Abstract
OBJECTIVE
To determine the optimal treatment for patients with acute type A aortic dissection (ATAAD) and previous cardiac surgery (PCS).
METHODS
545 patients underwent open repair of an ATAAD (July 1996-January 2017), including patients with (n=50) and without PCS (n=495). Data were collected through the University of Michigan Cardiac Surgery Data Warehouse, medical record review, and the National Death Index database.
RESULTS
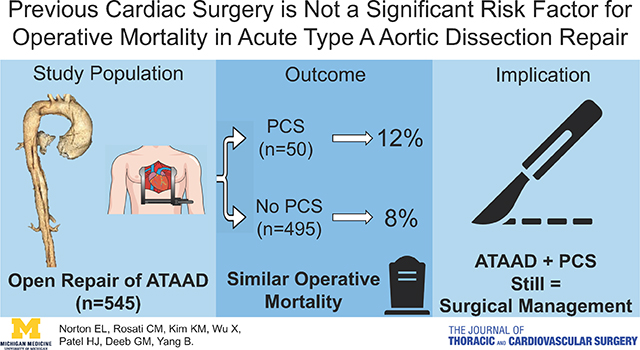
Compared to patients without PCS, patients with PCS were older (62 vs. 59, p=0.24) and had significantly more coronary artery disease (48% vs. 14%, p<0.001), peripheral arterial disease (24% vs. 11%, p=0.01), connective tissue disorders (15% vs. 4.5%, p=0.004), and acute renal failure on presentation (28% vs. 15%, p=0.02); and significantly more concomitant mitral or tricuspid procedures, longer cardiopulmonary bypass time, and more intraoperative blood transfusions. There were no statistically significant differences in post-operative major complications between PCS and no PCS groups, including stroke, myocardial infarction, new-onset dialysis, and 30-day mortality (8.9% vs. 6.3%, p=0.55). Multivariable logistic model showed the significant risk factors for operative mortality were cardiogenic shock (odds ratio (OR)=9.6, p<0.0001) and male gender (OR=3.7, p=0.006). The 5- and 10-year unadjusted survival were significantly lower in the PCS group compared to the no PCS group (66% vs. 80% and 42% vs. 66%, respectively, p=0.02). However, PCS itself was not a significant risk factor for operative (OR=1.6, p=0.36) or all-time mortalities (hazard ratio=1.3, p=0.33).
CONCLUSIONS
ATAAD in patients with PCS can be repaired with favorable operative mortality and long-term survival, and should be treated surgically.
Graphical Abstract
Previous cardiac surgery itself was not a significant risk factor for operative mortality or long-term mortality; therefore, acute type A aortic dissection in patients with previous cardiac surgery can be and should be treated surgically for favorable outcomes.
INTRODUCTION
Acute type A aortic dissection (ATAAD) is a surgical emergency with high associated morbidity and mortality. Open surgical aortic repair is particularly challenging in the case of a patient with previous cardiac surgery (PCS), with the added risks of sternal re-entry and operating in a “hostile” mediastinum. There are a few reports in the literature about the outcomes of redo ATAAD repair in patients with PCS, with an in-hospital mortality around 25–38% at single institutions1–4 and the international registry of aortic dissection (IRAD)5. Because of the suboptimal outcome of emergent surgical repair in this patient population, one study recommends elective repair instead of emergent repair4. Some studies even recommend medical management rather than surgical repair in ATAAD patients with PCS6–8.
In this study, we compare the short- and long-term outcomes of ATAAD patients with vs. without PCS who underwent surgical repair of an ATAAD at our institution (University of Michigan, Ann Arbor, MI) over two decades. Our hypothesis was that previous cardiac surgery was not a risk factor for short- or long-term mortality in ATAAD patients treated at a high volume aortic center by aortic surgeons.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board at the University of Michigan, Michigan Medicine (Ann Arbor, MI) and was in compliance with Health Insurance Portability and Accountability Act regulations.
Study Population
From July 1996 to January 2017, 545 patients underwent open repair of an acute type A aortic dissection, including patients with previous cardiac surgery (PCS, n=50) and patients without PCS (no PCS, n=495). Patients with previous cardiac surgery included patients with a prior sternotomy with additional operation on the heart or aorta, including CABG, valve surgery, aortic root repair or replacement, and/or partial ascending replacement. Patients (n=4) who had patent ductus arteriosus ligation (n=1) or distal arch/descending TAA repair (n=3) through left thoracotomy without violating the pericardium were not categorized as patients with previous cardiac surgery in this study. Investigators leveraged the Society of Thoracic Surgeons data elements from the University of Michigan Cardiac Surgery Data Warehouse to identify the cohort and determine pre-operative, operative, and post-operative characteristics. Electronic medical record review was conducted to confirm previous cardiac surgery and to supplement data collection. Investigators utilized the National Death Index database through December 31, 20159 to determine survival. Further long-term survival data were collected from a thorough medical record review of patients’ return visits as well as surveys (including letters and phone calls, January 2018). Loss of follow-up or end of the study period were treated as censors during the time to events analysis.
