Abstract
SOX4 is an essential developmental transcription factor that regulates stemness, differentiation, progenitor development, and multiple developmental pathways including PI3K, Wnt, and TGFβ signaling. The SOX4 gene is frequently amplified and overexpressed in over 20 types of malignancies, and multiple lines of evidence support that notion that SOX4 is an oncogene. Its overexpression is due to both gene amplification and to activation of PI3K, Wnt, and TGFβ pathways that SOX4 regulates. SOX4 interacts with multiple other transcription factors, rendering many of its impacts on gene expression context and tissue-specific. Nevertheless, there are common themes that run through many of the effects of SOX4 hyperactivity, such as the promotion of cell survival, stemness, the epithelial to mesenchymal transition, migration, and metastasis. Specific targeting of SOX4 remains a challenge for future cancer research and drug development.
Keywords: SOX4, Cancer, Metastasis, EMT, Wnt, TGFβ, PI3K
SOX4 in normal development
The SRY-related High Mobility Group (HMG) box or SOX family of transcription factors plays numerous roles in normal development. The founding member of the family, SRY, is located on the Y-chromosome, and is essential for sex fate determination and male sexual development [1-3]. There are 20 members of the SOX family, which are found in all vertebrates, and the family is subdivided into nine subgroups ranging from SOXA to SOXH [4]. SOX family transcription factors play critical developmental roles in most organs and tissues in the endoderm, ectoderm, mesoderm, and germline, including the central nervous system, the retina, bone, cartilage, heart, pancreas, hematopoietic system, vasculature, and lymphatic system (reviewed in [4, 5]). A number of developmental genetic syndromes, or ‘SOXopathies,’ have been described [6] that include effects on the muscular system, central and peripheral nervous systems, cardiovascular system, auditory and ocular systems, reproductive system, hair and skin, kidney, and bone. Many of these syndromes are highly sensitive to genetic dosage and result from haploinsufficiency of SOX proteins [6]. SOX proteins are also key regulators of cell fate and stemness [7-9]. SOX2, in particular, has been shown to be essential for generation of induced pluripotent stem cells [8, 9], and alterations in expression levels of SOX2 can impact proper differentiation of multiple tissues including Schwann cells [10] in the CNS and the foregut endoderm [11, 12]. The two SOX factors most extensively studied in relation to cancer are SOX9 [13-15] and SOX4 [16-20], although many other SOX proteins, including SOX2 [21], SOX7 [22, 23], and SOX17 [24] have also been associated with various cancers (reviewed in [25]).
SOX4 is an essential developmental transcription factor that regulates stemness, differentiation, progenitor development, and multiple developmental pathways [24, 26]. The SOX4 protein is encoded by a single-exon gene and contains a highly conserved high-mobility group (HMG) DNA binding domain related to the TCF/LEF family of transcription factors that play important roles in the Wnt pathway. SOX4 is an essential gene, as embryonic total knockout of murine Sox4 is lethal due to cardiac developmental defects [27]. SOX4 null embryos also show impaired B lymphocyte development with a block at the pro-B cell stage [27]. SOX4 is expressed in stem cells [28], and modulates stem cell activation [29] and likely also plays a role in stem cell maintenance, possibly through activation of expression of SOX2 [30, 31]. Overexpression of Sox4 in the murine myeloid cell line 32Dcl3 markedly inhibited cytokine-induced granulocyte maturation, suggesting a differentiation block [32]. Moreover, prolonged Sox4 expression in cells of the oligodendrocyte lineage in the central nervous system (CNS) inhibits development of a fully mature phenotype, suggesting that Sox4 may normally prevent premature terminal differentiation [33]. In the adult mouse hippocampus, expression of Sox4, and its close relative Sox11, is initiated around the time of neuronal commitment of adult neural stem cells (NSCs) and is maintained in immature neurons, and expression of Sox4 promotes and is necessary for in vitro neurogenesis from adult NSCs [34]. Sox4 functions as a pro-survival factor during spinal cord development [35], and ensures the survival of tyrosine hydroxylase-expressing cells in the developing sympathetic nervous system [36]. Thus, the normal function of SOX4 appears to be to promote early differentiation and expansion and survival of transit amplifying progenitor cells, while inhibiting terminal differentiation, which are functions highly compatible with the development of malignancies.
Amplification and overexpression of SOX4 in cancer
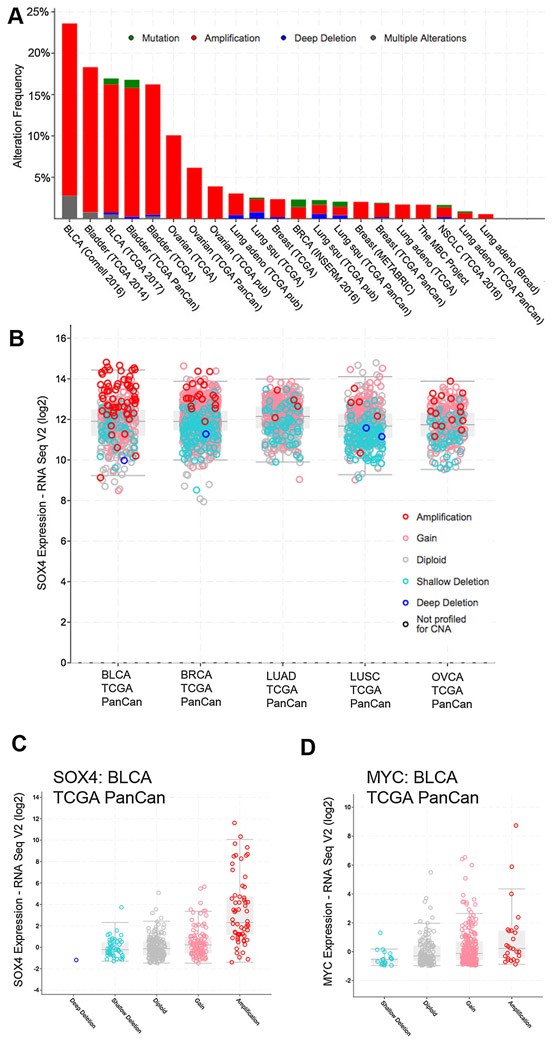
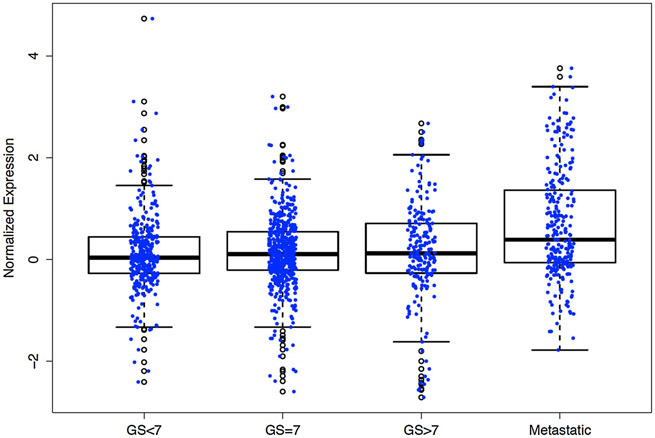
Although SOX4 is rarely mutated, the SOX4 gene, located on chromosome 6p22.3 is amplified in 1-3% of lung cancers [37], 10% of ovarian cancers, 16-24% of bladder cancers (BLCA), and roughly 10% of triple-negative breast cancers (TNBC) [38-40] (Figure 1A), strongly supporting its role as an oncogene. Much like the MYC oncogene, SOX4 expression is increased in cancers with SOX4 amplification (Figure 1B-D). While amplification does not always result in increased expression, in general, mRNA expression in amplified cases is higher than in diploid cases [41]. In lung cancer, SOX4 is overexpressed due to gene amplification and SOX4 exhibits functional oncogenic properties by significantly increasing the transforming ability of the weakly oncogenic RHOA-Q63L mutation [37]. The SOX4 6p22.3 amplification is the most significant focal amplification in BLCA [42-44]. Moreover, cBioPortal