Key Points
Question
Is primary intraocular lens implantation associated with improvements in the long-term visual outcome for infants undergoing unilateral congenital cataract surgery?
Findings
This randomized clinical trial of 114 patients who underwent treatment for unilateral congenital cataract, with or without intraocular lens implantation, between ages 1 and 6 months found that visual acuity at age 10.5 years was not significantly different between the 2 treatment groups.
Meaning
Results of this randomized clinical trial showed that implanting an intraocular lens at the time of cataract surgery extraction was neither beneficial nor detrimental to the visual outcome in children with unilateral congenital cataract.
Abstract
Importance
Although intraocular lenses (IOLs) are often implanted in children, little is known whether primary IOL implantation or aphakia and contact lens correction results in better long-term visual outcomes after unilateral cataract surgery during infancy.
Objective
To compare long-term visual outcomes with contact lens vs IOL correction following unilateral cataract surgery during infancy.
Design, Setting, and Participants
This multicenter randomized clinical trial enrolled 114 infants with a unilateral congenital cataract who underwent cataract surgery with or without primary IOL implantation between 1 and 6 months of age. Data on long-term visual outcomes were collected when the children were age 10.5 years (July 14, 2015, to July 12, 2019) and analyzed from March 30 through August 6, 2019.
Interventions
Intraocular lens implantation at the time of cataract surgery.
Main Outcomes and Measures
Best-corrected visual acuity using the electronic Early Treatment Diabetic Retinopathy Study (E-ETDRS) testing protocol. Analysis was performed on an intention-to-treat basis.
Results
Best-corrected visual acuity was measured at age 10.5 years for 110 of the 114 patients (96%) enrolled as infants. The participants included 58 girls (53%) and 52 boys (47%). Overall, 27 of the children (25%) had good (logMAR 0.30 [Snellen equivalent, 20/40] or better) visual acuity in the treated eye (12 [22%] in the IOL group and 15 [27%] in the aphakia group), but 50 children (44%) had a visual acuity of logMAR 1.00 (Snellen equivalent, 20/200) or worse (25 [44%] in the IOL group and 25 [44%] in the aphakia group). The median logMAR acuity in the treated eye was similar in children randomized to receive an IOL at the time of cataract extraction (0.89; interquartile range [IQR], 0.33-1.43 [Snellen equivalent, 20/159]) and those who remained aphakic (0.86; IQR, 0.30-1.46 [Snellen equivalent, 20/145]) (IQR, 0.30-1.46; P = .82). Although the overall difference in median visual acuity between the 2 groups was small, the estimate was imprecise (99% CI for the difference in medians was −0.54 to 0.47).
Conclusions and Relevance
As in previous phases of the study, visual acuity outcomes were highly variable with only 27 children (25%) achieving excellent visual acuity in their treated eye and 50 children (44%) having poor vision in the treated eye. Implanting an IOL at the time of cataract extraction was neither beneficial nor detrimental to the visual outcome.
Trial Registration
ClinicalTrials.gov Identifier: NCT00212134
This randomized clinical trial compares the effects of intraocular lens vs contact lens correction in visual acuity in children 10.5 years after undergoing cataract surgery as infants.
Introduction
Intraocular lenses (IOLs) are being used increasingly to focus eyes in children after cataract surgery.1,2,3 Compared with aphakic contact lenses and eyeglasses, IOLs have the advantage that partial optical correction is worn at all times. However, there is a general reluctance to implant IOLs in young children because their eyes can develop large refractive errors later in childhood and they have a higher risk of developing visual axis opacities after IOL implantation.4,5
The Infant Aphakia Treatment Study (IATS) is a multicenter randomized clinical trial comparing visual outcomes following cataract surgery with or without IOL implantation in infants aged 1 to 6 months with a unilateral congenital cataract.6 The IATS previously reported that grating acuities at age 12 months and HOTV acuity testing system results at age 4.5 years were similar for children randomized to receive an IOL at the time of cataract extraction compared with those who did not receive an IOL.7,8 However, the sensitive period for visual development, particularly in eyes affected by congenital cataracts, can extend beyond age 4.5 years.9,10,11 Therefore, this article extends our earlier results by comparing visual acuity outcomes in patients randomized to these 2 treatments at age 10.5 years using the electronic Early Treatment of Diabetic Retinopathy Study (E-ETDRS) testing protocol after the sensitive period for visual development had been completed. The trial protocols are available in Supplement 1.