Surgical Techniques
In the whole cohort, including patients with and without previous cardiac surgery, peripheral cannulations were used for 96% of the cases. In patients with previous cardiac surgery, 98% of cases had peripheral cannulations, including right axillary artery/intra-thoracic right subclavian artery/innominate artery (56%) and femoral artery (42%). (Supplemental Table 1) In certain cases, when patients had a large aneurysm, pseudoaneurysm, or contained rupture directly underneath the sternum and we anticipated a high risk of injury to the aorta causing massive hemorrhage during re-entry, we cooled the patient to 18 °C and performed the sternotomy with circulatory arrest. If there was enough space between the aorta and sternum, we performed redo sternotomy in the usual fashion after sewing a graft to the peripheral artery for arterial cannulation, in most cases. The indication for aortic root replacement in ATAAD patients included: (1) intimal tear at the aortic root, (2) root diameter ≥4.5 cm, (3) connective tissue disease, and (4) unrepairable aortic valve pathology10, 11. The root procedures include direct repair11, 12 or replacement as inclusion root, Bentall procedure, or David procedure10, 11, 13. Indications for zone 1–3 arch replacement included an arch aneurysm >4 cm or intimal tear located in the arch, which could not be resected by a hemiarch replacement; or dissection of arch branch vessels with malperfusion syndrome14. Malperfusion syndrome is defined as tissue or organ necrosis and dysfunction due to inadequate blood flow. Malperfusion syndrome due to dissection of arch branch vessels frequently manifests as stroke (cerebral malperfusion) or upper extremity necrosis and dysfunction (upper extremity malperfusion). We treated those patients with malperfusion syndrome with more aggressive aortic arch replacement and replacement of the dissected arch branch vessels individually to resolve the malperfusion14. All arch branch vessels were reimplanted/replaced individually to branch grafts. If needed, a frozen elephant trunk (cTAG 10 cm, manufactured by Gore) was placed into the true lumen of the descending thoracic aorta distal to the left subclavian artery as we described14. The detailed surgical technique, strategy of arch replacement with hypothermic circulatory arrest (HCA)14, and the root procedures11 were performed as previously described. We do not routinely perform coronary angiogram before ATAAD repair. Based on the CTA and intraoperative examination of the coronary artery bypass grafts for back bleeding of retrograde cardioplegia, we determine the patency of the previous coronary bypass graft. Patent coronary artery bypass vein grafts were reimplanted to the ascending Dacron graft as buttons including native aorta around the vein grafts. Patent left internal mammary artery (LIMA) bypassed to left anterior descending artery was left unclamped when the aorta was clamped. Coronary, cerebral, and spinal cord malperfusion syndrome or any malperfusion syndrome with hemodynamic instability (n=43) were treated with emergent open aortic repair. Visceral and limb malperfusion syndrome (n=83) without aortic rupture or cardiac tamponade were treated with endovascular fenestration and stenting first and delayed open central aortic repair15, 16.
Statistical Analysis
Continuous variables were summarized by median (25 percentile, 75 percentile) and categorical variables were reported as n (%) in frequency tables. Univariate comparisons between PCS and no PCS groups were performed using chi-square tests or fisher exact tests for categorical data and Wilcoxon rank sum tests for continuous data. Multivariable logistic regression was used to assess the risk factors for operative mortality by adjusting for age, gender, coronary artery disease, acute myocardial infarction, acute stroke, tamponade, malperfusion syndrome, and cardiogenic shock. Crude survival curves since operation were estimated using the non-parametric Kaplan-Meier method. Log-Rank test was used to compare the survival between groups. Cox proportional hazard regression was performed to calculate the hazard ratio (HR) for all-time mortality by adjusting age, gender, coronary artery disease, NYHA class III/IV, acute stroke, acute myocardial infarction, pre-operative renal failure, acute paralysis, and cardiogenic shock. The variables for the logistic and Cox models were chosen based on clinical judgement. All statistical calculations used SAS 9.4 (SAS Institute, Cary, NC) and were considered significant at p<0.05.
RESULTS
Demographics and Preoperative Data
The median time from last previous cardiac surgery to acute type A aortic dissection repair was 4 (IQR: 0, 10) years. The PCS group appeared to have significantly more coronary artery disease, peripheral vascular disease, and acute renal failure from aortic dissection, and also had significantly more patients with connective tissue disease and malperfusion syndrome (i.e. end organ necrosis and dysfunction due to inadequate blood flow resulted from aortic dissection); but fewer current smokers; there was no significant difference in other comorbidities. Cardiac tamponade was less frequent in the PCS group (2.0% vs. 9.7%, p=0.07). (Table 1)
Table 1.
Demographics, comorbidities, and clinical condition on admission
| All patients n = 545 | PCS n = 50 | no PCS n = 495 | p value | |
|---|---|---|---|---|
| Demographics and chronic comorbidities | ||||
