Abstract
Background
Studies on the hypertensive effect of long-term air pollution exposure were inconclusive and showed scarce evidence from rural areas in developing countries. In this context, we examined the associations of air pollution exposure with hypertension and blood pressure, and their effect modifiers in rural Chinese adults.
Methods
We studied 39,259 participants from a cohort established in five rural regions of central China. Individual exposures to PM2.5 and PM10 (particulate matter with an aerodynamic diameter less than or equal to 2.5 μm and 10 μm) and nitrogen dioxide (NO2) was evaluated using satellite-based spatiotemporal models. Mixed-effect regression models were applied to examine the associations of long-term exposure to air pollution with hypertension and four blood pressure component measurements, including systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP) and pulse pressure (PP). Several potential effect modifiers related to demographic and behavioral factors were also examined.
Results
The results showed that for each 1 μg/m3 increase in PM2.5, PM10 and NO2, the adjusted odds ratio of hypertension was 1.029 (95%CI: 1.001,1.057), 1.015 (95%CI: 1.001, 1.029) and 1.069 (95%CI: 1.038, 1.100), respectively. These three air pollutants were also associated with increased SBP (except for PM10), DBP and MAP. The hypertensive effects of air pollution were more pronounced among males, smokers, drinkers, individuals with a high-fat diet, and those with high-level physical activity.
Conclusion
Long-term exposure to PM2.5, PM10 and NO2 was associated with increased blood pressure and hypertension in rural Chinese adults, and the associations were modified by several behavioral factors.
Keywords: Air pollution, Blood pressure, Hypertension, Rural China, Effect modification
Graphical abstract
Highlights
-
•
The study aimed at rural population in developing countries.
-
•
The effect estimates of NO2 was observed to be larger than PM2.5 and PM10.
-
•
Pulse pressure was negatively associated with air pollution exposure.
-
•
Several behavioral factors can modify the hypertensive effect of air pollution.
1. Introduction
High systolic blood pressure (SBP) was ranked as the leading risk factor for mortality and disability globally (Global Burden Disease Risk Factor Collaborators, 2018). Particularly in China, high SBP was the top risk factor for mortality and accounted for 2.54 million deaths in 2017, of which 95.7% were due to cardiovascular diseases (Zhou et al., 2019). Moreover, particulate matter pollution was the fourth leading contributor to both deaths and DALYs in China in 2017 (Zhou et al., 2019). The associations of air pollution exposure with increased cardiovascular morbidity and mortality have been revealed by a large amount of epidemiological evidence, and changes in blood pressure were proposed to be a pathway to explain the association at the functional level.
Accumulated epidemiological studies also evaluated associations of long-term exposure to air pollution with blood pressure and hypertension (Xie et al., 2018; Yang et al. 2018a, 2019a, 2019b; Zhang et al., 2019). However, most of the research were primarily concerned with urban areas and developed countries, and the results remained inconsistent. A recent review focusing on vulnerable populations for adverse cardiovascular outcomes from air pollution indicated that more researches in rural settings and developing countries were warranted, in particular because of high concentration of air pollutants and large burden of cardiovascular disease in such areas (Tibuakuu et al., 2018). Rural China is faced with worsening air pollution with the rapid urbanization and economic progress. In addition, the hypertension prevalence among rural Chines adults has shown a persistent upward trend since 1993, and disparity between urban and rural region in hypertension prevalence has gradually narrowed (Li et al., 2017). As rural residents account for more than half of the Chinese population, it could be beneficial for hypertension prevention and public health improvement to explore the long-term effects of air pollution exposure on blood pressure and prevalent hypertension among rural Chinese.
The adverse effects of air pollution were not the same for all individuals (Tibuakuu et al., 2018). It is therefore imperative to identify effect modifiers of the associations between air pollution exposure and health outcomes, and to understand the factors that increase vulnerability to the health effects of air pollution. Studies show inconsistency in examining effect modifiers in the relationships between long-term air pollution exposure and cardiovascular outcomes. For example, some studies observed higher risks for cardiovascular outcomes for smokers (Pope et al., 2009; Turner et al., 2017; Xie et al., 2018), whereas some observed higher risks for nonsmokers (Lin et al., 2017a; Pope et al., 2002; Zhang et al., 2018), and some did not find such effect modification by smoking status (Liu et al., 2017).
In this context, we investigated the associations of three-year exposure to air pollutants (PM2.5, PM10 and NO2) with hypertension and four blood pressure components, including SBP, diastolic blood pressure (DBP), mean arterial pressure (MAP) and pulse pressure (PP), based on the Henan Rural Cohort Study in central China.
2. Methods
2.1. Study population
The study population were from a Cohort Study carried out in Henan Province, China in 2015. This province had a relatively high density of population and severe air pollution. The study design and inclusion criteria of the Henan Rural Cohort Study have been described in our previous publications (Tian et al. 2018a, 2018b). In brief, a multi-stage stratified cluster sampling method was employed to recruit participants from the general adult residents. First, we selected one rural county from each southern, central, northern, eastern, and western region of Henan Province using a simple cluster sampling approach. They are Tongxu county, Yima county, Suiping county, Xinxiang county and Yuzhou county. Second, rural communities (referred to as a “township”) between one and three in each county were selected according to the stability of population, local medical conditions and the compliance of the residents. Third, we investigated all eligible candidates in each rural village (the administrative unit) of the selected townships. Overall, we sent out a total of 41,893 invitations to those who met the inclusion criteria. However, 2634 individuals did not response to the baseline survey, resulting 39, 259 participants finally included (response rate: 93.7%) (Liu et al., 2018). All the participants signed the informed consents before interviews and data collection.
2.2. Assessment of hypertension and blood pressure
Hypertension cases were defined as systolic blood pressure of 140 mmHg or higher or diastolic blood pressure of 90 mmHg or higher, or either self-reported physician-diagnosed hypertension or current intake of any antihypertensive medication, or both (Liu, 2011). We measured blood pressure for each participant using an electronic sphygmomanometer (HEM-770A Fuzzy, Omron, Kyoto, Japan), according to the American Heart Association's standardized protocol (Perloff et al., 1993). Participants were asked not to smoke, drink alcohol, have coffee or tea, and to abstain from exercising for at least 30 min before measuring blood pressure. The mean of three consecutive measurements was calculated as the blood pressure measurement of each participant. MAP was calculated by DBP +1/3 (SBP − DBP) and PP was calculated as SBP minus DBP (Darne et al., 1989).
