Abstract
Pharmaceutical and personal care products (PPCPs) are commonly used chemicals that are increasingly detected in urban-impacted environments, particularly those receiving treated wastewater. PPCPs may have toxicological effects on the macrofauna that are exposed through contaminated water; thus, there is interest in microbially mediated transformations that may degrade PPCPs. This review discusses specific examples of PPCP transformations that may occur in anoxic environments, including O-methylation and O-demethylation.
Keywords: Biotransformation, anaerobic O-demethylation, anaerobic O-methylation, pharmaceutical biodegradation
Introduction
Pharmaceutical and personal care products (PPCPs) contain chemicals that are widely distributed in surface waters, sediment, and soil 1, 2. Pharmaceuticals enter wastewater treatment plants through ingestion and subsequent excretion 3, through improper disposal down a household drain 4, or from pharmaceutical manufacturing plant discharge 5. Wastewater treatment plants are not designed to remove these complex organic contaminants, which can result in incomplete PPCP removal. A major concern is, therefore, that treated effluent may contain low concentrations of PPCPs that can enter receiving waters or soils when biosolids are used as fertilizer 6– 8.
A range of adverse effects has been reported for wildlife that is exposed to treated effluent. When released into the environment, pharmaceuticals can be toxic 9 or can cause unwanted physiological responses to non-target organisms, including endocrine disruption (e.g. feminization of fish), altered development of aquatic organisms including fish and frogs, and changes to behavior 10– 13. In addition, bioaccumulation in aquatic organisms is a concern, particularly in fish intended for human consumption 14. Not only are these findings a potential public health problem, but they also raise concerns about the health of the ecosystem and overall water quality. While the concentrations of an individual chemical may be in the ng L -1 range (for example, see 15), PPCPs are typically found in wastewater as complex mixtures and may have additive effects that remain to be understood.
Microbial toxicity
Some PPCPs are designed specifically to have antagonist effects against microorganisms. Notably, this includes antibiotics, preservatives (e.g. parabens), and antimicrobial compounds (e.g. triclosan and triclocarban). Others may have unexpected inhibitory effects. Ibuprofen, for example, has been shown to inhibit the growth of a variety of microorganisms 16. Pharmaceuticals such as propranolol, diphenhydramine, and diclofenac sodium have also been reported to have inhibitory effects on the methanogenic microbial community found in anaerobic digesters 17, 18. Furthermore, the metabolites produced during microbial transformation of pharmaceuticals are not always further degraded 18– 20 and could also have negative effects on the microbial community. Alternatively, the microbial community may still carry out the desired function, such as methanogenesis, but the microbial community composition may be altered or enriched for antibiotic resistance genes 21, 22. In addition, these metabolites can still be pharmacologically active and can exhibit toxicity to eukaryotic organisms, although these effects have not yet been documented in prokaryotic organisms such as bacteria and archaea 23, 24. As prokaryotes provide ecosystem services for all environments, the effects of PPCPs and their metabolites on prokaryotes are valuable to know.
Biodegradation
PPCPs enter changing environmental conditions and encounter diverse microbial communities as they pass from households through the wastewater treatment process and ultimately into the environment. The initial stages of wastewater treatment are designed to first use well-oxygenated units to support aerobic degradation. Later in the process, further degradation of the sludge solids takes place in anaerobic digester units that promote a fermenting and methanogenic community operating under low oxidation-reduction potential (<–350 mV). Treated wastewater effluent is released into oxic surface water; however, some PPCPs will eventually migrate into anoxic sediments 25– 28. In freshwater systems, nitrate, iron, or carbonate are predominant electron acceptors available for respiration, whereas coastal marine waters would additionally have sulfate as a respiratory electron acceptor. These different conditions, therefore, support diverse microbial communities that may also be capable of divergent biochemical mechanisms for biodegradation in surface waters and anoxic sediments. This must all be taken into account when modeling the environmental fate of PPCPs, as degradation might be more likely to occur, proceed to a greater extent, or produce different intermediates depending on the location. Naproxen transformation, for example, has been shown to occur under sulfate-reducing and methanogenic conditions in constructed wetlands, estuarine sediment, and anaerobic digester sludge, yet nitrate-reducing conditions in constructed wetlands yielded little transformation activity 20, 29. Oxybenzone, in contrast, was transformed under aerobic, nitrate-reducing, iron-reducing, sulfate-reducing, and methanogenic conditions 30, 31. PPCPs have diverse chemical structures that underscore the need for a broader understanding of how microbes in different environments will metabolize the different classes of compounds. This is valuable for predicting potential activity in the environment, as partial microbial transformations may make the original PPCP undetectable by standard methods, yet the new transformation product may have ecotoxicological effects.
