Abstract
Background and aims
Recent studies suggest that sedation provided by anaesthesia professionals may be less protective against serious adverse events than previously believed, however, data are lacking regarding endoscopic retrograde cholangiopancreatography (ERCP). Using the clinical outcomes research initiative national endoscopic database (CORI-NED), we aimed to assess whether mode of sedation was associated with rates of unplanned interventions (UIs) during ERCP.
Patients and methods
All subjects from CORI-NED undergoing ERCP from 2004 to 2014 were identified and stratified into three groups based on the initial mode of anaesthesia: endoscopist-directed sedation (EDS), monitored anaesthesia care without an endotracheal tube (MAC-WET) and general endotracheal anaesthesia (GEA). The primary outcome was UIs. To assess the impact of sedation mode on UIs, multivariable logistic regression models were created adjusting for demographic, physician and procedure-level variables.
Design
Population-based study.
Results
26 698 ERCPs were analysed (7588 EDS, 8395 MAC-WET, 10 715 GEA). UIs occurred in 320 ERCPs (1.2%). EDS was associated with a higher risk of UIs compared with sedation administered by an anaesthesia professional (OR 1.86, 95% CI 1.44 to 2.42). Additional factors associated with a higher risk of UIs included ASA class IV compared with class II (OR 3.18, 95% CI 2.00 to 5.06) and ERCPs done in community (OR 1.41, 1.04 to 1.91) and health maintenance organisations (OR 3.75, 2.01 to 6.99) hospitals.
Conclusion
EDS is associated with a higher risk of UIs during ERCP compared with sedation administered by an anaesthesia professional. Higher ASA class and procedures performed in non-university hospitals were also associated with a higher risk of UIs. This study suggests that, when available, sedation using an anaesthesia professional should be utilised for ERCP.
Keywords: endoscopic retrograde pancreatography, endoscopy, anesthesia, sedation, adverse events
Significance of this study.
What is already known on this subject?
The use of anaesthesia professionals for sedation during endoscopic retrograde cholangiopancreatography (ERCP) has increased in the USA, however, moderate sedation ERCP remains a common practice in the UK.
Single centre studies have shown lower rates of procedure and sedation failure with anaesthesia-administered sedation (AAS) versus moderate sedation.
It is unclear whether serious adverse event rates are affected by mode of anaesthesia for ERCP.
What are the new findings?
ERCPs performed with endoscopist-directed sedation (EDS) are associated with significantly higher rates of unplanned interventions (UIs) when compared with AAS.
ERCPs performed at university medical centres are associated with fewer UIs than those performed at community and health maintenance organisations hospital settings.
There was no difference in UIs between ERCPs done with general endotracheal anaesthesia and monitored anaesthesia care without an endotracheal tube.
How might it impact on clinical practice in the future?
This large population-based study showing that ERCP with AAS is safer than EDS, may expedite the adoption of anaesthesia services for ERCP in centres where this practice may not yet be commonplace.
Introduction
Recent studies suggest that sedation provided by anaesthesia professionals may be less protective against serious adverse events (SAEs) than previously believed.1 While these data are fairly convincing for general gastrointestinal (GI) endoscopic procedures, there is little evidence evaluating how these conclusions translate to endoscopic retrograde cholangiopancreatography (ERCP). Currently, no standard of care exists with regard to mode of sedation during ERCP and anaesthesia practice patterns vary widely.2 There are three commonly used methods of sedation during ERCP: endoscopist-directed sedation (EDS), monitored anaesthesia care which typically involves the administration of deep sedation without an endotracheal tube by an anaesthesia professional (for simplicity, herein referred to as MAC-WET) and general endotracheal anaesthesia (GEA). The decision on which mode of anaesthesia to administer during ERCP is based on many variables at many different levels. In addition to regional and institutional variations in anaesthesia practice, and anaesthesia service allocation, variables such as individual physician preference and patient variables such as body mass index (BMI), sex and medical comorbidities also play a major role into this decision-making process.
