Abstract
Background
Traumatic brain injury is a leading cause of death and disability. Corticosteroids have been widely used in treating people with traumatic brain injury.
Objectives
To quantify the effectiveness and safety of corticosteroids in the treatment of acute traumatic brain injury.
Search methods
We searched: CENTRAL (The Cochrane Library 2007, Issue 4), MEDLINE (Ovid SP), PubMed [www.ncbi.nlm.nih.gov/sites/entrez/], EMBASE (Ovid SP) and PsycINFO (Ovid SP). The searches were last updated in January 2008.
Selection criteria
All randomised controlled trials of corticosteroid use in acute traumatic brain injury with adequate or unclear allocation concealment.
Data collection and analysis
Both authors independently scored quality of allocation concealment. Study authors were contacted for additional information. One author independently extracted data on numbers of participants randomised, numbers lost to follow up, length of follow up, case fatality rates, disablement, infections and gastrointestinal bleeds and this was checked by the other author.
Main results
We identified 20 trials with 12,303 randomised participants. The effect of corticosteroids on the risk of death was reported in 17 included trials. Due to significant heterogeneity we did not calculate a pooled estimate of the risk of death. The largest trial, with about 80% of all randomised participants, found a significant increase in the risk ratio of death with steroids 1.15 (95% CI 1.07 to 1.24) and a relative risk of death or severe disability of 1.05 (95% CI 0.99 to 1.10). For infections the pooled risk ratio from five trials was 1.03 (95% CI 0.99 to 1.07) and for the ten trials reporting gastrointestinal bleeding 1.23 (95% CI 0.91 to 1.67).
Authors' conclusions
In the absence of a meta‐analysis, we feel most weight should be placed on the result of the largest trial. The increase in mortality with steroids in this trial suggest that steroids should no longer be routinely used in people with traumatic head injury.
Plain language summary
Corticosteroids to treat brain injury
Traumatic brain injury is a leading cause of death and disability. After the injury the brain may swell, causing a potentially fatal condition called raised intracranial pressure (ICP). Corticosteroid drugs have been widely used, for many years, to treat patients with brain injury because they are thought to reduce intracranial pressure. Some examples of corticosteroids are dexamethasone and methylprednisolone.
The review authors searched the medical literature to determine how effective and safe corticosteroids are for treating brain injury. They focused their search on randomised controlled trials in which one group of people received a medical treatment (corticosteroids) and was compared with a similar group who received a different treatment or no treatment other than standard care. The review authors found 20 of these studies with 12,303 participants. When the review was first done the results of the research were inconclusive. A new large study with about 80% of the total participants was completed by the time of the 2006 update of this review. This study, called CRASH, showed a significant increase in number of deaths in patients given steroids compared with patients who received no treatment. The significant increase in deaths with steroids suggests that steroids should no longer be routinely used in people with traumatic head injury.
Background
Traumatic brain injury is a leading cause of premature death and disability. Road crashes account for the majority of fatal head injuries (Jennett 1996). Although road death rates are falling in most industrialised countries, in the rapidly motorising Asian countries they are rising, and will almost certainly continue to do so. Road death rates per head in China are already similar to those in the United States, in spite of the fact that there are only five vehicles per 1,000 population in China, compared with 770 vehicles per 1,000 population in the US (Roberts 1995). Overall, about 75% of the estimated 850,000 road crash deaths each year occur in the developing world (Murray 1994).
In the US, the incidence of brain injury related disability is estimated to be 33 new cases/100,000 people per year (Kraus 1993). Since this often occurs in young people and is long term, traumatic brain injury related disability is a major cause of ill health worldwide.
In 1961 Galicich and French reported rapid and significant improvement in response to corticosteroids in 28 of 34 people with cerebral oedema either due to brain tumours, or post‐operative (Galicich 1961). This led to their use in other intracranial problems characterized by raised intracranial pressure, including their use in severe head injury (Pickard 1993). Eighty percent of patients with fatal head injuries show evidence of increased intracranial pressure at necropsy (Miller 1992).
For a problem as common as brain injury, even a moderate reduction in mortality or disability from an intervention as widely practicable as corticosteroids would be important. There have been a number of randomised controlled trials of corticosteroids in head injury with apparently conflicting findings. Continuing uncertainty about the effects of corticosteroids for this indication is reflected in substantial variation in their use. A recent UK study found that corticosteroids were used in just under half of the intensive care units surveyed (Jeevaratnam 1996).
Objectives
To quantify the effectiveness of corticosteroids in reducing mortality and morbidity in people with acute traumatic brain injury.
To quantify the incidence of side effects of the use of corticosteroids.
To quantify the economic effects of corticosteroid use in this situation.
Methods
Criteria for considering studies for this review
Types of studies
We sought to identify all randomised controlled trials of a corticosteroid drug versus any control in the treatment of acute traumatic brain injury. Studies using a quasi random form of allocation were excluded from the review.
Types of participants
People of all ages with clinically diagnosed acute traumatic brain injury secondary to head injury who were treated with steroids or control within seven days of the injury. All severities of head injury were included.
Types of interventions
The experimental intervention was corticosteroids (those steroids with predominantly glucocorticoid effects, namely prednisolone, betamethasone, cortisone, dexamethasone, hydrocortisone, methylprednisolone, prednisone and triamcinolone) administered in any dose by any route for any duration started within seven days of the injury. Trials with these interventions were included irrespective of other treatments used.
Types of outcome measures
All causes of case fatality, any valid and reliable measure of neurological functioning, any other valid and reliable quality of life measures and economic outcomes were considered relevant if available. We sought numbers of infections (however defined) and significant gastrointestinal bleeds (however defined).
Search methods for identification of studies
The searches were not restricted by date, language or publication status.
Electronic searches
We searched the following databases:
CENTRAL (The Cochrane Library 2007, Issue 4);
MEDLINE (Ovid SP) 1950 to Nov (week 2) 2007;
PubMed [www.ncbi.nlm.nih.gov/sites/entrez/] (searched 7 Jan 2008: added to PubMed in the last 60 days);
EMBASE (Ovid SP) 1980 to (week 1) Jan 2008;
PsycINFO (Ovid SP) 1806 to April 2007.
