Abstract
Recent studies suggest that murine invariant natural killer T (iNKT) cell development culminates in three terminally differentiated iNKT cell subsets denoted as NKT1, 2, and 17 cells. Although these studies corroborate the significance of the subset division model, less is known about the factors driving subset commitment in iNKT cell progenitors. In this review, we discuss the latest findings in iNKT cell development, focusing in particular on how T-cell receptor signal strength steers iNKT cell progenitors toward specific subsets and how early progenitor cells can be identified. In addition, we will discuss the essential factors for their sustenance and functionality. A picture is emerging wherein the majority of thymic iNKT cells are mature effector cells retained in the organ rather than developing precursors.
Keywords: invariant natural killer T cells, subsets, development, T cell receptor signalling, thymus, CD1d, lipid, thymus, agonist selection
Introduction
Identified by their T-cell receptor (TCR) specificity for lipids, invariant natural killer T (iNKT) cells are innate-like αβ T cells capable of releasing cytokines almost instantly upon stimulation without the need for prior activation 1, 2. Like conventional αβ T cells, iNKT cells arise from common lymphoid progenitors and run through their developmental program in the thymus. At the double-positive (DP) stage, their developmental programs bifurcate: While conventional αβ T cells get positively and negatively selected by thymic epithelial cells presenting peptide antigens by classical class I and II major histocompatibility complex (MHC) molecules 3, 4, iNKT cell progenitors are selected by other DP thymocytes presenting lipid antigens by CD1d, a non-classical MHC-like molecule 5– 8. Strong TCR signaling is required at this stage (referred to as agonist selection) 9 for upregulation of Egr2 10, 11 and PLZF 12, 13, the latter of which is a master regulator of iNKT cell development. This consequently commits the DP αβ T-cell progenitor with the “right” TCR rearrangement to the iNKT cell pathway 14, 15. In addition to the strong TCR stimulation, auxiliary co-stimulatory signals are required by engaging CD80/CD86 16 and via homotypic interactions between signaling lymphocyte activation molecule family (SLAMF) receptors, Slamf1 and Slamf6 17. Following selection, iNKT cells complete their developmental program in the thymus and can egress to peripheral tissues. However, a substantial number are retained in the thymus, ending up as terminally differentiated functional subsets in this organ.
Despite the latest insights in the field of iNKT cell biology, the development of iNKT cell subsets and their differentiation pathways remain puzzling 14, 15, 18– 21. In this review, we will consider the contemporary understanding of iNKT cell subset development and in parallel we will discuss factors required for their maintenance and proper function. Moreover, we will focus on TCR signal strength involvement in iNKT cell lineage commitment and stability.
The developmental map of iNKT cells
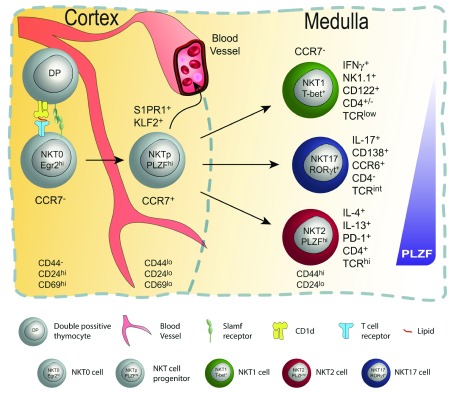
The initial studies investigating iNKT cell development postulated that all iNKT cells execute the same developmental program divided into four stages (S0–S3). According to this model, iNKT cells progress from the most immature stage S0 (CD24 +CD44 −NK1.1 −) to their final mature stage S3 (CD24 −CD44 +NK1.1 +) by losing CD24 expression and subsequently upregulating first CD44 (in stage S2) and lastly natural killer NK1.1 (in stage S3) 22, 23. Although this holds true for some iNKT cells, the latest data demonstrate that this model does not apply to all iNKT cells. For instance, this model fails to incorporate interleukin-17 (IL-17)-producing iNKT cells 24– 26, it does not account for iNKT cells that produce high levels of IL-4 but never express NK1.1, and it cannot be employed with mouse strains that do not express NK1.1 27. Therefore, a new functional classification of iNKT cells into three terminally differentiated subsets, which is based on the expression pattern of characteristic cytokines and transcription factors, was proposed 28, 29. In this model, all iNKT cells arise from a common progenitor designated as NKT0 cells (Egr2 hiCD24 +) and further differentiate into NKT1, NKT2, or NKT17 cell subsets. NKT1 cells (PLZF loTbet +) produce interferon gamma (IFNγ) and low levels of IL-4 upon stimulation. In addition, they are the only subset expressing NK cell signature proteins like NK1.1, NKG2D, Nkp46, and a cytotoxic gene expression program 30– 32. NKT2 cells express the highest levels of PLZF and IL-4. Lastly, NKT17 (PLZF intRORγt +) cells produce IL-17. Of note, only NKT2 cells are shown to actively produce and secrete IL-4 under steady-state conditions, an essential process for CD8 innate-like T-cell generation in the thymus and periphery 28, 33– 38. In this model, NKT1 cells, IL-4–producing NKT2 cells, and NKT17 cells are considered terminally differentiated cells which generally do not give rise to any of the other subsets 24, 25, 28. Subsequently, three independent groups performed transcriptome analysis of thymic-derived iNKT cell subsets and congruently observed distinct gene expression patterns for each subset 30– 32. Only NKT1 cells pass through all the stages of development S0–S3 defined by the original model. In contrast, NKT2 and NKT17 cells finish their maturation as terminally differentiated effector cells at stage 2. Taken together, these data widely validate the foundations of the NKT1/2/17 concept ( Figure 1).
