Abstract
Objective
To understand the effectiveness of ustekinumab in treating Crohn’s disease (CD) in a UK real-world setting.
Design
Retrospective cohort study using prospectively maintained clinical records.
Setting
Single UK inflammatory bowel disease centre.
Patients
Adult patients with an established diagnosis of CD prescribed ustekinumab outside of clinical trials at University Hospital Southampton (UHS).
Interventions
Ustekinumab, a monoclonal antibody to the shared p40 subunit of interleukin (IL) 12 and IL-23 as part of routine clinical care.
Main outcome measures
Effectiveness as measured by an improvement in physician’s global assessment, drug persistence and improvement in biomarkers (C-reactive protein (CRP), albumin and calprotectin).
Results
84 patients were included, 72 had a postinduction review and 49 had 1-year data. At postinduction clinical review, clinical response occurred in 53% of patients and clinical remission occurred in 8%. For patients on ustekinumab at 1 year, clinical response occurred in 71% and remission in 14%. Adverse events included four patients with infections requiring admission, one drug-related rash, five CD surgeries and two CD exacerbations.
Conclusions
Ustekinumab was well tolerated in a complex UK CD population and demonstrated benefit to patients in terms of clinical response and improvement of biomarkers and with some patients attaining clinical remission. No unexpected safety signals were seen.
Keywords: crohn's disease, ibd clinical, inflammatory bowel disease
Significant of this study.
What is already known about this subject
The UNITI phase III randomised controlled trial programme demonstrated the efficacy of ustekinumab for the induction and maintenance of remission in moderate to severely active Crohn’s disease (CD).
The safety of ustekinumab has been demonstrated in large registry studies on psoriasis.
What this study adds
We demonstrate the real-world effectiveness of ustekinumab in inducing and maintaining clinical and biochemical response in a complex cohort of UK CD patients.
How might it impact on clinical practice in the foreseeable future
Ustekinumab should be considered by clinicians for induction and maintenance of remission in patients with CD who have failed anti-TNF therapy.
Ustekinumab should be considered as primary treatment for patients with contraindications for anti-TNF therapy (eg, heart failure or factors such as psoriasis or recurrent infections) which make anti-TNF therapy less desirable.
In patients who have not responded at week 16, a further dose should be considered as some patients show late response.
Introduction
Crohn’s disease (CD) is a chronic relapsing remitting inflammatory disorder of the gastrointestinal tract characterised by transmural discontinuous intestinal inflammation, progressive tissue injury, constitutional symptoms and extra-intestinal manifestations.
Anti-tumour necrosis factor (Anti-TNF) therapies are now first-line treatment for those with moderate to severe disease activity or an aggressive disease phenotype,1 however 40% do not respond and up to 50% lose response to anti-TNF in the first year.2 3 Vedolizumab (anti-α4β7 monoclonal antibody (mAb)) was licensed for CD in 2015, offering an alternate treatment modality.4 Meta-analysis of real world effectiveness in CD demonstrated 30% corticosteroid-free remission at 1 year.5 While need for surgery has decreased, population-based studies show over a third of patients will still require surgery in the first 5 years.6 7 The need for effective novel treatments therefore remains.
Ustekinumab is a human IgG1 kappa mAb targeting the shared p40 subunit of interleukin (IL) 12 and IL-23 which are involved in TH1 and TH17 mediated pathways of CD pathogenesis.8 9 It has been licensed in the UK for use in psoriasis and psoriatic arthritis since 2009 and 2015, respectively. Following the successful UNITI phase III programme, ustekinumab was approved by the National Institute for Health and Care Excellence for moderate to severely active CD in June 2017.10
Induction studies UNITI 1 and 2 recruited anti-TNF failed and biologic naïve patients, respectively, with active CD as judged by CRP, faecal calprotectin (FC) or endoscopy.11 The primary endpoint was a decrease in the Crohn’s Disease Activity Index (CDAI) score of ≥100 points or a CDAI score <150 at week 6. Response rates between ustekinumab 6 mg/kg or placebo were 33.7% and 21.5% in UNITI1 and 55.5% and 28.7% in UNITI2, respectively. In IM-UNITI, responders were randomised to either 90 mg subcutaneous (SC) every 8 weeks, every 12 weeks or placebo; week 44 remission (CDAI <150) was met by 53.1%, 48.8% and 35.9%, respectively. Steroid-free remission was only statistically significant in the 8-weekly group. Subsequently, 2-year follow-up data have been published for the IM-UNITI cohort showing maintenance of response and remission to 92 weeks.12
Here we present a large cohort of UK CD patients treated with ustekinumab. University Hospital Southampton (UHS) is a tertiary centre with an intestinal failure unit therefore many of our cohort would have been excluded from the above clinical trials. Our aim is to supplement the efficacy data of clinical trials with evidence of ‘real-word’ effectiveness.
