Key Points
Andexanet alfa demonstrated a rapid reversal of rivaroxaban- and edoxaban-induced anticoagulation in healthy human subjects.
Andexanet alfa was well tolerated, with no clinical evidence of prothrombotic activity.
Abstract
As with any anticoagulant, factor Xa (FXa) inhibitors are associated with a risk of major bleeding. Andexanet alfa is a recombinant modified human FXa lacking enzymatic activity, developed for reversal of FXa inhibitor–induced anticoagulation. In two phase 2, randomized, double-blind, placebo-controlled, single-center studies, different regimens of andexanet alfa were administered to healthy volunteers after therapeutic anticoagulation with rivaroxaban or edoxaban, and multiple anticoagulation reversal and safety end points were evaluated. Andexanet alfa rapidly and effectively reversed anticoagulation with both rivaroxaban and edoxaban. Within 2 minutes after bolus, anti-FXa activity decreased significantly, with maximum decreases of ≈93% (P < .05) and ≈82% (P < .05), respectively, compared with placebo. The stoichiometric ratios of andexanet alfa:total anticoagulant at maximum reversal of anti-FXa activity ranged from 1:1 to 1.3:1 for rivaroxaban and 1.41:1 to 2.58:1 for edoxaban. Sustained normalization of thrombin generation for ≈2 hours and sustained decrease in unbound anticoagulant (maximum ≈80%) for up to ≈4 hours following completion of andexanet alfa administration, compared with placebo, were observed when andexanet was administered as a bolus or as a bolus followed by continuous infusion. Andexanet alfa was well tolerated, and there were no serious adverse events or thrombotic events. Andexanet alfa has been approved in the United States and Europe for reversal of anticoagulation in patients treated with rivaroxaban or apixaban who experience life-threatening or uncontrolled bleeding. These studies were registered with clinicaltrials.gov (#NCT03578146 and #NCT03551743).
Visual Abstract
Introduction
Direct factor Xa (FXa) inhibitors are approved for the management of multiple indications, including prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation, prophylaxis/treatment of venous thromboembolism, prevention of recurrent arterial vascular disease, and thromboprophylaxis after hip or knee replacement surgery.1 As with other anticoagulants, however, FXa inhibitors are associated with a risk of bleeding.2-4
Andexanet alfa (Andexxa [Portola Pharmaceuticals Inc., South San Francisco, CA]; coagulation factor Xa [recombinant], inactivated-zhzo) has been approved in the United States, and granted a conditional marketing authorization by the European Medicines Agency’s human medicines committee, for patients treated with rivaroxaban and apixaban when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding.5,6 Andexanet alfa is a recombinant human FXa that lacks the catalytic activity of native FXa but retains high-affinity binding to FXa inhibitors. In animal models, andexanet alfa reduced anti-FXa activity and bleeding associated with edoxaban, rivaroxaban, enoxaparin, and fondaparinux, and helped restore hemostasis.7,8 Phase 2 studies in healthy volunteers using pharmacodynamic (PD) markers have shown that andexanet alfa also reverses the anticoagulant effects of enoxaparin and apixaban.9,10 In a phase 3 study in older healthy volunteers, andexanet alfa reversed the anticoagulant activity of rivaroxaban and apixaban, as assessed according to thrombin generation and anti-FXa activity.11 A phase 3b/4 study (ANNEXA-4 [Prospective, Open-Label Study of Andexanet Alfa in Patients Receiving a Factor Xa Inhibitor who have Acute Major Bleeding]; #NCT02329329) in patients anticoagulated with an FXa inhibitor who presented with acute major bleeding established the clinical efficacy and safety of andexanet in this patient population.12-14
Our early studies with other FXa inhibitors suggested that dosing requirements for andexanet alfa may vary for different FXa inhibitors due to their various pharmacokinetic (PK) properties, especially volumes of distribution.7,10 We therefore performed 2 safety and dose-ranging phase 2 clinical studies in participants receiving rivaroxaban or edoxaban to characterize the PK/PD parameters of andexanet alfa during and after administration of an intravenous bolus or bolus plus infusion. In addition, we analyzed the anticoagulant PK profiles of rivaroxaban and edoxaban following andexanet alfa administration. Safety and tolerability data were also collected. These data, along with previously published phase 2 data with apixaban, were used to establish the andexanet alfa dosing regimens for the phase 3 healthy participants (ANNEXA-A [A Phase 3 Randomized, Double-blind, Placebo-controlled Study in Older Subjects to Assess Safety and the Reversal of Apixaban Anticoagulation with Intravenously Administered Andexanet Alfa; #NCT02207725] and ANNEXA-R [A Phase 3 Randomized, Double-blind, Placebo-controlled Study in Older Subjects to Assess Safety and the Reversal of Rivaroxaban Anticoagulation with Intravenously Administered Andexanet Alfa; #NCT02220725) and phase 3b/4 study in patients with bleeding (ANNEXA-4).
Methods
Subjects
Healthy male or female adult subjects (between 18 and 45 years of age) were considered eligible if their medical history, physical examination, electrocardiogram (ECG), and vital signs were clinically unremarkable. Laboratory values for coagulation and results of hematology and liver function tests had to be within normal ranges. Subjects with a personal or family history, or with risk factors for bleeding or for a hypercoagulable or thrombotic condition, were excluded. Full eligibility criteria are presented in the supplemental Appendix. The study was approved by the Chesapeake Institutional Review Board (Columbia, MD), and all subjects provided written informed consent.
Study design, treatments, and assessments
We report here 2 modules of a randomized, double-blind, placebo-controlled, single-center, phase 2 clinical trial; these modules were registered individually as #NCT03578146 and #NCT03551743. The studies were conducted 18 March 2013 to 14 April 2014 for rivaroxaban (#NCT03578146) and 7 March 2014 to 5 August 2015 for edoxaban (#NCT03551743). Subjects were inpatients starting on the day before anticoagulant dosing (day –1) for up to 13 days (day 13) and then followed up as outpatients from days 14 to 48. After confirmation that all inclusion and exclusion criteria were met (supplemental Appendix), subjects were randomized to receive andexanet alfa or placebo in a 2:1 ratio on day 1. The randomization was performed by an unblinded research pharmacist using a predetermined randomization schedule. Subjects received 6 days (days 1-6) of oral rivaroxaban (20 mg daily, with food) or edoxaban (60 mg daily, in a fasted state) to achieve steady-state plasma concentrations; anticoagulant administration was open-label. The last anticoagulant dose was administered on the morning of day 6. Andexanet alfa or placebo was administered on a single day (day 6) after the last dose of anticoagulant. Several different dosing regimens were investigated, both within and between the rivaroxaban- and edoxaban-treated subjects, and each subject participated in only 1 andexanet alfa dosing regimen (supplemental Table 1). Subjects in the placebo arms received a single IV bolus or continuous infusion of vehicle in the same volume and rate as the corresponding andexanet alfa dose. Andexanet alfa doses were administered so that the bolus portion of the regimen ended at 3 hours after the last anticoagulation dose for all cohorts in the rivaroxaban study and for cohorts 1 and 2 in the edoxaban study. For cohort 3 in the edoxaban study, the bolus andexanet alfa dose ended 5 hours after the last edoxaban dose.
