Key Points
NF-κB and its activator, IκB kinase 2 (IKK2, IKKβ), are central inflammatory molecules in various cell types, including anucleate platelets.
Neither platelet-specific deletion of IKK2 by excision of exon 3 nor pharmacological inhibition of IKK2 affects platelet function.
Abstract
Platelets are small anucleate cells that release a plethora of molecules to ensure functional hemostasis. It has been reported that IκB kinase 2 (IKK2), the central enzyme of the inflammatory NF-κB pathway, is involved in platelet activation, because megakaryocyte/platelet-specific deletion of exons 6 and 7 of IKK2 resulted in platelet degranulation defects and prolonged bleeding. We aimed to investigate the role of IKK2 in platelet physiology in more detail, using a platelet-specific IKK2 knockout via excision of exon 3, which makes up the active site of the enzyme. We verified the deletion on genomic and transcriptional levels in megakaryocytes and were not able to detect any residual IKK2 protein; however, platelets from these mice did not show any functional impairment in vivo or in vitro. Bleeding time and thrombus formation were not affected in platelet-specific IKK2-knockout mice. Moreover, platelet aggregation, glycoprotein GPIIb/IIIa activation, and degranulation were unaltered. These observations were confirmed by pharmacological inhibition of IKK2 with TPCA-1 and BMS-345541, which did not affect activation of murine or human platelets over a wide concentration range. Altogether, our results imply that IKK2 is not essential for platelet function.
Visual Abstract
Introduction
Platelets are key players in hemostasis, and granule secretion is essential for their function. Although platelets lack a nucleus, it has been postulated that the pathway that leads to activation of the inflammatory transcription factor NF-κB is important for their activation and degranulation.1 In general, NF-κB is kept inactive by binding to inhibitory molecules (IκBs). A plethora of stimuli leads to phosphorylation of IκBs by IκB kinases (IKKs), triggering their proteasomal degradation and the release of NF-κB. Most of these activating pathways converge at IKK2, which is the main IκB-phosphorylating enzyme in the course of NF-κB activation.2,3 In platelets, adenosine 5′-diphosphate (ADP), thrombin, epinephrine, and collagen have been reported to cause activation of the IKK2/IκB/NF-κB axis.3 However, although some investigators claim an activating role for this pathway,1,4 others suggest that it has inhibitory effects,5 leaving its role in platelet activation incompletely understood.
We aimed to resolve these conflicting findings for the nongenomic link between the NF-κB pathway and platelet signaling by using a mouse model with a platelet-specific deletion of IKK2,6 combined with in-depth analysis of hemostatic and immunomodulatory platelet functions in vitro and in vivo.
Methods
Detailed information is provided in supplemental Methods.
Mice and human samples
Mice with a loxP-flanked exon 3 of the IKK2 gene6 were crossed with PF4-iCre+/− mice7 (IKK2fl/fl PF4-iCre+/−; referred to as IKK2ΔPlt) (both from The Jackson Laboratory on a C57BL/6 background). IKK2fl/fl PF4-iCre−/− littermates were referred to as wild-type (WT). All animal experiments were conducted according to institutional guidelines. The Animal Care and Use Committee of the Medical University of Vienna, as well as the Austrian Federal Ministry of Education, Science and Research, approved all animal experiments (authorization number BMWFW-66.009/0246-WF/V/3b/2016). Human blood samples were taken from healthy volunteers with informed consent based on an approval by the ethics commission of the Medical University of Vienna (allowance number 1738/2015).
Statistical analysis
If not stated otherwise, data are depicted as mean ± standard deviation. Calculations were performed using GraphPad Prism 6.01 software. Comparison of 2 groups was done using an unpaired Student t test or Mann-Whitney U test if data were not distributed normally. Two or more groups were compared with the respective control group using 1-way analysis of variance with Dunnett correction. Two groups with ≥1 condition were compared by 2-way analysis of variance with Sidak correction.