The use of an anaesthesia professional for sedation during ERCP has become increasingly popular over the last decade in the USA. The most recent American Society for Gastrointestinal Endoscopy guidelines recommend that anaesthesia-administered sedation (AAS) be considered in all complex endoscopic procedures.3 In single centre studies, the use of AAS for ERCP has been associated with lower rates of procedure and sedation failure.4 5 Additionally, AAS has been associated with decreased hospital days and lower cost when compared with gastroenterologist-administered sedation.4 While these single centre studies provide some evidence to suggest an enhanced safety profile for AAS during ERCP, it is unclear how mode of sedation affects SAEs and the use of unplanned interventions (UIs) during ERCP across a wide array of practice settings.
The national endoscopic database (NED) contains procedural data collected by the clinical outcomes research initiative (CORI) from 1995 to 2014.6 Over the course of the data collection, sites in all regions of the USA, representing a wide array of practice settings (eg, academic medical centres, community hospitals, Veterans Affairs Medical Centres, health maintenance organisations (HMO)) participated in this database. Using the CORI-NED, we aimed to assess whether mode of sedation was associated with the occurrence of UIs during ERCP. Additionally, we also aimed to assess for other predictors of the need for UIs during ERCP (eg, hospital setting, patient variables).
Methods and materials
Study subjects
We identified all subjects ≥18 years of age from the CORI-NED undergoing ERCP from 2004 to 2014. The subjects were stratified into three groups based on the initial mode of anaesthesia chosen for the ERCP; EDS, MAC-WET and GEA. The EDS group was defined as any sedation which was directly administered by the endoscopist without an anaesthesia professional. The MAC group was defined as sedation administered by an anaesthesia professional (eg, nurse anaesthetist, anaesthesiologist) without the use of an endotracheal tube. The GEA group was defined as anaesthesia administered by an anaesthesia professional with the use of an endotracheal tube. Patients were excluded from analysis if more than one type of anaesthesia was listed for the procedure, if anaesthesia other than EDS, MAC, or GEA was used, or if there were missing physician variables.
Data management
The time frame of our study spanned two versions of the CORI-NED, V.3 (V3) and V.4 (V4). Within a version, we used a masked unique procedure identifier to gather information from many relational datasets. Variables were identified using the data dictionary and consulting the user interface screen shots for comparability between V3 and V4. In some instances, we had to search free text variables in V3 in order to match discrete data entered in V4 (eg, foregut surgical history). Some patient variables, most importantly BMI, were not adequately documented and therefore could not be analysed.
Outcomes
The primary outcome was the use of UIs in response to a sedation-related adverse event (SRAE). Within CORI-NED, adverse events were entered by body system and include cardiac, pulmonary, GI and other events. Required interventions were entered into the health record according to previously defined check boxes. These interventions included the use of cautery, cardiopulmonary resuscitation, intravenous fluids administered, oxygen administered, unplanned hospital or emergency department admission, premature cessation of the procedure, reversal of sedation, unplanned surgery, blood transfusion, death and other. The ‘other’ variable in V3 was not accompanied by a free text qualifier. Because of the wide variation in the free-text descriptions of ‘other interventions’ in V4, we did not further subclassify this variable. UIs are entered into the database more reliably than SAEs and thus, we used UIs as the primary outcome.
Statistical analysis
A Cochran-Armitage Trend Test was used to assess overall trends in sedation type over time. To assess predictors of UIs, we performed univariate analyses using χ2 and Fisher’s exact tests, where appropriate. Multivariable logistic regression analyses were performed with backward selection, using predictor variables with p<0.2 from the univariate analysis and p<0.05 for retention. Two separate multivariable logistic regression models were created to assess risk factors for UIs. The first (model 1) compared EDS versus AAS (defined as GEA or MAC-WET) as the primary exposure. A second multivariable model (model 2) compared the three anaesthesia arms separately using the GEA group as the reference variable. The final models adjusted for race, ASA class, hospital setting, endoscopist graduation year (in 5 year increments), stent placement, stent removal, stent replacement, stone removal and patient age. All data management and analyses were performed using SAS V.9.3.
