Abstract
Background
Acute otitis media (AOM) is one of the most common diseases in early infancy and childhood. Antibiotic use for AOM varies from 56% in the Netherlands to 95% in the USA, Canada and Australia. This is an update of a Cochrane review first published in The Cochrane Library in Issue 1, 1997 and previously updated in 1999, 2005, 2009 and 2013.
Objectives
To assess the effects of antibiotics for children with AOM.
Search methods
We searched CENTRAL (2015, Issue 3), MEDLINE (1966 to April week 3, 2015), OLDMEDLINE (1958 to 1965), EMBASE (January 1990 to April 2015), Current Contents (1966 to April 2015), CINAHL (2008 to April 2015) and LILACS (2008 to April 2015).
Selection criteria
Randomised controlled trials (RCTs) comparing 1) antimicrobial drugs with placebo and 2) immediate antibiotic treatment with expectant observation (including delayed antibiotic prescribing) in children with AOM.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data.
Main results
For the review of antibiotics against placebo, 13 RCTs (3401 children and 3938 AOM episodes) from high‐income countries were eligible and had generally low risk of bias. The combined results of the trials revealed that by 24 hours from the start of treatment, 60% of the children had recovered whether or not they had placebo or antibiotics. Pain was not reduced by antibiotics at 24 hours (risk ratio (RR) 0.89, 95% confidence interval (CI) 0.78 to 1.01) but almost a third fewer had residual pain at two to three days (RR 0.70, 95% CI 0.57 to 0.86; number needed to treat for an additional beneficial outcome (NNTB) 20). A quarter fewer had pain at four to seven days (RR 0.76, 95% CI 0.63 to 0.91; NNTB 16) and two‐thirds fewer had pain at 10 to 12 days (RR 0.33, 95% CI 0.17 to 0.66; NNTB 7) compared with placebo. Antibiotics did reduce the number of children with abnormal tympanometry findings at two to four weeks (RR 0.82, 95% CI 0.74 to 0.90; NNTB 11), at six to eight weeks (RR 0.88, 95% CI 0.78 to 1.00; NNTB 16) and the number of children with tympanic membrane perforations (RR 0.37, 95% CI 0.18 to 0.76; NNTB 33) and halved contralateral otitis episodes (RR 0.49, 95% CI 0.25 to 0.95; NNTB 11) compared with placebo. However, antibiotics neither reduced the number of children with abnormal tympanometry findings at three months (RR 0.97, 95% CI 0.76 to 1.24) nor the number of children with late AOM recurrences (RR 0.93, 95% CI 0.78 to 1.10) when compared with placebo. Severe complications were rare and did not differ between children treated with antibiotics and those treated with placebo. Adverse events (such as vomiting, diarrhoea or rash) occurred more often in children taking antibiotics (RR 1.38, 95% CI 1.19 to 1.59; number needed to treat for an additional harmful outcome (NNTH) 14). Funnel plots do not suggest publication bias. Individual patient data meta‐analysis of a subset of included trials found antibiotics to be most beneficial in children aged less than two years with bilateral AOM, or with both AOM and otorrhoea.
For the review of immediate antibiotics against expectant observation, five trials (1149 children) from high‐income countries were eligible and had low to moderate risk of bias. Four trials (1007 children) reported outcome data that could be used for this review. From these trials, data from 959 children could be extracted for the meta‐analysis of pain at three to seven days. No difference in pain was detectable at three to seven days (RR 0.75, 95% CI 0.50 to 1.12). One trial (247 children) reported data on pain at 11 to 14 days. Immediate antibiotics were not associated with a reduction in the number of children with pain (RR 0.91, 95% CI 0.75 to 1.10) compared with expectant observation. Additionally, no differences in the number of children with abnormal tympanometry findings at four weeks, tympanic membrane perforations and AOM recurrence were observed between groups. No serious complications occurred in either the antibiotic or the expectant observation group. Immediate antibiotics were associated with a substantial increased risk of vomiting, diarrhoea or rash compared with expectant observation (RR 1.71, 95% CI 1.24 to 2.36; NNTH 9).
Results from an individual patient data meta‐analysis including data from six high‐quality trials (1643 children) that were also included as individual trials in our review showed that antibiotics seem to be most beneficial in children younger than two years of age with bilateral AOM (NNTB 4) and in children with both AOM and otorrhoea (NNTB 3).
Authors' conclusions
This review reveals that antibiotics have no early effect on pain, a slight effect on pain in the days following and only a modest effect on the number of children with tympanic perforations, contralateral otitis episodes and abnormal tympanometry findings at two to four weeks and at six to eight weeks compared with placebo in children with AOM. In high‐income countries, most cases of AOM spontaneously remit without complications. The benefits of antibiotics must be weighed against the possible harms: for every 14 children treated with antibiotics one child experienced an adverse event (such as vomiting, diarrhoea or rash) that would not have occurred if antibiotics were withheld. Therefore clinical management should emphasise advice about adequate analgesia and the limited role for antibiotics. Antibiotics are most useful in children under two years of age with bilateral AOM, or with both AOM and otorrhoea. For most other children with mild disease in high‐income countries, an expectant observational approach seems justified.
Plain language summary
Antibiotics for acute middle ear infection (acute otitis media) in children
Review questions
This review compared 1) the clinical effectiveness and safety of antibiotics against placebo in children with an acute middle ear infection (acute otitis media (AOM)) and 2) the clinical effectiveness and safety of antibiotics against expectant observation (observational approaches in which prescriptions may or may not be provided) in children with AOM.
Background
AOM is one of the most common infections in early infancy and childhood, causing pain and general symptoms of illness such as fever, irritability and problems feeding and sleeping. By three years of age, most children have had at least one AOM episode. Though AOM usually resolves without treatment, it is often treated with antibiotics.
Study characteristics
The evidence in this review is current to 26 April 2015.
For the review of antibiotics against placebo we included 13 trials (3401 children aged between two months and 15 years) from high‐income countries with generally low risk of bias. Three trials were performed in a general practice (GP) setting, six in an outpatient hospital setting and four in both settings.
For the review of antibiotics against expectant observation, five trials (1149 children) from high‐income countries were eligible with low to moderate risk of bias. Two trials were performed in a GP setting and three in an outpatient hospital setting. Four trials (1007 children) reported outcome data that could be used for this review.
Key results
We found that antibiotics were not very useful for most children with AOM; antibiotics did not decrease the number of children with pain at 24 hours (when 60% of children were better anyway), only slightly reduced the number of children with pain in the days following and did not reduce the number of children with late AOM recurrences and hearing loss (that can last several weeks) at three months compared with placebo. However, antibiotics did slightly reduce the number of children with perforations of the eardrum and AOM episodes in the initially unaffected ear compared with placebo. Results from an individual patient data meta‐analysis including data from six high‐quality trials (1643 children), which were also included as individual trials in our review, showed that antibiotics seem to be most beneficial in children younger than two years of age with infection in both ears and in children with both AOM and a discharging ear.
We found no difference between immediate antibiotics and expectant observational approaches in the number of children with pain three to seven days and 11 to 14 days after assessment. Furthermore, no differences in the number of children with hearing loss at four weeks, perforations of the eardrum and late AOM recurrences were observed between groups.
There was not enough information to know if antibiotics reduced rare complications such as mastoiditis (infection of the bones around the ear). All of the studies included in this review were from high‐income countries. Data are lacking from populations in which the AOM incidence and risk of progression to mastoiditis is higher.
Antibiotics caused unwanted effects such as diarrhoea, vomiting and rash and may also increase resistance to antibiotics in the community. It is difficult to balance the small benefits against the small harms of antibiotics in children with AOM. However, for most children with mild disease in high‐income countries, an expectant observational approach seems justified.
Quality of the evidence
We judged the quality of the evidence to be high for most of the outcomes in the review of antibiotics against placebo (this means that further research is very unlikely to change our confidence in the estimate of effect).
For the review of immediate antibiotics versus expectant observation, we judged the evidence to be of moderate quality for most of the outcomes (this means that further research is likely to have an important impact on how confident we are in the results and may change those results). Quality was affected by concerns about sample size (perforation of the eardrum, rare complications) and the large number of children who are 'lost to follow‐up' (pain at days 11 to 14, hearing loss at four weeks and late AOM recurrences).
Summary of findings
Summary of findings for the main comparison. Antibiotics versus placebo for acute otitis media in children.
| Antibiotics versus placebo for acute otitis media in children | ||||||
| Patient or population: children with acute otitis media Settings: primary care and secondary care Intervention: antibiotics versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotics versus placebo | |||||
| Pain ‐ pain at 24 hours | Study population | RR 0.89 (0.78 to 1.01) | 1394 (5 studies)1 | ⊕⊕⊕⊕ high | ||
| 426 per 1000 | 379 per 1000 (332 to 431) | |||||
| Pain ‐ pain at 2 to 3 days | Study population | RR 0.70 (0.57 to 0.86) | 2320 (7 studies) | ⊕⊕⊕⊕ high | ||
| 159 per 1000 | 111 per 1000 (90 to 137) | |||||
| Pain ‐ pain at 4 to 7 days | Study population | RR 0.76 (0.63 to 0.91) | 1347 (7 studies)1 | ⊕⊕⊕⊕ high | ||
| 241 per 1000 | 183 per 1000 (152 to 220) | |||||
| Pain ‐ pain at 10 to 12 days | Study population | RR 0.33 (0.17 to 0.66) | 278 (1 study) | ⊕⊕⊕⊝ moderate2 | ||
| 216 per 1000 | 71 per 1000 (37 to 142) | |||||
| Abnormal tympanometry ‐ 2 to 4 weeks | Study population | RR 0.82 (0.74 to 0.90) | 2138 (7 studies) | ⊕⊕⊕⊕ high | ||
| 481 per 1000 | 395 per 1000 (356 to 433) | |||||
| Abnormal tympanometry ‐ 3 months | Study population | RR 0.97 (0.76 to 1.24) | 809 (3 studies) | ⊕⊕⊕⊕ high | ||
| 241 per 1000 | 234 per 1000 (183 to 299) | |||||
| Vomiting, diarrhoea or rash | Study population | RR 1.38 (1.19 to 1.59) | 2107 (8 studies) | ⊕⊕⊕⊕ high | ||
| 196 per 1000 | 270 per 1000 (233 to 311) | |||||
| *The basis for the assumed risk for ‘Study population’ was the average risk in the control groups (i.e. total number of participants with events divided by total number of participants included in the meta‐analysis). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1The number of studies reported in the 'Summary of findings' table for the outcomes 'Pain at 24 hours' and 'Pain at 4 to 7 days' differ slightly from those reported in the Data Analysis Table 1 ‐ Antibiotics versus placebo (five versus six studies and seven versus eight studies, respectively). This is due to the van Buchem trial. This trial is included as one study in our review (and in the 'Summary of findings' table), but we included data from two different comparisons from this 2 x 2 factorial design trial in our analyses (van Buchem 1981a; van Buchem 1981b).
2We downgraded the evidence for pain at days 10 to 12 from high quality as this outcome was not specified a priori in this trial (secondary analysis).
Background
Description of the condition
Acute otitis media (AOM) is one of the most frequent diseases in early infancy and childhood. AOM is defined as the presence of middle‐ear effusion and a rapid onset of signs or symptoms of middle‐ear inflammation, such as ear pain, otorrhoea or fever (AAP 2013), and has a high morbidity and low mortality (Stool 1989). Approximately 10% of children have an episode of AOM by three months of age and, by three years of age, approximately 50% to 85% of all children have experienced at least one AOM episode (Teele 1989). The peak age‐specific incidence is between six and 15 months (Klein 1989).
Description of the intervention
Despite a large number of published clinical trials, there is no consensus regarding the most appropriate therapy for AOM; for example, the rates of use of antibiotics for AOM vary from 56% in the Netherlands (Akkerman 2005) to 95% in the USA and Canada (Froom 2001). One meta‐analysis emphasises that AOM resolves spontaneously in most children (Rosenfeld 1994). However, one semi‐randomised trial of 1365 participants conducted in Sweden in 1954 reported a rate of mastoiditis of 17% in the untreated group versus none in the penicillin‐treated groups (Rudberg 1954). Over recent years, prescription strategies in which antibiotic treatment for acute respiratory infections such as AOM is delayed and instituted only if symptoms persist or worsen after several days have been advocated (AAP 2013).
How the intervention might work
AOM has a multifactorial pathogenesis. Mucosal swelling of the nasopharynx and Eustachian tube due to a viral upper respiratory tract infection can lead to Eustachian tube dysfunction with impaired clearance and pressure regulation of the middle ear. Prolonged dysfunction may be followed by aspiration of potential viral and bacterial pathogens from the nasopharynx to the middle ear. These pathogens might in turn provoke a host inflammatory response, which leads to the clinical manifestations of AOM such as ear pain, otorrhoea, fever and irritability. Streptococcus pneumoniae (S. pneumoniae) has been the predominant pathogen related to AOM for many years, next to Moraxella catarrhalis (M. catarrhalis) and non‐typeable Haemophilus influenzae (H. influenzae). However, recent studies suggest that widespread implementation of pneumococcal conjugate vaccination has changed the frequency of otopathogens related to AOM with non‐typeable H. influenzae and non‐vaccine S. pneumoniae serotypes becoming more prevalent (Casey 2013; Coker 2010). Additionally, viral (co‐)infection is known to worsen the clinical and bacteriological outcome of AOM (Arola 1990; Chonmaitree 1992). As bacteria are considered to play a predominant role in the causation of AOM‐related symptoms, antibiotic treatment may accelerate clinical recovery and may reduce the number of complications related to AOM.
Why it is important to do this review
Although numerous randomised clinical trials (RCTs) on the effectiveness of antibiotic treatment in children with AOM have been performed over the decades, consensus regarding the most appropriate treatment strategy is lacking. As symptoms consistent with AOM resolve spontaneously in the majority of children, an expectant observational approach might be justified. We therefore performed a systematic review to examine the effects of both immediate antibiotic treatment and an expectant observational approach in children with AOM. This is an update of a Cochrane review first published in The Cochrane Library in Issue 1, 1997 (Glasziou 1997) and updated in 1999 (Glasziou 1999), 2005 (Glasziou 2005), 2009 (Sanders 2009), and 2013 (Venekamp 2013).
Objectives
To assess the effects of antibiotics for children with AOM.
We attempted to determine to what extent antibiotic therapy was more effective than placebo and what, if any, advantages it offered to children in terms of symptom relief (pain), avoidance of complications (such as tympanic membrane perforations and severe complications such as mastoiditis) and longer‐term hearing problems from middle‐ear effusion (as measured by tympanometry or audiometry). We also assessed the effect of immediate antibiotic versus expectant observation on AOM. Moreover, we aimed to provide information on subgroups of children with AOM that benefit more or less from antibiotics.
Methods
Criteria for considering studies for this review
Types of studies
RCTs of antimicrobial drugs versus placebo control. We also included RCTs comparing immediate antibiotic versus expectant observation.
Types of participants
Studies including children (aged from one month to 15 years) of either gender without ventilation tubes, suffering from AOM irrespective of the setting from which they were recruited.
Types of interventions
Antimicrobial drugs versus placebo control.
Immediate antibiotic versus expectant observation (also known as 'wait and see' or 'watchful waiting' or 'observation therapy'). This includes expectant observational approaches in which prescriptions may or may not be provided.
Types of outcome measures
We focused our data extraction on patient‐relevant outcomes, that is, those symptoms or problems that are important to the patient's sense of well‐being. While other endpoints, such as microbiological cure, may enhance medical understanding of the disease process, decisions about treatment should focus on helping the patient. We analysed the outcomes listed below in this review, but these outcomes were not used as a basis for including or excluding studies.
Primary outcomes
Proportion of children with pain at various time points (24 hours, two to three days, four to seven days, 10 to 14 days).
Adverse effects likely to be related to the use of antibiotics such as vomiting, diarrhoea or rash.
Secondary outcomes
Abnormal tympanometry findings at various time points (two to four weeks, six to eight weeks, and three months) as a surrogate measure for hearing problems caused by middle‐ear fluid.
Tympanic membrane perforation.
Contralateral otitis (in unilateral cases).
AOM recurrences.
Serious complications related to AOM such as mastoiditis and meningitis.
Long‐term effects (including the number of parent‐reported AOM‐symptom episodes, antibiotic prescriptions and health care utilisation as assessed at least one year after randomisation).
Search methods for identification of studies
Electronic searches
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 3) (accessed 26 April 2015), which contains the Acute Respiratory Infections (ARI) Group's Specialised Register, MEDLINE (October 2012 to April week 3, 2015), EMBASE (November 2012 to April 2015), Current Contents (2012 to April 2015), CINAHL (October 2012 to April 2015) and LILACS (2012 to April 2015). Our previous update using the same search strategies covered the period 2008 to November 2012. See Appendix 1 for details of earlier searches.
We used the search strategy described in Appendix 2 to search CENTRAL and MEDLINE. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the search strategy to search EMBASE (Appendix 3), Current Contents (Appendix 4), CINAHL (Appendix 5) and LILACS (Appendix 6).
There were no language or publication restrictions.
Searching other resources
We checked ClinicalTrials.gov (clinicaltrials.gov/) for ongoing trials (11 May 2015). To increase the yield of relevant studies, we inspected the reference lists of all identified studies and reviews.
Data collection and analysis
Selection of studies
One review author (RPV) screened titles and abstracts obtained from the database searches. Two review authors (RPV, MMR) reviewed the full text of the potentially relevant titles and abstracts against the inclusion criteria.
Data extraction and management
Two review authors (RPV, MMR) extracted data from the included studies. We resolved disagreements by discussion.
Assessment of risk of bias in included studies
Two review authors (RPV, MMR) independently assessed the methodological quality of the included trials. We resolved any disagreements by discussion. We assessed the methodological quality of the included studies as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). As a consequence, methodological quality assessment was based on random sequence generation, allocation concealment, blinding, completeness of data and outcome assessment. Results of the 'Risk of bias' assessment are presented in a 'Risk of bias' summary (Figure 1) and a 'Risk of bias' graph (Figure 2).
1.
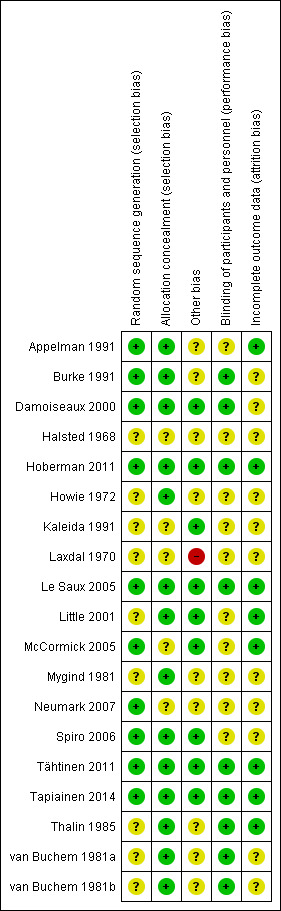
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
2.
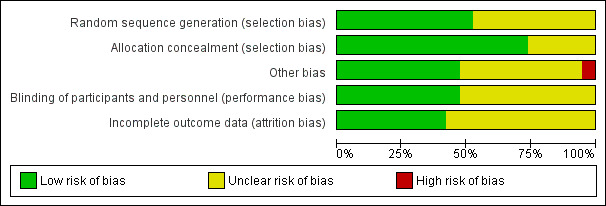
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Measures of treatment effect
We expressed dichotomous outcomes as risk ratio (RR) and risk difference (RD) with 95% confidence intervals (CIs). Additionally, we calculated the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH) (1/(absolute risk in exposed minus absolute risk in unexposed)).
Unit of analysis issues
We did not identify any studies with non‐standard designs, such as cross‐over trials and cluster‐randomised trials.
Dealing with missing data
We tried to contact the trial authors to provide additional information in case of missing data.
Assessment of heterogeneity
We assessed the level of clinical heterogeneity between the trials by reviewing differences across trials in study population, setting, intervention and outcome measures used. In the absence of substantial clinical heterogeneity, we performed meta‐analyses. We used the Chi2 test, the I2 statistic and visual inspection of the forest plots to assess statistical heterogeneity. When statistical heterogeneity was present (P value < 0.1), we re‐analysed the data using the random‐effects model. For the outcome of pain, we explored the magnitude of baseline risk and heterogeneity using L'Abbé plots (a graph of the proportion of participants with an outcome by the proportion of participants without an outcome).
Assessment of reporting biases
We assessed reporting bias using a funnel plot.
Data synthesis
We analysed the data according to the intention‐to‐treat (ITT) principle, whereby all participants are analysed in the groups to which they were randomly allocated. We performed meta‐analysis where we judged clinical heterogeneity to be minimal, to ensure that we would derive clinically meaningful results. We calculated treatment differences by the Mantel‐Haenszel method using a fixed‐effect or random‐effects (when statistical heterogeneity was present) model. We presented results separately for the reviews of antibiotics against placebo and immediate antibiotics versus expectant observation.
