Abstract
Purpose of Review
Disruptions in fetal development (via genetic and environmental pathways) have been consistently associated with risk for schizophrenia in a variety of studies. Although multiple obstetric complications (OCs) have been linked to schizophrenia, this review will discuss emerging evidence supporting the role of prenatal maternal stress (PNMS) in the etiology of schizophrenia spectrum disorders (SSD). In addition, findings linking PNMS to intermediate phenotypes of the disorder, such as OCs and premorbid cognitive, behavioral, and motor deficits, will be reviewed. Maternal immune and endocrine dysregulation will also be explored as potential mechanisms by which PNMS confers risk for SSD.
Recent Findings
PNMS has been linked to offspring SSD; however, findings are mixed due to inconsistent and retrospective assessments of PNMS and lack of specificity about SSD outcomes. PNMS is also associated with various intermediate phenotypes of SSD (e.g., prenatal infection/inflammation, decreased fetal growth, hypoxia-related OCs). Recent studies continue to elucidate the impact of PNMS while considering the moderating roles of fetal sex and stress timing, but it is still unclear which aspects of PNMS (e.g., type, timing) confer risk for SSD specifically.
Summary
PNMS increases risk for SSD, but only in a small portion of fetuses exposed to PNMS. Fetal sex, genetics, and other environmental factors, as well as additional pre- and postnatal insults, likely contribute to the PNMS-SSD association. Longitudinal birth cohort studies are needed to prospectively illuminate the mechanisms that account for the variability in outcomes following PNMS.
Keywords: Prenatal stress, Obstetric complications, Maternal inflammation, Premorbid deficits, Schizophrenia
Introduction
Schizophrenia spectrum disorders (SSD) are considered neurodevelopmental disorders marked by positive (hallucinations and delusions) and negative (volitional and expressive deficits) symptoms [1–3]. They occur in about 1% of the population and are more common in males [4, 5]. Although genetic factors clearly play a role in the etiology of the disorder, with concordance rates between monozygotic twins at approximately 50%, environmental factors also are involved [6]. Obstetric complications (OCs) have been among the most well-replicated environmental risk factors for SSD, with one OC, fetal hypoxia, occurring in approximately 20–30% of cases who develop schizophrenia [7•, 8]. A variety of other OCs have been linked to schizophrenia risk, such as prenatal infection, preeclampsia, and decreased fetal growth [9, 10•]. Importantly, one OC, prenatal maternal stress (PNMS), appears to also increase risk for a range of prenatal insults and premorbid disruptions linked to schizophrenia [7•, 11••]. Hence, it is critical to understand how PNMS operates within the course of SSD.
This review will explore human literature linking PNMS to risk for SSD in offspring, as well as intermediate phenotypes for SSD such as OCs and premorbid deficits. Furthermore, this paper will examine potential mechanistic underpinnings of the relationship between PNMS and the developmental cascade of risk to SSD including maternal activation of glucocorticoids and inflammation. Emphasis will also be given to how fetal sex and stress timing may moderate the relationship between PNMS and SSD outcomes.
Ecologic Studies of PNMS and SSD
Ecologic studies were among the first to associate prenatal incidence of a number of severe stressors, including warfare [12, 13], famine [14, 15], and natural disaster [16], with SSD outcomes in offspring. Loss or severe illness of a spouse during pregnancy has also been associated with offspring SSD outcomes [17, 18]; however, findings are inconsistent with respect to timing of exposure [19, 20]. One particular study identified increased risk for SSD following parental, sibling, or spousal loss during only the postnatal period [19], whereas other studies have identified increased SSD risk related to losses in the first trimester [18] or mid to late pregnancy [17]. Recent research from Israeli national registries has investigated the impact of genocide and terrorism on offspring risk for psychotic disorders. Offspring of mothers living in Holocaust-affected countries had significantly increased risk for schizophrenia [21, 22], and worsened course for the disorder [23] compared to offspring of mothers who had already immigrated to Israel prior to the Holocaust but likely still had ties to friends and family in affected countries. Offspring of mothers living in Holocaust-affected countries during their pregnancy and postnatally also had increased risk [22]. Greater risk for SSD in offspring was also observed in mothers who witnessed a terror attack in Israel within 270 days prior to birth [24].
Incidence of population-level traumatic life events provides a unique opportunity to examine the impact of PNMS on offspring; however, incidence of such extreme stressors is quite low and lacks generalizability to the experience of most pregnant women. Also, many of these studies fail to examine subjective response to these stressors, which may contribute to inconsistent findings. Furthermore, there are confounding environmental insults resulting from such adversities that can also confer mental health risk on offspring, such as malnutrition [25, 26] or exposure to environmental contaminants [27, 28]. Finally, there is evidence that maternal lifetime trauma, such as adverse childhood experiences, may impact the mother’s response to stress during pregnancy [29]. As such, examinations of the timing of stressors during the pregnancy may not be as impactful as examining the chronicity and severity of perceived stress.
The impact of less severe prenatal stressors on offspring outcomes of SSD has also been explored. Unwanted pregnancy can be considered a stressful life event and maternal unwantedness of pregnancy has been linked to increased risk of SSD among offspring [30, 31]. Another study found an association between unwantedness of pregnancy and risk of SSD, but the association failed to reach significance (p = .06), though the magnitude of the relationship was similar to other reports [32]. Paternal wantedness and concordance of wantedness between partners has not yet been explored with respect to offspring SSD outcomes and remains an area for future study.
Individual-Level Prenatal Stressors and SSD
A handful of studies have examined individual reports of PNMS with respect to offspring SSD outcomes using varying methodologies and both subjective (e.g., perceived stress) and objective (e.g., incidence of stressful life events) exposures. In one of the Prenatal Determinants of Schizophrenia (PDS) studies, various life stressors, including traumatic life events, daily life stress, and subjective reports of stress, including pregnancy-specific anxiety, were qualitatively coded from open-ended interviews completed in the second trimester [33••]. Daily life stress (e.g., chronic illness of a family member, financial stress) was associated with increased risk for SSD only in male offspring (OR = 2.091) [33••]. Alternatively, findings from the Helsinki Longitudinal Temperament Cohort observed no significant association between subjective PNMS and offspring psychosis [34•]. Mothers were asked “have you been mentally stressed?” and responded on a Likert scale every 2–3 weeks. The modal PNMS score was used as the predictor variable, perhaps washing out timing effects of PNMS. Additionally, among participants in the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort, there was only a weak association between PNMS, measured by a life events questionnaire, and childhood psychotic symptoms at age 12 [35]. The weak association may result from low base rates of childhood psychosis. Additionally, this study cites their life events questionnaire as a potential limitation. Specific events (e.g., suicide attempts, medical events) were included that also impact the fetus beyond the psychosocial stress imposed. Finally, a 2019 study of an Italian psychiatric sample did find a significant relationship between PNMS and SSD when assessing stress by tallying the incidence of stressful life events (e.g., job change, loss of loved one) [36]. Mothers of these participants (mean age = 39.5) retrospectively reported on PNMS during their pregnancies, which may impact the validity of these results [37]. The impact of fetal sex was not examined in either of these studies. Variability in assessment of stress [e.g., perceived stress (subjective), life events (objective)], timing of assessment (for both PNMS and SSD outcomes), lack of examination of important moderators (e.g., fetal sex), and type of outcome [e.g., SSD, schizophrenia, psychosis (affective or non-affective)] may account for inconsistency in findings.