Figure 1. Invariant natural killer T (iNKT) cell development in the thymus.
In the cortex of the thymus (left), double-positive (DP) iNKT progenitors are positively selected by other DP thymocytes presenting lipids via CD1d. This results in survival and lineage commitment; only those rare DP thymocytes bearing T-cell receptors (TCRs) (invariant Vβ14 chain paired with a limited set of beta chains) with the right specificity are selected and committed to the iNKT cell lineage. This step requires strong TCR signaling in combination with co-stimulatory signals via homotypic interactions between SLAM (signaling lymphocyte activation molecule) family members. Signaling leads to upregulation of Egr1 and Egr2, which are needed for PLZF induction and stable expression. Immediate post-selection iNKT cells are Egr2 hiCD24 hiCD44 −CD69 hi and are designated as NKT0 cells. Subsequently, NKT0 cells downregulate CD24 and transition into CCR7 + multi-potent NKT cell progenitors (NKTp). At that stage, NKTp cells can egress from the thymus or continue their differentiation into one of the effector subsets (iNKT1, 2, or 17). Mature NKT cells are CCR7 − and reside in the medulla region as terminally differentiated tissue-resident NKT cell subsets.
Two additional subsets—NKT10 and NKT follicular helper (NKT FH) cells—have recently been proposed as an extension to the iNKT cell subset family; IL-10–producing E4BP4 + NKT10 cells were resident in the adipose tissue 39– 41, and Bcl-6 +IL-21 + NKT FH cells were found in germinal centers 42, 43. Notably, these two functional subsets have been described only in the periphery and are not present in the thymus.
The complexity of iNKT cell subsets
Although current data indicate that NKT1, 2, and 17 cells are terminally differentiated functional iNKT cell subsets, the latest data bring a further level of complexity and reflect advances in the field. For instance, NKT1 cells segregate into CD4 + and CD4 − fractions. CD4 − NKT1 cells were shown to display a more NK-like phenotype with preferential expression of NK cell signature receptors and soluble cytotoxic mediators (for example, granzyme a, b, and perforin) 30. In contrast, CD4 + NKT1 cells express higher levels of NK cell–unrelated genes like IL-4 and CD81 30, 44. In light of these findings, new questions arise about iNKT cell subset development and function. For example, are both NKT1 fractions distinct terminally differentiated cell subsets with divergent functions or do they represent intermediate versus fully matured stages of the NKT1 cell subset? Along the same line, a recent study described an alternative iNKT cell developmental pathway where a small CD4 − NKT1 population can arise from double-negative (DN) stage thymocytes 45. However, this pathway seems to contribute in only a minor way to the mature CD4 − NKT1 cell pool.
Even though less is known about NKT17 cell development 46, a recent study provided valuable insight into NKT17 biology 47. By evaluating the expression pattern of the NKT17 characteristic genes CD138 48 and CCR6 26, the investigators suggested the final steps of the NKT17 developmental pathway from RORγt + NKT17 committed progenitors which progressively gain CD138 followed by CCR6 to become CD138 +CCR6 + DP mature NKT17 cells 47.
Expectedly, the PLZF hi iNKT cells display the highest level of heterogeneity 28, 30 since this cell fraction encompasses both mature NKT2 and immature NKT progenitor cells 28, 49 ( Figure 1). Yet, in a 2016 study, Engel et al. performed a single-cell RNA-sequencing analysis on each of the iNKT cell subsets from thymic origin 31. In addition to including NKT1, 2, and 17 cells, this study included the most immature NKT0 cells which are still CD24 hi and have recently undergone selection. Despite that, authors still detected some NKT2 cells with transcripts of characteristic genes known to be highly expressed in recently selected cells (for example, Itm2a, Ccr9, and Ldhb) 31, 50, 51. In line with that, principal component analysis of NKT0, 1, 2, and 17 cells showed that each of these fractions segregated as different subsets with only marginal overlap between NKT0 and NKT2 cells 31, 32. Taken together, these data argue for a missing link in the development of the terminally differentiated iNKT cell subsets from the CD24 hi NKT0 stage. As mentioned above, there are several reported genes with a shared expression pattern between NKT0 and some CD24 −PLZF hi cells. Hence, they might be suitable as markers for iNKT cell subset progenitors “hiding” within the PLZF hi iNKT cell fraction. Such a candidate is CCR7, which was shown to be expressed on both cell fractions at RNA 30, 31 and protein 30, 52 levels. Indeed, a recent study by Wang et al. described CCR7 as a characteristic marker for multi-potent iNKT cell progenitors (NKTp) 52 ( Figure 1). In that study, the authors took advantage of the KN2 mouse model in which IL-4–secreting cells can be identified by human CD2 (hCD2) expression 53. Those experiments demonstrated that the small fraction of CCR7 + iNKT cells did not produce IL-4 yet could give rise to all iNKT effector subsets in the thymus and periphery.