Subgroup analysis and investigation of heterogeneity
The publication of Rovers 2006 describes the results of an individual patient data (IPD) meta‐analysis that was performed on a subset of trials included in this review (six trials including 1643 children aged six months to 12 years with AOM) to identify subgroups of children with AOM who might benefit more than others from treatment with antibiotics. Extensive details on the methods and results of this IPD meta‐analysis can be found in the original article (Rovers 2006). The primary outcome was a prolonged course of AOM defined as having either residual pain or fever (> 38 ºC) at three to seven days. Potential subgroups were selected on the basis of a multivariable prediction tool. The independent baseline predictors, that is, age (< two years versus > two years), fever and bilateral AOM (yes versus no), were used to study whether those at risk of a prolonged course also benefited more from treatment with antibiotics. In addition, otorrhoea (yes versus no) at baseline was studied as this is a clinically relevant outcome that occurred too infrequently to be identified as an independent predictor. To assess whether the effect of antibiotics was modified by age, bilateral disease, otorrhoea or a combination of these, a fixed‐effect logistic regression analysis. In this model, antibiotics (yes versus no), the potential effect modifier (age, bilateral disease, otorrhoea, or a combination of these), a dummy for the particular study and an interaction term (antibiotics * potential effect modifier) were included as independent variables and a prolonged course at three to seven days was the dependent variable. If a significant interaction effect was found, stratified analyses were performed to study the rate ratios and rate differences within each stratum of the subgroups.
Sensitivity analysis
We did not perform sensitivity analysis.
GRADE and ’Summary of findings'
For each outcome, we rated the overall quality of evidence as high, moderate, low and very low using the GRADE approach. Randomised controlled trials that do not have serious limitations are rated as high quality. However, we downgraded the evidence to moderate, low or very low depending on the presence of each of the following factors:
study limitations (risk of bias);
indirectness of evidence (directness of evidence);
imprecision (precision of results);
inconsistency (consistency of results); and
publication bias (existence of publication bias).
We included a 'Summary of findings' table (Table 1) for the review of antibiotics against placebo, constructed according to the descriptions as described in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We included our primary outcomes and important secondary outcomes in the 'Summary of findings' table:
pain at 24 hours;
pain at two to three days;
pain at four to seven days;
pain at 10 to 12 days;
adverse effects likely to be related to the use of antibiotics (vomiting, diarrhoea or rash);
abnormal tympanometry findings at two to four weeks;
abnormal tympanometry findings at three months.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies tables.
Results of the search
This is an update of a Cochrane review first published in The Cochrane Library in Issue 1, 1997 (Glasziou 1997) and updated in 1999 (Glasziou 1999), 2005 (Glasziou 2005), 2009 (Sanders 2009), and 2013 (Venekamp 2013). In the 2013 update of our review (Venekamp 2013), we identified 12 RCTs for the review of antibiotics against placebo (Appelman 1991; Burke 1991; Damoiseaux 2000; Halsted 1968; Hoberman 2011; Howie 1972; Kaleida 1991; Le Saux 2005; Mygind 1981; Tähtinen 2011; Thalin 1985; van Buchem 1981a and van Buchem 1981b), while we judged five RCTs eligible for the review of immediate antibiotics versus expectant observation (Laxdal 1970; Little 2001; McCormick 2005; Neumark 2007; Spiro 2006). We excluded a total of 11 studies for various reasons (Arguedas 2011; Casey 2012; Chaput 1982; Engelhard 1989; Liu 2011; Ostfeld 1987; Rudberg 1954; Ruohola 2003; Sarrell 2003; Tähtinen 2012; van Buchem 1985).
With the updated search (November week 2, 2012 to April week 3, 2015), we retrieved a total of 1065 records. Removing duplicates left 937. After screening titles and abstracts, we identified four potentially eligible articles. After reviewing the full text, all articles appeared to be relevant for this review. However, three articles were additional analyses of previously included trials (Damoiseaux 2000; Hoberman 2011; Little 2001), providing additional data on pain at 10 to 12 days (Hoberman 2011) and long‐term effects (Damoiseaux 2000) for the review of antibiotics against placebo and data on long‐term effects (Little 2001) for the review of immediate antibiotics versus expectant observation. We did not identify any additional trials after reviewing the reference lists of the full‐text papers and relevant systematic reviews. This left one new trial eligible for inclusion in the review of antibiotics against placebo (Tapiainen 2014). We identified one ongoing trial (ACTRN12608000424303).
Included studies
Methods, participants, interventions and outcomes of the included studies are described in more detail in the table of Characteristics of included studies.
Antibiotics versus placebo
Thirteen trials including 3401 children (3938 AOM episodes) were eligible for the review of antibiotics against placebo (Appelman 1991; Burke 1991; Damoiseaux 2000; Halsted 1968; Hoberman 2011; Howie 1972; Kaleida 1991; Le Saux 2005; Mygind 1981; Tähtinen 2011; Tapiainen 2014; Thalin 1985; van Buchem 1981a and van Buchem 1981b).
Design
Twelve trials were double‐blind, placebo‐controlled, parallel‐group randomised clinical trials (Appelman 1991; Burke 1991; Damoiseaux 2000; Halsted 1968; Hoberman 2011; Howie 1972; Kaleida 1991; Le Saux 2005; Mygind 1981; Tähtinen 2011; Tapiainen 2014; Thalin 1985), while one trial had a 2 x 2 factorial design (van Buchem 1981a and van Buchem 1981b).
Participants and settings
The sample size of the 13 individual trials ranged from 84 children (Tapiainen 2014) to 536 children (Kaleida 1991). The children were aged between two months and 15 years and 50% to 60% of included children were male. Three trials were performed in primary care (Burke 1991; Damoiseaux 2000; Tähtinen 2011), six in secondary care (Halsted 1968; Hoberman 2011; Howie 1972; Kaleida 1991; Le Saux 2005; Thalin 1985), and four in both primary and secondary care (Appelman 1991; Mygind 1981; Tapiainen 2014; van Buchem 1981a and van Buchem 1981b). AOM was diagnosed by the presence of acute symptoms and otoscopic signs in nine trials (Appelman 1991; Burke 1991; Damoiseaux 2000; Halsted 1968; Hoberman 2011; Howie 1972; Kaleida 1991; Mygind 1981; van Buchem 1981a and van Buchem 1981b), and by the presence of middle‐ear effusion at pneumatic otoscopy and/or tympanometry in three trials (Le Saux 2005; Tähtinen 2011; Tapiainen 2014), while the criteria were not clearly described in one trial (Thalin 1985).
Interventions and comparators
Two trials compared penicillin for seven days with placebo (Mygind 1981; Thalin 1985), four trials compared amoxicillin for seven to 14 days with or without myringotomy with placebo (Burke 1991; Damoiseaux 2000; Kaleida 1991; Le Saux 2005), and four trials compared amoxicillin/clavulanate for seven to 10 days with placebo (Appelman 1991; Hoberman 2011; Tähtinen 2011; Tapiainen 2014). In one trial, ampicillin for 10 days was compared with pheneticillin and sulfisoxazole and placebo (Halsted 1968), while another trial compared erythromycin and triple sulphonamide with ampicillin, triple sulphonamide, erythromycin and placebo (Howie 1972). One trial, van Buchem 1981a and van Buchem 1981b, had a 2 x 2 factorial design resulting in four treatment groups: (1) sham myringotomy plus antibiotics; (2) sham myringotomy plus placebo; (3) myringotomy plus antibiotics; and (4) myringotomy plus placebo. We used all arms of this trial: van Buchem 1981a includes the sham myringotomy plus antibiotic and the sham myringotomy plus placebo arms, whereas van Buchem 1981b includes the myringotomy plus antibiotic and myringotomy plus placebo arms.
Outcomes
Pain
Five trials (1394 children) reported data on pain at 24 hours (Burke 1991; Le Saux 2005; Thalin 1985; Tähtinen 2011; van Buchem 1981a and van Buchem 1981b), seven (2320 children) on pain at two to three days (Appelman 1991; Halsted 1968; Kaleida 1991; Le Saux 2005; Mygind 1981; Tähtinen 2011; Thalin 1985), seven (1347 children) on pain at four to seven days (Burke 1991; Damoiseaux 2000; Mygind 1981; Tähtinen 2011; Tapiainen 2014; Thalin 1985; van Buchem 1981a and van Buchem 1981b), and one (278 children) on pain at 10 to 12 days (Hoberman 2011).
Adverse effects likely to be related to the use of antibiotics (vomiting, diarrhoea or rash)
Eight trials (2107 children) reported data on adverse events likely to be related to the use of antibiotics such as vomiting, diarrhoea or rash (Burke 1991; Damoiseaux 2000; Hoberman 2011; Le Saux 2005; Mygind 1981; Tähtinen 2011; Tapiainen 2014; Thalin 1985).
Abnormal tympanometry findings as a surrogate measure for hearing problems
Seven trials (2138 children) reported data on abnormal tympanometry findings at two to four weeks (Appelman 1991; Burke 1991; Kaleida 1991; Le Saux 2005; Mygind 1981; Tapiainen 2014; Thalin 1985), three (953 children) on abnormal tympanometry findings at six to eight weeks (Damoiseaux 2000; Kaleida 1991; Tapiainen 2014), and three (809 children) on abnormal tympanometry findings at three months (Burke 1991; Le Saux 2005; Mygind 1981), as a surrogate measure for hearing problems caused by middle‐ear fluid.
Tympanic membrane perforation
Five trials (1075 children) reported data on tympanic membrane perforation (Burke 1991; Hoberman 2011; Mygind 1981; Tähtinen 2011; Tapiainen 2014).
Progression of symptoms (contralateral otitis or late AOM recurrences)
Four trials (906 children) reported data on contralateral otitis (in unilateral cases) (Burke 1991; Hoberman 2011; Mygind 1981; Thalin 1985), while six trials (2200 children) reported data on late AOM recurrences (Hoberman 2011; Kaleida 1991; Le Saux 2005; Mygind 1981; Thalin 1985; van Buchem 1981a).
Serious complications
Ten trials reported on serious complications including mastoiditis or meningitis (Burke 1991; Damoiseaux 2000; Hoberman 2011; Howie 1972; Kaleida 1991; Le Saux 2005; Mygind 1981; Tähtinen 2011; Tapiainen 2014; van Buchem 1981a and van Buchem 1981b), while information on complications was not explicitly reported in three trials (Appelman 1991; Halsted 1968; Thalin 1985).
Long‐term effects
One trial reported data on secondary care referrals at one year after randomisation as assessed by reviewing the children's notes (Burke 1991). Four children in the antibiotic group (4%) and seven in the placebo group (6%) were lost to follow‐up.
One trial reported data on the proportion of children with AOM recurrences, secondary care referrals and ENT surgery at approximately 3.5 years after randomisation (Damoiseaux 2000). These long‐term outcome data were collected by questionnaires. Questionnaires were returned in 168 of the 240 children (70%) that were originally randomised.
Immediate antibiotics versus expectant observation
Five trials including a total of 1149 children were eligible for the review of immediate antibiotics versus expectant observation (Laxdal 1970; Little 2001; McCormick 2005; Neumark 2007; Spiro 2006).
Design
All trials were open‐label, parallel‐group randomised clinical trials.
Participants and settings
The sample size of the five individual trials ranged from 142 children (Laxdal 1970) to 315 children (Little 2001). The children were aged 15 years and younger and 50% to 60% of included children were male. Two trials were performed in primary care (Little 2001; Neumark 2007), and three in secondary care (Laxdal 1970; McCormick 2005; Spiro 2006). AOM was diagnosed by the presence of acute symptoms and otoscopic signs in three trials (Laxdal 1970; Little 2001; McCormick 2005), by pneumatic otoscopy or preferably an aural microscope in one trial (Neumark 2007), while diagnostic criteria were unclear in one trial (AOM diagnosis was made at the discretion of the clinician) (Spiro 2006).
Intervention and comparators
In two of these trials provision of an immediate antibiotic script was compared with an antibiotic script with instructions not to commence antibiotic treatment unless the child was not better or was worse at 48 hours (Spiro 2006) or 72 hours (Little 2001). In these trials, 24% (36/150) and 38% (50/132) of children in the delayed arms reported using antibiotics at some stage during the illness.
The other three trials compared immediate antibiotics with a watchful waiting approach (Laxdal 1970; McCormick 2005; Neumark 2007). In the Laxdal 1970 trial, children in the control group were closely monitored, especially during the first 48 hours and particularly when severe involvement was evident. In the McCormick 2005 trial, antibiotics were administered to the watchful waiting group if a child returned to the office with a treatment failure or recurrence (four children in the expectant observation group had received antibiotics by day four). In the Neumark 2007 trial, 5% (4/87) of children randomised to the watchful waiting group received antibiotics due to treatment failure.
Outcomes
One trial did not report any data on our primary or secondary outcomes (Laxdal 1970), leaving four trials from which relevant data could be extracted (Little 2001; McCormick 2005; Neumark 2007; Spiro 2006).
Pain
Data on pain at three to seven days could be derived from four trials (959 children) (Little 2001; McCormick 2005; Neumark 2007; Spiro 2006). The data on pain from the Little 2001 trial have been derived from data from the IPD meta‐analysis (Rovers 2006), while the data on pain from the McCormick 2005 trial have been provided by the author. One trial (247 children) reported data on pain at 11 to 14 days (Spiro 2006).
Adverse effects likely to be related to the use of antibiotics (vomiting, diarrhoea or rash)
Two trials (550 children) reported data on adverse events likely to be related to the use of antibiotics such as vomiting, diarrhoea or rash (Little 2001; Spiro 2006).
Abnormal tympanometry findings as a surrogate measure for hearing problems
One trial (207 children) reported data on abnormal tympanometry findings at two to four weeks (McCormick 2005).
Tympanic membrane perforation
One trial (179 children) reported data on tympanic membrane perforation (Neumark 2007).
Progression of symptoms (contralateral otitis or late AOM recurrences)
None of the trials reported data on contralateral otitis (in unilateral cases), while one trial (209 children) reported data on late AOM recurrences (McCormick 2005).
Serious complications
Three trials reported on serious complications including mastoiditis or meningitis (McCormick 2005; Neumark 2007; Spiro 2006), while information on complications was not explicitly reported in one trial (Little 2001).
Long‐term effects
One trial reported data on the further ear pain episodes at three months and one year after randomisation (Little 2001). These long‐term outcome data were collected by questionnaires. Questionnaires were returned in 219 of the 315 children (70%) that were originally randomised at one year.
Excluded studies
We excluded 11 studies after reviewing the full text. Three were non‐randomised studies (Ostfeld 1987; Rudberg 1954; van Buchem 1985), while three other studies had no comparison of antibiotic with placebo or expectant observation (Casey 2012; Engelhard 1989; Sarrell 2003). Two trials studied the effectiveness of short‐ versus long‐course antibiotics (Arguedas 2011; Chaput 1982), one trial studied a single‐dose antibiotic with slow versus immediate‐release formulations (Liu 2011), whereas another trial was conducted in children with ventilation tubes (Ruohola 2003). Moreover, we excluded one trial report as this study reported on the effectiveness of immediate versus delayed antibiotic prescription based on a secondary analysis of a placebo‐controlled trial (Tähtinen 2012).
Risk of bias in included studies
The methodological quality of the included studies was generally high. For further details on the risk of bias in included studies see the 'Risk of bias' summary (Figure 1) and 'Risk of bias' graph (Figure 2).
Allocation
Concealment of allocation was adequately described in 11 of the 13 included trials comparing antibiotics with placebo (Appelman 1991; Burke 1991; Damoiseaux 2000; Hoberman 2011; Howie 1972; Le Saux 2005; Mygind 1981; Tähtinen 2011; Tapiainen 2014; Thalin 1985; van Buchem 1981a and van Buchem 1981b), and two out of five trials comparing immediate antibiotics with expectant observation (Little 2001; Spiro 2006). Random sequence generation was adequate in seven of the 13 trials (Appelman 1991; Burke 1991; Damoiseaux 2000; Hoberman 2011; Le Saux 2005; Tähtinen 2011; Tapiainen 2014), and in three of the five included trials (McCormick 2005; Neumark 2007; Spiro 2006), respectively.
Blinding
All included trials in the review of antibiotics against placebo stated that they were double‐blinded. However, we judged blinding to be adequate in eight of the 13 included trials (Burke 1991; Damoiseaux 2000; Hoberman 2011; Le Saux 2005; Tähtinen 2011; Tapiainen 2014; Thalin 1985; van Buchem 1981a and van Buchem 1981b). All five trials comparing immediate antibiotics with expectant observation were open‐label trials (Laxdal 1970; Little 2001; McCormick 2005; Neumark 2007; Spiro 2006). As a consequence, reporting of the child's symptoms by parents was not blinded in these trials. However, investigators were blinded in two of the five trials (McCormick 2005; Spiro 2006).
Incomplete outcome data
The loss to follow‐up was below 5% in eight of the 13 trials comparing antibiotics with placebo (Appelman 1991; Burke 1991; Hoberman 2011; Howie 1972; Le Saux 2005; Tähtinen 2011; Tapiainen 2014; Thalin 1985). Loss to follow‐up was high in three trials with a total loss to follow‐up of 15% (van Buchem 1981a and van Buchem 1981b), 7% (Kaleida 1991), and 12% (Damoiseaux 2000), respectively. However, one of these trials included all randomised patients in the primary analysis at day four (Damoiseaux 2000). In two of the 13 trials the total number of loss to follow‐up/exclusions are described but it was unclear from which treatment group children were excluded (Halsted 1968; Mygind 1981). For the review of immediate antibiotics against expectant observation, the loss to follow‐up was below 5% in two of the five trials (McCormick 2005; Neumark 2007). The total loss to follow‐up in the other trials was 11% (Laxdal 1970), 10% (Little 2001), and 6% (Spiro 2006), respectively.
Selective reporting
Eight of the 13 included trials comparing antibiotics with placebo used intention‐to‐treat (ITT) analyses, while in the other five this was not clear (Halsted 1968; Howie 1972; Mygind 1981; Thalin 1985; van Buchem 1981a and van Buchem 1981b). For the review of immediate antibiotics versus expectant observation, three of the five included trials used ITT analyses, while this was not clear in the other two trials (Laxdal 1970; Neumark 2007).
Other potential sources of bias
No other potential sources of bias could be detected in the included trials, except for the Laxdal 1970 trial, which we judged as having a high risk of detection bias since children in the control group were subjected to very close scrutiny, especially during the first 48 hours and particularly when severe involvement was evident. However, this trial did not report any data on our primary or secondary outcomes.
Effects of interventions
See: Table 1
Antibiotics versus placebo
Primary outcomes
1. Proportion of children with pain at various time points
The combined results of the trials revealed that by 24 hours from the start of treatment, 60% of the children had recovered whether or not they had placebo or antibiotics. The proportion of children that recovered spontaneously at two to three days, four to seven days and 10 to 12 days was 84%, 76% and 78%, respectively. Antibiotics achieved a 30% (95% confidence interval (CI) 14% to 43%) relative reduction in the risk of pain at two to three days, 24% (95% CI 9% to 37%) relative reduction in the risk of pain at four to seven days and 67% (95% CI 34% to 83%) relative reduction in the risk of pain at 10 to 12 days (Analysis 1.1). This means 5% (95% CI 2% to 7%) fewer children had pain after two to three days (number needed to treat for an additional beneficial outcome (NNTB) 20, 95% CI 14 to 50), 6% (95% CI 2% to 9%) fewer children had pain after four to seven days (NNTB 16, 95% CI 11 to 50) and 14% (95% CI 6% to 22%) fewer children had pain after 10 to 12 days (NNTB 7, 95% CI 4 to 16), respectively. Plots of the event rate (pain) in the treatment and control groups for each study at 24 hours and two to three days are reported in Figure 3 and Figure 4. The funnel plot for pain at the various time points did not reveal asymmetry (Figure 5).
1.1. Analysis.
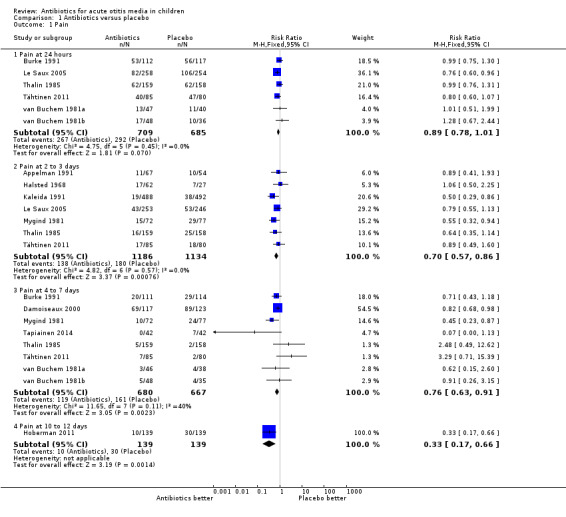
Comparison 1 Antibiotics versus placebo, Outcome 1 Pain.
