2018. Participants were adults (n = 236) with type 1 or 2 diabetes, 1 study eye with CI-DME, and VA letter score at least 79 (Snellen equivalent, 20/25 or better) assigned to initial observation. Data were analyzed from March 2019 to November 2019.
Interventions
Initial observation and follow-up with aflibercept only for VA loss of at least 10 letters from baseline at 1 visit or 5 to 9 letters at 2 consecutive visits. Follow-up occurred at 8 weeks and then every 16 weeks unless VA or optical coherence tomography central subfield thickness worsened.
Main Outcomes and Measures
Whether individuals received aflibercept.
Results
Among 236 eyes in 236 individuals (149 [63%] male; median age, 60 years [interquartile range, 53-67 years]) randomly assigned to initial observation, 80 (34%) were treated with aflibercept during 2 years of follow-up. At 2 years, the median VA letter score was 86.0 (interquartile range, 89.0-81.0; median Snellen equivalent, 20/20 [20/16-20/25]). Receipt of aflibercept was more likely in eyes with baseline central subfield thickness at least 300 μm (Zeiss-Stratus equivalent) vs less than 300 μm (45% vs 26%; hazard ratio [HR], 1.98 [95% CI, 1.26-3.13], continuous P = .005), moderately severe nonproliferative diabetic retinopathy (Early Treatment Diabetic Retinopathy Study retinopathy severity level 47) and above vs moderate nonproliferative diabetic retinopathy (retinopathy severity level 43) and below (51% vs 27%; HR, 2.22 [95% CI, 1.42-3.47], ordinal P < .001), and among participants whose nonstudy eye received DME treatment within 4 months of randomization vs not (52% vs 25%; HR, 2.55 [95% CI, 1.64-3.99], P < .001).
Conclusions and Relevance
Most eyes managed with initial observation plus aflibercept only if VA worsened maintained good vision at 2 years and did not require aflibercept for VA loss. However, the eyes in the trial were approximately twice as likely to receive aflibercept for VA loss if they had greater baseline central subfield thickness, worse diabetic retinopathy severity level, or a nonstudy eye receiving treatment for DME.
Trial Registration
ClinicalTrials.gov Identifier: NCT01909791
Introduction
The DRCR Retina Network Protocol V1 compared strategies of initial treatment using intravitreous aflibercept, focal/grid laser photocoagulation, and observation for eyes with center-involved diabetic macular edema (CI-DME) and visual acuity (VA) 20/25 or better. The groups initially managed with laser photocoagulation or observation received aflibercept treatment only if VA worsened during follow-up. Aflibercept treatment was not initiated if optical coherence tomography (OCT) central subfield thickness (CST) worsened in the absence of VA decline. There were no significant differences in rates of at least 5-letter VA loss after 2 years between the 3 treatment strategies, and each led to 20/20 mean VA at 2 years.1
These results suggest that eyes with CI-DME and good vision managed with initial observation vs immediate anti–vascular endothelial growth factor (anti-VEGF) therapy can achieve similarly good visual outcomes with reduced costs and lower risk of injection-associated adverse events, such as endophthalmitis (previous research estimates the risk to be <0.1% per injection).2 Of note, fewer eyes in the laser photocoagulation group (26%) received anti-VEGF injections compared with eyes in the observation group (36%) (P = .01) but obtained similar visual outcomes at the end of 2 years.1 However, laser can cause macular scarring and visual scotomas.3
Although each treatment strategy was effective, ophthalmologists may choose a strategy of initial observation for eyes with CI-DME and good vision given the aforementioned reasons. This report provides additional details and post hoc analyses of the observation strategy and outcomes beyond the scope of the primary report,1 including an analysis of baseline characteristics associated with receipt of aflibercept during follow-up.