CCR7 + cells (amongst NKT2) represent an undifferentiated precursor that emigrates from the thymus
Although previous studies had shown first that NK1.1 − iNKT 22, 23 cells and later that “NKT2-like” cells 30 can emigrate into the periphery, these reports did not investigate CCR7 expression on recent thymic emigrants (RTEs). Interestingly, Wang et al. also found that CCR7 + iNKT cells, despite representing only about 5% of total thymic iNKT cells, were prominent amongst RTEs, a process stringently dependent on Klf2 and S1PR1 52 ( Figure 1). This suggests that undifferentiated NKTp cells exit the thymus and can initiate or complete (or both) differentiation in peripheral tissues. Indeed, 5 days after adoptive transfer, CCR7 + NKTp cells differentiated into all three effector subsets whether they had been transferred directly into the thymus or intravenously into the periphery. Moreover, parabiosis experiments showed that more than 99% of all thymic iNKT cells were tissue-resident as opposed to arriving from circulation 52. Thus, only a small fraction of “developing” NKTp cells in the thymus are constantly replenishing the pool of iNKT cells in the thymus and periphery.
Overall, these data raise the logical questions: What are the precise signals specifying iNKT cell differentiation, and what are the checkpoints for subset commitment? Moreover, does commitment occur at the DP stage in the cortex or at a later developmental stage post-selection as the undifferentiated CCR7 + cell encounters a new cellular milieu in the thymic medulla? In fact, recent evidence suggests a combination of the two. These new findings are discussed in the following section.
TCR signaling: strength and context
It has long been known that TCR signaling is critical for iNKT cell development, as recognition of CD1d:lipid ligands is required for positive selection 5– 8. However, recent data suggest a critical role for TCR signaling in subset differentiation as well. Of interest in this regard is the observation that mature iNKT cell subsets exhibit different levels of TCR on their surface 30, 54 where NKT1 cells are low, NKT17 cells are intermediate, and NKT2 cells display the highest level of TCR expression. This also appears to correlate with their ongoing TCR signal strength under steady-state conditions 54, 55. Overall, this suggests a potential requirement for different TCR signal intensities in the development of each ( Figure 2). In fact, two independent groups recently addressed this question by exploiting the SKG mouse model, in which TCR signaling is weakened because of a hypo-morphic ZAP70 allele 54, 56. Hence, both studies showed that weakened TCR signaling led to abrogation in NKT2 and, to a lesser extent, NKT17 cell development while not reducing NKT1 cell development. In addition, genome-wide analysis of chromatin accessibility between NKT2 cells from SKG and wild-type control mice showed that gene regions coding MAPK/ERK and Notch pathway regulators were less accessible in the SKG mouse. Moreover, a recent study showed that a deficiency in TRAF3-interacting protein which facilitates MEK/ERK signaling at the trans-Golgi network led to a decrease in IL-4–producing NKT2 cells 57. This places TCR strength signaling as a possible modulator of MAPK/ERK and Notch signaling, which might influence iNKT cell subset development. Additionally, the Src homology2 domain-containing phosphatase 1 (Shp1)-deficient mouse showed an increase of NKT2 and NKT17 cells 58. Although the authors did not find direct evidence that this is due to altered TCR signaling, Shp1 was previously identified as a negative regulator of TCR signaling by targeting ZAP-70 59.
Figure 2. Model of T-cell receptor (TCR) signaling in the cortex for invariant natural killer T (iNKT) differentiation.
In this model, TCR binding strength (avidity) during the cortical selection process steers stage 0 iNKT cells to specific cell fates. For iNKT cells, which express a semi-invariant TCR alpha chain, TCR beta chain usage can influence TCR binding avidity. For example, Vβ2 and Vβ7 chains tend to confer higher binding avidity. TCRs with high avidity drive NKT2 differentiation preferentially, TCRs with intermediate binding avidity drive NKT17 differentiation, and those with low binding avidity drive commitment to the NKT1 pathway. NKT progenitor (NKTp) cells finish their differentiation in the medulla.
One critical gene target of TCR signaling in iNKT cells is PLZF 11. Indeed, PLZF expression in iNKT subsets mirrors TCR levels and signaling; NKT2 expresses the highest level, NKT17 expresses intermediate, and NKT1 expresses the lowest level 28 ( Figure 1). An interesting recent study showed that, like that of TCR, the quantity of PLZF expressed by a given cell is a critical factor in iNKT subset differentiation. In that study, a hypomorphic allele of PLZF was found to strongly reduce NKT2 and NKT17 numbers while relatively sparing NKT1 cells 60. Collectively, these findings corroborate the idea that the quantity of TCR signaling is a critical factor in iNKT cell subset differentiation.