Methods
This was a retrospective observational cohort study of assessments and investigations performed as part of routine care at one centre (UHS). All patients started on ustekinumab for clinically active CD outside of clinical trials and before August 2018 were included. Unless otherwise stated, patients were treated as per licence with a weight-based intravenous loading dose followed by 90 mg SC at 8 weeks and then 90 mg SC 8–12 weekly.13 See figure 1 for suggested dosing regimen.
Figure 1.
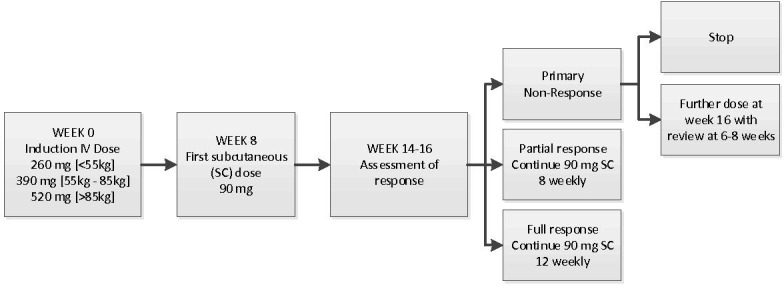
Suggested ustekinumab dosing regimen. IV, intravenous.
Electronic patient records were analysed up until December 2018. Patients were assessed at baseline, post-induction (median time 14.7 weeks, IQR 8.03) and at 1 year (median time 44.4 weeks, IQR 13.4). Clinical disease activity was scored by physician’s global assessment (PGA) using the categories remission, mild, moderate or severe.14
The primary end-point was effectiveness as measured by response (defined as an improvement in PGA category) and drug persistence. Secondary outcome measures were PGA-remission and improvement in biomarkers if abnormal at baseline (CRP >10 mg/L, FC >250 µg/g and serum albumin <35 g/L). Adverse events including admission to hospital, unplanned surgery (surgery arranged after starting ustekinumab) and infections were recorded.
Laboratory values outside the quantification limit were substituted with the upper/lower limit of quantification. Statistical analysis was performed in R V.3.4.4. The paired PGA measurements (ordinal) and the continuous variables (non-parametric) were assessed using Wilcoxon signed-rank test. Survival curves are used to show drug persistence. 95% CIs were calculated using the adjusted Wald method. One-year effectiveness calculations include all patients who had a 1-year review and all treatment failures in the first 12 months.
Results
Cohort characteristics
Eighty-four subjects were included with a total follow-up of 27 809 treatment days (table 1). Fifty-three (65%) had penetrating or perianal disease and 43 (51%) had undergone previous inflammatory bowel disease (IBD) surgery. Only three were anti-TNF naïve, with two naïve to any biological therapy. Thirty-five (42%) had previously failed both vedolizumab and at least one anti-TNF medication. Seventy-two (86%) had moderate or severe disease, with 12 treated for mild disease due to concomitant extraintestinal manifestations or an aggressive disease phenotype. Significant proportions were intolerant to thiopurines (52%), methotrexate (32%) or both (22%).
Table 1.
