Abstract
Purpose
The continuous growth of the lens throughout life may contribute to the onset of age-related conditions in the lens (i.e., presbyopia and cataract). Volumetric growth is the result of continuous proliferation of lens epithelial cells (LECs). The driving factors controlling LEC proliferation are not well understood. This study tested the hypothesis that mechanical stretching modulates LEC proliferation.
Methods
Biomechanical regulation of LEC proliferation was investigated by culturing whole porcine lenses and connective tissues ex vivo under varying physiologically relevant stretching conditions using a bespoke lens stretching device. Additionally, some lenses were treated with a YAP function inhibitor to determine the Hippo signaling pathway's role in regulating lens growth. Resulting changes in LEC labeling index were analyzed using EdU incorporation and flow cytometry for each lens.
Results
LEC proliferation was found to be modulated by mechanical strain. Increasing both the magnitude of static stretching and the stretching frequency in cyclic stretching resulted in a proportional increase in the labeling indices of the LECs. Additionally, treatment with the YAP function inhibitor effectively eliminated this relationship.
Conclusions
These data demonstrate that LEC proliferation is regulated in part, by the mechanotransduction of stresses induced in the lens capsule and that YAP plays an important role in mechanosensing. These results have important implications for understanding lens growth and morphogenesis. The model may also be used to identify and evaluate targets for modulating lens growth.
Keywords: lens epithelium, mechanotransduction, Hippo pathway, cell culture, cell proliferation
The lens is the pivotal tissue in accommodation—the process by which the eye alters its focal distance from far to near. The lens continues to grow in size throughout a person's lifetime while the size of the globe of the eye stays constant through adulthood.1,2 This growth is a result of lens epithelial cell (LEC) proliferation, which ultimately leads to an increase in the number of fiber cells.3 Since a lens fiber cell has a much larger volume than an LEC, the lens progressively becomes larger as a result of LEC proliferation.4 The age-related changes in lens size and shape contribute to presbyopia and cataracts.5–8
The driving force(s) for this continuous growth remain unknown, at least in part due to technical challenges with reproducing the lens' complex in vivo environment which includes biochemical and biomechanical influences. Flat-mount lens explants are frequently used to probe specific aspects of LEC behavior, but may not be appropriate for examining mechanobiological behavior due to altered cell morphology9 and mechanical environment. Similarly, species which accommodate using a human-like mechanism are few and prohibitively expensive for many basic scientific studies.
In disaccommodation, tension is applied to the lens capsule via the zonules due to relaxation of the ciliary muscle.10 During accommodation, this tension is released via ciliary muscle contraction, allowing the lens to elastically recoil to a rounder shape. The ciliary muscle remains active into old age11–14 and the mechanical properties of zonules are independent of age,15 implying that the human lens capsule may experience cyclic tension even after the onset of presbyopia. In the context of presbyopia, younger lenses exhibit a large magnitude in focal length changes during stretching, while lenses above the age of 60 showed no changes in focal length with stretching, such that the eye is only able to focus on distant objects.10,16–18 Axial strains acting upon the lens, similar to those experienced during disaccommodation, have been observed to lead to a reversible increase in LEC area indicating that mechanical loading of the lens is transduced onto its cells.19 This change in LEC area provides strong evidence that a lifetime of accommodation and disaccommodation and the resulting changes in LEC area would play a part in driving strain-responsive behaviors in LECs.
Recent work has demonstrated that the lens epithelium is sensitive to changes in its mechanical environment. LEC proliferation is altered during cataract surgery which disrupts both the biochemical and biomechanical homeostasis of the lens.20 Departure from equibiaxial stresses in the capsule may drive cell migration and morphological changes leading to posterior capsular opacification (PCO).21 Such anisotropic strains have been found to exist in the intact human lens capsule near the equator, coinciding with the region in which proliferation is known to occur in the mouse lens.3,22 PCO can be inhibited in vitro by using pharmaceutical agents targeting the cytoskeleton23; the cytoskeleton is known to convey information about mechanical stresses on a cell to the nucleus resulting in changes in protein expression.24 Together, these results suggest that LECs are strain-responsive cells (i.e., they can alter their behavior in response to mechanical cues from their environment). However, these studies have primarily focused on pathologic LEC differentiation leading to PCO and therefore did not examine whether proliferation rates or biomarker expression levels were directly influenced by mechanical stretching.
Activation of YAP/Taz signaling is a potential mechanism by which the axial loading of the lens by disaccommodation is transduced onto the LECs and upregulates proliferative activity. Earlier studies have observed the expression of YAP in rodent LEC explants and the role it plays in FGF-induced LEC proliferation.25 The role of YAP in the mechanoregulation of LEC stretch-induced proliferation is not well established; however, stretch-induced YAP activation and a resulting upregulation of cell proliferation has been observed in other cell types, including epithelial cells from other tissue types (i.e., mammary, lung, and skin epithelium26–29). It is well established that YAP has a pivotal role in transducing mechanical cues from stretching into increased cell proliferation. This study seeks to provide evidence that this same behavior is exhibited by LECs, thus identifying a contributing factor to the continuous growth of the lens throughout life.
In order to study the effects of lens stretching on the proliferation of LECs without disturbing the complex microenvironment present within the lens, it was necessary to culture whole lens tissues under different loading conditions for an extended period of time ex vivo. For this purpose, a lens stretching bioreactor was developed. Lens stretchers have been employed in previous studies; however, they were primarily used to provide insight on the biomechanical30–35 and optopmechanical36,37 properties of the lens and were unsuitable for use in the sterile environment of an incubator. The lens stretcher developed for this study was capable of applying both static and cyclic loading conditions on the lens ex vivo, while remaining autoclavable to ensure sterility.
This study was designed to determine whether the mechanical forces experienced by the lens during the process of accommodation contribute to lens growth by increasing the proliferation rate of LECs as well as to identify what role YAP signaling plays in the stretch-induced proliferation of LECs. By developing a lens organ culture bioreactor capable of reproducing the biochemical milieu and cyclic tension exerted on the human lens throughout life, we have overcome the technical challenges associated with studying the intact lens epithelium ex vivo. This technique allows detailed study of the mechanobiologic response of the lens epithelium to stretching ex vivo while closely mimicking that experienced by the human lens in vivo. Furthermore, agonists or inhibitors of specific molecular pathways may be used to elucidate the underlying mechanobiologic mechanisms involved.
Methods
All animal tissues were used in accordance with institutionally approved protocols.
