Abstract
The symposium “Mechanisms, Biomarkers and Targets for Therapy in Alcohol-associated Liver Injury: From Genetics to Nutrition” was held at the 19th Congress of International Society for Biomedical Research on Alcoholism on September 13th, 2018 in Kyoto, Japan. The goal of the symposium was to discuss the importance of genetics and nutrition in alcoholic liver disease (ALD) development from mechanistic and therapeutic perspectives. The following is a summary of this session addressing the gene polymorphisms in ALD, the role of zinc in gut-liver axis perturbations associated with ALD, highlighting the importance of dietary fat in ALD pathogenesis and hepatic n6 and n3 PUFA oxylipin pattern associated with ethanol-induced liver injury, and finally deliberating on new biomarkers for alcoholic hepatitis and their implications for diagnosis and therapy. This summary of the symposium will benefit junior and senior faculty currently investigating alcohol-induced organ pathology as well as undergraduate, graduate and post-graduate students and fellows.
Keywords: alcoholic liver disease, genetic mutations, Lipid droplets, perilipins, phosphatidylcholine, phosphatidyethanolamine, oxylipins, resolvins, zinc, defensin 5, zinc transporter, acrolein-adducts, 3-hydroxypropyl-mercapturic acid, Porphyromonas gingivalis
1. Introduction
Alcohol consumption is an important social, economic, and health problem (Axley et al., 2019). Heavy alcohol consumption results in a wide range of multi-organ pathology, including alcoholic liver disease (ALD) which is a major cause of alcohol-related morbidity and mortality in the United States and Worldwide (Farooq and Bataller, 2016;Rehm et al., 2017). ALD is one of the main causes of chronic liver diseases worldwide and accounts for up to 48% of all cirrhosis-associated deaths in the United States (Lucey, 2019). The clinical spectrum of ALD ranges from fatty liver to alcoholic hepatitis, fibrosis and cirrhosis, which may progress even further to hepatocellular carcinoma (Farooq and Bataller, 2016;Roth and Qin, 2019). ALD has limited prevention and therapeutic options (Farooq and Bataller, 2016;Singh et al., 2017;Osna et al., 2017). Therefore, understanding the molecular mechanism(s) that mediate the development and progression of alcohol-induced organ pathology, as well as identifying new biomarkers, therapeutic targets and promising novel therapeutic agents to prevent, manage, or reverse the disease progression, is of paramount importance.
The advanced stages of ALD develop only in a subset of long-term heavy drinkers. Genetic polymorphisms, epigenetic changes, intestinal dysbiosis, alterations in methionine metabolism, various environmental factors (e.g., smoking and dietary habits), are some of the factors that influence the pathogenesis and progression of ALD (Osna et al., 2017;Sugimoto and Takei, 2017;Kourkoumpetis and Sood, 2019). Gender and drinking patterns are among other well-known risk factors for ALD (Osna et al., 2017). The goal of the symposium was to discuss the newer insights into the importance of genetics and nutrition in ALD development from mechanistic and therapeutic perspectives, and to analyze new biomarkers and their implications for diagnosis and therapy for ALD.
Overall, this scientific session reiterated that ALD is a complex disease and emphasized the important role that dietary and genetic factors play in the pathogenesis of ALD. The session was comprised of the following presentations:
Relevance of Genetics in Alcoholic Liver Disease;
Impaired Phospholipid Methylation Results In Decreased Lipid Droplet Lipolysis: Role In Hepatic Steatosis;
Hepatic ω−6 and ω−3 PUFA Oxylipin Patterns Associated with Ethanol-Induced Liver Injury in Mice;
Paneth Cell Dysfunction Mediates Alcohol-Induced Dysbiosis and Hepatitis in Mice: Role of Zinc Deficiency and
Biomarkers for Acute Alcoholic Hepatitis: Implications for diagnosis and therapy
The highlights of these presentation were that: (i) The novel mutations identified in Genome-wide association studies (GWAs) of ALD patients were all in genes involved in lipid metabolism/processing; (ii) The specialized cellular organelles that store lipids, lipid droplets, are altered structurally and functionally by the alcohol-induced methylation defects which impairs the breakdown of the stored lipids that ultimately contributes to the development of alcoholic steatosis; (iii) A targeted lipidomic approach revealed that the dietary fats generate distinct hepatic n6 and n3 PUFA oxylipin pattern which may contribute to exacerbation of alcoholic liver injury; (iv) Cellular zinc deficiency mediates the deleterious effects of alcohol on Paneth cells dysfunction which results in the development of alcohol-induced intestinal dysbiosis, endotoxemia and hepatitis and (v) urine 3-hydroxypropyl-mercapturic acid (HPMA) and blood P. gingivalis IgA levels may serve as good biomarkers for severe acute alcoholic hepatitis (AAH).
2. Summary of Presentations at the Symposium
2.1. Relevance of Genetics in Alcoholic Liver Disease - Devanshi Seth, PhD
It is unknown why only a proportion of heavy and prolonged alcohol misusers develop cirrhosis and others who drink similar levels don’t. In addition to the amount, type and pattern of alcohol intake, factors such as female gender and ethnicity increase the risk for ALD/alcoholic liver cirrhosis (ALC). For example, with 10 standard drinks per day, the relative risk of ALD in females reaches 10.69 compared to 4.38 for males and inheritance of risk for ALD is ~3-fold higher in monozygotic twins than dizygotic twins (Hrubec and Omenn, 1981). Furthermore, ALD-related mortality varies considerably among ethnicities, supporting a genetic basis for this disease. The role of genetics has been investigated for specific genes known to operate during ALD pathogenesis. The best examples how genetic mutations can alter the risk of ALD, comes from mutations altering enzyme kinetics in the alcohol dehydrogenase (ADH) and acetaldehyde dehydrogenase (ALDH). ADH1B rs1229984 induces ADH activity and acetaldehyde formation, whereas ALDH2*2 rs671 reduces ALDH activity impairing its ability to clear acetaldehyde. Both increase the accumulation of toxic acetaldehyde, resulting in profound rise in arterial blood flow to the face causing flushing and nausea. These SNPs are associated with reduced risk of alcoholism in carriers of one or both mutations and confer protection against alcohol use disorders including ALD. East Asians are doubly protected as these SNPs are particularly prevalent in this population. Other candidate gene studies regulating alcohol metabolism (ADH, ALDH, CYP2E1), inflammation (TNFα, IFNγ, IL10, IL1B, CD14), oxidative stress (CYP2E1, GST, MnSOD), ECM and fibrosis (TGFβ, angiotensin, CTGF, leptin, TIMPs, MMPs) (Stickel and Osterreicher, 2006) remain largely unconfirmed due to underpowered and flawed study designs.
Since ALD is a complex multifactorial disease, and its susceptibility is controlled by many genes, each gene mutation makes a small overall contribution towards the disease phenotype. In complex traits, simultaneous search for global mutations using GWAs has identified novel mutations in unsuspected genes. The only GWAs in ALD so far included a moderate size retrospective cohort of drinkers with and without cirrhosis (Buch et al., 2015). Interestingly, SNPs identified in this study (PNPLA3, TM6SF2, MBOAT7) have also been reported in nonalcoholic fatty liver disease (NAFLD)/steatohepatitis (NASH) (Romeo et al., 2008;Kozlitina et al., 2014;Mancina et al., 2016).
Patatin-like phospholipase domain containing 3 (PNPLA3) variant rs739409: C>G on chromosome 22 is the single most replicated variant in liver diseases. It is a non-synonymous single nucleotide mutation altering a highly conserved amino acid isoleucine to methionine at residue 148. PNPLA3 encodes for adiponutrin, a transmembrane protein highly expressed in the liver and adipose tissues. Previous independent studies reported a strong association of I148M with ALC (Seth et al., 2010;Tian et al., 2010), increasing the ancestry adjusted odds ratio by 1.79 per G allele (Seth et al., 2010). Our meta-analysis of 10 studies (excluding the GWAS by Buch et al), showed a significant correlation with the entire spectrum of ALD, with the magnitude of association increasing with disease severity (Salameh et al., 2015). PNPLA3 has lipogenic transacetylase and triglyceride hydrolase activities and is strongly linked with lipid droplets. It is suspected that I148M promotes hepatic intracellular lipid accumulation by reducing the breakdown of TG trapped in the lipid droplets (Pirazzi et al., 2012).
