Abstract
In recent years, biomaterial- and scaffold-based immunomodulation strategies were implemented in tissue regeneration efforts for manipulating macrophage polarization (a.k.a. phenotype or lineage commitment, or differentiation). Yet, most of our understanding of macrophage phenotype commitment and phagocytic capacity is limited to how physical cues (extracellular matrix stiffness, roughness, and topography) and soluble chemical cues (cytokines and chemokines released from the scaffold) influence macrophage polarization. In the context of immune response-tissue interaction, the mechanical cues experienced by the residing cells within the tissue also play a critical role in macrophage polarization and inflammatory response. However, there is no compiled study discussing the effect of the dynamic mechanical environment around the tissues on macrophage polarization and the innate immune response. The aim of this comprehensive review paper is twofold; a) to highlight the importance of mechanical cues on macrophage lineage commitment and function and b) to summarize the important studies dedicated to understand how macrophage polarization changes with different mechanical loading modalities. For the first time, this review paper compiles and compartmentalizes the studies investigating the role of dynamic mechanical loading with various modalities, amplitude, and frequency on macrophage differentiation. A deeper understanding of macrophage phenotype in mechanically dominant tissues (i.e. musculoskeletal tissues, lung tissues, and cardiovascular tissues) provides mechanistic insights into the design of mechano- immunomodulatory tissue scaffold for tissue regeneration.
Keywords: Macrophages, Polarization, Immunomodulation, Mechanical Strain, Mechanotransduction, Tissue Engineering, Mechanoimmunomodulation, Anti-inflammatory, Pro-inflammatory, Phagocytic Activity
1. The Intertwined Concepts of Tissue Engineering and the Innate Immune Response
The field of tissue engineering has made great strides in repairing damaged or diseased tissues using stem cells, smart biomaterials, and scaffold design strategies. However, the success of any tissue engineering strategy is also dependent on the initiation of the host foreign body response (FBR), which is mediated by innate immune cells. In response to tissue scaffold implantation, platelets begin to form a clot at injury sites and cytokines get caught in the newly formed clot. Activated by these cytokines, circulating monocytes in the blood infiltrate the site and differentiate into macrophages (1). Macrophages are one of the core innate immune system cell types that perform a variety of immune responses including metabolic regulation, tissue homeostasis, and defense against pathogens.
Initially, macrophages were thought to only execute pro-inflammatory activities, such as phagocytosis and the release of pro-inflammatory factors to the surrounding tissue. However, studies have now demonstrated that upon tissue extravasation macrophages polarize to a spectrum of functional phenotypes due to the surrounding environmental stimuli in the tissue (physical, chemical, and mechanical) (2). Macrophages have two main functional phenotypes: classically activated and alternatively activated subsets, which have different regulatory roles (3). The classically activated (M1) macrophages exhibit a pro-inflammatory response and are commonly identified by secretion of interleukin (IL)-12, inducible Nitric Oxide Synthase (iNOS), Prostaglandin E2 (PGE2), cyclooxygenase (COX)-2, and tumor necrosis factor-alpha (TNF-α). The alternatively activated (M2) macrophages are found to have a pro-healing phenotype, such as wound healing, tissue repair, and anti-inflammatory responses. M2 macrophages and are characterized by secretion of IL-10, IL-4, chemokine ligand (CCL)1, and CCL18 (4, 5). The M2 phenotype differentiates further into subsets M2a, M2b, M2c, and M2d according to their specific markers (6, 7). The functions of M2 sub-phenotypes and others are tabulated in Table 1. Table 1 compiles the information on M1, M2, and M2 sub-phenotypes including the inducers, transcriptions factors, expressed markers, secretions, and their functions.
Table 1.
Macrophage subsets and their functions
| Macrophage Phenotype | Inducers | Secretions | Expressed Markers | Functions | References | |
|---|---|---|---|---|---|---|
| M1 | LPS, IFN-γ | iNOS, IL-1β, IL-6, IL-12, IL-15, IL-18, TNF-α, CCL-15, -20, CXCL-9, -10, -11 | MHCII, CD86, TLR, IFN-γR | Phagocytose bacteria and debris, attract immune cells, tissue damage, clearance of intracellular pathogens, pro-inflammatory | (8–12) | |
| M2 | M2a | IL-4, IL-13, IL-33 | IL-10, IL-12, CCL-13, -14, -17, -18, 22, 23, 24, 26, IGF-1, arginase, FIZZ1, YM1 | MR, GR, SR, CD 163, CD23 | Support ECM deposition wound closure, angiogenesis, promotion of allergic responses, anti-inflammatory | (13–15) |
| M2b | Immune complexes, IL-1β, LPS | IL-10, CCL-1, -20, CXCL-1, -2, -3 | IL-1R, IL-10R, TLR4, FcγR | Suppress inflammation via high IL-10, immuneregulation, | (16–19) | |
| M2c | IL-10, GC, TGF-β | CCL-16, 18, CXCL-13, TGF-β, BMP-2 | CD163, CD206, IL-10R, PD-1 | Suppress inflammation via IL-10, produce ECM and MMPs for vascular/matric remodeling, tissue remodeling | (20–22) | |
| M2d | IL-6 and adenosine | VEGF, IL-10, IL-12, TNF-α, TGF-β | --- | Aids in angiogenesis via VEGF | (23–25) | |
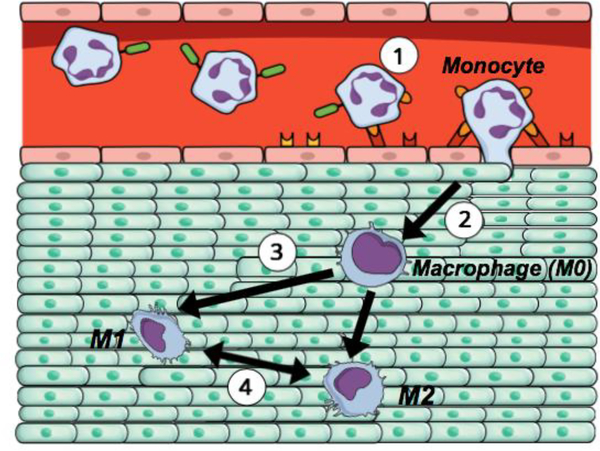
Figure 1 also demonstrates the schematic representation of monocyte extravasation into tissue and its differentiation. Both the classically and alternatively activated macrophage phenotypes are transient, meaning polarized macrophages can re-polarize to a different phenotype depending on the environmental need. Since the initial macrophage polarization and its transiency are interrelated with the chemical, physicochemical, structural, and mechanical cues received from the microenvironment, this provides a great avenue for scientists to tailor the tissue microenvironment to suit their needs.
Figure 1.
The schematic representation of a monocyte extravasation into a tissue and macrophage polarization (1) A circulating monocyte from the bloodstream attaches to the endothelial layer and begins to infiltrate into the targeted tissue, (2) Upon extravasation, monocyte differentiates into macrophage (M0), which further exposes to the chemical, physical, and mechanical cues experienced within the targeted tissue, (3) Macrophage polarizes into classically activated (pro-inflammatory; M1) and alternatively activated (anti-inflammatory; M2) phenotype depending on the tissue milieu, (4) Macrophage differentiation is temporal. Macrophage may change its polarization state by time depending on the needs of the tissue.
A significant effort has been made in modulating macrophage polarization, also known as immunoinformed techniques, towards tissue regeneration using biomaterial- and scaffold-based design strategies (26, 27). As part of these immunoinformed strategies, the biomaterial’s or scaffolds’ physicochemical properties (surface energy, hydrophilicity) and physical properties (roughness, stiffness, porosity, and viscosity) are designed with or without soluble cytokines to create scaffolds that have polarized macrophages into the M2 lineage (28–30).
The immunoinformed biomaterials for tissue regeneration are generally synthesized by introducing anti-inflammatory cytokines to the biomaterial using absorption or encapsulation strategies. Primarily collagen-based and FDA-approved synthetic biomaterials are used in this field because of their already proven tissue regeneration capacities. Studies have shown that the release of IL-4 from a biomaterial will polarize macrophages to M2 across a variety of scaffold materials and that the release of IL-4 can be sustained for 6 days (31–33).
In addition to the aforementioned cytokines and physiochemical stimulants-based immunomodulation, a biomaterial’s physical properties such as surface topography, roughness, and stiffness play a key role in modulating the innate immune response. Topography such as surface grooves and surface roughness can modulate macrophage polarization in a dose-dependent manner. For instance, Hotchkiss et al. (34) induced M2 polarization of C57BL/6 bone marrow derived macrophages (BMDMs) through 3.61μm surface roughness of titanium. On the rougher surface, an increase of anti-inflammatory gene expression of IL-4 (2 fold) and IL-10 (1.75 fold) was observed. The expression of pro-inflammatory markers including IL-1β, IL-6, and TNF-α increased when surface roughness dropped from 3.61μm to 0.59μm. Other studies observed elongation of the macrophages – a morphological characteristic of macrophages – and increased gene expression of IL-13 and IL-4 with down-regulation of iNOS, and Arginase (Arg1) with topographical patterns ranging from 0.15μm to 50μm (2, 35).
The biomaterial stiffness is another factor affecting macrophage phenotype upon tissue scaffold implantation. There are several important studies conducted to understand the role of matrix stiffness on macrophage polarization. For instance, Friedemann et al. (36) found that human blood monocyte-derived macrophages exhibited anti-inflammatory (M2) phenotype (increased expression of IL-10 and IL-12) when cultured on a stiffer matrix. Blakney et al (37) observed how substrate stiffness plays a role in the activation of RAW264.7 murine macrophages and found that stiffer matrices promoted the anti-inflammatory (M2) response. In contrast, Friedemann et al. (36) concluded that the macrophages differentiated to pro-inflammatory (M1) in stiffer matrices. The discrepancy between studies could be explained by the different inherent chemical stimuli of the scaffolds in which the macrophages were grown and different source of macrophages used in the studies. Despite these differences in experimental methods, the results indicate that macrophages are sensitive to the overall stiffness of the material. Further studies need to be conducted with various biomaterial forms (i.e hydrogel, solid) and stiffnesses ranging from soft tissue stiffness to bone stiffness to truly understand the role of matrix stiffness on macrophage phenotype commitment. There are more in-depth reviews that summarize the effect of tissue stiffness and disease progression on macrophage presence, activation, and accumulation in an affected area (4, 38).
Our current understanding on immunomodulation and macrophage polarization towards tissue regeneration is still limited to how soluble factors such as cytokines influence polarization and how alterations in the physical environment (stiffness, roughness, topography) change macrophage differentiation. It is surprising that the role of mechanical cues that macrophages experience upon extravasation and macrophage polarization has been largely ignored in immunological or tissue engineering studies. In this comprehensive review paper, we highlight the importance of mechanical cues on macrophage lineage commitment and function and summarize the important studies dedicated to understanding how macrophage polarization changes with different mechanical loading modalities and magnitudes experienced within the body.