Since it is known that iNKT cell selection happens in the cortex 61, it seems likely that the interaction of stage 0 iNKT cells with CD1d/lipid-presenting DP thymocytes is the stage at which signal strength is critical ( Figure 2). In this regard, it is curious that cells immediately following the CCR7 + stage do not yet show signs of differentiation to distinct subsets and express a uniformly high level of TCR 52. Furthermore, the CCR7 + iNKT cell population has a transcriptome that does not yet resemble any of the differentiated subsets 32. Thus, it remains an open question whether CCR7 + cells are already “committed” to a specific cell subset or are multi-potent at the single-cell level. Future studies will need to address this by, for example, using single-cell adoptive transfer assays of cell fate or single-cell epigenomics analysis of CCR7 + cells ( Figure 2).
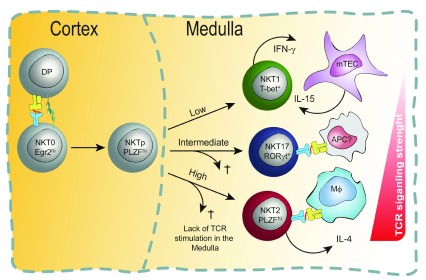
Another (non-mutually exclusive) possibility is that TCR signaling is critical during and following the CCR7 + stage ( Figure 3). CCR7 is a chemokine receptor that facilitates the movement of iNKT cells from the cortex to the medulla and is crucial for proper maturation and maintenance of resident iNKT cells in the thymus 52. It is possible that NKT cells require continued interaction with CD1d-expressing antigen-presenting cells in the medulla, particularly to maintain expression of survival factors, including bcl2 family members 60 ( Figure 3). Of note, medulla-derived factors have already been shown to play an essential role in iNKT cell subset homeostasis. For instance, it has been shown that IL-15 is required for terminal maturation and survival of NKT1 cells 62 and that IL-25 is implicated in NKT2 cell development and effector functions 24, 63. A new study by Wang et al. sought to discern whether steady-state IL-4 production by mature NKT2 cells is TCR–CD1d interaction-dependent 55. Indeed, this work showed that intrathymic transfer of NKT2 cells into CD1d-deficient recipient mice resulted in a loss of IL-4 production within 9 days after transfer 55. Moreover, using inducible knockout (KO) models, the authors identified the CD1d-presenting cell subset as macrophages. At this point, it is unknown whether the medullary macrophages that activate NKT2 cells present the same or distinct self-lipid ligands compared with the cortical DP thymocytes that initially select NKT cells ( Figure 2 and Figure 3).
Figure 3. Model of T-cell receptor (TCR) signaling in the medulla for invariant natural killer T (iNKT) subset activation and survival.
According to this non-mutually exclusive model, subset commitment decisions are made or reinforced (or both) in the thymic medulla. At the CCR7 + stage, developing natural killer cell progenitors (NKTp) may still be uncommitted to any particular subset (as shown). Alternatively, signaling at the double-positive (DP) stage may have altered their epigenomes such that they have a propensity to differentiate into one of the three major subsets (as depicted in Figure 2). Either way, following migration into the medulla, NKTp cells experience different strengths of TCR signaling—based on their TCR:CD1d/lipid binding avidity with distinct antigen-presenting cells (APCs) they stochastically encounter in the medulla—which governs their subset commitment or survival or both. Cells with the highest binding avidity survive as NKT2 cells, those with intermediate affinity survive as NKT17 cells, and only NKT1 cells survive without continued TCR stimulation. In the medulla, NKT2 cells require TCR–CD1d interaction with macrophages in order to produce interleukin-4 (IL-4) in the steady state, and NKT1 cells need IL-15 produced by medullary thymic epithelial cells (mTECs) for their proper differentiation and survival. It is unknown what cell types present lipids to NKT17 cells, although it is reported that they encounter intermediate TCR signaling in the steady state, which is crucial for their differentiation.
Although NKT cells are often assumed to be mono-specific given the semi-invariant nature of their TCR alpha chain, evidence suggests that the particular TCR beta chain used can influence recognition 64– 67. In this context, it is interesting that the different NKT subsets have reproducible differences in TCR beta usage 54. Furthermore, retrogenic experiments showed that TCR:CD1d/lipid binding avidity positively correlated with selection efficiency. These avidity differences were conferred by different TCR beta chains. In addition, a longer half-life of binding favored the development of PLZF hi NKT2 cells over the other subsets 68. Further deep sequencing of the TCR alpha and beta repertoires of iNKT subsets could provide insight in the future.
