Abstract
Purpose:
Individuals with Parkinson disease (PD) present with complex and variable symptoms, with recent findings suggesting that the etiology of PD extends beyond the involvement of just the basal ganglia. These symptoms include significant impairments in the speech and swallowing domains, which can greatly affect quality of life and therefore require therapeutic attention. This research-based update reviews the neurophysiological basis for swallowing and speech changes in PD, the effectiveness of various types of treatments, and implications for symptom evaluation and management.
Conclusion:
The mechanisms responsible for swallowing and speech symptoms in PD remain largely unknown. Dopaminergic medication and deep-brain-stimulation do not provide consistent benefits for these symptoms suggesting a non-dopaminergic network is involved. Importantly, evidence suggests that symptoms of dysphagia and hypokinetic dysarthria may be early indications of PD, so it is critical to investigate the cause of these changes.
Background
Parkinson Disease (PD) is a highly prevalent neurodegenerative disease that affects up to 2% of adults over the age of 65 (Forsaa, Larsen, Wentzel-Larsen, Herlofson, & Alves, 2008) and impacts nearly 10 million people worldwide (de Lau et al., 2004). More people are diagnosed with PD than multiple sclerosis, muscular dystrophy and Amyotrophic Lateral Sclerosis (ALS) combined, and the prevalence of PD is projected to double in the next 20 years (Kowal, Dall, Chakrabarti, Storm, & Jain, 2013), affirming this disease as a major public health concern. As PD is currently incurable and progressive (Beitz, 2014; Braak et al., 2003), individuals living with PD and their caregivers face great challenges to manage the features of the disease and maintain their quality of life (Forsaa et al., 2008; Müller, Assmus, Herlofson, Larsen, & Tysnes, 2013; Opara, Brola, Leonardi, & Błaszczyk, 2012).
PD is characterized by hallmark motor dysfunction and dysregulation, manifesting as gross motor features including rigidity, bradykinesia, tremor, postural instability, and disturbances in gait (Berardelli et al., 2018; Shahed & Jankovic, 2007; Sprenger & Poewe, 2013). These motor impairments contribute to increased risk of falls and limitations in ability to complete activities of daily living, resulting in a loss of independence and an increased need of assistance from others, placing burden on caregivers (Hariz & Forsgren, 2011; Yousefi, Tadibi, Khoei, & Montazeri, 2009).
Although the motor deficits are primarily caused by progressive dopaminergic depletion in the substantia nigra (Chu & Kordower, 2007; Davie, 2008; Kirik et al., 2002; Kish, Shannak, & Hornykiewicz, 1988; Lo Bianco, Ridet, Schneider, Deglon, & Aebischer, 2002), the pathology of PD is complex. Experts now appreciate that this disease involves multiple neurotransmitter pathways throughout the central and peripheral nervous systems (Chaudhuri & Schapira, 2009; Schapira, Chaudhuri, & Jenner, 2017) and pathology has been found in peripheral muscles critical for swallow and voice function (Mu et al., 2012). In a recent review, Schapira and colleagues illustrated the lengthy and gradual progression of PD (Figure 1), noting that many features appear up to a decade prior to the presentation of the hallmark motor symptoms (Schapira et al., 2017). These are often subtle features including anosmia, mood changes, and autonomic dysfunction and can also involve changes in communication and swallowing function. Unfortunately, these features are often missed or attributed to the natural aging process, so these go undiagnosed and untreated in the early stages of the disease.
Figure 1. Time courses of the onset of the motor and non-motor features of Parkinson Disease.
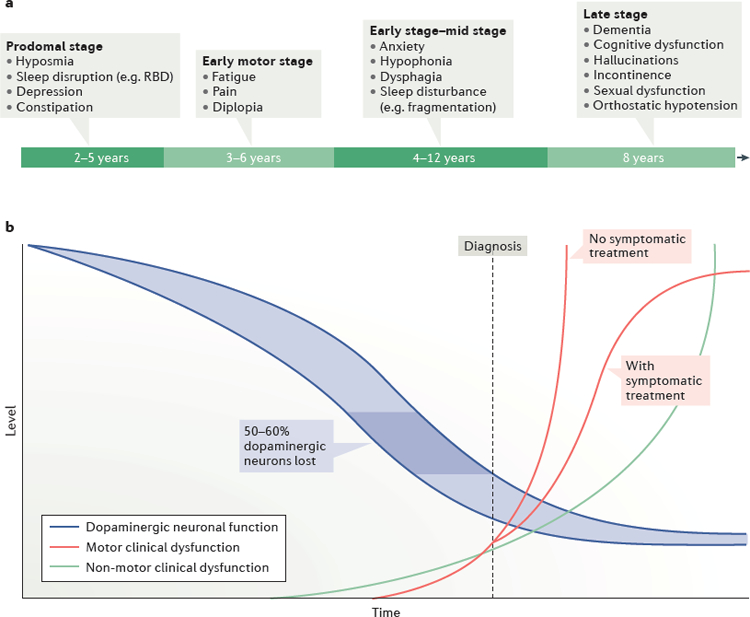
a) A schematic representation of the potential timeline the non-motor features of PD may manifest. b) A depiction of the rates of development and progression of the motor and non-motor features in relation to the decline in dopaminergic neuronal function (Schapira et al., 2017).
Because of the multifaceted nature of the underlying neuropathology associated with PD, the clinical presentation of this disease is complex and varied. There are genetic and inherited forms of PD (Bonifati et al., 2002; Guo et al., 2011), as well as idiopathic presentations of the disease. Each displays its own unique expression and progression of features. These variations in causation and presentation of symptoms are thought to be associated with differences in underlying pathology and result in distinctive clinical subtypes or phenotypes (Klingelhoefer & Reichmann, 2017). The subtypes of PD are described by the presentation of the primary motor feature: tremor dominant, akinetic or rigid dominant, and mixed phenotypes (Schiess, Zheng, Soukup, Bonnen, & Nauta, 2000) or tremor-dominant and non-tremor-dominant (Jankovic et al., 1990). Other PD researchers suggest further subtyping the disease by variations seen in non-motor symptom presentation such as disturbances in cognitive function, apathy, sleep, pain, fatigue, depression/anxiety and autonomic function (Sauerbier, Jenner, Todorova, & Chaudhuri, 2016). Defining these distinct phenotypes appreciates the heterogeneity and widespread underlying pathology associated with PD and encourages the possibility of developing subtype-specific treatment approaches. Currently, however, we do not fully understand the onset, progression, or etiology of many PD deficits, including speech and swallowing dysfunction, which complicates the diagnosis and management of these features.
No specific test exists to diagnose PD. A neurologist comprehensively considers the individual’s medical history, administers a clinical evaluation and neurologic imaging, trials medications, and rules out other diseases in order to make a clinical diagnosis. The most widely used clinical rating scale for PD is the Unified Parkinson’s Disease Rating Scale (UPDRS) which was developed in the 1980s by Fahn and colleagues (Fahn, Elton, & UPDRS Program Members, 1987) and then revised and expanded to the Movement disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) in 2008 (Goetz et al., 2008). The MDS-UPDRS involves participation by patients/caregivers and a clinical investigator and was designed to assess the presence and severity of both motor and non-motor aspects of PD and how these impact activities of daily living. The MDS-UPDRS has been assessed for validity, reliability, and can be quickly administered (Goetz et al., 2008). While this assessment tool is commonly used to track the progression and severity of many motor aspects of PD, it does not gather qualitative data and it does not adequately incorporate assessments of speech or swallowing function. These limitations hinder the clinical utility of this assessment to guide goals and treatment plans for dysarthria and dysphagia in this population.
Clinical Features of Dysphagia in PD
Clinician-administered swallowing assessments revealed that 82% of individuals with PD demonstrate signs of dysphagia (Kalf, de Swart, Bloem, & Munneke, 2012). Swallowing deficits are often significantly debilitating in the later stages of the disease, reducing quality of life (Han et al., 2011; Plowman-Prine et al., 2009) and contributing to dehydration, malnutrition, and aspiration pneumonia, the leading cause of death of patients with PD (Beyer, Herlofson, Arsland, & Larsen, 2001; Mehanna & Jankovic, 2010; Morgante et al., 2000). Shockingly, the mean survival time after the onset of a complaint of dysphagia is only 15–24 months (Müller et al., 2001). Further, the strongest predictor of mortality for nursing home residents with PD was a diagnosis of aspiration pneumonia (Fernandez & Lapane, 2002). While dysphagia in PD is often thought of as an end-of-disease complication, signs of oropharyngeal and esophageal dysphagia can often be seen earlier in the disease when objective measures are used in assessment (Jones & Ciucci, 2016; Sung et al., 2010; Volonté, Porta, & Comi, 2002). Signs manifesting from esophagus the gut such as achalasia, motility dysfunction, and reflux, as well as oropharyngeal swallowing changes, are often the first to present themselves as a sign of PD (Jones & Ciucci, 2016).
In addition to the serious health complications that results from dysphagia in PD, swallowing difficulty can diminish quality of life (Plowman-Prine et al., 2009). Dining is a social activity that revolves around communication and functional swallowing skills. Individuals with PD and dysphagia often experience reluctance to eat in public due to embarrassment about drooling, slowness of eating, or fear of choking (Rosenbek & Jones, 2009). Patients may also have difficulty with reach-to-eat movements which can negatively impact feeding experience (Doan, Melvin, Whishaw, & Suchowersky, 2008). These are all important aspects to consider when determining how best to manage the features of the disease.
Due to the complex sensorimotor sequencing required for swallowing, it is not surprising that all four phases of the swallow (oral preparatory, oral, pharyngeal, and esophageal) can be disrupted by PD. PD-related dysphagia presents as repetitive tongue elevations (tongue pumping), reduced mastication speed and coordination, prolonged oropharyngeal transit time, decreased control of the bolus with premature spilling into the pharynx, delayed initiation of the swallow, increased number of swallows necessary to clear the pharynx, slowed and reduced hyolaryngeal movement, esophageal dysmotility and reflux, and oropharyngeal residue (Ali et al., 1996; Bird, Woodward, Gibson, Phyland, & Fonda, 1994; Fuh et al., 1997; Leopold & Kagel, 1996; Potulska, Friedman, Królicki, & Spychala, 2003; Stroudley & Walsh, 1991; Sung et al., 2010). It is argued that one of the first patient-reported signs of dysphagia in individuals with PD could be excessive drooling, occurring in 56% of patients, (Kalf, Swart, Borm, Bloem, & Munneke, 2009) and is thought to result from swallowing and sensory impairment (Bagheri et al., 1999; Edwards, Quigley, & Pfeiffer, 1992) There is also a direct correlation between drooling and dysphagia severity; patients who have more severe dysphagia exhibit greater drooling (Nóbrega et al., 2008). Others highlight that PD-related dysphagia begins with esophageal dysfunction and reflux when objective measures are taken (Edwards et al., 1992; Noyce et al., 2012; Potulska et al., 2003; Sung et al., 2010).
Neurophysiological Basis for Dysphagia in PD
While the classic gross motor deficits associated with PD are primarily linked to dopaminergic loss in the substantia nigra pars compacta area of the brainstem (Kish et al., 1988; Plowman & Kleim, 2011), the pathophysiology underlying dysphagia in PD is not well understood. There is not a direct correlation between dysphagia severity and extent of motor impairment or disease duration (Monte, da Silva-Júnior, Braga-Neto, Nobre e Souza, & Sales de Bruin, 2005; Nilsson, Ekberg, Olsson, & Hindfelt, 1996; Volonté et al., 2002). Additionally, the pharmacologic interventions used to alleviate gross motor features of PD that target dopaminergic depletion, do not consistently improve swallow function (Baijens & Speyer, 2009; Hunter, Crameri, Austin, Woodward, & Hughes, 1997; Menezes & Melo, 2009). These findings indicate that non-dopaminergic neural networks are also involved in the features of dysphagia observed in PD.
Pharyngeal swallowing is a complex, semi-automatic, repetitive medullary program that can be altered by voluntary control, to some degree, and relies on involvement of peripheral, afferent, and central feedback mechanisms (Simons, 2017). In PD-related dysphagia, there is noted impairment in the dopaminergic basal ganglia system that disrupts the supramedullary swallow system (Leopold & Daniels, 2010), as well as pathology that exists in the brainstem that impacts the medullary swallowing central pattern generator (Suntrup et al., 2013). This swallowing program includes the dorsal motor nucleus of the glossopharyngeal and vagus nerves as well as the reticular activating system, all of which demonstrate neuronal loss early in the progression of PD (Braak, Ghebremedhin, Rüb, Bratzke, & Del Tredici, 2004; Hawkes, Del Tredici, & Braak, 2010). There is also evidence that distinct cortical regions in the brain such as the supplementary motor area, which is involved in motor programming, initiation and execution, demonstrate reduced activity measured by magnetoencephalography (MEG) in individuals with PD during volitional swallowing tasks compared to healthy controls (Suntrup et al., 2013). In addition to the central nervous system, pathology in the peripheral nerves and muscles that control communication and swallowing was also discovered, albeit in post-mortem studies so when this pathology occurs in unknown (Mu et al., 2012).
