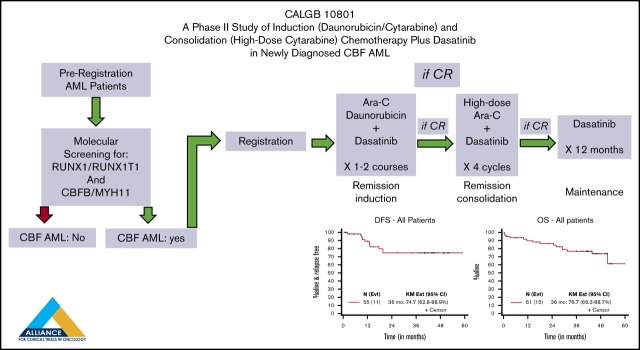
Key Points
The KIT inhibitor dasatinib can be safely added to conventional chemotherapy for CBF AML.
Chemotherapy plus dasatinib provides excellent outcomes for both younger and older patients with or without KITmut.
Abstract
Acute myeloid leukemia (AML) with either t(8;21)(q22;q22) or inv(16)(p13q22)/t(16;16)(p13;q22) is referred to as core binding factor (CBF) AML. Although categorized as favorable risk, long-term survival for these patients is only ∼50% to 60%. Mutated (mut) or overexpressed KIT, a gene encoding a receptor tyrosine kinase, has been found almost exclusively in CBF AML and may increase the risk of disease relapse. We tested the safety and clinical activity of dasatinib, a multi-kinase inhibitor, in combination with chemotherapy. Sixty-one adult patients with AML and CBF fusion transcripts (RUNX1/RUNX1T1 or CBFB/MYH11) were enrolled on Cancer and Leukemia Group B (CALGB) 10801. Patients received cytarabine/daunorubicin induction on days 1 to 7 and oral dasatinib 100 mg/d on days 8 to 21. Upon achieving complete remission, patients received consolidation with high-dose cytarabine followed by dasatinib 100 mg/d on days 6 to 26 for 4 courses, followed by dasatinib 100 mg/d for 12 months. Fifteen (25%) patients were older (aged ≥60 years); 67% were CBFB/MYH11–positive, and 19% harbored KITmut. There were no unexpected or dose-limiting toxicities. Fifty-five (90%) patients achieved complete remission. With a median follow-up of 45 months, only 16% have relapsed. The 3-year disease-free survival and overall survival rates were 75% and 77% (79% and 85% for younger patients [aged <60 years], and 60% and 51% for older patients). Patients with KITmut had comparable outcome to those with wild-type KIT (3-year rates: disease-free survival, 67% vs 75%; overall survival, 73% vs 76%), thereby raising the question of whether dasatinib may overcome the negative impact of these genetic lesions. CALGB 10801 was registered at www.clinicaltrials.gov as #NCT01238211.
Visual Abstract
Introduction
In acute myeloid leukemia (AML), t(8;21)(q22;q22) and inv(16)(p13q22)/t(16;16)(p13;q22) [hereafter referred to as t(8;21) and inv(16), respectively] are predictive of a more favorable outcome compared with most other cytogenetic/molecular subtypes, especially in patients receiving consolidation with high-dose cytarabine (HiDAC) after achieving complete remission (CR).1-3 However, despite being categorized as a favorable cytogenetic risk group, 40% to 50% of these patients still relapse, underscoring the need for more effective therapeutic approaches.4-7
At the molecular level, t(8;21) and inv(16) result in fusion genes (ie, RUNX1-RUNX1T1 and CBFB-MYH11, respectively) that lead to the disruption of the RUNX1 and CBFB genes encoding the subunits of the core binding factor (CBF) complex, a heterodimeric transcription factor involved in the regulation of myeloid hematopoiesis.8 However, transgenic, knock-in, and transduction RUNX1-RUNX1T1 and CBFB-MYH11 mouse models have shown that the fusion genes alone are not sufficient to cause a leukemic phenotype and that additional genetic lesions are necessary for the development of overt leukemia.9-12
Recent molecular analyses have identified gene signatures and recurrent gene mutations associated with CBF AML. Mutations in the KIT gene have been found in ∼25% of CBF AML patients and are among the most frequent recurrent mutations.13-17 KIT is located at chromosome band 4q12 and encodes a predicted 110-kD transmembrane glycoprotein that is a member of the type III receptor tyrosine kinase family. After ligand binding, the receptor dimerizes, becomes phosphorylated, and activates downstream signaling pathways involved in cell proliferation, differentiation, and survival. Ligand-independent activation of KIT can be caused by gain-of-function mutations.8,18 In several studies, but not all, KIT mutations have been associated with an inferior outcome in patients with CBF AML, especially in t(8;21) where KIT mutations confer a higher risk of relapse.14-19 In addition, regardless of mutation status, KIT is also highly expressed in CBF AML blasts,20 and in vitro and in vivo models support cooperation of activated KIT and CBF fusion proteins to induce and sustain AML growth.21,22 Therefore, despite some uncertainties regarding the prognostic role of KIT mutations and KIT overexpression, there is a mechanistic rationale for incorporating KIT inhibitors into the treatment of CBF AML.
We report here the results of a prospective clinical trial that tested the feasibility of adding dasatinib, an oral multi-kinase inhibitor with activity on KIT and SRC activated proteins,23 to combination therapy with a previously optimized Cancer and Leukemia Group B (CALGB) cytarabine-daunorubicin/HiDAC-based chemotherapy regimen in patients with CBF AML. The study also assessed the safety and the clinical activity of this approach. CALGB is now part of the Alliance for Clinical Trials in Oncology (Alliance).
