Key Points
Question
Is a decline in both memory and gait speed with aging associated with a higher risk of dementia than no decline or a decline in memory or gait only in older adults?
Findings
In this meta-analysis of 6 studies including 8699 participants from the United States and Europe, a decline in both memory and gait was associated with 6.28 times higher risk of developing dementia than no decline.
Meaning
Older adults without dementia with parallel declines in memory and gait are associated with high risk of developing dementia and may be a group to target for prevention.
Abstract
Importance
Dual decline in both memory and gait speed may characterize a group of older individuals at high risk for future dementia.
Objective
To assess the risk of dementia in older persons who experience parallel declines in memory and gait speed compared with those who experience no decline or decline in either memory or gait speed only.
Design, Setting, and Participants
A multicohort meta-analysis was performed of 6 prospective cohort studies conducted between 1997 and 2018 in the United States and Europe. Participants were 60 years or older, had an initial gait speed of more than 0.6 m/s (ie, free of overt dismobility), with repeated measures of memory and gait speed before dementia diagnosis during a mean follow-up of 6.6 to 14.5 years. Within each study, participants were divided into 4 groups: memory decline only, gait speed decline only, dual decline, or no decline (hereafter referred to as usual agers). Gait decline was defined as a loss of 0.05 m/s or more per year; memory decline was defined as being in the cohort-specific lowest tertile of annualized change.
Main Outcomes and Measures
Risk of incident dementia according to group membership was examined by Cox proportional hazards regression with usual agers as the reference, adjusted for baseline age, sex, race/ethnicity, educational level, study site, and baseline gait speed and memory.
Results
Across the 6 studies of 8699 participants, mean age ranged between 70 and 74 years and mean gait speed ranged between 1.05 and 1.26 m/s. Incident dementia ranged from 5 to 21 per 1000 person-years. Compared with usual agers, participants with only memory decline had 2.2 to 4.6 times higher risk for developing dementia (pooled hazard ratio, 3.45 [95% CI, 2.45-4.86]). Those with only gait decline had 2.1 to 3.6 times higher risk (pooled hazard ratio, 2.24 [95% CI, 1.62-3.09]). Those with dual decline had 5.2 to 11.7 times the risk (pooled hazard ratio, 6.28 [95% CI, 4.56-8.64]).
Conclusions and Relevance
In this study, dual decline of memory and gait speed was associated with increased risk of developing dementia among older individuals, which might be a potentially valuable group for preventive or therapeutic interventions. Why dual decline is associated with an elevated risk of dementia and whether these individuals progress to dementia through specific mechanisms should be investigated by future studies.
This meta-analysis assesses whether parallel declines in memory and gait speed among older adults, compared with those who experience no decline or decline in either memory or gait speed only, are associated with risk of developing dementia.
Introduction
Impaired mobility, such as slow gait, is associated with an increased risk of dementia, but the effect size of this association is generally modest.1,2,3,4,5,6 Identifying persons who experience both mobility decline and memory decline, a main symptom in the early stage of dementia, may have a greater prognostic value in assessing risk of dementia because the combination could identify a group in whom gait speed decline is at least in part caused by neurodegenerative pathologic conditions of the central nervous system rather than local musculoskeletal problems, such as sarcopenia or osteoarthritis.7,8,9 A recent study of 154 participants with mild cognitive impairment reported that those who declined in both cognition and gait speed had the highest risk of dementia.10 However, this study population was limited to a relatively small clinical sample admitted to geriatric clinics. Whether this association occurs in general aging populations initially free of dismobility and cognitive impairment is unknown. If such an association is confirmed, it may help to identify older individuals who are free of dismobility and cognitive impairment but at high risk of developing dementia. It could also motivate investigations into whether this group develops dementia through specific pathophysiological mechanisms.
A growing line of research suggests that motoric cognitive risk syndrome, a combination of cognitive decline, slow gait, and preserved activities of daily living, is associated with high risks of cognitive impairment and dementia.11,12,13,14 However, motoric cognitive risk syndrome is typically operationalized at 1 time point. Recent evidence suggests that assessing gait speed longitudinally rather than with a 1-time assessment better distinguishes individuals who develop dementia.10
To address these knowledge gaps, we examined whether the extreme phenotype of dual decline in memory and gait with aging compared with no decline or decline in gait or memory only would be associated with an increased risk of dementia among community-dwelling older adults. We used data from 6 prospective cohort studies in the United States and Europe to create an individual-level meta-analysis. To capture early memory and gait changes with aging, we identified participants aged 60 years or older who were initially free of dismobility, cognitive impairment, and dementia; tracked changes in memory and gait speed during the dementia-free period; and then captured incident dementia. We hypothesized that those who experienced a decline in both memory and gait speed would have a higher risk of dementia than those with no decline or with a decline in gait or memory only.
Methods
Study Population
We used individual-level data from 6 prospective cohort studies: the Baltimore Longitudinal Study of Aging (BLSA)15; the Health, Aging and Body Composition Study (Health ABC)16; the Mayo Clinic Study of Aging (MCSA)17; the Age, Gene/Environment Susceptibility-Reykjavik Study (AGES-RS)18; the InCHIANTI (Invecchiare in Chianti, Aging in the Chianti Area) study19; and the Swedish National Study on Aging and Care-Kungsholmen Population Study (SNAC-K).20 The years of data collection ranged between 1997 and 2018. All studies were conducted under the oversight and approval of the institutional review boards of the institutions that conducted them. All participants consented to participate after receiving a comprehensive description of the study, including possible risks. This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.