The underlying pathology that causes PD-related dysphagia disrupts a combination of complex, multifaceted systems that incorporate both dopaminergic and non-dopaminergic neural networks. The underlying pathways and their interactions during this disease process has not fully been described and needs to be further investigated. Exploring the underlying neural networks impacted by PD would yield a better understanding of the causes of dysphagia, allowing for the development of more targeted and effective pharmacological and behavioral intervention approaches to clinically manage this disease.
Translational Research
Individuals with PD display a large amount of inherent variability due to age, onset, phenotype, social situation, medication, and motivation to participate in intervention and research. This is problematic in that it not only inhibits our ability to assess the underlying neurobiological mechanisms that cause swallowing problems in PD, but also limits investigations into treatment outcomes with necessary experimental control (Barbe et al., 2014; van Rooden et al., 2011). In order to address the confounds inherent in human research, animal models of communication and swallowing impairment in PD have been developed. One such well-established model of PD is a genetic knockout of the Pink1 gene in rats (Pink1−/−). Recent research has expanded the use of this model to investigate neurobiological and behavioral constructs contributing to signs manifested in PD progression (Cullen et al., 2018; Johnson et al., 2011; Kelm-Nelson, Brauer, & Ciucci, 2016; Kelm-Nelson, Yang, & Ciucci, 2015). In humans, Pink1 genetic variants contribute to early onset and progressive deficits over time and extensive nigrostriatal dopamine depletion in the later stages (Guo et al., 2011). Individuals with PD show early, subtle deficits in swallowing and communication. Early and progressive sensorimotor and oromotor deficits in the preclinical and early stages of the Pink1−/− model have been demonstrated (Cullen et al., 2018; Grant et al., 2015; Johnson et al., 2011; Kelm-Nelson et al., 2015), confirming the construct validity of this animal model. Ongoing research uses this model to study brain-behavior relationships, and to develop and test interventions for the treatment of the disease with necessary experimental control.
Diagnosis
In light of the high prevalence of dysphagia in PD, as well as the mortality resulting from aspiration pneumonia in this population, it is essential that the entire multi-disciplinary medical team is aware of both the high risk for dysphagia and the fact that many patients may not be aware of their own swallowing difficulties. While patient questionnaires and interviews may function as a reasonable “first line” of screening for swallowing impairment, it is important for the medical team to realize that many patients do not often report dysphagia. Less than 10% of patients spontaneously report difficulty with swallowing and when asked by health care professionals, only 50% admit they have problems swallowing (Robbins, Logemann, & Kirshner, 1986). In a recent meta-analysis to determine prevalence of dysphagia in PD, clinician-administered swallowing assessments concluded that 82% of patients exhibited impairments in swallowing (Kalf et al., 2012). In contrast, only 35% of patients demonstrated dysphagia when patient questionnaires or interviews were used in diagnosis (Kalf et al., 2012).
Evaluation of swallowing function in individuals with PD should include patient questionnaires/interviews, as well as both clinical and instrumented assessments. A clinical evaluation includes collection of a case history with a patient and/or their primary care givers, an oral mechanism exam, and observation of oral trials of multiple solids and liquids, particularly those with which the patient reports having difficulty. The clinical assessment affords the clinician an opportunity to evaluate the functional and cognitive status of the patient and provides direction for instrumented assessments.
Because of the often-subtle externally appreciable signs of dysphagia in PD, instrumented assessment is essential for accurate diagnosis of dysphagia and for meaningful description of swallowing pathophysiology in order to provide appropriate treatment. Imaging studies, such as videofluoroscopic swallow studies (VFSS) and fiberoptic endoscopic evaluations of swallowing (FEES) are “gold-standard” assessments, allowing for description of normal or disordered movement of oral and pharyngeal structures during swallowing, particularly as they relate to swallowing efficiency and swallowing safety (Giraldo-cadavid et al., 2017; Langmore, 2017; Pisegna & Langmore, 2016). Spatio-temporal pressure measurements obtained with high-resolution pharyngeal manometry (HRPM) and bolus flow measurement obtained with impedance testing provide quantitative description of the pressure differentials necessary for safe swallowing (Omari et al., 2011).
Swallowing Treatment
Because of the heterogeneity of swallowing impairment in PD, treatment of dysphagia must be tailored to the individual physiologic deficits presented by each patient. Before deciding upon a course of treatment, clinicians must be informed by history and physical exam with attention to potential comorbidities associated swallow impairment, and a combination of clinical and instrumented swallowing assessments. Treatment goals should be focused toward helping the patient to meet nutritional and hydrational needs in the safest, most-efficient, and least-restrictive manner possible, with particular attention paid to patient-informed quality of life.
The impact of apomorphine and levodopa on swallowing function in PD has been investigated. While some studies have demonstrated improvements in swallowing outcomes of reduction of frequency of penetration and aspiration, others have shown little change or exacerbation (Fuh et al., 1997; Hunter et al., 1997; A. Lim, Leow, Huckabee, Frampton, & Anderson, 2008; Menezes & Melo, 2009; Warnecke et al., 2016). It has been suggested that the reduced mortality associated with levodopa treatment in PD is evidence for the positive influence of levodopa on swallowing function (Sutton, 2013). Such an interpretation should be considered cautiously, particularly in light of the fact that research that has directly investigated swallow function in on- and off-levodopa conditions has been equivocal.
Deep brain stimulation (DBS) involves surgical placement of electrodes in the basal ganglia (typically in the globus pallidus or the subthalamic nucleus) with subsequent stimulation of target nuclei. While improvements in motor functions such as gate, tremor, and bradykinesia have been well-documented, evaluation of the impact of DBS on swallow function has shown marked variability across studies, described in a thorough review by Troche and colleagues (Troche, Brandimore, Foote, & Okun, 2013).
The use of expiratory muscle strength training (EMST) has been shown to improve swallowing kinematics and to reduce frequency of airway invasion in PD (Troche et al., 2010). The underlying physiologic deficit that is being targeted by EMST is reduced elevation and anterior excursion of the hyolaryngeal complex. EMST increases submental muscle activity, which in turn increases hyolaryngeal elevation and excursion, and thus swallow safety. This intervention is attractive in that it involves a very tangible therapy target that can be performed in essentially any physical setting. These factors are likely to be important when considering the fact that adherence to swallow treatment recommendations is, at best, challenging (Krekeler, Broadfoot, Johnson, Connor, & Rogus-Pulia, 2018).
Lee Silverman Voice Treatment (LSVT® -LOUD) is a therapeutic intervention targeted at modulating speech and voice function in PD via high-effort recruitment of respiratory musculature and vocal fold adduction. Sharkawi and colleagues investigated the impact of LSVT® -LOUD on swallow function (Sharkawi et al., 2002) They hypothesized that LSVT® -LOUD would improve swallowing function, based on clinician observations that reported such. In this study of 8 patients, improvements in oral and pharyngeal transit times and reductions in oral and pharyngeal residue were observed. A study of 20 patients with PD assessed the influence of the LSVT® -LOUD on cough function and swallow performance (Miles et al., 2017). Treatment resulted in increased pharyngo-esophageal segment opening and duration of opening as well as decreased pharyngeal residue. In addition, cough-related measures of involuntary peak expiratory flow rate and peak expiratory flow rise time were increased following treatment. In both studies, airway protection was minimally compromised at baseline, with absent aspiration and mild or absent penetration, resulting in now observable change in airway protection with treatment. While these initial explorations of the relationship between LSVT® -LOUD and swallow function are encouraging, larger studies with more severely dysphagic populations must be conducted in order to further-validate the intervention. This is particularly relevant when considering the relative burden of the standard dosage for LSVT® -LOUD, which includes one-hour-long therapy sessions for four consecutive days in a week for four consecutive weeks in addition to 30 minutes of home practice on every day that the patient does not receive therapy (Ramig, Halpern, Spielman, Fox, & Freeman, 2018).
In addition to exercise-, medication-, and surgically-based treatments of swallow dysfunction in PD, implementation of compensatory maneuvers and diet modifications can help to improve functional swallow outcomes. Upright positioning with a chin-tuck to chest posture is often recommended in order to promote airway protection via anterior positioning of the larynx and shortening of the pharynx (Logemann, 1998). When necessary, the texture of solid foods and the viscosity of liquids can be modified to accommodate impairments in oral and pharyngeal bolus control, reduced oral and pharyngeal sensation, and increased oral and pharyngeal transit time (Logemann et al., 2008). Prior to diet modification, effectiveness of such modifications should be evaluated with videofluoroscopy and/or fiberoptic endoscopic evaluation of swallowing.
Hypokinetic Dysarthria
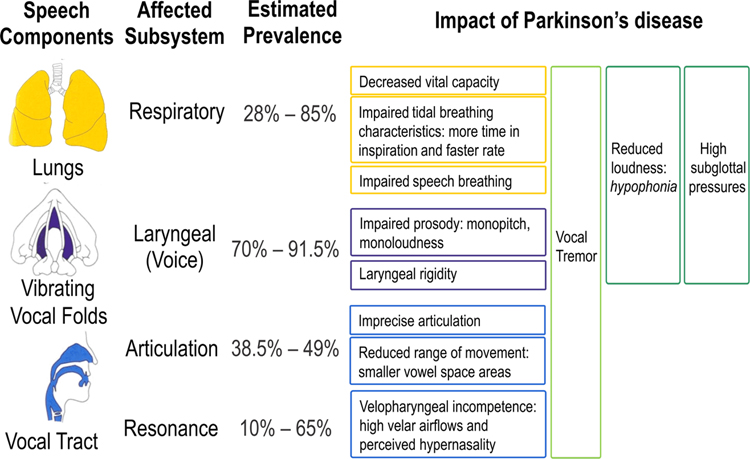
In addition to the hallmark motor symptoms, 90% of individuals with PD develop a motor speech disorder known as hypokinetic dysarthria, characterized by impairments in voice as well as articulation (Duffy, 2013). Hypokinetic dysarthria has been classically defined as, “a perceptually distinctive motor speech disorder associated with basal ganglia control circuit pathology,” based on the initial understanding of PD (Duffy, 2013). However, it is now clear that PD involves many parts of the brain (reviewed earlier) so hypokinetic dysarthria is not solely a basal ganglia based disorder. Speech symptoms of PD can encompass all the laryngeal, articulatory, and respiratory subsystems of speech (see Figure 2). The most predominant changes in hypokinetic dysarthria are typically reduced loudness (i.e., hypophonia (Duffy, 2013)) and reduced pitch fluctuations (i.e., the perception of monopitch (Bowen, Hands, Pradhan, & Stepp, 2013)). Articulatory impairments can also occur, including decreased vowel space area (McRae, Tjaden, & Schoonings, 2002), dysfluencies (Goberman, Blomgren, & Metzger, 2010), and speech festination (sudden bursts of increased speech rate; (Moreau et al., 2007)). Together, these multi-subsystem speech changes can impact functional communication outcomes, such as speech intelligibility (Anand & Stepp, 2015; Miller et al., 2007; Tjaden, Sussman, & Wilding, 2014) and naturalness (Anand & Stepp, 2015), and negatively affect quality of life in PD (Miller, Noble, Jones, & Burn, 2006a). Dopaminergic therapy is widely accepted as the gold standard for reduction of global motor symptoms in PD (Ferreira et al., 2013) and is typically administered as levodopa medication. However, as reviewed below, the improvement of speech symptoms from dopaminergic treatment is limited.
Figure 2. Impact of Parkinson disease on speech subsystems.
The specific impact of Parkinson disease on each speech subsystem is listed by speech component, affected subsystem, and estimated prevalence in the patient population.
Assessment of Speech Impairment and Function in PD
Subsystem impairments: Respiratory
Respiratory abnormalities in PD can present in approximately 28% – 85% of patients (Izquierdo-Alonso, Jimenez-Jimenez, Cabrera-Valdivia, & Mansilla-Lesmes, 1994; Sabaté, González, Ruperez, & Rodríguez, 1996). Studies of respiration in PD have shown specific impairments that respond to dopaminergic medication and other symptoms which do not. In particular, decreased vital capacity, increased speech inspiration time, and faster tidal breathing rate have been reported in speakers with PD compared to controls, and these features have been shown to improve with dopaminergic medication (De Letter, Santens, De Bodt, et al., 2007; Solomon & Hixon, 1993; Vercueil, Linard, Wuyam, Pollak, & Benchetrit, 1999). Speakers with PD have also been found to use abdominal breathing to a greater degree than controls during speech breathing and speak fewer words per breath group with longer pauses and these changes remain present regardless of medication status (Darling & Huber, 2011; Solomon & Hixon, 1993).