Methods
Patients
Patients with previously untreated AML according to the World Health Organization classification24 were eligible for molecular screening for the CBF fusion transcripts RUNX1/RUNX1T1 or CBFB/MYH11 in a CALGB/Alliance Molecular Pathology central laboratory. All screened patients signed consent forms in compliance with the Declaration of Helsinki for the Institutional Board Review–approved protocols for molecular screening on CALGB 20202 and for treatment on CALGB 10801 (#NCT01238211). Patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| Characteristic | Patients (N = 61) |
|---|---|
| Male sex, n (%) | 31 (51) |
| Age, median (range), y | 51 (20-85) |
| Race, n (%) | |
| White | 46 (75) |
| African American | 5 (8) |
| Other | 4 (7) |
| Unknown/not reported | 6 (10) |
| Days from diagnosis to study entry, median (range) | 4 (0-11) |
| CBF results (from RT-PCR screening), n (%) | |
| RUNX1-RUNX1T1 | 20 (33) |
| CBFB-MYH11 | 41 (67) |
Molecular screening for CBF fusion transcripts
Patients were centrally screened for the RUNX1/RUNX1T1 or CBFB/MYH11 fusion transcripts by reverse transcription polymerase chain reaction (RT-PCR). Bone marrow aspirate samples (or blood with at least 20% circulating blasts) were shipped to the Alliance Leukemia Tissue Bank. Samples were subjected to Ficoll density gradient to collect mononuclear cells, which were resuspended in RPMI 1640 with 20% albumin and submitted to the Alliance Molecular Pathology central laboratory. RNA extraction and RT-PCR assays for RUNX1/RUNX1T1 or CBFB/MYH11 were performed as previously reported.25 The presence of a CBF fusion transcript was confirmed in all RT-PCR–positive samples by using Sanger sequencing–based assays.
Molecular characterization of KIT status
KIT mutations and expression analyses were performed as previously reported.14,18 The median value of KIT expression levels measured by using quantitative RT-PCR was employed as a cutoff to define higher vs lower expressers.20
Treatment
Treatment is summarized in the Visual Abstract and Table 2. Patients received induction chemotherapy with cytarabine 200 mg/m2 per day continuous IV infusion on days 1 to 7, daunorubicin 60 mg/m2 per day IV bolus on days 1 to 3, and dasatinib (Sprycel, Bristol-Myers Squibb) 100 mg/d orally on days 8 to 21. Patients with residual disease (>5% blasts) in a day 21 marrow aspirate were re-induced with the same doses of cytarabine on days 1 to 5, daunorubicin on days 1 to 3, and dasatinib on days 6 to 19 of re-induction treatment. Patients who achieved CR after 1 or 2 cycles of induction therapy received consolidation with HiDAC 3000 mg/m2 (if aged <60 years) or 1000 mg/m2 (if older), given IV over 3 hours every 12 hours on days 1, 3, and 5, and dasatinib 100 mg/d on days 6 to 26 for 4 courses. Patients in CR after consolidation received dasatinib 100 mg daily for 12 months.
Table 2.
Treatment
| Drug | Dose | Route | Schedule |
|---|---|---|---|
| Induction | |||
| Cytarabine | 200 mg/m2/d | CIV | Days 1-7 |
| Daunorubicin | 60 mg/m2/d | IV | Days 1-3 |
| Dasatinib | 100 mg/d | Oral | Days 8-21 |
| Consolidation | |||
| Cycle #1, 2, 3, and 4 | |||
| HiDAC* | 3000 mg/m2 every 12 h | IV | Days 1,3,5 |
| Dasatinib | 100 mg/d | Oral | Days 6-28 |
| Maintenance | |||
| Dasatinib | 100 mg/d | Oral | 12 (28-d) cycles |
CIV, continuous IV infusion.
1000 mg/m2 every 12 hours for patients aged ≥60 years.
Definition of clinical end points and statistical analysis
The primary end point was safety as assessed by 30-day survival after starting induction therapy. This two-stage phase 2 design provided 90% power to identify a true 30-day survival rate of ≥85% vs the null hypothesis that it is at most 70%. At least 59 evaluable patients were required for this two-stage trial, with an interim analysis after the first 20 evaluable patients. Overall, the regimen would be declared not feasible due to toxicity if ≥14 patients (≥24%) among the first 59 evaluable patients experienced early or hypoplastic death, and stopped early if ≥6 hypoplastic deaths were observed in the first 20 patients. Efficacy end points included CR rate with at least 90% power to detect a true rate of ≥90% (vs the null hypothesis that this rate was at most 75%). If ≥50 (≥85%) patients attained a CR, the treatment would be considered promising. These conservative assumptions were based on the limited knowledge available at the time of the study design of the possibly toxic effects of the combination of a KIT inhibitor on the posttreatment recovery of normal hematopoiesis and thus a potentially high risk of early hypoplastic death after induction. Furthermore, the enrolled cohort included older patients with no upper age limit, for whom it was anticipated that treatment-related toxicity could be relatively high given the intensity of the proposed cytotoxic regimen.
Efficacy end points included overall survival (OS) and disease-free survival (DFS). Response and relapse were defined by using previously published criteria.26 The distributions of OS (time from study entry to death; patients who were alive were censored at the time of their last follow-up evaluation) and DFS (time from documentation of CR to relapse or death; patients who were alive and relapse-free were censored at the time of their last follow-up evaluation or at initiation of subsequent therapy, including allogeneic transplantation) were estimated by using the Kaplan-Meier method. Log-rank statistics were used to compare survival distributions between subsets. Simple descriptive statistics were used to summarize baseline characteristics. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. Toxicity was graded by using the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.0. The database was locked on 25 October 2016.
