Key Points
Question
Is sustained inflation a more effective intervention than standard intermittent positive pressure ventilation or continuous positive airway pressure for preterm infants who require respiratory support after birth?
Findings
In this systematic review and meta-analysis, sustained inflation was associated with a similar risk of in-hospital mortality compared with standard therapy. Sustained inflation was associated with an increased risk of mortality in the first 2 days compared with standard therapy, and there were no differences in the risk of any other secondary outcomes.
Meaning
These results do not support the use of sustained inflation after birth to improve outcomes for preterm infants.
Abstract
Importance
Most preterm infants require respiratory support to establish lung aeration after birth. Intermittent positive pressure ventilation and continuous positive airway pressure are standard therapies. An initial sustained inflation (inflation time >5 seconds) is a widely practiced alternative strategy.
Objective
To conduct a systematic review and meta-analysis of sustained inflation vs intermittent positive pressure ventilation and continuous positive airway pressure for the prevention of hospital mortality and morbidity for preterm infants.
Data Sources
MEDLINE (through PubMed), Embase, the Cumulative Index of Nursing and Allied Health Literature, and the Cochrane Central Register of Controlled Trials were searched through June 24, 2019.
Study Selection
Randomized clinical trials of preterm infants born at less than 37 weeks’ gestation that compared sustained inflation (inflation time >5 seconds) vs standard resuscitation with either intermittent positive pressure ventilation or continuous positive airway pressure were included. Studies including other cointerventions were excluded.
Data Extraction and Synthesis
Two reviewers assessed the risk of bias of included studies. Meta-analysis of pooled outcome data used a fixed-effects model specific to rarer events. Subgroups were based on gestational age and study design (rescue vs prophylactic sustained inflation).
Main Outcomes and Measures
Death before hospital discharge.
Results
Nine studies recruiting 1406 infants met inclusion criteria. Death before hospital discharge occurred in 85 of 736 infants (11.5%) treated with sustained inflation and 62 of 670 infants (9.3%) who received standard therapy for a risk difference of 3.6% (95% CI, −0.7% to 7.9%). Although analysis of the primary outcome identified important heterogeneity based on gestational age subgroups, the 95% CI for the risk difference included 0 for each individual gestational age subgroup. There was no difference in the primary outcome between subgroups based on study design. Sustained inflation was associated with increased risk of death in the first 2 days after birth (risk difference, 3.1%; 95% CI, 0.9%-5.3%). No differences in the risk of other secondary outcomes were identified. The quality-of-evidence assessment was low owing to risk of bias and imprecision.
Conclusions and Relevance
There was no difference in the risk of the primary outcome of death before hospital discharge, and there was no evidence of efficacy for sustained inflation to prevent secondary outcomes. These findings do not support the routine use of sustained inflation for preterm infants after birth.
This systematic review and meta-analysis compares sustained inflation vs intermittent positive pressure ventilation and continuous positive airway pressure for the prevention of hospital mortality and morbidity for preterm infants.
Introduction
Almost all very preterm infants require support to achieve lung aeration immediately after birth. The current standard practice is to provide intermittent positive pressure ventilation (IPPV) with positive end-expiratory pressure for infants with apnea and continuous positive airway pressure (CPAP) for spontaneously breathing infants who require respiratory support.1 The optimal inflation time during IPPV to aerate the newborn lung after birth is unknown because airway resistance is higher in the presence of fetal fluid compared with air. Strategies to overcome this resistance include using higher pressures or longer inflation times.2 Sustained inflation (SI), in which an inflating pressure is held for a prolonged duration greater than 5 seconds,1 is an alternative approach to clear lung liquid and aerate the newborn lung.
Preclinical studies have demonstrated that SI leads to rapid and homogenous lung aeration.3,4 In preliminary observational studies, preterm infants treated with SI experienced improved short-term outcomes, such as less frequent delivery room intubation and less exposure to mechanical ventilation in the first 72 hours of life compared with historical controls.5,6,7 A recent Cochrane systematic review of 8 randomized clinical trials enrolling 941 infants found no evidence of benefit for SI for the primary outcome of mortality or for important secondary clinical outcomes.8
The recently completed Sustained Aeration of Infant Lungs (SAIL) randomized clinical trial (RCT) was the largest trial to date, to our knowledge, designed to compare SI with IPPV on the composite outcome of bronchopulmonary dysplasia or death at 36 weeks’ postmenstrual age among extremely preterm infants.9 The SAIL trial included more extremely preterm infants than previous trials and unexpectedly showed a higher rate of death in the first 2 days after birth in the experimental group. It was important to perform this systematic review to include the SAIL trial results and to investigate for evidence of differential treatment outcomes based on specified gestational age (GA) subgroups. The primary objective was to determine the effectiveness of SI vs standard resuscitation for the outcome of mortality prior to hospital discharge among preterm infants enrolled in RCTs.
Methods
This systematic review and meta-analysis followed the standard methods of the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.010 and the Cochrane Neonatal Review Group.11 Reporting followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.12 The review was registered on the International Prospective Register of Systematic Reviews (PROSPERO; identifier CRD42019133858).
We conducted a comprehensive search of MEDLINE (through PubMed), Embase, the Cumulative Index of Nursing and Allied Health Literature (CINAHL), and the Cochrane Central Register of Controlled Trials (CENTRAL) using the search terms (sustained inflation) OR (sustained AND inflation). We used database-specific filters for preterm infants and RCTs as provided by the Cochrane Neonatal Group. We searched for ongoing or unpublished trials using ClinicalTrials.gov and the World Health Organization International Trials Registry and Platform, and we identified abstracts from the Pediatric Academic Society annual meetings from the available archived years (2014-2019) by searching for the key terms sustained inflation and clinical trial. The search was last conducted on June 24, 2019.
