Abstract
Objective
To establish the relationship between trough vedolizumab levels and outcomes during maintenance therapy.
Design
Cross-sectional service evaluation was performed on patients with inflammatory bowel disease (IBD) receiving maintenance vedolizumab therapy (minimum of 12 weeks following induction). Prior to infusion, data on clinical activity (Harvey-Bradshaw Index or partial Mayo score), trough C-reactive protein (CRP)/vedolizumab levels and faecal calprotectin were collected. Endoscopic data (±8 weeks from vedolizumab level testing) were obtained by review of medical records. Vedolizumab levels were processed using the Immundiagnostik monitor ELISA.
Setting
The Edinburgh IBD Unit, Western General Hospital (tertiary IBD referral centre).
Patients
Seventy-three patients (30 ulcerative colitis and 43 Crohn’s disease) were identified who fulfilled inclusion criteria and had vedolizumab levels matched with clinical activity scores, CRP and faecal calprotectin. Of these, 40 patients also had matched endoscopic data.
Main outcome measures
The association of trough vedolizumab levels with clinical remission (Harvey-Bradshaw Index <5 or partial Mayo <2), biologic remission (faecal calprotectin <250 µg/g+CRP <5 mg/L) and endoscopic remission (Mayo score 0/no inflammation and ulceration on colonoscopy).
Results
The median trough vedolizumab levels were similar between patients in and not in clinical remission (10.6 vs 9.9 µg/mL, p=0.54); biologic remission (10.6 vs 9.8 µg/mL, p=0.35) and endoscopic remission (8.1 vs 10.2 µg/mL, p=0.21). Quartile analysis revealed no significant increase in the proportion of patients in clinical remission, biologic remission or endoscopic remission with increasing trough vedolizumab levels (p<0.05).
Conclusions
In this cohort, trough vedolizumab levels were not associated with clinical, biological or endoscopic outcomes during maintenance therapy.
Keywords: vedolizumab, therapeutic drug monitoring, ulcerative colitis, crohn’s disease
Summary box.
What is already known about this subject?
Therapeutic drug monitoring (TDM) is now standard of care when prescribing anti-tumour necrosis factor treatment for inflammatory bowel disease (IBD).
The role of TDM in the context of vedolizumab therapy remains unclear. Some initial studies have shown associations of post-induction levels with long-term outcomes; however, data ascertaining the utility of vedolizumab TDM during maintenance therapy is limited.
What are new findings?
In our cohort of vedolizumab-treated patients, trough vedolizumab levels during maintenance therapy for IBD were not associated with clinical, biological or endoscopic outcomes.
How might it impact on clinical practice in the foreseeable future?
Further data are required to establish the clinical utility of vedolizumab TDM during maintenance therapy prior to its use in routine clinical practice.
Introduction
Vedolizumab is the first gut selective biologic agent licensed for the treatment of moderate to severe inflammatory bowel disease (IBD).1 2 Its mechanism of action is via blockade of a4b7-integrin on memory T cells, thus preventing binding to mucosal vascular addressin cell adhesion molecule 1 (MADCAM1) receptors and migration into the gut.3 A number of studies have shown that vedolizumab is an effective induction and maintenance treatment for patients with Crohn’s disease (CD) and ulcerative colitis (UC).4 However, as is the case with anti-tumour necrosis factor (TNF) agents, a number of patients will not respond or lose response with time.5 Studies of anti-TNF agents have demonstrated that non-response may be due to sub-therapeutic drug levels.5 Furthermore, higher circulating anti-TNF levels have been associated with improved outcomes.6 7 Therefore, therapeutic drug monitoring (TDM) has become standard clinical practice in order to help monitor and optimise anti-TNF therapy.8 In contrast, limited data exist on the utility of TDM in the context of vedolizumab treatment.
In the original GEMINI trials, an exposure response relationship was demonstrated for both UC and CD.1 2 In both GEMINI 1 and 2, patients with UC and CD with week 6 vedolizumab levels in the highest quartile had higher rates of clinical response and remission at week 6 compared to patients with levels in the lowest quartile.1 2 Similar observations were made during maintenance therapy at week 52. However, no analysis was carried out to determine whether the differences were statistically significant. Furthermore, in the era of treating beyond symptoms, data is lacking on associations of vedolizumab levels with mucosal healing (MH) or normalisation of biomarkers like faecal calprotectin (FC). This study, therefore, aimed to establish the relationship between trough serum vedolizumab levels and objective clinical outcomes during maintenance therapy.
