Abstract
Purpose:
The aim of the present study was to compare the effects of the preoperative doses of betamethasone acetate 0.1% and placebo on controlling dry eye after cataract surgery.
Methods:
This randomized triple-blind clinical trial was conducted on 62 patients. For the purpose of the study, the participants were assigned into two groups of betamethasone (n = 28) and placebo (n = 34). The groups were administered with drops A or B three days before the operation, four times a day. These drops contained either betamethasone 0.1% or normal saline (placebo). Postoperative follow-up was performed 1, 7, and 30 days after the surgery. Dry eye symptoms were evaluated by means of the ocular surface disease index (OSDI) using the meniscometry test. Repeated measures analysis was also used to study the effect of the interaction between betamethasone and time on meniscometry and OSDI variables.
Results:
A total of 62 patients, including 51.6% female and 48.4% male, were investigated in this study with a mean age of 69.19 ± 12.80 years. The results of the analysis of variance of the repeated measures plot indicated that the OSDI and meniscometry dry eye variables were not affected by the interaction between time and betamethasone (P = 0.192 and P = 0.578, respectively).
Conclusion:
As the findings indicated, the use of betamethasone acetate 0.1% prior to cataract surgery had no significant effect on postoperative dry eye indices.
Keywords: Betamethasone acetate, cataract surgery, dry eye
Cataract is considered the most important reason for blindness in the world.[1] According to a recent report released by the World Health Organization in 2012, the first cause of visual impairment is refractive error, followed by cataract, resulting in 33% visual impairment. Surgery is the best-known method to solve this problem,[1] with the phacoemulsification technique being the gold standard approach for cataract surgery.[2]
The incidence of cataracts increases with aging; accordingly, the prevalence rate of this disease in people over 85 years of age is 65% in such countries as the United States and England.[3] Studies targeting the incidence of cataracts in Iran have suggested that this is a very common condition. For instance, in a study conducted in Shahrood and Tehran, Iran, 36% of visual impairment was attributed to cataracts.[4,5]
Dry eye is one of the most important side effects after such operations as refractive surgery, vitrectomy, and cataract surgery mostly occurring owing to the exacerbation of symptoms or as a result of surgery.[6,7] The prevalence of dry eye following cataract surgery was reported to be as high as 55.7%,[8] with the peak intensity (9.8%) being observed in the first 7 days.[9]
Many causes justify the mechanisms of dry eye development after cataract surgery. Some of these causes include damage to the corneal nerves, neurogenic inflammation that can change the action of the corneal nerves and reduce corneal sensitivity, disruption of the normal corneal innervation or lacrimal functional unit, intraoperative irrigation of the tear film, intraoperative application of topical anesthesia, and postoperative administration of topical eye drops entailing preservatives.[9]
Although the exacerbation of dry eye after the modern phacoemulsification surgery seems to be self-limiting, this process takes 3-6 months and is unpleasant for the patients. In addition, ocular lubricants are considered the main therapeutic agents. Cyclosporine A is approved by the Food and Drug Administration. Accordingly, the topical form of cyclosporine 0.05 (RESTASIS) has been used in severe and resistant dry eye. This prescription is limited for people with inflammation associated with dry eye.[10]
Additionally, several studies have been performed on the role of inflammation in the development and exacerbation of dry eye and the effect of steroidal and nonsteroidal anti-inflammatory drugs on the improvement of this condition. In this regard, Badooin et al. (2017) showed that the inflammatory cycle, including intrinsic and acquired immunity, is a part of chronic dry eye pathophysiology. They also demonstrated that such drugs as cyclosporine and corticosteroids that break the cycle are effective in improving dry eye.[11]