The search strategies used for previous versions of this review can be found in Appendix 1. The strategies used for this update can be found in Appendix 2.
Searching other resources
We also searched specialised databases, handsearched journals and contacted trialists.
Data collection and analysis
We each extracted the following information independently from each trial: strategy for allocation concealment, number of randomised patients, duration of follow up and number lost to follow up. The major outcome data sought were numbers of deaths and numbers of people disabled at the end of the study period, using the Glasgow Outcome Scale (Jennett 1975) to assess the neurological outcome; the categories for persistent vegetative state and moderate disability were combined into 'disability' for this review. This enabled inclusion of the one trial which did not use the Glasgow Outcome Scale but a similar ordinal categorisation of function. We also extracted data on side effects or complications where these were reported, using the authors' definitions of these complications.
Since there is evidence that the quality of allocation concealment particularly affects the results of studies (Higgins 2008), each of us scored this quality on the scale used by Higgins (Higgins 2008) as shown below, assigning 'No' to poorest quality and 'Yes' to best quality:
No = trials in which concealment was inadequate (such as alternation or reference to case record numbers or to dates of birth);
Unclear = trials in which the authors either did not report an allocation concealment approach at all or reported an approach that did not fall into one of the other categories;
Yes = trials deemed to have taken adequate measures to conceal allocation (i.e. central randomizations; numbered or coded bottles or containers; drugs prepared by the pharmacy; serially numbered, opaque, sealed envelopes; or other description that contained elements convincing of concealment).
If the method used to conceal allocation was not clearly reported, we contacted the author whenever possible, for clarification. We then compared the scores allocated and resolved differences by discussion.
We calculated relative risks and 95% confidence intervals for mortality for each trial on an intention to treat basis. Heterogeneity between trials was tested using a chi‐squared test, where P less than or equal to 0.05 was taken to indicate significant heterogeneity. As long as statistical heterogeneity did not exist, for dichotomous data, we calculated summary relative risks and 95% confidence intervals using a fixed‐effect model.
2004 update
For the October 2004 update of the review, the Cochrane Injuries Group staff searched the Group's register for more trials but found none. In October 2004, the initial results of the CRASH trial, previously listed as an ongoing study, were published. Since Ian Roberts was a principal investigator on this trial, Phil Alderson extracted data and updated the review, with Ian Roberts checking for correctness.
The inclusion of the CRASH trial introduced significant heterogeneity, and the original review's methods section did not clearly specify how heterogeneity would be investigated. It was therefore decided that the only investigation of heterogeneity would be to undertake a sensitivity analysis, removing those trials with less than adequate allocation concealment. If that failed to remove heterogeneity, the trials would not be pooled in the review, and reasons for heterogeneity suggested but not examined formally.
2006 update
Searches were repeated by the Cochrane Injuries Group in November 2005. The final results of CRASH are now available and have been included. No other new data were identified by the searches. Phil Alderson added the new data and updated the text of the review. As the results of CRASH dominate the other trial results for death or severe disability, the results were not pooled.
2009 update
The search was updated by the Cochrane Injuries Group in January 2008. No new trials were found; the results and conclusions remain the same.
Results
Description of studies
The combined search strategies identified 19 trials which satisfied the inclusion criteria. The earliest was from 1972 and the most recent from 1995. There were two reports of the same trial (see Fanconi 1988). Two were previously unpublished studies for which outcome data were obtained (Hernesniemi 1979; Pitts 1980). For one other unpublished study (Tahara 1972) the authors were unable to provide outcome data, and in one other we have been unable to trace the author (Hoyt 1972). The 2004 update added one more trial (CRASH 2005), which is the largest of all the trials in the review.
A total of 11,792 participants are included in outcome of death.
Risk of bias in included studies
Methodological quality was variable − see table 'Characteristics of included studies' for details.
Effects of interventions
The effect of corticosteroids on the risk of death was reported in 17 included trials. There was significant heterogeneity for this outcome when using a fixed‐effect risk ratio model (Chi2 26.46, P = 0.03, I2 43%). Excluding the trials with less than adequate allocation concealment failed to remove the heterogeneity, and so the trials were not pooled. The largest single trial (CRASH 2005) reported a risk ratio for death of 1.18 (95% CI 1.09 to 1.27), indicating a significant increase in death with steroids.
The CRASH trial reported a relative risk of 1.05 (95%CI 0.99 to 1.10) for death or severe disability. There was no significant heterogeneity between the results from the 10 trials reporting this outcome, but it was decided not to pool the results as the data from CRASH dominate the other results.
Five trials reported infections, and the pooled relative risk was 1.03 (95% CI 0.99 to 1.07) making a decrease in infectious complications unlikely with steroids. For the ten trials reporting gastrointestinal bleeding the pooled relative risk was 1.23 (95% CI 0.91 to 1.67) which neither confirms nor excludes an important increase or decrease in this complication. For both these outcomes, data on about 2% of the CRASH trial participants were missing, and these patients have been excluded from the analysis. Sensitivity analysis was not undertaken due to the low proportion and because it had not been prespecified.
No study included economic data.
Discussion
This systematic review summarises the evidence from randomised controlled trials of corticosteroids in acute traumatic brain injury.
Methodological issues
The inclusion of an EMBASE search identified one study not found on MEDLINE (Tahara 1972). Contact with trialists enabled us to include data from two large unpublished studies (Hernesniemi 1979; Pitts 1980), but not from others (Hoyt 1972; Tahara 1972).
The addition of the CRASH 2005 trial's early results introduced significant heterogeneity. A sensitivity analysis based on the quality of allocation concealment failed to remove this. The most obvious reason for the presence of heterogeneity is the contrasting results of the Faupel 1976 and CRASH 2005 trials. It is difficult to come up with a convincing reason post hoc for excluding either of these trials from a meta‐analysis. We noted in a previous version of this review that in the Faupel trial "...the outcome was assessed at discharge", yet overall 19% of the participants were classified as "unconscious stabilized". The apparently short follow up period may account for the incongruous result. Other sources of variation between trials may include severity and pathology of the head injury, variations in corticosteroid regimens (e.g. drug, dose, route) and temporal trends in the use of other interventions. The CRASH trial was stopped early because of the apparent harmful effect of steroids, and stopping trials because of extreme results may select extreme results by chance. Trials of the use of corticosteroids in spinal cord injury suggest that the timing of administration is important (Bracken 1990), but the CRASH trial followed the protocol from spinal cord trials closely, so this is unlikely to explain the excess mortality in the CRASH trial. However, as none of these hypotheses was listed in the original review methods, we think it is safer not to pool the trial results.