PNMS and OCs
Additional support for the role of PNMS in the etiology of SSD comes from studies that link PNMS to a range of OCs previously associated with SSD. As highlighted below, PNMS has been associated with increases in prenatal inflammation and infection, fetal hypoxia, decreased fetal growth, and adverse health behaviors, which are all OCs that have been linked to SSD. Although there is a paucity of data examining PNMS in conjunction with these OCs in schizophrenia studies, we will delineate evidence that supports the plausible role of PNMS in increasing risk for a range of OCs associated with SSD.
Infection and Inflammation
Prenatal exposure to an array of infections, including influenza, has repeatedly been demonstrated to increase risk for SSD in offspring [38, 39]. Observational and experimental studies have revealed that a variety of stressors and perceived stress increase susceptibility to many infections and immune dysfunction in non-pregnant samples [40–42]. Similarly, in pregnant samples, PNMS has been linked to elevations in inflammatory markers [43, 44] and increased susceptibility to infection [45]. A rodent study identified that those exposed to PNMS had increased levels of proinflammatory cytokines in the placenta, which were then suppressed when the rodents were given an anti-inflammatory agent, but only in the males [46]. This study suggests inflammation in the placenta may contribute to sex differences observed in offspring following PNMS [47]. Moreover, we previously found that prenatal infection during the second trimester was associated with offspring depressive symptoms only if the mother also reported daily life stress [48]. These findings cumulatively support the possibility that PNMS may not only increase the likelihood of getting an infection, but also increase the vulnerability of the fetus to the damaging effects of the infection, subsequently increasing the possibility of neurodevelopmental sequelae.
Importantly, not all infections are able to pass through the placenta to reach the fetus, so other mechanisms have been examined as a means for transmission of risk for offspring SSD [10•, 49]. Proinflammatory cytokines are signaling proteins that become elevated in a variety of circumstances, including infectious threat [50] and stress [43, 44], that are indicators of the body’s inflammatory response; elevations in maternal inflammation have been associated with increased risk of SSD [51, 52, 53•]. Specifically, several studies have identified an association between elevations in certain cytokines and diagnostic outcomes of SSD in offspring. Elevated interleukin-8 in the second trimester [51], tumor necrosis factor-α at childbirth [54], and C-reactive protein in early pregnancy [55] were associated with schizophrenia outcomes in separate pregnancy cohorts. A recent study of mid-aged offspring from the Boston and Providence sites of the Collaborative Perinatal Cohort (CPP), also known as the New England Family Study (NEFS), demonstrated that elevated interleukin-6 early in the third trimester was associated with increased risk for SSD in male offspring [52]. Another study from the Collaborative Perinatal Project (NCPP) found elevated levels of anti-inflammatory cytokines at childbirth may be protective against SSD, but found no association between elevated proinflammatory cytokines and incidence of SSD [56]. These findings indicate that elevations in maternal cytokines during various points in the pregnancy may impact risk for SSD; however, findings are still inconsistent and must be carefully considered within the scope of the complicated inflammatory milieu of a pregnant woman.
Emerging evidence also shows that the gut microbiome may contribute to PNMS-immune associations. Specifically, one study prospectively showed that PNMS and elevations in maternal cortisol in the third trimester were related to alterations in the infant gut microbiome in days following childbirth [57]. Another study found that maternal early childhood stress is related to changes in the gut microbiome during pregnancy that were linked to elevations in cortisol and cytokines in response to a laboratory stressor, demonstrating the relationship between the maternal microbiome and stress response [29]. Hence, examination of the maternal microbiome with respect to both PNMS and maternal infection/inflammation may be another important piece in understanding risk for SSD following PNMS.
Hypoxia-Related OCs
Fetal hypoxia, or deprivation of oxygen to the fetus, is a by product of many maternal health conditions linked to incidence of SSD, such as preeclampsia [36, 58, 59]. It is possible that hypoxia may be a mechanism by which PNMS threatens fetal development. For example, incidence of preeclampsia has been associated with PNMS [60••, 61]. A relationship between PNMS and maternal biomarkers of stress (e.g., cortisol and corticotropin-releasing hormone) late in pregnancy with impaired placental blood flow has also been identified, although results are inconsistent [62–65]. Thus, hypoxia-related OCs may be related, in part, to PNMS, but the evidence is still unclear.
Fetal Growth
A variety of studies have found that decreased fetal growth and low birth weight (LBW) are associated with increased risk of SSD [9, 66]; however, findings have been somewhat mixed, potentially due to variability in the prevalence of prenatal exposures that affect fetal growth [67, 68]. In our previous study, we found that only infants who later developed schizophrenia and were exposed to OCs (fetal hypoxia and/or maternal influenza during pregnancy) exhibited decreased fetal growth, suggesting that liability associated with schizophrenia rendered fetuses more susceptible to OCs [67]. These findings are of particular relevance to the role PNMS in risk for offspring SSD, because multiple studies have demonstrated that elevated prenatal maternal cortisol, PNMS, and exposure to traumatic life events are associated with decreases in fetal growth, especially among male fetuses [69–71]. Moreover, a recent study from Ellman and colleagues [72] shows that elevated prenatal maternal cortisol levels do not directly predict SSD but, instead, predict decreases in fetal growth among infants who later develop SSD. This relationship was observed only in male offspring who developed SSD, consistent with prior findings [73]. Therefore, PNMS and its biological correlates (e.g., increases in cortisol) may be associated with intermediate phenotypes found in the course of SSD. These findings also suggest that infant sex and liability associated with schizophrenia could contribute to increased fetal vulnerability when exposed to OCs such as PNMS.
Maternal Health Behaviors
PNMS may also threaten maternal health by promoting engagement in adverse health behaviors, such as caffeine use, smoking, and disruptions in sleep, and decreasing engagement in positive behaviors, like vitamin use and exercise [74, 75]. Some of these health-impairing behaviors have been linked to OCs and other intermediate phenotypes of SSD. For example, smoking during pregnancy has been associated with LBW [76] as well as offspring diagnostic SSD outcomes [77, 78]. Similarly, sleep disruptions have been consistently linked with stress and increases in inflammation [79]. Furthermore, interpersonal stress and discordance in health behaviors between parents in the prenatal period have been demonstrated to impact prenatal maternal health behaviors [80, 81]. Therefore, an important area for future research is clarifying how health behaviors might moderate or mediate associations between PNMS and risk for offspring SSD.
Nutrition, Eating Behaviors, and BMI
Elevated pre-pregnancy BMI and obesity during early and late pregnancy have been associated with SSD in offspring [82–84], as well as OCs related to SSD such as gestational diabetes[85].Evidence from non-pregnant samples points to a relationship between stress and unhealthy eating behaviors (e.g., large quantities, high-fat) [86, 87] which may in turn increase BMI. Interestingly, a recent study did not find a significant difference in perceived stress or pregnancy-specific stress between obese and non-obese pregnant women [88]; however, obesity has been linked to low-grade inflammation [85, 89, 90] and, therefore, remains an important variable to consider in SSD studies. Furthermore, prenatal folate and vitamin D deficiencies have been linked to increased risk for SSD [91]; however, omega-3 fatty acids have been demonstrated to be protective against SSD, as well as the adverse effects of PNMS on the fetus in human studies, perhaps by acting as anti-inflammatory agent [29, 92]. Despite these findings, no study has examined the relationships between PNMS, nutrition, eating behaviors, and/or BMI in the course of SSD, which may be a fruitful area of research for future studies.