The initial andexanet alfa dosing regimens for these studies were based on results from a previous phase 1 single-ascending dose study in healthy human volunteers, showing that andexanet alfa was well tolerated and reversed the anticoagulant effect of exogenously added FXa inhibitors (ie, FXa inhibitor was added ex vivo to the subject’s plasma); subsequent dosing regimens were selected based on emerging data from prior cohorts.15 Dose escalation decisions between cohorts were made based on accumulating safety and PD data on the recommendation of the unblinded independent safety committee.
The studies were conducted in accordance with the International Conference on Harmonisation Good Clinical Practice guideline and the Declaration of Helsinki. Safety and tolerability were monitored by an independent safety committee composed of clinicians, including coagulation/thrombosis specialists, and a statistician.
PK and PD parameters
The PK parameters of andexanet alfa, rivaroxaban, and edoxaban were characterized by half-life (distribution and terminal), time to maximum plasma concentration, maximum plasma concentration (Cmax), area under the plasma concentration–time curve, systemic clearance, volume of distribution at steady state, and elimination rate constant.
The PD effects of andexanet alfa on rivaroxaban and edoxaban were assessed by measuring anti-FXa activity by using a modified anti-FXa assay to minimize the effect of sample dilutions, total and unbound levels of rivaroxaban and edoxaban (in addition to the human-specific metabolite D-21-2393), thrombin generation, prothrombin time (PT), activated partial thromboplastin time (aPTT), and activated clotting time (ACT). The “Methods” in the supplemental Appendix provides blood sample collection and assay details for specified PK and PD parameters.
Safety
Safety assessments included standard laboratory blood and urine tests, physical examinations, vital signs, 12-lead ECGs, coagulation markers, and testing for antibodies to andexanet alfa, factor X (FX), and FXa. Adverse events (AEs) were coded according to the Medical Dictionary for Regulatory Activities Version 15.0. AEs occurring after anticoagulant dosing but before dosing with andexanet alfa/placebo were separately summarized from those occurring after administration of andexanet alfa/placebo.
To assess immunogenicity, blood samples for anti–andexanet alfa, anti-FX, and anti-FXa antibodies were collected on day 1 (0 hour; predose) and then on days 20, 34, and 48. Antibody presence determination was performed by a central laboratory using an electrochemiluminescent assay of citrated plasma. Neutralizing antibody activity was further assessed for samples with positive anti–andexanet alfa antibodies.
Markers of coagulation and fibrinolysis were analyzed by using commercially available kits in a central laboratory. The “Methods” in the supplemental Appendix provides details on the assays, including measurement of D-dimer and prothrombin fragment 1 + 2 (F1+2) levels.
Statistical analysis
For PK/PD data, descriptive summary statistics, including mean and standard deviation or standard error of the mean, median and maximum/minimum, and coefficient of variation, were calculated according to cohort. Geometric mean and geometric coefficient of variation were calculated for areas under the plasma concentration–time curve and Cmax.
PD data were normalized to baseline and expressed as percent change from baseline or percentage of baseline. The baseline for PD markers measured after anticoagulant administration but before administration of andexanet alfa/placebo was defined as the day 1 value before anticoagulant dosing, whereas the baseline for markers measured after administration of andexanet alfa/placebo was defined as the 3-hour (except for cohort 3 in the edoxaban study) post-anticoagulant value (before andexanet alfa/placebo dosing) on day 6, which would be close to the maximum anticoagulation at steady state. For cohort 3 in the edoxaban study, the baseline for markers after administration of andexanet alfa/placebo was defined as the value measured 5 hours’ post-anticoagulant.
Analysis of covariance was used to compare results between groups inferentially after adjusting for baseline values. The least squares means (adjusted means) were reported for inferential comparisons. The paired Student t test was used for within-group comparisons. Statistical summaries were performed by using SAS version 9.3 or higher (SAS Institute, Inc., Cary, NC).
Because demographic information and PK/PD data were available from the inpatient study period, patients lost to extended follow-up (ie, after discharge from the research unit) were included in the analyses.
Results
Baseline characteristics
A total of 48 subjects were administered rivaroxaban. Of these, 33 received andexanet alfa and 15 received placebo. Three subjects from the andexanet alfa group were lost to extended follow-up after discharge from the inpatient research unit. Forty-five subjects who received andexanet alfa or placebo were included in the PD and safety analyses, and all 30 subjects who received andexanet alfa were included in the andexanet alfa PK analysis.
A total of 28 healthy subjects were administered edoxaban. Of these, 18 received andexanet alfa and 10 received placebo. Two subjects did not complete 6 days of dosing with edoxaban (one due to AEs occurring on day 5 and the other due to personal reasons) and thus were not treated with andexanet alfa/placebo. Both of these subjects had been randomized to the placebo group on day 1. All 28 subjects were included in the safety analysis, 26 subjects were included in the PD analysis, and 18 subjects were included in the andexanet alfa PK analyses.
The subject characteristics are shown in supplemental Table 2.
PK variables
Andexanet alfa PK variables.
In both studies, the Cmax of andexanet alfa occurred very close to the end of the bolus administration (time to maximum plasma concentration), even for those cohorts who received subsequent infusions of andexanet alfa. Andexanet alfa concentrations declined in a biexponential manner after the end of the bolus dose administration. Table 1 presents the andexanet alfa plasma PK data on day 6 after anticoagulant dosing. In both studies, the plasma concentrations of andexanet alfa were dose proportionate with increasing andexanet alfa doses, whereas andexanet alfa urine levels were below the limit of quantitation. The mean terminal half-life of andexanet alfa ranged from 3.91 to 6.47 hours and from 8.06 to 8.21 hours in the rivaroxaban and edoxaban studies, respectively.