Another non-synonymous single nucleotide variant in the transmembrane 6 superfamily 2 (TM6SF2) rs58542926: C>T (E167K) on chromosome 19, was modestly associated with an increased the risk of ALD (Buch et al., 2015). This variant, also first identified in NAFLD, is associated with hepatic triglyceride content (Kozlitina et al., 2014), lipid transportation and as an independent risk factor for liver fibrosis. In vitro, the TM6SF2 inhibition was associated with increased cellular triglyceride concentration and lipid droplet content and its overexpression reduced lipid droplet size and number (Mahdessian et al., 2014).
Buch et al also reported a variant rs641738: C>T in the Membrane Bound O-AcylTransferase domain-containing 7 gene (MBOAT7) as moderately associated with ALC (Buch et al., 2015). MBOAT7 is expressed in the liver and encodes an enzyme with lysophosphatidylinositol acyltransferase (LPIAT1) activity. It is implicated in neutrophil mediated anti-inflammatory processes and inflammation-driven liver fibrogenesis. Again, this variant is associated with hepatic fat in NAFLD (Mancina et al., 2016).
Most recently, association between a variant rs72613567:T>A in the Hydroxy-Steroid 17-beta Dehydrogenase 13 (HSD17B13) gene, on chromosome 4, was reported to be associated with reduced hepatic transaminases in ALC and NAFLD (Abul-Husn et al., 2018). Previous association with plasma liver enzymes in a large functional genomic approach, was reported by the same group (Chambers et al., 2011). HSD17B13 rs72613567:TA is associated with reduced risk of all stages of liver diseases, including ALD and NAFLD. Most interestingly, the T allele mitigated the risk conferred by the risk allele of PNPLA3 (Abul-Husn et al., 2018).
It is worth noting, that all polymorphisms identified so far in ALD/are shared with NAFLD/NASH, indicating that in addition to histopathology, disease progression and other common risk factors such as obesity and diabetes, both diseases also have a common genetic basis. These shared risks indicate non-specificity to alcohol-induced liver disease.
Most intriguingly, all reported polymorphisms are involved in lipid metabolism/processing perhaps supporting inflammation and fibrogenesis during ALD progression. Despite widespread recognition that liver steatosis is the earliest and most consistent morphologic feature in heavy drinkers, the mechanism of how excessive alcohol consumption-induced liver fat accumulation plays a crucial role in disease chronicity remains unclear.
2.2. Impaired Phospholipid Methylation Results In Decreased Lipid Droplet Lipolysis: Role In Hepatic Steatosis - Kusum K. Kharbanda, PhD
With the aim of identifying factors that contribute to fatty liver, Kharbanda laboratory characterized lipid droplets (LDs). These are specialized cell organelles that store fat and are composed of a core of neutral lipid including triglycerides and cholesteryl esters. The neutral lipid core of a LD is shielded from the surrounding cytosol by a phospholipid monolayer consisting mostly of phosphatidylcholine (PC) and phosphatidylethanolamine (PE) (Bartz et al., 2007;Chitraju et al., 2012). The LD surface is further decorated with LD associated proteins whose functions vary widely and include roles in lipid metabolism, trafficking, and signaling (Fujimoto et al., 2004;Khan et al., 2015;Turro et al., 2006). Kharbanda laboratory and others have consistently observed that Wistar rats fed the ethanol Lieber DeCarli diet for 4–5 weeks exhibit dramatic increases in hepatic lipid accumulation as compared to the rats fed the control diet (Kharbanda et al., 2007). An increase in both LD size and number as revealed by BODIPY-staining was observed in the livers of ethanol-fed rats (Kharbanda et al., 2012;Listenberger et al., 2018). Differences in hepatic lipid metabolism following ethanol feeding were also detected by examining the ability of the isolated hepatocytes to catabolize accumulated fat. Hepatocytes of rats fed ethanol for 4–5 weeks displayed an approximately 50% decrease in the rate of lipolysis as compared to hepatocytes isolated from control diet fed animals (Listenberger et al., 2018). As the liver exports triglycerides and cholesterol only as constituents of very low-density lipoprotein (VLDL) particles, its secretion is one of the major mechanisms for removing liver fat. Since up to 70% of the triglycerides packaged and secreted by hepatocytes in VLDLs are derived via lipolysis of preformed triglyceride stores in LDs (Gibbons et al., 2002;Salter et al., 1998;Wiggins and Gibbons, 1992), the reduction in lipolysis observed in their recently published study (Listenberger et al., 2018) is consistent with our previous report showing a decreased in vivo VLDL secretion rate in the ethanol-fed rats compared to control rats (Kharbanda et al., 2009).
Interestingly, triacylglycerol hydrolase (TGH), a major lipase in hepatocytes that has been shown to mobilize LD triglycerides for VLDL assembly (Gilham et al., 2003;Wang et al., 2010;Wei et al., 2010), was not altered by ethanol exposure (Listenberger et al., 2018). To understand the reason for reduced lipolysis, isolated populations of LDs were prepared by differential centrifugations and the composition of these specialized structures examined. An increase in the amount of triglyceride in the hepatic LDs isolated from ethanol fed rats was detected which was consistent with the greater overall accumulation of triglyceride in the livers of these animals (Listenberger et al., 2018). Additionally, the amount of PC relative to PE was significantly decreased in the LD fractions from ethanol fed rats compared to hepatic LDs from control rats (Listenberger et al., 2018). This difference is likely due to the reduction in the generation of PC from PE via the phosphatidylethanolamine methyltransferase (PEMT)-catalyzed methylation because of the lowering of the hepatocellular SAM:SAH ratio in the ethanol fed (2.49±0.22) compared to control (4.40±0.59) rats as reported before (Barak et al., 2003;Kharbanda et al., 2007). In addition, LD fractions from the livers of ethanol fed rats contained elevated levels of perilipin 2 and 3 (Listenberger et al., 2018). These specific LD proteins have been associated with the onset of alcohol-induced hepatic steatosis (Carr and Ahima, 2016;Ikura and Caldwell, 2015) facilitating over-accumulation of lipid in the liver by preventing lipolysis (Chang et al., 2010;Carr et al., 2014;Carr et al., 2012).
How these anti-lipolytic proteins are recruited to the LD surface is largely unknown. Many organelles use special lipids (e.g., phosphoinositides, phosphatidylserine, phosphatidic acid) for mediating protein targeting to membranes (Walther and Farese, 2012;Thiam et al., 2013). We were interested in examining whether LDs utilize a similar strategy for recruiting surface proteins. Since the phospholipid ratio (PC and PE) and surface protein association (perilipins 2 and 3) in hepatic LDs are both altered by ethanol exposure (Listenberger et al., 2018), we considered the possibility that these major LD surface phospholipids were playing a role in recruiting LD proteins. To test this conjecture, we utilized an in vitro binding assay previously developed to explore perilipin 2 targeting to lipid droplet surfaces (Sletten et al., 2014).
Large unilamellar vesicles (LUVs), that mimicked the PC:PE ratios observed in the livers of control and ethanol-fed rats, were constructed and incubated with perilipin 2 from the cytosolic fraction of perilipin 2 overexpressing HEK 293 cells (Listenberger et al., 2007) as previously described (Sletten et al., 2014). Next, unbound perilipin 2 was separated from the LUVs by sucrose gradient centrifugation and the amount of perilipin 2 that fractionated with the LUVs of each phospholipid composition was determined. These experiments showed that the relative levels of PC and PE influenced the amount of perilipin 2 bound to LUVs (Listenberger et al., 2018). Specifically, perilipin 2 association increased as the ratio of PC to PE decreased. This difference was significant for PC:PE ratios that modeled the hepatic lipid droplets of control (3.5:1) and ethanol fed (2.5:1) rats.
Collectively, these studies reveal significant differences in the overall composition of hepatic LDs from control and ethanol fed rats. Especially relevant were the alterations in the relative amounts of PC and PE, along with association of specific LD proteins in hepatic LDs from ethanol fed rats. Modeling the changes to the phospholipid composition of LDs in cultured cells and in vitro experiments revealed similar changes in the association of specific LD proteins led to the conclusions that changes to the LD phospholipid composition impact protein association and LD function to impair lipolysis and subsequent VLDL secretion contributing to the development of alcoholic steatosis.
