Abstract
This article has a companion Counterpoint by Gibson and Lindsley.
Introduction
Clonal hematopoiesis of indeterminate potential (CHIP) is a common age-related condition affecting over 10% of healthy adults older than age 65 years. Its prevalence gradually increases with age. The entity is characterized by the presence of somatic mutations in the blood of otherwise healthy adults and has been associated with an increased risk of hematologic malignancies, cardiovascular events, and adverse survival outcomes.1 With the advent of reduced intensity conditioning regimens, the age of blood or bone marrow hematopoietic stem cell transplantation (HSCT) recipients continues to rise, with many centers extending eligibility up to age 80 years.2-4 The effect of patients’ age on transplant outcomes has been well studied.2,5,6 However, with the extension of recipient age has come the extension of donor age, especially with use of related donors. Several studies have demonstrated that donor age has an important impact on the outcome of allogeneic HSCT (alloHSCT). The utilization of older donors is consistently associated with worse overall survival and increased nonrelapse mortality.6-11 This negative impact on transplant outcomes may be due to several different mechanisms, but a potential etiology could be the increasing prevalence of CHIP in the donor pool when donors of advancing age are used. An obvious medical goal with HSCT is to give the recipient every possible advantage to achieve the best outcome from the procedure; this goal encompasses selection of the most appropriate donor. Therefore, screening for a potentially detrimental condition, such as CHIP in donors, could minimize the risk of adverse events in the bone marrow recipients.
Recognition and diagnosis of CHIP is important before assessment and selection of a donor, especially one older than 50 to 60 years because donor-derived malignancies, occult malignancies, and premalignant conditions such as monoclonal gammopathy of unknown significance (MGUS) and clonal hematopoiesis, increase with age.12 Unexplained cytopenias, recurrent/severe infections, or failure to mobilize stem cells in a donor may be due to an underlying marrow failure syndrome necessitating additional investigation. CHIP, by definition, is a condition present in asymptomatic individuals with unremarkable hemograms and the clonal population of peripheral blood leukocytes. When the term CHIP was coined, the authors proposed the 2% variant allele frequency (VAF) for the definition, based on the lower limit of reliable detection of small somatic variants using whole-exome sequencing.1,13 This was reasonable in this context but may not fully detail the biological and clinical relevance of the CHIP because hematologic malignancies may arise from clones smaller than 1% VAF and cardiovascular complications are seen with VAF >10%. The entity of CHIP will go underrecognized in potential donors without dedicated next-generation molecular screening. Results of this testing are critical, both for decision on use as a donor (to protect the patient) as well as to identify the donor’s potential medical predisposition and risk. In this short opinion piece, we provide compelling evidence to support screening for CHIP in older stem cell donors. For the purpose of this manuscript, we focus specifically on CHIP as defined by the presence of somatic mutations present at VAF >2% affecting genes associated with hematologic malignancies. This is biologically relevant for the following reasons: (1) the clinical consequences of clonal hematopoiesis (CH) at large are unclear; (2) most commercially available tests are limited only to <100 genes known to be mutated in hematologic malignancies; (3) testing for any aberrant CH would require whole-genome sequencing approach that presently would carry analytical challenges and relatively high cost14; and (4) mutations at very low VAF (<1%) can be present in most individuals and rarely lead to expansion of the affected clone.15
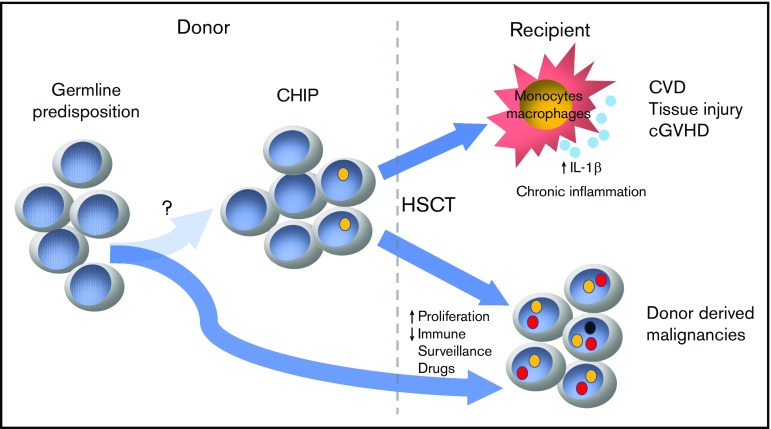
Donor-derived malignancies