Table 1.
PK parameters of andexanet alfa administered on day 6 at 3 hours following the last anticoagulant dose
| PK parameter | Rivaroxaban study, mean ± SD | Edoxaban study (n = 6/cohort), mean ± SD | ||||||
|---|---|---|---|---|---|---|---|---|
| Cohort 1 (210-mg bolus; n = 6) | Cohort 2 (420-mg bolus; n = 6) | Cohort 3 (600-mg bolus; n = 6) | Cohort 4* (720/4 × 60 min; n = 6) | Cohort 5† (800/8 × 120 min; n = 6) | Cohort 1 (600-mg bolus) | Cohort 2‡ (800/8 × 60 min) | Cohort 3 (800-mg bolus) | |
| Cmax, ng/mL | 50 400 ± 12 100 | 105 000 ± 28 500 | 110 000 ± 7070 | 123 000 ± 28 900 | 161 000 ± 40 400 | 111 000 ± 20 000 | 176 000 ± 16 600 | 235 000 ± 106 000 |
| AUC0-∞, ng • h/mL | 51 400 ± 7520 | 111 000 ± 35 900 | 133 000 ± 11 500 | 216 000 ± 41 300 | 429 000 ± 85 300 | 129 000 ± 26 900 | 315 000 ± 39 600 | 241 000 ± 77 200 |
| t1/2, h | 5.0 ± 0.74 | 3.9 ± 0.43 | 4.5 ± 1.65 | 6.5 ± 3.59 | 4.4 ± 0.58 | 8.2 ± 6.08 | 6.9 ± 1.57 | 8.1 ± 3.24 |
| CL, L/h | 4.2 ± 0.56 | 4.2 ± 1.51 | 4.5 ± 0.40 | 4.6 ± 0.84 | 4.2 ± 0.81 | 4.8 ± 0.87 | NC | 3.6 ± 0.995 |
| Vss, L | 5.2 ± 1.09 | 4.7 ± 1.69 | 5.4 ± 0.55 | 4.2 ± 0.80 | 3.3 ± 1.20 | 6.2 ± 1.96 | NC | 4.3 ± 1.61 |
AUC0-∞, area under the plasma concentration–time curve from 0 to infinity; CL, systemic clearance; Cmax, maximum plasma concentration; NC, not calculable; SD, standard deviation; t1/2, half-life; Vss, volume at steady state.
A 720-mg bolus followed by a continuous infusion at 4 mg/min for 60 minutes.
An 800-mg bolus followed by a continuous infusion at 8 mg/min for 120 minutes.
An 800-mg bolus followed by a continuous infusion at 8 mg/min for 60 minutes.
Anticoagulant PK variables after andexanet alfa administration.
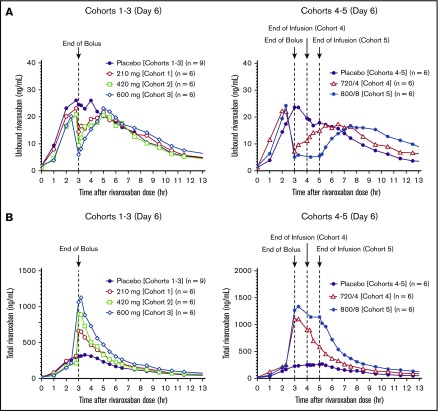
In subjects receiving anticoagulation with rivaroxaban, there was an immediate (within 2 minutes after end of bolus) dose-dependent decrease in unbound rivaroxaban relative to baseline and placebo values, with the maximum decrease seen in cohort 5 (80%) (range, 34%-80%) (Figure 1A). The decrease was significant from 2 minutes to 3.5 hours for cohort 5 (all, P < .05). Simultaneously, andexanet alfa increased total plasma rivaroxaban concentrations at steady state on day 6 (up to about fivefold relative to placebo) (Figure 1B). Similarly, there was an immediate (within 2 minutes after the end of the bolus) dose-dependent decrease in unbound edoxaban and D21-2393, by up to 76% (range, 56%-76%) and up to 78% (range, 45%-78%), respectively, relative to baseline and placebo values, with the maximum decrease seen in cohort 3 (Figures 2A-B). The decrease was sustained for up to ≈4 hours after the end of andexanet alfa bolus in cohort 2. Simultaneously, andexanet alfa increased mean total plasma levels of edoxaban and its pharmacologically active metabolite, D21-2393, at steady state on day 6 (up to 13-fold relative to placebo) (Figure 2C). In contrast to the rapid reduction of unbound anticoagulant concentration in the andexanet alfa groups, unbound rivaroxaban concentration in the respective placebo groups did not decrease at the end of the placebo bolus, whereas unbound edoxaban and D21-2393 concentrations decreased by <20% at the end of the bolus. The day 6 observed and calculated PK parameters for total rivaroxaban and edoxaban and D21-2393 are shown in Tables 2 and 3, respectively.
Figure 1.
Unbound and total rivaroxaban plasma concentrations on day 6 after the last dose of rivaroxaban with different andexanet alfa dosing regimens. (A) Unbound rivaroxaban (cohorts 1-3, upper left panel; cohorts 4-5, upper right panel). (B) Total rivaroxaban (cohorts 1-3, lower left panel; cohorts 4-5, lower right panel). Andexanet alfa was dosed at 3 hours after the last dose of rivaroxaban with a 210-, 420-, and 600-mg bolus (cohorts 1-3), 720-mg bolus plus 4 mg/min × 1-hour infusion (cohort 4), and 800-mg bolus plus 8 mg/min × 2-hour infusion (cohort 5), respectively.
Figure 2.
Unbound and total edoxaban and D21-2393 plasma concentrations on day 6 after the last dose of edoxaban with different andexanet alfa dosing regimens. (A) Unbound edoxaban. (B) Unbound D21-2393. (C) Total edoxaban (lower left panel) and total D21-2393 (lower right panel). Andexanet alfa was dosed at 3 hours (cohorts 1-2) or 5 hours (cohort 3) after the last dose of edoxaban with the 600-mg bolus (cohort 1), 800-mg bolus plus 8 mg/min × 1-hour infusion (cohort 2), and 800-mg bolus (cohort 3), respectively.