We excluded participants who, at baseline, had prevalent cognitive impairment, dementia, and dismobility (gait speed ≤0.6 m/s)21 and who had baseline Mini-Mental State Examination (MMSE) scores of less than 2422 (BLSA, MCSA, AGES-RS, InCHIANTI, and SNAC-K) or Modified Mini-Mental State Examination scores of less than 80 (Health ABC).23 Mild cognitive impairment or cognitive impairment was diagnosed by consensus conferences following published criteria in BLSA,24 MCSA,25 AGES-RS,26 and InCHIANTI.19 We further excluded participants who did not have repeated measures of memory and gait speed prior to diagnoses of dementia, last follow-up, or death because, for these individuals, we could not estimate annualized changes in memory and gait speed.
Phenotypic Groups
Within each study, participants were classified into 4 phenotypic groups based on annualized changes in memory and gait speed prior to diagnoses of dementia, last follow-up, or death. Usual gait speed for a distance of 7.6 m (25 ft) in MCSA or a distance of 6 m in all other studies was used for analysis. Gait speed was harmonized to the same unit in meters per second. Specifically, in InCHIANTI, we converted a 4-m gait speed to a 6-m gait speed.27 In Health ABC, we converted a 20-m gait speed assessed at years 2, 3, 5, and 8 to a 6-m gait speed.28 In MCSA, gait speed was simply expressed as meters (25 ft; 7.62 m) divided by time in seconds.29 Except in MCSA and SNAC-K, which conducted only 1 trial, usual gait speed was assessed in 2 trials, and the faster trial was used for analysis.
Across the 6 studies, we focused on verbal episodic memory when available, especially immediate recall, because it was among the first cognitive functions to decline among Alzheimer disease–related cognitive measures.30 Specifically, memory was assessed using the California Verbal Learning Test in BLSA,31 the Buschke Selective Reminding Test in Health ABC,32 the Rey Verbal Learning Test in MCSA,17,33 the modified California Verbal Learning Test in AGES-RS,31 the MMSE memory subscore in InCHIANTI,34 and a free recall test in SNAC-K.35
Outcome
Dementia was ascertained based on the Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised)36 in BLSA; the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) in MCSA and SNAC-K; race-specific decline in Modified Mini-Mental State Examination scores, medication use, and hospital records in Health ABC37; a 3-step consensus adjudication following DSM-IV criteria in AGES-RS18; and a 2-stage screening procedure following DSM-IV criteria in InCHIANTI.38 Within each study, the end point was at diagnoses of dementia for those who developed dementia or at last follow-up or death for those who did not develop dementia. Detailed assessment and study designs are presented in eTable 1 in the Supplement.
Statistical Analysis
Within each study, annual rates of change in memory and gait speed prior to diagnoses of dementia, last follow-up, or death were first computed using simple linear regression. Participants were then classified into 4 phenotypic groups, using cut points of annualized decline in gait speed equal to or greater than 0.05 m/s39 and the lowest tertile of annualized decline in memory performance. Specifically, those with less than 0.05 m/s annualized decline in gait speed and in the middle and highest tertiles (ie, less decline) of annualized decline in memory were referred to as usual agers. Those with less than 0.05 m/s annualized decline in gait speed but in the lowest tertile of annualized decline in memory were referred to as participants with memory decline only. Those with equal to or greater than 0.05 m/s annualized decline in gait speed and in the middle and highest tertile of annualized decline in memory were referred to as participants with gait decline only. Those with equal to or greater than 0.05 m/s annualized decline in gait speed and in the lowest tertile of annualized decline in memory were referred to as participants with dual decline. In InCHIANTI, individual slopes of MMSE memory subscores were estimated using a random-effects Poisson model owing to its distribution.
We first examined whether baseline gait speed and memory differed between groups, using multiple linear regression with usual agers being the reference group, adjusting for baseline age, sex, educational level, race/ethnicity, and study site. We then examined associations of baseline gait speed and memory performance with incident dementia using Cox proportional hazards regression, accounting for demographic characteristics.
We examined associations of phenotypic groups with incident dementia using Cox proportional hazards regression, with usual agers being the reference group, adjusting for demographic characteristics and baseline gait speed and memory performance. In Health ABC, because Buschke Selective Reminding Test began at year 3, the estimated intercept at baseline by simple linear regression was used as the covariate.
The mean risk of dementia among participants with memory decline, participants with gait decline, and participants with dual decline across the 6 studies was examined using random-effects meta-analysis, following guidelines from the Cochrane Handbook for Systematic Reviews of Interventions.40 The findings are presented as forest plots and reported following published guidelines.41 Heterogeneity among studies was examined using the Cochrane χ2 test. The amount of variation across studies that was due to heterogeneity was indicated by I2, expressed as a percentage. We performed sensitivity analyses by excluding 2 studies in which dementia was ascertained a posteriori (Health ABC and InCHIANTI). We performed additional sensitivity analyses by adjusting for baseline multimorbidity and specific diseases important for declines in memory and gait, including hypertension, cardiovascular disease, and stroke.
To evaluate whether changes in gait speed and memory added prognostic value beyond baseline gait speed and memory, we compared 2 models using likelihood ratio tests. To examine whether the risk of dementia associated with gait decline was similar for those with and those without memory decline, we added an interaction term between slope of memory decline and slope of gait decline in Cox proportional hazards regression. A significant interaction would reject the null hypothesis that the risk of developing dementia associated with gait decline was the same for participants with and participants without memory decline.
Statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc) and R, version 3.5.0 (R Project for Statistical Computing). All P values were from 2-sided tests and results were deemed statistically significant at P < .05.
Results
All 6 studies of 8699 participants included both men and women (Table 1). The BLSA and Health ABC study populations were racially/ethnically diverse and included a substantial proportion of black participants. Other studies had almost all or exclusively white participants. At baseline, the mean age across studies ranged between 70 and 74 years. The mean gait speed ranged between 1.05 and 1.26 m/s. The mean follow-up time ranged between 6.6 and 14.5 years. Across the 6 studies of 8699 participants, incident dementia ranged from 5 to 21 per 1000 person-years.