3.
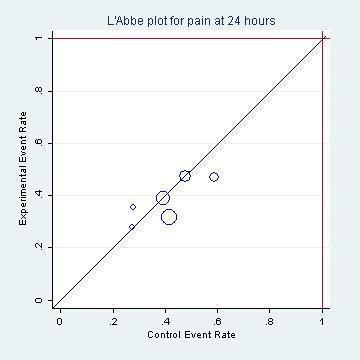
L'Abbé plot of the rates of pain at 24 hours for the placebo (control) versus antibiotic (experimental) group.
4.
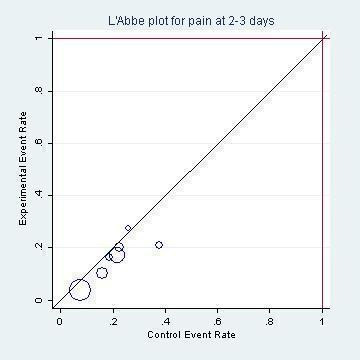
L'Abbé plot of the rates of pain at two to three days for the placebo (control) versus antibiotic (experimental) group.
5.
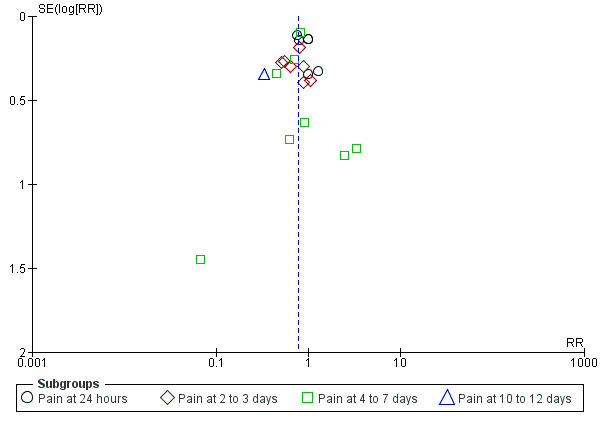
Funnel plot of comparison: 1 Antibiotic versus placebo, outcome: 1.1 Pain.
Quality of the evidence
We judged the data on pain at 24 hours, two to three days and four to seven days to be of high quality, while we judged the data on pain at 10 to 12 days to be of moderate quality. We downgraded the evidence for pain at days 10 to 12 from high quality as this outcome was not specified a priori in this trial (secondary analysis).
2. Adverse effects likely to be related to the use of antibiotics
Antibiotics resulted in a 38% (95% CI 15% to 73%) relative increase in the risk of adverse effects likely to be related to the use of antibiotics (defined as vomiting, diarrhoea or rash) compared with placebo; 27% (283/1044) of children treated with antibiotics versus 20% (208/1063) of children treated with placebo experienced vomiting, diarrhoea or rash (Analysis 1.2). The number needed to treat for an additional harmful outcome (NNTH) was 14 (9 to 26).
1.2. Analysis.
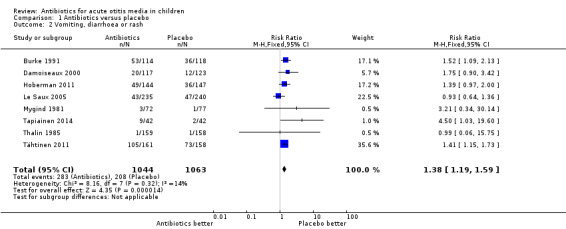
Comparison 1 Antibiotics versus placebo, Outcome 2 Vomiting, diarrhoea or rash.
Quality of the evidence
We judged the evidence for adverse effects likely to be related to the use of antibiotics (vomiting, diarrhoea or rash) to be of high quality.
Secondary outcomes
1. Abnormal tympanometry findings at various time points
Antibiotics achieved an 18% (95% CI 10% to 26%) relative reduction in the risk of abnormal tympanometry findings at two to four weeks, and a 12% (95% CI 0% to 22%) relative reduction in the risk of abnormal tympanometry findings at six to eight weeks (Analysis 1.3). This means 9% (95% CI 5% to 13%) fewer children had abnormal tympanometry findings at two to four weeks (NNTB 11, 95% CI 7 to 20) and 6% (95% CI 0% to 12%) fewer children had abnormal tympanometry findings at six to eight weeks (NNTB 16, 95% CI 8 to 277), respectively.
1.3. Analysis.
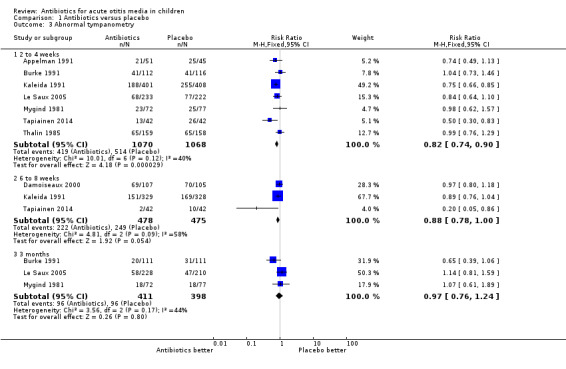
Comparison 1 Antibiotics versus placebo, Outcome 3 Abnormal tympanometry.
However, antibiotics were not associated with a statistically significant reduction in the risk of abnormal tympanometry findings at three months compared with placebo (Analysis 1.3). Furthermore, audiometry was done in only two studies and incompletely reported. The two studies that used audiograms were van Buchem 1981a and Kaleida 1991: (i) van Buchem 1981a reported that, "After one month, 31% of the patients showed an air/bone gap of more than 20 dB. After two months, this was still the case with 19% of the patients. Here again, there were no significant differences between the groups"; (ii) Kaleida 1991 stated that "Analysis of hearing acuity in children two years of age and older indicated that elevated hearing thresholds ... bore no apparent relationship ... to mode of treatment (amoxicillin versus placebo)".
Quality of the evidence
We judged the evidence for abnormal tympanometry findings at the various time points to be of high quality.
2. Tympanic membrane perforation
Antibiotic treatment was associated with a 63% (95% CI 24% to 82%) relative reduction in the risk of tympanic membrane perforation compared with placebo (Analysis 1.4). However, absolute benefits of antibiotics appeared to be small: 3% (95% CI 1% to 5%) fewer children had a tympanic membrane perforation. Therefore, 33 children (95% CI 20 to 100) needed to be treated to prevent one child experiencing a tympanic membrane perforation.
1.4. Analysis.
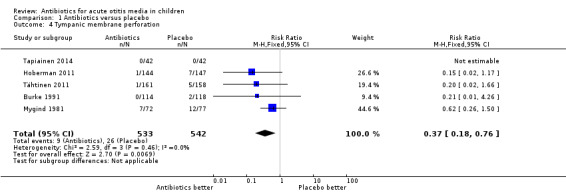
Comparison 1 Antibiotics versus placebo, Outcome 4 Tympanic membrane perforation.
Quality of the evidence
We judged the evidence for tympanic membrane perforation to be of high quality.
3. Contralateral otitis
Antibiotics were associated with a 51% (5% to 75%) relative reduction in the development of contralateral otitis compared with placebo (Analysis 1.5). This means 9% (95% CI 5% to 13%) fewer children had contralateral otitis (NNTB 11, 95% CI 7 to 20).
1.5. Analysis.
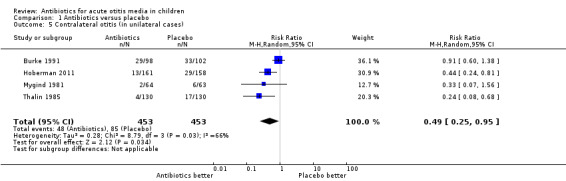
Comparison 1 Antibiotics versus placebo, Outcome 5 Contralateral otitis (in unilateral cases).
Quality of the evidence
We judged the evidence for contralateral otitis to be of high quality.
4. AOM recurrences
Antibiotics were not associated with a statistically significant reduction in the occurrence of late AOM recurrences compared with placebo (Analysis 1.6). AOM recurrences were common. Burke 1991 stated "The mean number of recorded recurrences of otitis media or acute red ear was 0.70 (range 0 to 4) in the antibiotic group and 0.63 (range 0 to 7) in the placebo group and this difference was not significant (difference 0.06, 95% CI ‐0.22 to 0.339)." Six other trials reported the proportions who relapsed; combined, these give a risk ratio (RR) of 0.93 (95% CI 0.78 to 1.10), which is consistent with Burke's findings.
1.6. Analysis.
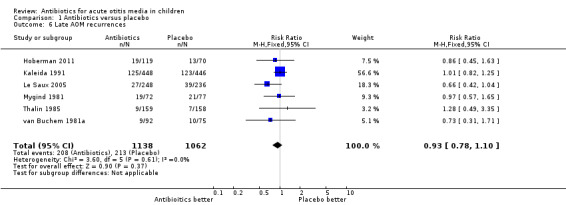
Comparison 1 Antibiotics versus placebo, Outcome 6 Late AOM recurrences.
Quality of the evidence
We judged the evidence for late AOM recurrences to be of high quality.
5. Serious complications related to AOM
Few serious complications occurred in either the antibiotic treatment group or the control group. In just over 3000 children studied, only one case of mastoiditis occurred in both the antibiotic group (Mygind 1981) and the placebo group (Hoberman 2011). Moreover, one child suffered from meningitis (Damoiseaux 2000), pneumococcal bacteraemia and radiologically confirmed pneumonia (Hoberman 2011) in the placebo group and one child had transient facial paralysis in the antibiotic group (Kaleida 1991). Hence, the applicability of these findings to groups of children in whom serious complications such as mastoiditis is common is uncertain. One of the excluded studies did report high rates of mastoiditis (Rudberg 1954). This was an open, semi‐randomised study conducted in Sweden in 1954. Participants were randomised by case‐sheet number but a proportion (about 30 of 220) requested, and were granted, entry to the penicillin group. The rate of mastoiditis was 17% in the untreated group versus 1.5% in the sulphonamide‐treated group and 0% in the penicillin‐treated group. The biases of this study (semi‐randomisation and unblinded outcome assessment) are unlikely to explain such a large difference.
Quality of the evidence
We judged the evidence for serious complications to be of moderate quality. We downgraded the evidence from high quality as we considered the sample size to be insufficient to draw any definite conclusions based on these data and due to the conflicting results found in an open, semi‐randomised study that was not included in our review.
6. Long‐term effects
Based on reviewing children's notes, antibiotics were not associated with a statistically significant reduction in the number of secondary care referrals at one year after randomisation: 7/110 (6%) in the antibiotic group and 9/111 (8%) in the placebo group (RR 0.78, 95% 0.30 to 2.03).
Based on questionnaires returned by parents approximately 3.5 years after initial randomisation, antibiotics were associated with a 46% (95% CI 8% to 97%) relative increase in the risk of AOM recurrences. This means 20% (95% CI 5% to 35%) fewer children had AOM recurrences (NNTB 5, 95% CI 2 to 20). No between‐group differences were observed for secondary care referrals. Furthermore, antibiotics were not associated with a statistically significant reduction in the number of ear, nose and throat surgeries (RR 0.68, 95% CI 0.40 to 1.17).
Quality of the evidence
We judged the evidence for long‐term effects at one year to be of high quality, while we judged the 3.5 years data to be of moderate quality. We mainly downgraded the evidence from high quality because of the high proportion of children that were not included in the analysis (30%), which introduced a significant risk of (attrition) bias.
Immediate antibiotics versus expectant observation
Primary outcomes
1. Proportion of children with pain at various time points
Immediate antibiotics were not associated with a statistically significant reduction in the risk of pain at three to seven days (RR 0.75, 95% CI 0.50 to 1.12) and 11 to 14 days (RR 0.91, 95% CI 0.75 to 1.10) compared with expectant observation (observation with or without an antibiotic prescription) (Analysis 2.1).
2.1. Analysis.
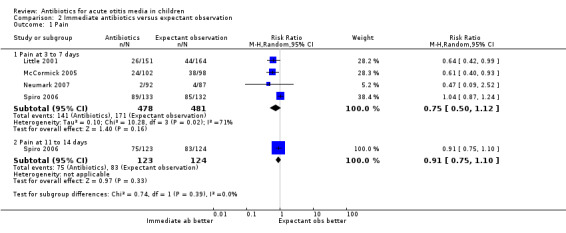
Comparison 2 Immediate antibiotics versus expectant observation, Outcome 1 Pain.
Quality of the evidence
We judged the data on pain at three to seven days to be of high quality, while we judged the data on pain at 11 to 14 days to be of moderate quality. We downgraded the evidence for pain at days 11 to 14 from high quality because of the substantial number of children that were 'lost to follow‐up' (13%), which introduced a risk of (attrition) bias.
2. Adverse effects likely to be related to the use of antibiotics
Immediate antibiotics were associated with a 71% (95% CI 24% to 136%) relative increase in the risk of adverse effects likely to be related to the use of antibiotics (defined as vomiting, diarrhoea or rash) compared with expectant observation; 29% (77/268) of children treated with immediate antibiotics versus 17% (47/282) of children treated with expectant observation experienced vomiting, diarrhoea or rash (Analysis 2.2). The NNTH was 9 (6 to 20).
2.2. Analysis.
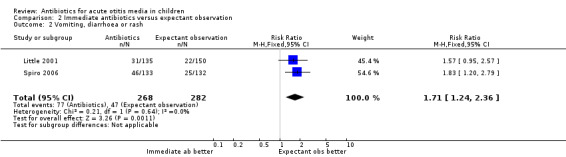
Comparison 2 Immediate antibiotics versus expectant observation, Outcome 2 Vomiting, diarrhoea or rash.
Quality of the evidence
We judged the evidence for adverse effects likely to be related to the use of antibiotics (vomiting, diarrhoea or rash) to be of high quality.
Secondary outcomes
1. Abnormal tympanometry findings at various time points
In one trial (207 children), the proportion of children with abnormal tympanometry findings at four weeks did not substantially differ between those receiving immediate antibiotics and expectant observation (RR 1.03, 95% CI 0.78 to 1.35) (Analysis 2.3).
2.3. Analysis.
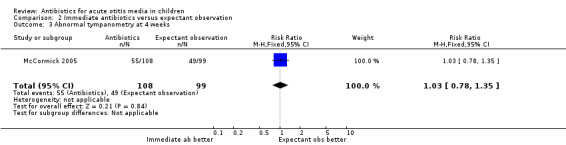
Comparison 2 Immediate antibiotics versus expectant observation, Outcome 3 Abnormal tympanometry at 4 weeks.
Quality of the evidence
We judged the data on abnormal tympanometry findings at four weeks to be of moderate quality. We downgraded the evidence from high quality as the number of children that were 'lost to follow‐up' in the immediate antibiotics group was substantially lower than in the expectant observation group (4% versus 11%), thereby introducing a risk of (attrition) bias.
2. Tympanic membrane perforation
No tympanic membrane perforations were observed in either group in the only trial (179 children) reporting on this outcome (Analysis 2.4).
2.4. Analysis.
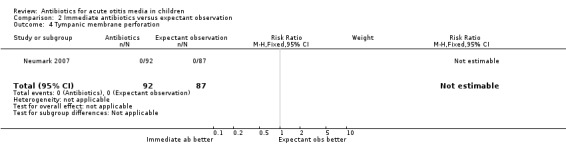
Comparison 2 Immediate antibiotics versus expectant observation, Outcome 4 Tympanic membrane perforation.
Quality of the evidence
We judged the data on tympanic membrane perforation to be of moderate quality. We downgraded the evidence from high quality as we considered the sample size to be insufficient to draw any definite conclusions based on these data
3. Contralateral otitis
None of the trials reported data on contralateral otitis.
4. AOM recurrences
In one trial (209 children), immediate antibiotics were associated with a non‐statistically significant 41% (95% CI ‐26% to 169%) relative increase in the risk of AOM recurrences (Analysis 2.5).
2.5. Analysis.
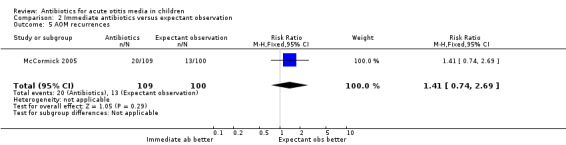
Comparison 2 Immediate antibiotics versus expectant observation, Outcome 5 AOM recurrences.
Quality of the evidence
We judged the data on late AOM recurrences to be of moderate quality. We downgraded the evidence from high quality as the number of children that were 'lost to follow‐up' in the immediate antibiotics group was substantially lower than in the expectant observation group (3% versus 10%), thereby introducing a risk of (attrition) bias.
5. Serious complications related to AOM
No serious complications occurred in either the immediate antibiotic group or the expectant observation group.
Quality of the evidence
We judged the evidence for serious complications to be of moderate quality. We downgraded the evidence from high quality as we considered the sample size to be insufficient to draw any definite conclusions based on these data.
6. Long‐term effects
No statistically significant differences were observed between the immediate antibiotics and the delayed antibiotics group in parent‐reported ear pain episodes at one year (odds ratio (OR) 1.03, 95% 0.60 to 1.78).
Quality of the evidence
We judged the evidence for long‐term effects to be of moderate quality. We mainly downgraded the evidence from high quality as this evidence was derived from a secondary analysis and because of the high proportion of children that were not included in the analysis at one year (30%), which introduced a significant risk of (attrition) bias.
Individual patient data (IPD) meta‐analysis to identify children most likely to benefit from antibiotic treatment
In 2006, an individual patient data (IPD) meta‐analysis was performed, Rovers 2006, using data from six high‐quality RCTs, including a total of 1643 children, which were also included in this review as individual trials (Appelman 1991; Burke 1991; Damoiseaux 2000; Le Saux 2005; Little 2001; McCormick 2005). The main findings of this IPD meta‐analysis were that significant effect modifications were noted for age and bilateral AOM and for otorrhoea; in children aged less than two years with bilateral AOM, 55% of the control group and 30% of the antibiotics group still had pain, fever or both at three to seven days (absolute risk reduction of 25%, 95% CI 14% to 36%; NNTB 4). In children aged two years or older with bilateral AOM the absolute risk reduction was 12% (95% CI ‐1% to 25%; P value for interaction = 0.022). Among children with otorrhoea, 60% of those in the control group had pain, fever or both at three to seven days versus 24% in the antibiotics group (risk reduction of 36%, 95% CI 19% to 53%; NNTB 3). The absolute reduction in risk among those without otorrhoea was 14% (95% CI 5% to 23%; NNTB 8; P value for interaction = 0.039). No differences were identified for age alone.
Quality of the evidence
We judged the evidence for subgroup analyses based on the IPD meta‐analysis to be of high quality.
Discussion
Summary of main results
This review reveals that antibiotics have no early effect on pain, a slight effect on pain in the days following and only a modest effect on the number of children with tympanic perforations, contralateral otitis episodes and abnormal tympanometry findings at two to four weeks and at six to eight weeks, compared with placebo in children with acute otitis media (AOM). However, in applying these results, there are a number of issues to consider, including the individual potential for serious complications and subgroups of children in whom there may be greater benefits.
Overall completeness and applicability of evidence
Does the effect vary in different clinical groups? Our number needed to treat for an additional beneficial outcome (NNTB) of 20 for pain at days two to three days, 16 for pain at four to seven days and seven for pain at 10 to 12 days is for the 'average' case and may vary in subgroups. Several studies reported higher rates of failure of placebo treatment among children less than two years of age and those with bilateral disease (Appelman 1991; Burke 1991; Damoiseaux 2000; Hoberman 2011; Tähtinen 2011), and another trial has suggested that most benefit is seen in children with high fever or vomiting (Little 2001). Moreover, some studies found that children with bilateral AOM differ with regards to clinical and microbiological (increased presence of (non‐typeable) H. influenzae) characteristics compared with children with unilateral AOM (Barkai 2009; McCormick 2007). However, the individual patient data (IPD) meta‐analysis demonstrated that the relative effects of antibiotics were not significantly modified by either age or bilateral disease alone but the absolute differences were larger in the younger patients (less than two years) with bilateral disease and in children with both AOM and otorrhoea (Rovers 2006). Further analysis of these data has shown that age younger than two years is an independent predictor of the development of asymptomatic middle‐ear effusion (Koopman 2008). This analysis also found that antibiotic therapy has a marginal effect on the development of asymptomatic middle‐ear effusion in children with AOM.
Does the impact vary by duration and dose of antibiotics? Most trials use seven days of antibiotic treatment. One recent meta‐analysis of a short (less than seven days) versus long (more than seven days) course of antibiotics reported that risk of treatment failure at one month was higher with short courses of antibiotics (odds ratio (OR) 1.34, 95% confidence interval (CI) 1.15 to 1.55) (Kozyrskyj 2010). However, the absolute difference in treatment effect was small (3%) and short courses of antibiotics were associated with a statistically significant reduction in gastrointestinal adverse events compared with longer courses. A recommendation regarding the most appropriate dose of antibiotics is not possible due to a lack of sufficient data.