There is no clear evidence of a difference in the occurrence of infectious complications and the risk of gastrointestinal bleeding with steroids, making these an unlikely explanation of the increased mortality in the CRASH trial.
In the absence of a meta‐analysis, interpretation of the whole body of evidence has to be qualitative. The high methodological quality and large size of the CRASH trial suggest that its result should be the main basis of a summary. This is reinforced by its finding of a significant increase in mortality, which should not be ignored despite the more optimistic results in some other trials.
Authors' conclusions
Implications for practice.
The results of the large CRASH trial suggest that steroids should not be used in head injury, as they appear to increase mortality. Despite the heterogeneity, we feel that the results of this trial are the most relevant to current practice and should be the basis for clinical decisions, rather than any of the earlier trials or their meta‐analysis.
Implications for research.
The mechanism of harm is unclear and there will need to be a reassessment of the understanding of the pathophysiology of traumatic brain injury. There seems no reason to start other trials.
What's new
| Date | Event | Description |
|---|---|---|
| 7 January 2008 | New search has been performed | New studies sought but none found. |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 3, 1997
| Date | Event | Description |
|---|---|---|
| 7 July 2008 | Amended | Converted to new review format. |
| 14 February 2006 | New search has been performed | The finalised data for CRASH 2005 have been incorporated. |
| 1 November 2005 | New search has been performed | New studies sought but none found. |
Acknowledgements
We wish to acknowledge help and advice from Dr Iain Chalmers in preparing this review, Julia Langham, Leah Lepage and Yoichi Nagayama in identifying trials, Dr Anthony Rodgers and Dr Colin Baigent for comments on a draft, Liz Norman and Jini Hetherington for proof reading and those trialists who we were able to contact for their cooperation.
Appendices
Appendix 1. Previous search strategies
CENTRAL SEARCH STRATEGY (The Cochrane Library 2005, Issue 4) #1 (head or crani* or capitis or brain* or forebrain* or skull* or hemisphere* or intracran* or orbit*) in ab or ti #2 (injur* or trauma* or lesion* or damag* or wound* or destruction* or oedema* or edema* or fracture* or contusion* or commotion* or pressur*) in ab or ti #3 (#1 and #2) #4 BRAIN INJURIES #5 DIFFUSE AXONAL INJURY #6 CRANIOCEREBRAL TRAUMA #7 #3 or #4 or #5 or #6 #8 (steroid* or glucocorticoid* or prednisolone* or betamethasone* or cortisone* or dexamethasone* or hydrocortisone* or methylprednisolone* or prednisone* or triamcinolone* or corticosteroid*) in ab or ti #9 GLUCOCORTICOIDS #10 ADRENAL CORTEX HORMONES #11 (#8 or #9 or #10) #12 (#7 and #11)
MEDLINE SEARCH STRATEGY (1966 to November 2005) 1. explode "Craniocerebral‐Trauma" / all SUBHEADINGS in MIME,MJME 2. (head or crani* or capitis or brain* or forebrain* or skull* or hemisphere* or intracran* or orbit*) near (injur* or trauma* or lesion* or damag* or wound* or destruction* or oedema* or edema* or fracture* or contusion* or commotion* or pressur*) 3. 1 or 2 4. explode "Adrenal‐Cortex‐Hormones" / all SUBHEADINGS in MIME,MJME 5. explode "Glucocorticoids‐" / all SUBHEADINGS in MIME,MJME 6. steroid* or glucocorticoid* or prednisolone* or betamethasone* or cortisone* or dexamethasone* or hydrocortisone* or methylprednisolone* or prednisone* or triamcinolone* or corticosteroid* 7. 4 or 5 or 6 8. 3 and 7 9. 8 and (Cochrane highly sensitive RCT strategy)
EMBASE SEARCH STRATEGY (1980 to November 2005, week 46) 1. exp Head Injury/ 2. ((head or crani$ or capitis or brain$ or forebrain$ or skull$ or hemisphere$ or intracran$ or orbit$) adj3 (injur$ or trauma$ or lesion$ or damag$ or wound$ or destruction$ or oedema$ or edema$ or fracture$ or contusion$ or commotion$ or pressur$)).mp.[title,abstract] 3. 1 or 2 4. exp CORTICOSTEROID THERAPY/ 5. (steroid$ or glucocorticoid$ or prednisolone$ or betamethasone$ or cortisone$ or dexamethasone$ or hydrocortisone$ or methylprednisolone$ or prednisone$ or triamcinolone$ or corticosteroid$).mp. [title, abstract] 6. Glucocorticoid/dt [Drug Therapy] 7. 4 or 5 or 6 8. 3 and 7
Appendix 2. Search strategy: 2008 update
CENTRAL (The Cochrane Library 2007, Issue 4) #1MeSH descriptor Craniocerebral Trauma explode all trees #2MeSH descriptor Cerebrovascular Trauma explode all trees #3MeSH descriptor Brain Edema explode all trees #4(brain or cerebral or intracranial) near3 (oedema or edema or swell*) #5MeSH descriptor Glasgow Coma Scale explode all trees #6MeSH descriptor Glasgow Outcome Scale explode all trees #7MeSH descriptor Unconsciousness explode all trees #8glasgow near3 (coma or outcome) near3 (score or scale) #9(Unconscious* or coma* or concuss* or 'persistent vegetative state') near 3 (injur* or trauma* or damag* or wound* or fracture*) #10"Rancho Los Amigos Scale" #11(head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or inter‐cran*) near3 (injur* or trauma* or damag* or wound* or fracture* or contusion*) #12Diffuse near3 axonal near3 injur* #13(head or crani* or cerebr* or brain* or intra‐cran* or inter‐cran*) near3 