| Age | 59 (49,68) | 62 (52,69) | 59 (49,68) | 0.24 |
| Female gender | 163 (30) | 9 (18) | 154 (31) | 0.07 |
| Body mass index - kg/m2 | 28 (25,32) | 28 (24,32) | 28 (25,32) | 0.42 |
| Body surface area - m2 | 2.1 (1.9,2.2) | 2.0 (1.9,2.2) | 2.1 (1.9,2.2) | 0.70 |
| NYHA Class | 0.92 | |||
| I or II | 395 (77) | 38 (76) | 357 (72) | |
| III or IV | 121 (23) | 12 (24) | 109 (22) | |
| Missing | 29 (5.3) | 0 (0) | 29 (5.9) | |
| Coronary artery disease | 98 (19) | 25 (50) | 73 (15) | <0.001 |
| Missing | 23 (4.2) | 0 (0) | 23 (4.6) | |
| Previous MI | 26 (4.8) | 5 (10) | 21 (4.2) | 0.08 |
| Hypertension | 387 (71) | 40 (80) | 347 (70) | 0.19 |
| Peripheral arterial disease | 68 (12) | 12 (24) | 56 (11) | 0.01 |
| COPD | 49 (9.0) | 4 (8.0) | 45 (9.1) | 1.0 |
| Tobacco Use | 0.006 | |||
| Never | 245 (45) | 21 (42) | 224 (46) | |
| Former | 142 (26) | 22 (44) | 120 (24) | |
| Current | 154 (28) | 7 (14) | 147 (30) | |
| Missing | 4 (0.7) | 0 (0) | 4 (0.8) | |
| Diabetes | 36 (6.6) | 4 (8.0) | 32 (6.5) | 0.56 |
| Chronic kidney disease | 23 (4.2) | 3 (6.0) | 20 (4.0) | 0.46 |
| On dialysis | 8 (1.5) | 1 (2.0) | 7 (1.4) | 0.30 |
| Missing | 1 (0.2) | 0 (0) | 1 (0.2) | |
| Previous stroke | 15 (2.8) | 2 (4.0) | 13 (2.6) | 0.64 |
| Connective tissue disorder | 30 (5.5) | 8 (16) | 22 (4.4) | <0.001 |
| Age at ATAAD | 34.5 (26, 46) | 43.5 (35, 57.5) | 33 (23, 42) | 0.06 |
| Clinical condition on admission | ||||
| Aortic Insufficiency | 0.22 | |||
| None | 148 (29) | 19 (38) | 129 (28) | |
| Trace/trivial | 58 (11) | 6 (12) | 52 (11) | |
| Mild | 99 (19) | 9 (18) | 90 (19) | |
| Moderate | 85 (17) | 4 (8.0) | 81 (17) | |
| Severe | 125 (24) | 8 (16) | 117 (25) | |
| Missing | 30 (5.5) | 4 (8.0) | 26 (5.3) | |
| Acute MI | 16 (2.9) | 3 (6.0) | 13 (2.6) | 0.17 |
| Rupture/Tamponade | 49 (9.0) | 1 (2.0) | 48 (9.7) | 0.07 |
| Pre-operative CPR | 16 (2.9) | 2 (10) | 14 (2.8) | 0.65 |
| Acute stroke | 23 (4.2) | 0 (0) | 23 (4.6) | 0.25 |
| Acute paralysis | 9 (1.7) | 1 (2.0) | 8 (1.6) | 0.58 |
| Acute renal failure | 74 (14) | 13 (26) | 61 (12) | 0.01 |
| Creatinine on admission - mg/dL | 1.0 (0.8,1.3) | 1.0 (0.8,1.4) | 1.0 (0.8,1.3) | 0.95 |
| GFR on admission - mg/dL/1.73 m2 | 88 (65,117) | 90 (63, 121) | 88 (65,117) | 0.95 |
| Malperfusion syndrome* | 126 (23) | 18 (36) | 108 (22) | 0.03 |
| Emergent operation | 43 (7.9) | 3 (6.0) | 40 (8.1) | 0.60 |
| Delayed operation | 83 (15) | 15 (30) | 68 (14) | 0.002 |
| Waiting time (days) | 3 (1, 9) | 10.5 (4, 24) | 2 (1, 7) | 0.01 |
Data reported as n (%) or median (interquartile range).
COPD=chronic obstructive pulmonary disease; CPR=cardiopulmonary resuscitation; GFR=glomerular filtration rate (Cockcroft-Gault formula); MI=myocardial infarction; NYHA=New York Heart Association; PCS=previous cardiac surgery.
Malperfusion syndrome is defined as organ necrosis and/or dysfunction due to inadequate blood flow. Emergent operation was performed for cerebral or coronary malperfusion syndrome. Delayed operation for visceral, renal and extremity malperfusion syndrome.
Intraoperative Data
The PCS group had significantly more aortic root replacement and less aortic root repair, but similar extent of aortic arch procedures with more antegrade cerebral perfusion and more concomitant mitral valve or tricuspid valve procedures (Table 2). Seventeen out of 19 patients with previous coronary artery bypass grafting had reimplantation of previous bypass grafts. The cardiopulmonary bypass and aortic cross-clamp times were significantly longer in the PCS group compared to the no PCS group. The PCS group also had a significantly larger amount of intraoperative transfusion of packed red blood cells (PRBCs). (Table 2)
Table 2.
Intra-operative outcomes
| All patients n = 545 | PCS n = 50 | no PCS n = 495 | p value | |
|---|---|---|---|---|
| Intra-operative death | 7 (1.3) | 1 (2.0) | 6 (1.2) | 0.49 |
| CPB time (minutes) | 222 (181,272) | 269.5 (216,326) | 219 (177,267) | <0.001 |
| Cross clamp time (minutes) | 156 (115,201) | 187.5 (137,239) | 154 (115,198) | 0.002 |
| HCA | 515 (94) | 47 (94) | 468 (95) | 0.75 |
| HCA time (minutes)* | 35 (28,45) | 37 (27,54) | 35 (28,45) | 0.55 |
| Type of cerebral perfusion | 0.015 | |||
| ACP only | 170 (32) | 22 (44) | 148 (30) | |
| RCP only | 202 (36) | 16 (32) | 186 (38) | |
| Both ACP and RCP | 139 (26) | 6 (12) | 133 (27) | |
| Neither | 33 (5.8) | 6 (12) | 27 (5.5) | |
| Lowest Temp (°C) | 18 (17, 21) | 18 (16, 19) | 18 (17, 22) | 0.12 |
| Type of aortic root procedure | <0.001 | |||
| None | 44 (10) | 11 (22) | 33 (6.7) | |
| Aortic valve replacement | 10 (1.8) | 3 (6.0) | 7 (1.4) | |
| Aortic root replacement | 184 (34) | 20 (40) | 164 (33) | |
| Aortic root repair | 307 (54) | 16 (32) | 291 (59) | |
| Type of aortic arch procedure | 0.26 | |||
| None | 32 (6.1) | 4 (8.0) | 28 (5.7) | |
| Hemiarch | 322 (59) | 30 (60) | 292 (59) | |
| Zone 1 | 41 (7.3) | 2 (4.0) | 39 (7.9) | |
| Zone 2 | 109 (20) | 7 (14) | 102 (21) | |
| Zone 3 | 41 (7.5) | 7 (14) | 34 (6.9) | |
| Frozen elephant trunk | 33 (6.1) | 6 (12) | 27 (5.5) | 0.11 |
| Concomitant procedure | ||||
| CABG | 32 (5.9) | 6 (12) | 26 (5.3) | 0.10 |
| Mitral or tricuspid valve procedure | 10 (1.8) | 3 (6.0) | 7 (1.4) | 0.02 |
| Need for intra-op PRBC transfusion | 415 (80) | 43 (93) | 372 (79) | 0.09 |
| Missing | 27 (5.0) | 4 (8.0) | 23 (4.6) | |
| Intra-operative PRBC units | 4 (1,8) | 7.5 (4,11) | 4.0 (1,7) | <0.001 |
Data reported as n (%) or median (interquartile range).
