Abstract
Methylmercury (MeHg) is a well-known neurotoxicant; however, its role in metabolic diseases has been gaining wider attention. We have previously shown that MeHg causes metabolic alterations in Caenorhabditis elegans, leading to decreased nicotinamide adenine dinucleotide cofactor, mitochondrial dysfunction, and oxidative stress. We were, therefore, interested in whether MeHg also affects nutrient metabolism, particularly lipid homeostasis, which may contribute to the development of metabolic conditions such as obesity or metabolic syndrome (MS). RNA from wild-type worms exposed to MeHg was collected immediately after treatment and used for gene expression analysis by DNA microarray. MeHg differentially regulated 215 genes, 17 genes involved in lipid homeostasis, and 12 genes involved in carbohydrate homeostasis. Of particular interest was cebp-1, the worm ortholog to human C/EBP, a pro-adipogenic transcription factor implicated in MS. MeHg increased the expression of cebp-1 as well as pro-adipogenic transcription factors sbp-1 and nhr-49, triglyceride synthesis enzyme acl-6, and lipid transport proteins vit-2 and vit-6. Concurrent with the altered gene expression, MeHg increased triglyceride levels, lipid storage, and feeding behaviors. Worms expressing mutant cebp-1 were protected from MeHg-induced alterations in lipid content, feeding behaviors, and gene expression, highlighting the importance of this transcription factor in the worm’s response to MeHg. Taken together, our data demonstrate that MeHg induces biochemical, metabolic, and behavioral changes in C. elegans that can lead to metabolic dysfunction.
Keywords: methylmercury, C/EBP, cebp-1, lipid homeostasis
Obesity and metabolic syndrome (MS) are worldwide health concerns. MS is a multifactorial condition, which includes obesity, insulin resistance, diabetes mellitus, and dyslipidemia. While increased caloric intake, sedentary lifestyles, and genetic factors influence an individual’s weight, there is considerable data that support a role for environmental factors in its etiology (Chamorro-Garcia and Blumberg, 2019; Kassotis and Stapleton, 2019). Recently, heavy metals have been implicated in lipid dysregulation involved in obesity and MS. Several studies have shown associations between an increase in blood essential metal levels (copper, iron, and manganese) in childhood obesity, elevated cholesterol, and elevated serum triglyceride levels in human populations (Fan et al., 2017; Lima et al., 2006; Ma et al., 2017; Orr et al., 2014). The ability of metals to increase fat stores and cause dyslipidemia is corroborated by biochemical and animal studies. Manganese has been shown to activate acetyl-CoA carboxylase in the liver of exposed rats, leading to an induction of fatty acid biosynthesis (Scorpio and Masoro, 1970). In addition, genetic models of obesity, such as the KK/HIJ polygenic obese mouse model, have elevated serum metal levels (Ma et al., 2017). These associations between metal exposure and fat accumulation extend evolutionarily into Caenorhabditis elegans where elevated iron levels increase serum and glucocorticoid-inducible kinase to promote fat accumulation (Wang et al., 2016) and manganese exposure impairs dopaminergic signaling leading to increased lipid accumulation (Gubert et al., 2018).
Methylmercury (MeHg) is a well-known neurotoxic metal that is linked to long-term, latent health effects. MeHg exposure occurs primarily through fish consumption, with the highest concentrations present in predatory species, such as swordfish, tuna, and shark. Developmental exposure to MeHg causes cognitive and behavioral dysfunction in children, and cumulative exposure to MeHg over an adult’s lifetime has been linked to the development of neurodegenerative diseases, such as Parkinson’s disease (Clarkson and Magos, 2006; Landrigan et al., 2005). The non-neuronal effects of MeHg have been gaining wider attention. There is growing evidence that there is a link between MeHg exposure and development of MS. Historically, MeHg poisonings have been associated with anorexic effects on weight in humans (Berthoud et al., 1976; Schroeder and Mitchener, 1975) and experimental animals (Berthoud et al., 1976; Ferrer et al., 2018; Magos, 1982). However, the effects of dietary levels of MeHg on weight are only recently being investigated. Evaluation of National Health and Nutrition Examination Survey (NHANES) and Koran NHANES (KNHANES) data from 2003–2014 to 2011–2013, respectively, support an association between blood mercury levels, alone or in combination with other heavy metals, with MS, obesity, and lipid dysregulation (Bulka et al., 2019; Lee, 2018; Wang et al., 2018). Elevated blood mercury levels are associated with increased visceral adipose tissue (Park et al., 2017). In addition, toenail mercury levels have been associated with development of MS (Park and Seo, 2016). Blood mercury levels are dependent on recent exposures to MeHg, whereas hair and toenail MeHg levels reveal longer-term exposures to the metal. It remains to be determined whether there is a causal relationship between MeHg and obesity or MS; current weight of evidence analysis of 34 epidemiological studies describes MeHg as a contributing factor in the development of metabolic diseases, due to the lack of prospective cohort studies and the multiple confounding variables in current literature (Roy et al., 2017).
Mechanistic and biochemical analyses of MeHg exposure and lipid dysregulation have been limited in animal models. Moreira et al. (2012) have investigated the effects of MeHg on cholesterol levels in mice, showing that MeHg altered total cholesterol and nonhigh-density lipoprotein levels. Caenorhabditis elegans offers a simplified platform to explore the relationship between lipid metabolism and MeHg exposure. There are key differences between mammalian and nematode lipid homeostasis, such as C. elegans being a cholesterol auxotroph and that triglycerides are stored in intestinal cells and the hypodermis rather than specialized adipocytes as in mammals (Lemieux and Ashrafi, 2015). However, worms and mammals both accumulate and store lipids as triglycerides, and share conserved pathways in synthesizing and oxidizing fatty acids, and in regulating lipid homeostasis. In mammals, transcriptional regulators of fat homeostasis are linked with the hypertrophy and hyperplasia of adipocytes, leading to dyslipidemia and obesity (Goto et al., 2011; Lee et al., 2019; Tang et al., 2003). These include the transcription factors CCAAT-enhancer-binding proteins (C/EBPs), peroxisome proliferator activated receptor gamma (PPARγ), and sterol response element binding protein (SREBP), all of which have orthologs in the worm (Nomura et al., 2010; Van Gilst et al., 2005; Xu et al., 2015). Finally, both nematodes and mammals regulate energy balance through the use of neuroendocrine signaling pathways (Barros et al., 2014; Gubert et al., 2018; Hu et al., 2018; Marraudino et al., 2018; Ramos-Lopez et al., 2018). These include serotonin, dopamine, glutamate, and adiponectin. Both glutamate and dopamine levels are significantly affected by MeHg (Culbreth and Aschner, 2016; Dare et al., 2003; Farina et al., 2003; Martinez-Finley et al., 2013a; Mutkus et al., 2005b), suggesting that MeHg may influence the worm’s caloric intake or energy expenditure. Therefore, we hypothesized that MeHg may alter lipid homeostasis, leading to increased lipid accumulation and altered feeding behaviors. We tested this hypothesis by performing DNA microarray analysis for worms treated with MeHg to identify genes involved in lipid homeostasis that are altered by exposure. After identifying the pro-adipogenic transcription factor cebp-1 as being upregulated by MeHg, we investigated triglyceride levels, lipid accumulation, and pro-adipogenic gene expression in wild type and cebp-1 mutant worms. Finally, feeding and locomotor behaviors linked to dopamine, serotonin, and glutamate neurotransmitter activity were assessed in the wild type and cebp-1 mutant worms.
MATERIALS AND METHODS
Reagents
Unless otherwise stated all reagents were obtained from Sigma-Aldrich (St. Louis, Missouri). TaqMan® gene expression assays utilized include acl-6 (Ce02475286_g1), ama-1 (Ce02462735_g1), ctl-3 (Ce02406266_g1), sod-4 (Ce02451138_g1), cebp-1 (Ce02491882_g1), sbp-1 (Ce02453000_m1), nhr-49 (Ce02412670_g1), vit-2 (Ce02505533_g1), and vit-6 (Ce02456459_g1) (Thermo Fisher Scientific, Waltham, Massachusetts).
Caenorhabditis elegans strains and handling of the worms
Caenorhabditis elegans strains were handled and maintained at 20˚C on Nematode Growth Medium (NGM) plates seeded with OP-50 strain of Escherichia coli, as previously described (Brenner, 1974). The following strains were used in this study: N2, CZ8920 (cebp-1 [tm2807]), and CB1112 (cat-2 [e1112]). All strains were provided by the Caenorhabditis Genetic Center (CGC; University of Minnesota). Synchronous L1 populations were obtained as previously described (Stiernagle, 1999). In brief, embryos were isolated from gravid worms using a bleaching solution (1% NaOCl and 0.25 M NaOH) followed by a sucrose gradient to segregate eggs from worm and bacterial debris. Unless otherwise stated, synchronized L1 worms were treated with 10 or 20 μM MeHg for 30 min in M9 liquid buffer at 25˚C on an Eppendorf tube rotator. We have previously shown these concentrations are below the LD50 for MeHg in C. elegans and mean intraworm concentration of MeHg immediately following treatment is 1 ppb.(Caito and Aschner, 2016; Martinez-Finley et al., 2013b).