Patient characteristics
| Variable | n=84 |
| Female, n (%) | 53 (63.1) |
| Age in years, mean (SD) | 41.6 (14.7) |
| Smoker, n (%) | 10 (11.9) |
| Disease duration, mean (SD) | 12.3 (8.9) |
| Previous resection, n (%) | 43 (51.0) |
| Location, n (%) | |
| L1 | 28 (33.7) |
| L2 | 14 (16.9) |
| L3 | 38 (45.8) |
| L4 | 3 (3.6) |
| Behaviour, n (%) | |
| B1 | 37 (44.6) |
| B2 | 27 (32.5) |
| B3 | 19 (22.9) |
| P | 21 (25.0) |
| Previous biologics, n (%) | |
| Infliximab | 69 (82.1) |
| Adalimumab | 69 (82.1) |
| Golimumab* | 6 (7.1) |
| Certolizumab* | 4 (4.8) |
| Vedolizumab | 36 (42.9) |
| Vedolizumab and anti-TNF | 35 (41.7) |
| Nil | 2 (2.4) |
| Combination therapy, n (%) | 39 (46.4) |
| Current treatment, n (%) | |
| 6-MP | 5 (6.0) |
| Azathioprine | 14 (16.7) |
| Methotrexate | 19 (22.6) |
| Prednisolone | 6 (7.1) |
| CRP, mean (SD) | 14.7 (21.1) |
| Calprotectin, mean (SD) | 1343 (1747) |
| PGA, n (%) | |
| Mild | 11 (13.3) |
| Moderate | 39 (47.0) |
| Severe | 33 (39.8) |
*Off-licence for anti-TNF responsive disease via individual funding requests.
PGA, physician's global assessment.
After induction, 9 were treated 12 weekly and 74 patients 8 weekly. One patient with extensive disease was treated 6 weekly (off licence) on account of proven on-going disease activity following a partial response. One 12-weekly patient was dose escalated and did not respond.
Effectiveness
Of 72 patients with documented postinduction review, clinical response occurred in 38 (53%, CI: 41% to 64%) and clinical remission in 6 (8%, CI: 4% to 17%). Of 49 patients with data at 1 year, clinical response occurred in 35 (71%, CI: 58% to 82%) and remission in 7 (14%, CI: 7% to 27%) (figure 2). Steroid-free response occurred in 35 (48%, CI: 37% to 60%) postinduction and 31 (65%, CI: 49% to 75%) at 1 year. Of the patients in remission, all but one were steroid-free.
Figure 2.

Response and remission at each review.
Twenty patients with PGA assessments at baseline, postinduction and 1 year showed no improvement postinduction. Five subsequently underwent surgery. The remaining 15 who had no alternative licensed medical options were continued on ustekinumab; nine subsequently responded. See online supplementary data for the range of PGA at each review.
flgastro-2019-101237supp001.pdf (761.3KB, pdf)
flgastro-2019-101237supp002.pdf (9.7KB, pdf)
flgastro-2019-101237supp003.pdf (11.3KB, pdf)
flgastro-2019-101237supp004.pdf (21.8KB, pdf)
flgastro-2019-101237supp005.pdf (58.6KB, pdf)
Four of 6 patients on steroids at the start of the study stopped during the induction period. Five who did not respond to ustekinumab required a new course of prednisolone during the follow-up period. Of eight patients with active perianal disease at baseline, three had a documented response at postinduction review, two were de-functioned and three had ongoing disease.
We treated two biologic-naïve patients, who both responded by 12 weeks and were in clinical and biochemical remission at 1 year.
Drug persistence
Eight patients stopped ustekinumab within the first year (median 256 days, range 62–329) (figure 3). One stopped due to a cutaneous drug reaction and seven due to primary non-response or secondary loss of response (LOR). There was a non-significant trend to higher drug persistence in patients on concomitant immunosuppressant therapy (see online supplementary data).
Figure 3.
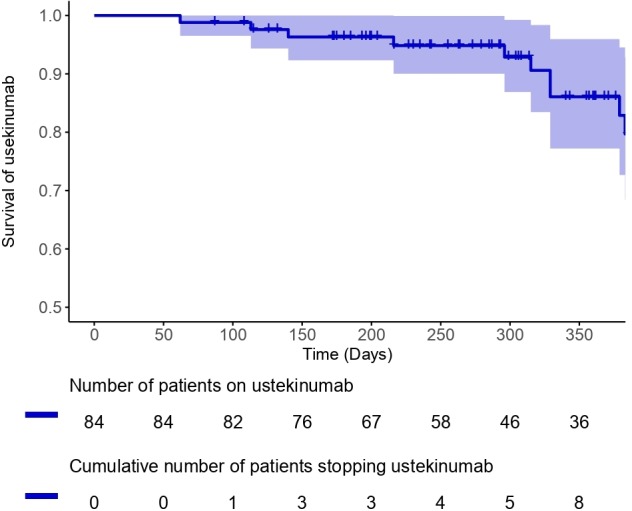
Survival curve for ustekinumab showing drug persistence and time patients stopped.