Materials and methods
Patients and design
This was a single-centre cross-sectional service evaluation. Adult patients (>16 years) on vedolizumab therapy were identified from our hospitals infusion suite records. Inclusion criteria were a diagnosis of IBD (based on standard clinical, radiological, endoscopic and histological criteria), minimum of 12 weeks vedolizumab therapy following standard induction of 300 mg at week 0, 2, 6, ±10 and no steroid use in the preceding 4 weeks. Immediately prior to patients’ next vedolizumab infusion, disease activity score (Harvey-Bradshaw Index (HBI) or partial Mayo score) was calculated; serum for routine biochemistry including CRP and trough vedolizumab levels were obtained and a stool sample for FC requested as part of routine clinical care. All samples were collected proactively irrespective of disease activity. Endoscopic data (±8 weeks from vedolizumab level collection) was obtained via review of endoscopy records. Patient demographics and disease characteristics were obtained following review of electronic medical health records (TrakCare). The primary outcome was the association of trough vedolizumab levels with clinical remission (HBI <5 or partial Mayo<2) and biologic remission (CRP <5 mg/L plus FC <250 µg/g). The definition for biologic remission was selected as it has been shown to correlate with mucosal healing.9 10 Secondary outcomes were the association of trough vedolizumab levels with endoscopic remission (Mayo score 0 or no inflammation/ulceration on colonoscopy), deep remission (clinical plus endoscopic remission) and discontinuation of treatment. In patients who had more than one sample for vedolizumab levels, only matched results at the first TDM sample were used when assessing associations so that clinicians were blinded to test results.
FC assay
Stool collection kits with instructions were given to patients at their infusion. Patients were advised to collect a sample from their first bowel motion of the day and return samples within 24 hours of collection. On arrival to the laboratories, samples were stored at −20°C. A standard ELISA technique (Calpro AS, Norway) was used to measure FC. All assays were performed in the Department of Clinical Biochemistry at the Western General Hospital, Edinburgh which now performs over 4000 assays per year.
Vedolizumab drug assay
Vedolizumab drug levels were processed at the Exeter Hospital Laboratories, UK, using the Immundiagnostik monitor ELISA as per manufacturer’s protocol. Drug levels were expressed in µg/mL.
Statistical analysis
SPSS V.24 (IBM, Chicago, Illinois, USA) and Prism V.7.0 (Graphpad Software, San Diego, California, USA) were used for statistical analyses and generation of graphs. Descriptive statistics are presented as medians with IQR for continuous variables and frequencies with percentages for categorical variables. Spearman’s correlation analysis was used to assess the association between vedolizumab levels and albumin. For non-parametric continuous and categorical variables, the Mann-Whitney and χ2 test were used, respectively. Receiver operator characteristic (ROC) curves were generated to assess the predictive ability of vedolizumab levels. Vedolizumab levels were also categorised into quartiles and rates of remission were compared across quartiles using the χ2 test of trend. Raw and false discovery rate (FDR) corrected p values (Benjamini-Hochberg test) were reported for multiple comparisons. A p value of <0.05 was considered significant for all statistical tests.
Ethics
Following local review, this study was considered a service evaluation by the local scientific officer therefore formal ethical approval was not required as per departmental policy and Health Research Authority guidance. Caldicott Guardian (NHS Lothian) approval was granted for anonymised patient data collection, analysis and submission for publication without the need for formal written consent (date: 27 September 18, application ID: 18100).
Results
Study population
A total of 110 patients were identified as receiving vedolizumab for the treatment of IBD. Of these, 73 patients (CD, n=43 and UC, n=30) fulfilled our inclusion criteria and had matched samples obtained. Patient demographics and clinical characteristics are shown in tables 1 and 2. The median age of the cohort was 35.6 years (IQR 28.9–56.4) with a median disease duration of 12.0 years (IQR 7.0–18.9). The median duration of vedolizumab therapy was 1.6 years (IQR 0.8–2.2) with 20.5% of the cohort receiving a concomitant immunomodulator (IMM).
Table 1.