Results and discussion
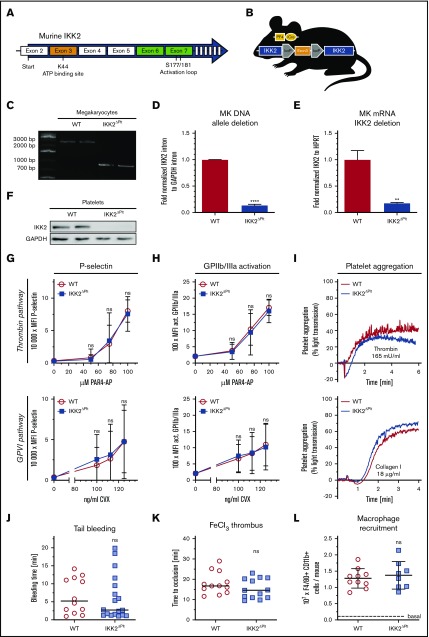
We used an IKK2-knockout mouse model in which the region that contains exon 3, coding for the catalytic adenosine triphosphate (ATP) binding site, is flanked by loxP sites (Figure 1A). We crossed these mice with the megakaryocyte/platelet-specific PF4 iCre strain (Figure 1B). Expression of Cre-recombinase results in excision of exon 3 and, thereby, a premature stop codon in exon 4.6 Knockout of IKK2 in megakaryocytes and platelets was confirmed on multiple levels. First, we observed the expected recombination-mediated shift of a genomic sequence in IKK2ΔPlt megakaryocytes (Figure 1C). Consistently, only remnant levels of recombined intron DNA between exon 2 and 3, and megakaryocytic IKK2 messenger RNA, were present (Figure 1D-E). Furthermore, we were not able to detect IKK2 protein in IKK2ΔPlt platelets (Figure 1F).
Figure 1.
Deletion of platelet IKK2 does not alter their function. (A) Graphical scheme of exons and their functional sites targeted by exon 3 and exon 6 and 7 deletion of IKK2. (B) Genetic scheme of IKK2ΔPlt mice. (C) Polymerase chain reaction (PCR) of WT megakaryocyte DNA of exon 3 and parts of adjacent introns generated an ∼2400–base pair (bp) band, whereas PCR of IKK2ΔPlt megakaryocyte DNA, with exon 3 deletion, generated an ∼730-bp band. (D) Quantitative PCR (qPCR) of an intron sequence between exon 2 and 3 of IKK2 that is excised during Cre recombination, normalized to a GAPDH intron sequence (n = 3). (E) qPCR of exon 3 of IKK2 messenger RNA, normalized to HPRT (n = 3). (F) Western blot of IKK2 and GAPDH (as loading control) of washed WT and IKK2ΔPlt platelets. P-selectin (G) and activated GPIIb/IIIa (H) median fluorescence intensity (MFI) of CD41+ events in diluted whole blood stimulated with PAR4-AP (upper panels) or CVX (lower panels) (n = 12). (I) Representative light-transmission aggregometry curves of washed WT and IKK2ΔPlt platelets, stimulated with 165 mU/mL thrombin (upper panel) or 18 µg/mL collagen (lower panel). (J) Tail tips (5 mm) of WT and IKK2ΔPlt mice were cut, and time until cessation of bleeding was recorded (n = 12-18). The horizontal line represents the median. (K) Mesenteric arteries of WT and IKK2ΔPlt mice were treated topically with 1.0 M FeCl3, and time until vessel occlusion was determined by intravital microscopy. Each symbol represents a mesenteric arteriole (n = 12-13). The horizontal line represents the median. (L) Recruitment of peritoneal macrophages after intraperitoneal injection of 4% thioglycollate. After 3 days, cells were harvested and analyzed by flow cytometry (n = 8-10). Dashed line represents basal count of untreated WT mice. **P ≤ .01, ****P ≤ .0001. ns, not significant.
