Abstract
Objective
To examine the comparative efficacy and safety of interventions for preventing chemotherapy-induced oral mucositis (OM) in adult cancer patients.
Methods
We searched PubMed, Embase and the Cochrane Central systematically for the randomised control trials (RCTs) of interventions for preventing OM. Network meta-analysis (NMA) was performed to estimate risk ratios (RR) and 95% confidence intervals (CI) from both direct and indirect evidence. The primary outcome was any grade of OM. Secondary outcomes were mild-moderate OM, severe OM and adverse events, such as taste disturbance and gastrointestinal adverse events. This study was registered with PROSPERO, number CRD42016052489.
Results
A total of 29 RCTs with 2348 patients (median age, 56.1 years; 57.5% male) were included. Cryotherapy was associated with a significantly lower risk of OM than control (RR 0.51, 95% CI 0.38 to 0.68), and zinc sulphate (RR 0.47, 95% CI 0.23 to 0.97), but not significantly lower than sucralfate and palifermin. No significant differences were observed between cryotherapy and control for taste disturbance and gastrointestinal adverse events. Palifermin was associated with the highest risk of taste disturbance.
Conclusions
This NMA suggests that cryotherapy was the most effective intervention for preventing chemotherapy-induced OM with a safety profile similar to control, but not significantly lower than sucralfate and palifermin. Large RCTs are needed to confirm these findings.
Keywords: mucositis, prevention, chemotherapy, cancer, network meta-analysis
Introduction
Oral mucositis (OM) is one of the most debilitating side effects of chemotherapy, which is characterised by inflammatory and ulcerative reactions in the oral cavity.1 It occurs in approximately 20% to 40% of patients receiving conventional chemotherapy and up to 90% of patients administered high-dose chemotherapy.2 3 OM is associated with pain, malnutrition, oral lesions representing a gateway for opportunistic infections and significant reductions in quality of life.4 Patients with severe mucositis often require dose reduction, treatment delays and hospitalisation which can potentially compromise treatment response and increase mortality.5 6
The Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO) recommended cryotherapy for preventing OM in cancer patients receiving bolus 5-fluorouracil- (5-FU) based chemotherapy regimens and suggests use in those receiving high-dose melphalan regimens.7 Palifermin, a recombinant human keratinocyte growth factor, is also suggested for preventing OM in patients receiving high-dose chemotherapy.7 Emerging evidence suggests that amifostine is also potentially effective in the prevention of OM.8 9 To date, there is no direct evidence from RCTs comparing different interventions used for preventing OM for patients with cancer.
A previous meta-analysis based on randomised control trials (RCTS) demonstrated cryotherapy is effective in reducing the incidence of OM compared with control.10–12 Palifermin also demonstrated efficacy in preventing OM.10 Glutamine significantly reduces the risk and severity of OM during radiotherapy or chemotherapy.13 However, most of these studies considered only direct comparisons of the intervention and included patient populations receiving multimodal therapy including surgery and radiation, in addition to chemotherapy.14
Network meta-analysis is a methodology suited to assessing multiple interventions using indirect comparisons. This allows comparisons of interventions for which there have been no head-to-head comparison.15 16 Therefore, we performed a systematic review and network meta-analysis to comprehensively compare and rank the efficacy and safety of interventions used for preventing OM in adult cancer patients receiving chemotherapy.
Methods
Study design
A systematic review (SR) and network meta-analysis (NMA) of RCTs is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement extension for network meta-analysis.17 This study was conducted following an a priori-established protocol registered with PROSPERO, number CRD42016052489.18 We also appraised quality of evidence and strength of recommendation by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology.19 20
Search strategy and selection criteria
We searched publicly available databases including, PubMed, Embase, the Cochrane Central Register of Controlled Trials and Clinicaltrials.gov, for relevant RCTs comparing interventions preventing OM in adult cancer patients receiving chemotherapy. The literature search was performed in November 2016. We included RCTs evaluating interventions assessing prevention of OM. Comparisons of the following interventions were considered: amifostine (910 mg/m2 intravenous infusion 15 mins); cryotherapy (ice pieces or ice-cold water held in the mouth 5–30 mins before chemotherapy, during chemotherapy and within 5–30 mins after chemotherapy); chlorhexidine 0.10%–0.15% mouthwash every 8–12 hours; glutamine 4–10 g orally every 8–12 hours; granulocyte-macrophage colony-stimulating factor (GM-CSF) 1–1.5 mcg/ml mouthwash every 6 hours; misoprostol 250 mcg orally every 8 hours; recombinant human keratinocyte growth factor-1 (KGF-1/palifermin) 40–180 mg/kg/day; sucralfate 15% 10 mL mouthwash every 6 hours; and zinc sulphate 220 mg orally every 12 hours. There was no restriction on language, publication date and publication status. Details of the search strategy and study selection procedures are shown in the supplementary materials.
Studies with the following characteristics were excluded: assessments of patients receiving radiotherapy or chemoradiotherapy and studies conducted to evaluate the treatment effects of interventions by non-RCTs designs such as systematic review and meta-analysis, review article, guideline, observational study and non-human studies.
Participants
Adult patients (18 years' of age or older) receiving treatment with chemotherapy for any invasive cancer type, including haematological malignancies, solid tumours or mixed cancer types were included.21
Study selection and data extraction
Four investigators (J.K., T.K., S.R. and C.S.) screened the titles and abstracts of retrieved citations independently to identify potentially eligible trials. Disagreements were resolved through discussions with a third reviewer (P.W. or K.K.). All identified potentially eligible citations were accessed in full text and reviewed by investigators (J.K., T.K., S.R., C.S. and P.W.) against the eligibility criteria. Final decisions regarding eligibility were independently, double-checked by a third investigator (P.W. or K.K.). Studies in the non-English-language were formally translated before assessment. Extracted data included the characteristics of the studies (first author, publication year, country, study size), characteristics of patients (cancer type, chemotherapy regimen, mean age, proportion of males, the Eastern Cooperative Oncology Group (ECOG)), characteristics of interventions (details of interventions, co-interventions, treatment duration], and outcomes (definition of mucositis, time to measurement)). If studies were performed over many cycles, we extracted a number of events in the first cycle only. Data from the intention-to-treat analysis were used. All extracted data were independently verified by two investigators (P.W. and K.K.).