Patient demographics and clinical characteristics of entire cohort at time of therapeutic drug monitoring for vedolizumab
| n= 73 | |
| Median age, years (IQR) | 35.6 (28.9–56.4) |
| Male gender, n (%) | 39 (53.4) |
| Median disease duration, years (IQR) | 12.0 (7.0–18.9) |
| Diagnosis of CD, n (%) | 43 (58.9) |
| Active smoking, n (%) | 8 (11.0) |
| Previous anti-TNF exposure, n (%) | 40 (54.8) |
| Concomitant immunomodulator, n (%) | 15 (20.5) |
| Median duration on vedolizumab, years (IQR) | 1.6 (0.8–2.2) |
| Dosing regimen, n (%) | |
| 8 weekly | 59 (80.8) |
| 4 weekly | 14 (19.2) |
| Median trough vedolizumab level, µg/mL (IQR) | 10.6 (7.9–16.1) |
| Median BMI (IQR) | 25.4 (22.5–29.7) |
| Median albumin, g/dL (IQR) | 36.0 (34.0–38.0) |
| Median CRP, mg/L (IQR) | 4.0 (2.0–8.3) |
| Median FC, µg/g (IQR) | 182.0 (54.0–569.0) |
BMI, body mass index; CRP, C-reactive protein; FC, faecal calprotectin; TNF, tumour necrosis factor.
Table 2.
Patient demographics and clinical characteristics of cohort separated by disease subtype at time of therapeutic drug monitoring for vedolizumab
| Crohn’s disease (n= 43) | Ulcerative colitis (n= 30) | |
| Median age, years (IQR) | 31.5 (24.5–59.8) | 39.9 (31.7–55.5) |
| Male gender, n (%) | 19 (44.2) | 20 (66.7) |
| Median disease duration, years (IQR) | 12.9 (7.0–27.7) | 9.5 (7.0–18.2) |
| Montreal behaviour/distribution, n (%) | ||
| L1 | 12 (27.9) | – |
| L2 | 6 (14.0) | – |
| L3 | 25 (58.1) | – |
| B1 | 20 (46.5) | – |
| B2 | 17 (39.5) | – |
| B3 | 6 (14.0) | – |
| +Perianal disease | 6 (14.0) | – |
| Montreal extent, n (%) | ||
| E1 | – | 3 (10.0) |
| E2 | – | 9 (30.0) |
| E3 | – | 18 (60.0) |
| Active smoking, n (%) | 5 (11.6) | 3 (10) |
| Previous anti-TNF exposure, n (%) | 30 (69.8) | 10 (33.3) |
| Concomitant immunomodulator, n (%) | 8 (18.6) | 7 (23.3) |
| Median duration on vedolizumab, years (IQR) | 1.4 (0.8–2.3) | 1.6 (0.7–2.2) |
| 4-weekly dosing, n (%) | 9 (20.9) | 5 (16.7) |
| Median BMI (IQR) | 24.9 (22.1–29.1) | 26.3 (22.7–30.9) |
| Median HBI/partial Mayo (IQR) | 3 (1–5) | 0 (0–1) |
| Median albumin, g/dL (IQR) | 35.0 (33.0–38.0) | 37.0 (34.8–39.0) |
| Median CRP, mg/L (IQR) | 3.0 (1.0–8.0) | 3.0 (1.0–4.3) |
| Median FC, µg/g (IQR) | 95.0 (38.0–422.0) | 43.5 (20.0–594.0) |
BMI, body mass index; CRP, C-reactive protein; FC, faecal calprotectin; HBI, Harvey-Bradshaw Index; TNF, tumour necrosis factor.
Vedolizumab trough levels
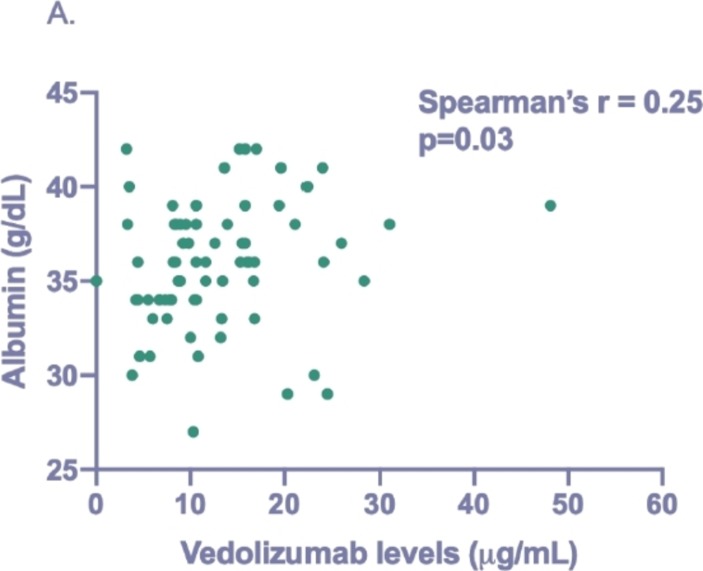
The majority of patients had detectable levels (n=71/73, 97.3%) with a median trough vedolizumab level of 10.6 µg/mL (IQR 7.9–16.1). No significant difference was observed in median levels between patients with UC and CD (11.1 µg/mL vs 10.4 µg/mL, p=0.55). Individuals on 4-weekly therapy had significantly higher median levels than those on 8 weekly (16.1 µg/mL vs 10.4 µg/mL, p=0.02). A statistically significant but weak correlation was observed between vedolizumab levels and albumin (Spearman’s r=0.25, p=0.03) (figure 1). There was no difference in median levels between patients receiving concomitant IMM (8.2 vs 11.2 µg/mL, p=0.07) or in patients receiving first-line treatment (10.6 µg/mL vs 10.6 µg/mL, p=0.84).