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressure) #14(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13) PubMed [www.ncbi.nlm.nih.gov/sites/entrez/] (searched 7 Jan 2008 (added to PubMed in the last 60 days) #1Craniocerebral Trauma [mh] OR Brain Edema [mh] OR Glasgow Coma Scale [mh] OR Glasgow Outcome Scale [mh] OR Unconsciousness [mh] OR Cerebrovascular Trauma [mh] OR ((head OR cranial OR cerebral OR brain* OR intra‐cranial OR inter‐cranial) AND (haematoma* OR hematoma* OR haemorrhag* OR hemorrhage* OR bleed* OR pressure)) OR (Glasgow AND scale) OR ("diffuse axonal injury" OR "diffuse axonal injuries") OR ("persistent vegetative state") OR ((unconscious* OR coma* OR concuss*) AND (injury* OR injuries OR trauma OR damage OR damaged OR wound* OR fracture* OR contusion* OR haematoma* OR hematoma* OR haemorrhag* OR hemorrhag* OR bleed* OR pressure)) #2(randomised OR randomized OR randomly OR random order OR random sequence OR random allocation OR randomly allocated OR at random OR randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh]) NOT ((models, animal[mh] OR Animals[mh] OR Animal Experimentation[mh] OR Disease Models, Animal[mh] OR Animals, Laboratory[mh]) NOT (Humans[mh])) #3Search #1 AND #2 Limits: published in the last 60 days MEDLINE (Ovid SP) 1950 to Nov (week 2) 2007 1.exp Craniocerebral Trauma/ 2.exp Brain Edema/ 3.exp Glasgow Coma Scale/ 4.exp Glasgow Outcome Scale/ 5.exp Unconsciousness/ 6.exp Cerebrovascular Trauma/ 7.((head or crani$ or cerebr$ or capitis or brain$ or forebrain$ or skull$ or hemispher$ or intra‐cran$ or inter‐cran$) adj3 (injur$ or trauma$ or damag$ or wound$ or fracture$ or contusion$)).ab,ti. 8.((head or crani$ or cerebr$ or brain$ or intra‐cran$ or inter‐cran$) adj3 (haematoma$ or hematoma$ or haemorrhag$ or hemorrhag$ or bleed$ or pressure)).ti,ab. 9.(Glasgow adj3 (coma or outcome) adj3 (scale$ or score$)).ab,ti. 10."rancho los amigos scale".ti,ab. 11.("diffuse axonal injury" or "diffuse axonal injuries").ti,ab. 12.((brain or cerebral or intracranial) adj3 (oedema or edema or swell$)).ab,ti. 13.((unconscious$ or coma$ or concuss$ or 'persistent vegetative state') adj3 (injur$ or trauma$ or damag$ or wound$ or fracture$)).ti,ab. 14.or/1‐13 15. (randomised or randomized or randomly or random order or random sequence or random allocation or randomly allocated or at random or controlled clinical trial$).tw,hw. 16.clinical trial.pt. 17.randomized controlled trial.pt. 18.17 or 18 or 19 19.exp models, animal/ 20.exp Animals/ 21.exp Animal Experimentation/ 22.exp Disease Models, Animal/ 23.exp Animals, Laboratory/ 24.or/21‐25 25.Humans/ 26.20 not 25 27.18 not 26 28.14 and 27 29.2007$.ed. 30.28 and 29 EMBASE (Ovid SP) 1980 to (week 1) Jan 2008 1.exp Brain Injury/ 2.exp Brain Edema/ 3.exp Glasgow Coma Scale/ 4.exp Glasgow Outcome Scale/ 5.exp Rancho Los Amigos Scale/ 6.exp Unconsciousness/ 7.((brain or cerebral or intracranial) adj3 (oedema or edema or swell$)).ab,ti. 8.((head or crani$ or cerebr$ or capitis or brain$ or forebrain$ or skull$ or hemispher$ or intra‐cran$ or inter‐cran$) adj3 (injur$ or trauma$ or damag$ or wound$ or fracture$ or contusion$)).ab,ti. 9.(Glasgow adj3 (coma or outcome) adj3 (scale$ or score$)).ab,ti. 10.Rancho Los Amigos Scale.ab,ti. 11.((unconscious$ or coma$ or concuss$ or 'persistent vegetative state') adj3 (injur$ or trauma$ or damag$ or wound$ or fracture$)).ti,ab. 12.Diffuse axonal injur$.ab,ti. 13.((head or crani$ or cerebr$ or brain$ or intra‐cran$ or inter‐cran$) adj3 (haematoma$ or hematoma$ or haemorrhag$ or hemorrhag$ or bleed$ or pressure)).ab,ti. 14.or/1‐13 15.exp animal model/ 16.Animal Experiment/ 17.exp ANIMAL/ 18.exp Experimental Animal/ 19.1 or 2 or 3 or 4 20.Human/ 21.5 not 6 22.(randomised or randomized or randomly or random order or random sequence or random allocation or randomly allocated or at random or controlled clinical trial$).tw,hw. 23.exp clinical trial/ 24.8 or 9 25.10 not 7 26.14 and 25 27.2007$.em. 28.26 and 27 PsycINFO (Ovid SP) 1806 to April 2007 1.explode "Head‐Injuries" in MJ,MN 2.explode "Brain‐Damage" in MJ,MN 3.explode "Traumatic‐Brain‐Injury" in MJ,MN 4.explode "Brain‐Concussion" in MJ,MN 5.explode "Coma‐" in MJ,MN 6.Unconscious* or coma* or concuss* or "persistent vegetative state" 7.(head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or inter‐cran*) near (injur* or trauma* or damag* or wound* or fracture* or contusion*) 8.(head or crani* or cerebr* or brain* or intra‐cran* or inter‐cran*) near (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressure) 9.#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8
Data and analyses
Comparison 1. Any steroid administered in any dose against no steroid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death at end of follow up period | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Death or severe disability at the end of the study period | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Infections of any type | 5 | 10798 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.99, 1.07] |
| 4 Major or significant gastrointestinal bleed | 10 | 11302 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.91, 1.67] |
1.1. Analysis.
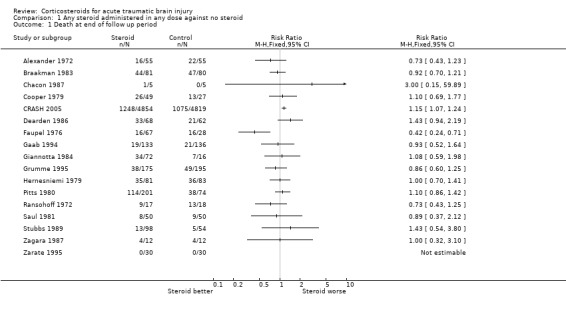
Comparison 1 Any steroid administered in any dose against no steroid, Outcome 1 Death at end of follow up period.