Outcomes
The primary outcome was any grade OM. The mucositis scale was defined according to WHO criteria,22 and the National Cancer Institute (NCI) common terminology criteria for adverse events (CTCAE).23 Secondary outcomes were mild to moderate OM (grade 1–2), severe OM (grade 3–4) and adverse events were taste disturbance, gastrointestinal adverse events (nausea, vomiting or diarrhoea) and skin reaction.
Quality of evidence
The risk of bias of individual studies was independently assessed by investigators (P.W., J.K., T.K., S.R. and C.S.) using the Cochrane Risk of Bias assessment tool.24
The GRADE approach was also assessed to rate the quality of evidence for articles included in the network meta-analysis. RCTs could be downgraded from a high-quality rating based on risk of bias, inconsistency (heterogeneity), indirectness, imprecision and publication bias for direct estimates and network meta-analysis estimates.19 20
Statistical analysis
First, we used pairwise meta-analysis to analyse direct treatment comparisons with a random-effects model (DerSimonian and Laird) to estimate pooled risks ratios (RR) with 95% confidence intervals.25 The statistical heterogeneity among studies was assessed by the I2 statistic.26
Then, we used random effects NMA to combine direct and indirect evidence of all treatment effects using STATA 14 (College station, TX: StataCorp LP).27–29 Placebo, normal saline or no treatment were combined into the same node and used as a reference (control) in the analyses. Inconsistency between direct and indirect sources of evidence was assessed for global inconsistency using design-by-treatment interaction models. Loop inconsistency was assessed using an inconsistency factor to examine the presence of inconsistency in loop and significant inconsistency between direct and indirect evidence (P<0.10) calculations of the difference between direct and indirect estimates in closed loops of the network.30 31 The node splitting method was used to explore within network inconsistency.32 We estimated the ranking probabilities for all interventions and hierarchy reported as surface under the cumulative ranking curve (SUCRA), with higher scores reflecting inventions with a greater probability of preventing mucositis. The small-study effects were tested using a comparison-adjusted funnel plot and Egger regression test (P<0.05).27 28 33
To determine whether the results were affected by study characteristics, we carried out subgroup network meta-analyses for primary outcome according to the following variables: cancer type; chemotherapy regimens: 5-FU-based or antimetabolite regimens; and dose of chemotherapy: high or low dose chemotherapy. Additionally, we did a sensitivity NMA for primary outcome by: omitting studies with non-WHO criteria; inappropriate of time to measurement; small sample size (less than 25th percentile); and studies with high risk of bias. Statistical testing with P<0.05 was considered statistically significant.
Results
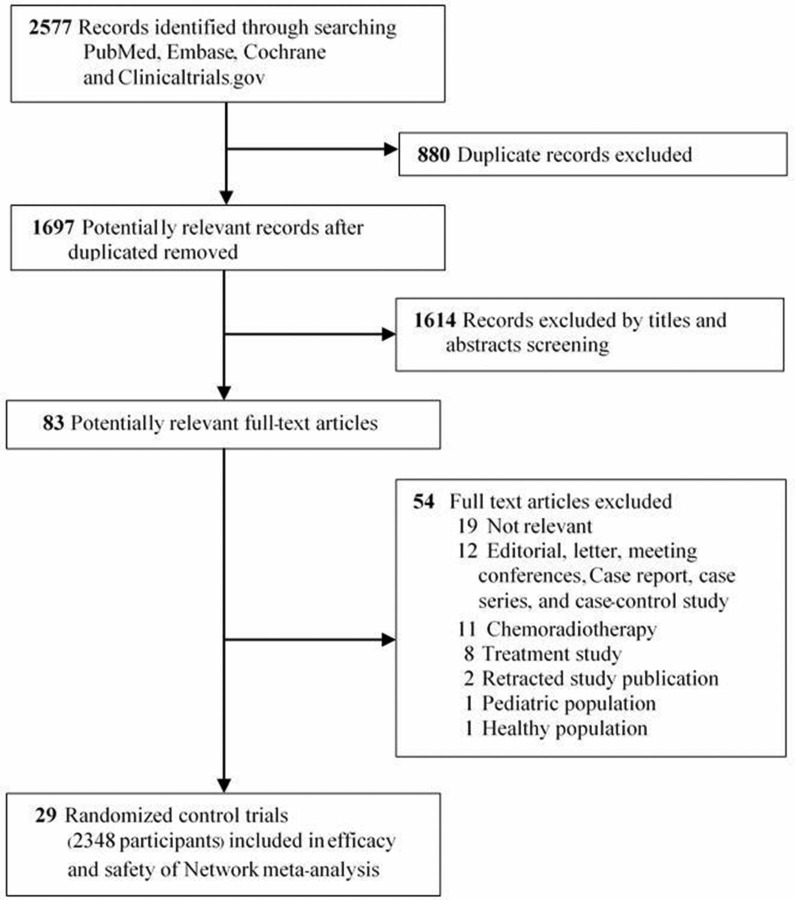
Overall, 2577 records were identified. After removing duplicated articles, 1697 eligible articles were screened by title and abstract from which 1622 articles were excluded. There were 83 articles retrieved in full text (figure 1). We excluded 54 articles and included 29 studies in the systematic review.8 9 34–59 Finally, 29 studies were incorporated in the network meta-analysis.8 9 34–60
Figure 1.
Study identification and selection.
Characteristics and quality of included studies
The characteristics of included studies are described in table 1. Overall, these 29 trials included 2348 participants (the range of size was 16 to 225 participants). The median age of study participants was 56.1 years (IQR, 45.0–60.8) and 57.5% of participants were males (IQR, 51.6%–66.7%). Twenty trials (69.0%) were performed exclusively in solid cancer,8 34 35 38–42 45 46 48 51–54 56–60 six trials (20.7%) in haematological cancer9 36 37 47 49 50 and three trials (10.3%) in mixed cancer types.43 44 55 Most trials (18 trials, 62.1%) were conducted in patients receiving antimetabolite therapy,34 35 37–40 42 46 48 50–54 56–58 60 16 trials (55.2%) receiving 5-FU-based regimens34 35 38–40 42 46 48 51–54 56–58 60 and 11 trials (31.9%) receiving other chemotherapy regimens.8 9 36 41 43–45 47 49 55 59 The most commonly investigated interventions were cryotherapy (nine trials, 31.0%),35 39 45–48 54 57 60 palifermin (five trials, 17.2%)36 37 51 56 59 and chlorhexidine (five trials, 17.2%).32 44 50 55 57
Table 1.