We included RCTs enrolling preterm infants younger than 37 weeks’ gestation that compared SI (inflation time >5 seconds) vs standard resuscitation with either IPPV using inflation times of 5 seconds or less or CPAP. We excluded studies with cointerventions outside of SI between the control and intervention groups. Protocolized differences in respiratory devices between treatment groups were considered cointerventions based on the differential consistency in pressure delivery between devices13 as well as emerging clinical evidence of the superiority of a T-piece device over a self-inflating bag to prevent pulmonary morbidity.14,15 Observational studies, cluster RCTs, and quasi-RCTs were excluded.
The primary outcome was death during hospitalization. Secondary outcomes included cardiopulmonary resuscitation (chest compressions or epinephrine) in the delivery room (DR), intubation in the DR, death in the DR, death in the first 2 days of life, intubation and mechanical ventilation in the first 72 hours of life, surfactant administration in the first 72 hours of life, air leaks (pneumothorax or pulmonary interstitial emphysema), grade 3 or 4 intraventricular hemorrhage, bronchopulmonary dysplasia (as defined by primary trial), medical or surgical treatment for patent ductus arteriosus, stage 3 or higher retinopathy of prematurity, or requiring therapy in either eye.
Two of us (E.E.F. and A.B.t.P.) independently assessed titles and abstracts to determine eligibility of all studies identified in the search. Reviewers retrieved full-text versions of all potentially eligible articles and articles for which the abstract contained insufficient information to determine eligibility. Any differences were resolved through consensus.
For each included trial, the following details were collected: study authors, calendar years in which the trial was conducted, publication details, trial design, duration and completeness of follow-up, single site vs multisite and location(s) of study, informed consent approach (antenatal, retrospective, or combination), devices and interfaces used, definition of SI (number, peak pressures, or duration), definition of control therapy, details and demographic characteristics of trial participants, and details of outcomes reported. Data were abstracted from published trial protocols as available. We contacted the trial authors to request missing data when needed. In addition, all authors of eligible studies provided additional pooled mortality data (death before hospital discharge, death in the DR, and death in the first 2 days) stratified by the following groupings: 23 to 24 6/7 weeks’ GA, 25 to 26 6/7 weeks’ GA, 27 to 31 6/7 weeks’ GA, and 32 to 36 6/7 weeks’ GA.
Two of us (E.E.F. and A.B.t.P.) assessed the risk of bias at the study level using the Cochrane Collaboration tool.10 Disagreements between the reviewers were resolved through consensus after discussion. The GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) method16 was used to assess the strength of evidence across studies for the primary outcome and for the following prespecified clinically relevant secondary outcomes: cardiopulmonary resuscitation in the DR, intubation in the first 72 hours, pneumothorax, grade 3 or 4 intraventricular hemorrhage, and bronchopulmonary dysplasia. Consistent with the GRADE method, the assessment of inconsistency was based on the relative treatment effects rather than absolute differences (ie, risk difference [RD]). When applicable, the importance of each outcome was assigned consistently with the rating of the International Liaison Committee on Resuscitation.17
Statistical Analysis
The primary meta-analysis was performed using a fixed-effects model because the limited degree of observed heterogeneity across trials supported the assumption of a common underlying treatment effect. A direct aggregate data meta-analysis was performed. The incidence and 95% CIs of each outcome were calculated for each study for each treatment group. For studies with zero events, exact CIs were calculated. Because events are rare, the approach of Böhning et al18 was used to estimate RDs in both the aggregate and cumulative data meta-analyses. Mantel-Haenszel relative risk (RR),19 with Sweeting correction of the reciprocal of the opposite group size applied to groups with 0 events,20 was calculated for the primary outcome and specified secondary outcomes included in the GRADE assessment. Random-effects models with a Hartung-Knapp correction were used for confirmatory analyses for all outcomes. The Cochrane Q statistic and the Higgins I2 index21 were used to evaluate heterogeneity. All analyses were performed using Stata, version 15.1 software (StataCorp LLC).
We preplanned subgroup analyses based on prespecified GA subgroups for all mortality outcomes (death before hospital discharge, death in the DR, and death in the first 2 days of life). Because few studies enrolled infants aged 23 to 24 6/7 weeks, post hoc subgroup analyses using 2 GA groups (<27 weeks’ GA and ≥27 weeks’ GA) were also performed for the primary mortality outcome. We prespecified 2 additional subgroup analyses of all outcomes based on 2 elements of trial design. The first was study design, characterized as rescue vs prophylactic based on the type of support provided in the standard resuscitation control group. Studies were considered to use a rescue approach if the infants in the control group of those trials were treated with IPPV. Trials were designated prophylactic if the infants who were allocated to the control intervention and required respiratory support received CPAP with or without IPPV. A second additional subgroup analysis compared SI defined as 15 seconds or more with SI defined as less than 15 seconds.
Results
The search yielded 129 original references. Full-text reviews were performed for 41 studies, and 9 studies9,22,23,24,25,26,27,28,29 of 1406 infants were included in this review (eFigure 1 in the Supplement). Published study protocols for 3 included trials were also reviewed.30,31,32
One trial was excluded because SI was defined as 5 seconds or less.33 Four trials were excluded on the basis of a trial design that allowed for cointerventions in addition to SI. In the trial by te Pas and Walther,34 SI was part of a package of interventions that included DR CPAP, a T-piece device that generates positive end-expiratory pressure, and a novel nasopharyngeal interface. Infants in the control group were treated with IPPV without positive end-expiratory pressure or CPAP using a self-inflating bag and face mask. The trial by El-Chimi et al35 and the registered Sustained Lung Inflation of Preterms trial36 were excluded based on protocolized differences in respiratory devices between treatment groups, with a T-piece device in the intervention group and a self-inflating bag used for the control group. Last, 1 excluded trial compared continuous vs coordinated chest compressions.37
Characteristics of Study Design
There were important differences between trials with regard to the number and GA of included participants and the study design (Table).9,22,23,24,25,26,27,28,29 In most studies, antenatal consent was obtained for infant participation, increasing the risk of recruitment of a nonrepresentative study population and limited generalizability.38 The studies by Ngan et al28 and Hunt et al29 used a retrospective consent approach, in which the parents were approached for informed consent after the infants had received the randomized study intervention. In the multisite study by Kirpalani and colleagues,9 a combination of antenatal and retrospective consent was used based on ethical approvals at each site.