Because patients with CHIP have an expanded clonal population of cells carrying somatic mutations in putative oncogenic drivers, the association between CHIP and hematologic malignancies is unsurprising.16-18 Despite the small absolute risk (0.5% to 1% per year), patients with CHIP are 4 to 15 times more likely to develop hematologic malignancies.19 Transformation to malignancy, in most cases, requires sequential acquisition of multiple mutations; thus, under homeostatic conditions, this process may take years or even decades and the majority of patients will likely succumb to other medical conditions. However, this process could be significantly enhanced by the tremendous proliferative stress required to reestablish hematopoiesis in the recipient.20 Additionally, posttransplant immunosuppression as well as constantly growing armamentarium of maintenance therapies may negatively influence immunological surveillance and promote oncogenic transformation of primed hematopoietic clones present in CHIP graft (Figure 1). This donor-derived CHIP could potentially progress to myeloid and, less often, lymphoid neoplasms through acquisition of a strong driver mutation posttransplant, as has been seen in other contexts.21 The published incidence of donor cell leukemia (DCL) varies greatly, affecting up to 5% of alloHSCT recipients.22,23 It may be relatively more common when older donors are used. In fact, the cumulative incidence of DCL extends beyond 6% in alloHSCT from donors older than age 60 years.24 Even though the etiology of DCL is not entirely clear, the growing body of literature suggest that preexisting somatic or predisposing germline mutations play a key role in this process.25-31
Figure 1.
Potential complications of donor CHIP. CVD, cardiovascular disease.
In addition to clinically apparent donor-derived malignancies, donor age was also associated with significantly higher rate of unexplained posttransplant cytopenias, particularly when donors with preexisting CHIP were used.32 In both instances, low-level CHIP clones not only engrafted but also outcompeted nonclonal counterparts during early hematopoietic reconstitution. Recently published studies demonstrated that the incidence of DCL was exclusively associated with preexisting donor CHIP.20 At the current stage of knowledge and in the absence of larger prospective studies, we are inclined to recommend screening older donors (>50 years) for the presence of CHIP to minimize the risk of DCL, an uncommon, yet frequently lethal, sequela of alloHSCT. Given the lack of definitive data on the impact of clonal size and/or type of mutations on the incidence of donor-derived leukemia, we recommend applying the commonly accepted threshold for CHIP of >2% VAF in genes known to be associated with hematologic malignancies.
Risk of inflammation and graft-versus-host disease
In addition to their malignant potential and altered differentiation, CHIP clones may result in chronic inflammation and end-organ damage.33-35 Even though it is postulated that chronic inflammation is mainly the result of transcriptional activation of proinflammatory cytokines (interleukin-1β, interleukin-6) by mutated monocytes, the exact pathophysiology and involvement of immune cells in this process is not entirely clear. In alloHSCT, both innate and adaptive immune systems in the recipient become entirely replaced by donor cells, including resident tissue macrophages that integrate signals derived from tissue infection or damage and present processed antigen from these sites to naïve T cells.36,37 It is then plausible that engrafted pro-inflammatory monocytes with altered epigenetic control will result in uncontrolled inflammation and organ damage. In fact, a recent study suggests that the prevalence of chronic graft-versus-host disease (cGVHD) is significantly increased in recipients engrafted with donor CHIP, particularly with DNMT3A mutations. This study looked at CHIP in 500 related HSCT donors (age ≥55 years) and revealed that at the time of graft donation 16% of donors had CHIP. In recipients allografted with donor CHIP, there was a higher cumulative incidence of cGVHD (hazard ratio, 1.73; 95% confidence interval, 1.21-2.49; P = .003).20 Even though donor CHIP had no effect on overall survival, the observed nearly twofold increase in cGVHD is nontrivial, especially given that this type of post-alloHSCT complication confers a significant morbidity that can markedly affect patients’ quality of life. Interestingly, the same study showed that donor CHIP was also associated with significantly lower cumulative incidence of relapse. Even though thought-provoking, such observation has to be approached with reservation, especially that study groups differed significantly with regard to hematologic malignancies leading to alloHSCT and disease risk index for neither group was available.