1.2. Analysis.
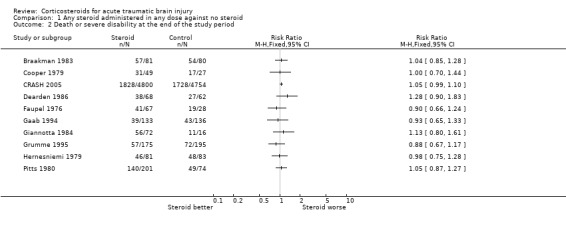
Comparison 1 Any steroid administered in any dose against no steroid, Outcome 2 Death or severe disability at the end of the study period.
1.3. Analysis.
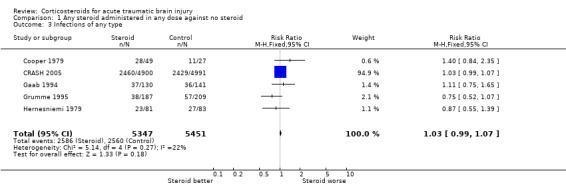
Comparison 1 Any steroid administered in any dose against no steroid, Outcome 3 Infections of any type.
1.4. Analysis.
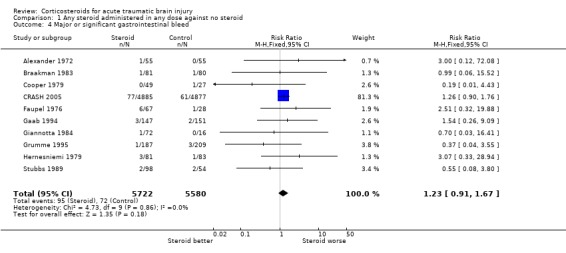
Comparison 1 Any steroid administered in any dose against no steroid, Outcome 4 Major or significant gastrointestinal bleed.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alexander 1972.
| Methods | Paper states that patients were randomized, half of them receiving dexamethasone. | |
| Participants | Those admitted to hospital with acute non missile head injuries who responded to painful stimuli by withdrawal or rigidity but who would not respond to voice command. | |
| Interventions |
|
|
| Outcomes | Death (time of assessment not stated). Complications. | |
| Notes | States all patients uniformly supervised. Author contacted, no further details of trial available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Unclear |
Braakman 1983.
| Methods | Randomized into two groups using identical vials in identical boxes prepared by a Pharmacy Department. Code not broken until all patients were evaluated for outcome. | |
| Participants | Patients of any age with severe non missile related head injury who were in a coma on admission to hospital. Coma was defined as no eye opening, no spoken response to painful stimuli and not obeying commands. Exclusions were those already brain dead (apnoea, flaccidity, dilated pupils not reacting to light, absence of reflex eye movements) or expected to become so within 1 hour, those who regained consciousness during initial examination, those who had already been given steroids and those with diabetes mellitus or a history of peptic ulcer. | |
| Interventions |
|
|
| Outcomes | Glasgow Coma Scale at 6 months after injury. Complication rate. | |
| Notes | Other care was determined by the result of a CT scan: those with a mass lesion had immediate operation, those without had monitored control of intracranial pressure using controlled ventilation and/or osmotic diuretic. Cerebrospinal fluid was drained in some patients. Barbiturates were not used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Adequate |
Braun 1986.
| Methods | Patients randomly assigned in emergency room to one of four groups. | |
| Participants | Those head trauma victims admitted from the emergency room to the adult neurosurgical intensive care unit. | |
| Interventions |
|
|
| Outcomes | Incidence of pneumonia defined as a new alveolar or alveolar‐interstitial infiltrate identified by a radiologist and one of the authors and two of:
|
|
| Notes | Otherwise treated with a standardised protocol including ICP monitoring, initial hyperventilation, hyperosmotic agents. Thiopentone in some. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Unclear |
Chacon 1987.
| Methods | Patients randomized 'by lottery method' to two groups. Blinding not clear. | |
| Participants | Children admitted to hospital with severe head trauma, a Glasgow Coma Score of 7 or less and evidence of cerebral oedema on computerised tomography. | |
| Interventions |
|
|
| Outcomes | Mortality at discharge. | |
| Notes | Other interventions ‐ fluid restriction guided by central venous pressure and urine output, hyperventilation to PCO2 of 25 to 30 torr, head up position of 35 to 40 degrees. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Unclear |
Cooper 1979.
| Methods | Random allocation using identical coded vials prepared and supplied by a drug company, who retained the code until the 6 month outcome assessment had been made. | |
| Participants | All patients with head injury admitted with a Grady Coma Grade of 3, 4 or 5. Glasgow Coma Scale also measured and in all except two patients GCS was 8 or less. Patients were excluded due to inability to obtain informed consent, previous administration of steroids or arrival at hospital more than 6 hours after the injury. | |
| Interventions |
All doses were contained in the same volume of fluid. Medication decreased gradually from day 7 and all were finished by day 11. Children aged 16 or younger given weight related doses. |
|
| Outcomes | GOS at 6 months after injury. | |
| Notes | CT scan used or diagnosis in most patients followed by operation for mass lesions. Other co‐interventions not specifically described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Adequate |
CRASH 2005.
| Methods | Stratified random allocation via central randomisation service or identical coded treatment packs. | |
| Participants | Adults (16 or older) less than 8 hours after head trauma with GCS of 14 or less on admission to hospital. | |
| Interventions |
|
|
| Outcomes | Mortality and GOS at 6 months. Complications (admission to intensive care, gastrointestinal bleeding, infections, neurosurgical operation). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Adequate |
Dearden 1986.