Table. Characteristics of Included Studies.
| Source | Setting | Gestational Age, wk |
Infants, No. |
Time of Consent | Rescue Approacha | SI Intervention | Control Intervention | Primary Outcome |
|---|---|---|---|---|---|---|---|---|
| Lindner et al,22 2005 | Single site | 25-28 6/7 | 61 | Antenatal | Yes | ≤3 SIs, 15 s each; 20, 25, and 30 cm H2O via ventilator and NP tube | IPPV, initial settings 20/4-6 cm H2O via ventilator and NP tube | Treatment failure requiring mechanical ventilation within 48 h |
| Lista et al,23 2015 | Multisite | 25-28 6/7 | 291 | Antenatal | No | ≤2 SIs, 15 s each; 25 cm H2O via TPR | CPAP 5 cm H2O with or without IPPV via TPR | Mechanical ventilation in first 72 h of life |
| Jiravisitkul et al,24 2017 | Single site | 25-32 | 81 | Antenatal | No | ≤2 SIs, 15 s each; 25 cm H2O via TPR | CPAP 6 cm H2O; IPPV, initial settings 15-20/5 cm H2O via TPR | FiO2, HR, and SpO2 during resuscitation; FiO2 at 10 min of life, DR intubation |
| Schwaberger et al,25 2015 | Single site | 28-33 6/7 | 40 | Antenatal | No | ≤3 SIs, 15 s each; 30 cm H2O via TPR | CPAP 5 cm H2O with or without IPPV via TPR | Change in CBV and cTOI |
| Mercadante et al,26 2016 | Single site | 34-36 6/7 | 185 | Antenatal | No | ≤2 SIs, 15 s each; 25 cm H2O via TPR | NRP, starting with initial steps, respiratory support via TPR | Need for respiratory support |
| Abd El-Fattah et al,27 2017 | Single site | <32 | 100 | Antenatal | No | 1 SI, 4 definitions used: 10-20 s, 15-20 cm H2O via TPR | CPAP 5 cm H2O, IPPV if needed via TPR | DR intubation |
| Ngan et al,28 2017 | Single site | 23-32 6/7 | 162 | Postnatal | Yes | ≤2 SIs: 24 cm H2O for 20 s, 24 cm H2O for 10-20 s via TPR | IPPV, initial settings 24/6 cm H2O via TPR | BPD at 36 wk PMA |
| Hunt et al,29 2019 | Single site | <34 | 60 | Postnatal | Yes | ≤2 SIs, 15 s each; 25 cm H2O via TPR | ≤2 Sequences of 5 inflations, 2-3 s each, initial settings 25/5 cm H2O via TPR | Minute volume in first minute of ventilation |
| Kirpalani et al,9 2019 | Multisite | 23-26 6/7 | 426 | Antenatal and postnatal, varied by site | Yes | ≤2 SIs, 15 s each; 20 cm H2O and 25 cm H2O via TPR | IPPV, initial settings 20/5-7 cm H2O via TPR | BPD or death at 36 wk PMA |
Abbreviations: BPD, bronchopulmonary dysplasia; CBV, cerebral blood volume; CPAP, continuous positive airway pressure; cTOI, cerebral tissue oxygenation index; DR, delivery room; FiO2, fraction of inspired oxygen; HR, heart rate; IPPV, intermittent positive pressure ventilation; NP, nasopharyngeal; NRP, neonatal resuscitation program; PMA, postmenstrual age; SI, sustained inflation; SpO2, oxygen saturation as measured by pulse oximetry; TPR, T-piece resuscitator.
Rescue approach: infants had to be deemed to require positive pressure ventilation to be enrolled.
Four trials7,9,28,29 used a rescue approach, in which the infants in the control group received IPPV. The remaining trials used a prophylactic approach. The pressures used during SI varied across studies from 10 to 30 cm H2O, and the duration of SI ranged from 10 to 20 seconds. In all trials, inflations of 15 seconds or greater were provided to at least some of the infants allocated to receive SI. In 1 RCT only, 1 SI was delivered,27 while the remaining trial designs allowed for up to 2 to 3 SIs. Treatment provided to infants in the control group varied across studies and included IPPV, “inflation breaths,” CPAP, or “routine resuscitation.”