Figure 1.
Correlation between trough vedolizumab levels and albumin levels.
Associations of vedolizumab trough levels with clinical outcomes
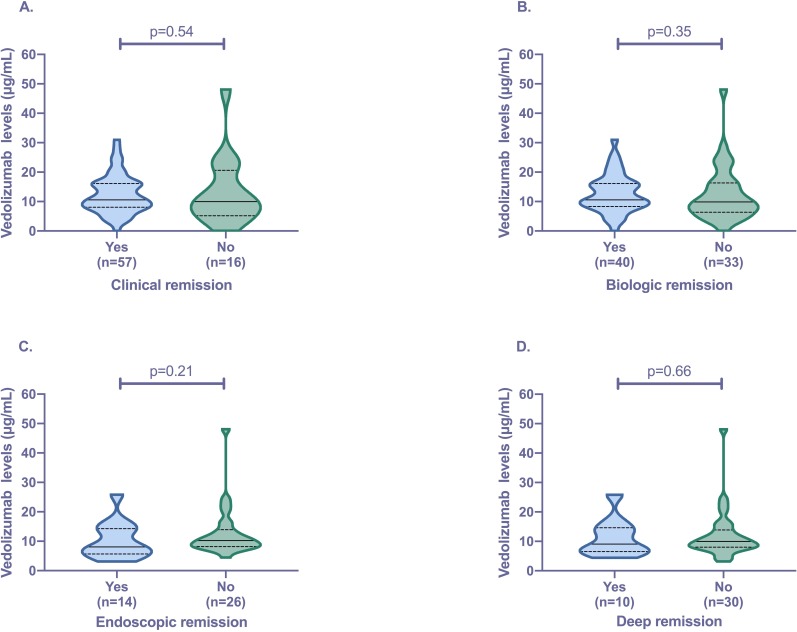
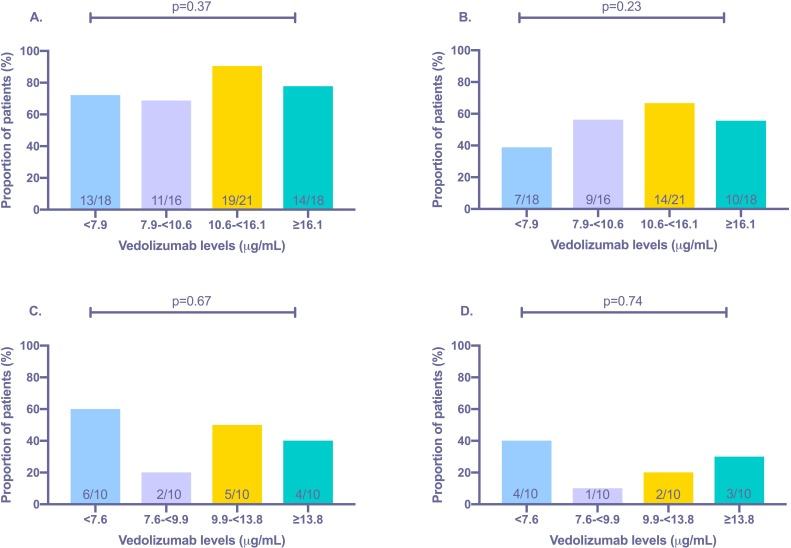
Clinical remission was present in 78.1% (n=57/73) of the cohort. There was no difference observed in median trough vedolizumab levels between patients in and not in clinical remission (10.6 vs 9.9 µg/mL, p=0.54) (figure 2). ROC analysis revealed an area under the curve (AUC) of 0.55 (95%CI 0.37 to 0.73, p=0.54) for predicting clinical remission. Quartile analysis also revealed no significant association between higher vedolizumab levels and rates of clinical remission (figure 3). Subgroup analysis, separating patients with CD and UC, again revealed no significant association between vedolizumab levels and clinical remission (table 3).