Table 1. Baseline Sample Characteristics.
| Characteristic | BLSA (N = 664) | Health ABC (N = 727) | MCSA (N = 2633) | AGES-RS (N = 2563) | InCHIANTI (N = 553) | SNAC-K (N = 1559) |
|---|---|---|---|---|---|---|
| Study entry | 2006a | 1997/1998 | 2004 | 2002 | 1998 | 2001-2004 |
| Study site | Baltimore, MD | Pittsburgh, PA; Memphis, TN | Olmsted County, MN | Iceland | Greve in Chianti, Bagno a Ripoli, Italy | Stockholm, Sweden |
| Demographic | ||||||
| Age, mean (SD) [range], y | 73.3 (8.2) [60-95] | 73.5 (2.7) [69-81] | 74.3 (7.0) [60-91] | 74.4 (4.5) [66-91] | 71.8 (5.3) [65-91] | 70.4 (8.9) [60-97] |
| Women, No. (%) | 329 (50) | 371 (51) | 1266 (48) | 1491 (58) | 293 (53) | 947 (61) |
| Black race/ethnicity, No. (%) | 140 (21) | 315 (43) | 5 (0.2) | 0 | 0 | 0 |
| Educational level, mean (SD) [range], y | 17.6 (2.7) [7-32] | 13.7 (2.8) [3-18] | 14.5 (2.7) [5-20] | No. (%), 783 (31) >high school | 6.3 (3.4) [0-22] | No. (%), 658 (42) >high school |
| Body mass index, mean (SD) [range]b | 26.8 (4.4) [17.8-45.6] | 27.0 (4.5) [15.6-48.0] | 28.3 (5.2) [16.9-57.8] | 27.3 (4.1) [15.6-47.5] | 27.4 (3.9) [18-47] | 26.0 (3.7) [16-47] |
| Global mental statusc | MMSE | 3MS | MMSE | MMSE | MMSE | MMSE |
| Median (IQR) score [range] | 29 (28-30) [24-30] | 94 (90-97) [80-100] | 28 (27-29) [24-30] | 28 (27-29) [24-30] | 27 (26-28) [24-30] | 29 (29-30) [25-30] |
| Memory performancec | CVLT | SRT | AVLT | Modified CVLT | MMSE memory subscore | Word recall |
| Mean (SD) score [range] | 51.0 (12.0) [6-80] | 46.8 (11.3) [2-72] | 40.7 (9.3) [13-69] | 28.6 (7.1) [0-57] | 5 (4-6) [3-6]d | 7.5 (2.3) [0-16] |
| Gait speed, m/se | ||||||
| Mean (SD) speed [range] | 1.16 (0.21) [0.61-1.97] | 1.26 (0.24) [0.69-2.0] | 1.12 (0.23) [0.64-1.91] | 1.05 (0.18) [0.61-1.74] | 1.19 (0.22) [0.62-2.0] | 1.21 (0.29) [0.61-2.00] |
| Incident dementia | ||||||
| No. (%) | 45 (7) | 130 (18) | 92 (3) | 254 (10) | 123 (22) | 165 (11) |
| Per 1000 person-years | 8 | 14 | 5 | 9 | 21 | 10 |
| No. of visits, mean (SD) [range]f | 4.2 (1.8) [2-12] | 8 (1) [3-9] | 4.5 (2.1) [2-11] | 2 (0)g | 3.6 (1.1) [2-5] | 3 (1) [2-5] |
| Follow-up time, mean (SD) [range], y | 8.3 (2.8) [2.0-13.1] | 12.2 (3.0) [3.9-17.1] | 6.6 (2.9) [1.3-13.0] | 10.7 (1.6) [4.7-13.0] | 14.5 (4.4) [3-20] | 10.0 (2.7) [2-14] |
| No. of visits per year, mean (SD) [range] | 1 (0.3) [0.2-2.0] | 1 (0.1) [0.5-1.5] | 1 (0.3) [0.2-2.2] | 0.4 (0.02) [0.2-0.8] | 0.5 (0.1) [0.2-0.7] | 0.3 (0.1) [0.1-1.0] |
| Phenotypic groups, No. (%) | ||||||
| No memory or gait speed decline | 375 (56) | 422 (58) | 1383 (53) | 1519 (59) | 291 (53) | 674 (43) |
| Memory decline only | 170 (26) | 202 (28) | 616 (23) | 743 (29) | 164 (30) | 309 (20) |
| Gait decline only | 67 (10) | 63 (9) | 373 (14) | 177 (7) | 61 (11) | 365 (23) |
| Dual decline in memory and gait speed | 52 (8) | 40 (6) | 261 (10) | 124 (5) | 37 (7) | 211 (14) |
Abbreviations: 3MS, Modified Mini-Mental State Examination; AGES-RS, Age, Gene/Environment Susceptibility-Reykjavik Study; AVLT, Auditory Verbal Learning Test; BLSA, Baltimore Longitudinal Study of Aging; CVLT, California Verbal Learning Test; Health ABC, Health, Aging and Body Composition Study; InCHIANTI, Invecchiare in Chianti, Aging in the Chianti Area; IQR, interquartile range; MCSA, Mayo Clinic Study of Aging; MMSE, Mini-Mental State Examination; SNAC-K, The Swedish National Study on Aging and Care in Kungsholmen; SRT, Buschke Selective Reminding Test.
The initial assessment of 6-m gait speed started in 2006, although the BLSA began in 1958. The sample in the Health ABC is from the Cognitive Vitality Substudy.
Calculated as weight in kilograms divided by height in meters squared.