Ray et al. (2017), reviewing all PubMed articles published from 1973 to 2017 about the role of inflammation in dry eye, emphasized the role of inflammation in dry eye and the effect of anti-inflammatory drugs on the improvement of this condition.[12] Specialists have performed comprehensive research on the effects of cataract surgery on dry eye and the different prevention and treatment methods of this condition. For example, Nistor et al. (2007), Li et al. (2007), and Kasetsuwan et al. (2013) studied dry eye and concluded that cataract surgery leads to dry eye. They also reported that in case of non-implementation of prompt dry eye treatment, this complication will be worsened.[9,13,14] Sanchez et al. (2010), Wan et al. (2012), and Sheppard et al. (2014) confirmed the effectiveness of some drugs, such as hydroxypropyl guar, loteprednol etabonate, and cyclosporine, on reducing the symptoms of postoperative dry eye.[15,16,17]
Although corticosteroids are highly effective in controlling postoperative complications after cataract surgery, they require time to exert their effects completely. Steroids have a dual effect; in this regard, on the one hand, they act on membrane receptors (rapid mechanism), and on the other hand, they have a slow and stable effect by changing the expression of the gene (quick effect become apparent within a few minutes and slow effect after 6-8 h).[18,19] With this background in mind, the present study was conducted to examine whether the pre-surgical administration of steroids when given enough time for effect initiation would cause more complete and better anti-inflammatory effects or not.
To the best of our knowledge, there is no study addressing the effect of preoperative administration of corticosteroid drops on controlling dry eye after cataract surgery. Therefore, the purpose of this study was to compare the effect of preoperative administration of betamethasone acetate (0.1%) and placebo in controlling dry eye after cataract surgery.
Methods
This randomized triple-blind clinical trial was conducted on 80 patients presenting for cataract surgery during winter 2016 to summer 2017. The eligible participants were selected using the non-probability sampling method and then randomly divided into two groups of 40 patients. However, only 62 patients (28 patients in the betamethasone group and 34 patients in the placebo group) completed the project.
The review of the literature revealed no study explicitly comparing dry eye between the two groups of steroids and placebo according to which we can determine the sample size. However, because eye pain is one of the symptoms and problems associated with dry eye, the sample size was calculated based on this variable. According to previous studies, the sample size was estimated at 62 cases depending on the ratio difference tests in the two communities (i.e., steroid and placebo use), compared to those without pain on the eighth day.[20]
x̄1=0/73 x̄2=0/27 α=0/05 β=0/2
The inclusion criterion was affliction with age-related cataract requiring ophthalmic surgery by an eye surgeon. On the other hand, the exclusion criteria were: 1) presence of a significant eye disease, 2) risk of systemic rheumatologic diseases or diabetes, 3) use of drugs that dry out the eye, 4) history of prolonged use of eye drops (e.g., glaucoma drugs), and 5) intra- or post-operative complications of cataract surgery. In line with a triple-blind design, the drops were filled by a pharmacologist blind to the researchers, patients, and statisticians. The type of each drop was revealed after performing all the steps and follow-up, at the end of the design.
Following the baseline visit that was performed before the preoperative treatment (i.e., 3-10 days before the surgery), drops A or B were administered 3 days prior to the surgery, four times a day, one drop each time. As mentioned earlier, corticosteroids exert rapid anti-inflammatory effects within a few minutes, while the delayed and sustained effects of this medication, preventing the development of side effects, take 6-8 h to emerge.[19] Regarding this, 3 days (short-term use) was considered a sufficient amount of time for the emergence of anti-inflammatory effects that control dry eye. The drops contained either betamethasone 0.1% or normal saline 0.9% (used in many studies as a placebo).[21,22] All patients were treated surgically on the scheduled dates according to phacoemulsification treatment by an anterior segment surgeon with temporal incision and Mani surgical blades.