2.3. Hepatic ω−6 and ω−3 PUFA Oxylipin Patterns Associated with Ethanol-Induced Liver Injury in Mice - Irina A. Kirpich, PhD
A primary focus of Kirpich laboratory is to understand the role of different types of dietary fatty acids (FAs, e.g., saturated vs. unsaturated; and ω−6 polyunsaturated fatty acids (PUFA) vs. ω−3 PUFA) in gut-liver axis alterations associated with ALD. Kirpich laboratory investigated the potential mechanisms by which dietary FAs may exacerbate or attenuate alcohol-induced intestinal and liver injury. They postulated that oxidized PUFA metabolites (oxylipins), among other critical factors, play significant roles in ALD pathogenesis. Oxylipins are diverse bioactive lipid mediators synthesized from ω−3 and ω−6 PUFAs and may exert pro-inflammatory or dual anti-inflammatory and pro-resolution of inflammation activities (Moghaddam et al., 1996;Gabbs et al., 2015). Previous studies using a mouse model demonstrated that the combination of ethanol and a diet with a high content of linoleic acid (LA) exacerbated liver injury through increased lipoxygenase (LOX) pathway production of oxidized LA metabolites (OXLAMs) that shift RAW cells/macrophages toward an M1 pro-inflammatory phenotype (Warner et al., 2017). However, less is known regarding the role of oxidized PUFA metabolites generated via other metabolic pathways (e. CYP and soluble epoxide hydrolase [sEH]). In addition, the role of lipid mediators derived from ω−3 PUFAs such as α-linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in the ALD pathogenesis is understudied.
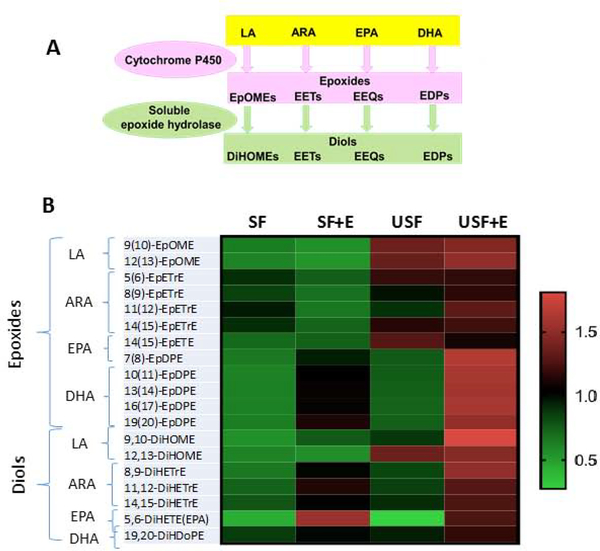
Using a targeted lipidomic approach, the pattern of ω−6 and ω−3 PUFA metabolites associated with liver injury caused by high dietary LA and ethanol consumption was examined. In this study a chronic-binge experimental mouse model of ALD (10+1 NIAAA model), which mimics the pathology of human alcoholic hepatitis (AH). Mice were fed Lieber–DeCarli liquid diets containing either saturated fat (SF, enriched in beef tallow/medium chain triglycerides) or unsaturated fat (USF, enriched in corn oil/LA). These data support a role for the CYP/sEH axis in ALD development and/or progression. CYPs metabolize numerous PUFAs and generate a variety of different signaling lipids, including epoxy-FAs, which are rapidly hydrolyzed by sEH to their corresponding vicinal diols, dihydroxy-FAs (Figure 1A). While epoxy-FAs possess potent cytoprotective, anti-oxidant and anti-inflammatory properties and mediate the resolution phase of inflammation (Gilroy et al., 2016), diols have long been considered to be less active than their parent epoxides. However, recent evidence demonstrated significant biological activity (Greene et al., 2000;Bettaieb et al., 2013;Fang et al., 2006;Oltman et al., 1998;Sisemore et al., 2001;Thompson and Hammock, 2007). In this study, the levels of epoxy-FAs were, in general, similar across all precursors and among all experimental groups; however, there were significant changes in their hydroxylated metabolites (diols, Figure 1B, adapted from (Warner et al., 2018)). One important observation was the increased levels of the LA dihydroxy metabolites, 9,10-DiHOME and 12,13-DiHOME, and α-linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) -derived 8,9-DiHETrE and 11,12-DiHETrE, in USF+ethanol-fed mice (the group with increased hepatic neutrophil-mediated inflammation and injury) compared to USF-fed control and SF+ethanol-fed animals. Further studies will be necessary to elucidate the functional significance of the increase in these dihydroxy-FAs in the context of ALD.
Figure 1.
Diet-specific alterations in oxylipin profiles in response to EtOH administration. A. Schematic representation of the CYP and sEH metabolic pathways of epoxide and diol production. B: Changes in the abundance of individual epoxides and diols between experimental groups. Results are expressed as a matrix view (heat map) where rows represent individual oxylipins and columns represent group distribution. The intensity of each color denotes the standardized ratio between each sample value and the average levels of each individual oxylipin across all samples. ARA, arachidonic acid; DHA, docosahexaenoic acid; DiHDoPEs, dihydroxy-docosapentaenoic acids; DiHETEs, dihydroxy-eicosatetraenoic acids; DiHOMEs, dihydroxy-octadecenoic acid; EPA, eicosapentaenoic acids; EpDPEs, epoxy-docosapentaenoic acids; EpETE, epoxy-eicosatetraenoic acids; EpETrEs=EETs, epoxy-eicosatrienoic acids; EpOMEs, epoxy-octadecenoic acid; Et, ethanol; LA – linoleic acid, SF, saturated fat; USF, unsaturated fat. P < 0.05 a: SF vs SF+Et; b: USF vs USF+Et; c: SF+ET vs USF+Et; d: SF vs USF
EPA and DHA are precursors to numerous metabolites, including resolvins, protectins, and maresins, that possess anti-inflammatory and pro-resolving properties (Lopez-Vicario et al., 2016;Serhan, 2014;Serhan et al., 2008). Ethanol-fed mice had altered hepatic concentrations of several hydroxy- metabolites of EPA and DHA. For example, 5-HEPE (LOX product), which is known to reduce the inflammatory response of macrophages (Wang et al., 2017), was increased in the USF+ethanol group vs. USF alone. 18-HEPE (a cyclooxygenase product and precursor to the resolvin E series (RvE), was significantly increased in both the SF+ethanol and USF+ethanol groups compared to pair-fed controls. Several DHA metabolites related to the pro-resolving lipid mediators, such as 10S,17S-dihydroxy-docosahexaenoic acid (10S,17S-DiHDoHE, a protectin D1 isomer), and 4,17-DiHDoHE, were increased by ethanol in the USF+ethanol fed mice. Interestingly, elevated levels of pro-resolving molecules in the USF+ethanol group were associated with marked liver inflammation and injury, suggesting that the resolution of inflammation likely was initiated as a natural/adaptive response to inflammation in these mice. It is possible that the deleterious effects of pro-inflammatory oxylipins outweigh the benefits of anti-inflammatory and pro-resolving bioactive lipid mediators and tip the balance toward liver damage. In a preliminary study, we found that mice treated with resolvin D1 (a DHA metabolite) had attenuated ethanol and LPS-induced liver injury (data not shown) suggesting beneficial effects of resolvins in ALD, but more research into this area is needed.
In summary, a complex diet-specific pattern of pro- and anti-inflammatory metabolites associated with ethanol-induced liver pathology was identified which may contribute to specific mechanisms of liver injury exacerbation or attenuation, and which may represent potential biomarkers and/or therapeutic targets for ALD.
