Abstract
Objective:
The underlying mechanism of interstitial cystitis/bladder pain syndrome (IC/BPS) is not well understood and evaluation of current therapeutic interventions have not identified any generally effective treatments. Physical activity has shown beneficial effects on individuals suffering from chronic pain. Anxiety-prone rats exposed to water avoidance stress (WAS) develop urinary frequency and lower bladder sensory thresholds with high face and construct validity for the study of IC/BPS. The aim of this study was to evaluate the role of chronic voluntary exercise on urinary frequency, voiding function, and hyperalgesia in animals exposed to WAS.
Materials and Methods:
Twenty-six female Wistar-Kyoto rats were exposed to WAS and thereafter randomized to either voluntary exercise for 3 weeks or sedentary groups. Voiding parameters were assessed at baseline, post-WAS, and weekly for 3 weeks. Prior to euthanasia, the animals underwent cystometrogram (CMG), external urinary sphincter electromyography (EUS EMG), and assessment of visceromotor response (VMR) to isotonic bladder distension (IBD).
Results:
WAS exposure resulted in adverse changes in voiding parameters. Compared to sedentary animals, animals in the voluntary exercise group had improved voiding parameters during metabolic cage and CMG testing, as well as improved bladder sensory thresholds as determined by VMR during IBD.
Conclusion:
Voluntary exercise in an animal model of chronic stress leads to improvement in voiding function and visceral bladder hyperalgesia.
Keywords: animal model, psychological stress, exercise, interstitial cystitis/bladder pain syndrome
Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic bladder syndrome characterized by bladder pain and increased frequency of urination. It is prevalent, affecting 3.3–7.9 million adult American females1 and costly, with expenditures of $34–750 million annually2. It has proven to be difficult to define, diagnosis, and treat, with no known etiology or standard treatment regimen3.
Numerous studies have documented a complex interplay between IC/BPS and stress. IC/BPS patients have increased incidences of childhood trauma and current life stress compared to healthy controls, both of which correlate with worse urinary symptoms, increased pain, and worse quality of life4, 5, 6. Patients additionally report that acute stress exacerbates their symptoms in a dose-dependent fashion7, 8. IC/BPS patients also have an increased incidence of post-traumatic stress disorder compared to chronic pain controls, which similarly correlates with increased pain and worse quality of life9. Based on these correlations, the American Urologic Association (AUA) recommends that stress management be considered as first-line therapy for all patients10.
Physical exercise has been shown to have numerous health benefits, including reduction of depression and anxiety and promoting resilience to stress11. In addition, exercise is currently recommended by the Center for Disease Control as one of the initial non-pharmacologic steps in management of chronic pain disorders, such as IC/BPS12. While there is growing evidence to support the use of exercise as first-line non-pharmacologic management of chronic pain disorders13 and patients with IC/BPS report beneficial effects of exercise6, 14, there is still limited evidence regarding the effectiveness of exercise as a therapeutic intervention specifically for IC/BPS patients.
While human literature is scant, numerous studies have demonstrated that exercise can either prevent or improve pain and stress metrics, such as autonomic dysfunction and corticosterone levels, in a variety of animal models of chronic pain15, 16, 17. Exercise was additionally able to improve bladder function and bladder hypersensitivity in an animal model of chronic stress, however this study did not evaluate the relationship between the amount of exercise and degree of symptom improvement18.
In the current study we propose to evaluate the effects of daily voluntary exercise on an animal model of stress induced bladder hyperalgesia and urinary frequency. WAS utilizing anxiety-prone rats (Wistar-Kyoto strain) is a model of chronic stress that replicates IC/BPS with face and construct validity and results in demonstration of anxiety-like behaviors19, increased urinary frequency19, increased visceral hypersensitivity20 and histologic alterations similar to the disease state21.
Materials and Methods
Animals
Twenty-six adult female Wistar-Kyoto rats aged 11–12 weeks (200–300g) were purchased from a commercial vendor (Charles River, Wilmington, MA, USA). This particular strain was utilized due to their genetic predisposition for anxiety22. We only studied female rats as IC/BPS occurs with greater frequency in women. We did not control for estrous cycle during these experiments.
Rats were housed under standard vivaria conditions (lights off from 6 pm to 6 am) with dry bedding and ad libitum access to standard lab chow (Laboratory Rodent Diet 5001, Constant Nutrition) and water (internal reverse osmosis system). We allowed an adjustment period of one to two weeks between the animals’ arrival and the start of experimental procedures.
Experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) (protocol #20651) and were conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals23.
Chronic WAS protocol
After collection of baseline voiding parameters, all of the animals were exposed to chronic WAS as previously described19, 20, 24. Briefly, the rat was placed on a standing cube (5.75 × 5.75 × 10.75cm3) in the middle of a Plexiglas cage (25×50×25cm3). The container was filled with room temperature water, 1cm below the top of the cube. The rat was placed on top of the cube for 1 hour every day for 10 consecutive days. The protocol was performed between the hours of 8:00am and 12:00pm to minimize circadian effects.
Exercise protocol
Voiding parameters were assessed after completion of the stress protocol. Animals were then randomized into either exercise (n=12) or sedentary groups (n=14). Sedentary animals were housed in standard cages. Exercise animals were housed in cages equipped with a single voluntary running wheel connected to a sensor to count revolutions (Scurry Activity Wheel, 35.6cm diameter, Lafayette Instrument, Lafayette, IN, USA). Given that preliminary studies demonstrated mostly nocturnal running, animals were individually housed at night in order to quantify the amount of exercise performed per animal. In order to control for the possible effects of single housing at night, sedentary animals were also individually housed at night in standard cages. During the daytime, animals in the exercise and sedentary arms were separately housed in assigned groups of two to reduce the stress of social isolation during the nighttime25. Exercise occurred Monday through Saturday with a day of rest and dual housing for all animals on Sunday.
