Abstract
Background
A previous Cochrane systematic review has shown that antibiotic drug treatment of asymptomatic bacteriuria in pregnant women substantially decreases the risk of pyelonephritis and reduces the risk of preterm delivery. However, it is not clear whether single‐dose therapy is as effective as longer conventional antibiotic treatment.
Objectives
To assess the effects of different durations of treatment for asymptomatic bacteriuria in pregnancy.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 August 2015) and reference lists of identified articles.
Selection criteria
Randomized and quasi‐randomized trials comparing antimicrobial therapeutic regimens that differed in duration (particularly comparing single dose with longer duration regimens) in pregnant women diagnosed with asymptomatic bacteriuria.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. We assessed the quality of the evidence using the GRADE approach.
Main results
We included 13 studies, involving 1622 women. All were comparisons of single‐dose treatment with short‐course (four‐ to seven‐day) treatments. The risk of bias of trials included in this review was largely unclear, and most trials were at high risk of performance bias. The quality of the evidence was assessed using the GRADE approach. When the any antibiotic agent was used, the 'no cure' rate for asymptomatic bacteriuria in pregnant women was slightly lower for the short‐course treatment over the single‐dose treatment, although there was evidence of statistical heterogeneity (average risk ratio (RR) 1.28, 95% confidence interval (CI) 0.87 to 1.88; women = 1502, studies = 13; I² = 56%; very low quality evidence). Data from only good quality trials also showed better cure rates with short (four‐ to seven‐day) regimens of the same microbial agent (average RR 1.72, 95% CI 1.27 to 2.33; women = 803, studies = two; I² = 0%; high quality evidence). There was no clear difference in the recurrence of asymptomatic bacteriuria rate between treatment and control groups, whether the same or different microbial agents were used (RR 1.13, 95% CI 0.77 to 1.66; 445 women studies = eight; I² = 0%; very low quality evidence). Differences were detected for low birthweight babies, favoring a short course (four‐ to seven‐day treatment) of the same microbial agent, although the data come from a single trial (RR 1.65, 95% CI 1.06 to 2.57; 714 women; high quality evidence), but no differences were observed for preterm delivery (RR 1.17, 95% CI 0.77 to 1.78; women = 804; studies = three; I² = 23%; moderate quality) or pyelonephritis (RR 3.09, 95% CI 0.54 to 17.55; women = 102; studies = two; I² = 0%; very low quality evidence). Finally, single‐dose treatment of any microbial agent was associated with a decrease in reports of 'any side effects' (RR 0.70, 95% CI 0.56 to 0.88; 1460 women, studies = 12; I² = 9%; low quality evidence). Evidence was downgraded for risk of bias concerns in trials contributing data and for imprecise effect estimates (wide confidence intervals crossing the line of no effect, and in some cases, small studies with few events).
Authors' conclusions
A single‐dose regimen of antibiotics may be less effective than a short‐course (four‐ to seven‐day) regimen, but more evidence is needed from large trials measuring important outcomes, such as cure rate. Women with asymptomatic bacteriuria in pregnancy should be treated by the standard regimen of antibiotics until more data become available testing seven‐day treatment compared with shorter courses of three‐ or five‐day regimens.
Plain language summary
Duration of treatment for asymptomatic bacteriuria during pregnancy
Asymptomatic bacteriuria is a urinary tract infection (without symptoms) common in pregnancy. If untreated, it can lead to pyelonephritis (kidney infection). Antibiotic treatment is recommended. This review aimed to identify whether single‐dose antibiotic treatments are as effective as longer ones for maternal and newborn outcomes. In general, the risk of bias of trials included in this review was largely unclear. The overall quality of the evidence was assessed using the GRADE approach. The review of 13 studies, involving over 1622 women, found that a seven‐day regimen is more effective than a one‐day course, especially for the outcome of low birthweight (high quality evidence), but this result is based on just one study. There were no clear differences between a single dose and a four‐ to seven‐day short course of antibiotics for other review outcomes, including kidney infection (very low quality evidence) and preterm birth (moderate quality evidence). Women with a single‐dose regimen reported fewer side effects (low quality evidence). More trials are needed to confirm which length of treatment is best for women and babies.
Summary of findings
Background
Description of the condition
Asymptomatic bacteriuria, defined as bacterial colonization of the urinary tract without symptomatology, is a common and potentially serious medical complication when it occurs during pregnancy. The incidence of asymptomatic bacteriuria during pregnancy has been reported to be between 2% and 10% (Andrews 1992; Sweet 1977). Escherichia coli is the most common causative organism followed by organisms such as Staphylococcus saprophyticus, Klebsiella spp, Enterobacter spp, Proteus spp, Enterococcus spp, and others. Between 15% and 45% of pregnant women with asymptomatic bacteriuria, if left untreated, will develop pyelonephritis (Wang 1989). Pyelonephritis is associated with an increase in maternal and fetal morbidity.
Description of the intervention
There is evidence to show that screening (and treatment) of all pregnant women for asymptomatic bacteriuria is both effective and cost‐beneficial when compared to no treatment in reducing the risk of pyelonephritis. A meta‐analysis of 11 randomized controlled trials (RCTs) found that treatment of asymptomatic bacteriuria reduced the risk of the development of pyelonephritis when compared to no treatment (Smaill 2015). Using decision analysis modeling to compare 'no screening' to 'screening' for asymptomatic bacteriuria, pyelonephritis was shown to decrease from 23.2 cases per 1000 among unscreened pregnant women to 16.20 cases per 1000 in those screened with leukocyte esterase‐nitrite dipstick, to 11.2 cases per 1000 among those screened with the more sensitive test of urine culture (Rouse 1995). In addition, both dipstick and culture screening were shown to be more cost‐beneficial when compared to no screening, and had a high level of agreement in the diagnosis of asymptomatic bacteriuria (Rouse 1995).
The association between asymptomatic bacteriuria and preterm delivery has also been studied. Findings from the Cardiff Birth Survey, which prospectively studied 25,844 births, reported that asymptomatic bacteriuria, adjusted for demographic and social factors, was not associated with preterm delivery (odds ratio (OR) 1.20, 95% confidence intervals (CI) 0.90 to 1.50) (Meis 1995a). However, when preterm births were categorized into 'indicated' or 'spontaneous' preterm births (Meis 1995b), a significant association between bacteriuria and indicated preterm birth was found (OR 2.03, 95% CI 1.50 to 2.80). In an overview of antimicrobial interventions to prevent preterm birth (Villar 1997), risk of preterm delivery/low birthweight was found to be significantly decreased for pregnant women who had received antibiotic treatment for asymptomatic bacteriuria when compared to women who did not receive treatment (risk ratio (RR) 0.67, 95% CI 0.52 to 0.85). There was an even greater decrease in risk when the only three trials which had categorized preterm delivery separately from low birthweight were considered (RR 0.53, 95% CI 0.33 to 0.86). An earlier meta‐analysis of eight RCTs showed that antibiotic treatment significantly reduced the risk of low birthweight (RR 0.56, 95% CI 0.43 to 0.73); however, preterm delivery was not reported independently (Romero 1989). Another meta‐analysis of six RCTs found that antibiotic treatment was associated with a reduction in the incidence of low birthweight babies (RR 0.64, 95% CI 0.45 to 0.93), and also a difference in preterm delivery (Smaill 2015). These findings have the limitations that the antibiotic regimens varied, and that many of the antimicrobials may no longer be prescribed in routine clinical practice. Nonetheless, we think that the evidence supports the view that all pregnant women with asymptomatic bacteriuria should be treated to prevent the development of acute pyelonephritis and reduce the risk of preterm delivery.
How the intervention might work
The relatively higher risk of ascending urinary tract infections and pyelonephritis during pregnancy may result from the decrease of ureteral peristalsis due to a progesterone‐mediated effect on smooth muscle contractility, on one hand, and the ureteral compression of the gravid uterus on the other. The resulting urinary stasis may facilitate the migration of uropathogens to the upper urinary tract. With eradication of the infection by antibiotic treatment, it is expected to prevent ascending urinary tract infections and the development of clinical pyelonephritis (Ramsey 2000).
The relationship of asymptomatic bacteriuria with low birthweight and preterm delivery is controversial and the possible mechanism involved has not been well established yet. Although both, the microbial colonization of the amniotic fluid and the inflammatory process resulting from the presence of bacteria in neighboring organs may induce uterine contractions, the population of women affected by lower urinary or genital tract infections usually have other risk factors for preterm birth and low birthweight (Goldenberg 2000) that may act as confounders. However, if the presence of bacteria predispose to premature uterine contractions, the rationale of antibiotic treatment to treat and clear the infection seems reasonable to prevent these adverse neonatal outcomes.
Why it is important to do this review
The question remains, what is the most effective treatment at the lowest cost, with the fewest side effects? These answers will depend on the pathogen, the choice and duration of antimicrobial, and the available healthcare services. An earlier meta‐analysis of seven RCTs reported that a single dose compared to a four‐ to seven‐day antibiotic treatment for bacteriuria showed no statistically significant difference in effectiveness when measuring outcomes of 'cure' and 'recurrence' (Smaill 1992a). However, these studies lacked a uniform definition for 'cure' and 'recurrence', and lacked consistent protocols for follow‐up of treatment, making comparisons difficult. Nonetheless, the available data do suggest that single‐dose therapy may be as effective as longer, conventional antibiotic treatment. Evidence regarding duration of therapy is especially important because single‐dose therapy offers the benefits of greater compliance of women during pregnancy at lower cost. In under‐resourced settings, the screening and treatment can be done during the same antenatal care visit.
Objectives
The objective of the review is to determine the clinical effectiveness of different durations of treatment for asymptomatic bacteriuria in pregnancy.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials (RCTs) and quasi‐RCTs comparing treatment regimens for bacteriuria during pregnancy that differ in duration including those that compared different duration of different antimicrobial agents as well as different durations of the same agent. We expected that the majority of trials would attempt to show equivalence between treatments. We have not included trials comparing different therapeutic agents with the same duration of administration in this review, nor those presented only as abstracts.
Types of participants
Women identified during pregnancy as having asymptomatic bacteriuria.
Types of interventions
Antimicrobials of varying duration. Antimicrobial therapy regimens tend to show large variations in duration. For the purposes of this review, we have considered the following interventions distinct and compared to each other:
single dose (including one‐day treatment with divided doses);
short course (four to seven days);
long course (14 days);
continuous (treatment continued until delivery).
We will group interventions that we identify in future that do not fall into one of the categories listed above in the category that is closest in duration. We will make this allocation without any consideration of the trial results.
Types of outcome measures
Primary outcomes
The primary outcome is maternal cure rate defined as the woman having negative culture (test of cure) following initial treatment for asymptomatic bacteriuria.
Secondary outcomes
(1) Maternal
(a) Recurrent asymptomatic bacteriuria (in this review, recurrence includes relapse (the recurrence of bacteriuria caused by the same organism, usually within six weeks of the initial infection); and reinfection (the recurrence of bacteriuria involving a different strain of bacteria after successful eradication of the initial infection, limited to the bladder, and occurring at least six weeks after therapy (Davison 1992)). (b) Pyelonephritis.
(2) Newborn
(a) Preterm delivery (gestational age less than 37 weeks). (b) Low birthweight (birthweight less than 2500 g). (c) Preterm delivery or low birthweight (if reported together). (d) Other birth outcomes.
(3) Side effects
Any side effect related to the antibiotic treatment.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 August 2015).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference lists of identified articles.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeWidmer 2011.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
For this update, we assessed the quality of the evidence using the GRADE approach as outlined in the GRADE Handbook in order to assess the quality of the body of evidence relating to the following outcomes for the comparisons 'Single‐dose antibiotic versus short‐course (four‐ to seven‐day) antibiotic', 'Single‐dose antibiotic versus long‐course (14‐day) antibiotic', and 'Single‐dose antibiotic versus continuous (treatment continued until delivery) antibiotic'. However, all included trials were of single‐dose treatments with short‐course (four‐ to seven‐day) treatments and so we were only able to produce 'Summary of findings' tables for this comparison.