2.4. Paneth Cell Dysfunction Mediates Alcohol-Induced Dysbiosis and Hepatitis in Mice: Role of Zinc Deficiency - Zhanxiang Zhou, PhD
Increasing evidence suggest that intestinal dysbiosis and gut-derived pathogen-associated molecular patterns (PAMPs) contribute to the pathogenesis of ALD through induction of hepatic inflammation (Hendrikx and Schnabl, 2018). However, the mechanisms involved in the development of intestinal dysbiosis and PAMP translocation are still not fully defined. Intestinal antimicrobial peptides (AMPs) play a critical role in regulating microbiota homeostasis and limiting bacterial translocation (Chung and Raffatellu, 2018). Paneth cells are professional AMP-producing innate immune cells and, of note, a major source of an important type of AMPs, α-defensins (Adolph et al., 2018). It is estimated that α-defensins contribute to about 70% of total bactericidal activity of the small intestine. Moreover, given the facts that ALD is associated with zinc deficiency (Mohammad et al., 2012b) and Paneth cells are enriched in zinc (Giblin et al., 2006), the role of alcohol-induced zinc deficiency in Paneth cell function and its pathophysiological consequences necessitate investigation. The present study aimed to determine if chronic alcohol exposure perturbs AMP production by Paneth cells via zinc deficiency, and if α-defensin dysfunction mediates alcohol-induced intestinal dysbiosis, PAMP translocation, and hepatitis. Mouse models of chronic alcohol exposure, genetic α-defensin dysfunction, and dietary, experimental and genetic zinc depletions were conducted. Wild type mice after 8 weeks of alcohol consumption developed intestinal dysbiosis as showed by perturbed microbiome, PAMP translocation as indicated by elevated levels of plasma endotoxins, and hepatic inflammation as indicated by increased cytokine expression and inflammatory cell infiltration. Meanwhile, the expression and bactericidal activity of Paneth cell AMPs were significantly reduced in the ileum of the alcohol-fed mice. The mechanistic link between Paneth cell AMP reduction and alcohol-induced dysbiosis as well as hepatitis was further determined using the matrix metallopeptidase 7 knockout (Mmp7−/−) mice that are deficient in active α-defensins. The Mmp7−/− mice showed more severe intestinal dysbiosis, endotoxemia and hepatitis along with reduced intestinal bactericidal activity, compared to the wild type mice after 8 weeks of alcohol feeding. Importantly, elevations of hepatic lipocalin 2 (LCN2), an immune mediator, and KC, a chemokine that attracts neutrophil infiltration, were observed to be a direct result of PAMP translocation instead of alcohol intoxication. The expression levels of both LCN2 and KC were significantly higher in the alcohol-fed MMP7−/− mice compared to the alcohol-fed wild type mice. Lipopolysaccharide (LPS) treatment directly induced LCN2 and KC production in hepatocytes, whereas ethanol or ethanol metabolites did not. Antibiotic treatment reduced PAMP translocation and prevented LCN2 and KC induction in these mice. To test if restoring Paneth cell antimicrobial activity would reverse the detrimental effects of alcohol, recombinant human defensin 5 (rHD5) was administrated to the alcohol-fed mice for the least 2 weeks of the 8-week feeding. rHD5 treatment effectively attenuated alcohol-induced intestinal dysbiosis and hepatic inflammation. Intriguingly, rHD5 upregulated Paneth cell AMP expression. To determine the role of zinc deficiency in alcohol-induced Paneth cell dysfunction, a mouse model of chronic alcohol feeding plus dietary zinc deficiency and an ex vivo model of zinc chelation of intestinal crypts was utilized. Dietary zinc deficiency exaggerated chronic alcohol-impaired Paneth cell bactericidal activity in association with downregulation of AMP gene expression. Ex vivo experiments showed that chelation of zinc from Paneth cell-secreted AMPs led to a reduced bactericidal activity. Bacterial killing experiment further showed that addition of zinc to rHD5 enhanced the bactericidal activity of both oxidized and reduced forms of rHD5, suggesting a role of zinc coordination in bactericidal activity of α-defensins. Moreover, we found that a Paneth cell-predominant zinc transporter, ZIP8, was significantly decreased by chronic alcohol feeding; the reduction was through acetaldehyde rather than zinc deficiency per se. To define the role of ZIP8 in Paneth cell zinc homeostasis and AMP activity, a Paneth cell-specific ZIP8 knockout mouse model (Zip8PC−/−) was generated. Knock out of Paneth cell ZIP8 led to decreased cellular zinc levels in Paneth cells and reduced production of α-defensins. As a result, Zip8PC−/− mice exhibited more severe hepatic inflammation. Taken together, our study suggest that Paneth cell AMP dysfunction is a pathophysiological factor in the development of alcohol-induced intestinal dysbiosis, endotoxemia and hepatitis, whereas cellular zinc deficiency may mediate the deleterious effects of alcohol on Paneth cells. The study also suggests that rHD5 has the potential to be a new class of therapeutics for treating alcoholic hepatitis.
2.5. Biomarkers for Acute Alcoholic Hepatitis: Implications for diagnosis and therapy - Craig J. McClain, MD
Biomarkers are increasingly used in evaluating and treating acute alcoholic hepatitis (AAH). Some biomarkers, such as the discriminant function (DF) and the model for end-stage liver disease (MELD) are used for inclusion of patients into treatment arms of clinical trials for severe AAH. Traditionally, DF >32 and MELD ≥20 have been criteria for inclusion in these studies (Crabb et al., 2016;Mitchell et al., 2017). These scoring systems and a variety of other static biomarkers utilize liver-related tests such as bilirubin and INR that are elevated with increasing severity of liver injury or non-hepatic factors such as creatinine that also tend to increase with very severe liver injury. Multiple other static scoring systems have been developed using logistic regression models for these types of laboratory tests and demographic factors such as age (Bissonnette et al., 2017).
The sources of biomarkers are typically serum, plasma or urine. Less commonly, saliva, breath analysis and cell-based functional assays are used. Easily-obtained specimens are optimal. Biomarkers can serve multiple functions including disease diagnosis, disease severity or prognosis, mechanisms of disease, response to therapy, or they can be a component of personalized medicine. Our group has been particularly interested in biomarkers that can also serve as indications of mechanisms of disease. We highlight below two of our recent observations on mechanistic biomarkers.
Endoplasmic Reticulum (ER) stress-Unfolded Protein Response (UPR) has recently been established as an important mechanism contributing to hepatic injury in AAH; however, the mediators and mechanisms of alcohol-induced ER stress and liver injury in AAH are not fully understood. Alcohol consumption and metabolism increase lipid peroxidation and generate acrolein, a reactive and toxic aldehyde which is known to cause ER stress and damage hepatocytes (Mohammad et al., 2012a). Acrolein exposure can also come from exogenous sources, such as cigarette smoke, fried foods, or environmental contact. Acrolein is primarily metabolized by the enzyme glutathione-S-transferase Pi (GSTP), and unless cleared, acrolein can form irreversible covalent adducts with proteins, thereby interfering with their function. Our preliminary in vivo (mice) data demonstrate that alcohol consumption results in significant hepatic acrolein generation and accumulation of acrolein-protein adducts, which correlates with ER stress, apoptosis and liver (Chen et al., 2016). Also, our data show that alcohol consumption decreases GSTP levels in mice livers, suggesting that GSTP downregulation may lead to a pathogenic accumulation of acrolein. Further, our pilot research showed that removal of acrolein by the scavenger hydralazine, significantly attenuated alcohol-induced liver injury in mice (Chen et al., 2016).
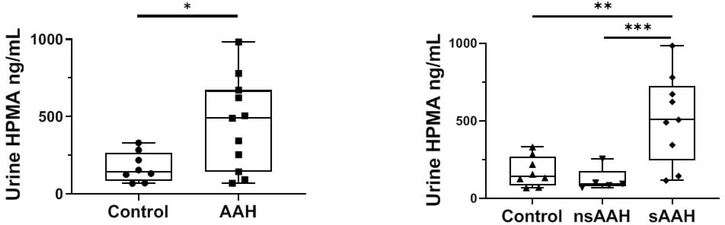
Based on these compelling observations, we extended our in vitro and mouse studies to humans with AAH. We obtained human urine specimens from individuals with AAH of varying severity and selected suitable matched non-alcoholic control specimens. We compared urine HPMA (3-hydroxypropyl-mercapturic acid, a metabolite of acrolein) levels in healthy controls and AAH patients, as a single collective group, or as divided into non-severe (MELD ≤ 19) and severe (MELD ≥ 20) groups (Fig. 2). HPMA levels were significantly higher in AAH patients as a group compared to healthy controls (Fig. 2A). There was no statistical difference found in HPMA values between non-severe AAH patients and healthy controls. When we compared HPMA levels in healthy volunteers and severe AAH patients, we found significant elevation of 3.23-fold in HPMA with a moderate effect size, adjusted R2=0.427 (Fig. 2B); with age as a covariate, the effect size was augmented to R2=0.465. We postulate that urine HPMA may be a good non-invasive biomarker for severe AAH, and that acrolein likely plays an etiologic role in AAH. These findings highlight the importance of lipids and lipid peroxidation products in AAH.