RNA isolation, DNA synthesis, and microarray hybridization
RNA from 20,000 worms per treatment was isolated using Trizol followed by chloroform extraction, as previously described (Chomczynski and Mackey, 1995). RNA quality was analyzed with the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, California) using the RNA 6000 nano kit. All RNA samples were of sufficient quality with RNA integrity number (RIN) >7. One hundred nanograms of RNA was used as the template for cDNA synthesis and in vitro transcription to synthesized biotin-modified aRNA using the GeneChip WT PLUS Reagent Kit (Affymetrix, 902281). A total of 15 μg of biotin-labeled aRNA was fragmented and hybridized to C. elegans Gene 1.0 ST Array (Affymetrix, 902160). The samples were processed and scanned using Affymetrix instrumentation and with hybridization, washing and scanning parameters provided by the manufacturer.
Microarray gene expression data analysis
Following hybridization and scanning, the microarray intensity data from CEL files were read using the oligo package from Bioconductor v3.0 and RMA preprocessing of probe level data was performed. Summarized data were filtered to retain transcript clusters belonging to the C. elegans GeneChip ST 1.0 main platform design that hybridized exclusively to target. Each of the treatment groups was tested for differential expression against the control samples. Moderated t-tests, followed by FDR correction using the Benjamini Hochberg method, available from limma package in Bioconductor v3.0 were performed. The differential expression cutoff criterion consisted of adjusted p-value <.05. DAVID was used for functional annotation enrichment for the resulting genes shared between and unique to the 3 contrasts. Gene Ontology was performed using WEB-based PANTHER Classification System (http://www.pantherdb.org/) (Mi et al., 2019).The entire data set is available at https://datadryad.org/review? doi=doi:10.5061/dryad.j9r1j26.
Real-time qPCR gene expression
To validate the microarray hybridizations and analysis, we chose 3 genes that were upregulated by MeHg treatment (ctl-3, sod-4, and cebp-1) and one gene that was downregulated by MeHg treatment (acl-6), and quantified their relative mRNA expression level by qPCR, normalizing against ama-1 gene as a housekeeping gene. Following RNA extraction, cDNA was synthesized from 1 mg of total RNA using the Appled Biosystems’ High-Capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific). Specific TaqMan® gene expression assays for the genes were used according to manufacturer’s instructions.
In addition, RNA was isolated from N2 and cebp-1 mutant worms 24 h post-MeHg exposure to assess gene expression profiles for lipid homeostatic genes (cebp-1, sbp-1, nhr-49, acl-6, vit-2, and vit-6) by TaqMan® gene expression assays.
Triglyceride quantification
Total triglycerides were measured using the Enzychrom™ triglyceride quantification kit according to the manufacturer’s instructions (BioAssay Systems, Hayward, California). In brief, 48 h post-MeHg treatment, 20,000 worms were homogenized in triglyceride assay buffer. Extracts were incubated for 30 min at room temperature with the triglyceride assay reagent mix and absorbency (optical density: 570 nm) was read. Data are expressed as mmol triglycerides/μg protein.
BODIPY and Nile Red staining
Visualization of fat stores in MeHg-treated worms was performed by BODIPY 493/503 (Invitrogen, Carlsbad, California) vital staining, which stains neutral and nonpolar lipids, and Nile Red staining of fixed nematodes. Twenty thousand L1 N2 and cebp-1 mutant worms were incubated with MeHg for 30 min, washed and were transferred to agar plates. Seventy-two hours after treatment worms were washed off the plates and incubated with BODIPY 493/503 (6.7 μg/ml in M9) for 20 min, as previously described (Klapper et al., 2011) or were fixed for Nile Red staining, as previously described (Pino et al., 2013). For the BODIPY 493/503 staining, worms were washed 3 times in M9, placed on OP-50-spread NGM plates for 2 h, allowing for excess dye to be excreted. Twenty worms were mounted on 4% agarose pads in M9 and anesthetized with 0.2% tricaine/0.02% tetramisole in M9. Fluorescence observations were performed with an epifluorescence microscope (Nikon Eclipse 80i) equipped with a Lambda LS Xenon lamp and Nikon Plan Apo 60× 1.3 oil objective. Quantification of BODIPY 493/503 fluorescence was performed using ImageJ 1.52 software as previously described (Gavet and Pines, 2010). For the Nile Red staining, 1000 worms were washed with 0.1% Triton X-100 in PBS, fixed in 40% isopropanol for 3 min, and incubated with 3 μg/ml Nile Red in 40% isopropanol for 30 min. Worms were washed with M9 and loaded onto a 96 well microtiter plate. Nile Red fluorescence was read at excitation 560, emission 590. Data were normalized to worm number and protein levels.
Feeding behavioral analysis
L1 worms were seeded on OP-50-spread NGM plates following MeHg treatment and 72 h post-treatment worms were assessed for behaviors associated with feeding, including pharyngeal pumping, locomotion, and basal slowing response. For pharyngeal pumping, 10 worms were transferred to fresh OP-50-seeded NGM plates and number of pharynx pumps were scored for 30 s. Locomotion was assessed by the body-bend assay. Worms were plated on an NGM plate without bacteria and scored for the number of body-bends scored for 30 s. The basal slowing response assay measures dopamine-dependent behavior that mediates the worm slowing movement to consume food. The basal slowing response assay was performed as previously described (Sawin et al., 2000). The number of body-bends was assessed for worms either placed on NGM plates seeded or unseeded with OP-50 E. coli. Worms deficient in cat-2 (homolog of mammalian tyrosine hydroxylase [TH]) were used as a positive control. Data for the basal slowing response are presented as the change in body-bends, calculated by subtracting the number of body-bends of worms plated on OP-50-seeded plates from the number of body-bends of worms plated on unseeded plates.
Statistics
Statistical analyses were performed using Prism 8 software (Graphpad, San Diego, California).
Statistical analysis of significance was carried out either by Student’s t-test (Figure 1) or 2-way analysis of variance (ANOVA). Values of p < .05 were considered statistically significant.
Figure 1.
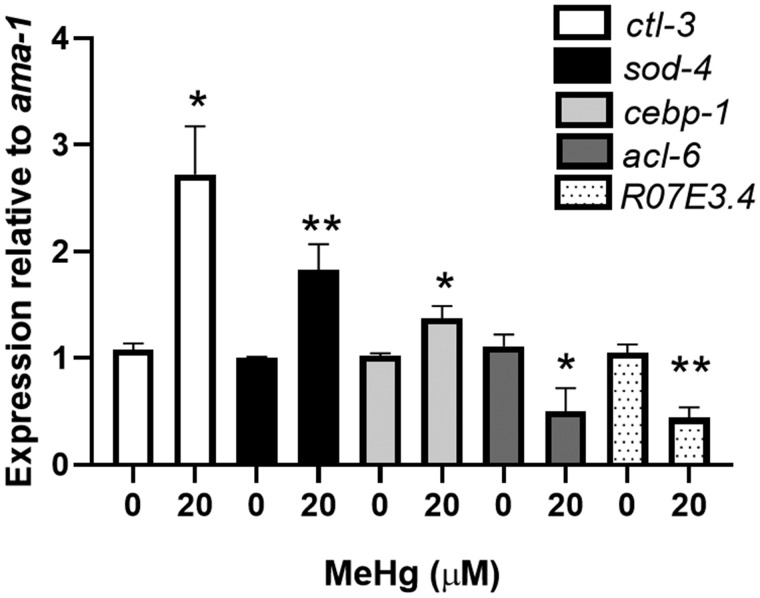
Real-time RT-PCR validation of microarray results of selected genes. N2 worms were treated with 20 μM MeHg for 30 min and RNA was extracted immediately following treatment. Levels of ctl-3, sod-4, cebp-1, and acl-6 were measured by quantitative PCR and normalized to ama-1 as a housekeeping gene. Data are expressed as mean relative expression ± SEM from 5 independent experiments. *p < .05, **p < .01 as compared with untreated control.
RESULTS
Identification of Lipid Metabolic Genes Dysregulated by MeHg
Xenobiotic metabolism genes are known to be upregulated by MeHg exposure in C. elegans and mammalian organisms (Arantes et al., 2016; Helmcke and Aschner, 2010; Hwang and Naganuma, 2006; Ni et al., 2011; Rudgalvyte et al., 2017); however, the effect of MeHg on nutrient metabolism has not been well described. To assess whether MeHg alters lipid metabolic genes, we carried out microarray analysis on L1 larval N2 worms exposed to 20 μM MeHg for 30 min. Gene expression arrays showed that similar to xenobiotic metabolic genes, genes associated with both lipid and carbohydrate metabolism are upregulated immediately following MeHg exposure. Of the 8902 probe sets with UniGene identifiers, 169 genes were upregulated immediately following MeHg exposure and 46 were downregulated (Supplementary data). To validate the microarray results, we performed real-time RT-PCR on 5 genes differentially expressed in the microarray results (Figure 1). The genes ctl-3 (catalase ortholog), sod-4 (superoxide dismutase 3 ortholog), and cebp-1 (C/EBP ortholog) showed upregulation in N2 worms immediately following MeHg treatment and acl-6 (glycerol-3-phosphate acyltransferase ortholog) showed downregulation by MeHg in the microarray results. Messenger RNA levels for ctl-3, sod-4, and cebp-1 were increased by 170%, 82%, and 38%, respectively, and acl-6 message levels were decreased by 50% immediately following MeHg treatment.