Genetic predisposition/genomic instability
Although the vast majority of cancers are sporadic, it is estimated that ∼3% of cancers (or >300 000 cancers) per year worldwide are due to cancer-predisposing gene mutations.38 This is likely an underestimate because the contribution of known genes has been poorly characterized and not all genes have been identified to date.38 Even though CHIP seems to be exclusively an age-related phenomenon arising from accumulation of random somatic mutations,12 there is an emerging body of literature suggesting the possibility of genetic predisposition to CHIP. For instance, the risk of TET2 somatic mutation in siblings >55 years of age is two- to threefold higher than in the general population.39 Additionally, genome-wide association studies revealed a possible constitutive CH predisposition within the TERT gene locus.14 It is then conceivable that shared genetic traits may result in CHIP and hematologic malignancies. That allogeneic bone marrow transplantation is frequently performed from related donors raises the possibility that heritable predisposition is present in related individuals.6 Interestingly, recent data that CHIP is more common in individuals with first-degree relatives affected by myeloid malignancies supports the hypothesis that there could be a common and potentially genetic origin of both hematologic malignancy and CHIP.39 Conversely, a population-based study focused on older mono- and dizygotic twins showed no difference in the prevalence of CHIP between the groups.40
Conscientious monitoring for patient and donor
In addition to its potential impact on the recipient, the identification of CHIP may have both emotional and medical consequences for the donor. As expected, the presence of cancer-associated mutations could be anxiety-provoking and may lead to unnecessary interventions. Thus, it is important to emphasize that the risk of hematologic malignancies is very small. As such, we recommend against repeat next-generation screening to monitor clonal dynamics and against bone marrow evaluation in otherwise asymptomatic individuals with normal hemograms. Complete blood count with differential should be monitored during routine health maintenance visits.
Conclusions
In the current era, great efforts are ongoing to expand access to alloHSCT to more patients through reduced intensity conditioning,41 increasing recipient age,5 and expansion of the donor pool.42,43 With this broader availability and constantly diminishing early transplant-related mortality, we must continue every effort to minimize potential long-term complications including, but not limited to, donor-derived malignancies, debilitating GVHD, or cardiovascular events. It has become a standard practice in some large-volume HSCT centers to perform serum protein electrophoresis in potential donors as screening for monoclonal gammopathy or myeloma (MGUS). Because MGUS has a very similar prevalence to CHIP in the general population and comparable rate of progression to clinically apparent hematologic malignancy,44 screening for CHIP in a similar fashion is reasonable. Moreover, unlike MGUS, the clinical consequences of CHIP may reach far beyond its oncogenic potential. Thus, we advocate for screening for CHIP in potential allogeneic donors (related and unrelated) older than age 50 years. The prevalence of CHIP is negligible in general population younger than 40 years of age and is present in <2% of individuals in 40 to 50 years. Thus, it is reasonable to screen all older related donors (>50 years of age) for CHIP and then exclude any CHIP donors, especially when alternative younger donors are available. This will allow for optimization of the graft choice to avoid potential late complications such as malignancies, ongoing cytopenias, increased rates of GVHD, or inflammation that could adversely affect patients’ overall morbidity, mortality, and health-related quality of life.
Nearly 9000 alloHSCTs are performed in the United States annually, 50% of which are unrelated donors followed by matched sibling and half-matched related donor transplants. Because the National Matched Donor Program prioritizes donors <45 years of age, the prevalence of CHIP in this donor pool is negligible. For haploidentical transplants, most patients <50 years receive grafts from a sibling donor, whereas older patients use offspring donors, prioritizing younger donors. Consequently, screening for CHIP would be primarily limited to older matched sibling donors where the age of the donor and recipient are usually within the same decade. In 2017, this estimate would affect approximately 2000 potential donors (Center for International Blood and Marrow Transplant Research, last updated June 26, 2019) screened for CHIP.
With a rapidly expanding donor pool including partially matched donors or umbilical cord blood, exclusion of a small fraction of CHIP-positive individuals would have a trivial effect on overall donor availability. Similarly, in the context of alloHSCT evaluations, the cost of CHIP evaluation in potential donors is fiscally comparable to standard metaphase karyotyping or fluorescence in situ hybridization.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (K08HL136894 [L.P.G.], R21HL143096 [L.P.G.], and 1R03HL136797 [A.E.D.]), and NIH, National Cancer Institute (CA006973) (A.E.D.).