Table 2.
Total and unbound plasma anticoagulant PK parameters on day 6: rivaroxaban
| PK parameter | Andexanet alfa dose | Placebo* (n = 15) | ||||
|---|---|---|---|---|---|---|
| Cohort 1 (210-mg bolus; n = 6) | Cohort 2 (420-mg bolus; n = 6) | Cohort 3 (600-mg bolus; n = 6) | Cohort 4 (720/4 × 60 min; n = 6) | Cohort 5 (800/8 × 120 min; n = 6) | ||
| Total rivaroxaban, mean ± SD | ||||||
| Cmax, ng/mL | 676 ± 77.9 | 915 ± 112 | 1170 ± 267 | 1110 ± 178 | 1380 ± 296 | 325 ± 76.6 |
| C3.03,† ng/mL | 662 ± 84.6 | 898 ± 126 | 1060 ± 344 | 1100 ± 167 | 1270 ± 288 | 271 ± 68.4 |
| Tmax,‡ h (range) | 3.1 (3.06, 3.30) | 3.2 (3.05, 3.38) | 3.2 (3.18, 4.04) | 3.2 (2.97, 3.28) | 3.3 (3.09, 4.36) | 3.5 (2.42, 5.61) |
| AUC0-24, ng • h/mL | 3050 ± 395 | 3030 ± 488 | 4040 ± 503 | 4760 ± 861 | 7100 ± 1180 | 2250 ± 412 |
| CL/F, L/h | 6.6 ± 0.81 | 6.7 ± 0.97 | 5.0 ± 0.60 | 4.3 ± 0.65 | 2. 9 ± 0.55 | 9.2 ± 1.60 |
| t1/2, h | 7.3 ± 1.43 | 5.8 ± 0.97 | 7.7 ± 2.96 | 9.7 ± 5.52 | 7.4 ± 1.50 | 7.3 ± 2.09 |
| Unbound rivaroxaban, mean ± SD | ||||||
| Pre–andexanet alfa/placebo | 23.1 ± 5.59 | 20.9 ± 7.13 | 20.2 ± 9.83 | 22.1 ± 7.38 | 24.2 ± 8.50 | 21.8 ± 6.59 |
| C3.03,† ng/mL | 14.5 ± 3.06 | 9.42 ± 2.91 | 6.02 ± 5.25 | 7.3 ± 5.68 | 5.1 ± 3.32 | 24.3 ± 5.88 |
AUC, area under the plasma concentration–time curve from 0 to 24 hours; Cmax, maximum plasma concentration; C3.03, concentration at 3.03 hours postdose; CL/F, total clearance after oral administration; Tmax, time to maximum plasma concentration.
Pooled placebo from cohorts 1 to 5.
Anticoagulant plasma concentration measured 2 minutes after the end of the andexanet alfa bolus dose (3.03 hours after rivaroxaban dose).
Median (minimum, maximum); time relative to rivaroxaban dose, τ = 24 hours.
Table 3.
Total and unbound plasma anticoagulant PK parameters on day 6: edoxaban and D21-2393
| PK parameter | Andexanet alfa dose (n = 6/cohort) | Placebo* (n = 8) | ||
|---|---|---|---|---|
| Cohort 1 (600-mg bolus) | Cohort 2 (800/8 × 60 min) | Cohort 3 (800-mg bolus) | ||
| Total edoxaban, mean ± SD | ||||
| Cmax, ng/mL | 1420 ± 384 | 1720 ± 327 | 1240 ± 225 | 212 ± 62.1 |
| C3.03,† ng/mL | 1450 ± 424 | 1680 ± 340 | NA | 164 ± 91.4‡ |
| C5.03,§ ng/mL | NA | NA | 1200 ± 228 | 94.9 ± 14.9ǁ |
| Tmax,¶ h (range) | 3.1 (3.04, 3.21) | 3.3 (3.05, 3.27) | 5.2 (5.03, 5.40) | 2.0 (1.00, 2.47) |
| AUC0-24, ng • h/mL | 3550 ± 970 | 4950 ± 1050 | 3280 ± 438 | 1520 ± 358 |
| CL/F, L/h | 17.9 ± 4.24 | 12.6 ± 2.80 | 18.6 ± 2.33 | 41.3 ± 8.77 |
| t1/2, h | 15.4 ± 6.24 | 9.3 ± 2.90 | 13.3 ± 4.14 | 9.5 ± 4.20 |
| Unbound edoxaban, mean ± SD | ||||
| Pre–andexanet alfa/placebo | 130 ± 52.9 | 114 ± 37.1 | 63.6 ± 13.3 | 125 ± 57.5‡ |
| C3.03,† ng/mL | 59.6 ± 33.1§ | 41.1 ± 19.7 | NA | 103 ± 55.4‡ |
| C5.03,§ ng/mL | NA | NA | 15.1 ± 7.52 | 65.6 ± 10.5ǁ |
| Total D21-2393, mean ± SD | ||||
| Cmax, ng/mL | 61.9 ± 11.9 | 85.3 ± 53.7 | 45.6 ± 12.3 | 14.4 ± 5.43 |
| C3.03,† ng/mL | 60.0 ± 14.8¶ | 75.6 ± 46.1 | NA | 14.5 ± 5.48# |
| C5.03,§ ng/mL | NA | NA | 43.2 ± 13.2 | 5.00 ± 1.63ǁ |
| Tmax,¶ h (range) | 3.2 (3.05, 4.02) | 3.3 (3.25, 3.30) | 5.2 (5.03, 5.54) | 2.2 (2.00, 3.00) |
| AUC0-24, ng • h/mL | 205 ± 47.9 | 290 ± 191 | 164 ± 38.0 | 98.6 ± 34.5 |
| t1/2, h | 10.4 ± 4.77§ | 8.4 ± 2.53 | 12.8 ± 4.83 | 8.7 ± 3.35 |
| Unbound D21-2393, mean ± SD | ||||
| Pre–andexanet alfa/placebo | 5.9 ± 3.03 | 7.5 ± 2.82 | 3.6 ± 1.25 | 7.7 ± 2.89# |
| C3.03,† ng/mL | 3.07 ± 1.07¶ | 2.7 ± 0.96§ | Not applicable | 7.8 ± 4.26# |
| C5.03,§ ng/mL | NA | NA | 0.73 ± 0.32 | 2.7 ± 0.98ǁ |
C5.03, concentration at 5.03 hours postdose; NA, not available.