Assessment of Potential Sources of Bias
The assessment of potential sources of bias is presented in eTable 1 in the Supplement. As noted, many studies obtained informed consent antenatally, increasing the risk of a nongeneralizable population. Three studies were considered to have an unclear risk of selection bias because the method of generating the random sequence was not specified. In the trials by Ngan et al28 and Hunt et al,29 randomization envelopes were opened prior to the determination of eligibility for the trial, increasing the risk of selection bias related to inadequate allocation concealment. All RCTs were considered to be at high risk of performance bias because the caregivers were not blinded, but this factor did not introduce a serious risk of bias for the assessment of the primary outcome of hospital-based mortality. Three RCTs reported a substantial number of postrandomization exclusions. In the trial by Kirpalani et al,9 these exclusions were distributed equally between treatment groups, while there were more infants in the SI group who were excluded after randomization in the trial by Ngan et al.28 The allocation of infants excluded after randomization was not reported by Jiravisitkul and colleagues.24 In that trial, the number of infants in the control group (n = 38) did not reach the target (n = 40), although the overall study recruitment goal was met. Early trial closure occurred in the trials of Lindner and colleagues22 (for poor recruitment and projected futility) and Kirpalani et al9 (for increased risk of the prespecified safety outcome of death in the first 48 hours after birth). We did not evaluate funnel plot asymmetry to assess for publication bias because fewer than 10 trials were included in this review.10
Primary Outcome: In-Hospital Mortality
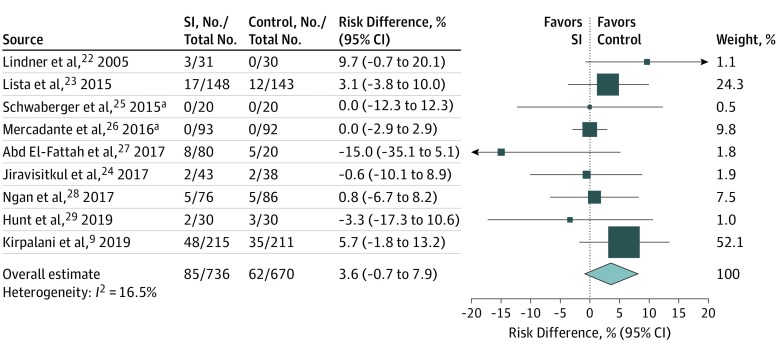
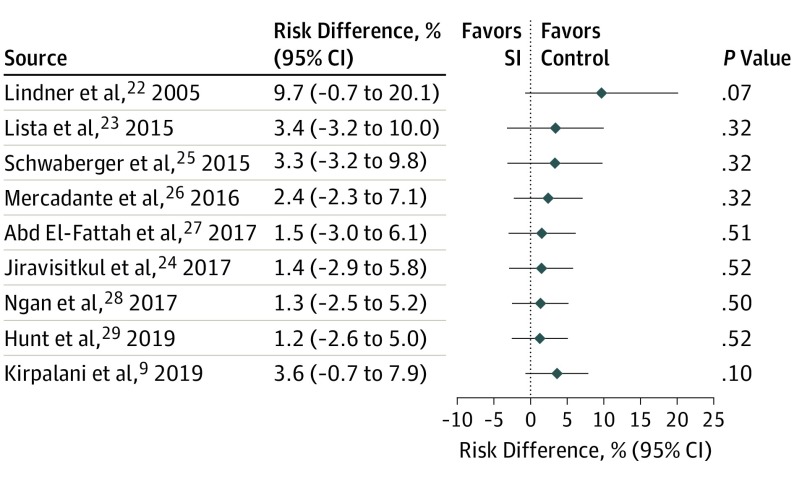
A total of 9 studies were included in the primary meta-analysis. Death before hospital discharge occurred in 85 of 736 infants (11.5%) treated with SI and 62 of 670 infants (9.3%) who received standard therapy for an RD of 3.6% (95% CI, −0.7% to 7.9%) and an RR of 1.16 (95% CI, 0.86-1.57) (Figure 1; eFigure 2 in the Supplement). Heterogeneity of 17% was found in the RD model and 0% in the RR model. Confirmatory analyses using random-effects models produced similar estimates, with I2 statistics of 0% for both RD and RR. Cumulative meta-analysis for the primary outcome (Figure 2) demonstrates a consistent point estimate favoring the control intervention.
Figure 1. Fixed-Effects Meta-analysis of Risk Difference of Primary Outcome, Death During Hospitalization.
Study weights are indicated by the box sizes. Overall estimate and 95% CI are indicated by the diamond. SI indicates sustained inflation.
aExact 95% CIs are shown.
Figure 2. Fixed-Effects Cumulative Meta-analysis of Risk Difference of Primary Outcome, Death During Hospitalization.
SI indicates sustained inflation.
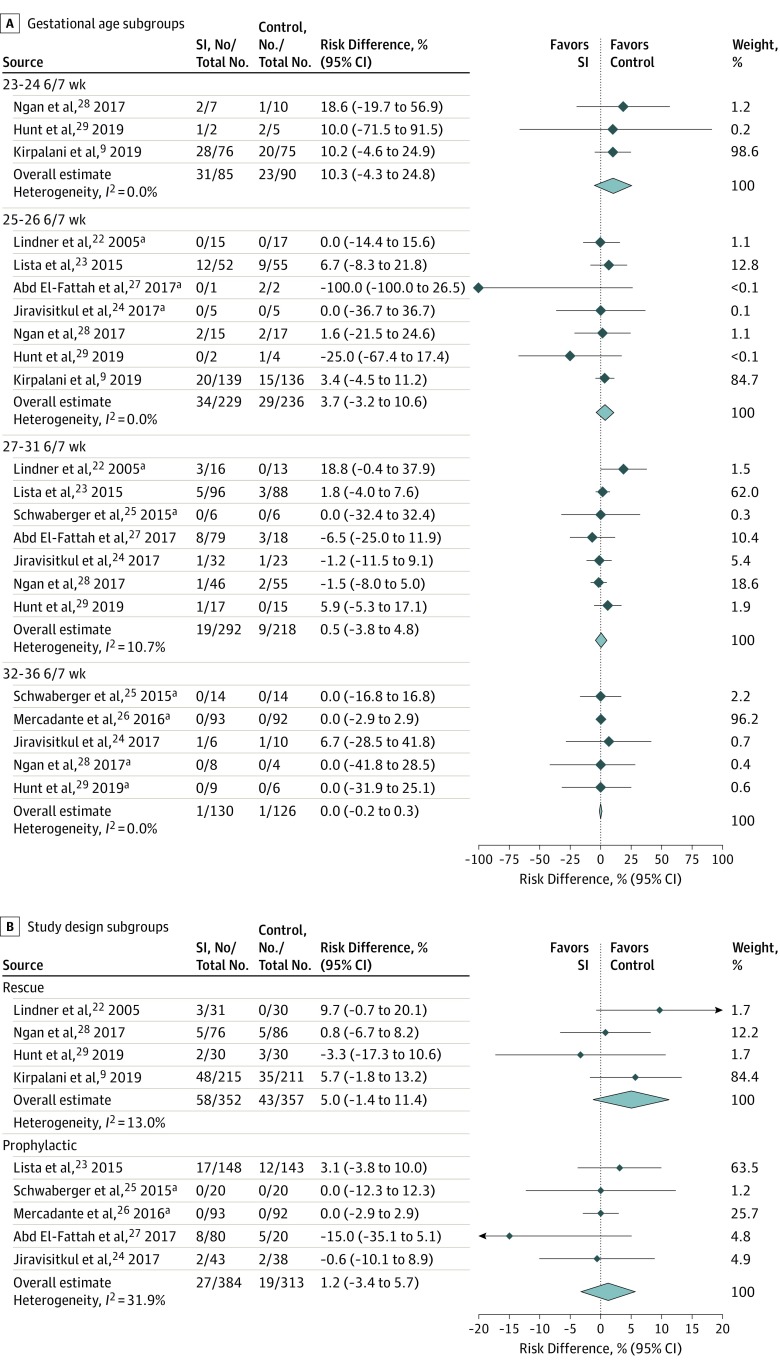
Figure 3A shows the deaths during hospitalization by GA subgroups. The combined RD estimates were highest among infants of 23 to 24 6/7 weeks’ GA (RD, 10.3%; 95% CI, −4.3% to 24.8%) and decreased to 0.0% (95% CI, −0.2% to 0.3%) among infants of 32 to 36 6/7 weeks’ GA. The Mantel-Haenszel Q statistic for heterogeneity showed important differences between GA subgroups (Q = 15.9, df = 3; P < .001). In post hoc subgroup analysis based on only 2 GA strata, there was no difference in the outcome of mortality before hospital discharge among either stratum (eFigure 3 in the Supplement). The results for the pooled analysis of the primary outcome based on the study design subgroups (rescue vs prophylactic) are shown in Figure 3B. Because SI lasting 15 seconds or more was provided to at least some participants in the SI group of all trials, subgroup analysis based on duration of SI (<15 seconds vs ≥15 seconds) was not performed.