Lens Stretching Organ Culture
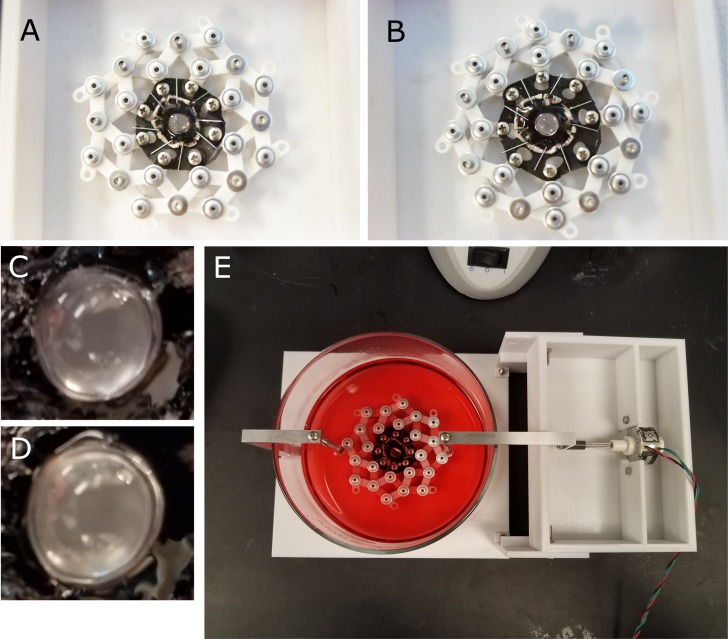
Freshly enucleated porcine eyes were obtained from a local abattoir (Delaware Meats, Delaware, OH, USA). Extraocular tissue was removed and the whole globe was disinfected by submersion in 0.5% povidone-iodine (Sigma-Aldrich Corp., St. Louis, MO, USA) in PBS for 5 minutes, then transferred into PBS. The globe was removed from the PBS and partial-thickness incisions were made with a scalpel along the limbus and the equator. The cornea and the iris were carefully removed. The globe was bisected along the equatorial incision and the posterior half was discarded. The posterior portion of the vitreous was removed and the anterior portion of the vitreous was left attached to the anterior section of the eye. Eight radial cuts were made through the sclera spaced 45° apart to create eight flaps surrounding the lens. To ensure uniform stretching about the lens' circumference a stapler was used to attach each of the scleral flaps to a 40-mm diameter silicone disk (McMaster-Carr, Elmhurst, IL, USA) with a 10-mm diameter hole in the center with the lens positioned in the central hole (Fig. 1).
Figure 1.
The anterior portion of the eye was isolated, and the cornea and iris removed; leaving the lens, ciliary body, and a ring of sclera intact. Eight flaps were cut into the sclera and each flap was affixed to the silicone disk using staples. The silicone disk was mounted onto the stretching ring which, when expanded, would stretch the lens equally along eight axes. Disk mounted onto stretching ring in unstrained (A) and strained (B) configurations. A close up of the lens in the unstretched (C) and stretched conditions (D). The lens and accessory tissue mounted onto the motorized lens stretching device and submerged in culture media (E). The amplitude and frequency of the lens stretching regime can be customized and run for the entire duration of the tissue culture period.
The silicone disks were mounted onto a bespoke stretching ring device which attached to eight equally-spaced peripheral holes in the silicone disk. Lenses mounted in this way were submerged in prewarmed, serum-free medium 199 (M199) with Earle's salts L-glutamine, and sodium bicarbonate, supplemented with 0.1% bovine serum albumin, 100 IU/mL penicillin, 100 mg/mL streptomycin and 2.5 mg/mL Amphotercin B (Sigma-Aldrich Corp.). Some lenses were exposed to the Yes-associated protein (YAP) function inhibitor verteporfin (5 μM verteporfin, Sigma-Aldrich Corp.). Lens tissue was incubated at 37°C, 5% CO2 for 24 hours. After 23 hours in culture, 0.1% of 10 mM EdU (5-ethynyl-2′-deoxyuridine; Thermo-Fisher, San Jose, CA, USA) in DMSO was added to the cell culture media for the remaining 1 hour.
Lenses were subjected to static stretching at 0% (control), 6%, and 12% strain (defined as percent change in the equatorial diameter of the lens). These strain amplitudes were chosen so as to remain close to the in vivo physiological range.38–40 For lenses undergoing cyclic stretching, the stretching ring was mounted onto a motorized rig and stretched at 6% strain amplitude with a frequency of 0 (control), 0.05, 0.1, and 0.2 Hz. These frequencies were chosen with 0.2 Hz as the highest because previous studies have observed proliferation in other cell types is inhibited at higher frequencies.41 The strain amplitude was validated by comparing images of the lenses in the stretched and unstretched configuration in ImageJ (NIH, Bethesda, MD, USA).42
Flow Cytometry
Lenses were removed from culture, immediately isolated from the surrounding tissue, and rinsed in PBS. The lens capsule was isolated by peeling the capsule open from the posterior pole using jeweler's forceps then the fiber cell bundle removed. The lens capsules were rinsed twice with PBS and submerged in 0.25% trypsin with 0.04% ethylenediaminetetraacetic acid (EDTA) (VWR, Radnor, PA, USA) at 4°C for 18 hours. Excess trypsin was removed and the capsules were placed in a 37°C water bath for 30 minutes. We added 10 mL of M199 supplemented with 0.1% BSA to quench the trypsin activity. Capsule fragments were filtered using a 70-μm cell strainer and the solution was centrifuged for 10 minutes at 180 rcf to collect the LECs.
The LECs were immediately fixed in 10% neutral buffered formalin (Sigma-Aldrich Corp.) and stained for EdU detection with AlexaFluor 488-azide using a commercial kit for 30 minutes according to manufacturer instructions (Click-iT; Invitrogen, Carlsbad, CA, USA). LECs were also stained for nuclear detection using a commercial reagent for 30 minutes (NucRed Live 647 ReadyProbes; Invitrogen). LECs were analyzed using a BD LSR II flow cytometer using the 488- and 640-nm lasers (BD Biosciences, Franklin Lakes, NJ, USA). The labeling index was calculated from the percentage of cells that had a positive signal for the AlexaFluor 488 stain using commercial software (Flowing Turku, Finland).
The resulting raw data were analyzed by collecting measurements from unstained cells to establish autofluorescence thresholds and cells from the same lens stained with both dyes. The dataset from each stained sample was gated such that the threshold would exclude 95% of the signal from the unstained control.
Fluorescent Microscopy
In order to image the intact LEC monolayer in situ, a flat-mounting technique was used43 rather than imaging an intact lens.44 This avoids the potential complications arising from imaging a curved surface. Lenses were removed from culture, and immediate isolated from surrounding tissue. The intact lens was fixed in 10% neutral buffered formalin for 10 minutes in order prevent sloughing off of the LEC monolayer during flat-mounting. Lenses were then dissected and flat-mounts of the lens capsule were prepared.43 Lens flat-mounts were then fixed in 10% neutral buffered formalin for a further 10 minutes. The LECs were permeabilized in 0.05% (vol/vol) Triton X-100 and blocked in 1% (wt/vol) bovine serum albumin for 30 minutes.43 Flat-mounts were rinsed in PBS and then incubated with fluorescent stains. AlexaFluor 488-azide with a commercial kit (Invitrogen) were used to visualize proliferative activity in the lens. To visualize YAP localization, flat mounts were incubated with primary and secondary antibodies for 60 minutes each. Primary antibodies included YAP1 Rabbit Polyclonal Antibody (Thermo Fisher Scientific). The secondary antibody used was Goat anti-Rabbit, AlexaFluor 488 (Thermo Fisher Scientific). For both tests, following incubation with the antibodies and commercially available kits, samples were then counterstained with Hoechst (Thermo Fisher Scientific). Lenses stained for proliferation were imaged using a confocal microscope (Nikon Eclipse Ti2-E; Nikon Instruments Inc., Melville, NY, USA) and those stained for YAP localization were imaged using a fluorescent microscope (Nikon Eclipse Ts2; Nikon Instruments Inc.).
Confocal images were processed to objectively remove background signal, assumed to arise from either autofluorescence or residual dye, as follows. Z-stacks were flattened by keeping the maximum channel intensity value for each pixel location in the horizontal plane. Pixels corresponding to Hoechst-positive nuclei were determined using Otsu's threshold method to produce a binary mask.45 Morphologic closing and opening operations were applied to avoid the loss of real connections or development of spurious connections between pixels. Areas with an area >50 pixels were excluded.