Subsystem impairments: Laryngeal
Several studies have established laryngeal abnormalities in PD, and voice symptoms are often the most prominent speech problem in PD (~70% of patients (Ho, Iansek, Marigliani, Bradshaw, & Gates, 1999; Logemann, Fisher, Boshes, & Blonsky, 1978). A high incidence of vowel fold bowing is reported in PD (~87% of patients (Blumin, Pcolinsky, & Atkins, 2004)), as well as incomplete glottal phase closure, reduced abductory gestures during phonation, and abnormalities in the mucosal waveform (Stelzig, Hochhaus, Gall, & Henneberg, 1999). Vocal tremor can also be a symptom of PD (Logemann et al., 1978), but there is conflicting evidence for when it may present relative to disease progression (Holmes, Oates, Phyland, & Hughes, 2000; Perez, Ramig, Smith, & Dromey, 1996) and the source of the tremor is not clear. Intrinsic laryngeal muscle rigidity appears to be a widespread symptom of PD (73% – 91.5% of patients (Zarzur, de Campos Duprat, Cataldo, Ciampi, & Fonoff, 2014; Zarzur, Duprat, Shinzato, & Eckley, 2007), as measured by abnormal intrinsic muscle firing during voice rest. However, abnormal firing during vocal rest is not associated with disease severity (Zarzur et al., 2014) and doesn’t indicate the presence of intrinsic laryngeal muscle tremor, even when vocal tremor is observed clinically during videolaryngoscopic examination (Zarzur et al., 2014; Zarzur et al., 2007). This implicates other mechanisms of vocal tremor in PD, such as respiratory and/or vocal tract tremor.
Impairments of voice in PD often include decreased vocal prosody, reduced loudness, and perception of breathiness. These impairments have been reflected in speech acoustics. Mean speaking fundamental frequency (fo: the acoustic correlate of vocal pitch) has been reported as both higher (Canter, 1963) or not different (Zwirner, Murry, & Woodson, 1991) in PD relative to controls. Differences between male and female speaking fo have been observed as well (Holmes et al., 2000; Skodda & Schlegel, 2008), but these may be influenced by other factors such as aging. Reduced variability of fo (the acoustic correlate of vocal prosody) has been reported in several studies (Bowen et al., 2013; Flint, Black, Campbell-Taylor, Gailey, & Levinton, 1992; Holmes et al., 2000; Skodda, Rinsche, & Schlegel, 2009) and presents both on and off dopaminergic medication (Bowen et al., 2013). Variability in fo has also been found to differ between male and female speakers, but results are inconsistent and do not relate to PD progression (Holmes et al., 2000; Skodda & Schlegel, 2010). However, fo variability has shown sensitivity to early stages of PD (5 years pre-diagnosis (Harel, Cannizzaro, & Snyder, 2004)). Measures reflecting vocal quality, such as intensity (the acoustic correlate of loudness) and harmonics-to-noise ratio (HNR; the acoustic correlate of breathiness (Skodda, Grönheit, Mancinelli, & Schlegel, 2013)) also show deterioration in PD. Reduced intensity (Canter, 1963; Holmes et al., 2000; Illes, Metter, Hanson, & Iritani, 1988) and HNR (reflecting increased breathiness (Shrivastav & Sapienza, 2003)) are found in PD relative to controls. Similar trends are found for perceptual measures of loudness and breathiness (Holmes et al., 2000; Logemann et al., 1978) and may present more frequently in late-stage compared to early-stage PD (Holmes et al., 2000). In support of this, a longitudinal study by Rusz et al. found that reduced speech intensity correlated with increased symptoms of bradykinesia (Rusz, Tykalová, Klempíř, Čmejla, & Růžička, 2016).
Prior work shows little-to-no benefit of both short-term or long-term dopaminergic medication on acoustic measures of mean fo, variability of fo, and mean speech intensity (Skodda, Visser, & Schlegel, 2010). Perception of vocal prosody following dopaminergic medication has been found to both improve (De Letter, Santens, Estercam, et al., 2007) or show no changes (Plowman-Prine et al., 2009). Acoustic measures of prosody, as variability in fo, show either small benefits from dopaminergic medication (Bowen et al., 2013; De Letter, Santens, De Bodt, et al., 2007) and no benefits (Ho, Bradshaw, & Iansek, 2008; Skodda, Grönheit, & Schlegel, 2011; Skodda et al., 2010). Similarly, speech intensity shows both improvement (Kompoliti, Wang, Goetz, Leurgans, & Raman, 2000; Viallet et al., 2002) or no change (Goberman, Coelho, & Robb, 2002; Ho et al., 2008; Jiang, Lin, Wang, & Hanson, 1999) with dopaminergic medication. However, at the laryngeal level, there is evidence for decreased laryngeal rigidity following dopaminergic treatment (Jiang et al., 1999). One study also reported that vocal fold bowing and vocal onset and offset impairments in PD were more pronounced when patients were off medication compared to on medication (Ludlow, Connor, & Bassich, 1987).
Subsystem impairments: Articulatory and Resonatory
Articulatory impairments are common in PD (occurring in 38.5% – 49% of patients (Ho, Bradshaw, Iansek, & Alfredson, 1999; Logemann et al., 1978)) and show high variability. Measures of vowel space area in PD show conflicting results in the literature (Goberman & Elmer, 2005; Sapir, Ramig, Spielman, & Fox, 2010; Skodda, Flasskamp, & Schlegel, 2011; Tjaden, Lam, & Wilding, 2013). However, novel metrics such as articulatory acoustic-acoustic vowel space (AAVS; (Whitfield & Goberman, 2014)) and vowel articulation index (VAI; (Skodda, Grönheit, & Schlegel, 2012)) have shown sensitivity to tracking articulatory changes in PD compared to controls. These measures, which incorporate information about vowel space, show reduced values in PD relative to controls (Skodda et al., 2013; Skodda, Flasskamp, et al., 2011; Whitfield & Goberman, 2014). In addition, AAVS shows promise for tracking within-speaker increases in speech clarity in PD (Whitfield & Goberman, 2014) and VAI shows significant reductions as PD progresses (Skodda, 2012).
It is not clear whether dopaminergic therapy consistently improves symptoms of articulation in PD, but some studies report benefits of medication. Benefits to lip muscle rigidity (Cahill et al., 1998; Leanderson, Meyerson, & Persson, 1971) and mandibular mobility (Svensson, Henningson, & Karlsson, 1993) have been shown, which would positively impact articulation. In contrast, another study found no overall improvements of articulatory measures with short-term and long-term dopaminergic treatment (Skodda et al., 2010). One study found that speakers with PD demonstrated significantly more dysfluencies after 3–6 years of dopaminergic medication compared to off medication and controls (Tykalová et al., 2015), yet other studies have found no differences with medication (De Letter, Santens, De Bodt, Boon, & Van Borsel, 2006; Goberman & Blomgren, 2003). Examining the same speakers during a 6-year course of dopaminergic medication, greater levodopa dosage led to slight improvements in stop consonant articulation (Rusz et al., 2016).
Speech rate, although not purely articulatory, has also been examined in PD. While some studies have found no differences in speaking rate between speakers with PD and controls (Lowit, Brendel, Dobinson, & Howell, 2006), other work reports slower syllable repetition (Dworkin & Aronson, 1986; Ludlow et al., 1987) or faster connected speech (Skodda & Schlegel, 2008) in speakers with PD. A possible explanation for the variability in speech rate is that the underlying cause is not purely resulting from motor symptoms of PD. Difficulty in monitoring the timing of speech movements in PD could explain the combination of slower, typical, and faster speech (Ackermann, Konczak, & Hertrich, 1997; Ludlow et al., 1987). There is also evidence that slower speech rate can result from interactions with cognitive decline (Lowit et al., 2006). Further, studies show both no differences (De Letter et al., 2006; Goberman, Coelho, & Robb, 2005) and increased rate (Ho et al., 2008) with dopaminergic medication. Individual speakers also may reduce their speaking rate when on medication compared to off (Spencer, Morgan, & Blond, 2009). Therefore, changes in speech rate in PD may result from several different mechanisms that include motor changes to articulators.
In some individuals with PD, resonance impairments can present in the form of velopharyngeal incompetence (Hoodin & Gilbert, 1989b; Robbins et al., 1986). Resonatory impairments in PD are reported less frequently in PD, but have been considered as a deviant feature of speech in hypokinetic dysarthria (Duffy, 2013). Perceptually, hypernasality has been reported in as few as 10% of patients (Logemann et al., 1978) and as many as 65% of patients (Novotný et al., 2016). In a study using acoustic analyses, 27% of patients with PD demonstrated excessive nasal energy in the speech signal (Novotný et al., 2016). Increased nasal airflow (Hoodin & Gilbert, 1989a; Logemann et al., 1978) and decreased oral pressure have also been found during speech in PD (Solomon & Hixon, 1993), suggesting incomplete closure of the velopharyngeal port. The magnitude of nasal airflow in PD further increases when speaking at a speed compatible with fluent speech (Hoodin & Gilbert, 1989a). The effect of dopaminergic medication on resonatory impairment in PD has not been characterized, but perception of hypernasality is unrelated to motor symptom severity (Novotný et al., 2016).
Functional speech outcomes
Speech intelligibility, measured both by listener perceptions (Stipancic, Tjaden, & Wilding, 2015; Tjaden et al., 2014) and patient self-reports (Miller, Noble, Jones, & Burn, 2006b), is found to decline in PD. Problems with speech intelligibility are common: studies report that 65% to 70% of speakers with PD are below a typical range (Coates & Bakheit, 1997; Miller et al., 2007). Importantly, patient self-reports of voice changes do not always correlate with listener’s perceived intelligibility (Miller et al., 2007). There is evidence that speech intelligibility may worsen with disease duration (Miller et al., 2007; Skodda et al., 2013), and perceptual measures have suggested a greater intelligibility decline in males relative to female speakers (Midi et al., 2008).
Studies of speech intelligibility as a function of dopaminergic treatment show conflicting results in the literature. Prior work has shown amelioration of categorical ratings of speech intelligibility by speech language pathologists (De Letter, Santens, Estercam, et al., 2007) and untrained listeners (Nakano, Zubick, & Tyler, 1973) with levodopa, but no group differences were found on and off medication in visual-analog-scale ratings of speech intelligibility, naturalness, and vocal quality by experienced listeners (Spencer et al., 2009). Dyskinesias due to dopaminergic medication effects may also affect individuals’ self-perceived speech intelligibility (Nakano et al., 1973).
Treatments that impact speech in PD
As reviewed earlier, the speech symptoms of PD are very heterogeneous and do not show clear improvements with dopaminergic medication. One possible explanation for that speech symptoms may be specific to PD phenotype (reviewed above). This is supported by prior work reporting greater communication and speech problems (Hariz & Forsgren, 2011; Wu et al., 2016) in postural instability and gait dominant PD (PIGD) compared to tremor dominant PD (TD) speakers. However, speech intelligibility does not appear to be associated with PD phenotype (Miller et al., 2007). Given the wide range of speech problems, individual variability, and minimal benefit of dopaminergic medication, individually targeted treatments are necessary to improve communication.
Surgical therapy through deep brain stimulation (DBS) has shown to substantially improve global motor symptoms in PD (Deuschl et al., 2006; Fasano, Daniele, & Albanese, 2012; Limousin et al., 1998), but its effect on speech symptoms is less clear. Following subthalamic nucleus (STN) DBS implantation, 9.3% of patients are reported to develop speech problems resulting from the DBS (Kleiner‐Fisman et al., 2006). However, these impairments may vary by speech subsystem. Speech problems at the laryngeal level can improve with STN DBS implantation. For instance, a case study found that STN DBS resulted in improvements in vocal HNR (Sidtis, Cameron, Bonura, & Sidtis, 2012) and abductory movement for an individual with vocal fold immobility resulting from PD (Arocho-Quinones, Hammer, Bock, & Pahapill, 2017). Vocal intensity during a sustained vowel has also shown increases with right STN DBS stimulation (the same effects were not seen with the left STN DBS; (Wang, Metman, Bakay, Arzbaecher, & Bernard, 2003)). In further support of positive impacts of DBS on voice, one study found improvements in vocal motor control in speakers with PD when their DBS was on compared to off (through attenuation of abnormally large vocal responses to altered auditory feedback; (Behroozmand et al., 2019)). Resonatory features may also improve: one study found increased oral pressure and velopharyngeal closure in a subset of participants with PD with STN DBS (Hammer, Barlow, Lyons, & Pahwa, 2011). Instead, articulatory changes with STN DBS show conflicting results in the literature. Positive effects of STN DBS have also been found on severity of the categorical speech ratings (Limousin et al., 1998; Rousseaux et al., 2004) and the strength of oral articulators (Pinto, Gentil, Fraix, Benabid, & Pollak, 2003). However, worsening of stuttering symptoms (Burghaus et al., 2006; Toft & Dietrichs, 2011) and reductions in vowel space (Sidtis, Alken, Tagliati, Alterman, & Van Lancker Sidtis, 2016) following DBS implantation have also been observed. Speech intelligibility has been consistently reported to reduce with STN DBS. Studies using both categorical speech ratings (Gervais-Bernard et al., 2009; Piboolnurak et al., 2007; Romito, Scerrati, Contarino, & Iacoangeli, 2003; Thobois et al., 2002) and measures of intelligibility (Tripoliti et al., 2011; Yorkston, Beukelman, & Traynor, 1984) report negative effects of STN DBS that increase in frequency with the number of years post-implantation (Gervais-Bernard et al., 2009; Piboolnurak et al., 2007; Thobois et al., 2002; Tripoliti et al., 2011). While some patients can ameliorate speech symptoms of DBS by turning it off, individuals with bilateral STN DBS implantation may not see improvement in speech symptoms even when the DBS stimulation is off (Aldridge, Theodoros, Angwin, & Vogel, 2016; Robertson et al., 2011; Tripoliti et al., 2011). Taken together, these studies suggest the interaction of DBS with speech symptoms is not well understood.