Characteristics of included studies
| Author (year) | Type of cancer | Chemotherapy regimen | Intervention (n) | Study size (n) | Mean age (years) | Male (%) |
ECOG performance status |
| Ala et al (2016)34 | Solid cancer | 5-FU-based regimens | Sucralfate (26) Placebo (26) |
52 | 56.8 | 68.5 | NA |
| Baydar et al (2005)35 | Solid cancer | 5-FU-based regimens | Cryotherapy (45) Control (54) |
99 | 54.2 | NA | NA |
| Blijilevens et al (2013)36 | Haematological cancer | HDM regimens | Palifermin (115) Placebo (57) |
172 | 56.1 | 56.5 | 0 (45.0%) 1 (43.3%) |
| Bradstock et al (2014)37 | Haematological cancer | ICE regimens | Palifermin (79) Placebo (81) |
160 | 45.0 | 64.0 | 0 (52%) 1 (43%) |
| Cartee et al (1995)38 | Solid cancer | AFM regimens | GM-CSF (36) 0.1% albumin (9) |
45 | 43.5 | NA | NA |
| Cascinu et al (1994)39 | Solid cancer | 5-FU-based regimens | Cryotherapy (44) Control (40) |
84 | 57.9 | 69.1 | 0 (50%) 1 (33.3%) 2 (16.7%) |
| Choi et al (2007)40 | Solid cancer | 5-FU/leucovorin regimens | Glutamine (22) Supportive care (29) |
51 | 52.5 | 64.6 | 0 (29.7%) 1 (70.3%) |
| Dazzi et al (2003)41 | Solid cancer | Thiotepa and melphalan, Mitoxantrone, thiotepa and cyclophosphamide, Busulfan and melplalan |
GM-CSF (46) Placebo (44) |
90 | 33.3 | 56.6 | NA |
| Dodd et al (1996)42 | Solid cancer | Doxorubicin, bleomycin, etoposide, 5-FU, MTX, paclitaxel or fludarabine | Chlorhexidine (112) Placebo (110) | 222 | 56.7 | 32.5 | NA |
| Duenas-Gonzalez et al
(1996)43 |
Mixed cancer | ICE regimens | Misoprostol (9) Placebo (7) |
16 | 39.3 | 36.5 | NA |
| Ferretti et al (1990)44 | Mixed cancer | High-dose chemotherapy | Chlorhexidine (19) Placebo (21) | 40 | 32.0 | NA | NA |
| Hartmann et al (2001)8 | Solid cancer | VIC regimens | Amifostine (20) Control (20) |
40 | 45.8 | 10.0 | 0–1 (100%) |
| Heydari et al (2012)60 | Solid cancer | 5-FU-based regimens | Cryotherapy (40) No intervention (40) |
80 | 61.4 | NA | NA |
| Karagozoglu (2005)45 | Solid cancer | Etoposide and cisplatin regimens | Cryotherapy (30) Control (30) |
60 | NA | NA | NA |
| Katranci et al (2012)46 | Solid cancer | 5-FU/leucovorin regimens | Cryotherapy (30) Control (30) |
60 | NA | 50.0 | NA |
| Lilleby et al (2006)47 | Haematological cancer | HDM regimens | Cryotherapy (21) Normal saline (19) |
40 | 57.4 | 69.7 | NA |
| Mahood et al (1991)48 | Solid cancer | 5-FU/leucovorin regimens | Cryotherapy (50) Control (45) |
95 | NA | NA | NA |
| Mansouri et al (2012)49 | Haematological cancer | Busulfan-based regimens | Zinc sulphate (30) Placebo (30) |
60 | 29.0 | 66.7 | NA |
| McGaw (1985)50 | Haematological cancer | Cytarabine, doxorubicin and amsacrine | Chlorhexidine (8) Control (8) | 16 | NA | NA | NA |
| Meropol et al (2003)51 | Solid cancer | 5-FU/leucovorin regimens | KGF (54) Placebo (27) |
81 | 63.8 | 58.4 | 0–2 (100%) |
| Nottage et al (2003)52 | Solid cancer | 5-FU/leucovorin regimens | Sucralfate (41) Placebo (40) |
81 | 60.8 | 55.0 | 0 (63.9 %) 1 (31.3%) |
| Okuno et al (1999)53 | Solid cancer | 5-FU-based regimens | Glutamine (66) Placebo (68) |
134 | 62.7 | 53.8 | NA |
| Papadeas et al (2007)54 | Solid cancer | 5-FU/leucovorin regimens | Cryotherapy (36) Placebo (40) |
76 | 62.4 | 34.2 | NA |
| Pitten et al (2003)55 | Mixed cancer | NA | Chlorhexidine (24) Placebo (23) |
47 | 51.5 | 63.8 | NA |
| Rosen et al (2006)56 | Solid cancer | 5-FU/leucovorin regimens | Palifermin (36) Placebo (28) |
64 | 65.0 | 64.7 | 0 (46%) 1 (44%) 2 (8.5%) Other (2.5%) |
| Sorensen et al (2008)57 | Solid cancer | 5-FU/leucovorin regimens | Chlorhexidine (75) Placebo (75) Cryotherapy (75) |
225 | 59.3 | 55.4 | NA |
| Spencer et al (2005)9 | Haematological cancer | HDM regimens | Amifostine (43) Control (47) |
90 | 51.8 | 67.2 | 2 (100%) |
| Tanaka et al (2016)58 | Solid cancer | DCF regimens | Glutamine (10) Control (10) |
20 | 70.0 | 80.0 | NA |
| Vadhan-Raj et al (2010)59 | Solid cancer | Doxorubicin-based regimens | Palifermin (32) Placebo (16) |
48 | 40.13 | 51.6 | NA |
AFM regimens, Adriamycin (doxorubicin) +5-FU+MTX; CALGB criteria, Cancer and Leukaemia Group B; CTCAE v3.0, Common Terminology Criteria for Adverse Events version 3.0; DCF regimens, Docetaxel+cisplatin+5 FU; DGS regimens; Docetaxel+nedaplatin+ S-1; HDM regimens, High-dose melphalan; ICE regimens, Idarubicin +cytarbine+ etoposide or Ifosfamide +carboplastin+ etoposide; NA, not available; NCI-CTC, National Cancer Institute Common Toxicity Criteria; VIC regimens, High-dose etoposide +ifosfamide+ carboplastin; WHO, World Health Organization grading system.