Fate in wastewater treatment plants and receiving aquatic habitats
There have been many reports of PPCP removal during the biological stages of wastewater treatment (for reviews, see 32, 33) or quantifying PPCPs in effluent-impacted water and sediment 2, 34. Treated wastewater effluents are one of the main pathways by which PPCPs enter watersheds. While some removal during wastewater treatment can be attributed to the biological activity of microorganisms, there are few simplified consortia or pure cultures available to demonstrate the biochemistry involved in PPCP transformation. Without biochemical evidence, it is difficult to determine if the PPCP in question has been mineralized 35, lost due to abiotic processes such as sorption to solids 36– 38, or transformed into unknown metabolites 31, 39.
Some anaerobic transformation reactions may lead to persistent metabolites that present additional environmental problems. Nonylphenol and octylphenol, for example, are produced from the sequential removal of ethoxyl groups from the nonionic surfactants nonylphenol polyethoxylate and octylphenol polyethoxylate, as shown in Table 1 40, 41. These metabolites have been shown to mimic estrogen 13 and are frequently detected in wastewater treatment systems and in the aquatic environment 2. The genes and biochemical intermediates for nonylphenol and octylphenol degradation have been reported under aerobic conditions 42– 44; however, only limited data exist regarding their fate under anaerobic conditions and the biochemical pathways are largely unknown 46– 49. With the identification of nonylphenol and octylphenol as persistent metabolites with toxicological effects, it is now imperative to monitor their concentrations in the environment and quantify their potential estrogenic impact.
Table 1. Anaerobic and aerobic transformation reactions may lead to persistent metabolites.
Specific examples of pharmaceutical and personal care products with corresponding transformation products are shown.
| Parent compound | Transformation product | References |
|---|---|---|
| O-Demethylation | ||
|
Naproxen
Over-the-counter non-steroidal anti- inflammatory drug |
6-O-Desmethylnaproxen
|
20, 31 |
|
Guaifenesin
Expectorate 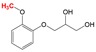
|
3-(2-hydroxyphenoxy) propane-1,2-diol
|
31 |
|
Oxybenzone
UV light absorber Found in sunscreens and plastics |
2,4-Dihydroxybenzophenone
|
31 |
|
Methylparaben
Preservative in cosmetics, pharmaceuticals, and food 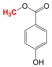
|
4-Hydroxybenzoic acid
|
31 |
| N-Demethylation | ||
|
Diphenhydramine
Anti-histamine 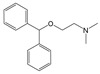
|
N-Desmethyl diphenhydramine
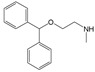
|
18 |
| O-Methylation | ||
|
Bisphenol A (BPA)
Plastic precursor |
BPA monomethyl ether (left),
BPA dimethyl ether (right) |
45 |
| De-ethoxylation | ||
|
Octylphenol and
nonylphenol polyethoxylate Nonionic surfactant 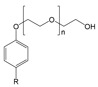
|
Octylphenol or nonylphenol
|
40 |
Other types of anaerobic biotransformation reactions include O-demethylation. Recently, we reported on the complex microbial strategy of naproxen transformation by a methanogenic consortium enriched from anaerobic digester sludge 20. The methanogenic consortium O-demethylated naproxen to form the persistent metabolite 6-O-desmethylnaproxen, which is illustrated in Table 1. Acetogenic bacteria were responsible for this step and produced acetate that subsequently enriched for a population of syntrophic acetate-oxidizing bacteria. The latter supported a methanogenic community that produced the amount of methane that was consistent with O-demethylation 20. This model is an example of an anaerobic microbial food web that was supported through pharmaceutical biotransformation.
Similarly, diphenhydramine can be transformed by anaerobic digester sludge microbes via N-demethylation to N-desmethyl diphenhydramine (see Table 1; 18), a metabolite formerly known to be generated only by mammals and fungi 50. While the parent compound, diphenhydramine, suppressed both fermentative and methanogenic activity in the anaerobic digester community, the metabolite suppressed only methanogenic activity. In contrast, there was negligible toxicity of naproxen and 6-O-desmethylnaproxen to the same community 20. These differences highlight how chemically different PPCPs and their transformation products may have different effects on the same microbial community, further underscoring the complexity of the fate and effect of the PPCPs.