No cure
Preterm delivery or low birthweight
Pyelonephritis
Recurrent asymptomatic bacteriuria
Any side effect related to the antibiotic treatment
We used GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
No continuous data were analyzed. In future updates, if appropriate, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardized mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
The unit of analysis was individual randomized women.
Cluster‐randomized trials
No cluster‐randomized trials were found for this review. If we had identified cluster‐randomized trials, they would have been eligible for inclusion.
In future updates, we will include cluster‐randomized trials in the analyses along with individually‐randomized trials. We will adjust either their sample sizes or standard errors using the methods described in the Handbook [Section 16.3.4 or 16.3.6] using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomized trials and individually‐randomized trials, we plan to synthesize the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomization unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomization unit and perform a sensitivity analysis to investigate the effects of the randomization unit.
Cross‐over trials
Given the objectives of this review, cross‐over trials were not eligible for inclusion.
Other unit of analysis issues
No other units of analyses were used in this review.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomized to each group in the analyses. The denominator for each outcome in each trial was the number randomized minus any participants whose outcomes were known to be missing. Trials were excluded if it was not possible to enter data on an intention‐to‐treat basis.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Had we identified substantial heterogeneity (above 30%) in the primary outcomes, we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
We investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we attempted to explain and interpret the finding.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. If we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity in the primary outcomes, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it.
All analyses in the review were set up according to the following clinical subgroups of trials.
Same antimicrobial agent
Different antimicrobial agent
Where we had sufficient evidence, we reported a pooled effect estimate. Where there were only data for one of type of antimicrobial, we reported only the evidence we had. We reported positive results of the Test for Subgroup differences where relevant and meaningful. We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result.
We carried out the following sensitivity analyses according to trial quality, saved as our Comparison 2.
Good quality studies
Poor quality studies
Trial quality was assessed according to whether a trial reported taking measures to prevent selection, detection, performance and attrition bias. Trials reporting these domains were considered of high quality.
Results
Description of studies
Results of the search
We identified two new reports from an updated search in 2015 for the same trial (Rafalsky 2013), which we subsequently added to Excluded studies.
Included studies
We included 13 studies, involving 1622 women. For a detailed description of studies, seeCharacteristics of included studies.
Eleven trials included in the review were conducted in high‐income countries (Austria, Belgium, Denmark, Italy, New Zealand, Spain, the United Kingdom, and the United States), and two were conducted in low‐ and middle‐income ones (one multicenter trial conducted in Argentina, Philippines, Thailand, and Viet Nam, and one was conducted in Turkey). The laboratory measurements of selected outcomes required facilities in which urine culture and antibiotic sensitivity testing were possible. Three trials were published recently (Bayrak 2007; Estebanez 2009; Lumbiganon 2009); one of the trials was published in 1990 (Thoumsin 1990); eight of the trials were published in the 1980s; and the remaining trial was published in 1975 (Reeves 1975). The antimicrobial drugs used in the trials included: ampicillin, nitrofurantoin, cephalexin, fosfomycin trometamol, fosfomycin, amoxicillin‐clavulanate, amoxicillin, co‐trimoxazole, trimethoprim, and other sulfonamides. Bayrak 2007 compared a single dose of fosfomycin trometamol with five‐day treatment of cefuroxime axetyl; Thoumsin 1990 compared a single dose of fosfomycin trometamol with seven‐day treatment of nitrofurantoin; and Estebanez 2009 compared a single dose of fosfomycin with seven‐day treatment of amoxicillin‐clavulanate. The remaining 10 trials compared different durations of the same antimicrobial family. The duration of antimicrobial used in the experimental group was either a single dose or one‐day treatment with divided dose, and in the control groups, varied between four and seven days' duration. We included Brumfitt 1982 in spite of inclusion of 24% of symptomatic women in both groups, and Thoumsin 1990 in spite of the state of publication (preliminary results).
Excluded studies
For details of the excluded studies, see table of Characteristics of excluded studies.
Risk of bias in included studies
In general, the trials lacked evidence of sufficient rigor in the design, conduct and analysis of results (Figure 1; Figure 2).
1.
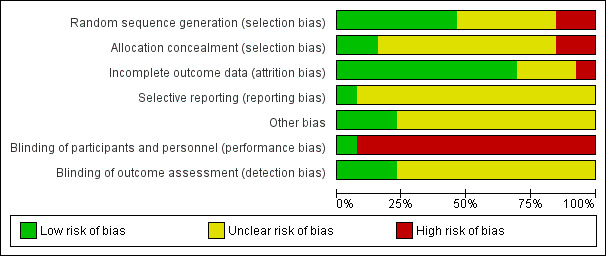
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
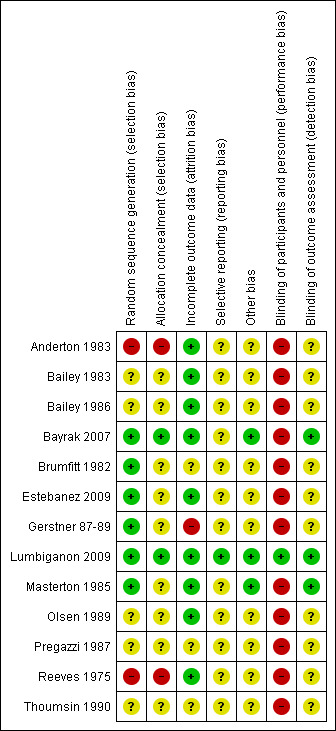
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
For detailed information on methods see table of Characteristics of included studies.
Allocation
Generation methods for randomization in six of the 13 trials included computerized process for simple randomization (Masterton 1985), randomized tables (Brumfitt 1982;Estebanez 2009), and blocked randomization (Bayrak 2007; Gerstner 87‐89; Lumbiganon 2009); all assessed as low risk of bias. Two trials described alternate methods (every other woman) and were assessed as of high risk of bias (Anderton 1983; Reeves 1975). The remaining five trials provided no description of the generation method and were assessed as having an unclear risk of bias (Bailey 1983; Bailey 1986; Olsen 1989; Pregazzi 1987; Thoumsin 1990).
Seven trials did not describe the mechanism used for allocation concealment (Brumfitt 1982; Estebanez 2009; Gerstner 87‐89; Masterton 1985; Olsen 1989; Pregazzi 1987; Thoumsin 1990) and were assessed as unclear risk of bias. One trial used numbered treatment bags (Bayrak 2007), another used sealed, opaque treatment boxes numbered sequentially (Lumbiganon 2009) and were considered as low risk of bias; and two described concealment as 'envelopes' only (Bailey 1983; Bailey 1986); these trials were assessed as having an unclear risk of bias. The remaining two trials (Anderton 1983; Reeves 1975) used alternated methods for allocation and were assessed as at high risk of bias.
Blinding
In all 13 trials, there was inadequate description to determine whether 'contamination' in the short‐course treatment group, co‐interventions, or protocol deviation occurred. Blinding for outcome assessment was reported in three trials (Bayrak 2007; Lumbiganon 2009; Masterton 1985). No women were blinded to treatment in any of the trials except in the Lumbiganon 2009 trial. Informed consent was mentioned in the majority of trials.
Incomplete outcome data
Loss to follow‐up was described in all trials except Brumfitt 1982, Pregazzi 1987 and Thoumsin 1990; all assessed as of unclear risk of bias. The rates of loss to follow‐up were, as expected given the longer duration, generally higher in the comparison group as compared to the experimental (single‐dose) group. Although the numbers are small, when two treatments produce a different pattern of withdrawal, then this offers evidence that the groups are not entirely comparable (Jones 1996). Several trials had small differences in rates of loss between treatment arms and were assessed as of low risk of bias (Anderton 1983; Bailey 1983; Bailey 1986; Bayrak 2007; Estebanez 2009; Lumbiganon 2009; Masterton 1985; Olsen 1989; Reeves 1975). Gerstner 87‐89, though, we assessed as of high risk due to 13% loss in the treatment arm and 26% loss in the control arm.
Selective reporting
The only trial that reported on the demographics or number of women who met the study eligibility criteria, but were not included in the study and also on compliance with the treatment regimen, was Lumbiganon 2009; this trial was assessed as of low risk of reporting bias. All other trials were assessed as of unclear risk of bias due to lack of information.
Other potential sources of bias
An explanation of the sample size calculation and power calculation was provided in the Bayrak 2007, Lumbiganon 2009 and Masterton 1985 trials; these trials were assessed as of low risk of other sources of bias.
All of the remaining trials were assessed as of unclear risk of bias due to lack of information in the trial report or to specific factors where we were unclear of the impact of potential bias. For example, randomization is less effective in achieving comparable groups in studies with small sample sizes, which many of our studies had. Also, in one trial women in the experimental group were more likely to have a history of urinary tract infection as compared to the control group (50% versus 35%) (Bailey 1983). Disparity in baseline characteristics between treatment groups suggests selection bias due to inadequate randomization or too small a sample size, or both (Villar 1996). We felt there was an increased likelihood of selection bias in most of the trials, but we were unclear of the impact of this bias on results. The total number of women enrolled in these studies ranged from 41 to 778.
Effects of interventions
Summary of findings for the main comparison. Single‐dose antibiotic versus short‐course (four‐ to seven‐day) antibiotic for asymptomatic bacteriuria during pregnancy.
| Single dose antibiotic versus short‐course (four‐ to seven‐day) antibiotic for asymptomatic bacteriuria during pregnancy | ||||||
|
Patient or population: patients with asymptomatic bacteriuria during pregnancy
Settings: high‐, low‐ and middle‐income countries
Intervention: single‐dose antibiotic Comparison: short‐course (four‐ to seven‐day) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Single‐dose antibiotic versus short‐course (4‐ to 7‐day) antibiotic for asymptomatic bacteriuria | |||||
| No cure ‐ all trials | Study population | RR 1.28 (0.87 to 1.88) | 1502 (13 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 166 per 1000 | 212 per 1000 (144 to 312) | |||||
| Moderate | ||||||
| 138 per 1000 | 177 per 1000 (120 to 259) | |||||
| Recurrent asymptomatic bacteriuria ‐ all trials | Study population | RR 1.13 (0.77 to 1.66) | 445 (8 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 169 per 1000 | 191 per 1000 (130 to 281) | |||||
| Moderate | ||||||
| 139 per 1000 | 157 per 1000 (107 to 231) | |||||
| Pyelonephritis ‐ Same antimicrobial agent only4 | Study population | RR 3.09 (0.54 to 17.55) | 102 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 21 per 1000 | 64 per 1000 (11 to 366) | |||||
| Moderate | ||||||
| 28 per 1000 | 87 per 1000 (15 to 491) | |||||
| Low birthweight ‐ Same antimicrobial agent only | Study population | RR 1.65 (1.06 to 2.57) | 714 (1 study) | ⊕⊕⊕⊕ high | ||
| 80 per 1000 | 132 per 1000 (85 to 206) | |||||
| Preterm delivery ‐ Same antimicrobial agent only | Study population | RR 1.17 (0.77 to 1.78) | 804 (3 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 91 per 1000 | 99 per 1000 (47 to 206) | |||||
| Moderate | ||||||
| 89 per 1000 | 97 per 1000 (46 to 201) | |||||
| Side effects ‐ all trials | Study population | RR 0.70 (0.56 to 0.88) | 1460 (12 studies) | ⊕⊕⊝⊝ low1 | ||
| 194 per 1000 | 136 per 1000 (109 to 171) | |||||
| Moderate | ||||||
| 182 per 1000 | 127 per 1000 (102 to 160) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded twice due to serious risk of bias concerns. Most of the pooled effect provided by studies “B” or “C” (as specified by WHO criteria)* with a substantial proportion (i.e. > 40%) from studies “C” (‐2). 2 Downgraded once for imprecision. Wide confidence interval crossing the line of no effect (‐1). 3 Downgraded once for imprecision. Small sample size and few events (‐1).