Methods
This study, supported through a cooperative agreement with the National Eye Institute of the National Institutes of Health, was conducted by the IATS Group at 12 clinical sites. The hypothesis of the study was that primary IOL implantation in an infant with a unilateral congenital cataract would result in a better visual outcome than leaving the eye aphakic and optically correcting it with a contact lens. The study design, eligibility criteria, method of randomization, sample size determination, surgical techniques, patching and optical correction regimens, evaluation methods, and patient characteristics at baseline have been reported previously.6,7,12 To maintain consistency within the randomized groupings, patients left aphakic were not eligible for an IOL implant until age 5 years without approval from the IATS executive committee. Intraocular lens implantation after age 5 years was at the discretion of the investigator. This study was approved by the institutional review boards at all participating institutions and was in compliance with the Health Insurance Portability and Accountability Act and Declaration of Helsinki.13 Written informed parental consent was obtained from all parents and written assent was obtained from all patients. Participants received financial compensation.
Clinical Examination
A follow-up clinical examination was performed by IATS-certified investigators (including S.R.L., S.J.K., and D.R.W.) at age 10.5 years ±3 months. To ensure that the visual acuity obtained represented the best-corrected visual acuity, a cycloplegic refraction was performed on the date of testing for both the treated and untreated eyes at least 30 minutes after the topical instillation of cyclopentolate, 1.0%, and phenylephrine, 2.5%, drops. When possible, an autorefraction was performed and the prescription was verified with subjective refinement. Eyes wearing a contact lens were refracted both with and without the contact lens on the eye. If the child was wearing a contact lens, the type and power of the contact lens was documented. If the child was wearing eyeglasses, the spectacle correction was documented.
Visual Acuity Assessment
Optotype acuity was assessed using the E-ETDRS testing protocol.14 Patients wearing a contact lens were tested wearing their current contact lens. Any residual refractive error was corrected in trial frames. The child wore his or her aphakic correction in trial frames for visual assessment if a child was randomized to contact lens correction and had discontinued contact lens wear and had not received a secondary IOL. Children with pseudophakia had their vision tested wearing their cycloplegic refraction in trial frames. Visual acuity was initially assessed using both eyes. Monocular visual acuity was then tested starting with the aphakic/pseudophakic eye. The eye not being tested was occluded using frosted occluder glasses (Good-Lite) to minimize the amplitude of latent nystagmus under monocular conditions. The initial testing distance was 3 m. If the child was unable to see a 20/800 letter at this distance, the distance was decreased to 1 m. If the child was unable to detect a 20/800 letter at 1 m, then E-ETDRS testing was stopped for this eye and the tester proceeded to test for hand motion at 0.66 m. If hand motion was not present, the eye was assessed for light perception or no light perception following standard protocols.12 The letter scores were then converted to logMAR acuity.14 Hand motion acuity was assigned a logMAR acuity of 2.64; light perception acuity, 2.78; and no light perception acuity, 2.93.15
Statistical Analysis
Monocular visual acuities in the treated and untreated eyes and binocular visual acuity were compared between the treatment groups using the Wilcoxon rank sum test. The patients were analyzed on an intention-to-treat basis. The categorized difference between binocular vision and the monocular visual acuity in the best-seeing eye of the same patient was compared between treatment groups using a χ2 test. A nonparametric test was used because of the skewed distribution of the data in the treated eye and because of the assignment of visual acuity values for patients with low vision below the level detectable with the E-ETDRS testing protocol. Bootstrap methods were used to calculate the 99% CI for the difference between the median visual acuities in the 2 treatment groups. The Kruskal-Wallis test was used to compare visual acuities in the aphakia group between patients who had a secondary IOL, those wearing a contact lens, those only wearing eyeglasses, and those wearing no optical correction. Age at the time of testing was compared between treatment groups using an independent-group t test. In the aphakia group, private insurance rates were compared between patients who continued to wear a contact lens and those who did not, using a χ2 test. In the aphakia group, mean reported adherence to contact lens wear and patching therapy from the age of 4 to 5 years was compared between patients who continued to wear contact lenses and those who did not using an independent group t test. Adherence to contact lens use and patching therapy was assessed using caregiver adherence interviews.16,17 All reported P values are 2-sided without adjustment for multiple testing. Findings were considered significant at P < .05. Statistical analysis was conducted using SAS, version 9.4 (SAS Institute Inc); data were analyzed from March 30 through August 6, 2019.