Pooled placebo subjects from cohorts 1 to 3.
Anticoagulant plasma concentration measured 2 minutes after the end of the andexanet alfa bolus dose (3.03 hours after edoxaban dosing).
N = 5.
Anticoagulant plasma concentration measured 2 minutes after the end of the andexanet alfa bolus dose (5.03 hours after edoxaban dosing).
N = 3.
Median (minimum, maximum); time relative to edoxaban dosing.
N = 4.
Urine PK data for the FXa inhibitors showed that andexanet alfa decreased the renal clearance of rivaroxaban and edoxaban on day 6 in a dose-dependent manner by up to ≈65% (range, 56%-69%) and ≈70% (range, 68%-74%), respectively, compared with both the day 5 and placebo values. This is likely due to the sequestering of the anticoagulant by andexanet alfa in plasma, thus temporarily delaying its availability for renal elimination.
PD variables
Reversal of Anti-FXa activity.
In the rivaroxaban study, administration of andexanet alfa resulted in a significant decrease in the anti-FXa activity in each active group compared with baseline (Figure 3A). In subjects receiving andexanet alfa, there was a rapid decrease within 2 minutes (cohorts 2-5, P < .05 vs placebo), whereas the placebo group showed increased anti-FXa activity at the same time point. The magnitude of decrease was dose and regimen dependent (range, 18%-93%; the greatest decrease [≈93%] was observed in cohort 5, P < .05 vs placebo). The longest duration of effect in anti-FXa activity was also seen in cohort 5, in which decreased anti-FXa activity persisted for 3 hours after the end of bolus.
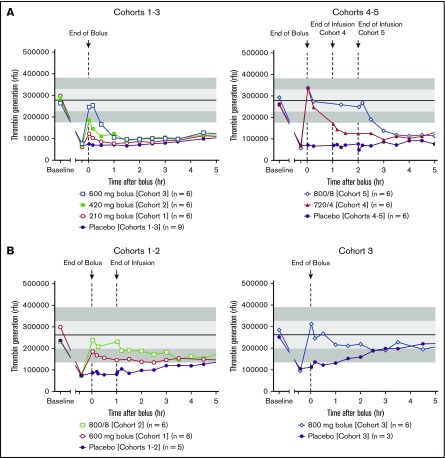
Figure 3.
Arithmetic mean anti-FXa activity vs time on day 6 after the last dose of rivaroxaban or edoxaban following andexanet alfa/placebo administration. (A) Rivaroxaban studies (cohorts 1-3, upper left panel; cohorts 4-5, upper right panel). Andexanet alfa was dosed at 3 hours after the last dose of rivaroxaban. (B) Edoxaban studies (cohorts 1-2, lower left panel; cohort 3, lower right panel). Andexanet alfa was administered at 3 hours (cohorts 1-2) or 5 hours (cohort 3) after the last dose of edoxaban.
In the edoxaban study, anti-FXa activity decreased after andexanet alfa administration compared with baseline (Figure 3B). In each active group, anti-FXa activity declined rapidly (within 2 minutes) in subjects receiving andexanet alfa (P < .05 vs placebo). The greatest reduction in anti-FXa activity (≈82%) was observed in cohort 3 (range across cohorts, 52%-82%). Subjects receiving placebo exhibited a <40% decrease in anti-FXa activity. The decrease in anti-FXa activity with andexanet alfa was sustained the longest in cohort 2 until 1.5 hours after the end of bolus (P < .05 vs the placebo group).
There was a linear positive relationship between unbound rivaroxaban and anti-FXa activity (Figure 4A) and unbound edoxaban and anti-FXa activity (Figure 4B). These results are consistent with the hypothesis that binding of andexanet alfa to unbound rivaroxaban or unbound edoxaban results in a reversal of anti-FXa activity. In the rivaroxaban study, rapid reversal of anti-FXa activity (89% and 93% decrease in cohorts 4 and 5, respectively) was achieved with a ≈1:1 to 1.3:1 molar ratio of andexanet alfa:total plasma rivaroxaban. The unbound rivaroxaban concentration corresponding to this reversal ranged from 5.1 to 7.3 ng/mL. Similarly, in the edoxaban study, a >70% decrease in anti-FXa activity was achieved at a molar ratio (andexanet alfa:edoxaban and andexanet alfa:total anticoagulant) of 1.41:1 to 2.58:1 in cohorts 2 and 3 (75% and 82% decrease). The unbound edoxaban and unbound D21-2393 concentrations corresponding to the reversal of anticoagulant effect ranged from 15.1 to 41.1 ng/mL and 0.7 to 2.7 ng/mL.
Figure 4.
Relationship between mean unbound FXa inhibitor plasma concentration and anti-FXa activity on day 6. Group mean of anti-FXa activity is plotted against unbound rivaroxaban (A) and edoxaban (B) for andexanet alfa–treated subjects at each time point on day 6, between pre–andexanet alfa and 21 hours post–andexanet alfa bolus. Day 6 was the last dose of the inhibitor and the day when andexanet alfa was administered. Total inhibitor concentrations were increased after andexanet alfa administration, as shown in Figures 1 and 2. The solid line represents the best linear regression; the dashed lines indicate the 95% confidence intervals.
Restoration of thrombin generation.
In both studies, restoration of thrombin generation was defined as a value that was at or above the mean −1 standard deviation day 1 pre-anticoagulant value. In the rivaroxaban study, administration of andexanet alfa resulted in a reversal of rivaroxaban-induced inhibition of thrombin generation and a rapid (within 2 minutes after completion of the andexanet alfa bolus) restoration of thrombin generation in all active groups, except cohort 1 (Figure 5A). In contrast, no placebo subjects achieved restoration at the end of the bolus dose. The magnitude and duration of thrombin generation following andexanet alfa administration were dose and regimen dependent. The most prolonged restoration was observed in cohort 5 with bolus followed by a 2-hour infusion of andexanet alfa. Thrombin generation was maintained within the normal range during the infusion, and the effect lasted for ≈2 hours after infusion before returning to the placebo level. In the placebo group, thrombin generation was not restored until 1 to 2 days after the bolus dose, following plasma clearance of rivaroxaban. In the edoxaban study, thrombin generation decreased after administration of edoxaban and was rapidly restored (within 2 minutes after completion of the andexanet alfa bolus) in 100% of subjects in all active groups, except in cohort 1 (Figure 5B). The restoration of thrombin generation was maintained for 2 hours after the bolus plus 1-hour infusion in cohort 2. Thrombin generation was gradually returned to placebo levels several hours after secession of andexanet alfa infusion. Thrombin generation was not restored until ≈33 hours after the bolus among placebo subjects from cohorts 1 to 2, following plasma clearance of edoxaban. In cohort 3, when andexanet alfa was administered 5 hours after the last edoxaban dose, restoration of thrombin generation was maintained for ≈2 hours after the andexanet alfa bolus before it returned to the placebo level. Mean thrombin generation in placebo subjects from cohort 3 returned to predose levels ≈4.5 hours after bolus dosing.