EdU-positive nuclei were detected as follows. Autofluorescence or residual dye intensity in the green channel was estimated on the basis of the green channel intensity for all pixels not corresponding to a nucleus (as defined above). An empirical (Kaplan-Meier) cumulative distribution function was determined by including all such pixels. A probability that the mean pixel intensity within a given nucleus was not a result of autofluorescence or residual dyes was then calculated. If this probability exceeded 95%, the corresponding nucleus was considered to be EdU-positive.
Statistical Analysis
A paired t-test was conducted on paired lenses cultured for either 1 or 24 hours to determine whether the labeling indices of LECs, defined as the number of EdU-labeled LECs to the total number of LECs, varied with respect to time spent in culture. Simple linear regression analysis was used to determine the effects of stretch amplitude and frequency on LEC proliferation. Analysis of covariance (ANCOVA) was performed to investigate the effects of verteporfin on LEC proliferation across different stretching conditions. A post-hoc Tukey's honestly significant difference (HSD) test was used to compare the verteporfin group with the control. Statistical analysis was performed using JMP Pro 13 (SAS Institute, Cary, SC, USA).
Results
Labeling Index of LECs Varies with Culture Time
The microenvironment LECs experience within the eye in vivo differs from that experienced in vitro. In order to investigate whether the stresses caused by a change in environment affect the labeling index of LECs, paired whole lenses mounted on silicon rings and were cultured for either 1 hour or 24 hours, under null stretch conditions with each lens exposed to EdU for 1 hour. LECs were then analyzed using flow cytometry to determine labeling index.
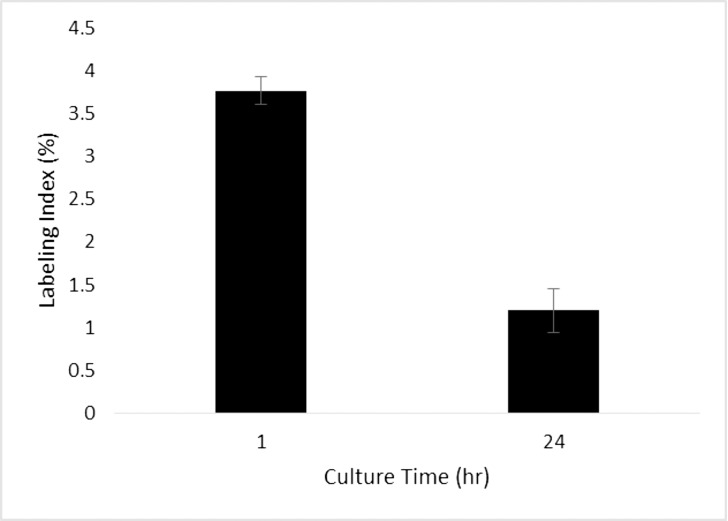
A paired t-test was used to determine if the labeling index of LECs cultured for 1 hour immediately following dissection was significantly different than those cultured for 24 hours (Fig. 2). A significant difference was found (P = 0.0055). The data show that the labeling index of LECs in whole lens cultures decreased with time in culture. The higher initial labeling index followed by a decrease over time may be due to a stress response induced by changing the LEC microenvironment and the subsequent acclimation of the LECs to the new environment. Longer culture times could therefore be preferable for later studies in order to avoid any initial confounding cellular response to a new microenvironment.
Figure 2.
Variation of labeling index under null stretch with different times spent in culture. A paired t-test showed a significant difference in means (P = 0.0055). The labeling indices of lenses cultured without strain for one hour was higher than those cultured for 24 hours.
Increasing Stretch Amplitude Increases LEC Proliferation in Whole Lens Cultures
To determine if the amplitude of static stretching affected LEC proliferation, a total of eight pairs of whole lenses were cultured for a period of 24 hours under various static stretch conditions. For each pair, one was subjected to 6% (4 pairs) or 12% (4 pairs) strain while the other (control) was held at 0%. During the final hour of the culture period the lenses were exposed to EdU, which would be incorporated into any newly synthesized DNA. The LECs were analyzed using flow cytometry and the labeling index was calculated from the percentage of the total population of cells that had synthesized new DNA during the hour-long EdU pulse. The labeling index values for the different stretch conditions are presented in Table 1.
Table 1.
Labeling Indices of Lenses Cultured Under Varying Static Strain Amplitudes
|
Strain Amplitude, % |
Labeling Index, % |
n |
| 0 | 1.14 ± 0.37 | 8 |
| 6 | 3.23 ± 0.27 | 4 |
| 12 | 6.57 ± 0.46 | 4 |
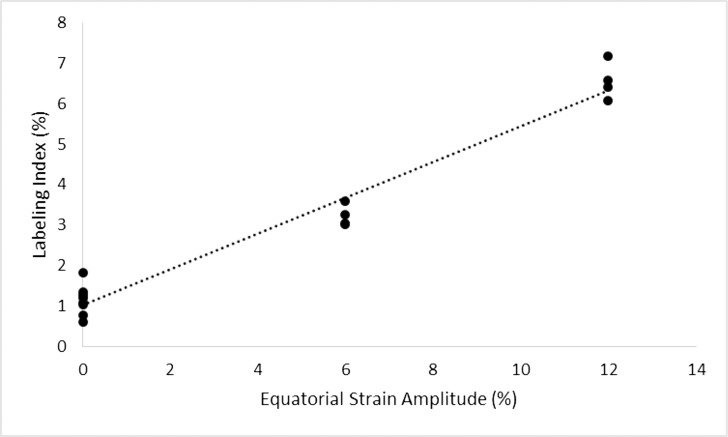
Simple linear regression analysis was used to determine if stretch amplitude was a significant predictor of LEC proliferation (Fig. 3). A significant regression equation was found (Labeling Index = 1.03% + 0.44% × Percent Stretch, R2 = 0.963, P < 0.0001). The data show that the proliferation of LECs increased proportionally to stretch amplitude. These findings indicate LEC proliferation is driven, at least in part, by mechanotransduction. Therefore, the accommodative process may contribute to the growth of the lens.
Figure 3.
Variation of labeling index with respect to strain amplitude. Linear regression analysis predicted the relationship to follow: Labeling Index = 1.03 + 0.44 × Percent Strain Amplitude (R2 = 0.963, P < 0.0001). Lenses were cultured for 24 hours under varying static strain amplitudes and exposed to a one-hour EdU pulse before LECs were isolated and analyzed using flow cytometry. The labeling index increased proportionally with strain amplitude, suggesting a strong relationship between lens stretching and LEC proliferation.
LEC Proliferation Increases with Stretching Frequency in Whole Lens Cultures
Once a link between LEC proliferation and stretch amplitude was established it was necessary to determine if a change in labeling index occurred in response to changes in stretching frequency as well as stretch amplitude. Whole lens tissues were cultured and analyzed as described above. Stretch amplitude was held at 6% and the triangular stretch waveform was applied cyclically, oscillating from 0% to 6%, at frequencies of 0, 0.05, 0.1, and 0.2 Hz. A total of 16 unpaired lenses were used. The labeling index values are described in Table 2.
Table 2.