The patients whose cataract surgery terminated without any complications were followed up for further study. The post-surgical drug regimen was similar in all patients and consisted of steroid drops (betamethasone 0.1% for 4 weeks) and antibiotics (Levofloxacin eye drops for 10 days). Regular post-surgical monitoring was performed for all patients by colleagues 1, 7, and 30 days after the surgery between 5:00 and 8:00 pm and at least one hour after using the last drop. Despite the fact that the self-limiting process of dry eye lasts 3-6 months, owing to the gradual improvement and emergence of most symptoms in the first month, in the present study, the patients were followed up for 30 days. Therefore, supplementary studies are recommended to be performed using a 6month follow-up period.
A standard ocular surface disease index (OSDI) was adopted to assess the symptoms of dry eyes. The validity and reliability of this tool have been already confirmed in various studies [Supplementary Material 1]. In addition, tear volume was measured using the meniscometry test.[23] The meniscometry test was performed using the Strip Meniscometry Tube (SMTube; Echo Electricity Co., Ltd). The sides of the SMTube marked with R and L are used for the right and left eyes, respectively. To use this device, first, the SMTube strip was removed from the sterile pouch; then, the tip of the device was applied on the eye for 5 sec to the lateral lower lid tear meniscus. Given the fact that the cornea and conjunctiva were not contacted, the length of the stained tear column was rapidly measured as the amount of meniscometry test.
Ethical considerations
The present study was approved by the Ethics Committee of Mashhad University of Medical Sciences (REC.1395.76) and registered by the Iranian Registry of Clinical Trials (IRCT2017103037105N1).
Statistical analysis
The data were analyzed using SPSS software, version 11.5. Descriptive statistics, including the mean, standard deviation, minimum, and maximum of the variables, were calculated. To compare the dry eye changes in each group among the pre-intervention and follow-up stages, ANOVA and parametric repeated measures tests were used. Additionally, Bonferroni follow-up test was run to compare the two groups.
Results
A total of 62 patients, including 51.6% females and 48.4% males, were candidates for the surgery with a mean age of 69.19 ± 12.80 years. The dry eye variable was evaluated by the meniscometry and OSDI indices. Table 1 presents the demographic characteristics of the research groups. According to the OSDI, some patients had severe dry eye. However, all patients did not have severe dry eye criteria when examined with the slit lamp, such as filamentary keratitis and punctate epithelial erosion. This may be due to the limitations of the questionnaire entailing some questions about visibility interfering with cataract symptoms. It should be noted that preoperative vision may have problems as a result of cataract, rather than dry eye. This results in a positive response to questions; therefore, it was not contraindicated to perform the cataract surgery. Furthermore, as the surgery was uncomplicated, no severe postoperative inflammation or pain was reported. The test was performed 60 min before using the therapeutic postoperative drops; consequently, the results of the meniscometry test would be reliable a day after the surgery.
Table 1.
Basic demographic characteristics of patients
| Case group Betamethasone 0.01% | Control group Normal saline 0.9% | Total | P | ||
|---|---|---|---|---|---|
| Sex | Female (%) | 14 (50%) | 18 (52.9%) | 32 (51.6) | P=1.000 |
| Male (%) | 14 (50%) | 16 (47.1%) | 30 (48.4%) | ||
| Age (year): Med±SD | 65.96±12.923 | 64.56±12.863 | 65.19±12.803 | P=0.671 | |
| Meniscometry (mm) | Normal (>5 mm) (%) | 14 (50%) | 21 (61.8%) | 35 (56.5%) | P=0.797 |
| Abnormal (<5 mm) (%) | 14 (50%) | 13 (38.2%) | 27 (43.5%) | ||
| OSDI | Normal (0-12) (%) | 10 (40%) | 15 (44.1%) | 25 (42.4%) | P=0.623 |
| Mild dry eye (13-22) (%) | 8 (32%) | 6 (17.6%) | 14 (23.7%) | ||
| Moderate dry eye (23-32) (%) | 3 (12%) | 6 (17.6%) | 9 (15.3%) | ||
| Severe dry eye (33-100) (%) | 4 (16%) | 7 (20.6%) | 11 (18.6%) |
OSDI=Ocular surface disease index
Basic assumptions, such as the normal distribution of errors, were investigated for all variables. According to the normal probability diagram for the residues, the distribution of each of the four errors generated by the four study periods was normal.