2.3. Assessment of air pollution exposure
We estimated daily concentration of PM2.5, PM10 and NO2 at a spatial resolution of 0.1° (approximately equal to 10 km) with machine learning algorithm using ground-monitored air pollution data, satellite-retrieved aerosol optical depth (AOD) and the information on other spatial and temporal predictors (urban cover, forest cover, weather data, and calendar month, etc.). The detailed description of the estimation has been previously published (Chen et al. 2018a, 2018b). The ability of prediction was tested by a ten-fold cross-validation, and the results showed that R2 for daily PM2.5, PM10 and NO2 prediction was 83%, 78% and 64%, respectively. Each participant's daily exposure to air pollutants during the three years prior to the baseline survey was estimated according to the geocoded address of their natural village (latitude and longitude) through AutoNavi Map ([Anonymous], 2019). Then the estimated daily concentrations were aggregated into three-year average concentrations that was used in the analyses.
2.4. Assessment of covariates
All the variables in the current analyses were collected through face-to-face interviews by trained investigators. Sociodemographic variables included age, sex and home address. Socioeconomic variables were educational attainment, marital status and average monthly income. Educational attainment was categorized into three groups: low (illiteracy or primary school), medium (junior school), and high (senior high school or above). Behavioral variables included smoking status, alcohol drinking, high fat diet, more vegetables and fruits intake and physical activity. Smoking was defined as a person who smoked more than one cigarette per day in the past six months. Drinking was defined as consumption of alcoholic drinks for twelve or more times in the past one year, whether spirits, beer, wine, or other forms of alcohol beverage. Smoking status was grouped into “never smoked” and “ever smoked” (those who were former or current smokers). Alcohol drinking was also divided into “never drunk” and “ever drunk”. A high fat diet referred to consumption of 75 g or more meat from livestock and poultry per day, and “more vegetables and fruits intake” referred to average intake of 500 g or more vegetables and fruits per day, on recommendations of Chinese dietary guidelines (Wang et al., 2016). The level of physical activity was categorized according to the international physical activity questionnaire (IPAQ) (Lee et al., 2011). The diagnosis of type 2 diabetes was based on the American Diabetes Association recommendations (American Diabetes Association, 2013).
2.5. Statistical analysis
Participants with missing blood pressure measurements, hypertension status, or other key covariate data (n = 52) were excluded from the analyses, resulting a final study population of 39,207 rural Chinese adults for the current analyses.
We employed generalized linear mixed models with a random effect term for survey sites to examine associations of air pollutants with blood pressure measurements and hypertension prevalence. The effect estimates for four blood pressure component measurements were calculated from linear regression and reported as changes in mmHg for each 1 μg/m3 increase in three-year average concentration. For hypertension, the effect estimates were reported as odds ratios (ORs) (per 1 μg/m3 increase) and 95%CI. We controlled for potential confounders based on the previous literature on air pollution and blood pressure. We initiated the model development with a crude model (no adjustment), and then added a range of covariates into regression models based on previous literature. All the models were adjusted for sex, age, educational level, marital status, monthly individual income, smoking, drinking, high-fat diet, more vegetables and fruits intake, physical activity, BMI, family history of hypertension, type 2 diabetes and survey sites. Among these covariates, survey site was included as random effect term and the remaining cofounders were incorporated as fixed effect terms. This method has also been widely applied in previous studies exploring the long-term effect of air pollution on health (Lin et al., 2017b; Yang et al., 2018b; Zhang et al., 2019). In view of the high co-linearity among three air pollutants, only single-pollutant models were applied in our analysis (Spearman correlation coefficients were 0.95 for PM2.5 and PM10, 0.90 for PM2.5 and NO2, 0.96 for PM10 and NO2. See Table S1 in supplementary material). In addition, we performed several stratified analyses to test potential effect modifications of sex, age, smoking, drinking, high-fat diet, more vegetables and fruits intake and physical activity by including an interaction term. (Liu et al., 2017).
We conducted several sensitivity analyses. First, we excluded participants taking anti-hypertensive medicines to examine the associations. Second, we restricted study participants to those who were free of obesity and type 2 diabetes to reduce the influence from these factors that are potentially on the causal pathway between air pollution and hypertension.
All analyses were completed using R version 3.5.0.
3. Results
3.1. Descriptive statistics
The location of five survey sites in the Henan Rural Cohort Study was displayed in Fig. S1 in supplementary material. The basic characteristics of all participants are shown in Table 1, and characteristics of participants by study sites were shown in Table S1. We excluded 34 participants because of the missing information on blood pressure measurement or hypertension status, and a total of 39,207 participants were finally included. There were 12,823 hypertension cases identified, with the prevalence of 32.7%. Among the hypertension cases, 7879 (61.4%) were self-reported and 4955 (38.6%) were diagnosed in the baseline survey. Moreover, 6319 hypertensive participants (49.3% of the whole hypertensive participants) had taken anti-hypertensive medication during the two weeks prior to the survey.
Table 1.
Baseline characteristics of study participants.