analysis of public datasets indicates that SOX4 is amplified or overexpressed in 41% of metastatic PCa [45, 46], although SOX4 amplification is less common in studies of primary localized prostate cancer (e.g. TCGA). SOX4 mRNA is overexpressed in many types of human cancers, including leukemias [47], melanomas [48], glioblastomas [49], medulloblastomas [50], and cancers of the prostate [51], bladder [52], lung [53], and breast [54, 55]. When comparing cancer to normal tissues in the Oncomine database [56], SOX4 is overexpressed in 107 (23%) of 462 unique studies in over 20 types of cancer (Table 1), whereas MYC and SOX9 are each upregulated in only 41 (9%) of these studies. In prostate cancer, upregulation of SOX4 mRNA and protein is correlated with Gleason score or tumor grade [51]. This observation was confirmed in a meta-analysis of 1,321 human prostate cancer gene expression profiles [57] and is shown in Figure 2. SOX4 also plays an important role in myeloid leukemias by cooperating with Mef2c [58] and with CREB [59] in myeloid leukemogenesis. C/EBPα inactivation results in Sox4 overexpression, which contributes to the development of leukemia with a distinct acute myeloid leukemia (AML) leukemia-initiating cells (LICs) phenotype [60]. A meta-analysis examining the transcriptional profiles of over 3700 human cancers found SOX4 to be one of 64 genes uniquely upregulated as a general “Cancer Signature” [61], suggesting that it has a fundamental role in multiple tumor types. SOX4 expression is induced by many pathways that are commonly activated in cancers, including PI3K signaling [18], Wnt signaling [62], TGFβ signaling [63, 64], and deletion of the BMP1 receptor [65], which activates Wnt signaling and the PI3K-AKT pathway.
Figure 1:
(A) cBioPortal analysis of SOX4 amplification in Bladder, Ovarian, Lung, and Breast Cancers. Amplifications are shown in red, mutations in green, deletions in blue, and multiple alterations in grey. (B) cBioPortal analysis of SOX4 mRNA expression in TCGA PanCan data shows strong correlation of SOX4 mRNA with gene amplification. (C) cBioPortal analysis of SOX4 mRNA in BLCA supports association of SOX4 amplification with gene expression. (D) Similar cBioPortal analysis of MYC amplification and mRNA in BLCA.
Table 1:
Cancer vs. Normal studies in the Oncomine database showing significant SOX4 changes in mRNA expression. SOX4 is upregulated in 107 of 462 unique studies across over 20 types of malignancies. In contrast, MYC was found upregulated in only 41 studies, as was SOX9. The SOX factor SOX3, which has little association with cancer, was upregulated in only two studies, and downregulated in three (not shown).
| Cancer Type | # Studies with SOX4 Upregulated |
# Studies with SOX4 Downregulated |
# Studies with MYC Upregulated |
# Studies with MYC Downregulated |
# Studies with SOX9 Upregulated |
# Studies with SOX9 Downregulated |
|---|---|---|---|---|---|---|
| Bladder | 4 | - | - | - | 1 | - |
| Brain | 10 | - | 7 | 1 | 5 | - |
| Breast | 1 | - | 1 | 10 | - | |
| Cervical | 4 | - | - | - | - | - |
| Colorectal | 13 | - | 20 | - | 17 | - |
| Esophageal | 6 | - | - | - | 2 | - |
| Gastric | 2 | - | - | - | 1 | - |
| Head & Neck | 8 | - | 2 | - | - | - |
| Kidney | 2 | 1 | 2 | - | 2 | - |
| Leukemia | 15 | 5 | - | 3 | - | - |
| Liver | 6 | - | - | 1 | 6 | - |
| Lung | 9 | - | - | - | 1 | - |
| Lymphoma | 1 | 2 | 2 | 2 | - | 1 |
| Melanoma | 1 | - | - | - | - | 1 |
| Myeloma | - | 1 | 1 | - | - | - |
| Other | 9 | - | 1 | 1 | - | 5 |
| Ovarian | - | - | - | - | 4 | - |
| Pancreatic | 3 | - | - | - | - | - |
| Prostate | 6 | - | 5 | - | - | - |
| Sarcoma | 8 | - | - | 4 | 2 | 1 |
| Significant Unique Analyses | 107 | 9 | 41 | 22 | 41 | 8 |
| Total Unique | 462 | 470 | 437 | |||
Figure 2:
Normalized SOX4 expression across 1,321 prostate cancer samples. Indolent prostate cancers have gleason scores (GS) < 7, while aggressive prostate cancers have GS ≥ 7. SOX4 expression is sharply increased in prostate cancer metastases.
SOX4 in cancer progression and PI3K signaling
Our laboratory was the first to demonstrate that SOX4 can act as an oncogene in human prostate cancer, showing that SOX4 expression was essential for anchorage independent growth of immortalized RWPE-1 prostate epithelial cells [51]. Moreover, conditional deletion of Sox4 in adult stem cells of stratified epithelia results in increased skin stem cell quiescence and resistance to chemical carcinogenesis [29], suggesting a role of SOX4 in development and initiation of cancers. Our lab has also shown that tissue-specific homozygous deletion of Sox4 in the adult murine prostate epithelium strongly inhibits tumor progression initiated by homozygous loss of the Pten tumor suppressor gene [18]. Mechanistically, Sox4 ablation reduced activation of both AKT and β-catenin, leading to an attenuated invasive phenotype, and arresting cancer progression at the high-grade prostatic intraepithelial neoplasia (HGPIN) precancerous stage [18]. Furthermore, in the mouse prostate, SOX4 expression was induced by Pten loss as a result of the activation of PI3K-AKT-mTOR signaling, suggesting a positive feedback loop between SOX4 and PI3K-AKT-mTOR activity [18]. In acute lymphoblastic leukemia (ALL), mouse genetic studies have also demonstrated that Sox4 is a critical activator of PI3K/AKT and MAPK signaling in ALL cells [66]. Also, in silico analyses of breast cancer patients followed by in vitro confirmation using RNAi in breast cancer cells have also shown that SOX4 amplification promotes PI3K/AKT signaling [67]. Recently, several studies have identified PTEN as not only a critical tumor suppressor gene, but also as a metastasis-suppressor gene [68, 69], which is consistent with many of the functional roles of SOX4 in cancer.