4 All data available for this outcome came from trials testing the same microbial agents.
* "A" studies are of low risk of bias; "B" studies (‐1) are those with serious design limitations, including lack of allocation concealment, blinding (where relevant) or other problems with the conduct of the study; and "C" studies (‐2) have the same problems as B studies but to a greater degree.
Summary of findings 2. Single‐dose antibiotic versus short‐course (four‐ to seven‐day) antibiotic for asymptomatic bacteriuria during pregnancy (subgrouped by trial quality).
| Single‐dose antibiotic versus short‐course (four‐ to seven‐day) antibiotic for asymptomatic bacteriuria during pregnancy (subgrouped by trial quality) | ||||||
|
Patient or population: patients with asymptomatic bacteriuria during pregnancy
Settings: high‐ middle‐ and low‐income countries
Intervention: single‐dose antibiotic Comparison: short‐course (4‐7 day) antibiotic | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Single‐dose antibiotic versus short‐course (4‐ to 7‐day) antibiotic for asymptomatic bacteriuria (subgrouped by trial quality) | |||||
| Same antimicrobial agent ‐ No cure (good quality studies) | Study population | RR 1.72 (1.27 to 2.33) | 803 (2 studies) | ⊕⊕⊕⊕ high | ||
| 137 per 1000 | 236 per 1000 (175 to 320) | |||||
| Moderate | ||||||
| 134 per 1000 | 230 per 1000 (170 to 312) | |||||
| Different antimicrobial agents ‐ No cure (good quality studies) | Study population | RR 1.36 (0.24 to 7.75) | 84 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 50 per 1000 | 68 per 1000 (12 to 388) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded once for imprecision. Wide confidence interval crossing the line of no effect (‐1). 2 Downgraded once for imprecision. Small sample size and few events (‐1).
Comparison 1: Single‐dose antibiotic versus short‐course (four‐ to seven‐day) antibiotic for asymptomatic bacteriuria
We included 13 trials, involving 1622 women. After reporting overall results, we report individual results for two groups: (a) trials that compared different duration regimens of the same agent; and (b) trials that compared different duration regimens of different antimicrobial agents.
Comparison 1: overall pooled totals for all trials
Primary outcome
No cure
Cure rates were similar whether women received a single dose or a short course of any antibiotic (average risk ratio (RR) 1.28, 95% confidence interval (CI) 0.87 to 1.88; women = 1502; studies = 13; I² = 56%; Analysis 1.1). There was no evidence of differences in cure rates for our subgroups of same antimicrobial agent or different microbial agent (Test for subgroup differences: Chi² = 0.55, (P = 0.46), I² = 0%) and moderate heterogeneity even with a random‐effects model (Heterogeneity: Tau² = 0.23; Chi² = 27.00, I² = 56%). We graded the evidence for the outcome of no cure as very low quality due to risk of bias in the contributing trials and wide CIs crossing the line of no effect.
1.1. Analysis.
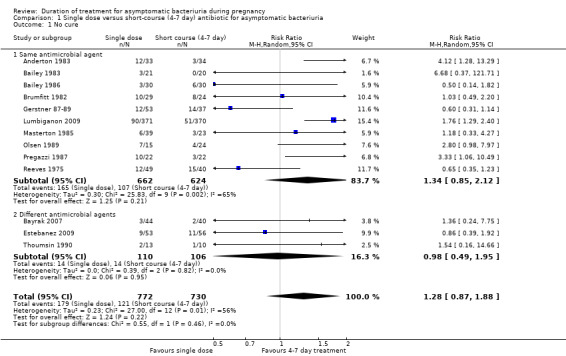
Comparison 1 Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria, Outcome 1 No cure.
When assessing forest plots for publication bias, we found suggested asymmetry in the funnel plot for this outcome (Figure 3). However, the single outlier trial was extremely small, and so the source of asymmetry may well be a small‐study effect rather than publication bias. This trial also contribute very little to the overall pooled effect estimate (1.6%). We have not downgraded evidence for this outcome for publication bias.
3.
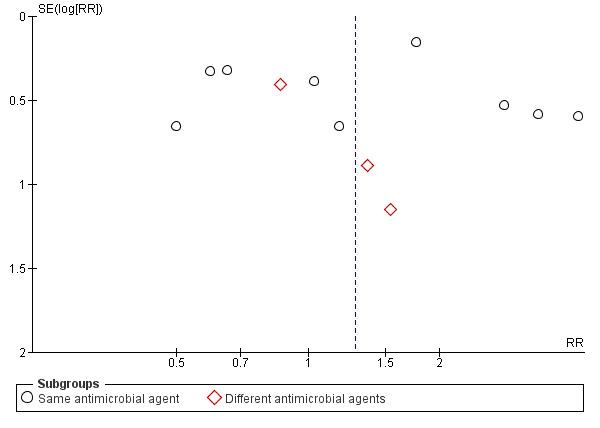
Funnel plot of comparison: 1 Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria, outcome: 1.6 No cure.
Secondary outcomes
Maternal and newborn outcomes
Recurrent asymptomatic bacteriuria
Rates of recurrence were similar between treatment groups (RR 1.13, 95% CI 0.77 to 1.66; women = 445; studies = eight; I² = 0%), Analysis 1.2. There was no evidence of any difference between subgroups of the same or different agents (Test for subgroup differences: Chi² = 0.03, (P = 0.86), I² = 0%). We graded evidence for recurrence as very low due to risk of bias concerns in the contributing trials and wide CIs crossing the line of no effect.
1.2. Analysis.
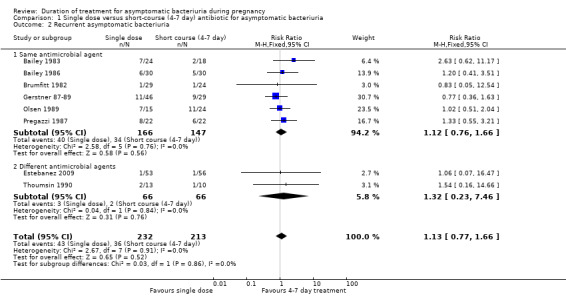
Comparison 1 Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria, Outcome 2 Recurrent asymptomatic bacteriuria.
Results for outcomes (pyelonephritis, preterm delivery and low birthweight) Analysis 1.3; Analysis 1.4; Analysis 1.5 were not pooled due to each outcome only having one subgroup. Results for these outcomes are found below for trials with either the same or different agents.
1.3. Analysis.
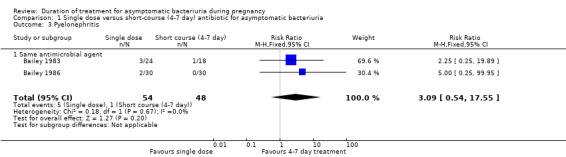
Comparison 1 Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria, Outcome 3 Pyelonephritis.
1.4. Analysis.
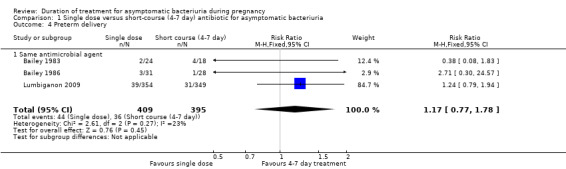
Comparison 1 Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria, Outcome 4 Preterm delivery.
1.5. Analysis.
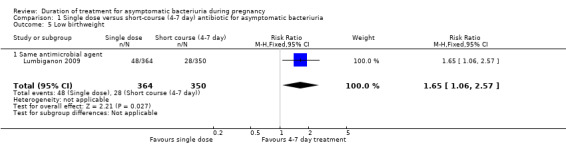
Comparison 1 Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria, Outcome 5 Low birthweight.
Side effects
Fewer women who had the single dose experienced side effects (RR 0.70, 95% CI 0.56 to 0.88; women = 1460; studies = 12; I² = 9%), Analysis 1.6. Evidence for side effects was graded to be of low quality due to risk of bias concerns in the trials contributing data. There was evidence of a difference between using the same or different microbial agents noted; however, we would not emphasize this finding due to the small number of trials and events in the different antimicrobial group. The results for both subgroups fall in the same direction with minimal heterogeneity (Test for subgroup differences: Chi² = 5.42, df = 1 (P = 0.02), I² = 81.5%; Heterogeneity: Chi² = 10.93, I² = 9%).
1.6. Analysis.
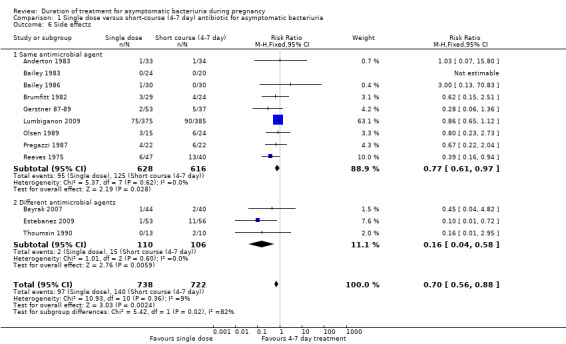
Comparison 1 Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria, Outcome 6 Side effects.
Comparison 1: (a) different duration of the same antimicrobial agent
Primary outcome
No cure
All trials reported bacteriological success of treatment by repeat cultures (Anderton 1983; Bailey 1983; Bailey 1986; Brumfitt 1982; Gerstner 87‐89; Lumbiganon 2009; Masterton 1985; Olsen 1989; Pregazzi 1987; Reeves 1975) The 'no cure' rate was similar for the one‐day and the four‐ to seven‐day treatment (average RR 1.34, 95% CI 0.85 to 2.12; women = 1286; studies = 10; Analysis 1.1). There was moderate heterogeneity for this outcome even with a random‐effects model (Heterogeneity: Tau² = 0.30; Chi² = 25.83, df = 9 (P = 0.002); I² = 65%).
Secondary outcomes
(a) Maternal outcomes
The risk of recurrent asymptomatic bacteriuria (Analysis 1.2) in one‐day treatment was not significantly different to that with longer treatment (RR 1.12, 95% CI 0.76 to 1.66; women = 313; studies = six; I² = 0%).
Pyelonephritis (Analysis 1.3) was reported only by Bailey 1983 and Bailey 1986, with 102 women included in the two trials together. There were four more women with pyelonephritis following single‐dose treatment (5/54 versus 1/48; RR 3.09, 95% CI 0.54 to 17.55; women = 102; studies = two; I² = 0%). We graded evidence for this outcome as of very low quality due to risk of bias concerns in the contributing trials, wide CIs and limited data.
(b) Newborn outcomes
Preterm birth
Only three of the 10 trials included in this review reported preterm birth rates (Bailey 1983; Bailey 1986; Lumbiganon 2009). In total, 804 women were studied in these trials (Analysis 1.4), and there is no evidence of a difference between a single‐dose antibiotic and a four‐ to seven‐day course (RR 1.17, 95% CI 0.77 to 1.78; women = 804; studies = three; I² = 23%). We graded evidence for preterm birth to be of moderate quality due to imprecision, or wide CIs crossing the line of no effect.
Low birthweight
Just one trial Lumbiganon 2009 reported low birthweight rates (Analysis 1.5) with 48 cases out of 364 babies in the one‐day arm compared to 28 cases in the 350 babies from the longer‐treatment group (RR 1.65, 95% CI 1.06 to 2.57; women = 714; studies = one; I² = 0%). Evidence for low birthweight was graded as of high quality.
(c) Side effects
The meta‐analysis regarding any side effects (Analysis 1.6) shows a lower incidence of side effects with single‐dose treatment. For those trials that tested the same microbial agent the result is similar (RR 0.77, 95% CI 0.61 to 0.97; women = 1244; studies = nine; I² = 0%). The Reeves 1975 study included in this analysis was stopped prematurely due to the side effects (mainly nausea, vomiting, diarrhea) of sulfadimidine in seven‐day treatment group. A sensitivity analysis including all trials comparing the same antimicrobial but excluding Reeves 1975 also showed higher, yet statistically non‐significant, rates of similar side effects with longer treatment (RR 0.81, 95% CI 0.64 to 1.04).