Figure 2.
Association of trough vedolizumab levels with (A) clinical remission (Harvey-Bradshaw Index<5/partial Mayo score <2); (B) biologic remission (C-reactive protein <5 mg/L plus faecal calprotectin <250 µg/g); (C) endoscopic remission (Mayo score 0/absence of inflammation and ulceration) and (D) deep remission (clinical remission plus endoscopic remission). Violin plots show median (solid line), IQR (dotted line), maximum and minimum.
Figure 3.
Associations between quartile trough vedolizumab levels and proportion of patients in (A) clinical remission (Harvey-Bradshaw Index<5/partial Mayo<2); (B) biologic remission (C-reactive protein <5 mg/L plus faecal calprotectin <250 µg/g); (C) endoscopic remission (Mayo score 0/absence of inflammation and ulceration) and (D) deep remission (clinical remission plus endoscopic remission).
Table 3.
Associations of trough vedolizumab levels with outcomes stratified by disease subtype
| Vedolizumab levels in Crohn’s disease | Yes | n | No | n | P value |
| Clinical remission (median, IQR) | 10.6 µg/mL (7.5–16.2) | 31 | 9.9 µg/mL (5.4–20.6) | 12 | 0.85 |
| Biologic remission (median, IQR) | 10.5 µg/mL (7.9–15.5) | 22 | 9.8 µg/mL (6.5–18.5) | 21 | 0.99 |
| Endoscopic remission (median, IQR) | 6.5 µg/mL (4.1–11.3) | 6 | 9.9 µg/mL (8.1–13.3) | 16 | 0.13 |
| Deep remission (median, IQR) | 10.4 µg/mL (4.4–13.9) | 3 | 8.9 µg/mL (7.9–13.2) | 19 | 0.99 |
| Vedolizumab levels in ulcerative colitis | Yes | n | No | n | P value |
| Clinical remission (median, IQR) | 12.1 µg/mL (8.6–16.2) | 26 | 8.5 µg/mL (5.0–38.7) | 4 | 0.50 |
| Biologic remission (median, IQR) | 13.1 µg/mL (9.1–16.9) | 18 | 9.5 µg/mL (6.2–15.2) | 12 | 0.14 |
| Endoscopic remission (median, IQR) | 10.7 µg/mL (6.8–16.4) | 8 | 10.6 µg/mL (8.2–15.8) | 10 | 0.88 |
| Deep remission (median, IQR) | 7.8 µg/mL (6.7–16.8) | 7 | 10.8 µg/mL (8.7–15.5) | 11 | 0.64 |
After correcting for multiple comparisons, the only significant difference between patients in clinical remission versus those not in clinical remission was a lower rate of 4-weekly dosing (supplementary table 1). Considering the significantly higher trough vedolizumab levels, as well as the lower clinical remission rates observed in those on 4-weekly dosing, we performed a subanalysis omitting these patients (n=14). However, this again did not result in any significant association between levels and clinical outcomes (supplementary figure 1)
flgastro-2019-101197supp001.pdf (175.6KB, pdf)
Association of vedolizumab trough levels with biologic remission
Biologic remission was present in 54.8% (n=40/73) of the cohort. There was no difference observed in median trough vedolizumab levels between patients in and not in biologic remission (10.6 vs 9.8 µg/mL, p=0.35) (figure 2). ROC analysis revealed an AUC of 0.56 (95%CI 0.43 to 0.70, p=0.35) for predicting biologic remission. Quartile analysis also revealed no significant association between higher vedolizumab levels and rates of biologic remission (figure 3). Reducing the FC level to <150 µg/g and <50 µg/g again resulted in no significant difference in vedolizumab levels in patients achieving the cut-offs (supplementary figure 2). Subgroup analysis, separating patients with CD and UC, again revealed no significant association between vedolizumab levels and biologic remission (table 3).
After correction for multiple comparisons, we found that patients in biologic remission had significantly lower levels of albumin and a lower rate of 4-weekly dosing versus those not in biologic remission (supplementary table 2). Considering the higher trough vedolizumab levels as well as the lower biologic remission rates in those on 4-weekly treatment, we performed a subanalysis omitting these patients (n=14). However, this again did not result in any significant association between levels and biologic remission (supplementary figure 1).