A higher score indicates higher performance. The range of possible scores is 0 to 30 for the MMSE and 0 to 100 for the 3MS.
The MMSE memory subscore is shown as median (IQR).
A higher value indicate higher performance.
Only visits where memory function and gait speed were assessed and used to define the phenotypic classification.
In AGES-RS, gait and memory were assessed only twice with a mean (SD) interval of 5 (0.3) years.
Compared with usual agers, after adjustment, participants with gait decline and participants with dual decline had faster baseline gait speed in all studies except InCHIANTI. Baseline gait speed did not differ between participants with memory decline and usual agers in all studies (eTable 2 in the Supplement).
Compared with usual agers, participants with memory decline and participants with dual decline had higher baseline memory performance in all studies except InCHIANTI and BLSA. Baseline memory did not differ between participants with gait decline and usual agers except in Health ABC, in which participants with gait decline had poorer baseline memory performance (eTable 2 in the Supplement).
After adjustment, poorer baseline memory performance was associated with a higher risk of developing dementia in all studies except InCHIANTI. Slower baseline gait speed was associated with a higher risk of dementia in all studies except BLSA (Table 2; model 1).
Table 2. Associations of Phenotypic Groups With Subsequent Dementia Riska.
| Phenotype | BLSA (N = 664) | Health ABC (N = 727) | MCSA (N = 2633) | AGES-RS (N = 2563) | InCHIANTI (N = 553) | SNAC-K (N = 1559) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Model 1 | ||||||||||||
| Baseline memory | 0.951 (0.926-0.977) | <.001 | 0.983 (0.966-1.000) | .04 | 0.942 (0.917-0.969) | <.001 | 0.946 (0.928-0.965) | <.001 | 1.008 (0.836-1.215) | .93 | 0.923 (0.867-0.982) | .01 |
| Baseline gait speed, m/s | 1.270 (0.261-6.190) | .76 | 0.259 (0.104-0.643) | .004 | 0.228 (0.080-0.650) | .005 | 0.293 (0.135-0.633) | .001 | 0.279 (0.106-0.734) | .009 | 0.440 (0.236-0.818) | .009 |
| Model 2 | ||||||||||||
| No memory or gait speed decline | [Reference] | NA | [Reference] | NA | [Reference] | NA | [Reference] | NA | [Reference] | NA | [Reference] | NA |
| Memory decline only | 4.473 (2.036-9.826) | <.001 | 4.323 (2.786-6.709) | <.001 | 4.362 (2.603-7.310) | <.001 | 2.619 (1.965-3.492) | <.001 | 2.252 (1.478-3.432) | <.001 | 4.633 (2.916-7.359) | <.001 |
| Gait decline only | 2.418 (0.789-7.411) | .12 | 2.166 (1.108-4.233) | .02 | 1.889 (0.915-3.902) | .08 | 1.558 (0.945-2.568) | .08 | 3.629 (1.971-6.683) | <.001 | 2.503 (1.564-4.006) | <.001 |
| Dual decline in memory and gait speed | 5.912 (2.252-15.524) | <.001 | 11.722 (6.346-21.651) | <.001 | 5.810 (3.040-11.104) | <.001 | 5.206 (3.484-7.781) | <.001 | 6.993 (3.654-13.385) | <.001 | 5.332 (3.166-8.982) | <.001 |
| Baseline memory | 0.932 (0.904-0.960) | <.001 | 0.957 (0.939-0.975) | <.001 | 0.928 (0.903-0.955) | <.001 | 0.930 (0.911-0.949) | <.001 | 1.030 (0.856-1.239) | .75 | 0.849 (0.797-0.904) | <.001 |
| Baseline gait speed, m/s | 0.997 (0.200-4.965) | .99 | 0.196 (0.077-0.503) | <.001 | 0.183 (0.063-0.536) | .001 | 0.241 (0.109-0.533) | <.001 | 0.200 (0.078-0.513) | <.001 | 0.302 (0.150-0.605) | <.001 |
Abbreviations: AGES-RS, Age, Gene/Environment Susceptibility-Reykjavik Study; BLSA, Baltimore Longitudinal Study of Aging; Health ABC, Health, Aging and Body Composition Study; HR, hazard ratio; InCHIANTI, Invecchiare in Chianti, Aging in the Chianti Area; MCSA, Mayo Clinic Study of Aging; NA, not applicable; SNAC-K, The Swedish National Study on Aging and Care in Kungsholmen.
All models were adjusted for baseline age, sex, and educational level in all studies; additionally adjusted for race/ethnicity in BLSA, Health ABC, and MCSA; and additionally adjusted for site in Health ABC and InCHIANTI.