Following secondary LOR, eight patients already on 90 mg SC 8 weekly were ‘reloaded’ with a further weight-based intravenous dose in an attempt to recapture response. This approach resulted in sustained PGA improvement in only one patient.
Biomarkers
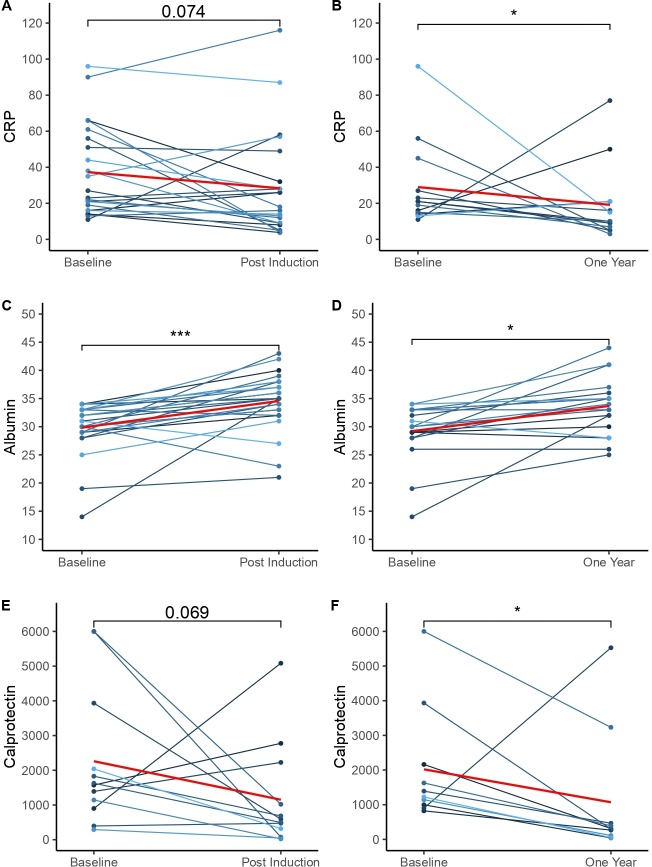
For analysis of change in CRP, albumin and FC, patients who had resections between reviews were excluded. In those with a baseline abnormal result (CRP >10 mg/L, albumin <35 g/L, FC>250 µg/g) a statistically significant improvement in all these markers was seen for patients who continued ustekinumab to their 1-year review; only albumin reached significance postinduction (figure 4). Postinduction mean CRP improved from 37 (11–96) to 28 (3.8–116) (p=0.074), mean albumin from 30 (14–43) to 35 (21–43) (p=0.0001) and FC 2260 (292–6000) to 1152 (24–5084) (p=0.089). For paired results at baseline and 1-year mean CRP improved from 29 (11–96) to 19 (3–77) (p=0.045), mean albumin from 29 (14–34) to 34 (25–44) (p=0.013) and mean FC 2019 (823–6000) to 1070 (42–5528) (p=0.011). There was no significant change in biomarkers at any time-point for patients who had a normal baseline result (see supplementary data).
Figure 4.
Changes in CRP (A, B), albumin (C, D) and calprotectin (E, F) for patients with abnormal baseline results.
Adverse events
Admissions to UHS within the first year (24 231 treatment days) were compared with the same duration prior to treatment. 22 patients were admitted post-treatment (total 400 days), compared with 29 (total 454 days) prior. Two notified the IBD team of admissions elsewhere, both for infections (fever of unknown origin and respiratory infection). Five patients treated with ustekinumab to downstage inflammatory disease had planned resection or defunctioning surgery. Five further patients had unforeseen surgery (right hemicolectomy, anastomotic resection, small bowel resection, abscess drainage and panproctocolectomy). All but one were restarted on ustekinumab following surgery. Two patients were admitted to UHS with infections; one Hickman line infection and one viral gastroenteritis. Four were admitted for nutritional support. Two were admitted with active CD, treated with intravenous steroids and subsequently stopped ustekinumab. Four had ustekinumab-unrelated admissions.