Methods
This was a post hoc secondary analysis of a randomized clinical trial of the DRCR Retina Network Protocol V. Protocol V1 was conducted at 91 clinical sites in the United States and Canada from November 2013 to September 2018. The protocol and statistical analysis plan are published elsewhere1 and are available with the primary outcome report. This study adhered to the tenets of the Declaration of Helsinki.4 The ethics board associated with each site provided approval and participants provided written informed consent (available in the primary report).1
Baseline VA of 20/25 or better using Electronic-Early Treatment Diabetic Retinopathy Study (E-ETDRS) testing5 after protocol refraction was confirmed at both screening and randomization visits no more than 4 weeks apart. At the screening visit, OCT CST was at least 290 μm in women and at least 305 μm in men on Zeiss Cirrus (Zeiss International) or at least 305 μm in women and at least 320 μm in men on Heidelberg Spectralis (Heidelberg Engineering).6,7 At the randomization visit, OCT CST was at least 275 μm in women and at least 290 μm in men on Zeiss Cirrus or at least 290 μm in women and at least 305 μm in men on Heidelberg Spectralis. Investigators confirmed definite retinal thickening due to DME involving the center of the macula on clinical examination.
Initial Observation Strategy in Protocol V
At each visit, protocol-defined best-corrected E-ETDRS VA was measured after protocol refraction, and OCT CST was measured. Eyes assigned to initial observation had follow-up visits at least every 16 weeks (±4 weeks) through 2 years with an additional visit 8 weeks (±2 weeks) from randomization to check for early VA deterioration (Figure 1). Follow-up remained at 16-week intervals unless VA or CST worsened. Even when CST did not worsen, treatment with aflibercept was initiated only if either of the following VA worsening criteria were met: (1) best-corrected VA decreased at least 10 letters (2 lines) from baseline because of DME at any visit or (2) best-corrected VA decreased 5 to 9 letters (1 line) from baseline because of DME at any visit and was confirmed at a second visit 4 weeks (±2 weeks) later. Baseline VA was defined as the mean of the screening and randomization VAs. Once initiated, aflibercept was given according to the DRCR Retina Network anti-VEGF for DME regimen8 described below. If an eye with 5- to 9-letter VA decrease did not have a decrease from baseline by 5 or more letters at the subsequent 4-week visit, follow-up was extended to 8 weeks and then every 16 weeks.
Figure 1. DRCR Retina Network Initial Observation Treatment Algorithm.
anti-VEGF indicates anti–vascular endothelial growth factor.
aVisual acuity was considered worse if it was 5 to 9 letters (1 line) worse from baseline at 2 consecutive visits every 4 weeks (±2 weeks) apart or at least 10 letters (2 lines or more) worse at any visit. Of note, increasing central subfield thickness or change in any other parameter did not initiate injections if the visual acuity was not worse.
bOnce injections were initiated, the DRCR Retina Network anti-VEGF for diabetic macular edema regimen was followed.8
cIf central subfield thickness worsened by at least 10% from the last visit or became greater than 400 μm (Zeiss-Stratus equivalent) without vision loss, the follow-up interval was halved with a minimum interval of 4 weeks. If central subfield thickness subsequently stabilized at 2 consecutive visits without vision loss, follow-up could be extended to 8 weeks and then to 16 weeks.
In the absence of VA worsening, follow-up remained at 16-week intervals unless CST increased by at least 10% from the previous visit or became greater than 400 μm (time-domain [Zeiss Stratus] equivalent),9 at which point the follow-up interval was halved to every 8 weeks. If CST further increased at least 10% compared with the previous visit without meeting criteria for initiating aflibercept, the follow-up interval was reduced to every 4 weeks. If CST stabilized (ie, did not worsen at 2 consecutive visits) without meeting VA worsening criteria, the follow-up interval was doubled to a maximum of 16-week intervals. If the follow-up interval determined by VA and CST criteria differed, the shorter interval was used. Injections were initiated only by worsening of VA regardless of CST.
DRCR Retina Network Anti-VEGF for DME Regimen
The anti-VEGF retreatment regimen was identical in the aflibercept, laser photocoagulation, and observation groups (after loss of VA in the laser photocoagulation and observation groups). Aflibercept was given every 4 weeks (±1 week) for 24 weeks (6 injections) with 1 exception: injections were deferred if neither VA nor CST improved or worsened after at least 2 consecutive injections and the CST was less than the spectral-domain OCT threshold for CI-DME (defined by OCT machine and sex: Heidelberg Spectralis CST of at least 305 μm in women and at least 320 μm in men and Zeiss Cirrus CST of at least 290 μm in women and at least 305 μm in men) and the VA letter score was at least 84 (Snellen equivalent, 20/20 or better) (Figure 1). Improvement and worsening for retreatment determination were defined as at least a 5-letter VA or 10% CST change.