Figure 2:
Urine levels of the acrolein metabolite HPMA in healthy controls and patients with acute alcoholic hepatitis (AAH). A: HPMA levels in healthy controls and all patients with AAH, P = 0.01. B: HPMA levels in healthy controls and patients with non-severe and severe AAH. **P = 0.002 between control and patients with severe AAH, ***P = 0.001 between patients with non-severe and severe AAH. HPMA, 3-hydroxypropylmercapturic acid.
Translocation of bacteria/bacterial proteins across the gastrointestinal tract plays a critical mechanistic role in AAH. Bacterial infection is frequently observed in patients with AAH (discussed elsewhere in this manuscript). P. gingivalis is a major periodontal pathogen that plays a predominant role in chronic periodontitis, and it can also affect remote organs such as the liver. We evaluated the role of P. gingivalis in the development/progression and severity of disease in patients with AAH (Zhou et al., 2019).
Plasma specimens from 47 AAH patients (16 moderate [MELD < 20] and 31 severe [MELD ≥20]) and 22 healthy controls (HC) were collected. Antibody tests for IgG, IgM and IgA against two P. gingivalis strains were performed.
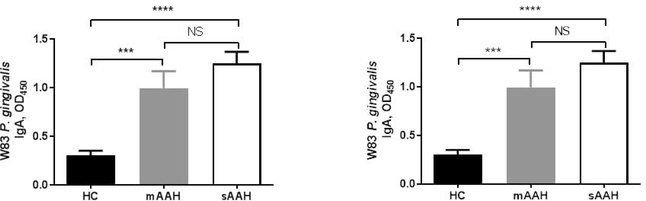
Severe AAH patients showed significantly higher plasma levels of IgG, IgA and IgM against both P. gingivalis strains (W83 and 33277) compared to HC (Zhou et al., 2019). Moderate AAH patients also had significantly-elevated anti-P. gingivalis IgA concentrations for both strains compared to HC (Fig. 3). These significantly-elevated plasma anti-P. gingivalis IgG, IgA and IgM in severe AAH provide preliminary data that P. gingivalis could be a novel risk factor in the development/progression or severity of AAH, and we postulate that P. gingivalis IgA levels serve as biomarkers for severity of AAH. This study highlights the potential importance of the oral microbiome in AAH, and it presents a unique therapeutic target.
Figure 3.
Anti-P. gingivalis IgA and AAH severity. Anti-P. gingivalis Wi3IgA levels in HCs, patients with moderate AAH, and patients with severe AAH.
In summary, the advent of molecular tools, such as proteomics and metabolomics as well as large well-characterized specimens/data sets from AAH trials should provide new biomarkers that may serve multiple functions, ranging from disease severity/prognosis to mechanisms and comparison diagnostics.
3. FINAL SUMMARY
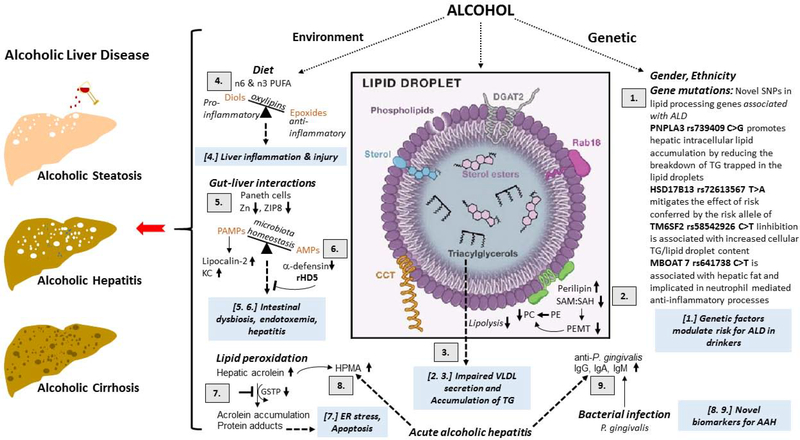
Overall, this symposium provided an update on the mechanistic and therapeutic viewpoint on the role of genetics and nutrition in the development of ALD which is schematically highlighted in Figure 4. The following summarizes the take-home message of the session’s presentations: (i) GWAS of ALD patients have identified novel mutations in many genes which are involved in lipid metabolism/processing; (ii) Alcohol-induced methylation defects alter the structure and function of the specialized cellular organelles that store lipids, lipid droplets, which impairs the breakdown of the stored lipids that ultimately contributes to the development of alcoholic steatosis; (iii) A targeted lipidomic approach revealed that the dietary fats generate distinct hepatic n6 and n3 PUFA oxylipin pattern which may contribute to exacerbation of alcoholic liver injury; (iv) Cellular zinc deficiency mediates the deleterious effects of alcohol on Paneth cells dysfunction which results in the development of alcohol-induced intestinal dysbiosis, endotoxemia and hepatitis and (v) urine 3-hydroxypropyl-mercapturic acid (HPMA) and blood P. gingivalis IgA levels may serve as good biomarkers for severe acute alcoholic hepatitis (AAH).
Figure 4.
Schematic of the update on the mechanistic and therapeutic viewpoint on the role of genetics and nutrition in the development of alcoholic liver disease (ALD).
[1.] GWAS identified SNPs in lipid processing pathways are associated with risk of progressing ALD. PNPLA3 rs739409 C>G promotes hepatic intracellular lipid accumulation by reducing the breakdown of TG trapped in the lipid droplets. HSD17B13 rs72613567 T>A mitigates the effect of risk conferred by the risk allele of PNPLA3. TM6SF2 rs58542926 C>T (E167K) inhibition is associated with increased cellular TG and lipid droplet content. MBOAT7 rs641738: C>T is associated with hepatic fat and implicated in neutrophil mediated anti-inflammatory processes.
[2.] Alcohol consumption decreases the phosphatidylethanolamine methyltransferase (PEMT)-catalyzed generation of the PC from PE that lowers the ratio of these two major classes of phospholipids on the lipid droplet (LD) surface monolayer. This change in the PC:PE ratio promotes the generation of enlarged LDs as well as recruits’ specific proteins such as perilipins 2 to the LD surface.
[3.] Supersized LDs and increase in specific perilipins prevents the access of lipases to LD triglyceride stores to inhibit their lipolysis thus facilitating lipid over-accumulation in the liver contributing to the development of alcoholic steatosis.
[4.] Ethanol-induced liver injury is associated with distinct profile of bioactive n6 and n3 PUFA metabolites. CYPs metabolize numerous PUFAs and generate a variety of different signalling oxidised lipids (oxylipins), including anti-inflammatory epoxy-FAs, which are rapidly hydrolyzed by sEH to their corresponding vicinal diols, dihydroxy-FAs, which are less active or might be pro-inflammatory. Possibly the deleterious effects of pro-inflammatory oxylipins outweigh the benefits of anti-inflammatory and pro-resolving lipid mediators and tip the balance toward liver damage.
[5.] Chronic alcohol impairs zinc transporter, ZIP8, activity leading to zinc deficiency in Paneth cells. The consequent reduction in antimicrobial peptides (AMPs, a-defensin) allows for pathogen associated molecular patterns (PAMPS) translocation from the intestine to the liver to induce pro-inflammatory lipocalin-2 and KC chemokine production, thereby contributing to alcoholic hepatitis.
[6.] Restoring a-defensin by recombinant human defensin 5 (rDH5) restores AMP, reduces intestinal dysbiosis, endotoxemia and hepatitis and is potentially a new class of therapeutic for treating alcoholic hepatitis.
[7.] Alcohol consumption and metabolism increase lipid peroxidation and generate acrolein. The concomitant alcohol-reduced reduction in glutathione-S-transferase Pi (GSTP) impairs the clearance of acrolein facilitating its accumulation and protein adduct formation causing ER stress.
[8.] Acrolein metabolite 3-hydroxypropyl-mercapturic acid (HMPA) is increased in urine of acute alcoholic hepatitis (AAH) patients. Urine HPMA may be a good non-invasive biomarker for severe AAH. These findings highlight the importance of lipids and lipid peroxidation products in AAH.
[9.] P. gingivalis is a major periodontal pathogen that can also affect remote organs such as the liver. Plasma levels of IgG, IgA and IgM against P. gingivalis strains are increased in severe acute alcoholic hepatitis and can serve as biomarkers for severity of AAH.
The symposium “Mechanisms, Biomarkers and Targets for Therapy in Alcohol-associated Liver Injury: From Genetics to Nutrition” was held at the 19th Congress of International Society for Biomedical Research on Alcoholism on September 13th, 2018 in Kyoto, Japan. The goal of the symposium was to discuss the importance of genetics and nutrition in alcoholic liver disease (ALD) development from mechanistic and therapeutic perspectives. Overall, this scientific session reiterated that ALD is a complex disease and highlighted the important role that dietary and genetic factors play in the pathogenesis of alcoholic liver disease.
Acknowledgments
GRANT SUPPORT
The work presented in this summary was supported by Merit Review grants BX004053 (KKK) and BX002996 (CJM) from the U.S. Department of Veterans Affairs and NIH grants, R01AA026723 (KKK), R01AA024102 (IAK), R01 026936 (WF), R01AA023190 (WF), R01AA020212 (ZZ), R01AA023681 (CJM), U01AA022489 (CJM), 1U01AA021901 (CJM), 1U01 AA021893 (CJM), P20GM113226 (CJM), P50AA024337 (CJM).
Abbreviations
- HPMA
3-hydroxypropyl-mercapturic acid
- ALDH
acetaldehyde dehydrogenase
- AAH
acute alcoholic hepatitis
- ALD
alcoholic liver disease
- ALC
alcoholic liver cirrhosis
- ADH
alcohol dehydrogenase
- ALA
α-linolenic acid
- AMPs
antimicrobial peptides
- CTGF
connective tissue growth factor
- CYP
cytochrome P450
- DF
discriminant function
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- ER
endoplasmic reticulum
- FA
fatty acids
- GWAs
genome-wide association studies
- GST
glutathione S-transferase
- GSTP
glutathione-S-transferase Pi
- HSD17B13
hydroxy-steroid 17-beta dehydrogenase 13
- LUVs
large unilamellar vesicles
- LA
linoleic acid
- LDs
lipid droplets
- LCN2
lipocalin 2
- LOX
lipoxygenase
- LPIAT1
lysophosphatidylinositol acyltransferase
- MnSOD
manganese superoxide dismutase
- MBOAT7
membrane bound O-acyltransferase domain-containing 7 gene
- MMP
metallopeptidase
- MELD
model for end-stage liver disease
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- OXLAMs
oxidized LA metabolites
- PNPLA3
Patatin-like phospholipase domain containing 3
- PAMPs
pathogen-associated molecular patterns
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PEMT
phosphatidylethanolamine methyltransferase
- PUFA
polyunsaturated fatty acids
- rHD5
recombinant human defensin 5
- RvE
resolvin E series
- SF
saturated fat
- she
soluble epoxide hydrolase
- TIMPs
tissue inhibitors of metalloproteinases
- TM6SF2
transmembrane 6 superfamily 2
- TGHL
triacylglycerol hydrolase
- UPR
unfolded protein response
- USF
unsaturated fatty acids
- VLDL
very low-density lipoprotein
Footnotes
Conflicts of Interest: The authors have no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Global status report on alcohol and health 2018, in Series Global status report on alcohol and health 2018, Geneva: World Health Organization; 2018. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Abul-Husn NS, Cheng X, Li AH, Xin Y, Schurmann C, Stevis P, Liu Y, Kozlitina J, Stender S, Wood GC, Stepanchick AN, Still MD, McCarthy S, O’Dushlaine C, Packer JS, Balasubramanian S, Gosalia N, Esopi D, Kim SY, Mukherjee S, Lopez AE, Fuller ED, Penn J, Chu X, Luo JZ, Mirshahi UL, Carey DJ, Still CD, Feldman MD, Small A, Damrauer SM, Rader DJ, Zambrowicz B, Olson W, Murphy AJ, Borecki IB, Shuldiner AR, Reid JG, Overton JD, Yancopoulos GD, Hobbs HH, Cohen JC, Gottesman O, Teslovich TM, Baras A, Mirshahi T, Gromada J, Dewey FE (2018) A Protein-Truncating HSD17B13 Variant and Protection from Chronic Liver Disease. N. Engl. J. Med 378:1096–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph TE, Mayr L, Grabherr F, Tilg H (2018) Paneth Cells and their Antimicrobials in Intestinal Immunity. Curr. Pharm. Des 24:1121–1129. [DOI] [PubMed] [Google Scholar]
- Axley PD, Richardson CT, Singal AK (2019) Epidemiology of Alcohol Consumption and Societal Burden of Alcoholism and Alcoholic Liver Disease. Clin Liver Dis 23:39–50. [DOI] [PubMed] [Google Scholar]
- Barak AJ, Beckenhauer HC, Mailliard ME, Kharbanda KK, Tuma DJ (2003) Betaine lowers elevated S-adenosylhomocysteine levels in hepatocytes from ethanol-fed rats. J. Nutr. 133:2845–2848. [DOI] [PubMed] [Google Scholar]
- Bartz R, Li WH, Venables B, Zehmer JK, Roth MR, Welti R, Anderson RG, Liu P, Chapman KD (2007) Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J. Lipid Res 48:837–847. [DOI] [PubMed] [Google Scholar]
- Bettaieb A, Nagata N, AbouBechara D, Chahed S, Morisseau C, Hammock BD, Haj FG (2013) Soluble epoxide hydrolase deficiency or inhibition attenuates diet-induced endoplasmic reticulum stress in liver and adipose tissue. J. Biol. Chem 288:14189–14199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette J, Altamirano J, Devue C, Roux O, Payance A, Lebrec D, Bedossa P, Valla D, Durand F, Ait-Oufella H, Sancho-Bru P, Caballeria J, Gines P, Boulanger CM, Bataller R, Rautou PE (2017) A prospective study of the utility of plasma biomarkers to diagnose alcoholic hepatitis. Hepatology 66:555–563. [DOI] [PubMed] [Google Scholar]
- Buch S, Stickel F, Trepo E, Way M, Herrmann A, Nischalke HD, Brosch M, Rosendahl J, Berg T, Ridinger M, Rietschel M, McQuillin A, Frank J, Kiefer F, Schreiber S, Lieb W, Soyka M, Semmo N, Aigner E, Datz C, Schmelz R, Bruckner S, Zeissig S, Stephan A-M, Wodarz N, Deviere J, Clumeck N, Sarrazin C, Lammert F, Gustot T, Deltenre P, Volzke H, Lerch MM, Mayerle J, Eyer F, Schafmayer C, Cichon S, Nothen MM, Nothnagel M, Ellinghaus D, Huse K, Franke A, Zopf S, Hellerbrand C, Moreno C, Franchimont D, Morgan MY, Hampe J (2015) A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat. Genet 47:1443–1448. [DOI] [PubMed] [Google Scholar]
- Carr RM, Ahima RS (2016) Pathophysiology of lipid droplet proteins in liver diseases. Exp. Cell Res 340:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RM, Patel RT, Rao V, Dhir R, Graham MJ, Crooke RM, Ahima RS (2012) Reduction of TIP47 improves hepatic steatosis and glucose homeostasis in mice. Am J Physiol Regul Integr Comp Physiol 302:R996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RM, Peralta G, Yin X, Ahima RS (2014) Absence of perilipin 2 prevents hepatic steatosis, glucose intolerance and ceramide accumulation in alcohol-fed mice. PLoS One 9:e97118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JC, Zhang W, Sehmi J, Li X, Wass MN, Van der Harst P, Holm H, Sanna S, Kavousi M, Baumeister SE, Coin LJ, Deng G, Gieger C, Heard-Costa NL, Hottenga J-J, Kuhnel B, Kumar V, Lagou V, Liang L, Luan Ja, Vidal PM, Leach IM, O’Reilly PF, Peden JF, Rahmioglu N, Soininen P, Speliotes EK, Yuan X, Thorleifsson G, Alizadeh BZ, Atwood LD, Borecki IB, Brown MJ, Charoen P, Cucca F, Das D, de Geus EJC, Dixon AL, Doring A, Ehret G, Eyjolfsson GI, Farrall M, Forouhi NG, Friedrich N, Goessling W, Gudbjartsson DF, Harris TB, Hartikainen A-L, Heath S, Hirschfield GM, Hofman A, Homuth G, Hypponen E, Janssen HLA, Johnson T, Kangas AJ, Kema IP, Kuhn JP, Lai S, Lathrop M, Lerch MM, Li Y, Liang TJ, Lin J-P, Loos RJF, Martin NG, Moffatt MF, Montgomery GW, Munroe PB, Musunuru K, Nakamura Y, O’Donnell CJ, Olafsson I, Penninx BW, Pouta A, Prins BP, Prokopenko I, Puls R, Ruokonen A, Savolainen MJ, Schlessinger D, Schouten JNL, Seedorf U, Sen-Chowdhry S, Siminovitch KA, Smit JH, Spector TD, Tan W, Teslovich TM, Tukiainen T, Uitterlinden AG, Van der Klauw MM, Vasan RS, Wallace C, Wallaschofski H, Wichmann HE, Willemsen G, Wurtz P, Xu C, Yerges-Armstrong LM, Abecasis GR, Ahmadi KR, Boomsma