Results
Patient demographic characteristics and molecular features
Between April 2011 and January 2013, we screened 779 patients newly diagnosed with AML from CALGB/Alliance member institutions for CBF AML by using RT-PCR. The average turnaround time to report the molecular diagnosis of CBF AML was 48 hours. Treatment began within a median of 4 days from collection of the diagnostic sample and shipment to the CALGB/Alliance central molecular laboratory (interquartile range, 3-5 days). Patients who had an FLT3 mutation were offered another study.
Sixty-one patients were found positive for RUNX1-RUNX1T1 or CBFB-MYH11 and were eligible for the CALGB 10801 trial. All patients (Table 1) were evaluable; of these, 20 and 40 were subsequently confirmed to harbor t(8;21) or inv(16), respectively, according to central review of the results of cytogenetic analyses performed by the CALGB/Alliance–approved institutional laboratories; 1 patient was classified as CBF [inv(16)] based on local diagnosis. Of the 60 patients whose karyotype was centrally reviewed, 32 (53%) had additional cytogenetic abnormalities, which are listed in Table 3. The median age of the treated patients was 51 years (range, 20-85 years); 15 (25%) patients were older (aged ≥60 years), 31 (51%) were male, 46 (75%) were white, 41 (67%) were CBFB/MYH11–positive, and 20 (33%) were RUNX1/RUNX1T1–positive. Eleven (19%) of the 57 patients who were molecularly evaluable harbored mutated (mut) KIT.
Table 3.
Additional cytogenetic abnormalities in patients with previously untreated CBF AML
| UPN | Sex | Secondary chromosome abnormality(s) |
|---|---|---|
| Patients with t(8;21) | ||
| 1 | Female | ‒X |
| 5 | Female | ‒X |
| 48 | Female | ‒X |
| 58 | Female | ‒X |
| 6 | Male | ‒Y |
| 7 | Male | ‒Y |
| 10 | Male | ‒Y |
| 23 | Male | ‒Y |
| 4 | Male | del(9)(q13q22) |
| 12 | Female | del(9)(q13q22) |
| 47 | Male | del(9)(q31q33) |
| 13 | Female | ‒X,del(9)(q13q22) |
| 16 | Male | ‒Y,del(9)(q12q22) |
| 45 | Male | ‒Y,del(9)(q13q22) |
| 34 | Male | +4 |
| 43 | Female | add(5)(q33) |
| 27 | Male | +8/del(12)(p12) |
| 11 | Female | Tetraploid clone with t(8;21)x2 |
| Patients with inv(16) or t(16;16) | ||
| 20 | Male | +8 |
| 46 | Female | +8 |
| 8 | Female | +8/add(17)(q23) |
| 14 | Male | +6,+8,+19,+20,+21,+22 |
| 37 | Female | t(11;20)(p15;q11.2)/+8,t(11;20)(p15;q11.2) |
| 52 | Male | +21/+6,+8,+13,+21/+13,t(13;16)(q14;q24),+20,+21/+13,+21 |
| 56 | Female | del(7)(q32q36)/+8,+14,+14,+21/+22 |
| 42 | Male | +6 |
| 28 | Female | +9 |
| 22 | Male | +22 |
| 33 | Female | del(16)(q22q22)/del(16)(q22q22),+22 |
| 36 | Female | +9,+14,+22 |
| 30 | Male | del(5)(q15q34) |
| 29 | Female | der(1)inv(1)(p13.3q11)del(1)(q41q43),t(4;6)(q34;p12),add(7)(q34), ‒10,+r,+mar |
Toxicity
Of the 61 patients, 4 died while receiving this treatment: 3 during induction (2 were older and had CBFB/MYH11 and 1 was younger with RUNX1/RUNX1T1), and 1 in CR during consolidation (a younger CBFB/MYH11 patient). Of these 4 deaths, 2 were within 30 days of start of treatment (1 younger, 1 older). Overall, 10 patients had an adverse event resulting in treatment discontinuation (1, 7, and 2 during induction, consolidation, and maintenance, respectively). Ten patients declined to complete the planned treatment (7 during consolidation, and 3 during maintenance), but data for the reasons of these voluntary discontinuations were not consistently reported. “Treatment fatigue” has been reported in other leukemia trials involving prolonged courses of therapy.27 Among patients who achieved CR and underwent consolidation treatment, the median number of consolidation cycles received was 3 (range, 1-4).
The observed hematologic and nonhematologic adverse events were as expected and similar to those previously reported with this chemotherapy regimen and/or dasatinib. Pleural effusions (four with grade 3 and two with grade 4) and abnormal liver function test results (seven with grade 3 and three with grade 4) possibly related to dasatinib were observed, although other causes such as chemotherapy, infection, or antifungal drugs used during the patient’s treatment may have contributed. Sepsis with respiratory and/or multiorgan failure was the cause of death in the 4 patients who died while on protocol treatment.
Fifty-four patients (89%) completed most or all of the planned consolidation therapy. Thirty-two of all enrolled patients (53%) started the maintenance therapy, and 19 (53%) of these completed all 12 monthly cycles. Altogether, 55 (90%), 42 (76%), and 19 (31%) patients completed induction, consolidation, and maintenance, respectively. Allogeneic transplantation was performed in 1 patient during first CR and in 5 others after relapse.
Outcomes
The 30-day survival rate was 97% (59 of 61; 95% confidence interval [CI], 89-100), with 98% and 93% 30-day survival rates in the younger and older patients, respectively. Fifty-five patients (90%; 95% CI, 80-96) achieved CR: 93% of younger patients and 80% of older patients (Table 4). Six patients failed to achieve CR because of early death (n = 3) or refractory disease (n = 3). Of these 6 patients, 2 had RUNX1/RUNX1T1 and 4 had CBFB/MYH1. Four patients relapsed during consolidation, five during continuation and one after protocol treatment was discontinued.