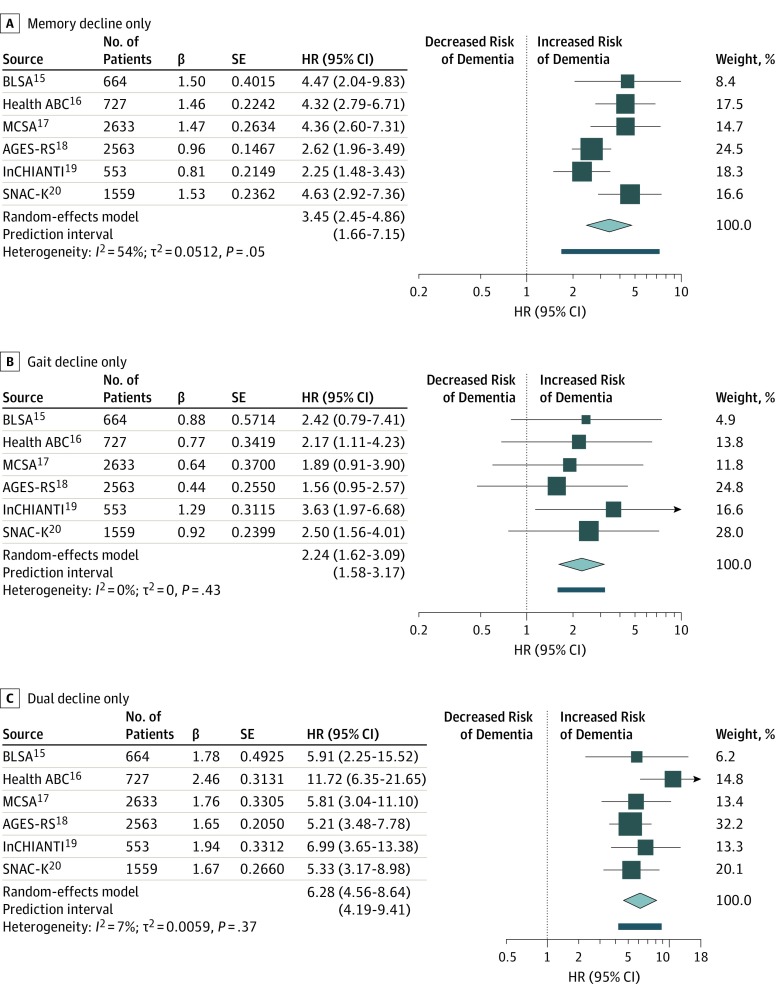
Compared with usual agers, participants with dual decline and participants with memory decline had a significantly higher risk of developing dementia consistently across all studies after adjustment for demographic characteristics and baseline memory and gait speed (Table 2; model 2; eFigure in the Supplement). Specifically, participants with dual decline had 5.2 to 11.7 times higher risk of developing dementia compared with usual agers, with a pooled hazard ratio (HR) of 6.28 (95% CI, 4.56-8.64) (Figure, C).15,16,17,18,19,20 Participants with only memory decline had 2.2 to 4.6 times higher risk, with a pooled HR of 3.45 (95% CI, 2.45-4.86) (Figure, A).15,16,17,18,19,20 Participants with only gait decline had 2.1 to 3.6 times the risk (pooled HR, 2.24; 95% CI, 1.62-3.09) (Figure, B).15,16,17,18,19,20 Associations of gait decline with dementia risk were significant in Health ABC, InCHIANTI, and SNAC-K but not significant in BLSA, MCSA, and AGES-RS. Meta-analyses results remained similar after excluding 2 studies in which dementia was ascertained a posteriori: compared with usual agers, participants with dual decline had a pooled HR of 5.40 (95% CI, 4.95-5.89), participants with only memory decline had a pooled HR of 3.62 (95% CI, 2.22-5.90), and participants with only gait decline had a pooled HR of 2.01 (95% CI, 1.36-2.98). Adjustment for multimorbidity and specific diseases did not substantially alter the results (eTable 3 in the Supplement).
Figure. Forest Plots of Hazard Ratios (HRs) for Dementia Risk in Phenotypic Groups.
A, Participants with only memory decline compared with those with no decline in memory or gait speed. B, Participants with gait decline only compared with those with no decline in memory or gait speed. C, Participants with dual decline compared with those with no decline in memory or gait speed. The sizes of the data markers indicate the size of each study; the larger the data marker, the more participants were in the study. AGES-RS indicates the Age, Gene/Environment Susceptibility-Reykjavik Study; BLSA, the Baltimore Longitudinal Study of Aging; Health ABC, the Health, Aging and Body Composition Study; InCHIANTI, Invecchiare in Chianti, Aging in the Chianti Area; MCSA, the Mayo Clinic Study of Aging; and SNAC-K, the Swedish National Study on Aging and Care-Kungsholmen Population Study. SE indicates standard error.
Based on likelihood ratio tests performed consistently across the 6 studies, models including information on change over time yielded a lower Akaike information criterion and a significant model change compared with models including information on baseline gait speed and baseline memory only, indicating a better fit (eTable 4 in the Supplement).
In BLSA, Health ABC, and SNAC-K, the interaction between slope of memory decline and slope of gait decline was significant in Cox proportional hazards regression models associated with dementia risk (BLSA: β [SE], –1.285 [0.495]; P = .01; Health ABC: β [SE], –3.071 [1.137]; P = .006; and SNAC-K: β [SE], –7.590 [2.403]; P = .001). In MCSA, findings were not significant (β [SE], 0.595 [0.362]; P = .10). In AGES-RS and InCHIANTI, the interaction term was also not significant (AGES-RS: β [SE], 2.10 [1.51]; P = .16; InCHIANTI: β [SE], –0.053 [0.053]; P = .31 [in InCHIANTI, the slope of gait and slope of memory (by Poisson) were multiplied by 100 owing to small values]).
Discussion
Across 6 large cohort studies of aging, dual decline in both memory and gait speed during the dementia-free period was associated with high risk of developing dementia. Our findings are consistent with the recent report by Montero-Odasso and colleagues.10 Our data further contribute to the literature by confirming these findings in 6 geographically and culturally diverse aging studies, focusing on memory function, rather than global cognition, and by characterizing early phenotype when participants are free of dismobility, cognitive impairment, and dementia.
Poorer baseline memory was associated with a higher risk of dementia except in InCHIANTI. The lack of an association in InCHIANTI may be due to the use of the MMSE memory subscore, a poorly sensitive measure of memory. In all studies except BLSA, slower baseline gait speed was associated with a higher risk of dementia, consistent with prior findings.1 The lack of an association in BLSA supports the notion that a 1-time gait speed assessment may be less able than change over time to assess risk of dementia.10 In all 6 studies, models with change over time significantly improved the assessment of the risk of dementia than models with only information on baseline gait speed and memory. Thus, tracking changes over time may add important information in assessing the risk of developing dementia beyond obtaining a 1-time assessment.