SOX4 protein-protein interactions and target genes
SOX4 has been shown to have many protein interaction partners and a wide variety of target genes. Transcription factors that have been shown to interact with SOX4 include p53 [70], β-catenin [24], plakoglobin [62], TCF4 [24], KLF5 [71], SMAD3 [72], ERG [73], EVI1 [32], and NSD3 [74]. In hepatocellular carcinoma, SOX4 modulates the transcriptional activity of p53 and leads to the inhibition of p53-mediated apoptosis [75], including significant repression of p53-induced Bax expression and subsequent repression of p53-mediated apoptosis induced by gamma-irradiation [76]. On the other hand, in pancreatic cancer, Klf5 cooperates with Sox4 in oncogenesis and prevents Sox4-induced apoptosis [71], suggesting context-dependent functions of Sox4 that are tissue-specific. As another example of context specific function, in colon cancer cells, Sox4 may function to stabilize β-catenin protein [24], whereas when prostate and breast cancer cells are stimulated with WNT3A, the interaction between SOX4 and plakoglobin is significantly increased and the activity of β-catenin reporter constructs is reduced [62]. Recently, it has been shown by ChIP-seq that SOX4 and SMAD3 co-occupy a large number of genomic loci [72], and that SOX4 expression was required for TGFβ-mediated induction of a subset of SMAD3/SOX4-co-bound genes that regulate migration and extracellular matrix-associated processes in claudinlow MDA-MB-231 breast cancer cells [72]. Protein-protein interactions screens have also identified NSD3 (WHSC1L1), DACH1, CDKN2A, and AURKA as protein-protein interaction partners with SOX4, although the functional significance of these interactions have yet to be demonstrated [74].
Regarding downstream targets of SOX4, a number of studies have identified genes regulated by SOX4 [20, 71, 72, 77, 78], but there is relatively little overlap between them, which is consistent with the variety of protein partners and context-specific nature of SOX4 transcriptional activation activity. Tenascin C (TNC) is an extracellular matrix protein associated with TGFβ signaling and migration that has been identified as a SOX4 target in five separate studies [16, 71, 72, 77, 79]. Another direct transcriptional target of SOX4 in breast cancer cells is the transmembrane protein TMEM2 [80], which mediates pro-migratory and pro-invasive phenotypes. Sox4 also directly regulates the expression of Ezh2 [17], the catalytic subunit of the polycomb repressive complex that mediates tri-methylation of H3K27 residues in repressive chromatin. We identified 282 high-confidence direct SOX4 target genes in prostate cancer cells, including DICER, EGFR, FOXA1, NKX3-1, and many regulators of pivotal cancer signaling networks of differentiation, cell survival, and apoptosis including the Wnt (e.g. FZD4, FZD5, FZD8) and PI3K pathways (e.g. PIK3R1, PIK3R4) [77]. These results are supported by another recent study in mouse models of leukemia showing that SOX4 regulates expression of Pik3r2, Pik3r3, and Mtor [66]. In SOX4-amplified lung cancer cells, SOX4 regulates genes involved in neuronal development such as PCDHB, MYB, RBP1, and TEAD2 [78], whereas in endothelial cells, SOX4 directly regulates endothelin-1 (ET-1) expression, potentially promoting tumor-induced angiogenesis [20].
SOX4 in Wnt signaling
As noted above, SOX4 is important for Wnt signaling [24, 26, 81], and interacts directly with β-catenin [24, 62, 77, 82], although there are clearly SOX4-independent aspects of the Wnt pathway that are mediated by TCF/LEF factors. While the precise role of SOX4 in the Wnt pathway is still unclear, SOX4 can interact directly with β-catenin in a cooperative way to activate gene expression [24, 77]. Induction of β-catenin/TCF activity by Sox4 is caused by stabilization of the β-catenin protein, not by induction of β-catenin transcription [82]. We recently demonstrated that combined inhibition of Wnt signaling and SOX4 inhibits proliferation and migration and induces apoptosis of triple negative BT-549 breast cancer cell lines [83]. This same Wnt inhibitor (iCRT3) was also shown to interfere with androgen receptor (AR) activity and to inhibit growth of prostate cancer xenografts [84]. Several lines of evidence suggest that Wnt signaling is important for the progression and metastasis of castration-resistant prostate cancer (CRPC) based on numerous studies [85-97]. Clinical studies indicate that at least a subset of patients with CRPC exhibit activation of Wnt signaling [86, 88-90, 92, 94, 97]. Our data [18] show that genetic deletion of Sox4 reduces levels of active β-catenin in vivo in prostate cancers, but the mechanisms of how Sox4 loss inhibits β-catenin activation remains to be determined. One potential mechanism is that SOX4 stimulates β-catenin activity indirectly via maintenance of active AKT. It is well established that there is crosstalk between the PI3K-AKT and Wnt-β-catenin pathways via AKT phosphorylation and inhibition of GSK3β [98-100]. Moreover, PI3K-AKT and β-catenin can cooperate to stimulate AR signaling in CRPC [101-103], and β-catenin can interact directly with AR [104, 105]. Thus, SOX4 may play an important role at the intersection of PI3K and Wnt signaling in metastatic prostate cancer.
SOX4, TGFβ, and the Epithelial to Mesenchymal Transition (EMT)
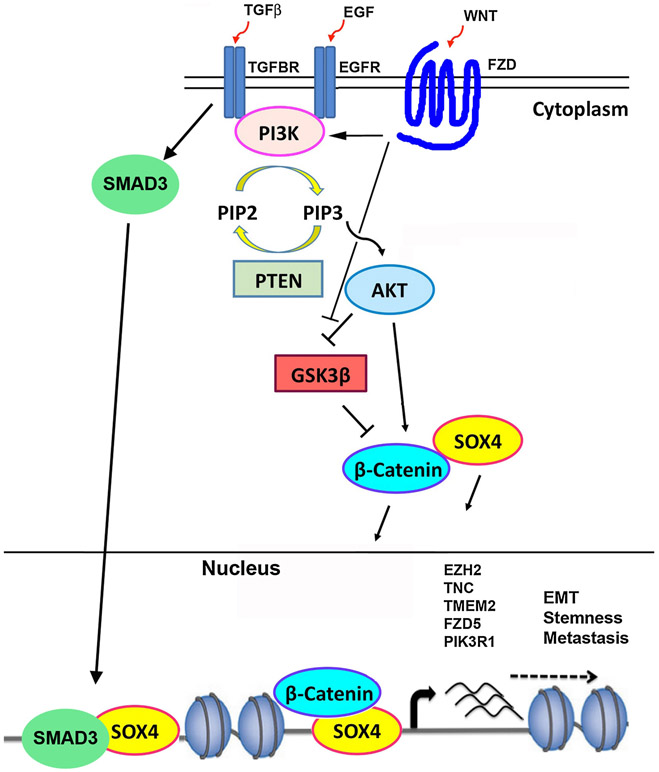
Several studies have indicated that SOX4 also plays a critical role in regulation of the Epithelial to Mesenchymal Transition, or EMT, which is a frequent (although not absolutely necessary [106]) step for metastasis of solid tumors. In breast cancers, Sox4 is essential for EMT and cell survival in vitro and for primary tumor growth and metastasis in vivo [17]. Moreover, Sox4 regulation of Ezh2 is critical to Sox4 control of EMT in this model [17]. Other groups have also shown that SOX4 overexpression induces EMT in breast cancer cells [107], which in turn induces stem cell markers and enhances formation of mammospheres [108]. SOX4 positively regulates expression of known EMT inducers, and plays an important function in mediating activation of the TGFβ pathway to contribute to EMT [72, 107]. Several studies have also shown that SOX4 itself is induced by TGFβ [63, 64, 107]. SOX4’s role in EMT has also been demonstrated in prostate cancer cells, where ERG and SOX4 have cooperative roles in TGFβ1-induced EMT [73] and SOX4 inhibition reversed EMT [109]. A model of the role of SOX4 in EMT and cancer is depicted in Figure 3. Consistent with this model, nuclear expression of SOX4 has been shown to be associated with depth of invasion, metastasis, stage, and poor disease free survival in colon cancer patients [110]. In fact, a meta-analysis of 1348 cancer patients found that SOX4 overexpression is a poor prognostic factor for human cancers including colon and gastric cancers [111].