Comparison 1 (b): different durations of different antimicrobial agents
Primary outcome
No cure
The three trials included in this outcome (216 women) reported bacteriological success of treatment by repeat cultures (Bayrak 2007; Estebanez 2009; Thoumsin 1990). There was no difference in the 'no cure' rate for the single‐dose treatment versus the short‐course treatment, but with wide CIs (RR 0.98, 95% CI 0.49 to 1.95; women = 216; studies = three; I² = 0%), Analysis 1.1.
Secondary outcomes
(a) Maternal outcomes
Recurrent asymptomatic bacteriuria
The incidence of recurrent bacteriuria was reported in two of the three included trials (Estebanez 2009; Thoumsin 1990) (132 women), and there was no significant difference between the two groups, again with wide CIs (RR 1.32, 95% CI 0.23 to 7.46), Analysis 1.2.
Pyelonephritis
No trial included in this subgroup of different agents reported the outcomes of pyelonephritis.
(b) Newborn outcomes
No trial included in this subgroup of different agents reported the outcomes of preterm birth or low birthweight.
(c) Side effects
The three trials (Bayrak 2007; Estebanez 2009; Thoumsin 1990) showed fewer side effects in the single‐dose treatment group than in the short‐course treatment group (RR 0.16, 95% CI 0.04 to 0.58; women = 216; studies = three; I² = 0%), Analysis 1.6.
Comparison 2: Single‐dose antibiotic versus short‐course (four‐ to seven‐day) antibiotic for asymptomatic bacteriuria
Subgroup analysis by trial quality
Comparison 2 (1): same antimicrobial agent ‐ 'no cure'
When grouped by trial quality (Analysis 2.1), the two trials of good quality (reporting measures to prevent selection, detection, performance and attrition bias) show a significant increase in 'no cure' rates with single‐dose treatment when compared with short‐course (four‐ to seven‐day) treatment (Lumbiganon 2009; Masterton 1985; two trials, 803 women, average RR 1.72, 95% CI 1.27 to 2.33; Heterogeneity: Chi² = 0.35, df = 1 [P = 0.55]; I² = 0%). Heterogeneity remains in the subgroup of poor quality studies (eight trials, 483 women, average RR 1.32, 95% CI 0.73 to 2.42, Heterogeneity: Chi² = 20.84, df = 7 (P = 0.004); I² = 66%). There was some evidence that the intervention worked differently, and better, in trials of higher quality, although the test for subgroup was not significant (Test for subgroup differences: Chi² = 0.58, df = 1 (P = 0.44), I² = 0%). However, there were only two studies in the good quality subgroup and the CIs for both pooled results do overlap.
2.1. Analysis.
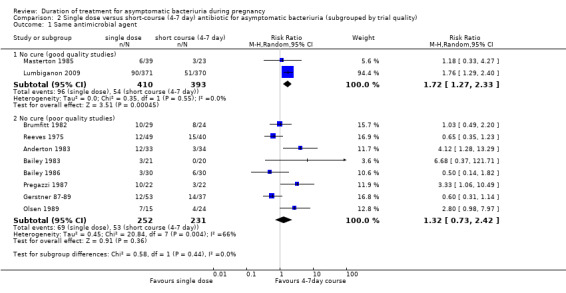
Comparison 2 Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria (subgrouped by trial quality), Outcome 1 Same antimicrobial agent.
We have graded the evidence for no cure (good quality studies) as of high quality. See Table 2.
Comparison 2 (2): different antimicrobial agent ‐ 'no cure'
When grouped by trial quality, only Bayrak 2007 shows relatively good quality. There was no difference in rates of no cure with a single dose or short‐course doses (one study, RR1.36, 95% CI 0.24 to 7.75), Analysis 2.2. The remaining two trials were ranked as of poor quality; these trials also show no group differences in rates of no cure (two studies, RR 0.93, 95% CI 0.44 to 1.96. Heterogeneity: Chi² = 0.22, df = 1 (P = 0.64); I² = 0%). There was no evidence of a difference between subgroups of high or low quality studies (Test for subgroup differences: Chi² = 0.16, df = 1 (P = 0.69), I² = 0%), but there were too few trials in each subgroup to make this analysis meaningful.
2.2. Analysis.
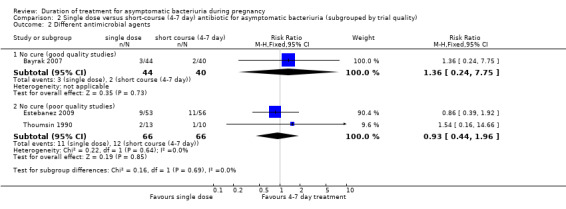
Comparison 2 Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria (subgrouped by trial quality), Outcome 2 Different antimicrobial agents.
We have graded the evidence for no cure (good quality studies) as of low quality, due to the estimate coming from a single small trial and having wide CIs crossing the line of no effect.
Comparison 3: Single‐dose antibiotic versus long‐course (14 days) antibiotic for asymptomatic bacteriuria
No trials found.
Comparison 4: Single‐dose antibiotic versus continuous (treatment continued until delivery) antibiotic for asymptomatic bacteriuria
No trials found.
Discussion
Summary of main results
Asymptomatic bacteriuria during pregnancy has serious consequences if it is not treated. Routine screening and antibiotic treatment of positive cases is generally recommended. The optimal duration of the treatment has both cost and practical implications.The standard treatment is a short course of four to seven days. Single‐dose treatment, if effective, could increase compliance (as it can be administered at the healthcare site), and it is likely to be cheaper. These advantages are important in lower‐income countries where women attend antenatal clinics irregularly and where approximately 90% of all preterm deliveries around the world take place (Villar 1994). The objective of this review was to determine the clinical effectiveness of different durations of treatment for asymptomatic bacteriuria in pregnancy. Only trials comparing a single dose with a short course of four to seven days were found for inclusion in this review.
For the outcome of no cure, meta‐analysis of all trials comparing a single dose versus a short course of treatment of the same or different antibiotics showed a wide range of effects (from 50% reduction to a six‐fold increase), with no conclusive difference between treatment groups and moderate heterogeneity between trials. The evidence for no cure was graded as of very low quality due to risk of bias concerns in the contributing trials and the imprecision of treatment effects (wide confidence intervals crossing the line of no effect). Fewer babies of low birthweight were born to women who had treatment with a short course of the same microbial agent than to women receiving a single dose, but this result was based on a single study with 714 women and graded to be of moderate quality. A single course of antibiotics also resulted in fewer side effects in 12 trials (1460 women), regardless of the type of antibiotic used; however, this evidence was graded as of low quality due to serious risk of bias concerns in the contributing trials. There were no clear benefits of either a single dose or a short course of antibiotics for any of the following review outcomes, whether the same antibiotic or different antibiotics were used: recurrence (very low quality evidence), pyelonephritis (very low quality evidence), or preterm delivery (moderate quality evidence). Evidence was downgraded due to risk of bias concerns in contributing trials and to imprecision in effect estimates.
When analysis was restricted to two high quality trials, results from the two trials for the outcome of no cure favored short‐course treatment of the same microbial agent. No conclusions about cure rates can be drawn for different antimicrobial drugs as this analysis included only one small trial.
Overall completeness and applicability of evidence
In general, the trials in this review have several methodological limitations, which makes the interpretation of pooled results difficult. Heterogeneity could presumably be explained by trial design and conduct, but baseline risks of the population studied, differences in the laboratory definition of positive cases (i.e. cultures of > 10,000 or 100,000 CFU/mL), and the different pharmacokinetics and specificities of the antimicrobial agents used could not be ruled out. The Lumbiganon 2009 trial provides about half of the data to the meta‐analysis and is methodologically sound. We therefore think that overall, longer duration of treatment is likely to be more effective. However, there is a drawback that non‐adherence may be higher with treatments of longer duration.
Quality of the evidence
In general, the risk of bias of trials included in this review was largely unclear, with all trials being at high risk of performance bias. Most trials were conducted in the 1980s and 1990s and did not describe methods to avoid selection, detection or performance bias. Only three trials (Lumbiganon 2009; Masterton 1985 in the same agent group, and in Bayrak 2007 in the different agent group) were rated as low risk of bias.
The quality of the evidence was assessed using the GRADE approach, see Table 1; Table 2. The evidence was graded as: very low quality for the outcomes no cure rate, recurrence of asymptomatic bacteriuria and pyelonephritis; high quality for the outcome low birthweight; moderate quality for preterm delivery; and low quality for side effects. Evidence was downgraded for risk of bias concerns in trials contributing data and for imprecise effect estimates (wide confidence intervals crossing the line of no effect, and in some cases, small studies with few events).
Potential biases in the review process
We attempted to minimise bias during the review process by having two people assess the eligibility of studies, assess risk of bias and extract data. We attempted to be as inclusive as possible in our search. The asymmetric funnel plot may suggest publication bias (Figure 3) that could result from the small numbers in the included trials. Many trials included in the review were very small. Small trials not only can predispose to publication bias, but also to misleading results as they tend to be conducted and analyzed with less methodological rigor than larger trials and may overestimate the effect of one group. This is of greater concern in equivalence trials where larger sample sizes are required than comparative trials (Jones 1996). Most trials were not blinded and the risk of performance and detection bias was high, especially for clinical outcomes. Morover, few studies reported efforts to blind the outcome assessment, even those performed at the bacteriology lab. Thus, the poor methodological quality of these trials may obscure any important clinical and laboratory differences between duration of treatment regimens. We explored these issues as a plausible source of heterogeneity by analyzing good and poor quality studies separately.
Agreements and disagreements with other studies or reviews
Currently there is a consensus in most settings that asymptomatic bacteriuria during pregnancy should be universally screened and treated (NICE 2008; Nicolle 2005). However, there are increasing concerns about the emergence of resistant bacterial strains due to high rates of antibiotic use in hospitals, the community and agriculture (Laxminarayan 2014). Because of these worries and the lack of convincing evidence for an universal screening and treating policy, some professional associations such as the Dutch Society of Obstetrics and Gynaecology (NVOG 2011), and the Dutch General Practitioners Society (Van Haaren 2005) recommend screening for asymptomatic bacteriuria only in high‐risk women (i.e. women with congenital urinary tract defects, a previous history of urinary tract infections, diabetes mellitus, sickle cell disease or neurological disorders, and reduced immunity). A recently published cohort study from the Netherlands (Kazemier 2015) also questions the universal routine screen‐treat‐policy for asymptomatic bacteriuria in pregnancy as it showed that, if untreated, asymptomatic bacteriuria had low (although significant) absolute risks for pyelonephritis (2.4% in 208 women with untreated asymptomatic bacteriuria versus 0.6% in 4035 women without bacteriuria). An embedded small randomized controlled trial within this cohort (40 women randomly assigned to nitrofurantoin and 45 to placebo) failed to demonstrate significant differences in the rates of low birthweight, preterm birth or pyelonephritis. However, this study is small, included only very low‐risk women and it is uncertain whether these results are generalizable.
Authors' conclusions
Implications for practice.
The evidence suggests that the standard short course of four‐ to seven‐day treatment regimen for treating asymptomatic bacteriuria in pregnant women is more effective than single‐dose regimens. More evidence is needed from large trials measuring important outcomes, such as cure rate.
Implications for research.
There is a need for a randomized controlled trial designed to test whether shorter courses, i.e. three‐day antimicrobial therapy of first‐line of choice drug is as effective as longer treatment regimens in prevention of preterm birth, pyelonephritis, and recurrent infection during the index pregnancy. Future trials should, therefore, be designed taking the following factors into account.
(1) Trial size
The trials should be appropriately sized taking into account the 'equivalence' nature of the comparison.
(2) Trial design
Ideally, these trials should be double‐blinded and placebo‐controlled to prevent bias.