Figure 3. Subgroup Analysis of Risk Difference for Death During Hospitalization.
A, Gestational age subgroups. B, Study design subgroups. Overall estimate and 95% CI are indicated by the diamond. SI indicates sustained inflation.
aExact 95% CIs are shown.
Secondary Outcomes
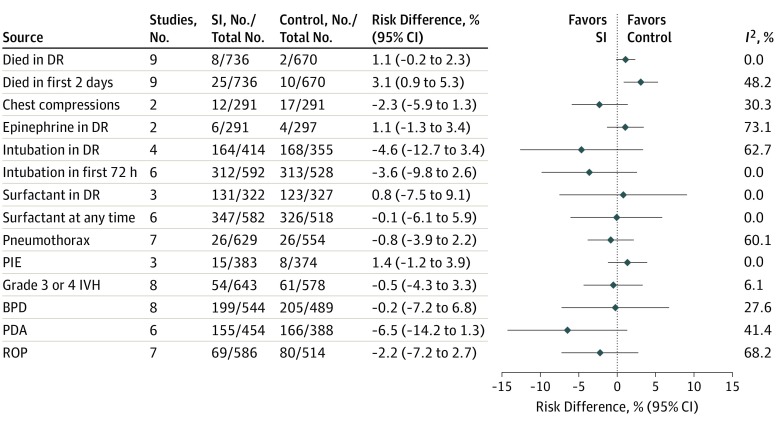
Figure 4 shows the results for the fixed-effect meta-analysis combined RD for all of the secondary outcomes and using all possible studies for each outcome, ranging from 2 to 9 studies. Death in the first 2 days of life showed an increased risk with SI (RD, 3.1%; 95% CI, 0.9%-5.3%) but with moderate heterogeneity (I2 = 48%). Stratification by the 4 GA subgroups (eFigure 4 in the Supplement) provided an explanation for this heterogeneity. The pooled RD was likely associated with the infants of 23 to 24 6/7 weeks’ GA (RD, 11.9%; 95% CI, 3.3%-20.5%). Cumulative meta-analysis demonstrates a substantial association between the SAIL trial data and this outcome (eFigure 5 in the Supplement). Subgroup analysis for the outcome of mortality in the DR based on GA is shown in eFigure 6 in the Supplement. Analysis of secondary outcomes based on the study design subgroups is shown in eFigure 7 in the Supplement.
Figure 4. Fixed-Effects Meta-analysis for Risk Difference of All Secondary Outcomes.
BPD indicates bronchopulmonary dysplasia; DR, delivery room; IVH, intraventricular hemorrhage; PDA, patent ductus arteriosus; PIE, pulmonary interstitial emphysema; ROP, retinopathy of prematurity; and SI, sustained inflation.
The GRADE Assessment of Evidence table for key prespecified outcomes is shown in eTable 2 in the Supplement, with fixed-effects and random-effects models for these outcomes in eTable 3 in the Supplement. The outcome of cardiopulmonary resuscitation in the DR is presented as individual components of chest compressions and epinephrine. The quality of data for specified outcomes was downgraded to low owing to risk of bias and imprecision.
Discussion
Lung aeration is essential for the successful transition to the extrauterine environment after birth, and almost all extremely preterm infants require respiratory support during this process. Only limited data inform the choice of inflation times and pressures used during positive pressure ventilation in the DR.39 In this pooled analysis of 1406 preterm infants enrolled in 9 RCTs of SI compared with standard resuscitation, there was no significant difference in the risk of the primary outcome of death before hospital discharge. However, SI was associated with an increased risk of mortality in the first 2 days of life, especially in the least mature GA subgroup. There were no observed differences between SI and control therapy in the risk of any other specified secondary outcomes.
Previous observational studies and RCTs of SI provided limited but promising evidence favoring SI over IPPV.2 The SAIL trial was the largest trial to date, contributing 30% of the infants included in this review.9 The SAIL trial enrolled only the most extremely preterm infants (23-26 6/7 weeks’ GA), a more immature population than in previous studies. The SAIL trial was closed early based on an interim, blinded, case-by-case clinical analysis that found an increased risk of death in the first 48 hours after birth among infants in the SI group. We therefore conducted this pooled analysis of SI trials (including SAIL) to examine for evidence of harm with SI, particularly among the most extremely preterm infants.
This study specifically includes preplanned subgroup analyses based on GA. We obtained aggregate data from all included trials to examine for differences in the mortality risk based on uniformly defined GA subgroups. Although there were no differences in the primary outcome for any subgroup, there was important heterogeneity between subgroups for this outcome, favoring control therapy in the least mature subgroup (23-24 6/7 weeks’ GA) of infants, who experience high mortality event rates.