| Methods | Randomly allocated to receive treatment or placebo. | |
| Participants | All ages, patients with severe head injury (no definition given). Exclusions were due to incorrect administration of steroid or placebo during the trial and those receiving steroids before admission. | |
| Interventions |
|
|
| Outcomes | Glasgow Outcome Scale at 6 months after injury. Duration of artificial ventilation. | |
| Notes | Other interventions guided by CT and ICP monitoring. Controlled hypocapeic ventilation and osmotic diuretics used to control ICP. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Adequate |
Faupel 1976.
| Methods | Randomly allocated using identical, coded vials containing the same volumes of clear solution. | |
| Participants | All adult patients with severe (not defined) closed head injury. Exclusions were due to being a child (age cut off not given), impression fractures, missile injuries, imminent brain death and open head injuries. After randomization, 3 patients considered to be brain dead on angiography, one given steroids outside the trial and one who died of other injuries were excluded. | |
| Interventions |
|
|
| Outcomes | Disability status scale at discharge from hospital. Frequency of complications. | |
| Notes | Mass lesions removed following angiography. Other interventions not specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Adequate |
Gaab 1994.
| Methods | Patients were randomized in blocks of 6. Stated to be double blind design. | |
| Participants | Age 15 to 55 with moderate central nervous system injury (two or more of disturbed consciousness, eye opening to stimulation, no adequate verbal response, disorientation in time and place) or severe central nervous system injury (comatose on injury and/or on admission to hospital, no eye opening to painful stimuli). Patients also were required to have obvious neurological symptoms (e.g. hemiparesis or hemiplegia) or CT evidence of lesions requiring surgical intervention or hypodense area(s) in the brain. Reasons for exclusion were: time to treatment was more than 3 hours, they had already had steroids, penetrating head injury, primary bulbar symptoms present, prognosis considered hopeless, known malignancy, peptic ulcer, tuberculosis, Cushing's syndrome, non traumatic neurological or psychiatric disease, spinal injury, suspected coagulation defects. |
|
| Interventions |
|
|
| Outcomes | Modified GOS at 10 to 14 months (good recovery classified as fit for work or for rehabilitation, extra category indicating degree of care required for disabled). GCS on day 5. Interval to regaining consciousness. | |
| Notes | Co‐interventions are not specified, but the study states that other care was not controlled. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Adequate |
Giannotta 1984.
| Methods | Randomly allocated to treatment or placebo using identical, coded vials. Code not broken until all outcomes had been collected. | |
| Participants | Patients with blunt head trauma and a GCS of 8 or less 6 hours after the injury. Exclusions were due to history of peptic ulcer, an undiagnosed or untreated medical condition or who had been taking steroids during the two weeks before injury, penetrating brain injuries, other injuries expected to cause rapid death, and pregnancy. | |
| Interventions |
NB Patients randomized in 2:2:1 ratio for these groups. |
|
| Outcomes | Glasgow Outcome Scale at 6 months. | |
| Notes | CT guided management. Controlled ventilation, cerebrospinal fluid drainage, osmotic diuretics and barbiturates were used to control ICP. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Adequate |
Grumme 1995.
| Methods | Random allocation to treatment or control using identical vials. | |
| Participants | Patients of all ages admitted with head injury. Excluded groups were those with contraindication to steroids (not specified), impaired level of consciousness not due to trauma and absence of relevant brain damage (not specified). | |
| Interventions |
|
|
| Outcomes | GOS at discharge from hospital and at approximately 1 year from the injury. | |
| Notes | CT was used to guide treatment. Osmotic diuretics, controlled ventilation and cerebrospinal fluid drainage were used to control ICP. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Adequate |
Hernesniemi 1979.
| Methods | Random allocation using sealed opaque envelopes. Double blind. | |
| Participants | Age 15 or above with severe closed brain injury. Five exclusions; three not head injury, one 14 years old, one imminent death. | |
| Interventions |
|
|
| Outcomes | Glasgow Outcome Scale at 6 or 12 months. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Adequate |
Hoyt 1972.
| Methods | Assigned "sequentially in random order". | |
| Participants | Patients with cranial trauma. | |
| Interventions |
|
|
| Outcomes | Proportion demonstrating a "marked improvement". | |
| Notes | Unable to contact author for further details. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Unclear |
Pitts 1980.
| Methods | "Prospectively randomized" into three groups. | |
| Participants | Head injured adults admitted to hospital who were comatose on admission or who lapsed into coma for six hours or more. | |
| Interventions |
|
|
| Outcomes | Glasgow Outcome Scale at 6 months. Gastrointestinal bleeding. Infections (signs of pneumonia, wound infection, urinary infection, blood culture, CSF culture). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Adequate |
Ransohoff 1972.
| Methods | Stated to be "random steroid studies" and a double blind study of steroids against placebo. | |
| Participants | Critically ill acute closed head injury patients without evidence of angiographic shift or significant clots, but with documented increase in intracranial pressure. Age not stated. | |
| Interventions |
|
|
| Outcomes | Death. Complications (no data presented). | |
| Notes | Also received fluid restriction. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Unclear |
Saul 1981.
| Methods | States that 100 patients were "randomized into two groups of 50". One group received steroid, there was no placebo. | |
| Participants | Patients admitted with craniocerebral trauma within 6 hours of injury. No other body systems injured, Glasgow Coma Scale 7 or less on admission. Average age 31 years. | |
| Interventions |
|
|
| Outcomes | Glasgow Outcome Scale at 6 months. | |
| Notes | Also received mechanical hyperventilation and surgery if indicated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Unclear |
Stubbs 1989.
| Methods | "Randomized and double‐blind" study. Allocation concealment not described. Separate randomization for patients over and under 40 years old. | |
| Participants | Patients were more than 6 years old with closed head trauma within the previous 48 hours and a GCS of 9 to 12. The first dose had to be administered within 6 hours of the GCS measurement. Exclusions were pregnancy, hypersensitivity to steroids and presence of infectious disease. | |
| Interventions |
|
|
| Outcomes | Glasgow Outcome Scale, Glasgow Coma Scale, Karnofsky Rating Scale at discharge, 3 and 6 months. Complications listed. | |
| Notes | Glasgow Outcome Scale reported as mean score. Attempting to locate authors for further information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Unclear |
Tahara 1972.