Assessment of voiding parameters
All animals underwent metabolic cage assessment at baseline (pre-WAS), post-WAS, and at weeks 1, 2, and 3 thereafter, prior to termination. Rats were placed individually in metabolic cages (Model 3600M024, Techniplast West Chester, PA, USA) with ad libitum access to water. After 30 minutes of behavioral accommodation, urine output was quantified in real time over a 12-hour period (6pm to 6am) using a data acquisition system (MP150; Biopac Systems Inc. Goleta, CA, USA). Total volume of urine, number of urinations, water consumption, and fecal pellets were measured. Volume per void was calculated.
Assessment of bladder function and hyperalgesia during bladder filling and distension
After 3 weeks of either the exercise or sedentary protocol, the animals underwent sedated cystometrogram (CMG), external urinary sphincter electromyography (EUS EMG), and visceromotor response (VMR) to isotonic bladder distension (IBD) prior to euthanasia.
Animals were anesthetized with isoflurane (1–2% in the gas mixture of 30% oxygen and 70% nitrogen) and a percutaneous venous catheter was placed in the right external jugular vein. Forty mg/mL of α-chloralose (Sigma Aldrich, St. Louis, MO, USA) was dissolved in 20% β-cyclodextrin (Sigma Aldrich, St. Louis, MO, USA) and administered intravascularly as a bolus (40 mg/kg). Isoflurane anesthesia was maintained at 1% for 30 minutes to allow the α-chloralose to take effect. A PE-50 catheter was inserted into the bladder through the urethra. The bladder catheter was connected to an infusion pump (KD Scientific. Holliston, MA, USA) and a pressure sensor (MP150, Biopac Systems Inc., Goleta, CA, USA) via a 3-way connector. The EUS EMG was recorded by placing two PFA-insulated platinum-iridium wire electrodes (50 μm in diameter, A-M systems, Everett, WA, USA) with exposed tips into the EUS. The VMR was recorded by embedding 2 additional insulated wire electrodes with exposed tips into the left abdominal external oblique muscle (EOM). The wire electrodes were connected to an amplifier (MP150, Biopac Systems Inc., Goleta, CA, USA). The sampling rate was set to 1kHz for both EMG channels. Measurements from the urethral catheter and electrodes were recorded and analyzed by a data acquisition system (MP150, Biopac Systems Inc., Goleta, CA, USA).
After all of the surgical procedures were completed the animals were maintained under conscious sedation with α-chloralose infusion (15mg/kg/hr, IV). The CMG, IBD, EUS EMG, and VMR procedures were begun 30 minutes after discontinuation of isoflurane anesthesia. The bladder was drained via the urethral catheter prior to beginning the CMG. To obtain the CMG, the bladder was continuously infused with room temperature normal saline (0.9%, 0.1 mL/min) via the urethral catheter and the animal was allowed to void spontaneously per urethra around the catheter. Three consecutive voiding cycles were recorded, and the following parameters were obtained: latency to void or leak (LV), maximum intravesical pressure (IVPmax), and pressure threshold to void or leak (PT). Bladder capacity was calculated from the first void (as the bladder had been previously empty) as LV × infusion rate (0.1mL/min). The EUS EMG activity during voiding was also analyzed and the following parameters were obtained: maximum amplitude, duration of activity, and area under the curve (AUC).
After completion of the CMG, the IBD was performed. The urethra was occluded with a 5–0 silk suture and the animal was allowed to accommodate for 30 minutes. Using a saline-filled reservoir, the bladder was distended through the urethral catheter to a pressure of 10cmH20 for 2 minutes. During this time the VMR latency, VMR area under the curve (AUC) and maximum amplitude (AMPmax) were recorded. After 2 minutes the bladder was drained, and the animal was allowed to rest. This procedure was repeated for pressures of 20, 30, and 40cm H20. Animals were euthanized after completion of the IBD and VMR recording.
Statistical analysis
Means and standard error of the mean (SEM) were calculated to describe the distribution of metabolic cage variables, exercise variables, urodynamic data, and IBD data. Animals were classified into four groups during the analyses (sedentary (14 rats), low exercise (4 rats), medium exercise (4 rats), and high exercise (4 rats)) based on the tertile cut points of total distance run across three weeks. Baseline and post-WAS metabolic cage variables compared all animals prior to randomization. Kruskal-Wallis test was used to test differences in non-normally distributed continuous variables between/among groups and subgroups in the setting of univariate and stratified analyses. Multivariable mixed modeling was performed to identify the effects/influences of main interested features (e.g. amount of exercise, time points) on outcomes (e.g. urinary frequency), while controlling for water consumption. Tukey-Kramer method was used to adjust for the multiple/pairwise comparisons based on least square means. Multivariable linear regression using least square means while controlling for water consumption was used to determine the dose effect of exercise on urinary frequency at various time points. Pearson’s correlation coefficient was calculated to assess correlation between total distance run and exercise-induced changes in urinary frequency (Week 3 – Post-WAS). The coefficient was transformed into Z-value using Fisher’s transformation for statistical analysis. All p-values were 2-sided and p<0.05 was considered statistically significant. Statistical software package SAS, Version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for analyses of these variables and Prism 6 (GraphPad Software, San Diego, CA, USA) was used for creation of graphs.