The analysis of the variance of the parametric duplicate sizes was performed for the OSDI and meniscometry variables. Although the results in the intervention group were better than those in the control group (especially for the OSDI variable and on the first day post-surgery), at 5% error level, the results showed no significant difference between the two groups in terms of time and group interaction (P = 0.192 and P = 0.578, respectively; Table 2 and Figs. 1, 2).
Table 2.
Comparing OSDI and meniscometry test scores before the preoperative treatment and 1, 7 and 30 days after surgery
| Before the preoperative treatment (1) | 1 day after surgery (2) | 7 days after surgery (3) | 30 days after surgery (4) | P | |||
|---|---|---|---|---|---|---|---|
| Dry eye | Meniscometry (mm) | Case group Betamethasone 0.01% | 5.46±2.94 | 6.79±2.43 | 5.68±2.67 | 6.11±2.21 | P=0.578 |
| Control group Normal saline 0.9% | 5.38±2.42 | 5.85±2.48 | 4.94±2.24 | 5.18±2.05 | |||
| Total | 5.42±2.65 | 6.27±2.48 | 5.27±2.45 | 5.59±2.16 | |||
| OSDI | Case group Betamethasone 0.01% | 17.12±16.79 | 10.88±15.65 | 11.82±18.54 | 13.46±17.68 | P=0.192 | |
| Control group Normal saline 0.9% | 17.99±17.20 | 20.84±19.60 | 14.48±15.22 | 17.79±16.66 | |||
| Total | 17.59±16.87 | 16.28±18.44 | 13.26±16.72 | 15.81±17.13 | |||
OSDI=Ocular Surface Disease Index
Figure 1.
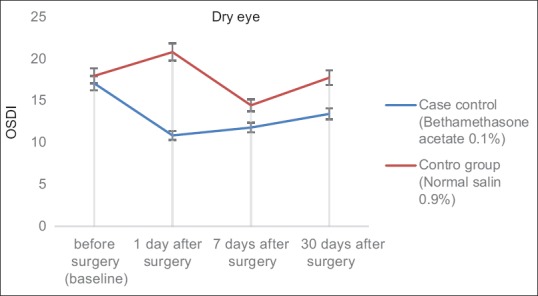
The mean of control and intervention groups scores in OSDI (Ocular Surface Disease Index) for different follow up times
Figure 2.
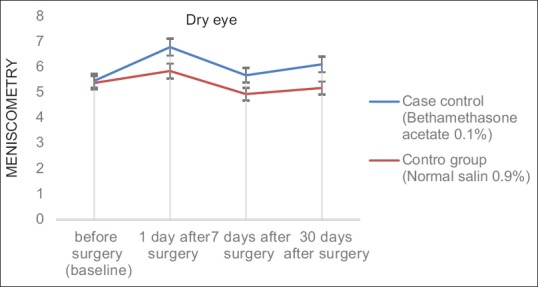
The mean of control and intervention groups scores for Meniscometry index on different follow up times
Discussion
The aim of this study was to evaluate the effects of preoperative betamethasone acetate 0.1% eye drops on dry eyes after cataract surgery. Observations indicated that the use of betamethasone acetate 0.1% drop had no significant effect on dry eye indices (OSDI and meniscometry) 1, 7, and 30 days after the cataract surgery. According to the results of previous studies, there is a significant relationship between increased prevalence and severity of dry eye and cataract surgery. In this regard, the severity of these complications can be considered a criterion for success in cataract surgery.[24]
Several studies have been performed on the role of inflammation in the development and exacerbation of dry eye and the effect of steroidal and nonsteroidal anti-inflammatory drugs on the improvement of this condition.[11,12] Therefore, the current study was targeted toward investigating the effects of preoperative betamethasone acetate 0.1% eye drops on controlling dry eye after cataract surgery.