| Methods | Random allocation to one of three groups. | |
| Participants | Seriously head injured. | |
| Interventions |
|
|
| Outcomes | None reported. | |
| Notes | Trial of 100 participants; author contacted but unable to provide details. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Unclear |
Zagara 1987.
| Methods | Randomization by table into steroid or no therapy groups. | |
| Participants | Average age 29. Severe isolated head trauma with average GCS of 5.7 (sd 1.2) in one group and 5.8 (sd 1.2) in the other. | |
| Interventions |
|
|
| Outcomes | Glasgow Outcome Scale at 3 months. Nitrogen balance. | |
| Notes | Also received mechanical hyperventilation, surgery if required, mannitol infusion and benzodiazepine sedation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Adequate |
Zarate 1995.
| Methods | "Randomly allocated", no further details given. 60 patients, 30 in each group. | |
| Participants | Children admitted with head injury with a Glasgow Coma Scale of 9 to 15. | |
| Interventions |
|
|
| Outcomes | Mortality at discharge. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Unclear |
CT: computerised tomography GCS: Glasgow Coma Scale CSF: Cerebrospinal Fluid GOS: Glasgow Outcome Scale ICP: intracranial pressure IM: Intramuscular IV: Intravenous MPSS: methylprednisolone sodium succinate
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cheng 1991 | Randomised trial of high versus low dose steroids. No group with no steroids. |
| Fanconi 1988 | Judged to have inadequate allocation concealment after contact with authors. |
| Gobiet 1976 | Study was retrospective. |
| James 1979 | Allocation consisted of the first four patients being given no or low dose steroids and the next five given high dose steroids. There was therefore no concurrent control group and no concealment of allocation. |
| Robertson 1985 | Patients were alternately allocated. |
Contributions of authors
Both authors selected studies for inclusion, extracted data and wrote the text. Both authors contacted trialists for further information.
Sources of support
Internal sources
Institute of Child Health, University of London, UK.
London School of Hygiene and Tropical Medicine, UK.
NHS R&D Programme, UK.
External sources
NHS R&D Programme, Mother and Child Health, UK.
Declarations of interest
Ian Roberts collaborated on the design and conduct of the CRASH trial of corticosteroids in head injury, funded by the UK Medical Research Council.
Philip Alderson has no known conflict of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Alexander 1972 {published data only}
- Alexander E. Medical management of closed head injuries. Clinical Neurosurgery 1972;19:210‐50. [DOI] [PubMed] [Google Scholar]
Braakman 1983 {published data only}
- Braakman R, Schouten HJA, Blaauw‐van Dishoeck M, Minderhoud JM. Megadose steroids in severe head injury. Journal of Neurosurgery 1983;58:326‐30. [DOI] [PubMed] [Google Scholar]
Braun 1986 {published data only}
- Braun SR, Levin AB, Clark KL. Role of corticosteroids in the development of pneumonia in mechanically ventilated head‐trauma victims. Critical Care Medicine 1986;14:198‐201. [DOI] [PubMed] [Google Scholar]
Chacon 1987 {published data only}
- Chacon L. Edema cerebral en traumatismo craneoencefalico severo en ninos tatados con y sin dexametasona. Medicina Critica Venezolana 1987;2:75‐9. [Google Scholar]
Cooper 1979 {published data only}
- Cooper PR, Moody S, Clark WK, Kirkpatrick J, Maravilla K, Gould AL, et al. Dexamethasone and severe head injury. Journal of Neurosurgery 1979;51:307‐16. [DOI] [PubMed] [Google Scholar]
CRASH 2005 {published data only}
- CRASH Trial Collaborators. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo‐controlled trial. Lancet 2004;364:1321‐8. [DOI] [PubMed] [Google Scholar]
- CRASH Trial Collaborators. Final results of MRC CRASH, a randomised placebo‐controlled trial of intravenous corticosteroid in adults with head injury ‐ outcomes at 6 months. Lancet 2005;365:1957‐9. [DOI] [PubMed] [Google Scholar]
Dearden 1986 {published data only}
- Dearden NM, Gibson JS, McDowall DG, Gibson RM, Cameron MM. Effect of high dose dexamethasone on outcome from severe head injury. Journal of Neurosurgery 1986;64:81‐8. [DOI] [PubMed] [Google Scholar]
Faupel 1976 {published data only}
- Faupel G. The influence of dexamethasone on the midbrain syndrome after severe head injury. In: Hartmann A, Brock M editor(s). Treatment of cerebral edema. Berlin: Springer‐Verlag, 1982:107‐14. [Google Scholar]
- Faupel G, Reulen HJ, Muller D, Schurmann. Clinical double blind study on the effects of dexamethasone on severe closed head injuries. Acta neurochirurgica 1977;36:277‐8. [Google Scholar]
- Faupel G, Reulen HJ, Muller D, Schurmann K. Double‐blind study on the effects of steroids on severe closed head injury. In: Pappius MM, Feindel W editor(s). Dynamics of Brain Edema. Berlin: Springer‐Verlag, 1976:337‐43. [Google Scholar]
Gaab 1994 {published data only}
- Gaab MR, Trost HA, Alcantara A, Karimi‐Nejad A, Moskopp D, Schultheiss R, et al. "Ultrahigh" dexamethasone in acute brain injury. Zentralblatt fur Neurochirurgie 1994;55:135‐43. [PubMed] [Google Scholar]
Giannotta 1984 {published data only}
- Giannotta SL, Weiss MH, Apuzzo MLJ, Martin E. High dose glucocorticoids in the management of severe head injury. Neurosurgery 1984;15:497‐501. [DOI] [PubMed] [Google Scholar]
Grumme 1995 {published data only}
- Grumme T, Baethmann A, Kolodziejczyk D, Krimmer J, Fischer M, Eisenhart Rothe BV, et al. Treatment of patients with severe head injury by triamcinolone: a prospective, controlled multicenter trial of 396 cases. Research in Experimental Medicine 1995;195:217‐29. [DOI] [PubMed] [Google Scholar]
Hernesniemi 1979 {unpublished data only}