Table 4.
Clinical results
| End point | All patients (N = 61) | Younger, <60 y (n = 46) | Older, ≥60 y (n = 15) | RUNX1/RUNX1T1 (n = 20) | CBFB/MYH11 (n = 40) | RUNX1/RUNX1T1, Younger (n = 15) | CBFB/MYH11, Younger (n = 30) |
|---|---|---|---|---|---|---|---|
| 30 d Survival (95% CI) | 97 (89-100) | 98 (88-100) | 93 (68-100) | 95 (75-100) | 97.5 (87-100) | 93 (68-100) | 100 (88-100) |
| CR | 90 | 93 | 80 | 90 | 90 | 93 | 93 |
| 36-mo DFS (95% CI) | 75 (63-89) | 79 (66-94) | 60 (36-100) | 71 (50-100) | 76 (62-93) | 80 (58-100) | 78 (63-97) |
| 36-mo OS (95% CI) | 77 (66-89) | 85 (75-97) | 51 (30-85) | 68 (50-93) | 81 (68-95) | 78 (59-100) | 89 (77-100) |
Values are percentages.
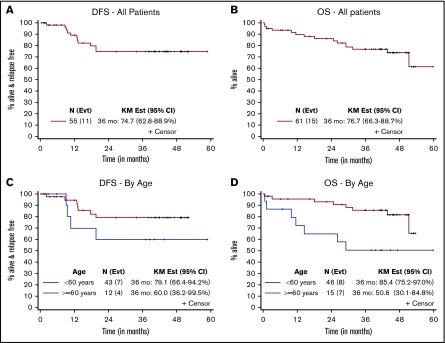
With a median follow-up of 45 months for living patients (range, 1.4-60 months), 10 (16%) patients have relapsed (4 with RUNX1/RUNX1T1 and 6 with CBFB/MYH11; 6 younger and 4 older). The 3-year DFS and OS rates were 75% (95% CI, 63-89) and 77% (95% CI, 66-89), respectively, for all patients; 79% (95% CI, 66-94) and 85% (95% CI, 75-97) for younger patients; and 60% (95% CI, 36-100) and 51% (95% CI, 30-85) for older patients (Table 4; Figure 1).
Figure 1.
Disease-free and overall survival. DFS (A) and OS (B) for all patients. DFS (C) and OS (D) for patients according to age. Outcomes at 36 months were calculated by using the Kaplan-Meier (KM) method.
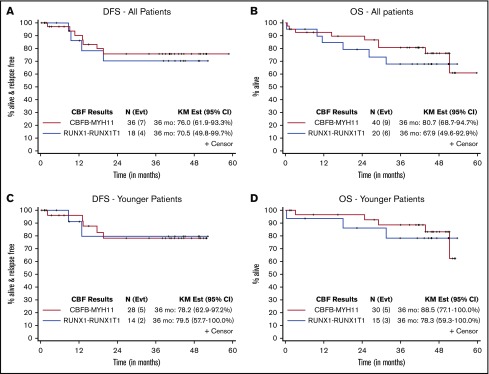
The 3-year DFS and OS rates are shown in Table 4 and Figure 2. For patients with RUNX1/RUNX1T1, the 3-year DFS was 71% (95% CI, 50-100) (80% for younger patients), and the 3-year OS was 68% (95% CI, 50-93) (78% for younger patients). For those with CBFB/MYH11, the 3-year DFS was 76% (95% CI, 62-93) (78% for younger patients), and the 3-year OS was 81% (95% CI, 68-95) (89% for younger patients). Of note, the seemingly longer DFS than OS in this relatively small study was because 2 patients went on to non-protocol therapies and were therefore censored for DFS but continued to be evaluable for OS.
Figure 2.
Disease-free and overall survival according to CBF fusion transcripts. DFS (A) and OS (B) for all patients. DFS (C) and OS (D) for younger patients. Outcomes at 36 months were calculated by using the Kaplan-Meier (KM) method.
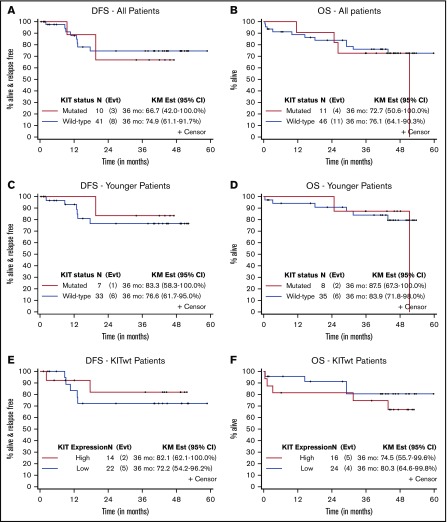
In a post hoc analysis, we assessed the impact of KITmut or high expression of wild-type (wt) KIT (KITwt) on treatment response. The 3-year DFS and OS rates for patients with KITmut compared with KITwt were 67% vs 75% (83% vs 77% for younger patients) and 73% vs 76% (88% vs 84% for younger patients), respectively (Table 5; Figure 3). The 3-year DFS and OS rates for patients with higher vs lower levels of KITwt expression were 82% vs 72% and 75% vs 80%, respectively.
Table 5.