Labeling Indices of Lenses Cultured Under Varying Cyclic Strain Frequencies
|
Strain Frequency, Hz |
Labeling Index, % |
n |
| 0 | 3.23 ± 0.27 | 4 |
| 0.05 | 3.80 ± 0.51 | 4 |
| 0.10 | 5.52 ± 0.27 | 4 |
| 0.20 | 7.56 ± 0.23 | 4 |
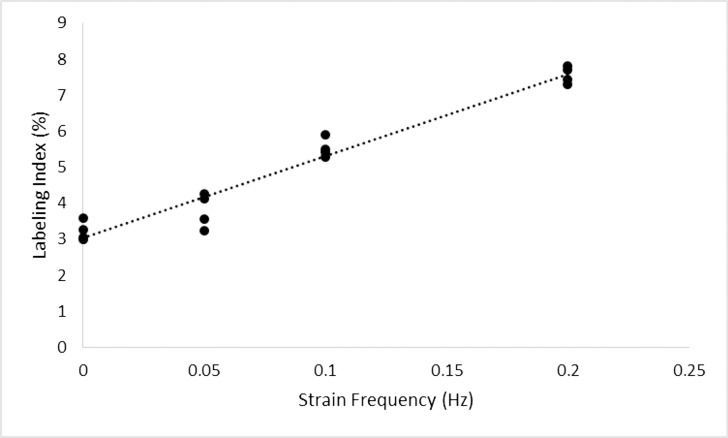
Linear regression analysis was used to determine if LEC labeling index is predicted by changes in stretching frequency (Fig. 4). A significant regression was found (Labeling Index = 3.05% + 22.62% × Stretching Frequency (Hz), R2 = 0.954, P < 0.0001). Results show that the proliferation of LECs increased proportionally to stretching frequency. As such, lens growth is not only affected by the amplitude but also the frequency of the stretch applied.
Figure 4.
Variation of labeling index with respect to strain frequency. Linear regression analysis predicted the relationship to follow: Labeling Index = 3.05 + 22.62 × Strain Frequency (Hz; R2 = 0.954, P < 0.0001). Lenses were cultured for 24 hours under 6% cyclic strain amplitude at varying strain frequencies with one hour of exposure to EdU. Labeling index was highly correlated to strain frequency.
Stretching Alters the Localization of LEC Proliferation Across the Lens Capsule
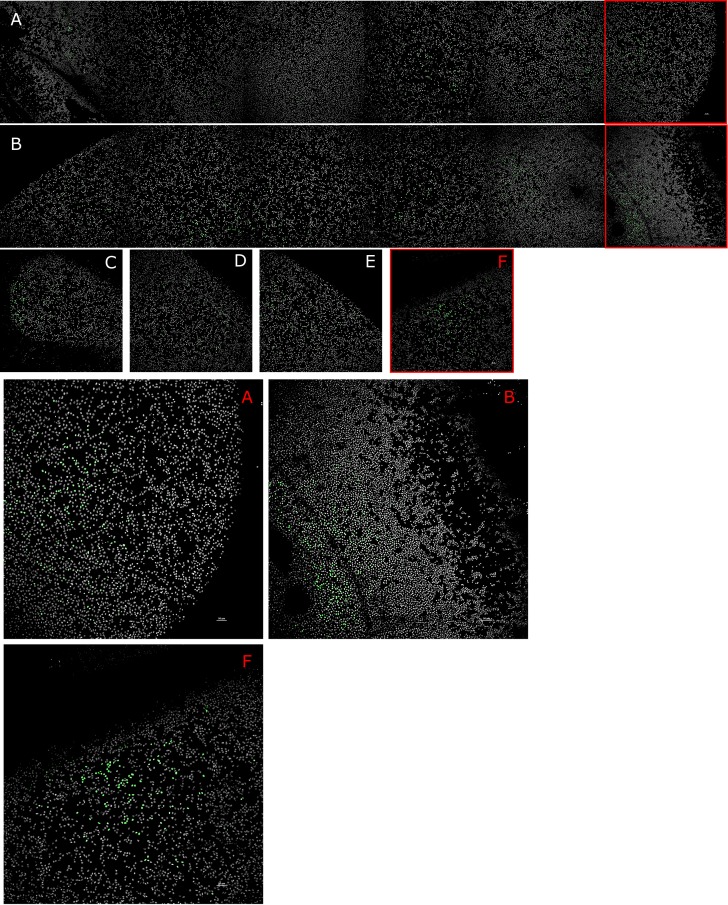
Qualitative analysis of the effects of different stretching regimes on the localization of LEC proliferative activity was performed by staining flat-mounted lens capsules for the thymine analog, EdU, and counterstaining LEC with the nuclear stain Hoechst. Representative images of lenses cultured under null strain (Fig. 5A), 12% static stretch (Fig. 5B), and cyclic stretch at 6% amplitude and 0.20 Hz (Fig. 5C) were used (Fig. 5). Low levels of EdU staining were observed in the null stretch lens (Fig. 5A), primarily near the equator. The static stretch lens appeared to have a higher labeling index (Fig. 5B), with the majority of EdU staining also occurring near the equator. In the cyclic lens, EdU labeling was observed near the anterior pole (Fig. 5C) and at different points along the equator (Fig. 5D–F).
Figure 5.
Variation in EdU labeling localization under different strain conditions. Qualitative analysis of representative images of lenses cultured under null strain (A), static strain (B), and cyclic strain (C–F); and stained for the EdU proliferative marker (green). The null (A) and static (B) stretch lenses are shown as mosaics going from one side of the equator to the other. The cyclic stretch lens (C–F) shows discrete images taken at different points along the anterior capsule: the anterior pole (C) and points along the equator (D–F). Lenses cultured under null strain (A) showed little reactivity with the EdU stain. In static strain (B), and cyclic strain (C) lenses, EdU labeling was primarily in the germinative zones (GZ). Magnified images of the GZ of null (A), static (B), and cyclic (F) stretched lenses are shown.
Inhibition of YAP Function Blocks Mechanotransductive Effects of Stretching on LEC Proliferation
The effects of the YAP function inhibitor verteporfin on LEC proliferation was determined by exposing both paired eyes to identical static stretching conditions: 0%, 6%, or 12%. Both members of each pair were cultured in enhanced M199 and the treatment group was supplemented with verteporfin. A total of 10 pairs of eyes were used with 4 pairs of eyes cultured under null stretch, and three pairs each with 6% and 12% static strain. The labeling index values are described in Table 3.
Table 3.
Labeling Indices of Verteporfin Treated and Paired Control Lenses Cultured Under Varying Static Strain Amplitudes
|
Strain Amplitude (%) |
Labeling Index (%) |
n |
|
|
Verteporfin |
Control |
||
| 0 | 0.925 ± 0.24 | 1.21 ± 0.10 | 4 |
| 6 | 1.27 ± 0.20 | 3.86 ± 0.21 | 4 |
| 12 | 1.14 ± 0.14 | 5.92 ± 0.59 | 4 |
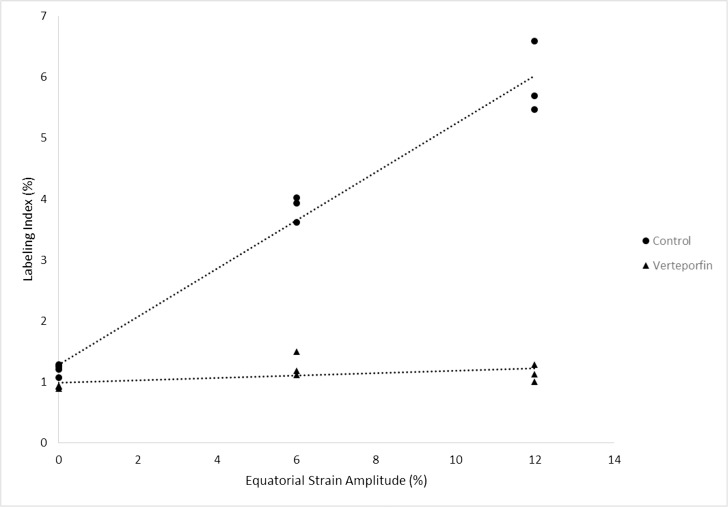
Linear regression analysis was performed on both treatment groups. A significant correlation was found for the control group (Labeling Index = 1.28% + 0.39% × Stretch Amplitude, R2 = 0.975, P < 0.0001), but no significant relationship was found for the group treated with verteporfin (Labeling Index = 0.99% + 0.02% × Stretch Amplitude, R2 = 0.292, P = 0.107; Fig. 6). To confirm the difference between the treatment groups, ANCOVA was used to determine whether there was a significant difference between groups; a significant difference was observed (F = 282.34, P < 0.0001). A post-hoc Tukey's HSD test indicated a significant difference between the regression lines of the verteporfin and control groups (P < 0.0001).