Figure 5.
Arithmetic mean thrombin generation vs time following andexanet alfa/placebo administration. Rivaroxaban studies (A, cohorts 1-3, upper left panel; cohorts 4-5, upper right panel); edoxaban studies (B, cohorts 1-2, lower left panel; cohort 3, lower right panel). Shaded regions represent day 1 pre-anticoagulant (mean ± 1 standard deviation [light shading]; ± 2 standard deviation [dark shading]) values. rfu, relative fluorescence unit.
PT, aPTT, and ACT.
Both rivaroxaban and edoxaban increased PT, aPTT, and ACT values (relative to day 1 pre-anticoagulant level). Prolongation of PT and aPTT was relatively small after rivaroxaban compared with prolongation of ACT (supplemental Table 3A). Administration of andexanet alfa resulted in a dose-dependent decrease in mean PT, aPTT, and ACT values within 2 minutes following the end of bolus dosing in all active groups (but not placebo) on day 6 of the rivaroxaban study. Following administration of andexanet alfa, the greatest mean percent changes from baseline values for PT, aPTT, and ACT were approximately –17% (cohort 4 at 2 minutes after end of bolus), –16% (cohort 5 at 1.5 hours after end of continuous infusion), and –47% (cohort 4 at 2 minutes after end of bolus), respectively.
Edoxaban increased PT, aPTT, and ACT at 3 hours post-edoxaban dosing on days 5 or 6 (relative to the day 1 pre-edoxaban level) (supplemental Table 3B). Administration of andexanet alfa resulted in small and modest dose-dependent decreases in mean PT and ACT values, respectively, within 2 minutes following the end of bolus dosing relative to just before administration of andexanet alfa. Andexanet alfa administration did not result in decreases in aPTT values. In the placebo groups, there was effectively no change in mean PT or ACT levels at the end of the bolus dose.
Safety
Andexanet alfa was generally well tolerated, and no significant safety signals were observed (supplemental Table 4). There was no apparent effect of andexanet alfa on clinical laboratory, ECG, vital signs, or physical examination findings. There were no deaths, serious AEs, severe AEs, AEs resulting in early termination, thrombotic events, or major bleeding events in either study. In the rivaroxaban study, the overall incidence of AEs was similar in the andexanet alfa and placebo groups, whereas in the edoxaban study, the overall incidence of AEs was higher in subjects treated with andexanet alfa (61%) than in subjects receiving placebo (38%). There was no apparent relation between any AE and andexanet alfa dose in either study. In both studies, the most frequently reported AE after treatment was infusion-related reaction, defined as any AE occurring during or shortly after study drug administration unless believed not to be related to the study drug. These events occurred more frequently in active subjects than in placebo subjects (33% vs 7% in the rivaroxaban study, and 17% vs 13% in the edoxaban study, respectively) and were highly variable, including feelings of warmth and flushing. None was consistent with an anaphylactic or anaphylactoid reaction. No subject in the rivaroxaban study developed antibodies to andexanet alfa, FX, or FXa. In the edoxaban study, no subject developed antibodies to FX or FXa, but 4 subjects developed low titer nonneutralizing antibodies to andexanet alfa.
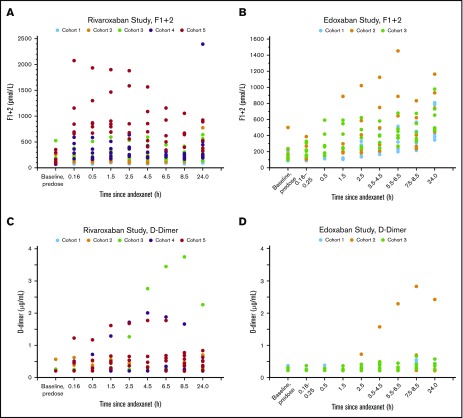
D-Dimer levels.
In the rivaroxaban study, there were dose-dependent increases in D-dimer values, relative to day 1 pre-anticoagulant levels, following andexanet alfa administration; the greatest mean D-dimer value was seen in cohort 3 at 8.5 hours after the end of the andexanet alfa bolus dose (Figure 6). In the edoxaban study, increases in the mean D-dimer values relative to day 1 levels were also observed following andexanet alfa administration; however, dose-dependent increases were not observed in the 2 bolus dosing regimens (cohorts 1 and 3). The greatest increase in mean D-dimer value was seen with cohort 2, 9.5 hours after the end of the andexanet alfa bolus dose. The mean D-dimer post–andexanet alfa treatment values in the active groups in both studies declined to those below the day 1 pre-anticoagulant levels within 48 hours after andexanet alfa administration, whereas the mean D-dimer post–placebo treatment values remained at or below the day 1 pre-anticoagulant levels at most time points sampled.
Figure 6.
Time courses of median prothrombin fragments 1 and 2 and D-dimer levels before and after the administration of andexanet alfa. Rivaroxaban: F1+2 (A), D-dimer (C). Edoxaban: F1+2 (B), D-dimer (D).
Prothrombin fragment 1+2 levels.
In the rivaroxaban study, there were dose-dependent increases in the mean F1+2 levels, relative to day 1 pre-anticoagulant levels, following andexanet alfa bolus administration. The greatest increase was observed 3.5 hours after the andexanet alfa bolus in cohort 5. In the edoxaban study, dose-dependent increases in the mean F1+2 levels after andexanet alfa administration were observed. The greatest increase was observed 24 hours after the andexanet alfa bolus in cohort 2. Within 5 days after andexanet alfa administration, the mean F1+2 post–andexanet alfa treatment values in the active groups in both studies declined to below the day 1 pre-anticoagulant value. The placebo values following treatment remained near or below the day 1 pre-anticoagulant levels at most time points sampled. Figure 6 presents the time courses of prothrombin F1+2 and D-dimer levels before and after the administration of andexanet alfa.