Clinical results according to KIT status
| End point | KITmut (n = 11) | KITwt (n = 46) | KITmut, younger (n = 8) | KITwt, younger (n = 35) | KITwt/high (>4.67) (n = 16) | KITwt/low (≤4.67) (n = 24) |
|---|---|---|---|---|---|---|
| CR | 91 | 89 | 88 | 94 | 88 | 91 |
| 36-mo DFS (95% CI) | 67 (42-100) | 75 (61-92) | 83 (58-100) | 77 (63-95) | 82 (62-100) | 72 (54-96) |
| 36-mo OS (95% CI) | 73 (51-100) | 76 (64-90) | 88 (67-100) | 84 (72-98) | 75 (56-100) | 80 (65-100) |
Values are percentages.
Figure 3.
Disease-free and overall survival according to KIT status. DFS (A) and OS (B) for all patients (according to KIT mutation status). DFS (C) and OS (D) for younger patients (according to KIT mutation status). DFS (E) and OS (F) for KITwt patients (according to KIT expression level). Outcomes at 36 months were calculated by using the Kaplan-Meier (KM) method.
Discussion
Patients with CBF AML have been classified in a favorable cytogenetic/genetic risk group, but a relatively large subset of these patients is not cured despite optimal dosing of induction and consolidation chemotherapy. Emerging data show that a high frequency of mutations and/or high expression of KIT in CBF AML likely result in aberrant tyrosine kinase activity, leukemia cell growth and survival, and treatment resistance. Thus, we hypothesized that pharmacologic inhibition of KIT would lead to significant antileukemia activity if combined with an optimized chemotherapy regimen in patients with CBF AML. Dasatinib is a multi-kinase inhibitor that has been shown to inactivate KIT at nanomolar concentrations. It has been previously incorporated into relatively intensive chemotherapy regimens in Philadelphia chromosome–positive acute lymphoblastic leukemia.28 Furthermore, recent mechanistic findings support the potential clinical benefit of KIT inhibition in CBF AML.29 Thus, we elected to conduct a phase 2, open-label, multicenter trial that evaluated the safety and the clinical activity of dasatinib given in combination with cytarabine/daunorubicin induction therapy (“7+3”) and HiDAC consolidation therapy, and as a single agent for 1 year of maintenance treatment in patients newly diagnosed with CBF AML.
The rationale for choosing standard-dose cytarabine (200 mg/m2 by continuous infusion over 7 days) and daunorubicin (60 mg/m2 IV for 3 days) as induction chemotherapy was based on the high CR rates (∼90%) observed in previous CALGB/Alliance trials (ie, CALGB 9621 and CALGB 19808) that used a similar chemotherapy regimen without a kinase inhibitor.30,31 Furthermore, although daunorubicin 90 mg/m2 has been shown to be superior to 45 mg/m2 dosing at least in younger patients,32 this higher dose had no advantage over 60 mg/m2, which had been previously administered safely and effectively to patients with CBF AML.33,34 For postremission therapy, repetitive courses of HiDAC have been shown to be an optimal treatment of patients with CBF AML.35-37 The dose of dasatinib (ie, 100 mg daily) was based on previous experience combining dasatinib with chemotherapy.28 The administration of chemotherapy and dasatinib sequentially rather than concurrently leveraged data supporting potentially synergistic activity of administration of tyrosine kinase inhibitors following chemotherapy38; however, whether this is indeed the optimal drug sequence remains to be fully investigated. A maintenance phase of dasatinib as a single agent for an additional 12 months after completion of consolidation chemotherapy was included. Although standard therapy for adults with AML does not routinely involve maintenance treatment, a small but consistent proportion of patients with CBF AML have molecular evidence for persistent minimal residual disease (MRD) following treatment.39 These patients have an increased risk for later relapse, thereby justifying the prolonged use of dasatinib maintenance.
The main objectives of this feasibility study were achieved. We showed that molecular diagnostic evaluation for CBF AML could be performed in a central molecular pathology laboratory of an National Cancer Institute–sponsored adult cooperative oncology group with a short turnaround time between sample submission and result reporting (median time of 48 hours). The large pool of patients accessible to a cooperative oncology group allowed complete accrual for this relatively uncommon subset of CBF AML to be achieved in only 22 months, despite the low frequency of this disease (∼12% of all AML cases). We showed that the chemotherapy/dasatinib regimen was tolerable in all patients with CBF AML, including older individuals. The CR rate was seemingly equivalent to that reported in previous CALGB/Alliance studies that used cytarabine/anthracycline/HiDAC–based regimens administered without a KIT inhibitor.30,31 DFS and OS were also seemingly comparable and perhaps superior to those from previous CALGB/Alliance studies. DFS and OS 3-year rates for CBF patients on CALGB 10801 were 79% and 93% in younger patients compared with 41% and 55% for similarly aged CBF patients treated on CALGB 9621, and 56% and 67% for those treated on CALGB 19808. However, although these data provide historical context for the results of CALGB 10801, it is important to underscore that this study was not designed to produce a direct comparison of the current vs historical cohorts of CALGB/Alliance CBF AML patients. Furthermore, although only a minority of CBF patients harbor FLT3 mutations, and the prognostic impact of these molecular aberrations in CBF AML remains uncertain, Alliance CBF AML patients with FLT3 mutations were offered another study (ie, CALGB 10603).40 Thus, it should be noted that patients with CBF AML and FLT3 mutations were likely underrepresented in CALGB 10801 with respect to CALGB 9621 and CALGB 19808 (no FLT3 data are available for these 2 protocols). Nevertheless, the prognostic value of FLT3 mutations in CBF AML remains to be fully elucidated. Thus, these results need to be interpreted cautiously, and they require confirmation in larger, randomized trials.