As expected, participants with memory decline had an increased risk of dementia consistently across the 6 cohorts. Our focus on verbal episodic memory, especially immediate recall, rather than global cognition or mental status, allows us to capture early memory decline among Alzheimer disease–related cognitive measures within aging. Focusing on memory, rather than executive function or processing speed, also allows us to better differentiate phenotypic groups because gait speed is associated with executive function within cognitive domains.42
Although participants with gait decline also showed some increased risk of dementia, when accompanied by a parallel decline in objective memory, the risk of developing dementia tended to be the highest among phenotypic groups. Our finding that participants with dual decline are at particularly high risk of developing dementia supports the importance of the unique occurring symptom of cognitive and motor deficits outlined in previous research on motoric cognitive risk syndrome and further establishes its clinical relevance. Among older adults living in the community free of cognitive impairment and dismobility, the dual decline phenotype can be captured early in clinical settings by routinely administering gait speed assessment and a free recall memory test.43 At this stage, this group should be carefully evaluated for potentially reversible risk factors for dementia. However, research focusing on biological and physiological characteristics that explain why individual with dual decline are at such high risk of developing dementia may lead to new opportunities for prevention.
Our findings add weight to the hypothesis that dual decline with aging represents some shared underlying processes associated with declines in both gait and memory that are associated with ultimate overt dementia. Our study may provide a possible explanation for the heterogeneity of reported findings.1,3 Perhaps gait speed decline is associated with dementia most particularly when it is at least in part due to incipient central nervous system pathologic characteristics. Our findings that participants with dual decline have the highest risk of dementia capture the critical need for tracking gait and memory and may help isolate potential central nervous system causes of gait decline. An interesting hypothesis is that dual decline may characterize older persons who will eventually progress to dementia through specific pathophysiological mechanisms. Although the nature of these mechanisms is not yet known, we propose that vascular, metabolic, or energetic dysfunction is the likely factor.44,45,46,47,48,49,50,51,52,53,54,55 This hypothesis should be tested in further studies by examining the particular metabolic, vascular, and neuroimaging features that characterize this specific group.
Our findings do not necessarily address the question as to whether the high risk of dementia associated with dual decline is simply due to the combination of 2 independent factors, gait decline and memory decline, or whether the increased risk represents a shared underlying pathologic condition. Findings varied across the 6 cohorts. Specifically, while the relative risk of dementia associated with dual decline was always higher than the sum of risks due to gait decline and memory decline, the interaction between gait decline and memory decline was significant in 3 studies (BLSA, Health ABC, and SNAC-K). In studies that demonstrated a significant interaction, the period in which gait and memory were assessed was relatively long (mean period, 6-9 years) vs AGES-RS and MCSA (5 years). Although InCHIANTI also had a relatively long period of time assessing gait and memory (9 years), it was somewhat limited by the insensitive measure of memory, based only on the MMSE memory subscore.
Strengths and Limitations
This study has numerous strengths: use of multiple large longitudinal cohort studies, efforts to harmonize measures, careful delineation of individual study approaches to dementia diagnosis, and use of rigorous meta-analytic measures. Accounting for multimorbidity and specific diseases, such as hypertension, cardiovascular disease, and stroke, enriched the analyses, and the findings remained robust. Although a posteriori ascertainment of dementia in 2 studies may have overestimated incident cases, the results remained largely unchanged after excluding these 2 studies.
This study also has some limitations. We acknowledge a lack of information on subtypes of dementia in some studies; thus, we could not evaluate the risk of dementia subtypes. Second, the specific memory measures that we examined in InCHIANTI and MCSA are partly used in dementia diagnostic procedures. However, in InCHIANTI, the MMSE is not the only assessment used to ascertain dementia, and our study used the memory subscore. In MCSA, information from the Rey Verbal Learning Test among all other neuropsychological tests contributed to the determination of a dementia diagnosis.17 In the other 4 studies, the memory measures that we used in this study are not used in their dementia diagnostic algorithms. Another potential limitation is the possibility of regression to the mean. In some studies, participants with dual decline had higher baseline gait speed and memory performance than usual agers. Participants with dual decline who had higher baseline gait and memory may have had more room to decline than those with lower baseline performance. However, by adjusting for baseline gait speed and memory, we minimized this possibility.
Conclusions
Our findings have relevant clinical implications and provide important contributions to research on early signs of an underlying dementia process. Older persons with dual decline in memory and gait speed should receive further attention to address issues that may increase dementia risk, including evaluation of cardiovascular and metabolic risk factors. Studying the cardiovascular and metabolic characteristic of this subgroup may provide clues to the specific mechanisms that confer high risk of dementia. Dual decline in memory and gait speed was associated with high risk of developing dementia. These individuals might serve as a valuable target for preventive interventions. Why individual with dual decline are at such a high risk of developing dementia and whether they progress to dementia through distinct mechanisms are not yet known and deserve further investigation.
eFigure. Kaplan-Meier Survival Curve Between Phenotypic Groups and Dementia Risk
eTable 1. Study Design and Detailed Assessment
eTable 2. Associations of Baseline Gait Speed and Baseline Memory Performance With Phenotypic Groups
eTable 3. Associations of Phenotypic Groups With Dementia Risk After Adjustment for Disease Conditions
eTable 4. Likelihood Ratio Estimates for Model Comparisons
eReferences.