In terms of study quality (risk of bias), 16 trials (55.2%) were rated as high risk of bias in blinding of outcome assessment,8 9 35 39 40 42 44–48 50 54 55 57 60 10 trials (34.5%) were considered unclear risk because they could not be graded due to inadequate information36–38 41 43 51–53 56 58 and three trials (10.4%) had low risk of bias.34 49 59
Direct meta-analysis
Treatment effects in pairwise meta-analyses are shown in online supplementary appendix. Evidence of statistical heterogeneity was identified for some pairwise comparisons.
ejhpharm-2018-001649supp001.pdf (1.6MB, pdf)
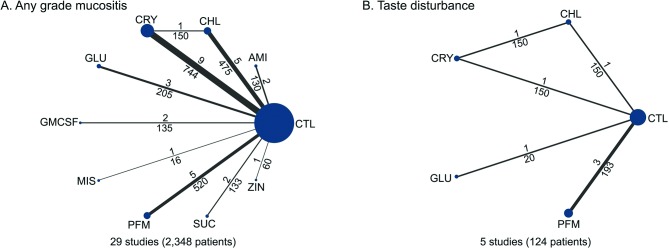
Network consistency
The network plots of treatment outcomes for any grade of mucositis and adverse events (taste disturbance) are presented in figure 2. Inconsistencies between direct and indirect evidence are noted for some network comparisons. The design-by-treatment interaction model did not identify global inconsistency within any network, except for taste disturbance.
Figure 2.
Network comparisons of included studies in the analyses. The size of the nodes and the thickness of the lines are weighted according to the number of studies assessing each treatment and direct comparison. Numbers above and below the lines indicate studies and patients respectively. AMI, amifostine; CHL, chlorhexidine; CRY, cryotherapy; CTL, control; GLU, glutamine; GM-CSF, granulocyte-monocyte colony-stimulating factor; MIS, misoprostol; PFM, palifermin; SUC, sucralfate; ZIN, zinc sulphate.
NMA results
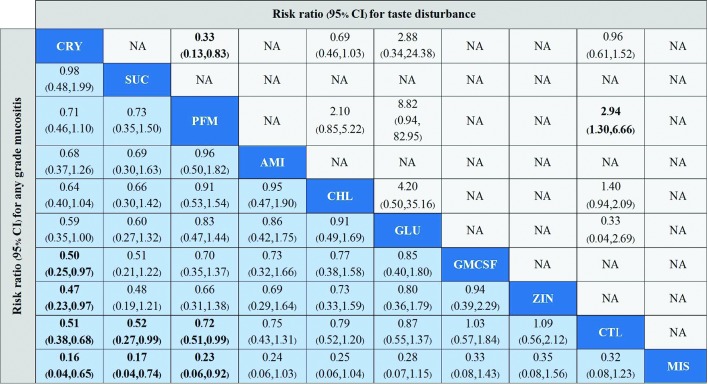
Any grade OM
A total of 29 studies involving 2348 patients evaluated any grade OM of nine interventions. NMA suggests that cryotherapy is the best intervention to prevent OM (figure 3). Cryotherapy was associated with a significantly lower risk of OM than GM-CSF (RR 0.50, 95% CI 0.25 to 0.97; moderate-quality evidence), zinc sulphate (RR 0.47, 95% CI 0.23 to 0.97; low-quality evidence), misoprostol (RR 0.16, 95% CI 0.04 to 0.65) and control (0.51, 95% CI 0.38 to 0.68; moderate-quality evidence); and no significant differences were noted in cryotherapy compared with palifermin and sucralfate. Sucralfate (RR 0.52, 95% CI 0.27 to 0.99; moderate-quality evidence) and palifermin (RR 0.72, 95% CI 0.51 to 0.99; high-quality evidence) were significantly more effective in preventing any grade OM than control. Misoprostol was significantly less effective in preventing OM than sucralfate and palifermin. No significant differences were found in the other comparisons. The ranking of interventions based on SUCRAs and clustered ranking are presented in online supplementary appendix.
Figure 3.
Comparison incidence of any grade mucositis and adverse events (taste disturbance) in network meta-analysis. Summary estimate represents risk ratio of any grade of mucositis (light blue background) and adverse events (taste disturbance) (light grey background). Interventions are ordered by ranking for any grade of mucositis. Risk ratio for comparisons are in the cell in common between the column-defining and row-defining Intervention. For any grade mucositis outcome, column intervention is compared with row intervention (ie, row treatment is reference). For adverse events (taste disturbance), row intervention is compared with column intervention (ie, column intervention is reference). Numbers in bold represent statistically significant results. AMI, amifostine; CHL, chlorhexidine; CRY, cryotherapy; CTL, control; GLU, glutamine; GM-CSF, granulocyte-monocyte colony-stimulating factor; MIS, misoprostol; NA, not available; PFM, palifermin; SUC, sucralfate; ZIN, zinc sulphate.
Mild-moderate OM
Mild-moderate OM was evaluated in 20 studies involving 1776 patients and eight interventions. NMA suggests that only cryotherapy was associated with statistically significant reductions in mild-moderate OM compared with control (RR 0.66, 95% CI 0.47 to 0.93; moderate-quality evidence). No significant differences were found in the other comparisons.
Severe OM
Severe mucositis was assessed in 20 studies involving 1807 patients and eight interventions. NMA suggests that sucralfate is the best intervention to prevent severe grade OM. Sucralfate (RR 0.31, 95% CI 0.10 to 0.93; moderate-quality evidence), cryotherapy (RR 0.37, 95% CI 0.24 to 0.58; moderate-quality evidence), amifostine (RR 0.42, 95% CI 0.20 to 0.86; high-quality evidence) and chlorhexidine (RR 0.46, 95% CI 0.22 to 0.95; moderate-quality evidence) significantly prevented severe mucositis compared with control. Cryotherapy was significantly more effective than GM-CSF (RR 0.37, 95% CI 0.16 to 0.83). No other statistically significant differences in the other comparison were observed.