Results
Voluntary exercise data
Animals ran an average daily distance across the three-week exercise period of 9,127 ± 364.2 m/24hr. The average daily distance during Week 1 was 4,337 ± 301 m/24hr and during Week 3 it was 13,211 ± 771 m/24hr. The low exercise group (n=4) ran 110,320 ± 4956m. The medium exercise group (n=4) ran 152,991 ± 2641m. The high exercise group (n=4) ran 174,645 ± 4090m. These groups were statistically different on univariate analysis (p=0.007).
Metabolic cage data
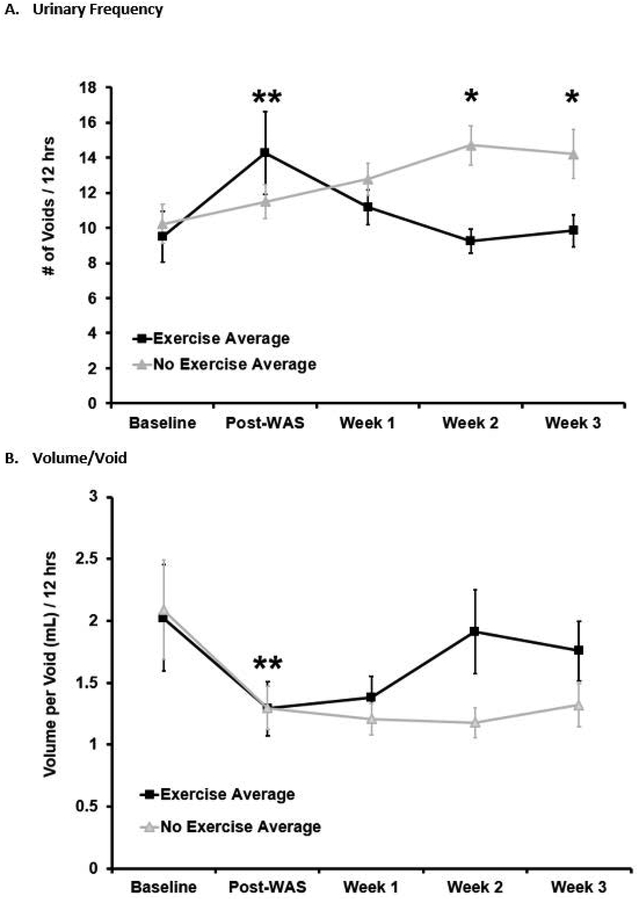
Prior to undergoing WAS, the sedentary group had 10.2 ± 1.1 voids/12hr and the exercise group had 9.5 ± 1.4 voids/12hr, which was not significantly different between groups on univariate analysis (p=0.48). After undergoing WAS, but prior to randomization, both groups had an increase in voiding frequency from baseline (Sedentary group 11.5 ± 0.9 voids/12hr, Exercise group 14.3 ± 2.4 voids/12hr) that was significant in the exercise group on univariate analysis (p=0.03) and significant in both groups on multivariable mixed modeling (p=0.009), but was not significantly different between groups on univariate analysis (p=0.6). After initiation of the voluntary exercise protocol, the exercise group had a progressive decline in voiding frequency back to the baseline that was statistically significant compared to the sedentary group (Week 1: Sedentary 12.8 ± 0.9 voids/12hr vs. Exercise 11.2 ± 0.9 voids/12hr, p=0.2; Week 2: Sedentary 14.7 ± 1.1 voids/12hr vs. Exercise 9.3 ±1.2 voids/12hr, p=0.02; Week 3: Sedentary 14.2 ± 1.4 vs. Exercise 9.8 ± 0.9, p=0.02). This finding was confirmed on multivariable mixed modeling as well (p=0.02). Figure 1A.
Figure 1:
Urinary frequency and volume per void by group over time (average ± standard error). Water avoidance stress (WAS) increased urinary frequency and decreased volume per void (average ± standard error, Kruskal-Wallis test performed between baseline and each time point within the group and across groups). Significant changes from baseline (** = p <0.05) were noted for both groups in the frequency variable, and for the sedentary group in the volume/void variable, with no significant group difference. With voluntary exercise, the exercise group (WAS/EX, n=12) showed a decline in urinary frequency and increase in volume per void back to baseline compared to the no-exercise group (WAS/no-EX, n=14). Significant group differences for the frequency variable were noted at weeks 2 and 3 (* = p <0.02 for both weeks), which was confirmed with multivariable mixed modeling (p=0.02). For the volume/void variable, there was a trend towards significance (p =0.08) at week 3, with significant group differences demonstrated on multivariable mixed modeling (p=0.02).
Prior to undergoing WAS, both groups had a volume per void of 2.1 ± 0.4 mL/void (p=1). After undergoing WAS, both groups had a decrease in the volume per void (Sedentary 1.3 ± 0.2 mL/void, Exercise 1.3 ± 0.2 mL/void), which significant in the sedentary group on univariate analysis (p=0.02) and significant in both groups on multivariable mixed modeling (p=0.02) but was not significantly different between groups on univariate analysis (p=0.9). After initiation of the voluntary exercise protocol, the exercise group had a progressive increase in the volume per void compared to the sedentary group with a trend towards significance on univariate analysis (Week 2 p=0.4, Week 3 p=0.08). Exercise accounted for an increase of 0.4 ± 0.2mL/void in the volume per void independent of time and water consumption on multivariable mixed modeling (p=0.02). Figure 1B.