Results
Patient Characteristics
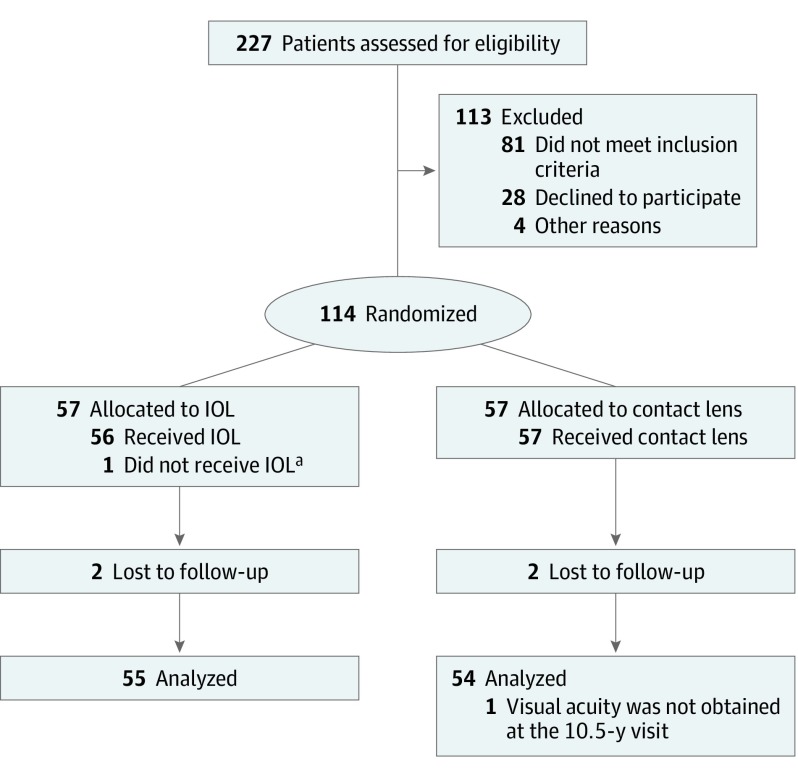
Of the original 114 participants, 4 children (4%), 2 from each treatment arm, were lost to follow-up. The remaining 110 participants (96%) were examined between July 14, 2015, and July 12, 2019, at a mean (SD) age of 10.6 (0.3) years in the IOL group (n = 55) and 10.6 (0.3) years in the aphakia group (n = 55) (P = .86) (Figure 1). The participants included 58 girls (53%) and 52 boys (47%). Overall, 78 of the patients (71%) were tested in the window (10.5 years ±3 months), 9 patients (8%) were tested before age 123 months, and 23 children (21%) were tested after age 129 months. Visual acuity was not obtained for 1 participant in the aphakia group.
Figure 1. CONSORT Diagram for the Infant Aphakia Treatment Study.
IOL indicates intraocular lens.
aIntraoperatively, stretching of ciliary processes was found; the investigator decided that an IOL could not be safely implanted. The patient remained aphakic and was treated with a contact lens.
Seven children in the IOL group underwent an IOL exchange. A secondary IOL was implanted in 24 of the 55 children (44%) tested who were randomized to the aphakia group; the other 31 children did not receive an IOL for aphakia. For the 31 children who remained aphakic, 18 continued to wear a contact lens (silicone elastomer, n = 6; rigid gas permeable, n = 6; hydrogel, n = 5; and silicone hydrogel, n = 1); 9 were wearing only eyeglasses, and 4 were not wearing any optical correction device. All 18 of the children in the aphakia group who continued to wear a contact lens had their vision tested wearing their contact lens correction.
Monocular Visual Acuity
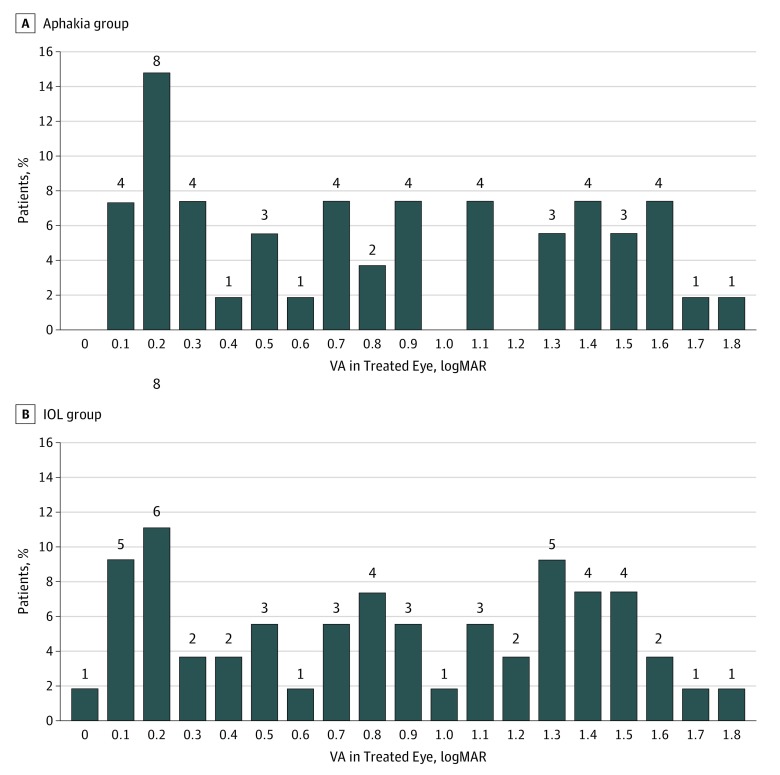
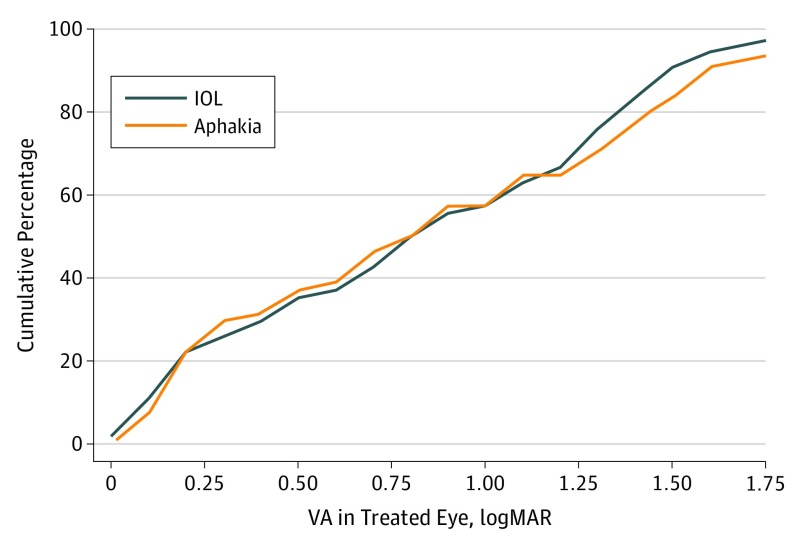
Twelve treated eyes (22%) randomized to receive an IOL and 15 eyes (27%) originally left aphakic had a visual acuity of 20/40 or better, but 25 eyes (44%) randomized to receive an IOL and 25 eyes (44%) eyes originally remaining aphakic had a visual acuity of logMAR 1.00 (Snellen equivalent 20/200) or worse (P = .97). Overall, the logMAR best-corrected visual acuity for the treated eyes was variable, ranging from 0.00 to 2.93 with a median of 0.89 (Snellen equivalent, 20/159) (interquartile range [IQR], 0.33-1.43). The