Pathway Analysis using Gene Ontology with PANTHER Classification System Toolkit highlighted xenobiotic metabolism, lipid metabolism, and carbohydrate metabolism, among others, as being significantly altered by MeHg exposure (Table 1). Seventeen differentially expressed genes identified in the microarray results were identified as being associated with lipid metabolism (Table 2). Ten genes were involved in modifications and processing of lipids, 3 genes with lipid storage, 2 genes with lipid catabolism, and 2 genes were transcription factors involved in lipid homeostasis. The transcription factor cebp-1 is the worm ortholog to human C/EBP-1 (Yan et al., 2009), an important regulator of lipid biogenesis and adipocyte differentiation. Dysregulation of C/EBP-1 in humans is known to occur in obesity and MS. Therefore, the upregulation of cebp-1 transcription factor by MeHg immediately following treatment suggested the potential for long-term changes in lipid homeostasis in C. elegans.
Table 1.
Occurrence of gene ontology for biological processes that show differential regulation following MeHg treatment.
| Gene family | Count | % of total | p-Value |
|---|---|---|---|
| Increased expression | |||
| Lipid metabolism | 16 | 7.4 | 5.89 × 10−6 |
| Glutathione metabolism | 14 | 6.5 | 1.14 × 10−13 |
| Xenobiotic metabolism | 12 | 5.5 | 2.55 × 10−3 |
| Antioxidant response | 12 | 5.6 | 2.79 × 10−3 |
| Carbohydrate metabolism | 10 | 4.7 | 1.60 × 10−3 |
| Gene expression | 10 | 4.7 | 4.65 × 10−3 |
| ER stress/unfolded protein response | 6 | 2.8 | 2.96 × 10−3 |
| Amino acid metabolism | 5 | 2.3 | 5.46 × 10−3 |
| Cell signaling | 5 | 2.3 | 7.49 × 10−3 |
| Mitochondrial function | 3 | 1.4 | 3.52 × 10−2 |
| Decreased expression | |||
| Amino acid metabolism | 5 | 2.3 | 3.53 × 10−5 |
| Transition metal binding | 5 | 2.3 | 4.19 × 10−2 |
Table 2.
Identities of genes related to lipid homeostasis that are altered by MeHg expression.
| Gene | LogFC | Human ortholog | Function |
|---|---|---|---|
| ugt-63 | 2.95 | UGT3A2/UGT3A1 | Glycosyltransferase, lipid modification |
| ugt-8 | 2.74 | UGT3A2/UGT3A1 | Glycosyltransferase, lipid modification |
| ugt-13 | 2.73 | UGT3A2/UGT3A1 | Glycosyltransferase, lipid modification |
| ugt-25 | 2.70 | UGT3A2/UGT3A1 | Glycosyltransferase, lipid modification |
| ugt-9 | 2.34 | UGT3A2/UGT3A1 | Glycosyltransferase, lipid modification |
| stdh-2 | 2.16 | HSD17B3 | Steroid dehydrogenase |
| ugt-14 | 1.93 | UGT3A2/UGT3A1 | Glycosyltransferase, lipid modification |
| nhr-178 | 1.73 | ROR-α | Transcription factor |
| ugt-11 | 1.52 | UGT3A2/UGT3A1 | Glycosyltransferase, lipid modification |
| ugt-41 | 1.37 | UGT3A2/UGT3A1 | Glycosyltransferase, lipid modification |
| cebp-1 | 1.17 | C/EBP-1 | Transcription factor |
| dod-20 | 1.17 | Lipid storage | |
| acl-14 | 1.07 | LPGAT1 | Lysophosphatidylglycerol acyltransferase |
| ipla-2 | 0.78 | Phospholipase A2 | Phospholipase |
| C56E10.3 | 0.78 | Lipid storage | |
| acl-12 | 0.66 | LPGAT1 | Lysophosphatidylglycerol acyltransferase |
| C17G10.7 | −1.29 | Lipid storage |
Bolded text is the gene investigated in the remainder of the paper.
Intercellular Lipid Levels Are Increased by MeHg
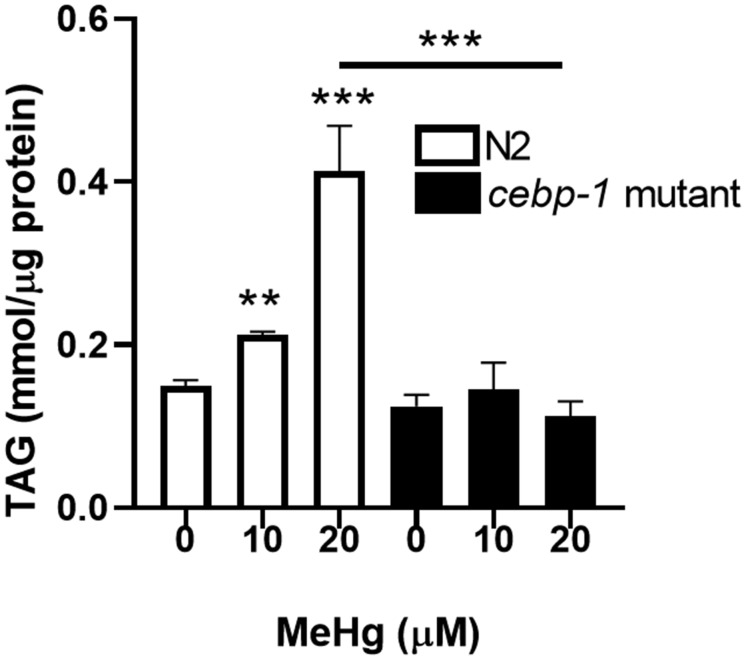
We have previously shown that early-life exposure to MeHg has both neurotoxic and metabolic sequellae in C. elegans (Caito and Aschner, 2016; Martinez-Finley et al., 2013a,b). Therefore, we decided to investigate whether early-life upregulation of cebp-1 by MeHg could lead to increased lipid levels later in life. Intracellular lipids were analyzed through analytical quantification of triglycerides and visualization of lipid stores in wild-type N2 worms and in worms deficient in cebp-1. Mutant cebp-1 worms were equally sensitive to MeHg as N2 worms; the dose-response survival curves for N2 and cebp-1 mutant worms were not significantly different (data not shown). Triglycerides are the major storage lipid in both C. elegans and in humans; therefore, if MeHg increased lipid storage, we hypothesized the intercellular triglyceride content would increase. L1 N2 and cebp-1 mutant worms were treated with 10 or 20 μM MeHg for 30 min, and were allowed to feed and mature for 48 h before harvesting for extraction of triglycerides. MeHg dose-dependently increased total triglyceride content in N2 worms (Figure 2). Mutant cebp-1 worms contained lower basal levels of triglycerides, likely due to the importance of cebp-1 transcription factor in lipid biosynthesis. Triglyceride levels were significantly lower in the cebp-1 mutants treated with MeHg than the N2 worms, suggesting the accumulation of triglycerides in the N2 worms is due to the activation of the cebp-1 transcription factor by MeHg.
Figure 2.
Triglyceride content is increased after MeHg treatment. N2 and cebp-1 mutant worms were treated with 10 or 20 μM MeHg for 30 min. Whole worm extracts were used to measure total triglyceride levels 48 h after MeHg treatment using a spectrophotometric triglyceride quantification kit. Data are expressed as mean triglycerides mmol/μg protein ± SEM. All data are representative of 5 independent experiments. **p < .01,***p < .001 as compared with untreated control.
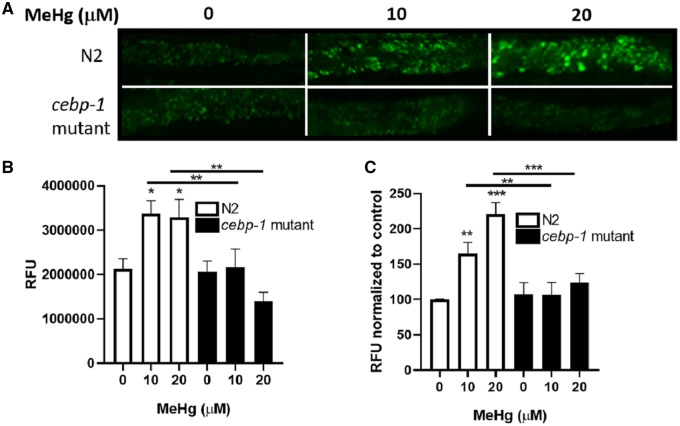
We next investigated whether worms treated with MeHg had increased fat stores. Caenorhabditis elegans store lipids in their intestine and hypodermis. Lipid stores were visualized using BODIPY 493/503 or Nile Red 72 post-MeHg treatment. N2 worms treated with MeHg had increased BODIPY 493/503 staining (Figures 3A and 3B), suggesting that there was an increase in fat stores following MeHg exposure. Mutant cebp-1 worms did not have increased fat storage following MeHg exposure. Similarly, MeHg increased the amount of Nile Red fluorescence in N2 worms (Figure 3C), which was absent in the cebp-1 mutant worms. Combined, these data suggest that there are increased numbers of lipid droplet stores in MeHg-treated worms and that the formation of these droplets is dependent on the cebp-1 transcription factor.