Adverse events
Few studies reported the adverse events of the interventions. Eight trials8 37 43 51 52 56 58 59 examined GI adverse events, while five51 56–59 and four trials36 37 51 56 reported taste disturbances and skin reactions, respectively. The results from NMA demontrated no statistically significant differences were found between cryotherapy and control (RR 0.96, 95% CI 0.61 to 1.52) in terms of taste disturbance. However, palifermin was associated with a significantly increased risk of taste disturbance compared with controls (RR 2.94, 95% CI 1.30 to 6.66) and cryotherapy (RR 3.06, 95% CI 1.20 to 7.82) (figure 3). No statistically significant differences in gastrointestinal and skin reaction adverse events were observed between treatment options.
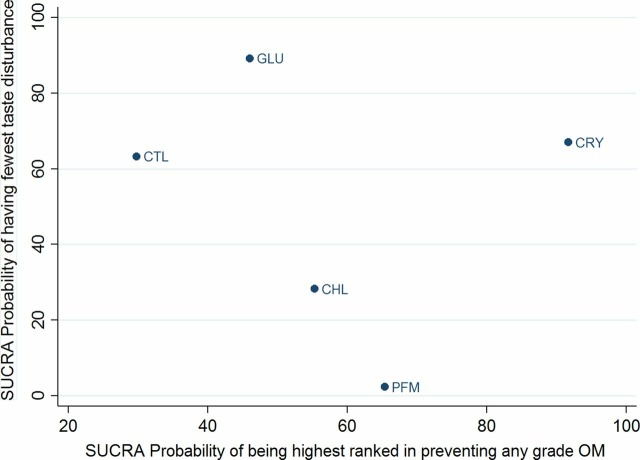
NMA suggested that cryotherapy was associated with the highest probability of preventing any grade OM (SUCRA, 0.92), followed by sucralfate (SUCRA, 0.85) and palifermin (SUCRA, 0.65). Similarly, glutamine was associated with the highest probability of the fewest taste disturbances (SUCRA, 0.89), followed by cryotherapy (SUCRA, 0.67). Palifermin was associated with the highest risk of taste disturbance (SUCRA, 0.02) (figure 4). The individual ranking of interventions based on cumulative probability plots and SUCRAs are presented in online supplementary appendix.
Figure 4.
SUCRAs for preventing any grade mucositis and adverse events (taste disturbance). Surface under the cumulative rankings (SUCRAs) between 0 and 100 represent the probability of being ranked highest. For the prevention of any grade OM outcomes, higher score corresponds to higher proportion preventing OM with a particular therapy. For the taste disturbance outcome, higher scores reflect lower probability of taste disturbance.
Subgroup and sensitivity analyses
The results from subgroup analyses confirm cryotherapy is the best intervention in preventing any grade OM in any type of cancer and all chemotherapy regimens. Sensitivity analyses demonstrated similar results to the main analysis.
Publication bias
There was no evidence of small-study effects, either qualitatively based on funnel-plot asymmetry or quantitatively (Egger regression test, P>0.05 for all comparisons), although the number of studies included in each comparison was small.
Discussion
This is the first SR and NMA comparing the effectiveness of interventions for the prevention of OM in adult cancer patients. The study has several key findings. First, cryotherapy was the best intervention to reduce the incidence of OM with few side effects, except mild discomfort during administration. Our findings suggest that cryotherapy should be considered the first-line intervention for preventing OM. Second, palifermin compared with cryotherapy was not statistically nor significantly different in preventing OM. Based on this review, palifermin may be used as an alternative intervention for preventing OM in patients with a contraindication to cryotherapy such as those receiving oxaliplatin-containing regimens. However, palifermin was associated with an increased risk of taste disturbance, a known side effect of the therapy and the cost effectiveness of this agent has yet to be demonstrated.61 62 Third, the most surprising finding from our NMA found that cancer patients treated with sucralfate mouthwash before receiving chemotherapy significantly reduced the incidence of severe OM compared with control and the reduction was similar to cryotherapy. These results indicate that sucralfate may be the best intervention for preventing severe OM. Finally, zinc sulphate did not significantly reduce the incidence of OM compared with control and therefore should not be recommended for the prevention of OM.
Similar to previous meta-analyses, our findings suggested that cryotherapy was significantly better than control for preventing OM.1011 Oral cryotherapy also significantly decreased the incidence of severe OM.12 When compared with chlorhexidine, oral cryotherapy did not significantly reduce in the incidence of OM.11 Cryotherapy causes local vasoconstriction in the oral mucosa, leading to reduced blood flow and delivery of cytotoxic drugs to the oral mucous membrane.35 Based on this review, cryotherapy had the highest probability of being the best intervention for preventing OM in patients receiving chemotherapy and is currently recommended for use in the MASCC/ISOO guidelines for preventing OM in those receiving bolus 5-fluorouracil and suggested use for high-dose melphalan.7
Moreover, palifermin can prevent OM in patients receiving high-dose chemotherapy.7 Palifermin has potent epithelial cell proliferative activity and induces epithelial thickening of the non-keratinocyte layers of the oral mucosa and gastrointestinal tract.63 Palifermin is recommended for use by MASCC/ISOO guidelines and is the only agent approved for use by regulatory agencies in the USA and EU to prevent OM in patients receiving high-dose chemotherapy followed by autologous stem cell transplant.7
On the contrary, previous meta-analyses have demonstrated that sucralfate has no significant advantage for preventing OM in patients receiving chemoradiotherapy, and current guidelines recommend against this agent for the prevention of chemotherapy-induced OM.7 10 64 Our NMA found that sucralfate does provide significant protective effects for chemotherapy-induced OM of all grades. Furthermore, we demonstrated no statistically significant differences between sucralfate and cryotherapy. Sucralfate, a sulphated disaccharide which is not absorbed, acts as a mucoprotective agent to shield nerve endings in the oral mucosa from cytotoxic agents.34 52 Thus, sucralfate mouthwash could be an alternative intervention for preventing OM. However, the number of included trials in this analysis was limited and this finding should be confirmed by high-quality RCTs.34 52 Current clinical practice guidelines suggests that zinc sulphate, an antioxidant and trace element involved in tissue repair, may prevent OM in patients receiving radiotherapy or chemoradiotherapy.7 However, our findings demonstrate zinc sulphate had no benefit for preventing OM in patients receiving chemotherapy.