Discussion
The phase 2 clinical studies reported here evaluated the safety, tolerability, and anticoagulation reversal effects of andexanet alfa in healthy subjects receiving rivaroxaban or edoxaban. Andexanet alfa rapidly reversed rivaroxaban- and edoxaban-induced anti-FXa activity and inhibition of thrombin generation after steady-state dosing of the anticoagulants. Furthermore, the anticoagulation reversal effects were dose dependent and sustained when andexanet alfa was administered as a bolus followed by a continuous infusion. Together with previous studies showing reversal of apixaban anticoagulation therapy,10 these data show that andexanet alfa administration allows for a rapid reversal of FXa inhibition. The phase 2 studies presented here informed dosing regimens for the phase 3 studies in older healthy volunteers and a phase 3b/4 (ANNEXA-4) study in patients with acute major bleeding.10,12-14
PK results showed that mean total exposure to andexanet alfa rose with increasing andexanet alfa doses, consistent with previous studies.10 Andexanet alfa had a dose-related effect on the PK profile of the FXa inhibitors, causing an increase in total plasma concentration of rivaroxaban and edoxaban (and D21-2393) and a simultaneous decrease in the unbound fraction of rivaroxaban and edoxaban (and D21-2393). The observed increase in total plasma levels of rivaroxaban and edoxaban is likely related to redistribution of the FXa inhibitors from the extravascular space to plasma following binding of the inhibitor in plasma by andexanet alfa. Upon elimination of andexanet alfa, the equilibrium of the FXa inhibitors between the central and peripheral compartments is restored and FXa inhibitor elimination resumed at pre–andexanet alfa levels that were related to the unbound levels in plasma. These findings are consistent with the mechanism of action of andexanet alfa, which binds to the FXa inhibitor with high affinity within the vasculature and reduces the free inhibitor concentration.
The present studies enrolled subjects who were young and healthy and thus may not accurately reflect the patient population in whom FXa inhibitors are routinely prescribed. It is important to note, however, that results from a large phase 3 trial conducted in older healthy volunteers has shown that andexanet alfa produces a similar rapid reversal of apixaban- and rivaroxaban-induced anticoagulation.11 Although the present studies did not assess bleeding outcomes, they have demonstrated normalization of hemostasis based on currently used biomarkers of coagulation integrity (anti-FXa activity, unbound anticoagulant concentration, and thrombin generation assays). Furthermore, the correlation of anti-FXa activity with correction of blood loss by andexanet alfa in a rabbit liver injury model and a porcine polytrauma model provides additional support for use of anti-FXa activity as a biomarker likely to predict the clinical benefit of andexanet alfa for reversal of bleeding due to FXa inhibitor–induced anticoagulation.8,16
The clinical efficacy and safety of andexanet alfa were investigated in the ANNEXA-4 study in patients with acute major bleeding.15 In the final analysis of this study (efficacy population, n = 254), an andexanet alfa bolus followed by a 2-hour infusion reduced anti-FXa activity by 86% from baseline among patients receiving rivaroxaban and by 91% among patients receiving apixaban. Clinical hemostasis was adjudicated as excellent or good in 82% of patients 12 hours after andexanet alfa infusion. The incidence of thrombotic events during the 30-day follow-up period was 10% (n/N = 34/352 in the safety population). Anticoagulation with FXa inhibitors was immediately discontinued upon enrollment into the ANNEXA-4 study. One hundred (28%) patients restarted oral anticoagulation during the follow-up period, and no patient experienced a thrombotic event after their therapeutic-dose anticoagulants were restarted.
In the phase 2 studies presented here, andexanet alfa was generally well tolerated, with no significant safety signals. The most frequently reported AE following andexanet alfa treatment was infusion-related reactions. An elevation of D-dimer and F1+2 levels was observed without thromboembolic events, which was also reported for andexanet alfa infusion following apixaban-mediated anticoagulation.10 These elevations are likely related to the binding of andexanet alfa to tissue factor pathway inhibitor (TFPI), an endogenous inhibitor of FXa in a manner analogous to its binding to FXa, and these findings are consistent with the results from preclinical studies in animal models, as well as phase 2 and 3 studies in healthy volunteers.10,11,17 In healthy subjects, the D-dimer elevation may reflect the ability of normal fibrinolytic activity that could counterbalance the increased coagulation activity associated with the andexanet alfa–TFPI interaction.18
Sequestration of the FXa inhibitors is the major contributor in restoring thrombin generation both during and immediately after the administration of andexanet alfa. The andexanet alfa–TFPI interaction may contribute to the duration of the sustained reversal of TF-initiated thrombin generation, which might be affected by the TF concentration used in the assay. In the present study, thrombin generation was initiated with a high TF concentration (100 pM) and was less sensitive to the andexanet alfa–TFPI interaction. Therefore, the thrombin generation profile is similar to the anti-FXa reversal, and the duration of thrombin generation following andexanet alfa administration was shorter compared with the ANNEXA-A and ANNEXA-R studies, which used a commercially available calibrated automated thrombogram with low TF concentration (5 pM).19
In conclusion, andexanet alfa is characterized by a well-defined mechanism of action, validated by multiple anticoagulation reversal end points, and exhibits a predictable and consistent PK and PD response after administration as either a bolus alone or as a bolus followed by an infusion. Clinical data from patients with bleeding in ANNEXA-4 has provided further efficacy and safety data for reversal of anticoagulation in patients receiving direct or indirect FXa inhibitors.14 Comparison of andexanet alfa vs usual care for acute intracranial hemorrhage in patients receiving an oral FXa inhibitor will be studied in a phase 4 randomized trial that began recruitment in 2019 (#NCT03661528). Additional studies are needed to evaluate andexanet alfa as a therapeutic option for patients receiving FXa inhibitors who require urgent procedures or surgery.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Accuverus, Inc., and the Lockwood Group for editorial assistance with the manuscript.
The study was funded by Portola Pharmaceuticals, Inc. Financial support for medical editorial assistance was provided by Portola Pharmaceuticals, Inc.
Footnotes
Requests for access to additional data and documents related to the results reported in this article should be sent to medinfo@portola.com. Key clinical data for each module are available at clinicaltrials.gov. Individual participant data will not be shared. The study inclusion/exclusion criteria and additional details on study methods are included in the supplemental Appendix.