The cumulative meta-analysis demonstrates point estimates that consistently favored control therapy for the primary outcome of mortality prior to hospital discharge. Explanations for this finding are speculative. Sustained inflation may have exacerbated cardiorespiratory failure after birth in this vulnerable population by delaying initiation of effective ventilation, leading to end organ injury. Alternatively, because rapid lung inflation with SI can lead to regional lung overdistention and injury,40 it is possible that SI as operationalized in the included RCTs contributed to volutrauma and acute lung injury among extremely preterm infants. However, there were no differences in air leaks or other secondary outcomes in pooled analysis to suggest a unified causal pathway for increased mortality.
Sustained inflation was associated with an increased risk of mortality in the first 2 days of life in pooled analysis, but this finding was not consistently evident in the cumulative meta-analysis prior to the addition of the SAIL trial data.9 This finding may reflect the fact that the SAIL trial enrolled the largest number of the least mature infants and had higher event rates of early mortality than most other trials. Alternatively, it is possible that the increased mortality in the first 2 days of life among infants treated with SI in the SAIL trial was a chance finding, particularly because this end point was 1 of 34 prespecified secondary and safety outcomes assessed in that study.
Ultimately, the association of SI and IPPV with lung aeration, gas exchange, and volutrauma likely depends on how effectively the interventions are applied. Most of the included trials were pragmatic and did not include respiratory recordings to assess the actual pressures and volumes delivered. Although some preclinical studies found SI to be a superior approach to lung aeration, respiratory interventions in those studies were delivered via endotracheal tubes to anesthetized animals.3,4 Study results may not apply to SIs delivered via face mask to preterm infants. Known technical impediments, such as mask leak and airway obstruction, reduce effective tidal volume delivery during face mask ventilation.41,42,43 It is possible that there was diminished gas volume delivered for infants treated with both noninvasive SI and IPPV.
In addition, laryngeal closure impedes effective noninvasive ventilation.44 In previous preterm studies, very little air volume entered the lung unless breathing occurred during SI.45,46 Therefore, we performed a subgroup analysis based on study design for the likelihood of glottis opening with spontaneous breathing among enrolled infants. In the 4 rescue trials, all infants in the control group received IPPV, suggesting absent or insufficient respiratory effort and a closed glottis among enrolled infants. In the 5 prophylactic trials, infants in the control group could have received CPAP, which suggests that many enrolled participants had sufficient respiratory effort and therefore an open glottis. In this subgroup analysis, mortality favored the control in both the rescue and prophylactic trials, although the 95% CI included 0 for both subgroups.
Limitations
We acknowledge the limitations of our study. Only 9 available trials met the eligibility criteria, contributing to the imprecision of the results. However, the pooled analysis suggests that additional data from further trials would not demonstrate evidence of efficacy for SI for the critical outcome of in-hospital mortality. Although the number of included trials precluded the ability to conduct formal tests to assess for publication bias, our comprehensive search strategy included both published and unpublished sources to reduce this risk of bias. In addition, there were important differences between studies in the maturity of enrolled infants, definition of SI, and interventions applied in the control group. Subgroup analyses to account for some of these differences show little evidence of additional harm nor added benefit associated with SI.
Conclusions
This pooled analysis of 1406 preterm infants presents some evidence that favors standard resuscitation over SI for the outcome of death during hospitalization. Sustained inflation is associated with an increased risk of death in the first 2 days after birth, and there is no evidence of efficacy for SI to prevent other secondary outcomes. These findings do not support the routine use of SI for preterm infants after birth.
eFigure 1. Flow Diagram
eFigure 2. Fixed Effects Meta-Analysis of Relative Risk for Death During Hospitalization
eFigure 3. Fixed Effects Meta-Analysis of Death During Hospitalization by Post Hoc Gestational Age Subgroups
eFigure 4. Fixed Effects Meta-Analysis for Death in the First 2 Days by Gestational Age Subgroups
eFigure 5. Fixed Effects Cumulative Meta-Analysis for Death in the First 2 Days
eFigure 6. Fixed Effects Meta-Analysis for Death in the Delivery Room by Gestational Age Subgroups
eFigure 7. Subgroup Analysis of All Secondary Outcomes Based on Study Design Subgroups
eTable 1. Assessment of Risk of Bias for Included Trials
eTable 2. GRADE Quality of Evidence Assessment for Primary and Prespecified Secondary Outcomes
eTable 3. Results From Fixed Effects and Random Effects Models for Risk Difference and Relative Risk for All Outcomes Reported in the GRADE Assessment of Quality of Evidence
References
- 1.Perlman JM, Wyllie J, Kattwinkel J, et al. ; Neonatal Resuscitation Chapter Collaborators . Part 7: neonatal resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2015;132(16)(suppl 1):-. doi: 10.1161/CIR.0000000000000276 [DOI] [PubMed] [Google Scholar]
- 2.Foglia EE, te Pas AB. Sustained lung inflation: physiology and practice. Clin Perinatol. 2016;43(4):633-646. doi: 10.1016/j.clp.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 3.te Pas AB, Siew M, Wallace MJ, et al. . Effect of sustained inflation length on establishing functional residual capacity at birth in ventilated premature rabbits. Pediatr Res. 2009;66(3):295-300. doi: 10.1203/PDR.0b013e3181b1bca4 [DOI] [PubMed] [Google Scholar]
- 4.Klingenberg C, Sobotka KS, Ong T, et al. . Effect of sustained inflation duration; resuscitation of near-term asphyxiated lambs. Arch Dis Child Fetal Neonatal Ed. 2013;98(3):F222-F227. doi: 10.1136/archdischild-2012-301787 [DOI] [PubMed] [Google Scholar]
- 5.Lista G, Fontana P, Castoldi F, Cavigioli F, Dani C. Does sustained lung inflation at birth improve outcome of preterm infants at risk for respiratory distress syndrome? Neonatology. 2011;99(1):45-50. doi: 10.1159/000298312 [DOI] [PubMed] [Google Scholar]
- 6.Grasso C, Sciacca P, Giacchi V, et al. . Effects of sustained lung inflation, a lung recruitment maneuver in primary acute respiratory distress syndrome, in respiratory and cerebral outcomes in preterm infants. Early Hum Dev. 2015;91(1):71-75. doi: 10.1016/j.earlhumdev.2014.12.002 [DOI] [PubMed] [Google Scholar]
- 7.Lindner W, Vossbeck S, Hummler H, Pohlandt F. Delivery room management of extremely low birth weight infants: spontaneous breathing or intubation? Pediatrics. 1999;103(5, pt 1):961-967. doi: 10.1542/peds.103.5.961 [DOI] [PubMed] [Google Scholar]
- 8.Bruschettini M, O’Donnell CP, Davis PG, et al. . Sustained versus standard inflations during neonatal resuscitation to prevent mortality and improve respiratory outcomes. Cochrane Database Syst Rev. 2017;7:CD004953. doi: 10.1002/14651858.CD004953.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirpalani H, Ratcliffe SJ, Keszler M, et al. ; SAIL Site Investigators . Effect of sustained inflations vs intermittent positive pressure ventilation on bronchopulmonary dysplasia or death among extremely preterm infants: the SAIL randomized clinical trial. JAMA. 2019;321(12):1165-1175. doi: 10.1001/jama.2019.1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. https://training.cochrane.org/handbook/archive/v5.1/. Updated March 2011. Accessed June 28, 2018.