As previously mentioned, SLAM family receptors (SFRs) play a crucial role in iNKT cell development 17. This family includes six members that can convey both activating or inhibitory signals depending on the adaptor proteins that are recruited upon receptor engagement 69. Lu et al. recently addressed the role of SLAM receptor proteins (SRPs) in iNKT cell development by generating a KO mouse strain for all six members of this family 70. They showed that loss of SFRs led to higher TCR signaling in developing iNKT cells, as judged by higher Nur77 and Egr2 levels, and to reduced numbers of all mature iNKT cells. In addition, they reported that the number of CD24 + immature iNKT cells was not altered. Considering these findings, the authors concluded that the initial positive selection of iNKT cells was unaffected in SFR-deficient mice but that the observed iNKT cell loss was due to augmented TCR signal strength driving apoptosis. The authors also conclude that inhibitory signals provided by SFRs attenuate TCR signal strength after positive selection to promote NKT cell development, as opposed to previous studies proposing that SFRs contributes with a positive signal that complements TCR signaling to support NKT cell development 71. Interestingly, however, Lu et al. provided evidence that Vβ usage was altered, whereby Vβ7 clones were preferentially enriched in SFR-deficient mice 70. Of note, it was reported that Vβ7 usage confers higher avidity binding to CD1d and is expressed mostly by NKT2 cells 28, 54, 66. As mentioned above, NKT2 cells are thought to experience the strongest TCR signaling in the steady state 54, 55. Hence, this raises the possibility that mainly clones with high-avidity Vβ chain rearrangements, which result in stronger TCR signaling, are able to survive in the absence of SFR co-stimulation. Strikingly, a previous study showed that in conditions of limited endogenous ligand concentrations, thymic selection favors Vβ7 + iNKT cells over iNKT cells expressing other Vβ chains 66. Thus, although Lu et al. show that iNKT cells from SFR-deficient mice have higher ongoing TCR signal strength in comparison with wild-type mice, this effect might be due to selective survival of high-avidity Vβ7-expressing NKT2 cells and not to lack of SFR-mediated inhibition. Therefore, further studies are needed to understand the precise role of SFR signaling in NKT cell development or subset differentiation or both.
Concluding remarks
A couple of technical points regarding the development of iNKT cells can be gleaned from the studies we discussed here. First is that the paradigm of “staging” NKT cell development using CD44 and NK1.1 is relevant only to NKT1 lineage cells in B6 (C57BL/6) mice and thus should be employed with caution. Second, a high level of PLZF expression is not sufficient to distinguish functionally differentiated NKT2 cells from multi-potent NKTp cells. Therefore, CCR7, PD1, or other markers are needed to discriminate between developing and differentiating iNKT subsets. In addition, NKT2 cells were shown to actively produce and secrete high levels of IL-4 whereas multi-potent NKTp cells did not. Furthermore, most studies investigating cytokine production in iNKT cells use PMA/ionomycin as a stimulus, which might be misleading in this regard. Hence, reporter mouse models currently represent the best way to identify cells actively producing IL-4. In the future, it will be interesting and important to determine when NKT1 or NKT17 or both actively produce cytokine in uninfected animals.
Despite the recent progress in the field of iNKT cell biology, many questions remain unanswered. For instance, assuming surface TCR levels dictate TCR signaling quantity, what drives the high TCR expression on NKT2 and 17 cells? Is it a signal received during selection owing to specific Vβ chain usage or rather an environmental consequence such as high presence of endogenous cognate ligand? Moreover, what are the relevant self-lipid ligands in the thymus and do they differ between cortical and medullary antigen-presenting cells? How diverse are they and is this diversity detected? In this context, a recent study showed that non-agonist CD1d-associated lipids could alter lipid presentation and impact iNKT development in a complex way 72. Thus, many interesting questions remain open about the complex biology induced by the T-cell recognition of lipids.
Abbreviations
DP, double-positive; iNKT, invariant natural killer T; MHC, major histocompatibility complex; NK, natural killer; NKT FH, invariant natural killer T follicular helper; NKTp, multi-potent invariant natural killer T-cell progenitor; RTE, recent thymic emigrant; SFR, SLAM family receptor; Shp1, Src homology2 domain-containing phosphatase 1
Acknowledgments
We thank all present and past members of the Kristin Hogquist and Stephen Jameson labs and members of the Center for Immunology at the University of Minnesota for the inspiring discussions and assistance.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Kamel Benlagha, INSERM, UMR-1160, Institut de Recherche St-Louis (IRSL), Paris, France; Université Paris Diderot, Sorbonne Paris Cité, Paris, France
Laurent Gapin, Department of Immunology and Microbiology, University of Colorado School of Medicine, Aurora, Colorado, USA
Kayuza Iwabuchi, Department of Immunology, Kitasato University School of Medicine, Sagamihara, Japan