There is no study that has specifically investigated the effects of preoperative administration of this type of drop on eye dryness after cataract surgery; therefore, the current study was the first attempt in this domain. Li et al. (2007) investigated the pathogenic factors involved in dry eye in patients after cataract surgery. They examined the symptoms of dry eye in patients at four stages before the surgery, namely 1 week, 1 month, and 3 months after the operation. Their observations showed that the misuse and inappropriate administration of eye drops were among the pathogenic factors in the development of post-surgical dry eye. Consequently, they concluded that this agent should be carefully administered to prevent or reduce the occurrence of postoperative dry eye.
This study also involved the investigation of a drug that could reduce dry eye after cataract surgery. Since at least with our current design and sample size, betamethasone was found to have no proven useful effects, it seems that the results of the mentioned study are indirectly consistent with those of the present study. It also seems that the administration of betamethasone drops prior to cataract surgery is not logical and even prescribing a preservative-containing form may exacerbate dry eye in patients.[25]
In another study, Ghanbari et al. examined the use of diclofenac 1% prior to surgery as one of the preventive methods of postoperative inflammation. Their findings showed that this method only reduced pain, redness, and eye irritation. However, it had no effect on intraoperative miosis, amount of intraocular pressure, and frequency and severity of postoperative anterior chamber reaction in the follow-ups performed after the first day[26] given that inflammation is one of the causes of dry eye. In general, anti-inflammatory agents are effective in improving dry eye, and steroidal and nonsteroidal anti-inflammatory drugs have almost similar effects.[27,28]
In 2016, Abadia et al. examined the application of topical dexamethasone to age-related eye problems and concluded that dexamethasone is effective in controlling inflammation and dry eye after cataract surgery. However, they also reported such side effects as increased intraocular pressure.[29] Yasushi Inou et al. (2017) investigated the effects of diquafosol sodium 3% (DQS) and artificial tears (AT) on higher-order aberrations in patients with dry eye after cataract surgery.[30] Patients received 3% sodium ophthalmic diquafosol and artificial tears for 4 weeks after the surgery. The results showed that dry eye rate significantly reduced in the DQS group, compared to that in the AT group, which is inconsistent with our results. Researchers found that DQS is effective in the treatment of dry eye after cataract surgery through improving visual performance.
Jee et al. (2015) investigated the effect of preservative-free sodium hyaluronate 0.1% and fluorometholone 0.1% eye drops on dry eye after cataract surgery. In the mentioned study, patients with dry eye after cataract surgery were treated with these drugs four times a day in the first month and twice a day in the second month. The results at the end of the second month showed that preservative-free sodium hyaluronate 0.1% and fluorometholone 0.1% eye drops were effective in improving dry eye after cataract surgery.[31]
The reason for the discrepancy between the results of our study with those of others is that in the current study, only the meniscometry test and OSDI questionnaire were used for evaluating dry eye. Furthermore, since most of the patients undergoing cataract surgery were elderly and usually lived alone and suffered from other illnesses (e.g., dementia), it seems that their drug compliance was lower.[32,33]
There are various mechanisms to explain the insignificant effect of betamethasone acetate 0.1% on dry eye after cataract surgery. For instance, if the patients undergoing cataract surgery do not have severe dry eye, and the surface tear layer is preserved, they may have clinical symptoms; however, the tests, such as meniscometry, may be normal. In this case, the administration of an additional drug does not seem logical.
Cataract surgery by itself and regardless of the pre-surgical state of the eye can cause or exacerbate the dry eye.[9] This raises the hypothesis that surgery can neutralize the effects of preoperative drug given its invasive nature. In addition, the prescription of multiple drops with preservatives after cataract surgery may have neutralized the positive effects of the preoperative doses of betamethasone, and despite its administration, the patients still have dry eyes or exacerbated symptoms.[30]
According to the results of the present study, the use of preoperative doses of betamethasone had no significant effect on dry eye control after the cataract surgery. Accordingly, the new cataract surgery method seemed to be largely safe and harmless. Therefore, it seems logical to use fewer drops containing preservatives. This would, in turn, result in the reduction of treatment costs and enhancement of medication compliance in patients.