Older patients enrolled on CALGB 10801 had worse DFS and OS than younger patients although toxicities did not differ markedly. Several factors may be responsible, including age-related biological differences or the lower doses of HiDAC given to older patients (1000 mg/m2 compared with 3000 mg/m2 given to younger patients). Nevertheless, with DFS and OS rates of 60% and 65% at 2 years, and 60% and 51% at 3 years, respectively, older patients with CBF AML seemingly benefited from the CALGB 10801 combined treatment approach; they had clinical outcomes comparable to those observed for younger patients on the earlier CALGB 9621 and CALGB 19808 trials.
Although the presence of KITmut has been linked to a worse outcome in some, but not all, studies (see also Chen et al19), we noted very similar outcomes for those patients with and those without an adverse molecular profile enrolled on CALGB 10801, especially when only the younger patients were considered. Similar considerations applied for patients presenting with higher KITwt expression. However, these were post hoc analyses, and the numbers of subjects with these molecular features were small; therefore, these results need to be confirmed.
Although the clinical results observed with the dasatinib/chemotherapy combination are encouraging, we recognize that the CALGB 10801 study had some intrinsic limitations to achieve definitive conclusions given the current state of the science. These include the single-arm study design, the relatively small cohort of treated patients, the uncertain role of dasatinib maintenance postchemotherapy (because only 19 patients completed this part of the treatment program), the absence of additional diagnostic studies to define the molecular landscape of each subject’s disease at diagnosis and relapse, and the lack of monitoring of molecular aberrations and/or MRD at sequential time points during and after treatment. These end points will be explored in future larger Alliance or Intergroup studies of CBF AML.
Of note, during the preparation of the manuscript, a study using a nearly identical regimen of dasatinib plus chemotherapy in CBF AML was reported by The Akute Myeloische Leukämie-Studiengruppe (AMLSG 11-08).41 This study accrued 89 patients with results very similar to CALGB 10801. The CR/CR with incomplete hematologic recovery rate was 94%, and the 4-year OS was 74%. However, in an exploratory analysis, the outcomes of KITmut patients (n = 19) seemed somewhat inferior to those of KITwt patients. The AMLSG is currently conducting a randomized clinical trial comparing a regimen nearly identical to CALGB 10801 (chemotherapy/dasatinib) vs chemotherapy alone (#NCT02013648), and that study will provide further insights.
Overall, our findings support further evaluation of KIT inhibitors in combination with chemotherapy in CBF AML through future larger, prospective randomized trials. Furthermore, additional pharmacodynamics studies that analyze the impact of dasatinib on aberrantly activated KIT-dependent pathways are necessary to define and quantify the pharmacologic activity of this agent on CBF AML blasts. However, despite our encouraging results, it is evident that even when dasatinib was added to chemotherapy, not all patients with CBF AML achieved long-term leukemia-free status. Thus, the treatment approach for patients with CBF AML should be further refined with the rational addition of other molecularly targeted agents, such as gemtuzumab ozogamicin, which has recently been reported in combination with chemotherapy to confer a clinical benefit to patients with a favorable or intermediate cytogenetic risk.42-45 Nevertheless, the goal of achieving cure in nearly all patients with CBF AML seems to be within reach of the next generation of clinical trials. These should incorporate novel study designs that include both clinical and molecular end points (ie, MRD) and possibly early intervention for patients with an impending risk of relapse either during the treatment or while in the posttreatment follow-up phase.
Acknowledgments
The authors thank patients and their families, collaborators and staff in the CALGB and Alliance member institutions/data center, the CALGB/Alliance Leukemia Tissue Bank, and Donna Bucci. They acknowledge the important contributions of Meir Wetzler (deceased) to this study. Michael J. Kelly and Samantha Sublett served as protocol coordinators.
This trial was sponsored by the National Cancer Institute Cancer Therapy Evaluation Program. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers U10CA180821 and U10CA180882 (Alliance for Clinical Trials in Oncology), P30CA033572, U10CA180833, U10CA180836, U10CA180850, U10CA180866, UG1 CA233338, U24 CA196171, and U10CA180867.
The following institutional networks participated in this study (principal investigator and grant numbers in parentheses, as applicable): Dana-Farber/Partners CancerCare LAPS, Boston, MA (Harold Burstein, U10CA180867); Eastern Maine Medical Center Cancer Care, Brewer, ME (Thomas Openshaw); Florida Hospital Orlando, Orlando, FL (Carlos Alemany); Northwell Health NCORP, Lake Success, NY (Daniel Budman, UG1CA189850); The Ohio State University Comprehensive Cancer Center LAPS, Columbus, OH (Claire Verschraegen, U10CA180850); Roswell Park Cancer Institute LAPS, Buffalo, NY (Ellis Levine, U10CA180866); Southeast Clinical Oncology Research Consortium NCORP, Winston-Salem, NC (James Atkins, UG1CA189858); University of Chicago Comprehensive Cancer Center LAPS, Chicago, IL (Hedy Kindler, U10CA180836); University of Maryland/Greenebaum Cancer Center, Baltimore, MD (Dan Zandberg); University of Vermont College of Medicine, Burlington, VT (Steven Ades); Wake Forest University Health Sciences, Winston-Salem, NC (Heidi Klepin); Washington University–Siteman Cancer Center LAPS, Saint Louis, MO (Nancy Bartlett, U10CA180833); and Weill Medical College of Cornell University, New York, NY (Scott Tagawa).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
For original data requests, please contact gmarcucci@coh.org and mandrekar.sumithra@mayo.edu for the Alliance Statistics and Data Center.