The major strengths of this study include the explicit eligibility criteria, a comprehensive search strategy, consideration of trials in languages other than English, and the independent and duplicate assessment of eligibility. Furthermore, this is the first NMA of RCTs that include interventions for preventing OM in adult cancer patients for combining direct and indirect evidence.
This study has limitations. First, in the quality of evidence (GRADE), many comparisons were assessed as low quality, which largely restricts the interpretation of these results. Second, this NMA was restricted to trials involving only patients receiving chemotherapy. We excluded trials involving radiotherapy or chemoradiotherapy regimens to reduce heterogeneity and inconsistency among trials in NMA, but we recognised that it restricts the external validity and applicability of the study findings. Thus, our results are less generalisable to adult patients receiving radiotherapy or chemoradiotherapy. Third, we were unable to assess the validity of NMA results because direct and indirect estimates were not available for the outcomes of the comparisons.31 We tested for the overall inconsistency in the network using a global method, yet we were unable to test loop-specific inconsistency since most pairwise comparisons only have one study. Hence, these results should be interpreted with caution. Fourth, patient characteristics were heterogeneous across the trials, which is a significant limitation of our NMA. Plausible confounder of this analysis includes imbalance of chemotherapy regimens, cancer types and co-interventions. However, we did subgroup and sensitivity analysis with these variables and the findings of which were not materially different from the primary analysis in most of these comparisons. Finally, although the different control interventions including routine care, placebo, normal saline or no treatments were combined, we found that the effect to prevent OM of these control interventions were not statistically different.
In conclusion, our NMA suggests that cryotherapy was the most effective intervention for preventing OM with a safety profile similar to control. Cryotherapy should be considered as the first-line intervention preventing chemotherapy-induced OM in adult cancer patients in the absence of contraindications. Palifermin and sucralfate did not differ significantly from cryotherapy for the prevention of chemotherapy-induced OM and would be reasonable alternatives, however cost needs to be considered especially with the use of palifermin. Further large RCTs are needed to confirm these findings.
What this paper adds.
What is already known on this subject
Oral mucositis (OM) is one of the most debilitating side effects of chemotherapy.
Interventions such as cryotherapy, palifermin, glutamine and sucralfate have demonstrated efficacy in preventing oral mucositis
Strong evidence with ranking of available interventions by safety and efficacy is needed to guide clinical practice.
What this study adds
This study provides the first comprehensive systematic review and network meta-analysis of randomised trials, comparing and ranking interventions for preventing chemotherapy- induced oral mucositis.
Cryotherapy was identified as the best intervention to prevent chemotherapy-induced oral mucositis, followed by palifermin and sucralfate mouthwash.
Therefore, cryotherapy should be considered a first-line intervention for the prevention of chemotherapy-induced OM in adult cancer patients in the absence of contraindications.
Acknowledgments
We would like to thank Wanpen Tanjankul (Library Resources and Educational Media Center, University of Phayao) for her support on full-text access.
Footnotes
Contributors: PW, KK and SS had the idea for, and designed, the research. PW, KK, TK, SR, JK and CS identified and acquired reports of trials and extracted data. PW and KK did all data analyses, checked for statistical inconsistency and interpreted data. PW, KK, TK, SR, JK and CS contributed to data interpretation. PW, KK, TK, SR, JK and CS drafted the report and DDS, SS, PW and KK critically reviewed the report.
Funding: The study was financially supported by the grants from the School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Presented at: This study was partially presented as abstract form in ISPOR 20th Annual European Congress Conference on 6 November 2017 in Glasgow, Scotland, UK.
References
- 1. Pico JL, Avila-Garavito A, Naccache P. Mucositis: Its occurrence, consequences, and treatment in the oncology setting. Oncologist 1998;3:446–51. [PubMed] [Google Scholar]
- 2. Jones JA, Avritscher EB, Cooksley CD, et al. Epidemiology of treatment-associated mucosal injury after treatment with newer regimens for lymphoma, breast, lung, or colorectal cancer. Support Care Cancer 2006;14:505–15. 10.1007/s00520-006-0055-4 [DOI] [PubMed] [Google Scholar]
- 3. Vera-Llonch M, Oster G, Ford CM, et al. Oral mucositis and outcomes of allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies. Support Care Cancer 2007;15:491–6. 10.1007/s00520-006-0176-9 [DOI] [PubMed] [Google Scholar]
- 4. Barkokebas A, Silva IH, de Andrade SC, et al. Impact of oral mucositis on oral-health-related quality of life of patients diagnosed with cancer. J Oral Pathology Med 2015;44:746–51. 10.1111/jop.12282 [DOI] [PubMed] [Google Scholar]
- 5. Gabriel DA, Shea T, Olajida O, et al. The effect of oral mucositis on morbidity and mortality in bone marrow transplant. Semin Oncol 2003;30(6 Suppl 18):76–83. 10.1053/j.seminoncol.2003.11.040 [DOI] [PubMed] [Google Scholar]
- 6. Bey A, Ahmed SS, Hussain B, et al. Prevention and management of antineoplastic therapy induced oral mucositis. National J Maxillofac Surg 2010;1:127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lalla RV, Bowen J, Barasch A, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014;120:1453–61. 10.1002/cncr.28592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hartmann JT, von Vangerow A, Fels LM, et al. A randomized trial of amifostine in patients with high-dose VIC chemotherapy plus autologous blood stem cell transplantation. Br J Cancer 2001;84:313–20. 10.1054/bjoc.2000.1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spencer A, Horvath N, Gibson J, et al. Prospective randomised trial of amifostine cytoprotection in myeloma patients undergoing high-dose melphalan conditioned autologous stem cell transplantation. Bone Marrow Transplant 2005;35:971–7. 10.1038/sj.bmt.1704946 [DOI] [PubMed] [Google Scholar]