PNMS and Premorbid Outcomes
Motor, cognitive, and behavioral deficits common in the premorbid period of SSD also have been associated with PNMS. The premorbid period of SSD is marked by motor delays, such as delays in sitting, standing, and walking, as well as issues with coordination [93•, 94]; evidence suggests that motor deficits as early as infancy may increase risk for SSD [95]. PNMS in mid to late pregnancy, in the forms of objective hardship and negative cognitive appraisals, have been linked to poor and delayed gross motor development in infancy [96–100]. Findings from the Queensland Flood Studies (QFS) identified post-traumatic stress as a mediator between PNMS and motor dysfunction in offspring [97]. Further follow-up of the offspring in the QFS demonstrated that, by age 2.5–4 years, affected offspring were able to catch up in development [99]. Deficits to motor development in later childhood and adolescence have also been observed in children of prenatally stressed mothers [101], which is consistent with findings that motor problems reemerge during the prodrome of psychosis [102]. A recent study identified adverse effects of parental postnatal stress on motor development, while PNMS improved motor development; however, this study specifically examined women using antidepressants in the prenatal period, which may point to a protective role of antidepressants against adverse effects of PNMS [103].
PNMS has also been linked to several other deficits common in the premorbid period of SSD. Cognitive difficulties including impaired language abilities [104], lower IQ [104–106], difficulties with regulation and shifting of attention [107, 108], and executive functioning difficulties [109] have been linked to PNMS. Various studies have examined the link between PNMS and difficult or irritable offspring temperament, with mixed results. Subjective distress has been related to difficult temperament, and neutral and positive cognitive appraisals of stressful events have been linked to irritable temperament [110, 111]. Fetal sex and timing of stress were identified as moderators of this relationship in one study, with male offspring more likely to experience irritable temperament following objective hardship and stress in early pregnancy related to arrhythmic temperament [110]. One study examining mother-offspring dyads of Hurricane Katrina did not find such an association but identified a relationship between poor maternal health (e.g., PTSD, depression) and difficult temperament [112]. These studies are consistent with findings that shyness, behavioral difficulties, and isolated play occur in the premorbid period of SSD [92]. PNMS, as well as lifetime trauma exposure, have also been linked to difficulties with emotion regulation and negative affectivity in offspring [113, 114]. Finally, PNMS has been associated with alterations in brain structure in human and preclinical studies, such as reductions in gray matter and hippocampal and dopaminergic abnormalities that are typical of those with SSD [115, 116].
Fetal Sex as a Potential Moderator
Fetal sex and timing of stress have emerged as potential moderators of the relationship between PNMS and offspring SSD [117]. Rodent and human models of prenatal stress indicate increased risk for male offspring, potentially mediated by the sex-regulated placenta [47, 118, 119]. Several of the referenced studies have specifically identified increased SSD risk for male offspring in response to various types of PNMS [13, 18, 33••].
Evidence for a viability-vulnerability tradeoff has been accumulating, such that prenatal insults are associated with morbidity, mortality, and more severe neurodevelopmental sequelae (including measures of growth and overall cognition), in males, but with more enduring behavioral or temperamental deficits (consistent with anxious/depressive phenotypes) in females [120••]. Although there are exceptions in which PNMS has been linked to increased risk of SSD in females, in those studies, there was greater mortality of male offspring, complicating findings and corroborating the aforementioned hypotheses [121]. There is also evidence of sex-specific risk for particular birth outcomes (i.e., shortened gestational age, preterm birth) that threaten morbidity and mortality of the fetus following PNMS that may also, in turn, increase risk for SSD [122, 123].
Timing of Exposure as a Potential Moderator
Findings examining the role of timing of PNMS in risk for offspring SSD are more mixed. First trimester PNMS, more often than later-term PNMS, has been linked to increased risk for SSD [13, 18, 33••]; however, depending on the type of stressor, PNMS in early and late [18] gestation have both been associated with increased risk for offspring. There is also evidence that PNMS in mid-pregnancy is associated with OCs that have been linked with SSD, including LBW and decreased fetal growth [124]. Nonetheless, a number of studies were limited by retrospective reporting of stressors [36], which complicates the examination of timing as a potential moderator.
The period directly prior to conception is an under-investigated period of risk, although initial findings indicate marginal and non-significant elevations in risk for offspring SSD associated with preconception stressors [20]. The preconception period may instead be of interest with respect to paternal stress. Evidence from rodent models indicates that preconception paternal stress may impact DNA methylation or miRNA coding so as to alter genetic material in sperm, which then may alter HPA-axis functioning in offspring in ways that confer risk for neuropsychiatric outcomes [125–127].
Conclusions
In sum, PNMS can be considered a “disease primer,” potentially programming subtle alterations in fetal development that make offspring vulnerable to additional risk or “second hits” in the postnatal period (Fig. 1) [128]. Support for this possibility comes from a study using the Danish register, which found that male offspring had a significantly increased risk of developing SSD after exposure to both prenatal infection and early childhood trauma [129]; this finding is consistent with preclinical literature [130] and highlights the plausibility of OCs interacting with other pre- and postnatal risk factors in the neurodevelopmental course of SSD. In studies considering the impact of PNMS in the course to neuropsychiatric disorders in offspring, it may be important (albeit challenging) to acquire data about the full scope of pre- and postnatal adversities to construct a developmental pathway of accumulating risk [33••, 131].
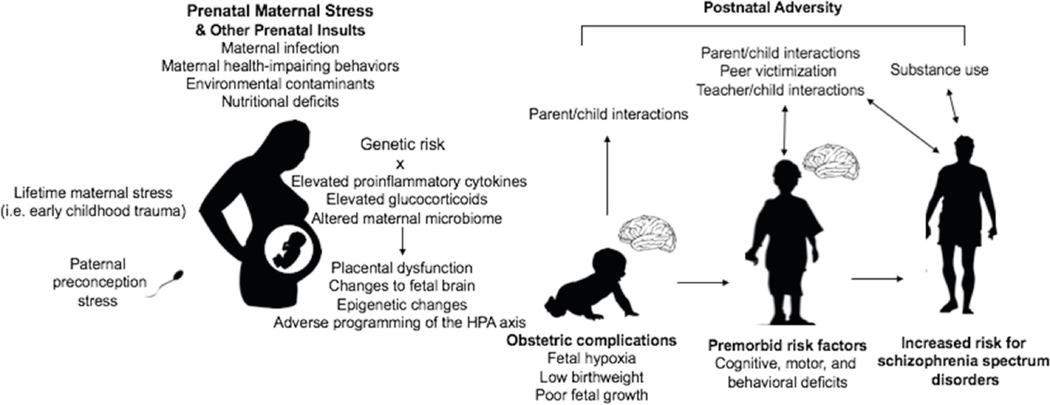
Fig. 1.