2. Mechano-immunomodulation: The role of mechanical environment on macrophage polarization
The aforementioned studies of illustrating polarization through chemical and physical microenvironments, the potential effect of mechanical environment on immune cell polarization, function, and their phagocytic activities have begun to be investigated. In fact, in vivo, and in vitro studies demonstrated that macrophages are mechanically responsive and alter their lineages according to mechanical stimuli around them. For instance, it is well established that macrophages lead to osteolysis and aseptic loosening following joint arthroplasties (39–41). The mechanical stimuli around the implant in the forms of tensile micromechanical strains, hydrostatic cyclic pressure, and compressive strains (42–44) further accelerate the inflammatory cytokine production and osteolytic potential of macrophages. In hypertensive patients, the fluctuating shear strain and pressure within the blood vessels also affect macrophages and its function, which have paramount importance in plaque stabilization (45, 46). Macrophages respond to shear stress and pressure within blood vessels by the selective release of matrix metalloproteinases (MMPs), which contribute to the degradation of the extracellular matrix (ECM). The degraded ECM then leads to instability of coronary-artery plaques in atherosclerotic lesions (47, 48). In the abdominal wall region, the mechanical strain in the context of stretching also induces changes in inflammatory gene expressions of macrophages residing in the peritoneal cavity (49).
These representative examples demonstrate that macrophages are mechanoresponsive and the mechanical environment around the host tissue may function as an immunomodulatory stimulus for macrophages. Upon extravasation, the macrophages establish extracellular matrix contacts and sense the mechanical cues experienced by the host tissue. Considering the fact that tissues within our body are exposed to a myriad of mechanical loading modalities (shear stress, hydrostatic pressure, uniaxial stretching, and biaxial stretching) with various strains and frequencies, it is important to understand the response of macrophages in each mechanical loading modalities for designing superior mechano-informed tissue scaffolds.
a). Role of Shear Stress and Pressure on Macrophage Polarization
The role of shear stress within the human body is primarily studied in the context of the vasculature. In healthy vascular models, the steady and laminar flowing blood creates shear stress on the blood vessel varying within the range of 1.5–7.0 Pa over the cardiac cycle for carotid arteries (50, 51) . Keeping the shear stress in this physiological range is very crucial for maintaining endothelial cell function and preventing atherosclerosis formation. Low and oscillatory shear stress ranging between 1.0–1.5 Pa as observed in atherosclerotic region activates pro-inflammatory phenotype under high oxidative stress (52). The low shear stress in atherosclerotic region induces nitric oxide production and endothelial NOS expression, which further induce reactive oxygen species production (O2− and H2O2) and expression of inflammatory molecules such as vascular cell adhesion molecule 1 (VCAM-1) and Intercellular Adhesion Molecule 1 (ICAM-1), C-reactive protein, Vascular Endothelial Growth Factor (VEGF), IL-6 (53–55). The pro-inflammatory environment around the atherosclerotic vessel further promotes pro-inflammatory lineage commitment of macrophages and exacerbates plaque formation in the inner lining of arteries (45, 47, 56). Thus, from a clinical perspective, investigating how macrophage functions (phagocytic capacity) and phenotype commitment may change under various shear stress conditions (athero-protective or athero-prone) is very important to understand the plaque formation and atherosclerotic plaque rupture (57). A great amount of effort has been put in this direction. Table 2 tabulates the representative studies investigating the changes in macrophage polarization state and function with altered shear stress condition.
Table 2.
Summarized studies investigating the role of shear stress or pressure on macrophage polarization and function for (A) blood vessels and (B) bone tissue.
| (A) | ||||
| Shear Stress or Pressure Parameters | Cell Origin | Targeted Tissue | Polarization State and Function | Ref |
| 40,60,90 mmHg pressure (static for 1 hr) | J774.16 (murine) | Blood vessels (hypertension) | M1 | (71) |
| 0, 40–45, 70–75, 95–100, and 125–130 mmHg pressure (static 2 hrs) | U 937 (human) | Blood vessels (hypertension) | Increased motility | (72) |
| Parameters were determined using the manufacture’s guidelines not listed in the study | Human donor blood: monocyte-derived macrophages | Blood vessels (hemophilia A) | Sensitivity | (58) |
| Low Shear Stress (LSS): 10 Pa Oscillatory Shear Stress (OSS): 14 Pa |
ApoE−‘− (murine) | Blood Vessels (atherosclerosis) | LSS → M1 OSS → M2 |
(62, 73) |
| 0.3, 1.0, 2.5 Pa (static 5–6 hrs) | IC21 (murine) | Blood Vessels | M2 or M1 | (74) |
| (B) | ||||
| Shear Stress or Pressure Parameters | Cell Origin | Targeted Tissue | Polarization State and Function | Ref |
| 70 and 150 mmHg pressure with 0.1 Hz | Rabbit | Bone | M1 | (75) |
| 0.138 MPa (20 PSI) with 0.05 Hz and 0.5 Hz | Human donor blood; buffy coats | Bone | M1 | (76) |
For instance, Nunez et al. (58) studied how changes in shear stress affect the macrophage phagocytic capacity on factor VIII (FVIII) / von Willebrand factor (vWF) complex circulating in blood plasma. The research group extracted macrophages from human peripheral blood and exposed them to the various shear stress (0.1, 0.2, 0.4, 0.6, and 0.8 Pa) using the parallel-plate flow chamber by GlycoTech. The data demonstrated that the macrophages cultured under static conditions could not clear the Factor VIII/vWF complex, while the macrophages exposed to 0.4 Pa shear stress cleared both Factor VIII and vWF. This study is significant because it demonstrates that changes in macrophage phagocytic capacity due to shear stress directly contribute to pathology such as abnormal blood clotting. It is well-established that vWF and FVIII are crucial factors for platelet adhesion and subsequently thrombosis (59). Yet, without macrophages and their phagocytic function, these factors accumulate at the injury site and create excessive platelet aggregation, which leads to abnormal blood circulation, capillary blockage, blood clots, acute myocardial infarction, and ischemic stroke (58, 60).
Rosenson-Schloss et al. (61) exposed IC21 murine macrophages to 2.5 kPa shear stress using a custom-built flow chamber system to study how shear stress affects the phagocytic capacity of macrophages. Their results demonstrated a significant elongation in the direction of the flow along with an increase in phagocytic uptake of macrophages upon shear stress. Further, across all applied stresses of 5.3, 8, and 12 kPa, the macrophages demonstrated increased uptake of IgG complexes. The increased phagocytic capacity of macrophages demonstrated a pro-inflammatory (M1) phenotype. While IgG uptake capacity was 3,087cpm/mg cellular protein (CP) under 5.3 kPa shear stress, it is 3,523cpm/mg CP, and 3,616cpm/mg CP under 8 kPa, and 12 kPa shear stress, respectively. They also demonstrated that the uptake of IgG complexes was further amplified by the incubation with calcium chloride. These results seem to indicate that under these loading conditions the macrophages polarized to M1.
In an in vivo study, Seneviratne et al. (48, 62) investigated how macrophage polarization under shear stress modulates atherosclerotic plaque vulnerability. They applied various types of shear stresses using a shear stress-altering cast to the carotid arteries of C57BL/6 mice. The shear stress within the arteries was manipulated with the cast. Two different settings were applied with 17.8% representing low shear stress and 28.1% representing oscillatory shear stress. At the designated time point, the carotid artery was harvested and cross sections of the artery were taken. In these cross-sections, there was an increased level of iNOS (1.4x) and Interferon gamma (IFN-γ) (1.9x) and decreased levels of IL-10 (0.6x) and Arg-1 (1.6x) in comparison to the oscillatory shear stress gene expression fold changes of 1.1x (IL-10), and 6.0x (Arg1). Their data suggested that low shear stress promoted M1 polarization, while oscillatory shear stress promoted M2 lineage commitment.
These studies and many others in the literature (63–66) demonstrated that shear stress is able to modulate macrophage phenotype commitment and phagocytic activities. However, it should be also pointed out that the same amplitude of shear stress may polarize the macrophages to M1 or M2 in different experiments. It is well-established that the source of macrophages dictates its function and activity, which means that human bone marrow-derived macrophages would behave and function differently than the human blood-derived macrophages and human tissue-resident macrophages such as Kupffer cells or Langerhans cells. Another reason for inconsistent data would be the fact that the species macrophages are derived from is typically different from experiment to experiment. Several prominent studies in the immunology literature emphasize that there are differences between mouse and human immunology, which can affect how macrophages respond to the same stimuli (67–70). Last but not least, the loading modality of shear stress is also very important in obtaining consistent results. The shear stress with the same amplitude can be applied in a continuous manner to the cells over a period of time or can be applied with a certain frequency. It is expected that the macrophages would experience different mechanical stimuli for each case and would behave differently. It is extremely important to consider these facts during data interpretation and design the experiments.
In hypertension, increased hydrostatic pressure along with the repetitive mechanical strain exerted on blood vessels may alter the cellular functions important for hemostasis. Several research groups have been studying the role of hydrostatic pressure on macrophage lineage commitment phagocytic activity, and migration.
Mattana et al. (71) studied the role of hydrostatic pressure on the phagocytic activity of macrophages in engulfing immunoglobulin G (IgG) complexes. The J774.16 murine macrophage cells were cultured on either 2D petri dish or Collagen type-1 substrate and exposed to 5.33, 8, and 12 kPa pressure for one hour. Their results demonstrated that the IgG phagocytic capacity of macrophages increased with increased pressure and the IgG engulfing was mediated by calcium ion channels. They also proved the role of calcium ion channels in phagocytosis and demonstrated that increased uptake of IgG complexes due to increased pressure could be attenuated in the presence of calcium ion channel blockers. From a clinical perspective, the phagocytosis of IgG immune complex studies is extremely important. Circulating immune complexes such as IgG play a role in numerous immune complex disorders including systemic lupus erythematosus and bacterial endocarditis. The IgG engulfing process is crucial to prevent their deposition in organs. The IgG deposits trigger inflammatory responses and create significant tissue damage (particularly in kidney and arterial wall), which further exacerbates the pathologies of these disorders. The IgG phagocytosis by macrophages from the blood circulation limits their possible deposition and subsequently limits IgG-mediated tissue damage (77–82).
Ferrier et al. (76) studied the role of two cyclic hydrostatic pressure regimes on human monocyte-derived macrophage phenotype commitment. They exposed macrophages to two different hydrostatic pressure regimes; the first one was 0.138 MPa pressure for 8 hours with 0.05 frequency, while the second regime was 0.138 MPa pressure for 1 hour with 0.5 Hz frequency. They identified that both regimes increased TNF-α and IL-6 production. Yet, only the high-frequency regime (0.5 Hz) promoted an increase in IL-1β production, which is a hallmark for the pro-inflammatory phenotype. They concluded that high-frequency cyclic pressure even with less exposure polarized monocyte-derived macrophages to the pro-inflammatory phenotype. Singhal et al. investigated the role of hydrostatic pressure on macrophage migration (72). They used a modified Boyden chamber placed in a pressure container maintained at various pressures (0, 5.33, 9.33, 12.67, and 16.67 kPa) and applied for 2 hours to monocytes. The compartmental system separated with 5 μm pore size was used to allow monocytes to migrate from one compartment to another under various pressures. They concluded that macrophage motility increased with increased pressure and demonstrated a pressure amplitude-dependent manner.