(3) Outcomes
Immediate bacteriological cure (in one to two weeks) is the relevant outcome as related to the treatments under study.
(4) Interventions
Antibiotics should be researched with regard to the following principles: (a) the antibiotic of choice should be safe in pregnancy; (b) in circumstances where culture and sensitivity are not feasible, empiric, broad‐spectrum treatment would be appropriate; (c) where susceptibility is known, narrow‐spectrum, specific antibiotic treatment would be appropriate.
What's new
| Date | Event | Description |
|---|---|---|
| 31 August 2015 | New search has been performed | Search updated and one study added to excluded studies (Rafalsky 2013). Methods updated. 'Risk of bias' tables reassessed. 'Summary of findings' tables (GRADE) added. |
| 31 August 2015 | New citation required but conclusions have not changed | No new included studies added, the conclusions remain the same. |
History
Protocol first published: Issue 4, 1997 Review first published: Issue 1, 1999
| Date | Event | Description |
|---|---|---|
| 31 August 2011 | New search has been performed | Search updated. Three new trials included (Bayrak 2007; Estebanez 2009; Lumbiganon 2009). |
| 9 September 2008 | New citation required and conclusions have changed | The inclusion of a well‐designed trial with a large sample size, published in 2009 (Lumbiganon 2009) contributed significantly to provide a definite answer to this review's question: the one‐day antimicrobial treatment is significantly less effective than the seven‐day one. |
| 2 September 2008 | Amended | Converted to new review format. |
| 31 July 2006 | New search has been performed | Search updated. We identified one new trial report of an ongoing WHO trial. |
| 1 May 2004 | New search has been performed | We added two new trials to the May 2004 update (Pregazzi 1987; Thoumsin 1990). |
Acknowledgements
Edgardo Abalos, who provided comments on the final draft of the 2015 update.
Nancy Medley helped with the editing of the 'Summary of findings' tables and provided feedback to the editorial office. Nancy Medley's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
The World Health Organization and Ivana Lopez, Luciano Mignini, Ariel Rogant retain copyright and all other rights in their respective contributions to the manuscript of this review as submitted for publication, including any revisions or updates to the manuscript which WHO may make from time to time
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No cure | 13 | 1502 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.87, 1.88] |
| 1.1 Same antimicrobial agent | 10 | 1286 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.85, 2.12] |
| 1.2 Different antimicrobial agents | 3 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.49, 1.95] |
| 2 Recurrent asymptomatic bacteriuria | 8 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.77, 1.66] |
| 2.1 Same antimicrobial agent | 6 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.76, 1.66] |
| 2.2 Different antimicrobial agents | 2 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.23, 7.46] |
| 3 Pyelonephritis | 2 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.54, 17.55] |
| 3.1 Same antimicrobial agent | 2 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.54, 17.55] |
| 4 Preterm delivery | 3 | 804 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.77, 1.78] |
| 4.1 Same antimicrobial agent | 3 | 804 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.77, 1.78] |
| 5 Low birthweight | 1 | 714 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.06, 2.57] |
| 5.1 Same antimicrobial agent | 1 | 714 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.06, 2.57] |
| 6 Side effects | 12 | 1460 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.56, 0.88] |
| 6.1 Same antimicrobial agent | 9 | 1244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.61, 0.97] |
| 6.2 Different antimicrobial agents | 3 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.04, 0.58] |
Comparison 2. Single dose versus short‐course (4‐7 day) antibiotic for asymptomatic bacteriuria (subgrouped by trial quality).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Same antimicrobial agent | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 No cure (good quality studies) | 2 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [1.27, 2.33] |
| 1.2 No cure (poor quality studies) | 8 | 483 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.73, 2.42] |
| 2 Different antimicrobial agents | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 No cure (good quality studies) | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.24, 7.75] |
| 2.2 No cure (poor quality studies) | 2 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.44, 1.96] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anderton 1983.
| Methods | Alternate allocation. It was unclear whether the following criteria were met: blinding of outcome assessment, measurement of contamination of control group, assessment of co‐interventions, any deviation from protocol. Providers and pregnant women were not blinded. Informed consent was obtained. No description of sample size or power calculation was provided. | |
| Participants | 67 women enrolled in study. Setting: out‐patient clinic in United Kingdom. Inclusion criteria: pregnant women > 16 years; confirmed asymptomatic bacteriuria with 2 consecutive positive bacteriologic count of identical organisms; urine culture sensitive to amoxicillin. Exclusion criteria: allergic to penicillin or cephalosporins; inability to take oral medications; requires parenteral antibiotics. | |
| Interventions | Experimental group: amoxicillin 3 g x 2 doses. Control group: amoxicillin 250 mg 3 times daily x 7 days. | |
| Outcomes | Clinical outcomes: medication side effects. Laboratory outcomes: rate of 'no cure'. | |
| Notes | Type of healthcare provider: unknown. Attrition bias: no loss to follow‐up from experimental group 0/33; loss of follow‐up for control group 2/34 (6%). Authors state that the 'majority' of study participants had asymptomatic bacteriuria; no description of distribution or breakdown by outcomes is provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The author used alternate allocation, which is not a good practice. |
| Allocation concealment (selection bias) | High risk | Inadequate. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up from experimental group 0/33; loss of follow‐up for control group 2/34 (6%). |
| Selective reporting (reporting bias) | Unclear risk | Not mentioned. |
| Other bias | Unclear risk | Lack of information in text to assess other sources of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
Bailey 1983.
| Methods | Randomized controlled trial. Method of randomization not described. Envelopes containing group assignment were used (no information re: sealed/opaque). No further description was provided regarding allocation. It was unclear whether the following criteria were met: blinding of outcome assessment, measurement of contamination of control group, assessment of co‐interventions, any deviation from protocol. Providers and pregnant women were not blinded. Consent process not described. No description of sample size or power calculation. | |
| Participants | 44 women enrolled in study. Setting: out‐patient clinic in New Zealand. Inclusion criteria: pregnant women < 30 weeks estimated gestational age; confirmed asymptomatic bacteriuria with mid‐stream urine culture of bacterial count > 100,000, and second urine specimen by suprapubic bladder aspiration showing infection regardless of bacterial count; urine culture was sensitive to co‐trimoxazole. Exclusion criteria: allergic to sulfonamides or co‐trimoxazole. | |
| Interventions | Experimental group: co‐trimoxazole 1.92 g x 1 dose. Control group: co‐trimoxazole 0.96 g twice daily x 5 days. | |
| Outcomes | Clinical outcomes: preterm delivery, pyelonephritis, medication side effects. Laboratory outcomes: no cure, recurrent asymptomatic bacteriuria. | |
| Notes | Type of healthcare provider: unknown. Attrition bias: no loss to follow‐up from experimental group 0/24; 2/20 (10%) women lost to follow‐up from control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. Envelopes, but no mention if they were sealed/opaque. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 of the women treated with a 5‐day course of cotrimoxazole dropped out of the study. |
| Selective reporting (reporting bias) | Unclear risk | Not mentioned. |
| Other bias | Unclear risk | Women in the experimental group were more likely to have a history of urinary tract infection as compared to the control group (50% versus 35%), but we were unclear of the impact of this on specific trial outcomes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
Bailey 1986.
| Methods | Randomized controlled trial. Method of randomization not described. Envelopes containing group assignment were used (no information re: sealed/opaque). No further description was provided regarding allocation. It was unclear whether the following criteria were met: blinding of outcome assessment, measurement of contamination of control group, assessment of co‐interventions, any deviation from protocol. Providers and pregnant women were not blinded. Consent process not described. No description of sample size or power calculation. | |
| Participants | 60 women enrolled in study. Population race/ethnicity 28% 'Polynesian'. Setting: out‐patient clinic in New Zealand. Inclusion criteria: pregnant women 16‐30 weeks' estimated gestational age; confirmed asymptomatic bacteriuria with mid‐stream urine culture of bacterial count > 100,000, and second urine specimen by suprapubic bladder aspiration showing infection regardless of bacterial count. Exclusion criteria: not described. | |
| Interventions | Experimental group: trimethoprim 600 mg x 1 dose. Control group: trimethoprim 300 mg once daily x 5 days. | |
| Outcomes | Clinical outcomes: preterm delivery, pyelonephritis, medication side effects. Laboratory outcomes: no cure, recurrent asymptomatic bacteriuria. | |
| Notes | Type of healthcare provider: unknown. Minimal attrition bias: no loss to follow‐up from experimental group 0/30; 2/30 (7%) women lost to follow‐up from control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. Envelopes were used but no mention if they were sealed/opaque. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 of the women treated with a 5‐day course of trimethoprim moved to another city after initial bacteriological follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Not mentioned. |
| Other bias | Unclear risk | Lack of information in text to assess other sources of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
Bayrak 2007.
| Methods | Randomized controlled trial. 90 pregnant women were randomized to receive either a single dose fosfomycin trometamol or a 5‐day course of cefuroxime axetyl. Pregnant women were not blinded to treatment assignment. It was unclear whether the following criteria were met: measurement of contamination of control group, assessments of co‐interventions. Power calculation described, sample size calculation conducted. | |
| Participants | 90 women were enrolled in the trial. Setting: women attending the department of Urology and ANC clinics of Faith University, Ankora, Turkey. 1 patient in the fosfomycin trometamol group and 5 patients in the cefuroxime axetyl group were lost to follow‐up and excluded from the trial. Inclusion criteria: pregnant women in the second trimester of gestation, confirmed asymptomatic bacteriuria with 2 consecutive clean‐catch urine specimens yielding positive cultures of the same uropathogen. Exclusion criteria: gravidas presenting leukocytosis, fever, urolithiasis, lower back pain, previous urologic surgery, anomalies of the urinary tract. | |
| Interventions | Experimental group: single dose of 3 g fosfomycin trometamol. Control group: cefuroxime axetyl 250 mg twice a day for 5 days. |
|
| Outcomes | Clinical outcome: side effects. Laboratory outcome: bacteriological eradication of uropathogens. |
|
| Notes | Cure rates are informed only as percentages. No ratios are informed. Side effects are informed in percentages. No risk ratios nor confidence intervals provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A block randomization method was used to ensure an equal number of patients in each group. |
| Allocation concealment (selection bias) | Low risk | The blocks were numbered, placed into a bag, and a staff member blinded to the research protocol selected the patients into the treatment groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 patient in the fosfomycin trometamol group and 5 patients in the cefuroxime axetyl group did not come to the follow‐up visit; therefore, they were excluded from the study. |
| Selective reporting (reporting bias) | Unclear risk | Not mentioned. |
| Other bias | Low risk | Sample size and power calculation provided. No other risk noted. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The staff members were blinded. |
Brumfitt 1982.
| Methods | Randomized controlled trial. Randomization tables were used to allocate participants. Unclear measurement of contamination of control group, assessment of co‐interventions, any deviation from protocol. Providers and pregnant women were not blinded. No description of sample size or power calculation. | |
| Participants | 54 women enrolled in a out‐patient antenatal clinic in England, UK. Inclusion criteria: pregnant women with culture confirmed asymptomatic bacteriuria during routine screening. Exclusion criteria: allergic to penicillin, infecting organisms were not sensitive to ampicillin. | |
| Interventions | Experimental group: oral amoxicillin 3 g x 2 doses during 1 day. Control group: oral amoxicillin 250 mg 3 times daily x 7 days. | |
| Outcomes | Clinical outcomes: birthweight, medication side effects. Laboratory outcome: cultures cure rates at 2 and 6 weeks. | |
| Notes | 24% were symptomatic in both groups, 65% were in the second trimester. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization tables. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | Not mentioned. |
| Other bias | Unclear risk | Lack of information in text to assess other sources of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
Estebanez 2009.