If the eye achieved sustained stability (ie, had not improved or worsened with respect to VA or CST for at least 2 consecutive injections) 24 weeks after initiation of aflibercept or later, injections were deferred. If neither VA nor CST improved from the previous 2 consecutive injections and the CST thickness was greater than the spectral-domain CST threshold, focal/grid laser photocoagulation to treat residual DME was optional based on investigator discretion. If the VA or CST worsened from the previous 2 consecutive injections, laser photocoagulation was administered unless complete laser photocoagulation had previously been administered or all treatable microaneurysms were within 500 μm of the fovea.
If injections were deferred at 3 consecutive visits 24 weeks after aflibercept initiation, follow-up intervals were extended to 8 and then 16 weeks if deferral criteria were still met. If VA or CST worsened from the most recent visit or the visit at which the last injection occurred, injections were resumed. The study protocol did not specify any treatment for systemic conditions, and treatment for diabetes was usual care.
Statistical Analysis
All analyses were post hoc, followed the intention-to-treat principle, and included observed data from all eyes randomly assigned to initial observation. To limit the influence of potential outliers, changes in VA and CST were truncated at 3 SDs from the mean. Cox proportional hazards regression was used to test for associations between baseline characteristics and time to first aflibercept injection.10 Visual inspection of Kaplan-Meier plots and martingale residuals were used to verify the proportional hazards assumption.11 Cumulative probabilities accounting for loss to follow-up were estimated using the Kaplan-Meier method.12 Poisson regression with robust variance estimation was used to model the relationship between baseline characteristics and at least 5-letter loss of vision at 2 years.13 All P values and 95% CIs are 2-sided. Characteristics with P < .05 in univariable analysis were entered into a multivariable model (priority was given to the P value for the continuous or ordinal version of the characteristic if available), and stepwise selection with backward elimination of variables with P > .05 was used to select a final model. SAS/STAT software, version 15.1 (SAS Institute Inc) was used for all analyses. There was no adjustment for multiplicity. Data were analyzed from March 2019 to November 2019.
Results
A total of 236 eyes in 236 individuals (149 [63%] male; median age, 60 years [interquartile range [IQR], 53-67 years]) were randomly assigned to initial observation. As reported previously, the cumulative probability (Kaplan-Meier estimate, adjusting for loss to follow-up) of receiving aflibercept in the observation group within 2 years was 36% (95% CI, 30%-43%).1 Median VA letter score at 2 years for the entire observation cohort was 86.0 (IQR, 89.0-81.0; median Snellen equivalent, 20/20 [20/16-20/25]); the percentage of eyes with no CI-DME and at least 10% CST decrease from baseline at 2 years was 36% (74 of 208).1
Table 1 presents the cumulative probability of receiving an aflibercept injection during 2 years of follow-up by baseline characteristics. Among eyes with baseline VA of 20/25, the probability of receiving aflibercept by 2 years was 42% vs 32% among eyes with VA of 20/20 or better (hazard ratio [HR], 1.42 [95% CI, 0.91-2.21], categorical P = .12, continuous P = .34). Among eyes with Zeiss-Stratus equivalent CST of at least 300 μm at baseline, the probability of receiving aflibercept was 45% vs 26% among eyes with CST less than 300 μm (HR, 1.98 [95% CI, 1.26-3.13], categorical P = .003, continuous P = .005). Among eyes with moderately severe nonproliferative or more severe diabetic retinopathy (ETDRS retinopathy severity level ≥47),14 the probability of receiving an aflibercept injection was 51% vs 27% among eyes with moderate nonproliferative or less severe diabetic retinopathy (ETDRS retinopathy severity level ≤43) (HR, 2.22 [95% CI, 1.42-3.47], categorical P < .001, ordinal P < .001). Among eyes in which DME treatment was planned or performed for the nonstudy eye within 4 months of randomization (at investigator discretion), the probability of receiving an aflibercept injection was 52% vs 25% among eyes for which the nonstudy eye did not have recent or planned DME treatment within 4 months of randomization (HR, 2.55 [95% CI, 1.64-3.99], P < .001).