The limitations of this study include small sample size, old age of the participants, and low patient cooperation in testing. In addition, the participants did not experience clinical symptoms and answered the questions in the OSDI, especially up to one week after the surgery, because of using the eye shield for up to one week. They also stayed in an enclosed environment for various reasons, such as loneliness and old age.
Conclusion
The use of betamethasone acetate 0.1% prior to cataract surgery has no significant effect on postoperative dry eye indices.
Financial consideration
Nil.
Conflicts of interest
There are no conflicts of interest
Supplementary Material 1
Ocular Surface Disease Index© (OSDI©)2
Ask your patients the following 12 questions, and circle the number in the box that best represents each answer. Then, fill in boxes A, B, C, D, and E according to the instructions beside each.
| Have you experienced any of the followingduring the last week? | All of the time | Most of the time | Half of the time | Some of the time | None of the time |
|---|---|---|---|---|---|
| 1. Eyes that are sensitive to light? .. | 4 | 3 | 2 | 1 | 0 |
| 2. Eyes that feel gritty? .......... | 4 | 3 | 2 | 1 | 0 |
| 3. Painful or sore eyes? ........... | 4 | 3 | 2 | 1 | 0 |
| 4. Blurred vision? ............ | 4 | 3 | 2 | 1 | 0 |
| 5. Poor vision? ........ | 4 | 3 | 2 | 1 | 0 |
| Subtotal score for answers 1 to 5 | (A) | ||||
| Have problems with your eyes limited you in performing any of the followinqduring the last week? | All of the time | Most of the time | Half of the time | Some of the time | None of the time | N/A |
|---|---|---|---|---|---|---|
| 6. Reading? ................... | 4 | 3 | 2 | 1 | 0 | N/A |
| 7. Driving at night? ............................. | 4 | 3 | 2 | 1 | 0 | N/A |
| 8. Working with a computer or bank machine (ATM)? .......................... | 4 | 3 | 2 | 1 | 0 | N/A |
| 9. Watching TV? | 4 | 3 | 2 | 1 | 0 | N/A |
| Subtotal score for answers 6 to 9 | (B) | |||||
| Have your eyes felt uncomfortable in any of the following situations durinq the last week? | All of the time | Most of the time | Half of the time | Some of the time | None of the time | N/A |
|---|---|---|---|---|---|---|
| 10. Windy conditions? ................. | 4 | 3 | 2 | 1 | 0 | N/A |
| 11. Races or areas with low humidity (very dry)? ...................... | 4 | 3 | 2 | 1 | 0 | N/A |
| 12. Areas that are air conditioned? ... | 4 | 3 | 2 | 1 | 0 | N/A |
| Subtotal score for answers 10 to 12 | (C) | |||||
Acknowledgments
This article is the result of a research project (941147) at “…” of Medical Sciences. The authors of the paper are grateful to all the patients participating in this study.