Results
A total of 26 698 ERCPs were analysed, including 7588 in the EDS group, 8395 in the MAC group and 10 715 in the GEA group. Over the 11-year study period, a significant trend was observed with an increase in AAS and a concomitant decrease in EDS (p<0.001) (figure 1). The trend of UIs over time is presented in figure 2. Baseline characteristics and demographic information are outlined in table 1. SAEs were observed in 320 individual ERCP procedures (1.2%). Multiple UIs were observed in 75 cases (0.3%) (table 2). The breakdown of UIs by category is presented in table 3. The most commonly documented UIs were the administration of higher levels of supplemental oxygen (82/320), the use of sedation reversal agents (62/320) and the unplanned cessation of a procedure (61/320). There were UIs documented as ‘other’ in 128 cases, but these could not be further subclassified due to unclear or absent free-text qualifying language.
Figure 1.
Trends in sedation for endoscopic retrograde cholangiopancreatography over time. GEA, general endotracheal anaesthesia; MAC-WET, monitored anaesthesia care without an endotracheal tube.
Figure 2.
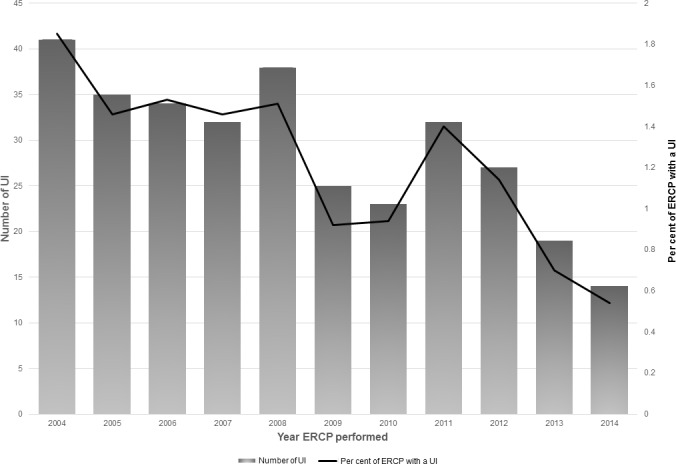
Rates of unplanned interventions (UIs) over time. ERCP, endoscopic retrograde cholangiopancreatography.
Table 1.
Baseline and demographic information on 26 698 endoscopic retrograde cholangiopancreatography procedures in the clinical outcomes research initiative national endoscopic database from 2004 to 2014
| GEA (10 715) | MAC-WET (8395) | EDS (7588) | P value | |
| Gender | <0.001 | |||
| Male | 5677 (53.0) | 3853 (45.9) | 4340 (57.2) | |
| Age (Mean±SD) | 58.3±17.1 | 58.4±18.5 | 58.2±18.1 | 0.63 |
| Race | <0.001 | |||
| White | 7974 (74.4) | 5946 (70.8) | 4841 (63.8) | |
| Black | 739 (6.9) | 544 (6.5) | 816 (10.8) | |
| Hispanic | 1382 (12.9) | 1470 (17.5) | 1206 (15.9) | |
| Other/Unknown | 620 (5.8) | 435 (5.2) | 725 (9.5) | |
| ASA Class | <0.001 | |||
| I | 613 (5.7) | 656 (7.8) | 623 (8.2) | |
| II | 4468 (41.7) | 3703 (44.1) | 4190 (55.2) | |
| III | 4428 (41.3) | 2886 (34.4) | 2255 (29.7) | |
| IV+ | 568 (5.3) | 212 (2.5) | 81 (1.1) | |
| Unknown | 638 (6.0) | 938 (11.2) | 439 (5.8) | |
| Status | <0.001 | |||
| Outpatient | 4917 (45.9) | 3753 (44.7) | 3595 (47.4) | |
| Inpatient | 4540 (42.4) | 3924 (46.7) | 3644 (48.0) | |
| Unknown | 1258 (11.7) | 718 (8.6) | 349 (4.6) | |
| Trainee involved | <0.001 | |||
| Yes | 4189 (39.1) | 2100 (25.0) | 3337 (44.0) | |
| Setting | <0.001 | |||
| University | 7134 (66.6) | 4188 (49.9) | 1547 (20.4) | |
| Community | 1634 (15.3) | 3623 (43.2) | 3645 (48.0) | |
| HMO | 716 (6.7) | 281 (3.3) | 12 (0.2) | |
| VA | 1231 (11.5) | 303 (3.6) | 2384 (31.4) |
EDS, endoscopist-directed sedation; GEA, general endotracheal anaesthesia; HMO, health maintenance organisation; MAC-WET, monitored anaesthesia care without an endotracheal tube; VA, Veterans Affairs.