- Hernesniemi J, Troupp H. A clinical retrospective and a double blind study of betamethasone in severe closed brain injuries. Acta Neurochrurgica 1979;28(2):499. [PubMed] [Google Scholar]
Hoyt 1972 {published data only}
- Hoyt HJ, Goldstein FP, Reigel DH, Holst R. Clinical evaluation of highly water‐soluble steroids in the treatment of cerebral edema of traumatic origin (a double blind study). Pharmacology and Therapeutics 1972;13:141. [Google Scholar]
Pitts 1980 {unpublished data only}
- Pitts LH, Kaktis JV. Effect of megadose steroids on ICP in traumatic coma. In: Shulman K, Marmarou A, Miller JD editor(s). Intracranial Pressure. Vol. IV, Berlin: Springer‐Verlag, 1980:638‐42. [Google Scholar]
Ransohoff 1972 {published data only}
- Ransohoff J. The effects of steroids on brain edema in man. In: Reulen H J, Schurmann K editor(s). Steroids and Brain Edema. Berlin/Heidelberg/New York: Springer‐Verlag, 1972:211‐3. [Google Scholar]
Saul 1981 {published data only}
- Saul TG, Ducker TB, Salcman M, Carro E. Steroids in severe head injury. Journal of Neurosurgery 1981;54:596‐600. [DOI] [PubMed] [Google Scholar]
Stubbs 1989 {published data only}
- Stubbs DF, Stiger TR, Harris WR. Multinational controlled trial of high‐dose methylprednisolone in moderately severe head injury. In: Capildeo editor(s). Steroids in diseases of the central nervous system. Chichester: John Wiley & Sons, 1989:163‐8. [Google Scholar]
Tahara 1972 {published data only}
- Tahara I, Fujii C, Tanaka N, Ogawa M, Katsurada K. Effects of steroid therapy on acute head injury. Neurologia medico‐chirurgica 1972;12:111‐2. [Google Scholar]
Zagara 1987 {published data only}
- Zagara G, Scaravilli P, Carmen Belluci M, Seveso M. Effect of dexamethasone on nitrogen metabolism in brain‐injured patients. Journal of Neurosurgery 1987;31:207‐12. [PubMed] [Google Scholar]
Zarate 1995 {published data only}
- Zarate IO, Guerrero JG. Coprticosteroids in paediatric patients with head and brain trauma [Corticosteroides en pacientes pediatricos con traumatismo craneoencefalico]. Practica Pediatrica 1995;4:7‐14. [Google Scholar]
References to studies excluded from this review
Cheng 1991 {published data only}
- Cheng Z. Effect of postoperative megadose dexamethasone on delayed traumatic brain edema. Chinese Journal of Traumatology 1991;8:65. [Google Scholar]
- Cheng Z. Effect of postoperative megadose dexamethasone on delayed traumatic brain edema. Chinese Medical Journal 1992;105:788. [Google Scholar]
Fanconi 1988 {published data only}
- Fanconi S, Kloti J, Meuli M, Zaugg H, Zachmann M. Dexamethasone therapy and endogenous cortisol production in severe pediatric head injury. Intensive Care Medicine 1988;14:163‐6. [DOI] [PubMed] [Google Scholar]
- Kloti J, Fanconi S, Zachmann M, Zaugg H. Dexamethasone therapy and cortisol excretion in severe paediatric head injury. Child's Nervous System 1987;3:103‐5. [DOI] [PubMed] [Google Scholar]
Gobiet 1976 {published data only}
- Gobiet W, Bock WJ, Liesegang J, et al. Treatment of acute cerebral edema with high dose of dexamethasone. In: Becks JWF, Bosch DA, Brock M editor(s). Intracranial Pressure. Vol. III, Berlin/Heidelberg/New York: Springer‐Verlag, 1976:231‐5. [Google Scholar]
James 1979 {published data only}
- James HE, Madauss WC, Tibbs PA, McCloskey JJ, Bean JR. The effect of high dose dexamethasone in children with severe closed head injury. Acta Neurochirurgica 1979;45:225‐36. [DOI] [PubMed] [Google Scholar]
Robertson 1985 {published data only}
- Robertson CS, Clifton GL, Goodman JC. Steroid administration and nitrogen excretion in the head‐injured patient. Journal of Neurosurgery 1985;63:714‐8. [DOI] [PubMed] [Google Scholar]
Additional references
Bracken 1990
- Bracken MB, Shepard MJ, Collins WF, Holford TR, et al. A randomised controlled trial of methylprednisolone or naloxone in the treatment of acute spinal cord injury. New England Journal of Medicine 1990;322:1405‐11. [DOI] [PubMed] [Google Scholar]
Galicich 1961
- Galicich J H, French L A. Use of dexamethasone in the treatment of cerebral edema resulting from brain tumours and brain surgery. American Practitioner and Digest of Treatment 1961;12:169‐74. [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Jeevaratnam 1996
- Jeevaratnam DR, Menon DK. Survey of intensive care of severely head injured patients in the United Kingdom. BMJ 1996;312:944‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jennett 1975
- Jennett B, Bond M. Assessment of outcome after severe brain damage. A practical scale. Lancet 1975;1:480‐4. [DOI] [PubMed] [Google Scholar]
Jennett 1996
- Jennett B. Epidemiology of head injury. Journal of Neurology, Neurosurgery, and Psychiatry 1996;60:362‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kraus 1993
- Kraus JF. Epidemiology of head injury. In: Cooper PR editor(s). Head injury. 3rd Edition. Baltimore: William Wilkins, 1993:1‐25. [Google Scholar]
Miller 1992
- Miller JD, Jones PA, Dearden NM, Tocher JL. Progress in the management of head injury. British Journal of Surgery 1992;79:60‐4. [DOI] [PubMed] [Google Scholar]
Murray 1994
- Murray CJL, Lopez AD, eds. Global comparative assessments in the health sector. Geneva: World Health Organisation, 1994. [Google Scholar]
Pickard 1993
- Pickard JD, Czosnyka M. Management of raised intracranial pressure. Journal of Neurology, Neurosurgery, and Psychiatry 1993;56:845‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 1995
- Roberts I. Letter from Chengdu: China takes to the roads. BMJ 1995;310:1311‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Alderson 1997
- Alderson P, Roberts I. Corticosteroids in acute traumatic brain injury: systematic review of randomised controlled trials. BMJ 1997;314:1855‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]