Figure 3:
Model of SOX4 role in cancer. TGFβ and Wnt activate PI3K/AKT to induce and stabilize SOX4 protein. SOX4 recruits β-catenin and interacts with SMAD3 at promoters of genes critical for EMT.
SOX4 and Metastasis
SOX4 knockdown has been shown to reduce migration of cancer cells in many studies [16, 72, 73, 83, 107, 112]. In addition to regulation of EMT and migration, SOX4 is critical for metastasis, since shRNA knockdown of SOX4 inhibits lung metastases of breast cancer xenografts [16]. The microRNA miR-335 also suppresses lung metastasis and migration of breast cancers through repression of SOX4 and TNC [16]. In hepatocellular carcinoma (HCC) tumor metastasis, RNAi knockdown of SOX4 reduces tumor cell migration, invasion, in vivo tumorigenesis and metastasis [112]. It is interesting that SOX4 is most frequently amplified in bladder cancer, which is defined by its ability to invade one of the most impenetrable barriers in the body, the basement membrane and stromal muscle of the bladder. Thus, it is clear that SOX4 is critical for several aspects of aggressive cancer progression including EMT, migration, and metastasis, making SOX4 a potential drug target for advanced disease.
Targeting SOX4 in cancer
While the literature strongly supports the notion that SOX4 is an oncogene that promotes stemness, cancer cell survival, angiogenesis, migration, EMT, and metastasis, targeting of SOX4 remains a challenge. Because SOX4 is relatively unstructured outside of its HMG DNA binding domain (DBD) [113], it is difficult to generate crystal structures that could lead to rational drug targeting. Moreover, the SOX4 DBD is highly homologous to other SOX family transcription factors, thus making target specificity an issue for any small compound that targets the SOX4 DBD. However, the FDA has recently approved the first siRNA treatment for the treatment of peripheral nerve diseases [114], and thus it is conceivable that with proper, targeted delivery to cancer cells, siRNA targeting of SOX4 could be effective. However, the fact that SOX4 regulates DICER and AGO1 [77], at least in prostate cancer cells, could make the siRNA approach challenging. Furthermore, even successful targeting of SOX4 could have undesirable side effects such as immunosuppression due to inhibition of proliferation of B lymphocytes.
Another approach to targeting SOX4 could be to identify compounds that disrupt SOX4 protein-protein interactions (PPIs). Such compounds might have more specificity than drugs that target SOX4-DNA interactions and could even target only a subset of the many functions that SOX4 plays within the cell, or that are important only in specific cell types of interest. Since SOX4 plays a critical role in such a wide variety of malignancies, high-throughput screening of compounds that disrupt SOX4 PPIs could ultimately lead to therapies that target many aggressive forms of cancer.
Acknowledgements
This work was supported in part by NIH grant U01CA217875.
Grant support: This work was supported in part by NIH grant U01CA217875.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Berta P, Hawkins JR, Sinclair AH, Taylor A, Griffiths BL, Goodfellow PN, Fellous M, Genetic evidence equating SRY and the testis-determining factor, Nature 348(6300) (1990) 448–50. [DOI] [PubMed] [Google Scholar]
- [2].Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN, A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif, Nature 346(6281) (1990) 240–4. [DOI] [PubMed] [Google Scholar]
- [3].Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Munsterberg A, Vivian N, Goodfellow P, Lovell-Badge R, A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes, Nature 346(6281) (1990) 245–50. [DOI] [PubMed] [Google Scholar]
- [4].She ZY, Yang WX, SOX family transcription factors involved in diverse cellular events during development, Eur J Cell Biol 94(12) (2015) 547–63. [DOI] [PubMed] [Google Scholar]
- [5].Sarkar A, Hochedlinger K, The sox family of transcription factors: versatile regulators of stem and progenitor cell fate, Cell Stem Cell 12(1) (2013) 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Angelozzi M, Lefebvre V, SOXopathies: Growing Family of Developmental Disorders Due to SOX Mutations, Trends Genet (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Julian LM, McDonald AC, Stanford WL, Direct reprogramming with SOX factors: masters of cell fate, Curr Opin Genet Dev 46 (2017) 24–36. [DOI] [PubMed] [Google Scholar]
- [8].Takahashi K, Yamanaka S, Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors, Cell 126(4) (2006) 663–76. [DOI] [PubMed] [Google Scholar]
- [9].Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA, Induced pluripotent stem cell lines derived from human somatic cells, Science 318(5858) (2007) 1917–20. [DOI] [PubMed] [Google Scholar]
- [10].Le N, Nagarajan R, Wang JY, Araki T, Schmidt RE, Milbrandt J, Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell differentiation and myelination, Proc Natl Acad Sci U S A 102(7) (2005) 2596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, Taranova O, Pevny LH, Hogan BL, Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm, Development 134(13) (2007) 2521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Que J, Luo X, Schwartz RJ, Hogan BL, Multiple roles for Sox2 in the developing and adult mouse trachea, Development 136(11) (2009) 1899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Thomsen MK, Ambroisine L, Wynn S, Cheah KS, Foster CS, Fisher G, Berney DM, Moller H, Reuter VE, Scardino P, Cuzick J, Ragavan N, Singh PB, Martin FL, Butler CM, Cooper CS, Swain A, G. Transatlantic Prostate, SOX9 elevation in the prostate promotes proliferation and cooperates with PTEN loss to drive tumor formation, Cancer Res 70(3) (2010) 979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Comprehensive molecular characterization of human colon and rectal cancer, Nature 487(7407) (2012) 330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Aguilar-Medina M, Avendano-Felix M, Lizarraga-Verdugo E, Bermudez M, Romero-Quintana JG, Ramos-Payan R, Ruiz-Garcia E, Lopez-Camarillo C, SOX9 Stem-Cell Factor: Clinical and Functional Relevance in Cancer, J Oncol 2019 (2019) 6754040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, Gerald WL, Massague J, Endogenous human microRNAs that suppress breast cancer metastasis, Nature 451(7175) (2008) 147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tiwari N, Tiwari VK, Waldmeier L, Balwierz PJ, Arnold P, Pachkov M, Meyer-Schaller N, Schubeler D, van Nimwegen E, Christofori G, Sox4 is a master regulator of epithelial-mesenchymal transition by controlling ezh2 expression and epigenetic reprogramming, Cancer Cell 23(6) (2013) 768–83. [DOI] [PubMed] [Google Scholar]