References
- 1.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–8. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 2.Gogate P. Comparison of various techniques for cataract surgery, their efficacy, safety, and cost. Oman J Ophthalmol. 2010;3:105–6. doi: 10.4103/0974-620X.71880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puri BK, Singh I. Prevalence of cataract in adult Down's syndrome patients aged 28 to 83 years. Clin Prat Epidemiol Ment Health. 2007;3:26. doi: 10.1186/1745-0179-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashemi H, Khabazkhoob M, Emamian MH, Shariati M, Fotouhi A. Visual impairment in the 40- to 64-year-old population of Shahroud, Iran. Eye (Lond) 2012;26:1071–7. doi: 10.1038/eye.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shahriari HA, Izadi S, Rouhani MR, Ghasemzadeh F, Maleki AR. Prevalence and causes of visual impairment and blindness in Sistan-va-Baluchestan Province, Iran: Zahedan Eye Study. Br J Ophthalmol. 2007;91:579–84. doi: 10.1136/bjo.2006.105734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banaee T, Gharaee H, KhajehDaluee M, Shokoohi-Rad S, Abrishami M. Alteration of Tear Film after Vitrectomy and its Influencing Factors. Iran J Ophthalmol. 2008;20:32–6. [Google Scholar]
- 7.Wang B, Naidu RK, Chu R, Dai J, Qu X, Zhou H. Dry Eye Disease following Refractive Surgery: A 12-Month Follow-Up of SMILE versus FS-LASIK in High Myopia. J Ophthalmol. 2015;2015:132417. doi: 10.1155/2015/132417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao X, Xia S, Chen Y. Comparison of the efficacy between topical diquafosol and artificial tears in the treatment of dry eye following cataract surgery: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017;96:e8174. doi: 10.1097/MD.0000000000008174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasetsuwan N, Satitpitakul V, Changul T, Jariyakosol S. Incidence and pattern of dry eye after cataract surgery. PloS One. 2013;8:e78657. doi: 10.1371/journal.pone.0078657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daneshvar R. Cataract surgery and dry eye Cataract Surg. (1st ed) 2013:339–52. [Google Scholar]
- 11.Baudouin C, Irkec M, Messmer EM, Benitez-Del-Castillo JM, Bonini S, Figueiredo FC, et al. Clinical impact of inflammation in dry eye disease: Proceedings of the ODISSEY group meeting. Acta Ophthalmol. 2017 doi: 10.1111/aos.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhee MK, Mah FS. Inflammation in Dry Eye Disease: How Do We Break the Cycle? Ophthalmology. 2017;124:S14–9. doi: 10.1016/j.ophtha.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 13.Li XM, Zhao X, Hu LZ, Wang W. Clinical observation of dry eye in patients before and after cataract surgery. Zhonghua Yan Ke Za Zhi. 2007;43:10–3. [PubMed] [Google Scholar]
- 14.Nistor MC, Nistor C. Clinical correlations between dry eye and cataract surgery. Oftalmologia. 2007;51:79–82. [PubMed] [Google Scholar]
- 15.Sanchez MA, Arriola-Villalobos P, Torralbo-Jimenez P, Giron N, de la Heras B, Herrero Vanrell R, et al. The effect of preservative-free HP-Guar on dry eye after phacoemulsification: A flow cytometric study. Eye (Lond) 2010;24:1331–7. doi: 10.1038/eye.2010.24. [DOI] [PubMed] [Google Scholar]
- 16.Sheppard JD, Donnenfeld ED, Holland EJ, Slonim CB, Solomon R, Solomon KD, et al. Effect of loteprednol etabonate 05% on initiation of dry eye treatment with topical cyclosporine 005% Eye Contact Lens. 2014;40:289–96. doi: 10.1097/ICL.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 17.Wan PX, Wang XR, Song YY, Li ZY, Duan HC, Zhang W, et al. Study on the treatment of dry eye with Loteprednol Etabonate. Zhonghua Yan Ke Za Zhi. 2012;48:142–7. [PubMed] [Google Scholar]