While anaerobic O-demethylation of aromatic compounds has been well established (see 51), less is known about this transformation in PPCPs. We have evidence that PPCPs with diverse uses and chemical structures but share a phenylmethyl ether functional group can be transformed via O-demethylation 31. Microbial communities enriched under both methanogenic and sulfate-rich conditions showed this capability when provided with naproxen, guaifenesin, methylparaben, or oxybenzone 31. The sulfate-rich cultures formed O-demethylated metabolites, shown in Table 1, that were not further degraded. A similar pattern was observed in the methanogenic cultures 31.
In contrast, many phenolic compounds can be transformed by microbial O-methylation (see 52). For example, bacteria are able to O-methylate bisphenol A (BPA) to its monomethyl and dimethyl ether derivatives, as pictured in Table 1 (BPA MME and BPA DME, respectively) 45, resulting in metabolites with increased toxicity as shown from differences in survival and occurrence of developmental lesions in developing zebrafish embryos exposed to BPA, BPA MME, and BPA DME. The monomethyl and dimethyl ether derivatives were more toxic than BPA, resulting in increased mortality. Furthermore, exposure to either of the O-methylated metabolites resulted in an increase in the incidence of developmental lesions as compared to BPA exposure 45. These data illustrate a new mechanism for the microbial transformation of BPA, producing metabolites warranting further study to understand their prevalence and effects in the environment. In addition, the O-methylated transformation products could serve as potential substrates for O-demethylation by the organisms described above 31, 51. The interconversion between O-methylated and O-demethylated forms thus presents a mechanism by which a PPCP compound can be transformed in one environment and the original parent compound regenerated by microbes that are active in another environment. This is similar to reports of flame-retardant and antimicrobial compound transformations that have been described in plants 53, 54.
Predicting anaerobic biodegradation
Identifying common functional groups may serve as a basis for predicting transformation products. Gulde et al. 55 used a systematic approach to identify potential metabolites of PPCPs that contain an amine group, applying this method to predicting reactions in aerobic activated sludge. Alternatively, we used a culture-based approach to examine the range of O-demethylation substrates in anoxic sediments and anaerobic wastewater digestion 31. Gonzalez-Gil et al. 56 used enzyme assays to examine co-metabolic transformations of diverse PPCPs, including naproxen, nonylphenol, octylphenol, triclosan, and BPA, that were mediated by acetate kinase. The extent to which transformation occurred varied with the substrate from 10–90% and suggested the involvement of additional transformation pathways 56, which could lead to a mixture of different transformation products existing from the same parent compound. Laboratory-based assays, such as those conducted by Gonzalez-Gil et al. 56 and Wolfson et al. 31, represent a starting point for the identification of potential metabolites, although it may not be representative of the dominant transformation mechanism that occurs in the environment. Likewise, the microbial community composition may have an effect on PPCP transformations, especially under methanogenic conditions 20, 57. Additionally, the effects that mixtures of PPCPs and associated transformation products will have on microbial community function cannot be overlooked.
Future directions for PPCP removal
Recognition of the expanding extent of PPCP contamination has stimulated the search for solutions that remove pharmaceuticals from wastewater before they can reach the environment, including technologies like advanced oxidation processes and membrane bioreactors 58– 62. These new technologies have shown promise with higher removal rates in pilot treatment plants than with conventional treatment 61, 63. In combination with increased removal efficiencies, re-designing PPCPs to promote biodegradability could lead to a reduction in the environmental load in the future 64, 65. Understanding transformation products is important not only to the health of impacted aquatic ecosystems and humans but also for monitoring the safety of reclaimed wastewater reuse (for review, see 66) and for the accuracy of wastewater-based epidemiology to follow human health and pharmaceutical use 67– 70.