Authorship
Contribution: A.E.D. and L.P.G. both performed a literature search and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lukasz P. Gondek, Division of Hematologic Malignancies, Department of Oncology, 1650 Orleans St, CRBI Room 290, Baltimore, MD 21287; e-mail: lgondek1@jhmi.edu.
References
- 1.Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126(1):9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28(11):1878-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorror ML, Sandmaier BM, Storer BE, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA. 2011;306(17):1874-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chevallier P, Szydlo RM, Blaise D, et al. Reduced-intensity conditioning before allogeneic hematopoietic stem cell transplantation in patients over 60 years: a report from the SFGM-TC. Biol Blood Marrow Transplant. 2012;18(2):289-294. [DOI] [PubMed] [Google Scholar]
- 5.Kasamon YL, Bolaños-Meade J, Prince GT, et al. Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol. 2015;33(28):3152-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCurdy SR, Zhang MJ, St Martin A, et al. Effect of donor characteristics on haploidentical transplantation with posttransplantation cyclophosphamide. Blood Adv. 2018;2(3):299-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kollman C, Howe CW, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98(7):2043-2051. [DOI] [PubMed] [Google Scholar]
- 8.Bastida JM, Cabrero M, Lopez-Godino O, et al. Influence of donor age in allogeneic stem cell transplant outcome in acute myeloid leukemia and myelodisplastic syndrome. Leuk Res. 2015;39(8):828-834. [DOI] [PubMed] [Google Scholar]
- 9.Mehta J, Gordon LI, Tallman MS, et al. Does younger donor age affect the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies beneficially [published correction appears in Bone Marrow Transplant. 2006;38(5):397]? Bone Marrow Transplant. 2006;38(2):95-100. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Chang YJ, Xu LP, et al. Who is the best donor for a related HLA haplotype-mismatched transplant? Blood. 2014;124(6):843-850. [DOI] [PubMed] [Google Scholar]
- 11.Kollman C, Spellman SR, Zhang MJ, et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood. 2016;127(2):260-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steensma DP. Clinical implications of clonal hematopoiesis. Mayo Clin Proc. 2018;93(8):1122-1130. [DOI] [PubMed] [Google Scholar]
- 14.Zink F, Stacey SN, Norddahl GL, et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood. 2017;130(6):742-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young AL, Challen GA, Birmann BM, Druley TE. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat Commun. 2016;7(1):12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shlush LI, Zandi S, Mitchell A, et al. ; HALT Pan-Leukemia Gene Panel Consortium . Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia [published correction appears in Nature. 2014;508(7496):420]. Nature. 2014;506(7488):328-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corces-Zimmerman MR, Majeti R. Pre-leukemic evolution of hematopoietic stem cells: the importance of early mutations in leukemogenesis. Leukemia. 2014;28(12):2276-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jan M, Snyder TM, Corces-Zimmerman MR, et al. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Sci Transl Med. 2012;4(149):149ra118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steensma DP. Clinical consequences of clonal hematopoiesis of indeterminate potential. Hematol Am Soc Hematol Educ Program. 2018;2018:264-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frick M, Chan W, Arends CM, et al. Role of donor clonal hematopoiesis in allogeneic hematopoietic stem-cell transplantation. J Clin Oncol. 2019;37(5):375-385. [DOI] [PubMed] [Google Scholar]
- 21.Tiacci E, Venanzi A, Ascani S, et al. High-risk clonal hematopoiesis as the origin of AITL and NPM1-mutated AML. N Engl J Med. 2018;379(10):981-984. [DOI] [PubMed] [Google Scholar]
- 22.Boyd CN, Ramberg RC, Thomas ED. The incidence of recurrence of leukemia in donor cells after allogeneic bone marrow transplantation. Leuk Res. 1982;6(6):833-837. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz-Argüelles GJ, Ruiz-Delgado GJ, Garces-Eisele J, Ruiz-Arguelles A, Perez-Romano B, Reyes-Nuñez V. Donor cell leukemia after non-myeloablative allogeneic stem cell transplantation: a single institution experience. Leuk Lymphoma. 2006;47(9):1952-1955. [DOI] [PubMed] [Google Scholar]