HCA time was the time for distal anastomosis at the aortic arch during circulatory arrest of the lower body
ACP=antegrade cerebral perfusion; CABG=coronary artery bypass graft; CPB=cardiopulmonary bypass; HCA=hypothermic circulatory arrest; PCS=previous cardiac surgery; PRBC=packed red blood cells; RCP=retrograde cerebral perfusion
Postoperative Outcomes
Overall, there were no significant differences in major postoperative outcomes between the PCS and no PCS groups, including post-operative stroke, myocardial infarction, new-onset renal failure on dialysis, and 30-day mortality [4 (8.9%) vs 31 (6.3%), p=0.55]. (Table 3) The operative mortality which includes mortality within 30 days after the operation and/or mortality in the hospital were not significantly different either [6 (12%) vs 41 (8.3%), p=0.42]. (Table 3) The significant risk factors for operative mortality were cardiogenic shock (odds ratio (OR)=9.6, 95% CI: [4.4, 21], p<0.0001) and male gender (OR=3.7, 95% CI: [1.5, 9.6], p=0.006). PCS was not a significant risk factor for operative mortality (OR=1.6, 95% CI: [0.6, 4.4], p=0.36); nor was age (OR=1.022, 95% CI: [0.996, 1.049], p=0.104), nor malperfusion syndrome (OR=1.5, 95% CI: [0.67, 3.45], p=0.31). Within the group of patients with PCS, non-survivors had significantly more preoperative cardiogenic shock and previous multi-valve surgery. (Table 4)
Table 3.
Post-operative outcomes
| All patients n = 545 | PCS n = 50 | no PCS n = 495 | p value | |
|---|---|---|---|---|
| Reoperation for bleeding | 47 (8.6) | 7 (14) | 40 (8.1) | 0.18 |
| Post-operative tamponade | 11 (2.0) | 3 (6.0) | 8 (1.6) | 0.04 |
| Peri-operative MI | 5 (1.1) | 1 (2.0) | 4 (0.8) | 0.38 |
| Atrial fibrillation | 193 (36) | 18 (36) | 175 (35) | 0.93 |
| Deep sternal wound infection | 13 (2.4) | 1 (2.0) | 12 (2.4) | 1.0 |
| Sepsis | 16 (2.9) | 1 (2.0) | 15 (3.0) | 1.0 |
| New-onset Stroke | 38 (7.2) | 1 (2.0) | 37 (7.5) | 0.24 |
| New-onset paralysis | 4 (0.9) | 2 (4.0) | 2 (0.4) | 0.005 |
| Prolonged ventilation | 292 (16) | 24 (48) | 268 (54) | 0.46 |
| Pneumonia | 97 (18) | 8 (16) | 89 (18) | 0.85 |
| Reintubation | 36 (6.7) | 5 (10) | 31 (6.3) | 0.36 |
| Tracheostomy | 19 (3.5) | 3 (6.0) | 16 (3.2) | 0.40 |
| New-onset acute renal failure needing dialysis | 24 (4.4) | 2 (4.0) | 22 (4.4) | 0.88 |
| Post-operative length of stay (days) | 11 (7,19) | 14.5 (9,28) | 11 (7,18) | 0.02 |
| In-hospital mortality | 44 (8.1) | 6 (12) | 38 (7.7) | 0.28 |
| 30-day mortality | 35 (6.4) | 4 (8.9) | 31 (6.3) | 0.55 |
| Operative mortalitya | 47 (8.6) | 6 (12) | 41 (8.3) | 0.42 |
Defined as mortality in-hospital or within 30 days after surgery.
Data reported as n (%) or median (interquartile range).
MI=myocardial infarction; PCS=previous cardiac surgery.
Table 4.
Admission variables and in-hospital outcomes for patients with previous cardiac surgery
| In-hospital outcome | ||||
|---|---|---|---|---|
| All patients n = 50 | Survivors n =44 | Non-survivors n = 6 | p value | |
| Years from last PCS to ATAAD repair | 4 (0,11) | 4 (1,10) | 15.5 (0,22) | 0.27 |
| Last PCS | ||||
| Isolated CABG | 15 (30) | 15 (34) | 0 (0) | 0.16 |
| Isolated valve | 14 (28) | 12 (27) | 2 (33) | 1.0 |
| Aortic valve | 7 (14) | 5 (11) | 2 (33) | |
| Mitral valve | 7 (14) | 7 (16) | 0 (0) | |
| Ascending aorta replacement | 2 (4.0) | 2 (4.5) | 0 (0) | 1.0 |
| CABG + AVR | 3 (6.0) | 3 (6.8) | 0 (0) | 1.0 |
| CABG + ascending aortoplasty | 1 (2.0) | 1 (2.2) | 0 (0) | 1.0 |
| ARR ± ascending replacement | 8 (16) | 8 (18) | 0 (0) | 0.57 |
| Multiple valvea | 2 (4.0) | 0 (0) | 2 (33) | 0.01 |
| Multiple valveb + ascending aorta | 1 (2.0) | 0 (0) | 1 (17) | 0.12 |
| Congenitalc | 2 (4.0) | 1 (2.3) | 1 (17) | 0.23 |
| Otherd | 2 (4.0) | 2 (4.5) | 0 (0) | 1.0 |
| Number of PCS | 1.0 | |||
| One | 47 (94) | 41 (93) | 6 (100) | |
| Two | 3 (6.0) | 3 (6.8) | 0 (0) | |
| Cardiogenic shock pre-operatively | 2 (4.0) | 0 (0) | 2 (33) | 0.01 |
AVR + mitral valve replacement (n=1) and mitral valve repair + tricuspid valve repair (n=1)
Mitral valve repair + AVR
PFO/VSD repair at the age of 3 (n=1) and ASD/VSD repair at the age of 5 (n=1)
Other includes a heart transplant (n=1) and trauma/coronary artery laceration (n=1)
All other admission variables: no statistically significant difference (all p > 0.05) between in-hospital survivors and non-survivors.
Data reported as n (%) or median (interquartile range). P-values indicate the difference of the incidence rate between survivors and non-survivors and were carried out with Fisher’s exact test.