| Methods | Randomized, prospective, longitudinal, unblinded trial. Allocation concealment done using random number tables. Method of randomization not described. | |
| Participants | 131 pregnant women enrolled in the study. Setting: out‐patient clinic in Spain. Inclusion criteria: pregnant women with asymptomatic bacteriuria (≧ 100,000 CFU/mL of the same micro‐organism in two consecutive cultures) without fever or symptoms of UTI. Exclusion criteria: having taken antibiotics 14 days prior to taking the culture for any reason other than having UTI; allergy to penicillins; high‐risk pregnancy; admitted to hospital; impossibility of performing follow‐up; anomalies in the urinary tract; infection due to microorganisms resistant to either of the 2 antibiotics and symptomatic UTI. | |
| Interventions | Experimental group: fosfomycin 3 g x 1 dose. Control group: amoxicillin‐clavulanate 500 mg/125 mg tablets every 8 hours for 7 days. |
|
| Outcomes | Clinical outcomes: microbiological cure, recurrences, reinfection, persistences, secondary effects, and therapeutic compliance. | |
| Notes | Authors reported that there were no losses to follow‐up, although outcomes reported for 109 out of 131 women recruited. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 17% LTFU. 22 women excluded from the analyses (10 Amoxiclav, 12 Fosfomycin). Reasons explained in the paper. |
| Selective reporting (reporting bias) | Unclear risk | Not mentioned. |
| Other bias | Unclear risk | Lack of information in text to assess other sources of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
Gerstner 87‐89.
| Methods | Randomized controlled trial. Allocation concealment not described. Allocation to treatment done in blocks of 10‐5 per treatment group. It was unclear whether the following criteria were met: blinding of outcome assessment, measurement of contamination of control group, assessment of co‐interventions, any deviation from protocol. Providers and pregnant women were not blinded. Informed consent was obtained. No description of sample size or power calculation was provided. | |
| Participants | 91 women (53 single dose, 38 longer course) enrolled in study. Setting: multicenter out‐patient clinic in Austria. Inclusion criteria: pregnant women; confirmed asymptomatic bacteriuria with mid‐stream urine culture of bacterial count > 100,000 and bladder catheterization with bacterial count > 10,000 diagnosed with the dip‐slide method; urine culture sensitive to amoxicillin. | |
| Interventions | Experimental group: amoxicillin 3 g x single dose. Control group: amoxicillin 750 mg 3 times daily x 4 days. | |
| Outcomes | Clinical outcomes: medication side effects. Laboratory outcomes: 'no cure', recurrence of asymptomatic bacteriuria after 1 and 4 weeks following therapy. | |
| Notes | Healthcare providers: physicians and nurses. Attrition bias: loss to follow‐up from experimental group was 7/53 (13%); loss to follow‐up from control group was 10/38 (26%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization in groups of 10; 5 patients per regimen. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up from experimental group was 7/53 (13%); loss to follow‐up from control group was 10/38 (26%). |
| Selective reporting (reporting bias) | Unclear risk | Not mentioned. |
| Other bias | Unclear risk | Lack of information in text to assess other sources of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
Lumbiganon 2009.
| Methods | Randomized, double‐blind, placebo‐controlled non‐inferiority trial. Pregnant women were randomly allocated to receive either a 1‐day or a 7‐day course of nitrofurantoin. Compliance was of 98% in both groups. Power calculation described, sample size calculation conducted. Assessment of co‐interventions was done. Deviations from protocol were described. | |
| Participants | 778 women from ANC clinics from Thailand, The Phillippines, Vietnam and Argentina were enrolled in the trial. 9 women in the 1‐day regimen and 10 women in the 7‐day regimen were lost to follow‐up. Sociodemographic characteristics of each group were similar at entry. Inclusion criteria: pregnant women at gestational age 12‐32 weeks with no symptoms of urinary tract infection. Exclusion criteria: history of urinary tract infection during current pregnancy, under steroids and/or antibiotic treatment, presence of any hematological disease including glucose‐6‐phosphate dehydrogenase deficiency. | |
| Interventions | Experimental group: 1‐day nitrofurantoin 100 mg twice a day. Control group: 7‐day nitrofurantoin 100 mg twice a day. |
|
| Outcomes | Clinical outcomes: incidence of symptomatic urinary tract infection, pyelonephritis, preterm delivery, low birthweight, adverse effects. Laboratory outcomes: bacteriologic cure after antibiotic treatment assessed by a urine culture 14 days after the initiation of the treatment. |
|
| Notes | Eligible, but not randomized women reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It was generated using computer‐generated random numbers with randomly varying blocks of 6‐8 (SAS software, SAS Institute, Inc., Cary, NC). |
| Allocation concealment (selection bias) | Low risk | The random allocation was concealed by using sealed, opaque treatment boxes numbered sequentially. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Delivery outcomes were available in 91.7% and 89.0% of the women in the 1‐day and 7‐day regimen respectively. |
| Selective reporting (reporting bias) | Low risk | No. The protocol was registered in Clinical Trial Registration with N° ISRCTNISrctn.IERCTN11966088. |
| Other bias | Low risk | Sample size and power calculation provided. No other risk noted. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled trial. |
Masterton 1985.
| Methods | Randomized controlled trial. Allocation by computer‐generated randomization. There was blinding of outcome assessment and providers (treatment allocation by clinic secretary). Pregnant women were not blinded. It was unclear whether the following criteria were met: measurement of contamination of control group, assessment of co‐interventions, any deviation from protocol. Informed consent was obtained. Power calculation described; sample size calculation conducted, however, sample size achieved inadequate due to limited trial period. | |
| Participants | 102 women were enrolled in study, 90 completed the protocol, and 62 women were analyzed as the subgroup 'antenatal asymptomatic bacteriuria'. Wives (British nationality) of servicemen from United Kingdom stationed in Germany. Setting: out‐patient clinic. Inclusion criteria: pregnant women 28‐36 weeks' estimated gestational age; confirmed asymptomatic bacteriuria with 2 consecutive urine cultures of bacterial count ≧ 100,000 colonies/mL urine; urine culture was ampicillin sensitive. Exclusion criteria: pyelonephritis; history of drug sensitivity to beta‐lactam agents; or use of antibiotics within last 2 weeks. | |
| Interventions | Experimental group: amoxicillin 3 g single dose. Control group: ampicillin 500 mg 4 times daily x 7 days. | |
| Outcomes | Laboratory outcomes: 'no cure', recurrent asymptomatic bacteriuria. Re‐infection assessed at 1 week and 6 weeks after treatment. | |
| Notes | Type of healthcare provider: general practitioners and obstetricians. No attrition bias: no women lost from experimental group 0/39, or from control group 0/23. Ampicillin rather than amoxicillin was chosen because it is standard in this hospital. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list. |
| Allocation concealment (selection bias) | Unclear risk | Allocation done by secretary. Open‐label study. Not mention on how secretary was protected to introduce selection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 90 out of 102 women enrolled (88.2%) completed the protocol. |
| Selective reporting (reporting bias) | Unclear risk | Not mentioned. |
| Other bias | Low risk | Sample size and power calculation provided. No other risk noted. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women not blinded to trial treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Personal in charge of outcome assessment blinded to treatment. |
Olsen 1989.
| Methods | Randomized controlled trial. No description of method for generation and concealment of treatment. Providers and pregnant women were not blinded. It was unclear whether the following criteria were met: blinding of outcome assessment, measurement of contamination of control group, assessment of co‐interventions, any deviation from protocol. Informed consent was obtained. No description of sample size or power calculation. | |
| Participants | 41 women enrolled in study. Setting: out‐patient clinic in Denmark. Inclusion criteria: pregnant women < 36 weeks estimated gestational age; confirmed asymptomatic bacteriuria with 2 consecutive urine cultures of bacterial count ≧ 100,000 colonies/mL urine; urine culture was sensitive to sulfamethizole. Exclusion criteria: signs of urinary tract infection; chronic disease of the genitourinary tract; history of more than 2 urinary tract infections in previous 12 months; threatening preterm labour > 26 weeks estimated gestational age; allergy to sulfonamides; antibiotic therapy for any reason within 3 weeks prior to study. | |
| Interventions | Experimental group: sulfamethizole 2 g x single dose. Control group: sulfamethizole 1 g twice daily x 6 days. | |
| Outcomes | Clinical outcomes: medication side effects. Laboratory outcomes: 'no cure', recurrence of asymptomatic bacteriuria. | |
| Notes | Type of healthcare provider: not described. Attrition bias: no women lost from experimental group 0/15; 2/26 (8%) women lost to follow‐up from control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 dropouts in the longer treatment arm. |
| Selective reporting (reporting bias) | Unclear risk | Not mentioned. |
| Other bias | Unclear risk | Lack of information in text to assess other sources of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
Pregazzi 1987.
| Methods | Randomized controlled trial. 44 pregnant women were divided into 2 groups: 1 treated traditionally and the other with single dose. Allocation to treatment is not described. Pregnant women were not blinded. Consent process is not described. It was unclear whether the following criteria were met: measurement of contamination of control group, assessment of co‐interventions, any deviation from protocol. The traditional protocol produced an immediate response in 86.4% of the cases demonstrating its superiority over the single‐dose treatment that brought success in 54.5% of the cases. | |
| Participants | 44 women enrolled in the study. Setting: out‐patient clinic in Trieste University, Italy. October 1980 to December 1985. Inclusion criteria: pregnant women aged 17‐35 years old, confirmed asymptomatic bacteriuria with positive bacteriological count > 100,000 CFU/mL. Exclusion criteria: 1st bacteriological count < 100,000 CFU/mL or 2nd bacteriological count > 100,000 CFU/mL but different micro‐organism comparing with the first one. | |
| Interventions | Experimental group: single dose of amoxicillin 3 g, or ampicillin 3.5 g, or trimethoprim 320 mg, or sulfamethoxazole 1600 mg, or cephalexin 3 g. Control group: 2‐4 times daily x 1‐2 weeks of the antibiotics named in the experiment group. | |
| Outcomes | Clinical outcomes: medication side effects. Laboratory outcomes: no cure rate, recurrent asymptomatic bacteriuria. | |
| Notes | Type of health provider: not described. Attrition bias: no description of loss to follow‐up from experimental group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | Not mentioned. |
| Other bias | Unclear risk | Lack of information in text to assess other sources of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
Reeves 1975.
| Methods | Generation of allocation sequence was by alternation. It was unclear whether the following criteria were met: measurement of contamination of control group, assessment of co‐interventions, any deviation from protocol. Blinding of outcome assessment, providers and pregnant women was not done. Consent process not described. No description of sample size or power calculation. | |
| Participants | 100 women enrolled in study. Setting: antenatal clinics in England. Inclusion criteria: pregnant women attending the bacteriuria clinic; confirmed bacteriuria with 2 consecutive urine cultures of bacterial count greater than or equal to 100,000 colonies/mL urine; urine culture was sensitive to sulfonamides. Exclusion criteria: not described. | |
| Interventions | Experiment group: sulfonamide sulfametopyrazine 2 g single dose, orally in 50 mL of water in the clinic. Control group: sulfadimidine 1 g 4 times daily x 7 days. | |
| Outcomes | Clinical outcome: medication side effects. Laboratory outcome: 'no cure', 2 and 6 weeks after the first dose. Growth of different bacteria considered re‐infection. | |
| Notes | Type of healthcare provider: physicians. Attrition bias: loss to follow‐up from experimental group 5/54 (9% ); loss to follow‐up from control group 6/46 (13%). Unable to abstract data on 'recurrent asymptomatic bacteriuria': Reeves's operational definition of cure rate at 6 weeks was incomparable to the definition of recurrent asymptomatic bacteriuria used for review. No differentiation was made between asymptomatic and symptomatic bacteriuria in methods or results section. However, no indication or description was provided on the presence of any signs or symptoms of urinary tract infection, thus, the study was included in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternated. |
| Allocation concealment (selection bias) | High risk | Not used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/54 (9%) were lost to follow‐up from the experimental arm; 6/46 (13%) from the control one. |
| Selective reporting (reporting bias) | Unclear risk | Not mentioned. |
| Other bias | Unclear risk | Lack of information in text to assess other sources of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
Thoumsin 1990.