Association of vedolizumab trough levels with endoscopic and deep remission
A total of 40 patients had endoscopic data available ±8 weeks from vedolizumab level testing. Endoscopic remission and deep remission were present in 35.0% (n=14/40) and 25.0% (n=10/40) of patients, respectively. There was no difference observed in median trough vedolizumab levels between patients in and not in endoscopic remission (8.1 vs 10.2 µg/mL, p=0.21), as well as those in and not in deep remission (9.1 vs 9.9 µg/mL, p=0.66) (figure 2). ROC analysis revealed an AUC of 0.62 (95%CI 0.42 to 0.83, p=0.21) and 0.55 (95%CI 0.32 to 0.78, p=0.65) for predicting endoscopic and deep remission, respectively. Quartile analysis also revealed no significant association between higher vedolizumab levels and rates of endoscopic remission as well as deep remission (figure 3). Subgroup analysis, separating patients with CD and UC, again revealed no significant association between vedolizumab levels and endoscopic outcomes (table 3).
After correction for multiple comparisons, rates of biologic remission were significantly higher in patients in endoscopic remission/deep remission versus those who were not (supplementary tables 3 and 4). Again, we performed a subanalysis omitting patients on 4-weekly dosing (n=9), but this did not result in any significant association between levels and endoscopic outcomes (supplementary figure 1).
Association of vedolizumab trough levels with discontinuation of treatment
At 6 months post-vedolizumab level testing, 82.2% (n=60/73) remained on vedolizumab treatment. Reasons for discontinuation included: treatment failure (n=11), arthralgia (n=1) and pregnancy (n=1). Vedolizumab trough levels were similar between patients who discontinued therapy due to treatment failure and those who continued (data not shown).
Discussion
Here we present data from a cross-sectional cohort of vedolizumab-treated IBD patients, showing no clear association between maintenance vedolizumab trough levels and clinical, biological as well as endoscopic outcomes. To our knowledge, this is the only published study assessing the relationship of vedolizumab levels with faecal calprotectin and adds to the evidence that the utility of TDM during maintenance vedolizumab remains unclear.
From the studies published to date, post-induction vedolizumab levels appear to show the strongest association with outcomes.11–14 In a multicentre prospective observational study from France, a week 6 vedolizumab level of <18.5 µg/mL was shown to predict the need for dose escalation within 6 months of treatment.11 The same group also showed that week 6 levels could differentiate those with and without MH at week 52 (MH 26.8 µg/mL vs no MH, 15.1 µg/mL, p=0.035), with an optimum week 6 level of >18.0 µg/mL identified.12 Furthermore, Waljee et al showed a machine learning algorithm that incorporated week 6 levels was able to accurately predict steroid-free endoscopic remission at week 52.13 In contrast, the association between vedolizumab levels and outcomes during maintenance treatment remains unclear.
In our cohort, trough vedolizumab levels were similar in patients who were in clinical remission (HBI <5 or partial Mayo <2)/biologic remission (CRP <5 mg/L plus FC <250 µg/g) versus those who were not (figure 2, table 2). In addition, quartile analysis revealed no association between higher levels and outcomes (figure 3). Using a combination of CRP <5 mg/L plus FC <250 µg/g as a proxy for mucosal healing, our data would suggest no clear association between maintenance trough vedolizumab levels and mucosal outcomes. Furthermore, even reducing the cut-off to <150 µg/g and <50 µg/g still revealed no association (supplementary figure 2). This was further supported by our subanalysis of patients with endoscopic data available (figure 2, table 2). This data is in keeping with the recent study by Al-Bawardy et al that looked at 171 patients with IBD and showed no association between vedolizumab levels and clinical remission or mucosal healing.15
Within our cohort, we did observe higher trough vedolizumab levels in those on 4-weekly treatment (4 weekly, 16.1 µg/mL vs 8 weekly, 10.4 µg/mL, p=0.02). Furthermore, patients receiving 4-weekly vedolizumab were less likely to be in clinical or biologic remission (supplementary tables 1 and 2). This may have introduced bias, as this group of patients may represent those with more severe disease less likely to respond. However, even when these patients were omitted from our analysis, there continued to be no clear association between levels and outcomes (supplementary figure 1).
In contrast to our findings, Unagro et al recently reported significantly higher maintenance trough vedolizumab levels in patients with steroid-free clinical remission (defined by CRP <5 mg/L plus HBI <5/partial Mayo score ≤1) as well as in patients with steroid-free endoscopic remission.16 However, when the cohort was separated by disease type, the association of vedolizumab levels with clinical remission was not significant in CD and conversely the association with endoscopic remission was not significant in patients with UC. Furthermore, ROC analysis revealed the test quality of vedolizumab levels was only ‘satisfactory’ with an AUC of 0.62 and 0.67 for predicting clinical remission and endoscopic remission, respectively. Similarly, in a study from Leuven, vedolizumab trough levels of >14.0 µg/mL during maintenance therapy were associated with a higher probability of endoscopic remission.17 Although, no analysis was performed to determine whether this cut-off was independent of other confounders.