One patient had a cutaneous drug reaction which resolved on stopping ustekinumab. No other adverse events were recorded.
Discussion
Ninety-eight per cent of our patients had previously shown inadequate response or lost response to one or more anti-TNF therapies and nearly half to vedolizumab. This cohort reflects the biologic exposed, treatment-resistant patients in whom other centres may first use ustekinumab.
Our experience supports the findings of UNITI and IM-UNITI and is consistent with other real-world cohorts from the GETAID consortium, Spain and Canada.15–17 Clinical remission defined by PGA was only met in 14% who remained on ustekinumab at 1 year. For some with fibrostenotic disease, attaining PGA-defined clinical remission may be difficult, due to non-inflammatory symptoms. The proportion judged to have moderately or severely active disease was significantly lower both postinduction and at 1 year. For those with an elevated CRP or FC at baseline and a paired follow-up sample, the improvement in these biomarkers supports the more subjective clinical assessments.
Nine of 15 patients whose PGA was unchanged postinduction subsequently responded, including two to remission; indicating that clinical response can be delayed. Our data support continuation of ustekinumab beyond 3 months before defining primary non-response. The decision to continue at this point is complex and an important factor may be a paucity of alternative treatment options.
Treatment strategies to achieve maximal efficacy from ustekinumab are yet to be determined. Ustekinumab drug and anti-drug antibody (ADA) levels are not readily available in the UK and their utility has not been established. The incidence of ADAs to ustekinumab is reported to be low.18 The Canadian 1-year follow-on data from 104 responders demonstrated concomitant immunomodulation to be associated with a lower risk of secondary LOR, whereas longer follow-up data from GETAID did not find a significant difference at 3 years. The mechanisms for drug failure remain to be established. HLA DRA01*05 has been associated with the development of ADAs to both infliximab and adalimumab and should allow a more personalised approach to combination therapy.19 This association needs to be explored with other biological agents.
In IM-UNITI higher trough levels were associated with maintenance of endoscopic and clinical remission and were not affected by co-treatment with immunomodulators.18 A small prospective observational cohort study associated a week 26 trough level >4.5 µg/mL with endoscopic and biochemical response.20 The role of drug levels and dose escalation may be addressed in part by the on-going STARDUST study.21
The European licence for ustekinumab recommends maintenance dosing every 8–12 weeks. In both the GETAID and Spanish cohorts after initial IV loading some patients required dose escalation to 90 mg SC every 4–6 weeks, with success reported at 50%. Successful IV re-loading has been described after both interruption of treatment for surgery and after secondary LOR.22–24 In our cohort only one of eight patients reloaded for secondary LOR had a meaningful response.
From a safety perspective four patients were admitted with infections, which may have been related to immunosuppression with ustekinumab (one on concomitant immunomodulator). All were restarted after resolution. One patient with multiple previous drug reactions stopped ustekinumab after a generalised rash. As our analysis was conducted from hospital records, milder complications not requiring hospital input may have been missed. Safety data from the Psoriasis Longitudinal Assessment and Registry offer some reassurance. This reported over 40 000 patient-years of treatment with incidence of 0.68/100 patient-years for malignancy and 1.60/100 patient-years for serious infection.25 This malignancy rate is comparable to the general population and the unadjusted infection rate is numerically lower than for anti-TNF therapy. Likewise, the IM-UNITI 2-year follow-up data show no difference in adverse events between patients on active treatment or placebo.12
This study has significant limitations. This cohort includes patients from the regional intestinal failure service in whom assessment of clinical response to medical therapy maybe challenging. We also recognise that the lack of alternative therapeutic options may have contributed to the decision to persist with ustekinumab in patients with a partial or delayed response. Unlike the end-points required of clinical trials, patients did not systematically have CDAI or Harvey-Bradshaw Index documented. As with similar studies, we report the more subjective PGA. This reflects real-world clinical assessment and as such is a composite of patient symptoms, physician assessment, biomarkers and where available endoscopy. To allow more rigorous assessments of new treatments, there is a need for routine systematic monitoring of meaningful clinical assessments free of potential clinician bias such as validated patient-reported outcome measures.
