Abstract
Background
Prostaglandins have mainly been used for postpartum haemorrhage (PPH) when other measures fail. Misoprostol, a new and inexpensive prostaglandin E1 analogue, has been suggested as an alternative for routine management of the third stage of labour.
Objectives
To assess the effects of prophylactic prostaglandin use in the third stage of labour.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (7 January 2011). We updated this search on 25 May 2012 and added the results to the awaiting classification section.
Selection criteria
Randomised trials comparing a prostaglandin agent with another uterotonic or no prophylactic uterotonic (nothing or placebo) as part of management of the third stage of labour. The primary outcomes were blood loss 1000 mL or more and the use of additional uterotonics.
Data collection and analysis
Two review authors independently assessed eligibility and trial quality and extracted data.
Main results
We included 72 trials (52,678 women). Oral or sublingual misoprostol compared with placebo is effective in reducing severe PPH (oral: seven trials, 6225 women, not totalled due to significant heterogeneity; sublingual: risk ratio (RR) 0.66; 95% confidence interval (CI) 0.45 to 0.98; one trial, 661 women) and blood transfusion (oral: RR 0.31; 95% CI 0.10 to 0.94; four trials, 3519 women).
Compared with conventional injectable uterotonics, oral misoprostol was associated with higher risk of severe PPH (RR 1.33; 95% CI 1.16 to 1.52; 17 trials, 29,797 women) and use of additional uterotonics, but with a trend to fewer blood transfusions (RR 0.84; 95% CI 0.66 to 1.06; 15 trials; 28,213 women). Additional uterotonic data were not totalled due to heterogeneity. Misoprostol use is associated with significant increases in shivering and a temperature of 38º Celsius compared with both placebo and other uterotonics.
Authors' conclusions
Oral or sublingual misoprostol shows promising results when compared with placebo in reducing blood loss after delivery. The margin of benefit may be affected by whether other components of the management of the third stage of labour are used or not. As side‐effects are dose‐related, research should be directed towards establishing the lowest effective dose for routine use, and the optimal route of administration.
Neither intramuscular prostaglandins nor misoprostol are preferable to conventional injectable uterotonics as part of the management of the third stage of labour especially for low‐risk women; however, evidence has been building for the use of oral misoprostol to be effective and safe in areas with low access to facilities and skilled healthcare providers and future research on misoprostol use in the community should focus on implementation issues.
Keywords: Female; Humans; Pregnancy; Labor Stage, Third; Administration, Oral; Administration, Sublingual; Fever; Fever/chemically induced; Misoprostol; Misoprostol/adverse effects; Misoprostol/therapeutic use; Oxytocics; Oxytocics/adverse effects; Oxytocics/therapeutic use; Postpartum Hemorrhage; Postpartum Hemorrhage/prevention & control; Prostaglandins; Prostaglandins/therapeutic use; Randomized Controlled Trials as Topic; Shivering
Plain language summary
Prostaglandins for preventing postpartum haemorrhage
An injectable uterotonic is the drug of choice for routine third stage management when the placenta is delivered. Oral or sublingual misoprostol may be used where no injectable uterotonic is available.
After her baby is born, the woman's womb (uterus) contracts and bleeding decreases. If the womb does not contract, postpartum haemorrhage (heavy bleeding) can occur, which can be life threatening. A prostaglandin, oxytocin and ergometrine are all drugs that cause contractions of the womb (uterotonics). This review of 72 randomised controlled trials, involving 52,678 women, found that oral or sublingual prostaglandin (misoprostol) is effective in reducing severe haemorrhage after giving birth and the need for blood transfusions. Misoprostol is not as effective as oxytocin and has more side‐effects. The main side‐effects are shivering, high temperature and diarrhoea, occurring in a significant proportion of women. Twenty‐six of the trials included centres in low‐ and middle‐income countries only. Misoprostol may be useful in places where injectable uterotonics are not available, perhaps because of poor access to skilled healthcare providers. Injectable prostaglandin may be effective in reducing blood loss but has adverse effects of vomiting, abdominal pain and diarrhoea and costs more.
Background
Postpartum haemorrhage (PPH) is a major cause of morbidity and mortality during childbirth, especially in low‐ and middle‐income countries. The contribution of PPH to maternal death in low‐ and middle‐income countries is more marked in domiciliary or rural settings where trained staff are scarce, transport facilities are inadequate and the availability of uterotonic agents and blood are limited. According to the latest population‐based maternal mortality survey in Ghana, haemorrhage is the main cause, accounting for 24% of the maternal deaths (Ghana 2009).
The third stage of labour is defined as the period from birth of the baby until the delivery of the placenta and its membranes. This stage usually takes less than 10 minutes when active management is used. Active management of the third stage of labour is a term to express the use of uterotonics, early cord clamping and active efforts to deliver the placenta following birth. It is not always clearly defined and universally applied in a standard manner. PPH is usually defined as blood loss of 500 mL or more and severe PPH as 1000 mL or more in the third stage of labour. The 'normal' amount of blood loss is difficult to ascertain because different ways of managing the third stage and assessing the blood loss lead to markedly different amounts.
It has been well demonstrated that active management of the third stage of labour is associated with less blood loss. There seems to be general agreement that if the blood loss exceeds 500 mL close monitoring and additional measures such as administering uterotonics or checking for a cause of bleeding are prudent measures.
Traditionally, oxytocin and ergot preparations have been used as uterotonic agents for PPH prophylaxis mostly as part of active management of the third stage of labour. These agents, although effective in decreasing the blood loss, have the disadvantage of instability in tropical climates (Hogerzeil 1996) and also require the use of syringes and trained personnel for administration. Another disadvantage, mainly related to ergot preparations, is the relatively high incidence of side‐effects such as nausea, vomiting and increase in blood pressure.
Prostaglandins have strong uterotonic properties and are used widely in obstetric and gynaecological practice for cervical ripening, together with mifepristone for termination of pregnancy and for induction of labour. Prostaglandin preparations are available in injectable, tablet or gel forms according to their intended use. These agents do not cause hypertension, which enables them to be used in hypertensive patients. In the management of the third stage of labour, prostaglandins have been mainly used for intractable PPH as a last resort when other measures fail. To date, the main disadvantages of prostaglandins have been their cost and availability. Since the mid‐1990s, misoprostol, a prostaglandin E1 analogue used orally for the prevention of peptic ulcer disease has also been reported for use in the management of the third stage of labour (El‐Refaey 1997). Misoprostol is inexpensive, can be administered orally and is stable at ambient temperatures. There is considerable experience with misoprostol use, both for peptic ulcer disease and as a uterotonic in obstetrics and gynaecology. The main side‐effects of prostaglandins are nausea, vomiting and diarrhoea. Shivering and elevated body temperature have been reported with the use of misoprostol in the third stage of labour.
The use of prostaglandins in general, and of misoprostol in particular, could have implications for the efficacy and acceptability of active management of the third stage of labour. The rate and nature of side‐effects (nausea, vomiting, diarrhoea, shivering) could influence the immediate relationship between the mother and her baby in the hours following birth.
Active management of the third stage of labour (by use of uterotonics, early cord clamping and active efforts to deliver the placenta) decreases blood loss during the third stage of labour (Begley 2011).
This review is one in a series of reviews evaluating strategies to prevent PPH (Cotter 2001; McDonald 2004) and focuses on the role of prostaglandins in the active management of the third stage of labour.
Objectives
To determine the effectiveness of prophylactic prostaglandin use compared with placebo or conventional uterotonics as part of the routine management of the third stage of labour.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials with a comparison between a prostaglandin and either another uterotonic agent or no uterotonic agent (placebo or nothing) were considered for inclusion in the review. We excluded quasi‐random studies.
Types of participants
Women after the birth of their baby were the participants of this review. These women could be at high or low risk for postpartum haemorrhage. The definitions of high risk used by the trialists were accepted in general. These typically included having had a previous postpartum haemorrhage, grand multiparity and multiple pregnancy among others.
Studies including women with caesarean births were eligible.
Types of interventions
In the earlier version of this review we included the use of prostaglandins when used 'as part of active management of the third stage of labour'. Recently, there has been increasing interest in evaluating the individual components of the 'active management' package and at least one trial has evaluated the use of a uterotonic without other components of active management of the third stage of labour. We included the use of a prostaglandin alone within the scope of this review.
The experimental intervention evaluated in this review is the prophylactic use of prostaglandins in the management of the third stage of labour. Prostaglandin preparations are currently available in injectable and tablet forms, therefore different routes may be used and compared either with each other or with conventional injectable uterotonic agents. Different routes of administration are analyzed in separate comparisons.
The choice of routine uterotonic drug used during the third stage of labour varies greatly around the world. In this review, oxytocin (Syntocinon®), ergometrine‐oxytocin (Syntometrine®) and ergometrine are grouped together as 'conventional injectable uterotonics'. In cases where the comparison is made between two different types of conventional uterotonics, oxytocin is selected as the conventional uterotonic as it is the drug used in most of the studies included in this review.
The main categories of prostaglandins evaluated in the review are misoprostol (prostaglandin E1 analogue), which is available in tablets and PGF2alpha and E2 preparations that are administered parenterally for use in the third stage of labour. Misoprostol tablets are administered either orally, sublingually or rectally. Since the absorption of misoprostol from these two routes is currently unknown and likely to be different, these routes have been evaluated separately.
Injection of oxytocin or saline, or both, into the umbilical vein (reviewed elsewhere on retained placenta) and intramyometrial injection of prostaglandins other than at caesarean section (not used for routine active management) were not eligible for inclusion in this review.
The following comparisons have been used in the review:
oral misoprostol versus no uterotonic/placebo;
oral misoprostol versus injectable (conventional) uterotonics;
rectal misoprostol versus no uterotonic/placebo;
rectal misoprostol versus injectable uterotonics;
rectal misoprostol versus intramuscular prostaglandins;
sublingual misoprostol versus no uterotonics/placebo;
sublingual misoprostol versus injectable uterotonics;
intramuscular prostaglandins versus rectal misoprostol;
intramuscular prostaglandin versus no uterotonic/placebo;
intramuscular prostaglandin versus injectable uterotonics;
comparisons of different prostaglandins or different dose/routes of the same prostaglandin;
comparisons of different prostaglandins plus injectable uterotonics versus injectable uterotonics or other prostaglandins.
Types of outcome measures
The primary outcomes of this review are blood loss of 1000 mL or more and the use of additional uterotonics in the third stage of labour. Maternal death is included as an outcome but it is unlikely that the review will have power to evaluate this outcome.
Reported blood loss is influenced by the assessment technique. Measurement of blood and clots in jars and weighing of linen are likely to be more precise than clinical estimation used in some studies. The latter is known to underestimate blood loss (Andolina 1999). Also, the duration of measurement and reporting the amount as 'greater than' or 'greater than or equal to' a certain cut‐off level (e.g. 500 or 1000 mL) may affect the total reported amount of blood loss especially when this amount is estimated. This becomes less of a problem for comparison between treatment and control groups when the trials blind their assessment processes.
Primary outcomes
Severe postpartum haemorrhage (at least 1000 mL);
use of additional uterotonics in the third stage of labour.
Secondary outcomes
Postpartum haemorrhage (at least 500 mL);
mean blood loss (mL);
blood transfusion;
manual removal of placenta;
duration of third stage (minutes);
third stage longer than 30 minutes;
any side‐effect reported;
any side‐effect requiring treatment;
nausea;
vomiting;
diarrhoea;
headache;
abdominal pain;
high blood pressure;
shivering;
severe shivering;
pyrexia (at least 38 ºC);
severe pyrexia (at least 40 ºC);
other.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (7 January 2011). We updated this search on 25 May 2012 and added the results to the awaiting classification section.
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeAppendix 1.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, by consulting the third author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third author. We entered the data into Review Manager software (RevMan 2011) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'as treated' analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting bias (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5))
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We included cluster‐randomised trials in the analyses along with individually‐randomised trials. We adjusted their sample sizes and standard errors using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we used ICCs from other sources, we reported this and conducted sensitivity analyses to investigate the effect of variation in the ICC. If we identified both cluster‐randomised trials and individually‐randomised trials, we planned to synthesise the relevant information. We considered it reasonable to combine the results from both if there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely.
We also acknowledged heterogeneity in the randomisation unit and performed a subgroup analysis to investigate the effects of the randomisation unit.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analyzed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
In the protocol for this review, we specified that if heterogeneity was significant (i.e. P value less than 0.10 or I² greater than 50%), we would not use random‐effects right away and we would not total the results due to this heterogeneity. However, we discussed the trials and outcomes individually in the text.
Assessment of reporting biases
If there were 10 or more studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually, and used formal tests for funnel plot asymmetry. For continuous outcomes we used the test proposed by Egger 1997, and for dichotomous outcomes we used the test proposed by Harbord 2006. If asymmetry was detected in any of these tests or was suggested by a visual assessment, we performed exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using Review Manager software (RevMan 2011). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we planned to use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. We would have treated the random‐effects summary as the average range of possible treatment effects and discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
If we had used random‐effects analyses, we would have presented the results as the average treatment effect with its 95% confidence interval, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it. Subgroup analyses were restricted to the review's primary outcomes.
We carried out the following subgroup analyses.
Prespecified low‐ and high‐risk group pregnancies for studies comparing injectable prostaglandins.
Different doses of misoprostol used in the studies.
Data relating to high‐ and low‐risk women were analyzed separately as well as together (totals). Recent trials (mostly misoprostol) focused on a general population of women with vaginal or caesarean section delivery without specifying any risk status. Therefore, the high‐ and low‐risk subgroupings were not used in the misoprostol comparisons. However, if future trials falling into these comparisons specifically study a risk group these subgroups will be added to the list of comparisons.
If a particular (risk) group was not specified, this implied that all women are included in that analysis regardless of their risk status. Studies that did not specify the risk status of women included are put in the low‐risk category where such distinctions are made.
In meta‐analyses with significant heterogeneity (statistical or visual), we discuss the trials individually (i.e. without totals).
Sensitivity analysis
We did not conduct sensitivity analyses for this update, because all of the larger trials in this systematic review were at low risk of bias.
Results
Description of studies
Fifty‐three reports of 34 trials were identified and considered for inclusion in this updated review. Eight trials were excluded (seeCharacteristics of excluded studies table). Altogether, 26 trials were included in this 2011 update and this review now includes a total of 72 trials involving 52,678 women ‐ seeCharacteristics of included studies for details. Of these, 49 evaluated misoprostol, eight studies evaluated misoprostol plus oxytocin and the remainder evaluated injectable and intramuscular prostaglandins (12 PGF2alpha and three PGE2). One trial report remains in Studies awaiting classification (Yuan 2003). Two reports are in the Ongoing studies section. (Twelve additional reports from an updated search in May 2012 have been added to Studies awaiting classification for consideration at the next update.)
Settings
The review includes trials conducted in all continents from both low‐ to middle‐income countries and industrialized countries. Twenty‐six trials included centres in low‐ and middle‐income countries only. The WHO 2001 trial was conducted in nine countries in Africa, Asia, Europe and Latin America. The Africa 2011 trial was conducted in South Africa, Nigeria and Uganda. In Africa, 10 countries contributed 20 trials (five in South Africa). Sixteen trials were conducted in India and four trials were conducted in China.
The WHO 2001 trial is the largest trial in the review with 18,530 participants from nine countries. The WHO 1999 trial is a pilot dose‐finding trial which preceded the WHO 2001 trial and used the same protocol. Side‐effects of misoprostol during the first hour after delivery from the WHO 2001 trial are included in the meta‐analyses, but further data describing side‐effects in the first 24 hours after delivery were published in a separate article and are described in the results section.
Most trials (68/72) were conducted in hospitals where births were performed by skilled caregivers. Two trials (Gambia 2005; Pakistan 2011) were community‐based. In Gambia 2005 traditional birth attendants were trained in trial procedures and blood loss measurement provided the interventions (oral misoprostol and oral ergometrine as placebo). Traditional birth attendants were trained in the management of third stage of labour in the Pakistan 2011 trial and provided 600 mcg oral misoprostol to women delivering in the community. In the Guinea‐Bissau 2005 trial, midwives administered sublingual misoprostol or placebo to women delivering at primary care centres. In the India 2006c trial, auxiliary nurse‐midwives administered oral misoprostol or placebo tablets to women delivering either at primary care centres (approximately 55%) or at home (approximately 45%).
Management of the third stage of labour
In 48 trials, the third stage was managed actively (at least two of the components of active management described, or specified as 'active'); two trials used 'expectant management' (Holland 1991; India 2006c); 10 trials did not mention management of third stage and three were mixed with components of both active or passive management used. The remaining nine trials included women with caesarean section births and did not report any particular form of management.
Risk status
Four studies specifically studied women who were at high risk for postpartum haemorrhage (PPH) (China 2003b; Egypt 1997; Holland 1995; India 2001b). The participants were classified as high risk if they had a history of PPH or conditions such as multiple pregnancy and grand multiparity.
Mode of delivery
Nine trials included only caesarean section births (India 2006a; Iran 2009; Mexico 2009; Switzerland 2006; Tunisia 2009; United Kingdom 1994; United Kingdom 2001b; USA 1990; USA 2005). There were two trials which included both caesarean sections and vaginal births (China 2003b; Turkey 2010).
Blood loss assessment
The majority of the trials (n = 41) used some form of measurement, some using detailed weighing and hematin‐dye techniques. Clinical estimation was used in 25 trials, haemoglobin change or level, or both, was used in three and no method was mentioned in the remaining four trials (Colombia 2002; India 2001b; India 2005a; Mexico 2009). Overall, 11 trials used drapes to assess blood loss (China 2003b; Guinea‐Bissau 2005; India 2006c; India 2006f; India 2009d; India 2010; Iran 2009; Jamaica 2009; Tibet 2009; Tunisia 2009; United Kingdom 2001c).
Comparisons
Of the 72 trials included in the review, 36 evaluated misoprostol in doses ranging from 50 mcg to 800 mcg and using oral, sublingual, buccal and rectal routes. Misoprostol was compared with placebo in 11 trials (China 2003a; France 2001; Gambia 2005; Guinea‐Bissau 2005; India 2006c; Pakistan 2011; South Africa 1998b; South Africa 1998c; South Africa 1998d; South Africa 2001; Switzerland 1999) and with conventional injectable uterotonics in 25 trials. It should be noted that although Gambia 2005 is grouped under placebo trials, it used oral ergometrine as a placebo. In most of the trials, the uterotonic agent was oxytocin 10 international units (IU) intramuscularly or intravenously. In some trials, the uterotonic group received oxytocin or ergometrine‐oxytocin depending on the hospital routine (Australia 1999) or depending on whether the woman was hypertensive or not (United Kingdom 2000). Some trials had several treatment arms. One of the intramuscular prostaglandin trials (Holland 1991) and two misoprostol trials (France 2001; South Africa 1998d) had three arms, one of which was a placebo control group. The WHO 1999 trial was also a three‐arm trial comparing misoprostol 600 mcg, 400 mcg orally and oxytocin 10 IU. The United Kingdom 2003 trial had three arms comparing oral misoprostol 600 mcg, rectal misoprostol 600 mcg, and rectal misoprostol 400 mcg. The India 2008a trial compared intravenous oxytocin with sublingual misoprostol in doses of 100 mcg and 200 mcg, whereas the India 2009b compared it with sublingual misoprostol in doses of 400 mcg and 600 mcg.
One trial compared oral misoprostol with a Tibetan traditional medicine Zhi Byed 11 (Tibet 2009) and one trial used controlled release PGE2 intravaginal insert (Turkey 2010).
Concurrent routine uterotonic use
Two trials from Turkey had four arms, comparing misoprostol 400 mcg after cord clamp followed by misoprostol 100 mcg at four and eight hours postpartum; the same regimen of misoprostol combined with intravenous oxytocin; intravenous oxytocin only; and intramuscular methyl ergometrine only. For blood loss and other early outcomes assessed before the follow‐up doses of misoprostol were given, the dosage is regarded as 400 mcg. The only differences between these two trials were that Turkey 2002 used rectal misoprostol and Turkey 2003 used oral misoprostol. The USA 2004 and USA 2005 trials compared 200 mcg buccal misoprostol with placebo in women delivering vaginally and by caesarean section respectively. All women received 20 IU oxytocin infusion at a rate of 10 mL/minute for 30 minutes and then 125 mL/hour for eight hours. Africa 2011 and Nigeria 2011 both used 400 mcg misoprostol plus oxytocin versus only oxytocin.
The review includes unpublished data from Canada 2005, South Africa 1998d, WHO 1999, United Kingdom 2000, WHO 2001 and India 2008b trials.
Risk of bias in included studies
Figure 1 summarizes the risk of bias analysis for all the studies included in this review. Random sequence generation was considered adequate in 51 studies and unclear in 21 studies. Allocation concealment was considered adequate in 48 studies that used sealed envelopes, opaque containers, or identical numbered boxes containing trial medications. Holland 1995 excluded 15% of the women after randomisation, mostly due to women being randomised despite being ineligible (for augmentation of labour), and Turkey 2003 excluded 12.6% of the women after randomisation secondary to them requiring caesarean sections. There were an unspecified but small number of postrandomisation exclusions in South Africa 1998a. These were due to hypertension being discovered after randomisation, which resulted in the exclusion of some women allocated to ergometrine‐oxytocin.
1.
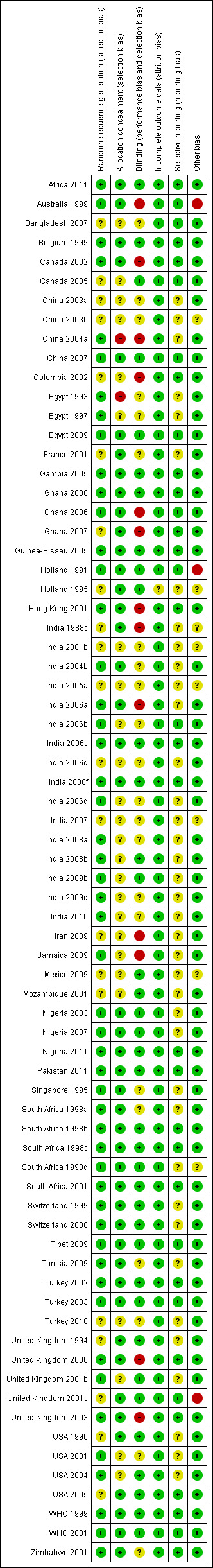
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
In trials evaluating different interventions in the third stage of labour, PPH is often the primary outcome. Assessment of PPH is prone to bias if the staff making the assessments are not blind to the intervention. In this review, all outcome assessments were blinded in 21 trials. Some outcome assessments were blinded in two trials.
In this review, trials comparing misoprostol with other uterotonics are, in essence, equivalence trials designed to evaluate whether misoprostol is as effective as others given its advantage of oral or rectal route of administration. The majority of such trials have set relatively large margins of equivalence and are therefore, in practical terms, underpowered to test an equivalence hypothesis. The WHO 2001 trial is the largest trial in the review which set an a priori clinical equivalence margin (within 35% efficacy of oxytocin). In this trial the primary outcomes were blood loss greater than or equal to 1000 mL and the use of additional uterotonics. Misoprostol versus placebo or no treatment trials are superiority trials and do not have the problem mentioned above.
The South African trials and the United Kingdom 2001b trial evaluating oral misoprostol used non‐identical placebos. The women participating in the South African trials took the medications out of an opaque container with care being taken to conceal the tablets from midwives. Although this method of blinding is not 100% safe, the authors provided the review authors with the information that unblinding was unlikely to occur in the settings in which the trials were conducted. In the United Kingdom 2001b trial, side‐effect assessments were blinded.
One study (Holland 1995) was stopped prematurely before reaching a prespecified interim analysis to determine an appropriate sample size. This was due to the manufacturer of the drug issuing a warning about serious cardiovascular side‐effects after intramuscular use of sulprostone, a synthetic PGE2 derivative. Another study (Australia 1999), was stopped after recruitment of 863/1862 women following the unsatisfactory results of an interim analysis.
Effects of interventions
The results are based on 37 misoprostol and nine intramuscular prostaglandin trials.
Misoprostol trials
Primary outcomes
Misoprostol versus placebo/no treatment (11 trials, comparisons 01, 02, 03, 04)
Oral misoprostol was used in seven trials (comparison 01: 6225 women total, 5325 in six 600 mcg trials), rectal (comparison 02), sublingual (03) and buccal (04). There were three maternal deaths in misoprostol and one in placebo groups overall in nine trials.
There was significant statistical heterogeneity for the outcome severe postpartum haemorrhage (PPH) in the oral misoprostol versus placebo comparison. Earlier trials (France 2001; South Africa 1998d; South Africa 2001) did not indicate any reduction in severe PPH while the more recent Gambia 2005 (risk ratio (RR) 0.48; 95% confidence interval (CI) 0.09 to 2.59, 2/629 versus 4/599) and India 2006c (RR 0.20; 95% CI 0.04 to 0.91, 2/812 versus 10/808) and Pakistan 2011 (RR 0.57; 95% CI 0.27‐1.22, 10/514 versus 19/558) trials suggest some protective effect of misoprostol on severe PPH. The use of additional uterotonics was less when misoprostol was used in four out of six trials but not in the South Africa 1998d trial that had both 600 and 400 mcg treatment arms. As South Africa 1998d is the only study in this comparison suggesting superiority of placebo over oral misoprostol, which is biologically implausible, we re‐conducted the analyses excluding this study for the primary outcomes. Oral misoprostol was protective against the use of additional uterotonics (RR 0.87; 95% CI 0.70 to 1.08, five trials, 3585 women) compared with the placebo group. Compared with placebo, oral misoprostol reduced the need for blood transfusions required (RR 0.31; 95% CI 0.10 to 0.94, four trials, 3519 women).
One rectal misoprostol trial South Africa 1998c using 400 mcg did not show a statistically significant difference in severe PPH (RR 0.69; 95% CI 0.35 to 1.37).
The Guinea‐Bissau 2005 trial used 600 mcg sublingual misoprostol and showed a statistically significant difference in reducing severe PPH (RR 0.66; 95% CI 0.45 to 0.98, 37/330 versus 56/331).
The USA 2004 and USA 2005 trials used 200 mcg buccal misoprostol in women undergoing vaginal delivery and caesarean section respectively. All women received 20 IU oxytocin infusion in 1 litre of saline. In the USA 2005 trial there were 24/173 versus 22/179 cases of severe PPH in the misoprostol and placebo groups respectively, whereas there were no cases of severe PPH in the USA 2004 trial. In both trials the protocol included oxytocin infusion after delivery of the placenta.
Misoprostol versus conventional injectable uterotonics (39 trials, comparisons 05, 06, 07)
Twenty‐one trials compared oral misoprostol (comparison 05), 10 compared rectal (comparison 06) and eight compared sublingual (comparison 07) with injectable uterotonics (oxytocin intramuscular or intravenous, ergometrine, ergometrine + oxytocin). Maternal deaths were reported only in the WHO 2001 trial (2/9264 versus 2/9266) and Ghana 2007 (0/224 versus 1/226). There were no deaths in the Ghana 2006, Canada 2005, Turkey 2002 and WHO 1999 trials. The other trials did not mention whether or not there were any deaths. The funnel plots for the primary outcomes suggest no publication bias (Figure 2; Figure 3).
2.
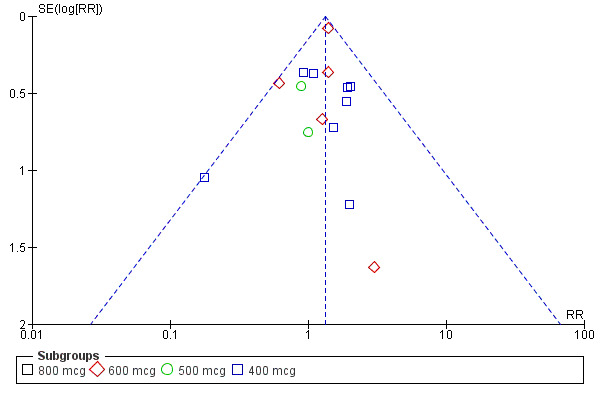
Funnel plot of comparison: 5 Oral misoprostol versus injectable uterotonics, outcome: 5.2 Severe postpartum haemorrhage (>= 1000 ml).
3.
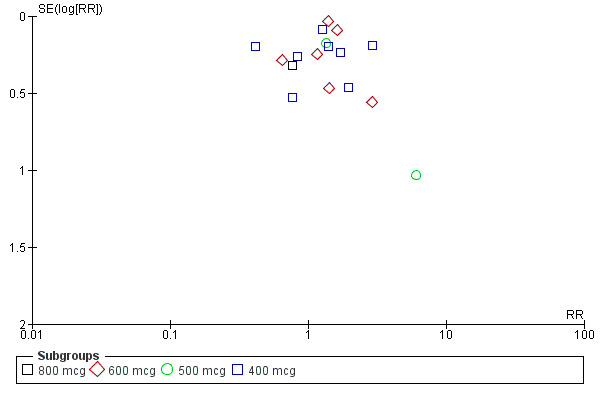
Funnel plot of comparison: 5 Oral misoprostol versus injectable uterotonics, outcome: 5.5 Use of additional uterotonics.
Oral misoprostol was associated with a statistically significant higher risk of severe PPH (RR 1.33; 95% CI 1.16 to 1.52, 17 trials, 29,797 women). While the large WHO 2001 trial results dominated the meta‐analysis, the majority of trials showed similar results with no statistically significant heterogeneity across different doses or trials. Although the results were not totalled due significant statistical heterogeneity, overall, the use of additional uterotonics showed a similar trend among different dose groups. There was also a trend towards fewer blood transfusions with misoprostol (RR 0.84; 95% CI 0.66 to 1.06, 15 trials, 28,213 women).
Three 400 mcg rectal misoprostol versus injectables trials reported on severe PPH and there were similar numbers of women with this outcome in the two groups (RR 1.14; 95% CI 0.70 to 1.85, three trials, 1780 women). More women who received misoprostol required additional uterotonics (RR 1.64; 95% CI 1.16 to 2.31). One study using 800 mcg rectal misoprostol (Ghana 2007) reported only one case of severe PPH reported in the control group.
Seven trials compared sublingual misoprostol versus injectables. Use of additional uterotonics reported by all the trials were less likely among misoprostol group compared with injectables (RR 0.61; 95% CI 0.44 to 0.85, seven trials, 1013 women).
Concurrent routine uterotonic use (comparisons 08, 09, 10 and 11)
Oral misoprostol combined with oxytocin were compared with conventional uterotonics in the Africa 2011, Nigeria 2011 and Turkey 2003 trials. Oral misoprostol when combined with oxytocin was more effective than placebo and oxytocin in decreasing PPH (RR 0.71; 95% CI 0.53 to 0.95, three trials, 3205 women) and although not totalled due to heterogeneity, a similar trend can be observed for severe PPH. It should be noted here that for outcomes assessed within four hours, including all blood loss outcomes, the effective dose was 400 mcg in Africa 2011 and Nigeria 2011, whereas Turkey 2003 added 100 mcg to the initial 400 mcg dose after four and eight hours.
The Turkey 2002 trial compared rectal misoprostol and oxytocin, Tunisia 2009 compared sublingual misoprostol and oxytocin with conventional uterotonics in women who had caesarean sections, whereas Mexico 2009 used a combination of intravaginal misoprostol and oxytocin.
Side‐effects
Oral misoprostol 600 mcg was consistently associated with higher rates of prostaglandin‐related side‐effects such as nausea, vomiting, diarrhoea as well as for 'any' shivering, severe shivering and pyrexia (greater than 38 °C) when compared with placebo as well as with conventional uterotonics. Not all of the side‐effects were totalled due to heterogeneity under Comparison 5.0, however, in the meta‐analyses both severe shivering and diarrhoea were more likely to occur among the misoprostol group participants: RR 7.24; 95% CI 4.74‐11.08, five trials, 20823 women and RR: 1.83; 95% CI 1.34‐2.50; 13 trials, 27011 women, respectively. For 'any' shivering, the individual trial RRs ranged between 1.43 and 69.10.
Further analysis of side‐effects during the first 24 hours in the WHO 2001 trial showed that in comparison to oxytocin, women who received misoprostol had a higher incidence of 'any' shivering (RR 4.70; 95% CI 1.90 to 11.20), and of pyrexia (RR 6.3; 95% CI 3.70 to 10.80) in the period two to six hours after delivery. Diarrhoea was also more common in the misoprostol group in the period two to six hours (RR 21.00; 95% CI 5.10 to 86.50) and seven to 12 hours (RR 7.70; 95% CI 2.30 to 25.40).
The results of three trials (India 2009b; South Africa 1998d; WHO 1999) where 600 mcg and 400 mcg doses of oral misoprostol were compared indicate that side‐effects are dose‐related (any shivering RR 1.37; 95% CI 1.12 to 1.69) (Analysis 16.14). This might not apply, however, to rectal misoprostol, as there were no significant differences in the one trial (United Kingdom 2003) that evaluated 600 mcg and 400 mcg doses of rectal misoprostol. A comparison of 600 mcg rectal versus 600 mcg oral misoprostol in the same trial showed that rectal misoprostol resulted in less pyrexia, 'any' shivering, and severe shivering (RR 0.27; 95% CI 0.16 to 0.46) (Analysis 18.8) than oral misoprostol. Severe shivering was also observed more frequently among all different doses of misoprostol compared with injectable uterotonics (Analysis 5.19).
16.14. Analysis.
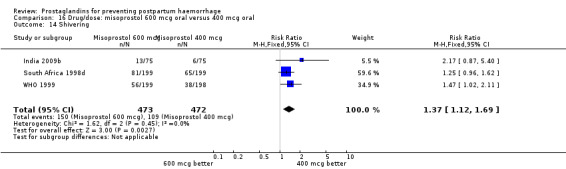
Comparison 16 Drug/dose: misoprostol 600 mcg oral versus 400 mcg oral, Outcome 14 Shivering.
18.8. Analysis.
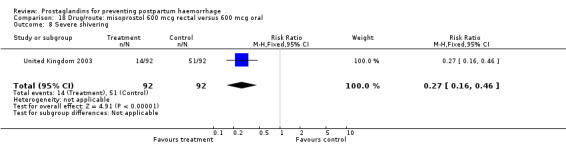
Comparison 18 Drug/route: misoprostol 600 mcg rectal versus 600 mcg oral, Outcome 8 Severe shivering.
5.19. Analysis.
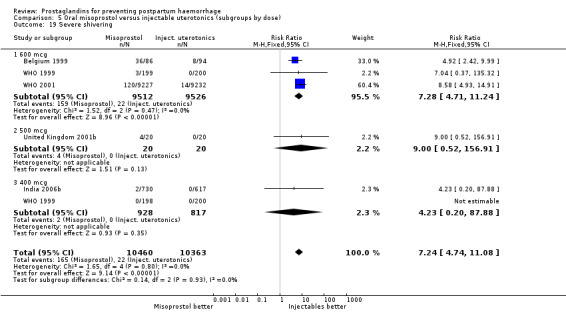
Comparison 5 Oral misoprostol versus injectable uterotonics (subgroups by dose), Outcome 19 Severe shivering.
Intramuscular prostaglandin trials (comparisons 12, 13, 14, 15)
One trial (Holland 1991) was a three‐arm trial with a placebo arm in addition to sulprostone and oxytocin. Thirteen trials compared injectable prostaglandins with conventional injectable uterotonics. The occurrence of primary outcomes such as a blood loss of 1000 mL or more and the use of additional uterotonics were too few to give reliable estimates.
Intramuscular prostaglandins had less mean blood loss when compared with no uterotonic use in the one trial with 46 women (Holland 1991) that examined this outcome (‐224 mL mean difference (MD); 95% CI ‐420.35 to ‐27.65 mL). Other outcomes evaluated in this study were not statistically significant.
When compared with conventional uterotonics, intramuscular prostaglandins resulted in less blood loss and shorter duration of the third stage of labour (‐1.25 minutes MD; 95% CI ‐1.42 to ‐1.08 minutes). Blood loss data were not totalled because of heterogeneity due to one small trial having the results in the opposite direction. Five other trials showed less blood loss with injectable prostaglandin.
Vomiting, abdominal pain and diarrhoea were more common with intramuscular prostaglandins.
Intramuscular prostaglandin F2alpha was compared with rectal misoprostol 400 mcg in one small trial with 120 women (India 2006d). There were more women requiring additional uterotonics (2/60 versus 10/60) but the study was too small to give any guiding evidence. Another small trial compared intramyometrial injection of PGF2alpha with intramyometrial oxytocin in women having caesarean section births (USA 1990).
One small study compared two different intramuscular prostaglandins and observed that intramuscular carboprost was less likely to result in PPH (less than 500 mL) but more likely to cause nausea and diarrhoea compared with intramuscular methylergometrine maleate (MEM) (India 2007).
Discussion
This review includes comparisons of intramuscularly, orally, and rectally administered prostaglandins with placebo, and with conventional injectable uterotonics. We did not combine misoprostol with other prostaglandins in the meta‐analyses. Misoprostol tablets are used via oral, rectal, sublingual or buccal routes while other prostaglandins are used intramuscularly (or intramyometrial during caesarean section). In terms of outcomes, we gave emphasis to blood loss of at least 1000 mL and the use of additional uterotonics as the most clinically relevant outcomes. We recorded maternal death data systematically but did not anticipate having sufficient power to analyse this outcome.
Misoprostol for PPH prevention in the community: While the results of earlier trials comparing misoprostol (used orally or rectally) with placebo or no treatment were somewhat equivocal, the results of the recent trials are more promising (Gambia 2005; Guinea‐Bissau 2005; India 2006c; Pakistan 2011). It is important to note that all four recent trials have design and setting differences that make the summing up of their results difficult. The Gambia 2005 trial had a lower than expected number of events and although the direction of effect favours misoprostol the trial is not powered adequately. In addition, oral ergometrine was assumed to be equivalent to placebo and although the value of oral ergometrine is questionable (WHO 1994), it may not be zero. However, we kept this comparison in the misoprostol versus placebo comparison, because the authors' a priori assumption was that the direction and the pharmacokinetics of oral ergometrine suggests that it is unlikely to be effective. The third stage management was 'active'. This trial is the one of the two trials that used traditional birth attendants to administer the trial interventions. The Guinea‐Bissau 2005 trial used sublingual misoprostol within the context of active management and showed greater effect with higher blood loss (i.e. 1000 mL compared to 500 mL). Almost half of the women in this trial (150/330 and 170/331 in the misoprostol and placebo groups) experienced blood loss of 500 mL or more which is unusual in postpartum haemorrhage (PPH) trials with active management. The India 2006c trial used oral misoprostol in the context of 'expectant' management of the third stage of labour. Therefore, its findings are more applicable to settings where this type of third stage management is the norm. It is not known whether with other components of active management being in place the same magnitude of effect would hold or not. The Pakistan 2011 trial used 600 mcg oral misoprostol versus placebo delivered via traditional birth attendants at home births and the third stage management was 'active'. Recent analysis by Hofmeyr et al. using the data from 46 studies included in the earlier version of this review concluded that 400 mcg of misoprostol were found to be safer than doses larger than 600 mcg and just as effective (Hofmeyr 2009).
With the addition of four non‐hospital based trials, it is possible to make some inferences for those settings although all four trials have important differences. All four trials were conducted either at home or at primary care centres and it is reassuring to see that there were no major adverse events related to misoprostol use. The Guinea‐Bissau, Pakistan and India trials were conducted by caregivers trained in third stage management although only the Guinea‐Bissau 2005 had fully qualified midwives.
The addition of several smaller misoprostol versus injectable uterotonic trials confirm the findings of the earlier version of the review. Overall, injectable uterotonics are more effective than misoprostol. Various injectables were used in the included trials. The data with regard to the comparative efficacy of oxytocin 10 international units (IU) versus ergometrine suggest that there are no major advantages of either of them (McDonald 2004). Ergot preparations seem to be somewhat more effective in reducing blood loss but are associated with a higher rate of side‐effects and the choice should be made according to the trade‐off between the benefit and harm (Carroli 2001).
The results of the large WHO 2001 trial, conducted in nine countries with the participation of 18,530 women, dominate the systematic review's comparison between misoprostol 600 mcg and injectable uterotonics, mostly 10 IU of oxytocin. This comparison demonstrates that oral misoprostol up to 600 mcg is associated with a higher risk of blood loss and the use of additional uterotonics (up to 16% of women will require additional uterotonic treatment) when compared with a policy of injectable uterotonics. There is a consistent increase in all prostaglandin‐related side‐effects. Considering that the observed rate of side‐effects is already high, it is unlikely that higher doses of oral misoprostol (to increase efficacy) could be used for the routine prevention of PPH among healthy women although the Ghana 2006 trial used 800 mcg oral misoprostol.
Although in almost all of the trials these side‐effects were reported as not severe, they cause discomfort. For example, women in the WHO 2001 trial rated to have severe shivering needed extra blankets or other comfort measures. Amant reported that women who had shivering had their teeth chattering for 10 to 20 minutes and had no control over their body movements during this period (Amant 2001). On the other hand, in the case of pyrexia (greater than 38 °C), the staff may be concerned for the woman about the risk of postpartum infections and the need for initiating any unnecessary antibiotic treatment. Furthermore, fever may delay blood transfusion.
The largest trial (WHO 2001), used oxytocin both intramuscularly or intravenously. While it is obvious that intravenous injection provides faster availability of the drug, pharmacokinetic data show that with the intramuscular route oxytocin is circulating in the blood within two to three minutes (Gibbens 1972). Furthermore, the pharmacokinetics of oral misoprostol demonstrate that misoprostol acid reaches its peak in the plasma between 20 to 30 minutes after oral administration (Zieman 1997), well after the mean time from delivery until placental expulsion observed in the WHO 2001 (8.3 minutes, standard deviation (SD) 14.6) and Mozambique 2001 (9.0 minutes, SD 3.6) trials. Therefore, we do not think that the route of administration of oxytocin will affect its efficacy.
The nine studies which enrolled women undergoing caesarean section births have been included together with the others in the analysis. The amount of blood loss during and after caesarean section may be different, due to additional bleeding not directly related to the contractility of the uterus and, due to inevitable contamination with other fluids. However, a differential effect between different uterotonics is unlikely. Therefore, a sensitivity analysis according to the mode of delivery was not conducted. The problems associated with measurement of blood loss at caesarean section may, however, obscure any smaller differences in efficacy and push the results towards 'no difference'. In this review, these studies were analyzed within the group of studies that included women at low risk for PPH, when appropriate. Different from the prior version of the review, there are two studies (China 2003b; Turkey 2010) which included both caesarean sections and vaginal births in their study and comparison groups. It should be noted that unlike all the other studies, Turkey 2010 compared a controlled release PGE2 vaginal insert with intravenous oxytocin.
With the data available so far, there do not seem to be major differences between intramuscular prostaglandins and conventional injectable uterotonics (oxytocin or ergometrine) in reducing blood loss in the third stage of labour. These trials had few women who experienced the primary outcomes of this review, although the mean blood loss (a secondary outcome) was reduced by 22 mL to 34 mL on average for women who received intramuscular prostaglandins, based on the dose. Vomiting and diarrhoea were common side‐effects with intramuscular prostaglandins. The studies reported, however, that side‐effects did not need treatment. The concerns of safety, cost and side‐effects are important limitations of intramuscular prostaglandins.
One article (Tibet 2009) compared a traditional Tibetan herbal medicine (ZB11) used for prevention of PPH with oral misoprostol and concluded that misoprostol is more effective in reducing the rates of PPH, however, there were no differences between the two groups in terms of severe PPH and mean blood loss.
Further evidence has been building around the use of oral misoprostol plus injectable uterotonics versus uterotonics. Three trials (Africa 2011; Nigeria 2011; Turkey 2003) have shown that the combination has been more effective in reducing severe PPH compared with uterotonics alone, although side‐effects like shivering and fever were more frequent with the combination. Based on the results of these studies, it is important to underline the effectiveness of a dose as low as 400 mcg versus placebo in women who received oxytocin. Given that in settings where oxytocin is available, it will reduce PPH significantly, it is not clear whether routine concurrent use should be a priority.
A WHO systematic review on the cause of maternal deaths identified obstetric haemorrhage as the largest cause of maternal death in Africa and Asia where the majority of maternal deaths occur (Khan 2006). Prevention of PPH with appropriate, evidence‐based interventions such as oxytocin and misoprostol when oxytocin is not available could prevent a substantial proportion of deaths in these two regions.
Authors' conclusions
Implications for practice.
The uterotonic of choice in settings where active management is practiced is oxytocin 10 IU administered intravenously or intramuscularly. Oxytocin retains more than 85% active drug after storage for one year at under 30 °Celsius. Getting oxytocin used as widely as possible should be the primary aim for births occurring outside hospitals at peripheral levels of the healthcare system. If these conditions for oxytocin use cannot be met then misoprostol could be used based on the current evidence. The empirical dosage most used in trials to date is 600 mcg orally. Promising results against placebo have also been reported in individual trials of 400 mcg orally (over and above the routine use of oxytocin) and 600 mcg sublingually.
More efforts should be devoted to making injectable uterotonics available, especially using strategies such as that of disposable prefilled syringes, e.g. Uniject (PATH 2001; Tsu 2009). Developing the skills to administer injections in areas where this is not currently available will have the additional benefit of enabling other effective treatments such as parenteral antibiotics or anticonvulsants to be used. However, recent studies have been promising to show that oral misoprostol use has been effective and safe in community settings with low access to facilities and skilled healthcare providers. These recommendations are in line with the most recent WHO Essential Medicines List which approved misoprostol “for the prevention of PPH in settings where oxytocin is not available or cannot be safely used” (WHO 2011).
Intramuscular prostaglandins are not preferable to conventional uterotonics in the routine management of the third stage of labour especially for low‐risk women.
Implications for research.
The recent misoprostol versus placebo trials conducted outside hospitals showed promising results and future research on misoprostol use in the community should focus on implementation issues such as giving misoprostol in advance during the antenatal period to women or to traditional birth attendants for use after childbirth or other means of improving access to and safe use of uterotonics in home births. As side‐effects are dose‐related and life‐threatening hyperpyrexia has been reported with 800 mcg orally (Chong 1997), research should be directed towards establishing the lowest effective dose for routine use, and the optimal route of administration.
For the settings in which active management of the third stage is the norm, there is no need for further trials comparing misoprostol with injectable uterotonics. Future research in the third stage of labour could focus on investigating the effectiveness of the particular components of active management.
Intramuscular prostaglandins may be studied for the management of high‐risk cases since they are unlikely to find widespread use in low‐risk cases due to their costs and side‐effects.
What's new
| Date | Event | Description |
|---|---|---|
| 20 June 2011 | New search has been performed | Search updated in January 2011. Number of included studies has increased from 46 to 72 studies. We updated the search in May 2012 and have added the results to Studies awaiting classification for consideration at the next update. |
| 20 June 2011 | New citation required but conclusions have not changed | Main conclusions remain unchanged. More evidence has accumulated on oral misoprostol versus placebo comparison in community‐based settings and misoprostol versus conventional oxytocics in facilities. New review author helped to prepare this update. |
History
Protocol first published: Issue 4, 1997 Review first published: Issue 4, 1997
| Date | Event | Description |
|---|---|---|
| 7 January 2011 | Amended | Search updated. Forty‐five new reports added to Studies awaiting classification. |
| 19 June 2008 | Amended | Converted to new review format. |
| 23 May 2007 | New search has been performed | Search updated on 28 February 2007. The current update includes 14 new trials bringing the total to 46 trials. The review now includes more evidence on misoprostol compared to placebo at non‐hospital, peripheral settings. The conclusions related to misoprostol comparison to conventional injectable uterotonics and that of intramuscular prostaglandins remain unchanged. Three papers from China are in the awaiting assessment section pending their translation. |
| 23 May 2007 | New citation required but conclusions have not changed | The statistics editor noticed some discrepancies in standard deviation figures of continuous data in some trials. In Switzerland 1999 the data were actually reported as standard error and this has been corrected. Continuous data from India 1988c, Nigeria 2003 and Ghana 2000 have ben excluded because they could not be reconciled by looking at the paper again. |
| 21 November 2003 | New search has been performed | The current update includes 5104 additional women from seven misoprostol trials (Canada 2002; Colombia 2002; France 2001; Nigeria 2003; Turkey 2002; Turkey 2003; United Kingdom 2003) and some excluded trials. The conclusions of the review have not changed. |
Acknowledgements
We are grateful Dr Hany Abdel‐Aleem, Dr Hazem El‐Refaey, Dr Antonio Bugalho, Dr Thomas Baskett, Dr Lars Høj and Dr Pralhad Kushtagi for providing additional data and to the referees for their comments.
Appendices
Appendix 1. Methods used to assess trials included in previous versions of this review
Two review authors independently evaluated trials under consideration for methodological quality and appropriateness for inclusion without consideration of their results. No language preferences were applied either during the search or selection of trials. Two authors independently extracted data regardless of whether they participated in a particular included trial or not.
We assessed methodological quality in terms of adequacy of allocation concealment as described in Higgins 2005.
In addition to the main outcomes, we systematically extracted the following data for each study:
trial entry criteria (high versus low risk, other specific exclusion criteria);
exclusions and missing data after randomization;
management of the third stage of labour;
the duration and technique of assessment of blood loss.
We evaluated statistical heterogeneity across trial results using the chi‐square test as calculated in MetaView. Whenever statistical (P < 0.1) or visual heterogeneity was encountered, we explored the possible reasons. In meta‐analyses with significant heterogeneity (statistical or visual), we discuss the trials individually (i.e. without totals).
It is not clear how components of third stage management, other than the uterotonic, affect the blood loss. While the comparison of the uterotonic might be valid, if other components of active management are effective, then the scope for any difference between a prostaglandin and a placebo or another uterotonic could be minimized if those components are used.
These factors are assessed as possible sources of heterogeneity where appropriate and if there are adequate numbers of studies to allow such assessments.
Because of the significant differences in pharmacokinetics and possibly other properties, we analysed oral, rectal, sublingual and buccal misoprostol and intramuscular prostaglandins (PGF2alpha and synthetic E2) separately.
We did not exclude trials on the basis of a predetermined cut‐off value for loss to follow‐ups and postrandomization exclusions. We systematically extracted this information and discussed as appropriate for each trial.
Data and analyses
Comparison 1. Oral misoprostol versus no uterotonic/placebo (subgroups by dose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal death | 3 | 3965 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.24, 8.81] |
| 1.1 600 mcg | 3 | 3965 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.24, 8.81] |
| 2 Maternal death or severe morbidity | 2 | 2848 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.36, 3.80] |
| 2.1 600 mcg | 2 | 2848 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.36, 3.80] |
| 3 Severe postpartum haemorrhage (>= 1000 ml) | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 600 mcg | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 400 mcg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Postpartum haemorrhage (>= 500 ml) | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 600 mcg | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 400 mcg | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Blood loss (ml) | 5 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 600 mcg | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 400 mcg | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Use of additional uterotonics | 5 | 3585 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.70, 1.08] |
| 6.1 600 mcg | 4 | 2685 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.66, 1.13] |
| 6.2 400 mcg | 2 | 900 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.26] |
| 7 Blood transfusion | 4 | 3519 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.10, 0.94] |
| 7.1 600 mcg | 3 | 2619 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.06, 0.94] |
| 7.2 400 mcg | 2 | 900 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.08, 4.52] |
| 8 Manual removal of placenta | 3 | 1900 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.27, 2.63] |
| 8.1 600 mcg | 2 | 1000 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.30, 5.93] |
| 8.2 400 mcg | 2 | 900 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.06, 2.89] |
| 9 Duration of third stage (minutes) | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐3.64, 1.64] |
| 9.1 600 mcg | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐3.64, 1.64] |
| 9.2 400 mcg | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Third stage >= 30 minutes | 3 | 1899 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.90, 4.44] |
| 10.1 600 mcg | 2 | 999 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.61, 5.34] |
| 10.2 400 mcg | 2 | 900 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.70, 7.26] |
| 11 Any side‐effect | 1 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [1.35, 3.20] |
| 11.1 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 400 mcg | 1 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [1.35, 3.20] |
| 12 Nausea | 4 | 3741 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.52, 1.64] |
| 12.1 600 mcg | 4 | 3343 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.49, 1.58] |
| 12.2 400 mcg | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 73.20] |
| 13 Vomiting | 5 | 4147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.52, 1.25] |
| 13.1 600 mcg | 5 | 3749 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.51, 1.25] |
| 13.2 400 mcg | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.88] |
| 14 Headache | 3 | 2512 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.65, 3.32] |
| 14.1 600 mcg | 3 | 2114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.54, 3.03] |
| 14.2 400 mcg | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 103.49] |
| 15 Abdominal pain | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.1 600 mcg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 400 mcg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Diarrhoea | 4 | 3741 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.42, 2.92] |
| 16.1 600 mcg | 4 | 3343 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.42, 2.92] |
| 16.2 400 mcg | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Any shivering | 8 | 6332 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.96 [2.66, 3.31] |
| 17.1 600 mcg | 7 | 5434 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [2.68, 3.39] |
| 17.2 400 mcg | 2 | 898 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [1.90, 3.63] |
| 18 Pyrexia (>= 38 degrees C) | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 600 mcg | 5 | 4140 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.39 [3.78, 7.69] |
| 18.2 400 mcg | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.6 [2.21, 14.21] |
1.1. Analysis.
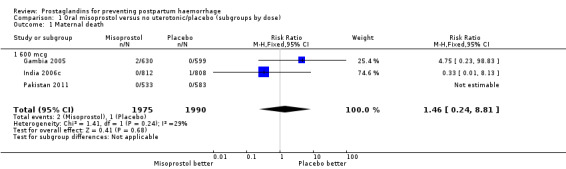
Comparison 1 Oral misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 1 Maternal death.
1.2. Analysis.
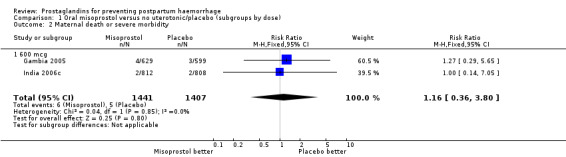
Comparison 1 Oral misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 2 Maternal death or severe morbidity.
1.3. Analysis.
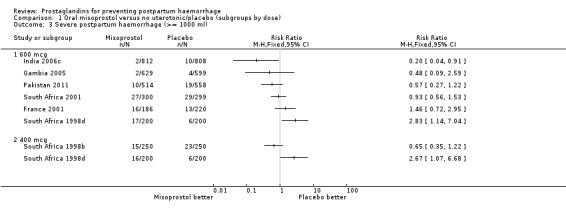
Comparison 1 Oral misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 3 Severe postpartum haemorrhage (>= 1000 ml).
1.4. Analysis.
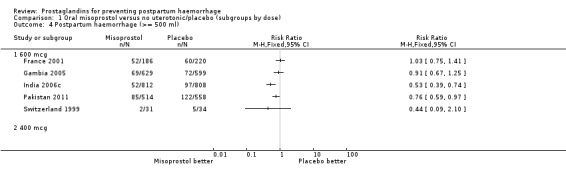
Comparison 1 Oral misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 4 Postpartum haemorrhage (>= 500 ml).
1.5. Analysis.
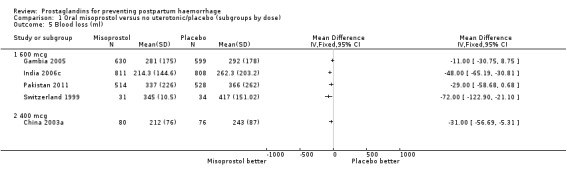
Comparison 1 Oral misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 5 Blood loss (ml).
1.6. Analysis.
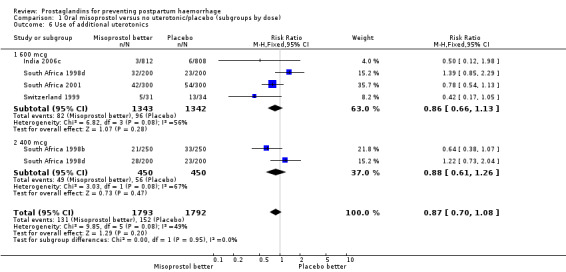
Comparison 1 Oral misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 6 Use of additional uterotonics.
1.7. Analysis.
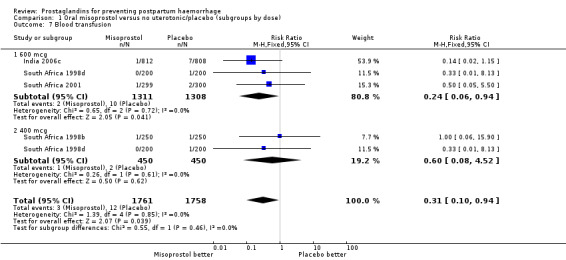
Comparison 1 Oral misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 7 Blood transfusion.
1.8. Analysis.
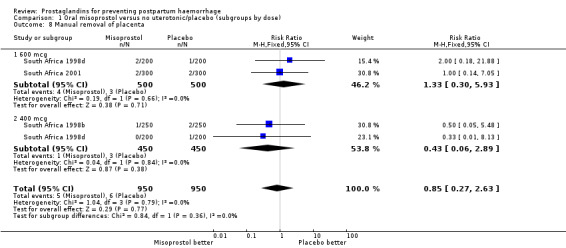
Comparison 1 Oral misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 8 Manual removal of placenta.
1.9. Analysis.
Comparison 1 Oral misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 9 Duration of third stage (minutes).
1.10. Analysis.
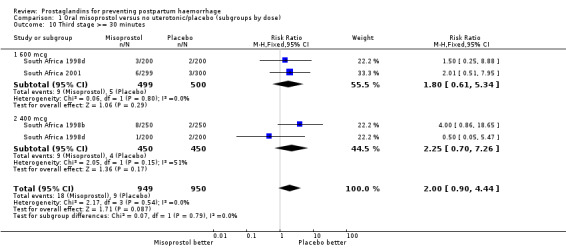
Comparison 1 Oral misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 10 Third stage >= 30 minutes.
1.11. Analysis.
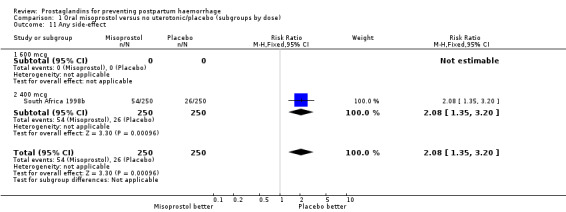
Comparison 1 Oral misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 11 Any side‐effect.
1.12. Analysis.
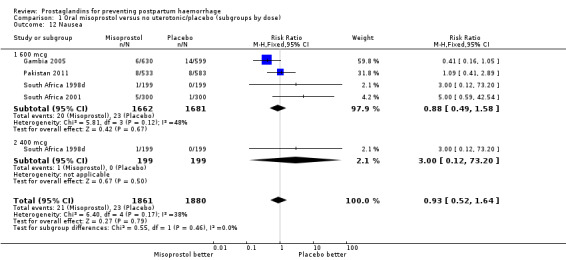
Comparison 1 Oral misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 12 Nausea.
1.13. Analysis.
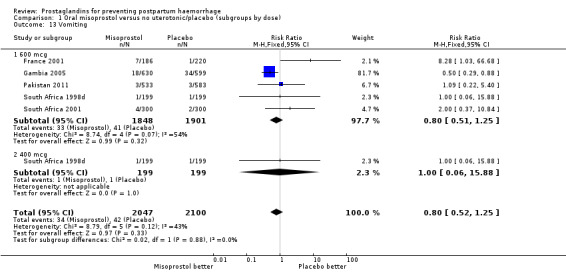
Comparison 1 Oral misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 13 Vomiting.
1.14. Analysis.
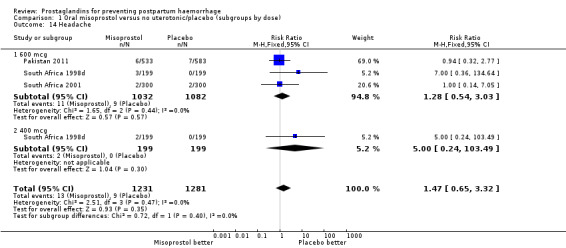
Comparison 1 Oral misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 14 Headache.
1.15. Analysis.
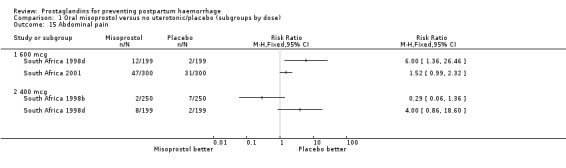
Comparison 1 Oral misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 15 Abdominal pain.
1.16. Analysis.
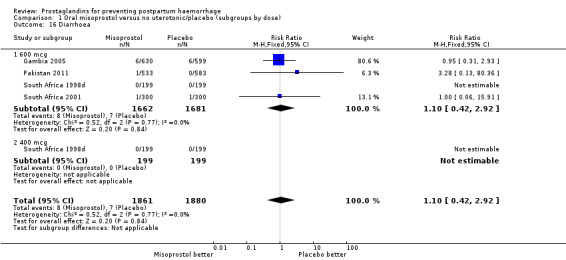
Comparison 1 Oral misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 16 Diarrhoea.
1.17. Analysis.
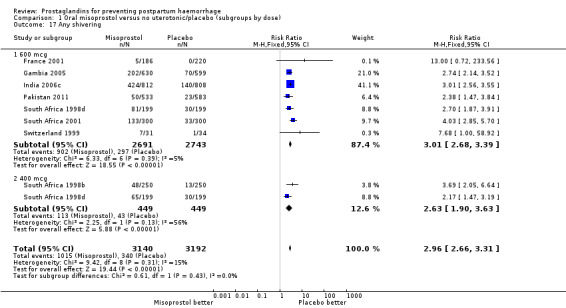
Comparison 1 Oral misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 17 Any shivering.
1.18. Analysis.
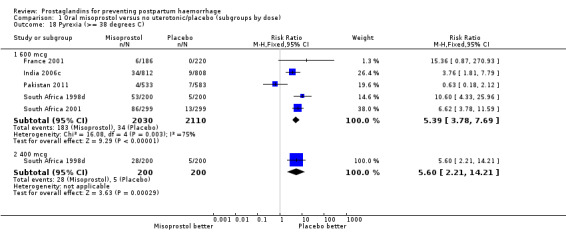
Comparison 1 Oral misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 18 Pyrexia (>= 38 degrees C).
Comparison 2. Rectal misoprostol versus no uterotonic/placebo (subgroups by dose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe postpartum haemorrhage (>= 1000 mL) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 400 mcg | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.35, 1.37] |
| 2 Use of additional uterotonics | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 400 mcg | 1 | 546 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.31, 1.62] |
| 3 Manual removal of placenta | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 400 mcg | 1 | 546 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.12, 74.40] |
| 4 Third stage >= 30 minutes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 400 mcg | 1 | 540 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.56] |
| 5 Vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 400 mcg | 1 | 546 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.06, 16.14] |
| 6 Abdominal pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 400 mcg | 1 | 546 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.12, 74.40] |
| 7 Any shivering | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 400 mcg | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.25] |
2.1. Analysis.
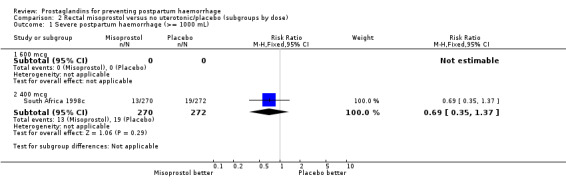
Comparison 2 Rectal misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 1 Severe postpartum haemorrhage (>= 1000 mL).
2.2. Analysis.
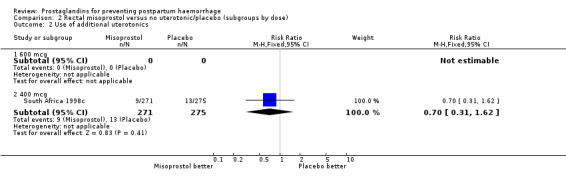
Comparison 2 Rectal misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 2 Use of additional uterotonics.
2.3. Analysis.
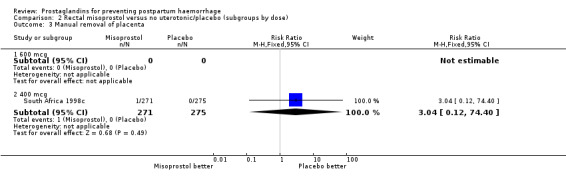
Comparison 2 Rectal misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 3 Manual removal of placenta.
2.4. Analysis.
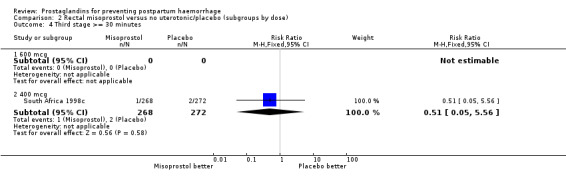
Comparison 2 Rectal misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 4 Third stage >= 30 minutes.
2.5. Analysis.
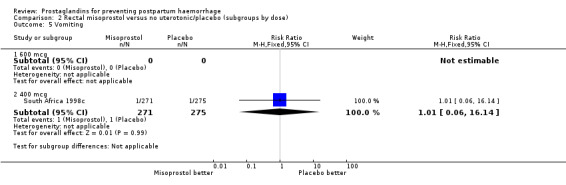
Comparison 2 Rectal misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 5 Vomiting.
2.6. Analysis.
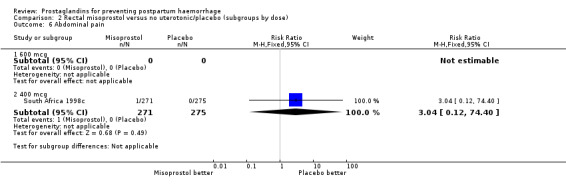
Comparison 2 Rectal misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 6 Abdominal pain.
2.7. Analysis.
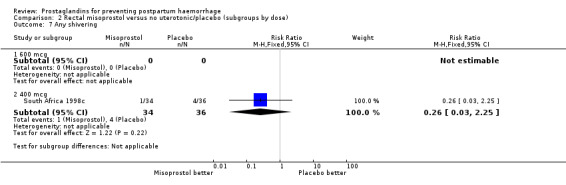
Comparison 2 Rectal misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 7 Any shivering.
Comparison 3. Sublingual misoprostol versus no uterotonic/placebo (subgroups by dose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Severe postpartum haemorrhage (>= 1000 mL) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 600 mcg | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.45, 0.98] |
| 2.2 400 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Postpartum haemorrhage (>= 500 mL) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 600 mcg | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.76, 1.04] |
| 3.2 400 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Nausea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 600 mcg | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.09, 2.72] |
| 4.2 400 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 600 mcg | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.51 [0.79, 7.92] |
| 5.2 400 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 600 mcg | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.51 [0.79, 7.92] |
| 6.2 400 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Any shivering | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 600 mcg | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [1.96, 3.01] |
| 7.2 400 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Pyrexia (>= 38 degrees C) | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.11 [3.85, 13.12] |
| 8.1 600 mcg | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.11 [3.85, 13.12] |
| 8.2 400 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
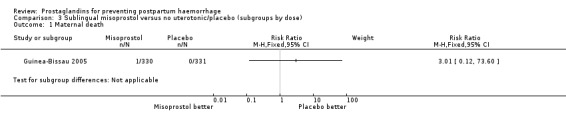
Comparison 3 Sublingual misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 1 Maternal death.
3.2. Analysis.
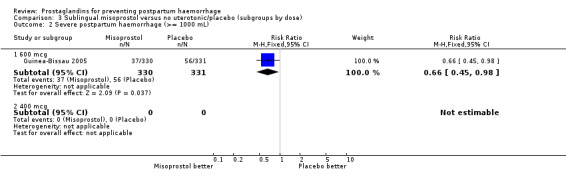
Comparison 3 Sublingual misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 2 Severe postpartum haemorrhage (>= 1000 mL).
3.3. Analysis.
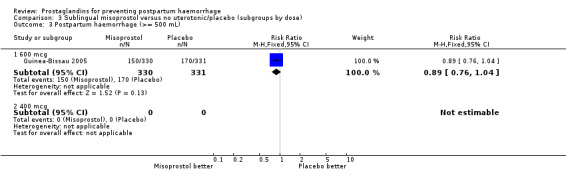
Comparison 3 Sublingual misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 3 Postpartum haemorrhage (>= 500 mL).
3.4. Analysis.
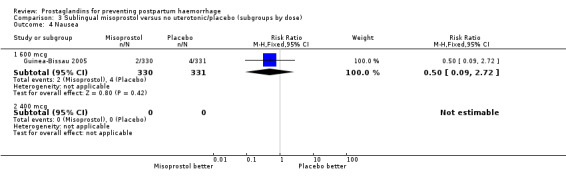
Comparison 3 Sublingual misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 4 Nausea.
3.5. Analysis.
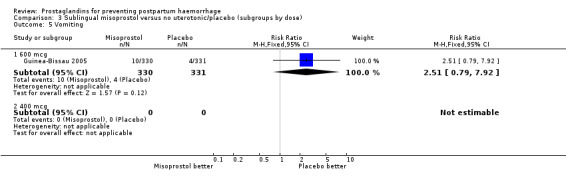
Comparison 3 Sublingual misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 5 Vomiting.
3.6. Analysis.
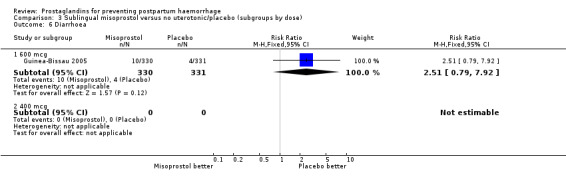
Comparison 3 Sublingual misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 6 Diarrhoea.
3.7. Analysis.
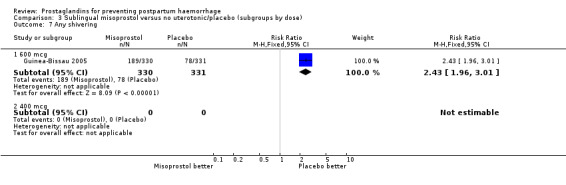
Comparison 3 Sublingual misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 7 Any shivering.
3.8. Analysis.
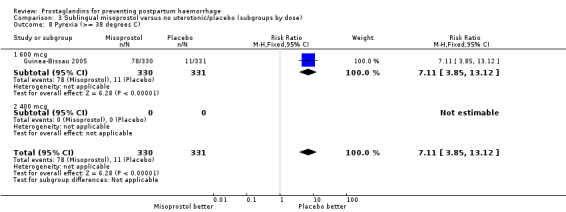
Comparison 3 Sublingual misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 8 Pyrexia (>= 38 degrees C).
Comparison 4. Buccal misoprostol versus no uterotonic/placebo (subgroups by dose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe postpartum haemorrhage (>= 1000 mL) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 400 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 200 mcg | 1 | 352 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.66, 1.94] |
| 2 Use of additional uterotonics | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 400 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 200 mcg | 2 | 1108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.48, 0.85] |
| 3 Blood transfusion | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 400 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 200 mcg | 2 | 1108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.24, 1.89] |
| 4 Blood loss (mL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 600 mcg | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 400 mcg | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 200 mcg | 1 | 352 | Mean Difference (IV, Fixed, 95% CI) | 24.0 [‐16.36, 64.36] |
4.1. Analysis.
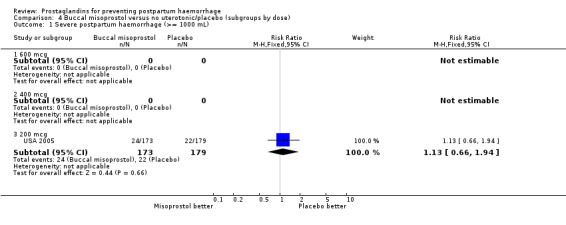
Comparison 4 Buccal misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 1 Severe postpartum haemorrhage (>= 1000 mL).
4.2. Analysis.
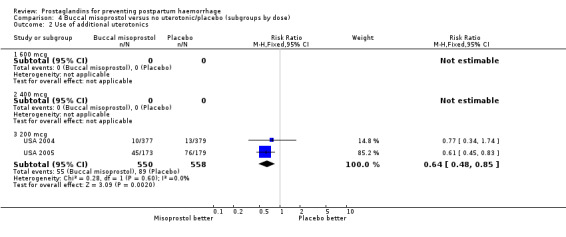
Comparison 4 Buccal misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 2 Use of additional uterotonics.
4.3. Analysis.
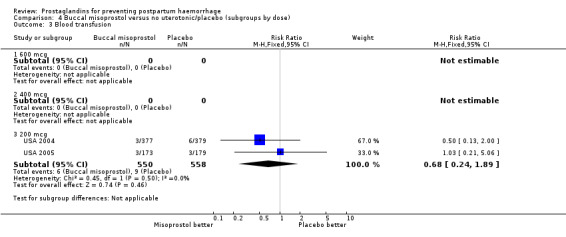
Comparison 4 Buccal misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 3 Blood transfusion.
4.4. Analysis.
Comparison 4 Buccal misoprostol versus no uterotonic/placebo (subgroups by dose), Outcome 4 Blood loss (mL).
Comparison 5. Oral misoprostol versus injectable uterotonics (subgroups by dose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal death | 4 | 20199 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.14, 7.10] |
| 1.1 800 mcg | 1 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 600 mcg | 2 | 18829 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.14, 7.10] |
| 1.3 400 mcg | 2 | 920 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Severe postpartum haemorrhage (>= 1000 mL) | 17 | 29797 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.16, 1.52] |
| 2.1 800 mcg | 1 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 600 mcg | 6 | 21977 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.17, 1.58] |
| 2.3 500 mcg | 2 | 1040 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.43, 1.98] |
| 2.4 400 mcg | 9 | 6330 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.89, 1.75] |
| 3 Postpartum haemorrhage (>= 500 mL) | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 800 mcg | 1 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.63] |
| 3.2 600 mcg | 7 | 22164 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.34, 1.52] |
| 3.3 500 mcg | 2 | 1040 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.82, 1.41] |
| 3.4 400 mcg | 8 | 5496 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.94, 1.34] |
| 4 Blood loss (mL) | 10 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 800 mcg | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐29.0 [‐158.67, 100.67] |
| 4.2 600 mcg | 3 | 20897 | Mean Difference (IV, Fixed, 95% CI) | 42.14 [35.44, 48.84] |
| 4.3 400 mcg | 7 | 4537 | Mean Difference (IV, Fixed, 95% CI) | ‐7.54 [‐16.16, 1.08] |
| 5 Use of additional uterotonics | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 800 mcg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 600 mcg | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 500 mcg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 400 mcg | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Blood transfusion | 15 | 28213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.66, 1.06] |
| 6.1 800 mcg | 1 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.45] |
| 6.2 600 mcg | 5 | 21600 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.59, 1.02] |
| 6.3 500 mcg | 2 | 1040 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.34, 1.95] |
| 6.4 400 mcg | 8 | 5130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.69, 1.91] |
| 7 Postpartum haemoglobin | 2 | 805 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.18, 0.28] |
| 7.1 800 mcg | 1 | 450 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.23, 0.43] |
| 7.2 400 mcg | 1 | 355 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.33, 0.33] |
| 8 Haematocrit drop 10% or more | 1 | 585 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.47, 2.52] |
| 8.1 400 mcg | 1 | 585 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.47, 2.52] |
| 9 Haemoglobin drop 30 mg/L or more | 1 | 585 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.69, 1.88] |
| 9.1 400 mcg | 1 | 585 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.69, 1.88] |
| 10 Manual removal of placenta | 15 | 26765 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.82, 1.12] |
| 10.1 800 mcg | 1 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 600 mcg | 6 | 21806 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.81, 1.16] |
| 10.3 500 mcg | 1 | 1000 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.34, 1.57] |
| 10.4 400 mcg | 8 | 3509 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.71, 1.41] |
| 11 Duration of third stage (minutes) | 6 | 22690 | Mean Difference (IV, Fixed, 95% CI) | 0.44 [0.22, 0.66] |
| 11.1 600 mcg | 2 | 18811 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.21, 0.58] |
| 11.2 400 mcg | 5 | 3879 | Mean Difference (IV, Fixed, 95% CI) | 0.56 [0.29, 0.82] |
| 12 Third stage >= 30 minutes | 8 | 5580 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.62, 1.42] |
| 12.1 600 mcg | 4 | 2954 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.53, 1.89] |
| 12.2 500 mcg | 1 | 1000 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.44, 1.95] |
| 12.3 400 mcg | 3 | 1626 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.36, 1.90] |
| 13 Any side‐effect | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 500 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 400 mcg | 1 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.16, 1.77] |
| 14 Nausea | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 800 mcg | 2 | 498 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.10, 1.94] |
| 14.2 600 mcg | 6 | 21793 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.90, 1.41] |
| 14.3 500 mcg | 1 | 846 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.59, 0.85] |
| 14.4 400 mcg | 6 | 3774 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.48, 1.07] |
| 15 Vomiting | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 800 mcg | 1 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.25] |
| 15.2 600 mcg | 7 | 22175 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.97, 1.58] |
| 15.3 500 mcg | 1 | 846 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.70, 1.23] |
| 15.4 400 mcg | 8 | 5439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.37, 0.86] |
| 16 Diarrhoea | 13 | 27011 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.34, 2.50] |
| 16.1 800 mcg | 1 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.85 [0.60, 195.06] |
| 16.2 600 mcg | 5 | 20326 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [1.60, 3.98] |
| 16.3 500 mcg | 1 | 846 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.55, 2.19] |
| 16.4 400 mcg | 8 | 5400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.67, 2.22] |
| 17 Headache | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 17.1 800 mcg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 600 mcg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.3 500 mcg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.4 400 mcg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 Any shivering | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 18.1 800 mcg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 600 mcg | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.3 500 mcg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.4 400 mcg | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 Severe shivering | 5 | 20823 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.24 [4.74, 11.08] |
| 19.1 600 mcg | 3 | 19038 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.28 [4.71, 11.24] |
| 19.2 500 mcg | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.52, 156.91] |
| 19.3 400 mcg | 2 | 1745 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.23 [0.20, 87.88] |
| 20 Pyrexia (>= 38 degrees C) | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 600 mcg | 7 | 22137 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.77 [5.55, 8.27] |
| 20.2 500 mcg | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 400 mcg | 5 | 2642 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.68 [3.74, 11.93] |
5.1. Analysis.
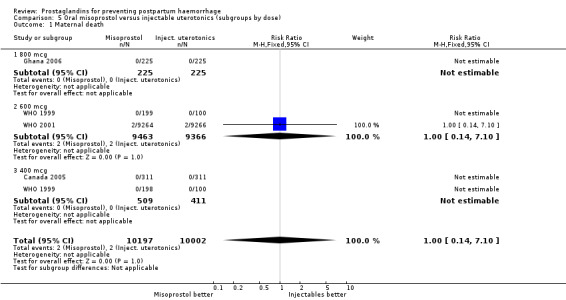
Comparison 5 Oral misoprostol versus injectable uterotonics (subgroups by dose), Outcome 1 Maternal death.
5.2. Analysis.
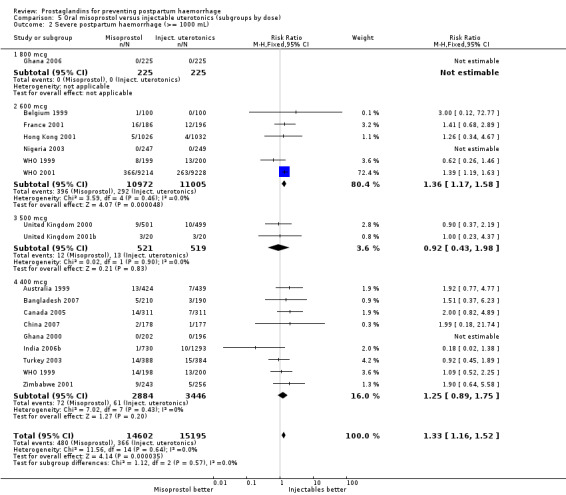
Comparison 5 Oral misoprostol versus injectable uterotonics (subgroups by dose), Outcome 2 Severe postpartum haemorrhage (>= 1000 mL).
5.3. Analysis.
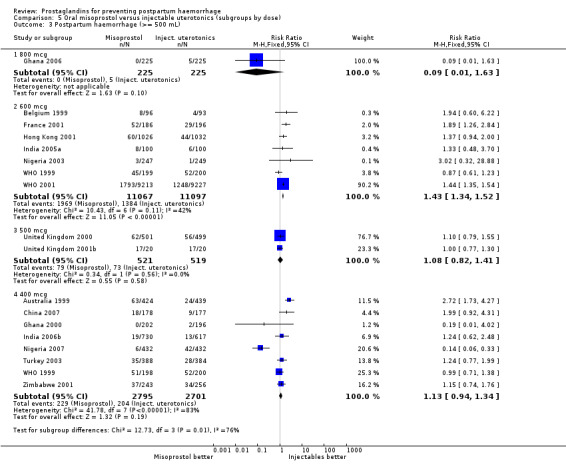
Comparison 5 Oral misoprostol versus injectable uterotonics (subgroups by dose), Outcome 3 Postpartum haemorrhage (>= 500 mL).
5.4. Analysis.
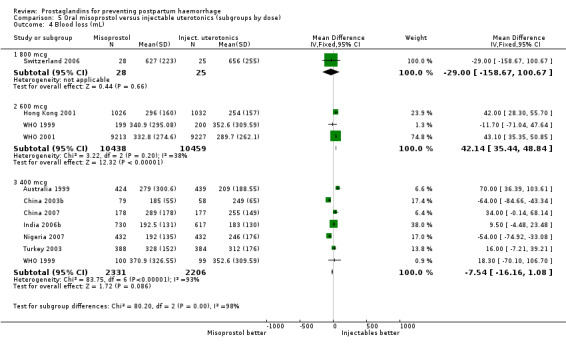
Comparison 5 Oral misoprostol versus injectable uterotonics (subgroups by dose), Outcome 4 Blood loss (mL).
5.5. Analysis.
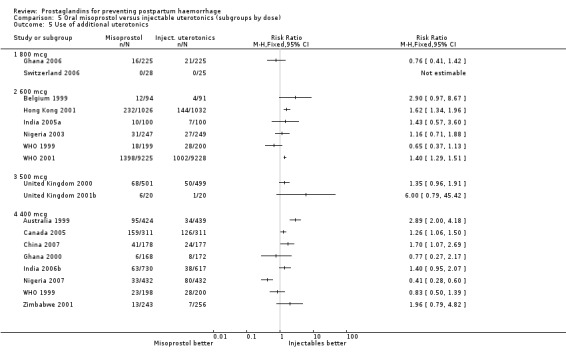
Comparison 5 Oral misoprostol versus injectable uterotonics (subgroups by dose), Outcome 5 Use of additional uterotonics.
5.6. Analysis.
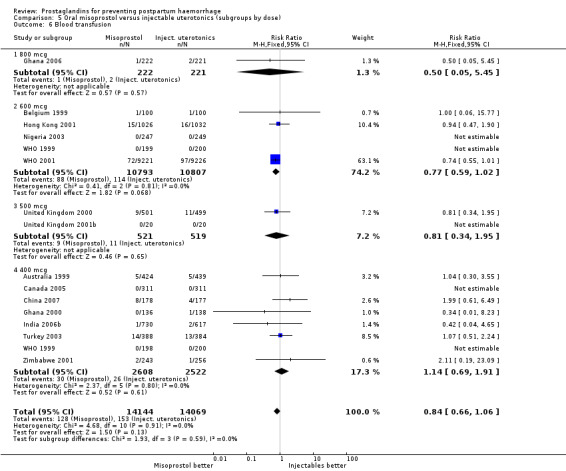
Comparison 5 Oral misoprostol versus injectable uterotonics (subgroups by dose), Outcome 6 Blood transfusion.
5.7. Analysis.
Comparison 5 Oral misoprostol versus injectable uterotonics (subgroups by dose), Outcome 7 Postpartum haemoglobin.
5.8. Analysis.
Comparison 5 Oral misoprostol versus injectable uterotonics (subgroups by dose), Outcome 8 Haematocrit drop 10% or more.
5.9. Analysis.
Comparison 5 Oral misoprostol versus injectable uterotonics (subgroups by dose), Outcome 9 Haemoglobin drop 30 mg/L or more.
5.10. Analysis.
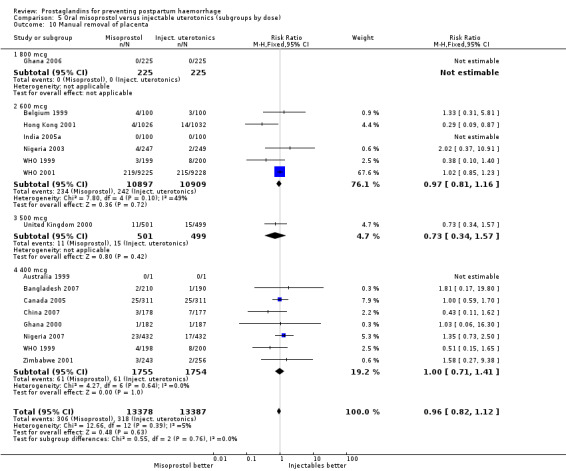
Comparison 5 Oral misoprostol versus injectable uterotonics (subgroups by dose), Outcome 10 Manual removal of placenta.
5.11. Analysis.
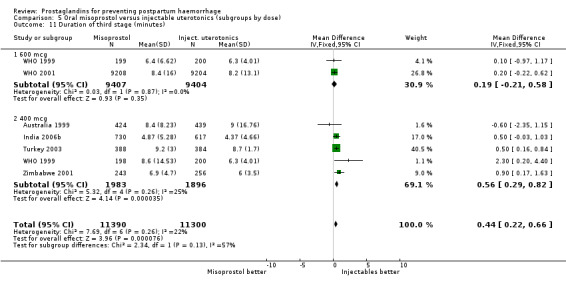
Comparison 5 Oral misoprostol versus injectable uterotonics (subgroups by dose), Outcome 11 Duration of third stage (minutes).
5.12. Analysis.
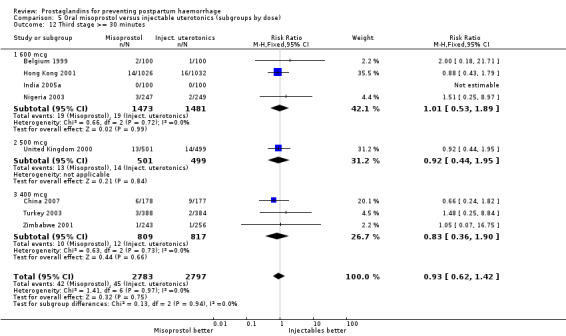
Comparison 5 Oral misoprostol versus injectable uterotonics (subgroups by dose), Outcome 12 Third stage >= 30 minutes.
5.13. Analysis.
Comparison 5 Oral misoprostol versus injectable uterotonics (subgroups by dose), Outcome 13 Any side‐effect.
5.14. Analysis.
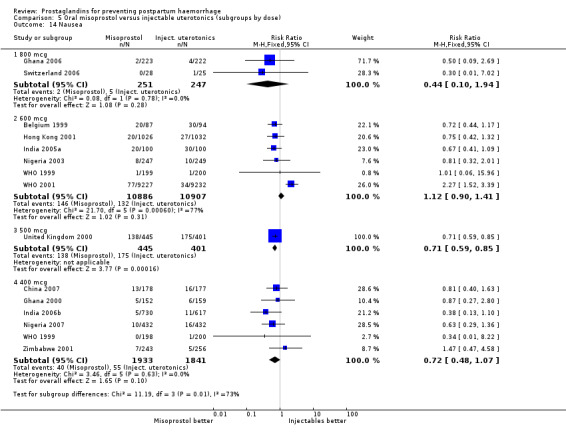
Comparison 5 Oral misoprostol versus injectable uterotonics (subgroups by dose), Outcome 14 Nausea.
5.15. Analysis.
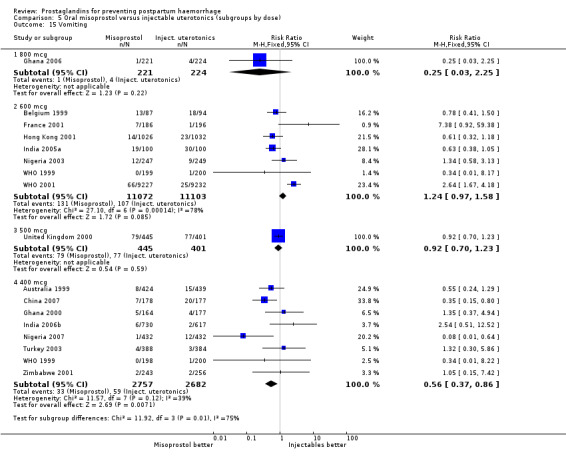
Comparison 5 Oral misoprostol versus injectable uterotonics (subgroups by dose), Outcome 15 Vomiting.
5.16. Analysis.
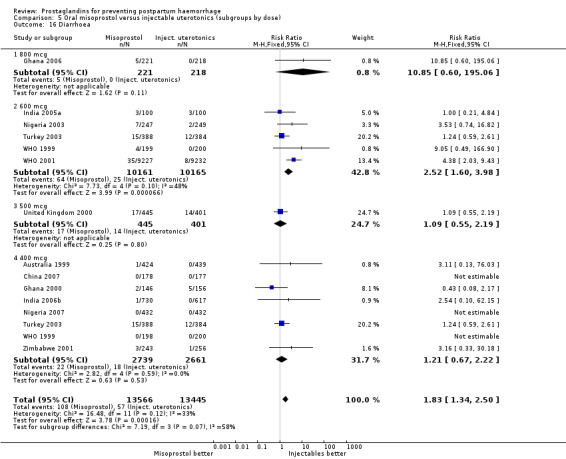
Comparison 5 Oral misoprostol versus injectable uterotonics (subgroups by dose), Outcome 16 Diarrhoea.
5.17. Analysis.
Comparison 5 Oral misoprostol versus injectable uterotonics (subgroups by dose), Outcome 17 Headache.
5.18. Analysis.
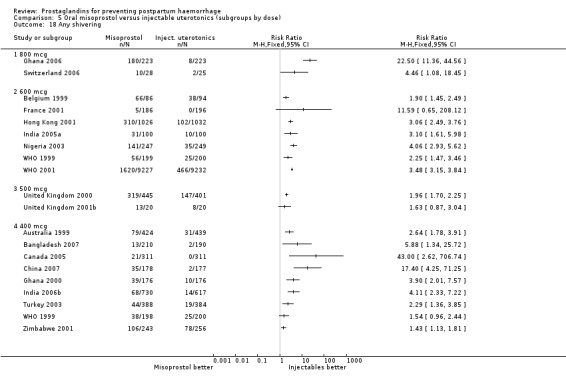
Comparison 5 Oral misoprostol versus injectable uterotonics (subgroups by dose), Outcome 18 Any shivering.
5.20. Analysis.
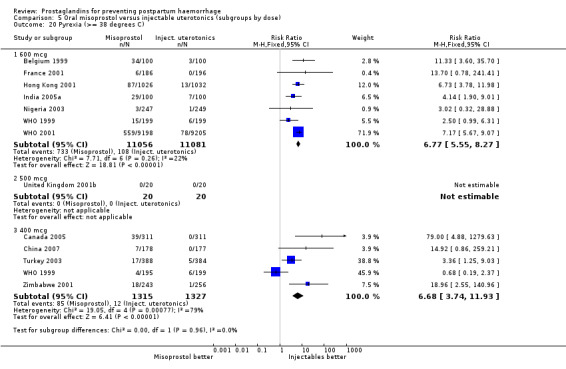
Comparison 5 Oral misoprostol versus injectable uterotonics (subgroups by dose), Outcome 20 Pyrexia (>= 38 degrees C).
Comparison 6. Rectal misoprostol versus injectable uterotonics (subgroups by dose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal death | 3 | 1393 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.21] |
| 1.1 800 mcg | 1 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.21] |
| 1.2 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 400 mcg | 2 | 943 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Postpartum haemorrhage (>= 500 mL) | 7 | 3399 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.41] |
| 2.1 800 mcg | 2 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.60, 2.09] |
| 2.2 600 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.77] |
| 2.3 400 mcg | 4 | 2244 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.92, 1.43] |
| 3 Severe postpartum haemorrhage (>= 1000 mL) | 4 | 2221 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.69, 1.77] |
| 3.1 800 mcg | 1 | 441 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.40] |
| 3.2 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 400 mcg | 3 | 1780 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.70, 1.85] |
| 4 Blood loss (mL) | 6 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 800 mcg | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 600 mcg | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 400 mcg | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Use of additional uterotonics | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 800 mcg | 2 | 961 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.35, 1.24] |
| 5.2 600 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.59, 42.04] |
| 5.3 400 mcg | 3 | 1210 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.16, 2.31] |
| 6 Blood transfusion | 7 | 3105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.59, 1.77] |
| 6.1 800 mcg | 2 | 952 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.40, 2.52] |
| 6.2 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 400 mcg | 5 | 2153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.52, 2.04] |
| 7 Manual removal of placenta | 3 | 563 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.17, 1.67] |
| 7.1 600 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.32, 28.35] |
| 7.2 400 mcg | 2 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.04, 1.16] |
| 8 Duration of third stage (minutes) | 7 | 3245 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.05, 0.46] |
| 8.1 800 mcg | 2 | 964 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.25, 0.56] |
| 8.2 600 mcg | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.25, 1.25] |
| 8.3 400 mcg | 4 | 2081 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.08, 0.58] |
| 9 Third stage >= 30 minutes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 400 mcg | 1 | 803 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.17 [1.39, 27.38] |
| 10 Postpartum haemoglobin | 2 | 954 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.16, 0.16] |
| 10.1 800 mcg | 2 | 954 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.16, 0.16] |
| 11 Nausea | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 800 mcg | 2 | 942 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.08] |
| 11.2 600 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 102.85] |
| 11.3 400 mcg | 2 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.41, 2.61] |
| 12 Vomiting | 6 | 2759 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.59, 2.26] |
| 12.1 800 mcg | 2 | 941 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.35, 2.82] |
| 12.2 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 400 mcg | 4 | 1818 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.53, 3.12] |
| 13 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 400 mcg | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [0.75, 7.42] |
| 14 Abdominal pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 400 mcg | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.46, 2.02] |
| 15 Diarrhoea | 3 | 1978 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.56, 2.11] |
| 15.1 800 mcg | 1 | 514 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.37, 3.88] |
| 15.2 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 400 mcg | 2 | 1464 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.46, 2.31] |
| 16 Any shivering | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 800 mcg | 2 | 940 | Risk Ratio (M‐H, Fixed, 95% CI) | 38.6 [11.04, 134.95] |
| 16.2 600 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.63, 2.42] |
| 16.3 400 mcg | 5 | 2143 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.34 [1.88, 2.92] |
| 17 Pyrexia (>= 38 degrees C) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 600 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 102.85] |
| 17.2 400 mcg | 2 | 1022 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [1.21, 3.57] |
6.1. Analysis.
Comparison 6 Rectal misoprostol versus injectable uterotonics (subgroups by dose), Outcome 1 Maternal death.
6.2. Analysis.
Comparison 6 Rectal misoprostol versus injectable uterotonics (subgroups by dose), Outcome 2 Postpartum haemorrhage (>= 500 mL).
6.3. Analysis.
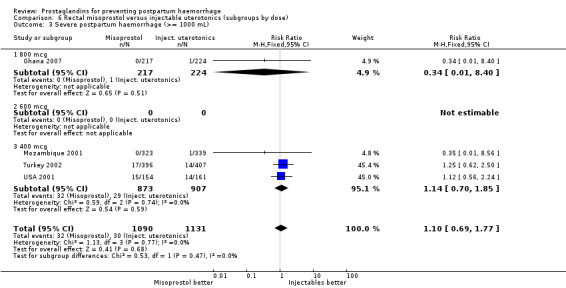
Comparison 6 Rectal misoprostol versus injectable uterotonics (subgroups by dose), Outcome 3 Severe postpartum haemorrhage (>= 1000 mL).
6.4. Analysis.
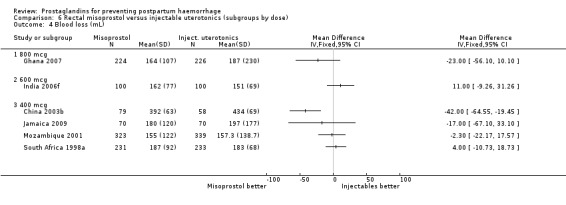
Comparison 6 Rectal misoprostol versus injectable uterotonics (subgroups by dose), Outcome 4 Blood loss (mL).
6.5. Analysis.
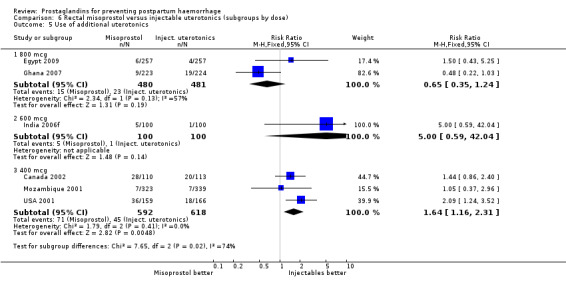
Comparison 6 Rectal misoprostol versus injectable uterotonics (subgroups by dose), Outcome 5 Use of additional uterotonics.
6.6. Analysis.
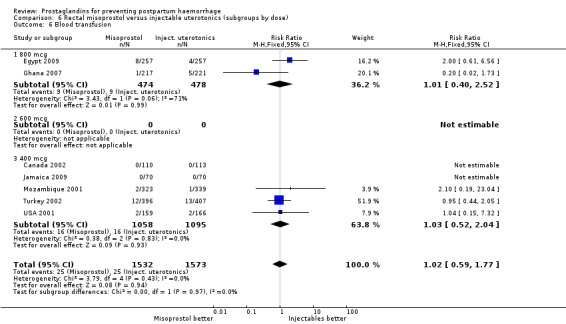
Comparison 6 Rectal misoprostol versus injectable uterotonics (subgroups by dose), Outcome 6 Blood transfusion.
6.7. Analysis.
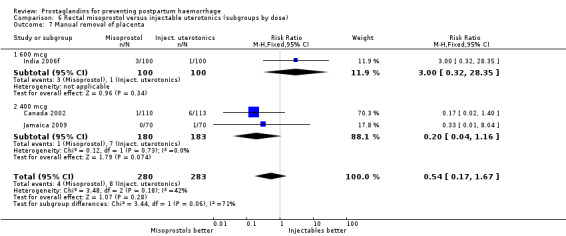
Comparison 6 Rectal misoprostol versus injectable uterotonics (subgroups by dose), Outcome 7 Manual removal of placenta.
6.8. Analysis.
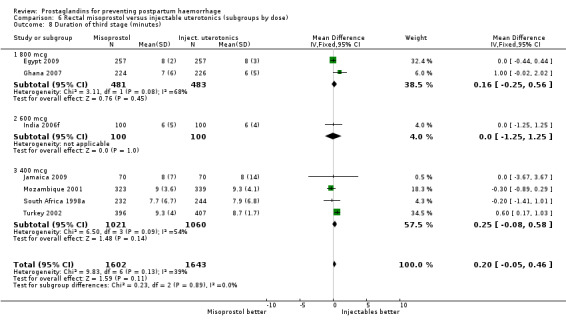
Comparison 6 Rectal misoprostol versus injectable uterotonics (subgroups by dose), Outcome 8 Duration of third stage (minutes).
6.9. Analysis.
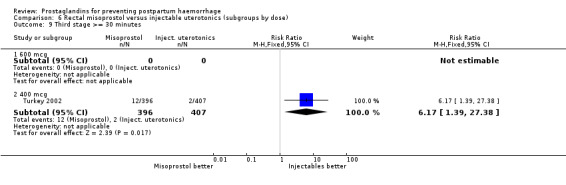
Comparison 6 Rectal misoprostol versus injectable uterotonics (subgroups by dose), Outcome 9 Third stage >= 30 minutes.
6.10. Analysis.
Comparison 6 Rectal misoprostol versus injectable uterotonics (subgroups by dose), Outcome 10 Postpartum haemoglobin.
6.11. Analysis.
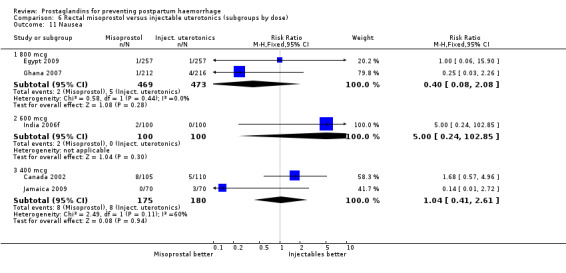
Comparison 6 Rectal misoprostol versus injectable uterotonics (subgroups by dose), Outcome 11 Nausea.
6.12. Analysis.
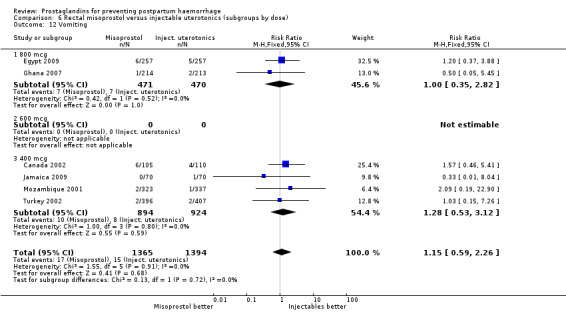
Comparison 6 Rectal misoprostol versus injectable uterotonics (subgroups by dose), Outcome 12 Vomiting.
6.13. Analysis.
Comparison 6 Rectal misoprostol versus injectable uterotonics (subgroups by dose), Outcome 13 Headache.
6.14. Analysis.
Comparison 6 Rectal misoprostol versus injectable uterotonics (subgroups by dose), Outcome 14 Abdominal pain.
6.15. Analysis.
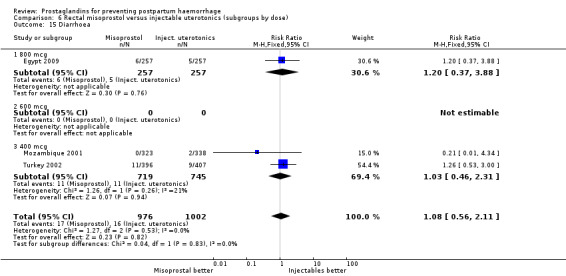
Comparison 6 Rectal misoprostol versus injectable uterotonics (subgroups by dose), Outcome 15 Diarrhoea.
6.16. Analysis.
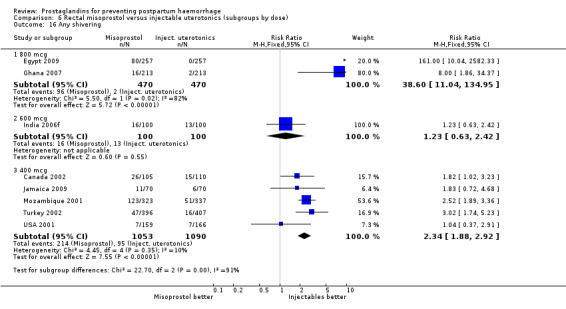
Comparison 6 Rectal misoprostol versus injectable uterotonics (subgroups by dose), Outcome 16 Any shivering.
6.17. Analysis.
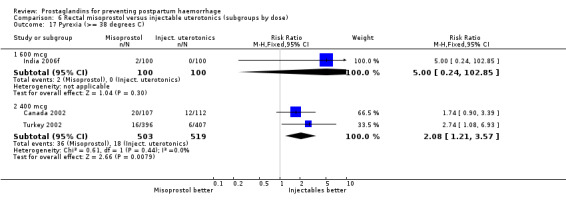
Comparison 6 Rectal misoprostol versus injectable uterotonics (subgroups by dose), Outcome 17 Pyrexia (>= 38 degrees C).
Comparison 7. Sublingual misoprostol versus injectable uterotonic (subgroups by dose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe postpartum haemorrhage (>= 1000 mL) | 3 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.23, 1.27] |
| 1.1 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 400 mcg | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.24, 1.53] |
| 1.3 50 mcg | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.99] |
| 2 Postpartum haemorrhage (>= 500 mL) | 6 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.83, 1.21] |
| 2.1 600 mcg | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.40, 10.11] |
| 2.2 400 mcg | 3 | 353 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.83, 1.17] |
| 2.3 200 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 50 mcg | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.37, 2.05] |
| 3 Blood loss (mL) | 5 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 600 mcg | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 400 mcg | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 200 mcg | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 50 mcg | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Use of additional uterotonics | 7 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.44, 0.85] |
| 4.1 600 mcg | 2 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.11, 1.02] |
| 4.2 400 mcg | 5 | 603 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.44, 0.90] |
| 4.3 200 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.81] |
| 5 Blood transfusion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 400 mcg | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Postpartum haemoglobin | 4 | 533 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.25, 0.21] |
| 6.1 600 mcg | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 400 mcg | 3 | 333 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.48, 0.36] |
| 6.3 200 mcg | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.28, 0.28] |
| 7 Manual removal of placenta | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.02] |
| 7.1 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 400 mcg | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.02] |
| 8 Duration of third stage (minutes) | 2 | 500 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.22, 0.22] |
| 8.1 600 mcg | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.32, 0.32] |
| 8.2 400 mcg | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.32, 0.32] |
| 8.3 200 mcg | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.39, 1.39] |
| 9 Any side‐effect | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.87, 2.29] |
| 9.1 400 mcg | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.87, 2.29] |
| 10 Nausea | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 400 mcg | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.09 [0.75, 49.22] |
| 10.2 200 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.22, 1.12] |
| 11 Vomiting | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.1 600 mcg | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 400 mcg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 50 mcg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.4 200 mcg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Headache | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.37, 1.52] |
| 12.1 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 400 mcg | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.28, 2.00] |
| 12.3 200 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.27, 2.08] |
| 13 Abdominal pain | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.07 [0.25, 103.73] |
| 13.1 400 mcg | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.07 [0.25, 103.73] |
| 14 Diarrhoea | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.13, 73.42] |
| 14.1 400 mcg | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.13, 73.42] |
| 15 Any shivering | 5 | 783 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.06 [4.46, 18.39] |
| 15.1 600 mcg | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 27.0 [1.63, 446.10] |
| 15.2 400 mcg | 3 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.53 [3.44, 16.49] |
| 15.3 200 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 17.0 [0.99, 290.62] |
| 15.4 50 mcg | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.30] |
| 16 Pyrexia >= 38 degrees C | 4 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.04 [4.77, 35.62] |
| 16.1 600 mcg | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 33.0 [2.02, 540.22] |
| 16.2 400 mcg | 4 | 503 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.18 [3.45, 30.06] |
7.1. Analysis.
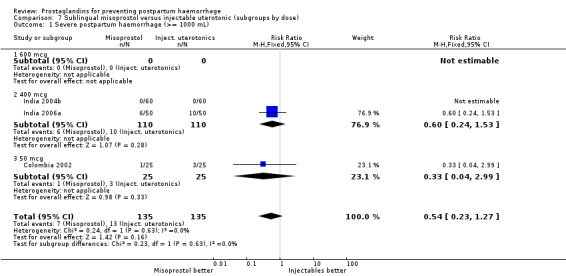
Comparison 7 Sublingual misoprostol versus injectable uterotonic (subgroups by dose), Outcome 1 Severe postpartum haemorrhage (>= 1000 mL).
7.2. Analysis.
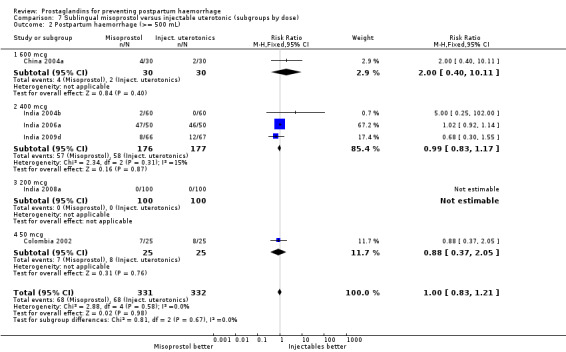
Comparison 7 Sublingual misoprostol versus injectable uterotonic (subgroups by dose), Outcome 2 Postpartum haemorrhage (>= 500 mL).
7.3. Analysis.
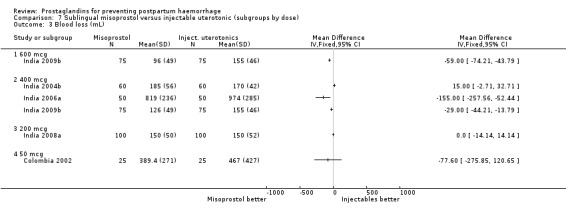
Comparison 7 Sublingual misoprostol versus injectable uterotonic (subgroups by dose), Outcome 3 Blood loss (mL).
7.4. Analysis.
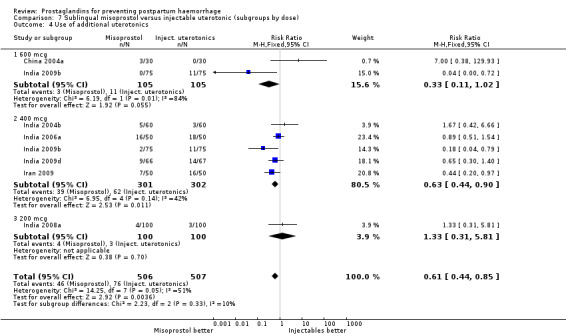
Comparison 7 Sublingual misoprostol versus injectable uterotonic (subgroups by dose), Outcome 4 Use of additional uterotonics.
7.5. Analysis.
Comparison 7 Sublingual misoprostol versus injectable uterotonic (subgroups by dose), Outcome 5 Blood transfusion.
7.6. Analysis.
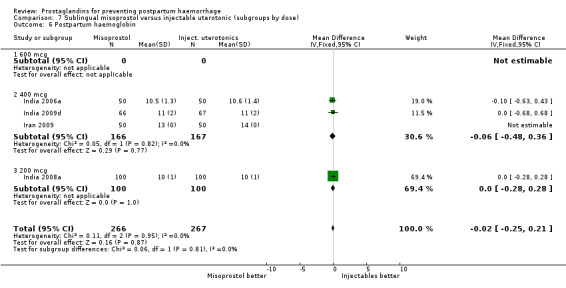
Comparison 7 Sublingual misoprostol versus injectable uterotonic (subgroups by dose), Outcome 6 Postpartum haemoglobin.
7.7. Analysis.
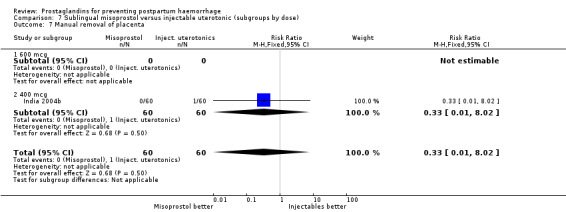
Comparison 7 Sublingual misoprostol versus injectable uterotonic (subgroups by dose), Outcome 7 Manual removal of placenta.
7.8. Analysis.
Comparison 7 Sublingual misoprostol versus injectable uterotonic (subgroups by dose), Outcome 8 Duration of third stage (minutes).
7.9. Analysis.
Comparison 7 Sublingual misoprostol versus injectable uterotonic (subgroups by dose), Outcome 9 Any side‐effect.
7.10. Analysis.
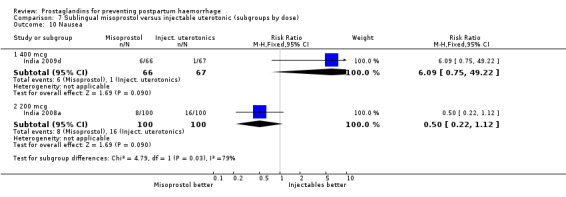
Comparison 7 Sublingual misoprostol versus injectable uterotonic (subgroups by dose), Outcome 10 Nausea.
7.11. Analysis.
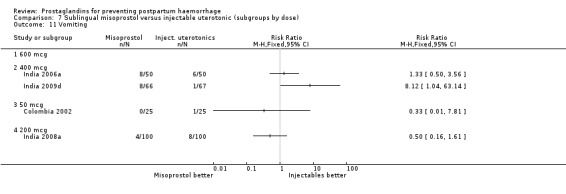
Comparison 7 Sublingual misoprostol versus injectable uterotonic (subgroups by dose), Outcome 11 Vomiting.
7.12. Analysis.
Comparison 7 Sublingual misoprostol versus injectable uterotonic (subgroups by dose), Outcome 12 Headache.
7.13. Analysis.
Comparison 7 Sublingual misoprostol versus injectable uterotonic (subgroups by dose), Outcome 13 Abdominal pain.
7.14. Analysis.
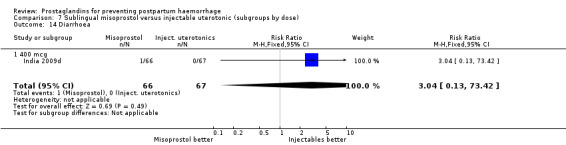
Comparison 7 Sublingual misoprostol versus injectable uterotonic (subgroups by dose), Outcome 14 Diarrhoea.
7.15. Analysis.
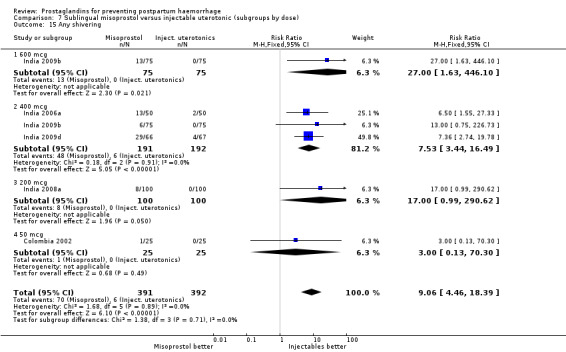
Comparison 7 Sublingual misoprostol versus injectable uterotonic (subgroups by dose), Outcome 15 Any shivering.
7.16. Analysis.
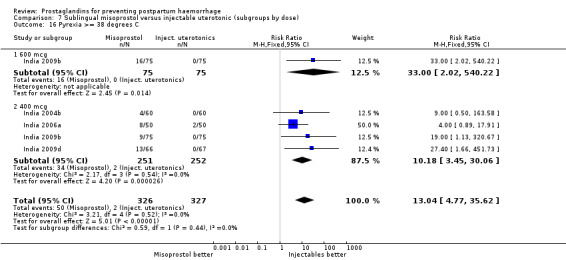
Comparison 7 Sublingual misoprostol versus injectable uterotonic (subgroups by dose), Outcome 16 Pyrexia >= 38 degrees C.
Comparison 8. Oral misoprostol plus injectable uterotonics versus injectable uterotonics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe postpartum haemorrhage (>= 1000 mL) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Postpartum haemorrhage (>= 500 mL) | 3 | 3205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.53, 0.95] |
| 3 Blood loss (mL) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Duration of third stage (mins) | 1 | 788 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.31, 0.51] |
| 5 Third stage >= 30 minutes | 1 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.24, 8.49] |
| 6 Manual removal of placenta | 2 | 2229 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.64, 1.35] |
| 7 Blood transfusion | 1 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.13, 1.02] |
| 8 Vomiting | 1 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.19, 4.68] |
| 9 Diarrhoea | 1 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.48, 2.23] |
| 10 Any shivering | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11 Pyrexia (>= 38 degrees C) | 2 | 2022 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [1.80, 5.28] |
8.1. Analysis.
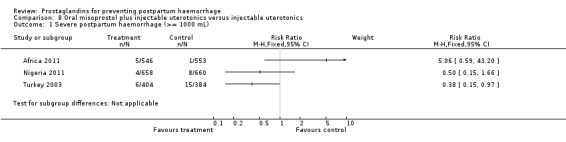
Comparison 8 Oral misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 1 Severe postpartum haemorrhage (>= 1000 mL).
8.2. Analysis.
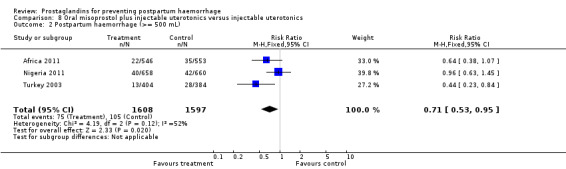
Comparison 8 Oral misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 2 Postpartum haemorrhage (>= 500 mL).
8.3. Analysis.
Comparison 8 Oral misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 3 Blood loss (mL).
8.4. Analysis.
Comparison 8 Oral misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 4 Duration of third stage (mins).
8.5. Analysis.
Comparison 8 Oral misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 5 Third stage >= 30 minutes.
8.6. Analysis.
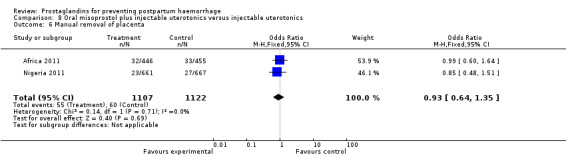
Comparison 8 Oral misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 6 Manual removal of placenta.
8.7. Analysis.
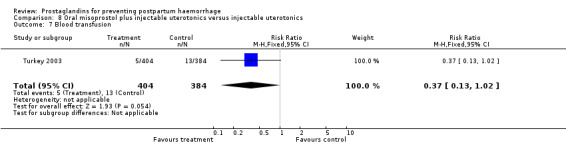
Comparison 8 Oral misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 7 Blood transfusion.
8.8. Analysis.
Comparison 8 Oral misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 8 Vomiting.
8.9. Analysis.
Comparison 8 Oral misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 9 Diarrhoea.
8.10. Analysis.
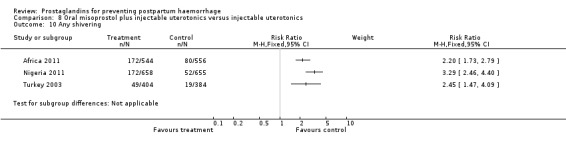
Comparison 8 Oral misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 10 Any shivering.
8.11. Analysis.
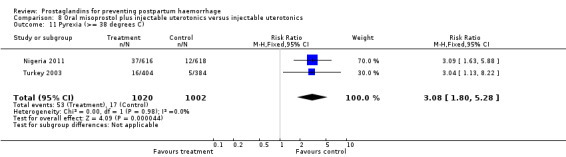
Comparison 8 Oral misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 11 Pyrexia (>= 38 degrees C).
Comparison 9. Rectal misoprostol plus injectable uterotonics versus injectable uterotonics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe postpartum haemorrhage (>= 1000 mL) | 1 | 808 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.37, 1.74] |
| 2 Postpartum haemorrhage (>= 500 mL) | 1 | 808 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.53, 1.40] |
| 3 Duration of third stage (minutes) | 1 | 808 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.46, 0.26] |
| 4 Third stage >= 30 minutes | 1 | 808 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.14, 7.17] |
| 5 Blood transfusion | 1 | 808 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.10, 0.95] |
| 6 Vomiting | 1 | 808 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.26, 9.06] |
| 7 Diarrhoea | 1 | 808 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.41, 2.53] |
| 8 Any shivering | 1 | 808 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.30 [1.92, 5.68] |
| 9 Pyrexia (>= 38 degrees C) | 1 | 808 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [1.30, 7.96] |
9.1. Analysis.
Comparison 9 Rectal misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 1 Severe postpartum haemorrhage (>= 1000 mL).
9.2. Analysis.
Comparison 9 Rectal misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 2 Postpartum haemorrhage (>= 500 mL).
9.3. Analysis.
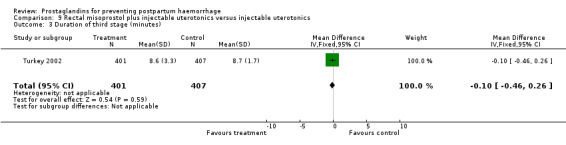
Comparison 9 Rectal misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 3 Duration of third stage (minutes).
9.4. Analysis.
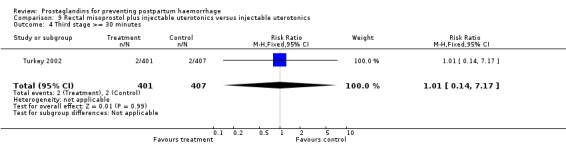
Comparison 9 Rectal misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 4 Third stage >= 30 minutes.
9.5. Analysis.
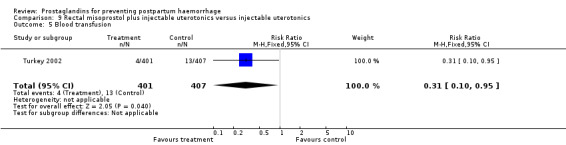
Comparison 9 Rectal misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 5 Blood transfusion.
9.6. Analysis.
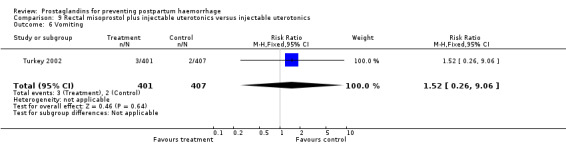
Comparison 9 Rectal misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 6 Vomiting.
9.7. Analysis.
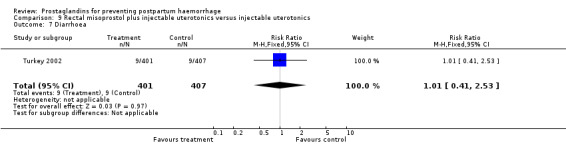
Comparison 9 Rectal misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 7 Diarrhoea.
9.8. Analysis.
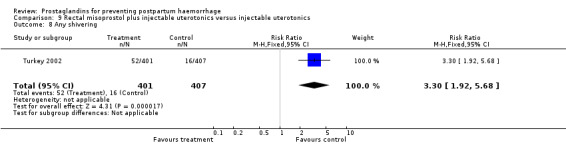
Comparison 9 Rectal misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 8 Any shivering.
9.9. Analysis.
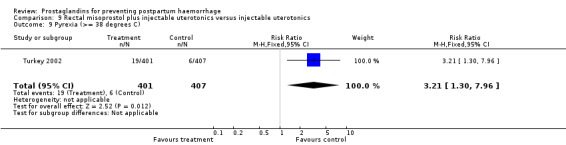
Comparison 9 Rectal misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 9 Pyrexia (>= 38 degrees C).
Comparison 10. Sublingual misoprostol plus injectable uterotonics versus injectable uterotonics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe postpartum hemorrhage (>=1000 mL) | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.46, 1.37] |
| 1.1 200 mcg | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.46, 1.37] |
| 2 Blood transfusion | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.04] |
| 2.1 200 mcg | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.04] |
| 3 Any side effects | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [1.58, 4.04] |
| 3.1 200 mcg | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [1.58, 4.04] |
| 4 Vomiting | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.70, 3.21] |
| 4.1 200 mcg | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.70, 3.21] |
| 4.2 200 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Nausea | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.5 [0.99, 20.41] |
| 5.1 200 mcg | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.5 [0.99, 20.41] |
| 6 Headache | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.37, 10.72] |
| 6.1 200 mcg | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.37, 10.72] |
| 7 Shivering | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.33 [1.85, 10.16] |
| 7.1 200 mcg | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.33 [1.85, 10.16] |
| 8 Pyrexia (>= 38 C) | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.87, 56.06] |
| 8.1 200 mcg | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.87, 56.06] |
10.1. Analysis.
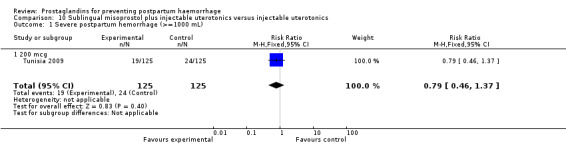
Comparison 10 Sublingual misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 1 Severe postpartum hemorrhage (>=1000 mL).
10.2. Analysis.
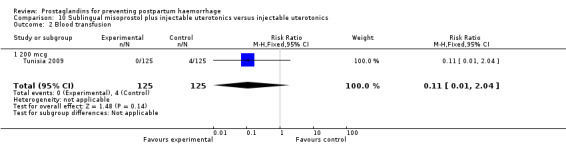
Comparison 10 Sublingual misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 2 Blood transfusion.
10.3. Analysis.
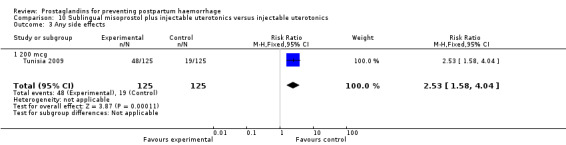
Comparison 10 Sublingual misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 3 Any side effects.
10.4. Analysis.
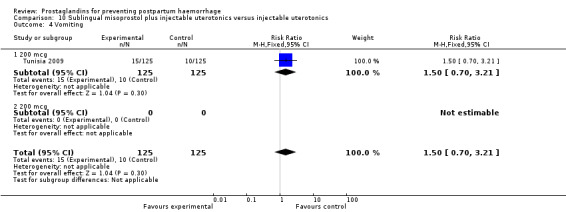
Comparison 10 Sublingual misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 4 Vomiting.
10.5. Analysis.
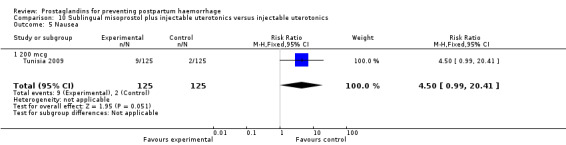
Comparison 10 Sublingual misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 5 Nausea.
10.6. Analysis.
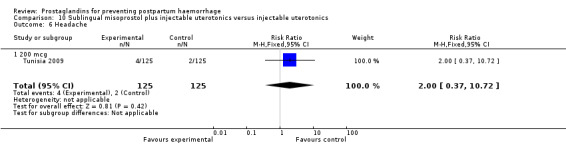
Comparison 10 Sublingual misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 6 Headache.
10.7. Analysis.
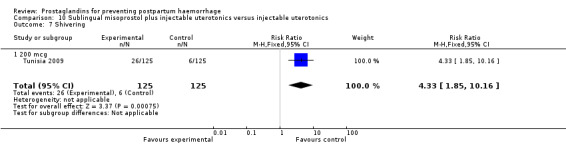
Comparison 10 Sublingual misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 7 Shivering.
10.8. Analysis.
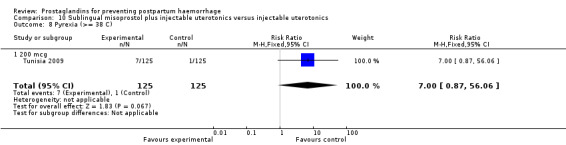
Comparison 10 Sublingual misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 8 Pyrexia (>= 38 C).
Comparison 11. Intravaginal misoprostol plus injectable uterotonics versus injectable uterotonics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postpartum haemoglobin | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [0.72, 1.28] |
| 1.1 800 mcg | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [0.72, 1.28] |
| 2 Use of Additional Uterotonics | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.32, 28.35] |
| 2.1 800 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.32, 28.35] |
| 3 Any shivering | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.50, 12.59] |
| 3.1 800 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.50, 12.59] |
| 4 Nausea | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.50, 12.59] |
| 4.1 800 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.50, 12.59] |
| 5 Vomiting | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.21, 4.84] |
| 5.1 800 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.21, 4.84] |
| 6 Pyrexia (>= 38 C) | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.62, 6.43] |
| 6.1 800 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.62, 6.43] |
11.1. Analysis.
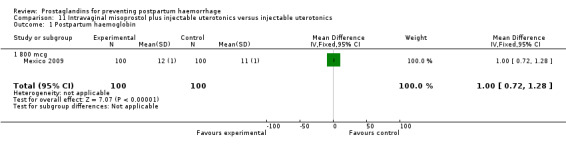
Comparison 11 Intravaginal misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 1 Postpartum haemoglobin.
11.2. Analysis.
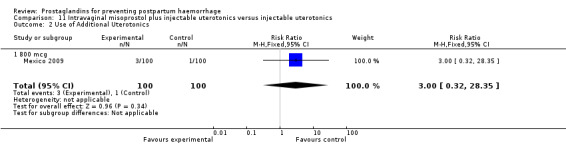
Comparison 11 Intravaginal misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 2 Use of Additional Uterotonics.
11.3. Analysis.
Comparison 11 Intravaginal misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 3 Any shivering.
11.4. Analysis.
Comparison 11 Intravaginal misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 4 Nausea.
11.5. Analysis.
Comparison 11 Intravaginal misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 5 Vomiting.
11.6. Analysis.
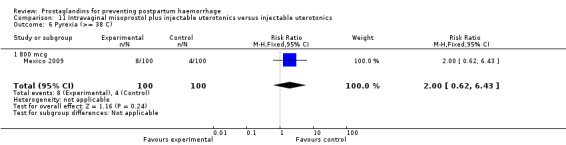
Comparison 11 Intravaginal misoprostol plus injectable uterotonics versus injectable uterotonics, Outcome 6 Pyrexia (>= 38 C).
Comparison 12. Intramuscular prostaglandin versus no uterotonic/placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postpartum haemorrhage (>= 500 mL) | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.22, 1.35] |
| 2 Severe postpartum haemorrhage (>= 1000 mL) | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.04, 3.24] |
| 3 Blood loss (mL) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐224.0 [‐420.35, ‐27.65] |
| 4 Use of additional uterotonics | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.29] |
| 5 Manual removal of placenta | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Duration of third stage (minutes) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐3.60 [‐7.65, 0.45] |
| 7 Any side‐effect | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.46] |
| 8 Nausea | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.46] |
12.1. Analysis.
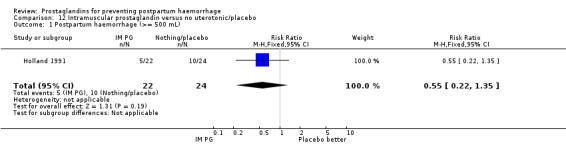
Comparison 12 Intramuscular prostaglandin versus no uterotonic/placebo, Outcome 1 Postpartum haemorrhage (>= 500 mL).
12.2. Analysis.
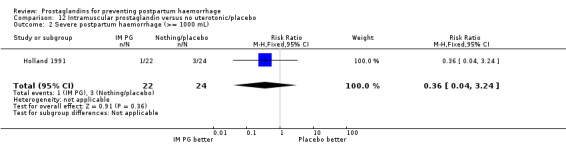
Comparison 12 Intramuscular prostaglandin versus no uterotonic/placebo, Outcome 2 Severe postpartum haemorrhage (>= 1000 mL).
12.3. Analysis.
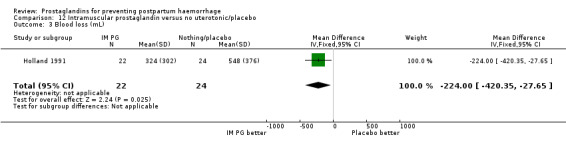
Comparison 12 Intramuscular prostaglandin versus no uterotonic/placebo, Outcome 3 Blood loss (mL).
12.4. Analysis.
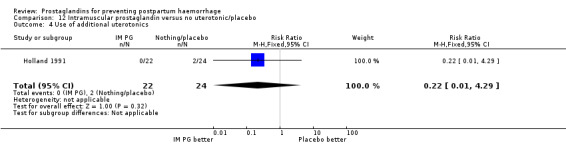
Comparison 12 Intramuscular prostaglandin versus no uterotonic/placebo, Outcome 4 Use of additional uterotonics.
12.5. Analysis.
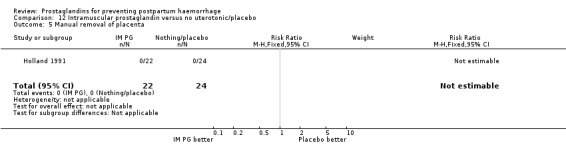
Comparison 12 Intramuscular prostaglandin versus no uterotonic/placebo, Outcome 5 Manual removal of placenta.
12.6. Analysis.
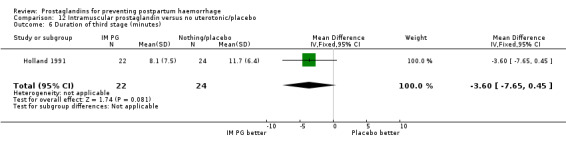
Comparison 12 Intramuscular prostaglandin versus no uterotonic/placebo, Outcome 6 Duration of third stage (minutes).
12.7. Analysis.
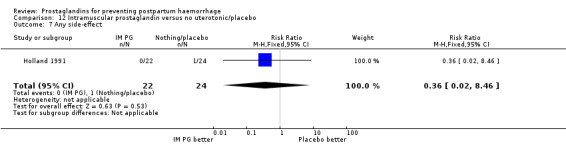
Comparison 12 Intramuscular prostaglandin versus no uterotonic/placebo, Outcome 7 Any side‐effect.
12.8. Analysis.
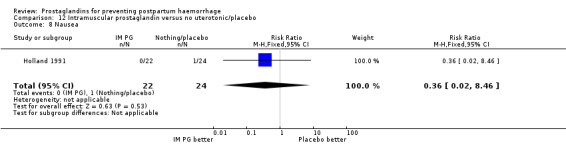
Comparison 12 Intramuscular prostaglandin versus no uterotonic/placebo, Outcome 8 Nausea.
Comparison 13. Intramuscular prostaglandin versus injectable uterotonics (subgroups by low‐ and high‐risk pregnancy).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postpartum haemorrhage (>= 500 mL) | 5 | 564 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.70, 1.61] |
| 1.1 Low‐risk women | 3 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.53, 2.37] |
| 1.2 High‐risk women | 2 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.62, 1.68] |
| 2 Severe postpartum haemorrhage (>= 1000 mL) | 2 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.14, 1.20] |
| 2.1 Low‐risk women | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.06, 6.57] |
| 2.2 High‐risk women | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.11, 1.23] |
| 3 Blood loss (mL) | 8 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Low‐risk women | 6 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 High‐risk women | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Use of additional uterotonics | 4 | 422 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.28, 3.68] |
| 4.1 Low‐risk women | 4 | 422 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.28, 3.68] |
| 4.2 High‐risk women | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Blood transfusion | 2 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.39, 2.86] |
| 5.1 Low‐risk women | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.40, 10.11] |
| 5.2 High‐risk women | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.17, 2.53] |
| 6 Manual removal of placenta | 5 | 631 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.31, 3.81] |
| 6.1 Low‐risk women | 4 | 562 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.22 [0.13, 77.34] |
| 6.2 High‐risk women | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.20, 3.39] |
| 7 Duration of third stage (minutes) | 7 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Low‐risk women | 5 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 High‐risk women | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Postpartum haemoglobin | 1 | 215 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.27, 0.27] |
| 8.1 Low‐risk women | 1 | 215 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.27, 0.27] |
| 8.2 High‐risk women | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Any side‐effect | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Low‐risk women | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 High‐risk women | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Nausea | 3 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [0.36, 16.09] |
| 10.1 Low‐risk women | 2 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.07, 16.55] |
| 10.2 High‐risk women | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 100.97] |
| 11 Vomiting | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.1 Low‐risk women | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 High‐risk women | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Headache | 2 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.28, 3.57] |
| 12.1 Low‐risk women | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.08] |
| 12.2 High‐risk women | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.39, 10.31] |
| 13 Abdominal pain | 3 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.99 [1.46, 17.05] |
| 13.1 Low‐risk women | 2 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.33 [1.40, 20.30] |
| 13.2 High‐risk women | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.26 [0.14, 77.46] |
| 14 Diarrhoea | 5 | 617 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.28 [4.47, 33.70] |
| 14.1 Low‐risk women | 4 | 537 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.88 [4.03, 35.03] |
| 14.2 High‐risk women | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.0 [0.89, 254.13] |
| 15 Pyrexia (>= 38 degrees C) | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Low‐risk women | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 High‐risk women | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
13.1. Analysis.
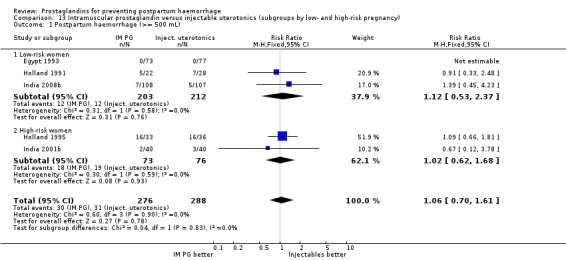
Comparison 13 Intramuscular prostaglandin versus injectable uterotonics (subgroups by low‐ and high‐risk pregnancy), Outcome 1 Postpartum haemorrhage (>= 500 mL).
13.2. Analysis.
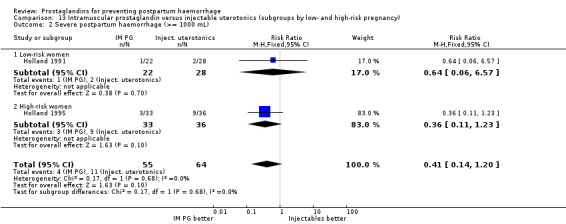
Comparison 13 Intramuscular prostaglandin versus injectable uterotonics (subgroups by low‐ and high‐risk pregnancy), Outcome 2 Severe postpartum haemorrhage (>= 1000 mL).
13.3. Analysis.
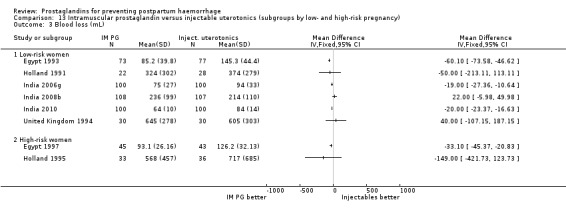
Comparison 13 Intramuscular prostaglandin versus injectable uterotonics (subgroups by low‐ and high‐risk pregnancy), Outcome 3 Blood loss (mL).
13.4. Analysis.
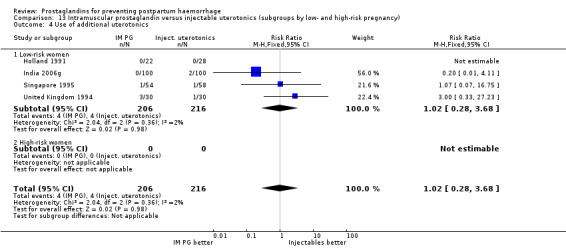
Comparison 13 Intramuscular prostaglandin versus injectable uterotonics (subgroups by low‐ and high‐risk pregnancy), Outcome 4 Use of additional uterotonics.
13.5. Analysis.
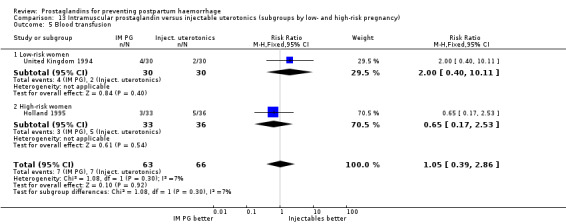
Comparison 13 Intramuscular prostaglandin versus injectable uterotonics (subgroups by low‐ and high‐risk pregnancy), Outcome 5 Blood transfusion.
13.6. Analysis.
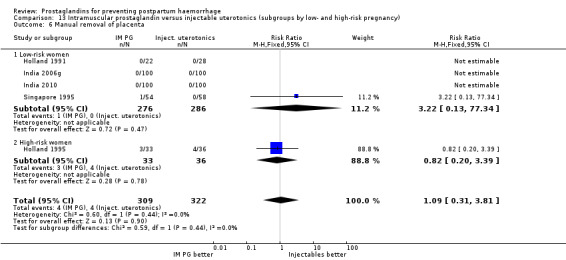
Comparison 13 Intramuscular prostaglandin versus injectable uterotonics (subgroups by low‐ and high‐risk pregnancy), Outcome 6 Manual removal of placenta.
13.7. Analysis.
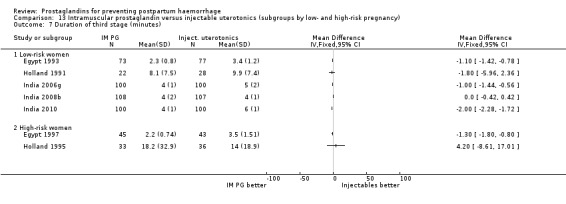
Comparison 13 Intramuscular prostaglandin versus injectable uterotonics (subgroups by low‐ and high‐risk pregnancy), Outcome 7 Duration of third stage (minutes).
13.8. Analysis.
Comparison 13 Intramuscular prostaglandin versus injectable uterotonics (subgroups by low‐ and high‐risk pregnancy), Outcome 8 Postpartum haemoglobin.
13.9. Analysis.
Comparison 13 Intramuscular prostaglandin versus injectable uterotonics (subgroups by low‐ and high‐risk pregnancy), Outcome 9 Any side‐effect.
13.10. Analysis.
Comparison 13 Intramuscular prostaglandin versus injectable uterotonics (subgroups by low‐ and high‐risk pregnancy), Outcome 10 Nausea.
13.11. Analysis.
Comparison 13 Intramuscular prostaglandin versus injectable uterotonics (subgroups by low‐ and high‐risk pregnancy), Outcome 11 Vomiting.
13.12. Analysis.
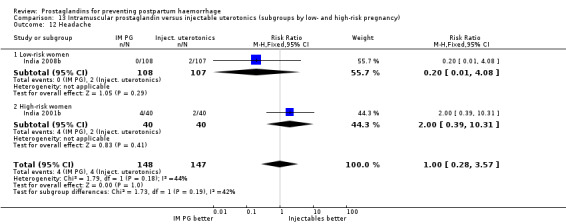
Comparison 13 Intramuscular prostaglandin versus injectable uterotonics (subgroups by low‐ and high‐risk pregnancy), Outcome 12 Headache.
13.13. Analysis.
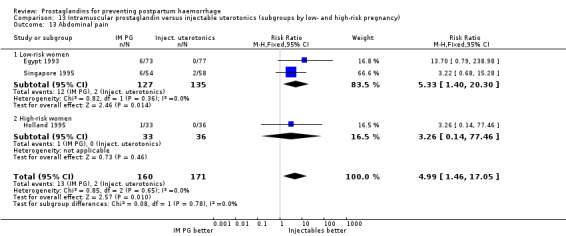
Comparison 13 Intramuscular prostaglandin versus injectable uterotonics (subgroups by low‐ and high‐risk pregnancy), Outcome 13 Abdominal pain.
13.14. Analysis.
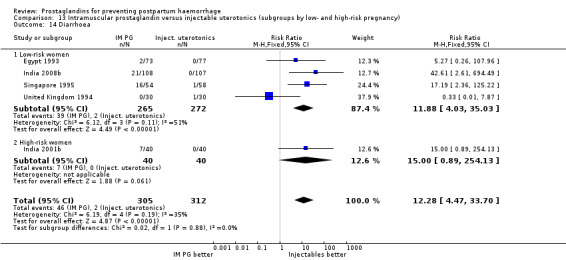
Comparison 13 Intramuscular prostaglandin versus injectable uterotonics (subgroups by low‐ and high‐risk pregnancy), Outcome 14 Diarrhoea.
13.15. Analysis.
Comparison 13 Intramuscular prostaglandin versus injectable uterotonics (subgroups by low‐ and high‐risk pregnancy), Outcome 15 Pyrexia (>= 38 degrees C).
Comparison 14. Intramuscular prostaglandin versus rectal misoprostol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postpartum haemorrhage (>= 500 mL) | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.18, 3.21] |
| 1.1 400 mcg | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.18, 3.21] |
| 2 Blood loss (mL) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐40.0 [‐99.66, 19.66] |
| 2.1 400 mcg | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐40.0 [‐99.66, 19.66] |
| 3 Use of additional uterotonics | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.05, 0.87] |
| 3.1 400 mcg | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.05, 0.87] |
| 4 Blood transfusion | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.02] |
| 4.1 400 mcg | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.02] |
| 5 Any shivering | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.61] |
| 5.1 400 mcg | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.61] |
14.1. Analysis.
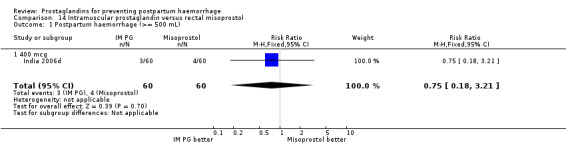
Comparison 14 Intramuscular prostaglandin versus rectal misoprostol, Outcome 1 Postpartum haemorrhage (>= 500 mL).
14.2. Analysis.
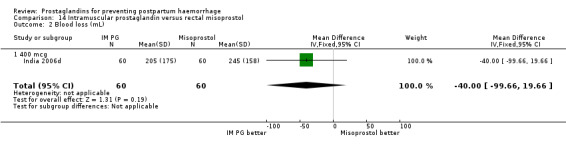
Comparison 14 Intramuscular prostaglandin versus rectal misoprostol, Outcome 2 Blood loss (mL).
14.3. Analysis.
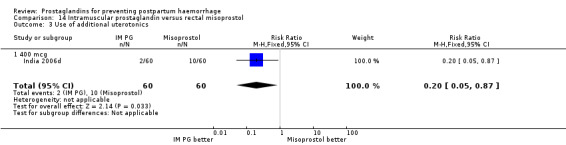
Comparison 14 Intramuscular prostaglandin versus rectal misoprostol, Outcome 3 Use of additional uterotonics.
14.4. Analysis.
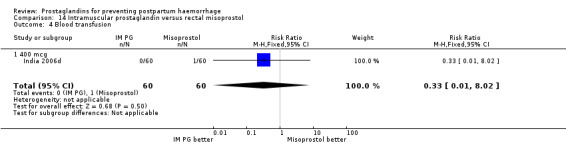
Comparison 14 Intramuscular prostaglandin versus rectal misoprostol, Outcome 4 Blood transfusion.
14.5. Analysis.
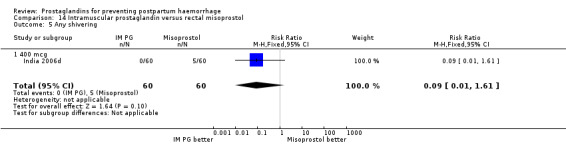
Comparison 14 Intramuscular prostaglandin versus rectal misoprostol, Outcome 5 Any shivering.
Comparison 15. Intramuscular uterotonics versus another intramuscular uterotonic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postpartum haemorrhage (>500 mL) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.06] |
| 2 Nausea | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.37, 132.10] |
| 3 Vomiting | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.34] |
| 4 Diarrhoea | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 101.58] |
| 5 High blood pressure | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.60] |
| 6 Pyrexia (>= 38 C) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
15.1. Analysis.
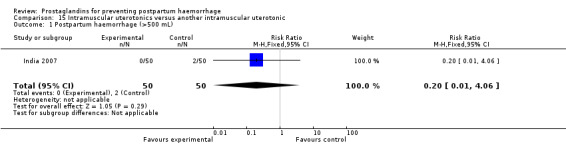
Comparison 15 Intramuscular uterotonics versus another intramuscular uterotonic, Outcome 1 Postpartum haemorrhage (>500 mL).
15.2. Analysis.
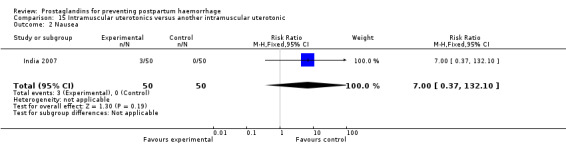
Comparison 15 Intramuscular uterotonics versus another intramuscular uterotonic, Outcome 2 Nausea.
15.3. Analysis.
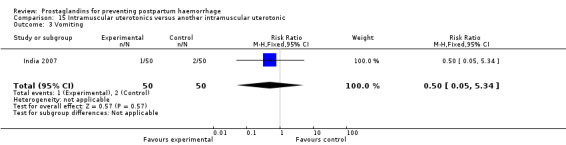
Comparison 15 Intramuscular uterotonics versus another intramuscular uterotonic, Outcome 3 Vomiting.
15.4. Analysis.
Comparison 15 Intramuscular uterotonics versus another intramuscular uterotonic, Outcome 4 Diarrhoea.
15.5. Analysis.
Comparison 15 Intramuscular uterotonics versus another intramuscular uterotonic, Outcome 5 High blood pressure.
15.6. Analysis.
Comparison 15 Intramuscular uterotonics versus another intramuscular uterotonic, Outcome 6 Pyrexia (>= 38 C).
Comparison 16. Drug/dose: misoprostol 600 mcg oral versus 400 mcg oral.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postpartum haemorrhage (>= 500 mL) | 1 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.62, 1.24] |
| 2 Severe postpartum haemorrhage (>= 1000 mL) | 2 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.50, 1.39] |
| 3 Blood loss (mL) | 2 | 547 | Mean Difference (IV, Fixed, 95% CI) | ‐30.00 [‐41.84, ‐18.16] |
| 4 Use of additional uterotonics | 3 | 947 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.66, 1.35] |
| 5 Blood transfusion | 2 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Manual removal of placenta | 2 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.35, 4.20] |
| 7 Duration of third stage (minutes) | 2 | 547 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.36, 0.27] |
| 8 Third stage >= 30 minutes | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.31, 28.60] |
| 9 Nausea | 2 | 792 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.22, 12.48] |
| 10 Vomiting | 2 | 792 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.88] |
| 11 Headache | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.25, 8.88] |
| 12 Abdominal pain | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.63, 3.59] |
| 13 Diarrhoea | 1 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.96 [0.49, 165.23] |
| 14 Shivering | 3 | 945 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.12, 1.69] |
| 15 Pyrexia (>= 38 degrees C) | 3 | 944 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [1.45, 2.88] |
16.1. Analysis.
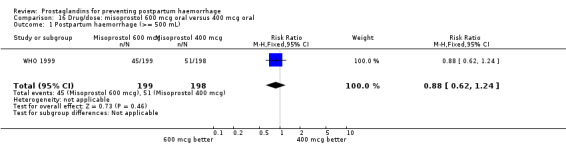
Comparison 16 Drug/dose: misoprostol 600 mcg oral versus 400 mcg oral, Outcome 1 Postpartum haemorrhage (>= 500 mL).
16.2. Analysis.
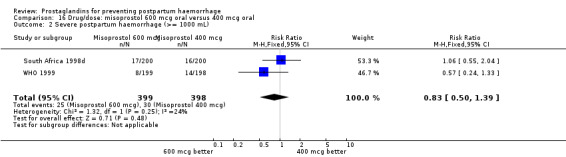
Comparison 16 Drug/dose: misoprostol 600 mcg oral versus 400 mcg oral, Outcome 2 Severe postpartum haemorrhage (>= 1000 mL).
16.3. Analysis.
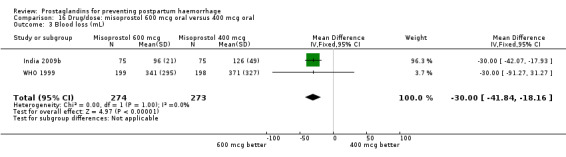
Comparison 16 Drug/dose: misoprostol 600 mcg oral versus 400 mcg oral, Outcome 3 Blood loss (mL).
16.4. Analysis.
Comparison 16 Drug/dose: misoprostol 600 mcg oral versus 400 mcg oral, Outcome 4 Use of additional uterotonics.
16.5. Analysis.
Comparison 16 Drug/dose: misoprostol 600 mcg oral versus 400 mcg oral, Outcome 5 Blood transfusion.
16.6. Analysis.
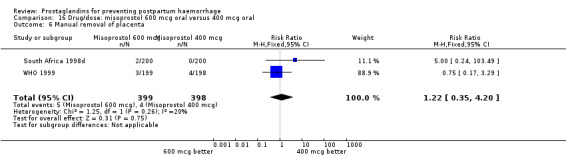
Comparison 16 Drug/dose: misoprostol 600 mcg oral versus 400 mcg oral, Outcome 6 Manual removal of placenta.
16.7. Analysis.
Comparison 16 Drug/dose: misoprostol 600 mcg oral versus 400 mcg oral, Outcome 7 Duration of third stage (minutes).
16.8. Analysis.
Comparison 16 Drug/dose: misoprostol 600 mcg oral versus 400 mcg oral, Outcome 8 Third stage >= 30 minutes.
16.9. Analysis.
Comparison 16 Drug/dose: misoprostol 600 mcg oral versus 400 mcg oral, Outcome 9 Nausea.
16.10. Analysis.
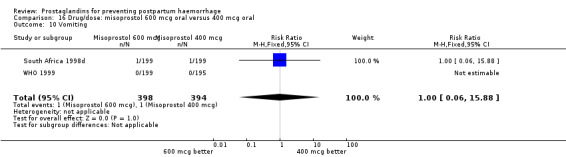
Comparison 16 Drug/dose: misoprostol 600 mcg oral versus 400 mcg oral, Outcome 10 Vomiting.
16.11. Analysis.
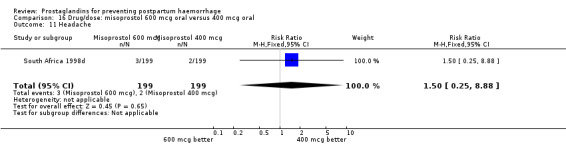
Comparison 16 Drug/dose: misoprostol 600 mcg oral versus 400 mcg oral, Outcome 11 Headache.
16.12. Analysis.
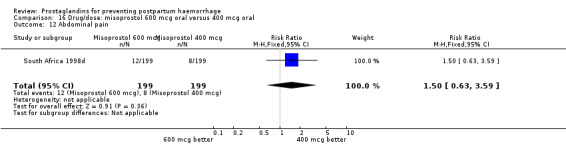
Comparison 16 Drug/dose: misoprostol 600 mcg oral versus 400 mcg oral, Outcome 12 Abdominal pain.
16.13. Analysis.
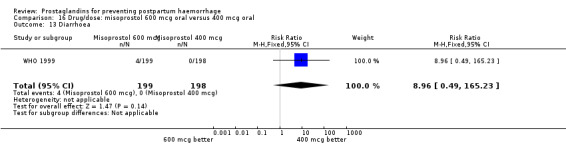
Comparison 16 Drug/dose: misoprostol 600 mcg oral versus 400 mcg oral, Outcome 13 Diarrhoea.
16.15. Analysis.
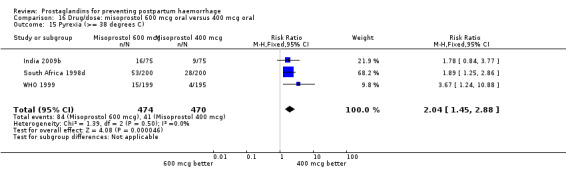
Comparison 16 Drug/dose: misoprostol 600 mcg oral versus 400 mcg oral, Outcome 15 Pyrexia (>= 38 degrees C).
Comparison 17. Drug/dose: misoprostol 600 mcg rectal versus 400 mcg rectal.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Manual removal of placenta | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.06] |
| 2 Nausea | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.27, 1.01] |
| 3 Vomiting | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.33, 1.91] |
| 4 Headache | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.29, 1.39] |
| 5 Abdominal pain | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.66, 1.12] |
| 6 Diarrhoea | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [0.12, 71.91] |
| 7 Any shivering | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.67, 1.56] |
| 8 Severe shivering | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.41, 1.45] |
| 9 Pyrexia | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.99] |
17.1. Analysis.
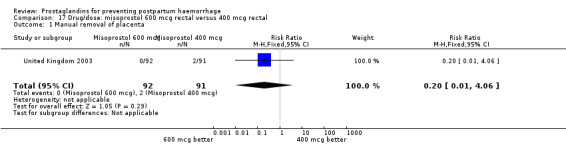
Comparison 17 Drug/dose: misoprostol 600 mcg rectal versus 400 mcg rectal, Outcome 1 Manual removal of placenta.
17.2. Analysis.
Comparison 17 Drug/dose: misoprostol 600 mcg rectal versus 400 mcg rectal, Outcome 2 Nausea.
17.3. Analysis.
Comparison 17 Drug/dose: misoprostol 600 mcg rectal versus 400 mcg rectal, Outcome 3 Vomiting.
17.4. Analysis.
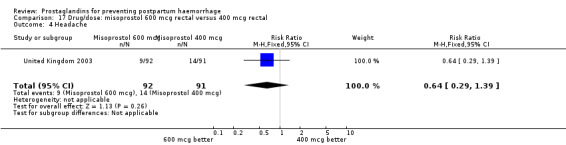
Comparison 17 Drug/dose: misoprostol 600 mcg rectal versus 400 mcg rectal, Outcome 4 Headache.
17.5. Analysis.
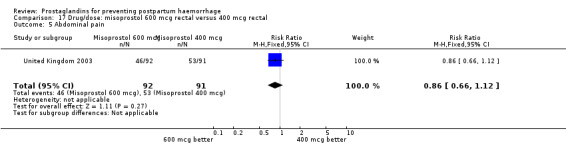
Comparison 17 Drug/dose: misoprostol 600 mcg rectal versus 400 mcg rectal, Outcome 5 Abdominal pain.
17.6. Analysis.
Comparison 17 Drug/dose: misoprostol 600 mcg rectal versus 400 mcg rectal, Outcome 6 Diarrhoea.
17.7. Analysis.
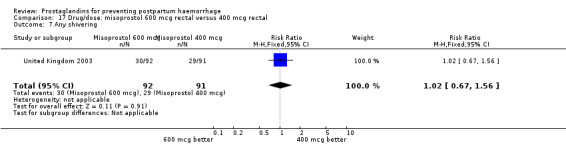
Comparison 17 Drug/dose: misoprostol 600 mcg rectal versus 400 mcg rectal, Outcome 7 Any shivering.
17.8. Analysis.
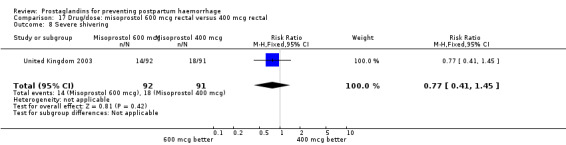
Comparison 17 Drug/dose: misoprostol 600 mcg rectal versus 400 mcg rectal, Outcome 8 Severe shivering.
17.9. Analysis.
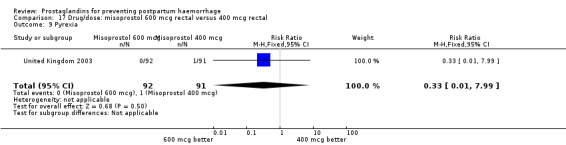
Comparison 17 Drug/dose: misoprostol 600 mcg rectal versus 400 mcg rectal, Outcome 9 Pyrexia.
Comparison 18. Drug/route: misoprostol 600 mcg rectal versus 600 mcg oral.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Manual removal of placenta | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.08] |
| 2 Nausea | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.28, 1.08] |
| 3 Vomiting | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [0.73, 9.74] |
| 4 Headache | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.56, 4.04] |
| 5 Abdominal pain | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.74, 1.30] |
| 6 Diarrhoea | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.70] |
| 7 Any shivering | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.33, 0.64] |
| 8 Severe shivering | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.16, 0.46] |
| 9 Pyrexia | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 1.00] |
18.1. Analysis.
Comparison 18 Drug/route: misoprostol 600 mcg rectal versus 600 mcg oral, Outcome 1 Manual removal of placenta.
18.2. Analysis.
Comparison 18 Drug/route: misoprostol 600 mcg rectal versus 600 mcg oral, Outcome 2 Nausea.
18.3. Analysis.
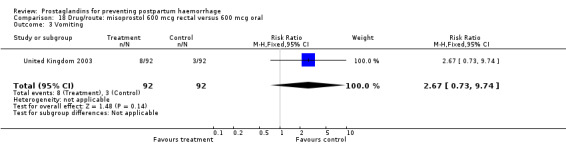
Comparison 18 Drug/route: misoprostol 600 mcg rectal versus 600 mcg oral, Outcome 3 Vomiting.
18.4. Analysis.
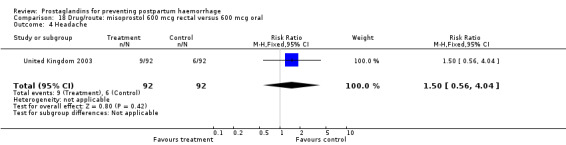
Comparison 18 Drug/route: misoprostol 600 mcg rectal versus 600 mcg oral, Outcome 4 Headache.
18.5. Analysis.
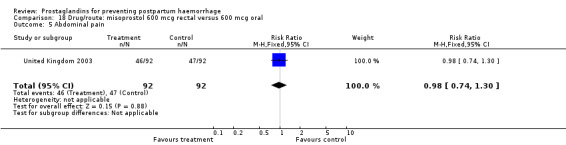
Comparison 18 Drug/route: misoprostol 600 mcg rectal versus 600 mcg oral, Outcome 5 Abdominal pain.
18.6. Analysis.
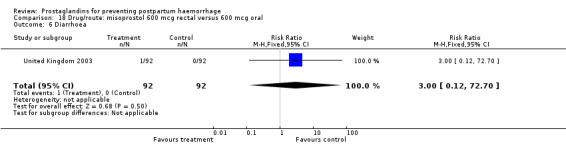
Comparison 18 Drug/route: misoprostol 600 mcg rectal versus 600 mcg oral, Outcome 6 Diarrhoea.
18.7. Analysis.
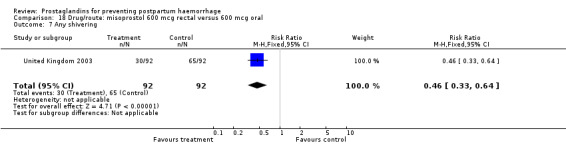
Comparison 18 Drug/route: misoprostol 600 mcg rectal versus 600 mcg oral, Outcome 7 Any shivering.
18.9. Analysis.
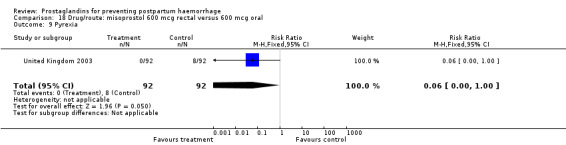
Comparison 18 Drug/route: misoprostol 600 mcg rectal versus 600 mcg oral, Outcome 9 Pyrexia.
Comparison 19. Drug/dose/route: misoprostol 400 mcg rectal versus 600 mcg oral.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Manual removal of placenta | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.06, 15.92] |
| 2 Nausea | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.62, 1.82] |
| 3 Vomiting | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.37 [0.96, 11.85] |
| 4 Headache | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [0.95, 5.87] |
| 5 Abdominal pain | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.87, 1.49] |
| 6 Diarrhoea | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Any shivering | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.32, 0.63] |
| 8 Severe shivering | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.23, 0.56] |
| 9 Pyrexia | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.06, 15.92] |
19.1. Analysis.
Comparison 19 Drug/dose/route: misoprostol 400 mcg rectal versus 600 mcg oral, Outcome 1 Manual removal of placenta.
19.2. Analysis.
Comparison 19 Drug/dose/route: misoprostol 400 mcg rectal versus 600 mcg oral, Outcome 2 Nausea.
19.3. Analysis.
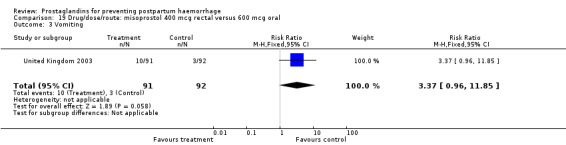
Comparison 19 Drug/dose/route: misoprostol 400 mcg rectal versus 600 mcg oral, Outcome 3 Vomiting.
19.4. Analysis.
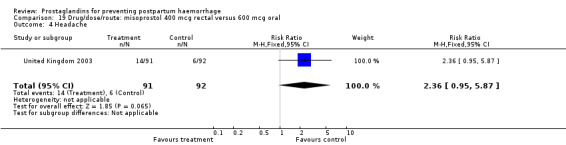
Comparison 19 Drug/dose/route: misoprostol 400 mcg rectal versus 600 mcg oral, Outcome 4 Headache.
19.5. Analysis.
Comparison 19 Drug/dose/route: misoprostol 400 mcg rectal versus 600 mcg oral, Outcome 5 Abdominal pain.
19.6. Analysis.
Comparison 19 Drug/dose/route: misoprostol 400 mcg rectal versus 600 mcg oral, Outcome 6 Diarrhoea.
19.7. Analysis.
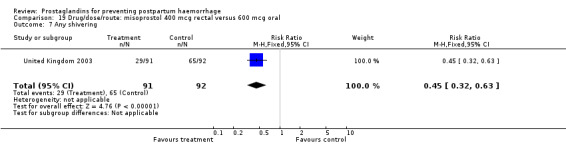
Comparison 19 Drug/dose/route: misoprostol 400 mcg rectal versus 600 mcg oral, Outcome 7 Any shivering.
19.8. Analysis.
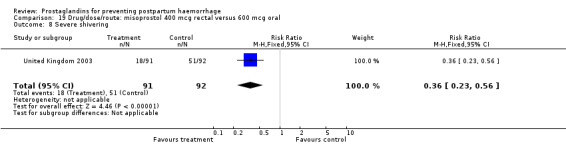
Comparison 19 Drug/dose/route: misoprostol 400 mcg rectal versus 600 mcg oral, Outcome 8 Severe shivering.
19.9. Analysis.
Comparison 19 Drug/dose/route: misoprostol 400 mcg rectal versus 600 mcg oral, Outcome 9 Pyrexia.
Comparison 20. Rectal misoprostol versus intramuscular prostaglandin (subgroups by dose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postpartum haemorrhage (>= 500 mL) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 400 mcg | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.70] |
| 2 Blood loss (mL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 600 mcg | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 400 mcg | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | 40.0 [‐19.66, 99.66] |
| 3 Use of additional uterotonics | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 600 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 400 mcg | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [1.14, 21.86] |
20.1. Analysis.
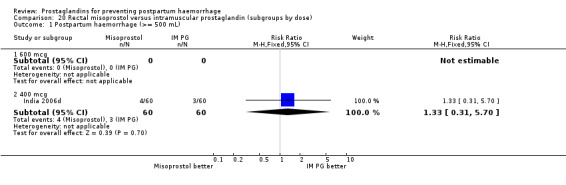
Comparison 20 Rectal misoprostol versus intramuscular prostaglandin (subgroups by dose), Outcome 1 Postpartum haemorrhage (>= 500 mL).
20.2. Analysis.
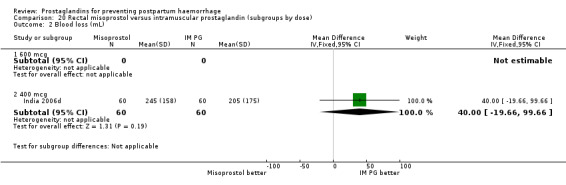
Comparison 20 Rectal misoprostol versus intramuscular prostaglandin (subgroups by dose), Outcome 2 Blood loss (mL).
20.3. Analysis.
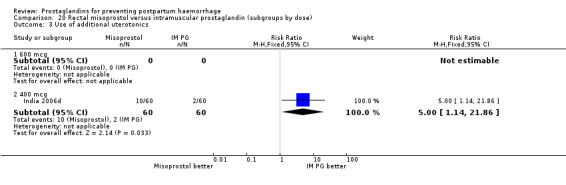
Comparison 20 Rectal misoprostol versus intramuscular prostaglandin (subgroups by dose), Outcome 3 Use of additional uterotonics.
Comparison 21. Controlled release prostaglandins (vaginal insert) versus injectable uterotonics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nausea | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.5 [1.00, 20.31] |
| 1.1 Dinoprostone 0.3 mg/hr | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.5 [1.00, 20.31] |
| 2 Diarrhoea | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.77] |
| 2.1 Dinoprostone 0.3 mg/hr | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.77] |
| 3 Pyrexia (>= 38 C) | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 75.28] |
| 3.1 Dinoprostone 0.3 mg/hr | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 75.28] |
| 4 Shivering | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.77] |
| 4.1 Dinoprostone 0.3 mg/hr | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.77] |
21.1. Analysis.
Comparison 21 Controlled release prostaglandins (vaginal insert) versus injectable uterotonics, Outcome 1 Nausea.
21.2. Analysis.
Comparison 21 Controlled release prostaglandins (vaginal insert) versus injectable uterotonics, Outcome 2 Diarrhoea.
21.3. Analysis.
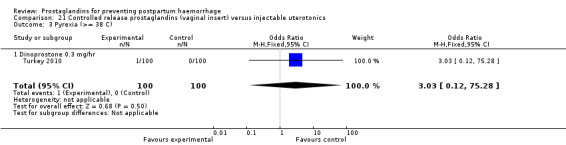
Comparison 21 Controlled release prostaglandins (vaginal insert) versus injectable uterotonics, Outcome 3 Pyrexia (>= 38 C).
21.4. Analysis.
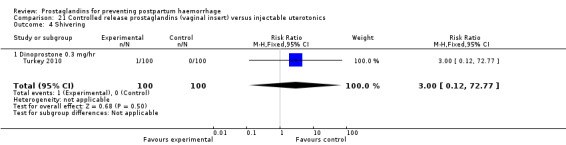
Comparison 21 Controlled release prostaglandins (vaginal insert) versus injectable uterotonics, Outcome 4 Shivering.
Comparison 22. Oral misoprostol versus traditional ZB11.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal death | 1 | 960 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 600 mcg | 1 | 960 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Postpartum haemorrhage (>=500 mL) | 1 | 960 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.03, 1.91] |
| 2.1 600 mcg | 1 | 960 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.03, 1.91] |
| 3 Severe postpartum hemorrhage (>=1000 mL) | 1 | 960 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.69, 3.36] |
| 3.1 600 mcg | 1 | 960 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.69, 3.36] |
| 4 Use of additional uterotonics | 1 | 960 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.91, 1.68] |
| 4.1 600 mcg | 1 | 960 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.91, 1.68] |
| 5 Nausea | 1 | 958 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.49, 2.11] |
| 5.1 600 mcg | 1 | 958 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.49, 2.11] |
| 6 Vomiting | 1 | 958 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.22, 3.01] |
| 6.1 600 mcg | 1 | 958 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.22, 3.01] |
| 7 Diarrhoea | 1 | 958 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.12, 1.15] |
| 7.1 600 mcg | 1 | 958 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.12, 1.15] |
| 8 Any shivering | 1 | 958 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.55, 1.05] |
| 8.1 600 mcg | 1 | 958 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.55, 1.05] |
| 9 Pyrexia (>= 38 C) | 1 | 958 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.10, 0.95] |
| 9.1 600 mcg | 1 | 958 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.10, 0.95] |
22.1. Analysis.
Comparison 22 Oral misoprostol versus traditional ZB11, Outcome 1 Maternal death.
22.2. Analysis.
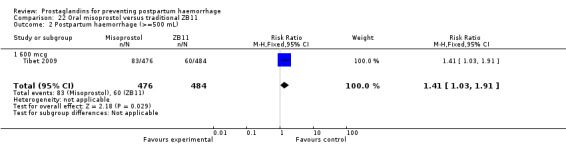
Comparison 22 Oral misoprostol versus traditional ZB11, Outcome 2 Postpartum haemorrhage (>=500 mL).
22.3. Analysis.
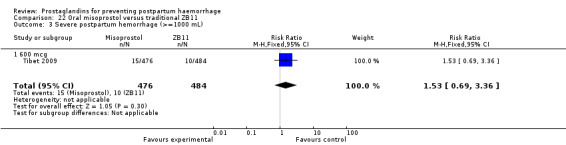
Comparison 22 Oral misoprostol versus traditional ZB11, Outcome 3 Severe postpartum hemorrhage (>=1000 mL).
22.4. Analysis.
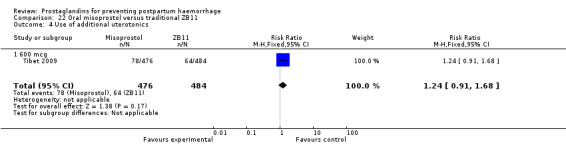
Comparison 22 Oral misoprostol versus traditional ZB11, Outcome 4 Use of additional uterotonics.
22.5. Analysis.
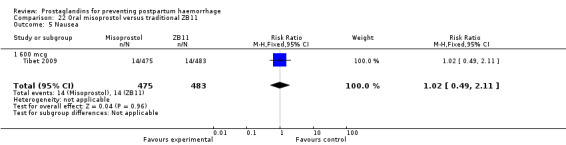
Comparison 22 Oral misoprostol versus traditional ZB11, Outcome 5 Nausea.
22.6. Analysis.
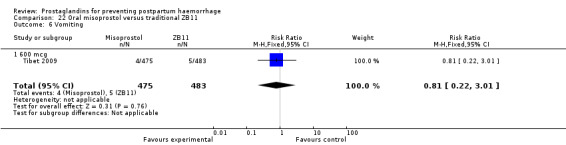
Comparison 22 Oral misoprostol versus traditional ZB11, Outcome 6 Vomiting.
22.7. Analysis.
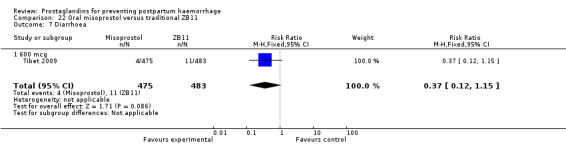
Comparison 22 Oral misoprostol versus traditional ZB11, Outcome 7 Diarrhoea.
22.8. Analysis.
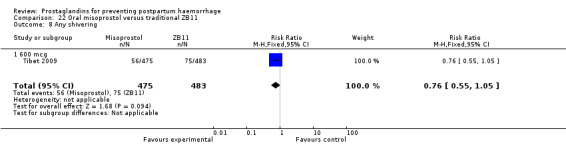
Comparison 22 Oral misoprostol versus traditional ZB11, Outcome 8 Any shivering.
22.9. Analysis.
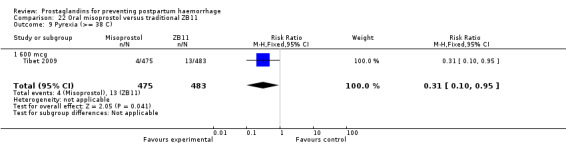
Comparison 22 Oral misoprostol versus traditional ZB11, Outcome 9 Pyrexia (>= 38 C).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Africa 2011.
| Methods | Multicentre, randomised, double blind, placebo‐controlled trial. | |
| Participants | 1099 women without significant obstetric complications expected to give birth vaginally at 6 different hospitals in South Africa, Uganda and Nigeria. Those who delivered via caesarean or had an assisted vaginal birth; those in whom sublingual administration of misoprostol was not possible; those in whom the pregnancy was not viable according to local gestational viability age limits; those who declined, or were too ill or distressed, to give consent; and those not entitled to give informed consent, such as minors were excluded. |
|
| Interventions | 400 mcg of misoprostol immediately after delivery, in addition to standard active management of the third stage of labour, as currently practiced in the collaborating centres (parenteral oxytocin in Uganda and South Africa, and oxytocin or ergometrine in Nigeria) versus placebo immediately after delivery, in addition to standard active management of the third stage of labour, as currently practiced in the collaborating centres. | |
| Outcomes | The primary outcome was the incidence of 500 mL or more of measured blood loss within 1 hour after the trial medication was administered. Secondary outcomes were the mean measured blood loss 1 hour after the trial medication was administered, a measured blood loss of 1000 mL or more, adverse effects such as shivering (mild and moderate/severe) and pyrexia, manual removal of the placenta, laparotomy, hysterectomy, and maternal morbidity and mortality. |
|
| Notes | Active management of third stage labour. Blood collection was started as soon as possible after delivery. A fresh, non‐absorbent sheet was placed under the buttocks of the woman. A low‐ profile, plastic bedpan was positioned below the woman's perineum in a position to collect all subsequent blood loss, for a period of 1 hour, measured by a standard clock. The blood collected in the bedpan and any spilled blood, which was collected from the non‐absorbent sheet and any blood‐soaked small gauze swabs, were transferred to a measuring jar and the volume was measured. Alternatively, the blood was collected into a plastic sheet placed below the woman immediately after delivery. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The random allocation sequence was generated by using computer‐generated random numbers and was stratified by country in blocks of 6‐8." |
| Allocation concealment (selection bias) | Low risk | "The packs were identical in shape, color, weight, and feel, and contained either 2 tablets of 200 mcg of misoprostol or 2 matching placebo tablets." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 people were lost to follow‐up due to data not recorded. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review were reported. |
| Other bias | Low risk | The 2 groups were similar in terms of baseline characteristics, except for a 2.8‐year difference in mean age. |
Australia 1999.
| Methods | Random allocation from a table of random numbers with sequentially numbered, sealed, opaque envelopes. Block randomisation was utilized. The study was not blinded. | |
| Participants | 930 women with vaginal delivery in 4 centres in Australia, China, and Papua New Guinea. Exclusion criteria: coagulation disorders, asthma, severe renal disease, epilepsy, elective caesarean section, severe hypertension. | |
| Interventions | Misoprostol 400 mcg orally vs IM injection of either oxytocin (10 IU) (1 centre) or ergometrine‐oxytocin (5 IU oxytocin + 0.5 mg ergometrine) (3 centres). | |
| Outcomes | Blood loss, duration of third stage, use of additional uterotonics, blood transfusion, side‐effects, haemoglobin level. Measurement of blood loss: by combining estimated (assessment by clinician) and measured (measuring volume with calibrated measuring jug, and weighing of linen). It is unclear if some centres used 1 or the other method. | |
| Notes | Management of third stage: no mention of third stage management technique. 31/455 (7%) were excluded after randomisation in the misoprostol group, and 36/475 (8%) were excluded after randomisation in the oxytocin/ergometrine‐oxytocin group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was by random number list in blocks of 20 with a separate randomization for each center." |
| Allocation concealment (selection bias) | Low risk | "Sequentially numbered sealed security (opaque) envelopes containing the appropriate drug label were provided for each center." |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Not blinded." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 94 patients were excluded prior to randomisation and 31 patients (6.8%) from the misoprostol arm and 36 patients (7.6%) from the control arm have been excluded after randomisation. The main reasons for exclusion prior to randomisation and following randomisation were the need for caesarean sections and the development of hypertension. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review have been reported. |
| Other bias | High risk | This trial was stopped after recruitment of 863/1862 women following the unsatisfactory results of an interim analysis. Patient characteristics prior to treatment showed no differences between the groups. |
Bangladesh 2007.
| Methods | Randomised controlled trial. | |
| Participants | 400 labouring women (nulliparous/multiparous) in vertex presentation with no known risk for excessive third stage blood loss. No exclusion criteria were reported. |
|
| Interventions | Oral 400 mg misoprostol versus IM 10 IU oxytocin just after cord clamping. | |
| Outcomes | Incidence of postpartum haemorrhage, estimation of average blood loss, the length of the third stage labour, manual removal of placenta, additional oxytocics, blood transfusion and side‐effects. | |
| Notes | Active management of third stage labour. Blood loss was estimated on approximate bases by the delivering physician after collecting blood within a plastic bowl. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clear on how the sequence generation was done. |
| Allocation concealment (selection bias) | Unclear risk | Not clear on how the allocation concealment was done. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not clear on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions have been reported. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review were reported. |
| Other bias | Low risk | At randomisation, the 2 groups were well‐balanced and comparable for demographic and labour characteristics. |
Belgium 1999.
| Methods | Random allocation from a computer‐generated list of study numbers. Randomisation in blocks. Identical numbered study boxes were used. Outcome assessments were blinded. | |
| Participants | 213 women with vaginal delivery in Leuven, Belgium. Exclusion criteria: caesarean section, hypertensive disorders, gestational age < 32 weeks, intrauterine death, uterine malformations, allergy to prostaglandins or alkaloids, inflammatory bowel disease, coronary disease, vascular disease, sepsis. | |
| Interventions | Misoprostol 600 mcg orally vs methylergometrine 200 mcg IV. Both oral and IV placebos were used. | |
| Outcomes | Blood loss, need for additional uterotonics, side‐effects. Blood loss was estimated. |
|
| Notes | Management of third stage: uterine massage, cord traction, manual removal of placenta after 30‐60 minutes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study number was taken from a computer‐generated list and randomization was in blocks." |
| Allocation concealment (selection bias) | Low risk | "The study boxes and capsules were indistinguishable in the two groups and both groups receives the full package of active management of the third stage equally." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 5 women (5%) from the misoprostol group and 8 women (7.4%) from the control group were excluded after randomisation due to having a caesarean section. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review were reported. |
| Other bias | Low risk | The demographic characteristics and labour variables are comparable in both groups. |
Canada 2002.
| Methods | Random allocation from a central centre statistician using block randomisation for each participating centre. Consecutively‐numbered opaque, sealed packets for allocation concealment. No blinding of treatment or outcome assessments. | |
| Participants | 223 women with vaginal delivery from 3 hospitals in Toronto, Canada. Exclusion criteria: parity > 6, gestational age < 32 weeks, clotting disorder, anticoagulant therapy, history of postpartum haemorrhage, previous caesarean delivery. | |
| Interventions | Misoprostol 400 mcg rectally after delivery vs oxytocin 5 IU IV or IM, or 10 IU IM given after delivery (sometimes given after placenta delivered). | |
| Outcomes | Blood loss was captured by measuring change in measured haemoglobin. Other outcomes were duration of third stage, need for additional uterotonics, manual removal of placenta, blood transfusion, side‐effects. | |
| Notes | No description of third stage management. 13 women excluded after randomisation secondary to having a caesarean section. 2 women lost to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The coordinating centre statistician developed blocked randomization tables for each participating centre." |
| Allocation concealment (selection bias) | Low risk | "consecutively numbered opaque, sealed packets that contained the group allocation and datasheets." |
| Blinding (performance bias and detection bias) All outcomes | High risk | "the allocation was revealed to the caregivers and the women." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13 women were excluded after randomisation due to having caesarean sections. 2 women were lost to follow‐up early in the trial. Analysis of 223 women was on intention‐to‐treat basis. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review were reported. |
| Other bias | Low risk | There were no significant differences between the 2 groups. |
Canada 2005.
| Methods | Randomised double blind, no further details. Unclear if outcome assessments were blinded. | |
| Participants | 622 women with vaginal delivery at a university hospital in Halifax, Nova Scotia, Canada. Women with multiple pregnancy, placenta previa, abruptio placentae, coagulation abnormalities, caesarean delivery and asthma were excluded. | |
| Interventions | Misoprostol 400 mcg orally after delivery of anterior shoulder vs oxytocin 5 IU IV. | |
| Outcomes | Blood loss measured by haematocrit drop greater than 10%, haemoglobin drop greater than 30%, additional uterotonics, blood loss greater than 1000 mL and 500 mL. | |
| Notes | Third stage management was 'active'. No mention of postrandomisation exclusion or loss to follow‐up. The authors attribute the high numbers of additional uterotonic use to most women having IV lines during labour and the threshold for bolus oxytocin administration being low. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of how the sequence generation was created. |
| Allocation concealment (selection bias) | Unclear risk | No mention of how the concealment occurred. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention of postrandomisation exclusion or loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review have been reported. |
| Other bias | Low risk | The baseline variables among 2 groups are comparable. |
China 2003a.
| Methods | Randomised controlled trial. | |
| Participants | 156 women in labour who gave birth without complications via the birth canal and had no coagulation disorders or contraindications regarding the use of misoprostol. | |
| Interventions | Oral 200 mcg dose of misoprostol immediately after delivery and another 200 mcg after 60 minutes versus no medication (a total of 400 mcg). | |
| Outcomes | Haemorrhage occurrence rate, amount 2 hours after delivery and side‐effects. | |
| Notes | No mention of active management of third stage labour. Blood loss was measured based on volume and weight. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear how the sequence generation was done. |
| Allocation concealment (selection bias) | Unclear risk | Unclear how the allocation concealment was done. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention of loss to follow‐up or postrandomisation exclusions. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | 2 groups were comparable. |
China 2003b.
| Methods | Randomised controlled trial. | |
| Participants | 137 women with risk factors for postpartum haemorrhage such as placenta previa, polyhydramnios, macrosomia, twin pregnancy and pregnancy‐induced hypertension. Control group had 28 women who had caesarean sections. No exclusion criteria specified. |
|
| Interventions | 400 mcg oral misoprostol versus 20 IU IM oxytocin immediately after the delivery of the baby. | |
| Outcomes | Amount of bleeding after delivery, blood pressure and pulse before and after taking the medication, side‐effects. | |
| Notes | AMSTL not mentioned and not applicable to caesarean section patients in the control group. Volume was measured with a blood collection drape until 2 hours after delivery, then poured into a scaled bottle to measure the volume. For caesarean patients, the volume of blood was directly measured in the suction bottle (after putting it aside all the visible amniotic fluid above the cleared blood was discarded and not counted). The weighing method for bleeding 2 hours after delivery used the dressings before and after which were weighed separately. The estimation method used surgical single contaminated blood stain range of 10 cm X 10 cm equals 5 mL blood estimation. The 3 methods mentioned above were added up to be the total amount of blood lost 2 hours after delivery. For 24 hours after delivery a paper pad was used to absorb vaginal bleeding, and then the weighing method was used to calculate the blood loss. Then finally added up the total amount of bleeding 24 hours after delivery. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clear on how the sequence generation was done. |
| Allocation concealment (selection bias) | Unclear risk | Unclear on how the allocation concealment was done. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear on the blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or postrandomisation exclusions were mentioned. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Unclear risk | General comparison of the 2 groups showed that there was no significant difference in age and gestational week, however, there are 28 caesarean sections in the control group only. |
China 2004a.
| Methods | Open, randomised trial. Randomisation generated by a random‐number table. Unclear if outcome assessments were blinded. | |
| Participants | 60 low‐risk women delivering vaginally in Hong Kong, China. | |
| Interventions | Misoprostol 600 mcg sublingually vs syntometrine IV. | |
| Outcomes | Blood loss, side‐effects. Blood loss was both estimated visually and measured using alkaline hematin technique. | |
| Notes | Third stage management was 'active' using early cord clamping and cord traction. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random number‐generated table." |
| Allocation concealment (selection bias) | High risk | Inadequate concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open trial. Unclear if outcome assessments are blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention of postrandomisation exclusion or loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review have been reported. |
| Other bias | Low risk | Demographic and labour characteristics of the 2 groups are comparable. |
China 2007.
| Methods | Randomised, double‐blind, placebo‐controlled trial. | |
| Participants | 355 women having a singleton pregnancy beyond 34 weeks of gestation, low risk for postpartum haemorrhage and a vaginal delivery. Women were considered low risk for postpartum haemorrhage if the index pregnancy was not complicated by presence of fibroids, polyhydramnios, fetal microsomia or any significant history of antepartum haemorrhage. Exclusion criteria included presence of contraindications for the use of either misoprostol or syntometrine, such as pre‐eclampsia, cardiac disease and asthma, and the presence of conditions requiring prophylactic oxytocin after delivery such as multiparity (parity >= 4) or presence of uterine fibroids. |
|
| Interventions | 400 mcg oral misoprostol versus 2 mL of syntometrine). | |
| Outcomes | Change in Hb levels before and 48 hr after delivery Blood loss, duration of 3rd stage labour, use of additional oxytocics, use of blood transfusion, manual removal of placenta and side‐effects (nausea, vomiting, headache, diarrhoea, shivering, pyrexia) |
|
| Notes | Active management of third stage of labour. Blood loss was assessed by clinical estimation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "table of computer generated random numbers." |
| Allocation concealment (selection bias) | Low risk | "All women were asked to swallow the tablets directly from the opaque cup without looking at them." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, placebo‐controlled. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 women were excluded from the analysis because of missing postdelivery haemoglobin level, all of them had postpartum blood loss of < 500 mL. Results from 355 women were analyzed on an intention‐to‐treat basis. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review were reported. |
| Other bias | Low risk | Baseline characteristics between the 2 groups are similar. |
Colombia 2002.
| Methods | Method of random allocation not stated. No placebo use or blinding of outcome assessments | |
| Participants | 75 women with vaginal delivery in Colombia. Exclusion criteria: asthma, coagulopathy, twins, stillbirth, lacerations, and "amniotic fluid in the blood collection". | |
| Interventions | Misoprostol 50 mcg sublingually after cord clamp vs oxytocin 16 m IU per minute intravenously after cord clamp vs methylergometrine 0.2 mg after placenta delivery. | |
| Outcomes | Blood loss, side‐effects, cost. Method of collection or estimation of blood loss not stated. | |
| Notes | Management of third stage: no mention of third stage management technique. No reported postrandomisation exclusions or loss to follow‐up. Analysis was based on the total population of 75 women. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random allocation not stated. |
| Allocation concealment (selection bias) | Unclear risk | No mention of how the concealment occurred. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo use or blinding of outcome assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up and postrandomisation exclusions have been reported. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review have been reported. |
| Other bias | Low risk | The characteristics of the 2 groups have been comparable. |
Egypt 1993.
| Methods | Random allocation from a table of random numbers. No mention of blinding or placebo use. | |
| Participants | 150 low‐risk women after vaginal delivery in Assiut, Egypt. Excluded: labour < 2 hours, prolonged labour (> 24 hours), magnesium sulphate therapy during labour, history of postpartum haemorrhage, chorioamnionitis, multiple pregnancy, antepartum haemorrhage and episiotomy. | |
| Interventions | Carboprost trometamol* 0.250 mg IM vs methylergometrine maleate 0.2 mg IV. | |
| Outcomes | Blood loss, duration of third stage, side‐effects. Measurement of blood loss: immediate blood loss was collected in trays and measured. Also, pads were used to collect blood for 4 hours and weighed. | |
| Notes | Management of third stage: reported as active but only uterotonic use is mentioned. No mention of exclusions or missing data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "using a table of random numbers." |
| Allocation concealment (selection bias) | High risk | Inadequate concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention of blinding or placebo use. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention of exclusions or missing data. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | There were no significant differences between the 2 groups in age, parity and duration of gestation. |
Egypt 1997.
| Methods | Randomisation using table of random numbers. No mention of blinding or placebo use. | |
| Participants | 132 high‐risk women after vaginal delivery in Assiut, Egypt. 'High‐risk' risk factors included: previous history of postpartum haemorrhage, high parity, uterine overdistention due to multiple pregnancy, polyhydramnios or fetal macrosomia, prolonged labour, placental abnormalities or chorioamnionitis. Exclusion criteria: organic heart disease, bronchial asthma, epilepsy, renal disease, caesarean section, episiotomy. |
|
| Interventions | Carboprost trometamol* 250 mcg IM vs methylergonovine maleate 0.4 mg IV, vs oxytocin 10 IU IV. | |
| Outcomes | Blood loss, duration of third stage. Measurement of blood loss ‐ blood collected in trays and measured. Sterile pads were weighed. |
|
| Notes | Management of third stage: reported only as active. No report of exclusion after randomisation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "using a table of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | No mention of how the concealment occurred. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention of blinding or placebo use. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No report of exclusion after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review have been reported. |
| Other bias | Low risk | "There were no statistically significant differences between the three groups in any of the variables mentioned except age." |
Egypt 2009.
| Methods | Double blind, randomised, placebo‐controlled trial. | |
| Participants | 514 women with spontaneous normal delivery of a live, singleton neonate, and absence of any contraindications for misoprostol or oxytocin use were included. The women were excluded if they delivered by caesarean, had a history of antepartum haemorrhage or bleeding tendency, were diagnosed with hypertensive disorder with pregnancy or had the need for anticoagulants. |
|
| Interventions | 800 mcg rectal misoprostol versus 5 IU of oxytocin in 5 mL lactated Ringer. | |
| Outcomes | The primary outcome was the number of patients estimated to have postpartum haemorrhage. Secondary outcomes were a haematocrit drop of 10% or more 24 hours postpartum; haemoglobin concentration 24 hours postpartum; changes in systolic and diastolic blood pressure; duration of the third stage of labour; need for manual removal of the placenta and/or blood transfusion; additional uterotonics, and nausea, shivering and fever (≥ 38 C) assessed 1 hour postpartum as the adverse effects of misoprostol. |
|
| Notes | Active management of third stage of labour. Estimation of blood loss was not done on a quantitative basis; diagnosis of blood loss greater than 500 mL and the decision to apply further measures to control postpartum blood loss were based on a subjective estimation of blood loss by the obstetrician. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated random allocation system." |
| Allocation concealment (selection bias) | Low risk | "sealed, opaque, consecutively numbered and coded envelopes." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. The randomisation code of data sheets was not broken until completion of the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or exclusions reported. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review have been reported. |
| Other bias | Low risk | 2 groups were comparable at baseline. |
France 2001.
| Methods | Randomly drawn envelopes containing the treatment codes. Placebos were not used. | |
| Participants | 602 women after vaginal delivery in France. Exclusion criteria: preterm birth (< 32 weeks), antepartum haemorrhage, intrauterine fetal death, uterine scar, caesarean section, multiple pregnancy, pre‐eclampsia. | |
| Interventions | Misoprostol 600 mcg orally vs oxytocin 2.5 IU IV given after cord clamp, vs no uterotonic. | |
| Outcomes | Blood loss, duration of third stage, side‐effects. Blood loss was measured. | |
| Notes | Management of the third stage: active with immediate cord clamping. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly drawn envelopes." |
| Allocation concealment (selection bias) | Low risk | Used opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention of loss to follow‐up or exclusion after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes have been reported. |
| Other bias | Low risk | The characteristics of all the groups are comparable. |
Gambia 2005.
| Methods | Randomisation generated by computer, allocation concealment by sealed, opaque envelopes. Power calculation made. Outcome assessments were blinded. | |
| Participants | 1229 women delivering vaginally at home by trained birth attendants in rural Gambia. | |
| Interventions | Misoprostol 600 mcg orally vs oral ergometrine 2 mg. | |
| Outcomes | Blood loss, postpartum haemoglobin. Blood loss was measured by collection of blood, pads and linen and weighing until 1 hour after delivery. | |
| Notes | Management of the third stage: controlled cord traction, delayed cord clamping (after cessation of pulsation), early suckling of the breast. No loss to follow‐up. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer randomization codes were generated in blocks of 10." |
| Allocation concealment (selection bias) | Low risk | "each sequentially numbered opaque sealed treatment packet." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or exclusion after randomisation was reported. |
| Selective reporting (reporting bias) | Low risk | All of the primary outcomes of the review which can be collected in the community setting were reported. |
| Other bias | Low risk | "The two groups were similar with regard to baseline characteristics and other factors potentially associated with the primary outcomes, except for men Hb level at last ANC visit." |
Ghana 2000.
| Methods | Randomised, double‐blind, controlled trial. Randomisation sequence generated by computer. Allocation by sequentially numbered, opaque packets containing active and placebo medications. The packets and misoprostol solution were prepared by a pharmacist not involved in the trial. Power calculation was based on a difference of drop in haemoglobin concentration (> 0.1 g/dL). | |
| Participants | 401 women delivering vaginally at the Korle Bu teaching hospital and its clinics in Accra, Ghana. Women were excluded if they were at risk of postpartum haemorrhage (grand multiparae, multiple gestation, gestation < 32 weeks, gestational hypertension with haemolysis‐elevated liver enzymes‐low platelets syndrome, hydramnios, previous postpartum haemorrhage, coagulation abnormalities, precipitous labour, chorioamnionitis and oxytocin induction or augmentation of labour. | |
| Interventions | Misoprostol 400 mcg in powdered form orally (in 50 mL of water) and 1 mL IM injection of normal saline (placebo) vs powdered lactose placebo orally (in 50 mL of water) and 1 mL IM injection of 10 IU oxytocin. | |
| Outcomes | Primary outcome: drop in haemoglobin concentration; side‐effects. Blood loss measurement: clinical estimation. |
|
| Notes | Management of third stage: active with cord traction. The authors mention that they report the data as intention to treat although outcome data are missing for 9/401 women. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "using computer generated random numbers." |
| Allocation concealment (selection bias) | Low risk | "sequentially numbered opaque packet." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind placebo controlled trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The outcome data is missing in 9 patients out of 401 women. The analysis was intention‐to‐treat. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review have been reported. |
| Other bias | Low risk | "There were no significant differences between the two groups with regards to maternal and neonatal demographics." |
Ghana 2006.
| Methods | Random‐number scheme generated by computer. Allocation concealment by opening the next sequentially‐numbered, sealed, opaque envelope. The study was not blinded. Power calculation is reported. | |
| Participants | 450 women delivering vaginally at Holy Family hospital, Techiman, Ghana. Women at both high and low risk for postpartum haemorrhage were included. | |
| Interventions | Misoprostol 800 mcg orally vs oxytocin 10 IU IM. | |
| Outcomes | Primary outcome: change in haemoglobin concentration, other measures of blood loss, side‐effects. Blood loss was estimated. | |
| Notes | Management of the third stage: 'active', no further details. No loss to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random, computer‐generated assignment." |
| Allocation concealment (selection bias) | Low risk | "sequentially numbered, opaque, sealed envelope." |
| Blinding (performance bias and detection bias) All outcomes | High risk | The study was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis was intent‐to‐treat. No loss to follow‐up and exclusion after randomisation was reported. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review have been reported. |
| Other bias | Low risk | "There was no difference between the groups regarding baseline characteristics or risk factors for postpartum haemorrhage." |
Ghana 2007.
| Methods | Randomised controlled trial. | |
| Participants | 440 women who had advanced labour and delivered vaginally at the 2 district hospitals in the Brong Ahafo region of Ghana were included. Exclusion criteria included any known contraindication to prostaglandin administration (hypersensitivity or medical conditions, including asthma or epilepsy). Women at perceived high risk for postpartum haemorrhage were not excluded, but the factors that increased the risk were recorded on the data sheet. They were as follows: grand multiparity (greater than para 5), multiple gestation, previous postpartum haemorrhage, precipitous labour (less than 3 hours), coagulation abnormality, chorioamnionitis, polyhydramnios, previous caesarean section, and oxytocin induction or augmentation of labour. |
|
| Interventions | Rectal misoprostol 800 mcg versus IM 10IU oxytocin with the delivery of the anterior shoulder. | |
| Outcomes | Primary outcome was the change in haemoglobin from before delivery to after delivery. Other outcomes were estimated blood loss, additional uterotonic use, clinical diagnosis of postpartum haemorrhage, blood transfusion and side‐effects (nausea, vomiting, shivering and temperature > 37.5 C). | |
| Notes | Active management of the third stage of labour. A subjective estimate of blood loss was recorded. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only mentions a "random assignment", not clear on how the sequence generation was done. |
| Allocation concealment (selection bias) | Low risk | "The next sequentially numbered, opaque, sealed envelope containing a standard data sheet with a random assignment to either the control group (intramuscular oxytocin) or the treatment group (rectal misoprostol) was opened." |
| Blinding (performance bias and detection bias) All outcomes | High risk | It was not mentioned in the text, however, the different administration methods of the 2 drugs and no use of placebo suggests that there was no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 women from the oxytocin group and 6 women from the misoprostol group were excluded from the analysis as hey did not have both pre and post delivery Hb concentration essays recorded. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review were reported. |
| Other bias | Low risk | There was no significant difference between the groups regarding baseline characteristics or risk factors for postpartum haemorrhage. |
Guinea‐Bissau 2005.
| Methods | Random‐number list used for randomisation scheme. Allocation concealment by sealed, opaque, consecutively‐numbered envelopes. Outcome assessments were blinded. | |
| Participants | 661 women delivering at a primary care centre in Guinea‐Bissau. | |
| Interventions | Misoprostol 600 mcg sublingual vs identical placebo. | |
| Outcomes | Blood loss, side‐effects. Blood loss was measured by collecting blood in swabs and absorbent drape and then weighing them. | |
| Notes | Management of the third stage: active with early cord clamping and controlled cord traction. The midwives were trained in these procedures before the start of the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "using a list of random numbers." |
| Allocation concealment (selection bias) | Low risk | "opaque envelopes were consecutively numbered and filled." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind placebo controlled. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up and exclusion after randomisation have been reported. All the women enrolled were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review have been reported. |
| Other bias | Low risk | Baseline characteristics were comparable between the 2 groups. |
Holland 1991.
| Methods | Random allocation was by allocating identical numbered boxes containing trial medications. Method of generation of numbers was not stated. Outcome assessments were not blinded. Saline injections were used as placebo. | |
| Participants | 74 low‐risk women with spontaneous labour and vaginal delivery in Nijmegen and Bergen op Zoom, Holland. | |
| Interventions | Sulprostone** 0.5 mg IM vs oxytocin 5 IU IM vs saline. | |
| Outcomes | Blood loss, duration of third stage, side‐effects. Measurement of blood loss: blood and clots collected in trays, swabs and linen weighed for the first hour after delivery. | |
| Notes | Management of third stage: 'conservatively', cord clamped within 1 minute, women asked to push after signs of separation, no cord traction or fundal pressure. 3/77 excluded (2 because of induction of labour, 1 vacuum delivery). There were more multiparous women with fewer episiotomies in the sulprostone group despite randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization was within each block of 10." |
| Allocation concealment (selection bias) | Low risk | "allocating identical numbered boxes containing trial medications." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, placebo controlled. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 women were excluded due to induction of labour and vacuum extraction. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review have been reported. |
| Other bias | High risk | There were more multiparous women with fewer episiotomies in the sulprostone group. To adjust for these factors, the authors used a linear regression model. The trial was stopped after 2 years for organisational reasons. |
Holland 1995.
| Methods | Random allocation to pharmacy coded identical boxes containing trial medications. Outcome assessments were blinded. Placebo use. | |
| Participants | 69 women with a history of previous postpartum blood loss of more than 1000 mL were eligible for this trial conducted in Leiden, Holland. Exclusion criteria: coagulation disorders, anticoagulant treatment, fibroids, multiple pregnancy, hypertension and induction of labour were excluded. | |
| Interventions | Sulprostone** 0.5 mg IM at delivery of anterior shoulder + placebo after delivery of placenta vs oxytocin 5 IU IM at delivery of anterior shoulder + methylergometrine 0.2 mg IM after delivery of placenta. | |
| Outcomes | Blood loss, duration of third stage, side‐effects. Measurement of blood loss: blood and clots were collected in trays and linen weighed. | |
| Notes | Management of third stage: fundal pressure while holding lower segment of the uterus after signs of placental detachment. 12/81 (15%) excluded after randomisation and before the intervention. No further exclusions after participation in the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "pharmacy coded boxes." |
| Allocation concealment (selection bias) | Low risk | Random allocation to pharmacy coded identical boxes containing trial medications. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12/81 (15%) excluded after randomisation and before the intervention. No further exclusions after participation in the trial. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Unclear risk | No mention of the comparability between groups. |
Hong Kong 2001.
| Methods | Random allocation was by sealed, consecutively‐numbered, opaque envelopes. Random allocation scheme was generated by computer. Outcome assessments were not blinded. Power calculation was done. | |
| Participants | 2058 women with singleton pregnancies and vaginal delivery in 3 hospitals in Hong Kong participated in the trial. Women with pre‐eclampsia, cardiac disease and asthma, conditions requiring prophylactic oxytocin infusion after delivery (uterine fibroids, grand multiparity) were excluded. | |
| Interventions | Misoprostol 600 mcg oral after delivery of the baby, vs oxytocin 5 IU + ergometrine 0.5 mg IM at delivery of anterior shoulder. | |
| Outcomes | Blood loss, duration of third stage, delayed haemorrhage, maternal haemoglobin after delivery, side‐effects. Shivering was assessed using a visual analogue scale. Blood loss was estimated. | |
| Notes | Management of third stage: controlled cord traction after signs of placental separation. No loss to follow‐up or postrandomisation exclusions were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "table of computer‐generated blocks of random numbers." |
| Allocation concealment (selection bias) | Low risk | "sealed consecutively numbered opaque envelopes." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Outcome assessments were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or postrandomisation exclusions were reported. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review were reported. |
| Other bias | Low risk | "There was no significant difference the two groups in terms of their demographic characteristics both within and among the three individual analysis." |
India 1988c.
| Methods | Random allocation by serially numbered, sealed envelopes. There was no placebo use or blinding of outcome assessments. | |
| Participants | 300 women in 3 centres in India. No mention of risk status. No note of exclusion criteria. | |
| Interventions | PGF2alpha 0.125 mg IM vs methylergometrine 0.2 mg IV. | |
| Outcomes | Blood loss, duration of third stage, side‐effects. Measurement of blood loss: blood was collected in trays for 4 hours postpartum and measured. | |
| Notes | Management of third stage: no mention of the third stage management technique. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated." |
| Allocation concealment (selection bias) | Low risk | Random allocation by serially numbered, sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention of any loss to follow‐up or exclusions after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Unclear risk | "The age, parity and the incidence of episiotomy were not significantly different between the two groups." |
India 2001b.
| Methods | Randomised trial. No further details. Unclear if outcome assessments were blinded. | |
| Participants | 120 women with at least 1 risk factor for atonic haemorrhage at Jawaharial Institute of Medical Education and Research Hospital in Pondicherry, India. | |
| Interventions | Group A: methylergometrine 0.2 mg IV. Group B: oxytocin 10 IU in 10 mL saline into the umbilical cord. Group C: carboprost 0.250 mg IM. | |
| Outcomes | Blood loss, side‐effects. Blood loss measurement not mentioned. | |
| Notes | Management of third stage: 'active' with controlled cord traction following signs of separation. No loss to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear if outcome assessments were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or exclusion after randomisation was mentioned. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Unclear risk | No mention of comparability of the groups. |
India 2004b.
| Methods | Random allocation by sealed, consecutively‐numbered envelopes. Unclear if outcome assessments were blinded. | |
| Participants | 120 low‐risk women at a rural health centre in New Delhi, India. | |
| Interventions | Misoprostol 400 mcg sublingually vs 0.2 mg methylergometrine IV. | |
| Outcomes | Blood loss, side‐effects. Blood loss was measured collecting all blood and weighing the linen and swabs. | |
| Notes | Management of the third stage: active with cord traction. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "using a random number table." |
| Allocation concealment (selection bias) | Low risk | "sequentially numbered sealed opaque envelopes." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear if outcome assessments were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention of loss to follow‐up or exclusion after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | The 2 maternal groups were similar in maternal and neonatal demographics. |
India 2005a.
| Methods | Random allocation, no further details. Unclear if outcome assessments were blinded. | |
| Participants | 200 primiparous women with singleton births at Lok Nayak Hospital, New Delhi, India. | |
| Interventions | Misoprostol 600 mcg orally immediately after delivery vs 0.2 mg methylergometrine IV at delivery of anterior shoulder. | |
| Outcomes | Blood loss, side‐effects. Blood loss measurement method not mentioned. | |
| Notes | Management of the third stage: early cord clamping but no mention of placental delivery. No mention of missing data or loss to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "1:1 ratio by random number sequence." |
| Allocation concealment (selection bias) | Unclear risk | Unclear how the allocation concealment was done. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear if outcome assessments were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention of loss to follow‐up or exclusion after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Unclear risk | The information on the comparability of 2 groups were not provided. |
India 2006a.
| Methods | Randomisation by computer‐generated random‐number list, allocation concealment by opening sealed opaque envelopes. Unclear if outcome assessments were blinded. | |
| Participants | 100 women undergoing caesarean section at the All India Institute of Medical Sciences, New Delhi, India. Women with risk factors for postpartum haemorrhage were not eligible. | |
| Interventions | Misoprostol 400 mcg sublingually vs 20 IU oxytocin in 1 litre lactated Ringer's solution at 125 mL/h. All women had spinal anaesthesia. | |
| Outcomes | Blood loss, side‐effects. Blood loss measurement: volume of blood in the suction bottle + weighing of blood‐soaked linen. | |
| Notes | Management of the third stage: not applicable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated random number." |
| Allocation concealment (selection bias) | Low risk | "sealed opaque envelopes." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐labeled. Unclear if outcome assessments were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no withdrawals following randomisation. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | "There were no significant differences in demographic data in relation to age, parity, gestation, history of previous cesarean section and neonatal birth weight." |
India 2006b.
| Methods | Randomisation achieved by computer‐generated numbers. No details regarding allocation concealment available. | |
| Participants | 2023 women delivering at the Christian Medical College Hospital, Vellore, India. Women with cardiac disease, bronchial asthma, rhesus factor incompatibility, pregnancy‐induced or pregnancy‐aggravated hypertension and caesarean delivery were excluded. | |
| Interventions | Misoprostol 400 mcg orally vs oxytocin 10 IU IM versus ergometrine 0.2 mg IV. | |
| Outcomes | Blood loss, haemoglobin levels, side‐effects. Blood loss measurement: large plastic bag placed under the buttocks following drainage of amniotic fluid. The blood was then transferred to a measuring jar. | |
| Notes | Management of the third stage: active management. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Unclear on how the allocation concealment was done. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention of loss to follow‐up or exclusion after randomisation. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review were reported. |
| Other bias | Low risk | Baseline characteristics were similar in all the groups. |
India 2006c.
| Methods | Computer‐generated, random‐number schedule with a random block list. Random allocation by giving the next of a series of non‐distinguishable envelopes containing active or placebo tablets. Identical placebos were used. Outcome assessments were blinded. | |
| Participants | 1620 women delivering at home or primary care centre in 4 primary health centre areas of Belgaum District, Karnataka State, India. Women were delivered by ANMs who were trained in the trial procedures and the intervention. 2 sets of midwives were involved in the study. 18 at the beginning and 12 leaving and replaced by 7 new ANMs. | |
| Interventions | Misoprostol 600 mcg orally vs identical placebos. | |
| Outcomes | Blood loss, side‐effects. Blood loss measurement: A calibrated blood collection drape placed under the buttocks following delivery. Blood loss was measured after 1 hour and 2 hours. | |
| Notes | Management of the third stage: the ANMs practised expectant management of the third stage of labour apart from the uterotonic in the intervention arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generation randomization list with a random block size." |
| Allocation concealment (selection bias) | Low risk | "non‐distinguishable envelopes." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled. Outcome assessments were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention of loss to follow‐up or exclusion after randomisation. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review were reported. |
| Other bias | Low risk | "The two groups did not differ across any characteristics." |
India 2006d.
| Methods | Randomised study, no further details presented. Unclear if outcome assessments were blinded. | |
| Participants | 120 low‐risk women delivering at the Comprehensive Rural Health Services Project, a rural health centre affiliated with the All India Institute of Medical Sciences, New Delhi, India. Women who received oxytocin during labour, caesarean section delivery, multiple pregnancy and Hb < 8 g/dL were excluded. | |
| Interventions | Misoprostol 400 mcg rectally vs PG‐F2alpha 125 mcg IM. | |
| Outcomes | Blood loss. Blood loss measurement: by clinical estimation. | |
| Notes | Management of the third stage: not mentioned. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear on how the adequate sequence generation was done. |
| Allocation concealment (selection bias) | Unclear risk | Unclear on how the allocation concealment was done. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear if blinding occurred. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention of loss to follow‐up or exclusion after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review have been reported. |
| Other bias | Low risk | "The two treatment groups were similar with respect to demographic and labour characteristics." |
India 2006f.
| Methods | Randomised, placebo‐controlled study. | |
| Participants | 200 women in spontaneous labour. No exclusion criteria were reported. |
|
| Interventions | 600 mcg rectal misoprostol versus 10 IU IM oxytocin. | |
| Outcomes | Estimated blood loss (mL), blood loss ≥ 500 mL, change in Hb (g/dL), length of third stage of labour (min), need for additional oxytocic drugs, need for manual removal of placenta and side‐effects (shivering, nausea, temperature ≥ 38 C) | |
| Notes | Active management of third stage of labour. Blood loss was measured by using calibrated drapes (BRASSS‐V drape) and using preweighted gauzes to clean the perineal tears and episiotomy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated random tables." |
| Allocation concealment (selection bias) | Low risk | "sealed envelope with a code number was opened when vaginal delivery was imminent." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. The code was not broken until the end of the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up or postrandomisation exclusions were reported. |
| Selective reporting (reporting bias) | Low risk | All of the outcomes were reported. |
| Other bias | Low risk | The 2 groups were similar with regards to baseline characteristics. |
India 2006g.
| Methods | Randomised controlled trial. | |
| Participants | 200 women with singleton pregnancy, spontaneous onset of labour at term and vertex presentation admitted in active phase of labour were included. Women with hypertension, cardiac disease, renal disease, gastrointestinal disorders, respiratory disease, endocrinal problems, coagulation disorder, and sensitivity to prostaglandin or methergin were excluded. |
|
| Interventions | 125 mcg IM PGF2 versus 0.2 mg methergin IV at the time of the anterior shoulder delivery. | |
| Outcomes | Duration of third stage, blood loss, need for additional drugs, retained placenta and side‐effects (nausea and vomiting). | |
| Notes | Active management of third stage of labour. Blood loss was estimated by blood and blood clots collected in the kidney tray. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number tables." |
| Allocation concealment (selection bias) | Unclear risk | Unclear on how the allocation concealment was done. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not clear on if the blinding occurred. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up and exclusions after randomisation were reported. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | The 2 groups were well matched in the baseline. |
India 2007.
| Methods | Randomised controlled trial. | |
| Participants | 100 women aged 18‐32 years of age, gravida 1‐3 with singleton pregnancy and vertex presentation delivered normally. Women with heart disease, renal or hepatic disorder, previous caesarean section delivery and severe hypertension. |
|
| Interventions | Intramuscular 125 mcg carboprost tromethamine at the delivery of the anterior shoulder versus IM 0.2 mg methylergometrine after expulsion of the placenta. | |
| Outcomes | Postpartum haemorrhage (more than 500 mL), amount of blood loss (mL), duration of the third stage of labour (minutes), requiring blood transfusion and other uterotonics and side‐effects (high blood pressure, nausea, vomiting, diarrhoea, pyrexia). | |
| Notes | It is not clear whether all the components of AMTSL other than the uterotonics have been used in this study. The blood loss has been estimated by weighing the blood clots and vaginal pads before and after use following delivery. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear how the sequence generation was done. |
| Allocation concealment (selection bias) | Unclear risk | Unclear how the allocation concealment was done. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear if the blinding was done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions were reported. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Unclear risk | The authors mention that the groups were comparable in terms of gravida, but no other baseline characteristics are mentioned. |
India 2008a.
| Methods | Randomised controlled trial (2 arms of misoprostol and 1 arm of methylergometrine). | |
| Participants | 300 women with term gestation and spontaneous onset of labour (low‐risk, as identified by the authors) Exclusion criteria included grand multiparity (parity > 5), multiple gestation, pregnancy‐induced hypertension, antepartum haemorrhage, labour induction or augmentation, caesarean delivery (past/present), haemoglobin concentration of < 8 gm/dL or other obstetric problems and known hypersensitivity to prostaglandins. |
|
| Interventions | With the delivery of the anterior shoulder of the baby, women in group I received 100 mcg (1 tablet) of sublingual misoprostol, while the women in group II received 200 mcg (2 tablets) versus group III received a 200‐mcg IV injection of methylergometrine with the delivery of the anterior shoulder of the baby. | |
| Outcomes | The need for additional oxytocic drugs (If the blood exceeded 100 mL, an additional oxytocic was administered), blood loss ≥ 500 mL, change in haemoglobin levels and side‐effects (shivering, recorded temperature > 38 C, nausea, vomiting, headache, giddiness). | |
| Notes | Unclear whether all the components of active management of labour was performed other than the uterotonics. Blood loss was estimated by measuring blood and blood clots collected in sponges. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "using random number tables." |
| Allocation concealment (selection bias) | Unclear risk | Not clear how the allocation concealment was done. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not clear if the blinding occurred. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up or postrandomisation exclusions were mentioned. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | The 3 groups were similar in maternal and neonatal demographic profile and haemoglobin concentration at admission. |
India 2008b.
| Methods | Randomised controlled trial. | |
| Participants | 215 parturients who were at term(≥ 37 to < 41 weeks of gestation), with singleton pregnancy in cephalic presentation and vertex as presenting part, having haemoglobin concentration of at least 10g/dL. Parturients with active renal or hepatic disease, bronchial asthma, parity > 4 and bleeding disorders were excluded. |
|
| Interventions | Intramuscular carboprost tromethamine (0.5 mL containing125 mcg) versus intravenous methyl ergometrine (0.5 mL containing 200 mcg), both at the time of the delivery of the anterior shoulder. | |
| Outcomes | Duration of third stage of labour, measured blood loss, side‐effects (hypertension and diarrhoea). | |
| Notes | Active management of third stage labour. Amount of blood loss was quantified by noting the increment in weight of standardized tampons, which were placed high up in the vagina immediately after placental delivery. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation, using odd numbers for the control group and even numbers for the intervention group. |
| Allocation concealment (selection bias) | Unclear risk | Unclear how the allocation concealment was done. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Partial blinding. The patient was blinded to the kind of intervention but the clinician and outcome assessor knew. This could not be hidden as the clinician could tell which oxytocic was being given from the mode of administration‐ie intravenous for methyl ergometrine and intramuscular for carboprost |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up or postrandomisation exclusions are reported. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | Both groups were similar with regard to age distribution, parity and gestational age. |
India 2009b.
| Methods | Randomised, double‐blind, placebo‐controlled trial (2 arms of misoprostol and 1 arm of oxytocin). | |
| Participants | 300 women with healthy singleton pregnancies in spontaneous or induced labour at term were included. Exclusion criteria included known hypersensitivity/contraindication to prostaglandins, intrauterine fetal demise, antepartum haemorrhage, multiple pregnancy, malpresentation, cardiac disease, Rhesus‐negative mother, hypertensive disorders, and severe anaemia (haemoglobin < 7 g/dL). |
|
| Interventions | 400 mcg sublingual misoprostol, 600 mcg sublingual misoprostol versus 5 IU IV oxytocin. | |
| Outcomes | Blood loss during the 3rd and 4th stage of labour, duration of 3rd stage of labour, need for additional oxytocics, need for blood transfusion and adverse effects of the drugs. | |
| Notes | Active management of third stage of labour 1 hour after delivery, the preweighted, blood‐soaked linen‐saver sheet and preweighted sanitary pads were weighted to assess maternal blood loss. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Unclear how the allocation concealment was done. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or postrandomisation exclusions were reported. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | Baseline demographic and clinical characteristics of patients were similar among the groups. |
India 2009d.
| Methods | Randomised controlled trial. | |
| Participants | 200 pregnant women at ≥ 32 weeks of gestation with either spontaneous or induced labour (what authors identified as low‐risk) were included. Exclusion criteria included grandmultipara (≥ 5), multiple gestation, < 32 weeks of gestation, HELLP syndrome, hydramnios, known blood coagulation disorders, history of asthma or drug allergy, heart disease, severe renal disease, epilepsy, hypertension and haemoglobin concentration < 8 g%. |
|
| Interventions | 400 mcg sublingual misoprostol versus 0.2 mg IM methylergometrine | |
| Outcomes | Primary outcome measures were amount of blood loss in third stage of labour, postpartum haemorrhage (blood loss greater than 500 mL). Secondary outcome measures were change in haemoglobin concentration, need for additional oxytocic and side‐effects (nausea, temperature ≥ 38 C, shivering, pain in the abdomen and diarrhoea). |
|
| Notes | Active management of third stage of labour. Blood loss was estimated by measuring blood collected in the BRASS‐V drape, up to 1 hour after delivery of the baby, and blood soaked in gauze pieces. Blood loss in gauze pieces was calculated by subtracting weight of dry gauze from the weight of blood‐soaked gauze pieces. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random number." |
| Allocation concealment (selection bias) | Unclear risk | Unclear on how the allocation concealment was done. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not clear on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or exclusion postrandomisation were reported. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | Demographic variables were equally distributed in the groups. |
India 2010.
| Methods | Randomised controlled trial. | |
| Participants | 200 women with singleton pregnancies, spontaneous onset of labour at term and vertex position admitted in active phase of labour were included. Exclusion criteria included hypertension, cardiac disease, renal disease, gastrointestinal disorders, respiratory disease, endocrinal problems, coagulation disorder and sensitivity to prostaglandins. |
|
| Interventions | IM 125 mcg PGF2α versus IV 0.2 mg of methergin at the time of the delivery of the anterior shoulder. | |
| Outcomes | The interval between injection and expulsion of the placenta, amount of blood loss, third stage complications, side‐effects and need for second injection of additional drug. | |
| Notes | Active management of third stage of labour Blood loss was estimated by blood and blood clots collected in the kidney tray and adding the difference in the weight of the drapes before use and after delivery. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "using random tables." |
| Allocation concealment (selection bias) | Unclear risk | Unclear on how the allocation concealment was done. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear on the blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or postrandomisation exclusions were reported. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | The 2 groups were well matched in terms of gravidity, parity and age. |
Iran 2009.
| Methods | Open‐label randomised controlled trial. | |
| Participants | 100 term pregnant women between 37 and 40 weeks of pregnancy who were candidates for caesarean and underwent general anaesthesia were included. Exclusion criteria included an additional risk for postcaesarean haemorrhage, including: multiple gestation; prolonged labour of more than 12 hours; 2 or more previous caesarean sections; a history of uterine rupture and anaemia‐Hb (8 mg/dL). Also women who had a history of heart, renal or liver disorders or had a coagulopathy were excluded. |
|
| Interventions | Immediately after delivery, 2 misoprostol tablets (200 mcg) dissolved in 5 cc of distilled water (for better distribution and absorption) and taken sublingually (vs 20 units of oxytocin in 1 litre Ringer lactate at the rate of 10 cc/min was used until full contraction of the uterus). | |
| Outcomes | The need for additional oxytocin in surgery, the volume of infusion, need for blood transfusion, drug side‐effects (such as tachycardia, decrease in blood pressure, fever, shivering, headache and metallic taste) or any other significant complications were recorded. | |
| Notes | AMTSL not applicable, as this study includes women who had caesarean section only. The blood loss was measured in the suction bottle so that amniotic fluid was first suctioned and measured and then subtracted from the total volume of the suction bottle. The amount of blood on the patients' drapes and pads was measured by weighing them and then subtracting their known dry weight and this value was added to the amount of blood in the suction bottle. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear how the sequence generation was done. |
| Allocation concealment (selection bias) | Unclear risk | Unclear how the allocation concealment was done. |
| Blinding (performance bias and detection bias) All outcomes | High risk | This was an open‐label trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or exclusions were mentioned. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | "There were no significant differences in demographic data between the 2 groups in relation to age, weight, gestational age and parity." |
Jamaica 2009.
| Methods | Open‐label randomised controlled trial. | |
| Participants | 140 women delivering at the labour ward at the hospital during the 6‐month period were included. Exclusion criteria included previous postpartum haemorrhage, hypertensive disorders, previous caesarean section, intrauterine death in current pregnancy, sepsis/pyrexia ≥ 38 C), antepartum haemorrhage, symptomatic anaemia or haemoglobin below 8 gr/dL. |
|
| Interventions | Rectally administered 400 mcg misoprostol versus IM syntometrine in the AMTSL. | |
| Outcomes | Main outcome parameter was measured blood loss up to 1 hour postdelivery. Secondary outcome measures included the proportion of participants with postpartum haemorrhage, the proportion of women who need additional uterotonic agents and incidence of adverse events (shivering, nausea, vomiting). | |
| Notes | Active management of third stage labour. Blood loss was measured by the use of a modified plastic collection drape. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated block randomisation." |
| Allocation concealment (selection bias) | Unclear risk | Unclear how the allocation concealment was done. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or exclusions were reported. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | "The clinical characteristics of the sample showed no difference in the variables measured between the two treatment groups." |
Mexico 2009.
| Methods | Randomis double‐blind study. | |
| Participants | 200 patients requiring caesarean section with single or multiple pregnancy and not presenting placenta previa, blood dyscrasia or myomatosis. Patients with obstetrical haemorrhage caused by uterine lacerations and those who did not want to participate in the study were excluded. |
|
| Interventions | Misoprostol (800 mcg) intravaginally versus placebo (Manogen‐sorbitol with aerosol) Both groups received oxytocin after the birth of the baby. | |
| Outcomes | Hb and Hct levels before and after labour and the difference and side‐effects (pain, fever, shivers, nausea, vomiting, average systolic and diastolic blood pressure before and after caesarean section) were measured. | |
| Notes | AMTSL not applicable, as the study includes caesarean sections. Measurement of blood loss was not mentioned. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear how the sequence generation was done. |
| Allocation concealment (selection bias) | Unclear risk | Unclear how the allocation concealment was done. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. The tablets were placed in 200 separate envelopes and assigned a number that was chosen randomly. The content of the envelopes were not revealed until the end of the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up and postrandomisation exclusions were reported. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Unclear risk | Not clear on the comparability of the groups at the baseline. |
Mozambique 2001.
| Methods | Randomised double‐blind trial. Generation of allocation sequence unclear. Double placebos prepared by a pharmacist independent of the trial on a daily basis and provided to the investigators upon request. Outcome assessments were blinded. | |
| Participants | 663 women with uncomplicated vaginal delivery between 30 and 42 weeks of gestation at Central Hospital of Maputo, Mozambique. Women undergoing induction or augmentation of labour were excluded. | |
| Interventions | Misoprostol 400 mcg dissolved in 5 mL saline and administered rectally as a micro‐enema + 1 mL saline placebo IM versus oxytocin 10 IU administered IM + 5 mL saline micro‐enema (placebo). | |
| Outcomes | Blood loss, side‐effects. Blood loss measured by a metal collector placed under the buttocks after delivery until the woman was moved from the delivery room. | |
| Notes | Management of third stage not described. 26/350 (7.4%) in the misoprostol group and 11/350 (3.1%) in the oxytocin group were excluded after randomisation because of emergency caesarean section or incomplete data collection. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear on how the adequate sequence generation was done. |
| Allocation concealment (selection bias) | Unclear risk | Unclear on how the allocation concealment was done. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. Outcome assessments were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16 women in the misoprostol group and 11 women in the oxytocin group were excluded after randomisation due to having emergency caesarean section or incomplete data collection. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | The 2 groups were similar in baseline characteristics. |
Nigeria 2003.
| Methods | Randomised double‐blind trial with identical looking double placebos. Randomisation schedule generated using random‐number tables. Allocation concealment achieved by using sealed opaque packets containing both active and the corresponding placebo medication. | |
| Participants | 496 low‐risk women having vaginal births in 2 hospitals in Delta State, Nigeria. Women undergoing caesarean section and who had other risk factors for haemorrhage were excluded. | |
| Interventions | Misoprostol 600 mcg in powder form dissolved in 50 mL water per os vs oxytocin 10 IU IM at delivery of anterior shoulder. | |
| Outcomes | Blood loss, postdelivery haemoglobin, side‐effects. Blood loss estimated by the clinicians. | |
| Notes | Management of third stage: controlled cord traction, no other details. No loss to follow‐up or postrandomisation exclusions reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number‐generated tables." |
| Allocation concealment (selection bias) | Low risk | "opaque sealed packets containing a card indicating group allocation." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or postrandomisation exclusions reported. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | There were no significant differences between 2 groups. |
Nigeria 2007.
| Methods | Single‐blinded randomised controlled study. | |
| Participants | 864 women with singleton, low‐risk pregnancies with anticipated spontaneous vertex delivery in a secondary health centre located in a semi‐urban area of Ibadan, capitol city of Oyo State. Exclusion criteria included the presence of contraindications to the use of either misoprostol and methylergometrine, such as pre‐eclampsia and other hypertensive diseases in pregnancy, pre‐existing cardiac disease, severe anaemia, history of asthma, renal or hepatic disorders, allergy to prostaglandins, and the presence of conditions requiring prophylactic oxytocin infusion after delivery such as grand multiparity, multiple pregnancy, polyhydramnios, previous history of postpartum haemorrhage and uterine fibroid. |
|
| Interventions | 400 mcg of oral misoprostol versus 500 mcg of IM methylergometrine at the delivery of the anterior shoulder. | |
| Outcomes | Total estimated blood loss (mL), postpartum haemorrhage (blood loss > 500 mL), duration of third stage of labour (min), duration of third stage of labour > 15 min, postdelivery packed cell volume (PCV) (%), differences in PCV (%), percentage changes in PCV (%), percentage PCV change > 10%, manual placental removal, additional oxytocics and side‐effects (fever, shivering, nausea, vomiting, headache). | |
| Notes | Active management of third stage labour. Blood loss was estimated by a combination of careful measurement of blood collected in a receptacle after the delivery of the baby, visual estimation of blood loss and extrapolation of blood loss using weight difference of the total perineal pad used up to 24 hours in the postpartum period. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "An independent statistician generated sets of four random letters, which were in boxes, and each box contained four separate random allocations which was equivalent to an opaque sealed envelope stratified in a block for four." |
| Allocation concealment (selection bias) | Low risk | Adequate, see above. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Single‐blinded." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or postrandomisation exclusions were reported. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | None of the baseline variables are statistically significant between 2 groups. |
Nigeria 2011.
| Methods | Double‐blind, randomised, placebo‐controlled trial. | |
| Participants | 1345 pregnant multiparous women who deliver vaginally in 6 different hospitals in Nigeria were included. Only multiparous women were recruited because they were more likely to proceed to normal delivery. Exclusion criteria included any severe allergic condition or severe asthma, age below 18 years, temperature above 38 C, or abortion of the pregnancy. |
|
| Interventions | This is a trial where misoprostol is given in addition to the traditional oxytocics. In addition to 10‐IU oxytocin or 0.25 mg/0.5 mg ergometrine immediately after delivery of the child, 2 tablets of 200 mcg misoprostol (400 mcg dose) were provided versus only below traditional treatment and 2 placebo tablets. | |
| Outcomes | Primary: blood loss of at least 500 mL within 1 hour of administration of the trial medication. Secondary: total severe postpartum blood loss of at least 1000 mL within 1 hour of drug administration, average blood loss after drug administration, need for additional oxytocin or ergometrine, maternal morbidity, maternal mortality, and adverse effects. Adverse‐effect measures were self‐reported moderate/severe shivering and pyrexia (body temperature at least 38 C) within 1 hour of taking the trial drug. The occurrence of nausea, vomiting, and diarrhoea after administration of the trial drug was also assessed. |
|
| Notes | All centres had a policy of active management of the third stage of labour. Blood collection was initiated as soon as possible after administration of the trial medication. A low‐profile plastic fracture bedpan was placed below the woman's perineum to collect all subsequent blood loss for a period of 1 hour. Blood collected in the bedpan and all blood‐soaked small gauze swabs were emptied into a plastic measuring jar and the volume was measured. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatment was allocated in blocks of 6‐8 women by the research nurse, who used a computer‐generated randomization sequence." |
| Allocation concealment (selection bias) | Low risk | "The trial drugs were concealed in sealed, sequentially numbered opaque envelopes and were identical in shape, color, size and design." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or postrandomisation exclusions were reported. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review were reported. |
| Other bias | Low risk | "The baseline characteristics in the two arms of the study were similar with regard to age, proportion of primiparous women, proportion of labours induced with misoprostol, type of routine uterotonic administered, and birth weight of the infant." |
Pakistan 2011.
| Methods | Randomised, double‐blind, placebo‐controlled trial. | |
| Participants | 1119 pregnant women in general good health, residing in 1 of the 46 study villages and planning to deliver at home with a study TBA were eligible for inclusion. Women were not eligible if presenting with pregnancy complications such as hypertension, non‐cephalic presentation, polyhydramnios, previous caesarean section, suspected multiple pregnancy, suspected stillbirth, antepartum haemorrhage, and Hb < 8 g/dL. |
|
| Interventions | 600 mcg oral misoprostol versus placebo. The use of a placebo was considered ethical because the standard care for home births with TBAs in the study area is to give no prophylactic uterotonic at delivery. | |
| Outcomes | The primary outcomes were postpartum haemorrhage (defined as measured blood loss >= 500 mL) and drop in Hb > 2 g/dL. Secondary outcomes included intermediate and severe postpartum haemorrhage (blood loss 750 and 1000 mL), mean blood loss and postpartum Hb < 9 and < 11 g/dL. |
|
| Notes | Providers were asked to document how the third stage was managed for each participant. The TBAs are trained in management of the third stage of labour, including performing uterine massage, cord traction, delayed cutting of the cord, and immediate suckling at the breast. To collect postpartum blood loss, women were positioned on a perineal sheet and bedpan for a minimum of 1 hour or until active bleeding stopped whichever occurred last. Study TBAs were provided with a 1‐hour timer to track that blood was collected for 1 hour and were asked to estimate the time in minutes between delivery of the baby and of the placenta. Blood collected in the bedpan was transferred to a measuring jar, which was then closed, and the used perineal sheet and cotton roll were placed in a sealed plastic bag. The closed measuring jar and sealed plastic bag were then placed inside a plastic cooler which was tightly closed and stored in a secure place in the woman's home until the LHV/CHN arrived for weighing, 1‐2 days after delivery. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐ generated random code in blocks of 6 was maintained by Gynuity Health Projects in New York and not revealed until data collection and cleaning were completed." |
| Allocation concealment (selection bias) | Low risk | "Study medication was packed in numbered colour‐coded boxes to identify the randomization sequence. Study TBAs were provided with specially designed colour‐coded drug boxes to ensure that the sequence was maintained." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 patients (1 in the misoprostol group and 2 in the placebo group) could not be followed up. Blood loss data was not available for 19 patients in the misoprostol group and 25 patients in the placebo group. Hb measures were not available pre and post delivery for 5 patients in the misoprostol group and 12 patients in the placebo group. |
| Selective reporting (reporting bias) | Low risk | All of the primary outcomes of the review which can be collected in the community setting were reported. |
| Other bias | Low risk | 2 groups were comparable. |
Singapore 1995.
| Methods | Random allocation by a random‐number table. Blinding of some outcome assessments. | |
| Participants | 115 women with spontaneous labour and delivery in Singapore. Exclusion criteria: multiple pregnancy, any antenatal complications. | |
| Interventions | Carboprost trometamol* 125 mcg IM vs ergometrine‐oxytocin 0.5 mg IM. | |
| Outcomes | Blood loss, need for additional uterotonics, transfusion, haemoglobin levels, side‐effects. Measurement of blood loss: blood and clots in the first 2 hours after delivery mopped with absorbent paper, sanitary pads collected for the next 22 hours, and then measured. | |
| Notes | Management of third stage: controlled cord traction after placenta separation. 3/115 (2.6%) women were excluded after randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number table." |
| Allocation concealment (selection bias) | Low risk | Adequate. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear if blinding occurred. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 women were excluded as the births occurred before the blood loss measurement was set up. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | "The parity, gestation, mode of delivery and the mean duration of first and second stage of labor were similar in the two groups." |
South Africa 1998a.
| Methods | Random allocation by computer‐generated, random sequence for sealed opaque envelopes. No placebo use. Outcome assessments were not blinded. | |
| Participants | 491 women at low risk for postpartum haemorrhage at Natalspruit Hospital, Johannesburg, South Africa. Exclusion criteria: not noted. | |
| Interventions | Misoprostol 400 mcg rectally vs ergometrine‐oxytocin 1 ampoule IM. | |
| Outcomes | Blood loss, duration of third stage, side‐effects. Measurement of blood loss: by estimation. | |
| Notes | Third stage management was active. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random sequence." |
| Allocation concealment (selection bias) | Low risk | "sealed opaque envelopes." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not clear if blinding occurred. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was minimal for primary outcomes (2‐3%) with the exception of postpartum haemoglobin which was measured in 67% and 65% of women in the misoprostol and ergometrine‐oxytocin groups respectively. A small number of women (unspecified) allocated to ergometrine‐oxytocin were excluded because of high blood pressure discovered after randomisation. However, results were similar to the whole group when all hypertensives were excluded in a subgroup analysis. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | Baseline variables were comparable between 2 groups except systolic BP during labour. |
South Africa 1998b.
| Methods | Random allocation by computer‐generated random sequence. Double‐blinded, placebo‐controlled trial. Tablets kept in numbered, sealed, opaque containers. Non‐identical placebo tablets. | |
| Participants | 500 women after delivery at Coronation Hospital, Johannesburg, South Africa. No mention of risk status. Exclusion criteria: oxytocin infusion in progress at the time of delivery, hypertension, diabetes, previous caesarean section delivery. | |
| Interventions | Misoprostol 400 mcg orally vs placebo. | |
| Outcomes | Blood loss greater than or equal to 1000 mL within first hour of birth, use of additional uterotonics, side‐effects, third stage 30 minutes or longer, manual removal of the placenta, blood transfusion. Measurement of blood loss: blood and clots collected in bedpans and volume assessed. Linen weighed. | |
| Notes | Management of third stage: placenta removed by cord traction once firm uterine contraction diagnosed by palpation. No withdrawals after randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random sequence." |
| Allocation concealment (selection bias) | Low risk | "numbered, opaque test tubes." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions after the randomisation. Intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review have been reported. |
| Other bias | Low risk | The groups were well‐matched. |
South Africa 1998c.
| Methods | Random allocation by computer‐generated random numbers. Tablets kept in numbered, sealed, opaque containers. Non‐identical placebo tablets. Outcome assessments were blinded. | |
| Participants | 550 low‐risk women after delivery at Coronation Hospital Johannesburg, South Africa. Exclusion criteria: not noted. | |
| Interventions | Misoprostol 400 mcg rectally vs placebo. | |
| Outcomes | Blood loss greater than or equal to 1000 mL, use of additional uterotonics, spontaneous delivery of the placenta, third stage longer than or equal to 30 minutes, side‐effects. Measurement of blood loss: blood collected in bedpan until 1 hour after delivery. Linens weighed. | |
| Notes | Management of third stage: placenta delivered either by cord traction or spontaneous expulsion. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random sequence." |
| Allocation concealment (selection bias) | Low risk | "sealed, opaque containers." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Non‐identical placebo. The blinding of the midwife administering the tablets was not possible, as identical placebo tablets were not available. Outcome assessments were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions after randomisation: records for 4 allocations (all in placebo group), could not be traced. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review have been reported. |
| Other bias | Low risk | The 2 groups were well‐matched. |
South Africa 1998d.
| Methods | Random allocation according to a computer‐generated random sequence. Serially numbered, opaque test tubes. Outcome assessments were blinded. | |
| Participants | 600 women after delivery at Coronation Hospital, Johannesburg, South Africa. No mention of whether they are high or low risk. No mention of exclusion criteria. | |
| Interventions | Misoprostol 600 mcg orally vs misoprostol 400 mcg orally vs placebo. | |
| Outcomes | Shivering, pyrexia. Blood loss was measured using a flat bed pan. | |
| Notes | Management of third stage: placenta removed by cord traction after firm contraction of uterus. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random sequence." |
| Allocation concealment (selection bias) | Low risk | "Serially numbered, opaque test tubes." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded according to the authors' other published work. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes of the review have been reported. |
| Other bias | Unclear risk | Unclear if the baseline characteristics are comparable. |
South Africa 2001.
| Methods | Random allocation according to a computer‐generated random sequence. Serially numbered, opaque test tubes. Outcome assessments were blinded. | |
| Participants | 600 women after delivery at Coronation Hospital, Johannesburg, South Africa. Exclusion criteria: no mention of exclusion criteria. | |
| Interventions | Misoprostol 600 mcg oral vs placebo. | |
| Outcomes | Shivering, pyrexia. Measurement of blood loss: blood in bed pan measured, linen and sanitary towels weighed. | |
| Notes | Management of third stage: placenta removed by cord traction after firm contraction of uterus. No exclusions after randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated random sequence." |
| Allocation concealment (selection bias) | Low risk | "Serially numbered, opaque test tubes." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no withdrawals after randomisation and all outcomes were analysed in the allocated group. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review were reported. |
| Other bias | Low risk | The 2 groups were well‐matched. |
Switzerland 1999.
| Methods | Random allocation using random‐number tables. Trial was double blinded. | |
| Participants | 65 low‐risk women with vaginal births at Basel University Hospital, Basel, Switzerland. Exclusion criteria: multiple pregnancy, pre‐eclampsia, previous postpartum haemorrhage or antepartum haemorrhage, caesarean delivery. | |
| Interventions | Misoprostol 600 mcg orally vs placebo. | |
| Outcomes | Blood loss, length of third stage, use of additional uterotonics, side‐effects, haematocrit values. Measurement of blood loss: estimation by delivery physicians. | |
| Notes | Management of third stage: early cord clamping and cord traction. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number‐generated tables." |
| Allocation concealment (selection bias) | Low risk | Adequate. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | 2 groups were comparable in the baseline. |
Switzerland 2006.
| Methods | Randomised, double‐blind, placebo‐controlled trial. | |
| Participants | 56 pregnant women at low risk for postpartum haemorrhage who underwent indicated or elected caesarean section after the 37th week of pregnancy at the Basel Women's University Hospital in Switzerland. Indications for caesarean section were breech presentation, malposition, IUGR, placenta previa marginalis, twin pregnancy, previous caesarean section, maternal disease and failure of labour to progress. Exclusion criteria included emergency caesarean section within 30 minutes of admission, fetal distress, fetal malformations, preeclampsia or HELLP, hypersensitivity to prostaglandins, coagulopathy, severe systemic disorders, and American Society of Anesthesiologists physical status of 3 or greater, severe asthma, prior myomectomy, and fever (> 38.5 C). |
|
| Interventions | 800 mcg of oral misoprostol versus 20 IU of oxytocin in saline solution. | |
| Outcomes | Outcomes are estimated blood loss (mL), calculated blood loss (mL), Hb level 24 hours postoperatively, g/dL, Hb level 48 hours postoperatively, g/dL, hospital stay (days) and side‐effects (shivering, headache, nausea, flush, diarrhoea). | |
| Notes | Active management is not applicable, as the study is limited to caesarean sections. When the membranes had ruptured before the section, the amount of intraoperative and postoperative blood loss was calculated by determining the difference in weight of cloths and pads used to absorb blood during surgery and postoperatively in the intermediate care unit. When the membranes had not ruptured preoperatively, the amount of blood loss was assessed by collecting the blood in suction bottles and subtracting the estimated amniotic fluid volume. It should be noted that all participants received a 5 IU bolus of oxytocin intravenously and afterwards were randomised to receive either of the above medications immediately after cord clamping. This regimen was imposed by the institutional ethics committee as a consequence of the Cochrane Library Review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The hospital pharmacy performed 1:1 computer‐generated randomization that assigned the participants to their group." |
| Allocation concealment (selection bias) | Low risk | "The pharmacy also provided the study drugs and placebos in unidentifiable form. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 patients in the oxytocin group were excluded from statistical analysis because of errors in drug administration. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | The demographic and obstetric characteristics are similar between the 2 groups. |
Tibet 2009.
| Methods | Double‐blind, double placebo, randomised 2‐arm trial. | |
| Participants | 967 pregnant women 18 years of age or older with a viable intrauterine singleton pregnancy, ≥ 28 weeks' gestation at 3 obstetric units in Lhasa, TAR, PRC, were considered eligible for study participation. | |
| Interventions | Traditional dose of ZB11 at full dilation versus oral 600 mcg misoprostol immediately after delivery. | |
| Outcomes | Primary outcomes are postpartum haemorrhage (MBL >= 500 mL), administration of open label uterotonics within the 1 hour observation period after delivery, or maternal death. Secondary outcomes included: MBL >= 500 to 999 mL, MBL >= 1000 mL, mean and median MBL during the first hour postpartum, and side‐effects. |
|
| Notes | Active management of third stage of labour. Postpartum blood loss was measured using a closed‐end blood collection drape (BRASSS‐V drape blood collection receptacle) for 1 hour after delivery of the baby. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "a computer generated randomization list with a random block size for each hospital." |
| Allocation concealment (selection bias) | Low risk | "Sealed opaque study medication envelopes were distributed to the hospitals by research study staff." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 out of 480 ZB11 patients and 3 out of 487 misoprostol group patients have been excluded due to developing eclampsia, vomiting and unable to take medication. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review have been reported. |
| Other bias | Low risk | The 2 groups did not differ in any characteristic at the baseline. |
Tunisia 2009.
| Methods | Randomised controlled trial. | |
| Participants | 250 single low‐risk pregnant women undergoing caesarean section at gestational age > 32 weeks' gestation, under regional anaesthesia at the Department of Obstetrics Sousse, Tunisia. Exclusion criteria included patients with placenta previa, abruptio placentae, multiple gestation, preterm delivery (less than 32weeks), fetal death in utero, a caesarean under general anaesthesia, anaemia (Hb <8 g /dL), haemostasis disorders(congenital or anticoagulant therapy), HELLP syndrome, a history of postpartum haemorrhage, previous uterine rupture, women with more than 2 caesareans, prolonged labour (over 12 hours) and maternal fever(temperature ? 38 ? C). |
|
| Interventions | This study uses misoprostol on top of conventional oxytocics for prevention for 200 mcg sublingual misoprostol with 20 IU intravenous oxytocin (10 UI intravenous direct and 10 IU diluted in an infusion of 500 cc of Ringer lactate) versus only 20 IU intravenous oxytocin within 30 minutes after clamping umbilical cord. |
|
| Outcomes | The primary outcome: the fall of the haematocrit (difference between preoperative haematocrit value and the measured 48 hours after caesarean section). The secondary outcomes were the fall of the haemoglobin (difference between preoperative haemoglobin value and the measured 48 hours after caesarean section), blood loss estimated by direct method, the need for additional doses of oxytocin and the occurrence of adverse events related to administration of misoprostol. |
|
| Notes | AMTSL is not applicable as the study is limited to women with caesarean sections. Blood loss was estimated by the direct method (the sum of the volume of intraoperative blood draws in and the amount of blood found in gauze and surgical drapes). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed by computer and the result is marked on a notice placed in an opaque envelope and sealed. Just before surgery the responsible of the progress of the study will proceed to the opening of the envelope and thus the patient was assigned to one of two groups." |
| Allocation concealment (selection bias) | Low risk | Adequate. See above. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not clear on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or exclusion after randomisation were reported. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | Demographic, maternal and obstetric characteristics were similar in both groups. |
Turkey 2002.
| Methods | Randomisation based on computer‐generated random numbers. Sealed, consecutively‐numbered, opaque envelopes were used. Identical placebos were used except for the misoprostol tablets which were similar in size and colour but not in shape. There was blinding of outcome assessments. Midwives administered the misoprostol tablets, but residents that were blinded to the intervention, did the outcome assessments. | |
| Participants | 1633 women with vaginal births in Ankara, Turkey. Exclusion criteria: Gestational age < 32 weeks, caesarean delivery, hypersensitivity to prostaglandins. | |
| Interventions | Women randomised into 4 groups, all received corresponding placebos. Group 1: oxytocin 10 IU IV plus misoprostol 400 mcg rectally after cord clamp, followed by 2 doses 4 and 8 hours after delivery of 100 mcg misoprostol. Group 2: misoprostol 400 mcg rectally after cord clamp followed by 2 doses 4 hours apart of 100 mcg misoprostol. Group 3: oxytocin 10 IU IV. Group 4: oxytocin 10 IU IV plus 1 mL methylergometrine IM. | |
| Outcomes | Blood loss, transfusion, change in Hb, need for additional uterotonics, length of the third stage, subsequent evacuation of uterus, frequency of delayed haemorrhage, side‐effects. Clinical estimation of blood loss was done. | |
| Notes | Active management of third stage with early cord clamping, traction, and uterine massage. Concurrent study at this institution with similar design but evaluating oral misoprostol also published and is included in this meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "table of computer generated blocks of random numbers." |
| Allocation concealment (selection bias) | Low risk | "sealed, consecutively numbered opaque envelopes." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebos were used except for the misoprostol tablets which were similar in size and colour but not in shape. There was blinding of outcome assessments. Midwives administered the misoprostol tablets, but residents that were blinded to the intervention, did the outcome assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 27 exclusions after randomisation secondary to lack of Hb measurements. These were spread out among the 4 groups. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review have been reported. |
| Other bias | Low risk | There were no significant differences among the groups. |
Turkey 2003.
| Methods | Randomisation based on computer‐generated random numbers. Sealed, consecutively numbered, opaque envelopes were used. Identical placebos were used except for the misoprostol tablets which were similar in size and colour but not in shape. There was blinding of outcome assessments. Midwives administered the misoprostol tablets, but residents that were blinded to the intervention, did the outcome assessments. | |
| Participants | 1800 women with vaginal births in Ankara, Turkey. Exclusion criteria: Gestational age < 32 weeks, caesarean delivery, hypersensitivity to prostaglandins. | |
| Interventions | Women randomised into 4 groups, all received corresponding placebos. Group 1: oxytocin 10 IU IV plus misoprostol 400 mcg orally after cord clamp, followed by 2 doses 4 and 8 hours after delivery of 100 mcg misoprostol. Group 2: misoprostol 400 mcg orally after cord clamp followed by 2 doses 4 hours apart of 100 mcg misoprostol. Group 3: oxytocin 10 IU IV. Group 4: oxytocin 10 IU IV plus 1 mL methylergometrine IM. | |
| Outcomes | Blood loss, transfusion, change in Hb, need for additional uterotonics, length of the third stage, subsequent evacuation of uterus, frequency of delayed haemorrhage, side‐effects. Clinical estimation of blood loss was done. | |
| Notes | Active management of third stage with early cord clamping, traction, and uterine massage. 226 (12.6%) exclusions after randomisation secondary to lack of haemoglobin measurements. Concurrent study at this institution with similar design but evaluating oral misoprostol also published and is included in this meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐based random allocation." |
| Allocation concealment (selection bias) | Low risk | "sealed, consecutively numbered opaque envelopes." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebos were used except for the misoprostol tablets which were similar in size and colour but not in shape. There was blinding of outcome assessments. Midwives administered the misoprostol tablets, but residents that were blinded to the intervention, did the outcome assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 226 (12.6%) exclusions after randomisation secondary to having caesarean sections and lack of haemoglobin measurements. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review have been reported. |
| Other bias | Low risk | The groups were comparable. |
Turkey 2010.
| Methods | Randomised controlled study. | |
| Participants | 200 term singleton pregnancies undergoing spontaneous vaginal and elective caesarean section at the University Hospital in Eskisehir, Turkey. Exclusion criteria included known sensitivity to prostaglandins, excessive postpartum haemorrhage with haemodynamic instability that necessitated blood transfusion. Assisted vaginal delivery, use of epidural anaesthesia and cases with labour induction. |
|
| Interventions | Controlled release PGE2 vaginal insert (Dinoprostone) with a constant delivery of 0.3 mg/hr for 12 hours versus IV oxytocin infusion in balanced solution (10 IU for vaginal, 20 IU for caesarean delivery). | |
| Outcomes | Main outcome measures: amount of bleeding, need for additional oxytocins, haemoglobin and haematocrit level changes during the postpartum period and drug related side‐effects such as nausea, vomiting, shivering, pyrexia and diarrhoea and postpartum vaginal or endometrial infections. Some of these results have been reported by subgroup only (caesarean section, vaginal delivery). |
|
| Notes | Active management of third stage of labour for the vaginal births. In cases undergoing caesarean section, 20 IU intravenous oxytocin infusion was given after the delivery of the placenta. Estimated amount of postpartum blood was assessed via a gravimetric method by counting the blood‐filled pads within 24 hours of postpartum. Dry weight of the pads was assessed prior to delivery. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clear how the random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Not clear how the allocation concealment was done. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not clear on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or postrandomisation exclusions were reported. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported and some of them have only been reported within the subgroups (vaginal/caesarean section). |
| Other bias | Low risk | Demographic characteristics did not differ between the 2 groups. |
United Kingdom 1994.
| Methods | Method of random allocation not stated. Sealed opaque envelopes used for allocation concealment. Interventions prepared by someone not involved in the study, outside the intervention area (operating theatre). Outcome assessments were blinded. | |
| Participants | 60 low‐risk women undergoing elective caesarean section in an academic hospital in Oxford, UK. Exclusion criteria: hypertensive disease, asthma, heart disease. | |
| Interventions | Prostaglandin group: 15‐methyl prostaglandin F2alpha, 125 mcg intramyometrial + placebo. Oxytocin group: 5 IU oxytocin IV bolus injection followed by 15 IU in 500 mL of Ringer's lactate solution + placebo. Both interventions were started after delivery of the baby but before delivery of the placenta. | |
| Outcomes | Blood loss, use of additional uterotonics, blood transfusion, side‐effects, change in haemoglobin (subset of patients). Measurement of blood loss: clinical estimation. | |
| Notes | Management of third stage: not applicable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear if the sequence generation was adequate. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes used for allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. Outcome assessments were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up or postrandomisation exclusions reported. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | The groups were comparable. |
United Kingdom 2000.
| Methods | Random allocation by sealed, opaque, consecutively‐numbered envelopes. No blinding of outcome assessments. | |
| Participants | 1000 women delivering vaginally, in London, UK. Women with a history of asthma, planned caesarean section and water birth were excluded. | |
| Interventions | Misoprostol group: 500 mcg misoprostol orally after baby delivered and cord clamped. Uterotonic group: this group was given uterotonics at delivery of anterior shoulder. The choice of uterotonics varied according to the hospital policy for different groups of women. Women at high risk of haemorrhage received ergometrine (2%), those with hypertension received oxytocin (18%). All others received ergometrine‐oxytocin (80%). |
|
| Outcomes | Blood loss, side‐effects. Measurement of blood loss: clinical estimation by the midwives. | |
| Notes | Management of third stage: 'active': cord traction with signs of separation, oxytocics at anterior shoulder delivery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated block randomization." |
| Allocation concealment (selection bias) | Low risk | "opaque, sequentially numbered sealed envelopes." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention of postrandomisation exclusions or protocol violations. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review have been reported. |
| Other bias | Low risk | Both groups were comparable at entry to the trial. |
United Kingdom 2001b.
| Methods | Random allocation schedule generated by computer. Allocation made by opening sealed opaque envelopes which contained the names of the groups. No mention of consecutive numbering and opening. The obstetrician, surgical assistant, scrub nurse and recovery midwives were blinded to the group while anaesthetist was not. Double, non‐identical placebos were used. | |
| Participants | 40 women undergoing elective or emergency caesarean section in a university hospital in London, United Kingdom. Women with 2 or more caesarean sections or a history of previous ruptured uterus were excluded. Other eligibility criteria are not mentioned. | |
| Interventions | Misoprostol 500 mcg orally + 2 mL IV normal saline bolus vs 10 IU oxytocin bolus + 2 placebo tablets. | |
| Outcomes | Blood loss (clinical estimation), change in Hb levels, shivering (assessed in the recovery room), temperature within 1 hour. | |
| Notes | Management of third stage: 'active' during caesarean section. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Allocation made by opening sealed opaque envelopes which contained the names of the groups. No mention of consecutive numbering and opening. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The obstetrician, surgical assistant, scrub nurse and recovery midwives were blinded to the group while anaesthetist was not. Double, non‐identical placebos were used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals after caesarean section. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | The groups were comparable. |
United Kingdom 2001c.
| Methods | Open‐label, assessor‐blind, randomised controlled trial. | |
| Participants | Pregnant women over 16 years of age and delivering infants after 24 completed weeks of gestation at the Northwick Park Hospital, a district general hospital in the UK. Exclusion criteria included a known sensitivity to either prostaglandins, ergotrates or oxytocin, had a history of asthma, glaucoma, raised intraocular pressure or were known to have cardiac, pulmonary, renal or hepatic disease, hypertension, sepsis or obliterative vascular disorders. Women were excluded if they were currently taking anticoagulant treatment or participating in other clinical trials. |
|
| Interventions | 1 mL IM (250 mcg) of carboprost versus 1 mL of syntometrine (0.5 mg of ergometrine plus 5 units of syntocinon) intramuscularly at the time of presentation of the anterior shoulder. | |
| Outcomes | Main outcome measure was the occurrence of primary postpartum haemorrhage (500‐1000 mL). Other measures include maternal haemoglobin and haematocrit measured 3 days later, mode and timing of delivery of the placenta and maternal blood pressure after delivery of the placenta and 1 hour later. side‐effects (diarrhoea, nausea, vomiting) were also measured. | |
| Notes | Active management of third stage of labour. Blood loss was measured as accurately as possible, taking into consideration the liquor amnii and soiling of the surgical drapes. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clear on how the random sequence was generated. |
| Allocation concealment (selection bias) | Low risk | "The randomization slips were contained in envelopes which were opened by a person not involved in the postpartum assessments who resealed the envelope and drew 1 ml of the appropriate medication into a syringe." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The study is open‐labelled, however, the outcome assessments are blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 woman in the carboprost group was excluded from the analysis because she did not receive the medication. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review have been reported. |
| Other bias | High risk | This study has been suspended during the interim analysis due to higher percentage of GI adverse events, especially diarrhoea seen in the Carboprost group. The groups were comparable at the baseline. |
United Kingdom 2003.
| Methods | Random allocation prepared by independent statistician using computer‐generated random numbers with blocked randomisation. Sequentially numbered, sealed, opaque, envelopes used. No placebos used. No blinding of outcome assessments. | |
| Participants | 275 women with vaginal delivery in London, UK. Exclusion criteria: < 37 weeks' gestation, < 18 yrs old, multiple gestation, induced labour, asthma, cardiac, renal or hepatic disorder. Study was reported in conjunction with a misoprostol pharmacokinetics trial. | |
| Interventions | Misoprostol 600 mcg orally vs 600 mcg rectally vs 400 mcg rectally. | |
| Outcomes | Side‐effects, clinical estimation of blood loss, duration of third stage, manual removal of placenta. | |
| Notes | "Usual" management of third stage with cord traction. Blood loss estimated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated random numbers." |
| Allocation concealment (selection bias) | Low risk | "sequentially numbered, sealed, opaque envelopes." |
| Blinding (performance bias and detection bias) All outcomes | High risk | The study was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up or postrandomisation exclusions. |
| Selective reporting (reporting bias) | Low risk | Not all of the primary outcomes of the review were reported, however, this study was conducted to analyze the side‐effects of different doses. |
| Other bias | Low risk | There was a borderline statistically significant difference between the 2 groups with regard to the maximum plasma concentration. Otherwise the groups were comparable. |
USA 1990.
| Methods | Method of random allocation not stated. Double‐blinded trial. | |
| Participants | 46 women at low risk for postpartum haemorrhage undergoing delivery by caesarean section in Arkansas, USA. Exclusion criteria: hypertension, asthma, pre‐eclampsia, chorioamnionitis, multiple gestation or were receiving tocolytic agents. | |
| Interventions | Carboprost tromethamine* 0.125 mg intramyometrial vs oxytocin 20 IU intramyometrial. Both groups received 20 IU of oxytocin in 1 litre saline after delivery. | |
| Outcomes | Haematocrit change after delivery, blood loss not measured. | |
| Notes | Management of third stage: not applicable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear on how the adequate sequence generation was done. |
| Allocation concealment (selection bias) | Low risk | Adequate. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | The 2 groups were comparable. |
USA 2001.
| Methods | Random allocation sequence concealed until enrolment. Packs containing both active and placebo were made available after random allocation. It is not clear if the placebos are identical. No mention of blind outcome assessments. | |
| Participants | 400 women in active labour or undergoing induction of labour in Los Angeles, USA were enrolled. Women with multiple gestation, known coagulation disorders, contraindication to prostaglandin or oxytocin use, known initial haemoglobin below 7.0 mg/dL and an indication for caesarean section were excluded. | |
| Interventions | Misoprostol 400 mcg rectally + placebo (2 mL saline) vs oxytocin 20 IU + placebo (lactose tablets). Oxytocin (and its placebo) was administered as IV infusion in 1 L of Ringer's lactate solution. | |
| Outcomes | Blood loss (estimated and measured by weighing linen etc.), haematocrit, side‐effects. | |
| Notes | Management of the third stage not mentioned. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number sequence." |
| Allocation concealment (selection bias) | Unclear risk | Unclear on how the allocation concealment was done. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear if the blinding was not broken. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions after randomisation: 75/400 (18.75%), 73 had caesarean section during labour, 1 had Hb < 7.0 mg/dL and 1 was discharged home before delivery. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | 2 groups were comparable. |
USA 2004.
| Methods | Random allocation sequence generated by using a table of random numbers. Active and placebo (similar but not identical) were placed in opaque, numbered vials. Power calculation was made. Outcome assessments were not blinded. | |
| Participants | 756 women with anticipated vaginal delivery at a maternity hospital in Florida, USA. | |
| Interventions | Misoprostol 200 mcg buccal vs placebo. All women received intravenous infusion of 20 IU oxytocin in 1 litre of saline at 10 mL/min for 30 minutes (i.e. received approximately 6 IU oxytocin IV). | |
| Outcomes | Blood loss, haemoglobin measurements, side‐effects. Blood loss was estimated by the attending physician. |
|
| Notes | Management of the third stage: active management with early cord clamping. controlled cord traction and oxytocin after delivery of the placenta. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number table." |
| Allocation concealment (selection bias) | Unclear risk | Not clear on how the allocation concealment was done. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo‐controlled. It should be noted that the outcome assessors are not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 756/848 eligible women were randomised. 15 patients in the misoprostol group and 19 patients in the placebo group were excluded due to having caesarean section, refusing to participate and nursing or physician error. Analysis was intention to treat. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the primary outcomes of the review were reported. |
| Other bias | Low risk | There were no differences between the groups. |
USA 2005.
| Methods | Randomised, placebo‐controlled. No mention of random‐number generation scheme. Allocation concealment by pharmacy‐assigned numbers to opaque vials containing either misoprostol tablets or oxytocin ampoules. Outcome assessments were blinded. | |
| Participants | 352 women undergoing caesarean section in Orlando, Florida, USA. | |
| Interventions | Misoprostol 200 mcg buccal vs placebo at cord clamping. All women received 20 IU IV oxytocin in 1000 mL saline. | |
| Outcomes | Blood loss, additional uterotonics. Blood loss was estimated following 'standard' procedures. |
|
| Notes | No loss to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of random‐number generation scheme. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment by pharmacy‐assigned numbers to opaque vials containing either misoprostol tablets or oxytocin ampoules. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "At no time before the data analysis were the assignments available to anyone but the pharmacy." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up was reported. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review have been reported. |
| Other bias | Low risk | 2 groups were comparable. |
WHO 1999.
| Methods | Random allocation sequence, generated centrally. Sealed and numbered identical treatment packs taken consecutively from a dispenser. Double‐blinded, placebo controlled pilot trial. | |
| Participants | 597 women after delivery in Khon Kaen, Thailand and Johannesburg, South Africa. Risk status not stated. Exclusion criteria: asthma, other severe chronic allergic condition, if delivery considered an abortion, planned caesarean section, not willing or able to give informed consent. |
|
| Interventions | Misoprostol 600 mcg orally vs misoprostol 400 mcg orally vs oxytocin 10 IU IV. | |
| Outcomes | Shivering, pyrexia, side‐effects, blood loss from delivery to transferal of mother to postnatal care. Measurement of blood loss: collected blood poured in standard measuring jar. Linen not weighed. Small gauze swabs soaked with blood put into measuring jar and included in measurement. |
|
| Notes | Management of third stage: uterotonics, clamping and cutting of cord immediately after delivery, fundal or suprapubic pressure with cord traction after signs of placental separation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation sequence, generated centrally. |
| Allocation concealment (selection bias) | Low risk | Sealed and numbered identical treatment packs taken consecutively from a dispenser. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinded using double placebos. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion after randomisation: 8 women in the oxytocin group did not comply with treatment (6 had an emergency caesarean section, 1 was HIV positive and mistakenly excluded, 1 whose ampoule was not located). 1 woman in the 600 mcg group was excluded because her tablets could not be located, and 1 woman in the 400 mcg group was excluded because of an emergency caesarean section. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review were reported. |
| Other bias | Low risk | The groups were comparable at the trial entry and at delivery except for low birthweight, which was higher in the misoprostol 600 mch group. |
WHO 2001.
| Methods | Random allocation sequence, generated centrally. Sequentially‐numbered, identical treatment packs drawn from a treatment pack dispenser. Double blinding achieved by use of double placebos. | |
| Participants | 18,530 women expecting vaginal delivery in 9 countries. Countries were Argentina, China, Egypt, Ireland, Nigeria, South Africa, Switzerland, Thailand, and Vietnam. Exclusion criteria: pyrexia (> 38 degrees C) on admission to labour ward, severe asthma, bleeding disorders, elective caesarean section, no consent. | |
| Interventions | Misoprostol 600 mcg orally + placebo IV/IM, vs oxytocin 10 IU IV/IM + placebo tablets. | |
| Outcomes | Blood loss, shivering, pyrexia, nausea, vomiting, diarrhoea, need for transfusion, manual removal of placenta, exploration under general anaesthesia, hysterectomy, admission to ICU, maternal deaths. Measurement of blood loss: collected blood poured in standard measuring jar. Small gauze swabs soaked with blood put into measuring jar and included in measurement. Linen weighed in some centres. | |
| Notes | Management of third stage: uterotonics, clamping and cutting of cord immediately after delivery, fundal or suprapubic pressure with cord traction after signs of placental separation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation sequence, generated centrally. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, identical treatment packs drawn from a treatment pack dispenser. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinding achieved by use of double placebos. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 50/9264 (0.54%) excluded after randomisation in the misoprostol group, 37 because of an emergency caesarean section, and 13 for loss to follow‐up. 38/9226 (0.41%) excluded after randomisation in the oxytocin group, 34 for emergency caesarean section and 4 lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review were reported. |
| Other bias | Low risk | The 2 groups were comparable. |
Zimbabwe 2001.
| Methods | Random allocation sequence generated by computer, allocation by numbered, sealed, opaque envelopes. Placebos used but were not identical. It is not mentioned whether outcome assessments were blinded or not. | |
| Participants | 500 low‐risk women delivering at Harare Maternity Hospital, Zimbabwe were included. Women with a history of postpartum haemorrhage, disseminated intravascular coagulation, antepartum haemorrhage, coagulation disorders, operative delivery, multiple pregnancy, history of asthma and known allergies to misoprostol or oxytocin were excluded. | |
| Interventions | Misoprostol 400 mcg orally + 1 mL saline (placebo) vs oxytocin 10 IU IM + 2 placebo tablets. | |
| Outcomes | Blood loss, side‐effects. Measurement of blood loss: blood volume in jug + weighing of soiled linen. | |
| Notes | Management of the third stage not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation sequence generated by computer. |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed, opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear if the blinding was protected throughout the study or if the outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions after randomisation: 1 women excluded because of undiagnosed twin delivery. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of the review were reported. |
| Other bias | Low risk | The groups were comparable. |
* (15(S) 15 methyl PGF2alpha) ** Synthetic PGE2 derivative (16‐phenoxy‐17,18,19,20‐tetranor‐PGE2‐methylsulphonamide) ANM: auxiliary nurse midwives BP: blood pressure GI: gastrointestinal Hb: haemoglobin Hct: haematocrit ICU: intensive care unit IM: intramuscular(ly) IU: international unit(s) IUGR: intrauterine growth restriction IV: intravenous(ly) MBL: measured blood loss PCV: packed cell volume PPH: postpartum haemorrhage vs: versus wks: weeks
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Australia 2009 | This study was comparing 3 different regimens for third stage management after second‐trimester intravaginal misoprostol termination. |
| Austria 1983 | No clinically relevant outcomes reported. Healthy women delivering at term who had a normal duration of labour (< 12 hours) and without the use of oxytocics before delivery were recruited. Immediately following the separation of the placenta, a twin catheter was introduced into the cavity for intrauterine pressure measurement which was recorded on the cardiotocograph. The women were randomised to receive methergin (methylergometrine) 0.2 mg, or oxytocin 2 IU, or sulprostone 0.5 mg or saline, all administered intramuscularly. Sulprostone had the quickest onset of action and strongest increase in uterine contractility whereas methergin had the longest duration of action on uterine contractility. |
| Canada 2004 | Not a randomised controlled trial. A nested study within a randomised controlled trial to look at peripheral blood flow and temperature changes in women receiving misoprostol or oxytocin. |
| China 1997 | This trial was reported as randomised but no details of the method of randomisation were given. The 2 study groups were not balanced (260 versus 100), and they were further randomised into subgroups. |
| China 1998 | Randomised controlled trial of misoprostol versus oxytocin in caesarean section births only. Data are not presented in a form that can be extracted for the meta‐analysis. |
| China 1998b | This trial randomised 80 women to 1 mg carboprost methylate intravaginally versus sublingually vs ergometrine IV. The data were not in a form suitable for extraction for this meta‐analysis. |
| China 2000 | This trial randomised 102 women to receive 200 mcg rectal misoprostol versus Pitocin, however, there were not enough information in the article for the assessment of the methodology. |
| China 2001 | This trial randomised 348 women into 4 groups of misoprostol 200, 400, and 600 micrograms orally, and oxytocin 20 units intramuscularly. Data were presented only in means, and were not presented in a form suitable for extraction and inclusion in this meta‐analysis. |
| China 2002 | This study only includes women with pregnancy‐induced hypertension syndrome. |
| China 2003c | This study included only women with failure of induction and was excluded because there was very little information on the methodology and the outcomes were not reported properly. |
| China 2003d | This study was excluded because there was no information provided on the paper in regards to the methodology of the study (randomisation, allocation concealment, blinding, etc). |
| China 2004b | Randomised, double blind trial of 298 low‐risk women delivering vaginally in Hong Kong, China. Oral misoprostol vs IV oxytocin. The trial is excluded because the number of women in each group are not described and the report is available as an abstract. The authors have not responded to the request for additional information and clarification. There was no statistically significant difference in blood loss > 500 and 1000 mL. Additional oxytocics were used in 25.2 vs 7.5% in the misoprostol and oxytocin groups respectively. |
| China 2004c | Data are not in a usable format. Randomised controlled trial comparing misoprostol 400 mcg + syntometrine vs syntometrine. The author contacted but no response. |
| Egypt 1999 | 140 women were allocated to receive either 2 different doses of rectal misoprostol or 5 units of oxytocin and 0.2 mg ergometrine intramuscularly. There is no indication of any randomised comparison between the groups. |
| Hungary 1979 | The reason for exclusion is that the data are not presented in a usable form. The study is a randomised comparison of 1 mg intramyometrial prostaglandin F2alpha (47 women), 0.2 mg intravenous ergometrine (50) and no treatment (43). Prostaglandin F2alpha reduced the blood loss in the third stage of labour significantly when compared with ergometrine and no treatment. |
| India 1988a | 60 women were allocated to 125 microgram PGF2alpha intramuscularly or no uterotonic. There is no indication of any randomised comparison between the 2 groups. |
| India 1988b | Multicentre study carried out in 4 centres. Of these, 2 employed a random allocation scheme and 2 used a sequential scheme. The reason for exclusion is that the results are presented together and it is not possible to extract data for those utilising random allocation. |
| India 2000a | There are no data that can be extracted to evaluate the validity of the methods used and the outcome data in this study from the conference abstract. When the study is published in full it will be evaluated again. |
| India 2000b | There are no data that can be extracted to evaluate the validity of the methods used and the outcome data in this study from the conference abstract. When the study is published in full it will be evaluated again. |
| India 2000c | There are no data that can be extracted to evaluate the validity of the methods used and the outcome data in this study from the conference abstract. When the study is published in full it will be evaluated again. |
| India 2001a | This study is reported as randomised double blind but there is no mention of placebos. There is also a discrepancy in the results between the text and the tables. 200 women were assigned either misoprostol orally 400 mcg or methylergometrine. |
| India 2005b | The study is reported as a randomised controlled trial comparing carboprost with methylergometrine but the results are analyzed by risk subgroups only and they are imbalanced between the 2 random allocation groups. |
| India 2006e | This is a randomised trial (cluster) of an educational intervention to implement active management of the third stage of labour using misoprostol. The control group received standard practice which was 'no special training' and no use of misoprostol. |
| India 2006h | This study did not have sufficient information on the risk of bias and exclusion criteria. We have contacted the authors but have not received any response. |
| India 2009a | The study was reported in an abstract with not enough information on the methodology and outcomes. No contact information provided for the authors. |
| India 2009c | This study had a very high risk of bias on evaluation, therefore we have excluded the study. |
| Indonesia 2002 | Data to evaluate the validity of the methods used are not available in this published abstract. When the study is published in full it will be evaluated again. This study involves 196 women undergoing full term vaginal delivery. 98 women were randomly allocated to 600 micrograms of oral misoprostol or 10 IU of oxytocin intramuscularly immediately after the baby was born. The length of the third stage of labour was 8.122 minutes for the misoprostol group and 8.388 minutes for the oxytocin group. Third stage blood loss for the misoprostol and oxytocin group was respectively 144.286 mL and 131.020 mL. Shivering occurred in 13.3% in the misoprostol group and 2.0% in the oxytocin group. |
| Israel 1992 | This is a randomised controlled trial comparing intraumbilical PGF2alpha with saline injection. Although a prostaglandin was used for the management of the third stage of labour the mechanism of action may not be comparable to other routes of administration. This paper will be considered for inclusion in another review on the management of the third stage (intraumbilical uterotonics). |
| Italy 1988 | Data from this trial were published in an abstract. It is excluded because no full publication of the trial data could be located. |
| Japan 1976 | There does not seem to be a randomised comparison between study groups. 4 prostaglandin groups were studied: a. systemic: a.1. intramuscular (gluteal), a.2. continuous intravenous drip infusion, b. local: b.1. transabdominal intramyometrial injection, b.2. transvaginal intramyometrial injection. These groups were compared to ergot alkaloids. Number of participants are also not balanced (46 in prostaglandin vs 13 in ergot group). |
| Korea 2007 | The study was reported in an abstract with not enough information on the methodology and outcomes. No contact information provided for the authors. |
| Singapore 1990 | The outcome examined in this trial was serum prostaglandin levels. |
| Singapore 2001 | This trial has 57 women randomly assigned to receive oral misoprostol 200, 400, 500, 600, or 800 micrograms or ergometrine‐oxytocin. Uterine activity was the main outcome, but side‐effects were also reported. The data are incomplete and not in a suitable form for extraction. |
| South Africa 1999 | Data from this trial were published in an abstract. It is excluded because no further publication of complete trial data was located. This trial evaluates treatment of primary postpartum haemorrhage. |
| Thailand 2000 | The study was reported in an abstract with not enough information on the methodology and outcomes. No contact information provided for the authors. |
| Turkey 2005 | Randomised, placebo‐controlled trial comparing 400 mcg rectal vs 400 mcg vaginal misoprostol vs placebo after delivery of the placenta. Women with haemorrhage were excluded from the analysis after randomisation. Authors contacted for clarification. |
| United Kingdom 2001a | Randomised controlled trial of 400 mcg oral misoprostol versus 10 IU IV oxytocin. Primary outcome was 'intraoperative blood loss', which is not 1 of the outcomes for this review. |
| USA 1983 | 75 women were randomised to 3e groups of different doses of prostaglandin F2alpha (62.5, 125, 250 microgram intramuscularly). Then another 15 women were sequentially allocated to the same treatment groups, in groups of 5. The randomised and non‐randomised groups have been reported together in the paper to increase the sample size. It is not possible to extract data on the randomised women alone. |
| USA 1999 | Data from this trial were published in an abstract. It is excluded because no further publication of the completed trial data was located and the data presented in the abstract are incomplete. |
| Yemen 2009 | The study is not a randomised controlled trial. It used a "convenience sample" which was then divided into 2 groups by a "quasi‐random" allocation. |
IU: international unit IV: intravenous vs: versus
Characteristics of ongoing studies [ordered by study ID]
Kalahroudi 2010.
| Trial name or title | Comparison of the effect of rectal misoprostol and syntometrine in prevention of postpartum haemorrhage. |
| Methods | Randomised double blind clinical trial to investigate the effect of rectal misoprostol and syntometrine in prevention of postpartum haemorrhage. |
| Participants | 200 pregnant women referred to the Shabihkhany Hospital in Kashan for vaginal delivery. |
| Interventions | The first group received 1 mL syntometrine IM and the second group received 600 mgr misoprostol rectal after placental expulsion. |
| Outcomes | All patients were assessed 0.5 and 1 hour after delivery for uterine tonicity, pulse rate and blood pressure. 24 hours after delivery haemoglobin was checked for estimation of postpartum haemorrhage. Need for uterotonic drugs and rate of adverse effects were also compared between the intervention groups. |
| Starting date | 2010‐04‐21 |
| Contact information | Masoumeh Abedzadeh Kalahroudi Midwifery Faculty, Kashan University of Medical Sciences, Ravand Road, kashan Isfahan Iran, Islamic Republic Of 00983615550021 abedzadeh@kaums.ac.ir |
| Notes | As of June 14, 2011 recruitment has been completed. |
Moradi 2010.
| Trial name or title | Comparison of misoprostol and oxytocin in reduction of postpartum haemorrhage. |
| Methods | A randomised controlled trial (not blinded). |
| Participants | 300 pregnant women with these inclusion criteria: pregnant women with singleton pregnancy, cephalic, spontaneous and induced delivery, term pregnancy. |
| Interventions | To compare the effect of 400 µg of oral misoprostol with 10 IU of intravenous oxytocin in preventing postpartum haemorrhage. |
| Outcomes | Haemoglobin and haematocrit were measured in admission and 24 hours after delivery in order to compare drugs in reduction of postpartum haemorrhage. |
| Starting date | 2009‐12‐22 |
| Contact information | Simindokht Moradi Kosar Hospital Qazvin Iran, Islamic Republic Of 00982813666770 simindokht56@yahoo.com |
| Notes | As of June 14, 2011 recruitment has been complete. |
IM: intramuscular
Contributions of authors
Özge Tunçalp prepared the 2011 update, including completing the 'Risk of bias' tables for the studies included in earlier versions of the review. All other review authors contributed by assisting with the data extraction, analysis advice, writing and final approval of the text. Özge Tunçalp is the guarantor of the review.
Sources of support
Internal sources
UK Cochrane Centre, NHS R&D Programme, Oxford, UK.
HRP‐UNDP/UNFPA/WHO/World Bank Special Programme in Human Reproduction, Geneva, Switzerland.
Duke University School of Medicine, North Carolina, USA.
Department of Maternal and Child Health, University of North Carolina‐Chapel Hill, USA.
Effective Care Research Unit, University of the Witwatersrand, East London/Johannesburg, South Africa.
Department of Obstetrics and Gynecology, Emory University, Atlanta, USA.
External sources
No sources of support supplied
Declarations of interest
Two review authors (AM Gülmezoglu, GJ Hofmeyr) participated in the WHO 1999 and WHO 2001 trials and one review author (GJ Hofmeyr) participated in Africa 2011; South Africa 1998a; South Africa 1998b; South Africa 1998c; South Africa 1998d. GJ Hofmeyr did not assess the trials or extract the data for the trials in which he was involved. AM Gülmezoglu extracted the data but these were independently checked by Fatu Forna, an author on the earlier version of the review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Africa 2011 {published data only}
- Hofmeyr GJ. Misoprostol for preventing postpartum hemorrhage. ClinicalTrials.gov (www.clinicaltrials.gov) (accessed 20 February 2008).
- Hofmeyr GJ, Fawole B, Mugerwa K, Godi NP, Blignaut Q, Mangesi L, et al. Administration of 400mug of misoprostol to augment routine active management of the third stage of labor. International Journal of Gynecology & Obstetrics 2011;112(2):98‐102. [DOI] [PubMed] [Google Scholar]
Australia 1999 {published data only}
- Cook C, Spurrett B, Murray H. A randomized clinical trial comparing oral misoprostol with synthetic oxytocin or syntometrine in the third stage of labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 1999;39(4):414‐9. [DOI] [PubMed] [Google Scholar]
Bangladesh 2007 {published data only}
- Sultana N, Khatun M. Misoprostol versus oxytocin in the active management of the third stage of labour. Journal of Bangladesh College of Physicians and Surgeons 2007;25(2):73‐6. [Google Scholar]
Belgium 1999 {published data only}
- Amant F, Spitz B, Timmerman D, Corremans A, Assche FA. Misoprostol compared with methylergometrine for the prevention of postpartum haemorrhage: a double‐blind randomised trial. British Journal of Obstetrics and Gynaecology 1999;106:1066‐70. [DOI] [PubMed] [Google Scholar]
Canada 2002 {published data only}
- Karkanis S, Caloia D, Salenieks M, Kingdom J, Walker M, Meffe F, et al. Randomized controlled trial of rectal misoprostol versus oxytocin in third stage management. Journal of Obstetrics & Gynaecology of Canada: JOGC 2002;24(2):149‐54. [DOI] [PubMed] [Google Scholar]
Canada 2005 {published and unpublished data}
- Baskett TF, Persad V, Clough H, Young D. Prophylactic use of misoprostol in the third stage of labor [abstract]. Obstetrics & Gynecology 2005;105(4 Suppl):39S. [Google Scholar]
- Baskett TF, Persad VL, Clough HJ, Young DC. Misoprostol versus oxytocin for the reduction of postpartum blood loss. International Journal of Gynecology & Obstetrics 2007;97(1):2‐5. [DOI] [PubMed] [Google Scholar]
China 2003a {published data only}
- Fu YX, Ran KQ, Wang M. Prevention of early postpartum hemorrhage by way of oral misoprostol. Journal of Nursing Science 2003;18(12):910‐1. [Google Scholar]
China 2003b {published data only}
- Li D, Bei HZ. Clinical study on reduction of postpartum bleeding in the risk factors by misoprostol. Hainan Medical Journal 2003;14(11):11‐2. [Google Scholar]
China 2004a {published data only}
- Lam H, Tang OS, Lee CP, Ho PC. A pilot‐randomized comparison of sublingual misoprostol with syntometrine on the blood loss in third stage of labor. Acta Obstetricia et Gynecologica Scandinavica 2004;83:647‐50. [DOI] [PubMed] [Google Scholar]
China 2007 {published data only}
- Ng PS, Lai CY, Sahota DS, Yuen PM. A double‐blind randomized controlled trial of oral misoprostol and intramuscular syntometrine in the management of the third stage of labor. Gynecologic and Obstetric Investigation 2007;63(1):55‐60. [DOI] [PubMed] [Google Scholar]
Colombia 2002 {published data only}
- Penaranda W, Arrieta O, Yances B. Active management of the childbirth with sublingual misoprostol: a clinical controlled trial in the Hospital de Maternidad Rafael Calvo [Manejo activo del alumbramiento con misoprostol sublingual: un estudio clinico controlado en el Hospital de Maternidad Rafael Calvo de Cartagena]. Revista Colombiana de Obstetricia y Ginecologia 2002;53(1):87‐92. [Google Scholar]
Egypt 1993 {published data only}
- Abdel‐Aleem H, Abol‐Oyoun EM, Moustafa SAM, Kamel HS, Abdel‐Wahab HA. Carboprost trometamol in the management of the third stage of labor. International Journal of Gynecology & Obstetrics 1993;42:247‐50. [DOI] [PubMed] [Google Scholar]
Egypt 1997 {unpublished data only}
- Abdel‐Aleem H, Mostafa SAM, Makarem MH, Abol‐Oyoun EM, Shoukry M. Management of the third stage of labour with carboprost trometamol in high risk patients for postpartum hemorrhage. Assiut Medical Journal 1994;18:15. [Google Scholar]
- Abdel‐Aleem H, Mostafa SAM, Makarem MH, Abol‐Oyoun EM, Shoukry M. Management of the third stage of labour with carboprost trometamol in high risk patients for postpartum hemorrhage. The First World Congress on Maternal Mortality; 1997 March 8‐14; Morocco. 1997.
Egypt 2009 {published data only}
- Nasr A, Shahin AY, Elsamman AM, Zakherah MS, Shaaban OM. Rectal misoprostol versus intravenous oxytocin for prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2009;105(3):244‐7. [DOI] [PubMed] [Google Scholar]
France 2001 {published data only}
- Benchimol M, Gondry J, Mention J, Gagneur O, Boulanger J. Role of misoprostol in controlled delivery [Place du misoprostol dans la direction de la delivrance]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 2001;30(6):576‐83. [PubMed] [Google Scholar]
Gambia 2005 {published data only}
- Walraven G, Blum J, Dampha Y, Sowe M, Morison L, Winikoff B, et al. Misoprostol in the management of the third stage of labour in the home delivery setting in rural Gambia: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2005;112(9):1277‐83. [DOI] [PubMed] [Google Scholar]
Ghana 2000 {published data only}
- Walley RL, Wilson JB, Crane JM, Matthews K, Sawyer E, Hutchens D. A double‐blind placebo controlled randomised trial of misoprostol and oxytocin in the management of the third stage of labour. BJOG: an international journal of obstetrics and gynaecology 2000;107(9):1111‐5. [DOI] [PubMed] [Google Scholar]
Ghana 2006 {published data only}
- Parsons S, Ntumy YM, Walley RL, Wilson JB, Crane JMG, Matthews K, et al. Rectal misoprostol vs intramuscular oxytocin in the routine management of the third stage of labour. 30th British Congress of Obstetrics and Gynaecology; 2004 July 7‐9 Glasgow, UK. 2004:18.
- Parsons S, Walley RL, Crane JMG, Matthews K, Hutchens D. Oral misoprostol versus oxytocin in the management of the third stage of labour. Journal of Obstetrics & Gynaecology of Canada: JOGC 2006;28:20‐6. [DOI] [PubMed] [Google Scholar]
Ghana 2007 {published data only}
- Parsons SM, Walley RL, Crane JM, Matthews K, Hutchens D. Rectal misoprostol versus oxytocin in the management of the third stage of labour. Journal of Obstetrics and Gynaecology Canada 2007;29(9):711‐8. [DOI] [PubMed] [Google Scholar]
Guinea‐Bissau 2005 {published data only}
- Hoj L, Cardoso P, Nielsen BB, Hvidman L, Nielsen J, Aaby P. Effect of sublingual misoprostol on severe postpartum haemorrhage in a primary health centre in Guinea‐Bissau: randomised double blind clinical trial. BMJ 2005;331(7519):723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen BB, Hoj L, Hvidman LE, Nielsen J, Cardoso P, Aaby P. Reduced post‐partum bleeding after treatment with sublingual misoprostol: a randomized double‐blind clinical study in a developing country ‐ secondary publication. Ugeskrift for Laeger 2006;168(13):1341‐3. [PubMed] [Google Scholar]
Holland 1991 {published data only}
- Poeschmann RP, Doesburg WH, Eskes TKAB. A randomized comparison of oxytocin, sulprostone and placebo in the management of the third stage of labour. British Journal of Obstetrics and Gynaecology 1991;98:528‐30. [DOI] [PubMed] [Google Scholar]
- Poeschmann RP, Eskes TKAB, Doesburg WH. Oxytocin and sulprostone reduce postpartum blood los and shorten the third stage in low risk term women. Proceedings of 13th World Congress of Gynaecology and Obstetrics (FIGO); 1991 Sept 15‐20; Singapore. 1991:312.
- Poeschmann RP, Eskes TKAB, Doesburg WH, Lemmens WAJG, Benneker JCLH. Oxytocin and sulprostone reduce postpartum blood loss in low risk term women compared to saline. Proceedings of the 1st European Congress on Prostaglandins in Reproduction (ECPR); 1988 July 6‐9; Vienna, Austria. 1988:176.
Holland 1995 {published data only}
- Selm M, Kanhai HHH, Keirse MJNC. Preventing the recurrence of atonic postpartum hemorrhage: a double blind trial. Acta Obstetricia et Gynecologica Scandinavica 1995;74:270‐4. [DOI] [PubMed] [Google Scholar]
Hong Kong 2001 {published data only}
- Ng PS, Chan ASM, Sin WK, Tang LCH, Cheung KB, Yuen PM. A multicentre randomized controlled trial of oral misoprostol and i.m. syntometrine in the management of the third stage of labour. Human Reproduction 2001;16(1):31‐5. [DOI] [PubMed] [Google Scholar]
- Ng PS, Chan ASM, Sin WK, Tang LCH, Cheung KB, Yuen PM. Comparison of oral misoprostol and intramuscular syntometrine in the management of the third stage of labor a multicenter randomised controlled trial. XVI FIGO World Congress of Obstetrics and Gynecology. Book 4; 2000 Sept 3‐8; Washington DC, USA. 2000:29.
India 1988c {published data only}
- Bhattacharya P, Devi PK, Jain S, Kanthamani CR, Raghavan KS. Prophylactic use of 15(S)15 methyl PGF2alpha by intramuscular route for control of postpartum bleeding ‐ a comparative trial with methylergometrine. Acta Obstetricia et Gynecologica Scandinavica Supplement 1988;145:13‐5. [DOI] [PubMed] [Google Scholar]
India 2001b {published data only}
- Reddy R, Shenoy JV. Active management of third stage of labour. A comparative study in high risk patients for atonic postpartum haemorrhage. Journal of Obstetrics and Gynecology of India 2001;51(2):44‐7. [Google Scholar]
India 2004b {published data only}
- Vimala N, Mittal S, Kumar S, Dadhwal V, Mehta S. Sublingual misoprostol versus methlergometrine for active management of third stage of labor. International Journal of Gynecology & Obstetrics 2004;87:1‐5. [DOI] [PubMed] [Google Scholar]
India 2005a {published data only}
- Garg P, Batra S, Gandhi G. Oral misoprostol versus injectable methyergometrine in management of the third stage of labour. International Journal of Gynecology & Obstetrics 2005;91:160‐1. [DOI] [PubMed] [Google Scholar]
India 2006a {published data only}
- Vimala N, Mittal S, Kumar S. Sublingual misoprostol versus oxytocin infusion to reduce blood loss at cesarean section. International Journal of Gynecology & Obstetrics 2006;92:106‐10. [DOI] [PubMed] [Google Scholar]
India 2006b {published data only}
- Zachariah ES, Naidu M, Seshadri L. Oral misoprostol in the third stage of labour. International Journal of Gynecology & Obstetrics 2006;92:23‐6. [DOI] [PubMed] [Google Scholar]
India 2006c {published and unpublished data}
- Derman RJ, Kodkany BS, Goudar SS, Geller SE, Naik VA, Bellad MB, et al. Oral misoprostol in preventing postpartum hemorrhage in a community setting. International Conference. Evidence‐based interventions to prevent postpartum haemorrhage: translating research into practice; 2006 July 13‐14; Goa, India. 2006.
- Derman RJ, Kodkany BS, Goudar SS, Geller SE, Naik VA, Bellad MB, et al. Oral misoprostol in preventing postpartum hemorrhage in a community setting. Lancet 2006;368(9543):1248‐53. [DOI] [PubMed] [Google Scholar]
- Geller SE, Goudar SS, Adams MG, Naik VA, Patel A, Bellad MB, et al. Factors associated with acute postpartum hemorrhage in low‐risk women delivering in rural India. International Journal of Gynecology & Obstetrics 2008;101(1):94‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller SE, Patel A, Niak VA, Goudar SS, Edlavitch SA, Kodkany BS, et al. Conducting international collaborative research in developing nations. International Journal of Gynecology & Obstetrics 2004;87(3):267‐71. [DOI] [PubMed] [Google Scholar]
- Goudar SS, Chakraborty H, Edlavitch SA, Naik VA, Bellad MB, Patted SS, et al. Variation in the postpartum hemorrhage rate in a clinical trial of oral misoprostol. Journal of Maternal‐Fetal & Neonatal Medicine 2008;21(8):559‐64. [DOI] [PubMed] [Google Scholar]
- Kodkany BS, Derman RJ, Goudar SS, Geller SE, Edlavitch SA, Naik VA, et al. Initiating a novel therapy in preventing postpartum hemorrhage in rural India: a joint collaboration between the United States and India. International Journal of Fertility & Womens Medicine 2004;49(2):91‐6. [PubMed] [Google Scholar]
- Kodkany BS, Goudar SS, Derman RJ. The efficacy of oral misoprostol in preventing postpartum hemorrhage in a community setting: a randomized double‐blind placebo‐controlled trial. International Journal of Gynecology & Obstetrics 2006;94(Suppl 2):S141‐S142. [DOI] [PubMed] [Google Scholar]
- Moss NM. Oral misoprostol for prevention of postpartum hemorrhage. www.clinicaltrials.gov (accessed 21 March 2006).
- Patted SS, Goudar SS, Naik VA, Bellad MB, Edlavitch SA, Kodkany BS, et al. Side effects of oral misoprostol for the prevention of postpartum hemorrhage: results of a community‐based randomised controlled trial in rural India. Journal of Maternal‐Fetal & Neonatal Medicine 2009;22(1):24‐8. [DOI] [PubMed] [Google Scholar]
India 2006d {published data only}
- Nellore V, Mittal S, Dadhwal V. Rectal misoprostol vs. 15‐methyl prostaglandin F2‐alpha for the prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2006;94:45‐6. [DOI] [PubMed] [Google Scholar]
India 2006f {published data only}
- Gupta B, Jain V, Aggarwal N. Rectal misoprostol versus oxytocin in the prevention of postpartum hemorrhage ‐ a pilot study. International Journal of Gynecology & Obstetrics 2006;94(Suppl 2):S139‐S140. [DOI] [PubMed] [Google Scholar]
India 2006g {published data only}
- Nagaria T, Ekka M. Intramuscular PGF2 a 125 microg versus intravenous methyl ergometrine 0.2mg in the active management of third stage of labor. Journal of Obstetrics and Gynaecology of India 2006;56(4):396‐8. [Google Scholar]
India 2007 {published data only}
- Biswas A, Bal R, Kundu MK, Kyal A, Halder M. A study of prophylactic use of 15‐methyl prostaglandin f2alpha in the active management of third stage of labour. Journal of the Indian Medical Association 2007;105(9):506, 508‐9. [PubMed] [Google Scholar]
India 2008a {published data only}
- Chhabra S, Tickoo C. Low‐dose sublingual misoprostol versus methylergometrine for active management of the third stage of labor. Journal of Obstetrics and Gynaecology Research 2008;34(5):820‐3. [DOI] [PubMed] [Google Scholar]
India 2008b {published and unpublished data}
- Kushtagi P, Verghese LM. Evaluation of two uterotonic medications for the management of the third stage of labor. International Journal of Gynecology & Obstetrics 2006;94(1):47‐8. [DOI] [PubMed] [Google Scholar]
- Verghese L, Kushtagi P. Evaluation of carboprost as a prophylactic oxytocic in the management of third stage of labour. BJOG: an international journal of obstetrics and gynaecology 2008;115(s1):74. [Google Scholar]
India 2009b {published data only}
- Singh G, Radhakrishnan G, Guleria K. Comparison of sublingual misoprostol, intravenous oxytocin, and intravenous methylergometrine in active management of the third stage of labor. International Journal of Gynecology & Obstetrics 2009;107(2):130‐4. [DOI] [PubMed] [Google Scholar]
India 2009d {published data only}
- Vaid A, Dadhwal V, Mittal S, Deka D, Misra R, Sharma JB, et al. A randomized controlled trial of prophylactic sublingual misoprostol versus intramuscular methyl‐ergometrine versus intramuscular 15‐methyl PGF2alpha in active management of third stage of labor. Archives of Gynecology and Obstetrics 2009;280(6):893‐7. [DOI] [PubMed] [Google Scholar]
India 2010 {published data only}
- Khurshid R, Fatima K, Parveen S, Ul Shamas I, Salman R. A comparison between intramuscular PGF2 a125 mG and intravenous methyl ergometrine 0.2 Mg in the active management of third stage labor. Internet Journal of Gynecology and Obstetrics 2010;14(1):1‐6. [Google Scholar]
Iran 2009 {published data only}
- Eftekhari N, Doroodian M, Lashkarizadeh R. The effect of sublingual misoprostol versus intravenous oxytocin in reducing bleeding after caesarean section. Journal of Obstetrics and Gynaecology 2009;29(7):633‐6. [DOI] [PubMed] [Google Scholar]
Jamaica 2009 {published data only}
- Harriott J, Christie L, Wynter S, DaCosta V, Fletcher H, Reid M. A randomized comparison of rectal misoprostol with syntometrine on blood loss in the third stage of labour. West Indian Medical Journal 2009;58(3):201‐6. [PubMed] [Google Scholar]
Mexico 2009 {published data only}
- Quiroga Diaz R, Cantu Mata R, Tello Gutierrez HE, Puente Villalobos M, Montemayor Garza R, Martinez Mendoza A. Intrauterine misoprostol for the prevention of bleeding cesarean. Ginecologia y Obstetricia de Mexico 2009;77(10):469‐74. [PubMed] [Google Scholar]
Mozambique 2001 {published data only}
- Bugalho A, Daniel A, Faundes A, Cunha M. Misoprostol for prevention of postpartum haemorrhage. International Journal of Gynecology & Obstetrics 2001;73:1‐6. [DOI] [PubMed] [Google Scholar]
Nigeria 2003 {published data only}
- Oboro VO, Tabowei TO. A randomised controlled trial of misoprostol versus oxytocin in the active management of the third stage of labour. Journal of Obstetrics and Gynaecology 2003;23:13‐6. [DOI] [PubMed] [Google Scholar]
Nigeria 2007 {published data only}
- Enakpene CA, Morhason‐Bello IO, Enakpene EO, Arowojolu AO, Omigbodun AO. Oral misoprostol for the prevention of primary post‐partum hemorrhage during third stage of labor. Journal of Obstetrics and Gynaecology Research 2007;33(6):810‐7. [DOI] [PubMed] [Google Scholar]
Nigeria 2011 {published data only}
- Fawole AO, Sotiloye OS, Hunyinbo KI, Umezulike AC, Okunlola MA, Adekanle DA, et al. A double‐blind, randomized, placebo‐controlled trial of misoprostol and routine uterotonics for the prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2011;112(2):107‐11. [DOI] [PubMed] [Google Scholar]
Pakistan 2011 {published data only}
- Mobeen N, Durocher J, Zuberi NF, Jahan N, Blum J, Wasim S, et al. Administration of misoprostol by trained traditional birth attendants to prevent postpartum hemorrhage in homebirths in Pakistan: a randomised placebo controlled trial. BJOG: an international journal of obstetrics and gynaecology 2011;118:353‐61.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobeen N, Durocher J, Zuberi NF, Jahan N, Blum J, Wasim S, et al. Use of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage during home deliveries in Pakistan: a randomised placebo‐controlled trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobeen N, Walraven G. Misoprostol for the prevention of postpartum hemorrhage in rural Pakistan (ongoing trial). www.clinicaltrials.gov (accessed 21 March 2006).
Singapore 1995 {published data only}
- Chua S, Chew SL, Yeoh CL, Roy AC, Ho LM, Selamat N, et al. A randomized controlled study of prostaglandin 15‐methyl F2alpha compared with syntometrine for prophylactic use in the third stage of labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 1995;35(4):413‐6. [DOI] [PubMed] [Google Scholar]
South Africa 1998a {published data only}
- Bamigboye AA, Merrell DA, Hofmeyr GJ, Mitchell R. Randomized comparison of rectal misoprostol with syntometrine for management of third stage of labor. Acta Obstetricia et Gynaecologica Scandinavica 1998;77:178‐81. [PubMed] [Google Scholar]
South Africa 1998b {published data only}
- Hofmeyr GJ, Nikodem VC, Jager M, Gelbart BR. A randomised placebo controlled trial of oral misoprostol in the third stage of labour. British Journal of Obstetrics and Gynaecology 1998;105:971‐5. [DOI] [PubMed] [Google Scholar]
- Hofmeyr GJ, Jager M, Rose L, Nikodem VC, Lawrie T. Misoprostol for third stage of labour management: a double blind, placebo controlled clinical trial. Proceedings of the 16th Conference on Priorities in Perinatal Care in South Africa; 1997; South Africa. 1997:29‐31.
South Africa 1998c {published data only}
- Bamigboye AA, Hofmeyr GJ, Merrell DA. Rectal misoprostol in the prevention of postpartum haemorrhage: a placebo controlled trial. Proceedings of the 17th Conference on Priorities in Perinatal Care in South Africa; 1998; South Africa. 1998:49‐52. [DOI] [PubMed]
- Bamigboye AA, Hofmeyr GJ, Merrell DA. Rectal misoprostol in the prevention of postpartum hemorrhage: a placebo‐controlled trial. American Journal of Obstetrics and Gynecology 1998;179(4):1043‐6. [DOI] [PubMed] [Google Scholar]
South Africa 1998d {published and unpublished data}
- Hofmeyr GJ, Nikodem C, Jager M, Drakely A, Gilbart B. Oral misoprostol for labour third stage management: randomised assessment of side effects (part 2). Proceedings of the 17th Conference on Priorities in Perinatal Care; 1988; South Africa. 1998:53‐4.
South Africa 2001 {published and unpublished data}
- Hofmeyr G, Nikodem V, Jager M, Drakely A. Side effects of oral misoprostol in the third stage of labour: a random allocation placebo controlled trial. Journal of Obstetrics and Gynaecology 2000;20(Suppl 1):S40‐S41. [Google Scholar]
- Hofmeyr GJ, Nikodem VC, Jager M, Drakely A. Oral misoprostol for labour third stage management: randomised assessment of side effects (part 1). Proceedings of the 17th Conference on Priorities in Perinatal Care; 1998; South Africa. 1998:53‐4.
- Hofmeyr GJ, Nikodem VC, Jager M, Drakely A. Side‐effects of oral misoprostol in the third stage of labour: a randomised placebo controlled trial. South African Medical Journal 2001;91(5):432‐5. [PubMed] [Google Scholar]
Switzerland 1999 {published data only}
- Surbek DV, Fehr P, Hoesli I, Holzgreve W. Oral misoprostol for third stage of labor: a randomized placebo‐controlled trial. Obstetrics & Gynecology 1999;94:255‐8. [DOI] [PubMed] [Google Scholar]
- Surbek DV, Fehr PM, Hoesli I, Holzgreve W. Oral misoprostol vs placebo for third stage of labor [Orales misoprostol reduziert den postpartalen blutverlust]. Gynakologisch Geburtshilfliche Rundschau 1999;39:144. [Google Scholar]
Switzerland 2006 {published data only}
- Lapaire O, Schneider MC, Stotz M, Surbek DV, Holzgreve W, Hoesli IM. Oral misoprostol vs. intravenous oxytocin in reducing blood loss after emergency cesarean delivery. International Journal of Gynecology & Obstetrics 2006;95(1):2‐7. [DOI] [PubMed] [Google Scholar]
Tibet 2009 {published data only}
- Miller S, Tudor C, Thorsten V, Nyima, Kalyang, Sonam, et al. Randomized double masked trial of Zhi Byed 11, a Tibetan traditional medicine, versus misoprostol to prevent postpartum hemorrhage in Lhasa, Tibet. Journal of Midwifery & Women's Health 2009;54(2):133‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor C, Miller S, Nyima, Sonam, Droyoung, Varner M. Preliminary progress report: randomized double‐blind trial of Zhi Byed 11, a Tibetan traditional medicine, versus misoprostol to prevent postpartum hemorrhage in Lhasa, Tibet. International Journal of Gynecology & Obstetrics 2006;94(Suppl 2):S145‐S146. [DOI] [PubMed] [Google Scholar]
- Wright L. Preventing postpartum hemorrhage using a Tibetan traditional medicine. www.clinicaltrials.gov (accessed 21 March 2006).
Tunisia 2009 {published data only}
- Fekih M, Jnifene A, Fathallah K, Ben Regaya L, Memmi A, Bouguizene S, et al. Benefit of misoprostol for prevention of postpartum hemorrhage in cesarean section: a randomized controlled trial. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 2009;38(7):588‐93. [DOI] [PubMed] [Google Scholar]
Turkey 2002 {published data only}
- Caliskan E, Meydanli M, Dilbaz B, Aykan B, Sonmezer M, Haberal A. Is rectal misoprostol really effective in the treatment of third stage of labor? A randomized controlled trial. American Journal of Obstetrics and Gynecology 2002;187:1038‐45. [DOI] [PubMed] [Google Scholar]
Turkey 2003 {published data only}
- Caliskan E, Dilbaz B, Meydanli M, Ozturk N, Narin M, Haberal A. Oral misoprostol for the third stage of labor: a randomized controlled trial. Obstetrics & Gynecology 2003;101:921‐8. [DOI] [PubMed] [Google Scholar]
Turkey 2010 {published data only}
- Ozalp E, Tanir HM, Sener T. Dinoprostone vaginal insert versus intravenous oxytocin to reduce postpartum blood loss following vaginal or cesarean delivery. Clinical and Experimental Obstetrics and Gynecology 2010;37(1):53‐5. [PubMed] [Google Scholar]
- Tanir H, Sener T, Ozalp E. Dinoprostone vaginal insert versus intravenous oxytocin to reduce the postpartum blood loss following vaginal or cesarean delivery. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S506‐S507. [PubMed] [Google Scholar]
United Kingdom 1994 {published data only}
- Chou MM, MacKenzie IZ. A prospective, double blind, randomized comparison of prophylactic intramyometrial 15‐methyl prostaglandin F2alpha, 125 micrograms, and intravenous oxytocin, 20 units, for the control of blood loss at elective cesarean section. American Journal of Obstetrics and Gynecology 1994;171:1356‐60. [DOI] [PubMed] [Google Scholar]
United Kingdom 2000 {unpublished data only}
- El‐Refaey H, Nooh R, O'Brien P, Abdalla M, Geary M, Walder J, et al. The misoprostol third stage of labour study: a randomised controlled comparison between orally administered misoprostol and standard management. BJOG: an international journal of obstetrics and gynaecology 2000;107:1104‐10. [DOI] [PubMed] [Google Scholar]
United Kingdom 2001b {published data only}
- Lokugamage A, Paine M, Bassaw‐Balroop K, Sullivan K, el‐Refaey H, Rodeck C. Active management of the third stage at caesarean section: a randomised controlled trial of misoprostol versus syntocinon. Australian and New Zealand Journal of Obstetrics and Gynaecology 2001;41(4):411‐4. [DOI] [PubMed] [Google Scholar]
- Lokugamage A, Paine M, Bassaw‐Balroop K, Sullivan K, el‐Refaey H, Rodeck C. Active management of the third stage at caesarean section: misoprostol vs syntocinon. XVI FIGO World Congress of Obstetrics and Gynecology; 2000 Sept 3‐8; Washington DC, USA (Book 2). 2000:54.
United Kingdom 2001c {published data only}
- Lamont RF, Morgan DJ, Logue M, Gordon H. A prospective randomised trial to compare the efficacy and safety of hemabate and syntometrine for the prevention of primary postpartum haemorrhage. Prostaglandins & Other Lipid Mediators 2001;66(3):203‐10. [DOI] [PubMed] [Google Scholar]
United Kingdom 2003 {published data only}
- Khan R, El‐Refaey H. Pharmacokinetics and adverse‐effect profile of rectally administered misoprostol in the third stage of labor. Obstetrics & Gynecology 2003;101(5):968‐74. [DOI] [PubMed] [Google Scholar]
USA 1990 {published data only}
- Catanzarite VA. Prophylactic intramyometrial carboprost tromethamine does not substantially reduce blood loss relative to intramyometrial oxytocin at routine cesarean section. American Journal of Perinatology 1990;7:39‐42. [DOI] [PubMed] [Google Scholar]
USA 2001 {published data only}
- Gerstenfeld TS, Wing DA. Rectal misoprostol versus intravenous oxytocin for the prevention of postpartum hemorrhage after vaginal delivery. American Journal of Obstetrics and Gynecology 2001;185:878‐82. [DOI] [PubMed] [Google Scholar]
USA 2004 {published data only}
- Bhullar A, Carlan SJ, Hamm J, Lamberty N, White L, Richichi K. Buccal misoprostol to decrease blood loss after vaginal delivery: a randomized trial. Obstetrics & Gynecology 2004;104(6):1282‐8. [DOI] [PubMed] [Google Scholar]
USA 2005 {published data only}
- Hamm J, Russell Z, Botha T, Carlan SJ, Richichi K. Buccal misoprostol to prevent hemorrhage at cesarean delivery: a randomized study. American Journal of Obstetrics and Gynecology 2005;192:1404‐6. [DOI] [PubMed] [Google Scholar]
WHO 1999 {published and unpublished data}
- Lumbiganon P, Hofmeyr J, Gülmezoglu AM, Pinol A, Villar J. Misoprostol dose‐related shivering and pyrexia in the third stage of labour. British Journal of Obstetrics and Gynaecology 1999;106:304‐8. [DOI] [PubMed] [Google Scholar]
WHO 2001 {published and unpublished data}
- Gülmezoglu AM, Villar J, Ngoc NN, Piaggio G, Carroli G, Adetoro L, et al. The WHO multicentre double‐blind randomized controlled trial to evaluate the use of misoprostol in the management of the third stage of labour. Lancet 2001;358(9283):689‐95. [DOI] [PubMed] [Google Scholar]
- Lumbiganon P, Villar J, Piaggio G, Gülmezoglu AM, Adetoro L, Carroli G. Side effects of oral misoprostol during the first 24 hours after administration in the third stage of labour. BJOG: an international journal of obstetrics and gynaecology 2002;109:1222‐6. [DOI] [PubMed] [Google Scholar]
Zimbabwe 2001 {published data only}
- Kundodyiwa TW, Majoko F, Rusakaniko S. Misoprostol versus oxytocin in the third stage of labor. International Journal of Gynecology & Obstetrics 2001;75:235‐41. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Australia 2009 {published data only}
- Dickinson JE, Doherty DA. Optimization of third‐stage management after second‐trimester medical pregnancy termination. American Journal of Obstetrics & Gynecology 2009;201(3):303.e1‐303.e7. [DOI] [PubMed] [Google Scholar]
Austria 1983 {published data only}
- Baumgarten K, Schmidt J, Horvat A, Neumann M, Cerwenka R, Gruber W, et al. Uterine motility after post‐partum application of sulprostone and other oxytocics. European Journal of Obstetrics & Gynecology and Reproductive Biology 1983;16:181‐92. [DOI] [PubMed] [Google Scholar]
Canada 2004 {published data only}
- Chandra S, Persad V, Young D, Baskett T. A preliminary study of cutaneous blood flow associated with postpartum use of oral misoprostol. Journal of Obstetrics & Gynaecology Canada: JOGC 2004;26(12):1073‐6. [DOI] [PubMed] [Google Scholar]
China 1997 {published data only}
- Liu C, Wang D, Li X, Yuxia Y, Jian Z, Tao L. Clinical study on reduction of postpartum bleeding by methyl carprost suppository. Chinese Journal of Obstetrics and Gynecology 1997;32(1):22‐4. [PubMed] [Google Scholar]
China 1998 {published data only}
- Zhao Y, Li X, Peng Y. Clinical study on reduction of postpartum bleeding in cesarean section by misoprostol. Chung‐Hua Fu Chan Ko Tsa Chih (Chinese Journal of Obstetrics and Gynecology) 1998;33(7):403‐5. [PubMed] [Google Scholar]
China 1998b {published data only}
- Weihong H, Hanrong L, Linan C. Preventing of postpartum haemorrhage by Carboprost Methylate suppository administered through vagina or sublingually. Acta Academiae Medicinae Shangai 1998;25(2):137‐9. [Google Scholar]
China 2000 {published data only}
- Jin LJ, Zhou L. Application of anus misoprostol to decrease the volume of post partum hemorrhage. Journal of Practical Nursing 2000;16(2):9‐10. [Google Scholar]
China 2001 {published data only}
- Jiang Q, Wang P, Cao W. Effect on different doses of misoprostol to prevent postpartum hemorrhage. Chinese Nursing Research 2001;15(6):313‐4. [Google Scholar]
China 2002 {published data only}
- Li X, Wang H, Wang J, Cao X L, Ma Y. Prophylactic and therapeutic effect of misoprofil plus oxytocin on postpartum hemorrhage in patients with pregnancy‐induced hypertension syndrome. Journal of Postgraduates of Medicine 2002;25(7):34‐5. [Google Scholar]
China 2003c {published data only}
- Xu H. Misoprostol on preventing postpartum bleeding in cesarean. Hebei Medicine 2003;9(9):806‐7. [Google Scholar]
China 2003d {published data only}
- Zhao SF, Sun XF. Clinical study on preventing and curing postpartum hemorrhage in the third stage of labor. Journal of Practical Obstetrics and Gynecology 2003; Vol. 19, issue 5:278‐80.
China 2004b {published data only}
- Ng PS, Yuen PM, Sahota DS. Comparison of oral misoprostol and intravascular syntocinon in the management of the third stage of labour ‐ a double‐blind randomised controlled trial. 30th British Congress of Obstetrics and Gynaecology; 2004 July 7‐9 Glasgow, UK. 2004:69.
China 2004c {published data only}
- Yuen PM, Ng PS, Sahota DS. A double‐blind randomised controlled trial of oral misoprostol in addition to intra‐muscular syntometrine in the management of the third stage of labour. 30th British Congress of Obstetrics and Gynaecology; 2004 July 7‐9 Glasgow, UK. 2004:62.
Egypt 1999 {published data only}
- Diab KM, Ramy AR, Yehia MA. The use of rectal misoprostol as active pharmacological management of the third stage of labor. Journal of Obstetrics and Gynaecology Research 1999;25(5):327‐32. [DOI] [PubMed] [Google Scholar]
Hungary 1979 {published data only}
- Kerekes L, Domokos N. The effect of prostaglandin F2alpha on third stage of labour. Prostaglandins 1979;18:161‐6. [DOI] [PubMed] [Google Scholar]
India 1988a {published data only}
- Devi PK, Sutaria UD, Raghavan KS. Prophylactic use of 15(S)15 methyl PGF2alpha for control of postpartum bleeding. Acta Obstetricia et Gynaecologica Scandinavica Supplement 1988;145:7‐8. [DOI] [PubMed] [Google Scholar]
India 1988b {published data only}
- Anjaneyulu R, Devi PK, Jain S, Kanthamani CR, Vijaya R, Raghavan KS. Prophylactic use of 15(S)15 methyl PGF2alpha by intramuscular route ‐ a controlled clinical trial. Acta Obstetricia et Gynecologica Scandinavica Supplement 1988;145:9‐11. [DOI] [PubMed] [Google Scholar]
India 2000a {published data only}
- Chatterjee A. Misoprostol and the third stage. XVI FIGO World Congress of Obstetrics and Gynecology. Book 4; 2000 Sept 3‐8; Washington DC, USA. 2000:29.
India 2000b {published data only}
- Dutta DK, Saha KK. Comparative study on role of syntometrine and prostaglandin in the prevention of PPH. XVI FIGO World Congress of Obstetrics and Gynecology. Book 4; 2000 Sept 3‐8; Washington DC, USA. 2000:29.
India 2000c {published data only}
- Rajwani J, Survana K. Active management of third stage of labor ‐ a comparative study. XVI FIGO World Congress of Obstetrics and Gynecology. Book 4; 2000 Sept 3‐8; Washington DC, USA. 2000:54.
India 2001a {published data only}
- Ray A, Mukherjee P, Basu G, Chatterjee A. Misoprostol and third stage of labour. Journal of Obstetrics and Gynecology of India 2001;51(6):53‐4. [Google Scholar]
India 2005b {published data only}
- Singh N, Singh U. Methylergometrine and carboprost tromethamine prophylaxis for postpartum hemorrhage. Journal of Obstetrics and Gynaecology of India 2005;55(4):325‐8. [Google Scholar]
India 2006e {published data only}
- Chandhiok N, Dhillon BS, Datey S, Mathur A, Saxena NC. Oral misoprostol for prevention of postpartum hemorrhage by paramedical workers in India. International Journal of Gynecology & Obstetrics 2006;92:170‐5. [DOI] [PubMed] [Google Scholar]
India 2006h {published data only}
- Verma P, Aggarwal N, Jain V, Suri V. A double‐blind randomized controlled trial to compare sublingual misoprostol with methylergometrine for prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2006;94(Suppl 2):S137‐S138. [DOI] [PubMed] [Google Scholar]
India 2009a {published data only}
- Bellad M, Ganachari TDM, Mallapur M. Sublingual (SL) powdered misoprostol (400 mcg) vs IM oxytocin (10 IU) for prevention of postpartum blood loss ‐ a randomized controlled trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S124‐S125. [Google Scholar]
India 2009c {published data only}
- Tripti N, Balram S. 400 ug oral misoprostol versus 0.2mg intravenous methyl ergometrine for the active management of third stage of labor. Journal of Obstetrics and Gynecology of India 2009;59(3):228‐34. [Google Scholar]
Indonesia 2002 {published data only}
- Dasuki D, Emilia O, Harini S. Randomized clinical trial: the effectiveness of oral misoprostol versus oxytocin in prevention of postpartum hemorrhage. Journal of Obstetrics and Gynaecology Research 2002;28(1):46. [Google Scholar]
Israel 1992 {published data only}
- Bider D, Ben‐Rafael Z, Dulitzky M, Menashe Y, Mashiach S, Barkai G. Effect of intraumbilical prostaglandin F2alpha injection on the third stage of labour. Journal of Reproductive Medicine 1992;37:317‐9. [PubMed] [Google Scholar]
Italy 1988 {published data only}
- Norchi S, Beretta E, Zanini A, Bottino S. Prevention of primary post‐partum haemorrhage (PPH). Controlled clinical trial: sulprostone vs metilergometrina. World Congress of Gynecology and Obstetrics; 1988 October 23‐28; Brazil. 1988.
Japan 1976 {published data only}
- Takagi S, Yoshida T, Togo Y, Tochigi H, Abe M, Sakata H, et al. The effects of intramyometrial injection of prostaglandin F2alpha on severe post‐partum hemorrhage. Prostaglandins 1976;12:565‐79. [DOI] [PubMed] [Google Scholar]
Korea 2007 {published data only}
- Hong SC, Kim JW, Park HT, Seol HJ, Kim HJ, Kim SH, et al. Additional rectal misoprostol plus intravenous oxytocin versus intravenous oxytocin for the prevention of postpartum hemorrhage after cesarean section. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S99, Abstract no: 321. [Google Scholar]
Singapore 1990 {published data only}
- Ilancheran A, Ratnam SS. Effects of oxytocics on prostaglandin levels in the third stage of labour. Gynecologic and Obstetric Investigation 1990;29:177‐80. [DOI] [PubMed] [Google Scholar]
Singapore 2001 {published and unpublished data}
- Chong YS, Chua S, El‐Refaey H, Choo WL, Boonsri C, Koh BC, et al. Can oral misoprostol be used as an alternative to parenteral oxytocics in the active management of the third stage of labour? A preliminary study of its effect on the postpartum uterus. Personal Communication 1997.
- Chong YS, Chua S, El‐Refaey H, Choo WL, Chanrachakul B, Tai BC, et al. Postpartum intrauterine pressure studies of the uterotonic effect of oral misoprostol and intramuscular syntometrine. BJOG: an international journal of obstetrics and gynaecology 2001;108:41‐7. [DOI] [PubMed] [Google Scholar]
South Africa 1999 {published data only}
- Lokugamage AU, Moodley J, Sullivan K, Rodeck CH, Niculescu L, Tigere P. The Durham primary postpartum haemorrhage study. Women's Health ‐ into the new millennium. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 October 3‐6; Cape Town, South Africa. 1999:77‐8.
- Lokugamage AU, Sullivan KR, Niculescu I, Tigere P, Onyangunga F, El‐Refaey H, et al. A randomized study comparing rectally administered misoprostol versus Syntometrine combined with an oxytocin infusion for the cessation of primary post partum hemorrhage. Acta Obstetrica et Gynecologica Scandinavica 2001;80:835‐9. [DOI] [PubMed] [Google Scholar]
Thailand 2000 {published data only}
- Jirakulsawas J, Khooarmompattana S. Comparison of oral misoprostol and intramuscular methylergonovine for prevention of postpartum hemorrhage. Thai Journal of Obstetrics and Gynaecology 2000;12(4):332. [Google Scholar]
Turkey 2005 {published data only}
- Ozkaya O, Sezik M, Kaya H, Desdicioglu R, Dittrich R. Placebo‐controlled randomized comparison of vaginal with rectal misoprostol in the prevention of postpartum hemorrhage. Journal of Obstetrics and Gynaecology Research 2005;31:389‐93. [DOI] [PubMed] [Google Scholar]
United Kingdom 2001a {published data only}
- Acharya G, Al‐Sammarai M, Patel N, Al‐Habib, Kiserud T. A randomised, controlled trial comparing effect of oral misoprostol and intravenous syntocinon on intra‐operative blood loss during caesarean section. Acta Obstetrcia et Gynecologica Scandinavica 2001;80:245‐50. [DOI] [PubMed] [Google Scholar]
USA 1983 {published data only}
- Nelson GH. Use of 15‐Methyl prostaglandin F2alpha postpartum to contract the uterus in normal pregnant women. Journal of the Medical Association of Georgia 1983;72:703‐6. [PubMed] [Google Scholar]
USA 1999 {published data only}
- Daly S, Andolina K, Tolosa JE, Roberts N, Wapner R. A randomized controlled trial of misoprostol versus oxytocin in preventing postpartum blood loss. American Journal of Obstetrics and Gynecology 1999;180:S68. [Google Scholar]
Yemen 2009 {published data only}
- Al‐Harazi AH, Frass KA. Sublingual misoprostol for the prevention of postpartum hemorrhage. Saudi Medical Journal 2009;30(7):912‐6. [PubMed] [Google Scholar]
References to studies awaiting assessment
Afolabi 2010 {published data only}
- Afolabi EO, Kuti O, Orji EO, Ogunniyi SO. Oral misoprostol versus intramuscular oxytocin in the active management of the third stage of labour. Singapore Medical Journal 2010;51(3):207‐11. [PubMed] [Google Scholar]
Carbonell 2009 {published data only}
- Carbonell i Esteve JL, Hernandez JMR, Piloto M, Setien SA, Texido CS, Tomasi G, et al. Active management of the third phase of labour plus 400 mug of sublingual misoprostol and 200 mug of rectal misoprostol versus active management only in the prevention of post‐partum haemorrhage. A randomised clinical trial [Manejo activo de la tercera fase del parto mas 400 mug de misoprostol sublingual y 200 mug de misoprostol rectal frente a manejo activo solo en la prevencion de la hemorragia posparto. Ensayo clinico aleatorizado]. Progresos de Obstetricia y Ginecologia 2009;52(10):543‐51. [Google Scholar]
Chaudhuri 2012 {published data only}
- Chaudhuri P, Biswas J, Mandal A. Sublingual misoprostol versus intramuscular oxytocin for prevention of postpartum hemorrhage in low‐risk women. International Journal of Gynecology and Obstetrics 2012;116(2):138‐42. [DOI] [PubMed] [Google Scholar]
Dong 2011 {published data only}
- Dong Y. Effects of carboprost on prevention of hemorrhage after induced labor with scarred uterus. Journal of Shanghai Jiaotong University (Medical Science) 2011;31(8):1212‐5. [Google Scholar]
Elati 2011 {published data only}
- Elati A, Elmahaishi MS, Elmahaishi MO, Elsraiti OA, Weeks AD. The effect of misoprostol on postpartum contractions: a randomised comparison of three sublingual doses. BJOG: an international journal of obstetrics and gynaecology 2011;118(4):466‐73. [DOI] [PubMed] [Google Scholar]
Kumar 2011 {published data only}
- Kumar S. A study to determine the efficacy of 600 mcg misoprostol in prevention of post partum haemorrhage. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:305.
Le 2000 {published data only}
- Le J. Prevention of postpartum hemorrhage by carboprost and oxytocin in 90 cases analysis. Acta Medicinae Sinica 2000;13(2):140‐1. [Google Scholar]
Mansouri 2011 {published data only}
- Mansouri HA, Alsahly N. Rectal versus oral misoprostol for active management of third stage of labor: a randomized controlled trial. Archives of Gynecology and Obstetrics 2011;283(5):935‐9. [DOI] [PubMed] [Google Scholar]
Owonikoko 2011 {published data only}
- Owonikoko KM, Arowojolu AO, Okunlola MA. Effect of sublingual misoprostol versus intravenous oxytocin on reducing blood loss at cesarean section in Nigeria: A randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2011;37(7):715‐21. [DOI] [PubMed] [Google Scholar]
Sadiq 2011 {published data only}
- Sadiq UG, Kwanashie O, Mairiga G, Gamaniel S, Isa H, Abdu A, et al. A randomised clinical trial comparing the efficacy of oxytocin injection and oral misoprostol tablet in the prevention of postpartum haemorrhage in Maiduguri Nigeria. International Research Journal of Pharmacy 2011;2(8):76‐81. [Google Scholar]
Wang 2000 {published data only}
- Wang BI, Du JM. Clinical study on reduction of postpartum bleeding using carprost suppository. Henan Medical Research 2000;9(2):155‐6. [Google Scholar]
Yan 2000 {published data only}
- Yan WG, Ling MX, Mao HY. Clinical study on reduction of postpartum bleeding in cesarean operation by misoprostol. Journal of Zhenjiang Medical College 2000;10(3):440‐1. [Google Scholar]
Yuan 2003 {published data only}
- Yuan YM, Liu CF. Clinical observation of treatment for 60 cases of postpartum hemorrhage with misoprostol tablets combined with jiashenshenghuatang. Chinese Clinical New Medicine 2003;3(10):902. [Google Scholar]
References to ongoing studies
Kalahroudi 2010 {published data only}
- Kalahroudi MA. Comparison of the effect of rectal misoprostol and syntometrin in prevention of post partum hemorrhage. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 December 2010).
Moradi 2010 {published data only}
- Moradi S. Comparison of misoprostol and oxytocin in reduction of postpartum hemorrhage. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 December 2010).
Additional references
Amant 2001
- Amant F. The misoprostol third stage study: a randomised controlled comparison between orally administered misoprostol and standard management. [letter]. BJOG: an international journal of obstetrics and gynaecology 2001;108:338. [DOI] [PubMed] [Google Scholar]
Andolina 1999
- Andolina K, Daly S, Roberts N, Tolosa J, Wapner R. Objective measurement of blood loss at delivery: is it more than a guess?. American Journal of Obstetrics and Gynecology 1999;180:S69. [Google Scholar]
Begley 2011
- Begley CM, Gyte GML, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD007412.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carroli 2001
- Carroli G. RHL practical aspects: oxytocin or oxytocin+ergot alkaloids in active management of the third stage of labour. The WHO Reproductive Health Library. Oxford: Update Software. WHO/RHR/01.6 2001.
Chong 1997
- Chong YS, Chua S, Arulkumaran S. Severe hyperthermia following oral misoprostol in the immediate postpartum period. Obstetrics & Gynecology 1997;90:703. [DOI] [PubMed] [Google Scholar]
Cotter 2001
- Cotter AM, Ness A, Tolosa JE. Prophylactic oxytocin for the third stage of labour. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD001808] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Refaey 1997
- El‐Refaey H, O'Brien P, Morafa W, Walder J, Rodeck C. Use of misoprostol in the prevention of postpartum haemorrhage. British Journal of Obstetrics and Gynaecology 1997;104:336‐9. [DOI] [PubMed] [Google Scholar]
Ghana 2009
- Ghana Statistical Service (GSS), Ghana Health Service (GHS), and Macro International. Ghana Maternal Health Survey 2007. Calverton, Maryland, USA: GSS, GHS, and Macro International, 2009. [Google Scholar]
Gibbens 1972
- Gibbens D, Boyd NRH, Crocker S, Baumber S, Chard T. The circulating levels of oxytocin following intravenous and intramuscular administration of syntometrine. Journal of Obstetrics and Gynaecology of the British Commonwealth 1972;79:644‐6. [DOI] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.4 [updated March 2005]. In: The Cochrane Library, Issue 2, 2005. Chichester, UK: John Wiley & Sons, Ltd.
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Hofmeyr 2009
- Hofmeyr GJ, Gulmezoglu AM, Novikova N, Linder V, Ferreira S, Piaggio G. Misoprostol to prevent and treat postpartum haemorrhage: a systematic review and meta‐analysis of maternal deaths and dose‐related effects. Bulletin of the World Health Organization 2009;87(9):666‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hogerzeil 1996
- Hogerzeil HV, Walker GJA. Instability of (methyl)ergometrine in tropical climates: an overview. European Journal of Obsterics & Gynecology and Reproductive Biology 1996;69:25‐9. [DOI] [PubMed] [Google Scholar]
Khan 2006
- Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Look PFA. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367:1066‐74. [DOI] [PubMed] [Google Scholar]
McDonald 2004
- McDonald S, Abbott JM, Higgins SP. Prophylactic ergometrine‐oxytocin versus oxytocin for the third stage of labour. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD000201.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
PATH 2001
- PATH. Oxytocin in pre‐filled UnijectTM injection devices for management of third stage of labour: an introductory trial in Lombok, Indonesia. Final report to Program for Appropriate Technology in Health (PATH), Seattle, Washington, USA, May 2001. PATH, 2001. [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Tsu 2009
- Tsu VD, Luu HT, Mai TT. Does a novel prefilled injection device make postpartum oxytocin easier to administer? Results from midwives in Vietnam. Midwifery 2009;25(4):461‐5. [DOI] [PubMed] [Google Scholar]
WHO 1994
- Groot ANJA, Vree TB, Hogerzeil HV, Walker GJA. Stability of oral oxytocics in tropical climates (WHO/DAP/94.13). Geneva: World Health Organization, 1994. [Google Scholar]
WHO 2011
- WHO Expert Committee on Essential Medicines. Unedited report of the 18th Expert Committee on the Selection and Use of Essential Medicines. WHO, 2011. [Google Scholar]
Zieman 1997
- Zieman M, Fong SK, Benowitz NL, Banskter D, Darney PD, Zieman M, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstetrics & Gynecology 1997;90:88‐92. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gülmezoglu 1997
- Gülmezoglu AM. Prostaglandins in third stage of labour. Cochrane Database of Systematic Reviews 1997, Issue 3. [Google Scholar]
Gülmezoglu 1998
- Gülmezoglu AM. Prostaglandins in third stage of labour. Cochrane Database of Systematic Reviews 1998, Issue 4. [Google Scholar]
Gülmezoglu 2000
- Gülmezoglu AM. Prostaglandins for prevention of postpartum haemorrhage. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI] [PubMed] [Google Scholar]
Gülmezoglu 2002
- Gülmezoglu AM, Forna F, Villar J, Hofmeyr GJ. Prostaglandins for prevention of postpartum haemorrhage (Cochrane Review). Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD000494] [DOI] [PubMed] [Google Scholar]
Gülmezoglu 2003
- Gülmezoglu AM, Forna F, Villar J, Hofmeyr GJ. Prostaglandins for prevention of postpartum haemorrhage. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD000494] [DOI] [Google Scholar]
Gülmezoglu 2004
- Gülmezoglu AM, Forna F, Villar J, Hofmeyr GJ. Prostaglandins for prevention of postpartum haemorrhage. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD000494.pub2] [DOI] [PubMed] [Google Scholar]
Gülmezoglu 2007
- Gülmezoglu AM, Forna F, Villar J, Hofmeyr GJ. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD000494.pub3] [DOI] [PubMed] [Google Scholar]