Figure 3.
MeHg-induced fat accumulation is attenuated in cebp-1 mutant. N2 and cebp-1 mutant worms were treated with 10 or 20 μM MeHg for 30 min. 72 h after treatment, fat accumulation was measured by fluorescence staining of worms with BODIPY 493/503 and Nile Red. A, Representative fluorescence images are shown from 4 experiments, with (B) quantification of BODIPY 493/503 fluorescence. C, Nile Red fluorescence normalized. Data are expressed as mean fluorescence normalized to protein content ± SEM from 5 independent experiments. *p < .05, **p < .01, ***p < .001 as compared with untreated control.
MeHg Increases Pro-Adipogenic Gene Transcription
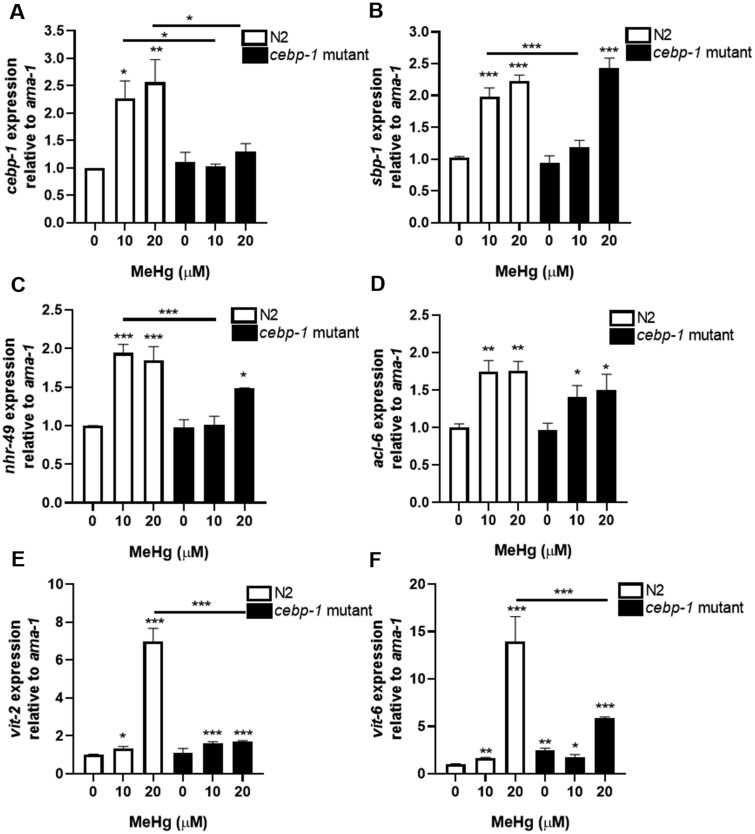
In humans, C/EBP is a known regulator of adipocyte differentiation, hyperplasia (increase in adipocyte cell numbers), and hypertrophy (adipocyte cell size) (Lee et al., 2019; Tang et al., 2003). Prolonged expression of C/EBP can lead to adipogenesis in response to xenobiotics such as ethanol and triphenyl phosphate (Cano-Sancho et al., 2017; Chen et al., 2009). As our initial characterization of increased cebp-1 gene expression occurred immediately following MeHg treatment and increased lipid content occurred in adulthood, we next determined whether cebp-1 gene expression remained elevated after MeHg exposure. Gene expression of cebp-1 was elevated in N2 worms 24 h post-treatment with 10 and 20 μM MeHg as compared with untreated control (Figure 4A). Mutant cebp-1 worms showed no induction of cebp-1 gene expression 24 h after MeHg treatment. These data suggest that the activity of cebp-1 transcription factor continues after initial treatment with MeHg, possibly contributing to the lipid accumulation observed as the worm matures.
Figure 4.
Increased expression of pro-adipogenic genes by MeHg exposure is partially inhibited in cebp-1 mutant. N2 and cebp-1 mutant worms were treated with MeHg for 30 min and RNA was extracted 24 h after treatment. Levels of (A) cebp-1 (ortholog of human C/EBP), (B) nhr-49 (functional homolog of human PPARγ), (C) sbp-1 (ortholog of human SREBP-1), (D) acl-6 (ortholog to glycerol-3-phosphate acyltransferase), (E) vit-2, and (F) vit-6 were measured by quantitative PCR and normalized to ama-1 as a housekeeping gene. Data are expressed as mean relative expression ± SEM from 4 independent experiments. *p < .05, **p < .01, ***p < .001 as compared with untreated control.
During adipogenesis C/EBP works in concert with multiple transcription factors to lead to adipocyte differentiation in mammals (Lee et al., 2019; Tang et al., 2003), 2 of which are PPARγ and SREBP. We investigated whether MeHg increased the expression of the worm homologs to PPARγ and SREBP, nhr-49 and sbp-1, respectively, in N2 worms. Gene expression analysis revealed that 24 h after treatment with either 10 or 20 μM MeHg, the levels of nhr-49 and sbp-1 were significantly increased compared with untreated control worms (Figures 4B and 4C). Mutation of cebp-1 prevented upregulation of nhr-49 and sbp-1 gene expression in response to 10 μM MeHg 24 h post-treatment. However, both sbp-1 and nhr-49 levels were elevated in the cebp-1 mutant worms 24 h post 20 μM MeHg treatment (Figures 4B and 4C). This suggests there are dose-dependent effects of MeHg on pro-adipogenic genes, at lower doses the levels of sbp-1 and nhr-49 are dependent on cebp-1 expression, but at higher levels of MeHg has effects on nhr-49 and sbp-1 that are not cebp-1 dependent.
Concurrent with upregulation of adipogenic transcription factors, MeHg increased the expression of acl-6, the worm ortholog of glycerol-3-phosphate acyltransferase, the rate-limiting enzyme in triglyceride biosynthesis (Figure 4D). Glycerol 3-phosphate acyltransferase plays critical roles in obesity and insulin resistance (Yu et al., 2018). Our data show a time-dependent expression of acl-6 in response to MeHg. Initially following exposure to MeHg, acl-6 is downregulated in C. elegans (Figure 1); however, 24 h postexposure, acl-6 is increased by MeHg as compared with untreated control. It is unclear why acl-6 would be initially downregulated by MeHg considering that triglyceride levels increase following MeHg exposure. However, this time-dependent observation may be explained due to acl-6 gene regulation. The transcription of glycerol-3-phosphate acyltransferase is dependent on SREBP (Ericsson et al., 1997). As MeHg increases sbp-1 expression, it is expected that sbp-1 target genes, such as acl-6 expression, would also change. There was no difference between cebp-1 mutant worms and N2 in acl-6 expression in response to MeHg. This may relate to only a partial decrease in sbp-1 expression in the cebp-1 mutants following MeHg treatment.
With increased levels of pro-adipogenic transcription factors and increased lipid content, we examined whether lipid transport proteins were increased in C. elegans in response to MeHg. Vitellogenins (vit-1 to vit-6) are yolk proteins with homology to human apolipoprotein B-100 (Baker, 1988) that deliver cholesterol to oocytes through receptor-mediated endocytosis mechanism mediated by RME-2, a member of the LDL receptor superfamily (Grant and Hirsh, 1999). The levels of vit-2 and vit-6 were increased in N2 worms 24 h post-treatment with 10 and 20 μM MeHg (Figures 4E and 4F). These data suggest that during MeHg exposure there were increased expression of lipid transport proteins in the worms to accommodate increased lipid accumulation. Mutation of cebp-1 prevented the upregulation of vit-2 and vit-6 gene expression following 20 μM MeHg treatment.
Feeding Behavioral Changes in Response to MeHg Are Dependent on Cebp-1
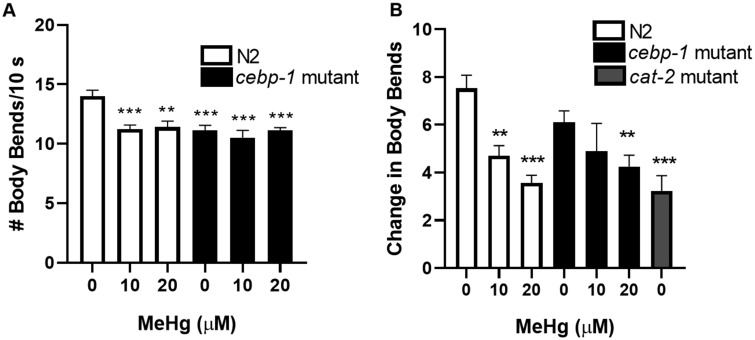
Caenorhabditis elegans continuously feed on bacteria, yet have refined feeding behaviors that are linked to specific neuronal activity. Since MeHg is a well-known neurotoxin, we hypothesized that the dysregulation in lipid levels may derive from MeHg interfering with feeding behaviors. Treatment of N2 worms with 10 or 20 μM MeHg decreased C. elegans locomotion 72 h after treatment (Figure 5A). These data suggest that worms treated with MeHg early in development have lower locomotive activity as adults. On a basal level, the untreated cebp-1 mutants had fewer body-bends than the untreated N2 worms; however, there was no further decrease in the locomotion of cebp-1 worms in response to MeHg (Figure 5A).