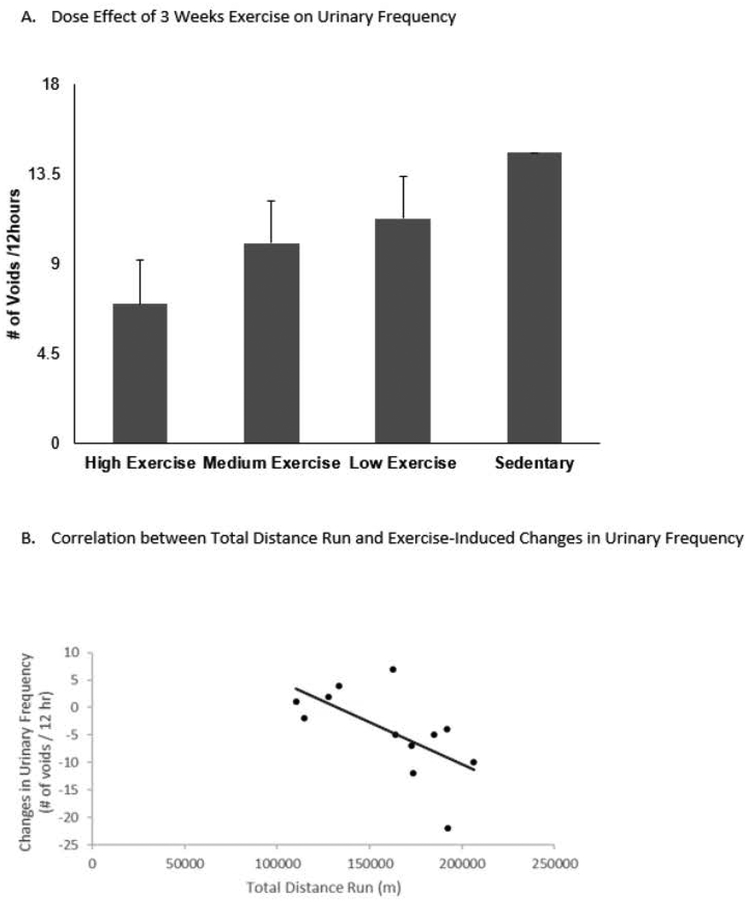
On univariate analysis stratifying for exercise, there was a trend over time for increasing amounts of exercise to be associated with lower frequency and large volume per void (Baseline: frequency p=0.6, volume/void p=0.8, Post-WAS: frequency p=1.0, volume/void p=0.6, Week 1: frequency p=0.6, volume/void p=0.3, Week 2: frequency p=0.1, volume/void p=0.3, Week 3: frequency p=0.06, volume/void p=0.08). On multivariable mixed modeling stratifying for exercise, increasing amounts of exercise were found to be related to decreasing urinary frequency independent of time. Animals that performed minimal amounts of exercise had 1.5 ± 1.5 fewer voids per 12 hours than sedentary animals (p=0.3). Animals that performed moderate amounts of exercise had 2.2 ± 1.5 fewer voids per 12 hours than sedentary animals (p=0.1). Animals that performed the most amount of exercise had 4.2 ± 1.5 fewer voids per 12 hours than sedentary animals (p=0.01). Multivariable linear regression comparing urinary frequency and exercise at different time points found a significant dose response with exercise in the medium exercise (p=0.04) and high exercise (p=0.0025) groups at Week 3. Exercised-induced changes in urinary frequency showed statistically significant, negative correlation with total distance run (Pearson’s r=− 0.62, p=0.03), suggesting that the greater distance run, the greater reversal of WAS-induced increase in urinary frequency. Figure 2.
Figure 2:
A. Dose effect of 3 weeks exercise on urinary frequency by comparing to sedentary group. After 3 weeks exercise, both the medium exercise and high exercise groups had significant decreases in urinary frequency related to the amount of exercise they performed in a multivariable linear regression model controlling for water consumption (p=0.003 high exercise, p=0.05 medium exercise). B. Correlation between total distance run and exercise-induced changes in urinary frequency. Exercise-induced changes in urinary frequency showed statistically significant, negative correlation with total distance run over 3 weeks (Pearson’s r=−0.62, p=0.03).
There was no difference between groups in water consumption, urinary volume, or fecal pellets at any time point.