ASD=atrial septal defect; ATAAD=acute type A aortic dissection; AVR=aortic valve replacement; ARR=aortic root replacement CABG=coronary artery bypass grafting; PCS=previous cardiac surgery; PFO=patent foramen ovale; VSD=ventricular septal defect.
Long-term Outcomes
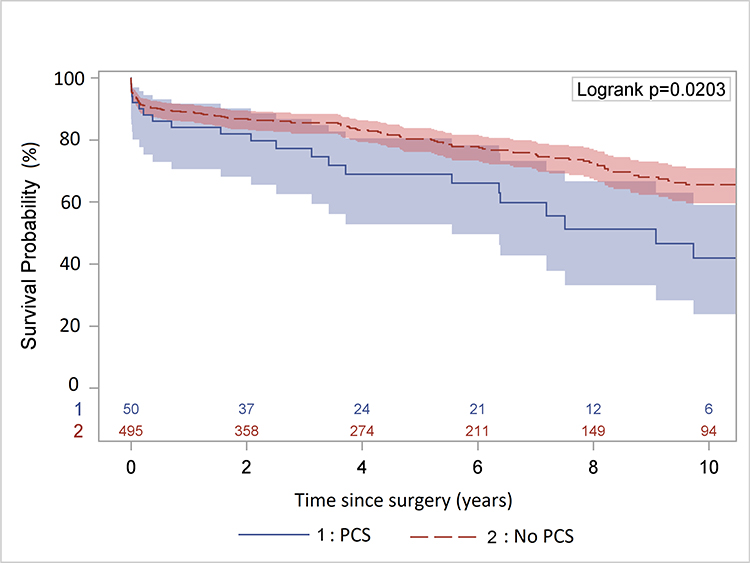
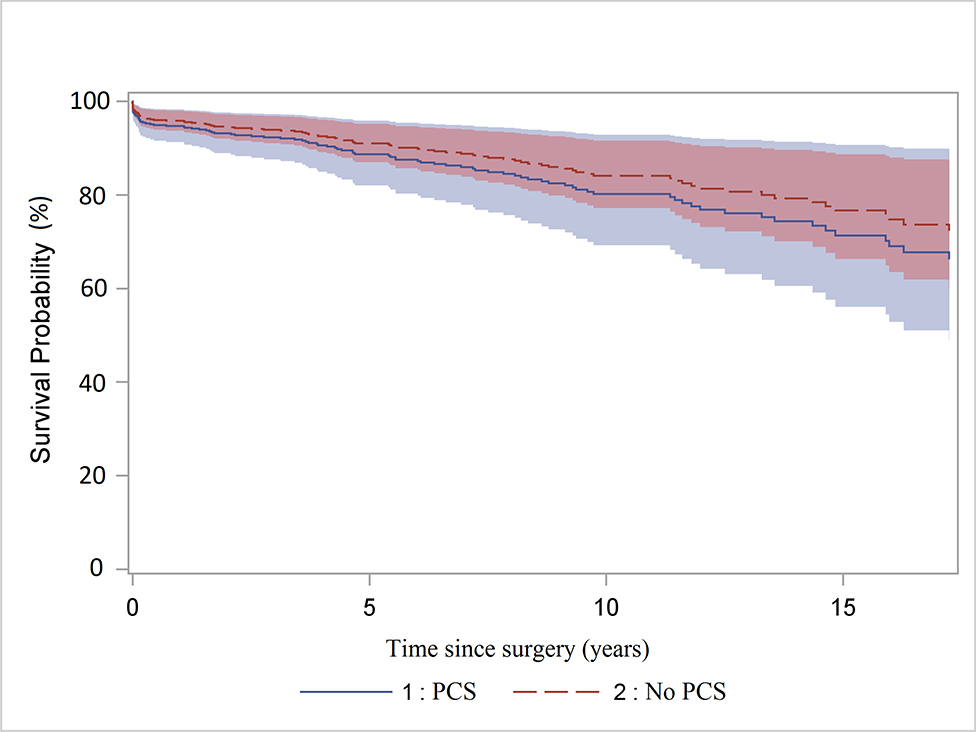
The mean follow-up time is 5.8 ± 5 years. Sixty-five patients (12%) were lost to follow-up at the end of the study period (January 2018). Among all patients, 159 deaths were identified during the follow-up period, 22 patients in the PCS group and 137 patients in the no PCS group. The raw 5- and 10-year survival were significantly lower in the PCS group (66% [95% CI: 50%, 78%] and 42% [95% CI: 24%, 59%]) than that in the no PCS group (80% [95% CI: 76%, 84%] and 66% [95% CI: 60%, 71%], p=0.02) (Figure 1A). However, the multi-variable analysis in the Cox model showed PCS was not a significant risk factor for all-time mortality after surgery compared to no PCS (hazard ratio (HR)=1.3, 95% CI: [0.8, 2.1], p=0.33). (Figure 1B) The significant risk factors for all-time mortality were age (HR=1.04, 95% CI:[1.02, 1.05], p<0.0001), year of surgery (HR=0.95, 95% CI: [0.92, 0.98], p=0.002), coronary artery disease (HR=1.5, 95% CI: [1.01, 2.2], p=0.04), and preoperative acute paralysis (HR=3.7, 95% CI: [1.5, 9.4], p=0.005).
Figure 1:
A. Kaplan-Meier survival analysis of patients with or without previous cardiac surgery (PCS) after acute type A aortic dissection repair. The raw 5- and 10-year survival were significantly lower in the PCS group (66% and 42%) compared to that in the no PCS group (80% and 66%) B. Cox proportional hazard regression of survival. The hazard ratio of PCS vs. no PCS was 1.3 (95% CI: 0.8, 2.1), p=0.33.