What are the potential consequences of not using antibiotics? Besides the immediate pain associated with AOM, there are some more serious complications. Though only two cases of mastoiditis were reported in the included trials (one child received antibiotics and one child was assigned to placebo), a semi‐randomised trial in Sweden in 1954 reported a rate of 17% in the untreated group versus none in the penicillin‐treated groups (Rudberg 1954). In populations or sub‐populations where mastoiditis is still judged a frequent problem, such as in some low‐income countries, antibiotic treatment would be strongly advised (Berman 1995).
Of note is an article that revealed that doctors commonly over‐diagnose AOM (Rothman 2003). What effect might this have on the efficacy of antibiotics (or any treatment)? One effect will be to blunt any treatment effect by dilution (from the cases of non‐AOM). The results of two recently performed trials (Hoberman 2011; Tähtinen 2011), in which AOM has been diagnosed with the use of stringent criteria (including pneumatic otoscopic examination in one trial (Tähtinen 2011), underline this phenomenon. Nevertheless, physicians in daily practice are likely to use the same diagnostic methods (perhaps even less stringent) as used in the majority of the included trials in this review. As a consequence, the effectiveness of antibiotics reported in this review is likely to be a true reflection of the effectiveness in actual clinical practice. However, if new and more accurate diagnostic procedures are introduced in future daily practice, then the current estimate of effectiveness will have to be reconsidered.
Quality of the evidence
The methodological quality of the included studies was generally high. We judged the evidence to be of high quality for most of the outcomes in the review of antibiotics against placebo. We judged the quality of evidence to be moderate for pain at 10 to 12 days, serious complications and long‐term effects (3.5 years data). We downgraded the evidence mainly because of the risk of reporting bias (pain at 10 to 12 days), sample size considerations (serious complications) and the risk of attrition bias (long‐term effects).
For the review of immediate antibiotics versus expectant observation, we judged the evidence to be of moderate quality for most of the outcomes. We downgraded the evidence mainly because of sample size considerations (tympanic membrane perforation, serious complications) and the risk of attrition bias (pain at days 11 to 14, abnormal tympanometry findings at four weeks, late AOM recurrences, long‐term effects). We judged the evidence to be of high quality for pain at days four to seven and adverse effects likely to be related to the use of antibiotics.
Potential biases in the review process
There was some clinical heterogeneity among the included trials. For example, patients were recruited from different settings (general practice, ear, nose and throat and paediatric clinics). However, the majority of included trials did use a diagnostic method (clinical diagnosis of AOM as inclusion criteria) that resembles daily clinical practice. Besides, duration and dosage of the antibiotic treatment varied to some extent. For the review of antibiotics against placebo, the duration of antibiotic treatment varied from seven to 14 days. However, we do not consider this as a major drawback since most trials used seven days of antibiotic treatment and current evidence indicates only a small absolute treatment difference (3%) in treatment failure at one month in favour of a long (more than seven days) versus a short (less than seven days) course of antibiotics. Moreover, the primary outcome of this review (proportions of children with pain) is reported within the first seven days of antibiotic treatment. In addition, we assessed funnel plots for potential reporting biases for the primary analysis (Figure 5). No asymmetry could be detected in the included trials.
Agreements and disagreements with other studies or reviews
This review demonstrated that at 24 hours pain had recovered spontaneously in 60% of children and that the majority had recovered in the following two to 12 days regardless of whether they had received placebo or antibiotics. However, the IPD meta‐analysis, which included six of the trials included in this review, revealed a slower rate of recovery (Figure 6) with only 22% of children experiencing spontaneous recovery at 24 hours (Rovers 2006). There are a number of possible explanations for this. First, data from older trials were not included in the IPD meta‐analysis and consequently the study population may reflect a higher threshold of doctor visitation; for example, the children may be 'sicker' or presenting to the doctor later in the course of their illness. Variation in the definitions of pain/no pain cut‐offs among the trials included in the reviews may also explain some of this variation. From the IPD meta‐analysis survival curve (Figure 6) it can be seen that antibiotics had greatest effect compared with placebo at day three.
6.
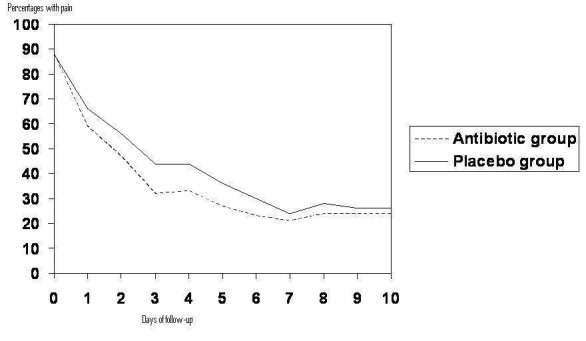
Percentage with pain based on the subset of six studies included in the IPD meta‐analysis (Rovers 2006).
A previous meta‐analysis has examined the question of whether antibiotics were indicated for AOM in children and concluded that the answer is a qualified 'yes' (Rosenfeld 1994). It estimated a NNTB of seven for "primary control" (complete clinical resolution), compared with our NNTB of 20 for symptom relief. The difference may be the consequence of our focus on patient‐oriented outcomes, such as pain, rather than clinical signs, such as eardrum appearance. The previous systematic review suggests that where mastoiditis is not a concern, primary care physicians could weigh the benefits against the risks of adverse effects from antibiotics with their patients. This statement is in agreement with the findings of our review as adverse events such as diarrhoea, vomiting or rash were more common in children receiving antibiotics. In the IPD meta‐analysis the most commonly described adverse effect of antibiotic treatment was diarrhoea, ranging from 2% to 14% in controls and from 4% to 21% in those given antibiotics (Rovers 2006). Occurrence of rash ranged from 2% to 6% in the control groups and from 1% to 8% in the antibiotic groups. A recent systematic review and meta‐analysis on common harms of amoxicillin revealed that harms were poorly reported in most placebo‐controlled trials (Gillies 2014). In this review, diarrhoea was attributed to amoxicillin only in the form of amoxicillin/clavulanate. Amoxicillin did increase the risk of candidiasis compared with placebo, but no association between amoxicillin and rash or vomiting was observed (Gillies 2014). Bacterial resistance to antibiotics is also a consideration, with an association between antibiotic use and resistant bacteria demonstrated for many important pathogens (Arnold 2005).
Several trials evaluated a management approach for AOM in which an expectant observational approach is used (Laxdal 1970; Little 2001; McCormick 2005; Neumark 2007; Spiro 2006). In one of these trials pain and malaise at day three were greater among those randomised to receive an antibiotic prescription with advice to fill it only if there was no improvement after 72 hours compared with those receiving immediate antibiotics (Little 2001). In a secondary analysis of the trial no difference was found between delayed and immediate treatment groups in ear function and ear pain at three and 12 months (Little 2006). Another study using a similar prescribing approach and examining clinical outcomes at four to six days found no difference between immediate and delayed antibiotic groups (Spiro 2006). In the third study (McCormick 2005), immediate antibiotic treatment was associated with decreased numbers of treatment failures and improved symptom control at day four and day 12 compared with those allocated to expectant observation with no prescription. Neumark 2007, in a similar comparison, found that immediate antibiotics provided some symptomatic benefit; children who received antibiotics had less pain, used fewer analgesics and consulted less during the first seven days. Meta‐analysis of data from these four trials found no difference in pain between immediate antibiotics and expectant observational approaches at three to seven days. Another review (Spurling 2013), which evaluated the effect of delayed versus immediate or no antibiotics for respiratory infections and which included two studies on AOM (Little 2001; Spiro 2006), concluded that immediate antibiotics was the strategy most likely to provide the best clinical outcomes for AOM. One randomised study found that observation therapy with or without a prescription in children with AOM was well accepted by parents (Chao 2008). Antibiotic use was less in those randomised to observation without prescription and no complications were reported.
Authors' conclusions
Implications for practice.
Antibiotics produce a (small) reduction in the number of children with pain at two to three days (number needed to treat for an additional beneficial outcome (NNTB) 20), four to seven days (NNTB 16) and 10 to 12 days (NNTB 7) from initial assessment, and reduce the number of children with tympanic membrane perforations (NNTB 33), contralateral otitis episodes (NNTB 11) and abnormal tympanometry findings at two to four weeks (NNTB 11) and six to eight weeks (NNTB 16) compared with placebo. However, in high‐income countries, most cases of acute otitis media (AOM) spontaneously remit without complications. The benefits of antibiotics must be weighed against the possible harms: for every 14 children treated with antibiotics one child experienced an adverse event (such as vomiting, diarrhoea or rash) that would not have occurred if antibiotics were withheld. Therefore management should emphasise advice about adequate analgesia and the limited role for antibiotics. Antibiotics are most useful in children under two years of age with bilateral AOM, or with both AOM and otorrhoea. For most other children with mild disease, an expectant observational approach seems justified. Cates has developed an appropriate handout and tested this together with an optional antibiotic prescription (Cates 1999). The handout is available at www.nntonline.net/ebm/main_pages/AOM.asp (accessed 22 November 2012).
Implications for research.
Further research is needed to determine if it is possible to predict which children are more likely to suffer from the complications of AOM and whether an expectant observation approach can be safely applied to children with mild AOM in low‐income countries.
Feedback
Antibiotics for AOM, 22 November 2000
Summary
1. Types of interventions includes surgical procedures versus placebo which are not dealt with in this review and should therefore be deleted.
2. The authors included only six studies in the analysis but in 1994 another meta‐analysis by Rosenfeld and colleagues to which the authors refer was published which included 33 randomized trials with 5400 children. Were any studies with a no‐treatment control excluded and if so why?
3. The meta‐analysis by Rosenfeld is only mentioned in the text; there is no reference to it. How many patients were included in the meta‐analysis?
4. It is stated that trials analysed on an intention to treat basis were preferred. This indicates that other trials were excluded which does not seem reasonable?
5. The description of the factorial trial is unclear; I suppose the authors excluded all patients who were randomised to myringotomy?
6. In the trial by Laxdal the control group was more closely monitored. The trial therefore violates the principle that all other Traitement etc. should be the same in the two randomised groups and it should therefore be excluded.
7. The strategy described by Dickersin lacks a publication year and it is not cited in the references.
8. The search was done in August 1994 and the Cochrane review was published in April 1997. The search should therefore have been updated before publication since Cochrane reviews are meant to be up‐to‐date.
9. There is no information whether the original authors and the pharmaceutical industry were contacted about additional data including unpublished trials and trials not registered in Medline. Useful trial data might be expected to be available in books published in connection with symposia arranged by the drug industry for example.
10. What is quality methodology?
11. The term blinded randomisation should be avoided since it may be confused with blinded treatments; the term concealed allocation should be used.
12. The elaborated quality assessment scale for the trials does not appear under Results and should therefore be deleted.
13. The authors refer to Rosenfeld's meta‐analysis when they state that 80% of the children have recovered spontaneously after 24 hours. Since such a percentage refers to untreated patients it raises the question why the authors did not use their own data? If these data are used in a meta‐analysis of the risk difference the NNTB will be 23 not 12 as stated in the Cochrane review.
14. For several of the excluded studies the authors gave no reason for the exclusion.
15. There should be a cross‐reference to the authors' nearly identical review in the BMJ (24 May 1997).
Reply
The changes made were:
1. We updated the search. (see Johansen criticism 7 & 8). No recent trials were found but we recognised that the Appelman trial qualifies (originally we had thought this was only prevention of recurrent otitis, rather than treatment of acute otitis in children with a recurrent episode).
2. We have corrected and updated the Relative Risk Reduction and consequent Number‐Needed‐to‐Treat (see Johansen criticism 13).
3. We have separate the four arms of the Van Buchem factorial trial, and treated this as "two" trials (i.e., two separate strata): (a) without myringotomy ‐ antibiotics versus placebo (b) with myringotomy ‐ antibiotics versus placebo. (see Johansen criticism 5)
4. As suggested by Andrew Herxheimer, we have added several references including (a) Chris Cates BMJ, and (b) Kozrskyj's meta‐analysis of short versus long duration of antibiotics (rather than just the de Saintonge paper).
5. We have made small text changes in response to Johansen's criticisms 5 (description added), 7 (dropped), 10 (‐ methodological quality), 11 (‐ allocation concealment), 13 (corrected in text), 14 (exclusions explained), and 15 (reference added).
6. As we have pointed out to Johansen in the BMJ correspondence, and point out in the discussion here, the Rosenfeld meta‐analysis is largely concerned with comparison between antibiotics. (see Johansen criticism 2 & 3).
Contributors
Helle Krogh Johansen Peter C. Gøtzsche
Antibiotic versus placebo for acute otitis media, 22 November 2010
Summary
This excellent and important review was completed in 1996, and I hope it will soon be updated. It is especially worth noting and discussing the new study by Christopher Cates (BMJ 13 March 1999, p715‐6), who has successfully tried a method in his general practice of substantially reducing the use of antibiotic in children with acute otitis media. This would considerably strengthen the 'implications for practice' in the conclusion.
I would like to suggest that in updating this review the objectives be amended and the trial by Chaput de Saintonge et al be added, because it contributes an important piece of evidence about the duration of amoxicillin therapy. The review concludes that some children will benefit from antibiotic treatment, and it would be valuable to say (as a result of the Chaput trial) that the evidence indicates that a 3‐day course is no less effective than a 10‐day course.
Reply
Chris and I have revised the acute otitis media review. We have made a number of modest changes, though none of these change the conclusions. However, because a new trial is included we've called it a "substantive update".
Contributors
Andrew Herxheimer
Antibiotic versus placebo for acute otitis media, 22 November 2000
Summary
1. I am glad to see this has been updated but the text does not explain what was updated, forcing the reader who wants to know to compare the previous version with the new one. Is it the sentence referring to Cates 99 [in implics for practice] or other points as well?
2. There are embarrassingly many typos in the refs to excluded and additional studies: Chaput de SaintoNGE, amoxyciillin, author not in bold in the first few additional refs, below that several authors' names begin in lower case when they should all begin with a capital.
3. It is implied that no comcrit was received before the final submission date for CL99 issue 3. Is this true? I think I sent one early this year.
CONFLICT OF INTEREST: None.
Reply
Excluded and additional references have been corrected and completed.
Contributors
Andrew Herxheimer
Antibiotic versus placebo for acute otitis media, 22 June 2000
Summary
1. The new study also reported diarrhoea and rashes. Shouldn't it be included in this outcome (side effects) also? 2. I think the methods used for calculating the NNTB should be made explicit. 3. The new trial is important because it looks at a sub‐group who were believed to be a greater risk of poor outcomes. In EBM OM Rosenfeld and Bluestone review the study inclusion criteria and state that the meta‐analysis 'most likely can be applied to children 2 years of age or older with non severe AOM, and most likely cannot be applied to infants with severe symptoms'. This study provides the best evidence that the conclusions of the meta‐analysis do appear to apply to this group. Perhaps this point needs to be emphasised (the peak incidence of AOM is 9 months). 4. I think the comment that 80% resolve spontaneously within 2 to 7 days is now slightly misleading as about 70% of the control children were clinical failures in this new study. 5. The entry in the table 'characteristics of included studies' should be consistent with previous entries. 6. Some typographical errors and inconsistent spelling.
Reply
Thank you for your comments and suggestions. The Absolute risk difference was used to calculate the NNTB in this systematic review. This has now been stated in the Results section of the review. A comment regarding the application of the conclusions to infants with severe symptoms has been added to the discussion section. The 70% incidence of clinical failure in the Damoiseaux, 2000 study have been included and typographical errors and inconsistencies have been corrected.
Contributors
Peter Morris
Antibiotics for acute otitis media, 19 February 2002
Summary
The second graph (comparison of outcome Abnormal Tympanometry) has wrong labels on the X‐axis.
It says 'antibiotics better' (left) and 'placebo worse' (right). The second should probably be 'placebo better'.
The other graphs are correctly labelled.
I certify that I have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
Reply
The label on the x‐axis has been corrected and now reads 'Placebo better'.
Contributors
Johannes C van der Wouden
Antibiotics reduce the risk of mastoiditis?, 26 August 2002
Summary
I agree with other commentators that this is a very good and important review. However, I would like some more clarity concerning one statement in your conclusions: Antibiotic treatment may play an important role in reducing the risk of mastoiditis in populations where it is more common.
What is the basis for this statement? In the included studies with more than 2000 children only one mastoiditis case occurred in a patient in a penicillin treated group. In the review you mention two articles concerning the mastoiditis. Firstly, the study of Rudberg (1954), which was excluded since it was not properly randomised. Even if it were, the rate of 17 % of mastoiditis cases is in these times highly unlikely, as is shown in the included studies. The second article by Berman (1995) is a literature review, where only the available literature concerning developing countries were reviewed. The goal of this review was to determine the extent to which otitis media impacts mortality and morbidity in developing countries, not to study the effect of antibiotics on (acute) otitis media or mastoiditis.
In neither of these studies evidence is shown that antibiotic treatment reduces the risk of mastoiditis, certainly not in developed countries. Since I think the rest of the review is excellent, I wonder if you could explain to me the reasons for including this statement in the conclusions.
I certify that I have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
Reply
Dear Markus,
We included the caveat about mastoiditis because we, and the reviewers, were concerned about misinterpretation of the results in situations with high rates of mastoiditis. We were mindful that "an absence of evidence is not equal to evidence of absence". Since the trials we analysed did not include high rates of mastoiditis, we can use them as the sole basis. Given that we have two weaker pieces of evidence:
1. The trials do show a modest reduction in other infective complications
2. The excluded Rudberg trial did show dramatic effects that we don't think explicable from the potential biases of that study.
Prudence would then suggest that antibiotics are advisable if there is a substantial risk of mastoiditis,
Regards,
Paul Glasziou
I certify that I have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
Contributors
Markus Oei (ENT surgeon)
Incorrect NNTB, 19 June 2005
Summary
I am a bit troubled by the way the conclusions of this review are written. By combining results of treatment at Days 2 to 7 in arriving at a NNTB of 15 one is going to underestimate treatment benefit after 2 days. In your abstract though you say the ARR is 7% and NNTB 15 for some pain after two days. This is simply not correct. If one carefully looks at trials that record pain at the end of day 2 the ARR is in fact 20% giving a NNTB of 5. Clearly acute otitis media is an acute condition and the main benefit of antibiotics is pain control and symptom relief. If this is measured at the end of 2 days the benefits are greater than one would surmise just from reading the review. It would be absurd to do a review of pain relief for biliary colic treated with pethidine and measuring the outcome 7 days later. For acute conditions symptom control in the first few days should be the outcome of interest. NNTB are meaningless unless giving a time period at which they apply. I think the review needs correcting. This is not just of academic interest but of direct relevance to parents and doctors faced with a child with AOM in pain. Unfortunately your review gets quoted uncritically and invariably the NNTB of 15 is given for symptom control after 2 days. I am currently trying to correct a brochure produced here in New Zealand for GPs to give to parents of children with AOM and it uncritically repeats this misleading information. If you want to comment on symptom control after Day 2 DO NOT pool it with data from Day 7 or later!
I certify that I have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
Reply
Thank you for your comment. We agree that we should be clearer about the time frame to which the ARR 7% and NNTB 15 applies. With the availability of results of the individual patient data meta‐analysis (Rovers 2006) we are able to obtain a clearer indication of the recovery pattern over time. We have reported this in the text and included an extra figure.
Contributors
Paul Corwin
Comment on two of the meta‐analyses, 9 June 2007
Summary
Summary Feedback: This is a comment on two of the meta‐analyses in the Cochrane Review, Glasziou et al. (2004). These analyses are for the outcomes "Vomiting, Diarrhea or Rash" and "Contralateral AOM."
1) Vomiting, Diarrhea or Rash
First we consider the meta‐analysis relating possible adverse effects of treatment. In Glasziou et al. (2004), this is done using the composite outcome "Vomiting, Diarrhea or Rash." The data used for this meta‐analysis are reproduced in the table below.
Outcome: Vomiting, Diarrhea or Rash Study Treatment Control Thalin et al. (1985) 1/159 1/158 Burke et al. (1991) 53/114 36/116 Mygind et al. (1981) 3/72 1/77 Damoiseaux et al. (2000) 20 12
We noted five major problems with this meta‐analysis. The first relates to clinical heterogeneity. This was manifested in variations in terms of the types of adverse effects recorded, who recorded them (parent or physician) and the time period over which they were recorded (from 3‐4 days to 21 days). In Thalin et al. (1985), the effects were recorded by an ENT physician on days 3‐4 or days 8‐10. In Burke et al. (1991), they were recorded by a parent in a 21‐day diary. In Mygind et al. (1981), it was done with 7 day parental score card. And in Damoiseaux et al. (2000), this was done by a physician on day 4 and day 11.