Urodynamic data
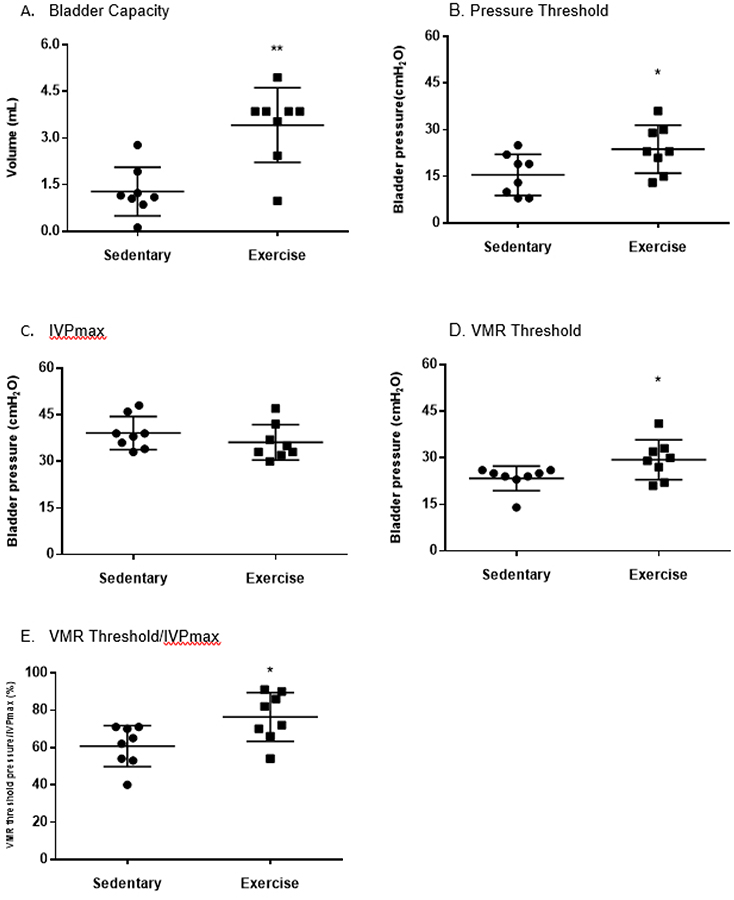
During the CMG, the exercise group had a statistically larger bladder capacity (Sedentary 1.3 ± 0.8 mL vs. Exercise 3.4 ± 1.2 p=0.01) and increased pressure threshold to initiate voiding (Sedentary 16 ± 7 cmH20 vs. Exercise 24 ± 8 cmH20, p=0.04). There was no difference in maximum IVP between groups. The VMR evoked by bladder distension appeared at a higher IVP in the exercise group compared to the sedentary group (Sedentary 23 ± 4 cmH20 vs. Exercise 29 ± 6 cmH20, p=0.04). The exercise group had increased latency to void based on the increased percentage of VMR threshold pressure/IVPmax compared to the sedentary group (Sedentary 61 ± 11% vs. Exercise 76 ± 13%, p=0.02). Figure 3.
Figure 3:
During the CMG, the exercise group had a statistically larger bladder capacity (Sedentary 1.3 ± 0.8 mL vs. Exercise 3.4 ± 1.2 p=0.01) (Figure 3A) and increased pressure threshold to initiate voiding (Sedentary 16 ± 7 cmH20 vs. Exercise 24 ± 8 cmH20, p=0.04) (Figure 3B). There was no difference in maximum IVP between groups (Figure 3C). The VMR evoked by bladder distension appeared at a higher IVP in the exercise group compared to the sedentary group (Sedentary 23 ± 4 cmH20 vs. Exercise 29 ± 6 cmH20, p=0.04) (Figure 3D). The exercise group had increased latency to void based on the increased percentage of VMR threshold pressure/IVPmax compared to the sedentary group (Sedentary 61 ± 11% vs. Exercise 76 ± 13%, p=0.02) (Figure 3E). * = p < 0.05
IBD data
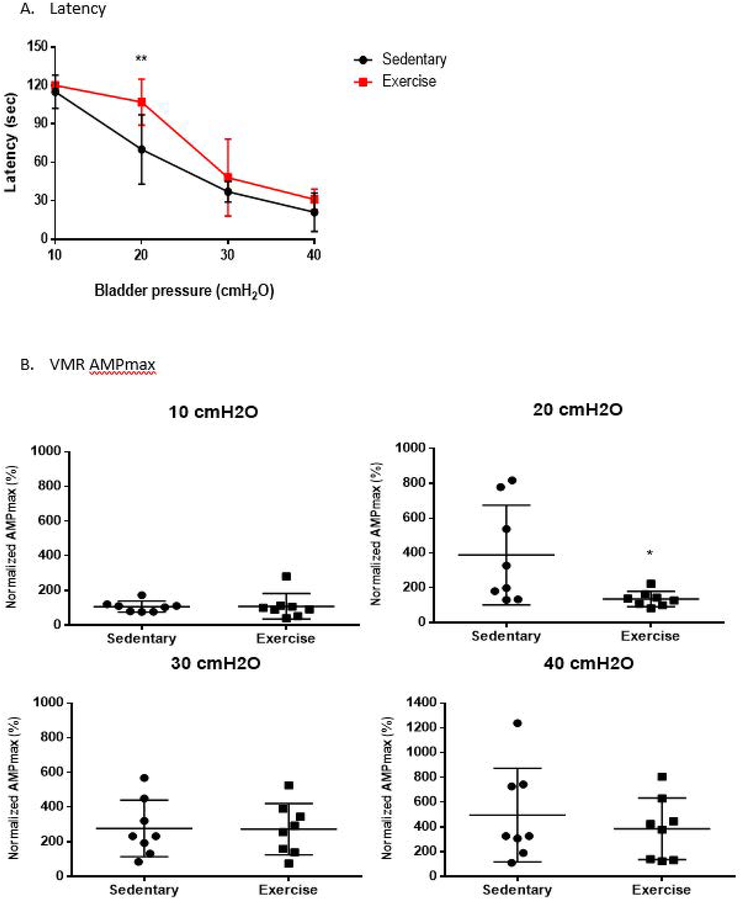
Bladder hyperalgesia was assessed by VMR during IBD. At 20 cmH2O, the exercise group had a significantly delayed VMR appearance compared to the sedentary group (Sedentary 70 ± 27 sec. vs. Exercise 107 ± 18 sec., p=0.007) with significantly decreased VMR AMPmax (Sedentary 389 ± 286% vs. Exercise 136 ± 43%, p=0.04) and VMR AUC (Sedentary 148 ± 57% vs. Exercise 101 ± 11%, p=0.04). This effect disappeared at bladder distensions greater than 30cmH20. Figure 4.