Funding Statement
This work was supported by Deutsche Forschungsgemeinschaft (DFG) fellowship GE 3062/1-1 (to HG) and by National Institutes of Health grant R01 AI140547 (to KAH).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 3 approved]
References
- 1. Stetson DB, Mohrs M, Reinhardt RL, et al. : Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198(7):1069–76. 10.1084/jem.20030630 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Crowe NY, Uldrich AP, Kyparissoudis K, et al. : Glycolipid antigen drives rapid expansion and sustained cytokine production by NK T cells. J Immunol. 2003;171(18):4020–7. 10.4049/jimmunol.171.8.4020 [DOI] [PubMed] [Google Scholar]
- 3. Starr TK, Jameson SC, Hogquist KA: Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–76. 10.1146/annurev.immunol.21.120601.141107 [DOI] [PubMed] [Google Scholar]
- 4. Palmer E: Negative selection--clearing out the bad apples from the T-cell repertoire. Nat Rev Immunol. 2003;3(5):383–91. 10.1038/nri1085 [DOI] [PubMed] [Google Scholar]
- 5. Bendelac A, Savage PB, Teyton L: The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. 10.1146/annurev.immunol.25.022106.141711 [DOI] [PubMed] [Google Scholar]
- 6. Godfrey DI, Stankovic S, Baxter AG: Raising the NKT cell family. Nat Immunol. 2010;11(3):197–206. 10.1038/ni.1841 [DOI] [PubMed] [Google Scholar]
- 7. Kronenberg M, Gapin L: The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2(8):557–68. 10.1038/nri854 [DOI] [PubMed] [Google Scholar]
- 8. Bendelac A: Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995;182(6):2091–6. 10.1084/jem.182.6.2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stritesky GL, Jameson SC, Hogquist KA: Selection of self-reactive T cells in the thymus. Annu Rev Immunol. 2012;30:95–114. 10.1146/annurev-immunol-020711-075035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lazarevic V, Zullo AJ, Schweitzer MN, et al. : The gene encoding early growth response 2, a target of the transcription factor NFAT, is required for the development and maturation of natural killer T cells. Nat Immunol. 2009;10(3):306–13. 10.1038/ni.1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seiler MP, Mathew R, Liszewski MK, et al. : Elevated and sustained expression of the transcription factors Egr1 and Egr2 controls NKT lineage differentiation in response to TCR signaling. Nat Immunol. 2012;13(3):264–71. 10.1038/ni.2230 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Kovalovsky D, Uche OU, Eladad S, et al. : The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9(9):1055–64. 10.1038/ni.1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Savage AK, Constantinides MG, Han J, et al. : The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29(3):391–403. 10.1016/j.immuni.2008.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Verykokakis M, Kee BL: Transcriptional and epigenetic regulation of innate-like T lymphocyte development. Curr Opin Immunol. 2018;51:39–45. 10.1016/j.coi.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Gapin L: Development of invariant natural killer T cells. Curr Opin Immunol. 2016;39:68–74. 10.1016/j.coi.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Chung Y, Nurieva R, Esashi E, et al. : A critical role of costimulation during intrathymic development of invariant NK T cells. J Immunol. 2008;180(4):2276–83. 10.4049/jimmunol.180.4.2276 [DOI] [PubMed] [Google Scholar]
- 17. Griewank K, Borowski C, Rietdijk S, et al. : Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27(5):751–62. 10.1016/j.immuni.2007.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Krovi SH, Gapin L: Invariant Natural Killer T Cell Subsets-More Than Just Developmental Intermediates. Front Immunol. 2018;9:1393. 10.3389/fimmu.2018.01393 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Wang H, Hogquist KA: How Lipid-Specific T Cells Become Effectors: The Differentiation of iNKT Subsets. Front Immunol. 2018;9:1450. 10.3389/fimmu.2018.01450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dashtsoodol N, Bortoluzzi S, Schmidt-Supprian M: T Cell Receptor Expression Timing and Signal Strength in the Functional Differentiation of Invariant Natural Killer T Cells. Front Immunol. 2019;10:841. 10.3389/fimmu.2019.00841 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Bennstein SB: Unraveling Natural Killer T-Cells Development. Front Immunol. 2017;8:1950. 10.3389/fimmu.2017.01950 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Benlagha K, Kyin T, Beavis A, et al. : A thymic precursor to the NK T cell lineage. Science. 2002;296(5567):553–5. 10.1126/science.1069017 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Pellicci DG, Hammond KJ, Uldrich AP, et al. : A natural killer T (NKT) cell developmental pathway iInvolving a thymus-dependent NK1.1 -CD4 + CD1d-dependent precursor stage. J Exp Med. 2002;195(7):835–44. 10.1084/jem.20011544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Watarai H, Sekine-Kondo E, Shigeura T, et al. : Development and function of invariant natural killer T cells producing T H2- and T H17-cytokines. PLoS Biol. 2012;10(2):e1001255. 10.1371/journal.pbio.1001255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Michel ML, Mendes-da-Cruz D, Keller AC, et al. : Critical role of ROR-γt in a new thymic pathway leading to IL-17-producing invariant NKT cell differentiation. Proc Natl Acad Sci U S A. 2008;105(50):19845–50. 10.1073/pnas.0806472105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Doisne JM, Becourt C, Amniai L, et al. : Skin and peripheral lymph node invariant NKT cells are mainly retinoic acid receptor-related orphan receptor (gamma)t + and respond preferentially under inflammatory conditions. J Immunol. 2009;183(3):2142–9. 10.4049/jimmunol.0901059 [DOI] [PubMed] [Google Scholar]