Another related problem is the use of the outcome "Vomiting, Diarrhea or Rash" as an entity. Vomiting is only reported in Burke et al. (1991). It is not clear whether it was not observed, or observed but not reported in the other studies. Also, in Burkeat al. (1991), as noted, such effects were recorded over a 21‐day period while the maximum recording period for the other studies was 11 days. The totals then gave a much higher weight to Burke et al. (1991) than may be appropriate.
A third problem is possible double or triple counting with the use of the composite outcome. For Burke et al. (1991), the group numerator is the sum of the cases for each effect. A number of children may well have had two or three of these effects at the same time.
A fourth problem is also with the numbers used. Damoiseaux et al. (2000) gives two sets of numbers for "de novo diarrhoea," for day 4 and for day 11. Glasziou et al. (2004) uses the day 4 numbers only. The reason for this choice is not clear. It may be better to use the sums of the numbers for the two days (provided this does not involve double counting.)
Further, the group denominators used for Burke et al. (1991) are perhaps not what they should be. In this study, the adverse effects were recorded by parents. Only 220 (treatment = 107, control = 113) out of a total of 232 (treatment = 114, control = 118) diaries were collected. Using the total group size in the numerator (also done in Burke at al. (1991)) is thus not appropriate.
Finally, it is not clear if the numbers for adverse effects in Burke et al. (1991) and Damoiseaux et al. (2000) included the cases known or suspected to have dropped out of the study due to an adverse effect.
In our view, this meta‐analysis should be modified as follows: First, do not use the data on vomiting until it is reported in at least one other study. Second, do not use a composite adverse effect outcome. Instead, perform separate meta‐analyses for diarrhoea and rash. Third, for Damoiseaux et al. (2000), use the total numbers for day 4 and day 11, with the above noted qualification in mind. Fourth, for Burke et al. (1991) change the denominators as noted above. Finally, include drop outs due to side effects in the meta‐analyses. The table below gives the possible numerators to be used for these meta‐analysis.
Separated Data on Side Effects Vomiting Diarrhea Rash Study T C T C T C Thalin et al. (1985) ? ? 0 0 1 1 Burke et al. (1991)+ 20 14 24 16 16 9 Mygind et al. (1981) ? ? 2 1 1/2? 0 Damoiseaux et al. (2000)*,+ ? ? 20 12 0 3 Damoiseaux et al. (2000)? ? ? 34 22 0 3 Note: ? Unclear if vomiting not observed or not reported. Note: ? = 2 if a dropout was not counted; else = 1. * Day 4; ? Day 4 and Day 11; + unclear if dropouts counted.
2) Contralateral AOM
The occurrence of contralateral AOM, as is made clear in Glasziou et al. (2004), is relevant for only the cases with unilateral AOM at the outset. This numbers in the table below are used for the meta‐analysis of this outcome in Glasziou et al. (2004).
Outcome: Contralateral AOM Study Treatment Control Thalin et al. (1985) 4/159 17/158 Burke et al. (1991) 29/98 33/102 Mygind et al. (1981) 2/72 6/77 Overall 35/329 56/337
The first problem is clinical heterogeneity, as noted in the table below. The issues in that respect are similar to those stated for the meta‐analysis of adverse effect.
Clinical Heterogeneity: Contralateral AOM Study Time Period Evaluator(s) Thalin et al. (1985) day 8‐10 or day 30 ENT Physician Burke et al. (1991) 21 days Parent Mygind et al. (1981) 1 week Physician
A further problem with this meta‐analysis is the denominators used. Consider this issue for each study.
Thalin et al. (1985): The denominators in Glasziou et al. (2004) include unilateral and bilateral cases. Only 82% of the episodes were unilateral at the start but the breakdown by group is not given in the paper. We obtained adjusted denominators as follows. Treatment: 0.82?159 = 130; Control: 0.82?158 = 130. The bias now remains the same but the precision level is now corrected.
Burke et al. (1991): The denominators represent the total unilateral cases for each group. The study authors used these denominators. Completed 21‐day diaries, the source of data on contralateral otitis, were, however, available only for 107 (of 114) in the treatment group and 113 (of 118) in the control group. So either one assumes that only the bilateral cases had missing diaries (which is unlikely) or that the rate of missingness in each group was not affected by laterality. In the latter case, the adjusted denominators are: Treatment: (98?107)/114 = 92; Control: (102?113)/118 = 98. The level of bias remains unknown but the precision level is possibly better.
Mygind et al. (1991): The denominators used include unilateral and bilateral cases. But there were 8 bilateral cases in the placebo group and 14 in the control group. So the appropriate denominators are Treatment: 72 ‐ 8 = 64; Control: 77 ‐ 14 = 65. The bias and precision levels are now corrected.
The appropriately adjusted data for this meta analysis are given below.
Contralateral AOM: Adjusted Data Study Treatment Control Thalin et al. (1985) 4/130 17/130 Burke et al. (1991) 29/92 33/98 Mygind et al. (1981) 2/64 6/65 Overall 35/286 56/294
References
1. Burke P, Bain J, Robinson D and Dunleavey J (1991) Acute red ear in children: Controlled trial of non‐antibiotic treatment in general practice, British Medical Journal, 303, 558?562.
2. Damoiseaux RAMJ, van Balen FAM, Hoes AW, Verheij TJM and de Melker RA (2000) Primary care based randomised, double blind trial of amoxicillin versus placebo for acute otitis media in children aged under 2 years, British Medical Journal, 320: 330?334.
3. Glasziou PP, Del Mar CB, HayemMand Sanders SL (2004) Antibiotics for acute otitis media in children, Cochrane Database of Systematic Reviews, 2004; (1): CD000219. Art. No: CD000219, DOI: 10.1002/14651858.CD000219.pub2 (21pages)
4. Mygind N, Meistrup‐Larsen K‐I, Thomsen J, Thomsen VF, Josefsson K and Sorenson H (1981) Penicillin in acute otitis media: a double‐blind placebo‐controlled trial, Clinical Otolaryngology, 6: 5?13.
5. Thalin A, Densert O, Larsson A, Lyden E and Ripa T (1986) Is penicillin necessary in the treatment of acute otitis media? In: Proceedings of the International Conference on Acute and Secretory Otitis Media, Amsterdam, The Netherlands, Kegler Publications, pages 441?446.
Submitter agrees with default conflict of interest statement: I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
1) We acknowledge the variation in methods of collecting and recording information on adverse events and in the types of adverse events reported in the included trials. We contend however, that considering vomiting, diarrhoea or rash as an entity is justified by the easier interpretation it provides. Though the events are biologically very different, they are of similar seriousness; irritating and difficult to manage but minor in nature. Also, as pointed out in the above comments, dividing the adverse events into each type would not be helpful as they are infrequently reported (i.e. vomiting is only reported in one study). We recognise that 'lumping' the adverse events together is a crude approach but believe the benefits in continuing to do so outweigh the drawbacks. In the discussion section of this update we have made reference to the results of the individual patient data meta analysis (Rovers 2006) (which included a subset [n = 6 ] of the trials included in this review [n = 10]) which reports separately on the frequency of diarrhoea and rash in the treatment and control groups. We appreciate your consideration and suggestions related to the inclusion of drop outs due to side effects in the Burke and Damoiseaux studies. Corrections to the data have been incorporated. 2) Thank you for pointing out the numerical errors in the meta analysis of contralateral AOM. We have corrected the analysis as suggested. This results in a minor changed to the pooled random effects OR (OR 0.44 95% CI 0.16, 1.26 versus 0.45 95% CI 0.16, 1.23) with antibiotics appearing to reduce contralateral AOM though the effect was not significant with the random effects model.
Contributors
Karim F. Hirji, D.Sc Peter C. Gøtzsche
Antibiotics for acute otitis media in children, 8 March 2011
Summary
The title and conclusion of the review need revising as it is just reviewing the effect of penicillin family antibiotic on the AOM and not other antibiotics. It is suggesting to changed the title to "Usage of penicillin family Antibiotics for acute otitis media in children".
Warm regards.
PS: The only included trials were too old and they just used the publish data:
Halsted 1968 ampicillin 100 mg/kg/day or phenethicillin 30 mg/kg/day plus sulphisoxazole 150 mg/kg/day Howie 1973 one of erythromycin, ampicillin, or triple sulphonamide plus erythromycin
Submitter agrees with default conflict of interest statement: I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
The title is our intention. However, as you point out, it just so happens that most (but not all) antibiotics trialled against placebo for acute otitis media were from the penicillin group. Moreover more trials might be undertaken using non‐penicillin antibiotics. So it is appropriate to retain the original title.
Chris Del Mar, 19 June, 2012
Contributors
Amirkambiz Hamedanizadeh, Medical Doctor
What's new
| Date | Event | Description |
|---|---|---|
| 26 April 2015 | New citation required but conclusions have not changed | The conclusions regarding the effectiveness and safety of antibiotics have essentially not changed, except for some new outcomes (e.g. long‐term effects on AOM recurrences) and minor changes to the risk of bias. |
| 26 April 2015 | New search has been performed | We updated the searches in April 2015. In this updated review, we now provide outcome data on:
The outcome 'Adverse effects likely to be related to the use of antibiotics such as vomiting, diarrhoea or rash' has been added to primary outcomes (in earlier versions this outcome was listed as a secondary outcome) according to the recommendations described in Chapter 5.4.2 of the Cochrane Handbook for Systematic Reviews of Interventions ("the primary outcomes should include at least one desirable and at least one undesirable outcome") (Higgins 2011). One new trial was identified for the review of antibiotics against placebo (Tapiainen 2014). This study included children aged between six months and 15 years and provided data on pain at days four to seven, adverse effects likely to be related to the use of antibiotics, abnormal tympanometry findings at two to four weeks and six to eight weeks, tympanic membrane perforation and serious complications. New data were added to the review from previously included trials. For the review of antibiotics against placebo:
For the review of immediate antibiotics against expectant observation:
We identified one ongoing trial (ACTRN12608000424303). The objective of this double‐blind, placebo‐controlled randomised clinical trial is to assess the effectiveness of azithromycin for seven days in aboriginal children with asymptomatic AOM, defined as a bulging tympanic membrane without associated symptoms at the time of diagnosis. The primary outcome is the proportion of children with a bulging tympanic membrane or ear discharge or withdrawn due to complications or side effects at 14 days. Quality of evidence is now described based on the GRADE framework. |
History
Protocol first published: Issue 1, 1995 Review first published: Issue 3, 1996
| Date | Event | Description |
|---|---|---|
| 8 November 2012 | New search has been performed | A new review author joined the team to update this review. We updated the searches in November 2012. Two new trials were identified for the review of antibiotics against placebo (Hoberman 2011; Tähtinen 2011). These studies included children < 35 months of age and provided data on pain (Tähtinen 2011), contralateral otitis, late recurrences (Hoberman 2011), perforation and adverse events (Hoberman 2011; Tähtinen 2011). The Laxdal 1970 trial has been removed from the review of antibiotics against placebo and added to the review of immediate antibiotics versus expectant observation. No new trials were identified for the review of immediate antibiotics compared with expectant observation. Furthermore, we did not identify ongoing trials. In this updated review, we now provide outcome data for pain at 24 hours, two to three days and four to seven days (in earlier versions outcome data for pain were presented at 24 hours and two to seven days). |
| 8 November 2012 | New search has been performed | The general conclusions and recommendations regarding the effectiveness of antibiotics on pain and adverse events remained unchanged. Antibiotic treatment led to a statistically significant reduction of children with AOM experiencing pain at two to seven days compared with placebo, but since most children (82%) settle spontaneously, about 20 children must be treated to prevent one suffering from ear pain at two to three and four to seven days. (In the previous version the number needed to treat to benefit (NNTB) was 16). However, in this updated review antibiotic treatment appeared to have a statistically significant beneficial effect on the number of tympanic membrane perforations (risk ratio (RR) 0.37, 95% confidence interval (CI) 0.18 to 0.76; NNTB 33) and contralateral acute otitis media (AOM) episodes (RR 0.49, 95% CI 0.25 to 0.95; NNTB 11) compared with placebo. For every 14 children treated with antibiotics one child experienced an adverse event (such as vomiting, diarrhoea or rash) that would not have been occurred if antibiotics were withheld. (In the previous version the number needed to treat to harm (NNTH) was 24). Antibiotics are most useful in children under two years of age with bilateral AOM, or with both AOM and otorrhoea. For most other children with mild disease, an expectant observational approach seems justified. We have no data on populations with higher risks of complications. |
| 19 June 2012 | Feedback has been incorporated | Feedback added to review. |
| 2 September 2009 | Amended | 95% confidence intervals corrected for the outcome pain at two to seven days and adverse events stated in the abstract and body of the review. |
| 2 July 2008 | New search has been performed | The search was updated in July 2008. Four new trials were identified and included in the review (Le Saux 2005; McCormick 2005; Neumark 2007; Spiro 2006). One of these trials compared antibiotics with placebo (Le Saux 2005). For the outcome pain at 24 hours and two to seven days, inclusion of this trial did not alter the overall conclusions of the primary analysis. The three other new trials compared immediate antibiotics with various observational approaches (McCormick 2005; Neumark 2007; Spiro 2006). One of the new trials compared immediate antibiotics with delayed prescribing (Spiro 2006). The other trials compared immediate antibiotics with 'watchful waiting', in which no prescription was supplied but advice on when to seek treatment was provided (McCormick 2005; Neumark 2007). Outcome data on pain at three to seven days from these trials were analysed with data from another trial of immediate versus delayed prescription (Little 2001). In earlier versions of the review data from the Little trial had been included in a sensitivity analysis (Little 2001). In this update, data from the four trials comparing immediate versus observational management strategies have been included in the main analysis. Information on subgroups of children who are most likely to benefit from treatment with antibiotics, obtained from a meta‐analysis of individual patient data, has been included in this review (Rovers 2006). Methods of the IPD meta‐analysis, conducted by two authors on this review (and others) are also included. Survival curves from the IPD meta‐analysis showing the pattern of recovery from acute otitis media over time has been included as an extra figure. Two ongoing trials comparing antibiotics with placebo in children < 35 months have been identified. |
| 17 January 2008 | Amended | Converted to new review format. |
| 4 September 2007 | Feedback has been incorporated | Feedback added. |
| 18 February 2005 | Feedback has been incorporated | Feedback and reply added. |
| 24 March 2003 | New search has been performed | Searches conducted. |
| 24 August 2002 | Feedback has been incorporated | Feedback added. |
| 17 February 2002 | Feedback has been incorporated | Feedback added. |
| 20 November 2000 | Feedback has been incorporated | Feedback comments and replies added. |
| 3 February 2000 | New search has been performed | Searches conducted. |
| 3 February 2000 | New citation required and conclusions have changed | Conclusions changed. |
| 30 December 1998 | New search has been performed | Searches conducted. |
| 30 July 1994 | New search has been performed | Searches conducted. |
Acknowledgements
We would like to thank Professor Charles Bridges‐Webb (deceased) for stimulating initial discussions and for constructive advice on the protocol for this review and Professor Steve Berman for helpful comments on the draft review. We would like to thank Bruce Arroll and Tom Fahey for peer refereeing the 2005 updated review and Dilip Raghavan, Brian Westerberg, Mark Jones and Peter Morris for peer refereeing the 2009 updated review. We also thank Dilip Raghavan, Brian Westerberg, Teresa Neeman and Peter Morris for commenting on the 2012 updated review and Ann Fonfa, Julie Gildie, Conor Teljeur, Bruce Arroll, Brian Westerberg and Peter Morris for peer refereeing the 2015 updated review.
Thank you to David McCormick and his colleagues for allowing us to access raw study data from the trial comparing immediate antibiotics and expectant observation (McCormick 2005).
Thank you to Chris Cates for noticing and advising us of an error in the confidence intervals presented in the review.
We gratefully thank Sarah Thorning for her support with the search strategy and searches.
Appendices
Appendix 1. Previous search
Several electronic databases were used to compile relevant published RCTs of antibiotic treatment of AOM in children. The Cochrane Controlled Trials Register, MEDLINE and Current Contents were searched from 1966 to January 2000 by an expert librarian in conjunction with one researcher, using combinations of "OTITIS MEDIA" and a search strategy described by (Dickersin 1994) for optimally identifying controlled trials. In addition, titles in Index Medicus were checked from 1958 to 1965. The references of all relevant retrieved trials were checked to identify other articles.
The search was updated in March 2003, and again in July 2008. We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 2008, Issue 2), which contains the ARI Group's Specialized Register; MEDLINE (1966 to June week 4 2008); OLDMEDLINE (1958 to 1965); EMBASE (January 1990 to July 2008); and Current Contents (1966 to July 2008). The bibliographies of relevant articles were checked. A forward search of relevant articles was conducted in Web of Science®.
The following search strategy was run on MEDLINE (Ovid) combined with terms from Phase 1 and 2 of the Cochrane highly sensitive search strategy for identifying reports of RCTs (Lefebvre 2011). Modified terms were used to search the other databases:
MEDLINE (Ovid)
#1 exp Otitis Media/ #2 exp Otitis Media with Effusion/ #3 exp Otitis Media, Suppurative/ #4 glue ear.mp. #5 otitis media.mp. #6 OME.mp. #7 AOM.mp. #8 #1 or #2 or #3 or #4 or #5 or #6 or #7 #9 exp Anti‐Bacterial Agents/ #10 exp Drug Therapy/ #11 exp Anti‐Infective Agents/ #12 antibiotic$.mp. #13 #9 or #10 or #11 or #12 #14 #8 and #13
There were no language or publication restrictions.