DISCUSSION
In this study, we found that despite the patients with PCS having significantly more comorbidities and the operation being more complex, the perioperative outcomes, including mortality, were comparable to those of patients without PCS. The long-term survival was significantly worse in patients with PCS; however, PCS itself was not a significant risk factor for operative mortality (OR= 1.6, p=0.36) or all-time mortality (HR=1.3, p=0.33). (Video)
When to operate on ATAAD patients with PCS? We operated on those patients with PCS emergently, just like patients with no PCS, if they did not have visceral or limb malperfusion syndrome, which we first treat endovascularly15, 16. There is less cardiac tamponade in ATAAD patients with PCS as demonstrated in our data (Table 1) and in the literature1, 3. However, there is still risk of rupture of the dissected proximal aorta. Because of the scar tissue from the previous cardiac surgery around the proximal aorta, the aorta may have less chance to rupture into the pericardial space causing tamponade3. However, the dissected aorta can rupture into the pulmonary artery1 and right atrium17, so we operate when the patients arrive at our institution.
We do not routinely perform coronary angiogram for ATAAD patients with PCS, even in those who had a previous coronary artery bypass. We examine the coronary artery bypass grafts on the CT angiogram to identify patent bypass grafts. During the operation, we use retrograde cardioplegia for all ATAAD patients. If we see back bleeding from the coronary bypass grafts, we reimplant those bypass grafts as buttons by sewing the aortic button(s) to the ascending aortic Dacron graft. We do not recommend to directly anastomose the saphenous vein graft to the Dacron graft for the risks of increasing medial hyperplasia and rupture of the vein graft from the Dacron graft and subsequent pseudoaneurysm, especially in patients with Marfan syndrome. We have performed at least two reoperations of repairing a pseudoaneurysm from a ruptured vein graft from the Dacron graft in patients who had an initial ATAAD repair by other surgeons. If the bypass graft appears to be like a cord, with no flow on CT angiogram, and no back bleeding of retrograde cardioplegia, then we do not reimplant that bypass graft. If in doubt, we always reimplant the coronary bypass graft. Some of us would like to dissect out the LIMA and clamp it during cardiac arrest. Others just leave the LIMA alone, but maintain the body temperature at 25 degrees Celsius during cardiac arrest. Both ways seemed to work equally well.
We found that previous cardiac surgery as a whole was not an independent significant risk factor for operative mortality (OR=1.6, 95% CI: [0.6, 4.5], p=0.36) or long-term mortality (HR=1.3, 95% CI: [0.8, 2.1], p=0.33) (Figure 1B). Despite patients with PCS having more comorbidities than patients with no PCS (Table 1) and the operations being much more complex with significantly longer cardiopulmonary bypass and aortic cross-clamp times (Table 2), the 30-day (8.9%) and in-hospital mortalities (12%) were relatively low and not significantly different from those in patients with no PCS (Table 3). Our operative mortality was similar or lower than those reported as single center outcomes1–4 or IRAD5; and much better than the mortality with just medical management which is 50%5. There were several potential reasons for the relatively low operative mortality in our study. Number one, we manage visceral/extremity malperfusion syndrome first in all hemodynamically stable ATAAD patients, including patients with PCS, to give those critically ill patients a chance to recover before open aortic repair 15, 16. Since patients with PCS had much lower risk of tamponade (Table 1) and rupture3, we really emphasize resolving the malperfusion syndrome before redo open aortic repair for ATAAD. We do not put patients through a very long redo operation when patients are suffering ongoing end-organ ischemia and necrosis, thereby giving patients with PCS and malperfusion syndrome a better chance of survival by resolving the malperfusion endovascularly and waiting for the patients to recover from malperfusion syndrome, such as necrotic bowel and limb, severe metabolic acidosis, acute respiratory distress syndrome, sepsis, etc.15, 16, and after patients recovered from malperfusion syndrome, their chance of survival was much improved. We speculate this might be a reason for lower operative mortality. Our multivariable logistic model showed malperfusion syndrome was not a significant risk factor, since patients all recovered from malperfusion syndrome when they had the open aortic repair (OR=1.5, p=0.31).
Number two, patient’s selection. After endovascular fenestration/stenting, we only performed open aortic repair in those patients who recovered from the complications of malperfusion syndrome, such as septic shock, acute respiratory distress syndrome (ARDS), severe metabolic acidosis and organ failure. There were ten patients who underwent endovascular fenestration/stenting, but never underwent open aortic repair, including 5 patients who could never recover from those complications despite the correction of malperfusion by endovascular fenestration/stenting and died, 3 patients died of aortic rupture while still suffering from multi-organ failure, and 2 patients survived without open repair. Most centers would categorize those 10 patients with severe malperfusion syndrome (end organ necrosis and failure) as non-operative candidates. We did not perform open aortic repair on those patients (n=10) and they were not included in this study. The PCS group in this study was a highly selected group of patients who underwent a reoperation of open aortic repair.
Number three, we have a subspecialty practice for ATAAD – 94.5% of all the ATAAD cases and 96% of the PCS patients in this study were operated on by 3 aortic surgeons. All aortic surgeons at our center are very familiar with complex aortic root and arch procedures, redo sternotomy with substernal ascending aortic aneurysm, sternotomy with hypothermic circulatory arrest, and emergent management of a ruptured ascending aorta during sternotomy. Some of those situations are not common cases for cardiac surgeons but the aortic surgeons deal with them often under elective and emergent circumstances. In this study, PCS patients had significantly more redo aortic root replacement most likely due to more patients with connective tissue disease and previous aortic valve replacement in the PCS group. Those redo aortic root replacements in ATAAD demanded surgeons who are more familiar and comfortable with aortic root procedures.
The significant risk factors for operative mortality were male sex (OR=3.7) and cardiogenic shock (OR=9.6). Those are consistent with previous findings at a single institution1 and IRAD5. Age and previous coronary artery bypass were not significant risk factors. No patients (0/19) with previous coronary bypass died after ATAAD repair in the PCS group. The causes of death in the 6 operative deaths in the PCS group were quite heterogeneous, including neurological damage (n=1) due to preoperative CPR, cardiogenic shock (n=2) with one patient due to coronary malperfusion syndrome and massive acute myocardial infarction, ischemic bowel, endocarditis, and multiorgan failure (Table 5), which are typically seen with ATAAD. Taken together, these perioperative results support that ATAAD patients with PCS should be treated with emergent surgical repair as are those without PCS. Surgeons, patients, and their families should be aware that patients with cardiogenic shock have substantial increased risk of operative mortality.