- 18.Becker DE. Basic and Clinical Pharmacology of Glucocorticosteroids. Anesth Prog. 2013;60:25–32. doi: 10.2344/0003-3006-60.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.So AY, Chaivorapol C, Bolton EC, Li H, Yamamoto KR. Determinants of cell- and gene-specific transcriptional regulation by the glucocorticoid receptor. PLoS Genet. 2007;3:e94. doi: 10.1371/journal.pgen.0030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajpal RK, Roel L, Siou-Mermet R, Erb T. Efficacy and safety of loteprednol etabonate 0.5% gel in the treatment of ocular inflammation and pain after cataract surgery. J Cataract Refract Surg. 2013;39:158–67. doi: 10.1016/j.jcrs.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Baek J, Doh SH, Chung SK. The Effect of Topical Diquafosol Tetrasodium 3% on Dry Eye After Cataract Surgery. Curr Eye Res. 2016;41:1281–5. doi: 10.3109/02713683.2015.1122813. [DOI] [PubMed] [Google Scholar]
- 22.Chung YW, Oh TH, Chung SK. The effect of topical cyclosporine 0.05% on dry eye after cataract surgery. Korean J Ophthalmol. 2013;27:167–71. doi: 10.3341/kjo.2013.27.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–21. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 24.Bazzari N, Eslami F, Pahlavan P, Akbarzadeh S. Dry Eye Following Cataract Surgery in Women Over 50 Years Old. Hamadan J Med Sci. 2016;23:221–6. [Google Scholar]
- 25.Li XM, Hu L, Hu J, Wang W. Investigation of dry eye disease and analysis of the pathogenic factors in patients after cataract surgery. Cornea. 2007;26:S16–20. doi: 10.1097/ICO.0b013e31812f67ca. [DOI] [PubMed] [Google Scholar]
- 26.Ghanbari H, Dehghani AR, Akhlaghi MR, Mostafaei S. Efficacy of Preoperative Topical Diclofenac for Prevention of Post-Cataract Surgery Inflammation. Bina J Ophthalmol. 2007;12:348–54. [Google Scholar]
- 27.Amuzadeh J, Soltani R, Beheshtnezhad AH. Comparison of the effect of diclofenac sodium 01% drop with betamethasone drop of 01% and co-administration of these two drops in controlling inflammation after cataract surgery Iranian. J Ophthalmol. 2006;18:1–5. [Google Scholar]
- 28.Hossain MM, Mohiuddin AA, Hossain MA, Aziz MA. Diclofenac sodium and prednisolone acetate ophthalmic solution in controlling inflammation after cataract surgery. Mymensingh Med J. 2010;19:343–7. [PubMed] [Google Scholar]
- 29.Abadia B, Calvo P, Ferreras A, Bartol F, Verdes G, Pablo L. Clinical Applications of Dexamethasone for Aged Eyes. Drugs Aging. 2016;33:639–46. doi: 10.1007/s40266-016-0392-z. [DOI] [PubMed] [Google Scholar]
- 30.Inoue Y, Ochi S. Effects of 3% diquafosol sodium ophthalmic solution on higher-order aberrations in patients diagnosed with dry eye after cataract surgery. Clin Ophthalmol. 2017;11:87–93. doi: 10.2147/OPTH.S122542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jee D, Park M, Lee HJ, Kim MS, Kim EC. Comparison of treatment with preservative-free versus preserved sodium hyaluronate 01% and fluorometholone 01% eyedrops after cataract surgery in patients with preexisting dry-eye syndrome. J Cataract Refract Surg. 2015;41:756–63. doi: 10.1016/j.jcrs.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez Lison LC, Baron Franco B, Vazquez Dominguez B, Martinez Garcia T, Urendes Haro JJ, Pujol de la Llave E. Medication errors and non-compliance in polymedicated elderly patients. Farm Hosp. 2006;30:280–3. doi: 10.1016/s1130-6343(06)73991-5. [DOI] [PubMed] [Google Scholar]
- 33.Pastor Climente IP, Ortiz de Urbina Sandomingo V, Perez Escoto I, Quintana Vargas I, Moreno Miralles A, Martinez Martinez M. The introduction of a programme to improve the treatment compliance of institutionalised elderly patients. Farm Hosp. 2007;31:106–11. doi: 10.1016/s1130-6343(07)75721-5. [DOI] [PubMed] [Google Scholar]