The studies investigating the interaction of shear stress and hydrostatic pressure with macrophages demonstrated that without exception shear stress increased the phagocytic capacity of macrophages and proinflammatory cytokine secretion. Understanding the interaction between innate immune cells and shear stress may help to regulate the inflammation in the affected area and create effective treatments for these conditions.
b). Role of Uniaxial and Biaxial Stretching on Macrophage Polarization
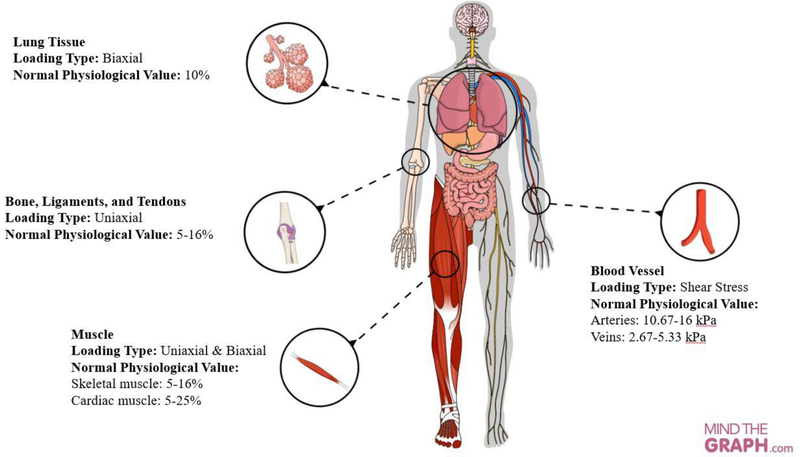
Uniaxial and biaxial loading modalities are dominant mechanical stimuli primarily for musculoskeletal tissues (muscle, ligaments, and tendons), myocardium tissue, and lung tissues. For instance, muscles experience a uniaxial strain with a range of 5–15% from moderate to vigorous physical activities (83). In walking, leg muscles are uniaxially stretched to 5–9% strain and during sprinting the uniaxial strain in muscle is 12% (84, 85). Tendons and ligaments also experience uniaxial strain with a range of 5–16% (86–91). The human myocardium is also a specialized muscle tissue that undergoes biaxial mechanical strains ranging from 5% to 25 % in all segments (apical, middle, basal) with almost 1Hz frequency (72 rpm) for 24 hours (92). Myocardial strain measurements taken from tagged magnetic resonance imaging (MRI) demonstrated that circumferential strain values were −23 ± 4%, −22 ± 3%, −16 ± 5%, and −16 ± 4% in the anterior, lateral, inferior, and septal sectors of an equatorial slice, respectively (93).
One of the early studies conducted by Mattana et al. (94) demonstrated that uniaxially strained macrophages have less phagocytic activity compared to unstrained counterparts. They applied uniaxial strains on J774.16 murine macrophage-cell line seeded on a 2D membrane stretched for 60 minutes at 1.67 Hz frequency using a commercial mechanical loading platform, Flexcell™. They applied a minimal 11–12.5% and a peak 15–17% strain corresponding to minimal and peak glomerular capillary pressures of 4 to 5.1 kPa and 7.1 to 8.1 kPa, respectively. They demonstrated that macrophages’ IgG complexes uptake capacity decreased substantially with the increased mechanical strain.
A different study researched how monocytes, recruited into the sub-endothelial space, experience the mechanical strain exerted on a blood vessel with each heartbeat (95). They used human macrophage-like cells (U937) and rat peritoneal macrophage (RPM) extracted from the peritoneal cavity of the male rat. The cells were seeded on a sulfuric acid treated 2D silicone elastomer membrane with 10% mechanical uniaxial strain for 24 or 48 hours at 1 Hz frequency. Upon mechanical loading, the morphological measurements of macrophages were conducted on stained human and mouse macrophages. They defined the differentiation based on cell morphology in three different ways; vacuolized cells, spindle cells, and stellate cells. Vacuolized cells represent M1 macrophages while spindle and stellate cells represent M2 macrophages due to their elongated nature. The mechanical strain application inhibited the macrophage differentiation into vacuolized cells, which is associated with the phagocytic capacity of the cells. On the other hand, mechanical strain application increased the percentage of spindle cells by almost 3-fold for U937 cells and 1.5-fold for RPM cells. For differentiation towards stellate cells, only RPM cells demonstrated significant differentiation upon 24-hour mechanical straining. The data also demonstrated that the macrophage responses on mechanical strain differ from species to species. The human macrophage-like cells (U937) were more sensitive to the strain application than the rat macrophages.
Miyazaki H. and Hatashi K. (96) investigated the role of uniaxial strain experienced within the tunica intima on macrophage morphology, polarization, and phagocytic activities. Their hypothesis was the pulsatile blood pressure-induced mechanical strain on tunica intima affected the infiltrated macrophages and changed its morphology and function. The primary murine peritoneal macrophages extracted from 6-week old female mice were seeded on a 2D silicone rubber sheets and strained with 5% amplitude and 1Hz frequency for 48 hours. They quantified the morphological changes using a shape index (SI), which was defined as the relationship between the adhesion areas of the cells to the construct to the perimeter of the cells. where A is the adhesion area and P is the perimeter. Their results suggested that cyclic uniaxial strain applied on the 2D macrophage monolayer decreased the SI and phagocytosis of latex particles. Ohki et al. (97) investigated how cyclic biaxial mechanical strain induces gene expression in human monocytic THP-1 cells. They applied biaxial strain to a thin and transparent membrane, on which cells were cultured using the custom-built stretching device. Various biaxial strain values with 0, 1, 2, and 3% were applied for 1, 3, and 6 hours with 1Hz frequency to the macrophages and the gene transcription were assessed using DNA microarray. The genes observed from DNA microarray were further confirmed by real-time reverse transcriptase polymerase chain reaction. Their data demonstrated that no genes were mechanically induced at 1 hour; yet at 3 and 6 hours, only three genes, immediate-early response gene (IEX-1), interleukin-8 (IL-8), and prostate apoptosis response-4 (Par-4) demonstrated significant expression. They concluded that THP-1 cells responded to biaxial mechanical strain with the induction of immediate-early and inflammatory genes, implying differentiation to M1, and the inflammatory gene expression was strain amplitude-dependent. Figure 3 shows the fluorescence and scanning microscopy images of the macrophages demonstrating the changes in the cells’ morphology prior to and after the various mechanical loading.
Figure 3.
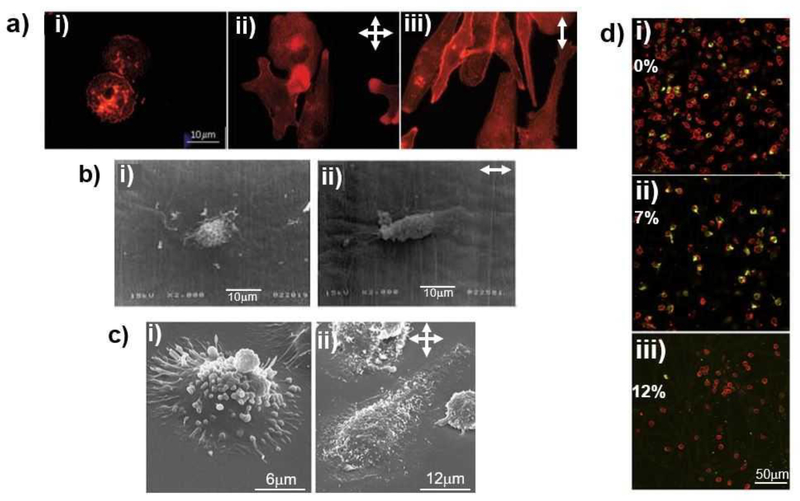
Mechanical strain-dependent changes in macrophage morphology (A, B, and C) and phenotype (D). (A) Fluorescence images of U937 human macrophages upon without mechanical strain (i), biaxial (ii) and uniaxial (iii) mechanical strain exposure (98–100), (B) Scanning electron microscope (SEM) images of murine macrophages cultured under without mechanical strain (i) and uniaxial mechanical strain (ii) conditions (96), (C) Magnified SEM images of U937 human macrophages cultured under without mechanical strain (i) and biaxial mechanical strain (ii) conditions (96), (D) Fluorescence images of human macrophages and its phenotypic state (M1 stained in red and M2 stained in green) under various uniaxial mechanical strain magnitude; 0% (i) , 7% (ii), and 12% (iii) (101). For images at A,B, and C, the arrows indicate the direction of applied mechanical strain.
Matheson et al. (98–100) conducted several studies on how cyclic biaxial strains alter the protein synthesis, intracellular esterase activity, acid phosphatase activities, and morphology of U937 human macrophage-like cells. They applied 10% biaxial strain with a 0.25 Hz frequency for 48 hours to U937 human macrophage-like cells cultured on a 2D flexible siloxane membrane using Flexcell™. Then, they run enzyme-linked immunosorbent assay (ELISA) for measuring protein IL-6 and IL-8 synthesis, p-nitrophenylbutyrate (PNB) for measuring esterase activity, and p-nitrophenylphosphate (PNP) for assessing acid phosphatase activity. They demonstrated that mechanical strain increased the release of IL-6, esterase, and acid phosphatase activity significantly (p<0.05) from U937 cells. Further, U937 cell demonstrated progressive reorganization of filamentous actin and morphology change from a round cell shape to an irregular, spread phenotype upon mechanical strain (98) (Figure 3). In another study (99), they compared the effects of two different mechanical strain types of uniform uniaxial and non-uniform biaxial mechanical strain on U937 cells’ in intracellular esterase and acid phosphatase (AP) activities, as well as monocyte-specific esterase (MSE) protein levels along with IL-6 and IL-8 levels. The 2D flexible siloxane membrane was coated with ECM proteins collagen type I or arginylycylaspartic acid (RGD) peptide and the study used U937 human macrophage-like cells. The membrane was then strained with 10% biaxial strain or uniaxial strain with a 0.25 Hz frequency. They found out that biaxial strain increased the intracellular esterase, AP activities, monocyte-specific esterase (MSE) protein levels, as well as IL-6 level but not IL-8. Upon uniaxial strain, intracellular esterase and IL-6 level increased but AP activity was not changed. These extensive studies demonstrated two important facts. First, 10% mechanical strain with 0.25 Hz frequency (whether it was applied uniaxially or biaxially) increased the proinflammatory-protein levels and intracellular activities selectively. Secondly, the method of applying mechanical strain (uniaxially or biaxially) affected the different functions of the cells.