Table 2.
Unplanned interventions (UIs) after ERCP by anaesthesia type
| Number of UIs per ERCP | Type of anaesthesia | |||
| GEA | MAC-WET | EDS | Total | |
| 1 | 80 | 57 | 108 | 245 |
| 2 | 16 | 19 | 17 | 52 |
| 3 | 2 | 4 | 11 | 17 |
| 4 | 1 | 0 | 3 | 4 |
| 5 | 0 | 0 | 2 | 2 |
| Total | 99 (30.9) | 80 (25.0) | 141 (44.1) | 320 |
Parenthetical numbers indicate the percentage of total UIs over the entire cohort stratified by anaesthesia type.
ERCP, endoscopic retrograde cholangiopancreatography; GEA, general endotracheal anaesthesia; MAC-WET, monitored anaesthesia care without an endotracheal tube.
Table 3.
Unplanned interventions (UIs) during ERCP by anaesthesia type*
| UI type | GEA | MAC-WET | EDS | Total |
| Oxygen administered | 10 | 36 | 36 | 82 |
| Sedation reversed | 2 | 8 | 52 | 62 |
| Procedure stopped prematurely | 11 | 23 | 27 | 61 |
| IV fluids/vasopressor for hypotension | 9 | 2 | 16 | 27 |
| Unplanned hospital admission | 12 | 3 | 11 | 26 |
| Cautery required | 4 | 2 | 12 | 18 |
| CPR | 6 | 1 | 3 | 10 |
| Emergent surgery | 4 | 2 | 3 | 9 |
| Patient death | 1 | 1 | 0 | 2 |
| Transfusion | 1 | 0 | 0 | 1 |
| Unplanned emergency department admission | 0 | 0 | 0 | 0 |
| Other | 62 | 29 | 37 | 128 |
*More than 1 UI occurred in 75/320 ERCPs.
CPR, cardiopulmonary resuscitation; ED, emergency department; EDS, endoscopist-directed sedation; ERCP, endoscopic retrograde cholangiopancreatography; MAC-WET, monitored anaesthesia care without an endotracheal tube.
Primary outcome
In multivariable analysis model A, ERCPs performed with EDS were 1.86 times as likely (95% CI 1.43 to 2.42) to have a UI when compared with ERCPs performed with AAS (figure 3A). In model B, ERCPs performed with EDS were 1.91 times as likely (95% CI 1.40 to 2.58) when compared with GEA (figure 3B). There was no significant difference in UIs observed between ERCPs performed with MAC-WET compared with GEA (OR 1.04, 95% CI 0.76 to 1.43).
Figure 3.

(A) Multivariable regression model a with the sedation reference variable=anesthetist-administered sedation (MAC-WET and GEA). (B) Multivariable regression model B with the sedation reference variable=general endotracheal anaesthesia. EDS, endoscopist-directed sedation; HMO, health maintenance organisation; MAC-WET, monitored anaesthesia care without an endotracheal tube; OR, odds ratio; VA, Veterans Affairs.
Several variables were independently associated with UIs during ERCP in multivariable analysis. In model A, African American patients were 1.45 times as likely (95% CI 1.02 to 2.06) to have an UI when compared with white patients. Persons with ERCPs performed in community hospitals (OR 1.41, 95% CI 1.04 to 1.91) and HMO hospitals (OR 3.75, 95% CI 2.01 to 6.99) were more likely to have an UI compared with persons seen in academic hospitals. Patients assessed to be ASA class IV were more likely to have an UI compared with ASA class II (OR 3.18, 95% CI 2.00 to 5.06), however, no significant difference in UIs was seen between class III (OR 1.30, 95% CI 1.00 to 1.69), or class I (OR 0.84, 95% CI 0.49 to 1.46) compared with ASA class II.
Discussion
Advanced endoscopic procedures carry a higher risk of SAEs compared with upper endoscopy and colonoscopy.7 This study is the first to utilise CORI-NED to evaluate the association between the type of anaesthesia during ERCP and the incidence of SAE resulting in the need for UIs, analysing more than 26 000 ERCP procedures performed at a wide variety of practice settings over an 11-year period. Prior studies using CORI-NED evaluated the incidence of SAE during ERCP, but have not stratified the results based on type of anaesthesia. A study by Sharma et al in 2007 used data from 1997 to 2002 in CORI-NED to evaluate cardiopulmonary unplanned events during various types of endoscopic procedures (eg, upper endoscopy, colonoscopy, ERCP, endoscopic ultrasound (EUS)).8 They found a 2.1% rate of unplanned cardiopulmonary events after 6092 ERCPs, and patients undergoing ERCP were 2.4 times more likely to have an unplanned cardiopulmonary event than those undergoing upper endoscopy.8 A 2013 study of 18 575 ERCP procedures by Enestvedt et al used CORI-NED V3 data from 2000 to 2008 to evaluate ASA class as a predictor for SAE.7 The authors defined SAE in the same way as we did in the current study, however, they excluded the interventions of intravenous fluids and supplemental oxygen administration. ERCP was associated with a 1.83% SAE rate, and although there was a general trend toward more SAEs with higher ASA class, the association in the multivariate model was not significant.7
The type of sedation used during ERCP has been shown in multiple studies to affect adverse event rates, however, existing data are conflicting and most studies have been performed in a single-centre, affecting the generalisability of the results. Some studies have suggested that the use of GEA for ERCP is associated with higher rates of haemodynamic instability and cardiac arrhythmia,9 10 however, in a recent randomised-controlled trial published by our group, we did not observe this association.11 Single-centre studies have also demonstrated lower rates of procedure and sedation failure using AAS for ERCP. In a non-randomised study, Buxbaum et al prospectively evaluated the use of AAS and EDS for ERCP and (EUS) at a single centre. The use of EDS was associated with an 8.2% sedation failure rate compared with 1.3% for AAS (multivariate OR 7.4, 95% CI 3.1 to 17.7, p<0.001) in 990 ERCPs.4 The authors estimated that failed ERCP led to an additional 93 inpatient hospital days and a cost of more than $67 000.4 Additionally, a German study by Raymondos et al demonstrated higher rates of procedure failure during ERCP with EDS when compared with GEA (14% vs 7% p=0.012), the majority of which were due to sedation failure.5 Four relatively small single-centre RCTs have evaluated the use of propofol versus conscious sedation with intermittent meperidine and midazolam.12–15 Results of these studies showed better patient cooperation13 14 and shorter recovery times12 13 15 in patients sedated with propofol. One trial demonstrated higher rates of hypoxaemia in the propofol groups, but no procedure interruption was required.14 No difference in SAE were seen, however, none of the studies were adequately powered to detect a difference in SAE of 1.2%, as we found in the present study.