Table 5.
Cause of death for patients with previous cardiac surgery who died in-hospital
| Case | PCS | Age at ATAAD Repair | Year of ATAAD Repair | Years Between | ATAAD Procedure | Preoperative MPS | ATAAD Preoperative Complications | ATAAD Postoperative Complications | Date of Death | Cause of Death |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MV repair, TV repair, Maze | 63 | 2003 | 0.12 | Root, ascending, and zone 2 arch replacement, CABG × 1 | No | coagulopathy; cardiogenic shock | - | POD # 0 | Operation required extended CPB time, unable to wean from bypass. |
| 2 | AVR | 39 | 2007 | 22.5 | Root, ascending, and hemiarch replacement | Yes | Mesenteric, celiac, and renal MPS | Required ECMO, he was oliguric, hyperkalemic, and required dialysis. Bowel ischemia with exploratory laparotomy and ileocecectomy | POD # 2 | Necrotic bowel, Multi-system organ failure. Withdrawal of care. |
| 3 | ASD/VSD repair | 37 | 2010 | 32 | Root repair, ascending, and hemiarch replacement | No | Acute MI, acute liver injury, new-onset acute renal failure, pneumonia, ITP with post-transfusion purpura | POD # 52 | Severe thrombocytopenia, mesenteric ischemia and necrosis, patient made DNR with continuation of care without escalation (no dialysis). Patient expired. | |
| 4 | MV repair, AVR, ascending replacement* | 78 | 2012 | 0.01 | Root repair | No | Preop CPR | Neuro exam revealed pupil and corneal reflex activity, and gag reflex only. He developed seizure like activity and myoclonic jerking. Head CTs negative for intracranial process. | POD # 6 | Remained neurologically unresponsive, made comfort care. |
| 5 | AVR, MVR, Cox-Maze | 57 | 2014 | 9 | Root, ascending, and hemiarch replacement | Yes | Marfan syndrome, acute MI, coronary MPS, cardiogenic shock | ECMO, paralysis probably due to dissection and thrombosis of intercostal arteries. | POD # 10 | After decannulation from ECMO patient became oliguric, hyperkalemic, extremities appeared dusky with pulses intact, family withdrew care. |
| 6 | AVR | 73 | 2016 | 22 | Ascending and hemiarch replacement | Yes | Renal and lower extremity MPS | Sepsis, pneumonia, tracheostomy, acute kidney injury on top of existing chronic kidney disease requiring dialysis, ischemic bowel, colectomy and end colostomy for necrotic bowel performed. Mental status declined, subacute cerebral ischemia, and endocarditis | POD # 79 | Given inability to treat pneumonia, new finding of endocarditis with concern for septic emboli to the brain, renal failure, and poor prognosis, patient was made comfort care. |
The patient developed aortic root dissection 3 days after initial MVR, AVR, and ascending aortic replacement.
ASD=atrial septal defect; ATAAD=acute type A aortic dissection; AVR=aortic valve replacement; CABG=coronary artery bypass graft; CPB=cardiopulmonary bypass; CPR=cardiopulmonary resuscitation; DNR=do not resuscitate; ECMO=extracorporeal membrane oxygenation; IR=interventional radiology; ITP=idiopathic thrombocytopenic purpura; MI=myocardial infarction; MPS=malperfusion syndrome; MV=mitral valve; MVR=mitral valve replacement; PCS=previous cardiac surgery; POD=postoperative day; TV=tricuspid valve; VSD=ventricular septal defect
The long-term survival in patients with PCS was compromised. The decreased survival was most likely due to significantly more comorbidities in PCS patients, including significantly more coronary artery disease (50% vs. 15%), previous myocardial infarction (10% vs. 4.2%), peripheral vascular disease (24% vs. 11%), more connective tissue disease which required more aortic root replacement, more acute renal failure (26% vs. 12%), and more malperfusion syndrome (36% vs. 22%). In the Cox model, PCS patients without those comorbidities had similar long-term survival as patients with no PCS (Figure 1B), though we had a small and highly selective subset of PCS patients in the Cox model. This finding also supports that ATAAD patients should not be turned down from surgical repair due to a previous cardiac surgery.
Our study is limited by a single-center and retrospective experience. The sample size of patients with PCS is relatively small, and the mortality is relatively low, our study may be under powered to achieve significant difference of operative mortality between and PCS and no PCS groups. However, the overall operative mortality was relatively low, even in the PCS group alone, which supports surgical management of ATAAD patients with PCS. The operative mortality underestimates the mortality of all ATAAD patients with PCS since the studied cohort only included the patients who underwent an open aortic repair. Patients who died from complications of malperfusion syndrome (necrotic viscera or limb) after revascularization by endovascular fenestration/stenting were not included in this study. The vast majority of cases were performed by aortic surgeons and we manage malperfusion syndrome endovascularly prior to open aortic repair; therefore, our experience may not apply to all centers operating on ATAAD patients.
CONCLUSION
In conclusion, both short- and long-term surgical outcomes in ATAAD patients with PCS were favorable, and PCS itself was not a risk factor for operative mortality or long-term mortality. We recommended ATAAD patients with PCS be treated with emergent open surgical repair just like ATAAD patients without PCS. (Graphical abstract)
Supplementary Material
Central Picture:
Cox proportional hazard regression of survival in acute type A aortic dissection patients.
Central Message:
Acute type A aortic dissection in patients with previous cardiac surgery can be and should be treated surgically for favorable short- and long-term outcomes.
Perspective Statement.
Patients with previous cardiac surgery often have significant comorbidities which adversely affect the perioperative and long-term survival after surgical repair of acute type A aortic dissection. However, previous cardiac surgery itself was not a significant risk factor for operative or long-term mortality and those patients should be considered for emergent surgical aortic repair.