Despite these limitations, our experiences from this challenging real-world cohort demonstrate that ustekinumab is a safe and effective treatment for UK patients with CD.
Footnotes
Contributors: RJH, MM and JRFC were responsible for the original concept and planning of the study. RJH, MM, DY, MB, LD and LP were responsible for data collection and analysis. RJH and MM contributed equally to this work and drafted the manuscript which RF, MG and JRFC critically reviewed and revised.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: Outside the submitted work the following interests are declared. RJH: personal fees from Abbvie & Janssen; non-financial support from Falk. MM: non-financial support from Falk, MSD, Janssen & Takeda. MB: Personal fees from Abbvie, Falk, Hospira, Janssen, MSD, Napp, Takeda & Pfizer. MG: personal fees and non-financial support from Abbvie & MSD; personal fees from Takeda; non-financial support from Falk & Janssen. JRFC: grants and personal fees from Samsung, Pfizer & Biogen; personal fees and non-financial support from Janssen & Abbvie; grants, personal fees and non-financial support from Takeda; personal fees from MSD, Sandoz, Celltrion & NAPP.
Patient consent for publication: Not required.
Ethics statement: The Health Research Authority does not consider postmarketing surveillance, research and therefore, suggests that NHS REC approval was not necessary.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Gomollón F, Dignass A, Annese V, et al. . 3rd European evidence-based consensus on the diagnosis and management of crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis 2017;11:3–25. 10.1093/ecco-jcc/jjw168 [DOI] [PubMed] [Google Scholar]
- 2. Adegbola SO, Sahnan K, Warusavitarne J, et al. . Anti-TNF Therapy in Crohn’s Disease. Int J Mol Sci 2018;19:2244 10.3390/ijms19082244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Papamichael K, Gils A, Rutgeerts P, et al. . Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD. Inflamm Bowel Dis 2015;21:182–97. 10.1097/MIB.0000000000000202 [DOI] [PubMed] [Google Scholar]
- 4. NICE Vedolizumab for treating moderately to severely active Crohn’s disease after prior therapy | Guidance and guidelines, 2015. Available: https://www.nice.org.uk/guidance/ta352/chapter/1-Guidance [Accessed 01 May 2019].
- 5. Schreiber S, Dignass A, Peyrin-Biroulet L, et al. . Systematic review with meta-analysis: real-world effectiveness and safety of vedolizumab in patients with inflammatory bowel disease. J Gastroenterol 2018;53:1048–64. 10.1007/s00535-018-1480-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mege D, Garrett K, Milsom J, et al. . Changing trends in surgery for abdominal Crohn’s disease. Colorectal Dis 2019;21:200–7. [DOI] [PubMed] [Google Scholar]
- 7. Lichtenstein GR, Yan S, Bala M, et al. . Infliximab maintenance treatment reduces hospitalizations, surgeries, and procedures in Fistulizing Crohn's disease. Gastroenterology 2005;128:862–9. 10.1053/j.gastro.2005.01.048 [DOI] [PubMed] [Google Scholar]
- 8. Benson JM, Peritt D, Scallon BJ, et al. . Discovery and mechanism of ustekinumab: a human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders. MAbs 2011;3:535–45. 10.4161/mabs.3.6.17815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moschen AR, Tilg H, Raine T. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol 2019;16:185–96. [DOI] [PubMed] [Google Scholar]
- 10. NICE Ustekinumab for moderately to severely active Crohn’s disease after previous treatment | Guidance and guidelines, 2017. Available: https://www.nice.org.uk/guidance/ta456/chapter/1-Recommendations [Accessed 01 May 2019].
- 11. Feagan BG, Sandborn WJ, Gasink C, et al. . Ustekinumab as induction and maintenance therapy for Crohn's disease. N Engl J Med 2016;375:1946–60. 10.1056/NEJMoa1602773 [DOI] [PubMed] [Google Scholar]
- 12. Sandborn WJ, Rutgeerts P, Gasink C, et al. . Long-term efficacy and safety of ustekinumab for Crohn's disease through the second year of therapy. Aliment Pharmacol Ther 2018;48:65–77. 10.1111/apt.14794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. EMA - European Medicines Agency Ustekinumab - Summary of product characteristics. 1–16 (2009). Available: https://www.ema.europa.eu/documents/product-information/stelara-epar-product-information_en.pdf [Accessed 20th December 2018].