- 10. Worthington HV, Clarkson JE, Bryan G, et al. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev 2011:Cd000978 10.1002/14651858.CD000978.pub5 [DOI] [PubMed] [Google Scholar]
- 11. Riley P, Glenny AM, Worthington HV, et al. Interventions for preventing oral mucositis in patients with cancer receiving treatment: oral cryotherapy. Cochrane Database Syst Rev 2015:Cd011552 10.1002/14651858.CD011552.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang L, Gu Z, Zhai R, et al. Efficacy of oral cryotherapy on oral mucositis prevention in patients with hematological malignancies undergoing hematopoietic stem cell transplantation: a meta-analysis of randomized controlled trials. PLoS One 2015;10:e0128763 10.1371/journal.pone.0128763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leung HW, Chan AL. Glutamine in alleviation of radiation-induced severe oral mucositis: a meta-analysis. Nutr Cancer 2016;68:734–42. 10.1080/01635581.2016.1159700 [DOI] [PubMed] [Google Scholar]
- 14. Sak K. Chemotherapy and dietary phytochemical agents. Chemother Res Pract 2012;2012:1–11. 10.1155/2012/282570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med 2002;21:2313–24. 10.1002/sim.1201 [DOI] [PubMed] [Google Scholar]
- 16. Kengkla K, Kongpakwattana K, Saokaew S, et al. Comparative efficacy and safety of treatment options for MDR and XDR Acinetobacter baumannii infections: a systematic review and network meta-analysis. J Antimicrob Chemother 2018;73:22–32. 10.1093/jac/dkx368 [DOI] [PubMed] [Google Scholar]
- 17. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–84. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 18. Kengkla K, Kaewthong J, Kaewpanan T, et al. Comparative efficacy and safety of interventions for preventing chemotherapy-induced oral mucositis in adult cancer patients: a systematic review and network meta-analysis. Secondary comparative efficacy and safety of interventions for preventing chemotherapy-induced oral mucositis in adult cancer patients: a systematic review and network meta-analysis, 2016. http://www.crd.york.ac.uk/prospero/display_record.asp?ID=CRD42016052489 [DOI] [PMC free article] [PubMed]
- 19. Puhan MA, Schünemann HJ, Murad MH, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630 10.1136/bmj.g5630 [DOI] [PubMed] [Google Scholar]
- 20. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 21. World Health Organization. Cancer. Secondary cancer, 2017. http://www.who.int/mediacentre/factsheets/fs297/en/
- 22. Sonis ST, Elting LS, Keefe D, et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004;100(9 Suppl):1995–2025. [DOI] [PubMed] [Google Scholar]
- 23. National Cancer Institiute. Common terminology criteria for adverse events (CTCAE). Secondary common terminology criteria for adverse events (CTCAE), 2010. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40
- 24. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 26. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One 2013;8:e76654 10.1371/journal.pone.0076654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105–24. 10.1002/sim.1875 [DOI] [PubMed] [Google Scholar]
- 30. Veroniki AA, Vasiliadis HS, Higgins JP, et al. Evaluation of inconsistency in networks of interventions. Int J Epidemiol 2013;42:332–45. 10.1093/ije/dys222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Higgins JP, Jackson D, Barrett JK, et al. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 2012;3:98–110. 10.1002/jrsm.1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932–44. 10.1002/sim.3767 [DOI] [PubMed] [Google Scholar]
- 33. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–71. 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 34. Ala S, Saeedi M, Janbabai G, et al. Efficacy of sucralfate mouth wash in prevention of 5-fluorouracil induced oral mucositis: a prospective, randomized, double-blind, controlled trial. Nutr Cancer 2016;68:456–63. 10.1080/01635581.2016.1153666 [DOI] [PubMed] [Google Scholar]
- 35. Baydar M, Dikilitas M, Sevinc A, et al. Prevention of oral mucositis due to 5-fluorouracil treatment with oral cryotherapy. J Natl Med Assoc 2005;97:1161–4. [PMC free article] [PubMed] [Google Scholar]
- 36. Blijlevens N, de Château M, Krivan G, et al. In a high-dose melphalan setting, palifermin compared with placebo had no effect on oral mucositis or related patient’s burden. Bone Marrow Transplant 2013;48:966–71. 10.1038/bmt.2012.257 [DOI] [PubMed] [Google Scholar]
- 37. Bradstock KF, Link E, Collins M, et al. A randomized trial of prophylactic palifermin on gastrointestinal toxicity after intensive induction therapy for acute myeloid leukaemia. Br J Haematol 2014;167:618–25. 10.1111/bjh.13086 [DOI] [PubMed] [Google Scholar]
- 38. Cartee L, Petros WP, Rosner GL, et al. Evaluation of GM-CSF mouthwash for prevention of chemotherapy-induced mucositis: a randomized, double-blind, dose-ranging study. Cytokine 1995;7:471–7. 10.1006/cyto.1995.0064 [DOI] [PubMed] [Google Scholar]
- 39. Cascinu S, Fedeli A, Fedeli SL, et al. Oral cooling (cryotherapy), an effective treatment for the prevention of 5-fluorouracil-induced stomatitis. Eur J Cancer B Oral Oncol 1994;30B:234–6. 10.1016/0964-1955(94)90003-5 [DOI] [PubMed] [Google Scholar]
- 40. Choi K, Lee SS, Oh SJ, et al. The effect of oral glutamine on 5-fluorouracil/leucovorin-induced mucositis/stomatitis assessed by intestinal permeability test. Clin Nutr 2007;26:57–62. 10.1016/j.clnu.2006.07.003 [DOI] [PubMed] [Google Scholar]
- 41. Dazzi C, Cariello A, Giovanis P, et al. Prophylaxis with GM-CSF mouthwashes does not reduce frequency and duration of severe oral mucositis in patients with solid tumors undergoing high-dose chemotherapy with autologous peripheral blood stem cell transplantation rescue: a double blind, randomized, placebo-controlled study. Ann Oncol 2003;14:559–63. 10.1093/annonc/mdg177 [DOI] [PubMed] [Google Scholar]