Potential role of prenatal maternal stress and other developmental risk factors in the etiology of schizophrenia spectrum disorders. This figure highlights that PNMS likely adds to and interacts with other pre- and postnatal risk factors in increasing risk for SSD [10, 11••, 92, 142]. For example, offspring affected by OCs are more likely to be difficult infants, impacting maternal attachment and parental interaction [60••]. The relationship between premorbid risk factors and other postnatal adversities such as adverse peer, parental, and teacher interactions are more likely to be reciprocal, such that premorbid deficits may both be the precedent and consequence of adverse interactions [134]. As risk likely accumulates across the lifespan in a transactional manner, an understanding of these developmental risk factors may provide insight into points for prevention and intervention efforts [92]
What has not been considered is the possibility that subtle changes following PNMS could lead to a cascade of developmental difficulties triggered by PNMS [11••]. Specifically, changes in motor functioning, cognition, language acquisition, and temperament could influence ways offspring interact with the environment and vice-versa. For example, parental, peer, and teacher interactions may differ for a child with learning difficulties [132–134]. Both peer victimization and cognitive difficulties in the premorbid period have been linked to adolescent and adult psychosis outcomes, and there is evidence that peer victimization occurs more often among children with psychological and cognitive difficulties [135, 136]. There is also evidence of a reciprocal relationship between bullying and cognitive deficits such that cognitive difficulties predispose victimization, which also is related to subsequent cognitive deficits [137]. The transactional nature of the organism and environment is critical to developmental outcomes; therefore, even small effects of PNMS on offspring may alter interplays that occur throughout development [11••]. Although exposure to PNMS is often unavoidable, childhood transactional experiences can be modified and serve as targets for early intervention [138]. For example, positive parental engagement has been demonstrated to counter effects of prenatal insults [139]. As such, the confluence of various pre- and postnatal environmental factors impacts risk for SSD across the developmental lifespan. Understanding that PNMS is a small piece of a complex puzzle of risk may also serve as comforting to pregnant mothers.
It is also important to note that the effect sizes in almost all studies of PNMS are small; therefore, the risk to an individual woman and her offspring is fairly small [11••]. For disorders such as schizophrenia that occur at a low base rate, the odds ratio of the association between PNMS and schizophrenia can be multiplied by the base rate of schizophrenia in the population to obtain a general sense of the risk of developing schizophrenia for a specific child whose mother experienced PNMS during their gestation (e.g., odds ratio of about 2.0 multiplied by base rate of 1% = 2% chance of offspring SSD) [140]. As such, even in the studies with the largest odds ratios, the risk to an individual child is still small following PNMS. Hence, it is critical for further studies to clarify how PNMS interacts with other pre- and postnatal contributors. This will both aid in pinpointing the offspring at the greatest risk for SSD and help in developing methods for early intervention.
Initial support for using such a model comes from studies that suggest an interaction of PNMS and maternal inflammation with existing genetic risk increases risk for the disorder [141]. Genes may also interact with other risk factors for SSD, such as OCs, to increase offspring risk [142]. As such, studies exclusively including families with known risk for psychosis should be considered separately due to the interaction of genetic risk.
Furthermore, PNMS has been demonstrated to confer risk for various psychopathological outcomes in offspring [34•, 117]; however, the current literature does not sufficiently elucidate the specificity of PNMS to SSD. This predicament is mirrored in genetics research, which also struggles to differentiate which genetic components may be predictive of specific or varied psychopathology [143]. The likelihood that PNMS interacts with several risk factors for SSD raises questions of equifinality and multifinality in the relationship between PNMS and SSD-specific risk [144•]. Future studies may seek to clarify this relationship, and perhaps investigate whether particular aspects of PNMS (type, timing, fetal sex, parental genetic risk, and polygenic risk scores) may specifically confer risk for SSD.
In summary, evidence shows that PNMS increases risk for SSD as well as related OCs and premorbid outcomes. In addition, there is some evidence that males are more vulnerable to the effects of PNMS in the course of SSD. Nonetheless, a variety of factors closely related to PNMS (e.g., sleep, inflammation, diet, postnatal factors) have yet to be studied in PNMS-SSD investigations. Delineating potential interacting variables with PNMS is essential for developing targeted early intervention strategies and is an important step for understanding the PNMS-SSD association.
Acknowledgments
We would like to thank Seth Maxwell for his helpful edits.
Funding Information This review was supported by the National Institute of Mental Health Grants MH096478 and MH118545 awarded to Lauren Ellman.
Footnotes
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Conflict of Interest The authors declare that they have no conflicts of interest.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Seidman LJ, Mirsky AF. Evolving notions of schizophrenia as a developmental neurocognitive disorder. J Int Neuropsychol Soc. 2017;23:881–92. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-V). 5th ed Washington: American Psychological Association; 2013. [Google Scholar]
- 3.Nuechterlein KH, Dawson ME. A heuristic vulnerability/stress model of schizophrenic episodes. Schizophr Bull. 1984;10: 300–12. [DOI] [PubMed] [Google Scholar]
- 4.Aleman A, Kahn RS, Selten J-P. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 2003;60:565–71. [DOI] [PubMed] [Google Scholar]
- 5.McGrath JJ, Susser ES. New directions in the epidemiology of schizophrenia. Med J Aust. 2009;190:S7–9. [DOI] [PubMed] [Google Scholar]
- 6.Cannon TD, Kaprio J, Lönnqvist J, Huttunen M, Koskenvuo M. The genetic epidemiology of schizophrenia in a Finnish twin cohort. A population-based modeling study. Arch Gen Psychiatry. 1998;55:67–74. [DOI] [PubMed] [Google Scholar]
- 7.• Iampietro MC, Ellman LM. Maternal stress during pregnancy and schizophrenia In: Brown AS, Patterson PH, editors. The origins of schizophrenia. New York: Columbia University Press; 2012. p. 120–39.Comprehensive book chapter examining the specific relationship between PNMS and risk for SSD with research through about 2012.
- 8.Mittal VA, Ellman LM, Cannon TD. Gene-environment interaction and covariation in schizophrenia: the role of obstetric complications. Schizophr Bull. 2008;34:1083–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. AJP. 2002;159:1080–92. [DOI] [PubMed] [Google Scholar]
- 10.• Fineberg AM, Ellman LM. Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biol Psychiatry. 2013;73:951–66.This article comprehensively reviews human research focused on the role of inflammation in the developmental life course for schizophrenia, from the prenatal period to fully-formed psychosis.
- 11.•• Ellman LM, Murphy SK, Maxwell SD. Pre- and perinatal risk factors for serious mental disorders: ethical considerations in prevention and prediction efforts. J Ethics Mental Health. 2018;10:1–14.This paper discusses ethical consideration in research examining obstetric complications and mental health outcomes, including discussion of how a cascade model framework could better inform prevention, prediction and intervention efforts for offspring psychopathology.