- 11.Cochrane Neonatal Resources for review authors. https://neonatal.cochrane.org/resources-review-authors. Accessed June 28, 2018.
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items For Systematic Reviews and Meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawkes CP, Ryan CA, Dempsey EM. Comparison of the T-piece resuscitator with other neonatal manual ventilation devices: a qualitative review. Resuscitation. 2012;83(7):797-802. doi: 10.1016/j.resuscitation.2011.12.020 [DOI] [PubMed] [Google Scholar]
- 14.Szyld E, Aguilar A, Musante GA, et al. ; Delivery Room Ventilation Devices Trial Group . Comparison of devices for newborn ventilation in the delivery room. J Pediatr. 2014;165(2):234-239.e3. doi: 10.1016/j.jpeds.2014.02.035 [DOI] [PubMed] [Google Scholar]
- 15.Guinsburg R, de Almeida MFB, de Castro JS, et al. . T-piece versus self-inflating bag ventilation in preterm neonates at birth. Arch Dis Child Fetal Neonatal Ed. 2018;103(1):F49-F55. doi: 10.1136/archdischild-2016-312360 [DOI] [PubMed] [Google Scholar]
- 16.Schünermann H, Brozek J, Guayatt G, Oxman A, eds. GRADE handbook: for grading the quality of evidence and strength of recommendations. http://gdt.guidelinedevelopment.org/app/handbook/handbook.html. Updated October 2013. Accessed May 24, 2019.
- 17.Strand ML, Simon WM, Wyllie J, Wyckoff MH, Weiner G. Consensus outcome rating for international neonatal resuscitation guidelines [published online March 29, 2019]. Arch Dis Child Fetal Neonatal Ed. doi: 10.1136/archdischild-2019-316942 [DOI] [PubMed] [Google Scholar]
- 18.Böhning D, Mylona K, Kimber A. Meta-analysis of clinical trials with rare events. Biom J. 2015;57(4):633-648. doi: 10.1002/bimj.201400184 [DOI] [PubMed] [Google Scholar]
- 19.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719-748. [PubMed] [Google Scholar]
- 20.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23(9):1351-1375. doi: 10.1002/sim.1761 [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 22.Lindner W, Högel J, Pohlandt F. Sustained pressure–controlled inflation or intermittent mandatory ventilation in preterm infants in the delivery room? a randomized, controlled trial on initial respiratory support via nasopharyngeal tube. Acta Paediatr. 2005;94(3):303-309. doi: 10.1080/08035250410023647 [DOI] [PubMed] [Google Scholar]
- 23.Lista G, Boni L, Scopesi F, et al. ; SLI Trial Investigators . Sustained lung inflation at birth for preterm infants: a randomized clinical trial. Pediatrics. 2015;135(2):e457-e464. doi: 10.1542/peds.2014-1692 [DOI] [PubMed] [Google Scholar]
- 24.Jiravisitkul P, Rattanasiri S, Nuntnarumit P. Randomised controlled trial of sustained lung inflation for resuscitation of preterm infants in the delivery room. Resuscitation. 2017;111:68-73. doi: 10.1016/j.resuscitation.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 25.Schwaberger B, Pichler G, Avian A, Binder-Heschl C, Baik N, Urlesberger B. Do sustained lung inflations during neonatal resuscitation affect cerebral blood volume in preterm infants? a randomized controlled pilot study. PLoS One. 2015;10(9):e0138964. doi: 10.1371/journal.pone.0138964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mercadante D, Colnaghi M, Polimeni V, et al. . Sustained lung inflation in late preterm infants: a randomized controlled trial. J Perinatol. 2016;36(6):443-447. doi: 10.1038/jp.2015.222 [DOI] [PubMed] [Google Scholar]
- 27.Abd El-Fattah N, Nasef N, Al-Harrass MF, Khashaba M. Sustained lung inflation at birth for preterm infants at risk of respiratory distress syndrome: the proper pressure and duration. J Neonatal Perinatal Med. 2017;10(4):409-417. doi: 10.3233/NPM-171760 [DOI] [PubMed] [Google Scholar]
- 28.Ngan AY, Cheung P-Y, Hudson-Mason A, et al. . Using exhaled CO2 to guide initial respiratory support at birth: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2017;102(6):F525-F531. doi: 10.1136/archdischild-2016-312286 [DOI] [PubMed] [Google Scholar]
- 29.Hunt KA, Ling R, White M, et al. . Sustained inflations during delivery suite stabilisation in prematurely-born infants—a randomised trial. Early Hum Dev. 2019;130:17-21. doi: 10.1016/j.earlhumdev.2019.01.005 [DOI] [PubMed] [Google Scholar]
- 30.Foglia EE, Owen LS, Thio M, et al. . Sustained Aeration of Infant Lungs (SAIL) trial: study protocol for a randomized controlled trial. Trials. 2015;16:95. doi: 10.1186/s13063-015-0601-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dani C, Lista G, Pratesi S, et al. . Sustained lung inflation in the delivery room in preterm infants at high risk of respiratory distress syndrome (SLI Study): study protocol for a randomized controlled trial. Trials. 2013;14:67. doi: 10.1186/1745-6215-14-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunt KA, Ali K, Dassios T, Milner AD, Greenough A. Sustained inflations versus UK standard inflations during initial resuscitation of prematurely born infants in the delivery room: a study protocol for a randomised controlled trial. Trials. 2017;18(1):569. doi: 10.1186/s13063-017-2311-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harling AE, Beresford MW, Vince GS, Bates M, Yoxall CW. Does sustained lung inflation at resuscitation reduce lung injury in the preterm infant? Arch Dis Child Fetal Neonatal Ed. 2005;90(5):F406-F410. doi: 10.1136/adc.2004.059303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.te Pas AB, Walther FJ. A randomized, controlled trial of delivery-room respiratory management in very preterm infants. Pediatrics. 2007;120(2):322-329. doi: 10.1542/peds.2007-0114 [DOI] [PubMed] [Google Scholar]