Yang et al. (102) studied how cyclic mechanical stimuli alter the immediate-early pro-inflammatory gene expression (c-fos and c-jun expression) and extracellular matrix degradation-related gene expression profiles including MMP-1, MMP-3, and TIMP-1 in macrophage cells. They isolated monocytes from human peripheral blood and underwent adhesion to create macrophages. The macrophage cells were then cultured on fibronectin-coated 2D thin and transparent membrane to apply 1, 4, and 9% biaxial mechanical strain at 1Hz for 24 hours. Phorbol myristate acetate (PMA) was also introduced to the cell culture media to understand the possible combinatorial effect of PMA and mechanical loading on MMP-1, MMP-3, and TIMP-1 expressions. Their results suggested that as early as half an hour after the mechanical loading, cells strained with 4% strain rapidly induced c-fos and c-jun expressions, while mechanical loading alone did not change the expression of matrix degradation-related genes (MMP-1, MMP-3, and TIMP-1). PMA inclusion (162 μmol/L) along with mechanical strain increased MMP-1 expression more than 5-fold, MMP-3 expression 1.6-fold, and TIMP-1 expression 1.3-fold. This study demonstrated that immediately after mechanical strain (within 30 min), pro-inflammatory gene expressions increased significantly compared to counterparts without mechanical strain.
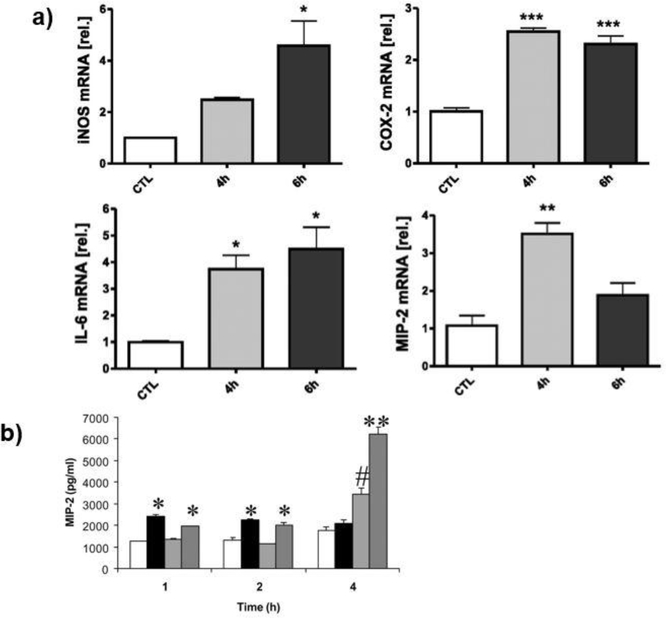
Other research groups studied how mechanical strain within the lung affects the macrophage functions and ventilation-induced lung injury (103, 104). Pugin et al. (103) studied how primary alveolar macrophages (AMs) and promonocytic human THP-1 cells were activated through prolonged cyclic biaxial strain resembling that induced by mechanical ventilation of lung. They also applied mechanical loading to endothelial cells, bronchial cells, and fibroblast cells to understand whether other lung cells were affected by a cyclic mechanical strain. Cells were seeded on a silastic membrane as a monolayer and exposed to 12–15% biaxial strain for 32 hours using Bio-flex six-well plates. They concluded that in response to strain mimicking the mechanical ventilation, macrophages produce proinflammatory mediators including IL-8 and MMP-9. Mourgeon et al. (104) applied 2% and 5% mechanical strains at various frequencies to organotypic cultures of fetal lung cells using Bio-Stretch™ for four hours. They measured the macrophage inflammatory protein (MIP)-2 production and expression using ELISA and RT-PCR, respectively. Their results suggested that MIP-2 production was significantly increased when cells strained with 5% strain and 40 cycles/min frequency. However, mRNA levels of MIP-2 were not elevated by mechanical stimulation. Yet, the lipopolysaccharide (LPS)-treated cells demonstrated both high MIP-2 mRNA level and MIP protein. They concluded that the major mechanical strain increased MIP-2 protein secretion, but not gene expression. Figure 4 shows the representative graphs from the studies investigating how mechanical strain and its application duration affected the mRNA levels of proinflammatory genes and protein secretion.
Figure 4.
The mechanical strain and its exposure time mediated mRNA level of proinflammatory genes in peritoneal macrophages (A) and MIP-2 protein secretion in fetal lung cells (B). (A) The changes in iNOS, COX-2, IL-1β, MIP-1α, and MIP-2 mRNA level with prolonged 20% mechanical strain exposure. * indicated p<0.05, ** indicated p<0.01, *** indicated p<0.001 versus unstretched controls. Error bars represented standard deviation with n=3. (49) (B) MIP-2 production of rat macrophages over time. White columns indicate control, Black columns indicated mechanical loading of 5% and 40 cycles/min, Light grey indicated LPS stimulation, and Dark grey columns indicate mechanical loading of 5% and 40 cycles/min and LPS stimulation. * indicated p<0.05 versus control, ** indicated p<0.05 versus all other groups, # indicated p<0.05 versus the rest of the groups (104)
Xu et al.(105) utilized mouse monocyte/macrophage cells (RAW264.7) as an osteoclast precursor and mechanically strained the cells using a custom-built uniaxial mechanical loading platform to understand whether osteoclast activation can be regulated by mechanical loading. They seeded the RAW264.7 cells as a monolayer on a cell-culture unit of the loading platform and applied various strain values to the cells with magnitudes of 0.001, 0.0015, 0.002, 0.0025, and 0.005% strain with 1Hz frequency for an hour each day for 3 days. Their results demonstrated that mechanical strain with lower magnitude suppressed RAW264.7 osteoclast differentiation while the higher strain acted as a stimulant for osteoclast fusion and activation.
Another important study investigated the effect of mechanical strain on human peripheral blood mononuclear cells (hPBMCs), which play a dominant role in early immune response (101). They applied 7% and 12% cyclic uniaxial strains on hPBMCs seeded on 2D electrospun thermoplastic elastomer polycaprolactone (PCL)-based tissue scaffold strip with a dimension of 25mm by 5mm. The mechanical strains were applied with 0.8 Hz frequency for 7 days using Flexcell™. They conducted gene expression analysis and immunohistochemistry to understand the expression of immune response markers upon mechanical strain applications. Their results suggested that 7% mechanical strain, considered as moderate amplitude, promoted polarization towards an anti-inflammatory and reparative M2 lineage and increased the expression of MCP-1, IL-6, IL-10, and MMP9. Further, 7% mechanical strain did not create cell loss. On the other hand, 12% strain promoted hPBMCs towards pro-inflammatory phenotype of M1 and resulted in cell loss over 7-days.
Wehner et al. (49) hypothesized that mechanical stress following intestinal manipulation during abdominal surgery initiates or aggravates proinflammatory responses of intestinal smooth muscle cells (iSMC) and macrophages. To test their hypothesis, they applied static and cyclic mechanical loading to monolayers of iSMC and macrophages cultured on collagen type-I coated Bioflex™ mechanical loading platform wells. Also, the biological relevance of applying cyclic loading was to mimic the peristaltic contraction of tunica muscularis (106). They assessed cell-specific proinflammatory gene expressions including iNOS, COX-2, IL-1β, MIP-1α, and MIP-2. The static mechanical strain was applied in repetitive 30-minute cycles with 20% strain for 23 minutes followed by 7 minutes break for 6 hours. The cyclic mechanical strain was applied with 0.5 Hz frequency with 20% strain for 6 hours with half sinus curve shape. Their results concluded that while cyclic strain applied for 6 hours did not increase the expression of proinflammatory genes; static strain significantly induced iNOS, COX-2, and IL-1β for iSMC as well as COX-2 and IL-6 expressions for macrophages. Although the applied mechanical strain was the same (20%) for static and cyclic loading conditions, only static loading promoted the pro-inflammatory gene expression. This can be attributed to the total exposure time of the cells to the mechanical strain. In static loading, the loading characteristic does not change with time, which means that the cells experienced 20% strain for continuous 6 hours (except 7 mins break in every hour).On the other hand, in cyclic loading (0.5 Hz frequency) with half sinus profile, the loading characteristic changes with a sinusoidal pattern, in which load increased, reached a peak point and decreased again. Thus, in cyclic loading, the cells exposed to the actual 20% strain at the peak point for a short period of time. The loading exposure time may affect the gene expression of intestinal smooth muscle cells and peritoneal macrophages.
These aforementioned studies demonstrated that regardless of their origin (human or murine) mechanical strain affects the lineage commitment of macrophages and polarizes them either to anti-inflammatory or pro-inflammatory lineages. Table 3 tabulates the representative studies investigating the relationship between uniaxial or biaxial mechanical strain and macrophage polarization state and function.
Table 3.
Summarized the studies investigating the relationship between uniaxial or biaxial mechanical strain and macrophage polarization state and function.
| Species | Mechanical Strain Parameters | Cell Origin | Targeted Tissue | Polarization State and Function | Ref |
|---|---|---|---|---|---|
| Human | 0–10% Strain for 48 hrs Frequency: 1 Hz |
U 937 | Arterial wall environment | M1 | (95) |
| 10% Strain for 48 hrs Frequency: 0.25 Hz |
U 937 | ECM | M1 | (99, 100) | |
| 7, 12% Strain for 7 days Frequency: 0.8 Hz |
Human donor blood; buffy coats | Arterial wall environment | M1 | (101) | |
| 0, 9, 18, and 27% strain for 96 hours | THP-1 | Bone | M1 | (42) | |
| 20% Strain for 16 hours Frequency: 1 Hz |
Human donor blood; buffy coats | Bone | M1 | (44) | |
| 12–15% Strain for 32 hrs Frequency: 0.33 Hz † |
THP-1 | Lung | M1 up to 24 hrs | (103) | |
| 10% Strain for 72 hrs Frequency: 0.25 Hz † |
U 937 | General immunological function – particularly with ECM | M1 | (99) | |
| 2.3–9.4% Strain for 12 hrs Frequency: 1 Hz † |
THP-1 | Arterial wall environment | M1 | (97, 107) | |
| 4% Strain for 24 hrs Frequency: 1 Hz |
Human donor blood; buffy coats | General immunological function | M2 | (102) | |
| 0, 7% Strain for 7 days Frequency: 0.8 Hz |
Human donor blood; buffy coats | Arterial wall environment | M2 (0 % and 7%) | (101) | |
| 0–10% Strain for 48 hrs Frequency: 1 Hz |
U 937 | Arterial wall environment | M2 (48 hrs) | (95) | |
| 12–15% Strain for 32 hrs Frequency: 0.33 Hz † |
THP-1 | Lung | M2 (after 24 hrs) | (103) | |
| Murine | 11–12.5% and 15–17% Strain for up to 2 hrs, Frequency: 1.67 Hz |
J774.16 | General immunological function | Increased phagocytic activity | (71) |
| 20% Strain for 3 days Frequency: 0.5 Hz |
iSMC | Intestinal tissue | M1 | (49) | |
| 1,000–5,000 με for 1 hr over 3 days Frequency: 0.5 Hz |
RAW264.7 | Bone | M1(1,000, 1,500, and 5,000 με) | (105) | |
| 1,000–5,000 με for 1 hr over 3 days Frequency: 0.5 Hz |
RAW264.7 | Bone | M2 (2,000 and 2,500 με) | (105) | |
| 5% Strain for 24 or 48 hrs Frequency: 1 Hz† |
C57BL/6J | General immunological function | M2 | (96) | |
| 0–10% Strain for 48 hrs Frequency: 1 Hz |
Rat Peritoneal Macrophage (RPM) | Arterial wall environment | M2 | (95) | |
Indicated biaxial loading, otherwise the parameters used were for uniaxial loading
3. Biomechanical-induced changes in Pro- and Anti-Inflammatory Cytokine Secretion
There is also an abundance of literature on how mechanical cues affect the pro- and anti-inflammatory cytokine production of cells (not necessarily immune cells), which can apply to macrophage lineage adaptation. These studies did not apply mechanical loading directly on macrophages, rather they studied how the cytokine dominated secretome from mechanically strained cells induced macrophage polarization and phagocytic activities.