- 12.Malaspina D, Corcoran C, Kleinhaus KR, Perrin MC, Fennig S, Nahon D, et al. Acute maternal stress in pregnancy and schizophrenia in offspring: a cohort prospective study. BMC Psychiatry. 2008;8:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Os J, Selten J-P. Prenatal exposure to maternal stress and subsequent schizophrenia. Br J Psychiatry. 1998;172:324–6. [DOI] [PubMed] [Google Scholar]
- 14.Roseboom TJ, Painter RC, van Abeelen AFM, Veenendaal MVE, de Rooij SR. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas. 2011;70:141–5. [DOI] [PubMed] [Google Scholar]
- 15.Song S, Wang W, Hu P. Famine, death, and madness: schizophrenia in early adulthood after prenatal exposure to the Chinese Great Leap Forward Famine. Soc Sci Med. 2009;68:1315–21. [DOI] [PubMed] [Google Scholar]
- 16.Selten JP, van der Graaf Y, van Duursen R, Gispen-de Wied CC, Kahn RS. Psychotic illness after prenatal exposure to the 1953 Dutch Flood Disaster. Schizophr Res. 1999;35:243–5. [DOI] [PubMed] [Google Scholar]
- 17.Huttunen MO, Niskanen P. Prenatal loss of father and psychiatric disorders. Arch Gen Psychiatry. 1978;35:429–31. [DOI] [PubMed] [Google Scholar]
- 18.Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, et al. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry. 2008;65:146–52. [DOI] [PubMed] [Google Scholar]
- 19.Abel KM, Heuvelman HP, Jörgensen L, Magnusson C, Wicks S, Susser E, et al. Severe bereavement stress during the prenatal and childhood periods and risk of psychosis in later life: population based cohort study. BMJ. 2014;348:f7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Class QA, Abel KM, Khashan AS, Rickert ME, Dalman C, Larsson H, et al. Offspring psychopathology following preconception, prenatal and postnatal maternal bereavement stress. Psychol Med. 2014;44:71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine SZ, Levav I, Goldberg Y, Pugachova I, Becher Y, Yoffe R. Exposure to genocide and the risk of schizophrenia: a population based study. Psychol Med. 2016;46:855–63. [DOI] [PubMed] [Google Scholar]
- 22.Levine SZ, Levav I, Yoffe R, Pugachova I. The effects of prenatal-, early-life- and indirectly-initiated exposures to maximum adversities on the course of schizophrenia. Schizophr Res. 2014;158:236–40. [DOI] [PubMed] [Google Scholar]
- 23.Levine SZ, Levav I, Pugachova I, Yoffe R, Becher Y. Transgenerational effects of genocide exposure on the risk and course of schizophrenia: a population-based study. Schizophr Res. 2016;176:540–5. [DOI] [PubMed] [Google Scholar]
- 24.Weinstein Y, Levav I, Gelkopf M, Roe D, Yoffe R, Pugachova I, et al. Association of maternal exposure to terror attacks during pregnancy and the risk of schizophrenia in the offspring: a population-based study. Schizophr Res. 2018;199:163–7. [DOI] [PubMed] [Google Scholar]
- 25.Susser E, Neugebauer R, Hoek HW, Brown AS, Lin S, Labovitz D, et al. Schizophrenia after prenatal famine: further evidence. Arch Gen Psychiatry. 1996;53:25–31. [DOI] [PubMed] [Google Scholar]
- 26.Xu M-Q, Sun W-S, Liu B-X, Feng G-Y, Yu L, Yang L, et al. Prenatal malnutrition and adult schizophrenia: further evidence from the 1959–1961 Chinese famine. Schizophr Bull. 2009;35: 568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sioen I, Den Hond E, Nelen V, Van de Mieroop E, Croes K, Van Larebeke N, et al. Prenatal exposure to environmental contaminants and behavioural problems at age 7–8years. Environ Int. 2013;59:225–31. [DOI] [PubMed] [Google Scholar]
- 28.Attademo L, Bernardini F, Garinella R, Compton MT. Environmental pollution and risk of psychotic disorders: a review of the science to date. Schizophr Res. 2017;181:55–9. [DOI] [PubMed] [Google Scholar]
- 29.Hantsoo L, Jašarević E, Criniti S, McGeehan B, Tanes C, Sammel MD, et al. Childhood adversity impact on gut microbiota and inflammatory response to stress during pregnancy. Brain Behav Immun. 2019;75:240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myhrman A, Rantakallio P, Isohanni M, Jones P, Partanen U. Unwantedness of a pregnancy and schizophrenia in the child. Br J Psychiatry. 1996;169:637–40. [DOI] [PubMed] [Google Scholar]
- 31.McNeil TF, Schubert EW, Cantor-Graae E, Brossner M, Schubert P, Henriksson KM. Unwanted pregnancy as a risk factor for offspring schizophrenia-spectrum and affective disorders in adulthood: a prospective high-risk study. Psychol Med. 2009;39:957–65. [DOI] [PubMed] [Google Scholar]
- 32.Herman DB, Brown AS, Opler MG, Desai M, Malaspina D, Bresnahan M, et al. Does unwantedness of pregnancy predict schizophrenia in the offspring? Findings from a prospective birth cohort study. Soc Psychiatry Psychiatr Epidemiol. 2006;41:605–10. [DOI] [PubMed] [Google Scholar]
- 33.•• Fineberg AM, Ellman LM, Schaefer CA, Maxwell SD, Shen L, Chaudhury HN, et al. Fetal exposure to maternal stress and risk for schizophrenia spectrum disorders among offspring: differential influences of fetal sex. Psychiatry Res. 2016;236:91–7.This study provides evidence from a prospective birth cohort study that subjective stress is associated with schizophrenia risk in offspring in a sex-dependent manner.
- 34.• Brannigan R, Cannon M, Tanskanen A, Huttunen MO, Leacy FP, Clarke MC. The association between subjective maternal stress during pregnancy and offspring clinically diagnosed psychiatric disorders. Acta Psychiatr Scand. 2019;139:304–10.One of the most recent studies examining the link between prospectively collected stress measures and risk for psychiatric disorders in offspring with a large sample from a national registry.
- 35.Dorrington S, Zammit S, Asher L, Evans J, Heron J, Lewis G. Perinatal maternal life events and psychotic experiences in children at twelve years in a birth cohort study. Schizophr Res. 2014;152:158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pugliese V, Bruni A, Carbone EA, Calabrò G, Cerminara G, Sampogna G, et al. Maternal stress, prenatal medical illnesses and obstetric complications: risk factors for schizophrenia spectrum disorder, bipolar disorder and major depressive disorder. Psychiatry Res. 2019;271:23–30. [DOI] [PubMed] [Google Scholar]
- 37.Wadhwa PD, Entringer S, Buss C, Lu MC. The contribution of maternal stress to preterm birth: issues and considerations. Clin Perinatol. 2011;38:351–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown AS. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev Neurobiol. 2012;72:1272–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khandaker GM, Zimbron J, Lewis G, Jones PB. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med. 2013;43: 239–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marsland AL, Bachen EA, Cohen S, Rabin B, Manuck SB. Stress, immune reactivity and susceptibility to infectious disease. Physiol Behav. 2002;77:711–6. [DOI] [PubMed] [Google Scholar]
- 42.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–51. [DOI] [PubMed] [Google Scholar]
- 43.Coussons-Read ME, Okun ML, Schmitt MP, Giese S. Prenatal stress alters cytokine levels in a manner that may endanger human pregnancy: psychosomatic medicine 2005;67:625–631. [DOI] [PubMed] [Google Scholar]
- 44.Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun. 2007;21:343–50. [DOI] [PubMed] [Google Scholar]
- 45.Culhane JF, Rauh V, McCollum KF, Hogan VK, Agnew K, Wadhwa PD. Maternal stress is associated with bacterial vaginosis in human pregnancy. Matern Child Health J. 2001;5:127–34. [DOI] [PubMed] [Google Scholar]
- 46.Bronson SL, Bale TL. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology. 2014;155:2635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bronson SL, Bale TL. The placenta as a mediator of stress effects on neurodevelopmental reprogramming. Neuropsychopharmacology. 2016;41:207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy SK, Fineberg AM, Maxwell SD, Alloy LB, Zimmermann L, Krigbaum NY, et al. Maternal infection and stress during pregnancy and depressive symptoms in adolescent offspring. Psychiatry Res. 2017;257:102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. N Engl J Med. 2014;370:2211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. [DOI] [PubMed] [Google Scholar]
- 51.Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, et al. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry. 2004;161:889–95. [DOI] [PubMed] [Google Scholar]
- 52.Goldstein JM, Cherkerzian S, Seidman LJ, Donatelli J-a L, Remington AG, Tsuang MT, et al. Prenatal maternal immune disruption and sex-dependent risk for psychoses. Psychol Med. 2014;44:3249–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.• Hantsoo L, Kornfield S, Anguera MC, Epperson CN. Inflammation: a proposed intermediary between maternal stress and offspring neuropsychiatric Risk. Biol Psychiatry. 2019;85: 97–106.This article proposes that poor coordination between inflammatory markers and glucocorticoids may mediate risk between prenatal maternal stress and offspring neuropsychiatric disorders, including schizophrenia. It also explores how this mechanism may vary across different types of maternal stressors.