Conclusions
An Organization for Economic Cooperation and Development report projects that sales of chemicals worldwide will increase by 3% annually between now and 2050 71, thus providing a steady stream of diverse chemical structures that may be entering wastewater treatment and the environment. Given that pharmaceuticals are used daily throughout the world, their release into the environment is both a public and an environmental health concern. Understanding not only the microbial transformation processes but also the metabolites that are formed is essential for comprehensive accounting of pharmaceuticals and potential pharmaceutically active compounds in the environment. The environmental context, be it the engineered anaerobic digester or freshwater or estuarine sediments that are impacted by treated wastewater, is critical for understanding potential microbial activities and biodegradation mechanisms to determine if biodegradation will occur or if potential metabolites may form and accumulate under the given redox conditions. This knowledge may provide solutions to remove these pharmaceuticals during wastewater treatment and prevent environmental deposition as well as to understand environmental processes that may occur to remove pharmaceuticals that have already entered the environment.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Ron Oremland, Department of Microbiology and Environmental Toxicology, University of California at Santa Cruz, Santa Cruz, CA, USA; U.S. Geological Survey, Menlo Park, California, USA
Adam C Mumford, U.S. Geological Survey, Reston, Virginia, USA
Funding Statement
This work was supported by the USDA National Institute of Food and Agriculture Hatch Multistate project 1007899 through the New Jersey Agricultural Experiment Station Hatch Multistate NJ07212 and Hatch project NJ01160. S.J.W. was supported by a U.S. National Science Foundation Fuels IGERT (NSF DGE 0903675) from Rutgers, the State University of New Jersey.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. Cantwell MG, Katz DR, Sullivan JC, et al. : Spatial patterns of pharmaceuticals and wastewater tracers in the Hudson River Estuary. Water Res. 2018;137:335–43. 10.1016/j.watres.2017.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Kolpin DW, Furlong ET, Meyer MT, et al. : Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999-2000: a national reconnaissance. Environ Sci Technol. 2002;36(6):1202–11. 10.1021/es011055j [DOI] [PubMed] [Google Scholar]
- 3. Xia K, Bhandari A, Das K, et al. : Occurrence and fate of pharmaceuticals and personal care products (PPCPs) in biosolids. J Environ Qual. 2005;34(1):91–104. 10.2134/jeq2005.0091 [DOI] [PubMed] [Google Scholar]
- 4. Jelic A, Rodriguez-Mozaz S, Barceló D, et al. : Impact of in-sewer transformation on 43 pharmaceuticals in a pressurized sewer under anaerobic conditions. Water Res. 2015;68:98–108. 10.1016/j.watres.2014.09.033 [DOI] [PubMed] [Google Scholar]
- 5. Fick J, Söderström H, Lindberg RH, et al. : Contamination of surface, ground, and drinking water from pharmaceutical production. Environ Toxicol Chem. 2009;28(12):2522–7. 10.1897/09-073.1 [DOI] [PubMed] [Google Scholar]
- 6. Biel-Maeso M, Corada-Fernández C, Lara-Martín PA: Removal of personal care products (PCPs) in wastewater and sludge treatment and their occurrence in receiving soils. Water Res. 2019;150:129–39. 10.1016/j.watres.2018.11.045 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Kinney CA, Furlong ET, Zaugg SD, et al. : Survey of organic wastewater contaminants in biosolids destined for land application. Environ Sci Technol. 2006;40(23):7207–15. 10.1021/es0603406 [DOI] [PubMed] [Google Scholar]
- 8. Sabourin L, Beck A, Duenk PW, et al. : Runoff of pharmaceuticals and personal care products following application of dewatered municipal biosolids to an agricultural field. Sci Total Environ. 2009;407(16):4596–604. 10.1016/j.scitotenv.2009.04.027 [DOI] [PubMed] [Google Scholar]