- [18].Bilir B, Osunkoya AO, Wiles W.G.t., Sannigrahi S, Lefebvre V, Metzger D, Spyropoulos DD, Martin WD, Moreno CS, SOX4 Is Essential for Prostate Tumorigenesis Initiated by PTEN Ablation, Cancer Res 76(5) (2016) 1112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lourenco AR, Coffer PJ, SOX4: Joining the Master Regulators of Epithelial-to-Mesenchymal Transition?, Trends Cancer 3(8) (2017) 571–582. [DOI] [PubMed] [Google Scholar]
- [20].Vervoort SJ, de Jong OG, Roukens MG, Frederiks CL, Vermeulen JF, Lourenco AR, Bella L, Vidakovic AT, Sandoval JL, Moelans C, van Amersfoort M, Dallman MJ, Bruna A, Caldas C, Nieuwenhuis E, van der Wall E, Derksen P, van Diest P, Verhaar MC, Lam EW, Mokry M, Coffer PJ, Global transcriptional analysis identifies a novel role for SOX4 in tumor-induced angiogenesis, Elife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gu W, Wang B, Wan F, Wu J, Lu X, Wang H, Zhu Y, Zhang H, Shi G, Dai B, Ye D, SOX2 and SOX12 are predictive of prognosis in patients with clear cell renal cell carcinoma, Oncol Lett 15(4) (2018) 4564–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Guo L, Zhong D, Lau S, Liu X, Dong XY, Sun X, Yang VW, Vertino PM, Moreno CS, Varma V, Dong JT, Zhou W, Sox7 Is an independent checkpoint for beta-catenin function in prostate and colon epithelial cells, Mol Cancer Res 6(9) (2008) 1421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhong WD, Qin GQ, Dai QS, Han ZD, Chen SM, Ling XH, Fu X, Cai C, Chen JH, Chen XB, Lin ZY, Deng YH, Wu SL, He HC, Wu CL, SOXs in human prostate cancer: implication as progression and prognosis factors, BMC Cancer 12 (2012) 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sinner D, Kordich JJ, Spence JR, Opoka R, Rankin S, Lin SC, Jonatan D, Zorn AM, Wells JM, Sox17 and Sox4 differentially regulate beta-catenin/T-cell factor activity and proliferation of colon carcinoma cells, Mol Cell Biol 27(22) (2007) 7802–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Grimm D, Bauer J, Wise P, Kruger M, Simonsen U, Wehland M, Infanger M, Corydon TJ, The role of SOX family members in solid tumours and metastasis, Semin Cancer Biol (2019). [DOI] [PubMed] [Google Scholar]
- [26].Cheung M, Abu-Elmagd M, Clevers H, Scotting PJ, Roles of Sox4 in central nervous system development, Brain Res Mol Brain Res 79(1–2) (2000) 180–91. [DOI] [PubMed] [Google Scholar]
- [27].Schilham MW, Oosterwegel MA, Moerer P, Ya J, de Boer PA, van de Wetering M, Verbeek S, Lamers WH, Kruisbeek AM, Cumano A, Clevers H, Defects in cardiac outflow tract formation and pro-B-lymphocyte expansion in mice lacking Sox-4, Nature 380(6576) (1996) 711–4. [DOI] [PubMed] [Google Scholar]
- [28].Fevr T, Robine S, Louvard D, Huelsken J, Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells, Mol Cell Biol 27(21) (2007) 7551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Foronda M, Martinez P, Schoeftner S, Gomez-Lopez G, Schneider R, Flores JM, Pisano DG, Blasco MA, Sox4 Links Tumor Suppression to Accelerated Aging in Mice by Modulating Stem Cell Activation, Cell Rep (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Peng X, Liu G, Peng H, Chen A, Zha L, Wang Z, SOX4 contributes to TGF-beta-induced epithelial-mesenchymal transition and stem cell characteristics of gastric cancer cells, Genes Dis 5(1) (2018) 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Weina K, Wu H, Knappe N, Orouji E, Novak D, Bernhardt M, Huser L, Larribere L, Umansky V, Gebhardt C, Utikal J, TGF-beta induces SOX2 expression in a time-dependent manner in human melanoma cells, Pigment Cell Melanoma Res 29(4) (2016) 453–8. [DOI] [PubMed] [Google Scholar]
- [32].Boyd KE, Xiao YY, Fan K, Poholek A, Copeland NG, Jenkins NA, Perkins AS, Sox4 cooperates with Evi1 in AKXD-23 myeloid tumors via transactivation of proviral LTR, Blood 107(2) (2006) 733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Potzner MR, Griffel C, Lutjen-Drecoll E, Bosl MR, Wegner M, Sock E, Prolonged Sox4 expression in oligodendrocytes interferes with normal myelination in the central nervous system, Mol Cell Biol 27(15) (2007) 5316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mu L, Berti L, Masserdotti G, Covic M, Michaelidis TM, Doberauer K, Merz K, Rehfeld F, Haslinger A, Wegner M, Sock E, Lefebvre V, Couillard-Despres S, Aigner L, Berninger B, Lie DC, SoxC transcription factors are required for neuronal differentiation in adult hippocampal neurogenesis, J Neurosci 32(9) (2012) 3067–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Thein DC, Thalhammer JM, Hartwig AC, Crenshaw EB 3rd, Lefebvre V, Wegner M, Sock E, The closely related transcription factors Sox4 and Sox11 function as survival factors during spinal cord development, J Neurochem 115(1) (2010) 131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Potzner MR, Tsarovina K, Binder E, Penzo-Mendez A, Lefebvre V, Rohrer H, Wegner M, Sock E, Sequential requirement of Sox4 and Sox11 during development of the sympathetic nervous system, Development 137(5) (2010) 775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Medina PP, Castillo SD, Blanco S, Sanz-Garcia M, Largo C, Alvarez S, Yokota J, Gonzalez-Neira A, Benitez J, Clevers HC, Cigudosa JC, Lazo PA, Sanchez-Cespedes M, The SRY-HMG box gene, SOX4, is a target of gene amplification at chromosome 6p in lung cancer, Hum Mol Genet 18(7) (2009) 1343–52. [DOI] [PubMed] [Google Scholar]