median visual acuity for children randomized to aphakia was 0.86 (Snellen equivalent, 20/145) (IQR, 0.30-1.46) and for children randomized to receive an IOL was 0.89 (Snellen equivalent, 20/159; IQR, 0.38-1.38) (P = .82) (Figure 2 and Figure 3). Although the overall difference in median visual acuity between the 2 groups was small, the estimate was imprecise (99% CI, −0.54-0.47 for the difference in medians).
Figure 2. LogMAR Visual Acuity (VA) of Treated Eyes at Age 10.5 Years.
The number above each bar refers to the number of patients in this acuity category. A, In the aphakia group, median VA was 0.86 (Snellen equivalent, 20/145) (interquartile range, 0.30-1.46; Snellen equivalent, 20/48-20/480). One patient had hand motion acuity (defined as logMAR, 2.64); 1, light perception acuity (defined as logMAR, 2.78); and 1, no light perception (defined as logMAR, 2.93). B, In the intraocular lens (IOL) group, median visual acuity was 0.89 (Snellen equivalent, 20/159) (interquartile range, 0.38-1.38; Snellen equivalent, 20/40-20/577) (P = .44). One patient had hand motion acuity.
Figure 3. Cumulative logMAR Visual Acuity (VA) of the Aphakia and Intraocular Lens (IOL) Groups at Age 10.5 Years.
Cumulative VA in patients with hand motion acuity (defined as logMAR, 2.64) was 2 (2%); light perception acuity (defined as logMAR, 2.78), 1 (1%); and no light perception (defined as logMAR, 2.93), 1 (1%).
In the aphakia group, the median logMAR acuity was best for children who continued to wear a contact lens correction (contact lens [n = 18], 0.37 [Snellen equivalent, 20/47]; IQR, 0.22-0.82) compared with children who underwent secondary IOL implantation (n = 24) (0.92 [Snellen equivalent, 20/166]; IQR, 0.36-1.44), wore eyeglasses (n = 9) (1.46 [Snellen equivalent, 20/577]; IQR, 1.18-1.88), or no correction (n = 4) (1.60 [Snellen equivalent, 20/796]; IQR, 1.44-2.22) (P < .001). Children in the aphakia group who continued to wear a contact lens correction were more likely to have private insurance (83% vs 54%; P = .03), report spending a higher percentage of their waking hours patching the untreated eye (3.7 vs 2.2 hours/d, P = .05), and report wearing their contact lens a higher percentage of their waking hours (85% vs 61%; P = .05) from age 4 to 5 years.