Figure 5.
Mutation of cebp-1 does not affect MeHg-induced locomotive dysfunction. N2 and cebp-1 mutant worms were treated with 10 or 20 μM MeHg, and locomotion behavioral analysis was performed 72 h post-treatment. (A) Number of body-bends were analyzed (B) Dopaminergic-dependent behavior was analyzed by the basal slowing response. Worms expressing mutant cat-2 were used as a positive control. Data are expressed as means ± SEM from 9 independent experiments. **p < .01,***p < .001 as compared with untreated control.
Previously we have demonstrated that MeHg decreases dopaminergic function in N2 worms, causing neurodegeneration, decreased dopamine neurotransmitter levels and decreases the basal slowing response (Caito and Aschner, 2016; Martinez-Finley et al., 2013a). The basal slowing response assay measures the worm’s ability to sense a food source and slow its motion to consume the food (Sawin et al., 2000). This behavior is dopamine-dependent, as worms that lack TH (cat-2 mutants), the rate-limiting enzyme in dopamine synthesis, do not exhibit this behavior (Sawin et al., 2000). N2 worms treated with 10 or 20 μM MeHg dose-dependently decrease the basal slowing response similar to the level of dopamine deficient cat-2 worms (Figure 5B). Treatment of 10 or 20 μM MeHg in cebp-1 mutant worms decreased the basal slowing response and was not significantly different from the N2 worms treated with MeHg (Figure 5B). These data suggest that MeHg-induced dopaminergic dysfunction in the basal slowing response is not influenced by cebp-1.
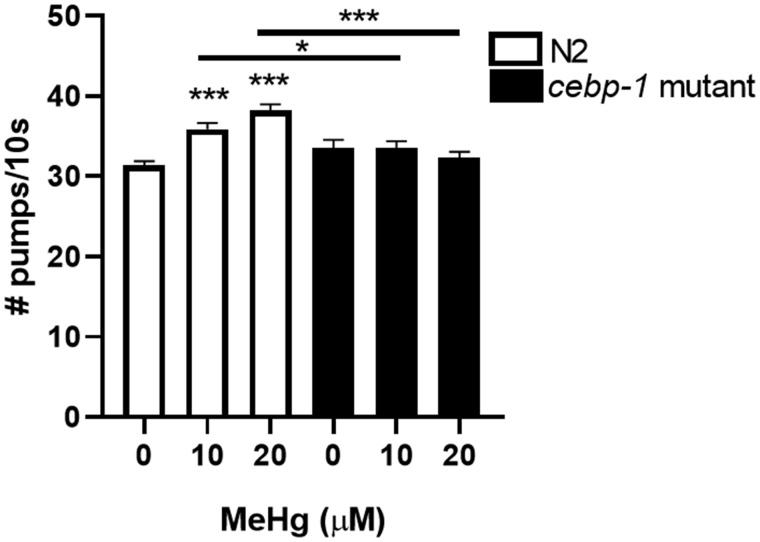
We next examined whether worms treated with MeHg consumed more bacteria than the untreated worms. The worm pharynx is an organ in the head of the animal that pulses in rhythm to draw food into the gastrointestinal tract. The pumping action is controlled by both serotonergic and glutamatergic neurotransmitters (Horvitz et al., 1982; Niacaris and Avery, 2003). While the effects of MeHg on serotonin release is not well characterized, MeHg is a known disruptor of glutamate signaling, leading to increased glutamate concentration in synapses and excitotoxicity of neurons (Culbreth and Aschner, 2016; Farina et al., 2003; Mutkus et al., 2005a). N2 worms were treated with 10 or 20 μM MeHg and pharynx pumping was assessed 72 h later. MeHg-treated worms had increased number of pharynx pumps as compared with the untreated worms (Figure 6). This is indicative that the MeHg-treated worms ate more food than the untreated worms. Mutant cebp-1 worms did not have increased pharynx pumps following MeHg treatment. This suggests that cebp-1 has additional roles in modulating behavior of the worm following MeHg treatment.
Figure 6.
Mutation in cebp-1 attenuates MeHg-induced increase in food consumption. N2 and cebp-1 mutant worms were treated with 10 or 20 μM MeHg, and food consumption was analyzed by the pharynx pump assay 72 h post-treatment. Data are expressed as means ± SEM from 9 independent experiments. ***p < .001 as compared with untreated control.
DISCUSSION
MeHg is a known neurotoxin that leads to cellular dysfunction and altered behavior; however, its role in metabolic diseases is poorly understood. Obesity and MS are multifactorial diseases, which are traditionally thought to be caused by genetic susceptibility, diet, and sedentary lifestyle. The ability of environmental agents to contribute to the development of metabolic conditions is gaining wider attention. MeHg has been shown to effect pathways important for the development of obesity and MS. MeHg induces mitochondrial dysfunction, decreasing total numbers of mitochondria and mitochondrial DNA (mtDNA) copy numbers, disrupts the mitochondrial membrane potential, leading to altered respiratory capacity and generation of reactive oxygen species (ROS) (Bragadin et al., 2002; Caito and Aschner, 2016; Dreiem et al., 2005). In addition, MeHg alters the circulating cholesterol profile in mice, which has implications for both metabolic disorders and cardiovascular disease (Moreira et al., 2012). Excess fat and carbohydrates present in obesity lead to mitochondrial dysfunction, which reduces lipid and glucose metabolism, causes the degradation of mtDNA and generates ROS (de Mello et al., 2018). This leads to metabolic imbalance because lipids and sugars cannot be oxidized efficiently. Accompanying metabolic dysfunction in obesity and MS is altered gene expression in adipocytes that promote hyperplasia and hypertrophy (Lee et al., 2019). MeHg is known to disrupt cell signaling pathways and gene regulation; however, its effects on the transcriptional regulators involved in adipocyte function have not previously been investigated. Herein, we demonstrate that MeHg exposure induced an increase in fat storage, triglyceride levels, and pro-adipogenic gene transcription in C. elegans. Furthermore, these metabolic changes were dependent on functioning cebp-1 transcription factor.
The effect of MeHg on metabolic gene expression has previously been concentrated on xenobiotic metabolic genes, such as the genes regulated by the Nuclear factor erythroid 2-related factor 2 (Nrf2, SKN-1 in C. elegans) transcription factor. Herein, we report a DNA microarray gene expression analysis in C. elegans that identified differential expression of not only xenobiotic metabolic genes, but also carbohydrate and lipid metabolic genes. The majority of the lipid and carbohydrate metabolic genes identified were in genes that modify these molecules; however, in the case of the lipid metabolic genes, genes associated with lipid storage (C17G10.7, C56E10.3, and dod-20) and lipid biosynthesis (cebp-1 and nhr-178) were also upregulated by MeHg. These data suggest that nutrient metabolism may be adversely effected by MeHg exposure in C. elegans. Our gene expression data were collected immediately after an acute treatment with MeHg; therefore, long-term effects of MeHg on gene expression cannot be inferred from this microarray data set. However, our data are in agreement with 2 reports that investigate gene expression following subchronic MeHg treatment in C. elegans (Rudgalvyte et al., 2013, 2017). In particular, Rudgalvyte et al. (2017) found MeHg treatment from L1 to L4 life stages increased histone H3K4 trimethylation of both cebp-1 and sbp-1. Histone H3K4 trimethylation is a marker of active transcription, suggesting that both cebp-1 and sbp-1 are upregulated in worms in response to MeHg throughout larval development. Although Rudgalvyte et al.’s data are from worms treated with MeHg for a longer duration, similar trends in activation of cebp-1 and sbp-1 are evident.