| Characteristicsa | Non-hypertension (n = 26,384) | Hypertension (n = 12,823) | Total (n = 39,207) | P value |
|---|---|---|---|---|
| Age, years | 53.27 ± 12.43 | 60.39 ± 10.09 | 55.6 ± 12.18 | <0.001 |
| Body mass index, kg/m3 | 24.25 ± 3.36 | 26.04 ± 3.67 | 24.83 ± 3.56 | <0.001 |
| Systolic blood pressure, mmHg | 115.82 ± 11.91 | 146.81 ± 16.86 | 125.95 ± 19.99 | <0.001 |
| Diastolic blood pressure, mmHg | 72.58 ± 8.14 | 88.22 ± 10.67 | 77.70 ± 11.64 | <0.001 |
| Mean arterial pressure, mmHg | 87.00 ± 8.64 | 107.75 ± 11.12 | 93.78 ± 13.62 | <0.001 |
| Pulse pressure, mmHg | 43.23 ± 8.68 | 58.59 ± 14.50 | 48.26 ± 13.09 | <0.001 |
| 3-year average exposure | ||||
| PM2.5, μg/m3 | 73.22 ± 2.61 | 73.86 ± 2.43 | 73.42 ± 2.57 | |
| PM10, μg/m3 | 131.95 ± 5.93 | 133.52 ± 5.42 | 132.46 ± 5.81 | |
| NO2, μg/m3 | 39.59 ± 3.66 | 40.45 ± 3.43 | 39.87 ± 3.60 | |
| Sex | 0.170 | |||
| male | 10,348 (39.2) | 51,272 (39.9) | 15,470 (39.5) | |
| female | 16,036 (60.8) | 7701 (60.1) | 23,737 (60.5) | |
| Educational level | <0.001 | |||
| Low | 10,722 (40.6) | 6826 (53.2) | 17,548 (44.8) | |
| Medium | 11,210 (42.5) | 4413 (34.4) | 15,623 (39.8) | |
| High | 4452 (16.9) | 1584 (12.3) | 6036 (15.4) | |
| Marital status | <0.001 | |||
| Married/Cohabitating | 24,023 (91.1) | 11,173 (87.1) | 35,196 (89.8) | |
| Widowed/Single/Divorced/Separation | 2361 (8.9) | 1650 (12.9) | 4011 (10.2) | |
| Income | <0.001 | |||
| ≤500 yuan | 8924 (33.8) | 5073 (39.6) | 13,997 (35.7) | |
| 500–1000 yuan | 8667 (32.8) | 4221 (32.9) | 12,888 (32.9) | |
| ≥1000 yuan | 8793 (33.3) | 3529 (27.5) | 12,322 (31.4) | |
| Smoking | 0.131 | |||
| Never | 19,145 (72.6) | 9395 (73.2) | 28,540 (72.8) | |
| Ever | 7239 (27.4) | 3428 (26.7) | 10,667 (27.2) | |
| Drinking | 0.010 | |||
| Never | 20,496 (77.7) | 9812 (76.5) | 30,308 (77.3) | |
| Ever | 5888 (22.3) | 3011 (23.5) | 8899 (22.7) | |
| Physical activity | <0.001 | |||
| Low | 7856 (29.8) | 4832 (37.7) | 12,688 (32.4) | |
| Moderate | 10,409 (39.5) | 4385 (34.2) | 14,794 (37.7) | |
| High | 8119 (30.8) | 3606 (28.1) | 11,725 (29.9) | |
| High fat diet | 5461 (20.7) | 2012 (15.7) | 7473 (19.1) | <0.001 |
| More vegetables and fruits intake | 11,748 (44.5) | 4622 (36.0) | 16,370 (41.8) | <0.001 |
| Family history of hypertension | 4055 (15.4) | 3532 (27.5) | 7587 (80.6) | <0.001 |
| Type 2 diabetes | 1808 (6.9) | 1892 (14.8) | 3700 (9.5) | <0.001 |
For continuous variables, numbers represent the mean ± standard deviation and for categorical variables, numbers represent count (percentage).
Table 2 presents the average concentration levels of three air pollutants. Mean (SD) concentrations were 73.4 (2.6) μg/m3 for PM2.5, 132.5 (5.8) μg/m3 for PM10 and 39.9 (3.6) μg/m3 for NO2. The annual mean levels of exposure to PM2.5 and PM10 for the all participants far exceeded the standards of World Health Organization air quality guidelines and the Class two limit values of Chinese ambient air quality guideline. We observed high correlations between three air pollutants (Table S1).
Table 2.
Three-year average exposure levels for the all participants.
| Pollutants | Mean ± SD | Median | Min | Max | IQR |
|---|---|---|---|---|---|
| PM2.5 (μg/m3) | 73.4 ± 2.6 | 73.3 | 68.0 | 84.9 | 4.5 |
| PM10 (μg/m3) | 132.5 ± 5.8 | 133.1 | 122.4 | 148.8 | 10.9 |
| NO2 (μg/m3) | 39.9 ± 3.6 | 40.3 | 31.0 | 49.8 | 6.4 |
Abbreviations: PM2.5, particulate matter with aerodynamic diameter equal to or less than 2.5 μm; PM10, particulate matter with aerodynamic diameter equal to or less than10μm; NO2, nitrogen dioxide; SD, standard deviation; Min, minimum; Max, maximum; IQR, interquartile range.
3.2. Associations of air pollution with hypertension and blood pressure
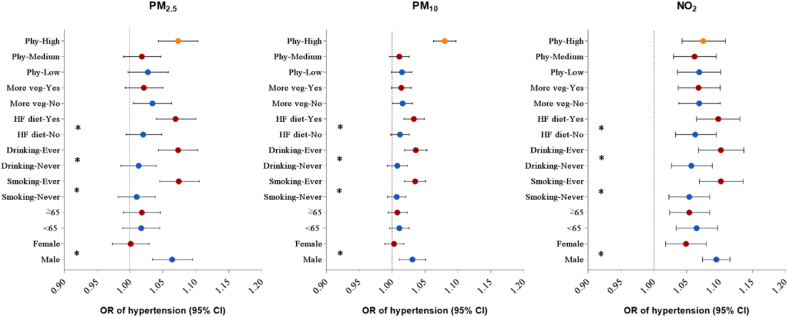
We observed positive associations of hypertension with all three pollutants (Fig. 2). In crude models, each 1 μg/m3 increase in PM2.5, PM10 and NO2 were associated with an 10.2% (OR: 1.102, 95%CI: 1.093,1.111), 4.8% (OR: 1.048, 95%CI: 1.044, 1.052) and 6.8% (OR: 1.068, 95%CI: 1.062, 1.075) increased odds of hypertension prevalence, respectively. After fully adjusting for potential confounders, each 1 μg/m3 increase in PM2.5, PM10 and NO2 was associated with 2.9% (OR: 1.029, 95% CI: 1.001,1.057), 1.5% (OR: 1.015, 95% CI: 1.033, 1.042) and 6.9% (OR: 1.069, 95% CI:1.038, 1.100) higher odds of hypertension prevalence, respectively.