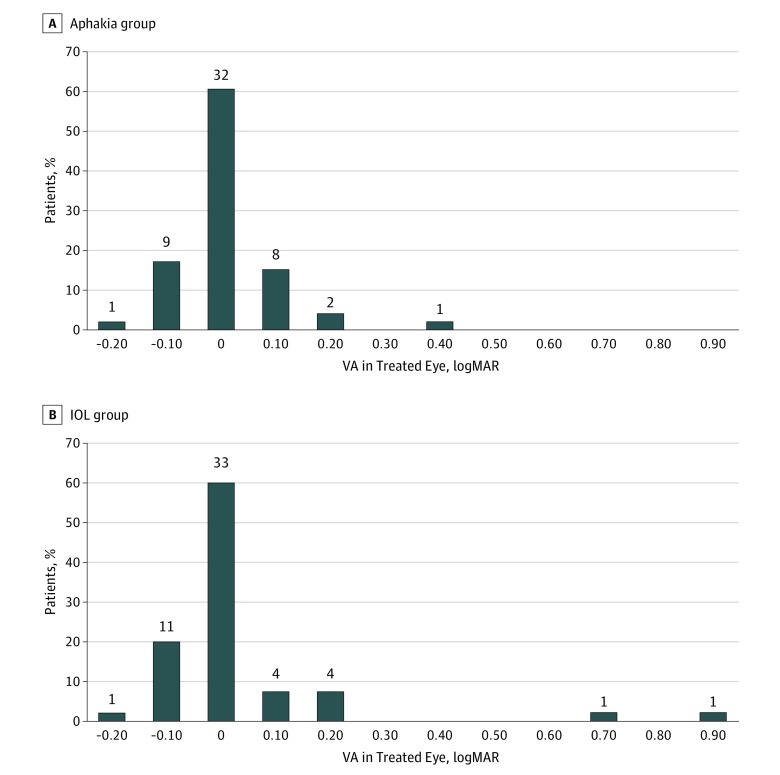
The median logMAR visual acuity for the untreated eyes was similar in both treatment groups (99% CI for the difference in medians between the aphakia and IOL groups, −0.04 to 0.10) (Figure 4). Three untreated eyes (6%) in the aphakia group and 6 untreated eyes (11%) in the IOL group had visual acuity of logMAR 0.2 (20/32) or worse (P = .32). Three children in the IOL group likely had anisometropic amblyopia in their untreated eyes. One of these children had hyperopic astigmatism (+5.25 + 2.25 × 78 diopters [D]) in their untreated eye and 2 had high myopia (−17.00 D, −8.75 + 4.00 × 85 D). The child with hyperopic astigmatism had better visual acuity in his treated eye (20/25) than his untreated eye (20/32) at age 10.5 years, and the child with high myopia had better vision in his treated eye until age 10 years when he developed a retinal detachment in his treated eye. The cause of the reduced vision in the untreated eyes of the other 6 children is uncertain.
Figure 4. LogMAR Visual Acuity (VA) of Untreated Eyes at Age 10.5 Years.
The number above each bar refers to the number of patients in this acuity category. Median visual acuity was 0.02 (20/21) (interquartile range, −0.06-0.08) (Snellen equivalent, 20/17-20/24) and −0.02 (20/19) (interquartile range, −0.08-0.08) (Snellen equivalent, 20/17-20/24) for the aphakia and intraocular lens (IOL) group, respectively (P = 44).
Binocular Visual Acuity
Binocular vision was evaluated in 53 children in both treatment groups. Median logMAR binocular vision was similar for both treatment groups (aphakia group: 0.02 [Snellen equivalent, 20/21]; IQR, −0.08 to 0.06 vs IOL group: −0.02 [Snellen equivalent, 20/19]; IQR, −0.08 to 0.08; P = .44) (99% CI for the difference in medians between the aphakia and IOL groups, −0.08 to 0.11). The difference between binocular vision and best-corrected monocular vision was within 0.1 logMAR (1 line) for 44 children (83%) in the aphakia group and 49 children (91%) in the IOL group, greater than or equal to 0.1 logMAR for 5 children (9%) in the aphakia group and 2 children (4%) in the IOL group, and less than or equal to −0.1 logMAR for 4 children (8%) in the aphakia group and 3 children (6%) in the IOL group (P = .43).
Discussion
At age 10.5 years, visual acuity outcomes in the treated eyes of children with unilateral congenital cataracts who underwent surgery during the first 6 months of life were variable and were similar in children who were initially left aphakic compared with those who underwent primary IOL implantation. Twenty-five percent of these children had sufficiently good visual acuity in their treated eye to drive using this eye if they were to lose vision in their untreated eye as a result of an illness or injury. However, in both groups, 44% of children had unilateral visual impairment of their treated eye. We did note, however, that the visual acuity of the subgroup of children in the aphakic group who wore a contact lens to age 10.5 years was better than in other subgroups.
These results are consistent with the previous work of IATS that showed substantial variability in visual acuity outcomes, with 25% of children having good visual outcomes and nearly 50% having poor visual outcomes.7,12,18 The similarity of these results to those presented earlier likely reflects that most adverse events occur in these eyes prior to age 4.5 years19 and there is little risk of deprivation amblyopia after age 7 years.20 However, it was important to confirm these findings in this cohort of children when they were visually mature, after the removal of study-imposed restrictions on secondary IOL implantation and after the study no longer provided financial support for contact lenses and glasses. These results likely represent the visual acuity that these patients will have as adults barring an injury or new disease process developing.