Figure 4:
Statistical analysis of visceromotor reflex during isotonic bladder distension (IBD). The exercise group has significantly delayed latency in VMR appearance at 20 cmH2O (Figure 4A). During IBD, VMR maximum amplitude (AMPmax) in the exercise group is significantly lower when compared to the sedentary group at 20 cmH2O (Figure 4B). During IBD, VMR AUC in the exercise group is also significantly decreased at 20 cmH2O (Figure 4C). * = p < 0.05
Discussion
Exercise has been shown to have numerous health benefits and therapeutic effects on stress, anxiety, and depression11. It may therefore be therapeutic in stress-sensitive conditions such as IC/BPS. A recent Cochrane systematic review evaluating 264 studies involving 19,642 participants with chronic pain conditions similar to IC/BPS (i.e. fibromyalgia, dysmenorrhea, low back pain, etc.) found that compared to no intervention, regular exercise had small to modest improvements in physical function, with generally favorable but inconsistent reductions in pain severity, and variable results in terms of psychological function and quality of life13. Although the quality of evidence included in the analysis was low due to small sample sizes (less than 50 participants per study) and short follow-up (less than 1 year), exercise is recommended as an intervention to pursue prior to more invasive therapies due to its minimal adverse event profile.
While there is growing evidence to support the use of exercise as first-line non-pharmacologic management of chronic pain disorders, there is still limited evidence regarding the effectiveness of exercise as a therapeutic intervention specifically for IC/BPS patients. To date, the only published study of the effect of exercise on IC/BPS in humans is an internet survey of 1,982 IC/BPS patients which found that exercise improved symptoms in 65% of patients14. There are a number of limitations with this study, including lack of physician confirmation of diagnosis, no description on which symptoms are improved, and no standardization of the amount or method of exercise. Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is similar to IC/BPS in terms of lifetime and current stress6. One randomized controlled trial of 85 men with CP/CPPS found that compared to stretching alone, walking for 40 minutes 3 times per week for 6 weeks resulted in improvements in pain, quality of life, anxiety and depression, but not urinary symptoms26. Exercise has additionally been shown to have therapeutic benefit in irritable bowel syndrome (IBS), which is a central sensitizing syndrome similar to IC/BPS or fibromyalgia27. After long-term follow-up with regular exercise, IBS patients reported improvements in IBS symptoms, anxiety, depression and quality of life28.
Given the strong relationship between stress and IC/BPS, we utilized a validated animal model of chronic stress that has been shows to demonstrate the sentinel symptoms of IC/BPS: urinary frequency and bladder hyperalgesia19, 20, 24, 29, 30. In the present study, animals exposed to WAS demonstrated increased urinary frequency, and decreased voided volumes.
After initiation of the voluntary exercise protocol, the exercise group had a progressive decline in voiding frequency back to the baseline that correlated with an increase in volume per void. Significant differences among groups were seen after 2 weeks of exercise, consistent with prior studies which demonstrated a delay in improved pain outcomes, as measured by tactile allodynia, after 5 days to 6 weeks of exercise15. While voiding frequency is clearly not the same as tactile allodynia, these findings support the idea that modulation of either pain or voiding dysfunction is not an immediate response and requires a period of time from the initiation of exercise possibly due to nerve modulation31. To our knowledge, there is only one publication examining the effect of exercise on a different stress induced model of IC/BPS. These authors examined the effect of 4 weeks of free access to a running wheel following neonatal maternal separation (NMS) in female mice. While there was a trend towards a decrease in number of voids in exercised stressed mice compared to sedentary stressed mice, the difference was not significant18. The differences between that study and our current one are many, including species, stress model, and methodology of exercise, and may account for some of the variation in outcomes. It is additionally possible that the study by Pierce et al. was not powered to detect a statistical difference (n=6) in spite of demonstrating trends of improvement in urinary frequency.
In the current study, we found that the amount of exercise performed correlated to improvement in urinary frequency, with those animals performing more exercise having less urinary frequency compared to animals that performed only low amounts or no amounts of exercise. While Pierce et al. did calculate the amount of exercise per animal pair, they did not calculate the amount of exercise per animal and thus was not able to report on the individual effect of the amount of exercise on their outcomes. Given these differences in findings and the wide range of distances run in our experiment, the results need to be substantiated in future work with more animals and longer time frames.
The normalization of voiding parameters seen in the exercised animals correlated with the urodynamic and IBD data. Exercised animals were able to hold large bladder volumes as demonstrated by their increased bladder capacity, wait longer to void as demonstrated by the increased percentage of VMR threshold pressure/IVP max, and had decreased sensory thresholds as demonstrated by VMR appearance at higher IVP and bladder volumes. Prior studies have used VMR responses to IBD to quantify bladder sensory thresholds as correlates of pain. Studies have demonstrated that IBD in control animals causes minimal VMR response at 10 cmH20, some VMR response at 20 cmH20 (which is the point of physiologic voiding in rodents), and a persistently elevated VMR response at 30 and 40 cmH20 (over-physiologic filling) that is attributed to ceiling effect. We have shown that animals exposed to WAS demonstrate decreased bladder sensory thresholds with increased VMR responses at 20 cmH20 compared to controls30. In the present study, we demonstrated that exercise in a stressed animal resulted a delayed VMR response at 20 cmH20 similar to an unstressed control. This implies that exercise increases bladder sensory thresholds back to the normal value and may result in reduced bladder hyperalgesia. Pierce et al. similarly found that exercised stressed animals had significantly less VMR response during urinary bladder distension than sedentary stressed animals that was not different from sedentary controls18.