| Methods | Randomized controlled study. Allocation to treatment not described. Pregnant women nor provider were blinded. Informed consent was taken. No description of sample size or power calculation was provided (the study did not conclude). It was unclear whether the following criteria were met: measurement of contamination of control group, assessment of co‐interventions, any deviation from protocol. | |
| Participants | 23 women enrolled in the study. Setting: out‐patient clinic in Belgium. Inclusion criteria: significant bacteriuria (10,000 CFU/mL or more) without symptoms. Exclusion criteria: not described. | |
| Interventions | Experimental group: single dose of fosfomycin trometamol 3 g. Control group: 7‐day course of nitrofurantoin 100 mg twice a day. | |
| Outcomes | Clinical outcome: medication side effect. Laboratory outcomes: rate of no cure. | |
| Notes | Type of healthcare provider: not described. Attrition bias: no description of loss to follow‐up. from experimental group nor for control group. This study shows preliminary results. No final results were published. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | Not mentioned. |
| Other bias | Unclear risk | Lack of information in text to assess other sources of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
ANC: antenatal care CFU: colony forming units g: grams LTFU: long‐term follow‐up mg: milligrams mL: milliliters UTI: urinary tract infections
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adelson 1992 | In this study, 66% of the experimental group were determined to have symptomatic bacteriuria as compared to 33% with asymptomatic bacteriuria. Similarly, in the control group 63% of women were determined to have symptomatic bacteriuria as compared to 37% with asymptomatic bacteriuria. Both symptomatic and asymptomatic bacteriuria were combined when outcomes were assessed; therefore, the study is excluded because of the inability to assess asymptomatic bacteriuria separately. Clarification of data is being sought from authors. |
| Bint 1979 | In this study 100 pregnant women were screened and randomly allocated in equal numbers to receive either 400 mg pivmecillinam 4 times daily for 7 days or 500 mg ampicillin 4 times daily for 7 days. The treatment did not differ in duration. No distinction was made between asymptomatic and symptomatic bacteriuria. Cure rates were 88% in the pivmecillinam group and 85% in the ampicillin group. Side effects were significantly more frequent in the pivmecillinam group. |
| Brumfitt 1973 | This paper includes 2 trials. The first trial compares a 7‐day course of treatment with trimethoprim‐sulfamethoxazole in 3 different combinations, but of the same duration. In the second trial, 4 drugs were administered at high doses to investigate the possibility that better results might be obtained with high blood levels of antibiotic than smaller dose. There was not a distinction between asymptomatic and symptomatic bacteriuria. |
| Campbell‐Brown 1983 | This is not a randomized controlled trial. In this study bacteriuria was diagnosed from suprapubic aspiration specimens of urine. All participants received the same treatment: single dose of 3 g cephalexin. There was no distinction between symptomatic and asymptomatic pregnant women. The cure rate achieved was 70%. 30% of the participants who were not cured received a 7‐day course of antibacterial according to the sensitivity of the organism isolated. 36% continued with the infection and were given continuous antibacterial therapy. |
| Davies 1975 | 4 drugs were compared but all were administered during a week. There were 53 women enrolled, 26 (49%) of whom had urinary symptoms at the first clinic attendance. |
| De Cecco 1987 | In this study no distinction was made between asymptomatic bacteriuria and symptomatic bacteriuria. The study participants included pregnant women > 8 weeks estimated gestational age who had any bacteriuria > 100,000. The terminology used in the paper to refer to the exposure of interest was 'lower urinary tract infections'. Therefore, this study was excluded. In addition, it was unclear whether randomization was carried out: there was a marked imbalance in the study groups (52 versus 31). If 1 of the 2 antimicrobial treatment regimens failed, women were switched to the other treatment regimen; no description was provided on how many or which women crossed over. |
| Harris 1982 | 86 pregnant women were sequentially assigned to 1 of 4 single‐dose, single‐antimicrobial treatment groups: ampicillin 2 g, plus probenimide 1 g; keflex 2 g, plus probenimide 1 g; macrodantin 200 mg; or, gantrisin 2 g. All participants were diagnosed as having asymptomatic bacteriuria if they had no symptoms of urinary tract infection and had 2 consecutive urine cultures > 100,000 colonies per mL of the same species of micro‐organism. The overall no cure rate was 31%, with a recurrence rate of 3.5%. |
| Jakobi 1987 | 50 asymptomatic pregnant women were treated with 3 g of amoxicillin or 2 g of cephalexin in accordance with the isolated micro‐organism disk sensitivity. The study was not based on randomization. There was no difference in duration of treatment. The immediate cure rate was 84% and the recurrence rate was 12%. The failure of treatment with single‐dose treatment was 16%, these participants were treated with the same drug administered for 7 days and were cured. It is possible that these participants had upper UTI or urinary tract malformations. |
| McFadyen 1987 | The main comparison of interest to the present review, single dose versus 3‐day regimen, was not based on randomization. 2 3‐day regimens that compared cephalexin 1 g with pivmecillinam‐pivampicillin in an independent randomized trial (reported separately) were grouped together in this paper and compared with a non‐randomized series of pregnant women who received a single dose of cephalexin 3 g orally. |
| Pathak 1969 | In this study, participants were randomized to nitrofurantoin or placebo, all of them during a 3‐week period. |
| Pedler 1985 | In this study no distinction was made between asymptomatic and symptomatic bacteriuria. Women were randomly allocated to receive either 1 tablet of amoxicillin‐clavulanic acid 3 times daily or 250 mg of cephalexin 3 times daily for 7 days. There was no difference in the duration of treatment. The study compared different choices of antimicrobials. Differences in cure rates were not statistically significant. No significant difference in the rate of side effects was found. No toxicity to the fetus was seen which could be ascribed to either drug. |
| Rafalsky 2013 | Prospective, multicenter and randomized trial. 58 women were randomized in group 1 (cefixime 400 mg once day, 7 days) and 54 women were included in group 2 (amoxicillin/clavulanate 625mg 3 times a day, 7 days). The treatment did not differ in duration. |
| Robertson 1968 | Women were alternately allocated into treatment with a 2‐week course of either cycloserine 250 mg 2 times daily or sulfadimidine 0.5 g 4 times daily. There was no difference in the duration of treatment; what was compared was the difference in choice of antimicrobial. |
| Sanderson 1984 | This study aimed at reducing reinfection. Participants whose urine was found to be sterile (after 7‐day course of pivmecillinam 1 tablet thrice daily), received at random pivmecillinam sachets prophylactically, 100 mg in the evening on alternates days, or were allocated to a control group, who were given no treatment. |
| Whalley 1977 | Randomized and non‐randomized study participants were combined together. This study was excluded because it was not possible to analyze the results of randomized and non‐randomized groups separately. |
| Zinner 1990 | Pregnant women with symptomatic and asymptomatic bacteriuria were combined. The majority of women in the study population were described as having symptomatic bacteriuria; therefore, the study is excluded because of the inability to assess asymptomatic bacteriuria separately. An additional concern was the introduction of bias through the sampling method; the sample size of n = 291 was obtained from 25 different study sites in Italy. |
n: number UTI: urinary tract infection
Differences between protocol and review
Methods updated to the current standards of the Cochrane Pregnancy and Childbirth Group. 'Summary of findings' tables have been incorporated.
Contributions of authors
J Villar and AM Gülmezoglu had the idea for the preparation of this review. MT Lydon‐Rochelle and A Roganti worked on the background, data extraction and writing the first version of the review.
M Widmer prepared the 2009 update. AM Gulmezoglu review drafts and provided suggestions for revisions. LE Mignini and A Roganti provided comments to the final draft.
I Lopez prepared the 2015 update, commented on and revised all drafts of the update, extracted data, assessed the new included studies, and prepared the 'Summary of findings' tables with the help of Virginia Diaz, Monica Chamilard and Edgardo Abalos. All authors revised and agreed the 2015 update. M Widmer is the guarantor of this review.
Sources of support
Internal sources
HRP‐UNDP/UNFPA/WHO/WORLD BANK, Switzerland.
External sources
UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Anderton 1983 {published data only}
- Anderton KJ, Abbas AM, Davey A, Ancill RJ. High dose, short course amoxycillin in the treatment of bacteriuria in pregnancy. British Journal of Clinical Practice 1983;37:212‐4. [PubMed] [Google Scholar]
Bailey 1983 {published data only}
- Bailey RR, Bishop V, Peddie BA. Comparison of single dose with a 5‐day course of co‐trimoxazole for asymptomatic (covert) bacteriuria of pregnancy. Australian and New Zealand Journal of Obstetrics and Gynaecology 1983;23:139‐41. [DOI] [PubMed] [Google Scholar]
Bailey 1986 {published data only}
- Bailey RR, Peddie BA, Bishop V. Comparison of single dose with a 5‐day course of trimethoprim for asymptomatic (covert) bacteriuria of pregnancy. New Zealand Medical Journal 1986;99:501‐3. [PubMed] [Google Scholar]
Bayrak 2007 {published data only}
- Bayrak O, Cimentepe E, Inegol I, Atmaca AF, Duvan CI, Koc A, et al. Is single‐dose fosfomycin trometamol a good alternative for asymptomatic bacteriuria in the second trimester of pregnancy?. International Urogynecology Journal 2007;18(5):525‐9. [DOI] [PubMed] [Google Scholar]
Brumfitt 1982 {published data only}
- Brumfitt W, Hamilton Miller JM, Franklin IN, Anderson FM, Brown GM. Conventional and two dose amoxycillin treatment of bacteriuria in pregnancy and recurrent bacteriuria: a comparative study. Journal of Antimicrobial Chemotherapy 1982;10:239‐48. [DOI] [PubMed] [Google Scholar]
Estebanez 2009 {published data only}
- Estebanez A, Pascual R, Gil V, Ortiz F, Santibanez M, Perez Barba C. Fosfomycin in a single dose versus a 7‐day course of amoxicillin‐clavulanate for the treatment of asymptomatic bacteriuria during pregnancy. European Journal of Clinical Microbiology & Infectious Diseases 2009;28(12):1457‐64. [DOI] [PubMed] [Google Scholar]
Gerstner 87‐89 {published data only}
- Gerstner GJ, Muller G, Nahler G. Amoxicillin in the treatment of asymptomatic bacteriuria in pregnancy: a single dose of 3g amoxicillin vs a 4‐day course of 3 doses 750mg amoxicillin. Gynecologic and Obstetric Investigation 1989;27:84‐7. [DOI] [PubMed] [Google Scholar]
- Gertsner GJ, Muller G, Nahler G. Amoxicillin in the treatment of asymptomatic bacteriuria in pregnancy‐‐3g single dose versus 3 times 750mg 4‐day therapy [Amoxicillin zur Behandlung der asymptomatischen Bakteriurie in der Schwangerschaft‐‐3g Einmal‐versus 3 x 750mg 4‐Tagestherapie]. Zeitschrift fur Geburtshilfe und Perinatologie 1987;191(5):202‐5. [PubMed] [Google Scholar]
Lumbiganon 2009 {published data only}
- Lumbiganon P, Villar J, Laopaiboon M, Widmer M, Thinkhamrop J, Carroli G, et al. One‐day compared with 7‐day nitrofurantoin for asymptomatic bacteriuria in pregnancy: a randomized controlled trial. Obstetrics & Gynecology 2009;113(2 Pt 1):339‐45. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Multicentre randomized placebo‐controlled trial to evaluate the effectiveness of one‐day versus seven‐day course of nitrofurantoin for the treatment of asymptomatic bacteriuria. World Health Organization (www.who.int/reproductive‐health/publications/highlights/hights_hrp_2005.html) (accessed 25 April 2006).