- 54.Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Wagner RL, Yolken RH. Maternal cytokine levels during pregnancy and adult psychosis. Brain Behav Immun. 2001;15:411–20. [DOI] [PubMed] [Google Scholar]
- 55.Canetta S, Sourander A, Surcel H-M, Hinkka-Yli-Salomäki S, Leiviskä J, Kellendonk C, et al. Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Am J Psychiatry. 2014;171:960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allswede DM, Buka SL, Yolken RH, Torrey EF, Cannon TD. Elevated maternal cytokine levels at birth and risk for psychosis in adult offspring. Schizophr Res. 2016;172:41–5. [DOI] [PubMed] [Google Scholar]
- 57.Zijlmans MAC, Korpela K, Riksen-Walraven JM, de Vos WM, de Weerth C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology. 2015;53:233–45. [DOI] [PubMed] [Google Scholar]
- 58.Sharma S, Norris WE, Kalkunte S. Beyond the threshold: an etiological bridge between hypoxia and immunity in preeclampsia. J Reprod Immunol. 2010;85:112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zornberg GL, Buka SL, Tsuang MT. Hypoxic-ischemia-related fetal/neonatal complications and risk of schizophrenia and other nonaffective psychoses: a 19-year longitudinal study. Am J Psychiatry. 2000;157:196–202. [DOI] [PubMed] [Google Scholar]
- 60.•• Coussons-Read ME. Effects of prenatal stress on pregnancy and human development: mechanisms and pathways. Obstet Med. 2013;6:52–7.This article describes inflammatory and endocrine pathways to obstetric complications and dysregulated offspring development in response to stress during pregnancy.
- 61.Schneider S, Freerksen N, Maul H, Roehrig S, Fischer B, Hoeft B. Risk groups and maternal-neonatal complications of preeclampsia—current results from the national German perinatal quality registry. J Perinat Med. 2011;39:257–65. [DOI] [PubMed] [Google Scholar]
- 62.Harville EW, Savitz DA, Dole N, Herring AH, Thorp JM, Light KC. Stress and placental resistance measured by Doppler ultrasound in early and mid-pregnancy. Ultrasound Obstet Gynecol. 2008;32:23–30. [DOI] [PubMed] [Google Scholar]
- 63.Helbig A, Kaasen A, Malt UF, Haugen G. Does antenatal maternal psychological distress affect placental circulation in the third trimester? PLoS One. 2013;8:e57071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Helbig A, Kaasen A, Malt UF, Haugen G. Maternal psychological distress and placental circulation in pregnancies after a previous offspring with congenital malformation. PLoS One. 2014;9: e86597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roos A, Geerts L, Koen N, Faure SC, Vythilingum B, Stein DJ. Psychosocial predictors of fetoplacental blood flow during pregnancy. Compr Psychiatry. 2015;57:125–31. [DOI] [PubMed] [Google Scholar]
- 66.Knud Larsen J, Bendsen BB, Foldager L, Munk-Jørgensen P. Prematurity and low birth weight as risk factors for the development of affective disorder, especially depression and schizophrenia: a register study. Acta Neuropsychiatr. 2010;22:284–91. [DOI] [PubMed] [Google Scholar]
- 67.Fineberg AM, Ellman LM, Buka S, Yolken R, Cannon TD. Decreased birth weight in psychosis: influence of prenatal exposure to serologically determined influenza and hypoxia. Schizophr Bull. 2013;39:1037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abel KM, Wicks S, Susser ES, Dalman C, Pedersen MG, Mortensen PB, et al. Birth weight, schizophrenia, and adult mental disorder: is risk confined to the smallest babies? Arch Gen Psychiatry. 2010;67:923–30. [DOI] [PubMed] [Google Scholar]
- 69.Cherak SJ, Giesbrecht GF, Metcalfe A, Ronksley PE, Malebranche ME. The effect of gestational period on the association between maternal prenatal salivary cortisol and birth weight: a systematic review and meta-analysis. Psychoneuroendocrinology. 2018;94: 49–62. [DOI] [PubMed] [Google Scholar]
- 70.Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, Garite TJ. The association between prenatal stress and infant birth weight and gestational age at birth: a prospective investigation. Am J Obstet Gynecol. 1993;169:858–65. [DOI] [PubMed] [Google Scholar]
- 71.Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30:724–43. [DOI] [PubMed] [Google Scholar]
- 72.Ellman LM, Murphy SK, Maxwell SD, Calvo EM, Cooper T, Schaefer CA, Bresnahan MA, Susser ES, Brown AS. Maternal cortisol during pregnancy and offspring schizophrenia: Influence of fetal sex and timing of exposure. Schizophr Res. 2019. 10.1016/j.schres.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thayer ZM, Feranil AB, Kuzawa CW. Maternal cortisol disproportionately impacts fetal growth in male offspring: evidence from the Philippines. Am J Hum Biol. 2012;24:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, Meyer BA. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol. 2008;27:604–15. [DOI] [PubMed] [Google Scholar]
- 75.Auerbach MV, Lobel M, Cannella DT. Psychosocial correlates of health-promoting and health-impairing behaviors in pregnancy. J Psychosom Obstet Gynaecol. 2014;35:76–83. [DOI] [PubMed] [Google Scholar]
- 76.Pereira PP d S, Da Mata FAF, Figueiredo ACG, de Andrade KRC, Pereira MG. Maternal active smoking during pregnancy and low birth weight in the Americas: a systematic review and meta-analysis. Nicotine Tob Res. 2017;19:497–505. [DOI] [PubMed] [Google Scholar]
- 77.Niemelä S, Sourander A, Surcel H-M, Hinkka-Yli-Salomäki S, McKeague IW, Cheslack-Postava K, et al. Prenatal nicotine exposure and risk of schizophrenia among offspring in a national birth cohort. Am J Psychiatry. 2016;173:799–806. [DOI] [PubMed] [Google Scholar]
- 78.Hunter A, Murray R, Asher L, Leonardi-Bee J. The effects of tobacco smoking, and prenatal tobacco smoke exposure, on risk of schizophrenia: a systematic review and meta-analysis. Nicotine Tob Res. 2018. [DOI] [PubMed] [Google Scholar]
- 79.Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015;66:143–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bloch JR, Webb DA, Mathews L, Dennis EF, Bennett IM, Culhane JF. Beyond marital status: the quality of the mother-father relationship and its influence on reproductive health behaviors and outcomes among unmarried low income pregnant women. Matern Child Health J. 2010;14:726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cornelius T, Desrosiers A, Kershaw T. Smoking concordance during pregnancy: are there relationship benefits? Soc Sci Med. 2017;192:30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khandaker GM, Dibben CRM, Jones PB. Does maternal body mass index during pregnancy influence risk of schizophrenia in the adult offspring? Obes Rev. 2012;13:518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schaefer CA, Brown AS, Wyatt RJ, Kline J, Begg MD, Bresnahan MA, et al. Maternal prepregnant body mass and risk of schizophrenia in adult offspring. Schizophr Bull. 2000;26:275–86. [DOI] [PubMed] [Google Scholar]
- 84.Simoila L, Isometsä E, Gissler M, Suvisaari J, Halmesmäki E, Lindberg N. Schizophrenia and pregnancy: a national register based follow-up study among Finnish women born between 1965 and 1980. Arch Womens Ment Health. :2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017;356:j1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Errisuriz VL, Pasch KE, Perry CL. Perceived stress and dietary choices: the moderating role of stress management. Eat Behav. 2016;22:211–6. [DOI] [PubMed] [Google Scholar]
- 87.Martins LB, Monteze NM, Calarge C, Ferreira AVM, Teixeira AL. Pathways linking obesity to neuropsychiatric disorders. Nutrition. 2019;66:16–21. [DOI] [PubMed] [Google Scholar]
- 88.Ruhstaller KE, Elovitz MA, Stringer M, Epperson CN, Durnwald CP. Obesity and the association with maternal mental health symptoms. J Matern Fetal Neonatal Med. 2017;30:1897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Segovia SA, Vickers MH, Reynolds CM. The impact of maternal obesity on inflammatory processes and consequences for later offspring health outcomes. J Dev Orig Health Dis. 2017;8:529–40. [DOI] [PubMed] [Google Scholar]