There are limitations in the interpretation of this study. This study lacks a non-stressed exercise control group which may help determine how exercise alone impacts the measured variables. Although social isolation has been shown to cause stress in rats, we attempted to control for this with periods of social interaction which has been reported to ameliorate isolated-induced behavioral changes25. We focused on exercise as a short-term intervention. Other studies have found that maximal results do not occur until 6 weeks of exercise15. Future studies should focus on exercise as long-term intervention to determine if the effects seen are sustainable. We do not know whether the improvement in voiding parameters and bladder hypersensitivity was due to reduction of stress or direct pain modulation. Future work could incorporate physiologic markers (i.e. serum corticosterone levels) and behavioral studies to assess stress (i.e. light-dark box transition test). Finally, we do not know if the effects were peripherally or centrally mediated and this would need to be explored in future work.
Conclusion
Voluntary exercise can improve voiding parameters and bladder hypersensitivity in an animal model of stress induced urinary frequency and bladder hyperalgesia. Exercise may be a beneficial treatment option in conditions affected by stress such as IC/BPS.
Acknowledgements
We would like to thank Dr. Jie Cai for his help with the multivariate statistical analysis.
Footnotes
Ethical Approval: Experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) (protocol #20651) and were conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals23.
References
- 1.Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, Nyberg L, Clemens JQ. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011. August;186(2):540–4. doi: 10.1016/j.juro.2011.03.132. Epub 2011 Jun 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Payne CK, Joyce GF, Wise M, Clemens JQ; Urologic Diseases in America Project. Interstitial cystitis and painful bladder syndrome. J Urol. 2007. June;177(6):2042–9. [DOI] [PubMed] [Google Scholar]
- 3.Rovner E, Propert KJ, Brensinger C, Wein AJ, Foy M, Kirkemo A, Landis JR, Kusek JW, Nyberg LM. Treatments used in women with interstitial cystitis: the interstitial cystitis data base (ICDB) study experience. The Interstitial Cystitis Data Base Study Group. Urology. 2000. December 20;56(6):940–5. [DOI] [PubMed] [Google Scholar]
- 4.Nickel JC, Tripp DA, Pontari M, Moldwin R, Mayer R, Carr LK, Doggweiler R, Yang CC, Mishra N, Nordling J. Childhood sexual trauma in women with interstitial cystitis/bladder pain syndrome: a case control study. Can Urol Assoc J. 2011. December;5(6):410–5. doi: 10.5489/cuaj.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nickel JC, Tripp DA, Pontari M, Moldwin R, Mayer R, Carr LK, Doggweiler R, Yang CC, Mishra N, Nordling J. Psychosocial phenotyping in women with interstitial cystitis/painful bladder syndrome: a case control study. J Urol. 2010. January;183(1):167–72. doi: 10.1016/j.juro.2009.08.133. [DOI] [PubMed] [Google Scholar]
- 6.Naliboff BD, Stephens AJ, Afari N, Lai H, Krieger JN, Hong B, Lutgendorf S, Strachan E, Williams D; MAPP Research Network. Widespread Psychosocial Difficulties in Men and Women With Urologic Chronic Pelvic Pain Syndromes: Case-control Findings From the Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research Network. Urology. 2015. June;85(6):1319–27. doi: 10.1016/j.urology.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothrock NE, Lutgendorf SK, Kreder KJ, Ratliff T, Zimmerman B. Stress and symptoms in patients with interstitial cystitis: a life stress model. Urology. 2001. March;57(3):422–7. [DOI] [PubMed] [Google Scholar]
- 8.Koziol JA, Clark DC, Gittes RF, Tan EM. The natural history of interstitial cystitis: a survey of 374 patients. J Urol. 1993. March;149(3):465–9. [DOI] [PubMed] [Google Scholar]
- 9.McKernan LC, Johnson BN, Reynolds WS, Williams DA, Cheavens JS, Dmochowski RR, Crofford LJ. Posttraumatic stress disorder in interstitial cystitis/bladder pain syndrome: Relationship to patient phenotype and clinical practice implications. Neurourol Urodyn. 2018. October 23. doi: 10.1002/nau.23861. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanno PM, Burks DA, Clemens JQ, Dmochowski RR, Erickson D, Fitzgerald MP, Forrest JB, Gordon B, Gray M, Mayer RD, Newman D, Nyberg L Jr, Payne CK, Wesselmann U, Faraday MM; Interstitial Cystitis Guidelines Panel of the American Urological Association Education and Research, Inc. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol. 2011. June;185(6):2162–70. doi: 10.1016/j.juro.2011.03.064. Epub 2011 Apr 16. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clin Psychol Rev. 2001. February;21(1):33–61. Review. [DOI] [PubMed] [Google Scholar]
- 12.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. JAMA. 2016. April 19;315(15):1624–45. doi: 10.1001/jama.2016.1464. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017. April 24;4:CD011279. doi: 10.1002/14651858.CD011279.pub3. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Hare PG 3rd, Hoffmann AR, Allen P, Gordon B, Salin L, Whitmore K. Interstitial cystitis patients’ use and rating of complementary and alternative medicine therapies. Int Urogynecol J. 2013. June;24(6):977–82. doi: 10.1007/s00192-012-1966-x. Epub 2012 Nov 14. [DOI] [PubMed] [Google Scholar]