The inconsistent findings of the effect of DBS on speech are not surprising considering the evidence that multiple neural mechanisms and neurotransmitters are affected in PD (Schapira et al., 2017). In addition, specific PD symptoms (Tsuboi et al., 2015), speech intelligibility prior to surgery, and surgical procedures can affect speech outcomes with DBS (Tripoliti et al., 2014). DBS implantation was initially developed to target motor symptoms (Limousin et al., 1995). However, due to the observed effects, subjective speech assessments during surgical procedures have become increasingly common. This recent shift might explain discrepancies in prior findings regarding the relationship between DBS and speech changes. Regardless, it is important to recognize that for some patients with PD the DBS implantation may result in motor symptom relief at the cost of deterioration in communication.
Behavioral speech therapy is currently the most effective treatment for improving speech in PD and, similar to gait therapies (Lim et al., 2005), has focused on leveraging external cueing. Speech therapy has shown improvements in both acoustic and auditory-perceptual measures when using external cues for vocal loudness (Lee Silverman Voice Treatment or LSVT® -LOUD; (Ramig et al., 2001; Sapir et al., 2002)) or when asking patients to speak with intent (SPEAK OUT!®; (Boutsen, Park, Dvorak, & Cid, 2018)).
LSVT® -LOUD has shown significant improvements in speech intensity both immediately following speech therapy and at follow-up examinations up to 2 years later. When examining the intensity of running speech immediately following treatment, LSVT® -LOUD shows average gains of 4 – 5 dB (Ramig, Halpern, Spielman, Fox, & Freeman, 2018; Ramig et al., 2001). LSVT® -LOUD has also shown larger effects compared to a therapy targeting increased movement in the orofacial and articulatory system (LSVT® -ARTIC; (Ramig et al., 2018)). Thus far, LSVT® -LOUD is also the only treatment to report follow-up information. Approximately 2 – 3 dB increases in speech intensity were found to remain when speakers were examined at 7 months and 2 years post-treatment (Ramig et al., 2018; Ramig et al., 2001). However, these changes in speech intensity at follow-up are quite small. Minor changes in speech intensity can certainly have meaningful perceptual consequences, but it is important to consider that speech intensity measurement error is at least 2.5 dB (based on mouth-to-microphone distance uncertainties and inherent error in sound level meters; (Švec & Granqvist, 2018)). Therefore, treatment effects observed below 2.5 dB are difficult to interpret. Vocal prosody, measured through fluctuations in voice fo, is also relevant for speech intelligibility. For example, enhancing fo contours of speakers with PD showed improvements in the accuracy of listener detection of vowels (Bunton, 2006) and treatment focused on rate and intonation in PD has resulted in increased speech intelligibility (Martens et al., 2015). LSVT® -LOUD has resulted in average increases in the standard deviation of fo (reflecting vocal prosody) of 0.35 to 0.58 semitones immediately post-treatment and 0.39 to 0.65 semitones at a 2-year follow-up (Ramig et al., 2001).
SPEAK OUT!® therapy for PD involves using cues to speak with intent. SPEAK OUT!® reports average increases of 7.0 – 8.2 dB for connected speech (Boutsen et al., 2018) directly post-treatment. These gains are larger than those reported for similar tasks in LSVT® -LOUD post-treatment, however, follow-up information is not yet available for SPEAK OUT!® to determine the degree of maintenance of voice changes. In addition to intensity gains, SPEAK OUT!® has shown significant average increases of 10 Hz in fo (vocal pitch) range following treatment which corresponded to improved clinical ratings (Boutsen et al., 2018). This change in fo range was not reported in semitones (which allows for comparison across different speakers), so it cannot be interpreted with respect to the LSVT® -LOUD outcomes.
When examining the effects of behavioral therapies on speech, patient self-reports and listener perception of intelligibility are also important to consider. LSVT® -LOUD and SPEAK OUT!® have both demonstrated improvements in patient self-reported voice complaints (Boutsen et al., 2018; Ramig et al., 2018). Clinical ratings of speech intensity, intonation, and speech intelligibility pre- and post- SPEAK OUT!® therapy have all shown improvements (Boutsen et al., 2018). Similarly, listener ratings of voice quality are significantly better following LSVT® -LOUD (Sapir et al., 2002). There is also support for increased speech intelligibility post-treatment for both SPEAK OUT!® (Boutsen et al., 2018) and LSVT® -LOUD (Cannito et al., 2012), although the methodologies for intelligibility measures contained various limitations (e.g., small numbers of listeners).
Overall, it is apparent that behavioral treatments of speech in PD are beneficial to patients and reduce speech difficulties in the short-term, but the effects lessen over time and are inherently limited due to the limited understanding of the neurophysiological mechanisms resulting in speech problems. Additionally, more work is needed to characterize how comprehensive acoustic measures of speech during treatment relate to an individual’s speech intelligibility using robust methods (Miller, 2013).
Neurophysiological basis of speech symptoms in PD
Speech symptoms in PD are largely attributed to the motor production in the laryngeal, respiratory, articulatory, and resonatory subsystems. However, there is also evidence for a deficit in sensorimotor integration (i.e., how sensory information is used for consequent motor actions) during speech production. Lewy-bodies, abnormal alpha-synuclein protein clusters, have been found in PD in the vocal tract (specifically, the tongue, larynx, and upper esophagus (Mu et al., 2015)) and are hypothesized to affect sensory axons in those regions. Changes to sensation in the vocal tract has implications for speech production given that reaching desired speech targets partially relies on somatosensory feedback (how self-produced speech feels in the vocal tract) (Houde & Nagarajan, 2011; Lametti, Nasir, & Ostry, 2012; Larson, Altman, Liu, & Hain, 2008; Tourville & Guenther, 2011). This is supported by prior work demonstrating decreased somatosentation to bursts of air applied to laryngeal musculature in PD compared to controls (Hammer & Barlow, 2010). Speech is also affected by the integration of sensory information through auditory feedback (how self-produced speech sounds to a speaker) (Houde & Nagarajan, 2011; Tourville & Guenther, 2011; Villacorta, Perkell, & Guenther, 2007), which may also be impacted by PD. In loudness perception tasks, individuals with PD did not differ from control subjects in their ability to make judgments about the loudness of external sounds (Abur, Lupiani, Hickox, Shinn-Cunningham, & Stepp, 2017; Dromey & Adams, 2000). However, when asked to make loudness judgments about a both active productions and passive playback of their own voice, individuals with PD significantly overestimated their vocal loudness compared to controls (Ho, Bradshaw, & Iansek, 2000). These studies collectively suggest that the recognition of the speech as a self-produced sound may differentially impact perception in PD. Given the role of auditory feedback in speech production, it is possible that impairments in the perception of speech are associated with the disordered production of speech in PD.
In line with this possibility, speakers with PD have been found to have impaired responses to experimental tasks which modify auditory feedback during speech production. These tasks involve unexpected or predictable changes, in near real-time, to how a speaker hears their own speech while speaking. In typical speakers, unexpected changes in speech feedback result in a quick, reflexive, response to the change (Burnett, Freedland, Larson, & Hain, 1998). When predictable changes are made in speech feedback, typical speakers respond gradually by learning from the changing feedback and adjusting their consequent speech productions (Houde & Jordan, 1998). Using measures involving the laryngeal subsystem, larger responses to unexpected changes (Liu, Wang, Metman, & Larson, 2012; Mollaei, Shiller, Baum, & Gracco, 2016) and variable responses to predictable changes (Abur et al., 2018; Mollaei et al., 2016) are seen in PD compared to controls. In contrast, using measures in the articulatory domain, smaller responses to both unexpected (Mollaei et al., 2016) and predictable changes (Mollaei, Shiller, & Gracco, 2013) are found in PD relative to controls. Thus, impairments in integrating auditory feedback of measures from the laryngeal and articulatory subsystems are seen in PD, but the responses are differentially impaired by subsystem. This suggests a separate basis for speech subsystem deficits in PD. It is also of note that both unexpected and predictable changes to auditory feedback show abnormal responses in PD. If solely the basal ganglia were involved, it is likely that deficits would only be present when new speech commands were being generated in response to predictable changes. Yet, during unexpected changes, larger responses are seen for measures of the laryngeal subsystem (Liu et al., 2012) and reduced responses are seen in measures of articulation (Mollaei et al., 2016). These deviant responses to unexpected changes in auditory feedback suggest that multiple neural regions play a role in speech motor control differences in PD.
Finally, although there are reports that speech impairments progress posterior to anterior in the oral cavity in PD (Logemann & Fisher, 1981; Logemann et al., 1978), recent work presents contrasting evidence (Read, Miller, & Kitsou, 2018). In terms of subsystem involvement, difficulties at the laryngeal level are found more frequently in early stage PD compared to articulatory symptoms (Ho, Bradshaw, et al., 1999), but some laryngeal symptoms may present only in late-stage PD (Holmes et al., 2000). Also, acoustic measures of articulation have shown sensitivity to the progression of PD, whereas measures of voice have not (Skodda et al., 2013). Collectively, these findings further imply that speech symptoms at the laryngeal and articulatory levels may have different underlying causes. It is likely that speech symptom progression is also affected by PD phenotype, specific motor symptom severity, and treatment factors (e.g., implantation of DBS and dopaminergic medication status).
Conclusion
In sum, the specific neural mechanisms responsible for symptoms of dysphagia and hypokinetic dysarthria in PD remain unknown. The presentation of these symptoms can be highly variable across individuals, however there is also evidence that some speech and swallowing changes might be early indications of PD (Harel et al., 2004; Jones & Ciucci, 2016). Given the inconsistency of findings in terms of the benefit of dopaminergic medication for hypokinetic dysarthria and dysphagia, it is likely that non-dopaminergic networks are involved in the presentation of these types of impairments. In general, pharmacological and DBS treatments do not provide clear improvements for swallowing or speech symptoms in PD. Behavioral therapies are currently the most effective treatment for symptom management of both dysphagia and hypokinetic dysarthria, but the degree of maintenance of therapy effects post-treatment is unclear. Thus, more work is needed to clarify the bases of these symptoms in order to create more individualized and targeted therapies for speech and swallowing difficulties in PD.
Acknowledgments
This work was supported by grant R01 DC016270 (C. Stepp and F. Guenther, PIs) and T32 DC013017 (C. Moore, PI).
Footnotes
The authors have no relevant conflicts of interest to declare.