- [38].N. Cancer Genome Atlas Research, Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA, Ellrott K, Shmulevich I, Sander C, Stuart JM, The Cancer Genome Atlas Pan-Cancer analysis project, Nat Genet 45(10) (2013) 1113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, Leiserson MD, Miller CA, Welch JS, Walter MJ, Wendl MC, Ley TJ, Wilson RK, Raphael BJ, Ding L, Mutational landscape and significance across 12 major cancer types, Nature 502(7471) (2013) 333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zack TI, Schumacher SE, Carter SL, Cherniack AD, Saksena G, Tabak B, Lawrence MS, Zhang CZ, Wala J, Mermel CH, Sougnez C, Gabriel SB, Hernandez B, Shen H, Laird PW, Getz G, Meyerson M, Beroukhim R, Pan-cancer patterns of somatic copy number alteration, Nat Genet 45(10) (2013) 1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Blancato J, Singh B, Liu A, Liao DJ, Dickson RB, Correlation of amplification and overexpression of the c-myc oncogene in high-grade breast cancer: FISH, in situ hybridisation and immunohistochemical analyses, Br J Cancer 90(8) (2004) 1612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ciriello G, Miller ML, Aksoy BA, Senbabaoglu Y, Schultz N, Sander C, Emerging landscape of oncogenic signatures across human cancers, Nat Genet 45(10) (2013) 1127–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Guo G, Sun X, Chen C, Wu S, Huang P, Li Z, Dean M, Huang Y, Jia W, Zhou Q, Tang A, Yang Z, Li X, Song P, Zhao X, Ye R, Zhang S, Lin Z, Qi M, Wan S, Xie L, Fan F, Nickerson ML, Zou X, Hu X, Xing L, Lv Z, Mei H, Gao S, Liang C, Gao Z, Lu J, Yu Y, Liu C, Li L, Fang X, Jiang Z, Yang J, Li C, Zhao X, Chen J, Zhang F, Lai Y, Lin Z, Zhou F, Chen H, Chan HC, Tsang S, Theodorescu D, Li Y, Zhang X, Wang J, Yang H, Gui Y, Wang J, Cai Z, Whole-genome and whole-exome sequencing of bladder cancer identifies frequent alterations in genes involved in sister chromatid cohesion and segregation, Nat Genet 45(12) (2013) 1459–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].N. Cancer Genome Atlas Research, Comprehensive molecular characterization of urothelial bladder carcinoma, Nature 507(7492) (2014) 315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL, Integrative genomic profiling of human prostate cancer, Cancer Cell 18(1) (2010) 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N, The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data, Cancer Discov 2(5) (2012) 401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Andersson A, Ritz C, Lindgren D, Eden P, Lassen C, Heldrup J, Olofsson T, Rade J, Fontes M, Porwit-Macdonald A, Behrendtz M, Hoglund M, Johansson B, Fioretos T, Microarray-based classification of a consecutive series of 121 childhood acute leukemias: prediction of leukemic and genetic subtype as well as of minimal residual disease status, Leukemia 21(6) (2007) 1198–203. [DOI] [PubMed] [Google Scholar]
- [48].Talantov D, Mazumder A, Yu JX, Briggs T, Jiang Y, Backus J, Atkins D, Wang Y, Novel genes associated with malignant melanoma but not benign melanocytic lesions, Clin Cancer Res 11(20) (2005) 7234–42. [DOI] [PubMed] [Google Scholar]
- [49].Sun L, Hui AM, Su Q, Vortmeyer A, Kotliarov Y, Pastorino S, Passaniti A, Menon J, Walling J, Bailey R, Rosenblum M, Mikkelsen T, Fine HA, Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain, Cancer Cell 9(4) (2006) 287–300. [DOI] [PubMed] [Google Scholar]
- [50].Lee CJ, Appleby VJ, Orme AT, Chan WI, Scotting PJ, Differential expression of SOX4 and SOX11 in medulloblastoma, J Neurooncol 57(3) (2002) 201–14. [DOI] [PubMed] [Google Scholar]
- [51].Liu P, Ramachandran S, Ali Seyed M, Scharer CD, Laycock N, Dalton WB, Williams H, Karanam S, Datta MW, Jaye DL, Moreno CS, Sex-determining region Y box 4 is a transforming oncogene in human prostate cancer cells, Cancer Res 66(8) (2006) 4011–9. [DOI] [PubMed] [Google Scholar]
- [52].Aaboe M, Birkenkamp-Demtroder K, Wiuf C, Sorensen FB, Thykjaer T, Sauter G, Jensen KM, Dyrskjot L, Orntoft T, SOX4 expression in bladder carcinoma: clinical aspects and in vitro functional characterization, Cancer Res 66(7) (2006) 3434–42. [DOI] [PubMed] [Google Scholar]
- [53].Friedman R, Bangur C, Zasloff E, Fan L, Wang T, Watanabe Y, Kalos M, Molecular and Immunological Evaluation of the Transcription Factor SOX-4 as a Lung Tumor Vaccine Antigen, J Immunol 172(5) (2004) 3319–3327. [DOI] [PubMed] [Google Scholar]
- [54].Yu K, Ganesan K, Tan LK, Laban M, Wu J, Zhao XD, Li H, Leung CH, Zhu Y, Wei CL, Hooi SC, Miller L, Tan P, A precisely regulated gene expression cassette potently modulates metastasis and survival in multiple solid cancers, PLoS Genet 4(7) (2008) e1000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].TCGA-Network, Comprehensive molecular portraits of human breast tumours, Nature 490(7418) (2012) 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM, ONCOMINE: a cancer microarray database and integrated data-mining platform, Neoplasia 6(1) (2004) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].You S, Knudsen BS, Erho N, Alshalalfa M, Takhar M, Al-Deen Ashab H, Davicioni E, Karnes RJ, Klein EA, Den RB, Ross AE, Schaeffer EM, Garraway IP, Kim J, Freeman MR, Integrated Classification of Prostate Cancer Reveals a Novel Luminal Subtype with Poor Outcome, Cancer Res 76(17) (2016) 4948–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Du Y, Spence SE, Jenkins NA, Copeland NG, Cooperating cancer-gene identification through oncogenic-retrovirus-induced insertional mutagenesis, Blood 106(7) (2005) 2498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sandoval S, Kraus C, Cho EC, Cho M, Bies J, Manara E, Accordi B, Landaw EM, Wolff L, Pigazzi M, Sakamoto KM, Sox4 cooperates with CREB in myeloid transformation, Blood 120(1) (2012) 155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhang H, Alberich-Jorda M, Amabile G, Yang H, Staber PB, Di Ruscio A, Welner RS, Ebralidze A, Zhang J, Levantini E, Lefebvre V, Valk PJ, Delwel R, Hoogenkamp M, Nerlov C, Cammenga J, Saez B, Scadden DT, Bonifer C, Ye M, Tenen DG, Sox4 is a key oncogenic target in C/EBPalpha mutant acute myeloid leukemia, Cancer Cell 24(5) (2013) 575–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM, Large-scale meta-analysis of cancer microarray data identifies common transcriptional profiles of neoplastic transformation and progression, Proc Natl Acad Sci U S A 101(25) (2004) 9309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lai YH, Cheng J, Cheng D, Feasel ME, Beste KD, Peng J, Nusrat A, Moreno CS, SOX4 interacts with plakoglobin in a Wnt3a-dependent manner in prostate cancer cells, BMC Cell Biol 12 (2011) 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ruebel KH, Leontovich AA, Tanizaki Y, Jin L, Stilling GA, Zhang S, Coonse K, Scheithauer BW, Lombardero M, Kovacs K, Lloyd RV, Effects of TGFbeta1 on gene expression in the HP75 human pituitary tumor cell line identified by gene expression profiling, Endocrine 33(1) (2008) 62–76. [DOI] [PubMed] [Google Scholar]