Next, we investigated potential effects of IKK2 deletion on platelet function. Degranulation was evaluated by surface expression of P-selectin and release of ATP after stimulation of the major platelet activation pathways with proteinase-activated receptor-4 agonist peptide (PAR4-AP), convulxin (CVX) or ADP. Platelet-specific deletion of IKK2 did not affect P-selectin surface expression, CXCL4 release (α-granules) (Figure 1G; supplemental Figure 1A,C), or ATP release (dense granules) (supplemental Figure 1D) at any agonist concentration, pointing toward unaffected degranulation in IKK2ΔPlt platelets. Furthermore, we could not detect any difference in glycoprotein (GP) IIb/IIIa activation, which is needed to sustain tight platelet-platelet interactions upon activation (Figure 1H; supplemental Figure 1B). In vitro aggregation of washed platelets revealed no impairment of IKK2ΔPlt compared with WT platelets (Figure 1I; supplemental Figure 1E-F). In concordance with these in vitro data, we observed no alterations in bleeding time, again indicating unaffected hemostatic functions (Figure 1J). Moreover, we tested platelet aggregation in vivo, as triggered by topical application of FeCl3 onto mesenteric arterioles. In line with the tail-bleeding results, thrombus formation remained unaltered in IKK2ΔPlt mice (Figure 1K). Because platelets exert hemostatic, as well as immunomodulatory, functions, we analyzed platelet-assisted macrophage extravasation upon sterile peritonitis.8 Once more, we could not detect any differences between IKK2ΔPlt mice and littermate controls (Figure 1L). Finally, we examined platelet structure, estimated the amount of young reticulated platelets by measuring RNA content, compared platelet microvesicle release, and evaluated megakaryocyte maturation and differentiation, none of which showed significant differences between WT and IKK2-knockout platelets (supplemental Figure 2).
In principle, some redundancy between IKK2 and related kinases might explain the lack of a clear phenotype after deletion of IKK2. However, the fact that a full knockout results in embryonic lethality9 and multiple observations of functional alterations after conditional deletion of IKK2 in specific cell types6,10-12 argue against such a possibility. In line with that, sequence alignment of IKK2 with the IKK-complex components IKK1 and NEMO and the 2 related kinases IKK-ε and TBK1 shows only moderate similarity (supplemental Figure 3).
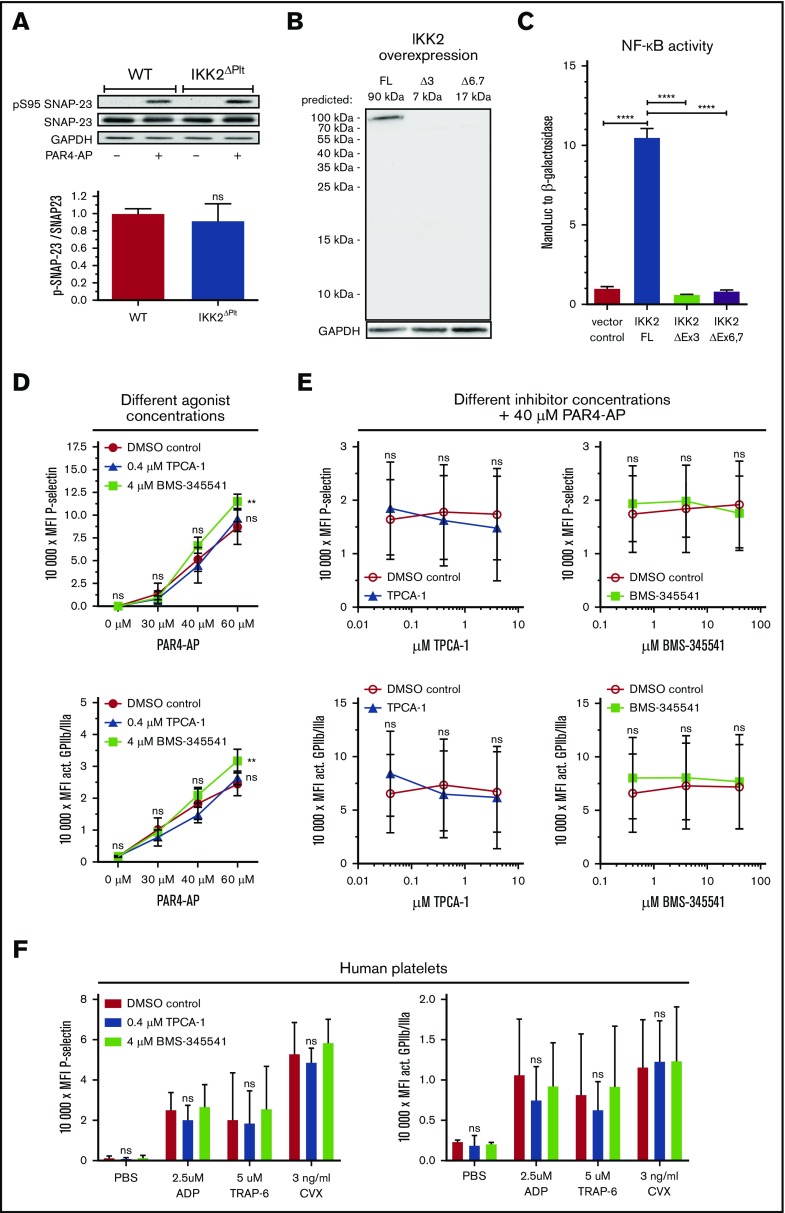