ACKNOWLEDGEMENT
The authors acknowledge the support of the Data Warehouse, Department of Cardiac Surgery, led by Dr. Donald Likosky, including Jeremy Wolverton, Amy Geltz, Mary Barry, Brett Cross, Mary Ryzak, and other team members. We appreciate the contributions of Dr. Panos N. Vardas and Dr. Annalisa Bernabei.
Sources of Funding: Dr. Yang is supported by the NHLBI of NIH K08HL130614 and R01HL141891, Phil Jenkins and Darlene & Stephen J. Szatmari Funds. Dr. Patel is supported by the Joe D. Morris Collegiate Professorship, the David Hamilton Fund, and the Phil Jenkins Breakthrough Fund in Cardiac Surgery. Dr. Deeb is supported by the Herbert Sloan Collegiate Professorship, Jamie Buhr Fund, and Richard Nerod Fund.
GLOSSARY OF ABBREVIATIONS
- ATAAD
acute type A aortic dissection
- CPR
cardiopulmonary resuscitation
- HR
hazard ratio
- HCA
hypothermic circulatory arrest
- IRAD
International Registry of Acute Aortic Dissection
- LIMA
left internal mammary artery
- NYHA
New York heart association
- OR
odds ratio
- PCS
previous cardiac surgery
- PRBCs
packed red blood cells
- 95% CI
95% confidence interval
Footnotes
Conflict of Interest: None related to this study
Date and Number of IRB Approval: 9/26/2016 and HUM00119716
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Estrera AL, Miller CC, Kaneko T, Lee TY, Walkes JC, Kaiser LR, et al. Outcomes of acute type a aortic dissection after previous cardiac surgery. Ann Thorac Surg. 2010;89:1467–1474. [DOI] [PubMed] [Google Scholar]
- 2.Klodell CT, Karimi A, Beaver TM, Hess PJ, Martin TD. Outcomes for acute type A aortic dissection: effects of previous cardiac surgery. Ann Thorac Surg. 2012;93:1206–1212; discussion 1212–1204. [DOI] [PubMed] [Google Scholar]
- 3.Rylski B, Desai ND, Bavaria JE, Moser W, Vallabhajosyula P, Pochettino A, et al. Type A aortic dissection after previous cardiac surgery: results of an integrated surgical approach. Ann Thorac Surg. 2014;97:1582–1588; discussion 1588–1589. [DOI] [PubMed] [Google Scholar]
- 4.Yang Z, Yang S, Wang F, Hong T, Lai H, Wang C. Type A Aortic Dissection Occurring After Previous Cardiac Surgery. J Card Surg. 2015;30:830–835. [DOI] [PubMed] [Google Scholar]
- 5.Collins JS, Evangelista A, Nienaber CA, Bossone E, Fang J, Cooper JV, et al. Differences in clinical presentation, management, and outcomes of acute type a aortic dissection in patients with and without previous cardiac surgery. Circulation. 2004;110:II237–242. [DOI] [PubMed] [Google Scholar]
- 6.Hassan M, Carvalho EM, Macedo FI, Gologorsky E, Salerno TA. Paradigm change in the management of patients with acute type A aortic dissection who had prior cardiac surgery. J Card Surg. 2010;25:387–389. [DOI] [PubMed] [Google Scholar]
- 7.Timek TA, Hooker R, Patzelt L, Bernath G. Conservative management and resolution of iatrogenic type A aortic dissection in a patient with previous cardiac surgery. J Thorac Cardiovasc Surg. 2012;144:e18–21. [DOI] [PubMed] [Google Scholar]
- 8.Elefteriades JA, Feldman M. Acute type A aortic dissection: surgical intervention for all: CON. Cardiol Clin. 2010;28:325–331. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention; National Center for Health Statistics. National Death Index Available at http://www.cdc.gov/nchs/ndi/index.htm December 27, 2017.
- 10.Yang B, Malik A, Waidley V, Kleeman KC, Wu X, Norton EL, et al. Short-term outcomes of a simple and effective approach to aortic root and arch repair in acute type A aortic dissection. J Thorac Cardiovasc Surg. 2018;155:1360–1370 e1361. [DOI] [PubMed] [Google Scholar]
- 11.Yang B, Norton EL, Hobbs R, Farhat L, Wu X, Hornsby WE, et al. Short- and Long-term Outcomes of Aortic Root Repair and Replacement in Patients Undergoing Acute Type A Aortic Dissection Repair: 20-Year Experience. J Thorac Cardiovasc Surg. 2019;157:2125–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang B Is biologic glue the inexperienced surgeon’s best friend? J Thorac Cardiovasc Surg. 2018;157:e122–e124. [DOI] [PubMed] [Google Scholar]
- 13.Yang B, Patel HJ, Sorek C, Hornsby WE, Wu X, Ward S, et al. Sixteen-Year Experience of David and Bentall Procedures in Acute Type A Aortic Dissection. Ann Thorac Surg. 2018;105:779–784. [DOI] [PubMed] [Google Scholar]
- 14.Yang B, Norton EL, Shih T, Farhat L, Wu X, Hornsby WE, et al. Late outcomes of strategic arch resection in acute type A aortic dissection. J Thorac Cardiovasc Surg. 2019;157:1313–1321 e1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang B, Rosati CM, Norton EL, Kim KM, Khaja MS, Dasika N, et al. Endovascular Fenestration/Stenting First Followed by Delayed Open Aortic Repair for Acute Type A Aortic Dissection With Malperfusion Syndrome. Circulation. 2018;138:2091–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang B, Norton EL, Rosati CM, Wu X, Kim KM, Khaja M, et al. Managing patients with acute type A aortic dissection and mesenteric malperfusion syndrome: 20-year experience. J Thorac Cardiovasc Surg. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanger O, Oberwalder P, Dacar D, Knez I, Rigler B. Late dissection of the ascending aorta after previous cardiac surgery: risk, presentation and outcome. Eur J Cardiothorac Surg. 2002;21:453–458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.