Appendix 2. MEDLINE (Ovid) search strategy
1 exp Otitis Media/ 2 otitis media.tw. 3 glue ear*.tw. 4 (middle ear adj5 (infect* or inflam*)).tw. 5 (ome or aom).tw. 6 or/1‐5 7 exp Anti‐Bacterial Agents/ 8 Drug Therapy/ 9 Anti‐Infective Agents/ 10 antibiotic*.tw. 11 antibacterial*.tw. 12 exp Ampicillin/ 13 exp Cephalosporins/ 14 exp Macrolides/ 15 exp Penicillins/ 16 (ampicillin* or cephalosporin* or macrolide* or penicillin* or amoxicillin* or amoxycillin* or cefdinir or cefpodoxime or cefuroxime or azithromycin or clarithromycin or erythromycin*).tw,nm. 17 or/7‐16 18 6 and 17
Appendix 3. Embase.com search strategy
18 #14 AND #17 17 #15 OR #16 16 random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross‐over':ab,ti OR 'cross over':ab,ti OR volunteer*:ab,ti OR assign*:ab,ti OR allocat*:ab,ti 15 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp 14 #4 AND #13 13 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 12 ampicillin*:ab,ti OR cephalosporin*:ab,ti OR macrolide*:ab,ti OR penicillin*:ab,ti OR amoxycillin*:ab,ti OR amoxicillin*:ab,ti OR cefdinir*:ab,ti OR cefpodoxime*:ab,ti OR cefuroxime*:ab,ti OR azithromycin*:ab,ti OR clarithromycin*:ab,ti OR erythromycin*:ab,ti 11 'penicillin g'/exp 10 'macrolide'/exp 9 'cephalosporin derivative'/exp 8 'ampicillin'/exp 7 antibiotic*:ab,ti OR antibacterial*:ab,ti 6 'drug therapy'/de OR 'antiinfective agent'/de 5 'antibiotic agent'/exp 4 #1 OR #2 OR #3 3 ('middle ear' NEAR/5 (inflam* OR infect*)):ab,ti 2 'otitis media':ab,ti OR 'glue ear':ab,ti OR 'glue ears':ab,ti OR ome:ab,ti OR aom:ab,ti 1 'otitis media'/exp
Appendix 4. Current Contents search strategy
| # 3 | 578 | #2 AND #1 Databases=CM, LS Timespan=All Years Lemmatization=On |
|
| # 2 | 528,401 | Topic=(random* or placebo* or crossover* or "cross over" or allocat* or ((doubl* or singl*) NEAR/1 blind*)) OR Title=(trial) Databases=CM, LS Timespan=All Years Lemmatization=On |
|
| # 1 | 2,624 | Topic=(otitis or "glue ear" or ("middle ear" NEAR/3 (infect* or inflam*)) or ome or aom) AND Topic=(antibiotic* or antibacterial* or antiinfective* or ampicillin* or cephalosporin* or macrolide* or amoxicillin* or amoxycillin* or penicillin* or cefdinir* or cefpodoxime* or cefuroxime* or azithromycin* or clarithromycin* or erythromycin*) Databases=CM, LS Timespan=All Years Lemmatization=On |
Appendix 5. CINAHL search strategy
S30 S19 and S29 S29 S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 S28 (MH "Quantitative Studies") S27 TI placebo* or AB placebo* S26 (MH "Placebos") S25 TI random* or AB random* S24 (MH "Random Assignment") S23 TI (singl* blind* or doubl* blind* or tripl* blind* or trebl* blind* or singl* mask* or doubl* mask* or tripl* mask* or trebl* mask*) or AB (singl* blind* or doubl* blind* or tripl* blind* or trebl* blind* or singl* mask* or doubl* mask* or tripl* mask* or trebl* mask*) S22 TI clinic* N1 trial* or AB clinic* N1 trial* S21 PT clinical trial S20 (MH "Clinical Trials+") S19 S7 and S18 S18 S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 S17 TI ( ampicillin* or cephalosporin* or macrolide* or amoxicillin* or amoxycillin* or penicillin* or cefdinir* or cefpodoxime* or cefuroxime* or azithromycin* or clarithromycin* or erythromycin* ) or AB ( ampicillin* or cephalosporin* or macrolide* or amoxicillin* or amoxycillin* or penicillin* or cefdinir* or cefpodoxime* or cefuroxime* or azithromycin* or clarithromycin* or erythromycin* ) S16 (MH "Penicillins+") S15 (MH "Antibiotics, Macrolide+") S14 (MH "Cephalosporins+") S13 (MH "Ampicillin+") S12 TI antibacterial* or AB antibacterial* S11 TI antibiotic* or AB antibiotic* S10 (MH "Antiinfective Agents") S9 (MH "Drug Therapy") S8 (MH "Antibiotics+") S7 S1 or S2 or S3 or S4 or S5 or S6 S6 TI ( aom or ome ) or AB ( aom or ome ) S5 TI middle ear inflam* or AB middle ear inflam* S4 TI middle ear infect* or AB middle ear infect* S3 AB glue ear* or TI glue ear* S2 TI otitis media or AB otitis media S1 (MH "Otitis Media+")
Appendix 6. LILACS search strategy
> Search > (MH:"otitis media" OR "otitis media" OR "Otite Média" OR MH:C09.218.705.633$) AND (MH:"Anti‐Bacterial Agents" OR antibiotic$ OR Antibacterianos OR Antibióticos OR MH:"Drug Therapy" OR Quimioterapia OR "Terapia por Drogas" OR Farmacoterapia OR MH:"Anti‐Infective Agents" OR Antiinfecciosos OR MH:ampicillin OR Ampicilina OR ampicillin$ OR MH:D02.065.589.099.750.750.050$ OR MH:D02.886.108.750.750.050$ OR MH:D03.438.460.825.750.050$ OR MH:D03.605.084.737.750.050$ OR D04.075.080.875.099.221.750.750.050$ OR MH:cephalosporins OR cephalosporin$ OR Cefalosporinas OR MH:D02.065.589.099.249$ OR D02.886.665.074$ OR D04.075.080.875.099.221.249$ OR MH:macrolides OR macrolide$ OR Macrólidos OR Macrolídeos OR D02.540.505$ OR D02.540.576.500$ OR D04.345.674.500$ OR MH:penicillins OR penicillin$ OR Penicilinas OR MH:D02.065.589.099.750$ OR D02.886.108.750$ OR D03.438.260.825$ OR D03.605.084.737$ OR D04.075.080.875.099.221.750$ OR amoxicillin$ OR Amoxicilina OR cefdinir OR cefpodoxim$ OR cefuroxim$ OR azithromycin$ OR Azitromicina OR clarithromycin$ OR Claritromicina OR erythromycin OR Eritromicina) > clinical_trials
Data and analyses
Comparison 1. Antibiotics versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Pain at 24 hours | 6 | 1394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.01] |
| 1.2 Pain at 2 to 3 days | 7 | 2320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.57, 0.86] |
| 1.3 Pain at 4 to 7 days | 8 | 1347 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.63, 0.91] |
| 1.4 Pain at 10 to 12 days | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.17, 0.66] |
| 2 Vomiting, diarrhoea or rash | 8 | 2107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.19, 1.59] |
| 3 Abnormal tympanometry | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 2 to 4 weeks | 7 | 2138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.74, 0.90] |
| 3.2 6 to 8 weeks | 3 | 953 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.78, 1.00] |
| 3.3 3 months | 3 | 809 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.76, 1.24] |
| 4 Tympanic membrane perforation | 5 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.18, 0.76] |
| 5 Contralateral otitis (in unilateral cases) | 4 | 906 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.25, 0.95] |
| 6 Late AOM recurrences | 6 | 2200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.78, 1.10] |
Comparison 2. Immediate antibiotics versus expectant observation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Pain at 3 to 7 days | 4 | 959 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.50, 1.12] |
| 1.2 Pain at 11 to 14 days | 1 | 247 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.75, 1.10] |
| 2 Vomiting, diarrhoea or rash | 2 | 550 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.24, 2.36] |
| 3 Abnormal tympanometry at 4 weeks | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.78, 1.35] |
| 4 Tympanic membrane perforation | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 AOM recurrences | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.74, 2.69] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Appelman 1991.
| Methods |
Randomised ‐ yes, computer‐generated random numbers Concealment of allocation ‐ adequate Double‐blind ‐ yes, blinding procedure not described Intention‐to‐treat (ITT) ‐ unclear Loss to follow‐up ‐ described Design ‐ parallel |
|
| Participants |
N ‐ 126 children (N = 121 children included in analysis) Age ‐ between 6 months and 12 years Setting ‐ general practice and secondary care in the Netherlands; confirmation of diagnosis and randomisation were done by otorhinolaryngologists Inclusion criteria ‐ recurrence of acute otitis media (AOM) characterised by a (sub)acute onset, otalgia and otoscopic signs of middle‐ear infection within 4 weeks to 12 months of the previous attack Exclusion criteria ‐ antibiotic treatment < 4 weeks prior to randomisation, previous participation in this study, contraindication for penicillin, serious concurrent disease that necessitated antibiotic treatment Baseline characteristics ‐ balanced |
|
| Interventions | Tx ‐ amoxicillin/clavulanate (weight tailored dose) for 7 days; N = 70 (N = 67 included in analysis) C ‐ matching placebo for 7 days; N = 56 (N = 54 included in analysis) Use of additional medication ‐ each child was given analgesics (paracetamol) as long as earache was present and decongestive nose drops for 1 week | |
| Outcomes |
Primary outcome ‐ treatment failure (i.e. presence of otalgia or fever > 38 °C or both at 3 days) Assessment by (blinded) general practitioner at 3 days on the presence or absence of fever (> 38 °C) and otalgia and 14 days on the presence or otorrhoea Assessment by otorhinolaryngologist at 1 month of otoscopy, tympanometry and in children > 3 years of age an audiogram |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Treatment allocated by otolaryngologist (independent to trial personnel); treatment code placed in sealed envelopes |
| Other bias | Unclear risk | ITT analysis ‐ unclear, baseline characteristics ‐ balanced |
| Blinding of participants and personnel (performance bias) | Unclear risk | Identical taste and appearance to amoxicillin/clavulanate and placebo not described |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up ‐ treatment: N = 3 (4%) and placebo: N = 2 (4%) due to loss of their registration forms |
Burke 1991.
| Methods |
Randomised ‐ yes, computer‐generated random numbers Concealment of allocation ‐ adequate Double‐blind ‐ yes Intention‐to‐treat (ITT) ‐ yes Loss to follow‐up ‐ not described Design ‐ parallel |
|
| Participants |
N ‐ 232 children Age ‐ between 3 and 10 years Setting ‐ general practice; 48 general practitioners in 17 general practices in Southampton, Bristol and Portsmouth (UK) Inclusion criteria ‐ acute earache and at least 1 abnormal eardrum Exclusion criteria ‐ antibiotic treatment or acute otitis media (AOM) < 2 weeks prior to randomisation, strong indication for antibiotic treatment according to general practitioner, contraindication for amoxicillin, serious chronic conditions Baseline characteristics ‐ slight imbalance in gender (boys treated with antibiotics versus boys treated with placebo = 52% versus 42%) and figure 1 appears to demonstrate that fewer children were crying at baseline (0 hours) in the amoxicillin arm compared with the placebo arm, suggesting a failure of randomisation |
|
| Interventions | Tx ‐ amoxicillin 250 mg 3 times daily for 7 days; N = 114 (N = 114 included in analysis for short‐term outcome) C ‐ matching placebo 3 times daily for 7 days; N = 118 (N = 118 included in analysis for short‐term outcome) Use of additional medication ‐ analgesics (paracetamol 120 mg/5 mL) for pain as needed | |
| Outcomes |
Main outcomes were divided into short‐term, middle‐term and long‐term: Short‐term ‐ (a) duration of symptoms; (b) use of analgesics (assessed by weighing bottles); (c) clinical signs at 1 week; (d) incidence of complications; (e) treatment failure (i.e. second‐line antibiotics were required) Middle‐term ‐ (a) tympanometry findings at 1 and 3 months Long‐term ‐ (b) number of AOM episodes in 12 months; (b) number of specialist referrals Home visits by researcher at day 1, days 4 to 6 and general practitioner visit at day 7 Symptom diary kept by parents for 21 days |
|
| Notes | It is not clear whether the "discharging ears" in Table 1 should be included as perforations, we now included the number of perforations as summarised in Table 2 in our analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Randomisation was carried out independently of the investigators; randomisation code was kept sealed and was unknown to any of the participants in the study |
| Other bias | Unclear risk | ITT analysis ‐ yes; baseline characteristics ‐ imbalance for gender and crying |
| Blinding of participants and personnel (performance bias) | Low risk | Each bottle was identified only by number |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up ‐ not described; all randomised patients included in short‐outcome analysis |
Damoiseaux 2000.
| Methods |
Randomised ‐ yes, computerised 2 block randomisation Concealment of allocation ‐ adequate Double‐blind ‐ yes Intention‐to‐treat (ITT) ‐ yes Loss to follow‐up ‐ described Design ‐ parallel |
|
| Participants |
N ‐ 240 children (N = 212 children included in analysis) Age ‐ between 6 months and 2 years Setting ‐ general practice; 53 general practitioners (GPs) in the Netherlands Inclusion criteria ‐ acute otitis media (AOM) defined as infection of the middle ear of acute onset and a characteristic eardrum picture (injection along the handle of the malleus and the annulus of the tympanic membrane or a diffusely red or bulging eardrum) or acute otorrhoea. In addition 1 or more symptoms of acute infection (fever, recent earache, general malaise, recent irritability) Exclusion criteria ‐ antibiotic treatment < 4 weeks prior to randomisation, contraindication for amoxicillin, comprised immunity, craniofacial abnormalities, Down's syndrome or being entered in this study before Baseline characteristics ‐ slight imbalance in the prevalence of recurrent AOM, regular attendance at a daycare centre and parental smoking; logistic regression was used to adjust for these imbalances |
|
| Interventions | Tx ‐ amoxicillin suspension 40 mg/kg/day 3 times daily for 10 days; N = 117 (N = 107 included in analysis for short‐term outcome) C ‐ matching placebo suspension for 10 days; N = 123 (N = 105 included in analysis for short‐term outcome) Use of additional medication ‐ all children received decongestive nose drops for 7 days; analgesics (paracetamol, children < 1 year: 120 mg suppository, > 1 year: 240 mg suppository) was allowed | |
| Outcomes |
Primary outcome ‐ persistent symptoms at day 4: assessed by the doctor and defined as persistent earache, fever > 38 °C, crying or being irritable. Additionally, prescription of another antibiotic because of clinical deterioration before the first follow‐up visit was to be considered a persistent symptom Secondary outcomes ‐ (a) clinical treatment failure at day 11 (i.e. persistent fever, earache, crying, being irritable or no improvement of tympanic membrane (including perforation); (b) duration of fever, pain or crying; (c) mean number of doses analgesics given; (d) adverse effects mentioned in diaries; (e) percentage of children with middle‐ear effusion at 6 weeks (i.e. combined otoscopy and tympanometry) Follow‐up visits at the GP's clinic were scheduled at day 4 and 11; home visit at 6 weeks by the researcher collecting data of symptoms, referrals and both otoscopy and tympanometry was performed Parents were instructed to keep a symptom diary for 10 days |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised 2 block randomisation |
| Allocation concealment (selection bias) | Low risk | Randomisation was carried out independently of the investigators; randomisation code was kept in pharmacy of the University Medical Centre Utrecht |
| Other bias | Low risk | ITT analysis ‐ yes, baseline characteristics ‐ slight imbalance, logistic regression was used to adjust for imbalances in prognostic factors |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo suspension with same taste and appearance as amoxicillin |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up/exclusion from analysis (received other antibiotics or had grommets inserted) ‐ treatment: N = 10 (9%) and placebo: N = 18 (15%). However, for primary analysis of symptoms at day 4 all randomised patients were included |
Halsted 1968.
| Methods |
Randomised ‐ yes, pre‐determined code, which was unknown to physician; method of random sequence generation unclear Concealment of allocation ‐ adequate Double‐blind ‐ yes Intention‐to‐treat (ITT) ‐ unclear Loss to follow‐up ‐ described, unclear from which treatment group patients were excluded Design ‐ parallel |
|
| Participants |
N ‐ 106 children (N = 89 children included in analysis; N = 12 children were excluded because they did not adhere to the double‐blind protocol; N = 5 children lost to follow‐up or excluded because of persistent fever, development of complication requiring antibiotic treatment or if group A streptococci was cultured from the middle ear) Age ‐ between 2 months and 5.5 years Setting ‐ secondary care: paediatric department of Cleveland (USA) Inclusion criteria ‐ AOM based on otoscopic findings; most of the cases had bulging membrane with loss of normal light reflex and landmarks, in a few the eardrum was only diffusely red Exclusion criteria ‐ antibiotic treatment < 10 days prior to randomisation, associated bacterial infection requiring antibiotic treatment, rupture of tympanic membrane, contraindication for study drugs Baseline characteristics ‐ not described |
|
| Interventions |
Tx 1 ‐ ampicillin 100 mg/kg/day 4 daily for 10 days; N = ? (N = 30 included in analysis) Tx 2 ‐ pheneticillin 30 mg/kg/day 4 daily and sulfisoxazole 150 mg/kg/day 4 daily for 10 days; N = ? (N = 32 included in analysis) C ‐ placebo for 10 days; N = ? (N = 27 included in analysis) Use of additional medication ‐ phenylephrine nose drops and aspirin for children over 6 months was prescribed as necessary; no other medications were employed |
|
| Outcomes |
Primary outcome ‐ early improvement defined as defervescence and decrease of symptoms at 24 to 72 hours Secondary outcomes ‐ (a) late improvement defined as resolution of symptoms and normal tympanic membrane at 14 to 18 days, (b) bacteriological cultures |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Pre‐determined code, which was unknown to physician; method of random sequence generation unclear |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Other bias | Unclear risk | ITT analysis ‐ unclear, baseline characteristics ‐ not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Identical taste and appearance to antibiotics and placebo not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Reasons described, unclear from which treatment group patients were excluded |
Hoberman 2011.
| Methods |
Randomised ‐ yes, stratified block randomisation with computer‐generated randomisation lists Concealment of allocation ‐ adequate Double‐blind ‐ yes Intention‐to‐treat (ITT) ‐ yes Loss to follow‐up ‐ described Design ‐ parallel |
|
| Participants |
N ‐ 291 (N = 291 included in analysis) Age ‐ between 6 months and 2 years Setting ‐ secondary care; children's hospital of Pittsburgh and a private paediatric clinic in Kittanning (USA) Inclusion criteria ‐ children needed to have received at least 2 doses of pneumococcal conjugate vaccine and to have acute otitis media (AOM) as defined on the basis of 3 criteria: (a) the onset (i.e. within the preceding 48 hours) of symptoms that parents rated with a score of at least 3 on the acute otitis media ‐ severity of symptoms (AOM‐SOS) scale (on which scores range from 0 to 14, with higher scores indicating greater severity of symptoms), (b) the presence of middle‐ear effusion and (c) moderate or marked bulging of the tympanic membrane or slight bulging accompanied by either otalgia or marked erythema of the membrane All the study clinicians were otoscopists who had successfully completed an otoscopic validation programme Exclusion criteria ‐ antibiotic treatment < 96 hours prior to randomisation, concomitant acute illness (e.g. pneumonia) or a chronic illness (e.g. cystic fibrosis), contraindication to amoxicillin, presence of otalgia for more than 48 hours, perforation of the tympanic membrane Baseline characteristics ‐ balanced |
|
| Interventions |
Tx ‐ amoxicillin‐clavulanate 90‐6.4 mg/kg daily in 2 doses for 10 days; N = 144 (N = 139 were assessed at day 4 to 5)
C ‐ matching placebo in 2 doses for 10 days; N = 147 (N = 142 were assessed at day 4 to 5)
Use of additional medication ‐ acetaminophen (paracetamol) as needed for symptom relief At each visit children were categorised as having met the criteria for either clinical success or clinical failure Children who met the criteria for clinical failure were treated with a standardised 10‐day regimen of orally administered amoxicillin (90 mg/kg daily) and cefixime (8 mg/kg daily) |
|
| Outcomes |
Primary outcomes ‐ (a) time to resolution of symptoms (i.e. time to the first recording of an AOM‐SOS score of 0 or 1 and the time to the second of 2 successive recordings of that score; (b) symptom burden over time (i.e. mean AOM‐SOS score over time each day for the first 7 days of follow‐up and groups' weighted mean scores for that period) Secondary outcomes ‐ (a) clinical failure at day 4 to 5; (b) clinical failure at day 10 to 12; (c) use of acetaminophen (paracetamol); (d) occurrence of adverse events; (e) nasopharyngeal colonisation rates; (f) use of healthcare resources; (g) relapses Clinical failure was defined at or before the day 4 to 5 visit as either a lack of substantial improvement in symptoms, a worsening of signs on otoscopic examination, or both and at the day 10 to 12 visit as the failure to achieve complete or nearly complete resolutions of symptoms and otoscopic signs, without regard to the persistence of resolution of middle‐ear effusion. Once a child had met the criteria for clinical failure, he or she remained in that category for the analysis Daily symptoms were assessed with the use of a structured interview of 1 of the child's parents until the first follow‐up visit; visits were scheduled at day 4 or 5, day 10 to 12 (end of treatment) and at day 21 to 25 Patients were asked to complete a diary twice a day for 3 days and once a day thereafter |
|
| Notes | This study did not report pain data that could be used for the review comparing antibiotics with placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block randomisation with computer‐generated randomisation lists |
| Allocation concealment (selection bias) | Low risk | A pharmacist (independent of the trial team) provided masked study medication bottles with amoxicillin/clavulanate or placebo |
| Other bias | Low risk | ITT analysis ‐ yes, baseline characteristics ‐ balanced |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo with same taste and appearance as amoxicillin‐clavulanate |
| Incomplete outcome data (attrition bias) | Low risk | Children not assessed at day 4 to 5 ‐ treatment: N = 5 (3%) and placebo: N = 5 (3%). All randomised patients included in analysis |
Howie 1972.
| Methods |
Randomised ‐ yes, method of randomisation not described Concealment of allocation ‐ adequate Double‐blind ‐ yes Intention‐to‐treat (ITT) ‐ unclear Loss to follow‐up ‐ not described Design ‐ parallel |
|
| Participants |
N ‐ 280 children Age ‐ 2.5 years or younger Setting ‐ secondary care: general paediatric practice in Huntsville (USA) Inclusion criteria ‐ acute otitis media (AOM) as clinically diagnosed by the participating paediatricians Exclusion criteria ‐ if researchers felt that parents would not accept diagnostic aspiration, when condition of the patient required immediate antibiotic treatment Baseline characteristics ‐ not described |
|
| Interventions |
Tx 1 ‐ erythromycin estolate 125 mg/5 mL ‐ triple sulphonamide suspension 0.5 g/5 mL; N = 80 Tx 2 ‐ ampicillin 250 mg/5 mL; N = 36 Tx 3 ‐ triple sulphonamide suspension 0.5 g/5 mL; N = 23 Tx 4 ‐ erythromycin estolate 125 mg/5 mL; N = 25 C 1 ‐ placebo ‐ equal parts acetaminophen (paracetamol) and chlorpheniramine maleate syrup; N = 33 C 2 ‐ placebo ‐ 4 parts Kaopectate and 1 part acetaminophen (paracetamol, Tylenol) plus food colouring; N = 83 Use of additional medication ‐ all children received decongestive nose drops for 7 days; analgesics (paracetamol, children < 1 year: 120 mg suppository, > 1 year: 240 mg suppository) was allowed |
|
| Outcomes |
Primary outcomes ‐ (a) presence or absence of exudate while on medication; (b) bacteriological findings of the exudate when present; no patient‐relevant outcomes were described At baseline and before treatment was started, the middle‐ear exudate was aspirated. The decision whether to collect exudate on the first repeat visit was made with no knowledge of the drug regimen to which the patient had been assigned |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by a collaborating pharmacist |
| Other bias | Unclear risk | ITT analysis ‐ unclear, baseline characteristics ‐ not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Identical taste and appearance to amoxicillin/clavulanate and placebo not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up ‐ not described |
Kaleida 1991.