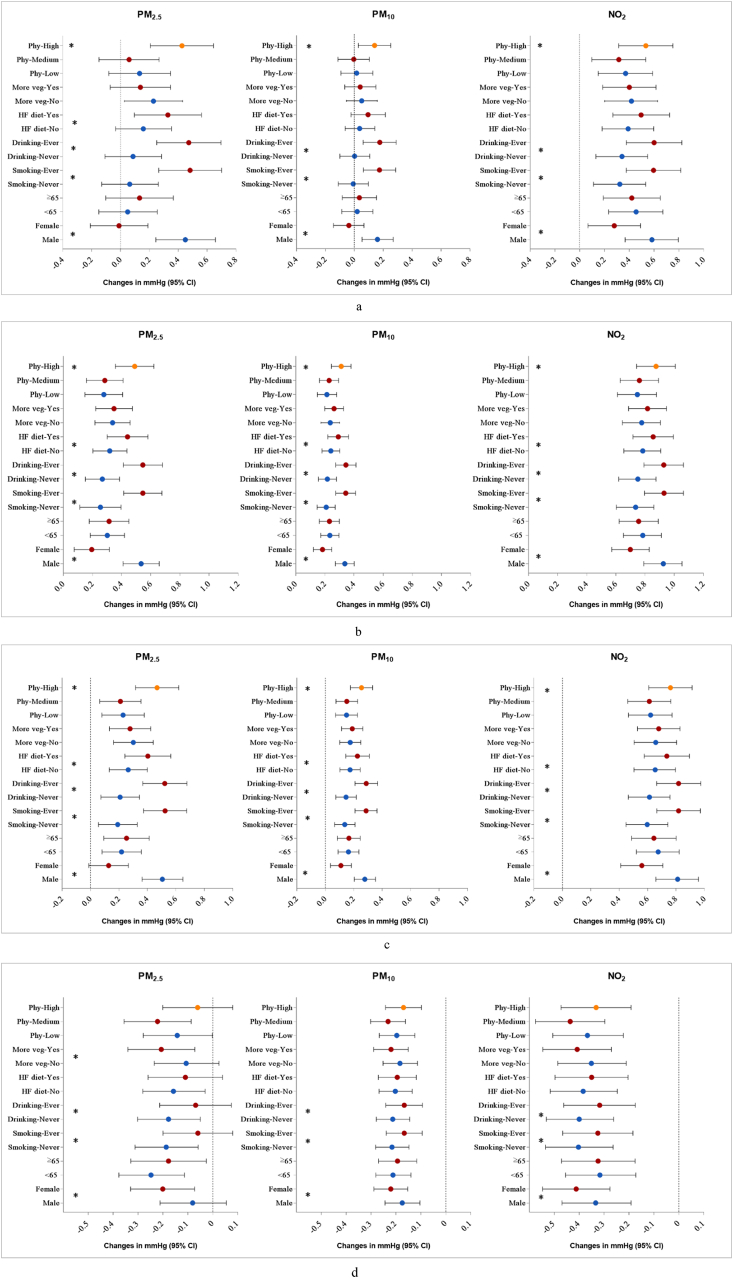
Fig. 2.
Associations between 1 μg/m3 increase in air pollutants and changes (95% CIs) in blood pressure measurements, stratified by potential modifiers. Abbreviations: HF diet, high fat diet; More veg: More vegetables and fruits intake; Phy: levels of physical activity. *P for interaction <0.05.
Table 3 summarizes the associations between air pollutants and four blood pressure measurements. In crude models, all three air pollutants were positively associated with systolic blood pressure, diastolic blood pressure, mean arterial pressure and pulse pressure. However, after adjusting for potential confounders, the association between PM10 and systolic blood pressure was not observed and all three air pollutants were negatively associated with pulse pressure. The associations of exposure to air pollutants with blood pressure components appeared to be stronger for NO2. For different blood pressure measurements, the effect of three air pollutants on diastolic blood pressure was greater than that on systolic blood pressure. In adjusted models, we found that SBP increased by 0.190 mmHg (95% CI: 0.001,0.382) and 0.409 mmHg (95% CI: 0.199, 0.614) associated with per 1 μg/m3 increment in PM2.5 and NO2, respectively, while each 1 μg/m3 increment in PM2.5, PM10 and NO2 was associated with 0.342 mmHg (95% CI: 0.228, 0.457), 0.249 mmHg (95% CI: 0.188, 0.311) and 0.797 mmHg (95% CI: 0.667, 0.920) increase in DBP, respectively.
Table 3.
Adjusted associations of long-term exposure to air pollutants with hypertension and blood pressure, presented per 1 μg/m3 of exposure concentration.
| Pollutants (μg/m3) | Hypertension, OR (95%CI) a | Changes in blood pressure, mmHg (95%CI) a |
|||
|---|---|---|---|---|---|
| SBP | DBP | MAP | PP | ||
| PM2.5 | 1.029 (1.001, 1.057) | 0.342 (0.228, 0.457) | 0.342 (0.228, 0.457) | 0.291 (0.159, 0.425) | −0.149 (-0.240,0.001) |
| PM10 | 1.015 (1.001, 1.029) | 0.249 (0.188, 0.311) | 0.249 (0.188, 0.311) | 0.182 (0.112, 0.253) | −0.201 (−0.266, −0.134) |
| NO2 | 1.069 (1.038, 1.100) | 0.797 (0.667, 0.920) | 0.797 (0.667, 0.920) | 0.667 (0.519, 0.810) | −0.378 (−0.509, −0.242) |
Abbreviations: PM2.5, particle matter with aerodynamic diameter ≤ 2.5 μm; PM10, particle matter with aerodynamic diameter ≤ 10 μm; NO2, nitrogen dioxide; OR, odds ratio; CI, confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure.
Adjusted for sex, age, marital status, education level, income, smoking, alcohol drinking, physical activity, high fat diet, vegetables and fruits intake, family history of hypertension, body mass index, type 2 diabetes.
3.3. Stratified analysis for air pollution and hypertension and blood pressure
In stratification analyses by potential modifiers, we observed stronger associations between air pollution exposure and hypertension in males, ever-smokers, ever-drinkers, and those with a high fat diet (Fig. 1). Similar trends were found for SBP, DBP, MAP and PP as well (Fig. 2). Besides, physical activity was also found to be an effect modifier in the associations of air pollution exposure with SBP, DBP and MAP, with greater effect estimates in those participants who had high-level physical activities.
Fig. 1.
Associations between 1 μg/m3 increase in air pollutants and odds ratio (95%CIs) of hypertension, stratified by potential modifiers. Abbreviations: HF diet, high fat diet; More veg: More vegetables and fruits intake; Phy: levels of physical activity. *P for interaction <0.05.
3.4. Sensitivity analysis
The sensitivity analyses showed that exclusion of the participants taking anti-hypertensive medicine did not change the results substantially. The results also remained robust after excluding the participants with obesity or T2DM (compare Table S5 with Table 3).
4. Discussion
In this large rural population-based study in Central China, we observed positive associations of long-term exposure to air pollution with hypertension prevalence and four blood pressure measurements. Additionally, we found stronger effects of exposure to PM2.5 and NO2 on blood pressure than PM10 exposure. Male participants, participants who ever smoked, participants who ever drunk and participants with a high-fat diet appeared to be more vulnerable to the adverse effects of air pollutants than female participants, never smokers, never drinkers and participants without a high-fat diet.