Figure 6.
Variation of labeling index with respect to strain amplitude of lenses treated with a YAP inhibitor, verteporfin, and their paired controls. Linear regression analysis predicted the relationships to follow: Labeling Index (%) = 0.99 + 0.02 × Strain Amplitude (R2 = 0.292, P = 0.107) and Labeling Index = 1.28 + 0.39 × Strain Amplitude (R2 = 0.975, P < 0.0001) for the treatment and control groups, respectively. Lenses were cultured for 24 hours under varying static strain amplitudes. After a 1-hour EdU pulse, LECs were analyzed using flow cytometry. The labeling index of the control group increased with static strain amplitude while the group treated with a YAP inhibitor showed no statistically significant relationship.
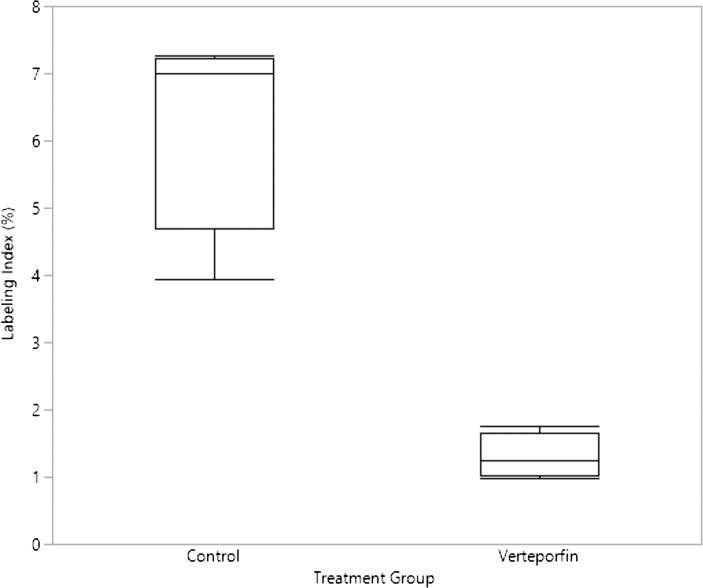
Analysis of the effects of YAP function inhibition on cyclic stretch proliferative response was also performed by culturing lenses at 6% stretch amplitude at 0.20 Hz with a verteporfin treated and untreated paired control (Fig. 7). The mean labeling index of the untreated control was 6.30 ± 1.58. The verteporfin treated group had a mean labeling index of 1.30 ± 0.33. A paired t-test showed a significant difference between the means (P < 0.0001).
Figure 7.
Labeling index of cyclically stretched lenses treated with and without verteporfin. Comparison of LEC labeling index of verteporfin and control lenses under a cyclic strain (6% strain amplitude, 0.20 Hz) regime. The control group had a mean labeling index of 6.30% ± 1.58%. The verteporfin treated group had a mean labeling index of 1.30% ± 0.33%. The results of a paired t-test had a P < 0.0001.
The data shows that when YAP function was inhibited by verteporfin, the correlation between LEC labeling index and stretch amplitude was effectively eliminated. This suggests that YAP plays a crucial role in the transduction of mechanical signals into an upregulation of LEC proliferation. Furthermore, when YAP function is inhibited those signals are blocked and LEC proliferation does not increase with mechanical stretching.
Stretching Alters YAP Localization in LECs
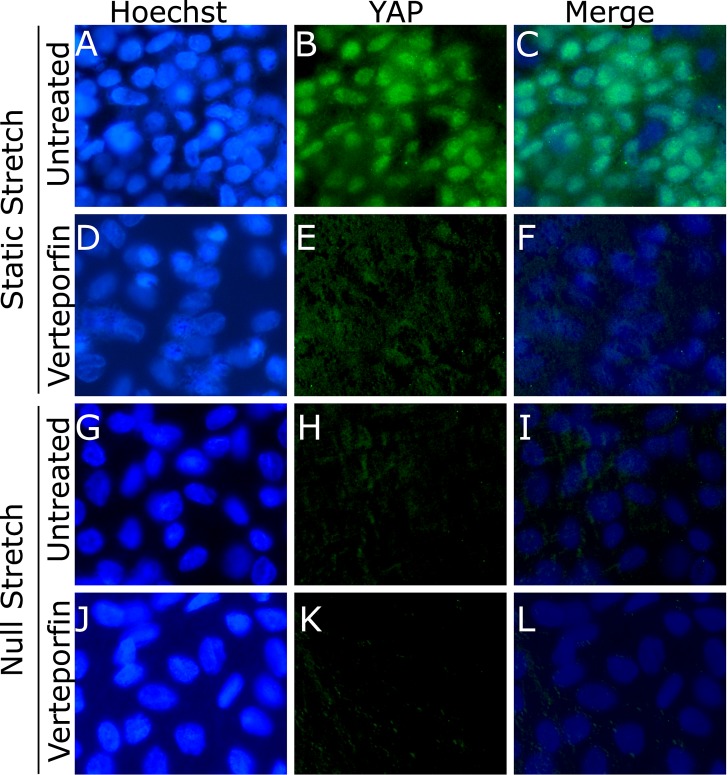
Qualitative analysis of the effects of YAP function inhibition by verteporfin on the localization of intracellular YAP was performed by staining flat-mounted lens capsules for YAP, and counterstaining them with the nuclear stain Hoechst. Paired verteporfin treated and untreated control lenses were cultured under static and null stretch conditions and were then stained and imaged using fluorescent microscopy (Fig. 8). All images were taken in the germinative zone near the equator of the lens. YAP nuclear localization was observed in the static stretched untreated lenses (Fig. 8C), suggesting the activation of YAP and translocation into the nucleus. YAP nuclear localization was not observed in the YAP function inhibited static stretched lens (Fig. 8F). YAP activation was not observed in both the null stretched untreated (Fig. 8I) and treated (Fig. 8L) lenses. The staining and microscopy techniques used were only able to visualize the nuclear YAP. This was potentially due to the more diffuse localization of cytoplasmic YAP being unable to generate a strong enough signal to be visualized over the autofluorescent background. These results suggest YAP can be activated by stretch in LECs and that its function can be inhibited by verteporfin.
Figure 8.
Localization of YAP in lenses cultured with and without verteporfin under static and null stretch conditions. Lenses were stained with the nuclear stain Hoechst (A, D, G, J) and for YAP (B, E, H, K). Nuclear localization of YAP was observed in the statically stretched untreated lens (C). This nuclear localization response was not observed in the statically stretched lens treated with verteporfin (F). YAP nuclear localization was also not detected in the untreated (I) or verteporfin treated (L) lenses cultured under null stretch conditions.