References
- Abur D, Lester-Smith RA, Daliri A, Lupiani AA, Guenther FH, & Stepp CE. (2018). Sensorimotor adaptation of voice fundamental frequency in Parkinson’s disease. PLoS ONE, 13(1), e0191839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abur D, Lupiani AA, Hickox AE, Shinn-Cunningham B, & Stepp CE. (2017). Loudness perception of pure tones in Parkinson’s disease. The Journal of the Acoustical Society of America, 141(5), 3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann H, Konczak J, & Hertrich I. (1997). The temporal control of repetitive articulatory movements in Parkinson’s disease. Brain and Language, 56(2), 312–319. [DOI] [PubMed] [Google Scholar]
- Aldridge D, Theodoros D, Angwin A, & Vogel AP. (2016). Speech outcomes in Parkinson’s disease after subthalamic nucleus deep brain stimulation: A systematic review. Parkinsonism & Related Disorders, 33, 3–11. [DOI] [PubMed] [Google Scholar]
- Ali G, Wallace K, Schwartz R, DeCarle D, Zagami A, & Cook I. (1996). Mechanisms of oral-pharyngeal dysphagia in patients with Parkinson’s disease. Gastroenterology, 110(2), 383–392. 10.1053/GAST.1996.V110.PM8566584 [DOI] [PubMed] [Google Scholar]
- Anand S, & Stepp CE. (2015). Listener Perception of Monopitch, Naturalness, and Intelligibility for Speakers With Parkinson’s Disease. J Speech Lang Hear Res, 58(4), 1134–1144. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/26102242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arocho-Quinones EV, Hammer MJ, Bock JM, & Pahapill PA. (2017). Effects of deep brain stimulation on vocal fold immobility in Parkinson’s disease. Surgical Neurology International, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagheri H, Damase-Michel C, Lapeyre-Mestre M, Cismondo S, O’Connell D, Senard JM, … Montastruc JL. (1999). A study of salivary secretion in Parkinson’s disease. Clinical Neuropharmacology, 22(4), 213–215. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10442250 [PubMed] [Google Scholar]
- Baijens LWJ, & Speyer R. (2009). Effects of Therapy for Dysphagia in Parkinson’s Disease: Systematic Review. Dysphagia, 24(1), 91–102. 10.1007/s00455-008-9180-1 [DOI] [PubMed] [Google Scholar]
- Barbe MT, Amarell M, Snijders AH, Florin E, Quatuor E-L, Schönau E, … Timmermann L. (2014). Gait and upper limb variability in Parkinson’s disease patients with and without freezing of gait. Journal of Neurology, 261(2), 330–342. 10.1007/s00415-013-7199-1 [DOI] [PubMed] [Google Scholar]
- Behroozmand R, Johari K, Kelley RM, Kapnoula EC, Narayanan NS, & Greenlee JDW. (2019). Effect of deep brain stimulation on vocal motor control mechanisms in Parkinson’s disease. Parkinsonism & Related Disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitz JM. (2014). Parkinson’s disease: a review. Frontiers in Bioscience (Scholar Edition), 6, 65–74. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24389262 [DOI] [PubMed] [Google Scholar]
- Berardelli I, Bloise MC, Bologna M, Conte A, Pompili M, Lamis DA, … Fabbrini G. (2018). Cognitive behavioral group therapy versus psychoeducational intervention in Parkinson’s disease. Neuropsychiatric Disease and Treatment, 14, 399–405. 10.2147/NDT.S152221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer MK, Herlofson K, Arsland D, & Larsen JP. (2001). Causes of death in a community-based study of Parkinson’s disease. Acta Neurologica Scandinavica, 103(1), 7–11. 10.1034/j.1600-0404.2001.00191.x [DOI] [PubMed] [Google Scholar]
- Bird MR, Woodward MC, Gibson EM, Phyland DJ, & Fonda D. (1994). Asymptomatic Swallowing Disorders in Elderly Patients with Parkinson’s Disease: A Description of Findings on Clinical Examination and Videofluoroscopy in Sixteen Patients. Retrieved from https://academic.oup.com/ageing/article-abstract/23/3/251/35713 [DOI] [PubMed] [Google Scholar]
- Blumin JH, Pcolinsky DE, & Atkins JP. (2004). Laryngeal findings in advanced Parkinson’s disease. Annals of Otology, Rhinology & Laryngology, 113(4), 253–258. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Dekker MCJ, Vanacore N, Fabbrini G, Squitieri F, Marconi R, … Italian Parkinson Genetics Network. (2002). Autosomal recessive early onset parkinsonism is linked to three loci: PARK2, PARK6, and PARK7. Neurological Sciences, 23(0), s59–s60. 10.1007/s100720200069 [DOI] [PubMed] [Google Scholar]
- Boutsen F, Park E, Dvorak J, & Cid C. (2018). Prosodic improvement in persons with parkinson disease receiving SPEAK OUT!® voice therapy. Folia Phoniatrica et Logopaedica, 70, 51–58. [DOI] [PubMed] [Google Scholar]
- Bowen LK, Hands GL, Pradhan S, & Stepp CE. (2013). Effects of Parkinson’s disease on fundamental frequency variability in running speech. Journal of Medical Speech-Language Pathology, 21(3), 235. [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, & Braak E. (2003). Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging, 24(2), 197–211. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12498954 [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, & Del Tredici K. (2004). Stages in the development of Parkinson’s disease-related pathology. Cell and Tissue Research, 318(1), 121–134. 10.1007/s00441-004-0956-9 [DOI] [PubMed] [Google Scholar]
- Bunton K. (2006). Fundamental frequency as a perceptual cue for vowel identification in speakers with Parkinson’s disease. Folia Phoniatrica et Logopaedica, 58(5), 323–339. [DOI] [PubMed] [Google Scholar]
- Burghaus L, Hilker R, Thiel A, Galldiks N, Lehnhardt FG, Zaro-Weber O, … Heiss W-D. (2006). Deep brain stimulation of the subthalamic nucleus reversibly deteriorates stuttering in advanced Parkinson’s disease. Journal of Neural Transmission, 113(5), 625–631. [DOI] [PubMed] [Google Scholar]
- Burnett TA, Freedland MB, Larson CR, & Hain TC. (1998). Voice F0 responses to manipulations in pitch feedback. J Acoust Soc Am, 103(6), 3153–3161. [DOI] [PubMed] [Google Scholar]
- Cahill LM, Murdoch BE, Theodoros DG, Triggs EJ, Charles BG, & Yao AA. (1998). Effect of oral levodopa treatment on articulatory function in Parkinson’s disease: preliminary results. Motor Control, 2(2), 161–172. [DOI] [PubMed] [Google Scholar]
- Cannito MP, Suiter DM, Beverly D, Chorna L, Wolf T, & Pfeiffer RM. (2012). Sentence intelligibility before and after voice treatment in speakers with idiopathic Parkinson’s disease. Journal of Voice, 26(2), 214–219. [DOI] [PubMed] [Google Scholar]
- Canter GJ. (1963). Speech characteristics of patients with Parkinson’s disease: Intensity, pitch, and duration. Journal of Speech & Hearing Disorders. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, & Schapira AH. (2009). Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. The Lancet Neurology, 8(5), 464–474. 10.1016/S1474-4422(09)70068-7 [DOI] [PubMed] [Google Scholar]
- Chu Y, & Kordower JH. (2007). Age-associated increases of α-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: Is this the target for Parkinson’s disease? Neurobiology of Disease, 25(1), 134–149. 10.1016/J.NBD.2006.08.021 [DOI] [PubMed] [Google Scholar]
- Coates C, & Bakheit AMO. (1997). The prevalence of verbal communication disability in patients with Parkinson’s disease. Disability and Rehabilitation, 19(3), 104–107. [DOI] [PubMed] [Google Scholar]
- Cullen KP, Grant LM, Kelm-Nelson CA, Brauer AFL, Bickelhaupt LB, Russell JA, & Ciucci MR. (2018). Pink1 −/− Rats Show Early-Onset Swallowing Deficits and Correlative Brainstem Pathology. Dysphagia. 10.1007/s00455-018-9896-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling M, & Huber JE. (2011). Changes to articulatory kinematics in response to loudness cues in individuals with Parkinson’s disease. Journal of Speech, Language, and Hearing Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie CA. (2008). A review of Parkinson’s disease. British Medical Bulletin, 86(1), 109–127. 10.1093/bmb/ldn013 [DOI] [PubMed] [Google Scholar]
- de Lau LML, Giesbergen PCLM, de Rijk MC, Hofman A, Koudstaal PJ, & Breteler MMB. (2004). Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology, 63(7), 1240–1244. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15477545 [DOI] [PubMed] [Google Scholar]
- De Letter M, Santens P, De Bodt M, Boon P, & Van Borsel J. (2006). Levodopa-induced alterations in speech rate in advanced Parkinson’s disease. Acta Neurologica Belgica, 106(1), 19. [PubMed] [Google Scholar]
- De Letter M, Santens P, De Bodt M, Van Maele G, Van Borsel J, & Boon P. (2007). The effect of levodopa on respiration and word intelligibility in people with advanced Parkinson’s disease. Clinical Neurology and Neurosurgery, 109(6), 495–500. [DOI] [PubMed] [Google Scholar]
- De Letter M, Santens P, Estercam I, Van Maele G, De Bodt M, Boon P, & Van Borsel J. (2007). Levodopa‐induced modifications of prosody and comprehensibility in advanced Parkinson’s disease as perceived by professional listeners. Clinical Linguistics & Phonetics, 21(10), 783–791. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, … Eisner W. (2006). A randomized trial of deep-brain stimulation for Parkinson’s disease. New England Journal of Medicine, 355(9), 896–908. [DOI] [PubMed] [Google Scholar]
- Doan JB, Melvin KG, Whishaw IQ, & Suchowersky O. (2008). Bilateral impairments of skilled reach-to-eat in early Parkinson’s disease patients presenting with unilateral or asymmetrical symptoms. Behavioural Brain Research, 194(2), 207–213. 10.1016/j.bbr.2008.07.015 [DOI] [PubMed] [Google Scholar]
- Dromey C, & Adams S. (2000). Loudness perception and hypophonia in Parkinson disease. Journal of Medical Speech-Language Pathology, 8(4), 255–259. [Google Scholar]
- Duffy J. (2013). Motor Speech Disorders Substrates, Differential Diagnosis, and Management (September 2012). [Google Scholar]
- Duffy JR. (2013). Motor speech disorders: Substrates, differential diagnosis, and management. Elsevier Health Sciences. [Google Scholar]
- Dworkin JP, & Aronson AE. (1986). Tongue strength and alternate motion rates in normal and dysarthric subjects. Journal of Communication Disorders, 19(2), 115–132. [DOI] [PubMed] [Google Scholar]
- Edwards LL, Quigley EM, & Pfeiffer RF. (1992). Gastrointestinal dysfunction in Parkinson’s disease: frequency and pathophysiology. Neurology, 42(4), 726–732. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1565224 [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton R, & UPDRS Program Members. (1987). Unified Parkinson’s disease rating scale. (Fahn S, Marsden C, Goldstein M, & Calne D, Eds.), Recent developments in Parkinson’s disease (Vol. 2). Florham Park, NJ: Macmillan Health Care Information; Retrieved from https://img.medscape.com/fullsize/701/816/58977_UPDRS.pdf [Google Scholar]
- Fasano A, Daniele A, & Albanese A. (2012). Treatment of motor and non-motor features of Parkinson’s disease with deep brain stimulation. The Lancet Neurology, 11(5), 429–442. [DOI] [PubMed] [Google Scholar]
- Fernandez HH, & Lapane KL. (2002). Predictors of mortality among nursing home residents with a diagnosis of Parkinson’s disease. Medical Science Monitor : International Medical Journal of Experimental and Clinical Research, 8(4), CR241–6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11951064 [PubMed] [Google Scholar]
- Ferreira JJ, Katzenschlager R, Bloem BR, Bonuccelli U, Burn D, Deuschl G, … Oertel WH. (2013). Summary of the recommendations of the EFNS/MDS-ES review on therapeutic management of Parkinson’s disease. European Journal of Neurology, 20(1), 5–15. 10.1111/j.1468-1331.2012.03866.x [DOI] [PubMed] [Google Scholar]
- Flint AJ, Black SE, Campbell-Taylor I, Gailey GF, & Levinton C. (1992). Acoustic analysis in the differentiation of Parkinson’s disease and major depression. Journal of Psycholinguistic Research, 21(5), 383–399. [DOI] [PubMed] [Google Scholar]
- Forsaa EB, Larsen JP, Wentzel-Larsen T, Herlofson K, & Alves G. (2008). Predictors and course of health-related quality of life in Parkinson’s disease. Movement Disorders, 23(10), 1420–1427. 10.1002/mds.22121 [DOI] [PubMed] [Google Scholar]
- Fuh JL, Lee RC, Wang SJ, Lin CH, Wang PN, Chiang JH, & Liu HC. (1997). Swallowing difficulty in Parkinson’s disease. Clinical Neurology and Neurosurgery, 99(2), 106–112. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9213054 [DOI] [PubMed] [Google Scholar]
- Gervais-Bernard H, Xie-Brustolin J, Mertens P, Polo G, Klinger H, Adamec D, … Thobois S. (2009). Bilateral subthalamic nucleus stimulation in advanced Parkinson’s disease: five year follow-up. Journal of Neurology, 256(2), 225. [DOI] [PubMed] [Google Scholar]