Previous epidemiology studies have assessed the adverse effect of long-term exposure to air pollution on hypertensive risk (Giorgini et al., 2016; Liang et al., 2014; Yang et al., 2018a). For example, Yang and colleagues included 20, 16 and 19 studies published before May 2017 in their meta analyses to investigate global associations of hypertension with long-term exposure to PM2.5, PM10 and NO2, respectively. They reported that the risk of hypertension increased by 5% per 10 μg/m3 increase in PM2.5, while no significant associations was reported for the PM10 and NO2. This study also showed that each 10 μg/m3 increment in PM2.5, PM10 and NO2 was associated with higher DBP, with increment ranging from 0.47 to 0.86 mmHg. Apart from reviews above and studies included in reviews, several studies published recently also supported positive associations between long-term exposure to ambient air pollution and hypertension risk and elevated blood pressure. In a study of 3.9 million reproductive-age Chinese adults, an OR of 1.01 (95%CI: 1.007, 1.012) for hypertension was related to a 10 μg/m3 increase in PM2.5 above an threshold concentration of 47.9 μg/m3 (Xie et al., 2018). A study of 15,477 Chinese adults showed that a 10 μg/m3 increase in three-year exposure to PM2.5, PM10 and NO2 was significantly associated with ORs of 1.07 (95%CI: 1.02, 1.13), 1.09 (95%CI:1.05,1.12) and 1.19 (95%CI:1.10,1.29) for hypertension, respectively (Yang et al., 2019b). Besides, the strongest associations with ORs of hypertension and increases in blood pressure appeared to be for exposure to NO2 in this study. This result is broadly consistent with several studies in which the adverse effect of NO2 exposure on health was found to be stronger than the effect of particulate matter exposure (Cesaroni et al., 2013; Hart et al., 2011; Hystad et al., 2013; Jerrett et al., 2013). This higher estimate may be attributed to more accurate exposure assessment, exposure assessment that captured both NO2 and PM2.5 influences because of the high correlation between air pollutants (Hystad et al., 2013), or better performance of NO2 exposure assessment models in identifying variability in exposure. Overall, our results were generally accordant with these studies and provided additional evidence in support of the adverse effects of air pollution on hypertension.
The findings that exposure to three air pollutants was associated with increased SBP (not PM10), DBP, and MAP, and decreased PP is consistent with some previous studies but not with all (Auchincloss et al., 2008; Chan et al., 2015; Honda et al., 2018; Zhang et al., 2018). Chan et al. reported a 10 μg/m3 increase in PM2.5 to be associated with increased SBP(1.4 mmHg), MAP (0.8 mmHg) and PP(1.0 mmHg), but not DBP(Chan et al., 2015). Among 4121 older Americans (older than 57 years), Honda et al. reported that each 3.91 μg/m3 increase in one-year exposure concentration of PM2.5 was associated with increased SBP (0.93 mmHg, 95% CI: 0.05, 1.80) and PP (0.89 mmHg, 95% CI: 0.21, 1.58), whereas no associations were observed for DBP and MAP (Honda et al., 2018). Compared with studies mentioned above, the magnitude of blood pressure changed in the present study is slightly larger. The inconsistency among these studies may be due to different study settings, the heterogeneity of the study populations, research methods and adjusted covariates. In addition, we observed that exposure to PM10 and NO2 was significantly associated with decreased PP. This finding is accordant with an animal toxicologic study reporting that concentrated ambient air particles exposure increased SBP, DBP, MAP, and decreased PP in conscious canines (Bartoli et al., 2009). PP was calculated as the difference of SBP and DBP, and a larger magnitude of elevation was observed for DBP than for SBP in our study, which may ultimately relate to decreased PP. Furthermore, Bartoli et al. pointed out that the decreased pulse pressure induced by concentrated ambient particles exposure in the context of preferential increases in DBP may be accounted by increased peripheral vascular resistance—one of the physiologic mechanisms of blood pressure regulation (Bartoli et al., 2009).
Evidence is inconsistent for sex difference in health effects of air pollution exposure. In the current study, stronger associations of long-term exposure to three air pollutants with hypertension and three blood pressure parameters (SBP, DBP and MAP) were observed among males, which is consistent with a previous study (Dong et al., 2013). Such discrepancy may be related to sex-related differences in the localization of air pollutants during deposition in the airways, the deposition rate decided by the size of body, the size of respiratory tract, and ventilatory parameters (Sacks et al., 2011). Specifically, it has been reported that airways are smaller and airway reactivity is slightly greater for females (Yunginger et al., 1992). Besides, males tend to get involved in more frequent and intense outdoor activities than females do, which may bring about greater pulmonary exposure to ambient air pollution (Abbey et al., 1998; Becklake and Kauffmann, 1999).
The stratified analyses also showed stronger effects of air pollution exposure on hypertension and blood pressure for ever-smokers than for never-smokers, and for ever-drinkers than for never-drinkers. Stronger associations between air pollution exposure and cardiovascular health outcomes in smokers have been observed in other epidemiologic studies as well. Pope et al. found that increases in risks of cardiovascular disease mortality associated with PM2.5 elevation were larger for smokers relative to nonsmokers in a prospective cohort study (Pope et al., 2004). Besides, a study carried out among adolescent girls reported that the combined action of cigarette smoking and air pollution exposure (total suspended particulate matter, sulfur dioxide and nitric dioxide) had more detrimental effect on some respiratory and cardiovascular functions than each alone (Turnovska et al., 2007). We consider that these findings are related to the potential biological mechanism of the air pollution-related health effects involving inflammation and oxidative stress. Smoking and drinking can promote responses and even trigger anatomical damage, which may exert additional harmful effect and exacerbate the adverse effects of ambient air pollution as well (Bazzano et al., 2003; O'Keefe et al., 2008; Piano, 2017). However, Pope et al. reported a larger risk of mortality for never smokers in a more recent research (Pope et al., 2019), which is in line with the results of several studies reporting smaller effects of air pollution exposure on blood pressure in smokers (Fuks et al., 2014; Kunzli et al., 2005; Zhang et al., 2018). A possible explanation for this result is that, as some researches indicated, the magnitude of the damaging health effects caused by smoking is relatively higher than that due to PM air pollution exposure (Englert, 2004). Another possible explanation is that smoking and air pollutants may mediate cardiovascular effects through oxidative stress and inflammation by the same pathway and exposure to air pollution might not exert additional effects along the same pathway for the dominant role of smoking in smokers (Zhang et al., 2018). The evidence on modifying effect of smoking on health effect of air pollution have been inconclusive, and well-designed researches are needed to explore the modifying role of such factors.
We also observed greater effects for those participants who had a high-fat diet than for those who did not. One plausible explanation for the result might be that high-fat diet was a key factor related with obesity (Schrauwen and Westerterp, 2000), which was regarded as one of chronic inflammatory conditions that may modulate health effects of air pollution (Sacks et al., 2011). Considerable research showed that obese or overweight individuals are more susceptible to the adverse health effects of particulate matter pollution relative to people of normal weight (Dubowsky et al., 2006; Miller et al., 2007; Zeka et al., 2006). The greater response observed in obese individuals could be attributed to higher dose rates of air pollutants in obese individuals, because individuals who are overweight or obese had an increased in tidal volume and resting minute ventilation, which had been demonstrated in children (Bennett and Zeman, 2004). Overall, the findings from stratified analyses suggest that some unhealthy behavior may aggravate the adverse effects of long-term air pollution exposure on blood pressure.