Authorship
Contribution: G.M. and R.A.L. were responsible for study conception and design; G.M., W.Z., D.B., G.L.U., W.B., A.-K.E., T.S.P., E.S.W., W.S., J.E.K., C.D.B., R.M.S., and R.A.L. acquired data; G.M., S.G., K.L., J.K., C.D.B., K.M., and R.A.L. analyzed and interpreted the data; G.M. drafted the manuscript; and all authors reviewed the draft manuscript and approved the final version for submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Guido Marcucci, Gehr Family Center for Leukemia Research, Department of Hematology and Hematopoietic Cell Transplantation, City of Hope Medical Center, 1500 East Duarte Rd, Duarte CA 91010; e-mail: gmarcucci@coh.org.
References
- 1.Grimwade D, Mrózek K. Diagnostic and prognostic value of cytogenetics in acute myeloid leukemia. Hematol Oncol Clin North Am. 2011;25(6):1135-1161, vii. [DOI] [PubMed] [Google Scholar]
- 2.Döhner H, Estey E, Grimwade D, et al. . Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mrózek K, Marcucci G, Nicolet D, et al. . Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol. 2012;30(36):4515-4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcucci G, Mrózek K, Ruppert AS, et al. . Prognostic factors and outcome of core binding factor acute myeloid leukemia patients with t(8;21) differ from those of patients with inv(16): a Cancer and Leukemia Group B study. J Clin Oncol. 2005;23(24):5705-5717. [DOI] [PubMed] [Google Scholar]
- 5.Appelbaum FR, Kopecky KJ, Tallman MS, et al. . The clinical spectrum of adult acute myeloid leukaemia associated with core binding factor translocations. Br J Haematol. 2006;135(2):165-173. [DOI] [PubMed] [Google Scholar]
- 6.Schlenk RF, Benner A, Krauter J, et al. . Individual patient data-based meta-analysis of patients aged 16 to 60 years with core binding factor acute myeloid leukemia: a survey of the German Acute Myeloid Leukemia Intergroup. J Clin Oncol. 2004;22(18):3741-3750. [DOI] [PubMed] [Google Scholar]
- 7.Ustun C, Marcucci G. Emerging diagnostic and therapeutic approaches in core binding factor acute myeloid leukaemia. Curr Opin Hematol. 2015;22(2):85-91. [DOI] [PubMed] [Google Scholar]
- 8.Goyama S, Mulloy JC. Molecular pathogenesis of core binding factor leukemia: current knowledge and future prospects. Int J Hematol. 2011;94(2):126-133. [DOI] [PubMed] [Google Scholar]
- 9.Yergeau DA, Hetherington CJ, Wang Q, et al. . Embryonic lethality and impairment of haematopoiesis in mice heterozygous for an AML1-ETO fusion gene. Nat Genet. 1997;15(3):303-306. [DOI] [PubMed] [Google Scholar]
- 10.Okuda T, Cai Z, Yang S, et al. . Expression of a knocked-in AML1-ETO leukemia gene inhibits the establishment of normal definitive hematopoiesis and directly generates dysplastic hematopoietic progenitors. Blood. 1998;91(9):3134-3143. [PubMed] [Google Scholar]
- 11.Castilla LH, Garrett L, Adya N, et al. . The fusion gene Cbfb-MYH11 blocks myeloid differentiation and predisposes mice to acute myelomonocytic leukaemia. Nat Genet. 1999;23(2):144-146. [DOI] [PubMed] [Google Scholar]
- 12.Rowe JM. The increasing genomic complexity of acute myeloid leukemia. Best Pract Res Clin Haematol. 2014;27(3-4):209-213. [DOI] [PubMed] [Google Scholar]
- 13.Cairoli R, Beghini A, Grillo G, et al. . Prognostic impact of c-KIT mutations in core binding factor leukemias: an Italian retrospective study. Blood. 2006;107(9):3463-3468. [DOI] [PubMed] [Google Scholar]
- 14.Schnittger S, Kohl TM, Haferlach T, et al. . KIT-D816 mutations in AML1-ETO-positive AML are associated with impaired event-free and overall survival. Blood. 2006;107(5):1791-1799. [DOI] [PubMed] [Google Scholar]
- 15.Paschka P, Marcucci G, Ruppert AS, et al. . Adverse prognostic significance of KIT mutations in adult acute myeloid leukemia with inv(16) and t(8;21): a Cancer and Leukemia Group B Study. J Clin Oncol. 2006;24(24):3904-3911. [DOI] [PubMed] [Google Scholar]
- 16.Pollard JA, Alonzo TA, Gerbing RB, et al. . Prevalence and prognostic significance of KIT mutations in pediatric patients with core binding factor AML enrolled on serial pediatric cooperative trials for de novo AML. Blood. 2010;115(12):2372-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin Y-Z, Zhu H-H, Jiang Q, et al. . Prevalence and prognostic significance of c-KIT mutations in core binding factor acute myeloid leukemia: a comprehensive large-scale study from a single Chinese center. Leuk Res. 2014;38(12):1435-1440. [DOI] [PubMed] [Google Scholar]
- 18.Solh M, Yohe S, Weisdorf D, Ustun C. Core-binding factor acute myeloid leukemia: Heterogeneity, monitoring, and therapy. Am J Hematol. 2014;89(12):1121-1131. [DOI] [PubMed] [Google Scholar]
- 19.Chen W, Xie H, Wang H, et al. . Prognostic significance of KIT mutations in core-binding factor acute myeloid leukemia: a systematic review and meta-analysis. PLoS One. 2016;11(1):e0146614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S, Wu LC, Pang J, et al. . Sp1/NFκB/HDAC/miR-29b regulatory network in KIT-driven myeloid leukemia. Cancer Cell. 2010;17(4):333-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang YY, Zhao LJ, Wu CF, et al. . C-KIT mutation cooperates with full-length AML1-ETO to induce acute myeloid leukemia in mice. Proc Natl Acad Sci U S A. 2011;108(6):2450-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao L, Melenhorst JJ, Alemu L, et al. . KIT with D816 mutations cooperates with CBFB-MYH11 for leukemogenesis in mice. Blood. 2012;119(6):1511-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schittenhelm MM, Shiraga S, Schroeder A, et al. . Dasatinib (BMS-354825), a dual SRC/ABL kinase inhibitor, inhibits the kinase activity of wild-type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies. Cancer Res. 2006;66(1):473-481. [DOI] [PubMed] [Google Scholar]