- 27. Carlyle JR, Mesci A, Ljutic B, et al. : Molecular and genetic basis for strain-dependent NK1.1 alloreactivity of mouse NK cells. J Immunol. 2006;176(12):7511–24. 10.4049/jimmunol.176.12.7511 [DOI] [PubMed] [Google Scholar]
- 28. Lee YJ, Holzapfel KL, Zhu J, et al. : Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14(11):1146–54. 10.1038/ni.2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Constantinides MG, Bendelac A: Transcriptional regulation of the NKT cell lineage. Curr Opin Immunol. 2013;25(2):161–7. 10.1016/j.coi.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Georgiev H, Ravens I, Benarafa C, et al. : Distinct gene expression patterns correlate with developmental and functional traits of iNKT subsets. Nat Commun. 2016;7:13116. 10.1038/ncomms13116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Engel I, Seumois G, Chavez L, et al. : Innate-like functions of natural killer T cell subsets result from highly divergent gene programs. Nat Immunol. 2016;17(6):728–39. 10.1038/ni.3437 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Lee YJ, Starrett GJ, Lee ST, et al. : Lineage-Specific Effector Signatures of Invariant NKT Cells Are Shared amongst γδ T, Innate Lymphoid, and Th Cells. J Immunol. 2016;197(4):1460–70. 10.4049/jimmunol.1600643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weinreich MA, Odumade OA, Jameson SC, et al. : T cells expressing the transcription factor PLZF regulate the development of memory-like CD8 + T cells. Nat Immunol. 2010;11(8):709–16. 10.1038/ni.1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kurzweil V, LaRoche A, Oliver PM: Increased peripheral IL-4 leads to an expanded virtual memory CD8 + population. J Immunol. 2014;192(12):5643–51. 10.4049/jimmunol.1301755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee YJ, Jameson SC, Hogquist KA: Alternative memory in the CD8 T cell lineage. Trends Immunol. 2011;32(2):50–6. 10.1016/j.it.2010.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carty SA, Koretzky GA, Jordan MS, et al. : Interleukin-4 regulates eomesodermin in CD8 + T cell development and differentiation. PLoS One. 2014;9(9):e106659. 10.1371/journal.pone.0106659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jameson SC, Lee YJ, Hogquist KA: Innate memory T cells. Adv Immunol. 2015;126:173–213. 10.1016/bs.ai.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Georgiev H, Ravens I, Shibuya A, et al. : CD155/CD226-interaction impacts on the generation of innate CD8 + thymocytes by regulating iNKT-cell differentiation. Eur J Immunol. 2016;46(4):993–1003. 10.1002/eji.201546073 [DOI] [PubMed] [Google Scholar]
- 39. Lynch L, Michelet X, Zhang S, et al. : Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of T reg cells and macrophages in adipose tissue. Nat Immunol. 2015;16(1):85–95. 10.1038/ni.3047 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Lynch L, Nowak M, Varghese B, et al. : Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37(3):574–87. 10.1016/j.immuni.2012.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sag D, Krause P, Hedrick CC, et al. : IL-10-producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J Clin Invest. 2014;124(9):3725–40. 10.1172/JCI72308 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Chang PP, Barral P, Fitch J, et al. : Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat Immunol. 2011;13(1):35–43. 10.1038/ni.2166 [DOI] [PubMed] [Google Scholar]
- 43. King IL, Fortier A, Tighe M, et al. : Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat Immunol. 2011;13(1):44–50. 10.1038/ni.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Crowe NY, Coquet JM, Berzins SP, et al. : Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202(9):1279–88. 10.1084/jem.20050953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dashtsoodol N, Shigeura T, Aihara M, et al. : Alternative pathway for the development of V α14 + NKT cells directly from CD4 -CD8 - thymocytes that bypasses the CD4 +CD8 + stage. Nat Immunol. 2017;18(3):274–82. 10.1038/ni.3668 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Tsagaratou A: Unveiling the regulation of NKT17 cell differentiation and function. Mol Immunol. 2019;105:55–61. 10.1016/j.molimm.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 47. Klibi J, Li S, Amable L, et al. : Characterization of the developmental landscape of murine RORγt + iNKT cells. Int Immunol. 2020;32(2):105–116. 10.1093/intimm/dxz064 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Dai H, Rahman A, Saxena A, et al. : Syndecan-1 identifies and controls the frequency of IL-17-producing naïve natural killer T (NKT17) cells in mice. Eur J Immunol. 2015;45(11):3045–51. 10.1002/eji.201545532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kwon DI, Lee YJ: Lineage Differentiation Program of Invariant Natural Killer T Cells. Immune Netw. 2017;17(6):365–77. 10.4110/in.2017.17.6.365 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Kirchner J, Bevan MJ: ITM2A is induced during thymocyte selection and T cell activation and causes downregulation of CD8 when overexpressed in CD4 +CD8 + double positive thymocytes. J Exp Med. 1999;190(2):217–28. 10.1084/jem.190.2.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Uehara S, Hayes SM, Li L, et al. : Premature expression of chemokine receptor CCR9 impairs T cell development. J Immunol. 2006;176(1):75–84. 10.4049/jimmunol.176.1.75 [DOI] [PubMed] [Google Scholar]