DI, Caulfield M, Cookson WO, van Duijn CM, Froguel P, Matsuda K, McCarthy MI, Meisinger C, Mooser V, Pietilainen KH, Schumann G, Snieder H, Sternberg MJE, Stolk RP, Thomas HC, Thorsteinsdottir U, Uda M, Waeber G, Wareham NJ, Waterworth DM, Watkins H, Whitfield JB, Witteman JCM, Wolffenbuttel BHR, Fox CS, Ala-Korpela M, Stefansson K, Vollenweider P, Volzke H, Schadt EE, Scott J, Jarvelin M-R, Elliott P, Kooner JS (2011) Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nat. Genet 43:1131–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BH, Li L, Saha P, Chan L (2010) Absence of adipose differentiation related protein upregulates hepatic VLDL secretion, relieves hepatosteatosis, and improves whole body insulin resistance in leptin-deficient mice. J. Lipid Res 51:2132–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Zhang J, Ghare S, Barve S, McClain C, Joshi-Barve S (2016) Acrolein Is a Pathogenic Mediator of Alcoholic Liver Disease and the Scavenger Hydralazine Is Protective in Mice. Cell Mol Gastroenterol Hepatol 2:685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitraju C, Trotzmuller M, Hartler J, Wolinski H, Thallinger GG, Lass A, Zechner R, Zimmermann R, Kofeler HC, Spener F (2012) Lipidomic analysis of lipid droplets from murine hepatocytes reveals distinct signatures for nutritional stress. J. Lipid Res 53:2141–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung LK, Raffatellu M (2018) G.I. pros: Antimicrobial defense in the gastrointestinal tract. Semin. Cell Dev. Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb DW, Bataller R, Chalasani NP, Kamath PS, Lucey M, Mathurin P, McClain C, McCullough A, Mitchell MC, Morgan TR, Nagy L, Radaeva S, Sanyal A, Shah V, Szabo G, Consortia NAH (2016) Standard Definitions and Common Data Elements for Clinical Trials in Patients With Alcoholic Hepatitis: Recommendation From the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology 150:785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Hu S, Xu B, Snyder GD, Harmon S, Yao J, Liu Y, Sangras B, Falck JR, Weintraub NL, Spector AA (2006) 14,15-Dihydroxyeicosatrienoic acid activates peroxisome proliferator-activated receptor-alpha. Am J Physiol Heart Circ Physiol 290:H55–63. [DOI] [PubMed] [Google Scholar]
- Farooq MO, Bataller R (2016) Pathogenesis and Management of Alcoholic Liver Disease. Dig. Dis 34:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto Y, Itabe H, Sakai J, Makita M, Noda J, Mori M, Higashi Y, Kojima S, Takano T (2004) Identification of major proteins in the lipid droplet-enriched fraction isolated from the human hepatocyte cell line HuH7. Biochim. Biophys. Acta 1644:47–59. [DOI] [PubMed] [Google Scholar]
- Gabbs M, Leng S, Devassy JG, Monirujjaman M, Aukema HM (2015) Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv Nutr 6:513–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons GF, Brown AM, Wiggins D, Pease R (2002) The roles of insulin and fatty acids in the regulation of hepatic very-low-density lipoprotein assembly. J. R. Soc. Med 95 Suppl 42:23–32. [PMC free article] [PubMed] [Google Scholar]
- Giblin LJ, Chang CJ, Bentley AF, Frederickson C, Lippard SJ, Frederickson CJ (2006) Zinc-secreting Paneth cells studied by ZP fluorescence. J. Histochem. Cytochem 54:311–316. [DOI] [PubMed] [Google Scholar]
- Gilham D, Ho S, Rasouli M, Martres P, Vance DE, Lehner R (2003) Inhibitors of hepatic microsomal triacylglycerol hydrolase decrease very low density lipoprotein secretion. FASEB J. 17:1685–1687. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Edin ML, De Maeyer RP, Bystrom J, Newson J, Lih FB, Stables M, Zeldin DC, Bishop-Bailey D (2016) CYP450-derived oxylipins mediate inflammatory resolution. Proc. Natl. Acad. Sci. U. S. A 113:E3240–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JF, Newman JW, Williamson KC, Hammock BD (2000) Toxicity of epoxy fatty acids and related compounds to cells expressing human soluble epoxide hydrolase. Chem. Res. Toxicol 13:217–226. [DOI] [PubMed] [Google Scholar]
- Hendrikx T, Schnabl B (2018) Antimicrobial proteins: intestinal guards to protect against liver disease. J. Gastroenterol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrubec Z, Omenn GS (1981) Evidence for genetic predisposition to alcoholic cirrhosis and psychosis: twin concordances for alcoholism and its biological end points by zygosity among male veterans. Alcohol. Clin. Exp. Res 5:207–215. [DOI] [PubMed] [Google Scholar]
- Ikura Y, Caldwell SH (2015) Lipid droplet-associated proteins in alcoholic liver disease: a potential linkage with hepatocellular damage. Int J Clin Exp Pathol 8:8699–8708. [PMC free article] [PubMed] [Google Scholar]
- Khan SA, Wollaston-Hayden EE, Markowski TW, Higgins L, Mashek DG (2015) Quantitative analysis of the murine lipid droplet-associated proteome during diet-induced hepatic steatosis. J. Lipid Res 56:2260–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharbanda KK, Mailliard ME, Baldwin CR, Beckenhauer HC, Sorrell MF, Tuma DJ (2007) Betaine attenuates alcoholic steatosis by restoring phosphatidylcholine generation via the phosphatidylethanolamine methyltransferase pathway. J. Hepatol 46:314–321. [DOI] [PubMed] [Google Scholar]
- Kharbanda KK, Todero SL, King AL, Osna NA, McVicker BL, Tuma DJ, Wisecarver JL, Bailey SM (2012) Betaine treatment attenuates chronic ethanol-induced hepatic steatosis and alterations to the mitochondrial respiratory chain proteome. International journal of hepatology 2012:962183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharbanda KK, Todero SL, Ward BW, Cannella JJ 3rd, Tuma DJ (2009) Betaine administration corrects ethanol-induced defective VLDL secretion. Mol. Cell. Biochem 327:75–78. [DOI] [PubMed] [Google Scholar]
- Kourkoumpetis T, Sood G (2019) Pathogenesis of Alcoholic Liver Disease: An Update. Clin Liver Dis 23:71–80. [DOI] [PubMed] [Google Scholar]
- Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjaerg-Hansen A, Vogt TF, Hobbs HH, Cohen JC (2014) Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet 46:352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listenberger L, Townsend E, Rickertsen C, Hains A, Brown E, Inwards EG, Stoeckman AK, Matis MP, Sampathkumar RS, Osna NA, Kharbanda KK (2018) Decreasing Phosphatidylcholine on the Surface of the Lipid Droplet Correlates with Altered Protein Binding and Steatosis. Cells 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listenberger LL, Ostermeyer-Fay AG, Goldberg EB, Brown WJ, Brown DA (2007) Adipocyte differentiation-related protein reduces the lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover. J. Lipid Res 48:2751–2761. [DOI] [PubMed] [Google Scholar]
- Lopez-Vicario C, Rius B, Alcaraz-Quiles J, Garcia-Alonso V, Lopategi A, Titos E, Claria J (2016) Pro-resolving mediators produced from EPA and DHA: Overview of the pathways involved and their mechanisms in metabolic syndrome and related liver diseases. Eur. J. Pharmacol 785:133–143. [DOI] [PubMed] [Google Scholar]
- Lucey MR (2019) Alcohol-Associated Cirrhosis. Clin Liver Dis 23:115–126. [DOI] [PubMed] [Google Scholar]