- Giraldo-cadavid LF, Leal-Leaño LR, Leon-Basantes GA, Bastidas AR, Garcia R, Ovalle S, & Abondano-Garavito JE. (2017). Accuracy of Endoscopic and Videofluoroscopic Evaluations of Swallowing for Oropharyngeal Dysphagia. The Laryngoscope, 127, 2002–2010. 10.1002/lary.26419 [DOI] [PubMed] [Google Scholar]
- Goberman AM, & Blomgren M. (2003). Parkinsonian speech disfluencies: Effects of L-dopa-related fluctuations. Journal of Fluency Disorders, 28(1), 55–70. [DOI] [PubMed] [Google Scholar]
- Goberman AM, Blomgren M, & Metzger E. (2010). Characteristics of speech disfluency in Parkinson disease. Journal of Neurolinguistics, 23(5), 470–478. [Google Scholar]
- Goberman, Coelho CA, & Robb MP. (2005). Prosodic characteristics of Parkinsonian speech: The effect of levodopa-based medication. Journal of Medical Speech-Language Pathology, 13(1), 51–69. [Google Scholar]
- Goberman, Coelho C, & Robb M. (2002). Phonatory characteristics of parkinsonian speech before and after morning medication: the ON and OFF states. J Commun Disord, 35(3), 217–239. [DOI] [PubMed] [Google Scholar]
- Goberman, & Elmer. (2005). Acoustic analysis of clear versus conversational speech in individuals with Parkinson disease. J Commun Disord, 38(3), 215–230. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, … LaPelle N. (2008). Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Movement Disorders, 23(15), 2129–2170. 10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- Grant LM, Kelm-Nelson CA, Hilby BL, Blue KV, Paul Rajamanickam ES, Pultorak JD, … Ciucci MR. (2015). Evidence for early and progressive ultrasonic vocalization and oromotor deficits in a PINK1 gene knockout rat model of Parkinson’s disease. Journal of Neuroscience Research, 93(11), 1713–1727. 10.1002/jnr.23625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Wang L, He D, Yang QO, Duan Z, Zhang X, … Tang B. (2011). Clinical features and [11C]-CFT PET analysis of PARK2, PARK6, PARK7-linked autosomal recessive early onset Parkinsonism. Neurological Sciences, 32(1), 35–40. 10.1007/s10072-010-0360-z [DOI] [PubMed] [Google Scholar]
- Hammer MJ, & Barlow SM. (2010). Laryngeal somatosensory deficits in Parkinson’s disease: implications for speech respiratory and phonatory control. Experimental Brain Research, 201(3), 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer MJ, Barlow SM, Lyons KE, & Pahwa R. (2011). Subthalamic nucleus deep brain stimulation changes velopharyngeal control in Parkinson’s disease. Journal of Communication Disorders, 44(1), 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Ohnishi H, Nonaka M, Yamauchi R, Hozuki T, Hayashi T, … Mori M. (2011). Relationship between dysphagia and depressive states in patients with Parkinson’s disease. Parkinsonism & Related Disorders, 17(6), 437–439. 10.1016/j.parkreldis.2011.03.006 [DOI] [PubMed] [Google Scholar]
- Harel B, Cannizzaro M, & Snyder PJ. (2004). Variability in fundamental frequency during speech in prodromal and incipient Parkinson’s disease: A longitudinal case study. Brain and Cognition, 56(1), 24–29. [DOI] [PubMed] [Google Scholar]
- Hariz G-M, & Forsgren L. (2011). Activities of daily living and quality of life in persons with newly diagnosed Parkinson’s disease according to subtype of disease, and in comparison to healthy controls. Acta Neurologica Scandinavica, 123(1), 20–27. 10.1111/j.1600-0404.2010.01344.x [DOI] [PubMed] [Google Scholar]
- Hawkes CH, Del Tredici K, & Braak H. (2010). A timeline for Parkinson’s disease. Parkinsonism & Related Disorders, 16(2), 79–84. 10.1016/j.parkreldis.2009.08.007 [DOI] [PubMed] [Google Scholar]
- Ho AK, Bradshaw JL, & Iansek R. (2000). Volume perception in parkinsonian speech. Movement Disorders, 15(6), 1125–1131. [DOI] [PubMed] [Google Scholar]
- Ho AK, Bradshaw JL, & Iansek R. (2008). For better or worse: The effect of levodopa on speech in Parkinson’s disease. Movement Disorders, 23(4), 574–580. 10.1002/mds.21899 [DOI] [PubMed] [Google Scholar]
- Ho AK, Bradshaw JL, Iansek R, & Alfredson R. (1999). Speech volume regulation in Parkinson ‘ s disease : effects of implicit cues and explicit instructions. Neuropsychologia, 37, 1453–1460. [DOI] [PubMed] [Google Scholar]
- Ho AK, Iansek R, Marigliani C, Bradshaw JL, & Gates S. (1999). Speech impairment in a large sample of patients with Parkinson’s disease. Behavioural Neurology, 11(3), 131–137. [PubMed] [Google Scholar]
- Holmes, Oates, Phyland, & Hughes. (2000). Voice characteristics in the progression of Parkinson’s disease. International Journal of Language & Communication Disorders, 35(3), 407–418. [DOI] [PubMed] [Google Scholar]
- Hoodin RB, & Gilbert HR. (1989a). Nasal airflows in parkinsonian speakers. Journal of Communication Disorders, 22(3), 169–180. [DOI] [PubMed] [Google Scholar]
- Hoodin RB, & Gilbert HR. (1989b). Parkinsonian dysarthria: An aerodynamic and perceptual description of velopharyngeal closure for speech. Folia Phoniatrica et Logopaedica, 41(6), 249–258. [DOI] [PubMed] [Google Scholar]
- Houde, & Jordan MI. (1998). Sensorimotor adaptation in speech production. Science, 279(5354), 1213–1216. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9469813 [DOI] [PubMed] [Google Scholar]
- Houde, & Nagarajan SS. (2011). Speech production as state feedback control. Frontiers in Human Neuroscience, 5, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter PC, Crameri J, Austin S, Woodward MC, & Hughes AJ. (1997). Response of parkinsonian swallowing dysfunction to dopaminergic stimulation. Journal of Neurology, Neurosurgery, and Psychiatry, 63(5), 579–583. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9408096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes J, Metter EJ, Hanson WR, & Iritani S. (1988). Language production in Parkinson’s disease: Acoustic and linguistic considerations. Brain and Language, 33(1), 146–160. [DOI] [PubMed] [Google Scholar]
- Izquierdo-Alonso JL, Jimenez-Jimenez FJ, Cabrera-Valdivia F, & Mansilla-Lesmes M. (1994). Airway dysfunction in patients with Parkinson’s disease. Lung, 172(1), 47–55. [DOI] [PubMed] [Google Scholar]
- Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, … Shoulson I. (1990). Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology, 40(10), 1529–1534. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2215943 [DOI] [PubMed] [Google Scholar]
- Jiang J, Lin E, Wang J, & Hanson DG. (1999). Glottographic measures before and after levodopa treatment in Parkinson’s disease. The Laryngoscope, 109(8), 1287–1294. [DOI] [PubMed] [Google Scholar]
- Johnson AM, Doll EJ, Grant LM, Ringel L, Shier JN, & Ciucci MR. (2011). Targeted Training of Ultrasonic Vocalizations in Aged and Parkinsonian Rats. Journal of Visualized Experiments, (54). 10.3791/2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CA, & Ciucci MR. (2016). Multimodal Swallowing Evaluation with High-Resolution Manometry Reveals Subtle Swallowing Changes in Early and Mid-Stage Parkinson Disease. Journal of Parkinson’s Disease, 6(1), 197–208. 10.3233/JPD-150687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalf JG, de Swart BJM, Bloem BR, & Munneke M. (2012). Prevalence of oropharyngeal dysphagia in Parkinson’s disease: A meta-analysis. Parkinsonism & Related Disorders, 18(4), 311–315. 10.1016/J.PARKRELDIS.2011.11.006 [DOI] [PubMed] [Google Scholar]
- Kalf JG, Swart BJM, Borm GF, Bloem BR, & Munneke M. (2009). Prevalence and definition of drooling in Parkinson’s disease: a systematic review. Journal of Neurology, 256(9), 1391–1396. 10.1007/s00415-009-5098-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm-Nelson CA, Brauer AFL, & Ciucci MR. (2016). Vocal training, levodopa, and environment effects on ultrasonic vocalizations in a rat neurotoxin model of Parkinson disease. Behavioural Brain Research, 307, 54–64. 10.1016/j.bbr.2016.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm-Nelson CA, Yang KM, & Ciucci MR. (2015). Exercise Effects on Early Vocal Ultrasonic Communication Dysfunction in a PINK1 Knockout Model of Parkinson’s Disease. Journal of Parkinson’s Disease, 5(4), 749–763. 10.3233/JPD-150688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N, … Björklund A. (2002). Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 22(7), 2780–2791. https://doi.org/20026246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, & Hornykiewicz O. (1988). Uneven Pattern of Dopamine Loss in the Striatum of Patients with Idiopathic Parkinson’s Disease. New England Journal of Medicine, 318(14), 876–880. 10.1056/NEJM198804073181402 [DOI] [PubMed] [Google Scholar]
- Kleiner‐Fisman G, Herzog J, Fisman DN, Tamma F, Lyons KE, Pahwa R, … Deuschl G. (2006). Subthalamic nucleus deep brain stimulation: summary and meta‐analysis of outcomes. Movement Disorders: Official Journal of the Movement Disorder Society, 21(S14), S290–S304. [DOI] [PubMed] [Google Scholar]
- Klingelhoefer L, & Reichmann H. (2017). Parkinson’s disease as a multisystem disorder. Journal of Neural Transmission, 124(6), 709–713. 10.1007/s00702-017-1692-0 [DOI] [PubMed] [Google Scholar]
- Kompoliti K, Wang QE, Goetz CG, Leurgans S, & Raman R. (2000). Effects of central dopaminergic stimulation by apomorphine on speech in Parkinson’s disease. Neurology, 54(2), 458. [DOI] [PubMed] [Google Scholar]
- Kowal SL, Dall TM, Chakrabarti R, Storm MV, & Jain A. (2013). The current and projected economic burden of Parkinson’s disease in the United States. Movement Disorders, 28(3), 311–318. 10.1002/mds.25292 [DOI] [PubMed] [Google Scholar]
- Krekeler BN, Broadfoot CK, Johnson S, Connor NP, & Rogus-Pulia N. (2018). Patient Adherence to Dysphagia Recommendations: A Systematic Review. Dysphagia, 33(2), 173–184. 10.1007/s00455-017-9852-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lametti DR, Nasir SM, & Ostry DJ. (2012). Sensory preference in speech production revealed by simultaneous alteration of auditory and somatosensory feedback. J Neurosci, 32(27), 9351–9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmore SE. (2017). History of Fiberoptic Endoscopic Evaluation of Swallowing for Evaluation and Management of Pharyngeal Dysphagia: Changes over the Years. Dysphagia, 32(1), 27–38. 10.1007/s00455-016-9775-x [DOI] [PubMed] [Google Scholar]
- Larson CR, Altman KW, Liu H, & Hain TC. (2008). Interactions between auditory and somatosensory feedback for voice F 0 control. Exp Brain Res, 187(4), 613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leanderson R, Meyerson BA, & Persson A. (1971). Effect of L-dopa on speech in Parkinsonism: An EMG study of labial articulatory function. Journal of Neurology, Neurosurgery & Psychiatry, 34(6), 679–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold NA, & Daniels SK. (2010). Supranuclear Control of Swallowing. Dysphagia, 25(3), 250–257. 10.1007/s00455-009-9249-5 [DOI] [PubMed] [Google Scholar]
- Leopold NA, & Kagel MC. (1996). Prepharyngeal dysphagia in Parkinson’s disease. Dysphagia, 11(1), 14–22. 10.1007/BF00385794 [DOI] [PubMed] [Google Scholar]
- Lim A, Leow L, Huckabee M-L, Frampton C, & Anderson T. (2008). A Pilot Study of Respiration and Swallowing Integration in Parkinson’s Disease: ‘‘On” and ‘“Off”‘ Levodopa. Dysphagia, 23, 76–81. 10.1007/s00455-007-9100-9 [DOI] [PubMed] [Google Scholar]
- Lim I, van Wegen E, de Goede C, Deutekom M, Nieuwboer A, Willems A, … Kwakkel G. (2005). Effects of external rhythmical cueing on gait in patients with Parkinson’s disease: a systematic review. Clinical Rehabilitation, 19(7), 695–713. [DOI] [PubMed] [Google Scholar]
- Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, & Benabid A-L. (1998). Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. New England Journal of Medicine, 339(16), 1105–1111. [DOI] [PubMed] [Google Scholar]
- Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas J-F, Perret JE, … Broussolle E. (1995). Effect on parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. The Lancet, 345(8942), 91–95. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang EQ, Metman LV, & Larson CR. (2012). Vocal responses to perturbations in voice auditory feedback in individuals with Parkinson’s disease. Public Library of Science ONE, 7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Bianco C, Ridet J-L, Schneider BL, Deglon N, & Aebischer P. (2002). alpha -Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America, 99(16), 10813–10818. 10.1073/pnas.152339799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann, Jeri A, Gensler G, Robbins J, Lindblad AS, Brandt D, Hind JA, … Miller Gardner PJ. (2008). A Randomized Study of Three Interventions for Aspiration of Thin Liquids in Patients With Dementia or Parkinson’s Disease. Journal of Speech, Language, and Hearing Research, 51(1), 173–183. 10.1044/1092-4388(2008/013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann, Jeri A, & Fisher HB. (1981). Vocal tract control in Parkinson’s disease. Journal of Speech and Hearing Disorders, 46(4), 348–352. [DOI] [PubMed] [Google Scholar]