- 9. Watanabe H, Tamura I, Abe R, et al. : Chronic toxicity of an environmentally relevant mixture of pharmaceuticals to three aquatic organisms (alga, daphnid, and fish). Environ Toxicol Chem. 2016;35(4):996–1006. 10.1002/etc.3285 [DOI] [PubMed] [Google Scholar]
- 10. Bringolf RB, Heltsley RM, Newton TJ, et al. : Environmental occurrence and reproductive effects of the pharmaceutical fluoxetine in native freshwater mussels. Environ Toxicol Chem. 2010;29(6):1311–8. 10.1002/etc.157 [DOI] [PubMed] [Google Scholar]
- 11. Dzieweczynski TL, Campbell BA, Kane JL: Dose-dependent fluoxetine effects on boldness in male Siamese fighting fish. J Exp Biol. 2016;219(Pt 6):797–804. 10.1242/jeb.132761 [DOI] [PubMed] [Google Scholar]
- 12. Weinberger J, 2nd, Klaper R: Environmental concentrations of the selective serotonin reuptake inhibitor fluoxetine impact specific behaviors involved in reproduction, feeding and predator avoidance in the fish Pimephales promelas (fathead minnow). Aquat Toxicol. 2014;151:77–83. 10.1016/j.aquatox.2013.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. White R, Jobling S, Hoare SA, et al. : Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994;135(1):175–82. 10.1210/endo.135.1.8013351 [DOI] [PubMed] [Google Scholar]
- 14. Huerta B, Rodriguez-Mozaz S, Lazorchak J, et al. : Presence of pharmaceuticals in fish collected from urban rivers in the U.S. EPA 2008-2009 National Rivers and Streams Assessment. Sci Total Environ. 2018;634:542–9. 10.1016/j.scitotenv.2018.03.387 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Archer E, Petrie B, Kasprzyk-Hordern B, et al. : The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere. 2017;174:437–46. 10.1016/j.chemosphere.2017.01.101 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Obad J, Šušković J, Kos B: Antimicrobial activity of ibuprofen: new perspectives on an "Old" non-antibiotic drug. Eur J Pharm Sci. 2015;71:93–8. 10.1016/j.ejps.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 17. Fountoulakis MS, Stamatelatou K, Lyberatos G: The effect of pharmaceuticals on the kinetics of methanogenesis and acetogenesis. Bioresour Technol. 2008;99(15):7083–90. 10.1016/j.biortech.2008.01.008 [DOI] [PubMed] [Google Scholar]
- 18. Wolfson SJ, Porter AW, Villani TS, et al. : The antihistamine diphenhydramine is demethylated by anaerobic wastewater microorganisms. Chemosphere. 2018;202:460–6. 10.1016/j.chemosphere.2018.03.093 [DOI] [PubMed] [Google Scholar]
- 19. Helbling DE, Hollender J, Kohler HP, et al. : High-throughput identification of microbial transformation products of organic micropollutants. Environ Sci Technol. 2010;44(17):6621–7. 10.1021/es100970m [DOI] [PubMed] [Google Scholar]
- 20. Wolfson SJ, Porter AW, Campbell JK, et al. : Naproxen Is Transformed Via Acetogenesis and Syntrophic Acetate Oxidation by a Methanogenic Wastewater Consortium. Microb Ecol. 2018;76(2):362–71. 10.1007/s00248-017-1136-2 [DOI] [PubMed] [Google Scholar]
- 21. Carey DE, Zitomer DH, Hristova KR, et al. : Triclocarban Influences Antibiotic Resistance and Alters Anaerobic Digester Microbial Community Structure. Environ Sci Technol. 2016;50(1):126–34. 10.1021/acs.est.5b03080 [DOI] [PubMed] [Google Scholar]
- 22. Fujimoto M, Carey DE, McNamara PJ: Metagenomics reveal triclosan-induced changes in the antibiotic resistome of anaerobic digesters. Environ Pollut. 2018;241:1182–90. 10.1016/j.envpol.2018.06.048 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Celiz MD, Tso J, Aga DS: Pharmaceutical metabolites in the environment: analytical challenges and ecological risks. Environ Toxicol Chem. 2009;28(12):2473–84. 10.1897/09-173.1 [DOI] [PubMed] [Google Scholar]
- 24. López-Serna R, Petrović M, Barceló D: Occurrence and distribution of multi-class pharmaceuticals and their active metabolites and transformation products in the Ebro River basin (NE Spain). Sci Total Environ. 2012;440:280–9. 10.1016/j.scitotenv.2012.06.027 [DOI] [PubMed] [Google Scholar]