- [64].Kuwahara M, Yamashita M, Shinoda K, Tofukuji S, Onodera A, Shinnakasu R, Motohashi S, Hosokawa H, Tumes D, Iwamura C, Lefebvre V, Nakayama T, The transcription factor Sox4 is a downstream target of signaling by the cytokine TGF-beta and suppresses T(H)2 differentiation, Nat Immunol 13(8) (2012) 778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kobielak K, Stokes N, de la Cruz J, Polak L, Fuchs E, Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling, Proc Natl Acad Sci U S A 104(24) (2007) 10063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ramezani-Rad P, Geng H, Hurtz C, Chan LN, Chen Z, Jumaa H, Melnick A, Paietta E, Carroll WL, Willman CL, Lefebvre V, Muschen M, SOX4 enables oncogenic survival signals in acute lymphoblastic leukemia, Blood 121(1) (2013) 148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mehta GA, Parker JS, Silva GO, Hoadley KA, Perou CM, Gatza ML, Amplification of SOX4 promotes PI3K/Akt signaling in human breast cancer, Breast Cancer Res Treat 162(3) (2017) 439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].McFadden DG, Papagiannakopoulos T, Taylor-Weiner A, Stewart C, Carter SL, Cibulskis K, Bhutkar A, McKenna A, Dooley A, Vernon A, Sougnez C, Malstrom S, Heimann M, Park J, Chen F, Farago AF, Dayton T, Shefler E, Gabriel S, Getz G, Jacks T, Genetic and clonal dissection of murine small cell lung carcinoma progression by genome sequencing, Cell 156(6) (2014) 1298–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Chen S, Sanjana NE, Zheng K, Shalem O, Lee K, Shi X, Scott DA, Song J, Pan JQ, Weissleder R, Lee H, Zhang F, Sharp PA, Genome-wide CRISPR screen in a mouse model of tumor growth and metastasis, Cell 160(6) (2015) 1246–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Pan X, Zhao J, Zhang WN, Li HY, Mu R, Zhou T, Zhang HY, Gong WL, Yu M, Man JH, Zhang PJ, Li AL, Zhang XM, Induction of SOX4 by DNA damage is critical for p53 stabilization and function, Proc Natl Acad Sci U S A 106(10) (2009) 3788–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].David CJ, Huang YH, Chen M, Su J, Zou Y, Bardeesy N, Iacobuzio-Donahue CA, Massague J, TGF-beta Tumor Suppression through a Lethal EMT, Cell 164(5) (2016) 1015–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Vervoort SJ, Lourenco AR, Tufegdzic Vidakovic A, Mocholi E, Sandoval JL, Rueda OM, Frederiks C, Pals C, Peeters JGC, Caldas C, Bruna A, Coffer PJ, SOX4 can redirect TGF-beta-mediated SMAD3-transcriptional output in a context-dependent manner to promote tumorigenesis, Nucleic Acids Res 46(18) (2018) 9578–9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wang L, Li Y, Yang X, Yuan H, Li X, Qi M, Chang YW, Wang C, Fu W, Yang M, Zhang J, Han B, ERG-SOX4 interaction promotes epithelial-mesenchymal transition in prostate cancer cells, Prostate 74(6) (2014) 647–58. [DOI] [PubMed] [Google Scholar]
- [74].Li Z, Ivanov AA, Su R, Gonzalez-Pecchi V, Qi Q, Liu S, Webber P, McMillan E, Rusnak L, Pham C, Chen X, Mo X, Revennaugh B, Zhou W, Marcus A, Harati S, Chen X, Johns MA, White MA, Moreno C, Cooper LA, Du Y, Khuri FR, Fu H, The OncoPPi network of cancer-focused protein-protein interactions to inform biological insights and therapeutic strategies, Nat Commun 8 (2017) 14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Yoon SK, Hur WH, Bae SH, Choi JY, Yang JM, Han NI, Kim CW, Lee CD, Jang JW, Choi SW, Lee YS, SOX4 Overexpression in Hepatocellular Carcinoma Inhibits P53 Transcriptional Activity Through Interaction with P53 Journal of Hepatology 48(Supplement 2) (2008) S169. [Google Scholar]
- [76].Hur W, Rhim H, Jung CK, Kim JD, Bae SH, Jang JW, Yang JM, Oh ST, Kim DG, Wang HJ, Lee SB, Yoon SK, SOX4 overexpression regulates the p53-mediated apoptosis in hepatocellular carcinoma: clinical implication and functional analysis in vitro, Carcinogenesis 31(7) (2010) 1298–307. [DOI] [PubMed] [Google Scholar]
- [77].Scharer CD, McCabe CD, Ali-Seyed M, Berger MF, Bulyk ML, Moreno CS, Genome-wide promoter analysis of the SOX4 transcriptional network in prostate cancer cells, Cancer Res 69(2) (2009) 709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Castillo SD, Matheu A, Mariani N, Carretero J, Lopez-Rios F, Lovell-Badge R, Sanchez-Cespedes M, Novel transcriptional targets of the SRY-HMG box transcription factor SOX4 link its expression to the development of small cell lung cancer, Cancer Res 72(1) (2012) 176–86. [DOI] [PubMed] [Google Scholar]
- [79].Moran JD, Kim HH, Li Z, Moreno CS, SOX4 regulates invasion of bladder cancer cells via repression of WNT5a, Int J Oncol (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lee H, Goodarzi H, Tavazoie SF, Alarcon CR, TMEM2 Is a SOX4-Regulated Gene That Mediates Metastatic Migration and Invasion in Breast Cancer, Cancer Res 76(17) (2016) 4994–5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Busslinger M, Transcriptional control of early B cell development, Annu Rev Immunol 22 (2004) 55–79. [DOI] [PubMed] [Google Scholar]
- [82].Lee AK, Ahn SG, Yoon JH, Kim SA, Sox4 stimulates beta-catenin activity through induction of CK2, Oncol Rep 25(2) (2011) 559–65. [DOI] [PubMed] [Google Scholar]
- [83].Bilir B, Kucuk O, Moreno CS, Wnt signaling blockage inhibits cell proliferation and migration, and induces apoptosis in triple-negative breast cancer cells, J Transl Med 11(1) (2013) 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lee E, Madar A, David G, Garabedian MJ, Dasgupta R, Logan SK, Inhibition of androgen receptor and beta-catenin activity in prostate cancer, Proc Natl Acad Sci U S A 110(39) (2013) 15710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Wu L, Zhao JC, Kim J, Jin HJ, Wang CY, Yu J, ERG is a critical regulator of Wnt/LEF1 signaling in prostate cancer, Cancer Res 73(19) (2013) 6068–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Jung SJ, Oh S, Lee GT, Chung J, Min K, Yoon J, Kim W, Ryu DS, Kim IY, Kang DI, Clinical Significance of Wnt/beta-Catenin Signalling and Androgen Receptor Expression in Prostate Cancer, World J Mens Health 31(1) (2013) 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Jiang Y, Dai J, Zhang H, Sottnik JL, Keller JM, Escott KJ, Sanganee HJ, Yao Z, McCauley LK, Keller ET, Activation of the Wnt pathway through AR79, a GSK3beta inhibitor, promotes prostate cancer growth in soft tissue and bone, Mol Cancer Res 11(12) (2013) 1597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lee SH, Luong R, Johnson DT, Cunha GR, Rivina L, Gonzalgo ML, Sun Z, Androgen signaling is a confounding factor for beta-catenin-mediated prostate tumorigenesis, Oncogene (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Yokoyama NN, Shao S, Hoang BH, Mercola D, Zi X, Wnt signaling in castration-resistant prostate cancer: implications for therapy, Am J Clin Exp Urol 2(1) (2014) 27–44. [PMC free article] [PubMed] [Google Scholar]