The transcription factor cebp-1 was of particular interest due to its known roles in lipid accumulation and adipocyte function in humans. Mammals have 6 isoforms of C/EBP, with the most important for adipogensis and lipid dysfunction being C/EBPα and C/EBPβ (Guo et al., 2015). Caenorhabditis elegans have 2 homologs to mammalian C/EBPs, cebp-1 and cebp-2; however, there is no consensus on whether cebp-1 and cebp-2 have distinct homology to only one specific mammalian C/EBP isoform. In our gene expression analysis only cebp-1, and not cebp-2, was responsive to MeHg treatment. C/EBP transcription factors play important roles in the growth and regulation of adipocytes, both under physiologic conditions and pathologically during the development of obesity and MS. In 3T3-L1 adipocyte cell line, C/EBPβ is induced early in differentiation and transactivates the expression of C/EBPα, PPARγ, and SREBP1c transcription factors allowing for terminal differentiation (Guo et al., 2015). Knockdown of either C/EBPα or C/EBPβ in vitro prevents adipogenesis, whereas over expression induces adipocyte differentiation in the absence of hormonal stimuli (Zhang et al., 2011). Likewise, in mice, knockdown of C/EBPβ or C/EBPδ impairs development of adipocytes, thereby decreasing fat mass (Tanaka et al., 1997). In obese individuals, C/EBP proteins regulate both lipid and glucose metabolisms, and genetic variants of C/EBPα are associated with increased serum triglycerides (Olofsson et al., 2008). Induction of cebp-1 in C. elegans by MeHg occurred immediately after exposure and persisted for 24 h after exposure. At 24 h following MeHg exposure, there was also an upregulation of pro-fat-associated genes, such as nhr-49 (functional homolog to PPARγ), sbp-1 (ortholog to SREBP), acl-6, vit-2, and vit-6 in wild-type worms. PPARγ and SREBP are important pro-adipogenic transcriptional factors that regulate the expression of many lipid metabolism genes. Importantly, SREBP generates endogenous PPARγ ligands, maintaining the adipogenic response (Payne et al., 2009). Upregulation of these genes by MeHg should promote lipid dysregulation. Mutant worms that did not express a functional cebp-1 could not upregulate cebp-1 and had reduced upregulation of nhr-49, sbp-1, vit-2, and vit-6 in response to MeHg. Although worms do not store lipids in adipocytes, our data show similar regulation of adipogenic genes following MeHg treatment in C. elegans is analogous to what occurs during adipocyte differentiation in mammalian cells in response to adipogenic stimuli. This suggests that MeHg may dysregulate lipid metabolism through altering transcriptional pathways involved in adipocyte function.
Analysis of triglyceride levels and fat depot staining revealed that the effects of MeHg on lipid metabolic gene expression in C. elegans had a significant phenotypic effect. Worms treated with MeHg as L1 larva had significantly higher intracellular triglyceride levels as adults. Likewise, adult worms demonstrated increased lipid stores following MeHg treatment than the untreated worms. Although there is naturally an increase in lipid storage as worms mature (Palikaras et al., 2017; Watts and Ristow, 2017), the levels of triglycerides and staining of lipid stores were significantly higher in MeHg-treated N2 worms than control. These findings are in agreement with a recent observation of environmental exposure of zebra finches (Taeniopygia guttata) to MeHg leading to increased adiposity (Gerson et al., 2019). The relationship between lipid metabolism and MeHg needs further clarification. High doses of MeHg that model MeHg poisoning have been shown to be anorexic in rodents (Berthoud et al., 1976; Magos, 1982), but the effects of lower environmentally relevant exposures have not been previously investigated. Genetically obese KK-Ay diabetic mice accumulate more mercury that nonobese mice and show enhanced neurological damage and inflammation than the nonobese mice (Yamamoto et al., 2014). Likewise, obese macaque monkeys exposed to MeHg have higher MeHg concentrations in their brain than normal weight monkeys (Vahter et al., 1995). These studies suggest that there is a relationship among body weight, MeHg exposure, and toxicity. However, these studies have not examined whether MeHg exposure can alter lipid metabolism or promote an obese phenotype.
Although lipid accumulation and energy expenditure are important aspects of developing metabolic diseases, neuronal control of metabolism and feeding are equally important. The central nervous system plays an important role in sensing nutrient status of the individual and integrating hormonal signals from the pancreas and adipocytes to regulate caloric intake and energy expenditure (Timper and Bruning, 2017). In humans, the hypothalamic-pituitary axis integrates multiple hormonal signals including leptin, insulin, ghrelin, and adiponectin. In vitro studies of hypothalamic neuronal cell lines treated with MeHg show increased expression of neuropeptides pro-omiomelanocortin (Pomc) and Agouti-related peptide (Agrp), key regulators of homeostasis (Ferrer et al., 2018). In C. elegans, the neuronal control of nutrient sensing is simplified, but uses conserved neurotransmitter systems (dopamine, glutamate, and serotonin) as in humans (Dalliere et al., 2017). Dopamine in C. elegans is implicated in sensing food source, increasing turn frequency when leaving food, and defecation (Hills et al., 2004; Sawin et al., 2000; Vidal-Gadea et al., 2011). Serotonin is the main regulator of the pharynx muscles that contract to draw food into the nematode (Horvitz et al., 1982). Serotonergic neurons coordinate the action of the cholinergic MC and glutamatergic M3 motor neurons that directly synapse on pharyngeal muscle cells (Niacaris and Avery, 2003). Glutamate released from M3 neurons, activate glutamate-gated chloride channel AVR-15 expressed on pm4 and pm5 pharyngeal muscle cells to modulate the duration and frequency of pharyngeal pumping (Dent et al., 1997; Niacaris and Avery, 2003). Mutants deficient in glutamate signaling lose the ability to terminate action potentials on the pm4 and pm5 cells, resulting in reduced pumping rate (Greer et al., 2008). MeHg disrupts both dopamine and glutamate signaling. We have previously shown that MeHg decreases dopamine levels and behaviors in C. elegans and increases glutamatergic signaling and excitotoxicity in primary cell culture in vitro (Caito and Aschner, 2016; Martinez-Finley et al., 2013a; Mutkus et al., 2005a,b). It is, therefore, possible that MeHg alters nutrient signaling in the worm. In our present study, we observed that MeHg decreased the worms’ ability to detect E. coli food source and slow its motion to consume food (dopaminergic controlled function), whereas concurrently the MeHg-treated worms consumed more food and moved less than untreated worms (serotonergic and glutaminergic controlled functions). It is important to note, that MeHg’s effects on locomotion may be influenced by direct toxicity to muscle development and function, as recent studies in Drosophila highlight the myotoxicity of MeHg (Montgomery et al., 2014; Prince and Rand, 2017, 2018). Similar to MeHg, manganese exposure in C. elegans also decreases dopaminergic signaling and increased lipid accumulation (Gubert et al., 2018). The CEBP-1 transcription factor in C. elegans has not only been linked to metabolic functions, but also in neuronal function; controlling neurodevelopment, axonal function, and regeneration (Sharifnia et al., 2017; Yan et al., 2009). Mutation of cebp-1 had no effect on the dopaminergic dependent behaviors in response to MeHg, but prevented the increased feeding behaviors in C. elegans. As glutamatergic signaling influences pharynx pumping in worms and MeHg increases glutamate release from neurons, cebp-1 may be involved in glutamate regulation in response to MeHg.
Altogether, this study demonstrates a role for MeHg in lipid dysregulation. MeHg increases the expression of genes involved in lipid synthesis, transport, storage, and modification, as well as increases lipid accumulation in the worm. Concurrent with the lipid dysregulation were alterations in nutrient sensing and feeding behaviors, suggesting a link between MeHg-induced neurotoxicity and metabolic effects. The CEBP-1 transcription factor was identified as a regulator of metabolic function, as mutants deficient in cebp-1 did not accumulate as much lipids as wild-type worms, nor had as increased pro-adipogenic gene expression in response to MeHg. Further investigation into the effects of MeHg on lipid dysregulation is warranted.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ACKNOWLEDGMENTS
The DNA microarray was performed by the Albert Einstein College of Medicine Genomics Core Facility and the subsequent analysis was performed by Swapna Menon, M. Phil.
FUNDING
NIEHS R01 ES007331 and NIEHS R01 ES020852 (MA), and Husson University School of Pharmacy Research Grant (S.W.C.).