Nevertheless, our observations differ from previous findings; deletion of IKK2 in platelets via excision of exons 6/7 on the same genetic background caused severe degranulation impairment and prolonged bleeding.1 It was postulated that absence of functional IKK2 interferes with phosphorylation of SNAP-23 at S95, which was claimed to be essential for fusion of granules with the cell membrane as a prerequisite for full platelet activation. However, in our study, stimulation of platelets resulted in the same degree of SNAP-23 phosphorylation in WT and IKK2ΔPlt platelets (Figure 2A). Similarly, conflicting results were reported for mast cells; an earlier publication claimed IKK2-mediated phosphorylation of SNAP-23,13 whereas a later study postulated that SNAP-23 phosphorylation and mast cell degranulation were, in fact, independent of IKK2.14
Figure 2.
SNAP-23 phosphorylation is not altered by IKK2 deletion and platelet activation is not reduced by pharmacological inhibition of IKK2. (A) Phosphorylation of S95 of SNAP-23 after stimulation of platelets with 100 µM PAR4-AP for 3 minutes and relative quantification of S95 of stimulated platelets (n = 5-6). (B) Overexpression of FLAG-tagged WT IKK2 or FLAG-tagged constructs mimicking exon 3 or exons 6 and 7 deletion in HeLa cells. Predicted band sizes for FLAG-tagged full-length (FL) IKK2: 90 kilodalton (kDa); for construct lacking exon 3 (Δ3): 7 kDa; without exons 6/7 (Δ6,7):17 kDa. (C) NF-κB reporter gene assay after transfection of the expression constructs. NanoLuc activity normalized to constitutively expressed β-galactosidase activity (n = 5-6). (D) Washed murine platelets were incubated with IKK inhibitors or respective solvent controls for 30 minutes and activated with the indicated concentrations of PAR4-AP. Median fluorescence intensity (MFI) of P-selectin (upper panel) and activated GPIIb/IIIa (lower panel) was analyzed (n = 3). (E) Washed murine platelets were incubated with the indicated concentrations of IKK inhibitors or respective dimethyl sulfoxide (DMSO) controls for 30 minutes and activated with 40 µM PAR4-AP. MFI of P-selectin (upper panels) and activated GPIIb/IIIa (lower panels) was analyzed (n = 6). (F) Human platelet-rich plasma was incubated with IKK inhibitors or respective DMSO control for 30 minutes and activated with 2.5 µM ADP, 5 µM thrombin receptor activator peptide 6 (TRAP-6), or 3 ng/mL CVX. MFI of P-selectin (left panel) and activated GPIIb/IIIa (right panel) was analyzed. n = 3. **P ≤ .01, ****P ≤ .0001.