- [90].Rajan P, Sudbery IM, Villasevil ME, Mui E, Fleming J, Davis M, Ahmad I, Edwards J, Sansom OJ, Sims D, Ponting CP, Heger A, McMenemin RM, Pedley ID, Leung HY, Nextgeneration sequencing of advanced prostate cancer treated with androgen-deprivation therapy, Eur Urol 66(1) (2014) 32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Lee GT, Kang DI, Ha YS, Jung YS, Chung J, Min K, Kim TH, Moon KH, Chung JM, Lee DH, Kim WJ, Kim IY, Prostate cancer bone metastases acquire resistance to androgen deprivation via WNT5A-mediated BMP-6 induction, Br J Cancer 110(6) (2014) 1634–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Barbieri CE, Rubin MA, Molecular characterization of prostate cancer following androgen deprivation: the devil in the details, Eur Urol 66(1) (2014) 40–1. [DOI] [PubMed] [Google Scholar]
- [93].Wan X, Liu J, Lu JF, Tzelepi V, Yang J, Starbuck MW, Diao L, Wang J, Efstathiou E, Vazquez ES, Troncoso P, Maity SN, Navone NM, Activation of beta-catenin signaling in androgen receptor-negative prostate cancer cells, Clin Cancer Res 18(3) (2012) 726–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kypta RM, Waxman J, Wnt/beta-catenin signalling in prostate cancer, Nat Rev Urol 9(8) (2012) 418–28. [DOI] [PubMed] [Google Scholar]
- [95].Li X, Martinez-Ferrer M, Botta V, Uwamariya C, Banerjee J, Bhowmick NA, Epithelial Hic- 5/ARA55 expression contributes to prostate tumorigenesis and castrate responsiveness, Oncogene 30(2) (2011) 167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kharaishvili G, Simkova D, Makharoblidze E, Trtkova K, Kolar Z, Bouchal J, Wnt signaling in prostate development and carcinogenesis, Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 155(1) (2011) 11–8. [DOI] [PubMed] [Google Scholar]
- [97].Parolia A, Cieslik M, Chu SC, Xiao L, Ouchi T, Zhang Y, Wang X, Vats P, Cao X, Pitchiaya S, Su F, Wang R, Feng FY, Wu YM, Lonigro RJ, Robinson DR, Chinnaiyan AM, Distinct structural classes of activating FOXA1 alterations in advanced prostate cancer, Nature 571(7765) (2019) 413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Fukumoto S, Hsieh CM, Maemura K, Layne MD, Yet SF, Lee KH, Matsui T, Rosenzweig A, Taylor WG, Rubin JS, Perrella MA, Lee ME, Akt participation in the Wnt signaling pathway through Dishevelled, J Biol Chem 276(20) (2001) 17479–83. [DOI] [PubMed] [Google Scholar]
- [99].Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S, Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase, Proc Natl Acad Sci U S A 95(19) (1998) 11211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Haq S, Michael A, Andreucci M, Bhattacharya K, Dotto P, Walters B, Woodgett J, Kilter H, Force T, Stabilization of beta-catenin by a Wnt-independent mechanism regulates cardiomyocyte growth, Proc Natl Acad Sci U S A 100(8) (2003) 4610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Yang X, Chen MW, Terry S, Vacherot F, Bemis DL, Capodice J, Kitajewski J, de la Taille A, Benson MC, Guo Y, Buttyan R, Complex regulation of human androgen receptor expression by Wnt signaling in prostate cancer cells, Oncogene 25(24) (2006) 3436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Sharma M, Chuang WW, Sun Z, Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3beta inhibition and nuclear beta-catenin accumulation, J Biol Chem 277(34) (2002) 30935–41. [DOI] [PubMed] [Google Scholar]
- [103].Mulholland DJ, Dedhar S, Wu H, Nelson CC, PTEN and GSK3beta: key regulators of progression to androgen-independent prostate cancer, Oncogene 25(3) (2006) 329–37. [DOI] [PubMed] [Google Scholar]
- [104].Amir AL, Barua M, McKnight NC, Cheng S, Yuan X, Balk SP, A direct beta-catenin-independent interaction between androgen receptor and T cell factor 4, J Biol Chem 278(33) (2003) 30828–34. [DOI] [PubMed] [Google Scholar]
- [105].Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B, Sun Z, Linking beta-catenin to androgen-signaling pathway, J Biol Chem 277(13) (2002) 11336–44. [DOI] [PubMed] [Google Scholar]
- [106].Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, Schwabe RF, Vahdat LT, Altorki NK, Mittal V, Gao D, Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance, Nature 527(7579) (2015) 472–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Zhang J, Liang Q, Lei Y, Yao M, Li L, Gao X, Feng J, Zhang Y, Gao H, Liu DX, Lu J, Huang B, SOX4 induces epithelial-mesenchymal transition and contributes to breast cancer progression, Cancer Res 72(17) (2012) 4597–608. [DOI] [PubMed] [Google Scholar]
- [108].Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA, The epithelial-mesenchymal transition generates cells with properties of stem cells, Cell 133(4) (2008) 704–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Liu Y, Zeng S, Jiang X, Lai D, Su Z, SOX4 induces tumor invasion by targeting EMT-related pathway in prostate cancer, Tumour Biol 39(5) (2017) 1010428317694539. [DOI] [PubMed] [Google Scholar]
- [110].Lin CM, Fang CL, Hseu YC, Chen CL, Wang JW, Hsu SL, Tu MD, Hung ST, Tai C, Uen YH, Lin KY, Clinical and Prognostic Implications of Transcription Factor SOX4 in Patients with Colon Cancer, PLoS One 8(6) (2013) e67128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Chen J, Ju HL, Yuan XY, Wang TJ, Lai BQ, SOX4 is a potential prognostic factor in human cancers: a systematic review and meta-analysis, Clin Transl Oncol 18(1) (2016) 65–72. [DOI] [PubMed] [Google Scholar]
- [112].Liao YL, Sun YM, Chau GY, Chau YP, Lai TC, Wang JL, Horng JT, Hsiao M, Tsou AP, Identification of SOX4 target genes using phylogenetic footprinting-based prediction from expression microarrays suggests that overexpression of SOX4 potentiates metastasis in hepatocellular carcinoma, Oncogene 27(42) (2008) 5578–89. [DOI] [PubMed] [Google Scholar]
- [113].Jauch R, Ng CK, Narasimhan K, Kolatkar PR, The crystal structure of the Sox4 HMG domain- DNA complex suggests a mechanism for positional interdependence in DNA recognition, Biochem J 443(1) (2012) 39–47. [DOI] [PubMed] [Google Scholar]
- [114].Mullard A, FDA approves landmark RNAi drug, Nature Reviews Drug Discovery 17 (2018) 613. [DOI] [PubMed] [Google Scholar]