Table 1. Receipt of Aflibercept During 2 Years of Follow-up by Baseline Characteristics.
| Characteristic | No. | Received Aflibercept, No. | Cumulative Probability, % (95% CI) | Hazard Ratio (95% CI) | P Value |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 149 | 48 | 34 (27-43) | 0.86 (0.55-1.34) | .50 |
| Female | 87 | 32 | 39 (29-50) | ||
| Age, y | |||||
| ≥60 | 122 | 32 | 28 (21-37) | 0.56 (0.36-0.88) | .01a |
| <60 | 114 | 48 | 44 (35-54) | ||
| Race/ethnicity | |||||
| Nonwhite | 73 | 23 | 33 (23-45) | 0.88 (0.54-1.43) | .60 |
| Non-Hispanic white | 161 | 56 | 37 (30-45) | ||
| HbA1c value, % | |||||
| ≥7.5 | 120 | 41 | 37 (29-47) | 1.18 (0.75-1.86) | .47b |
| <7.5 | 106 | 34 | 32 (24-42) | ||
| Visual acuity, Snellen equivalent (letter score) | |||||
| 20/25 (83-79) | 85 | 35 | 42 (32-53) | 1.42 (0.91-2.21) | .12c |
| 20/20 or better (≥84) | 151 | 45 | 32 (25-40) | ||
| OCT central subfield thickness (Zeiss-Stratus equivalent), μm | |||||
| ≥300 | 115 | 51 | 45 (37-55) | 1.98 (1.26-3.13) | .003d |
| <300 | 121 | 29 | 26 (19-35) | ||
| Diabetic retinopathy severity graded on color fundus photographs (ETDRS retinopathy severity level)14 | |||||
| ≥47 (Moderately severe NPDR and above) | 80 | 38 | 51 (40-63) | 2.22 (1.42-3.47) | <.001e |
| ≤43 (Moderate NPDR and below) | 149 | 39 | 27 (21-36) | ||
| Prior treatment for DME in the study eye | |||||
| Yes | 34 | 13 | 41 (26-60) | 1.22 (0.67-2.21) | .51 |
| No | 202 | 67 | 35 (29-42) | ||
| Recent or planned (within 4 mo) diabetic macular edema treatment in the nonstudy eye | |||||
| Yes | 92 | 47 | 52 (42-62) | 2.55 (1.64-3.99) | <.001 |
| No | 144 | 33 | 25 (18-33) |
Abbreviations: ETDRS, Early Treatment Diabetic Retinopathy Study; HbA1c, glycated hemoglobin; NPDR, nonproliferative diabetic retinopathy; OCT, optical coherence tomography.
Continuous age: HR for 10-year increase, 0.85 (95% CI, 0.70-1.04), P = .11.
Continuous HbA1c: HR for 1%-increase, 1.10 (95% CI, 0.96-1.26), P = .18.
Continuous visual acuity: HR for 5-letter decrease, 1.16 (95% CI, 0.85-1.59), P = .34.
Continuous OCT central subfield thickness: HR for 25-μm increase, 1.10 (95% CI, 1.03-1.18), P = .005.
Ordinal diabetic retinopathy severity level: HR for 1-step increase, 1.30 (95% CI, 1.14-1.49). P < .001.
After multivariable model selection, results were similar in a final model that included diabetic retinopathy severity, CST, and recent or planned DME treatment in the nonstudy eye (eTable 1 in the Supplement). The percentage of eyes that received injections during follow-up and the percentage that lost at least 5 letters of VA at 2 years (the primary outcome of Protocol V) by diabetic retinopathy severity level, CST, recent or planned DME treatment in the nonstudy eye, and the total number of risk factors are shown in eTable 2 in the Supplement. The likelihood of eyes receiving aflibercept increased as the number of risk factors present increased (ordinal P < .001); however, the risk of vision loss at 2 years did not appear to increase as the number of risk factors present increased (ordinal P = .52). Furthermore, none of the baseline characteristics associated with risk of aflibercept injection were associated with at least 5-letter vision loss at 2 years (eTable 3 in the Supplement); however, participants with higher glycated hemoglobin (HbA1c) levels more likely to lose vision (relative risk for 1% increase in baseline HbA1c level, 1.26 [95% CI, 1.09-1.46], P = .001).