- 25. Cantwell MG, Wilson BA, Zhu J, et al. : Temporal trends of triclosan contamination in dated sediment cores from four urbanized estuaries: evidence of preservation and accumulation. Chemosphere. 2010;78(4):347–52. 10.1016/j.chemosphere.2009.11.021 [DOI] [PubMed] [Google Scholar]
- 26. Hajj-Mohamad M, Aboulfadl K, Darwano H, et al. : Wastewater micropollutants as tracers of sewage contamination: analysis of combined sewer overflow and stream sediments. Environ Sci Process Impacts. 2014;16(10):2442–50. 10.1039/c4em00314d [DOI] [PubMed] [Google Scholar]
- 27. Lara-Martín PA, Renfro AA, Cochran JK, et al. : Geochronologies of pharmaceuticals in a sewage-impacted estuarine urban setting (Jamaica Bay, New York). Environ Sci Technol. 2015;49(10):5948–55. 10.1021/es506009v [DOI] [PubMed] [Google Scholar]
- 28. Li X, Doherty AC, Brownawell B, et al. : Distribution and diagenetic fate of synthetic surfactants and their metabolites in sewage-impacted estuarine sediments. Environ Pollut. 2018;242(Pt A):209–18. 10.1016/j.envpol.2018.06.064 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. He Y, Sutton NB, Rijnaarts HHM, et al. : Pharmaceutical biodegradation under three anaerobic redox conditions evaluated by chemical and toxicological analyses. Sci Total Environ. 2018;618:658–64. 10.1016/j.scitotenv.2017.07.219 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Liu YS, Ying GG, Shareef A, et al. : Biodegradation of the ultraviolet filter benzophenone-3 under different redox conditions. Environ Toxicol Chem. 2012;31(2):289–95. 10.1002/etc.749 [DOI] [PubMed] [Google Scholar]
- 31. Wolfson SJ, Porter AW, Villani TS, et al. : Pharmaceuticals and Personal Care Products Can Be Transformed by Anaerobic Microbiomes in the Environment and in Waste-Treatment Processes. Environ Toxicol Chem. 2019;38(7):1585–93. 10.1002/etc.4406 [DOI] [PubMed] [Google Scholar]
- 32. Alvarino T, Lema J, Omil F, et al. : Trends in organic micropollutants removal in secondary treatment of sewage. Rev Environ Sci Biotechnol. 2018;17:447–69. 10.1007/s11157-018-9472-3 [DOI] [Google Scholar]; F1000 Recommendation
- 33. Tiwari B, Sellamuthu B, Ouarda Y, et al. : Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour Technol. 2017;224:1–12. 10.1016/j.biortech.2016.11.042 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Bradley PM, Journey CA, Romanok KM, et al. : Expanded Target-Chemical Analysis Reveals Extensive Mixed-Organic-Contaminant Exposure in U.S. Streams. Environ Sci Technol. 2017;51(9):4792–802. 10.1021/acs.est.7b00012 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Wu Y, Sun Q, Wang YW, et al. : Comparative studies of aerobic and anaerobic biodegradation of methylparaben and propylparaben in activated sludge. Ecotoxicol Environ Saf. 2017;138:25–31. 10.1016/j.ecoenv.2016.12.017 [DOI] [PubMed] [Google Scholar]
- 36. Blair B, Nikolaus A, Hedman C, et al. : Evaluating the degradation, sorption, and negative mass balances of pharmaceuticals and personal care products during wastewater treatment. Chemosphere. 2015;134:395–401. 10.1016/j.chemosphere.2015.04.078 [DOI] [PubMed] [Google Scholar]
- 37. Cantwell MG, Katz DR, Sullivan JC, et al. : Selected pharmaceuticals entering an estuary: Concentrations, temporal trends, partitioning, and fluxes. Environ Toxicol Chem. 2016;35(11):2665–73. 10.1002/etc.3452 [DOI] [PubMed] [Google Scholar]
- 38. Martín J, Santos JL, Aparicio I, et al. : Pharmaceutically active compounds in sludge stabilization treatments: anaerobic and aerobic digestion, wastewater stabilization ponds and composting. Sci Total Environ. 2015;503-504:97–104. 10.1016/j.scitotenv.2014.05.089 [DOI] [PubMed] [Google Scholar]
- 39. Gasser G, Pankratov I, Elhanany S, et al. : Field and laboratory studies of the fate and enantiomeric enrichment of venlafaxine and O-desmethylvenlafaxine under aerobic and anaerobic conditions. Chemosphere. 2012;88(1):98–105. 10.1016/j.chemosphere.2012.02.074 [DOI] [PubMed] [Google Scholar]