- 42. Dodd MJ, Larson PJ, Dibble SL, et al. Randomized clinical trial of chlorhexidine versus placebo for prevention of oral mucositis in patients receiving chemotherapy. Oncol Nurs Forum 1996;23:921–7. [PubMed] [Google Scholar]
- 43. Dueñas-Gonzalez A, Sobrevilla-Calvo P, Frias-Mendivil M, et al. Misoprostol prophylaxis for high-dose chemotherapy-induced mucositis: a randomized double-blind study. Bone Marrow Transplant 1996;17:809–12. [PubMed] [Google Scholar]
- 44. Ferretti GA, Raybould TP, Brown AT, et al. Chlorhexidine prophylaxis for chemotherapy- and radiotherapy-induced stomatitis: a randomized double-blind trial. Oral Surg Oral Med Oral Pathol 1990;69:331–8. 10.1016/0030-4220(90)90295-4 [DOI] [PubMed] [Google Scholar]
- 45. Karagözoğlu S, Filiz Ulusoy M. Chemotherapy: the effect of oral cryotherapy on the development of mucositis. J Clin Nurs 2005;14:754–65. 10.1111/j.1365-2702.2005.01128.x [DOI] [PubMed] [Google Scholar]
- 46. Katrancı N, Ovayolu N, Ovayolu O, et al. Evaluation of the effect of cryotherapy in preventing oral mucositis associated with chemotherapy – a randomized controlled trial. Eur J Oncol Nurs 2012;16:339–44. 10.1016/j.ejon.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 47. Lilleby K, Garcia P, Gooley T, et al. A prospective, randomized study of cryotherapy during administration of high-dose melphalan to decrease the severity and duration of oral mucositis in patients with multiple myeloma undergoing autologous peripheral blood stem cell transplantation. Bone Marrow Transplant 2006;37:1031–5. 10.1038/sj.bmt.1705384 [DOI] [PubMed] [Google Scholar]
- 48. Mahood DJ, Dose AM, Loprinzi CL, et al. Inhibition of fluorouracil-induced stomatitis by oral cryotherapy. J Clin Oncol 1991;9:449–52. 10.1200/JCO.1991.9.3.449 [DOI] [PubMed] [Google Scholar]
- 49. Mansouri A, Hadjibabaie M, Iravani M, et al. The effect of zinc sulfate in the prevention of high-dose chemotherapy-induced mucositis: a double-blind, randomized, placebo-controlled study. Hematol Oncol 2012;30:22–6. 10.1002/hon.999 [DOI] [PubMed] [Google Scholar]
- 50. McGaw WT, Belch A. Oral complications of acute leukemia: prophylactic impact of a chlorhexidine mouth rinse regimen. Oral Surg Oral Med Oral Pathol 1985;60:275–80. 10.1016/0030-4220(85)90311-1 [DOI] [PubMed] [Google Scholar]
- 51. Meropol NJ, Somer RA, Gutheil J, et al. Randomized phase I trial of recombinant human keratinocyte growth factor plus chemotherapy: potential role as mucosal protectant. J Clin Oncol 2003;21:1452–8. 10.1200/JCO.2003.10.079 [DOI] [PubMed] [Google Scholar]
- 52. Nottage M, McLachlan SA, Brittain MA, et al. Sucralfate mouthwash for prevention and treatment of 5-fluorouracil-induced mucositis: a randomized, placebo-controlled trial. Support Care Cancer 2003;11:41–7. 10.1007/s00520-002-0378-8 [DOI] [PubMed] [Google Scholar]
- 53. Okuno SH, Woodhouse CO, Loprinzi CL, et al. Phase III controlled evaluation of glutamine for decreasing stomatitis in patients receiving fluorouracil (5-FU)-based chemotherapy. Am J Clin Oncol 1999;22:258–61. 10.1097/00000421-199906000-00009 [DOI] [PubMed] [Google Scholar]
- 54. Papadeas E, Naxakis S, Riga M, et al. Prevention of 5-fluorouracil-related stomatitis by oral cryotherapy: a randomized controlled study. Eur J Oncol Nurs 2007;11:60–5. 10.1016/j.ejon.2006.05.002 [DOI] [PubMed] [Google Scholar]
- 55. Pitten FA, Kiefer T, Buth C, et al. Do cancer patients with chemotherapy-induced leukopenia benefit from an antiseptic chlorhexidine-based oral rinse? A double-blind, block-randomized, controlled study. J Hosp Infect 2003;53:283–91. 10.1053/jhin.2002.1391 [DOI] [PubMed] [Google Scholar]
- 56. Rosen LS, Abdi E, Davis ID, et al. Palifermin reduces the incidence of oral mucositis in patients with metastatic colorectal cancer treated with fluorouracil-based chemotherapy. J Clin Oncol 2006;24:5194–200. 10.1200/JCO.2005.04.1152 [DOI] [PubMed] [Google Scholar]
- 57. Sorensen JB, Skovsgaard T, Bork E, et al. Double-blind, placebo-controlled, randomized study of chlorhexidine prophylaxis for 5-fluorouracil-based chemotherapy-induced oral mucositis with nonblinded randomized comparison to oral cooling (cryotherapy) in gastrointestinal malignancies. Cancer 2008;112:1600–6. 10.1002/cncr.23328 [DOI] [PubMed] [Google Scholar]
- 58. Tanaka Y, Takahashi T, Yamaguchi K, et al. Elemental diet plus glutamine for the prevention of mucositis in esophageal cancer patients receiving chemotherapy: a feasibility study. Support Care Cancer 2016;24:933–41. 10.1007/s00520-015-2864-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vadhan-Raj S, Trent J, Patel S, et al. Single-dose palifermin prevents severe oral mucositis during multicycle chemotherapy in patients with cancer: a randomized trial. Ann Intern Med 2010;153:358–67. 10.7326/0003-4819-153-6-201009210-00003 [DOI] [PubMed] [Google Scholar]
- 60. Heydari ASH, Salek R. Effect of oral cryotherapy on combination chemotherapy-induced oral mucositis: a randomized clinical trial. Middle East J Cancer 2012;3:55–64. [Google Scholar]
- 61. Nooka AK, Johnson HR, Kaufman JL, et al. Pharmacoeconomic analysis of palifermin to prevent mucositis among patients undergoing autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2014;20:852–7. 10.1016/j.bbmt.2014.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Elting LS, Shih YC, Stiff PJ, et al. Economic impact of palifermin on the costs of hospitalization for autologous hematopoietic stem-cell transplant: analysis of phase 3 trial results. Biol Blood Marrow Transplant 2007;13:806–13. 10.1016/j.bbmt.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 63. Farrell CL, Rex KL, Kaufman SA, et al. Effects of keratinocyte growth factor in the squamous epithelium of the upper aerodigestive tract of normal and irradiated mice. Int J Radiat Biol 1999;75:609–20. [DOI] [PubMed] [Google Scholar]
- 64. Cardona A, Balouch A, Abdul MM, et al. Efficacy of chlorhexidine for the prevention and treatment of oral mucositis in cancer patients: a systematic review with meta-analyses. J Oral Pathol Med 2017;46:680–8. 10.1111/jop.12549 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ejhpharm-2018-001649supp001.pdf (1.6MB, pdf)