One reason that may have contributed to the lack of association observed between vedolizumab levels and outcomes is the fact that a vedolizumab level of only 3 µg/mL has been shown to result in almost complete saturation of a4b7 on peripheral blood T-cells.14 Another reason could be the delayed onset of action of vedolizumab. In many studies of efficacy, maximum benefit was observed after 6 months of treatment.4 This may explain why, for example, post-induction levels have been shown to correlate with outcomes at 1 year, while cross-sectional analysis at a fixed time point shows no association. However, in our cohort, median duration of vedolizumab treatment was relatively long at 1.6 years (IQR 0.8–2.2). Furthermore, in the study by Al-Bawardy et al, they performed a subgroup analysis on patients who had vedolizumab levels checked after at least 6 months of treatment and still showed no significant association between levels and mucosal healing.15
Interestingly, we found a significant correlation between vedolizumab levels and albumin (figure 1). Initial pharmacokinetic studies have shown that vedolizumab clearance is higher at extremes of albumin.18 In the context of anti-TNF therapy, albumin has been shown to be a significant predictor of response to treatment, an observation thought to be partly mediated via its effect on circulating drug levels.19 Whether this is the case for vedolizumab remains unclear. In our analysis, we did observe lower albumin levels in those not in biologic remission. Considering this observation, as well as the positive correlation between albumin and vedolizumab levels, lower circulating vedolizumab levels may partly contribute towards lack of response but do not appear to be independently associated.
One of the main limitations of our study was that we were unable to assess for immunogenicity due to the unavailability of a commercial antibody assay. However, many studies have reported that immunogenicity rates with vedolizumab are low. In the GEMINI-1 and GEMINI-2 trials, approximately 4% of patients were antibody positive at any time point with only 1% reported as having persistently positive antibodies.1 2 Therefore, immunogenicity is likely less of a concern with vedolizumab treatment. Further limitations include the cross-sectional nature of our study. Analysis is based on a single time point during maintenance therapy and therefore only associations can be made. Furthermore, only a subset of our cohort had endoscopic data available at time of vedolizumab level testing. However, FC in combination with CRP has been shown to correlate well with mucosal healing.9 10 Finally, as this was a service evaluation, our sample size was limited and therefore may have been underpowered to detect differences.
In conclusion, we have shown that in our IBD cohort, trough vedolizumab levels are not associated with clinical, biological or endoscopic outcomes during maintenance treatment. Further prospective studies are required to establish the true utility of vedolizumab TDM in the context of maintenance therapy in IBD.
Acknowledgments
We would like to acknowledge all the staff at the IBD infusion suite, Western General Hospital, Edinburgh, for their help and assistance with this work.
Footnotes
Twitter: @PlevrisN
Contributors: NP contributed to the study design, data collection, analysis and writing of manuscript. PWJ, CSC, ML, LMM and RJP contributed to the data collection and critically reviewed the manuscript. IDA, GRJ and CWL contributed to the study design, analysis and critically reviewed the manuscript. All authors approved the final manuscript.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: PWJ, CSC, ML, RJP and GRJ have no personal interests to declare. NP has received consultancy fees from Takeda, speaker fees and/or travel support from Abbvie, Takeda, Norgine. LMM has received travel support from Janssen. IDA has received consultancy fees from Vifor and travel support from Shire. CWL has received research support from Abbvie and Shire, consultancy fees from Abbvie, Pfizer, Dr Falk, Hospira, MSD, Pharmacosmos, Takeda and Vifor, speaker fees and/or travel support from Abbvie, Pfizer, Dr Falk, Ferring, Hospira, MSD, Shire, Takeda and Warner-Chilcott.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1. Feagan BG, Rutgeerts P, Sands BE, et al. . Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med Overseas Ed 2013;369:699–710. 10.1056/NEJMoa1215734 [DOI] [PubMed] [Google Scholar]