Discussion
This study was designed to determine if radially stretching the lens had an effect on LEC proliferation and whether YAP was involved in the mechanotransductive signaling pathway driving stretch-induced LEC proliferation. The stretch response was found to be dependent on both stretching amplitude and frequency; qualitative analysis of the localization of LEC proliferative activity showed differences between static and cyclic stretch. YAP was found to play an important role in the signaling pathway.
These results have important implications for understanding lens growth and morphogenesis, as well as approaches for modulating LEC proliferation. Controlling LEC proliferation could allow for retarding lens growth as a means for delaying presbyopia or cataract, as well as prevention of PCO (i.e., regeneration of the lens material following cataract surgery). Our data suggest that behavioral, environmental, and therapeutic approaches may be feasible for limiting or encouraging lens growth.
The human lens continues to grow throughout life, with an apparent bi-phasic growth pattern.46 It may be that the initial, very rapid, prenatal growth phase is driven by a rapid increase in lens capsule surface area and constant stretching forces, whereas the later, much slower, growth phase is retarded by the partial relief of lens stretching during accommodation. This is supported by Augusteyn's observation that the transition between growth phases occurs near the time of birth, as does the ability to accommodate.46 In non- or minimally accommodating species, age-matched lenses tend to be much larger (e.g., a 6-month-old pig lens may be ∼400 mg, whereas an infant human lens is ∼150 mg), possibly due to persistent disaccommodation.
Earlier studies have identified YAP as playing an important role in the regulation of tissue size, including the lens.25,26,47 When YAP is unphosphorylated and active, it is localized in the nucleus, acting as a transcriptional coactivator promoting the expression of genes inducing cell proliferation, survival, and migration.48 YAP is primarily regulated by the Hippo-signaling pathway, which when activated, phosphorylates YAP and inhibits its activity. The Hippo pathway is regulated by various mechanisms, including cell-cell contact, cell polarity, cellular energy status, hormonal signals and, most relevant to this study, mechanical cues.26,48,49 Studies on other cell types have observed that Hippo pathway activity is downregulated by stretching of those cells via the phosphorylation of the LATS1 kinase, which is the primary negative regulator of YAP.26,50 While the role of YAP in mechanosensing and cell proliferation has been studied in other cell types,26,48–50 and its expression in the lens is well documented,25,47 this study is the first to establish the link between mechanical cues and the regulation of the YAP protein in the lens.
The data presented in this study support the hypothesis that stretching the lens results in the activation of YAP and a subsequent increase in proliferative activity. However, YAP regulation is controlled by several different pathways with a significant amount of crosstalk between them and several other mechanosensing pathways may also be at play.51,52 In addition to the Hippo signaling pathway, RHO and MAPK/ERK signaling have also been implicated in the mechanoregulation of YAP.51,53–56 In other tissue types the signaling pathways Wnt, TGF-β, and Notch have been also been shown to increase cell proliferation in reaction to shear stress without the mediation of YAP.52 Additionally, p38 and JNK signaling pathways have been reported to respond to stretch and increase cell proliferation.57,58 Further studies will be necessary to determine what role the different mechanotransduction pathways play in the regulation of LEC growth.
This study demonstrates that stretching the porcine lens and connective tissues ex vivo results in increased LEC proliferation. There are several factors which could modulate LEC behavior during stretching. First, the capsule experiences increased tension due to the increase of zonular tension; this increase in capsule surface area will necessarily increase the footprint of LECs. LECs will also presumably experience increased apical pressure from the fiber cell bundle in the stretched state which could vary with position. LEC-LEC tensile forces may increase as well. Stretching could also drive fluid flow in and out of the lens or drive an increased rate of transport via convection due to relative motion of the capsule to the surrounding media.59,60 Finally, it is also possible that alternate signaling molecule(s) are activated or transported due to the stretching motion. Further investigation is required to pinpoint the underlying mechanism(s) of the stretch-induced change in proliferation.
The microscopy results presented in this study suggest increased labeling in cyclic and static stretch lenses compared to static lenses. The labeling indices measured using flow cytometry for the different stretch conditions were in qualitative agreement with microscopy findings. When visualizing the distribution of proliferative activity, the majority of EdU staining was observed at the GZ (Fig. 5). Future work will quantitatively assess spatial variations in proliferation.
Fluorescent microscopy showed activated nuclear YAP in LECs in the GZ (Fig. 8). This activation of YAP was only observed in stretched lenses uninhibited with verteporfin; YAP activation was observed in neither the unstretched lenses nor lenses treated with verteporfin. In some regions of the GZ of the statically stretched, EdU stained LEC proliferative activity (Figs. 5B, 5E) was observed in a similar density as the static stretched lens stained for YAP nuclear localization (Fig. 8B); suggesting a correlation between YAP nuclear localization and DNA synthesis. The microscopy technique used was unable to visualize cytoplasmic YAP and as a result provides no information on the total YAP content of the LECs. Additionally, the distribution of YAP nuclear localization likely changes depending on the position on lens capsule, and potentially corresponds to the local strain profile of the LECs. Further studies are needed to more completely characterize the behavior of YAP in response to stretching the lens.
While mechanotransduction pathways are highly conserved and every effort was made to replicate the microenvironment of the lens in vitro, caution should be used in extrapolating these findings to predict the in vivo behavior of human LECs. We kept the lens and connective tissues intact and retained the anterior vitreous attached to the lens. Still, the conditions experienced by the lens during the study differed from those in vivo. For example, cell culture media composition, including oxygen content, could alter the magnitude of the change in proliferation that was observed. Stretch amplitudes were chosen to match physiological extents of stretching: the maximal stretch-induced change in equatorial radius in a young human lens has been found to be between 5%–10%.35,61–63 In the present study, porcine lenses were used, which have different geometric and mechanical properties when compared to human lenses.8,31,62,64 Therefore, the distribution of mechanical stresses and the deformation from stretching likely differs between species. Additionally, pigs do not accommodate, resulting in a smaller and less robust ciliary muscle than that found in a human or primate eye.65 However, the biomolecular composition and crystallin distribution in both the human and porcine lenses are very similar.66,67 Further, mechanotransduction signaling and gene expression are highly conserved between species.48 Thus, if the LECs in the porcine lens have an increased rate of proliferation in response to mechanical stretch, a similar response, albeit possibly with a different magnitude, would likely be observed in humans.
There are several possible explanations for the higher labeling index immediately after dissection relative to 24 hours of culturing after dissection. A stress response induced by changing the LEC microenvironment, subsequent acclimation of the LECs to the new environment, strain acting on the porcine lens in vivo and post mortem, and mechanical stimulation during the dissection process may contribute to the difference in labeling indices.32 Longer culture times could therefore be preferable for later studies in order to avoid any initial confounding cellular response to a new microenvironment or effects from mechanical loading prior to the culture period.
The results of the study establish a link between mechanical stretching and the upregulation of LEC proliferative activity, as well as identifying a target which, when inhibited, would reduce the growth of the lens. Overall this study provides new insights into the processes controlling lens growth and opens novel avenues by which to study the etiology of age-related vision disorders of the lens. Future work will map the localized proliferation changes with corresponding mechanical strains in the capsule.
Acknowledgments
The authors thank Keith Gooch for assistance early in the experimental design process, Wade Rich for assistance with protocol development, Rengasayee Veeraraghavan for microscopy support, as well as the Ohio State University Comprehensive Cancer Center Analytical Cytometry Shared Resource for technical support.