- 15.Pitcher MH. The Impact of Exercise in Rodent Models of Chronic Pain. Curr Osteoporos Rep. 2018. June 23. doi: 10.1007/s11914-018-0461-9. [Epub ahead of print] Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabharwal R, Rasmussen L, Sluka KA, Chapleau MW. Exercise prevents development of autonomic dysregulation and hyperalgesia in a mouse model of chronic muscle pain. Pain. 2016. February;157(2):387–98. doi: 10.1097/j.pain.0000000000000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitcher MH, Tarum F, Rauf IZ, Low LA, Bushnell C. Modest Amounts of Voluntary Exercise Reduce Pain- and Stress-Related Outcomes in a Rat Model of Persistent Hind Limb Inflammation. J Pain. 2017. June;18(6):687–701. doi: 10.1016/j.jpain.2017.01.006. Epub 2017 Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierce AN, Eller-Smith OC, Christianson JA. Voluntary wheel running attenuates urinary bladder hypersensitivity and dysfunction following neonatal maternal separation in female mice. Neurourol Urodyn. 2018. February 21. doi: 10.1002/nau.23530. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith AL, Leung J, Kun S, Zhang R, Karagiannides I, Raz S, Lee U, Glovatscka V, Pothoulakis C, Bradesi S, Mayer EA, Rodríguez LV. The effects of acute and chronic psychological stress on bladder function in a rodent model. Urology. 2011. October;78(4):967.e1–7. doi: 10.1016/j.urology.2011.06.041. Epub 2011 Aug 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee UJ, Ackerman AL, Wu A, Zhang R, Leung J, Bradesi S, Mayer EA, Rodríguez LV. Chronic psychological stress in high-anxiety rats induces sustained bladder hyperalgesia. Physiol Behav. 2015. February;139:541–8. doi: 10.1016/j.physbeh.2014.11.045. Epub 2014 Nov 20. [DOI] [PubMed] [Google Scholar]
- 21.Cetinel S, Ercan F, Cikler E, Contuk G, Sener G. Protective effect of melatonin on water avoidance stress induced degeneration of the bladder. J Urol. 2005. January;173(1):267–70. [DOI] [PubMed] [Google Scholar]
- 22.Paré WP. Learning behavior, escape behavior, and depression in an ulcer susceptible rat strain. Integr Physiol Behav Sci. 1992. Apr-Jun;27(2):130–41. [DOI] [PubMed] [Google Scholar]
- 23.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8th edition Washington (DC): National Academies Press (US); 2011. [PubMed] [Google Scholar]
- 24.Ackerman AL, Jellison FC, Lee UJ, Bradesi S, Rodríguez LV. The Glt1 glutamate receptor mediates the establishment and perpetuation of chronic visceral pain in an animal model of stress-induced bladder hyperalgesia. Am J Physiol Renal Physiol. 2016. April 1;310(7):F628–F636. doi: 10.1152/ajprenal.00297.2015. Epub 2015 Dec 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raz S. Ameliorative effects of brief daily periods of social interaction on isolation-induced behavioral and hormonal alterations. Physiol Behav. 2013. May 27;116–117:13–22. doi: 10.1016/j.physbeh.2013.03.009. Epub 2013 Mar 25. [DOI] [PubMed] [Google Scholar]
- 26.Giubilei G, Mondaini N, Minervini A, Saieva C, Lapini A, Serni S, Bartoletti R, Carini M. Physical activity of men with chronic prostatitis/chronic pelvic pain syndrome not satisfied with conventional treatments--could it represent a valid option? The physical activity and male pelvic pain trial: a double-blind, randomized study. J Urol. 2007. January;177(1):159–65. [DOI] [PubMed] [Google Scholar]
- 27.Verne GN, Price DD. Irritable bowel syndrome as a common precipitant of central sensitization. Curr Rheumatol Rep. 2002. August;4(4):322–8. Review. [DOI] [PubMed] [Google Scholar]
- 28.Johannesson E, Ringström G, Abrahamsson H, Sadik R. Intervention to increase physical activity in irritable bowel syndrome shows long-term positive effects. World J Gastroenterol. 2015. January 14;21(2):600–8. doi: 10.3748/wjg.v21.i2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao Y, Zhang R, Chang HH, Rodríguez LV. The role of C-fibers in the development of chronic psychological stress induced enhanced bladder sensations and nociceptive responses: A multidisciplinary approach to the study of urologic chronic pelvic pain syndrome (MAPP) research network study. Neurourol Urodyn. 2018. February;37(2):673–680. doi: 10.1002/nau.23374. Epub 2017 Aug 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Chang HH, Gao Y, Zhang R, Guo Y, Holschneider DP, Rodriguez LV. Effects of water avoidance stress on peripheral and central responses during bladder filling in the rat: A multidisciplinary approach to the study of urologic chronic pelvic pain syndrome (MAPP) research network study. PLoS One. 2017. September 8;12(9):e0182976. doi: 10.1371/journal.pone.0182976. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groover AL, Ryals JM, Guilford BL, Wilson NM, Christianson JA, Wright DE. Exercise-mediated improvements in painful neuropathy associated with prediabetes in mice. Pain. 2013. December;154(12):2658–67. doi: 10.1016/j.pain.2013.07.052. Epub 2013 Aug 6. [DOI] [PMC free article] [PubMed] [Google Scholar]