REFERENCES
- Arantes L. P., Peres T. V., Chen P., Caito S., Aschner M., Soares F. A. (2016). Guarana (Paullinia cupana mart.) attenuates methylmercury-induced toxicity in Caenorhabditis elegans. Toxicol. Res. (Camb) 5, 1629–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. E. (1988). Is vitellogenin an ancestor of apolipoprotein b-100 of human low-density lipoprotein and human lipoprotein lipase? Biochem. J. 255, 1057–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros A. G., Bridi J. C., de Souza B. R., de Castro Junior C., de Lima Torres K. C., Malard L., Jorio A., de Miranda D. M., Ashrafi K., Romano-Silva M. A. (2014). Dopamine signaling regulates fat content through beta-oxidation in Caenorhabditis elegans. PLoS One 9, e85874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud H. R., Garman R. H., Weiss B. (1976). Food intake, body weight, and brain histopathology in mice following chronic methylmercury treatment. Toxicol. Appl. Pharmacol. 36, 19–30. [DOI] [PubMed] [Google Scholar]
- Bragadin M., Marton D., Manente S., Grasso M., Toninello A. (2002). Methylmercury induces the opening of the permeability transition pore in rat liver mitochondria. J. Inorg. Biochem. 89, 159–162. [DOI] [PubMed] [Google Scholar]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulka C. M., Persky V. W., Daviglus M. L., Durazo-Arvizu R. A., Argos M. (2019). Multiple metal exposures and metabolic syndrome: A cross-sectional analysis of the national health and nutrition examination survey 2011-2014. Environ. Res. 168, 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caito S. W., Aschner M. (2016). Nad+ supplementation attenuates methylmercury dopaminergic and mitochondrial toxicity in Caenorhabditis elegans. Toxicol. Sci. 151, 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Sancho G., Smith A., La Merrill M. A. (2017). Triphenyl phosphate enhances adipogenic differentiation, glucose uptake and lipolysis via endocrine and noradrenergic mechanisms. Toxicol. In Vitro 40, 280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro-Garcia R., Blumberg B. (2019). Current research approaches and challenges in the obesogen field. Front. Endocrinol. (Lausanne) 10, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. H., Yang C. M., Chang S. P., Hu M. L. (2009). C/EBP beta and c/EBP delta expression is elevated in the early phase of ethanol-induced hepatosteatosis in mice. Acta Pharmacol. Sin. 30, 1138–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Mackey K. (1995). Short technical reports. Modification of the tri reagent procedure for isolation of RNA from polysaccharide- and proteoglycan-rich sources. Biotechniques 19, 942–945. [PubMed] [Google Scholar]
- Clarkson T. W., Magos L. (2006). The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 36, 609–662. [DOI] [PubMed] [Google Scholar]
- Culbreth M., Aschner M. (2016). Dysregulation of glutamate cycling mediates methylmercury-induced neurotoxicity. Adv. Neurobiol. 13, 295–305. [DOI] [PubMed] [Google Scholar]
- Dalliere N., Holden-Dye L., Dillon J., O’Connor V., Walker R. J. (2017). Caenorhabditis elegans Feeding Behaviors. Oxford Research Encyclopedia of Neuroscience. Oxford University Press, Oxford, UK. [Google Scholar]
- Dare E., Fetissov S., Hokfelt T., Hall H., Ogren S. O., Ceccatelli S. (2003). Effects of prenatal exposure to methylmercury on dopamine-mediated locomotor activity and dopamine d2 receptor binding. Naunyn Schmiedebergs Arch. Pharmacol. 367, 500–508. [DOI] [PubMed] [Google Scholar]
- de Mello A. H., Costa A. B., Engel J. D. G., Rezin G. T. (2018). Mitochondrial dysfunction in obesity. Life Sci. 192, 26–32. [DOI] [PubMed] [Google Scholar]
- Dent J. A., Davis M. W., Avery L. (1997). Avr-15 encodes a chloride channel subunit that mediates inhibitory glutamatergic neurotransmission and ivermectin sensitivity in Caenorhabditis elegans. EMBO J. 16, 5867–5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreiem A., Gertz C. C., Seegal R. F. (2005). The effects of methylmercury on mitochondrial function and reactive oxygen species formation in rat striatal synaptosomes are age-dependent. Toxicol. Sci. 87, 156–162. [DOI] [PubMed] [Google Scholar]
- Ericsson J., Jackson S. M., Kim J. B., Spiegelman B. M., Edwards P. A. (1997). Identification of glycerol-3-phosphate acyltransferase as an adipocyte determination and differentiation factor 1- and sterol regulatory element-binding protein-responsive gene. J. Biol. Chem. 272, 7298–7305. [DOI] [PubMed] [Google Scholar]
- Fan Y., Zhang C., Bu J. (2017). Relationship between selected serum metallic elements and obesity in children and adolescent in the U.S. Nutrients 9, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina M., Dahm K. C., Schwalm F. D., Brusque A. M., Frizzo M. E., Zeni G., Souza D. O., Rocha J. B. (2003). Methylmercury increases glutamate release from brain synaptosomes and glutamate uptake by cortical slices from suckling rat pups: Modulatory effect of ebselen. Toxicol. Sci. 73, 135–140. [DOI] [PubMed] [Google Scholar]
- Ferrer B., Peres T. V., Dos Santos A. A., Bornhorst J., Morcillo P., Goncalves C. L., Aschner M. (2018). Methylmercury affects the expression of hypothalamic neuropeptides that control body weight in c57bl/6j mice. Toxicol. Sci. 163, 557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavet O., Pines J. (2010). Progressive activation of cyclinb1-cdk1 coordinates entry to mitosis. Dev. Cell 18, 533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerson A. R., Cristol D. A., Seewagen C. L. (2019). Environmentally relevant methylmercury exposure reduces the metabolic scope of a model songbird. Environ. Pollut. 246, 790–796. [DOI] [PubMed] [Google Scholar]
- Goto T., Lee J. Y., Teraminami A., Kim Y. I., Hirai S., Uemura T., Inoue H., Takahashi N., Kawada T. (2011). Activation of peroxisome proliferator-activated receptor-alpha stimulates both differentiation and fatty acid oxidation in adipocytes. J. Lipid Res. 52, 873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B., Hirsh D. (1999). Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol. Biol. Cell 10, 4311–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer E. R., Perez C. L., Van Gilst M. R., Lee B. H., Ashrafi K. (2008). Neural and molecular dissection of a C. elegans sensory circuit that regulates fat and feeding. Cell Metab. 8, 118–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubert P., Puntel B., Lehmen T., Fessel J. P., Cheng P., Bornhorst J., Trindade L. S., Avila D. S., Aschner M., Soares F. (2018). Metabolic effects of manganese in the nematode Caenorhabditis elegans through DAergic pathway and transcription factors activation. Neurotoxicology 67, 65–72. [DOI] [PubMed] [Google Scholar]
- Guo L., Li X., Tang Q. Q. (2015). Transcriptional regulation of adipocyte differentiation: A central role for CCAAT/enhancer-binding protein (C/EBP) beta. J. Biol. Chem. 290, 755–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmcke K. J., Aschner M. (2010). Hormetic effect of methylmercury on Caenorhabditis elegans. Toxicol. Appl. Pharmacol. 248, 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills T., Brockie P. J., Maricq A. V. (2004). Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J. Neurosci. 24, 1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz H. R., Chalfie M., Trent C., Sulston J. E., Evans P. D. (1982). Serotonin and octopamine in the nematode Caenorhabditis elegans. Science 216, 1012–1014. [DOI] [PubMed] [Google Scholar]
- Hu S., Wang L., Yang D., Li L., Togo J., Wu Y., Liu Q., Li B., Li M., Wang G., et al. (2018). Dietary fat, but not protein or carbohydrate, regulates energy intake and causes adiposity in mice. Cell Metab. 28, 415–431.e4. [DOI] [PubMed] [Google Scholar]
- Hwang G. W., Naganuma A. (2006). DNA microarray analysis of transcriptional responses of human neuroblastoma imr-32 cells to methylmercury. J. Toxicol. Sci. 31, 537–538. [DOI] [PubMed] [Google Scholar]
- Kassotis C. D., Stapleton H. M. (2019). Endocrine-mediated mechanisms of metabolic disruption and new approaches to examine the public health threat. Front. Endocrinol. (Lausanne) 10, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapper M., Ehmke M., Palgunow D., Bohme M., Matthaus C., Bergner G., Dietzek B., Popp J., Doring F. (2011). Fluorescence-based fixative and vital staining of lipid droplets in Caenorhabditis elegans reveal fat stores using microscopy and flow cytometry approaches. J. Lipid Res. 52, 1281–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan P. J., Sonawane B., Butler R. N., Trasande L., Callan R., Droller D. (2005). Early environmental origins of neurodegenerative disease in later life. Environ. Health Perspect. 113, 1230–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. E., Schmidt H., Lai B., Ge K. (2019). Transcriptional and epigenomic regulation of adipogenesis. Mol. Cell Biol. 39, e00601-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. (2018). Blood mercury concentration in relation to metabolic and weight phenotypes using the KNHANES 2011-2013 data. Int. Arch. Occup. Environ. Health 91, 185–193. [DOI] [PubMed] [Google Scholar]
- Lemieux G. A., Ashrafi K. (2015). Insights and challenges in using C. elegans for investigation of fat metabolism. Crit. Rev. Biochem. Mol. Biol. 50, 69–84. [DOI] [PubMed] [Google Scholar]
- Lima S. C., Arrais R. F., Sales C. H., Almeida M. G., de Sena K. C., Oliveira V. T., de Andrade A. S., Pedrosa L. F. (2006). Assessment of copper and lipid profile in obese children and adolescents. Biol. Trace Elem. Res. 114, 19–29. [DOI] [PubMed] [Google Scholar]
- Ma X., Pham V. T., Mori H., MacDougald O. A., Shah Y. M., Bodary P. F. (2017). Iron elevation and adipose tissue remodeling in the epididymal depot of a mouse model of polygenic obesity. PLoS One 12, e0179889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magos L. (1982). Neurotoxicity, anorexia and the preferential choice of antidote in methylmercury intoxicated rats. Neurobehav. Toxicol. Teratol. 4, 643–646. [PubMed] [Google Scholar]