A potentially relevant difference between our study and the one that postulated an essential role for IKK2 in platelet activation is the mechanism of IKK2 deletion. Karim et al1 used deletion of exons 6 and 7, which contains the activation loop of IKK210 (Figure 1A), whereas our mouse strain deletes exon 3, coding for the active center of the enzyme.6 Therefore, a putative remnant protein of the exon 6/7 deletion would still possess the ATP binding site of exon 3, which could potentially affect platelet signaling in an unexpected manner. To test this possibility, we generated expression constructs of murine IKK2 mimicking deletion of exon 3 or exons 6 and 7, both with an N-terminal FLAG tag. However, we could only detect FLAG-tagged full-length IKK2 protein but no protein expressed from the deletion constructs (Figure 2B). Additionally, we performed a reporter gene assay to investigate transcriptional activity of NF-κB after expressing the different deletion mimics. Again, only full-length IKK2 was functionally active and induced strong NF-κB activity, whereas both deletion constructs were close to the negative control (Figure 2C). There was a slight difference between the 2 distinct deletion constructs; however, the low NF-κB activity in the range of the negative control would imply that both deletion strategies eliminate IKK2 function. Nevertheless, it cannot be excluded that, in vivo, some residual truncated protein can exert an inhibitory function. Interestingly, deletion of exon 7 alone has been reported to result in a remnant protein that integrates into the IKK complex,11 in line with such a possibility.
In addition to the IKK2-knockout strategy, we tested specific pharmacological inhibition of IKK2 using the compounds BMS-345541 and TPCA-1 at concentrations that inhibit PAR-mediated NF-κB activation in human platelets and basal NF-κB activity in HeLa cells (supplemental Figure 4). Platelets preincubated with BMS-345541 or TPCA-1 were activated with different concentrations of PAR4-AP; however, neither mild nor strong stimulation showed any inhibitory effect of these compounds on platelet degranulation or activation. Interestingly, BMS-345541 even increased platelet activation when high agonist concentrations were used (Figure 2D). Following this, we treated platelets with increasing concentrations of BMS-345541 or TPCA-1 before moderate stimulation with PAR4-AP. However, again we did not observe any effect of IKK2 inhibitors on platelet degranulation or activation (Figure 2E). This was also observed for human platelets treated with IKK2 inhibitors, which did not reduce P-selectin surface expression or GPIIb/IIIa activation after stimulation with 3 different agonists (Figure 2F). These findings differ from those of Karim et al,1 who found an inhibitory effect of these compounds on degranulation of platelets and bleeding times in mice. We have no explanation for this discrepancy. An ineffectiveness of the compounds can be excluded, as we could demonstrate the functionality of our inhibitors by in vitro assays showing that even low basal NF-κB activity in the absence of an activator can be clearly reduced by the compounds at the concentrations that we applied (supplemental Figure 4).
Taken together, we did not observe any effect of exon 3–mediated IKK2 deletion on platelet aggregation, bleeding time, in vivo thrombus formation, dense and α-granule degranulation, or SNAP-23 phosphorylation. Furthermore, pharmacological inhibition of human and murine IKK2 did not result in any degranulation or activation deficiencies. Thus, we provide substantial evidence to question an essential role for IKK2 in platelet degranulation. Nevertheless, this does not rule out that active IKK2, present under inflammatory conditions, has an influence on platelet function.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Hannah Paar, Susanne Humpeler, and Bhuma Wysoudil for excellent technical support.