- 24.Gondek LP, Zheng G, Ghiaur G, et al. Donor cell leukemia arising from clonal hematopoiesis after bone marrow transplantation. Leukemia. 2016;30(9):1916-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao H, Shi J, Luo Y, et al. First report of multiple CEBPA mutations contributing to donor origin of leukemia relapse after allogeneic hematopoietic stem cell transplantation. Blood. 2011;117(19):5257-5260. [DOI] [PubMed] [Google Scholar]
- 26.Diamond HR, Ornellas MH, Orfao A, et al. Acute myeloid leukemia of donor origin after allogeneic stem cell transplantation from a sibling who harbors germline XPD and XRCC3 homozygous polymorphisms. J Hematol Oncol. 2011;4(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasuda T, Ueno T, Fukumura K, et al. Leukemic evolution of donor-derived cells harboring IDH2 and DNMT3A mutations after allogeneic stem cell transplantation. Leukemia. 2014;28(2):426-428. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch P, Mamez AC, Belhocine R, et al. Clonal history of a cord blood donor cell leukemia with prenatal somatic JAK2 V617F mutation. Leukemia. 2016;30(8):1756-1759. [DOI] [PubMed] [Google Scholar]
- 29.Suárez-González J, Martínez-Laperche C, Martínez N, et al. Whole-exome sequencing reveals acquisition of mutations leading to the onset of donor cell leukemia after hematopoietic transplantation: a model of leukemogenesis. Leukemia. 2018;32(8):1822-1826. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi S, Kobayashi A, Osawa Y, et al. Donor cell leukemia arising from preleukemic clones with a novel germline DDX41 mutation after allogenic hematopoietic stem cell transplantation. Leukemia. 2017;31(4):1020-1022. [DOI] [PubMed] [Google Scholar]
- 31.Herold S, Kuhn M, Bonin MV, et al. Donor cell leukemia: evidence for multiple preleukemic clones and parallel long term clonal evolution in donor and recipient. Leukemia. 2017;31(7):1637-1640. [DOI] [PubMed] [Google Scholar]
- 32.Gibson CJ, Kennedy JA, Nikiforow S, et al. Donor-engrafted CHIP is common among stem cell transplant recipients with unexplained cytopenias. Blood. 2017;130(1):91-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mas-Peiro S, Hoffmann J, Fichtlscherer S, et al. Clonal haematopoiesis in patients with degenerative aortic valve stenosis undergoing transcatheter aortic valve implantation [published online ahead of print 3 September 2019]. Eur Heart J. doi: 10.1093/eurheartj/ehz591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Libby P, Sidlow R, Lin AE, et al. Clonal hematopoiesis: crossroads of aging, cardiovascular disease, and cancer: JACC review topic of the week. J Am Coll Cardiol. 2019;74(4):567-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savola P, Lundgren S, Keränen MAI, et al. Clonal hematopoiesis in patients with rheumatoid arthritis. Blood Cancer J. 2018;8(8):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakata K, Gotoh H, Watanabe J, et al. Augmented proliferation of human alveolar macrophages after allogeneic bone marrow transplantation. Blood. 1999;93(2):667-673. [PubMed] [Google Scholar]
- 37.Eisenbarth SC. Dendritic cell subsets in T cell programming: location dictates function. Nat Rev Immunol. 2019;19(2):89-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahman N. Realizing the promise of cancer predisposition genes [published correction appears in Nature. 2014;510:176]. Nature. 2014;505(7483):302-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buscarlet M, Provost S, Zada YF, et al. DNMT3A and TET2 dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood. 2017;130(6):753-762. [DOI] [PubMed] [Google Scholar]
- 40.Hansen JW, Pedersen DA, Larsen LA, et al. Clonal hematopoiesis in elderly twins: concordance, discordance and mortality [published online ahead of print 24 October 2019]. Blood. doi: 10.1182/blood.2019001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott BL, Pasquini MC, Logan BR, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35(11):1154-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dezern AE, Luznik L, Fuchs EJ, Jones RJ, Brodsky RA. Post-transplantation cyclophosphamide for GVHD prophylaxis in severe aplastic anemia. Bone Marrow Transplant. 2011;46(7):1012-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bolaños-Meade J, Fuchs EJ, Luznik L, et al. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012;120(22):4285-4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghobrial IM, Detappe A, Anderson KC, Steensma DP. The bone-marrow niche in MDS and MGUS: implications for AML and MM. Nat Rev Clin Oncol. 2018;15(4):219-233. [DOI] [PubMed] [Google Scholar]