- Logemann, Jeri A, Fisher HB, Boshes B, & Blonsky ER. (1978). Frequency and cooccurrence of vocal tract dysfunctions in the speech of a large sample of Parkinson patients. Journal of Speech and Hearing Disorders, 43(1), 47. [DOI] [PubMed] [Google Scholar]
- Logemann, Jerilyn A. (1998). The evaluation and treatment of swallowing disorders. Current Opinion in Otolaryngology & Head and Neck Surgery, 6, 395–400. [Google Scholar]
- Lowit A, Brendel B, Dobinson C, & Howell P. (2006). An investigation into the influences of age, pathology and cognition on speech production. Journal of Medical Speech-Language Pathology, 14, 253. [PMC free article] [PubMed] [Google Scholar]
- Ludlow CL, Connor NP, & Bassich CJ. (1987). Speech timing in Parkinson’s and Huntington’s disease. Brain and Language, 32(2), 195–214. [DOI] [PubMed] [Google Scholar]
- Martens H, Van Nuffelen G, Dekens T, Huici MH-D, Hernández-Díaz HAK, De Letter M, & De Bodt M. (2015). The effect of intensive speech rate and intonation therapy on intelligibility in Parkinson’s disease. Journal of Communication Disorders, 58, 91–105. [DOI] [PubMed] [Google Scholar]
- McRae PA, Tjaden K, & Schoonings B. (2002). Acoustic and perceptual consequences of articulatory rate change in Parkinson disease. Journal of Speech, Language, and Hearing Research. [DOI] [PubMed] [Google Scholar]
- Mehanna R, & Jankovic J. (2010). Respiratory problems in neurologic movement disorders. Parkinsonism & Related Disorders, 16(10), 628–638. 10.1016/j.parkreldis.2010.07.004 [DOI] [PubMed] [Google Scholar]
- Menezes C, & Melo A. (2009). Does levodopa improve swallowing dysfunction in Parkinson’s disease patients? Journal of Clinical Pharmacy and Therapeutics, 34(6), 673–676. 10.1111/j.1365-2710.2009.01031.x [DOI] [PubMed] [Google Scholar]
- Midi I, Dogan M, Koseoglu M, Can G, Sehitoglu MA, & Gunal DI. (2008). Voice abnormalities and their relation with motor dysfunction in Parkinson’s disease. Acta Neurologica Scandinavica, 117(1), 26–34. [DOI] [PubMed] [Google Scholar]
- Miles A, Jardine M, Johnston F, de Lisle M, Friary P, & Allen J. (2017). Effect of Lee Silverman Voice Treatment (LSVT LOUD®) on swallowing and cough in Parkinson’s disease: A pilot study. Journal of the Neurological Sciences, 383, 180–187. 10.1016/j.jns.2017.11.015 [DOI] [PubMed] [Google Scholar]
- Miller N. (2013). Measuring up to speech intelligibility. International Journal of Language & Communication Disorders, 48(6), 601–612. [DOI] [PubMed] [Google Scholar]
- Miller N, Allcock L, Jones D, Noble E, Hildreth AJ, & Burn DJ. (2007). Prevalence and pattern of perceived intelligibility changes in Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry, 78(11), 1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N, Noble E, Jones D, & Burn D. (2006a). Hard to swallow: dysphagia in Parkinson’s disease. Age and Ageing, 35(6), 614–618. 10.1093/ageing/afl105 [DOI] [PubMed] [Google Scholar]
- Miller N, Noble E, Jones D, & Burn D. (2006b). Life with communication changes in Parkinson’s disease. Age and Ageing, 35(3), 235–239. [DOI] [PubMed] [Google Scholar]
- Mollaei, Shiller DM, Baum SR, & Gracco VL. (2016). Sensorimotor control of vocal pitch and formant frequencies in Parkinson’s disease. Brain Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollaei, Shiller, & Gracco. (2013). Sensorimotor adaptation of speech in Parkinson’s disease. Mov Disord, 28(12), 1668–1674. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/23861349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte FS, da Silva-Júnior FP, Braga-Neto P, Nobre e Souza MÂ, & Sales de Bruin VM. (2005). Swallowing abnormalities and dyskinesia in Parkinson’s disease. Movement Disorders, 20(4), 457–462. 10.1002/mds.20342 [DOI] [PubMed] [Google Scholar]
- Moreau C, Ozsancak C, Blatt J, Derambure P, Destee A, & Defebvre L. (2007). Oral festination in Parkinson’s disease: biomechanical analysis and correlation with festination and freezing of gait. Movement Disorders: Official Journal of the Movement Disorder Society, 22(10), 1503–1506. [DOI] [PubMed] [Google Scholar]
- Morgante L, Salemi G, Meneghini F, Di Rosa AE, Epifanio A, Grigoletto F, … Savettieri G. (2000). Parkinson disease survival: a population-based study. Archives of Neurology, 57(4), 507–512. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10768625 [DOI] [PubMed] [Google Scholar]
- Mu L, Chen J, Sobotka S, Nyirenda T, Benson B, Gupta F, … Shill HA. (2015). Alpha-synuclein pathology in sensory nerve terminals of the upper aerodigestive tract of Parkinson’s disease patients. Dysphagia, 30(4), 404–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu L, Sobotka S, Chen J, Su H, Sanders I, Adler CH, … Beach TG. (2012). Altered Pharyngeal Muscles in Parkinson Disease. Journal of Neuropathology & Experimental Neurology, 71(6), 520–530. 10.1097/NEN.0b013e318258381b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller B, Assmus J, Herlofson K, Larsen JP, & Tysnes O-B. (2013). Importance of motor vs. non-motor symptoms for health-related quality of life in early Parkinson’s disease. Parkinsonism & Related Disorders, 19(11), 1027–1032. 10.1016/j.parkreldis.2013.07.010 [DOI] [PubMed] [Google Scholar]
- Müller, Wenning GK, Verny M, McKee A, Chaudhuri KR, Jellinger K, … Litvan I. (2001). Progression of Dysarthria and Dysphagia in Postmortem-Confirmed Parkinsonian Disorders. Archives of Neurology, 58(2), 259 10.1001/archneur.58.2.259 [DOI] [PubMed] [Google Scholar]
- Nakano KK, Zubick H, & Tyler HR. (1973). Speech defects of parkinsonian patients: Effects of levodopa therapy on speech intelligibility. Neurology. [DOI] [PubMed] [Google Scholar]
- Nilsson H, Ekberg O, Olsson R, & Hindfelt B. (1996). Quantitative assessment of oral and pharyngeal function in Parkinson’s disease. Dysphagia, 11(2), 144–150. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8721074 [DOI] [PubMed] [Google Scholar]
- Nóbrega AC, Rodrigues B, Torres AC, Scarpel RD, Neves CA, & Melo A. (2008). Is drooling secondary to a swallowing disorder in patients with Parkinson’s disease? Parkinsonism & Related Disorders, 14(3), 243–245. 10.1016/j.parkreldis.2007.08.003 [DOI] [PubMed] [Google Scholar]
- Novotný M, Rusz J, Čmejla R, Růžičková H, Klempíř J, & Růžička E. (2016). Hypernasality associated with basal ganglia dysfunction: evidence from Parkinson’s disease and Huntington’s disease. PeerJ, 4, e2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyce AJ, Silveira-Moriyama L, Gilpin P, Ling H, Howard R, & Lees AJ. (2012). Severe dysphagia as a presentation of Parkinson’s disease. Movement Disorders, 27(3), 457–458. 10.1002/mds.24006 [DOI] [PubMed] [Google Scholar]
- Omari TI, Dejaeger E, Van Beckevoort D, Goeleven A, De Cock P, Hoffman I, … Rommel N. (2011). A novel method for the nonradiological assessment of ineffective swallowing. American Journal of Gastroenterology, 106(10), 1796–1802. 10.1038/ajg.2011.143 [DOI] [PubMed] [Google Scholar]
- Opara JA, Brola W, Leonardi M, & Błaszczyk B. (2012). Quality of life in Parkinson’s disease. Journal of Medicine and Life, 5(4), 375–381. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/23346238 [PMC free article] [PubMed] [Google Scholar]
- Perez KS, Ramig LO, Smith ME, & Dromey C. (1996). The Parkinson larynx: tremor and videostroboscopic findings. Journal of Voice, 10(4), 354–361. [DOI] [PubMed] [Google Scholar]
- Piboolnurak P, Lang AE, Lozano AM, Miyasaki JM, Saint‐Cyr JA, Poon YW, … Moro E. (2007). Levodopa response in long‐term bilateral subthalamic stimulation for Parkinson’s disease. Movement Disorders, 22(7), 990–997. [DOI] [PubMed] [Google Scholar]
- Pinto S, Gentil M, Fraix V, Benabid A-L, & Pollak P. (2003). Bilateral subthalamic stimulation effects on oral force control in Parkinson’s disease. Journal of Neurology, 250(2), 179–187. [DOI] [PubMed] [Google Scholar]
- Pisegna JM, & Langmore SE. (2016). Parameters of Instrumental Swallowing Evaluations: Describing a Diagnostic Dilemma. Dysphagia, 31, 462–472. 10.1007/s00455-016-9700-3 [DOI] [PubMed] [Google Scholar]
- Plowman-Prine EK, Okun MS, Sapienza CM, Shrivastav R, Fernandez HH, Foote KD, … Rosenbek JC. (2009). Perceptual characteristics of Parkinsonian speech: a comparison of the pharmacological effects of levodopa across speech and non-speech motor systems. NeuroRehabilitation, 24(2), 131–144. 10.3233/NRE-2009-0462 [DOI] [PubMed] [Google Scholar]
- Plowman-Prine, Emily K, Sapienza CM, Okun MS, Pollock SL, Jacobson C, Wu SS, & Rosenbek JC. (2009). The relationship between quality of life and swallowing in Parkinson’s disease. Movement Disorders, 24(9), 1352–1358. 10.1002/mds.22617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowman EK, & Kleim JA. (2011). Behavioral and neurophysiological correlates of striatal dopamine depletion: A rodent model of Parkinson’s disease. Journal of Communication Disorders, 44(5), 549–556. 10.1016/j.jcomdis.2011.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potulska A, Friedman A, Królicki L, & Spychala A. (2003). Swallowing disorders in Parkinson’s disease. Parkinsonism & Related Disorders, 9(6), 349–353. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12853234 [DOI] [PubMed] [Google Scholar]
- Ramig L, Halpern A, Spielman J, Fox C, & Freeman K. (2018). Speech treatment in Parkinson’s disease: Randomized controlled trial (RCT). Movement Disorders, 33(11), 1777–1791. 10.1002/mds.27460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig LO, Sapir S, Countryman S, Pawlas AA, O’brien C, Hoehn M, & Thompson LL. (2001). Intensive voice treatment (LSVT®) for patients with Parkinson’s disease: a 2 year follow up. J Neurol Neurosurg Psychiatry, 71, 493–498. 10.1136/jnnp.71.4.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read J, Miller N, & Kitsou N. (2018). Is there an order of loss of sounds in speakers with Parkinson’s disease? Clinical Linguistics & Phonetics, 32(11), 997–1011. [DOI] [PubMed] [Google Scholar]
- Robbins JA, Logemann JA, & Kirshner HS. (1986). Swallowing and speech production in Parkinson’s disease. Annals of Neurology, 19(3), 283–287. 10.1002/ana.410190310 [DOI] [PubMed] [Google Scholar]
- Robertson LT, St George RJ, Carlson-Kuhta P, Hogarth P, Burchiel KJ, & Horak FB. (2011). Site of deep brain stimulation and jaw velocity in Parkinson disease. Journal of Neurosurgery, 115(5), 985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romito LM, Scerrati M, Contarino MF, & Iacoangeli M. (2003). Bilateral high frequency subthalamic stimulation in Parkinson’s disease: long-term neurological follow-up. Journal of Neurosurgical Sciences, 47(3), 119. [PubMed] [Google Scholar]
- Rosenbek J, & Jones H. (2009). Dysphagia in Movement Disorders. San Diego, CA: Plural Publishing. [Google Scholar]
- Rousseaux M, Krystkowiak P, Kozlowski O, Özsancak C, Blond S, & Destée A. (2004). Effects of subthalamic nucleus stimulation on parkinsonian dysarthria and speech intelligibility. Journal of Neurology, 251(3), 327–334. [DOI] [PubMed] [Google Scholar]
- Rusz J, Tykalová T, Klempíř J, Čmejla R, & Růžička E. (2016). Effects of dopaminergic replacement therapy on motor speech disorders in Parkinson’s disease: longitudinal follow-up study on previously untreated patients. Journal of Neural Transmission, 123(4), 379–387. [DOI] [PubMed] [Google Scholar]
- Sabaté M, González I, Ruperez F, & Rodríguez M. (1996). Obstructive and restrictive pulmonary dysfunctions in Parkinson’s disease. Journal of the Neurological Sciences, 138(1–2), 114–119. [DOI] [PubMed] [Google Scholar]
- Sapir S, Ramig LO, Hoyt P, Countryman S, O’Brien C, & Hoehn M. (2002). Speech loudness and quality 12 months after intensive voice treatment (LSVT®) for Parkinson’s disease: A comparison with an alternative speech treatment. Folia Phoniatrica et Logopaedica, 54(6), 296–303. [DOI] [PubMed] [Google Scholar]