This work was supported by the Austrian Science Fund (project SFB-F54 P07) (J.A.S.).
Footnotes
Data sharing requests should be sent to Johannes A. Schmid (johannes.schmid@meduniwien.ac.at) or Alice Assinger (alice.assinger@meduniwien.ac.at).
Authorship
Contribution: M.S. designed and performed experiments, analyzed data, and wrote the manuscript; S.B., B.M., M.M., B.H., W.C.S., J.B.K.-P., M.H., and K.S. performed experiments; M.I. provided the pS95 SNAP-23 antibody and scientific advice; and A.A. and J.A.S. supervised all research, contributed to the design and interpretation of experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Johannes A. Schmid, Medical University of Vienna, Institute of Vascular Biology and Thrombosis Research, Schwarzspanierstr 17, 1090 Vienna, Austria; e-mail: johannes.schmid@meduniwien.ac.at; and Alice Assinger, Medical University of Vienna, Institute of Vascular Biology and Thrombosis Research, Schwarzspanierstr 17, 1090 Vienna, Austria; e-mail: alice.assinger@meduniwien.ac.at.
References
- 1.Karim ZA, Zhang J, Banerjee M, et al. IκB kinase phosphorylation of SNAP-23 controls platelet secretion. Blood. 2013;121(22):4567-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witt A, Vucic D. Diverse ubiquitin linkages regulate RIP kinases-mediated inflammatory and cell death signaling. Cell Death Differ. 2017;24(7):1160-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mussbacher M, Salzmann M, Brostjan C, et al. Cell type-specific roles of NF-κB linking inflammation and thrombosis. Front Immunol. 2019;10:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malaver E, Romaniuk MA, D’Atri LP, et al. NF-kappaB inhibitors impair platelet activation responses. J Thromb Haemost. 2009;7(8):1333-1343. [DOI] [PubMed] [Google Scholar]
- 5.Gambaryan S, Kobsar A, Rukoyatkina N, et al. Thrombin and collagen induce a feedback inhibitory signaling pathway in platelets involving dissociation of the catalytic subunit of protein kinase A from an NFkappaB-IkappaB complex. J Biol Chem. 2010;285(24):18352-18363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li ZW, Omori SA, Labuda T, Karin M, Rickert RC. IKK beta is required for peripheral B cell survival and proliferation. J Immunol. 2003;170(9):4630-4637. [DOI] [PubMed] [Google Scholar]
- 7.Tiedt R, Schomber T, Hao-Shen H, Skoda RC. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109(4):1503-1506. [DOI] [PubMed] [Google Scholar]
- 8.Badrnya S, Schrottmaier WC, Kral JB, et al. Platelets mediate oxidized low-density lipoprotein-induced monocyte extravasation and foam cell formation. Arterioscler Thromb Vasc Biol. 2014;34(3):571-580. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Van Antwerp D, Mercurio F, Lee KF, Verma IM. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999;284(5412):321-325. [DOI] [PubMed] [Google Scholar]
- 10.Pasparakis M, Courtois G, Hafner M, et al. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature. 2002;417(6891):861-866. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt-Supprian M, Courtois G, Tian J, et al. Mature T cells depend on signaling through the IKK complex. Immunity. 2003;19(3):377-389. [DOI] [PubMed] [Google Scholar]
- 12.Mourkioti F, Kratsios P, Luedde T, et al. Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. J Clin Invest. 2006;116(11):2945-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki K, Verma IM. Phosphorylation of SNAP-23 by IkappaB kinase 2 regulates mast cell degranulation. Cell. 2008;134(3):485-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peschke K, Weitzmann A, Heger K, et al. IκB kinase 2 is essential for IgE-induced mast cell de novo cytokine production but not for degranulation. Cell Reports. 2014;8(5):1300-1307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.