- 40. Giger W, Brunner PH, Schaffner C: 4-Nonylphenol in sewage sludge: accumulation of toxic metabolites from nonionic surfactants. Science. 1984;225(4662):623–5. 10.1126/science.6740328 [DOI] [PubMed] [Google Scholar]
- 41. Lu J, Jin Q, He Y, et al. : Biodegradation of nonylphenol polyethoxylates by denitrifying activated sludge. Water Res. 2008;42(4–5):1075–82. 10.1016/j.watres.2007.09.031 [DOI] [PubMed] [Google Scholar]
- 42. Kolvenbach BA, Corvini PF: The degradation of alkylphenols by Sphingomonas sp. strain TTNP3 - a review on seven years of research N Biotechnol. 2012;30(1):88–95. 10.1016/j.nbt.2012.07.008 [DOI] [PubMed] [Google Scholar]
- 43. Porter AW, Hay AG: Identification of opdA, a gene involved in biodegradation of the endocrine disrupter octylphenol. Appl Environ Microbiol. 2007;73(22):7373–9. 10.1128/AEM.01478-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang Z, Yang Y, He T, et al. : Change of microbial community structure and functional gene abundance in nonylphenol-degrading sediment. Appl Microbiol Biotechnol. 2015;99(7):3259–68. 10.1007/s00253-014-6222-5 [DOI] [PubMed] [Google Scholar]
- 45. McCormick JM, Van Es T, Cooper KR, et al. : Microbially mediated O-methylation of bisphenol A results in metabolites with increased toxicity to the developing zebrafish (Danio rerio) embryo. Environ Sci Technol. 2011;45(15):6567–74. 10.1021/es200588w [DOI] [PubMed] [Google Scholar]
- 46. Chang BV, Lu ZJ, Yuan SY: Anaerobic degradation of nonylphenol in subtropical mangrove sediments. J Hazard Mater. 2009;165(1–3):162–7. 10.1016/j.jhazmat.2008.09.085 [DOI] [PubMed] [Google Scholar]
- 47. Chang BV, Chiang F, Yuan SY: Anaerobic degradation of nonylphenol in sludge. Chemosphere. 2005;59(10):1415–20. 10.1016/j.chemosphere.2004.12.055 [DOI] [PubMed] [Google Scholar]
- 48. de Weert J, Viñas M, Grotenhuis T, et al. : Aerobic nonylphenol degradation and nitro-nonylphenol formation by microbial cultures from sediments. Appl Microbiol Biotechnol. 2010;86(2):761–71. 10.1007/s00253-009-2394-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang Z, Yang Y, Dai Y, et al. : Anaerobic biodegradation of nonylphenol in river sediment under nitrate- or sulfate-reducing conditions and associated bacterial community. J Hazard Mater. 2015;286:306–14. 10.1016/j.jhazmat.2014.12.057 [DOI] [PubMed] [Google Scholar]
- 50. Moody JD, Heinze TM, Hansen EB, Jr, et al. : Metabolism of the ethanolamine-type antihistamine diphenhydramine (Benadryl) by the fungus Cunninghamella elegans. Appl Microbiol Biotechnol. 2000;53(3):310–5. 10.1007/s002530050026 [DOI] [PubMed] [Google Scholar]
- 51. Frazer AC: O-Demethylation and Other Transformations of Aromatic Compounds by Acetogenic Bacteria.In: Drake, H.L. (Ed.), Acetogenesis. Springer US,1994;445–483. 10.1007/978-1-4615-1777-1_17 [DOI] [Google Scholar]
- 52. Haggblom MM: Microbial breakdown of halogenated aromatic pesticides and related compounds. FEMS Microbiol Lett. 1992;9(1):29–71. 10.1111/j.1574-6968.1992.tb05823.x [DOI] [PubMed] [Google Scholar]
- 53. Fu Q, Liao C, Du X, et al. : Back Conversion from Product to Parent: Methyl Triclosan to Triclosan in Plants. Environ Sci Technol Lett. 2018;5:181–5. 10.1021/acs.estlett.8b00071 [DOI] [Google Scholar]; F1000 Recommendation
- 54. Hou X, Yu M, Liu A, et al. : Biotransformation of tetrabromobisphenol A dimethyl ether back to tetrabromobisphenol A in whole pumpkin plants. Environ Pollut. 2018;241:331–8. 10.1016/j.envpol.2018.05.075 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Gulde R, Meier U, Schymanski EL, et al. : Systematic Exploration of Biotransformation Reactions of Amine-Containing Micropollutants in Activated Sludge. Environ Sci Technol. 2016;50(6):2908–20. 10.1021/acs.est.5b05186 [DOI] [PubMed] [Google Scholar]