- Marraudino M., Bonaldo B., Farinetti A., Panzica G., Ponti G., Gotti S. (2018). Metabolism disrupting chemicals and alteration of neuroendocrine circuits controlling food intake and energy metabolism. Front. Endocrinol. (Lausanne) 9, 766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Finley E. J., Caito S., Slaughter J. C., Aschner M. (2013a). The role of skn-1 in methylmercury-induced latent dopaminergic neurodegeneration. Neurochem. Res. 38, 2650–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Finley E. J., Chakraborty S., Slaughter J. C., Aschner M. (2013b). Early-life exposure to methylmercury in wildtype and pdr-1/parkin knockout C. elegans. Neurochem. Res. 38, 1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H., Muruganujan A., Ebert D., Huang X., Thomas P. D. (2019). Panther version 14: More genomes, a new panther go-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 47, D419–D426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S. L., Vorojeikina D., Huang W., Mackay T. F., Anholt R. R., Rand M. D. (2014). Genome-wide association analysis of tolerance to methylmercury toxicity in drosophila implicates myogenic and neuromuscular developmental pathways. PLoS One 9, e110375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira E. L., de Oliveira J., Dutra M. F., Santos D. B., Goncalves C. A., Goldfeder E. M., de Bem A. F., Prediger R. D., Aschner M., Farina M. (2012). Does methylmercury-induced hypercholesterolemia play a causal role in its neurotoxicity and cardiovascular disease? Toxicol. Sci. 130, 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutkus L., Aschner J. L., Fitsanakis V., Aschner M. (2005a). The in vitro uptake of glutamate in GLAST and GLT-1 transfected mutant CHO-K1 cells is inhibited by manganese. Biol. Trace Elem. Res. 107, 221–230. [DOI] [PubMed] [Google Scholar]
- Mutkus L., Aschner J. L., Syversen T., Aschner M. (2005b). Methylmercury alters the in vitro uptake of glutamate in GLAST- and GLT-1-transfected mutant CHO-K1 cells. Biol. Trace Elem. Res. 107, 231–245. [DOI] [PubMed] [Google Scholar]
- Ni M., Li X., Yin Z., Sidoryk-Węgrzynowicz M., Jiang H., Farina M., Rocha J. B. T., Syversen T., Aschner M. (2011). Comparative study on the response of rat primary astrocytes and microglia to methylmercury toxicity. Glia 59, 810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niacaris T., Avery L. (2003). Serotonin regulates repolarization of the C. elegans pharyngeal muscle. J. Exp. Biol. 206, 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T., Horikawa M., Shimamura S., Hashimoto T., Sakamoto K. (2010). Fat accumulation in Caenorhabditis elegans is mediated by SREBP homolog SBP-1. Genes Nutr. 5, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson L. E., Orho-Melander M., William-Olsson L., Sjöholm K., Sjöström L., Groop L., Carlsson B., Carlsson L. M., Olsson B. (2008). CCAAT/enhancer binding protein alpha (C/EBPalpha) in adipose tissue regulates genes in lipid and glucose metabolism and a genetic variation in C/EBPalpha is associated with serum levels of triglycerides. J. Clin. Endocrinol. Metab. 93, 4880–4886. [DOI] [PubMed] [Google Scholar]
- Orr J. S., Kennedy A., Anderson-Baucum E. K., Webb C. D., Fordahl S. C., Erikson K. M., Zhang Y., Etzerodt A., Moestrup S. K., Hasty A. H. (2014). Obesity alters adipose tissue macrophage iron content and tissue iron distribution. Diabetes 63, 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palikaras K., Mari M., Petanidou B., Pasparaki A., Filippidis G., Tavernarakis N. (2017). Ectopic fat deposition contributes to age-associated pathology in Caenorhabditis elegans. J. Lipid Res. 58, 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. S., Ha K. H., He K., Kim D. J. (2017). Association between blood mercury level and visceral adiposity in adults. Diabetes Metab. J. 41, 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K., Seo E. (2016). Association between toenail mercury and metabolic syndrome is modified by selenium. Nutrients 8, 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne V. A., Au W. S., Lowe C. E., Rahman S. M., Friedman J. E., O’Rahilly S., Rochford J. J. (2009). C/EBP transcription factors regulate SREBP1c gene expression during adipogenesis. Biochem. J. 425, 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino E. C., Webster C. M., Carr C. E., Soukas A. A. (2013). Biochemical and high throughput microscopic assessment of fat mass in Caenorhabditis elegans. J. Vis. Exp. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince L. M., Rand M. D. (2017). Notch target gene E(spl)mdelta is a mediator of methylmercury-induced myotoxicity in Drosophila. Front. Genet. 8, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince L. M., Rand M. D. (2018). Methylmercury exposure causes a persistent inhibition of myogenin expression and c2c12 myoblast differentiation. Toxicology 393, 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Lopez O., Riezu-Boj J. I., Milagro F. I., Martinez J. A., and MENA Project. (2018). Dopamine gene methylation patterns are associated with obesity markers and carbohydrate intake. Brain Behav. 8, e01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy C., Tremblay P. Y., Ayotte P. (2017). Is mercury exposure causing diabetes, metabolic syndrome and insulin resistance? A systematic review of the literature. Environ. Res. 156, 747–760. [DOI] [PubMed] [Google Scholar]
- Rudgalvyte M., Peltonen J., Lakso M., Wong G. (2017). Chronic MeHg exposure modifies the histone H3K4me3 epigenetic landscape in Caenorhabditis elegans. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 191, 109–116. [DOI] [PubMed] [Google Scholar]
- Rudgalvyte M., VanDuyn N., Aarnio V., Heikkinen L., Peltonen J., Lakso M., Nass R., Wong G. (2013). Methylmercury exposure increases lipocalin related (lpr) and decreases activated in blocked unfolded protein response (abu) genes and specific miRNAs in Caenorhabditis elegans. Toxicol. Lett. 222, 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin E. R., Ranganathan R., Horvitz H. R. (2000). C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26, 619–631. [DOI] [PubMed] [Google Scholar]
- Schroeder H. A., Mitchener M. (1975). Life-term effects of mercury, methyl mercury, and nine other trace metals on mice. J. Nutr. 105, 452–458. [DOI] [PubMed] [Google Scholar]
- Scorpio R. M., Masoro E. J. (1970). Differences between manganese and magnesium ions with regard to fatty acid biosynthesis, acetyl-coenzyme a carboxylase activity and malonyl-coenzyme a decarboxylation. Biochem. J. 118, 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifnia P., Kim K. W., Wu Z., Jin Y. (2017). Distinct cis elements in the 3' UTR of the C. elegans cebp-1 mRNA mediate its regulation in neuronal development. Dev. Biol. 429, 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle T. (1999). Maintenance of C. elegans In C elegans: A Practical Approach (Hope I. A.). Oxford University Press, New York, NY. [Google Scholar]
- Tanaka T., Yoshida N., Kishimoto T., Akira S. (1997). Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J. 16, 7432–7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q. Q., Otto T. C., Lane M. D. (2003). Ccaat/enhancer-binding protein beta is required for mitotic clonal expansion during adipogenesis. Proc Natl Acad Sci U S A 100, 850–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timper K., Bruning J. C. (2017). Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Model Mech. 10, 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M. E., Mottet N. K., Friberg L. T., Lind S. B., Charleston J. S., Burbacher T. M. (1995). Demethylation of methyl mercury in different brain sites of Macaca fascicularis monkeys during long-term subclinical methyl mercury exposure. Toxicol. Appl. Pharmacol. 134, 273–284. [DOI] [PubMed] [Google Scholar]
- Van Gilst M. R., Hadjivassiliou H., Jolly A., Yamamoto K. R. (2005). Nuclear hormone receptor nhr-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol. 3, e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gadea A., Topper S., Young L., Crisp A., Kressin L., Elbel E., Maples T., Brauner M., Erbguth K., Axelrod A., et al. (2011). Caenorhabditis elegans selects distinct crawling and swimming gaits via dopamine and serotonin. Proc. Natl. Acad. Sci. U S A 108, 17504–17509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Jiang X., Wu J., Zhang L., Huang J., Zhang Y., Zou X., Liang B. (2016). Iron overload coordinately promotes ferritin expression and fat accumulation in Caenorhabditis elegans. Genetics 203, 241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Mukherjee B., Park S. K. (2018). Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among U.S. Adults in NHANES 2003-2014. Environ. Int. 121, 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts J. L., Ristow M. (2017). Lipid and carbohydrate metabolism in Caenorhabditis elegans. Genetics 207, 413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. Y., Hu J. P., Wu M. M., Wang L. S., Fang N. Y. (2015). CCAAT/enhancer-binding protein CEBP-2 controls fat consumption and fatty acid desaturation in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 468, 312–318. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Yanagisawa R., Motomura E., Nakamura M., Sakamoto M., Takeya M., Eto K. (2014). Increased methylmercury toxicity related to obesity in diabetic KK-Ay mice. J. Appl. Toxicol. 34, 914–923. [DOI] [PubMed] [Google Scholar]
- Yan D., Wu Z., Chisholm A. D., Jin Y. (2009). The DLK-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell 138, 1005–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Loh K., Song Z. Y., Yang H. Q., Zhang Y., Lin S. (2018). Update on glycerol-3-phosphate acyltransferases: The roles in the development of insulin resistance. Nutr. Diabetes 8, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Y., Li X., Qian S. W., Guo L., Huang H. Y., He Q., Liu Y., Ma C. G., Tang Q. Q. (2011). Transcriptional activation of histone H4 by C/EBPbeta during the mitotic clonal expansion of 3t3-L1 adipocyte differentiation. Mol. Biol. Cell 22, 2165–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]