| Methods |
Randomised ‐ yes, stratified randomisation, method of randomisation not described Concealment of allocation ‐ unclear, method not described Double‐blind ‐ yes, blinding procedure not described Intention‐to‐treat (ITT) ‐ yes Loss to follow‐up ‐ not described Design ‐ parallel |
|
| Participants |
N ‐ 536 children (representing 1049 non‐severe acute otitis media (AOM) episodes; 980 non‐severe AOM episodes included for primary analysis)
Age ‐ between 7 months and 12 years Setting ‐ secondary care: children's hospital and a private paediatric practice in Pittsburgh (USA) Inclusion criteria ‐ AOM based on presence of middle‐ear effusion, as determined otoscopically, in association with specified symptoms of acute middle‐ear infection (fever, otalgia or irritability), or signs of acute infection (erythema or white opacification, or both, accompanied by fullness or bulging and impaired mobility), or both Exclusion criteria ‐ children who recently received antibiotics, who had potential complicating or confounding conditions (e.g. eardrum perforation, asthma or chronic sinusitis) Baseline characteristics ‐ balanced |
|
| Interventions | Children were enrolled for a 1‐year period. At entry each child was assigned randomly to a treatment regimen that specified consistent treatments for episodes of non‐severe and severe AOM based on severity of otalgia and the presence of fever (> 39 °C orally or > 39.5 °C rectally within the 24‐hour period before presentation) Non‐severe AOM episodes were treated with: Tx ‐ amoxicillin 40 mg/kg/day 3 times daily for 14 days; N = 522 (N = 488 included in primary analysis) C ‐ placebo for 14 days; N = 527 (N = 492 included in primary analysis) Severe AOM episodes in children aged < 2 years were treated with: Tx 1 ‐ amoxicillin 40 mg/kg/day 3 times daily for 14 days Tx 2 ‐ amoxicillin 40 mg/kg/day 3 times daily for 14 days and myringotomy Severe AOM episodes in children aged ≥ 2 years were treated with: Tx 1 ‐ amoxicillin 40 mg/kg/day 3 times daily for 14 days Tx 2 ‐ amoxicillin 40 mg/kg/day 3 times daily for 14 days and myringotomy Tx 3 ‐ placebo and myringotomy |
|
| Outcomes |
Primary outcome ‐ initial treatment failure: in non‐severe episodes this was the case when either otalgia, fever or both was present more than 24 hours after treatment was initiated and when 48 hours or more after initial treatment was initiated the child's temperature reached 38 °C orally or 38.5 °C rectally or an otalgia score of ≥ 6 was present Secondary outcomes ‐ (a) recurrent AOM defined as the development of AOM 15 days or more after the initiation of treatment for a preceding episode, (b) new episodes of otitis media with effusion defined by otoscopy and tympanometry findings After initial visits, children were followed up by telephone to identify those with persistent symptoms and children younger than 2 years of age were re‐examined within 48 to 72 hours Follow‐up visits were scheduled routinely after 2 and 6 weeks after initial treatment and monthly thereafter |
|
| Notes | We included only the non‐severe AOM episodes in this review (N = 1049 of which 980 were included for primary analysis); children experiencing non‐severe AOM episodes were randomly allocated to either antibiotics or placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Other bias | Low risk | ITT analysis ‐ yes, baseline characteristics ‐ balanced |
| Blinding of participants and personnel (performance bias) | Unclear risk | Identical taste and appearance to amoxicillin and placebo not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Follow‐up/exclusion of non‐severe episodes for short‐term outcome ‐ treatment: N = 34 (7%) and placebo: N = 35 (7%). Reasons not described |
Laxdal 1970.
| Methods |
Randomised ‐ yes, method of randomisation not described Concealment of allocation ‐ unclear; method not described Double‐blind ‐ no; open‐label study, investigators not blinded Intention‐to‐treat (ITT) ‐ unclear Loss to follow‐up ‐ not described Design ‐ parallel |
|
| Participants |
N ‐ 142 children Age ‐ between 0 to 15 years Setting ‐ secondary care (private paediatric clinic) in Saskatoon (Canada) Inclusion criteria ‐ at least 1 eardrum had to show redness and loss of landmarks Exclusion criteria ‐ predominant respiratory symptoms, if allergy appeared to be a significant factor or if rupture of the eardrum had occurred Baseline characteristics ‐ not described |
|
| Interventions |
Tx 1 ‐ penicillin G 250 mg/m2/day 4 times daily (approximately 33 mg/kg/day) for at least 7 days; N = 45 Tx 2 ‐ ampicillin 250 mg/m2/day 4 times daily (approximately 33 mg/kg/day) for at least 7 days; N = 49 C ‐ symptomatic therapy (Auralgan ear drops, acetylsalicylic acid, decongestive nose drops); N = 48 Use of additional medication ‐ children in treatment groups also received symptomatic therapy as required |
|
| Outcomes |
Primary outcomes ‐ (a) treatment failure (i.e. either deterioration or no improvement observed at day 7) (b) relapses Results were evaluated at 7 days, except in cases where the ear inflammation was severe and the child appeared sufficiently ill (toxic) to warrant further examination 24 to 48 hours after treatment initiation Children in the control group were subjected to very close scrutiny, especially during the first 48 hours and particularly when severe involvement was evident (high risk of detection bias) |
|
| Notes | Open‐label trial comparing immediate antibiotics (penicillin G and ampicillin) versus expectant observation It was unclear whether otalgia played an important role in the definition of treatment failure Data on relapses: N = 126 included in analysis, no crude numbers for separate treatment groups provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Other bias | High risk | ITT analysis ‐ unclear, baseline characteristics ‐ not described, high risk of detection bias due to different follow‐up strategies between treatment groups |
| Blinding of participants and personnel (performance bias) | Unclear risk | Open‐label trial, outcome assessment not blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up ‐ not described for short‐term outcome. Loss to follow‐up for long‐term outcome (acute otitis media (AOM) relapses) ‐ N = 16 (11%), no crude numbers of separate treatment groups provided |
Le Saux 2005.
| Methods |
Randomised ‐ yes, computer‐generated randomisation sequence Concealment of allocation ‐ adequate Double‐blind ‐ yes Intention‐to‐treat (ITT) ‐ yes Loss to follow‐up ‐ described Design ‐ parallel |
|
| Participants |
N ‐ 531 children (N = 512 children included in analysis; N = 19 were excluded post hoc due to inappropriate randomisation (N = 4) or alternative clinical diagnosis (N = 15)) Age ‐ between 6 months and 5 years Setting ‐ secondary care: emergency department in Ottawa (Canada) Inclusion criteria ‐ new onset (< 4 days) of symptoms referable to the upper respiratory tract and either ear pain or fever (> 38 °C). In addition, all patients had to have evidence of middle‐ear effusion, defined by ≥ 2 of the following signs: opacity, impaired mobility on the basis of pneumatic otoscopy and redness or bulging (or both) of the tympanic membrane Exclusion criteria ‐ antibiotic treatment < 2 weeks prior to randomisation, contraindication to amoxicillin or penicillin or sensitivity to ibuprofen or aspirin, presence of otorrhoea, co‐morbid disease such as sinusitis or pneumonia, prior middle‐ear surgery, placement of a ventilation tube, history of recurrent acute otitis media (more than 4 episodes in 12 months), compromised immunity, craniofacial abnormalities, or any chronic or genetic disorder Baseline characteristics ‐ balanced |
|
| Interventions | Tx ‐ amoxicillin suspension (60 mg/kg) 3 times daily for 10 days; N = 258 (N = 253 included in analysis for day 3) C ‐ matching placebo for 10 days; N = 254 (N = 246 included in analysis for day 3) Use of additional medication ‐ parents were given a 5‐day supply of antipyretic and analgesic medication in the form of ibuprofen suspension as required for pain or fever and a 48‐hour supply of codeine elixir to be given as required for pain and fever | |
| Outcomes |
Primary outcome ‐ clinical resolution of symptoms, defined as absence of receipt of an antimicrobial (other than amoxicillin in the treatment group) at any time during the 14‐day period. The initiation of antimicrobial therapy was based on persistence or worsening of symptoms, fever or irritability associated with otoscopic signs of unresolving AOM, or development of symptoms indicative for mastoiditis or invasive disease Secondary outcomes ‐ (a) presence of symptoms (i.e. fever, pain, irritability, vomiting, activity level) on days 1, 2 and 3; (b) number of analgesic doses, codeine doses on days 1, 2 and 3; (c) occurrence of any rash or diarrhoea in the 14 days after randomisation; (d) presence of middle‐ear effusion assessed by tympanometry at 1 and 3 months after diagnosis The parents were contacted on days 1, 2 and 3 after randomisation and once between day 10 and day 14 for administration of a standard questionnaire. If the parents or research assistant felt that the symptoms were not improving or were worsening, a medical reassessment was advised and the child was seen by a physician in the emergency department or clinic or by the paediatrician The child was clinically assessed at 1 month and 3 months after randomisation to determine the number of subsequent episodes of acute otitis media (AOM) and to undergo tympanometry |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence stratified by study centre and age using random‐permuted blocks of sizes 4 and 6 |
| Allocation concealment (selection bias) | Low risk | Randomisation sequence was kept under secure conditions and was accessible only by the trial pharmacist |
| Other bias | Low risk | ITT analysis ‐ yes, baseline characteristics ‐ balanced |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo was similar to amoxicillin with regard to appearance and taste and was dispensed in identical opaque bottles, which were numbered sequentially |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up at day 3 ‐ treatment: N = 5 (2%) and placebo: N = 8 (3%) |
Little 2001.
| Methods |
Randomised ‐ yes, method of randomisation not described Concealment of allocation ‐ adequate Double‐blind ‐ no; open‐label study, investigators not blinded Intention‐to‐treat (ITT) ‐ yes Loss to follow‐up ‐ described Design ‐ parallel |
|
| Participants |
N ‐ 315 children (N = 285 children included in analysis) Age ‐ between 6 months and 10 years Setting ‐ general practice; 42 general practitioners in 3 health authorities in south‐west England Inclusion criteria ‐ acute otalgia and otoscopic evidence of acute inflammation of the eardrum (dullness or cloudiness with erythema, bulging or perforation). When children were too young for otalgia to be specifically documented from their history (under 3 years old) then otoscopic evidence alone was a sufficient entry criterion Exclusion criteria ‐ otoscopic appearances consistent with crying or a fever alone (pink drum alone), appearances and history more suggestive of otitis media with effusion and chronic suppurative otitis media, serious chronic disease (such as cystic fibrosis, valvular heart disease), use of antibiotics < 2 weeks prior to randomisation, previous complications (septic complications, hearing impairment) and if the child was unwell to be left to wait and see (e.g. high fever, floppy, drowsy, not responding to antipyretics) Baseline characteristics ‐ balanced |
|
| Interventions | Tx ‐ immediate treatment with antibiotics: amoxicillin syrup 125 mg/5 mL 3 times daily for 7 days (children who were allergic to amoxicillin received erythromycin 125 mg/5 mL 4 times daily; N = 151 (N = 135 included in analysis) C ‐ similar antibiotics were prescribed but parents were asked to wait for 72 hours before considering using the prescription. Parents were instructed that if their child still had substantial otalgia or fever after 72 hours, had discharge for > 10 days or was not starting to get better then they should collect the antibiotic prescription that was left at the practice; N = 164 (N = 150 included in analysis) Use of additional medication ‐ for both groups doctors emphasised the importance of paracetamol in full doses for relief of pain and fever | |
| Outcomes |
Primary outcomes ‐ (a) duration of symptoms (i.e. earache, ear discharge, night disturbance, crying); (b) daily pain score; (c) episodes of distress; (d) spoons of paracetamol used; (e) use of antibiotics Doctors were asked to provide information on days of illness, physical signs and antibiotic prescribing; parents were asked to complete a daily symptom diary |
|
| Notes | Open‐label trial comparing immediate versus delayed antibiotic prescription (prescription provided but advised to fill only if symptoms did not improve or worsened) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Sealed, numbered, opaque envelopes |
| Other bias | Low risk | ITT analysis ‐ yes, baseline characteristics ‐ balanced |
| Blinding of participants and personnel (performance bias) | Unclear risk | Open‐label trial, outcome assessment not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up/exclusion from analysis (intervention ineffective, did not use antibiotics or did not delay) ‐ treatment: N = 16 (12%) and placebo: N = 14 (9%); comparison of the baseline information for the 3 types of responders (those who provided diaries, those who gave information by telephone and those from whom no diary information could be collected) revealed no evidence of significant bias between treatment groups or between patients by age or severity of symptoms |
McCormick 2005.
| Methods |
Randomised ‐ yes, computer‐generated randomisation sequence Concealment of allocation ‐ unclear; method not described Double‐blind ‐ no, open‐label trial, investigators blinded, parents not blinded Intention‐to‐treat (ITT) ‐ yes Loss to follow‐up ‐ described Design ‐ parallel |
|
| Participants |
N ‐ 223 children (N = 218 children included in analysis at day 12) Age ‐ between 6 months and 12 years Setting ‐ secondary care: paediatric clinic of University of Texas Medical Branch (USA) Inclusion criteria ‐ children were required to have (a) symptoms of ear infection; (b) otoscopic evidence of acute otitis media (AOM), including middle‐ear effusion; (c) non‐severe AOM Exclusion criteria ‐ co‐morbidity requiring antibiotic treatment, anatomic defect of ear or nasopharynx, allergy to study medication, immunologic deficiency, major medical condition and/or indwelling ventilation tube or draining otitis in the affected ear(s) Baseline characteristics ‐ balanced |
|
| Interventions |
Tx ‐ immediate treatment with antibiotics: oral amoxicillin 90 mg/kg/day twice daily for 10 days; N = 112 (N = 110 included in analysis at day 12)
C ‐ expectant observation: no immediate antibiotics; N = 111 (N = 108 included in analysis at day 12) Children in the control group with AOM failure or recurrence received oral amoxicillin 90 mg/kg/day; children in Tx group with AOM failure or recurrence received amoxicillin‐clavulanate (90 mg/kg/day of amoxicillin component) Use of additional medication ‐ all parents received saline nose drops and/or cerumen‐removal drops (if needed), ibuprofen and over‐the‐counter decongestant/antihistamine to be given as needed |
|
| Outcomes |
Primary outcomes ‐ (a) parent satisfaction with AOM care; (b) resolution of AOM symptoms after treatment, including number of doses of symptom medication given; (c) AOM failure (days 0 to 12) or recurrence (days 13 to 30) defined as attending to the paediatrician clinic with acute ear symptoms, an abnormal tympanic membrane, or an AOM severity score higher than that at enrolment; (d) nasopharyngeal carriage of Streptococcus pneumoniae strains resistant to antibiotics Secondary outcomes ‐ (a) minor adverse events caused by medication (e.g. allergy, diarrhoea and candidal infection); (b) serious AOM‐related adverse events (e.g. invasive pneumococcal disease, mastoiditis, bacteraemia, meningitis, perforation of the tympanic membrane, hospitalisation and emergency ear surgery; (c) parent‐child quality of life measures related to AOM Parents were instructed to complete a symptom diary from day 1 to 10 and a satisfaction questionnaire on day 12 and day 30; routine follow‐up appointments for data collection were scheduled for day 12 and day 30. Patient‐initiated visits were scheduled on request by the parents for children who seemed to not be responding to treatment |
|
| Notes | Investigator‐blinded trial comparing immediate antibiotic prescribing versus expectant observation (no prescription provided) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Other bias | Low risk | ITT analysis ‐ yes, baseline characteristics ‐ balanced |
| Blinding of participants and personnel (performance bias) | Unclear risk | Investigator‐blinded study, parents not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up at day 12 ‐ treatment: N = 2 (2%) and expectant observation: N = 3 (3%) |
Mygind 1981.
| Methods |
Randomised ‐ yes, method of randomisation not described Concealment of allocation ‐ adequate Double‐blind ‐ yes Intention‐to‐treat (ITT) ‐ unclear Loss to follow‐up ‐ reasons described, unclear from which treatment group patients were excluded Design ‐ parallel |
|
| Participants |
N ‐ 158 children (N = 149 included in analysis) Age ‐ between 1 and 10 years Setting ‐ general practice and secondary care: confirmation of diagnosis and trial recruitment were done by otorhinolaryngologists in Copenhagen (Denmark) Inclusion criteria ‐ earache for 1 to 24 hours. The diagnosis was made if the child cried because of pain and if the tympanic membrane appeared to be red and inflamed Exclusion criteria ‐ antibiotic treatment < 4 weeks prior to randomisation, other treatment apart from acetylsalicylic acid already commenced, secretion in the external ear, suspected chronic otitis media, treatment for secretory otitis media within last 12 months, concurrent disease (e.g. pneumonia or severe tonsillitis), suspected penicillin allergy Baseline characteristics ‐ balanced |
|
| Interventions | Tx ‐ penicillin 50 mg/mL 4 times daily; children aged 1 to 2 years: 10 mL daily, children between 3 and 5 years: 20 mL daily, children between 6 and 10 years: 30 mL daily for 7 days; N = ? (N = 72 included in analysis) C ‐ placebo for 7 days; N = ? (N = 77 included in analysis) Use of additional medication ‐ acetylsalicylic acid tablets (maximum of 50 mg/kg/day for 3 days) were supplied as the only supplementary treatment permitted | |
| Outcomes |
Main outcomes: (a) mean symptoms (i.e. pain, fever) scores; (b) number of analgesic tables used; (c) contralateral otitis; (d) spontaneous perforation of tympanic membrane; (e) mean number of days of otorrhoea; (f) tympanometry results at 1 week, 4 weeks and 3 months Initial visits were performed at home: otoscopy and bacterial culture from nasopharynx were performed Score cards were given to parents Follow‐up visits at hospital at day 2 to 3, day 7, week 4 and week 12. If supplementary treatment was required at day 2 to 3, then myringotomy was performed. If supplementary treatment was required at day 7, then amoxicillin was given |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Randomisation performed by pharmaceutical company. Penicillin and placebo were supplied in coded bottles to study personnel |
| Other bias | Unclear risk | ITT analysis ‐ unclear, baseline characteristics ‐ balanced |
| Blinding of participants and personnel (performance bias) | Unclear risk | Identical taste and appearance to amoxicillin and placebo not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Patients not included in analysis ‐ N = 9 (6%). Reasons described, unclear from which treatment group patients were excluded |
Neumark 2007.
| Methods |
Randomised ‐ yes, Internet‐based random number generator Concealment of allocation ‐ unclear; method not described Double‐blind ‐ no, open‐label trial Intention‐to‐treat (ITT) ‐ unclear Loss to follow‐up ‐ reasons described, unclear from which treatment group patients were excluded Design ‐ parallel |
|
| Participants |
N ‐ 186 children (N = 179 patients were included in analysis; 7 patients were excluded due to non‐compliance with protocol) Age ‐ between 2 and 16 years Setting ‐ general practice: 32 healthcare centres and 72 general practitioners in Sweden Inclusion criteria ‐ acute otitis media (AOM) was based on direct inspection of the eardrum by pneumatic otoscope or preferably an aural microscope. Findings had to include a bulging, red eardrum displaying reduced mobility Exclusion criteria ‐ perforation of the eardrum, chronic ear conditions or impaired hearing, previous adverse reactions to penicillin, concurrent disease that should be treated with antibiotics, recurrent AOM (3 or more AOM episodes during the past 6 months), children with immunosuppressive conditions, genetic disorders and mental disease or retardation Baseline characteristics ‐ balanced |
|
| Interventions |
Tx ‐ immediate treatment with antibiotics: phenoxymethylpenicillin 25 mg/kg twice daily for 5 days; N = 92
C ‐ expectant observation: no immediate antibiotics; N = 87 The guardians received written information about how to act if the condition did not improve or got worse within 3 days after randomisation Use of additional medication ‐ symptomatic treatment with paracetamol or non‐steroidal anti‐inflammatory drugs (NSAIDs), drugs reducing the swelling of the nasal mucosa (e.g. decongestive nose drops) and nasal steroids were allowed |
|
| Outcomes |
Primary outcomes ‐ (a) pain at day 0, 1, 2 and 3 to 7; (b) use of analgesics at day 0, 1, 2, 3, 4 to 7; (c) fever > 38 °C at day 0, 1, 2 and 3 to 7; (d) subjective recovery at day 14 and 3 months; (e) perforations at 3 months; (f) serous otitis media at 3 months All participants were asked to complete a symptom diary for 7 days; a nurse telephoned all participants after approximately 14 days to supplement the information in the diary and to register all acute contacts that had occurred during the first week of treatment; the final follow‐up was performed after 3 months to register perforations and serous otitis media |
|
| Notes | Open‐label trial comparing immediate antibiotic prescribing versus expectant observation (no prescription provided but advice on what to do if symptoms did not improve or worsened) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Internet‐based random number generator |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Other bias | Unclear risk | ITT analysis ‐ unclear, baseline characteristics ‐ balanced |
| Blinding of participants and personnel (performance bias) | Unclear risk | Open‐label trial, outcome assessment not blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Patients not included in analysis ‐ N = 7 (4%). Reasons described, unclear from which treatment group patients were excluded |
Spiro 2006.