- 14. ImproveCareNow Physician global Assesment. Available: https://improvecarenow.org/sites/bmidrupalpimprovecarenow.chmcres.cchmc.org/files/media/documents/Care_Providers/PGAClinicalGuidelines.pdf [Accessed 30th April 2019].
- 15. Khorrami S, Ginard D, Marín-Jiménez I, et al. . Ustekinumab for the treatment of refractory Crohnʼs disease. Inflamm Bowel Dis 2016;22:1662–9. 10.1097/MIB.0000000000000842 [DOI] [PubMed] [Google Scholar]
- 16. Wils P, Bouhnik Y, Michetti P, et al. . Long-term efficacy and safety of ustekinumab in 122 refractory Crohn's disease patients: a multicentre experience. Aliment Pharmacol Ther 2018;47:588–95. 10.1111/apt.14487 [DOI] [PubMed] [Google Scholar]
- 17. Ma C, Fedorak RN, Kaplan GG, et al. . Long-term maintenance of clinical, endoscopic, and radiographic response to ustekinumab in moderate-to-severe Crohnʼs disease. Inflamm Bowel Dis 2017;23:833–9. 10.1097/MIB.0000000000001074 [DOI] [PubMed] [Google Scholar]
- 18. Adedokun OJ, Xu Z, Gasink C, et al. . Pharmacokinetics and exposure response relationships of ustekinumab in patients with Crohn's disease. Gastroenterology 2018;154:1660–71. 10.1053/j.gastro.2018.01.043 [DOI] [PubMed] [Google Scholar]
- 19. Sazonovs A, et al. OTU-002 HLA-DQA1 contributes to the development of antibodies to anti-TNF therapy in crohn’s disease. Gut 2018;67:A52–A53. [Google Scholar]
- 20. Battat R, Kopylov U, Bessissow T, et al. . Association between ustekinumab Trough concentrations and clinical, biomarker, and endoscopic outcomes in patients with Crohn's disease. Clin Gastroenterol Hepatol 2017;15:1427–34. 10.1016/j.cgh.2017.03.032 [DOI] [PubMed] [Google Scholar]
- 21. Study of Treat to Target Versus Routine Care Maintenance Strategies in Crohn’s Disease Patients Treated With Ustekinumab - Full Text View - ClinicalTrials.gov. Available: https://clinicaltrials.gov/ct2/show/NCT03107793 [Accessed : 20th December 2018].
- 22. Evers L, Bennett AL, Garrett A, et al. . Ustekinumab Reinduction for loss of response or interruption of treatment. Am J Gastroenterol 2018;113(Supplement):S377–S378. 10.14309/00000434-201810001-00667 [DOI] [Google Scholar]
- 23. Park S, Evans E, Sandborn WJ, et al. . Ustekinumab IV 6 mg/kg Loading Dose Re-induction Improves Clinical and Endoscopic Response in Crohn's disease: A Case Series. Am J Gastroenterol 2018;113:627–9. 10.1038/ajg.2018.22 [DOI] [PubMed] [Google Scholar]
- 24. Heron V, Restellini S, Bessissow T, et al. . P438 Reinduction with ustekinumab in Crohn’s disease patients with a loss of response to treatment. J Crohn’s Colitis 2018;12(supplement_1):S326 10.1093/ecco-jcc/jjx180.565 [DOI] [Google Scholar]
- 25. Papp K, Gottlieb AB, Naldi L, et al. . Safety surveillance for ustekinumab and other psoriasis treatments from the psoriasis longitudinal assessment and registry (PSOLAR). J Drugs Dermatol 2015;14:706–14. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
flgastro-2019-101237supp001.pdf (761.3KB, pdf)
flgastro-2019-101237supp002.pdf (9.7KB, pdf)
flgastro-2019-101237supp003.pdf (11.3KB, pdf)
flgastro-2019-101237supp004.pdf (21.8KB, pdf)
flgastro-2019-101237supp005.pdf (58.6KB, pdf)