The primary outcome of this randomized clinical trial was visual acuity. We had originally hypothesized that visual acuity would be better in the IOL group since these children would be wearing at least a partial correction for their treated eye at all times. However, we did not find a visual benefit to primary IOL implantation; this finding, coupled with the increased incidence of visual axis opacities developing in eyes with an IOL and the increased frequency of additional intraocular surgeries, led to our recommendation that IOLs not be routinely implanted in the eyes of children undergoing unilateral cataract surgery when aged 6 months or younger.12,21
We found that the subset of children in the aphakia group who continued to wear a contact lens to age 10.5 years had the best visual outcomes. It is likely that children who were successfully wearing a contact lens during the elementary school years did not perceive a need for secondary IOL implantation. This finding does not necessarily mean that a contact lens correction results in a better visual outcome than wearing eyeglasses or having a secondary IOL implanted, because there were likely many confounding factors that influenced the type of correction worn between ages 5 and 10.5 years. In future analyses, it will be important to understand the factors that influence contact lens wear. The IATS group previously reported that 81% of patients in the aphakia group wore a contact lens more than 80% of their waking hours up to age 5 years.17 The excellent contact lens adherence in the aphakia group may be partially due to the fact that contact lenses were provided to participants at no charge until age 5 years. Thus, it is not clear to what extent these findings are generalizable. Children who wore their contact lens for a greater proportion of their waking hours tended to have better visual acuity at age 4.5 years. We did not assess contact lens adherence between ages 5 and 10.5 years, but children who continued to wear a contact lens to age 10.5 years generally had excellent visual acuity in their treated eyes and likely perceived a visual benefit from wearing a contact lens. Since the study provided limited funding for contact lenses between the ages of 5 and 10.5 years, cost may have been a factor for some children discontinuing contact lens wear after age 5 years.22 Children who continued to wear contact lenses were more likely to have private health insurance. Although private health insurance does not usually cover the cost of contact lenses, contact lens use after age 5 years is likely a surrogate for higher socioeconomic status since low-income families would have a more difficult time paying for replacement contact lenses. The IATS group has argued that third-party payers should pay for the cost of contact lenses for children with aphakia.23
The types of contact lenses used changed during the course of the clinical trial. The IATS group previously reported that, at age 12 months, 74% of patients in the aphakia group were wearing silicone elastomer contact lenses, 21% were wearing rigid gas-permeable contact lenses, and 5% alternated between wearing both types of contact lenses.24 By age 5 years, the percentage of patients wearing silicone elastomer lenses had decreased to 46%.25 At age 10.5 years, only one-third of patients who continued to wear contact lenses were wearing silicone elastomer contact lenses, one-third were wearing rigid gas-permeable contact lenses, and one-third were wearing hydrogel or silicone-hydrogel contact lenses. Presumably, more patients were wearing hydrogel or silicone-hydrogel contact lenses at age 10.5 years because these lenses are available in the lower powers needed for older children. The decreased use of silicone elastomer lenses may have been due to the higher cost of these lenses and the higher incidence of deposits on these lenses in older children.26,27 The higher cost and increased incidence of deposits may have offset the convenience of these lenses being approved for extended wear.28 The use of rigid gas-permeable contact lenses may have increased between the ages of 5 and 10.5 years because they are less expensive than silicone elastomer lenses and are associated with fewer adverse events.29
Visual acuity was generally excellent in untreated eyes. However, it was reduced to 20/32 or worse in 9 patients. One of these patients had better visual acuity in their treated than untreated eye at age 10.5 years. This patient was myopic in the pseudophakic eye and had high hyperopic astigmatism in the untreated eye. Other studies have also reported worse vision in untreated eyes with hyperopic astigmatism in children who have undergone unilateral cataract surgery and IOL implantation during early childhood.30 This finding emphasizes the importance of striving to achieve the best possible visual outcome in an eye with a unilateral congenital cataract since, in some instances, the treated eye will become the better-seeing eye. The treated eye may become the better-seeing eye for more of these patients as they become adults owing to the cumulative risk to the fellow eye of disease or injury. Binocular vision was similar to best-corrected monocular vision in both treatment groups
Limitations
Limitations of this study include a lack of data regarding spectacle or contact lens adherence between ages 5 and 10.5 years and visual acuity was tested differently at each of the primary end points. At age 12 months, visual acuity was assessed by a masked examiner using forced preferential looking,7 at age 4.5 years, acuity was assessed using HOTV optotypes,12 and at age 10.5 years, acuity was assessed using the E-ETDRS protocol. Different tests were used at each age to ensure that the testing was developmentally appropriate. In addition, this study was not designed to compare different aphakic correction modalities (contact lenses vs eyeglasses vs secondary IOL implantation), so the visual results for these different subgroups should be interpreted with caution since there is a potential for confounding factors.