References
- 1.Beauchet O, Annweiler C, Callisaya ML, et al. . Poor gait performance and prediction of dementia: results from a meta-analysis. J Am Med Dir Assoc. 2016;17(6):-. doi: 10.1016/j.jamda.2015.12.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valkanova V, Ebmeier KP. What can gait tell us about dementia? review of epidemiological and neuropsychological evidence. Gait Posture. 2017;53:215-223. doi: 10.1016/j.gaitpost.2017.01.024 [DOI] [PubMed] [Google Scholar]
- 3.Peel NM, Alapatt LJ, Jones LV, Hubbard RE. The association between gait speed and cognitive status in community-dwelling older people: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. 2019;74(6):943-948. doi: 10.1093/gerona/gly140 [DOI] [PubMed] [Google Scholar]
- 4.Dumurgier J, Artaud F, Touraine C, et al. . Gait speed and decline in gait speed as predictors of incident dementia. J Gerontol A Biol Sci Med Sci. 2017;72(5):655-661. [DOI] [PubMed] [Google Scholar]
- 5.Kueper JK, Speechley M, Lingum NR, Montero-Odasso M. Motor function and incident dementia: a systematic review and meta-analysis. Age Ageing. 2017;46(5):729-738. doi: 10.1093/ageing/afx084 [DOI] [PubMed] [Google Scholar]
- 6.Quan M, Xun P, Chen C, et al. . Walking pace and the risk of cognitive decline and dementia in elderly populations: a meta-analysis of prospective cohort studies. J Gerontol A Biol Sci Med Sci. 2017;72(2):266-270. doi: 10.1093/gerona/glw121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosso AL, Sanders JL, Arnold AM, et al. . Multisystem physiologic impairments and changes in gait speed of older adults. J Gerontol A Biol Sci Med Sci. 2015;70(3):319-324. doi: 10.1093/gerona/glu176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci. 2014;69(11):1375-1388. doi: 10.1093/gerona/glu052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doi T, Makizako H, Tsutsumimoto K, et al. . Combined effects of mild cognitive impairment and slow gait on risk of dementia. Exp Gerontol. 2018;110:146-150. doi: 10.1016/j.exger.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 10.Montero-Odasso M, Speechley M, Muir-Hunter SW, et al. ; Canadian Gait and Cognition Network . Motor and cognitive trajectories before dementia: results from Gait and Brain Study. J Am Geriatr Soc. 2018;66(9):1676-1683. doi: 10.1111/jgs.15341 [DOI] [PubMed] [Google Scholar]
- 11.Doi T, Shimada H, Makizako H, Tsutsumimoto K, Verghese J, Suzuki T. Motoric cognitive risk syndrome: association with incident dementia and disability. J Alzheimers Dis. 2017;59(1):77-84. doi: 10.3233/JAD-170195 [DOI] [PubMed] [Google Scholar]
- 12.Verghese J, Annweiler C, Ayers E, et al. . Motoric cognitive risk syndrome: multicountry prevalence and dementia risk. Neurology. 2014;83(8):718-726. doi: 10.1212/WNL.0000000000000717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci. 2013;68(4):412-418. doi: 10.1093/gerona/gls191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chhetri JK, Chan P, Vellas B, Cesari M. Motoric cognitive risk syndrome: predictor of dementia and age-related negative outcomes. Front Med (Lausanne). 2017;4:166. doi: 10.3389/fmed.2017.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shock NW, Greulich RC, Andres R, et al. Normal human aging: the Baltimore Longitudinal Study of Aging. National Institutes of Health publication No. 84-2450. Washington, DC: US Government Printing Office; 1984.
- 16.Newman AB, Haggerty CL, Goodpaster B, et al. ; Health, Aging And Body Composition Research Group . Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51(3):323-330. doi: 10.1046/j.1532-5415.2003.51105.x [DOI] [PubMed] [Google Scholar]
- 17.Roberts RO, Geda YE, Knopman DS, et al. . The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58-69. doi: 10.1159/000115751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris TB, Launer LJ, Eiriksdottir G, et al. . Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165(9):1076-1087. doi: 10.1093/aje/kwk115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrucci L, Bandinelli S, Benvenuti E, et al. . Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48(12):1618-1625. doi: 10.1111/j.1532-5415.2000.tb03873.x [DOI] [PubMed] [Google Scholar]
- 20.Lagergren M, Fratiglioni L, Hallberg IR, et al. . A longitudinal study integrating population, care and social services data: the Swedish National study on Aging and Care (SNAC). Aging Clin Exp Res. 2004;16(2):158-168. doi: 10.1007/BF03324546 [DOI] [PubMed] [Google Scholar]
- 21.Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility—giving mobility clinical visibility: a Mobility Working Group recommendation. JAMA. 2014;311(20):2061-2062. doi: 10.1001/jama.2014.3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tombaugh TN, McIntyre NJ. The Mini-Mental State Examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922-935. doi: 10.1111/j.1532-5415.1992.tb01992.x [DOI] [PubMed] [Google Scholar]
- 23.Espeland MA, Rapp SR, Shumaker SA, et al. ; Women’s Health Initiative Memory Study . Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291(24):2959-2968. doi: 10.1001/jama.291.24.2959 [DOI] [PubMed] [Google Scholar]
- 24.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Kokmen E, Tangelos EG. Aging, memory, and mild cognitive impairment. Int Psychogeriatr. 1997;9(suppl 1):65-69. doi: 10.1017/S1041610297004717 [DOI] [PubMed] [Google Scholar]