- 56. Gonzalez-Gil L, Carballa M, Lema JM: Cometabolic Enzymatic Transformation of Organic Micropollutants under Methanogenic Conditions. Environ Sci Technol. 2017;51(5):2963–71. 10.1021/acs.est.6b05549 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Cetecioglu Z, Ince B, Orhon D, et al. : Anaerobic sulfamethoxazole degradation is driven by homoacetogenesis coupled with hydrogenotrophic methanogenesis. Water Res. 2016;90:79–89. 10.1016/j.watres.2015.12.013 [DOI] [PubMed] [Google Scholar]
- 58. Kanakaraju D, Glass BD, Oelgemöller M: Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J Environ Manage. 2018;219:189–207. 10.1016/j.jenvman.2018.04.103 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Ma J, Dai R, Chen M, et al. : Applications of membrane bioreactors for water reclamation: Micropollutant removal, mechanisms and perspectives. Bioresour Technol. 2018;269:532–43. 10.1016/j.biortech.2018.08.121 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Prasse C, Stalter D, Schulte-Oehlmann U, et al. : Spoilt for choice: A critical review on the chemical and biological assessment of current wastewater treatment technologies. Water Res. 2015;87:237–70. 10.1016/j.watres.2015.09.023 [DOI] [PubMed] [Google Scholar]
- 61. Radjenović J, Petrović M, Barceló D: Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009;43(3):831–41. 10.1016/j.watres.2008.11.043 [DOI] [PubMed] [Google Scholar]
- 62. Stasinakis AS: Use of Selected Advanced Oxidation Processes (AOPs) for Wastewater Treatment – a Mini Review. Global NEST Journal. 2008;10:376–85. Reference Source [Google Scholar]
- 63. Angeles LF, Mullen RA, Huang IJ, et al. : Assessing pharmaceutical removal and reduction in toxicity provided by advanced wastewater treatment systems. Environ Sci Water Res Technol. 2020;6:62–77. 10.1039/C9EW00559E [DOI] [Google Scholar]; F1000 Recommendation
- 64. Kümmerer K, Dionysiou DD, Olsson O, et al. : Reducing aquatic micropollutants - Increasing the focus on input prevention and integrated emission management. Sci Total Environ. 2019;652:836–50. 10.1016/j.scitotenv.2018.10.219 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Rastogi T, Leder C, Kümmerer K: Re-Designing of Existing Pharmaceuticals for Environmental Biodegradability: A Tiered Approach with β-Blocker Propranolol as an Example. Environ Sci Technol. 2015;49(19):11756–63. 10.1021/acs.est.5b03051 [DOI] [PubMed] [Google Scholar]
- 66. Christou A, Agüera A, Bayona JM, et al. : The potential implications of reclaimed wastewater reuse for irrigation on the agricultural environment: The knowns and unknowns of the fate of antibiotics and antibiotic resistant bacteria and resistance genes - A review. Water Res. 2017;123:448–67. 10.1016/j.watres.2017.07.004 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Baker DR, Barron L, Kasprzyk-Hordern B: Illicit and pharmaceutical drug consumption estimated via wastewater analysis. Part A: Chemical analysis and drug use estimates. Sci Total Environ. 2014;487:629–41. 10.1016/j.scitotenv.2013.11.107 [DOI] [PubMed] [Google Scholar]
- 68. Choi PM, Tscharke B, Samanipour S, et al. : Social, demographic, and economic correlates of food and chemical consumption measured by wastewater-based epidemiology. Proc Natl Acad Sci U S A. 2019;116(43):21864–73. 10.1073/pnas.1910242116 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Daughton CG: Monitoring wastewater for assessing community health: Sewage Chemical-Information Mining (SCIM). Sci Total Environ. 2018;619–620:748–64. 10.1016/j.scitotenv.2017.11.102 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. O'Brien JW, Banks APW, Novic AJ, et al. : Impact of in-Sewer Degradation of Pharmaceutical and Personal Care Products (PPCPs) Population Markers on a Population Model. Environ Sci Technol. 2017;51(7):3816–23. 10.1021/acs.est.6b02755 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. OECD, . OECD Environmental Outlook to 2050: The Consequences of Inaction. OECD Publishing, Paris. 2012 doi: 10.1787/9789264122246-en. [DOI] [Google Scholar]