- Mahdessian H, Taxiarchis A, Popov S, Silveira A, Franco-Cereceda A, Hamsten A, Eriksson P, van’t Hooft F (2014) TM6SF2 is a regulator of liver fat metabolism influencing triglyceride secretion and hepatic lipid droplet content. Proceedings of the National Academy of Sciences 111:8913–8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancina RM, Dongiovanni P, Petta S, Pingitore P, Meroni M, Rametta R, Borén J, Montalcini T, Pujia A, Wiklund O, Hindy G, Spagnuolo R, Motta BM, Pipitone RM, Craxì A, Fargion S, Nobili V, Käkelä P, Kärjä V, Männistö V, Pihlajamäki J, Reilly DF, Castro-Perez J, Kozlitina J, Valenti L, Romeo S (2016) The MBOAT7-TMC4 Variant rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology 150:1219–1230.e1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MC, Friedman LS, McClain CJ (2017) Medical Management of Severe Alcoholic Hepatitis: Expert Review from the Clinical Practice Updates Committee of the AGA Institute. Clin Gastroenterol Hepatol 15:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam M, Motoba K, Borhan B, Pinot F, Hammock BD (1996) Novel metabolic pathways for linoleic and arachidonic acid metabolism. Biochim. Biophys. Acta 1290:327–339. [DOI] [PubMed] [Google Scholar]
- Mohammad MK, Avila D, Zhang J, Barve S, Arteel G, McClain C, Joshi-Barve S (2012a) Acrolein cytotoxicity in hepatocytes involves endoplasmic reticulum stress, mitochondrial dysfunction and oxidative stress. Toxicol. Appl. Pharmacol 265:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad MK, Zhou Z, Cave M, Barve A, McClain CJ (2012b) Zinc and liver disease. Nutr. Clin. Pract 27:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltman CL, Weintraub NL, VanRollins M, Dellsperger KC (1998) Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation. Circ. Res 83:932–939. [DOI] [PubMed] [Google Scholar]
- Osna NA, Donohue TM Jr., Kharbanda KK (2017) Alcoholic Liver Disease: Pathogenesis and Current Management. Alcohol Res 38:147–161. [PMC free article] [PubMed] [Google Scholar]
- Pirazzi C, Adiels M, Burza MA, Mancina RM, Levin M, Ståhlman M, Taskinen M-R, Orho-Melander M, Perman J, Pujia A, Andersson L, Maglio C, Montalcini T, Wiklund O, Borén J, Romeo S (2012) Patatin-like phospholipase domain-containing 3 (PNPLA3) I148M (rs738409) affects hepatic VLDL secretion in humans and in vitro. J. Hepatol 57:1276–1282. [DOI] [PubMed] [Google Scholar]
- Rehm J, Gmel GE Sr., Gmel G, Hasan OSM, Imtiaz S, Popova S, Probst C, Roerecke M, Room R, Samokhvalov AV, Shield KD, Shuper PA (2017) The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction 112:968–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH (2008) Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet 40:1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth NC, Qin J (2019) Histopathology of Alcohol-Related Liver Diseases. Clin Liver Dis 23:11–23. [DOI] [PubMed] [Google Scholar]
- Salameh H, Raff E, Erwin A, Seth D, Nischalke HD, Falleti E, Burza MA, Leathert J, Romeo S, Molinaro A, Corradini SG, Toniutto P, Ulrich S, Daly A, Day CP, Kuo YF, Singal AK (2015) PNPLA3 Gene Polymorphism Is Associated With Predisposition to and Severity of Alcoholic Liver Disease. Am. J. Gastroenterol [DOI] [PubMed] [Google Scholar]
- Salter AM, Wiggins D, Sessions VA, Gibbons GF (1998) The intracellular triacylglycerol/fatty acid cycle: a comparison of its activity in hepatocytes which secrete exclusively apolipoprotein (apo) B100 very-low-density lipoprotein (VLDL) and in those which secrete predominantly apoB48 VLDL. Biochem. J 332:667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE (2008) Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 8:349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth D, Daly AK, Haber PS, Day CP (2010) PNPLA3 - a case in point linking genetic susceptibility for Alcoholic and Non-Alcoholic Liver Disease Hepatology Elsewhere 51:1463–1465. [DOI] [PubMed] [Google Scholar]
- Singh S, Osna NA, Kharbanda KK (2017) Treatment options for alcoholic and non-alcoholic fatty liver disease: A review. World J Gastroenterol 23:6549–6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisemore MF, Zheng J, Yang JC, Thompson DA, Plopper CG, Cortopassi GA, Hammock BD (2001) Cellular characterization of leukotoxin diol-induced mitochondrial dysfunction. Arch. Biochem. Biophys 392:32–37. [DOI] [PubMed] [Google Scholar]
- Sletten A, Seline A, Rudd A, Logsdon M, Listenberger LL (2014) Surface features of the lipid droplet mediate perilipin 2 localization. Biochem. Biophys. Res. Commun 452:422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickel F, Osterreicher CH (2006) The role of genetic polymorphisms in alcoholic liver disease. Alcohol Alcohol 41:209–224. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Takei Y (2017) Pathogenesis of alcoholic liver disease. Hepatol Res 47:70–79. [DOI] [PubMed] [Google Scholar]
- Thiam AR, Farese RV Jr., Walther TC (2013) The biophysics and cell biology of lipid droplets. Nat Rev Mol Cell Biol 14:775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DA, Hammock BD (2007) Dihydroxyoctadecamonoenoate esters inhibit the neutrophil respiratory burst. J Biosci 32:279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, Stokowski RP, Kershenobich D, Ballinger DG, Hinds DA (2010) Variant in PNPLA3 is associated with alcoholic liver disease. Nat. Genet 42:21–23. [DOI] [PubMed] [Google Scholar]
- Turro S, Ingelmo-Torres M, Estanyol JM, Tebar F, Fernandez MA, Albor CV, Gaus K, Grewal T, Enrich C, Pol A (2006) Identification and characterization of associated with lipid droplet protein 1: A novel membrane-associated protein that resides on hepatic lipid droplets. Traffic 7:1254–1269. [DOI] [PubMed] [Google Scholar]
- Walther TC, Farese RV Jr. (2012) Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem 81:687–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Liu W, Yao L, Zhang X, Zhang X, Ye C, Jiang H, He J, Zhu Y, Ai D (2017) Hydroxyeicosapentaenoic acids and epoxyeicosatetraenoic acids attenuate early occurrence of nonalcoholic fatty liver disease. Br. J. Pharmacol 174:2358–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wei E, Quiroga AD, Sun X, Touret N, Lehner R (2010) Altered lipid droplet dynamics in hepatocytes lacking triacylglycerol hydrolase expression. Mol. Biol. Cell 21:1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner DR, Liu H, Ghosh Dastidar S, Warner JB, Prodhan MAI, Yin X, Zhang X, Feldstein AE, Gao B, Prough RA, McClain CJ, Kirpich IA (2018) Ethanol and unsaturated dietary fat induce unique patterns of hepatic omega-6 and omega-3 PUFA oxylipins in a mouse model of alcoholic liver disease. PLoS One 13:e0204119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner DR, Liu H, Miller ME, Ramsden CE, Gao B, Feldstein AE, Schuster S, McClain CJ, Kirpich IA (2017) Dietary Linoleic Acid and Its Oxidized Metabolites Exacerbate Liver Injury Caused by Ethanol via Induction of Hepatic Proinflammatory Response in Mice. Am. J. Pathol 187:2232–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei E, Ben Ali Y, Lyon J, Wang H, Nelson R, Dolinsky VW, Dyck JR, Mitchell G, Korbutt GS, Lehner R (2010) Loss of TGH/Ces3 in mice decreases blood lipids, improves glucose tolerance, and increases energy expenditure. Cell Metab 11:183–193. [DOI] [PubMed] [Google Scholar]
- Wiggins D, Gibbons GF (1992) The lipolysis/esterification cycle of hepatic triacylglycerol. Its role in the secretion of very-low-density lipoprotein and its response to hormones and sulphonylureas. Biochem. J 284 ( Pt 2):457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Vatsalya V, Gobejishvili L, Lamont RJ, McClain CJ, Feng W (2019) Porphyromonas gingivalis as a possible risk factor in the development/severity of acute alcoholic hepatitis. Hepatology Commun 3:293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]