- 24.Arber DA, Brunning RD, Le Beau MM, et al. . Acute myeloid leukemia with recurrent genetic abnormalities In: Swerdlow SH, Campo E, Harris NL, et al., eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2008:110-123 [Google Scholar]
- 25.Mrózek K, Prior TW, Edwards C, et al. . Comparison of cytogenetic and molecular genetic detection of t(8;21) and inv(16) in a prospective series of adults with de novo acute myeloid leukemia: a Cancer and Leukemia Group B Study. J Clin Oncol. 2001;19(9):2482-2492. [DOI] [PubMed] [Google Scholar]
- 26.Cheson BD, Bennett JM, Kopecky KJ, et al. . Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia [published correction appears in J Clin Oncol. 2004;22(3):576]. J Clin Oncol. 2003;21(24):4642-4649. [DOI] [PubMed] [Google Scholar]
- 27.Blum W, Sanford BL, Klisovic R, et al. ; Alliance for Clinical Trials in Oncology . Maintenance therapy with decitabine in younger adults with acute myeloid leukemia in first remission: a phase 2 Cancer and Leukemia Group B Study (CALGB 10503). Leukemia. 2017;31(1):34-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravandi F, O’Brien S, Thomas D, et al. . First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Blood. 2010;116(12):2070-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kampa-Schittenhelm KM, Vogel W, Bonzheim I, et al. . Dasatinib overrides the differentiation blockage in a patient with mutant-KIT D816V positive CBFβ-MYH11 leukemia. Oncotarget. 2018;9(14):11876-11882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolitz JE, George SL, Marcucci G, et al. ; Cancer and Leukemia Group B . P-glycoprotein inhibition using valspodar (PSC-833) does not improve outcomes for patients younger than age 60 years with newly diagnosed acute myeloid leukemia: Cancer and Leukemia Group B study 19808. Blood. 2010;116(9):1413-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolitz JE, George SL, Dodge RK, et al. . Dose escalation studies of cytarabine, daunorubicin, and etoposide with and without multidrug resistance modulation with PSC-833 in untreated adults with acute myeloid leukemia younger than 60 years: final induction results of Cancer and Leukemia Group B Study 9621. J Clin Oncol. 2004;22(21):4290-4301. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez HF, Sun Z, Yao X, et al. . Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361(13):1249-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burnett AK, Russell N, Hills RK, et al. . A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood. 2015;125(25):3878-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prebet T, Bertoli S, Delaunay J, et al. . Anthracycline dose intensification improves molecular response and outcome of patients treated for core binding factor acute myeloid leukemia. Haematologica. 2014;99(10):e185-e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bloomfield CD, Lawrence D, Byrd JC, et al. . Frequency of prolonged remission duration after high-dose cytarabine intensification in acute myeloid leukemia varies by cytogenetic subtype. Cancer Res. 1998;58(18):4173-4179. [PubMed] [Google Scholar]
- 36.Byrd JC, Ruppert AS, Mrózek K, et al. . Repetitive cycles of high-dose cytarabine benefit patients with acute myeloid leukemia and inv(16)(p13q22) or t(16;16)(p13;q22): results from CALGB 8461. J Clin Oncol. 2004;22(6):1087-1094. [DOI] [PubMed] [Google Scholar]
- 37.Byrd JC, Dodge RK, Carroll A, et al. . Patients with t(8;21)(q22;q22) and acute myeloid leukemia have superior failure-free and overall survival when repetitive cycles of high-dose cytarabine are administered. J Clin Oncol. 1999;17(12):3767-3775. [DOI] [PubMed] [Google Scholar]
- 38.Sato T, Yang X, Knapper S, et al. . FLT3 ligand impedes the efficacy of FLT3 inhibitors in vitro and in vivo. Blood. 2011;117(12):3286-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duployez N, Willekens C, Marceau-Renaut A, Boudry-Labis E, Preudhomme C. Prognosis and monitoring of core-binding factor acute myeloid leukemia: current and emerging factors. Expert Rev Hematol. 2015;8(1):43-56. [DOI] [PubMed] [Google Scholar]
- 40.Stone RM, Mandrekar SJ, Sanford BL, et al. . Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paschka P, Schlenk RF, Weber D, et al. . Adding dasatinib to intensive treatment in core-binding factor acute myeloid leukemia-results of the AMLSG 11-08 trial. Leukemia. 2018;32(7):1621-1630. [DOI] [PubMed] [Google Scholar]
- 42.Hills RK, Castaigne S, Appelbaum FR, et al. . Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15(9):986-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borthakur G, Cortes JE, Estey EE, et al. . Gemtuzumab ozogamicin with fludarabine, cytarabine, and granulocyte colony stimulating factor (FLAG-GO) as front-line regimen in patients with core binding factor acute myelogenous leukemia. Am J Hematol. 2014;89(10):964-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taksin AL, Legrand O, Raffoux E, et al. . High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia. 2007;21(1):66-71. [DOI] [PubMed] [Google Scholar]
- 45.Appelbaum FR, Bernstein ID. Gemtuzumab ozogamicin for acute myeloid leukemia. Blood. 2017;130(22):2373-2376. [DOI] [PubMed] [Google Scholar]