- Sapir S, Ramig LO, Spielman JL, & Fox C. (2010). Formant centralization ratio: A proposal for a new acoustic measure of dysarthric speech. Journal of Speech, Language, and Hearing Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerbier A, Jenner P, Todorova A, & Chaudhuri KR. (2016). Non motor subtypes and Parkinson’s disease. Parkinsonism & Related Disorders, 22, S41–S46. 10.1016/j.parkreldis.2015.09.027 [DOI] [PubMed] [Google Scholar]
- Schapira AHV, Chaudhuri KR, & Jenner P. (2017). Non-motor features of Parkinson disease. Nature Reviews Neuroscience, 18(7), 435–450. 10.1038/nrn.2017.62 [DOI] [PubMed] [Google Scholar]
- Schiess, Zheng, Soukup, Bonnen, & Nauta. (2000). Parkinson’s disease subtypes: clinical classification and ventricular cerebrospinal fluid analysis. Parkinsonism & Related Disorders, 6(2), 69–76. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10699387 [DOI] [PubMed] [Google Scholar]
- Shahed J, & Jankovic J. (2007). Motor symptoms in Parkinson’s disease. Handbook of Clinical Neurology, 83, 329–342. [DOI] [PubMed] [Google Scholar]
- Sharkawi A, Ramig L, Logemann JA, Pauloski BR, Rademaker AW, Smith CH, … Werner C. (2002). Swallowing and voice effects of Lee Silverman Voice Treatment (LSVT®): A pilot study. Journal of Neurology, Neurosurgery & Psychiatry, 72(1), 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastav R, & Sapienza CM. (2003). Objective measures of breathy voice quality obtained using an auditory model. The Journal of the Acoustical Society of America, 114(4), 2217–2224. [DOI] [PubMed] [Google Scholar]
- Sidtis D, Cameron K, Bonura L, & Sidtis JJ. (2012). Speech intelligibility by listening in Parkinson speech with and without deep brain stimulation: Task effects. Journal of Neurolinguistics, 25(2), 121–132. [Google Scholar]
- Sidtis JJ, Alken AG, Tagliati M, Alterman R, & Van Lancker Sidtis D. (2016). Subthalamic stimulation reduces vowel space at the initiation of sustained production: Implications for articulatory motor control in Parkinson’s disease. Journal of Parkinson’s Disease, 6(2), 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JA. (2017). CHAPTER FORTY-THREE Swallowing Dysfunctions in Parkinson’s Disease. 10.1016/bs.irn.2017.05.026 [DOI] [PubMed] [Google Scholar]
- Skodda, Grönheit W, Mancinelli N, & Schlegel U. (2013). Progression of voice and speech impairment in the course of Parkinson’s disease: a longitudinal study. Parkinson’s Disease, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skodda, Rinsche H, & Schlegel U. (2009). Progression of dysprosody in Parkinson’s disease over time—a longitudinal study. Movement Disorders, 24(5), 716–722. [DOI] [PubMed] [Google Scholar]
- Skodda S. (2012). Effect of Deep Brain Stimulation on Speech Performance in Parkinson’s Disease. Parkinson’s Disease, 2012, 1–10. 10.1155/2012/850596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skodda S, Flasskamp A, & Schlegel U. (2011). Instability of syllable repetition as a marker of disease progression in Parkinson’s disease: a longitudinal study. Movement Disorders, 26(1), 59–64. [DOI] [PubMed] [Google Scholar]
- Skodda S, Grönheit W, & Schlegel U. (2011). Intonation and speech rate in Parkinson’s disease: General and dynamic aspects and responsiveness to levodopa admission. Journal of Voice, 25(4), e199–e205. [DOI] [PubMed] [Google Scholar]
- Skodda S, Grönheit W, & Schlegel U. (2012). Impairment of vowel articulation as a possible marker of disease progression in Parkinson’s disease. Public Library of Science ONE, 7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skodda S, & Schlegel U. (2008). Speech rate and rhythm in Parkinson’s disease. Movement Disorders: Official Journal of the Movement Disorder Society, 23(7), 985–992. [DOI] [PubMed] [Google Scholar]
- Skodda S, Visser W, & Schlegel U. (2010). Short-and long-term dopaminergic effects on dysarthria in early Parkinson’s disease. Journal of Neural Transmission, 117(2), 197–205. [DOI] [PubMed] [Google Scholar]
- Solomon NP, & Hixon TJ. (1993). Speech breathing in Parkinson’s disease. Journal of Speech, Language, and Hearing Research, 36(2), 294–310. [DOI] [PubMed] [Google Scholar]
- Spencer KA, Morgan KW, & Blond E. (2009). Dopaminergic medication effects on the speech of individuals with Parkinson’s disease. Journal of Medical Speech-Language Pathology, 17(3), 125–145. [Google Scholar]
- Sprenger F, & Poewe W. (2013). Management of Motor and Non-Motor Symptoms in Parkinson’s Disease. CNS Drugs, 27(4), 259–272. 10.1007/s40263-013-0053-2 [DOI] [PubMed] [Google Scholar]
- Stelzig Y, Hochhaus W, Gall V, & Henneberg A. (1999). Laryngeal manifestations in patients with Parkinson disease. Laryngo-Rhino-Otologie, 78(10), 544–551. [DOI] [PubMed] [Google Scholar]
- Stipancic KL, Tjaden K, & Wilding G. (2015). Comparison of intelligibility measures for adults with Parkinson’s disease, adults with multiple sclerosis, and healthy controls. Journal of Speech, Language, and Hearing Research, 59(2), 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroudley J, & Walsh M. (1991). Radiological assessment of dysphagia in Parkinson’s disease. The British Journal of Radiology, 64(766), 890–893. 10.1259/0007-1285-64-766-890 [DOI] [PubMed] [Google Scholar]
- Sung HY, Kim J-S, Lee K-S, Kim Y-I, Song I-U, Chung S-W, … Choi M-G. (2010). The prevalence and patterns of pharyngoesophageal dysmotility in patients with early stage Parkinson’s disease. Movement Disorders, 25(14), 2361–2368. 10.1002/mds.23290 [DOI] [PubMed] [Google Scholar]
- Suntrup S, Teismann I, Bejer J, Suttrup I, Winkels M, Mehler D, … Warnecke T. (2013). Evidence for adaptive cortical changes in swallowing in Parkinson’s disease. Brain, 136(3), 726–738. 10.1093/brain/awt004 [DOI] [PubMed] [Google Scholar]
- Sutton JP. (2013). Dysphagia in Parkinson’s disease is responsive to levodopa. Parkinsonism and Related Disorders, 19(3), 282–284. 10.1016/j.parkreldis.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Švec JG, & Granqvist S. (2018). Tutorial and guidelines on measurement of sound pressure level in voice and speech. Journal of Speech, Language, and Hearing Research, 61(3), 441–461. [DOI] [PubMed] [Google Scholar]
- Svensson P, Henningson C, & Karlsson S. (1993). Speech motor control in Parkinson’s disease: a comparison between a clinical assessment protocol and a quantitative analysis of mandibular movements. Folia Phoniatrica et Logopaedica, 45(4), 157–164. [DOI] [PubMed] [Google Scholar]
- Thobois S, Mertens P, Guenot M, Hermier M, Mollion H, Bouvard M, … Sindou M. (2002). Subthalamic nucleus stimulation in Parkinson’s disease. Journal of Neurology, 249(5), 529–534. [DOI] [PubMed] [Google Scholar]
- Tjaden K, Lam J, & Wilding G. (2013). Vowel acoustics in Parkinson’s disease and multiple sclerosis: Comparison of clear, loud, and slow speaking conditions. Journal of Speech, Language, and Hearing Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaden K, Sussman JE, & Wilding GE. (2014). Impact of clear, loud, and slow speech on scaled intelligibility and speech severity in Parkinson’s disease and multiple sclerosis. Journal of Speech, Language, and Hearing Research, 57(3), 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft M, & Dietrichs E. (2011). Aggravated stuttering following subthalamic deep brain stimulation in Parkinson’s disease-two cases. BMC Neurology, 11(1), 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourville JA, & Guenther FH. (2011). The DIVA model: A neural theory of speech acquisition and production. Lang Cogn Process, 26(7), 952–981. 10.1080/01690960903498424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripoliti E, Limousin P, Foltynie T, Candelario J, Aviles‐Olmos I, Hariz MI, & Zrinzo L. (2014). Predictive factors of speech intelligibility following subthalamic nucleus stimulation in consecutive patients with Parkinson’s disease. Movement Disorders, 29(4), 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripoliti E, Zrinzo L, Martinez-Torres I, Frost E, Pinto S, Foltynie T, … Hariz MI. (2011). Effects of subthalamic stimulation on speech of consecutive patients with Parkinson disease. Neurology, 76(1), 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troche MS, Okun MS, Rosenbek JC, Musson N, Rodriguez R, Romrell J, Pitts T, … Sapienza CM. (2010). Aspiration and swallowing in Parkinson disease and rehabilitation with EMST A randomized trial. Neurology, 75, 1912–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troche, Michelle S, Brandimore AE, Foote KD, & Okun MS. (2013). Swallowing and deep brain stimulation in Parkinson’s disease: A systematic review. Parkinsonism & Related Disorders, 19(9), 783–788. 10.1016/j.parkreldis.2013.05.001.Swallowing [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T, Watanabe H, Tanaka Y, Ohdake R, Yoneyama N, Hara K, … Atsuta N. (2015). Distinct phenotypes of speech and voice disorders in Parkinson’s disease after subthalamic nucleus deep brain stimulation. J Neurol Neurosurg Psychiatry, 86(8), 856–864. [DOI] [PubMed] [Google Scholar]
- Tykalová T, Rusz J, Čmejla R, Klempíř J, Růžičková H, Roth J, & Růžička E. (2015). Effect of dopaminergic medication on speech dysfluency in Parkinson’s disease: a longitudinal study. Journal of Neural Transmission, 122(8), 1135–1142. [DOI] [PubMed] [Google Scholar]
- van Rooden SM, Colas F, Martínez-Martín P, Visser M, Verbaan D, Marinus J, … van Hilten JJ. (2011). Clinical subtypes of Parkinson’s disease. Movement Disorders, 26(1), 51–58. 10.1002/mds.23346 [DOI] [PubMed] [Google Scholar]
- Vercueil L, Linard JP, Wuyam B, Pollak P, & Benchetrit G. (1999). Breathing pattern in patients with Parkinson’s disease. Respiration Physiology, 118(2–3), 163–172. [DOI] [PubMed] [Google Scholar]
- Viallet F, Teston B, Jankowski L, Purson A, Peragut J-C, Regis J, & Wiitjas T. (2002). Effects of pharmacological versus electrophysiological treatments on parkinsonian dysprosody. In Speech Prosody (pp. 679–682). Laboratoire Parole et Langage. [Google Scholar]
- Villacorta VM, Perkell JS, & Guenther FH. (2007). Sensorimotor adaptation to feedback perturbations of vowel acoustics and its relation to perception. J Acoust Soc Am, 122(4), 2306–2319. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17902866 [DOI] [PubMed] [Google Scholar]
- Volonté MA, Porta M, & Comi G. (2002). Clinical assessment of dysphagia in early phases of Parkinson’s disease. Neurological Sciences, 23(0), s121–s122. 10.1007/s100720200099 [DOI] [PubMed] [Google Scholar]
- Wang E, Metman LV, Bakay R, Arzbaecher J, & Bernard B. (2003). The effect of unilateral electrostimulation of the subthalamic nucleus on respiratory/phonatory subsystems of speech production in Parkinson’s disease—a preliminary report. Clinical Linguistics & Phonetics, 17(4–5), 283–289. [DOI] [PubMed] [Google Scholar]
- Warnecke T, Suttrup I, Schroder JB, Osada N, Oelenberg S, Hamacher C, … Dziewas R. (2016). Levodopa responsiveness of dysphagia in advanced Parkinson’s disease and reliability testing of the FEES-Levodopa-test. Parkinsonism and Related Disorders, 28, 100–106. 10.1016/j.parkreldis.2016.04.034 [DOI] [PubMed] [Google Scholar]
- Whitfield JA, & Goberman AM. (2014). Articulatory–acoustic vowel space: Application to clear speech in individuals with Parkinson’s disease. Journal of Communication Disorders, 51, 19–28. [DOI] [PubMed] [Google Scholar]
- Wu Y, Guo X, Wei Q, Ou R, Song W, Cao B, … Shang H. (2016). Non‐motor symptoms and quality of life in tremor dominant vs postural instability gait disorder Parkinson′ s disease patients. Acta Neurologica Scandinavica, 133(5), 330–337. [DOI] [PubMed] [Google Scholar]
- Yorkston KM, Beukelman DR, & Traynor C. (1984). Assessment of intelligibility of dysarthric speech. Pro-ed Austin, TX. [Google Scholar]
- Yousefi B, Tadibi V, Khoei AF, & Montazeri A. (2009). Exercise therapy, quality of life, and activities of daily living in patients with Parkinson disease: a small scale quasi-randomised trial. Trials, 10(1), 67 10.1186/1745-6215-10-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarzur, Ana P, de Campos Duprat A, Cataldo BO, Ciampi D, & Fonoff E. (2014). Laryngeal electromyography as a diagnostic tool for Parkinson’s disease. The Laryngoscope, 124(3), 725–729. [DOI] [PubMed] [Google Scholar]
- Zarzur Ana Paula, Duprat AC, Shinzato G, & Eckley CA. (2007). Laryngeal electromyography in adults with Parkinson’s disease and voice complaints. The Laryngoscope, 117(5), 831–834. [DOI] [PubMed] [Google Scholar]
- Zwirner P, Murry T, & Woodson GE. (1991). Phonatory function of neurologically impaired patients. Journal of Communication Disorders, 24(4), 287–300. [DOI] [PubMed] [Google Scholar]