The role of mechanical strain-induced changes in monocyte/macrophage molecular function and cytokine secretion in joint arthroplasties were investigated in terms of aseptic loosening of the implant (42–44). In these studies, it was shown that the mechanical environment around the implant affected the pro-inflammatory cytokine expression. Wear debris from the implant further increased cytokine expressions. The increased cytokine expressions then polarized monocyte-derived macrophages into pro-inflammatory lineage (M1) and promoted phagocytosis.
In another study by Dziki et al. (108), the effect of the secretome was investigated from mechanically-strained myoblasts (C2C12 cells) on macrophage polarization. They applied 10% strain with 1Hz frequency for 5 hours to myoblasts seeded on collagen type-I coated wells using Flexcell™ and collected the conditioned media from mechanically-strained cells for culturing BMDMs. They used immunolabeling to identify whether macrophages were polarized to M1 or M2 lineages when cultured in a conditioned media from mechanically-strained myoblast cells. In a different experimental setup, mechanically strained macrophages underwent the same loading condition as the myoblasts (10% strain, 1Hz, 5 hours). Conditioned media from the BMDMs were used to culture myoblasts and to examine the effects of myoblast migration, proliferation, and differentiation. They concluded that mechanically loaded myoblasts promote M2-like macrophage phenotype while mechanically loaded macrophages promote myoblast chemotaxis and differentiation.
Fujishiro et al.(42) investigated whether there was a synergistic effect between titanium particles (less than 5 μm diameter) from the implant and mechanical loading on macrophage prostaglandin E2 (PGE2) activity, which is highly associated with osteoclast differentiation. They cultured THP-1 cells on a 2D flexible siloxane membrane and applied 9, 18, and 27% strain with 0.1 Hz frequency for 96 hours using Flexcell™. Their results suggested that macrophages under mechanical strain or titanium particle exposure demonstrated significant elevated PGE2 activity. The most noteworthy difference; however, was observed for macrophages exposed to both mechanical strain and titanium particles. For these macrophages, the PGE2 activity increased 97-fold compared to non-stimulated counterparts. The mechanical strain and wear debris demonstrated the synergistic effect on macrophage differentiation towards anti-inflammatory linages and initiated osteolysis.
McEvoy et al. (43) also studied the synergistic effects of particles and hydrostatic cyclic pressure on inflammatory cytokine production in human monocyte-derived macrophages. They applied three different hydrostatic pressures (at 0.069, 0.035, at 0.017 MPa) on macrophages through culturing macrophage monolayers in a standard 24-well plate using pressurized wells with 5% CO2 for 10 days. The cells were cultured with and without particulate wear debris of polyethylene from an implant to understand the synergistic effect between hydrostatic pressure and particles on the aseptic loosening of an orthopedic implant. The inflammatory cytokines with an increased expression included IL-1β, IL-6, and TNF-α with increased pressure. The increased level of these cytokines further differentiates the macrophages into pro-inflammatory lineages and started osteolysis. However, their results also demonstrated that a combination of ultra-high molecular weight polyethylene particles and low pressure (at 0.017 MPa) resulted in a substantial elevation of IL-1β, IL-6, TNF-α cytokines compared with the levels found with either pressure or particles alone. They suggested that either reduction of hydrostatic pressure (through improving the implant fixation) or particulate wear debris would reduce the aseptic loosening.
Matthews et al.(44) investigated the synergistic effect of compressive strain and polyethylene (PE) microparticles on the osteolytic potential of macrophages through measuring pro-inflammatory cytokine secretion. Monocytes were isolated from human peripheral blood and co-cultured with PE particles (mean size 0.21 μm) with various suspension values of 7.5, 15, and 30 μm3 particles/cell on a commercially available crosslinked collagen type-I matrix, Permacol ®. The monocyte and PE particles-seeded matrix was then transferred to ComCell, a custom-built mechanical loading platform, for applying 20% compressive strain with 1Hz frequency for 16 hours. Upon compressive strain application, the pro-inflammatory cytokine TNF-α production was quantified using ELISA. Their data demonstrated that with lower amounts of PE particles in the system (7.5μm3/cells), there was no increase in TNF-α production with or without compressive strain application. However, when PE particle volume was increased to 15 μm3/cells, co-cultured monocytes exposed to 20% strain produced small amounts of TNF-α, while monocytes within a static culture (no compressive strain) did not. With an even higher number of PE particles (30 μm3/cells), both monocytes cultured under compressive strain and static conditions expressed TNF-α. Interestingly, the TNF-α secretion from compressively strained monocytes was 5-fold higher than the statically cultured ones. They concluded that at low PE particle presence, the mechanical strain may not contribute to pro-inflammatory cytokine secretion. However, high PE particle presence in combination with compressive strain led to increased TNF-α secretion which may significantly contribute to periprosthetic osteolysis.
Overall, it is clear that mechanical milieu has a great impact on cellular phenotype, commitment, and function. The knowledge gained from these studies can be utilized to understand mechanosensitive disease progression and to identify approaches for eliminating negative consequences from mechanical loading experienced by the tissue of interest.
4. Future Direction
The impactful studies mentioned in this review and many others in the literature shed light on biomechanical-induced changes on macrophages. They highlighted the importance of mechanical cues and demonstrated how these cues were dictating the innate immune cells activity and polarization. However, the study area of mechano-immunomodulation is in its infancy, which commands lots of new and exciting challenges. One of these challenges is utilizing more physiologically-relevant in vitro platforms to study the role of the tissue mechanical milieu on innate immune cells. Without any exception, the studies investigating the role of mechanical stimuli on macrophage phenotypic adaptation cultured and strained macrophages on two-dimensional (2D) surfaces (elastic membrane or petri dish). The three-dimensionality of the extracellular environment where macrophages reside and its effect on macrophage functions are largely ignored. Yet, seminal work in the past couple of decades has demonstrated that not only macrophages but many cell types differentiate, populate, and migrate differently in a three-dimensional (3D) microenvironment versus 2D surfaces (28).
Further, cells cultured on the 2D membrane may be exposed to additional shear forces due to the movement of fluid over the membrane in each strain application. This shear force over the 2D membrane is dependent on the depth of the media, which may change from study to study thereby creating inconsistencies in the data. This fact raises a considerable question for tissue engineering applications and perhaps is also suggestive of demand for biomimetic 3D mechano-immunomodulation studies. Several research groups designed and utilized biomimetic mechanical loading platforms, in which physiologically-relevant and homogeneous mechanical strain are applied to cells encapsulated within 3D collagen matrix using computer-controlled mechanisms (109–111). These mechanical loading platforms were computationally validated in how the applied strain was delivered to the matrix than to the cells across different parameters. Utilizing these kinds of computationally validated mechanical loading platforms to apply homogenous mechanical strain to 3D encapsulated innate immune cells will provide a more physiologically-relevant approach to study mechano-immunomodulation for tissue regeneration purposes.
Another challenge in this field is the data inconsistency. We can undoubtedly say that macrophages adapt their phenotype under mechanical loading; however, we are not able to precisely claim that a particular mechanical loading modality with particular strain and frequency can differentiate a macrophage to a certain phenotype. For instance, it is very hard to generalize and claim that 10% uniaxial mechanical strain with 1 Hz frequency differentiates macrophages to an anti-inflammatory phenotype. In fact, the literature presents incongruent findings. The discrepancies between the findings may be attributed to numerous factors including the macrophage cell line source, the differences between the mechanical loading platform, and the difference between the species.
The studies demonstrated that the macrophages isolated from different tissues behave differently since residing macrophages in each tissue adapt to the local environment. As mentioned before in this review, tissue-resident macrophages have specialized functions and external stimuli inducing mechanical loading may affect them differently. For instance, while adipose tissue associated macrophages regulate adaptive thermogenesis, insulin sensitivity, and glucose tolerance (112), the alveolar macrophages recycle of surfactant molecular, immune surveillance of inhaled pathogens, and clears the allergens (113). It is not reasonable to expect tissue-resistant macrophages behave the same way even they exposed to same external stimuli. The variety of mechanical loading platforms used in these studies can be another source for inconsistency. Surface and physical properties of the platforms used for culturing and mechanically loading macrophages can be different from platform to the platform, which may affect the outcome of the study. Further, the imprecise control or non-homogeneous strain distribution along the cultured cells can lead to conflicting results.
Last but not least, that data discrepancy between the studies may stem from the differences between the species. The utilization of the murine immune system in modeling the human immune system is a well-established technique in immunobiology and bioengineering. There are many similarities between the immune response of these two species; yet also many divergences (67, 70). While the differences between human and mice create data discrepancies between the experiments, more importantly, these differences pose a huge limitation in translating the findings towards clinical settings. The numerous comparative studies between human and mouse immune responses concluded that the scope of the study is the determinant factor in defining the relevancy of the mouse model. For instance, a comprehensive comparative study of gene expressions between human and mouse monocyte subsets demonstrated that 132 genes were regulated similarly between the monocyte subsets in both species, significantly strengthening the proposed homology between subsets (68). They also uncovered the gene expression patterns that were not conserved between human and mice. If the target of interest is among those 132 genes, then it can be said that the findings from that study are significant for clinical translation. Other than that, extreme caution needs to be demonstrated in the clinical interpretation of the data. In addition, when it comes to the macrophage polarization, in most cases, the mouse models can replicate the human macrophage response (114). However, for certain cases such as in iNOS and Arg-1 expression, there are huge differences between species for pro-inflammatory (M1) and anti-inflammatory (M2) markers (115). Thus, if the scope of a study involves iNOS and Arg-1, there is an increased risk associated with translation the findings to humans (69). This fact becomes really important in studies investigating atherosclerotic lesions (116). In sum, the clinical relevance of mice model depends on the objective of the study and what kind of genes are investigated.