- 2. Sandborn WJ, Feagan BG, Rutgeerts P, et al. . Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med 2013;369:711–21. 10.1056/NEJMoa1215739 [DOI] [PubMed] [Google Scholar]
- 3. Wyant T, Fedyk E, Abhyankar B. An overview of the mechanism of action of the monoclonal antibody Vedolizumab. J Crohns Colitis 2016;10:1437–44. 10.1093/ecco-jcc/jjw092 [DOI] [PubMed] [Google Scholar]
- 4. Schreiber S, Dignass A, Peyrin-Biroulet L, et al. . Systematic review with meta-analysis: real-world effectiveness and safety of vedolizumab in patients with inflammatory bowel disease. J Gastroenterol 2018;53:1048–64. 10.1007/s00535-018-1480-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roda G, Jharap B, Neeraj N, et al. . Loss of response to Anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol 2016;7:e135 10.1038/ctg.2015.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ungar B, Levy I, Yavne Y, et al. . Optimizing Anti-TNF-α Therapy: Serum Levels of Infliximab and Adalimumab Are Associated With Mucosal Healing in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 2016;14:550–7. 10.1016/j.cgh.2015.10.025 [DOI] [PubMed] [Google Scholar]
- 7. Plevris N, Lyons M, Jenkinson PW, et al. . Higher adalimumab drug levels during maintenance therapy for Crohn's disease are associated with biologic remission. Inflamm Bowel Dis 2019;25:1036–43. 10.1093/ibd/izy320 [DOI] [PubMed] [Google Scholar]
- 8. Feuerstein JD, Nguyen GC, Kupfer SS, et al. . American gastroenterological association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology 2017;153:827–34. 10.1053/j.gastro.2017.07.032 [DOI] [PubMed] [Google Scholar]
- 9. D'Haens G, Ferrante M, Vermeire S, et al. . Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012;18:2218–24. 10.1002/ibd.22917 [DOI] [PubMed] [Google Scholar]
- 10. Colombel J-F, Panaccione R, Bossuyt P, et al. . Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 2018;390:2779–89. 10.1016/S0140-6736(17)32641-7 [DOI] [PubMed] [Google Scholar]
- 11. Williet N, Boschetti G, Fovet M, et al. . Association between low Trough levels of Vedolizumab during induction therapy for inflammatory bowel diseases and need for additional doses within 6 months. Clin Gastroenterol Hepatol 2017;15:1750–7. 10.1016/j.cgh.2016.11.023 [DOI] [PubMed] [Google Scholar]
- 12. Yacoub W, Williet N, Pouillon L, et al. . Early vedolizumab Trough levels predict mucosal healing in inflammatory bowel disease: a multicentre prospective observational study. Aliment Pharmacol Ther 2018;47:906–12. 10.1111/apt.14548 [DOI] [PubMed] [Google Scholar]
- 13. Waljee AK, Liu B, Sauder K, et al. . Predicting corticosteroid-free endoscopic remission with vedolizumab in ulcerative colitis. Aliment Pharmacol Ther 2018;47:763–72. 10.1111/apt.14510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ungar B, Kopylov U, Yavzori M, et al. . Association of Vedolizumab level, anti-drug antibodies, and α4β7 occupancy with response in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2018;16:697–705. 10.1016/j.cgh.2017.11.050 [DOI] [PubMed] [Google Scholar]
- 15. Al-Bawardy B, Ramos GP, Willrich MAV, et al. . Vedolizumab drug level correlation with clinical remission, biomarker normalization, and mucosal healing in inflammatory bowel disease. Inflamm Bowel Dis 2019;25:580–6. 10.1093/ibd/izy272 [DOI] [PubMed] [Google Scholar]
- 16. Ungaro RC, Yarur A, Jossen J, et al. . Higher Trough Vedolizumab concentrations during maintenance therapy are associated with Corticosteroid-Free remission in inflammatory bowel disease. J Crohns Colitis 2019. doi: 10.1093/ecco-jcc/jjz041. [Epub ahead of print: 13 Feb 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dreesen E, Verstockt B, Bian S, et al. . Evidence to support monitoring of Vedolizumab Trough concentrations in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2018;16:1937–46. 10.1016/j.cgh.2018.04.040 [DOI] [PubMed] [Google Scholar]
- 18. Rosario M, Dirks NL, Milch C, et al. . A review of the clinical pharmacokinetics, pharmacodynamics, and immunogenicity of Vedolizumab. Clin Pharmacokinet 2017;56:1287–301. 10.1007/s40262-017-0546-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kopylov U, Seidman E. Predicting durable response or resistance to antitumor necrosis factor therapy in inflammatory bowel disease. Therap Adv Gastroenterol 2016;9:513–26. 10.1177/1756283X16638833 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
flgastro-2019-101197supp001.pdf (175.6KB, pdf)