This work was presented in part at the annual meeting of the Association for Research in Vision and Ophthalmology in Honolulu, Hawaii in 2018.
Disclosure: B. Kumar, None; H.L. Chandler, None; T. Plageman, None; M.A. Reilly, None
References
- 1.Levin LA, Adler FH. Adler's Physiology of the Eye. Edinburg, TX: Saunders; 2011. [Google Scholar]
- 2.Bassnett S. Cell biology of lens epithelial cells. In: Saika S, Werner L, Lovicu FJ, editors. Lens Epithelium and Posterior Capsular Opacification. Tokyo, Japan: Springer; 2014. pp. 25–38. [Google Scholar]
- 3.Shi Y, De Maria A, Lubura S, Šikić H, Bassnett S. The penny pusher: a cellular model of lens growth. Invest Ophthalmol Vis Sci. 2015;56:799–809. doi: 10.1167/iovs.14-16028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassnett S. Three-dimensional reconstruction of cells in the living lens: the relationship between cell length and volume. Exp Eye Res. 2005;81:716–723. doi: 10.1016/j.exer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Truscott RJW, Zhu X. Presbyopia and cataract: a question of heat and time. Prog Retin Eye Res. 2010;29:487–499. doi: 10.1016/j.preteyeres.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Shui Y-B, Beebe DC. Age-dependent control of lens growth by hypoxia. Invest Ophthalmol Vis Sci. 2008;49:1023–1029. doi: 10.1167/iovs.07-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel I, West SK. Presbyopia: prevalence, impact, and interventions. Community Eye Health. 2007;20:40–41. [PMC free article] [PubMed] [Google Scholar]
- 8.Reilly MA. A quantitative geometric mechanics lens model: Insights into the mechanisms of accommodation and presbyopia. Vis Res. 2014;103:20–31. doi: 10.1016/j.visres.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 9.O'Connor MD, Wederell ED, de Iongh R, Lovicu FJ, McAvoy JW. Generation of transparency and cellular organization in lens explants. Exp Eye Res. 2008;86:734–745. doi: 10.1016/j.exer.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Glasser A, Campbell MCW. Presbyopia and the optical changes in the human crystalline lens with age. Vision Res. 1998;38:209–229. doi: 10.1016/s0042-6989(97)00102-8. [DOI] [PubMed] [Google Scholar]
- 11.Fisher RF. The force of contraction of the human ciliary muscle during accommodation. J Physiol. 1977;270:51–74. doi: 10.1113/jphysiol.1977.sp011938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacskulin A, Gast R, Bergmann U, Guthoff R. Ultrasound biomicroscopy monitoring of human ciliary body changes during accommodation in presbyopia. Ophthalmologe-Berlin. 1996;93:199–204. [PubMed] [Google Scholar]
- 13.Strenk SA, Semmlow JL, Strenk LM, Munoz P, Gronlund-Jacob J, DeMarco JK. Age-related changes in human ciliary muscle and lens: a magnetic resonance imaging study. Invest Ophthalmol Vis Sci. 1999;40:1162–1169. [PubMed] [Google Scholar]
- 14.Glasser A. Restoration of accommodation: surgical options for correction of presbyopia. Clin Exp Optom. 2008;91:279–295. doi: 10.1111/j.1444-0938.2008.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michael R, Mikielewicz M, Gordillo C, Montenegro GA, Pinilla Cortés L, Barraquer RI. Elastic properties of human lens zonules as a function of age in presbyopes. Invest Ophthalmol Vis Sci. 2012;53:6109–6114. doi: 10.1167/iovs.11-8702. [DOI] [PubMed] [Google Scholar]
- 16.Glasser A. Accommodation: mechanism and measurement. Ophthalmol Clin North Am. 2006;19:1–12. doi: 10.1016/j.ohc.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Glasser A, Campbell MCW. Biometric, optical and physical changes in the isolated human crystalline lens with age in relation to presbyopia. Vision Res. 1999;39:1991–2015. doi: 10.1016/s0042-6989(98)00283-1. [DOI] [PubMed] [Google Scholar]
- 18.Glasser A, Hilmantel G, Calogero D, MacRae S, Masket S, Stark W. Special report: American Academy of Ophthalmology task force recommendations for test methods to assess accommodation produced by intraocular lenses. Ophthalmology. 2017;124:134–139. doi: 10.1016/j.ophtha.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 19.Parreno J, Cheng C, Nowak RB, Fowler VM. The effects of mechanical strain on mouse eye lens capsule and cellular microstructure. Mol Biol Cell. 2018;29:1963–1974. doi: 10.1091/mbc.E18-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang E, Reid B, Lois N, Forrester JV, McCaig CD, Zhao M. Electrical inhibition of lens epithelial cell proliferation: an additional factor in secondary cataract? FASEB J. 2005;19:842–844. doi: 10.1096/fj.04-2733fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedrigi RM, Dziezyc J, Kalodimos HA, Humphrey JD. Ex vivo quantification of the time course of contractile loading of the porcine lens capsule after cataract-like surgery. Exp Eye Res. 2009;89:869–875. doi: 10.1016/j.exer.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Burd HJ, Montenegro GA, Panilla Cortés L, Barraquer RI, Michael R. Equatorial wrinkles in the human lens capsule. Exp Eye Res. 2017;159:77–86. doi: 10.1016/j.exer.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Sureshkumar J, Haripriya A, Muthukkaruppan V, Kaufman PL, Tian B. Cytoskeletal drugs prevent posterior capsular opacification in human lens capsule in vitro. Graefes Arch Clin Exp Ophthalmol. 2012;250:507–514. doi: 10.1007/s00417-011-1869-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Humphrey JD, O'Rourke SL. Springer; 2015. An Introduction to Biomechanics: Solids and Fluids, Analysis and Design. [Google Scholar]
- 25.Dawes LJ, Shelley EJ, McAvoy JW, Lovicu FJ. A role for Hippo/YAP-signaling in FGF-induced lens epithelial cell proliferation and fibre differentiation. Exp Eye Res. 2018;169:122–133. doi: 10.1016/j.exer.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Codelia VA, Sun G, Irvine KD. Regulation of YAP by mechanical strain through Jnk and Hippo signaling. Curr Biol. 2014;24:2012–2017. doi: 10.1016/j.cub.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panciera T, Azzolin L, Cordenonsi M, Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017;758:758–770. doi: 10.1038/nrm.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin C, Yao E, Zhang K, et al. YAP is essential for mechanical force production and epithelial cell proliferation during lung branching morphogenesis. eLife. 2017;6:e21130. doi: 10.7554/eLife.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendoza-Reinoso V, Beverdam A. Epidermal YAP activity drives canonical WNT16/β-catenin signaling to promote keratinocyte proliferation in vitro and in the murine ski. Stem Cell Res. 2018;29:15–23. doi: 10.1016/j.scr.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Ehrmann K, Ho A, Parel JM, Ehrmann K, Ho A, Parel J-M. Biomechanical analysis of the accommodative apparatus in primates. Clin Exp Optom. 2008;91:302–312. doi: 10.1111/j.1444-0938.2008.00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reilly MA, Hamilton PD, Perry G, Ravi N. Comparison of the behavior of natural and refilled porcine lenses in a robotic lens stretcher. Exp Eye Res. 2009;88:483–494. doi: 10.1016/j.exer.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 32.Reilly MA, Hamilton PD, Ravi N. Dynamic multi-arm radial lens stretcher: a robotic analog of the ciliary body. Exp Eye Res. 2008;86:157–164. doi: 10.1016/j.exer.