Upon resolving these important challenges, we will be ready to dive deep into the exciting world of mechano-immunomodulation. For example, we can study how macrophages and other innate immune cells such as platelets, neutrophils, and dendritic cells communicate within 3D tissue-like structures cultured under physiologically relevant mechanical strain conditions; or how macrophage polarization changes with the presence of progenitor cells when cultured within 3D tissue-like structures cultured under physiologically relevant mechanical strain conditions. Furthermore, the studies discussed in section 3 demonstrated that macrophage phenotype commitment can be manipulated by cytokines secreted upon mechanical loading of non-macrophage cells. These findings are very exciting; however, what more exciting and interesting is whether there is a synergistic interaction between macrophages and non-macrophage cells (within the co-culture environment) under the mechanical loading condition and whether this synergistic interaction has an amplified effect on macrophage polarization. With well-designed experiments, this interesting research questions can be tackled and we will be able to identify the combination of the indirect effect of cytokine secretion and direct effect of mechanical loading on macrophage polarization and will be able to study innate immune cells- nonimmune cells communication under mechanically dynamic environment. Our exhausting effort in finding such studies, unfortunately, did not result positive. There are exciting research endeavors in this direction and it is worth for in detail investigation.
5. Conclusion
In response to the variety and magnitude of mechanical stimuli from extracellular spaces, macrophages polarize to a spectrum of functional phenotypes. In this review, we discussed some emerging evidence of mechanoresponsiveness of macrophages and that its phenotype commitment can be controlled by various mechanical stimuli. To this end, a deeper understanding of macrophage lineage adaptation under physiological mechanical strain is required to utilize the potential of macrophages as a therapeutic agent in tissue regeneration, especially for tissues under constant mechanical loading.
Figure 2.
Various mechanical loading modalities experienced across the human body
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Broughton G 2nd, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117(7 Suppl):12S–34S. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- 2.McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci U S A. 2013;110(43):17253–8. doi: 10.1073/pnas.1308887110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spiller KL, Wrona EA, Romero-Torres S, Pallotta I, Graney PL, Witherel CE, Panicker LM, Feldman RA, Urbanska AM, Santambrogio L, Vunjak-Novakovic G, Freytes DO. Differential gene expression in human, murine, and cell line-derived macrophages upon polarization. Exp Cell Res. 2016;347(1):1–13. doi: 10.1016/j.yexcr.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Vishwakarma A, Bhise NS, Evangelista MB, Rouwkema J, Dokmeci MR, Ghaemmaghami AM, Vrana NE, Khademhosseini A. Engineering Immunomodulatory Biomaterials To Tune the Inflammatory Response. Trends Biotechnol. 2016;34(6):470–82. doi: 10.1016/j.tibtech.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Brown BN, Sicari BM, Badylak SF. Rethinking regenerative medicine: a macrophage-centered approach. Front Immunol. 2014;5:510. doi: 10.3389/fimmu.2014.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiller KL, Wrona EA, Romero-Torres S, Pallotta I, Graney PL, Witherel CE, Panicker LM, Feldman RA, Urbanska AM, Santambrogio L. Differential gene expression in human, murine, and cell line-derived macrophages upon polarization. Experimental cell research. 2016;347(1):1–13. [DOI] [PubMed] [Google Scholar]
- 7.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6(13.10):12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kratochvill F, Neale G, Haverkamp JM, Van de Velde LA, Smith AM, Kawauchi D, McEvoy J, Roussel MF, Dyer MA, Qualls JE, Murray PJ. TNF Counterbalances the Emergence of M2 Tumor Macrophages. Cell Rep. 2015;12(11):1902–14. doi: 10.1016/j.celrep.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munder M, Eichmann K, Modolell M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J Immunol. 1998;160(11):5347–54. [PubMed] [Google Scholar]
- 10.Munder M, Eichmann K, Moran JM, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999;163(7):3771–7. [PubMed] [Google Scholar]
- 11.Schleicher U, Paduch K, Debus A, Obermeyer S, Konig T, Kling JC, Ribechini E, Dudziak D, Mougiakakos D, Murray PJ, Ostuni R, Korner H, Bogdan C. TNF-Mediated Restriction of Arginase 1 Expression in Myeloid Cells Triggers Type 2 NO Synthase Activity at the Site of Infection. Cell Rep. 2016;15(5):1062–75. doi: 10.1016/j.celrep.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minutti CM, Knipper JA, Allen JE, Zaiss DM. Tissue-specific contribution of macrophages to wound healing. Semin Cell Dev Biol. 2017;61:3–11. doi: 10.1016/j.semcdb.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Gundra UM, Girgis NM, Ruckerl D, Jenkins S, Ward LN, Kurtz ZD, Wiens KE, Tang MS, Basu-Roy U, Mansukhani A, Allen JE, Loke P. Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct. Blood. 2014;123(20):e110–22. doi: 10.1182/blood-2013-08-520619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169(5):2253–63. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 15.Ogle ME, Segar CE, Sridhar S, Botchwey EA. Monocytes and macrophages in tissue repair: Implications for immunoregenerative biomaterial design. Exp Biol Med (Maywood). 2016;241(10):1084–97. doi: 10.1177/1535370216650293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80(6):1298–307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hesketh M, Sahin KB, West ZE, Murray RZ. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int J Mol Sci. 2017;18(7). doi: 10.3390/ijms18071545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malissen B, Tamoutounour S, Henri S. The origins and functions of dendritic cells and macrophages in the skin. Nat Rev Immunol. 2014;14(6):417–28. doi: 10.1038/nri3683. [DOI] [PubMed] [Google Scholar]
- 19.Murray PJ. Macrophage Polarization. Annu Rev Physiol. 2017;79:541–66. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 20.Boyle JJ. Heme and haemoglobin direct macrophage Mhem phenotype and counter foam cell formation in areas of intraplaque haemorrhage. Curr Opin Lipidol. 2012;23(5):453–61. doi: 10.1097/MOL.0b013e328356b145. [DOI] [PubMed] [Google Scholar]
- 21.Roszer T Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garash R, Bajpai A, Marcinkiewicz BM, Spiller KL. Drug delivery strategies to control macrophages for tissue repair and regeneration. Exp Biol Med (Maywood). 2016;241(10):1054–63. doi: 10.1177/1535370216649444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans BJ, Haskard DO, Sempowksi G, Landis RC. Evolution of the Macrophage CD163 Phenotype and Cytokine Profiles in a Human Model of Resolving Inflammation. Int J Inflam. 2013;2013:780502. doi: 10.1155/2013/780502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrante CJ, Leibovich SJ. Regulation of Macrophage Polarization and Wound Healing. Adv Wound Care (New Rochelle). 2012;1(1):10–6. doi: 10.1089/wound.2011.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front Physiol. 2018;9:419. doi: 10.3389/fphys.2018.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sridharan R, Cameron AR, Kelly DJ, Kearney CJ, O’Brien FJ. Biomaterial based modulation of macrophage polarization: a review and suggested design principles. Materials Today. 2015;18(6):313–25. [Google Scholar]
- 27.Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Engineering Part A. 2008;14(11):1835–42. [DOI] [PubMed] [Google Scholar]
- 28.Bartneck M, Heffels KH, Pan Y, Bovi M, Zwadlo-Klarwasser G, Groll J. Inducing healing-like human primary macrophage phenotypes by 3D hydrogel coated nanofibres. Biomaterials. 2012;33(16):4136–46. Epub 2012/03/16. doi: 10.1016/j.biomaterials.2012.02.050. [DOI] [PubMed] [Google Scholar]
- 29.Koyal Garg NP, Oskeritzian Carole, Ryan John, Bowlin Gary. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds Biomaterials. 2013;34:4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stankevich Ksenia GA, Alexander Filimonov, Harald Kluter, Evgeniya Mamontova, Sergei Tverdokhlebov, Kzhyshkowska. Surface Modification of biomaterials based on hihg-molecular polylactic acid and their effect on inflammatory reactions of primary human monocyte-derived macrohage:Perpective of personalized theraphy Mater Sci Eng C 2015;51:117. [DOI] [PubMed] [Google Scholar]
- 31.Minardi S, Corradetti B, Taraballi F, Byun JH, Cabrera F, Liu X, Ferrari M, Weiner BK, Tasciotti E. IL-4 Release from a Biomimetic Scaffold for the Temporally Controlled Modulation of Macrophage Response. Ann Biomed Eng. 2016;44(6):2008–19. doi: 10.1007/s10439-016-1580-z. [DOI] [PubMed] [Google Scholar]
- 32.Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35(15):4477–88. doi: 10.1016/j.biomaterials.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taraballi F, Corradetti B, Minardi S, Powel S, Cabrera F, Van Eps JL, Weiner BK, Tasciotti E. Biomimetic collagenous scaffold to tune inflammation by targeting macrophages. J Tissue Eng. 2016;7:2041731415624667. doi: 10.1177/2041731415624667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hotchkiss KM, Reddy GB, Hyzy SL, Schwartz Z, Boyan BD, Olivares-Navarrete R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016;31:425–34. doi: 10.1016/j.actbio.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luu TU, Gott SC, Woo BW, Rao MP, Liu WF. Micro- and Nanopatterned Topographical Cues for Regulating Macrophage Cell Shape and Phenotype. ACS Appl Mater Interfaces. 2015;7(51):28665–72. doi: 10.1021/acsami.5b10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedemann M, Kalbitzer L, Franz S, Moeller S, Schnabelrauch M, Simon JC, Pompe T, Franke K. Instructing Human Macrophage Polarization by Stiffness and Glycosaminoglycan Functionalization in 3D Collagen Networks. Adv Healthc Mater. 2017;6(7). doi: 10.1002/adhm.201600967. [DOI] [PubMed] [Google Scholar]