- 35.El-Chimi MS, Awad HA, El-Gammasy TM, El-Farghali OG, Sallam MT, Shinkar DM. Sustained versus intermittent lung inflation for resuscitation of preterm infants: a randomized controlled trial. J Matern Fetal Neonatal Med. 2017;30(11):1273-1278. doi: 10.1080/14767058.2016.1210598 [DOI] [PubMed] [Google Scholar]
- 36.Sustained Lung Inflation of Preterms (SLIP). World Health Organization International Clinical Trials Registry Platform (ICTRP) Main ID: PACTR201707002434194. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201707002434194. Accessed June 24, 2019.
- 37.Schmölzer GM, O Reilly M, Fray C, van Os S, Cheung P-Y. Chest compression during sustained inflation versus 3:1 chest compression:ventilation ratio during neonatal cardiopulmonary resuscitation: a randomised feasibility trial. Arch Dis Child Fetal Neonatal Ed. 2018;103(5):F455-F460. doi: 10.1136/archdischild-2017-313037 [DOI] [PubMed] [Google Scholar]
- 38.Rich W, Finer NN, Gantz MG, et al. ; SUPPORT and Generic Database Subcommittees of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Enrollment of extremely low birth weight infants in a clinical research study may not be representative. Pediatrics. 2012;129(3):480-484. doi: 10.1542/peds.2011-2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foglia EE, te Pas AB. Effective ventilation: the most critical intervention for successful delivery room resuscitation. Semin Fetal Neonatal Med. 2018;23(5):340-346. doi: 10.1016/j.siny.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tingay DG, Pereira-Fantini PM, Oakley R, et al. . Gradual aeration at birth is more lung protective than a sustained inflation in preterm lambs. Am J Respir Crit Care Med. 2019;200(5):608-616. doi: 10.1164/rccm.201807-1397OC [DOI] [PubMed] [Google Scholar]
- 41.Schilleman K, Witlox RS, Lopriore E, Morley CJ, Walther FJ, te Pas AB. Leak and obstruction with mask ventilation during simulated neonatal resuscitation. Arch Dis Child Fetal Neonatal Ed. 2010;95(6):F398-F402. doi: 10.1136/adc.2009.182162 [DOI] [PubMed] [Google Scholar]
- 42.Schmölzer GM, Dawson JA, Kamlin COF, O’Donnell CP, Morley CJ, Davis PG. Airway obstruction and gas leak during mask ventilation of preterm infants in the delivery room. Arch Dis Child Fetal Neonatal Ed. 2011;96(4):F254-F257. doi: 10.1136/adc.2010.191171 [DOI] [PubMed] [Google Scholar]
- 43.Hartung JC, te Pas AB, Fischer H, Schmalisch G, Roehr CC. Leak during manual neonatal ventilation and its effect on the delivered pressures and volumes: an in vitro study. Neonatology. 2012;102(3):190-195. doi: 10.1159/000339325 [DOI] [PubMed] [Google Scholar]
- 44.Crawshaw JR, Kitchen MJ, Binder-Heschl C, et al. . Laryngeal closure impedes non-invasive ventilation at birth. Arch Dis Child Fetal Neonatal Ed. 2018;103(2):F112-F119. doi: 10.1136/archdischild-2017-312681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Vonderen JJ, Hooper SB, Hummler HD, Lopriore E, te Pas AB. Effects of a sustained inflation in preterm infants at birth. J Pediatr. 2014;165(5):903-908.e1. doi: 10.1016/j.jpeds.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 46.van Vonderen JJ, Lista G, Cavigioli F, Hooper SB, te Pas AB. Effectivity of ventilation by measuring expired CO2 and RIP during stabilisation of preterm infants at birth. Arch Dis Child Fetal Neonatal Ed. 2015;100(6):F514-F518. doi: 10.1136/archdischild-2014-307412 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flow Diagram
eFigure 2. Fixed Effects Meta-Analysis of Relative Risk for Death During Hospitalization
eFigure 3. Fixed Effects Meta-Analysis of Death During Hospitalization by Post Hoc Gestational Age Subgroups
eFigure 4. Fixed Effects Meta-Analysis for Death in the First 2 Days by Gestational Age Subgroups
eFigure 5. Fixed Effects Cumulative Meta-Analysis for Death in the First 2 Days
eFigure 6. Fixed Effects Meta-Analysis for Death in the Delivery Room by Gestational Age Subgroups
eFigure 7. Subgroup Analysis of All Secondary Outcomes Based on Study Design Subgroups
eTable 1. Assessment of Risk of Bias for Included Trials
eTable 2. GRADE Quality of Evidence Assessment for Primary and Prespecified Secondary Outcomes
eTable 3. Results From Fixed Effects and Random Effects Models for Risk Difference and Relative Risk for All Outcomes Reported in the GRADE Assessment of Quality of Evidence