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Sharma PK, Busscher HJ, Terwee T, Koopmans SA, van Kooten TG. A comparative study on the viscoelastic properties of human and animal lenses. Exp Eye Res. 2011:681–688. doi: 10.1016/j.exer.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Ziebarth NM, Borja D, Arrieta E, et al. Role of the lens capsule on the mechanical accommodative response in a lens stretcher. Invest Ophthalmol Vis Sci. 2008;49:4490–4496. doi: 10.1167/iovs.07-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pellegrino A, Burd HJ, Pinilla Cortés L, et al. Anterior lens capsule strains during simulated accommodation in porcine eyes. Exp Eye Res. 2018;168:19–27. doi: 10.1016/j.exer.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Augusteyn RC, Mohamed A, Nankivil D, et al. Age-dependence of the optomechanical responses of ex vivo human lenses from India and the USA, and the force required to produce these in a lens stretcher: The similarity to in vivo disaccommodation. Vision Res. 2011:1667–1678. doi: 10.1016/j.visres.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borja D, Manns F, Ho A, et al. Optical power of the isolated human crystalline lens. Invest Ophthalmol Vis Sci. 2008;49:2541–2548. doi: 10.1167/iovs.07-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson E. Amplitude of accommodation at different periods of life. Cal State J Med. 1907;5:163–166. [PMC free article] [PubMed] [Google Scholar]
- 39.Ziebarth NM, Arrieta E, Feuer WJ, Moy VT, Manns F, Parel JM. Primate lens capsule elasticity assessed using Atomic Force Microscopy. Exp Eye Res. 2011:490–494. doi: 10.1016/j.exer.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marussich L, Manns F, Nankivil D, et al. Measurement of crystalline lens volume during accommodation in a lens stretcher. Invest Ophthalmol Vis Sci. 2015;56:4239–4248. doi: 10.1167/iovs.15-17050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cui Y, Hameed FM, Yang B, et al. Cyclic stretching of soft substrates induces spreading and growth. Nat Commun. 2015;6:6333. doi: 10.1038/ncomms7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rueden CT, Schindelin J, Hiner MC, et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics. 2017;18:529. doi: 10.1186/s12859-017-1934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu JJ, Wu W, Tholozan FM, Saunter CD, Girkin JM, Quinlan RA. A dimensionless ordered pull-through model of the mammalian lens epithelium evidences scaling across species and explains the age-dependent changes in cell density in the human lens. J R Soc Interface. 2015;12:20150391. doi: 10.1098/rsif.2015.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiley LA, Shui YB, Beebe DC. Visualizing lens epithelial cell proliferation in whole lenses. Mol Vis. 2010;16:1253–1259. [PMC free article] [PubMed] [Google Scholar]
- 45.Otsu N. A Threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern Syst. 1979;9:62–66. [Google Scholar]
- 46.Augusteyn RC. On the growth and internal structure of the human lens. Exp Eye Res. 2010;90:643–654. doi: 10.1016/j.exer.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McAvoy JW, Dawes LJ, Sugiyama Y, Lovicu FJ. Intrinsic and extrinsic regulatory mechanisms are required to form and maintain a lens of the correct size and shape. Exp Eye Res. 2017;156:34–40. doi: 10.1016/j.exer.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nardone G, Oliver-De La Cruz J, Vrbsky J, et al. YAP regulates cell mechanics by controlling focal adhesion assembly. Nat Commun. 2017;8:15321–15321. doi: 10.1038/ncomms15321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aragona M, Panciera T, Manfrin A, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 51.Dupont S. Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp Cell Res. 2016;343:42–53. doi: 10.1016/j.yexcr.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 52.Abuammah A, Maimari N, Towhidi L, et al. New developments in mechanotransduction: cross talk of the Wnt, TGF-β and Notch signalling pathways in reaction to shear stres. Curr Opin Biomed Eng. 2018;5:96–104. [Google Scholar]
- 53.Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13:591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- 54.Wada K-I, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 55.Feng X, Degese Maria S, Iglesias-Bartolome R, et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated Rho GTPase signaling circuitry. Cancer Cell. 2014;25:831–845. doi: 10.1016/j.ccr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.You B, Yang Y-L, Xu Z, et al. Inhibition of ERK1/2 down-regulates the Hippo/YAP signaling pathway in human NSCLC cells. Oncotarget. 2015;6:4357–4368. doi: 10.18632/oncotarget.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kippenberger S, Bernd A, Loitsch S, et al. Signaling of mechanical stretch in human keratinocytes via MAP kinase. J Invest Dermatol. 2000;114:408–412. doi: 10.1046/j.1523-1747.2000.00915.x. [DOI] [PubMed] [Google Scholar]
- 58.Hofmann M, Žaper J, Bernd A, Bereiter-Hahn J, Kaufmann R, Kippenberger S. Mechanical pressure-induced phosphorylation of p38 mitogen-activated protein kinase in epithelial cells via Src and protein kinase. Biochem Biophys Res Commun. 2004;316:673–679. doi: 10.1016/j.bbrc.2004.02.101. [DOI] [PubMed] [Google Scholar]
- 59.Sheppard AL, Evans CJ, Singh KD, Wolffsohn JS, Dunne MCM, Davies LN. Three-dimensional magnetic resonance imaging of the phakic crystalline lens during accommodation. Invest Ophthalmol Vis Sci. 2011;52:3689–3697. doi: 10.1167/iovs.10-6805. [DOI] [PubMed] [Google Scholar]
- 60.Candia OA. Surface and volume changes in the lens during accommodation. Invest Ophthalmol Vis Sci. 2011;52:3698–3698. doi: 10.1167/iovs.11-7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manns F, Parel J-M, Denham D, et al. Optomechanical response of human and monkey lenses in a lens stretcher. Invest Ophthalmol Vis Sci. 2007;48:3260–3268. doi: 10.1167/iovs.06-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reilly MA, Ravi N. A geometric model of ocular accommodation. Vision Res. 2010;50:330–336. doi: 10.1016/j.visres.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 63.Burd HJ, Judge SJ, Cross JA. Numerical modelling of the accommodating lens. Vision Res. 2002;42:2235–2251. doi: 10.1016/s0042-6989(02)00094-9. [DOI] [PubMed] [Google Scholar]
- 64.Reilly M, Ravi N. Microindentation of the young porcine ocular lens. J Biomech Eng. 2009;131:044502. doi: 10.1115/1.3072891. [DOI] [PubMed] [Google Scholar]
- 65.Kammel R, Ackermann R, Mai T, Damm C, Nolte S. Pig lenses in a lens stretcher: implications for presbyopia treatment. Optom Vis Sci. 2012;89:908–915. doi: 10.1097/OPX.0b013e318255da87. [DOI] [PubMed] [Google Scholar]
- 66.De Korte CL, Van Der Steen AFW, Thijssen JM, Duindam JJ, Otto C, Puppels GJ. Relation between local acoustic parameters and protein distribution in human and porcine eye lenses. Exp Eye Res. 1994;59:617–627. doi: 10.1006/exer.1994.1147. [DOI] [PubMed] [Google Scholar]
- 67.Reilly MA, Rapp B, Hamilton PD, Shen AQ, Ravi N. Material characterization of porcine lenticular soluble proteins. Biomacromolecules. 2008;9:1519–1526. doi: 10.1021/bm701229t. [DOI] [PubMed] [Google Scholar]