Despite these data, others argue that EDS for ERCP remains appropriate in some circumstances. A recent study published in abstract form by Patel et al evaluated a selective sedation approach based on procedure indication and patient variables to compare EDS and GEA for ERCP.16 Indications for GEA included chronic medication (opiates or benzodiazepines) or alcohol use, illness severity, morbid obesity, prior failure of EDS and complexity of the ERCP. Lower rates of SAEs and shorter procedure times were observed in the EDS group, however, these differences are likely confounded by indication (ie, accounted for by the patient and procedure factors inherently used to determine which type of sedation was administered, with GEA preferentially used for more complex ERCPs). No differences in technical success or cannulation success were observed, however the authors note that 2.5% of ERCPs failed in the EDS group due to sedation AEs.16
Over time, the use of AAS for advanced endoscopic procedures in the USA has increased dramatically. During the 11-year period of our study, EDS usage for ERCP fell from over 50% of all procedures in 2004 to nearly 5% in 2014 (figure 1). In addition to the increase in anaesthesia resource allocation at many centres, this trend is due in part to the perception that advanced endoscopic procedures carry a higher incidence of adverse events compared with more routine endoscopy.7 Further, ERCP is commonly performed in the prone position, which is known to increase the risk of haemodynamic instability and can make obtaining an emergent advanced airway challenging, if required.17 18 Despite this trend in the USA, other countries such as the UK continue to perform the majority of ERCPs with EDS, in part due to the lack of available anaesthesia resources.19 20
Our study has several limitations, most of which are inherent to large database studies. First, this study is prone to site-selection bias. Sites that are willing to use the CORI-NED electronic health record and share data may differ in their clinical practice from those who are unwilling to participate.7 Second, V3 and V4 of CORI-NED contain different input variables that had to be merged by careful review and creation of clinical rules. This method may be prone to error, however, it was only utilised for a small fraction of data points and is unlikely to have a meaningful effect on the results. Third, delayed AE, namely post-ERCP pancreatitis, were not reliably entered into the CORI-NED and therefore, we cannot assess differences in this outcomes. There is also presumed under-reporting of some immediate UIs such as blood transfusions and unplanned emergency department admissions. This may alter the rates of UIs, however, this under-reporting is likely combatted to a certain degree by the large number of patient’s analysed. Additionally, V3 contained the UI category ‘other’ with no free-text explanation, which resulted in a large percentage of the UIs with ambiguous classification. We were not able to assess some meaningful patient variables such as BMI and position during ERCP as these were not entered into the database at an adequate frequency to be included in the analysis. Finally, the most common UI, ‘oxygen administered’, occurred at similar rates in the MAC-WET and EDS groups, but still occurred at a lower rate in the GEA group. We infer that this UI was primarily coded when supplemental oxygen levels were increased beyond the baseline rate at the beginning of the procedure. Unfortunately, we cannot assess to what degree the oxygen supplementation was increased or what threshold led to this particular UI.
In conclusion, this large database study suggests that the use of EDS for ERCP is associated with a higher rates of UIs when compared with sedation administered by an anaesthesia professional. Higher ASA class, and procedures performed at non-university hospitals were also associated with higher rates of UIs. These data suggest that, when available, sedation performed by an anaesthesia professional should be utilised for ERCP.
Acknowledgments
The authors wish to thank Vasian Markollari for his hard work throughout this project.
Footnotes
Contributors: ZLS: Study concept and design, database acquisition, acquisition of research funds, drafting of manuscript, critical review of manuscript, final approval of manuscript. KN: Database management, statistical analyses, critical review of manuscript, final approval of manuscript. MO: Database management, statistical analyses, critical review of manuscript, final approval of manuscript JV: Study concept and design, critical review of manuscript, final approval of manuscript. VK: Study concept and design, acquisition of research funds, critical review of manuscript, final approval of manuscript.