Additionally, our results indicated a modifying role for physical activity in the effects of air pollution exposure on blood pressure. The high level of physical activity was associated with stronger relationships between air pollutants and SBP, DBP and MAP, compared with low levels. Similar findings were reported in our previously published research (Li et al., 2019), and researches evaluating the effects of air pollution on respiratory outcomes (Strak et al., 2010) and other cardiovascular outcomes (Lin et al., 2017a). Individuals would be exposed to higher concentration of ambient air pollutants due to increased breathing rates and intensity during the outdoor physical activities (Weichenthal et al., 2014). Physically active ones thus suffered from an amplified adverse effect, induced by an increased inhaled quantity and deposition of air pollutants in the body (Strak et al., 2010). With such findings comes concerning about the balance between health gains from physical activity and potential health risks from increased air pollution exposures (Lu et al., 2015).
We acknowledged that our study was subject to several limitations. The primary limitation of this study was its cross-sectional design. We cannot obtain the onset date of hypertension cases, and blood pressure were measured at a single timepoint. Thus, temporal trend of the association and the causal relationships could not be examined. The longitudinal analysis with incidence of hypertension and repeated measurements of blood pressure are needed to confirm our results. Second, recall bias may exist and misclassification might have occurred as all the covariate information was collected using questionnaires. Third, we were not able to apply multi-pollutant models in the analyses because of high correlations among PM2.5, PM10 and NO2, which restricted us to evaluate the effects of multiple pollutants simultaneously. Forth, some important risk factors of elevated blood pressure such as intake of salty food and the food frequency (He et al., 2013; Korhonen et al., 1999; Stamler et al., 1989) were not evaluated in this study due to the lack of data. Another limitation is that our study substantially lacks exposure variability because of the insufficiency of observations with low-level exposure to air pollution. The concentrations of air pollutants in five study regions were much higher compared with some less polluted countries in western Europe or even North America, which suggested that the findings in this study may not be directly applicative to populations exposed to low levels of air pollution, but could provide references for some developing countries with server air pollution.
5. Conclusion
Our findings from rural Chinese population suggested that long-term exposure to PM2.5, PM10 and NO2 is significantly associated with increased risk of hypertension and elevation in blood pressure. And the associations can be modified by some behavioral factors including smoking, drinking, high-fat diet and high level of physical activity. Given the severe air pollution and epidemic status of hypertension in rural China, the positive associations observed in our study indicated an urgent need for policy makers to develop effective prevention and intervention policies, and that more attention should be paid to vulnerable population in rural areas.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
The authors acknowledge the cooperation of all the participants and administrators in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2019.109109.
Sources of Funding
This work was supported by the Foundation of National Key Program of Research and Development of China (Grant No. 2016YFC0900803), National Natural Science Foundation of China of China (Grant No. 81903279), the Bill & Melinda Gates Foundation (Grant No. OOP1148464), and the Natural Science Fund of Hubei Province (Grant No. 2018CFB634). Dr. Guo is supported by Career Development Fellowship APP1107107 from the Australian National Health and Medical Research Council (NHMRC). Dr. S. Li is supported by Early Career Fellowship APP1109193 from the Australian NHMRC.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- [Anonymous] 2019. https://lbs.Amap.Com/api/webservice/guide/api/georegeo
- Abbey D.E., Burchette R.J., Knutsen S.F., McDonnell W.F., Lebowitz M.D., Enright P.L. Long-term particulate and other air pollutants and lung function in nonsmokers. Am. J. Respir. Crit. Care Med. 1998;158:289–298. doi: 10.1164/ajrccm.158.1.9710101. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl. 1):S67–S74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchincloss A.H., Diez Roux A.V., Dvonch J.T., Brown P.L., Barr R.G., Daviglus M.L. Associations between recent exposure to ambient fine particulate matter and blood pressure in the multi-ethnic study of atherosclerosis (mesa) Environ. Health Perspect. 2008;116:486–491. doi: 10.1289/ehp.10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli C.R., Wellenius G.A., Diaz E.A., Lawrence J., Coull B.A., Akiyama I. Mechanisms of inhaled fine particulate air pollution-induced arterial blood pressure changes. Environ. Health Perspect. 2009;117:361–366. doi: 10.1289/ehp.11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzano L.A., He J., Muntner P., Vupputuri S., Whelton P.K. Relationship between cigarette smoking and novel risk factors for cardiovascular disease in the United States. Ann. Intern. Med. 2003;138:891–897. doi: 10.7326/0003-4819-138-11-200306030-00010. [DOI] [PubMed] [Google Scholar]
- Becklake M.R., Kauffmann F. Gender differences in airway behaviour over the human life span. Thorax. 1999;54:1119–1138. doi: 10.1136/thx.54.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett W.D., Zeman K.L. Effect of body size on breathing pattern and fine-particle deposition in children. J. Appl. Physiol. 2004;97:821–826. doi: 10.1152/japplphysiol.01403.2003. [DOI] [PubMed] [Google Scholar]
- Cesaroni G., Badaloni C., Gariazzo C., Stafoggia M., Sozzi R., Davoli M. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in rome. Environ. Health Perspect. 2013;121:324–331. doi: 10.1289/ehp.1205862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S.H., Van Hee V.C., Bergen S., Szpiro A.A., DeRoo L.A., London S.J. Long-term air pollution exposure and blood pressure in the sister study. Environ. Health Perspect. 2015;123:951–958. doi: 10.1289/ehp.1408125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Li S., Knibbs L.D., Hamm N.A.S., Cao W., Li T.T. A machine learning method to estimate pm2.5 concentrations across China with remote sensing, meteorological and land use information. Sci. Total Environ. 2018;636:52–60. doi: 10.1016/j.scitotenv.2018.04.251. [DOI] [PubMed] [Google Scholar]