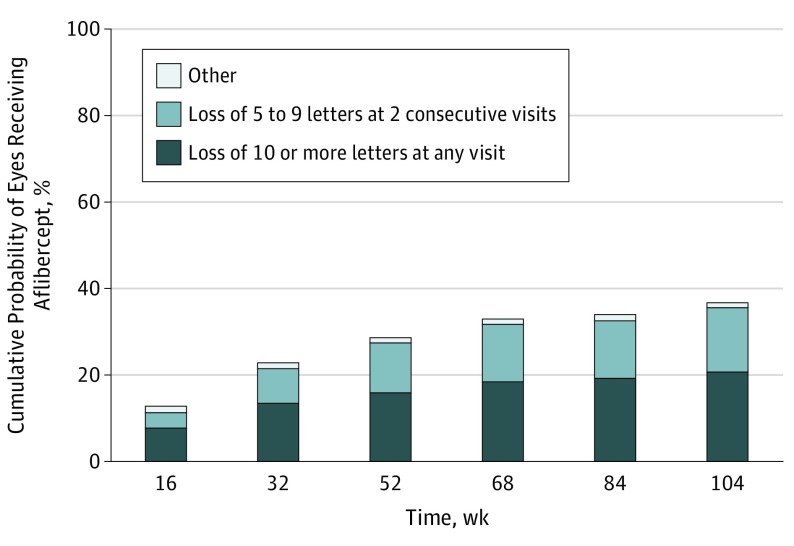
Figure 2 shows the cumulative probability of initiating aflibercept during 2 years of follow-up, by indication. Among 236 eyes, 44 (19%) initiated aflibercept injections because of at least 10-letter vision loss at a single visit, 34 eyes (14%) initiated aflibercept injections because of 5- to 9-letter vision loss at 2 consecutive visits, and 2 eyes (<1%) initiated injections counter to the protocol (1 for proliferative diabetic retinopathy and 1 for 7-letter VA loss with 150-μm CST increase).
Figure 2. Eyes Completing the 104-Week Visit in the Initial Observation Group.
Injections for “other” category were given for 1 case of proliferative diabetic retinopathy and 1 case of vision loss of 7 letters accompanied by a 150-μm increase in central subfield thickness. Group included 208 of 236 randomized; excluding 4 participants who died during follow-up, 90% of participants completed the 4-year visit.
While being observed, 92 of 236 eyes (39%) experienced a 5- to 9-letter loss at least once before their final visit. In this subgroup, 29 of 92 eyes (32%) had sustained VA loss because of DME at the next follow-up visit and began aflibercept treatment; 20 eyes (22%) received aflibercept for vision loss from DME later; and 42 eyes (46%) never received aflibercept. One eye received aflibercept for proliferative diabetic retinopathy after an initial loss of 5 letters.
Among 80 eyes in the observation group that began aflibercept therapy (63 eyes [79%] in year 1 and 17 [21%] in year 2), injections were deferred for success within the first 24 weeks after aflibercept initiation in 16 eyes (20%). Laser was administered per protocol in 5 of 236 eyes (2%) (all previously received aflibercept).1
Among eyes in the observation group that received at least 1 aflibercept injection (34% [80 of 236]), the percentage of eyes with a VA decrease of 5 or more letters at 2 years (Table 2) was 30% (23 of 76). Median letter change in VA from baseline at 2 years was –2.0 (IQR, –6.0 to 1.0), and median VA was 83.5 (87.0 to 78.5; median Snellen equivalent, 20/25 [20/20-20/32]). Median change in CST from baseline at 2 years was –53 μm (IQR, –117 to –14), and median CST (Zeiss-Stratus equivalent) was 250 μm (IQR, 218-315 μm); the percentage of eyes with no CI-DME and at least 10% CST decrease from baseline was 44% (33 of 75). Among 61 eyes without proliferative diabetic retinopathy at baseline (excluding eyes without gradable photos), 1 eye (2%) had proliferative diabetic retinopathy at 2 years.