Conclusions
Among children undergoing unilateral cataract surgery during infancy by expert surgeons, visual acuity was similar in children receiving an IOL and those who remained aphakic (99% CI, −0.54 to 0.47). These results are robust, having been replicated at 3 different ages (12 months, 4.5 years, and 10.5 years). Furthermore, these visual results will likely persist into adulthood barring an ocular injury or disease since these children are now beyond the sensitive period of visual development. The extent to which these findings can be generalized to intervention by surgeons with less experience or children whose families cannot afford the costs associated with contact lens wear should be taken into consideration when deciding on a course of treatment for an infant with a unilateral congenital cataract.
Trial Protocol
Data Sharing Statement
References
- 1.Solebo AL, Russell-Eggitt I, Cumberland PM, Rahi JS; British Isles Congenital Cataract Interest Group . Risks and outcomes associated with primary intraocular lens implantation in children under 2 years of age: the IoLunder2 cohort study. Br J Ophthalmol. 2015;99(11):1471-1476. doi: 10.1136/bjophthalmol-2014-306394 [DOI] [PubMed] [Google Scholar]
- 2.Solebo AL, Cumberland P, Rahi JS; British Isles Congenital Cataract Interest Group . 5-Year outcomes after primary intraocular lens implantation in children aged 2 years or younger with congenital or infantile cataract: findings from the IoLunder2 prospective inception cohort study. Lancet Child Adolesc Health. 2018;2(12):863-871. doi: 10.1016/S2352-4642(18)30317-1 [DOI] [PubMed] [Google Scholar]
- 3.Lambert SR, Aakalu VK, Hutchinson AK, et al. Intraocular lens implantation during early childhood: a report by the American Academy of Ophthalmology. Ophthalmology. 2019;126(10):1454-1461. doi: 10.1016/j.ophtha.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 4.Koo EB, VanderVeen DK, Lambert SR. Global practice patterns in the management of infantile cataracts. Eye Contact Lens. 2018;44(suppl 2):S292-S296. doi: 10.1097/ICL.0000000000000461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bothun ED, Wilson ME, Traboulsi EI, et al. ; Toddler Aphakia and Pseudophakia Study Group (TAPS) . Outcomes of unilateral cataracts in infants and toddlers 7 to 24 months of age: Toddler Aphakia and Pseudophakia Study (TAPS). Ophthalmology. 2019;126(8):1189-1195. doi: 10.1016/j.ophtha.2019.03.011 [DOI] [PubMed] [Google Scholar]
- 6.Lambert SR, Buckley EG, Drews-Botsch C, et al. ; Infant Aphakia Treatment Study Group . The infant aphakia treatment study: design and clinical measures at enrollment. Arch Ophthalmol. 2010;128(1):21-27. doi: 10.1001/archophthalmol.2009.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambert SR, Buckley EG, Drews-Botsch C, et al. ; Infant Aphakia Treatment Study Group . A randomized clinical trial comparing contact lens with intraocular lens correction of monocular aphakia during infancy: grating acuity and adverse events at age 1 year. Arch Ophthalmol. 2010;128(7):810-818. doi: 10.1001/archophthalmol.2010.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartmann EE, Lynn MJ, Lambert SR; Infant Aphakia Treatment Study Group . Baseline characteristics of the infant aphakia treatment study population: predicting recognition acuity at 4.5 years of age. Invest Ophthalmol Vis Sci. 2014;56(1):388-395. doi: 10.1167/iovs.14-15464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes JM, Melia M, Bradfield YS, Cruz OA, Forbes B; Pediatric Eye Disease Investigator Group . Factors associated with recurrence of amblyopia on cessation of patching. Ophthalmology. 2007;114(8):1427-1432. doi: 10.1016/j.ophtha.2006.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmes JM, Beck RW, Kraker RT, et al. ; Pediatric Eye Disease Investigator Group . Risk of amblyopia recurrence after cessation of treatment. J AAPOS. 2004;8(5):420-428. doi: 10.1016/S1091-8531(04)00161-2 [DOI] [PubMed] [Google Scholar]
- 11.Tailor V, Bossi M, Greenwood JA, Dahlmann-Noor A. Childhood amblyopia: current management and new trends. Br Med Bull. 2016;119(1):75-86. doi: 10.1093/bmb/ldw030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert SR, Lynn MJ, Hartmann EE, et al. ; Infant Aphakia Treatment Study Group . Comparison of contact lens and intraocular lens correction of monocular aphakia during infancy: a randomized clinical trial of HOTV optotype acuity at age 4.5 years and clinical findings at age 5 years. JAMA Ophthalmol. 2014;132(6):676-682. doi: 10.1001/jamaophthalmol.2014.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 14.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135(2):194-205. doi: 10.1016/S0002-9394(02)01825-1 [DOI] [PubMed] [Google Scholar]