- Chen G., Wang Y., Li S., Cao W., Ren H., Knibbs L.D. Spatiotemporal patterns of pm10 concentrations over China during 2005-2016: a satellite-based estimation using the random forests approach. Environ. Pollut. 2018;242:605–613. doi: 10.1016/j.envpol.2018.07.012. [DOI] [PubMed] [Google Scholar]
- Darne B., Girerd X., Safar M., Cambien F., Guize L. Pulsatile versus steady component of blood-pressure - a cross-sectional analysis and a prospective analysis on cardiovascular mortality. Hypertension. 1989;13:392–400. doi: 10.1161/01.hyp.13.4.392. [DOI] [PubMed] [Google Scholar]
- Dong G.H., Qian Z.M., Xaverius P.K., Trevathan E., Maalouf S., Parker J. Association between long-term air pollution and increased blood pressure and hypertension in China. Hypertension. 2013;61:578–584. doi: 10.1161/HYPERTENSIONAHA.111.00003. [DOI] [PubMed] [Google Scholar]
- Dubowsky S.D., Suh H., Schwartz J., Coull B.A., Gold D.R. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ. Health Perspect. 2006;114:992–998. doi: 10.1289/ehp.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englert N. Fine particles and human health - a review of epidemiological studies. Toxicol. Lett. 2004;149:235–242. doi: 10.1016/j.toxlet.2003.12.035. [DOI] [PubMed] [Google Scholar]
- Fuks K.B., Weinmayr G., Foraster M., Dratva J., Hampel R., Houthuijs D. Arterial blood pressure and long-term exposure to traffic-related air pollution: an analysis in the european study of cohorts for air pollution effects (escape) Environ. Health Perspect. 2014;122:896–905. doi: 10.1289/ehp.1307725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgini P., Giosia P.D., Grassi D., Rubenfire M., Brook R.D., Ferri C. Air pollution exposure and blood pressure: an updated review of the literature. Curr. Pharmaceut. Des. 2016;22:28–51. doi: 10.2174/1381612822666151109111712. [DOI] [PubMed] [Google Scholar]
- Global Burden Disease Risk Factor Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017. Lancet. 2018;vol. 392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart J.E., Garshick E., Dockery D.W., Smith T.J., Ryan L., Laden F. Long-term ambient multipollutant exposures and mortality. Am. J. Respir. Crit. Care Med. 2011;183:73–78. doi: 10.1164/rccm.200912-1903OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F.J., Li J., Macgregor G.A. Effect of longer term modest salt reduction on blood pressure: cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013;346:f1325. doi: 10.1136/bmj.f1325. [DOI] [PubMed] [Google Scholar]
- Honda T., Pun V.C., Manjourides J., Suh H. Associations of long-term fine particulate matter exposure with prevalent hypertension and increased blood pressure in older americans. Environ. Res. 2018;164:1–8. doi: 10.1016/j.envres.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hystad P., Demers P.A., Johnson K.C., Carpiano R.M., Brauer M. Long-term residential exposure to air pollution and lung cancer risk. Epidemiology. 2013;24:762–772. doi: 10.1097/EDE.0b013e3182949ae7. [DOI] [PubMed] [Google Scholar]
- Jerrett M., Burnett R.T., Beckerman B.S., Turner M.C., Krewski D., Thurston G. Spatial analysis of air pollution and mortality in California. Am. J. Respir. Crit. Care Med. 2013;188:593–599. doi: 10.1164/rccm.201303-0609OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen M.H., Litmanen H., Rauramaa R., Vaisanen S.B., Niskanen L., Uusitupa M. Adherence to the salt restriction diet among people with mildly elevated blood pressure. Eur. J. Clin. Nutr. 1999;53:880–885. doi: 10.1038/sj.ejcn.1600869. [DOI] [PubMed] [Google Scholar]
- Kunzli N., Jerrett M., Mack W.J., Beckerman B., LaBree L., Gilliland F. Ambient air pollution and atherosclerosis in los angeles. Environ. Health Perspect. 2005;113:201–206. doi: 10.1289/ehp.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P.H., Macfarlane D.J., Lam T., Stewart S.M. Validity of the international physical activity questionnaire short form (ipaq-sf): a systematic review. Int. J. Behav. Nutr. Phys. Act. 2011;8:115. doi: 10.1186/1479-5868-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Shi L., Li S., Xu L., Qin W., Wang H. Urban-rural disparities in hypertension prevalence, detection, and medication use among Chinese adults from 1993 to 2011. Int. J. Equity Health. 2017;16 doi: 10.1186/s12939-017-0545-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Chen G., Liu F., Mao S., Liu Y., Hou Y. Associations of long-term exposure to ambient pm1 with hypertension and blood pressure in rural Chinese population: the henan rural cohort study. Environ. Int. 2019;128:95–102. doi: 10.1016/j.envint.2019.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang R., Zhang B., Zhao X., Ruan Y., Lian H., Fan Z. Effect of exposure to pm2.5 on blood pressure: a systematic review and meta-analysis. J. Hypertens. 2014;32:2130–2140. doi: 10.1097/HJH.0000000000000342. discussion 2141. [DOI] [PubMed] [Google Scholar]
- Lin H., Guo Y., Di Q., Zheng Y., Kowal P., Xiao J. Ambient pm2.5 and stroke: effect modifiers and population attributable risk in six low- and middle-income countries. Stroke. 2017;48:1191–1197. doi: 10.1161/STROKEAHA.116.015739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Guo Y., Zheng Y., Di Q., Liu T., Xiao J. Long-term effects of ambient pm2.5 on hypertension and blood pressure and attributable risk among older Chinese adults. Hypertension. 2017;69:806–812. doi: 10.1161/HYPERTENSIONAHA.116.08839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Chen R., Zhao Y., Ma Z., Bi J., Liu Y. Associations between ambient fine particulate air pollution and hypertension: a nationwide cross-sectional study in China. Sci. Total Environ. 2017;584–585:869–874. doi: 10.1016/j.scitotenv.2017.01.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. 2010 Chinese guidelines for the management of hypertension. Chinese J. Hypertension. 2011;19:701–742. [PubMed] [Google Scholar]
- Liu X., Yu S., Mao Z., Li Y., Zhang H., Yang K. Dyslipidemia prevalence, awareness, treatment, control, and risk factors in Chinese rural population: the henan rural cohort study. Lipids Health Dis. 2018;17 doi: 10.1186/s12944-018-0768-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J.J., Liang L.C., Feng Y., Li R.N., Liu Y. Air pollution exposure and physical activity in China: current knowledge, public health implications, and future research needs. Int. J. Environ. Res. Public Health. 2015;12:14887–14897. doi: 10.3390/ijerph121114887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K.A., Siscovick D.S., Sheppard L., Shepherd K., Sullivan J.H., Anderson G.L. Long-term exposure to air pollution and incidence of cardiovascular events in women. N. Engl. J. Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- O'Keefe J.H., Gheewala N.M., O'Keefe J.O. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J. Am. Coll. Cardiol. 2008;51:249–255. doi: 10.1016/j.jacc.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Perloff D., Grim C., Flack J., Frohlich E.D., Hill M., McDonald M. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- Piano M.R. Alcohol's effects on the cardiovascular system. Alcohol Res. Curr. Rev. 2017;38:219–242. [PMC free article] [PubMed] [Google Scholar]