Table 2. Two-Year Outcomes by Receipt of Aflibercept During Follow-up in the Initial Observation Group.
| Outcome | Eyes, No. (%) | |
|---|---|---|
| Did Not Receive Aflibercept (n = 132) | Received Aflibercept (n = 76) | |
| Visual Acuity, Letter Score | ||
| Baselinea | ||
| Mean (SD) | 85.4 (3.5) | 84.8 (4.2) |
| Mean Snellen equivalent | 20/20 | 20/20 |
| Median (IQR) | 85.0 (88.0 to 83.0) | 84.0 (87.0 to 81.0) |
| Median Snellen equivalent (IQR) | 20/20 (20/20 to 20/25) | 20/20 (20/20 to 20/25) |
| 20/20 or better (≥84 letters) | 90 (68) | 43 (57) |
| 2 y | ||
| Mean (SD) | 86.6 (5.2) | 80.0 (14.4) |
| Mean Snellen equivalent | 20/20 | 20/25 |
| Median (IQR) | 87.0 (90.0 to 83.5) | 83.5 (87.0 to 78.5) |
| Median Snellen equivalent (IQR) | 20/20 (20/16 to 20/25) | 20/25 (20/20 to 20/32) |
| 20/20 or better (≥84 letters) | 99 (75) | 38 (50) |
| Change from baseline | ||
| Mean (SD) | 1.2 (4.5) | −3.0 (8.0) |
| Median (IQR) | 1.5 (−1.0 to 5.0) | −2.0 (−6.0 to 1.0) |
| ≥5-Letter decrease | 16 (12) | 23 (30) |
| ≥10-Letter decrease | 3 (2) | 11 (14) |
| ≥15-Letter decrease | 1 (<1) | 7 (9) |
| OCT Central Subfield Thickness (Zeiss-Stratus Equivalent), μm | ||
| Baselinea | ||
| Mean (SD) | 308 (59) | 331 (70) |
| Median (IQR) | 292 (273 to 324) | 312 (289 to 357) |
| 2 yb | ||
| Mean (SD) | 276 (69) | 267 (79) |
| Median (IQR) | 264 (229 to 306) | 250 (218 to 315) |
| Change from baselineb | ||
| Mean (SD) | −30 (66) | −62 (86) |
| Median (IQR) | −33 (−68 to −1) | −53 (−117 to −14) |
| ≥10% Central subfield thickness decrease | 61 (46) | 45 (60) |
| No center-involved diabetic macular edema and ≥10% central subfield thickness decreasec | 41 (31) | 33 (44) |
Abbreviations: CST, central subfield thickness; IQR, interquartile range; OCT, optical coherence tomography.
Mean of screening and randomization values.
Unavailable for 1 eye that received aflibercept.
Defined by OCT machine and sex: Heidelberg Spectralis CST at least 305 μm in women and at least 320 μm in men; Zeiss Cirrus CST at least 290 μm in women and at least 305 μm in men.
Among eyes in the observation group that did not receive aflibercept injections (66% [156 of 236]), the percentage with a VA decrease of at least 5 letters at 2 years was 12% (16 of 132) (Table 2). Median change in VA from baseline at 2 years was 1.5 (IQR, –1.0 to 5.0), and median VA was 87.0 (IQR, 90.0 to 83.5; median Snellen equivalent, 20/20 [20/16-20/25]). Median change in CST from baseline at 2 years was –33 μm (IQR, –68 to –1 μm), and median CST (Zeiss-Stratus equivalent) was 264 μm (IQR, 229-306 μm); the percentage of eyes with no CI-DME and at least 10% CST decrease from baseline was 31% (41 of 132). Among 112 eyes without proliferative diabetic retinopathy at baseline (excluding eyes without gradable photos), 8 (7%) had proliferative diabetic retinopathy at 2 years.
Examples of clinical scenarios with OCT scans and color fundus photographs are in the Supplement. eFigure 1 in the Supplement shows an eye that never experienced worsening of VA because of DME (defined as loss of ≥10 letters at any visit or loss of 5-9 letters at 2 consecutive visits) and therefore never received aflibercept; in addition, the CST never worsened (defined as ≥10% increase) before the final visit (42% [88 of 208] of eyes completing 104 weeks). eFigure 2 in the Supplement shows an eye that experienced worsening of VA and CST and therefore received aflibercept (25% [53 of 208] of eyes completing 104 weeks). eFigure 3 in the Supplement shows an eye that experienced worsening of CST but not VA and did not receive aflibercept (21% [44 of 208] of eyes completing 104 weeks). eFigure 4 in the Supplement shows an eye that experienced worsened VA even though CST never worsened and therefore received aflibercept (11% [23 of 208] of eyes completing 104 weeks).