Authorship
Contribution: G.L. contributed to the design of the studies, data analysis, and interpretation of the results, and provided critical review of the manuscript; P.B.C. contributed to the design of the studies, PK, PD, and safety biomarker data analysis, interpretation of results, and helped prepare and review the manuscript; J.M.L. provided input into the design of the studies, oversaw and interpreted the PK and PD analysis, and helped prepare and review the manuscript; M.J.K. contributed to the design of the studies, data analysis, and interpretation of the results, and review of the manuscript; G.G.L. contributed to the design of the studies, data analysis, interpretation of the results, and review of the manuscript; V.S.M. contributed to the study design, statistical analysis plan, study conduct, interpretation of the data, and critical review of the manuscript; J.C. provided input to the design of the studies, conduct of the study, and review of the manuscript; M.C. provided input into the design of the studies, interpretation of the results, and provided critical review of the manuscript; and J.T.C. contributed to the design of the studies, data analysis, and interpretation of the results, and provided critical review of the manuscript.
Conflict-of-interest disclosure: J.C., P.B.C., J.T.C., J.M.L., G.L., and M.J.K. are employees and stockholders of Portola Pharmaceuticals. G.G.L. was an employee and stockholder of Portola Pharmaceuticals at time of study. V.S.M. reports personal fees from Portola Pharmaceuticals during the conduct of the study. M.C. has served as a consultant and/or speaker for Shionogi, Alexion, Pfizer, Daiichi, Octapharma, BMS Canada, CSL Behring, Servier Canada, Diagnostica Stago, and Asahi Kasei; his institution has received research grants from Leo Pharma and the Heart and Stroke Foundation; and he owns stock in Alnylam Pharmaceuticals.
Correspondence: Mark Crowther, Department of Medicine, McMaster University and St. Joseph’s Healthcare, 4V32 Health Sciences Centre, Hamilton, ON L8N 4A6, Canada; e-mail: crowthrm@mcmaster.ca.
References
- 1.Oktay E. Will NOACs become the new standard of care in anticoagulation therapy. Int J Cardiovasc Acad. 2015;1(1):1-4. [Google Scholar]
- 2.Granger CB, Alexander JH, McMurray JJ, et al. ; ARISTOTLE Committees and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. [DOI] [PubMed] [Google Scholar]
- 3.Patel MR, Mahaffey KW, Garg J, et al. ; ROCKET AF Investigators . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891. [DOI] [PubMed] [Google Scholar]
- 4.Giugliano RP, Ruff CT, Braunwald E, et al. ; ENGAGE AF-TIMI 48 Investigators . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093-2104. [DOI] [PubMed] [Google Scholar]
- 5.Andexxa [prescribing information]. South San Francisco, CA: Portola Pharmaceuticals; 2018. [Google Scholar]
- 6.European Medicines Agency. First antidote for reversal of anticoagulation with factor Xa inhibitors apixaban and rivaroxaban. Press release March 1, 2019. https://www.ema.europa.eu/en/news/first-antidote-reversal-anticoagulation-factor-xa-inhibitors-apixaban-rivaroxaban. Accessed 16 April 2019.
- 7.Lu G, DeGuzman FR, Hollenbach SJ, et al. . A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013;19(4):446-451. [DOI] [PubMed] [Google Scholar]
- 8.Pine PR, Hollenbach SJ, Tan S, et al. Andexanet alfa reverses edoxaban-induced anticoagulation in a rabbit liver laceration model of acute bleeding. Presented at the European Society of Cardiology Congress. 29 August–2 September 2015. London, United Kingdom. [Google Scholar]
- 9.Crowther M, Levy G, Lu G, et al. . Reversal of enoxaparin-induced anticoagulation in healthy subjects by andexanet alfa (PRT064445), an antidote for direct and indirect fXa inhibitors—a phase 2 randomized, double-blind, placebo-controlled trial [abstract]. J Thromb Haemost. 2014;12(supp 1). Abstract 7. [Google Scholar]
- 10.Siegal D, Lu G, Leeds JM, et al. . Safety, pharmacokinetics, and reversal of apixaban anticoagulation with andexanet alfa. Blood Adv. 2017;1(21):1827-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegal DM, Curnutte JT, Connolly SJ, et al. . Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373(25):2413-2424. [DOI] [PubMed] [Google Scholar]
- 12.Connolly SJ, Gibson CM, Crowther M. Andexanet alfa for factor Xa inhibitor reversal. N Engl J Med. 2016;375(25):2499-2500. [DOI] [PubMed] [Google Scholar]
- 13.Connolly SJ, Milling TJ Jr., Eikelboom JW, et al. ; ANNEXA-4 Investigators . Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375(12):1131-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connolly SJ, Crowther M, Eikelboom JW, et al. ; ANNEXA-4 Investigators . Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380(14):1326-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crowther M, Kitt M, McClure M, et al. . Randomized, double-blind, placebo-controlled single ascending dose pharmacokinetic and pharmacodynamic study of PRT064445, a universal antidote for factor Xa inhibitors [abstract]. Arterioscler Thromb Vasc Biol. 2013;33(suppl 1). Abstract 10. [Google Scholar]
- 16.Grottke O, Braunschweig T, Rossaint R, et al. . Transient or extended reversal of apixaban anticoagulation by andexanet alfa is equally effective in a porcine polytrauma model. Br J Anaesth. 2019;123(2):186-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu G, Hollenbach SJ, Baker DC, et al. . Preclinical safety and efficacy of andexanet alfa in animal models. J Thromb Haemost. 2017;15(9):1747-1756. [DOI] [PubMed] [Google Scholar]
- 18.Lu G, Lin J, Curnette JT, Conley PB. Effect of andexanet-tissue factor pathway inhibitor (TFPI) interaction on in vitro clot formation and lysis via different coagulation pathways. Res Pract Thromb Haemost. 2018;2(suppl 1):183-184. [Google Scholar]
- 19.Lu G, Lin J, Bronson M, Crowther M, Conley PB, Curnutte JT. Reversal of apixaban and rivaroxaban anticoagulation by andexanet alfa in ANNEXA-A and ANNEXA-R as assessed by non-tissue factor-initiated thrombin generation independent of tissue factor pathway inhibitor. EMJ Cardiology. 2018;6(1):47-51. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.