- Pope C.A., Burnett R.T., Thun M.J., Calle E.E., Krewski D., Ito K. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA, J. Am. Med. Assoc. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C.A., 3rd, Burnett R.T., Thurston G.D., Thun M.J., Calle E.E., Krewski D. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Pope C.A., 3rd, Burnett R.T., Krewski D., Jerrett M., Shi Y., Calle E.E. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation. 2009;120:941–948. doi: 10.1161/CIRCULATIONAHA.109.857888. [DOI] [PubMed] [Google Scholar]
- Pope C.A., 3rd, Lefler J.S., Ezzati M., Higbee J.D., Marshall J.D., Kim S.Y. Mortality risk and fine particulate air pollution in a large, representative cohort of u.S. Adults. Environ. Health Perspect. 2019;127:77007. doi: 10.1289/EHP4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks J.D., Stanek L.W., Luben T.J., Johns D.O., Buckley B.J., Brown J.S. Particulate matter-induced health effects: who is susceptible? Environ. Health Perspect. 2011;119:446–454. doi: 10.1289/ehp.1002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauwen P., Westerterp K.R. The role of high-fat diets and physical activity in the regulation of body weight. Br. J. Nutr. 2000;84:417–427. doi: 10.1017/s0007114500001720. [DOI] [PubMed] [Google Scholar]
- Stamler J., Rose G., Stamler R., Elliott P., Dyer A., Marmot M. Intersalt study findings. Public health and medical care implications. Hypertension. 1989;14:570–577. doi: 10.1161/01.hyp.14.5.570. [DOI] [PubMed] [Google Scholar]
- Strak M., Boogaard H., Meliefste K., Oldenwening M., Zuurbier M., Brunekreef B. Respiratory health effects of ultrafine and fine particle exposure in cyclists. Occup. Environ. Med. 2010;67:118–124. doi: 10.1136/oem.2009.046847. [DOI] [PubMed] [Google Scholar]
- Tian Z., Li Y., Li L., Liu X., Shi Y., Yang K. Dose-response relationship between visceral fat index and untreated hypertension in Chinese rural population: the ruraldiab study. J Am Soc Hypertens. 2018;12:448–456. doi: 10.1016/j.jash.2018.03.009. e441. [DOI] [PubMed] [Google Scholar]
- Tian Z., Li Y., Mao Z., Yu S., Wang Y., Liu X. Sex-specific relationship between visceral fat index and dyslipidemia in Chinese rural adults: the henan rural cohort study. Prev. Med. 2018;116:104–111. doi: 10.1016/j.ypmed.2018.09.009. [DOI] [PubMed] [Google Scholar]
- Tibuakuu M., Michos E.D., Navas-Acien A., Jones M.R. Air pollution and cardiovascular disease: a focus on vulnerable populations worldwide. Curr Epidemiol Rep. 2018;5:370–378. doi: 10.1007/s40471-018-0166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M.C., Cohen A., Burnett R.T., Jerrett M., Diver W.R., Gapstur S.M. Interactions between cigarette smoking and ambient pm2.5 for cardiovascular mortality. Environ. Res. 2017;154:304–310. doi: 10.1016/j.envres.2017.01.024. [DOI] [PubMed] [Google Scholar]
- Turnovska T.H., Mandadzhieva S.K., Marinov B.I., Kostianev S.S. Respiratory and cardiovascular functions among smoking and nonsmoking girls from two regions with different air pollution degree. Int. J. Hyg Environ. Health. 2007;210:61–68. doi: 10.1016/j.ijheh.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Wang S.S., Lay S., Yu H.N., Shen S.R. Dietary guidelines for Chinese residents (2016): comments and comparisons. J. Zhejiang Univ. - Sci. B. 2016;17:649–656. doi: 10.1631/jzus.B1600341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichenthal S., Hatzopoulou M., Goldberg M.S. Exposure to traffic-related air pollution during physical activity and acute changes in blood pressure, autonomic and micro-vascular function in women: a cross-over study. Part. Fibre Toxicol. 2014;11 doi: 10.1186/s12989-014-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Wang Y., Yang Y., Xu J., Zhang Y., Tang W. Long-term effects of ambient particulate matter (with an aerodynamic diameter ≤2.5 lm) on hypertension and blood pressure and attributable risk among reproductive-age adults in China. J. Am. Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.008553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Qian Z., Howard S.W., Vaughn M.G., Fan S.J., Liu K.K. Global association between ambient air pollution and blood pressure: a systematic review and meta-analysis. Environ. Pollut. 2018;235:576–588. doi: 10.1016/j.envpol.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Yang B., Qian Z., Li S., Chen G., Bloom M.S., Elliott M. Ambient air pollution in relation to diabetes and glucose-homoeostasis markers in China: a cross-sectional study with findings from the 33 communities Chinese health study. The Lancet Planetary Health. 2018;2:e64–e73. doi: 10.1016/S2542-5196(18)30001-9. [DOI] [PubMed] [Google Scholar]
- Yang B.Y., Guo Y., Bloom M.S., Xiao X., Qian Z.M., Liu E. Ambient pm1 air pollution, blood pressure, and hypertension: insights from the 33 communities Chinese health study. Environ. Res. 2019;170:252–259. doi: 10.1016/j.envres.2018.12.047. [DOI] [PubMed] [Google Scholar]
- Yang B.Y., Guo Y., Markevych I., Qian Z.M., Bloom M.S., Heinrich J. Association of long-term exposure to ambient air pollutants with risk factors for cardiovascular disease in China. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunginger J.W., Reed C.E., Oconnell E.J., Melton L.J., Ofallon W.M., Silverstein M.D. A community-based study of the epidemiology of asthma - incidence rates, 1964-1983. Am. Rev. Respir. Dis. 1992;146:888–894. doi: 10.1164/ajrccm/146.4.888. [DOI] [PubMed] [Google Scholar]
- Zeka A., Sullivan J.R., Vokonas P.S., Sparrow D., Schwartz J. Inflammatory markers and particulate air pollution: characterizing the pathway to disease. Int. J. Epidemiol. 2006;35:1347–1354. doi: 10.1093/ije/dyl132. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Guo C., Lau A.K.H., Chan T.C., Chuang Y.C., Lin C. Long-term exposure to fine particulate matter, blood pressure, and incident hypertension in taiwanese adults. Environ. Health Perspect. 2018;126 doi: 10.1289/EHP2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Dong B., Li S., Chen G., Yang Z., Dong Y. Exposure to ambient particulate matter air pollution, blood pressure and hypertension in children and adolescents: a national cross-sectional study in China. Environ. Int. 2019;128:103–108. doi: 10.1016/j.envint.2019.04.036. [DOI] [PubMed] [Google Scholar]
- Zhou M., Wang H., Zeng X., Yin P., Zhu J., Chen W. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the global burden of disease study 2017. The Lancet. 2019;394(10204):1145–1158. doi: 10.1016/S0140-6736(19)30427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.