- 37.Blakney AK, Swartzlander MD, Bryant SJ. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J Biomed Mater Res A. 2012;100(6):1375–86. doi: 10.1002/jbm.a.34104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Li Y, Gao B, Qin C, He Y, Xu F, Yang H, Lin M. Engineering mechanical microenvironment of macrophage and its biomedical applications. Nanomedicine (Lond). 2018. doi: 10.2217/nnm-2017-0324. [DOI] [PubMed] [Google Scholar]
- 39.Rao AJ, Gibon E, Ma T, Yao Z, Smith RL, Goodman SB. Revision joint replacement, wear particles, and macrophage polarization. Acta Biomater. 2012;8(7):2815–23. doi: 10.1016/j.actbio.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purdue PE. Alternative macrophage activation in periprosthetic osteolysis. Autoimmunity. 2008;41(3):212–7. doi: 10.1080/08916930701694626. [DOI] [PubMed] [Google Scholar]
- 41.Santerre JP, Labow RS, Boynton EL. The role of the macrophage in periprosthetic bone loss. Canadian journal of surgery Journal canadien de chirurgie. 2000;43(3):173–9. [PMC free article] [PubMed] [Google Scholar]
- 42.Fujishiro T, Nishikawa T, Shibanuma N, Akisue T, Takikawa S, Yamamoto T, Yoshiya S, Kurosaka M. Effect of cyclic mechanical stretch and titanium particles on prostaglandin E2 production by human macrophages in vitro. J Biomed Mater Res A. 2004;68(3):531–6. doi: 10.1002/jbm.a.20098. [DOI] [PubMed] [Google Scholar]
- 43.McEvoy A, Jeyam M, Ferrier G, Evans CE, Andrew JG. Synergistic effect of particles and cyclic pressure on cytokine production in human monocyte/macrophages: proposed role in periprosthetic osteolysis. Bone. 2002;30(1):171–7. [DOI] [PubMed] [Google Scholar]
- 44.Matthews JB, Mitchell W, Stone MH, Fisher J, Ingham E. A novel three-dimensional tissue equivalent model to study the combined effects of cyclic mechanical strain and wear particles on the osteolytic potential of primary human macrophages in vitro. Proc Inst Mech Eng H. 2001;215(5):479–86. doi: 10.1243/0954411011536073. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto K, Ikeda U, Shimada K. Role of mechanical stress in monocytes/macrophages: implications for atherosclerosis. Curr Vasc Pharmacol. 2003;1(3):315–9. Epub 2004/08/24. [DOI] [PubMed] [Google Scholar]
- 46.Harwani SC. Macrophages under pressure: the role of macrophage polarization in hypertension. Transl Res. 2018;191:45–63. doi: 10.1016/j.trsl.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moreno PR, Falk E, Palacios IF, Newell JB, Fuster V, Fallon JT. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation. 1994;90(2):775–8. Epub 1994/08/01. [DOI] [PubMed] [Google Scholar]
- 48.Seneviratne AN, Cole JE, Goddard ME, Mohri Z, Krams R, Monaco C. Macrophage Polarisation in Shear Stress Modulated Atherosclerotic Plaque Vulnerability. Atherosclerosis. 2012;225(2):E2–E. [Google Scholar]
- 49.Wehner S, Buchholz BM, Schuchtrup S, Rocke A, Schaefer N, Lysson M, Hirner A, Kalff JC. Mechanical strain and TLR4 synergistically induce cell-specific inflammatory gene expression in intestinal smooth muscle cells and peritoneal macrophages. Am J Physiol Gastrointest Liver Physiol. 2010;299(5):G1187–97. doi: 10.1152/ajpgi.00452.2009. [DOI] [PubMed] [Google Scholar]
- 50.Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985;5(3):293–302. Epub 1985/05/01. [DOI] [PubMed] [Google Scholar]
- 51.Stone PH, Coskun AU, Kinlay S, Clark ME, Sonka M, Wahle A, Ilegbusi OJ, Yeghiazarians Y, Popma JJ, Orav J, Kuntz RE, Feldman CL. Effect of endothelial shear stress on the progression of coronary artery disease, vascular remodeling, and in-stent restenosis in humans: in vivo 6-month follow-up study. Circulation. 2003;108(4):438–44. Epub 2003/07/16. doi: 10.1161/01.CIR.0000080882.35274.AD. [DOI] [PubMed] [Google Scholar]
- 52.Heo KS, Fujiwara K, Abe J. Shear stress and atherosclerosis. Mol Cells. 2014;37(6):435–40. Epub 2014/05/02. doi: 10.14348/molcells.2014.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dhawan SS, Avati Nanjundappa RP, Branch JR, Taylor WR, Quyyumi AA, Jo H, McDaniel MC, Suo J, Giddens D, Samady H. Shear stress and plaque development. Expert Rev Cardiovasc Ther. 2010;8(4):545–56. Epub 2010/04/20. doi: 10.1586/erc.10.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG, Jo H. Oscillatory shear stress stimulates endothelial production of O2- from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. The Journal of biological chemistry. 2003;278(47):47291–8. Epub 2003/09/06. doi: 10.1074/jbc.M305150200. [DOI] [PubMed] [Google Scholar]
- 55.Lehoux S, Tedgui A. Cellular mechanics and gene expression in blood vessels. J Biomech. 2003;36(5):631–43. Epub 2003/04/16. [DOI] [PubMed] [Google Scholar]
- 56.van der Wal AC, Becker AE, van der Loos CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994;89(1):36–44. Epub 1994/01/01. [DOI] [PubMed] [Google Scholar]
- 57.Kwak BR, Back M, Bochaton-Piallat ML, Caligiuri G, Daemen MJ, Davies PF, Hoefer IE, Holvoet P, Jo H, Krams R, Lehoux S, Monaco C, Steffens S, Virmani R, Weber C, Wentzel JJ, Evans PC. Biomechanical factors in atherosclerosis: mechanisms and clinical implications. Eur Heart J. 2014;35(43):3013–20, 20a–20d. Epub 2014/09/19. doi: 10.1093/eurheartj/ehu353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castro-Nunez L, Dienava-Verdoold I, Herczenik E, Mertens K, Meijer AB. Shear stress is required for the endocytic uptake of the factor VIII-von Willebrand factor complex by macrophages. J Thromb Haemost. 2012;10(9):1929–37. Epub 2012/07/21. doi: 10.1111/j.1538-7836.2012.04860.x. [DOI] [PubMed] [Google Scholar]
- 59.Bryckaert M, Rosa JP, Denis CV, Lenting PJ. Of von Willebrand factor and platelets. Cell Mol Life Sci. 2015;72(2):307–26. Epub 2014/10/10. doi: 10.1007/s00018-014-1743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Denis CV, Lenting PJ. VWF clearance: it’s glycomplicated. Blood. 2018;131(8):842–3. Epub 2018/02/24. doi: 10.1182/blood-2018-01-824904. [DOI] [PubMed] [Google Scholar]
- 61.Rosenson-Schloss RS, Vitolo JL, Moghe PV. Flow-mediated cell stress induction in adherent leukocytes is accompanied by modulation of morphology and phagocytic function. Med Biol Eng Comput. 1999;37(2):257–63. [DOI] [PubMed] [Google Scholar]
- 62.Seneviratne AN, Cole JE, Goddard ME, Park I, Mohri Z, Sansom S, Udalova I, Krams R, Monaco C. Low shear stress induces M1 macrophage polarization in murine thin-cap atherosclerotic plaques. J Mol Cell Cardiol. 2015;89(Pt B):168–72. doi: 10.1016/j.yjmcc.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 63.De Wilde D, Trachet B, De Meyer GR, Segers P. Shear Stress Metrics and Their Relation to Atherosclerosis: An In Vivo Follow-up Study in Atherosclerotic Mice. Ann Biomed Eng. 2016;44(8):2327–38. Epub 2015/12/24. doi: 10.1007/s10439-015-1540-z. [DOI] [PubMed] [Google Scholar]
- 64.Olivon VC, Fraga-Silva RA, Segers D, Demougeot C, de Oliveira AM, Savergnini SS, Berthelot A, de Crom R, Krams R, Stergiopulos N, da Silva RF. Arginase inhibition prevents the low shear stress-induced development of vulnerable atherosclerotic plaques in ApoE−/− mice. Atherosclerosis. 2013;227(2):236–43. Epub 2013/02/09. doi: 10.1016/j.atherosclerosis.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 65.Seneviratne A, Hulsmans M, Holvoet P, Monaco C. Biomechanical factors and macrophages in plaque stability. Cardiovasc Res. 2013;99(2):284–93. Epub 2013/05/21. doi: 10.1093/cvr/cvt097. [DOI] [PubMed] [Google Scholar]
- 66.Seifert R, Kuhlmann MT, Eligehausen S, Kiefer F, Hermann S, Schafers M. Molecular imaging of MMP activity discriminates unstable from stable plaque phenotypes in shear-stress induced murine atherosclerosis. PLoS One. 2018;13(10):e0204305 Epub 2018/10/12. doi: 10.1371/journal.pone.0204305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. Journal of immunology (Baltimore, Md : 1950). 2004;172(5):2731–8. Epub 2004/02/24. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 68.Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F, Habenicht AJ, Ziegler-Heitbrock L, Randolph GJ. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115(3):e10–9. Epub 2009/12/08. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schneemann M, Schoedon G. Species differences in macrophage NO production are important. Nat Immunol. 2002;3(2):102 Epub 2002/01/29. doi: 10.1038/ni0202-102a. [DOI] [PubMed] [Google Scholar]
- 70.Schneemann M, Schoeden G. Macrophage biology and immunology: man is not a mouse. J Leukoc Biol. 2007;81(3):579 Epub 2007/03/01. doi: 10.1189/jlb.1106702. [DOI] [PubMed] [Google Scholar]
- 71.Mattana J, Sankaran RT, Singhal PC. Increased applied pressure enhances the uptake of IgG complexes by macrophages. Pathobiology. 1996;64(1):40–5. doi: 10.1159/000164004. [DOI] [PubMed] [Google Scholar]
- 72.Singhal PC, Sagar P, Gupta S, Arya M, Gupta M, Prasad A, Loona R, Sharma P, Mattana J. Pressure modulates monocyte migration. Am J Hypertens. 1997;10(11):1297–301. [DOI] [PubMed] [Google Scholar]
- 73.Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113(23):2744–53. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 74.Rosenson-Schloss RS, Vitolo JL, Moghe PV. Flow-mediated cell stress induction in adherent leukocytes is accompanied by modulation of morphology and phagocytic function. Med Biol Eng Comput. 1999;37(2):257–63. [DOI] [PubMed] [Google Scholar]
- 75.van der Vis H, Aspenberg P, de Kleine R, Tigchelaar W, van Noorden CJ. Short periods of oscillating fluid pressure directed at a titanium-bone interface in rabbits lead to bone lysis. Acta orthopaedica Scandinavica. 1998;69(1):5–10. [DOI] [PubMed] [Google Scholar]
- 76.Ferrier GM, McEvoy A, Evans CE, Andrew JG. The effect of cyclic pressure on human monocyte-derived macrophages in vitro. The Journal of bone and joint surgery British volume. 2000;82(5):755–9. [DOI] [PubMed] [Google Scholar]
- 77.Rojko JL, Evans MG, Price SA, Han B, Waine G, DeWitte M, Haynes J, Freimark B, Martin P, Raymond JT, Evering W, Rebelatto MC, Schenck E, Horvath C. Formation, clearance, deposition, pathogenicity, and identification of biopharmaceutical-related immune complexes: review and case studies. Toxicol Pathol. 2014;42(4):725–64. Epub 2014/04/08. doi: 10.1177/0192623314526475. [DOI] [PubMed] [Google Scholar]