- 90.Depino AM. Perinatal inflammation and adult psychopathology: from preclinical models to humans. Semin Cell Dev Biol. 2018;77:104–14. [DOI] [PubMed] [Google Scholar]
- 91.Brown AS, Susser ES. Prenatal nutritional deficiency and risk of adult schizophrenia. Schizophr Bull. 2008;34:1054–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lindsay KL, Buss C, Wadhwa PD, Entringer S. The interplay between maternal nutrition and stress during pregnancy: issues and considerations. Ann Nutr Metab. 2017;70:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.• Liu CH, Keshavan MS, Tronick E, Seidman LJ. Perinatal risks and childhood premorbid indicators of later psychosis: next steps for early psychosocial interventions. Schizophr Bull. 2015;41: 801–16.This article implements developmental framework to understand the impact of prenatal stress on premorbid deficits in those in high-risk families for psychosis. It proposes areas of intervention that could be implemented prior to the clinical high-risk phase of psychosis risk.
- 94.Burton BK, Hjorthøj C, Jepsen JR, Thorup A, Nordentoft M, Plessen KJ. Research review: do motor deficits during development represent an endophenotype for schizophrenia? A meta-analysis. J Child Psychol Psychiatry. 2016;57:446–56. [DOI] [PubMed] [Google Scholar]
- 95.Clarke MC, Tanskanen A, Huttunen M, Leon DA, Murray RM, Jones PB, et al. Increased risk of schizophrenia from additive interaction between infant motor developmental delay and obstetric complications: evidence from a population-based longitudinal study. Am J Psychiatry. 2011;168:1295–302. [DOI] [PubMed] [Google Scholar]
- 96.Cao X, Laplante DP, Brunet A, Ciampi A, King S. Prenatal maternal stress affects motor function in 5½-year-old children: project ice storm. Dev Psychobiol. 2014;56:117–25. [DOI] [PubMed] [Google Scholar]
- 97.Moss KM, Simcock G, Cobham V, Kildea S, Elgbeili G, Laplante DP, et al. A potential psychological mechanism linking disaster-related prenatal maternal stress with child cognitive and motor development at 16 months: the QF2011 Queensland Flood Study. Dev Psychol. 2017;53:629–41. [DOI] [PubMed] [Google Scholar]
- 98.Simcock G, Kildea S, Elgbeili G, Laplante DP, Stapleton H, Cobham V, et al. Age-related changes in the effects of stress in pregnancy on infant motor development by maternal report: the Queensland Flood Study. Dev Psychobiol. 2016;58:640–59. [DOI] [PubMed] [Google Scholar]
- 99.Simcock G, Laplante DP, Elgbeili G, Kildea S, King S. A trajectory analysis of childhood motor development following stress in pregnancy: the QF2011 flood study. Dev Psychobiol. 2018;60: 836–48. [DOI] [PubMed] [Google Scholar]
- 100.Buitelaar JK, Huizink AC, Mulder EJ, de Medina PGR, Visser GHA. Prenatal stress and cognitive development and temperament in infants. Neurobiol Aging. 2003;24(Suppl 1):S53–60 discussion S67–68. [DOI] [PubMed] [Google Scholar]
- 101.Grace T, Bulsara M, Robinson M, Hands B. The impact of maternal gestational stress on motor development in late childhood and adolescence: a longitudinal study. Child Dev. 2016;87:211–20. [DOI] [PubMed] [Google Scholar]
- 102.Walther S, Mittal VA. Motor system pathology in psychosis. Curr Psychiatry Rep. 2017;19:97. [DOI] [PubMed] [Google Scholar]
- 103.Karam F, Sheehy O, Huneau M-C, Chambers C, Fraser WD, Johnson D, et al. Impact of maternal prenatal and parental postnatal stress on 1-year-old child development: results from the OTIS antidepressants in pregnancy study. Arch Womens Ment Health. 2016;19:835–43. [DOI] [PubMed] [Google Scholar]
- 104.Laplante DP, Barr RG, Brunet A, Galbaud du Fort G, Meaney ML, Saucier J-F, et al. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatr Res. 2004;56:400–10. [DOI] [PubMed] [Google Scholar]
- 105.Laplante DP, Brunet A, Schmitz N, Ciampi A, King S. Project ice storm: prenatal maternal stress affects cognitive and linguistic functioning in 5 1/2-year-old children. J Am Acad Child Adolesc Psychiatry. 2008;47:1063–72. [DOI] [PubMed] [Google Scholar]
- 106.Virk J, Obel C,Li J, Olsen J.In-utero exposure to bereavement and offspring IQ: a Danish national cohort study. PLoS One. 2014;9: e88477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huizink AC, de Medina PGR, Mulder EJH, Visser GHA, Buitelaar JK. Psychological measures of prenatal stress as predictors of infant temperament. J Am Acad Child Adolesc Psychiatry. 2002;41:1078–85. [DOI] [PubMed] [Google Scholar]
- 108.Plamondon A, Akbari E, Atkinson L, Steiner M, Meaney MJ, Fleming AS, et al. Spatial working memory and attention skills are predicted by maternal stress during pregnancy. Early Hum Dev. 2015;91:23–9. [DOI] [PubMed] [Google Scholar]
- 109.Buss C, Davis EP, Hobel CJ, Sandman CA. Maternal pregnancy specific anxiety is associated with child executive function at 6–9 years age. Stress. 2011;14:665–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Simcock G, Elgbeili G, Laplante DP, Kildea S, Cobham V, Stapleton H, et al. The effects of prenatal maternal stress on early temperament: the 2011 Queensland Flood Study. J Dev Behav Pediatr. 2017;38:310–21. [DOI] [PubMed] [Google Scholar]
- 111.Laplante DP, Brunet A, King S. The effects of maternal stress and illness during pregnancy on infant temperament: Project Ice Storm. Pediatr Res. 2016;79:107–13. [DOI] [PubMed] [Google Scholar]
- 112.Tees MT, Harville EW, Xiong X, Buekens P, Pridjian G, Elkind-Hirsch K. Hurricane Katrina-related maternal stress, maternal mental health, and early infant temperament. Matern Child Health J. 2010;14:511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Enlow MB, Devick KL, Brunst KJ, Lipton LR, Coull BA, Wright RJ. Maternal lifetime trauma exposure, prenatal cortisol, and infant negative affectivity. Infancy. 2017;22:492–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Buthmann J, Ham J, Davey K, Finik J, Dana K, Pehme P, et al. Infant temperament: repercussions of Superstorm Sandy-related maternal stress. Child Psychiatry Hum Dev. 2019;50:150–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Buss C, Davis EP, Muftuler LT, Head K, Sandman CA. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinology. 2010;35:141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Straley ME, Van Oeffelen W, Theze S, Sullivan AM, O’Mahony SM, Cryan JF, et al. Distinct alterations in motor & reward seeking behavior are dependent on the gestational age of exposure to LPS-induced maternal immune activation. Brain Behav Immun. 2017;63:21–34. [DOI] [PubMed] [Google Scholar]
- 117.Kim DR, Bale TL, Epperson CN. Prenatal programming of mental illness: current understanding of relationship and mechanisms. Curr Psychiatry Rep. 2015;17:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.St-Pierre J, Laplante DP, Elgbeili G, Dawson PA, Kildea S, King S, et al. Natural disaster-related prenatal maternal stress is associated with alterations in placental glucocorticoid system: the QF2011 Queensland Flood Study. Psychoneuroendocrinology. 2018;94:38–48. [DOI] [PubMed] [Google Scholar]
- 119.Ursini G, Punzi G, Chen Q, Marenco S, Robinson JF, Porcelli A, et al. Convergence of placenta biology and genetic risk for schizophrenia. Nat Med. 2018;24:792–801. [DOI] [PubMed] [Google Scholar]
- 120.•• Sandman CA, Glynn LM, Davis EP. Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. J Psychosom Res. 2013;75:327–35.This article substantiates the viability-vulnerability tradeoff hypothesis with evidence, informing the evident increased risk for male offspring exposed to PNMS for risk for particular psychopathology.