Funding: The Center for Administrative Data Research is supported in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) and Grant Number R24 HS19455 through the Agency for Healthcare Research and Quality (AHRQ).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No data are available.
References
- 1. Vargo JJ, Niklewski PJ, Williams JL, et al. Patient safety during sedation by anesthesia professionals during routine upper endoscopy and colonoscopy: an analysis of 1.38 million procedures. Gastrointest Endosc 2017;85:101–8. 10.1016/j.gie.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 2. Goudra B, Singh PM. ERCP: the unresolved question of endotracheal intubation. Dig Dis Sci 2014;59:513–9. 10.1007/s10620-013-2931-3 [DOI] [PubMed] [Google Scholar]
- 3. Early DS, Lightdale JR, Vargo JJ, et al. Guidelines for sedation and anesthesia in GI endoscopy. Gastrointest Endosc 2018;87:327–37. 10.1016/j.gie.2017.07.018 [DOI] [PubMed] [Google Scholar]
- 4. Buxbaum J, Roth N, Motamedi N, et al. Anesthetist-Directed sedation favors success of advanced endoscopic procedures. Am J Gastroenterol 2017;112:290–6. 10.1038/ajg.2016.285 [DOI] [PubMed] [Google Scholar]
- 5. Raymondos K, Panning B, Bachem I, et al. Evaluation of endoscopic retrograde cholangiopancreatography under conscious sedation and general anesthesia. Endoscopy 2002;34:721–6. 10.1055/s-2002-33567 [DOI] [PubMed] [Google Scholar]
- 6. NIDDK Overview of the National endoscopic database, 2018. [Google Scholar]
- 7. Enestvedt BK, Eisen GM, Holub J, et al. Is the American Society of Anesthesiologists classification useful in risk stratification for endoscopic procedures? Gastrointest Endosc 2013;77:464–71. 10.1016/j.gie.2012.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sharma VK, Nguyen CC, Crowell MD, et al. A national study of cardiopulmonary unplanned events after GI endoscopy. Gastrointest Endosc 2007;66:27–34. 10.1016/j.gie.2006.12.040 [DOI] [PubMed] [Google Scholar]
- 9. Berzin TM, Sanaka S, Barnett SR, et al. A prospective assessment of sedation-related adverse events and patient and endoscopist satisfaction in ERCP with anesthesiologist-administered sedation. Gastrointest Endosc 2011;73:710–7. 10.1016/j.gie.2010.12.011 [DOI] [PubMed] [Google Scholar]
- 10. Perbtani YB, Summerlee RJ, Yang D, et al. Impact of endotracheal intubation on interventional endoscopy unit efficiency metrics at a tertiary academic medical center. Am J Gastroenterol 2016;111:800–7. 10.1038/ajg.2016.97 [DOI] [PubMed] [Google Scholar]
- 11. Smith ZL, Mullady DK, Lang GD, et al. A randomized controlled trial evaluating general endotracheal anesthesia versus monitored anesthesia care and the incidence of sedation-related adverse events during ERCP in high-risk patients. Gastrointest Endosc 2019;89 10.1016/j.gie.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 12. Kongkam P, Rerknimitr R, Punyathavorn S, et al. Propofol infusion versus intermittent meperidine and midazolam injection for conscious sedation in ERCP. J Gastrointestin Liver Dis 2008;17:291–7. [PubMed] [Google Scholar]
- 13. Riphaus A, Stergiou N, Wehrmann T. Sedation with propofol for routine ERCP in high-risk octogenarians: a randomized, controlled study. Am J Gastroenterol 2005;100:1957–63. 10.1111/j.1572-0241.2005.41672.x [DOI] [PubMed] [Google Scholar]
- 14. Schilling D, Rosenbaum A, Schweizer S, et al. Sedation with propofol for interventional endoscopy by trained nurses in high-risk octogenarians: a prospective, randomized, controlled study. Endoscopy 2009;41:295–8. 10.1055/s-0028-1119671 [DOI] [PubMed] [Google Scholar]
- 15. Vargo JJ, Zuccaro G, Dumot JA, et al. Gastroenterologist-administered propofol versus meperidine and midazolam for advanced upper endoscopy: a prospective, randomized trial. Gastroenterology 2002;123:8–16. 10.1053/gast.2002.34232 [DOI] [PubMed] [Google Scholar]
- 16. Patel RJ, Nelsen EM, Akhter A, et al. 939 general anesthesia versus moderate conscious sedation: a prospective study on outcomes with selective sedation in ERCP. Gastrointest Endosc 2018;87:AB136–AB137. 10.1016/j.gie.2018.04.1347 [DOI] [Google Scholar]
- 17. Sudheer PS, Logan SW, Ateleanu B, et al. Haemodynamic effects of the prone position: a comparison of propofol total intravenous and inhalation anaesthesia. Anaesthesia 2006;61:138–41. 10.1111/j.1365-2044.2005.04464.x [DOI] [PubMed] [Google Scholar]
- 18. Edgcombe H, Carter K, Yarrow S. Anaesthesia in the prone position. Br J Anaesth 2008;100:165–83. 10.1093/bja/aem380 [DOI] [PubMed] [Google Scholar]
- 19. Joshi D, Paranandi B, El Sayed G, et al. Experience of propofol sedation in a UK ERCP practice: lessons for service provision. Frontline Gastroenterol 2015;6:32–7. 10.1136/flgastro-2014-100495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garewal D, Waikar P. Propofol sedation for ERCP procedures: a dilemna? Observations from an anesthesia perspective. Diagn Ther Endosc 2012;2012:1–5. 10.1155/2012/639190 [DOI] [PMC free article] [PubMed] [Google Scholar]