Discussion
Based on the results of Protocol V, many clinicians and patients might choose initial observation for eyes with CI-DME and good VA while withholding anti-VEGF treatment unless vision worsens. These analyses explored whether select baseline characteristics within the initial observation group were associated with receiving aflibercept injections during 2 years of follow-up. Greater baseline CST in the study eye, more severe diabetic retinopathy in the study eye, and recent or planned treatment for DME in the nonstudy eye were associated with greater likelihood of initiating anti-VEGF treatment. Each of these characteristics approximately doubled the likelihood of receiving an injection; however, even among study eyes having greater CST or more severe retinopathy or a nonstudy eye receiving DME treatment, only approximately half received an injection during 2 years of follow-up. Furthermore, none of these characteristics were associated with loss of VA at 2 years; among the additional variables evaluated, only higher HbA1c level was associated with greater risk of 2-year VA loss.
The results of these post hoc analyses highlight the variability of VA sometimes seen from visit to visit among patients with DME. Among eyes in the observation group that lost 1 line of VA at any visit, 46% tested better at the next visit and did not require aflibercept at any subsequent time during the trial. Thus, when vision loss is less than 2 lines (10 letters), the approach of confirming sustained vision loss before initiating anti-VEGF therapy in eyes with good vision appears to be reasonable.
Eyes with good VA despite CI-DME often experience spontaneous resolution of retinal thickening, further supporting initial observation as a management approach. Approximately two-thirds of eyes in the observation group never received aflibercept. Among eyes not receiving aflibercept treatment, 46% of eyes had a 10% or greater reduction in CST and 31% had spontaneous resolution of CI-DME by 2 years. Although some eyes assigned to initial observation in Protocol V lost at least 1 line of VA at 2 years (19%; 95% CI, 14%-25%), mean VA at 2 years was 20/20 overall (inclusive of participants receiving aflibercept). For comparison, among eyes assigned to aflibercept, 16% lost at least 1 line of VA at 2 years (95% CI, 12%-22%).1
Among eyes that received aflibercept for vision worsening, mean 2-year VA was approximately 1-line worse than eyes not requiring aflibercept. Although whether starting aflibercept earlier in these eyes would have resulted in better VA outcomes is unknown, overall rates of at least 5-letter VA worsening were not different in eyes immediately treated with aflibercept vs those managed with initial observation (–3%; 95% CI, –11% to 4%; P = .77).1 Whether more frequent follow-up of such eyes would lead to earlier anti-VEGF therapy and alter the VA outcomes obtained in this study also is unknown. Without biomarkers that predict vision in individual eyes with DME, clinicians may guide their treatment decisions based on the overall group results from this study coupled with individual patient preferences.
Limitations
This study has several limitations. First, the analyses were conducted post hoc, and with multiple characteristics being evaluated, some associations could have been identified by chance. Second, a formal statistical comparison between eyes receiving vs not receiving aflibercept could not be undertaken without substantial bias because initiating injections was based on VA loss after randomization. Thus, visual outcomes in the subgroups of observation-group eyes that did vs did not receive aflibercept should not be compared. Third, treatment decisions were guided by VA obtained using E-ETDRS testing after protocol refraction, which may differ from VA obtained in clinical settings15; therefore, a strict application of this approach assumes careful clinical VA evaluation. Fourth, treatment in nonstudy eyes was at investigator discretion; however, randomization was stratified by recent or planned DME treatment in the nonstudy eyes because these participants were expected to be seen more frequently, increasing the likelihood of detecting a decrease in VA and initiating aflibercept. Fifth, follow-up time from initiation of aflibercept was variable (ie, eyes initiated aflibercept at various times, but follow-up ended at 2 years for all study participants). Furthermore, we cannot say whether results at 5 or 10 years after standard clinical care would differ substantially from what was seen at 2 years in this study cohort. Also, this analysis did not consider the initial laser photocoagulation group, which required fewer aflibercept injections during 2 years of follow-up than the initial observation group.