- 78.Barcelli U, Rademacher R, Ooi YM, Ooi BS. Modification of glomerular immune complex deposition in mice by activation of the reticuloendothelial system. The Journal of clinical investigation. 1981;67(1):20–7. Epub 1981/01/01. doi: 10.1172/JCI110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arazi A, Neumann AU. Modeling immune complex-mediated autoimmune inflammation. J Theor Biol. 2010;267(3):426–36. Epub 2010/09/14. doi: 10.1016/j.jtbi.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 80.Alpers CE, Hudkins KL, Pritzl P, Johnson RJ. Mechanisms of clearance of immune complexes from peritubular capillaries in the rat. Am J Pathol. 1991;139(4):855–67. Epub 1991/10/01. [PMC free article] [PubMed] [Google Scholar]
- 81.Gonzalez ML, Waxman FJ. Relationship between complement activation and renal deposition of immune complexes made with IgG2a monoclonal antibodies. Clin Immunol. 2001;100(3):362–71. Epub 2001/08/22. doi: 10.1006/clim.2001.5077. [DOI] [PubMed] [Google Scholar]
- 82.Miles SA, Conrad SM, Alves RG, Jeronimo SM, Mosser DM. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J Exp Med. 2005;201(5):747–54. Epub 2005/03/09. doi: 10.1084/jem.20041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Trumbull A, Subramanian G, Yildirim-Ayan E. Mechanoresponsive musculoskeletal tissue differentiation of adipose-derived stem cells. Biomed Eng Online. 2016;15:43 Epub 2016/04/23. doi: 10.1186/s12938-016-0150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fukunaga T, Kubo K, Kawakami Y, Fukashiro S, Kanehisa H, Maganaris CN. In vivo behaviour of human muscle tendon during walking. Proc Biol Sci. 2001;268(1464):229–33. doi: 10.1098/rspb.2000.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schache AG, Dorn TW, Blanch PD, Brown NA, Pandy MG. Mechanics of the human hamstring muscles during sprinting. Med Sci Sports Exerc. 2012;44(4):647–58. doi: 10.1249/MSS.0b013e318236a3d2. [DOI] [PubMed] [Google Scholar]
- 86.Hansen P, Bojsen-Moller J, Aagaard P, Kjaer M, Magnusson SP. Mechanical properties of the human patellar tendon, in vivo. Clin Biomech (Bristol, Avon). 2006;21(1):54–8. doi: 10.1016/j.clinbiomech.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 87.Johnson GA, Tramaglini DM, Levine RE, Ohno K, Choi NY, Woo SL. Tensile and viscoelastic properties of human patellar tendon. J Orthop Res. 1994;12(6):796–803. doi: 10.1002/jor.1100120607. [DOI] [PubMed] [Google Scholar]
- 88.Kongsgaard M, Nielsen CH, Hegnsvad S, Aagaard P, Magnusson SP. Mechanical properties of the human Achilles tendon, in vivo. Clin Biomech (Bristol, Avon). 2011;26(7):772–7. doi: 10.1016/j.clinbiomech.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 89.Maganaris CN, Paul JP. In vivo human tendon mechanical properties. J Physiol. 1999;521 Pt 1:307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Svensson RB, Hansen P, Hassenkam T, Haraldsson BT, Aagaard P, Kovanen V, Krogsgaard M, Kjaer M, Magnusson SP. Mechanical properties of human patellar tendon at the hierarchical levels of tendon and fibril. J Appl Physiol (1985). 2012;112(3):419–26. doi: 10.1152/japplphysiol.01172.2011. [DOI] [PubMed] [Google Scholar]
- 91.Wren TA, Yerby SA, Beaupre GS, Carter DR. Mechanical properties of the human achilles tendon. Clin Biomech (Bristol, Avon). 2001;16(3):245–51. Epub 2001/03/10. [DOI] [PubMed] [Google Scholar]
- 92.Cikes M, Sutherland GR, Anderson LJ, Bijnens BH. The role of echocardiographic deformation imaging in hypertrophic myopathies. Nature Reviews Cardiology. 2010;7(7):384–96. [DOI] [PubMed] [Google Scholar]
- 93.Genet M, Lee LC, Nguyen R, Haraldsson H, Acevedo-Bolton G, Zhang ZH, Ge L, Ordovas K, Kozerke S, Guccione JM. Distribution of normal human left ventricular myofiber stress at end diastole and end systole: a target for in silico design of heart failure treatments. J Appl Physiol. 2014;117(2):142–52. doi: 10.1152/japplphysiol.00255.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mattana J, Sankaran RT, Singhal PC. Repetitive mechanical strain suppresses macrophage uptake of immunoglobulin G complexes and enhances cyclic adenosine monophosphate synthesis. Am J Pathol. 1995;147(2):529–40. [PMC free article] [PubMed] [Google Scholar]
- 95.Matsumoto T, Delafontaine P, Schnetzer KJ, Tong BC, Nerem RM. Effect of uniaxial, cyclic stretch on the morphology of monocytes/macrophages in culture. J Biomech Eng. 1996;118(3):420–2. Epub 1996/08/01. [DOI] [PubMed] [Google Scholar]
- 96.Miyazaki H, Hayashi K. Effects of cyclic strain on the morphology and phagocytosis of macrophages. Biomed Mater Eng. 2001;11(4):301–9. [PubMed] [Google Scholar]
- 97.Ohki R, Yamamoto K, Mano H, Lee RT, Ikeda U, Shimada K. Identification of mechanically induced genes in human monocytic cells by DNA microarrays. J Hypertens. 2002;20(4):685–91. [DOI] [PubMed] [Google Scholar]
- 98.Matheson LA, Maksym GN, Santerre JP, Labow RS. Cyclic biaxial strain affects U937 macrophage-like morphology and enzymatic activities. Journal of biomedical materials research Part A. 2006;76(1):52–62. Epub 2005/10/15. doi: 10.1002/jbm.a.30448. [DOI] [PubMed] [Google Scholar]
- 99.Matheson LA, Maksym GN, Santerre JP, Labow RS. The functional response of U937 macrophage-like cells is modulated by extracellular matrix proteins and mechanical strain. Biochem Cell Biol. 2006;84(5):763–73. doi: 10.1139/o06-093. [DOI] [PubMed] [Google Scholar]
- 100.Matheson LA, Fairbank NJ, Maksym GN, Paul Santerre J, Labow RS. Characterization of the Flexcell Uniflex cyclic strain culture system with U937 macrophage-like cells. Biomaterials. 2006;27(2):226–33. doi: 10.1016/j.biomaterials.2005.05.070. [DOI] [PubMed] [Google Scholar]
- 101.Ballotta V, Driessen-Mol A, Bouten CV, Baaijens FP. Strain-dependent modulation of macrophage polarization within scaffolds. Biomaterials. 2014;35(18):4919–28. doi: 10.1016/j.biomaterials.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 102.Yang JH, Sakamoto H, Xu EC, Lee RT. Biomechanical regulation of human monocyte/macrophage molecular function. Am J Pathol. 2000;156(5):1797–804. doi: 10.1016/S0002-9440(10)65051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pugin J, Dunn I, Jolliet P, Tassaux D, Magnenat JL, Nicod LP, Chevrolet JC. Activation of human macrophages by mechanical ventilation in vitro. Am J Physiol. 1998;275(6 Pt 1):L1040–50. [DOI] [PubMed] [Google Scholar]
- 104.Mourgeon E, Isowa N, Keshavjee S, Zhang X, Slutsky AS, Liu M. Mechanical stretch stimulates macrophage inflammatory protein-2 secretion from fetal rat lung cells. Am J Physiol Lung Cell Mol Physiol. 2000;279(4):L699–706. Epub 2000/09/23. doi: 10.1152/ajplung.2000.279.4.L699. [DOI] [PubMed] [Google Scholar]
- 105.Xu XY, Guo C, Yan YX, Guo Y, Li RX, Song M, Zhang XZ. Differential effects of mechanical strain on osteoclastogenesis and osteoclast-related gene expression in RAW264.7 cells. Mol Med Rep. 2012;6(2):409–15. doi: 10.3892/mmr.2012.908. [DOI] [PubMed] [Google Scholar]
- 106.Yanagida H, Yanase H, Sanders KM, Ward SM. Intestinal surgical resection disrupts electrical rhythmicity, neural responses, and interstitial cell networks. Gastroenterology. 2004;127(6):1748–59. Epub 2004/12/04. [DOI] [PubMed] [Google Scholar]
- 107.Schaffer JL, Rizen M, L’Italien GJ, Benbrahim A, Megerman J, Gerstenfeld LC, Gray ML. Device for the application of a dynamic biaxially uniform and isotropic strain to a flexible cell culture membrane. J Orthop Res. 1994;12(5):709–19. doi: 10.1002/jor.1100120514. [DOI] [PubMed] [Google Scholar]
- 108.Dziki JL, Giglio RM, Sicari BM, Wang DS, Gandhi RM, Londono R, Dearth CL, Badylak SF. The Effect of Mechanical Loading Upon Extracellular Matrix Bioscaffold-Mediated Skeletal Muscle Remodeling. Tissue Eng Part A. 2018;24(1–2):34–46. doi: 10.1089/ten.TEA.2017.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Subramanian G, Elsaadany M, Bialorucki C, Yildirim-Ayan E. Creating homogenous strain distribution within 3D cell-encapsulated constructs using a simple and cost-effective uniaxial tensile bioreactor: Design and validation study. Biotechnol Bioeng. 2017;114(8):1878–87. Epub 2017/04/21. doi: 10.1002/bit.26304. [DOI] [PubMed] [Google Scholar]
- 110.Seo J, Shin JY, Leijten J, Jeon O, Ozturk AB, Rouwkema J, Li YC, Shin SR, Hajiali H, Alsberg E, Khademhosseini A. Interconnectable Dynamic Compression Bioreactors for Combinatorial Screening of Cell Mechanobiology in Three Dimensions. Acs Appl Mater Inter. 2018;10(16):13293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Elsaadany M, Harris M, Yildirim-Ayan E. Design and Validation of Equiaxial Mechanical Strain Platform, EQUicycler, for 3D Tissue Engineered Constructs. Biomed Res Int. 2017;2017:3609703 Epub 2017/02/09. doi: 10.1155/2017/3609703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jung SH, Saxena A, Kaur K, Fletcher E, Ponemone V, Nottingham JM, Sheppe JA, Petroni M, Greene J, Graves K, Baliga MS, Fayad R. The role of adipose tissue-associated macrophages and T lymphocytes in the pathogenesis of inflammatory bowel disease. Cytokine. 2013;61(2):459–68. Epub 2012/12/19. doi: 10.1016/j.cyto.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 113.Hong JE, Kye YC, Park SM, Cheon IS, Chu H, Park BC, Park YM, Chang J, Cho JH, Song MK, Han SH, Yun CH. Alveolar Macrophages Treated With Bacillus subtilis Spore Protect Mice Infected With Respiratory Syncytial Virus A2. Front Microbiol. 2019;10:447 Epub 2019/04/02. doi: 10.3389/fmicb.2019.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–25. Epub 2011/05/20. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 115.Raes G, Van den Bergh R, De Baetselier P, Ghassabeh GH, Scotton C, Locati M, Mantovani A, Sozzani S. Arginase-1 and Ym1 are markers for murine, but not human, alternatively activated myeloid cells. Journal of immunology (Baltimore, Md : 1950). 2005;174(11):6561; author reply −2. Epub 2005/05/21. doi: 10.4049/jimmunol.174.11.6561. [DOI] [PubMed] [Google Scholar]
- 116.Bentzon JF, Falk E. Atherosclerotic lesions in mouse and man: is it the same disease? Curr Opin Lipidol. 2010;21(5):434–40. Epub 2010/08/05. doi: 10.1097/MOL.0b013e32833ded6a. [DOI] [PubMed] [Google Scholar]