Masterton 1985 {published data only}
- Masterton RG, Evans DC, Strike PW. Single‐dose amoxycillin in the treatment of bacteriuria in pregnancy and the puerperium‐a controlled clinical trial. British Journal of Obstetrics and Gynaecology 1985;92:498‐505. [DOI] [PubMed] [Google Scholar]
Olsen 1989 {published data only}
- Olsen L, Nielsen IK, Zachariassen A, Sederberg‐Olsen J, Frimodt‐Moller N. Single‐dose vs six‐day therapy with sulfamethizole for asymptomatic bacteriuria during pregnancy. Danish Medical Bulletin 1989;36:486‐7. [PubMed] [Google Scholar]
Pregazzi 1987 {published data only}
- Pregazzi R, Mazzatenta E, Bouche C. Single‐dose antibiotic therapy of asymptomatic bacteriuria in pregnancy. Results and complications. Minerva Ginecologica 1987;39:289‐92. [PubMed] [Google Scholar]
Reeves 1975 {published data only}
- Reeves DS. Laboratory and clinical studies with sulfametopyrazine as a treatment for bacteriuria in pregnancy. Journal of Antimicrobial Chemotherapy 1975;1:171‐86. [DOI] [PubMed] [Google Scholar]
Thoumsin 1990 {published data only}
- Thoumsin H, Aghayan M, Lambotte R. Single dose fosfomycin trometamol versus multiple dose nitrofurantoin in pregnant women with bacteriuria: preliminary results. Infection 1990;18:S94‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adelson 1992 {published data only}
- Adelson MD, Graves WL, Osborne NG. Treatment of urinary infections in pregnancy using single dose vs 10 day dosing. Journal of the National Medical Association 1992;84:73‐5. [PMC free article] [PubMed] [Google Scholar]
Bint 1979 {published data only}
- Bint A, Bullock D, Reeves D, Wilkinson P. A comparative trial of pivmecillinam and ampicillin in bacteriuria in pregnancy. Infection 1979;7:290‐3. [DOI] [PubMed] [Google Scholar]
Brumfitt 1973 {published data only}
- Brumfitt W, Pursell R. Trimethoprim ‐ sulfamethoxazole in treatment of bacteriuria in women. Journal of Infectious Diseases 1973;128 Suppl:S657‐S663. [DOI] [PubMed] [Google Scholar]
Campbell‐Brown 1983 {published data only}
- Campbell‐Brown M, Fadyen IR. Bacteriuria in pregnancy treated with a single dose of cephalexin. British Journal of Obstetrics and Gynaecology 1983;90(11):1054‐9. [DOI] [PubMed] [Google Scholar]
Davies 1975 {published data only}
- Davies BI, Mummery RV, Brummfitt W. Ampicillin, carbenicillin indanyl ester, and nifurantel in treatment of urinary infection in domiciliary practice. British Journal of Urology 1975;47:335‐41. [DOI] [PubMed] [Google Scholar]
De Cecco 1987 {published data only}
- Cecco L, Ragni N. Urinary tract infections in pregnancy: monuril single‐dose treatment vs traditional therapy. European Urology 1987;13:108‐13. [DOI] [PubMed] [Google Scholar]
Harris 1982 {published data only}
- Harris RE, Gilstrap LC, Pretty A. Single‐dose antimicrobial therapy for asymptomatic bacteriuria during pregnancy. Obstetrics & Gynecology 1982;59:546‐8. [PubMed] [Google Scholar]
Jakobi 1987 {published data only}
- Jakobi P, Neiger R, Merzbach D, Paldi E. Single‐dose antimicrobial therapy in the treatment of asymptomatic bacteriuria in pregnancy. American Journal of Obstetrics and Gynecology 1987;156(5):1148‐52. [DOI] [PubMed] [Google Scholar]
McFadyen 1987 {published data only}
- McFadyen IR, Campbell BM, Stephenson M, Seal DV. Single‐dose treatment of bacteriuria in pregnancy. European Urology 1987;13 Suppl 1:22‐5. [DOI] [PubMed] [Google Scholar]
Pathak 1969 {published data only}
- Pathak UN, Tang K, Williams LL, Stuart KL. Bacteriuria of pregnancy: results of treatment. Journal of Infectious Diseases 1969;120:91‐103. [DOI] [PubMed] [Google Scholar]
Pedler 1985 {published data only}
- Pedler S, Bint A. Comparative study of amoxicillin‐clavulanic acid and cephalexin in the treatment of bacteriuria during pregnancy. Antimicrobial Agents and Chemotherapy 1985;27:508‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rafalsky 2013 {published data only}
- Rafal'skii VV, Dovgan' EV, Kozyrev IuV, Gustovarova TA, Khlybova SV, Novoselova AV, et al. The efficacy and safety of cefixime and amoxicillin/clavulanate in the treatment of asymptomatic bacteriuria in pregnant women: a randomized, prospective, multicenter study. Urologiia 2013;5(24):26‐8. [PubMed] [Google Scholar]
- Rafalskiy V, Dovgan E, Kozyrev Y, Gustovarova T, Khlybova S, Novoselova A, et al. Cefixime vs. amoxicillin/clavulanate in pregnant women with asymptomatic bacteriuria: Multicentre randomised study. Clinical Microbiology and Infection 2012;18(Suppl s3):425‐6. [Google Scholar]
Robertson 1968 {published data only}
- Robertson JG, Livingstone JRB, Isdale MH. The management and complications of asymptomatic bacteriuria in pregnancy. Journal of Obstetrics and Gynaecology of the British Commonwealth 1968;75:59‐65. [DOI] [PubMed] [Google Scholar]
Sanderson 1984 {published data only}
- Sanderson P, Menday P. Pivmecillinam for bacteriuria in pregnancy. Journal of Antimicrobial Chemotherapy 1984;13:383‐8. [DOI] [PubMed] [Google Scholar]
Whalley 1977 {published data only}
- Whalley PJ, Cunningham FG. Short‐term vs continuous antimicrobial therapy for asymptomatic bacteriuria in pregnancy. Obstetrics & Gynecology 1977;49:262‐5. [PubMed] [Google Scholar]
Zinner 1990 {published data only}
- Zinner S. Fosfomycin trometamol versus pipemidic acid in the treatment of bacteriuria during pregnancy. Chemotherapy 1990;36:50‐2. [DOI] [PubMed] [Google Scholar]
Additional references
Andrews 1992
- Andrews WW, Gilstrap LC. Urinary tract infections. In: Gleicher N editor(s). Principles and Practice of Medical Therapies in Pregnancy. Appleton and Lange, Norwalk, Co, 1992:913‐7. [Google Scholar]
Davison 1992
- Davison JM, Lindheimer MD. Chronic renal disease. In: Gleicher N editor(s). Principles and Practice of Medical Therapies in Pregnancy. Appleton and Lange, Norwalk, Co, 1992:928‐32. [Google Scholar]
Goldenberg 2000
- Goldberg RL, Hauth JC, Andrews WW. Mechanisms of disease: intrauterine infection and preterm delivery. New England Journal of Medicine 2000;342(20):1500‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jones 1996
- Jones B, Jarvis P, Lewis JA, Ebbutt AF. Trials to assess equivalence: the importance of rigorous methods. BMJ 1996;313:36‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kazemier 2015
- Kazemier BM, Koningstein FN, Schneeberger C, Ott A, Bossuyt PM, Miranda E, et al. Maternal and neonatal consequences of treated and untreated asymptomatic bacteriuria in pregnancy: a prospective cohort study with an embedded randomised controlled trial. Lancet Infectious Diseases 2015 August. [Epub ahead of print]. [DOI] [PubMed]
Laxminarayan 2014
- Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, et al. Antibiotic resistance ‐ the need for global solutions. Lancet Infectious Diseases 2013;13(2):1057‐98. [DOI] [PubMed] [Google Scholar]
Meis 1995a
- Meis PJ, Michielutte R, Peters TJ, Wells HB, Sands E, Coles EC, et al. Factors associated with preterm birth in Cardiff, Wales. I. Univariable and multivariable analysis. American Journal of Obstetrics and Gynecology 1995;173:590‐6. [DOI] [PubMed] [Google Scholar]
Meis 1995b
- Meis PJ, Michielutte R, Peters TJ, Wells HB, Sands E, Coles EC, et al. Factors associated with preterm birth in Cardiff, Wales. II. Indicated and spontaneous preterm birth. American Journal of Obstetrics and Gynecology 1995;173:597‐602. [DOI] [PubMed] [Google Scholar]
NICE 2008
- National Institute for Health and Clinical Excellence: Guidance. In: Jane Moody editor(s). Antenatal Care: Routine Care for the Healthy Pregnant Woman. London: RCOG Press, 2008. [PubMed] [Google Scholar]
Nicolle 2005
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clinical Infectious Diseases 2005;40(5):643‐54. [DOI] [PubMed] [Google Scholar]
NVOG 2011
- Dutch Society of Obstetrics and Gynecology. NVOG Guideline: Urinary Tract Infection in Pregnancy. Amsterdam: NVOG, 2011. [Google Scholar]
Ramsey 2000
- Ramsey P, Goldenberg R. Maternal Infections and their consequences. In: Newell, McIntyre editor(s). Congenital and Perinatal Infections. Prevention, Diagnosis and Treatment. 1st Edition. Cambridge: Cambridge University Press, 2000:32‐63. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Romero 1989
- Romero R, Oyarzun E, Mazor M, Sirtori M, Hobbins JC, Bracken M. Meta‐analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstetrics & Gynecology 1989;73:576‐82. [PubMed] [Google Scholar]
Rouse 1995
- Rouse DJ, Andrews WW, Goldenberg RL, Owen J. Screening and treatment of asymptomatic bacteriuria of pregnancy to prevent pyelonephritis: a cost‐effectiveness and cost‐beneficial analysis. Obstetrics & Gynecology 1995;86:119‐23. [DOI] [PubMed] [Google Scholar]
Smaill 2015
- Smaill FM, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD000490.pub3] [DOI] [PubMed] [Google Scholar]
Sweet 1977
- Sweet RL. Bacteriuria and pyelonephritis during pregnancy. Seminars in Perinatology 1977;1:25‐40. [PubMed] [Google Scholar]
Van Haaren 2005
- Haaren KAM, Visser HS, Vliet S, Timmermans AE, Yadava R, Geerlings SE, et al. Guideline of the Dutch College of General Practitioners on urinary tract infections: second revision [NHG‐standaard urineweginfecties: 2e herziening]. Huisarts en Wetenschap 2005;48(10):341‐52. [Google Scholar]
Villar 1994
- Villar J, Ezcurra EJ, Gurtner de la Fuente V, Campodonico L. Preterm delivery syndrome ‐ the unmet need. Research and Clinical Forums 1994;16:9‐39. [Google Scholar]
Villar 1997
- Villar J, Gülmezoglu AM, Onis M. Nutritional and antimicrobial interventions to prevent preterm birth: an overview of randomized control trials. Obstetrical & Gynecological Survey 1998;53:575‐85. [DOI] [PubMed] [Google Scholar]
Wang 1989
- Wang E, Smaill F. Infection in pregnancy. In: Chalmers I, Enkin MW, Keirse MJNC editor(s). Effective Care in Pregnancy and Childbirth. Oxford: Oxford University Press, 1989:534‐7. [Google Scholar]
References to other published versions of this review
Smaill 1992a
- Smaill F. Single dose vs 4‐7 day antibiotic for bacteriuria. [revised 02 April 1992]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Smaill 1992b
- Smaill F. Single dose vs 7‐day course of antibiotics for bacteriuria. [revised 02 April 1992]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration. Issue 2, Oxford: Update Software; 1995.
Smaill 1992c
- Smaill F. Two week vs continuous antibiotic for bacteriuria. [revised 02 April 1992]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration. Issue 2, Oxford: Update Software; 1995.
Villar 2000
- Villar J, Widmer M, Lydon‐Rochelle M, Gülmezoglu AM, Roganti A. Duration of treatment for asymptomatic bacteriuria during pregnancy. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000491] [DOI] [PubMed] [Google Scholar]
Widmer 2011
- Widmer M, Gülmezoglu AM, Mignini L, Roganti A. Duration of treatment for asymptomatic bacteriuria during pregnancy. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD000491.pub2] [DOI] [PubMed] [Google Scholar]