- 15.Roberts MF, Fishman GA, Roberts DK, et al. Retrospective, longitudinal, and cross sectional study of visual acuity impairment in choroideraemia. Br J Ophthalmol. 2002;86(6):658-662. doi: 10.1136/bjo.86.6.658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drews-Botsch CD, Hartmann EE, Celano M; Infant Aphakia Treatment Study Group . Predictors of adherence to occlusion therapy 3 months after cataract extraction in the Infant Aphakia Treatment Study. J AAPOS. 2012;16(2):150-155. doi: 10.1016/j.jaapos.2011.12.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cromelin CH, Drews-Botsch C, Russell B, Lambert SR; Infant Aphakia Treatment Study Group . Association of contact lens adherence with visual outcome in the infant aphakia treatment study: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2018;136(3):279-285. doi: 10.1001/jamaophthalmol.2017.6691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert SR, DuBois L, Cotsonis G, Hartmann EE, Drews-Botsch C. Factors associated with stereopsis and a good visual acuity outcome among children in the Infant Aphakia Treatment Study. Eye (Lond). 2016;30(9):1221-1228. doi: 10.1038/eye.2016.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plager DA, Lynn MJ, Buckley EG, Wilson ME, Lambert SR; Infant Aphakia Treatment Study Group . Complications in the first 5 years following cataract surgery in infants with and without intraocular lens implantation in the Infant Aphakia Treatment Study. Am J Ophthalmol. 2014;158(5):892-898. doi: 10.1016/j.ajo.2014.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hensch TK, Quinlan EM. Critical periods in amblyopia. Vis Neurosci. 2018;35:E014. doi: 10.1017/S0952523817000219 [DOI] [PubMed] [Google Scholar]
- 21.Plager DA, Lynn MJ, Buckley EG, Wilson ME, Lambert SR; Infant Aphakia Treatment Study Group . Complications, adverse events, and additional intraocular surgery 1 year after cataract surgery in the infant Aphakia Treatment Study. Ophthalmology. 2011;118(12):2330-2334. doi: 10.1016/j.ophtha.2011.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kruger SJ, DuBois L, Becker ER, et al. ; Infant Aphakia Treatment Study Group . Cost of intraocular lens versus contact lens treatment after unilateral congenital cataract surgery in the infant aphakia treatment study at age 5 years. Ophthalmology. 2015;122(2):288-292. doi: 10.1016/j.ophtha.2014.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kruger SJ, Vanderveen DK, Freedman SF, Bothun E, Drews-Botsch CD, Lambert SR; Infant Aphakia Study Group . Third-party coverage for aphakic contact lenses for children. Transl Vis Sci Technol. 2019;8(3):41. doi: 10.1167/tvst.8.3.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell B, Ward MA, Lynn M, Dubois L, Lambert SR; Infant Aphakia Treatment Study Group . The infant aphakia treatment study contact lens experience: one-year outcomes. Eye Contact Lens. 2012;38(4):234-239. doi: 10.1097/ICL.0b013e3182562dc0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell B, DuBois L, Lynn M, Ward MA, Lambert SR; Infant Aphakia Treatment Study Group . The Infant Aphakia Treatment Study Contact Lens Experience to Age 5 Years. Eye Contact Lens. 2017;43(6):352-357. doi: 10.1097/ICL.0000000000000291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurland JE. Use of silicone lenses in infants and children. Ophthalmology. 1979;86(9):1599-1604. doi: 10.1016/S0161-6420(79)35359-3 [DOI] [PubMed] [Google Scholar]
- 27.Nelson LB, Cutler SI, Calhoun JH, Wilson TW, Harley RD. Silsoft extended wear contact lenses in pediatric aphakia. Ophthalmology. 1985;92(11):1529-1531. doi: 10.1016/S0161-6420(85)33825-3 [DOI] [PubMed] [Google Scholar]
- 28.Lambert SR, Kraker RT, Pineles SL, et al. Contact Lens Correction of Aphakia in Children: A Report by the American Academy of Ophthalmology. Ophthalmology. 2018;125(9):1452-1458. doi: 10.1016/j.ophtha.2018.03.014 [DOI] [PubMed] [Google Scholar]
- 29.Amos CF, Lambert SR, Ward MA. Rigid gas permeable contact lens correction of aphakia following congenital cataract removal during infancy. J Pediatr Ophthalmol Strabismus. 1992;29(4):243-245. [DOI] [PubMed] [Google Scholar]
- 30.Ruth AL, Lambert SR. Amblyopia in the phakic eye after unilateral congenital cataract extraction. J AAPOS. 2006;10(6):587-588. doi: 10.1016/j.jaapos.2006.08.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Data Sharing Statement