- 25.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183-194. doi: 10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- 26.Lopez OL, Becker JT, Jagust WJ, et al. . Neuropsychological characteristics of mild cognitive impairment subgroups. J Neurol Neurosurg Psychiatry. 2006;77(2):159-165. doi: 10.1136/jnnp.2004.045567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Studenski S, Perera S, Patel K, et al. . Gait speed and survival in older adults. JAMA. 2011;305(1):50-58. doi: 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Best JR, Liu-Ambrose T, Metti AL, et al. ; Health, Aging and Body Composition Study . Longitudinal associations between walking speed and amount of self-reported time spent walking over a 9-year period in older women and men. J Gerontol A Biol Sci Med Sci. 2018;73(9):1265-1271. doi: 10.1093/gerona/glx129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mielke MM, Roberts RO, Savica R, et al. . Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2013;68(8):929-937. doi: 10.1093/gerona/gls256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bilgel M, An Y, Lang A, et al. . Trajectories of Alzheimer disease-related cognitive measures in a longitudinal sample. Alzheimers Dement. 2014;10(6):735-742.e4. doi: 10.1016/j.jalz.2014.04.520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. Research ed New York, NY: Psychological Corporation; 1987. [Google Scholar]
- 32.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24(11):1019-1025. doi: 10.1212/WNL.24.11.1019 [DOI] [PubMed] [Google Scholar]
- 33.Rey A. Auditory-Verbal Learning Test (AVLT). Paris, France: Press Universitaire de France; 1964. [Google Scholar]
- 34.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 35.Laukka EJ, Lövdén M, Herlitz A, et al. . Genetic effects on old-age cognitive functioning: a population-based study. Psychol Aging. 2013;28(1):262-274. doi: 10.1037/a0030829 [DOI] [PubMed] [Google Scholar]
- 36.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 3rd ed Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 37.Hong CH, Falvey C, Harris TB, et al. . Anemia and risk of dementia in older adults: findings from the Health ABC Study. Neurology. 2013;81(6):528-533. doi: 10.1212/WNL.0b013e31829e701d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cherubini A, Martin A, Andres-Lacueva C, et al. . Vitamin E levels, cognitive impairment and dementia in older persons: the InCHIANTI study. Neurobiol Aging. 2005;26(7):987-994. doi: 10.1016/j.neurobiolaging.2004.09.002 [DOI] [PubMed] [Google Scholar]
- 39.Kwon S, Perera S, Pahor M, et al. . What is a meaningful change in physical performance? findings from a clinical trial in older adults (the LIFE-P study). J Nutr Health Aging. 2009;13(6):538-544. doi: 10.1007/s12603-009-0104-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cumpston M, Li T, Page MJ, et al. . Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stroup DF, Berlin JA, Morton SC, et al. . Meta-analysis of observational studies in epidemiology: a proposal for reporting: Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 42.Morris R, Lord S, Bunce J, Burn D, Rochester L. Gait and cognition: mapping the global and discrete relationships in ageing and neurodegenerative disease. Neurosci Biobehav Rev. 2016;64:326-345. doi: 10.1016/j.neubiorev.2016.02.012 [DOI] [PubMed] [Google Scholar]
- 43.Rosano C, Snitz BE. Predicting dementia from decline in gait speed: are we there yet? J Am Geriatr Soc. 2018;66(9):1659-1660. doi: 10.1111/jgs.15368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66(3):300-305. doi: 10.1001/archneurol.2009.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frisardi V, Solfrizzi V, Seripa D, et al. . Metabolic-cognitive syndrome: a cross-talk between metabolic syndrome and Alzheimer’s disease. Ageing Res Rev. 2010;9(4):399-417. doi: 10.1016/j.arr.2010.04.007 [DOI] [PubMed] [Google Scholar]
- 46.Gorelick PB, Scuteri A, Black SE, et al. ; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia . Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672-2713. doi: 10.1161/STR.0b013e3182299496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grande G, Triolo F, Nuara A, Welmer AK, Fratiglioni L, Vetrano DL. Measuring gait speed to better identify prodromal dementia. Exp Gerontol. 2019;124:110625. doi: 10.1016/j.exger.2019.05.014 [DOI] [PubMed] [Google Scholar]
- 48.Hausdorff JM, Buchman AS. What links gait speed and MCI with dementia? a fresh look at the association between motor and cognitive function. J Gerontol A Biol Sci Med Sci. 2013;68(4):409-411. doi: 10.1093/gerona/glt002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson PT, Head E, Schmitt FA, et al. . Alzheimer’s disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 2011;121(5):571-587. doi: 10.1007/s00401-011-0826-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snyder HM, Corriveau RA, Craft S, et al. . Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement. 2015;11(6):710-717. doi: 10.1016/j.jalz.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neth BJ, Craft S. Insulin resistance and Alzheimer’s disease: bioenergetic linkages. Front Aging Neurosci. 2017;9:345. doi: 10.3389/fnagi.2017.00345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Penninx BW, Nicklas BJ, Newman AB, et al. ; Health ABC Study . Metabolic syndrome and physical decline in older persons: results from the Health, Aging And Body Composition Study. J Gerontol A Biol Sci Med Sci. 2009;64(1):96-102. doi: 10.1093/gerona/gln005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yin F, Sancheti H, Patil I, Cadenas E. Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic Biol Med. 2016;100:108-122. doi: 10.1016/j.freeradbiomed.2016.04.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cadonic C, Sabbir MG, Albensi BC. Mechanisms of mitochondrial dysfunction in Alzheimer’s disease. Mol Neurobiol. 2016;53(9):6078-6090. doi: 10.1007/s12035-015-9515-5 [DOI] [PubMed] [Google Scholar]
- 55.Love S, Miners JS. Cerebrovascular disease in ageing and Alzheimer’s disease. Acta Neuropathol. 2016;131(5):645-658. doi: 10.1007/s00401-015-1522-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Kaplan-Meier Survival Curve Between Phenotypic Groups and Dementia Risk
eTable 1. Study Design and Detailed Assessment
eTable 2. Associations of Baseline Gait Speed and Baseline Memory Performance With Phenotypic Groups
eTable 3. Associations of Phenotypic Groups With Dementia Risk After Adjustment for Disease Conditions
eTable 4. Likelihood Ratio Estimates for Model Comparisons
eReferences.