Conclusions
Most eyes with CI-DME and good VA initially managed with observation did not require subsequent aflibercept for VA loss. On average, eyes in the initial observation group that required aflibercept treatment because of VA worsening still achieved good visual outcomes at 2 years. These results were achieved using regular follow-up intervals and a strict treatment algorithm. Clinicians should be aware that participants with greater CST or more severe retinopathy in the study eye and those receiving DME treatment in the nonstudy eye were more likely to experience VA worsening that required treatment.
eFigure 1. DME Resolves and Vision Does Not Worsen—No Injection Given
eFigure 2. Central Subfield Thickness and Vision Worsen—Injection Given
eFigure 3. Central Subfield Thickness Worsens but Vision Does Not—No Injection Given
eFigure 4. Vision Worsens but Central Subfield Thickness Does Not—Injection Given
eTable 1. Multivariable Model for Time to First Aflibercept Injection
eTable 2. Receipt of Aflibercept During Follow-up and Loss of Visual Acuity at 2 Years by Select Baseline Characteristics
eTable 3. Loss of 5 or More Letter of Visual Acuity at 2 Years by Baseline Characteristics
References
- 1.Baker CW, Glassman AR, Beaulieu WT, et al. ; DRCR Retina Network . Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: a randomized clinical trial. JAMA. 2019;321(19):1880-1894. doi: 10.1001/jama.2019.5790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhavsar AR, Glassman AR, Stockdale CR, Jampol LM; Diabetic Retinopathy Clinical Research Network . Elimination of topical antibiotics for intravitreous injections and the importance of using povidone-iodine: update from the Diabetic Retinopathy Clinical Research Network. JAMA Ophthalmol. 2016;134(10):1181-1183. doi: 10.1001/jamaophthalmol.2016.2741 [DOI] [PubMed] [Google Scholar]
- 3.Schatz H, Madeira D, McDonald HR, Johnson RN. Progressive enlargement of laser scars following grid laser photocoagulation for diffuse diabetic macular edema. Arch Ophthalmol. 1991;109(11):1549-1551. doi: 10.1001/archopht.1991.01080110085041 [DOI] [PubMed] [Google Scholar]
- 4.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 5.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135(2):194-205. doi: 10.1016/S0002-9394(02)01825-1 [DOI] [PubMed] [Google Scholar]
- 6.Chalam KV, Bressler SB, Edwards AR, et al. ; Diabetic Retinopathy Clinical Research Network . Retinal thickness in people with diabetes and minimal or no diabetic retinopathy: Heidelberg Spectralis optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(13):8154-8161. doi: 10.1167/iovs.12-10290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross JG, Glassman AR, Jampol LM, et al. ; Writing Committee for the Diabetic Retinopathy Clinical Research Network . Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314(20):2137-2146. doi: 10.1001/jama.2015.15217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wells JA, Glassman AR, Ayala AR, et al. ; Diabetic Retinopathy Clinical Research Network . Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193-1203. doi: 10.1056/NEJMoa1414264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bressler SB, Edwards AR, Chalam KV, et al. ; Diabetic Retinopathy Clinical Research Network Writing Committee . Reproducibility of spectral-domain optical coherence tomography retinal thickness measurements and conversion to equivalent time-domain metrics in diabetic macular edema. JAMA Ophthalmol. 2014;132(9):1113-1122. doi: 10.1001/jamaophthalmol.2014.1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34(2):187-220. [Google Scholar]
- 11.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80(3):557-572. doi: 10.1093/biomet/80.3.557 [DOI] [Google Scholar]
- 12.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481. doi: 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 13.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199-200. doi: 10.1093/aje/kwi188 [DOI] [PubMed] [Google Scholar]
- 14.Early Treatment Diabetic Retinopathy Study Research Group Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology. 1991;98(5)(suppl):823-833. doi: 10.1016/S0161-6420(13)38014-2 [DOI] [PubMed] [Google Scholar]
- 15.Kaiser PK. Prospective evaluation of visual acuity assessment: a comparison of snellen versus ETDRS charts in clinical practice (an AOS thesis). Trans Am Ophthalmol Soc 2009;107:311-324. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. DME Resolves and Vision Does Not Worsen—No Injection Given
eFigure 2. Central Subfield Thickness and Vision Worsen—Injection Given
eFigure 3. Central Subfield Thickness Worsens but Vision Does Not—No Injection Given
eFigure 4. Vision Worsens but Central Subfield Thickness Does Not—Injection Given
eTable 1. Multivariable Model for Time to First Aflibercept Injection
eTable 2. Receipt of Aflibercept During Follow-up and Loss of Visual Acuity at 2 Years by Select Baseline Characteristics
eTable 3. Loss of 5 or More Letter of Visual Acuity at 2 Years by Baseline Characteristics