- 121.Selten J-P, Cantor-Graae E, Nahon D, Levav I, Aleman A, Kahn RS. No relationship between risk of schizophrenia and prenatal exposure to stress during the Six-Day War or Yom Kippur War in Israel. Schizophr Res. 2003;63:131–5. [DOI] [PubMed] [Google Scholar]
- 122.Rosa MJ, Nentin F, Bosquet Enlow M, Hacker MR, Pollas N, Coull B, et al. Sex-specific associations between prenatal negative life events and birth outcomes. Stress. 2019:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wainstock T, Shoham-Vardi I, Glasser S, Anteby E, Lerner-Geva L. Fetal sex modifies effects of prenatal stress exposure and adverse birth outcomes. Stress. 2015;18:49–56. [DOI] [PubMed] [Google Scholar]
- 124.Class QA, Lichtenstein P, Långström N, D’Onofrio BM. Timing of prenatal maternal exposure to severe life events and adverse pregnancy outcomes: a population study of 2.6 million pregnancies. Psychosom Med. 2011;73:234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McGowan PO, Matthews SG. Prenatal stress, glucocorticoids, and developmental programming of the stress response. Endocrinology. 2018;159:69–82. [DOI] [PubMed] [Google Scholar]
- 126.Bale TL. Sex differences in prenatal epigenetic programming of stress pathways. Stress. 2011;14:348–56. [DOI] [PubMed] [Google Scholar]
- 127.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci. 2013;33:9003–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, et al. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol. 2014;10:643–60. [DOI] [PubMed] [Google Scholar]
- 129.Debost J-CPG, Larsen JT, Munk-Olsen T, Mortensen PB, Meyer U, Petersen L. Joint effects of exposure to prenatal infection and peripubertal psychological trauma in schizophrenia. Schizophr Bull. 2017;43:171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Giovanoli S, Weber L, Meyer U. Single and combined effects of prenatal immune activation and peripubertal stress on parvalbumin and reelin expression in the hippocampal formation. Brain Behav Immun. 2014;40:48–54. [DOI] [PubMed] [Google Scholar]
- 131.Schlotz W, Phillips DIW. Fetal origins of mental health: evidence and mechanisms. Brain Behav Immun. 2009;23:905–16. [DOI] [PubMed] [Google Scholar]
- 132.Bryan TH. Social relationships and verbal interactions of learning disabled children. J Learn Disabil. 1978;11:107–15. [DOI] [PubMed] [Google Scholar]
- 133.McIntosh R, Vaughn S, Schumm JS, Haager D, Lee O. Observations of students with learning disabilities in general education classrooms. Except Child. 1993;60:249–61. [Google Scholar]
- 134.Haager D, Watson C, Willows DM. Parent, teacher, peer, and self-reports of the social competence of students with learning disabilities. J Learn Disabil. 1995;28:205–15. [DOI] [PubMed] [Google Scholar]
- 135.Schreier A, Wolke D, Thomas K, Horwood J, Hollis C, Gunnell D, et al. Prospective study of peer victimization in childhood and psychotic symptoms in a nonclinical population at age 12 years. Arch Gen Psychiatry. 2009;66:527–36. [DOI] [PubMed] [Google Scholar]
- 136.Lataster T, van Os J, Drukker M, Henquet C, Feron F, Gunther N, et al. Childhood victimisation and developmental expression of non-clinical delusional ideation and hallucinatory experiences: victimisation and non-clinical psychotic experiences. Soc Psychiatry Psychiatr Epidemiol. 2006;41:423–8. [DOI] [PubMed] [Google Scholar]
- 137.Danese A, Moffitt TE, Arseneault L, Bleiberg BA, Dinardo PB, Gandelman SB, et al. The origins of cognitive deficits in victimized children: implications for neuroscientists and clinicians. Am J Psychiatry. 2017;174:349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee S, Kim C-J, Kim DH. A meta-analysis of the effect of school-based anti-bullying programs. J Child Health Care. 2015;19:136–53. [DOI] [PubMed] [Google Scholar]
- 139.Schechter JC, Brennan PA, Smith AK, Stowe ZN, Newport DJ, Johnson KC. Maternal prenatal psychological distress and preschool cognitive functioning: the protective role of positive parental engagement. J Abnorm Child Psychol. 2017;45:249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Davies HT, Crombie IK, Tavakoli M. When can odds ratios mislead? BMJ. 1998;316:989–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Abbott PW, Gumusoglu SB, Bittle J, Beversdorf DQ, Stevens HE. Prenatal stress and genetic risk: how prenatal stress interacts with genetics to alter risk for psychiatric illness. Psychoneuroendocrinology. 2018;90:9–21. [DOI] [PubMed] [Google Scholar]
- 142.Mittal VA, Willhite R, Daley M, Bearden CE, Niendam T, Ellman LM, et al. Obstetric complications and risk for conversion to psychosis among individuals at high clinical risk. Early Interv Psychiatry. 2009;3:226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Harrison PJ. Recent genetic findings in schizophrenia and their therapeutic relevance. J Psychopharmacol (Oxford). 2015;29: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.• Huizink AC, de Rooij SR. Prenatal stress and models explaining risk for psychopathology revisited: generic vulnerability and divergent pathways. Dev Psychopathol. 2018;30:1041–62.A recent exploration of issues of equifinality and multifinality in research on prenatal stress and offspring outcomes, informative to the discussed cascade model in prenatal stressschizophrenia associations.