- 52. Wang H, Hogquist KA: CCR7 defines a precursor for murine iNKT cells in thymus and periphery. eLife. 2018;7: pii: e34793. 10.7554/eLife.34793 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Mohrs K, Wakil AE, Killeen N, et al. : A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity. 2005;23(4):419–29. 10.1016/j.immuni.2005.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Tuttle KD, Krovi SH, Zhang J, et al. : TCR signal strength controls thymic differentiation of iNKT cell subsets. Nat Commun. 2018;9(1): 2650. 10.1038/s41467-018-05026-6 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Wang H, Breed ER, Lee YJ, et al. : Myeloid cells activate iNKT cells to produce IL-4 in the thymic medulla. Proc Natl Acad Sci U S A. 2019;116(44):22262–8. 10.1073/pnas.1910412116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhao M, Svensson MND, Venken K, et al. : Altered thymic differentiation and modulation of arthritis by invariant NKT cells expressing mutant ZAP70. Nat Commun. 2018;9(1): 2627. 10.1038/s41467-018-05095-7 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Zhang X, Wang K, Zhao W, et al. : TRAF3IP3 at the trans-Golgi network regulates NKT2 maturation via the MEK/ERK signaling pathway. Cell Mol Immunol. 2019;16:1114. 10.1038/s41423-019-0234-0 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Cruz Tleugabulova M, Zhao M, Lau I, et al. : The Protein Phosphatase Shp1 Regulates Invariant NKT Cell Effector Differentiation Independently of TCR and Slam Signaling. J Immunol. 2019;202(8):2276–86. 10.4049/jimmunol.1800844 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Plas DR, Johnson R, Pingel JT, et al. : Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling. Science. 1996;272(5265):1173–6. 10.1126/science.272.5265.1173 [DOI] [PubMed] [Google Scholar]
- 60. Park JY, DiPalma DT, Kwon J, et al. : Quantitative Difference in PLZF Protein Expression Determines iNKT Lineage Fate and Controls Innate CD8 T Cell Generation. Cell Rep. 2019;27(9):2548–2557.e4. 10.1016/j.celrep.2019.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Benlagha K, Wei DG, Veiga J, et al. : Characterization of the early stages of thymic NKT cell development. J Exp Med. 2005;202(4):485–92. 10.1084/jem.20050456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gordy LE, Bezbradica JS, Flyak AI, et al. : IL-15 regulates homeostasis and terminal maturation of NKT cells. J Immunol. 2011;187(12):6335–45. 10.4049/jimmunol.1003965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Miller CN, Proekt I, von Moltke J, et al. : Thymic tuft cells promote an IL-4-enriched medulla and shape thymocyte development. Nature. 2018;559(7715):627–31. 10.1038/s41586-018-0345-2 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Cameron G, Pellicci DG, Uldrich AP, et al. : Antigen Specificity of Type I NKT Cells Is Governed by TCR β-Chain Diversity. J Immunol. 2015;195(10):4604–14. 10.4049/jimmunol.1501222 [DOI] [PubMed] [Google Scholar]
- 65. Clancy-Thompson E, Chen GZ, Tyler PM, et al. : Monoclonal Invariant NKT (iNKT) Cell Mice Reveal a Role for Both Tissue of Origin and the TCR in Development of iNKT Functional Subsets. J Immunol. 2017;199(1):159–71. 10.4049/jimmunol.1700214 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 66. Schümann J, Mycko MP, Dellabona P, et al. : Cutting edge: influence of the TCR Vbeta domain on the selection of semi-invariant NKT cells by endogenous ligands. J Immunol. 2006;176(4):2064–8. 10.4049/jimmunol.176.4.2064 [DOI] [PubMed] [Google Scholar]
- 67. Mallevaey T, Clarke AJ, Scott-Browne JP, et al. : A molecular basis for NKT cell recognition of CD1d-self-antigen. Immunity. 2011;34(3):315–26. 10.1016/j.immuni.2011.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cruz Tleugabulova M, Escalante NK, Deng S, et al. : Discrete TCR Binding Kinetics Control Invariant NKT Cell Selection and Central Priming. J Immunol. 2016;197(10):3959–69. 10.4049/jimmunol.1601382 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Veillette A: NK cell regulation by SLAM family receptors and SAP-related adapters. Immunol Rev. 2006;214:22–34. 10.1111/j.1600-065X.2006.00453.x [DOI] [PubMed] [Google Scholar]
- 70. Lu Y, Zhong MC, Qian J, et al. : SLAM receptors foster iNKT cell development by reducing TCR signal strength after positive selection. Nat Immunol. 2019;20(4):447–57. 10.1038/s41590-019-0334-0 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Dutta M, Kraus ZJ, Gomez-Rodriguez J, et al. : A role for Ly108 in the induction of promyelocytic zinc finger transcription factor in developing thymocytes. J Immunol. 2013;190(5):2121–8. 10.4049/jimmunol.1202145 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Melum E, Jiang X, Baker KD, et al. : Control of CD1d-restricted antigen presentation and inflammation by sphingomyelin. Nat Immunol. 2019;20(12):1644–55. 10.1038/s41590-019-0504-0 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation