Abstract
Purpose:
In patients with non–small cell lung cancer (NSCLC), concurrent chemoradiation therapy (CRT) exacerbates a cluster of difficult-to-manage symptoms, especially cancer-related fatigue. Minocycline is a readily available, low-cost antibiotic with anti-inflammatory properties. We conducted a phase II randomized, double-blinded, placebo-controlled trial to investigate the effect of minocycline in reducing CRT-symptom burden in NSCLC.
Methods and Materials:
Patients with NSCLC scheduled to receive CRT provided consent and were randomized to receive either minocycline (100 mg twice daily) or a matching placebo during 6 to 7 weeks of CRT. Patient-reported fatigue and other symptoms were assessed on MD Anderson Symptom Inventory weekly from the start of CRT for 12 weeks. The primary outcome was 12-week (±2 days) area under the curve (AUC) for symptom burden, which was compared between treatment groups.
Results:
Forty of 49 enrolled patients (80%) were evaluable (19 on minocycline and 21 on placebo). There were no grade 3+ adverse events related to the study medication. Fatigue was significantly reduced in the minocycline group compared to placebo group during the 12-weeks trial period (AUC=31.2±14.2 vs. 45.0±20.9, P=0.011), with a large effect size (Cohen’s d=0.77). Pain (Cohen’s d=0.54) and shortness of breath (Cohen’s d=0.55) were also significantly reduced in the minocycline group (all P<0.05).
Conclusion:
Minocycline during CRT for NSCLC was feasible, had a low toxicity profile, and yielded a clinically and statistically significant positive signal in reducing symptom burden related to NSCLC and CRT. This study is a proof of concept so a larger trial in CRT patients is warranted.
INTRODUCTION
Concurrent chemoradiation therapy (CRT), a standard treatment option for patients with locally advanced non–small cell lung cancer (NSCLC)(1), is often associated with acute side effects from the radiation and chemotherapy including both systemic symptoms (eg, fatigue, sadness, distress, disturbed sleep, drowsiness, and lack of appetite) and localized symptoms from lung cancer and radiation-induced toxic effects such as pneumonitis or esophagitis (eg, coughing, shortness of breath, sore throat, and pain)(2,3). Previous symptom research in patients with NSCLC, undergoing CRT, demonstrated that high severity of symptom burden contributes to the patient’s general distress during the course of treatment; fatigue is the most severe of these symptoms and is difficult to manage (4,5). The severity of CRT-related symptom burden is associated with an increased pro-inflammatory response (6,7). Our research goal has been to identify a new strategy for effective intervention against and/or prevention of the inflammation-driven fatigue symptom burden due to disease and aggressive therapy(8). Reduction of symptom burden impacts patient’s functional status and facilitates compilation of curative therapies.
Minocycline, a broad-spectrum tetracycline-antibiotics has the ability to cross the blood brain barrier and reduce anxiety-like behaviors through modulating neuroinflammation in preclinical studies (9,10). Minocycline prevents hyperoxia-induced brain changes, modulate inflammatory signaling pathways and inhibited hypoxia-induced cytokine release (11-13). The therapeutic effects of minocycline have been investigated in a number of diseases where inflammation plays a critical role (10). Minocycline had long-lasting effects in preventing neuropathic pain (14) and was safe and effective for patients with rheumatoid arthritis in a 48-week double-blind placebo-controlled trial (15). It is now widely used in the management of dermatitis associated with targeted therapy in cancer (16)(17); use of prophylactic oral minocycline significantly reduced grade ≥2 rash compared to use reactive topical steroids (44.0% vs. 84.6%, p=0.04) (18) and reduced moderate/severe itching compared to placebo (20% v 50%, P = .05) (19) in colorectal patients and it’s prophylactic use resulted in lower incidence of rash in pancreatic cancer compared to deferred treatment (47.7 vs. 80.8%, p<0.001) (20). Our preliminary studies and the published data by others showed that minocycline is safe with positive trend toward reducing the adverse events after cancer treatment (feeding tube, fatigue, diarrhea and neuropathy) (21-24). Those studies were either single arm Phase II or Phase III with small sample size and unpowered to detect the effect size or to show statistically significance reduction in the treatment-related toxicities in cancer patients after minocycline. To provide a proof of concept, we investigated minocycline’s ability to reduce treatment-related symptoms during CRT in a phase II randomized, double-blinded, placebo-controlled clinical trial in patients with NSCLC.
METHODS AND MATERIALS
Participants
Eligible patients for this single institution, prospective, blinded, and placebo-controlled randomized trial were adults with NSCLC who following multidisciplinary evaluation had been dispositioned to receive CRT in in the Division of Radiation Oncology at The University of Texas MD Anderson Cancer Center in Houston, Texas. Patients were approached and offered enrollment in the study. Eligible patients were at least 18 years old, had a pathologic diagnosis of primary or recurrent NSCLC, had adequate renal and hepatic function, had to have good performance status (Eastern Cooperative Oncology Group Performance Status 0-1), could read and understand English, were scheduled for CRT, and provided study-specific written informed consent. Patients were enrolled prior to therapy under a protocol approved by the Institutional Review Board. The chemotherapy regimen (standard platinum/taxane-based doublets) was consistent for all patients and radiation modality was intensity modulated radiation (IMRT). Data on patients’ characteristics, cancer therapy, comorbidities, and current medications were collected by research staff.
Randomization and Intervention
Prior to accruing the first patient, a randomization list for the entire sample was generated by the biostatistician stating into which group a patient will be randomized. This list containing the accrual number and treatment group information was set up in the Department of Biostatistics Clinical Trial Conduct website. Participants were randomized equally using a permuted block design pre-CRT to receive either minocycline (100 mg twice daily enterally) or matching placebo during the CRT course. The 6- to 7-week intervention period was chosen to align with the standard CRT period (from day 1 to the final day of the 6- to 7- week course of CRT). In this double blind study, patients, treating physicians and clinical research coordinators were unaware which intervention the patients received. The minocycline and placebo capsules were of identical size, color and shape.
The trial is registered at ClinicalTrials.gov: .
Patient-Reported Outcomes Tool: The MD Anderson Symptom Inventory–Lung Cancer
The MDASI is a patient-reported outcome (PRO) assessment tool validated for use in the cancer population(25). The severity of 13 common cancer-related symptoms during the previous 24 hours is assessed on a 0-10 numerical scale, with 0 being “not present” and 10 being “as bad as you can imagine.” Three lung cancer module items were also used in this study (MDASI–Lung Cancer)(26). The MDASI—Lung Cancer also contains six items that describe the extent to which symptoms have interfered with various aspects of the patient’s life during the past 24 hours, with 0 being “no interference” and 10 being “interfered completely.” The MDASI–Lung Cancer was administered before the start of CRT (baseline) and then weekly during and after therapy for up to 12 weeks from the start of therapy.
The quality of life (QoL) scores were reported by the patients, using single item QoL and EuroQol 5-dimensional questionnaire (EQ-5D).
Other Assessments
Both study medication–related and CRT–related adverse events were prospectively assessed weekly by the study team and treating physicians during the CRT period and graded weekly according to Common Terminology Criteria for Adverse Events version 3. The treating physicians determined the severity of other observed adverse events and attributed them to study medication or to CRT. Safety and futility monitoring was overseen by our institutional Data Safety and Monitoring Board.
Statistical Analysis
The primary endpoint was the 12-week (±2 days) area under the curve (AUC), which used trapezoidal approximation of five pre-specified patient-reported symptoms (pain, fatigue, sleep disturbance, lack of appetite, and sore throat) as assessed by the MDASI–Lung Cancer during the trial. These symptoms were selected a priori as a representative combination of known to contribute significantly to symptom burden based on a previous study (6). As the goal of this study was to detect the potential signal from the benefit of minocycline to inform future clinical trial design, we decided to detect a relatively large standardized effect size (ES) of 0.80 (one-sided, 5% significance level) with 80% for the primary endpoint, requiring 20 evaluable patients per arm. T-test was used to assess the statistical significance of differences between the minocycline and placebo groups in AUCs of the five pre-specified symptoms, the five most severe symptoms observed and each of the symptoms from the clusters taken individually. We pre-specified that evaluable patients would have at least 2 weeks of symptom intervention and data and that patients with less would be replaced. The QoL scores over time were compared between the two study groups, using Wilcoxon test. The Fisher’s exact Test (p-value) was used to compare the proportions of the Study Medication Satisfaction Scale between treatment arms. Forty-six percent of the patients indicated that they would be willing to use the symptom study medications if getting CRT again and 100% would recommend the symptom study medications to another patient undergoing CRT. Seventy-four percent of the patients reported that it was very easy taking the symptoms study medications in the form in which they were given and 66.67% rated their satisfaction with the symptom study medications as “very stratified”.
Patients’ clinical characteristics according to treatment group were compared using independent t-tests for continuous variables and chi-square tests for categorical variables. Aggregated adverse events were tabulated according to attribution and grade. Finally, to assess the impact of minocycline on symptoms over time, mixed-effects models were applied including a group by time interaction term. Baseline symptom score, age, race/ethnicity, education level, comorbid conditions, cancer stage, and previous treatment were included in the mixed-effects models. SAS 9.4 (Gary, NC).
RESULTS
Patient Characteristics
Table 1 presents the participants’ demographic and disease characteristics. The groups did not significantly differ in these characteristics except for the fact that the patients in minocycline group were statistically significantly younger compared to placebo group (median (range) 63 (47-83) vs 68 (56-77), P= 0.037).
Table 1.
Patient characteristics
| Minocycline (n=19) | Placebo (n=21) | P value | ||||
|---|---|---|---|---|---|---|
|
Continuous variables, mean (SD), median (range) |
Mean (SD) | Median (min-max) | Mean (SD) | Median (min-max) | ||
| Age, years | 62.4 (8.1) | 63 (47-83) | 67.3 (6.3) | 68 (56-77) | 0.037 | |
| Body mass index | 33.0 (12.6) | 28.3 (17.5-69.3) | 29.2 (7.8) | 28.7 (18.3-48.7) | 0.261 | |
| Total radiation dose, Gy | 62.9 (10.4) | 66 (25-72) | 62.5 (5.4) | 66 (54-74) | 0.229 | |
| Total fractions | 32.7 (4.8) | 33 (25-50) | 32.6 (2.6) | 33 (27-37) | 0.895 | |
| Categorical variables, n, % | ||||||
| Sex | Female | 10 | 52.6% | 10 | 47.6% | 0.752 |
| Male | 9 | 47.4% | 11 | 52.4% | ||
| Marriage | Married | 17 | 89.5% | 15 | 71.4% | 0.154 |
| Unmarried | 2 | 10.5% | 6 | 28.6% | ||
| Race/ethnicity | Non-Hispanic white | 17 | 89.5% | 19 | 90.5% | 0.916 |
| Other | 2 | 10.5% | 2 | 9.5% | ||
| Education | 13 years or more | 10 | 52.6% | 11 | 52.4% | 0.987 |
| 0-12 years | 9 | 47.4% | 10 | 47.6% | ||
| Cancer stage | I/II | 3 | 15.8% | 7 | 33.3% | 0.201 |
| III/IV | 16 | 84.2% | 14 | 66.7% | ||
| Charlson Comorbidity Index | 0 | 10 | 52.6% | 12 | 57.1% | 0.775 |
| 1 or more | 9 | 47.4% | 9 | 42.9% | ||
| ECOG PS | 0 | 11 | 57.9% | 6 | 28.6% | 0.061 |
| 1 | 8 | 42.1% | 15 | 71.4% | ||
| Recurrent disease | No | 18 | 94.7% | 20 | 95.2% | 0.942 |
| Yes | 1 | 5.3% | 1 | 4.8% | ||
| Prior treatment | No | 15 | 78.9% | 13 | 61.9% | 0.240 |
| Yes | 4 | 21.1% | 8 | 38.1% | ||
| Prior radiation | No | 19 | 100.0% | 21 | 100.0% | |
| Yes | 0 | 0.0% | 0 | 0.0% | ||
| Prior chemotherapy | No | 16 | 84.2% | 14 | 66.7% | 0.201 |
| Yes | 3 | 15.8% | 7 | 33.3% | ||
| Prior surgery | No | 17 | 89.5% | 17 | 81.0% | 0.451 |
| Yes | 2 | 10.5% | 4 | 19.0% | ||
| Radiotherapy | 0.115 | |||||
| IMRT | 16 | 84 | 13 | 62 | ||
| Proton | 3 | 16 | 8 | 38 | ||
SD, standard deviation; ECOG PS, Eastern Oncology Group Performance Status
Attrition, Compliance, and Toxicity
From January 2013 through August 2015, 56 eligible patients were approached, and 49 were enrolled and randomized: 25 to minocycline and 24 to placebo. Early drop-out or refusal to proceed after enrollment occurred for several reasons: 2 cancellation of the patient’s radiation or chemotherapy plan, 1 did not start the study drugs, 5 stopped the study drugs within 1 week, and 1 completed less than 2 MDASI assessments after treatment start. Thus, among the 49 patients enrolled, 40 (80%) were evaluable: 19 of those in the initial minocycline group and 21 of those in the initial placebo group. Medication compliance (90.5%) was adequate, and weekly PRO symptom assessments (489/520, 94% observations) were completed at a high rate. Supplementary Fig 1 presents the CONSORT diagram. There were no grade 3+ adverse events related to the study medication. Table 2 summarizes the observed adverse effects.
Table 2.
Toxicity by treatment group
| Attribution of AE to Study Medication | Total | |||||||
|---|---|---|---|---|---|---|---|---|
| Possible | Unlikely* | Unrelated** | ||||||
| Minocycline | Placebo | Minocycline | Placebo | Minocycline | Placebo | |||
| CTCAE grade, N1 | 1 | 0 | 0 | 1 | 2 | 1 | 5 | 9 |
| 2 | 0 | 0 | 13 | 9 | 3 | 4 | 29 | |
| 3 | 0 | 0 | 3 | 2 | 2 | 5 | 12 | |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total, N | 0 | 0 | 17 | 13 | 6 | 14 | 50 | |
Number of events
Grade 1 unlikely AEs include dermatitis, fever and weight loss.
Grade 2 unlikely AEs include anorexia, dehydration, diarrhea, dysgeusia, dysphagia, dyspnea, erythema, esophagitis, esophagtis, fatigue, fever, and odynophagia
Grade 3 unlikely AEs include cough, dehydration, dermatitis, dysphagia, and dyspnea.
Grade 1 unrelated AEs include alopecia, dermatitis, erythema, high bilirubin, and odynophagia.
Grade 2 unrelated AEs include AST increased, diarrhea, esophagitis, fatigue, odynophagia, rectal bleeding, and vomiting.
Grade 3 unrelated AEs include dehydration, dysphagia, dyspnea, nausea, pain in extremity, and pulmonary embolism
CTCAE, Common Terminology Criteria for Adverse Events version 3
Group Differences in Symptom Outcomes
At baseline, fatigue severity did not significantly differ between the minocycline and placebo groups. The MDASI AUC component score for the pre-specified primary outcomes (pain, fatigue, disturbed sleep, lack of appetite, and sore throat) significantly differed between the minocycline and placebo groups (mean MDASI composite score ±SD; 17.45±9.08 vs. 24.56±15.45 ), with a medium effect size (P=0.044, ES=0.56). For the five most severe symptoms observed in the study, which were fatigue, coughing, shortness of breath, pain, and poor appetite, we also observed significant symptom reduction in the minocycline group compared with the placebo group (mean MDASI composite score ±SD; 22.51±11.23 vs. 32.01±17.77 ) (P=0.026, ES=0.64; Table 3).
Table 3.
AUCs for symptom outcomes by treatment group
| Outcome | Placebo (n=21) | Minocycline (n=19) |
P value (one-sided t-test) |
Cohen’sd effect size |
||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Pre-specified primary outcomes: fatigue, pain, disturbed sleep, poor appetite, sore throat | 24.56 | 15.45 | 17.45 | 9.08 | 0.044 | 0.56 |
| Five most severe adverse effects in the study: fatigue, coughing, shortness of breath, pain, poor appetite | 32.01 | 17.77 | 22.51 | 11.23 | 0.026 | 0.64 |
| Total interference | 20.05 | 18.45 | 14.11 | 10.21 | 0.211 | 0.40 |
| Fatigue | 44.98 | 20.90 | 31.18 | 14.22 | 0.011 | 0.77 |
| Pain | 26.64 | 21.56 | 17.13 | 12.40 | 0.046 | 0.54 |
| Disturbed sleep | 19.50 | 16.41 | 17.34 | 14.29 | 0.331 | 0.14 |
| Poor appetite | 27.31 | 24.95 | 17.16 | 15.76 | 0.069 | 0.49 |
| Shortness of breath | 32.45 | 23.02 | 21.53 | 15.76 | 0.029 | 0.55 |
| Sore throat | 4.36 | 9.51 | 4.45 | 7.23 | 0.489 | −0.01 |
| Coughing | 28.69 | 19.01 | 25.55 | 16.28 | 0.353 | 0.18 |
| Drowsiness | 24.17 | 19.09 | 20.61 | 14.83 | 0.224 | 0.21 |
AUC, area under the curve; SD, standard deviation. Numbers in bold indicate statistically significant P values.
Among individual symptom outcomes, the most impacted was fatigue (Table 3 and Figure 1); the fatigue reduction in the minocycline group compared with that in the placebo group had the largest effect size (31.18±14.2 vs. 44.98±20.9, Cohen’s d=0.77, P=0.011). The minocycline group also showed significant reductions in shortness of breath (21.53±15.76 vs. 32.45±23.02, Cohen’s d=0.55, P=0.029) and pain (17.13±12.40 vs. 26.64±21.56, Cohen’s d=0.54, P=0.046). There was no significant difference in the MDASI interference items, although the minocycline group showed less symptom interference than did the placebo group, with a small effect size ((mean total MDASI interference score ±SD; 14.11±10.21 vs. 20.05±18.45), Cohen’s d=0.40, P=0.211). A similar trend was observed in the lesser severity of poor appetite in the minocycline group compared with the placebo group (mean MDASI score ±SD; 17.16±15.76 vs. 27.31±24.95), Cohen’s d=0.49, P=0.069). No difference between groups was found for the lung symptom items (coughing (mean MDASI score ±SD; 25.55±16.28 vs. 28.69±19.01), Cohen’s d=0.18, P=0.353) and sore throat (mean MDASI score ±SD; 4.45±7.23 vs. 4.36±9.51), Cohen’s d=−0.01, P=0.489)).
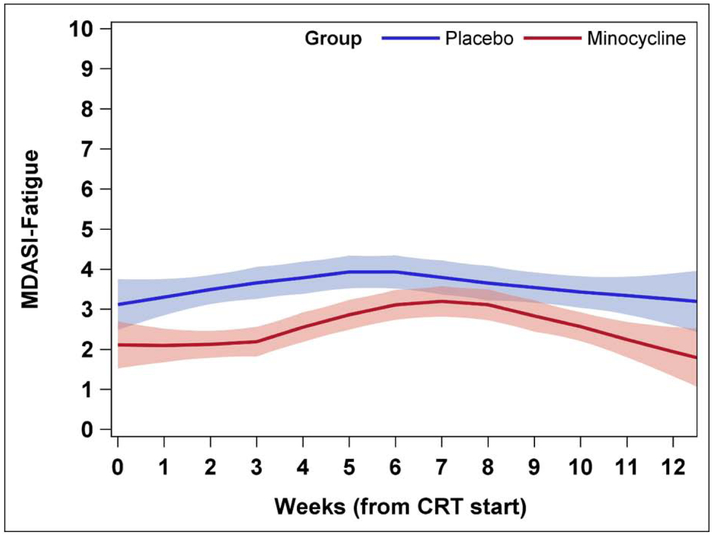
Figure 1. Mean severity of fatigue by treatment group.
Fatigue severity was measured on MD Anderson Symptom Inventory-Fatigue on 0-10 scale weekly during and after concurrent chemoradiation therapy. On longitudinal analysis, fatigue severity was significantly decreased in the minocycline group compared with the placebo group (est=−0.65, P=0.025), although fatigue significantly increased from baseline in both groups during the 7 weeks of chemoradiation therapy (est=0.18, P=0.0002) and decreased during weeks 7-12, after chemoradiation therapy (est=−0.38, P=0.025).
Longitudinal modeling of the entire sample revealed an expected significant increase in mean fatigue level from baseline during CRT (est=0.18, P=0.0002), a significant decrease in fatigue during weeks after completion of CRT (est=−0.38, P<0.0001), and a significantly lower mean fatigue level over the 12 weeks during and after CRT in the minocycline group compared with the placebo group (est=−0.65, P=0.025). On mixed modeling, there was a significant reduction in fatigue in the minocycline group compared with the placebo group over time (P=0.007), justified with covariance (baseline fatigue, age, race/ethnicity, cancer stage, comorbidity, ECOG-PS, education level, prior cancer treatment, and time in weeks).
Patients on minocycline reported better health-related quality of life (QoL) at week 12, measured by EQ-5D index (Mean±SD 0.89±0.14 compared to 0.82±0.13, P= 0.011) and single item QoL (Mean±SD 8.14±1.46 compared to 6.73±1.58, P=0.040). Overall, the patients were satisfied with the study medications, Supplementary Table 1.
DISCUSSION
In this phase II randomized, placebo-controlled trial in patients with NSCLC undergoing curative CRT, we observed a significant reduction in multiple patient-reported symptoms in those treated with minocycline compared with a placebo during the 12-week trial period. Minocycline was well tolerated, and compliance was high for the study medication and weekly PRO symptom assessments. Our results are consistent with the recent report of a significant symptom reduction in patients with head and neck cancer undergoing CRT or radiation therapy in a randomized phase II trial of minocycline versus placebo (21). Therefore, our findings encourage further clinical study of minocycline for symptom management in patients with NSCLC undergoing standard CRT.
Although we pre-specified a cluster of five PROs as the primary outcome of the trial, we learned that the five most severe symptoms in this trial were slightly different; coughing and shortness of breath were more severe than poor appetite and sore throat and so were included in a separate model also compared between groups individually. Nevertheless, for both outcome groupings, the minocycline group showed improvement in symptom severity compared with the placebo group, with similar effect sizes (Table 3). The consistency of the intervention’s beneficial effect on individual symptoms on MDASI items presents the underlines the strength of the effect of minocycline for managing the most troublesome CRT-induced symptom burden in this patient sample. Increased fatigue severity over time (Fig. 1) was driven by the accumulated dose of CRT, which is consistent with previous symptom research (3,4) ; however, this fatigue was significantly decreased in the treatment group compared with the placebo group in the weeks after CRT completion.
Mostly because of the difficulty of timing blood sample collection, the study was limited by a lack of a translational component to confirm the intervention’s benefit for inflammatory mechanisms. CRT-related fatigue has been associated with the inflammatory response (27) ; therefore, the mechanism of minocycline-induced fatigue reduction should be further studied. The second limitation of the study is lack of understanding of radiation parameters (such as PTV) associated effect of symptom reduction from minocycline. Although the randomized study design supports the result of this trial, it could be better reviewed in the future trial in these patients undergoing CRT.
The reduction in fatigue was the largest effect in our trial compared with the ES for other single symptoms on MDASI (Table 3). This result is encouraging after long frustration regarding fatigue reduction in oncology care with limited available pharmaceutical options (28-30). Furthermore, this study also demonstrated benefit from minocycline in the cluster of the most severe symptoms, including pain, poor appetite, and shortness of breath, all with a medium ES.
Adequate data on the appropriate timing of using the minocycline in relation to the cancer treatment course and the short/long-term effects of using minocycline on cancer treatment efficacy/resistance are lacking. Nevertheless, minocycline found to have a synergistic cytotoxicity effect (31-33), and its prophylactic use concurrent with anti-EGFR monoclonal antibodies was not associated with significant influence on tumor response in a retrospective study (18), thus safety and efficacy studies before concurrent use of minocycline and cancer treatment are warranted.
Although there is a growing role for immunotherapy in the treatment of NSCLC, the safety and efficacy of using minocycline concurrently or shortly after immunotherapy in patients with NSCLC have not yet been investigated. Yet, the immunomodulatory effect of minocycline has been successfully used in the treatment of the adverse events of immunotherapy in non-cancer conditions (34), and treated psychosis that may be associated with immunotherapy (35). However, minocycline or its metabolites could interact with the immune system and (36) triggers severe autoimmune conditions (37), thus, cancer patients on minocycline should be carefully monitored (38), especially if they are receiving immunotherapy. Underlying genetics mechanism was also proposed as another explanation for the minocycline-induced autoimmune phenomena (39).
The current trial has been conducted in the pre-immunotherapy era. Nevertheless, the drug interaction between minocycline and novel immunotherapy agents should be examined before moving to Phase III clinical study. Considering that steroids could be used in patients with NSCLC to relieve the RT-induced pneumonitis or immunotherapy-induced adverse events, concurrent use of minocycline with steroids also has not been adequately monitored in cancer patients, yet, minocycline found to have a steroid sparing effect in non-cancer conditions (40).
CONCLUSION
Minocycline for the management of CRT-related symptoms is feasible and has a low toxicity profile at 100 mg twice a day. Using minocycline at this dose yields a positive signal of its ability to reduce fatigue with a large effect size and to reduce the burden of the most severe symptoms overall with a medium effect size both during and after CRT. The results warrant a phase III trial of minocycline for CRT-related symptom reduction in patients with NSCLC.
Supplementary Material
Acknowledgments
Financial support: This study was partially by grants from the National Cancer Institute of the National Institutes of Health: NCI CA132109; 5P01CA021239, and MD Anderson Cancer Center Support Grant NCI P30 CA016672. The funding agency played no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Conflict of interest disclosure statement: These findings were partly presented at the American Society of Clinical Oncology 2016 Annual Conference, June 4, 2016, Chicago, IL. The MDASI is licensed to The University of Texas MD Anderson Cancer Center. The authors report no other conflicts of interest in this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov number:
References
- 1.Feliciano J, Feigenberg S, Mehta M. Chemoradiation for definitive, preoperative, or postoperative therapy of locally advanced non-small cell lung cancer. Cancer journal (Sudbury, Mass) 2013;19:222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hickok JT, Morrow GR, Roscoe JA, et al. Occurrence, severity, and longitudinal course of twelve common symptoms in 1129 consecutive patients during radiotherapy for cancer. J Pain Symptom Manage 2005;30:433–42. [DOI] [PubMed] [Google Scholar]
- 3.Wang XS, Shi Q, Williams LA, et al. Prospective study of patient-reported symptom burden in patients with non-small-cell lung cancer undergoing proton or photon chemoradiation therapy. J Pain Symptom Manage 2016;51:832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang XS, Fairclough DL, Liao Z, et al. Longitudinal study of the relationship between chemoradiation therapy for non-small-cell lung cancer and patient symptoms. J Clin Oncol 2006;24:4485–91. [DOI] [PubMed] [Google Scholar]
- 5.Barsevick AM, Whitmer K, Nail LM, et al. Symptom cluster research: Conceptual, design, measurement, and analysis issues. J Pain Symptom Manage 2006;31:85–95. [DOI] [PubMed] [Google Scholar]
- 6.Wang XS, Shi Q, Williams LA, et al. Inflammatory cytokines are associated with the development of symptom burden in patients with nsclc undergoing concurrent chemoradiation therapy. Brain Behav Immun 2010;24:968–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siva S, MacManus M, Kron T, et al. A pattern of early radiation-induced inflammatory cytokine expression is associated with lung toxicity in patients with non-small cell lung cancer. PloS one 2014;9:e109560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleeland CS. Cancer-related symptoms. Seminars in radiation oncology 2000;10:175–90. [DOI] [PubMed] [Google Scholar]
- 9.Majidi J, Kosari-Nasab M, Salari A-A. Developmental minocycline treatment reverses the effects of neonatal immune activation on anxiety- and depression-like behaviors, hippocampal inflammation, and hpa axis activity in adult mice. Brain Research Bulletin 2016;120:1–13. [DOI] [PubMed] [Google Scholar]
- 10.Liu H-Y, Yue J, Hu L-N, et al. Chronic minocycline treatment reduces the anxiety-like behaviors induced by repeated restraint stress through modulating neuroinflammation. Brain Research Bulletin 2018;143:19–26. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz T, Krabbe G, Weikert G, et al. Minocycline protects the immature white matter against hyperoxia. Experimental Neurology 2014;254:153–165. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Chen J, Zhan Y, et al. Low level segmentation of motion capture data based on cosine distance. 2015 3rd International Conference on Computer, Information and Application 2015. pp. 26–28. [Google Scholar]
- 13.Garrido-Mesa N, Zarzuelo A, Gálvez J. Minocycline: Far beyond an antibiotic. British Journal of Pharmacology 2013;169:337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padi SS, Kulkarni SK. Minocycline prevents the development of neuropathic pain, but not acute pain: Possible anti-inflammatory and antioxidant mechanisms. European journal of pharmacology 2008;601:79–87. [DOI] [PubMed] [Google Scholar]
- 15.Tilley BC, Alarcon GS, Heyse SP, et al. Minocycline in rheumatoid arthritis. A 48-week, double-blind, placebo-controlled trial. Mira trial group. Annals of internal medicine 1995;122:81–9. [DOI] [PubMed] [Google Scholar]
- 16.Melosky B, Anderson H, Burkes RL, et al. Pan canadian rash trial: A randomized phase iii trial evaluating the impact of a prophylactic skin treatment regimen on epidermal growth factor receptor-tyrosine kinase inhibitor–induced skin toxicities in patients with metastatic lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2016;34:810–5. [DOI] [PubMed] [Google Scholar]
- 17.Leporini C, Saullo F, Filippelli G, et al. Management of dermatologic toxicities associated with monoclonal antibody epidermal growth factor receptor inhibitors: A case review. Journal of Pharmacology and Pharmacotherapeutics 2013;4:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada M, Iihara H, Fujii H, et al. Prophylactic effect of oral minocycline in combination with topical steroid and skin care against panitumumab-induced acneiform rash in metastatic colorectal cancer patients. Anticancer research 2015;35:6175–81. [PubMed] [Google Scholar]
- 19.Scope A, Agero AL, Dusza SW, et al. Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2007;25:5390–6. [DOI] [PubMed] [Google Scholar]
- 20.Shinohara A, Ikeda M, Okuyama H, et al. Efficacy of prophylactic minocycline treatment for skin toxicities induced by erlotinib plus gemcitabine in patients with advanced pancreatic cancer: A retrospective study. American journal of clinical dermatology 2015;16:221–9. [DOI] [PubMed] [Google Scholar]
- 21.Gunn GB, Mendoza TR, Garden AS, et al. Minocycline for symptom reduction during radiation therapy for head and neck cancer: A randomized clinical trial. Support Care Cancer 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang XS, Shi Q, Bhadkamkar NA, et al. Minocycline for symptom reduction during oxaliplatin-based chemotherapy for colorectal cancer: A phase ii randomized clinical trial. J Pain Symptom Manage 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pachman DR, Dockter T, Zekan PJ, et al. A pilot study of minocycline for the prevention of paclitaxel-associated neuropathy: Accru study ru221408i. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 2017;25:3407–3416. [DOI] [PubMed] [Google Scholar]
- 24.Ichiki M, Wataya H, Yamada K, et al. Preventive effect of kampo medicine (hangeshashin-to, tj-14) plus minocycline against afatinib-induced diarrhea and skin rash in patients with non-small cell lung cancer. OncoTargets and therapy 2017;10:5107–5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: The m.D. Anderson symptom inventory. Cancer 2000;89:1634–46. [DOI] [PubMed] [Google Scholar]
- 26.Mendoza TR, Wang XS, Lu C, et al. Measuring the symptom burden of lung cancer: The validity and utility of the lung cancer module of the m. D. Anderson symptom inventory. The oncologist 2011;16:217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang XS, Williams LA, Krishnan S, et al. Serum stnf-r1, il-6, and the development of fatigue in patients with gastrointestinal cancer undergoing chemoradiation therapy. Brain Behav Immun 2012;26:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patrick DL, Ferketich SL, Frame PS, et al. National institutes of health state-of-the-science conference statement: Symptom management in cancer: Pain, depression, and fatigue, july 15-17, 2002. Journal of the National Cancer Institute 2003;95:1110–7. [DOI] [PubMed] [Google Scholar]
- 29.Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: A meta-analysis. JAMA oncology 2017;3:961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomlinson D, Robinson PD, Oberoi S, et al. Pharmacologic interventions for fatigue in cancer and transplantation: A meta-analysis. Current oncology (Toronto, Ont) 2018;25:e152–e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu FY, Wu YH, Zhou SJ, et al. Minocycline and cisplatin exert synergistic growth suppression on hepatocellular carcinoma by inducing s phase arrest and apoptosis. Oncology reports 2014;32:835–44. [DOI] [PubMed] [Google Scholar]
- 32.Ko JC, Wang TJ, Chang PY, et al. Minocycline enhances mitomycin c-induced cytotoxicity through down-regulating erk1/2-mediated rad51 expression in human non-small cell lung cancer cells. Biochemical pharmacology 2015;97:331–40. [DOI] [PubMed] [Google Scholar]
- 33.Teicher BA, Holden SA, Liu CJ, et al. Minocycline as a modulator of chemotherapy and hyperthermia in vitro and in vivo. Cancer letters 1994;82:17–25. [DOI] [PubMed] [Google Scholar]
- 34.Vinay K, Narang T, Saikia UN, et al. Minocycline successfully treats exaggerated granulomatous hypersensitivity reaction to mw immunotherapy. Dermatologic Therapy 2017;30:e12452. [DOI] [PubMed] [Google Scholar]
- 35.Xiang YQ, Zheng W, Wang SB, et al. Adjunctive minocycline for schizophrenia: A meta-analysis of randomized controlled trials. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 2017;27:8–18. [DOI] [PubMed] [Google Scholar]
- 36.Angulo JM, Sigal LH, Espinoza LR. Coexistent minocycline-induced systemic lupus erythematosus and autoimmune hepatitis. Seminars in arthritis and rheumatism 1998;28:187–92. [DOI] [PubMed] [Google Scholar]
- 37.Tehrani R, Nash-Goelitz A, Adams E, et al. Minocycline-induced cutaneous polyarteritis nodosa. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases 2007;13:146–9. [DOI] [PubMed] [Google Scholar]
- 38.Solmi M, Veronese N, Thapa N, et al. Systematic review and meta-analysis of the efficacy and safety of minocycline in schizophrenia. CNS Spectrums 2017;22:415–426. [DOI] [PubMed] [Google Scholar]
- 39.Dunphy J, Oliver M, Rands AL, et al. Antineutrophil cytoplasmic antibodies and hla class ii alleles in minocycline-induced lupus-like syndrome. The British journal of dermatology 2000;142:461–7. [DOI] [PubMed] [Google Scholar]
- 40.Daoud A, Gloria CJ, Taningco G, et al. Minocycline treatment results in reduced oral steroid requirements in adult asthma. Allergy and asthma proceedings 2008;29:286–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.