| Methods |
Randomised ‐ yes, computer‐assisted randomisation Concealment of allocation ‐ adequate Double‐blind ‐ no, open‐label study, investigators blinded Intention‐to‐treat (ITT) ‐ yes Loss to follow‐up ‐ described Design ‐ parallel |
|
| Participants |
N ‐ 283 children (N = 265 children included in analysis at days 4 to 6) Age ‐ between 6 months and 12 years Setting ‐ secondary care: paediatric emergency department of Yale‐New Haven Hospital in New Haven (USA) Inclusion criteria ‐ the diagnosis of acute otitis media (AOM) was made at the discretion of the clinician according to the diagnostic criteria in the evidence‐based guideline published in Pediatrics 2004 Exclusion criteria ‐ presence of additional intercurrent bacterial infection such as pneumonia, if the patient appeared to be "toxic" as determined by the clinician, hospitalisation, immunocompromised children, antibiotic treatment < 1 week prior to randomisation, children who had either myringotomy or a perforated tympanic membrane, uncertain access to medical care (e.g. no telephone access), primary language of parents was neither English nor Spanish, previous enrolment in the study Baseline characteristics ‐ balanced |
|
| Interventions | Tx ‐ immediate treatment with antibiotics; N = 145 (N = 133 included in analysis at days 4 to 6) C ‐ participants randomised to delayed prescription were given written and verbal instructions "not to fill the antibiotic prescription unless your child either is not better or is worse 48 hours (2 days) after today's visit"; N = 138 (N = 132 included in analysis at days 4 to 6) Use of additional medication ‐ all participants received complimentary bottles of ibuprofen suspension (100 mg/5 mL) and analgesic ear drops | |
| Outcomes |
Primary outcome ‐ proportion of each group that filled the prescription for an antibiotic. This was defined by whether the parent filled the prescription within 3 days of enrolment and was determined by the response to this question at the interview at day 4 to 6 Secondary outcomes ‐ (a) clinical course of the illness; (b) adverse effects of medications; (c) days of school or work missed; (d) unscheduled medical visits; (e) comfort of parents with management of AOM without antibiotics for future episodes 2 trained research assistants blinded to group assignment conducted standardised, structured telephone interviews with the parents at day 4 to 6, day 11 to 14, day 30 and day 40 after enrolment |
|
| Notes | Investigator‐blinded study comparing immediate versus delayed antibiotic prescribing (prescription provided and advised to fill only if symptoms worsen or do not improve) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐assisted randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Other bias | Low risk | ITT analysis ‐ yes, baseline characteristics ‐ balanced |
| Blinding of participants and personnel (performance bias) | Unclear risk | Investigator‐blinded study, parents not blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up at day 4 to 6 treatment: N = 12 (8%) and expectant observation: N = 6 (4%) |
Tapiainen 2014.
| Methods |
Randomised ‐ yes, block randomisation, computerised randomisation list Concealment of allocation ‐ adequate Double‐blind ‐ yes Intention‐to‐treat (ITT) ‐ yes Loss to follow‐up ‐ described Design ‐ parallel |
|
| Participants |
N ‐ 84 children (N = 84 children included in analysis at 8 weeks) Age ‐ between 6 months and 15 years Setting ‐ primary and secondary care: children in day care centres attending an AOM prevention trial at the Department of Pediatrics, Oulu University Hospital and children visiting the City of Oulu Health Care Center and Mehiläinen Pediatric Private Practice, Oulu (Finland) Inclusion criteria ‐ acute symptoms of respiratory infection and/or ear‐related symptoms and signs of tympanic membrane inflammation together with middle‐ear effusion at pneumatic otoscopy performed by a study physician Exclusion criteria ‐ ventilation tubes (grommets), AOM complication, amoxicillin allergy, Down syndrome, congenital craniofacial abnormality and immunodeficiency Baseline characteristics ‐ balanced |
|
| Interventions | Tx ‐ amoxicillin‐clavulanate for 7 days (amoxicillin 40 mg/kg/day divided into 2 daily doses); N = 42 (N = 42 included in analysis) C ‐ matching placebo in 2 doses for 7 days; N = 42 (N = 42 included in analysis) Use of additional medication ‐ not described | |
| Outcomes |
Primary outcome ‐ time middle‐ear effusion disappearance defined as a normal tympanogram finding (A curve) from both ears on 2 consecutive measurement days (either at home or at the study clinic) Secondary outcomes ‐ (a) time to improved tympanogram findings (i.e. A or C curve) from both ears; (b) time to normal pneumatic otoscopy or otomicroscopy findings from both ears; (c) proportions of children with persistent middle‐ear effusion on days 7, 14 and 60; (d) disappearance of pain; (e) disappearance of fever; (f) use of pain medication; (g) possible adverse effects of antimicrobial treatment Children were examined by the study physician with pneumatic otoscopy or otomicroscopy and tympanometry at study entry, after 3 and 7 days, and then weekly until both ears were healthy according to pneumatic otoscopy or otomicroscopy Families were trained to perform tympanometry using a handheld tympanometer for daily follow‐up at home |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation, computerised randomisation list |
| Allocation concealment (selection bias) | Low risk | Randomisation list was kept in the pharmacy, which delivered the study drugs to the families according to the consecutive study number |
| Other bias | Low risk | ITT analysis ‐ yes, baseline characteristics ‐ balanced |
| Blinding of participants and personnel (performance bias) | Low risk | Bottles containing amoxicillin‐clavulanate or placebo were indistinguishable, dosing was similar in both groups and placebo mixture was flavoured and sweetened to resemble the taste of amoxicillin‐clavulanate |
| Incomplete outcome data (attrition bias) | Low risk | All children were included in the analysis |
Thalin 1985.
| Methods |
Randomised ‐ yes, block randomisation, method of random sequence generation not described Concealment of allocation ‐ adequate Double‐blind ‐ yes Intention‐to‐treat (ITT) ‐ unclear Loss to follow‐up ‐ described Design ‐ parallel |
|
| Participants |
N ‐ 293 children (N = 293 children included in analysis) Age ‐ between 2 and 15 years Setting ‐ secondary care: department of otorhinolaryngology in Halmstad (Sweden) Inclusion criteria ‐ purulent acute otitis media (AOM) (no further criteria described) Exclusion criteria ‐ antibiotic treatment or AOM episode < 4 weeks prior to randomisation, suspected penicillin allergy, presence of ventilation tubes, sensorineural hearing loss, existence of concomitant infection for which antibiotic treatment was required and chronic diseases Baseline characteristics ‐ not described |
|
| Interventions | Tx ‐ phenoxymethyl penicillin 50 mg/kg/day twice daily for 7 days; N = 159 (N = 159 included in analysis) C ‐ matching placebo in 2 doses for 7 days; N = 158 (N = 158 included in analysis) Use of additional medication ‐ all children were given nose drops containing oxymetazoline chloride and, if needed, analgesics (paracetamol) | |
| Outcomes |
Primary outcome ‐ treatment failure (defined as remaining non‐negligible symptoms such as pain and fever, insufficient resolution of infectious signs during treatment period of 7 days, or both Secondary outcomes ‐ (a) resolution of symptoms over time; (b) AOM relapses; (c) tympanometry, audiometry, or both, results at 4 weeks The children were examined at day 0, days 3 to 4, days 8 to 10 and at 4 weeks Parents were instructed to record symptoms (i.e. temperature, otalgia, discharge from ear and consumption of supplied symptomatic drugs) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation, method of random sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Randomisation list was kept by the clinical pharmacologist of the hospital and not disclosed to the investigators until the clinical trial was completed |
| Other bias | Unclear risk | ITT analysis ‐ unclear; baseline characteristics ‐ not described |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo with same taste and appearance as penicillin |
| Incomplete outcome data (attrition bias) | Low risk | No children lost to follow‐up for primary analysis |
Tähtinen 2011.
| Methods |
Randomised ‐ yes, computerised random number generator with block length of 10 Concealment of allocation ‐ adequate Double‐blind ‐ yes Intention‐to‐treat (ITT) ‐ yes Loss to follow‐up ‐ described Design ‐ parallel |
|
| Participants |
N ‐ 322 children (N = 319 children were included in analysis)
Age ‐ between 6 months and 3 years Setting ‐ general practice: healthcare centre of Turku (Finland) Inclusion criteria ‐ acute otitis media (AOM) based on 3 criteria: (a) middle‐ear fluid had to be detected by means of pneumatic otoscopic examination that showed at least 2 of the following tympanic membrane findings: bulging position, decreased or absent mobility, abnormal colour or opacity not due to scarring, or air fluid interfaces; (b) at least 1 of the following acute inflammatory signs in the tympanic membrane had to be present: distinct erythematous patches or streaks or increased vascularity over full, bulging, or yellow tympanic membrane; (c) presence of acute symptoms such as fever, otalgia or respiratory symptoms Exclusion criteria ‐ ongoing antibiotic treatment; AOM with spontaneous perforation of the tympanic membrane; systemic or nasal steroid therapy within 3 preceding days; antihistamine, oseltamivir or a combination therapy within 3 preceding days; contraindication to penicillin or amoxicillin; presence of ventilation tube; severe infection requiring antibiotic treatment; documented Epstein‐Barr virus infection within 7 preceding days; Down's syndrome or other condition affecting middle‐ear diseases; known immunodeficiency Baseline characteristics ‐ balanced |
|
| Interventions | Tx ‐ amoxicillin‐clavulanate 40‐5.7 mg/kg daily in 2 doses for 7 days; N = 162 (N = 161 included in analysis) C ‐ matching placebo in 2 doses for 7 days; N = 160 (N = 158 included in analysis) Use of additional medication ‐ the use of analgesics and antipyretic agents was encouraged and the use of analgesic ear drops and decongestive nose drops or sprays was allowed | |
| Outcomes |
Primary outcome ‐ time to treatment failure (i.e. a composite endpoint consisting of 6 independent components: (a) no improvement in overall condition at day 2, (b) worsening of the child's overall condition at any time, (c) no improvement in otoscopic signs at day 7, (d) perforation of tympanic membrane at any time, (e) severe infection (e.g. mastoiditis or pneumonia) necessitating systemic open‐label antimicrobial treatment at any time, (f) any other reason for stopping the study drug at any time Secondary outcomes ‐ assessed by study physician ‐ (a) time to the initiation of rescue treatment; (b) time to development of contralateral AOM; ‐ diary symptom assessment; (c) resolution of symptoms; (d) use of analgesics Parents were given a diary to record symptoms, doses of study drugs and any other medications and adverse events First visit after enrolment (= day 0) was scheduled at day 2. End‐of‐treatment visit was scheduled at day 7 |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number generator with block length of 10 |
| Allocation concealment (selection bias) | Low risk | Concealment of allocation by the pharmacist (independent to trial team) by labelling the identical opaque study drug containers with allocation numbers; allocation list was kept at the paediatric infectious disease ward behind locked doors |
| Other bias | Low risk | ITT analysis ‐ yes, baseline characteristics ‐ balanced |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo with same taste and appearance as amoxicillin‐clavulanate |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up ‐ treatment: N = 1 (1%) and placebo: N = 2 (1%) |
van Buchem 1981a.
| Methods |
Randomised ‐ yes, method of randomisation not described Concealment of allocation ‐ adequate Double‐blind ‐ yes Intention‐to‐treat (ITT) ‐ unclear Loss to follow‐up ‐ reasons not described, unclear from which treatment group patients were excluded Design ‐ 2 x 2 factorial design |
|
| Participants |
N ‐ 202 children (N = 171 children included in analysis; N = 31 were excluded from the study) Age ‐ between 2 and 12 years Setting ‐ both general practice and secondary care: 12 general practitioners in or near Tilburg (the Netherlands) recruited patients and referred them to 1 of the 3 otorhinolaryngologists, which excluded those cases where there was disagreement with the diagnosis Inclusion criteria ‐ acute otitis media (AOM) was based on history and clinical picture (i.e. diffuse redness, bulging of the eardrum, or both) Exclusion criteria ‐ antibiotic treatment < 2 weeks prior to randomisation, chronic otitis or otitis media serosa, contraindication for antibiotic treatment Baseline characteristics ‐ balanced |
|
| Interventions | Tx ‐ sham myringotomy and amoxicillin 250 mg 3 times daily for 7 days; N = 47 C ‐ sham myringotomy and matching placebo for 7 days; N = 40 Use of additional medication ‐ all participants were allowed to use decongestive nose drops and analgesic suppositories (i.e. children aged 2 to 7 years: acetylsalicylic acid 50 mg, phenacetin 50 mg, phenobarbitone 15 mg, codeine phosphate 2.5 mg, caffeine 1.25 mg; children aged 8 to 12 years: acetylsalicylic acid 100 mg, phenacetin 100 mg, phenobarbitone 30 mg, codeine phosphate 5 mg, caffeine 2.5 mg | |
| Outcomes | Main outcomes ‐ (a) parent report of pain at day 0, 1 and 7; (b) otoscopic findings at day 0, 1 and 7; (c) discharge from ear at day 1, 7 and 14; (d) mean temperature at day 0, 1 and 7; (e) AOM relapses at 6 months; (f) audiogram findings after 4 and 8 weeks | |
| Notes | van Buchem 1981a is the 2 arms without myringotomy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Randomisation performed by otorhinolaryngologists; general practitioner and parent/child were outcome assessors and remained blinded |
| Other bias | Unclear risk | ITT analysis ‐ unclear, baseline characteristics ‐ balanced |
| Blinding of participants and personnel (performance bias) | Low risk | Sham myringotomy and placebo was similar with amoxicillin with regard to appearance and taste |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up/exclusions ‐ N = 31 (15%). Reasons not described |
van Buchem 1981b.
| Methods |
Randomised ‐ yes, method of randomisation not described Concealment of allocation ‐ adequate Double‐blind ‐ yes Intention‐to‐treat (ITT) ‐ unclear Loss to follow‐up ‐ reasons not described, unclear from which treatment group patients were excluded Design ‐ 2 x 2 factorial design |
|
| Participants |
N ‐ 202 children (N = 171 children included in analysis; N = 31 were excluded from the study) Age ‐ between 2 and 12 years Setting ‐ both general practice and secondary care: 12 general practitioners in or near Tilburg (the Netherlands) recruited patients and referred them to 1 of the 3 otorhinolaryngologists who excluded those cases where there was disagreement with the diagnosis Inclusion criteria ‐ acute otitis media (AOM) was based on history and clinical picture (i.e. diffuse redness, bulging of the eardrum, or both) Exclusion criteria ‐ antibiotic treatment < 2 weeks prior to randomisation, chronic otitis or otitis media serosa, contraindication for antibiotic treatment Baseline characteristics ‐ balanced |
|
| Interventions | Tx ‐ myringotomy and amoxicillin 250 mg 3 times daily for 7 days; N = 48 C ‐ myringotomy and matching placebo for 7 days; N = 36 Use of additional medication ‐ all participants were allowed to use decongestive nose drops and analgesic suppositories (i.e. children aged 2 to 7 years: acetylsalicylic acid 50 mg, phenacetin 50 mg, phenobarbitone 15 mg, codeine phosphate 2.5 mg, caffeine 1.25 mg; children aged 8 to 12 years: acetylsalicylic acid 100 mg, phenacetin 100 mg, phenobarbitone 30 mg, codeine phosphate 5 mg, caffeine 2.5 mg | |
| Outcomes | Main outcomes ‐ (a) parent report of pain at day 0, 1 and 7; (b) otoscopic findings at day 0, 1 and 7; (c) discharge from ear at day 1, 7 and 14; (d) mean temperature at day 0, 1 and 7; (e) AOM relapses at 6 months; (f) audiogram findings after 4 and 8 weeks | |
| Notes | van Buchem 1981b is the 2 arms with myringotomy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Randomisation performed by otorhinolaryngologists; general practitioner and parent/child were outcome assessors and remained blinded |
| Other bias | Unclear risk | ITT analysis ‐ unclear, baseline characteristics ‐ balanced |
| Blinding of participants and personnel (performance bias) | Low risk | Sham myringotomy and placebo was similar with amoxicillin with regard to appearance and taste |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up/exclusions ‐ N = 31 (15%). Reasons not described |
AOM: acute otitis media AOM‐SOS: otitis media ‐ severity of symptoms C: control ITT: intention‐to‐treat Tx: treatment
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arguedas 2011 | No comparison of antibiotic to placebo or expectant observation: trial comparing single‐dose, extended‐release azithromycin versus a 10‐day regimen of amoxicillin/clavulanate |
| Casey 2012 | No comparison of antibiotic to placebo or expectant observation: trial comparing high‐dose amoxicillin/clavulanate versus cefdinir |
| Chaput 1982 | Short versus long course of therapy |
| Engelhard 1989 | No comparison of antibiotic to placebo; the 3 arms were Augmentin, myringotomy, or both |
| Liu 2011 | No comparison of antibiotic to placebo or expectant observation: trial comparing single oral doses azithromycin in extended‐release versus immediate‐release formulations |
| Ostfeld 1987 | Non‐randomised study |
| Rudberg 1954 | Non‐randomised study: assigned "randomly" based on case number but then allowed to change groups |
| Ruohola 2003 | Conducted in children with ventilation tubes |
| Sarrell 2003 | No comparison of antibiotic to placebo. Method of randomisation not provided and groups appear to be unbalanced at baseline |
| Tähtinen 2012 | Secondary analysis of placebo‐controlled trial. This study included the total group of children allocated to immediate antimicrobial treatment (N = 161) and a subgroup of children from the placebo group that received delayed antibiotics (N = 53). As a consequence, comparability of prognosis achieved through randomisation is violated, producing groups of children that are incomparable, which may lead to biased effect estimates |
| van Buchem 1985 | Non‐randomised study |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12608000424303.
| Trial name or title | Antibiotics for asymptomatic acute otitis media |
| Methods | Double‐blind, placebo‐controlled randomised clinical trial |
| Participants | Aboriginal children aged between 6 and 30 months diagnosed with asymptomatic acute otitis media defined as a bulging tympanic membrane without associated symptoms (including ear pain, fever or ear discharge) at the time of diagnosis |
| Interventions | Azithromycin 30 mg/kg divided into 2 doses or placebo for 7 days |
| Outcomes |
Primary outcome ‐ proportion of children with a bulging tympanic membrane or ear discharge or withdrawn due to complications or side effects at 14 days (all children who are lost to follow‐up are considered clinical failures) Secondary outcomes ‐ (a) proportion of children with unresolved bulging at 7 and 30 days; (b) proportion of children with a bulging tympanic membrane or ear discharge or withdrawn due to complications or side effects at 7, 14 and 30 days (not including children who are lost to follow‐up); (c) proportion of children who develop an illness requiring additional medical treatment at 7, 14 and 30 days; (d) proportion of children who develop an illness requiring cessation of prescribed antibiotics at 30 days; (e) proportion of children who have no improvement in other conditions recorded, like skin sores and rhinosinusitis, at 7, 14 and 30 days; (f) microbiological outcomes including carriage and antibiotic resistance of Streptococcus pneumoniae and Haemophilus influenzae at 7, 14 and 30 days |
| Starting date | March 2007 |
| Contact information | Menzies School of Health Research, PO Box 41096, Casuarina NT 0811, Australia |
| Notes | ACTRN12608000424303 |
Differences between protocol and review
In this 2015 updated review, we now provide outcome data on:
pain at 24 hours, two to three days, four to seven days and 10 to 14 days (in earlier versions outcome data on pain were presented at 24 hours, two to three days and four to seven days);
abnormal tympanometry findings at two to four weeks, six to eight weeks and three months (in earlier versions outcome data on abnormal tympanometry findings were presented at four to six weeks and three months);
long‐term effects including number of parent‐reported AOM‐symptom episodes, antibiotic prescriptions and health care utilisation as assessed at least one year after randomisation (in earlier versions no data on long‐term effects were presented).
The outcome 'Adverse effects likely to be related to the use of antibiotics such as vomiting, diarrhoea or rash' has been added to primary outcomes (in earlier versions this outcome was listed as a secondary outcome) according to the recommendations described in Chapter 5.4.2 of the Cochrane Handbook for Systematic Reviews of Interventions ("the primary outcomes should include at least one desirable and at least one undesirable outcome") (Higgins 2011).
Contributions of authors
Chris Del Mar (CDM) and Paul P Glasziou (PPG) prepared the original version of the review. Sharon L. Sanders (SLS) conducted searches, identified studies, extracted data and prepared the manuscript for the updated reviews in 2003, 2007 and 2008. Maroeska M. Rovers (MMR) participated in the 2007 update by providing data and information from the individual patient data meta‐analysis that has been included in this update. Roderick P. Venekamp (RPV) conducted searches, identified studies, extracted data and prepared the manuscript for the updated review in 2012 and 2015. PPG, CDM, MMR, SLS and RPV have reviewed and provided comments on the updated version of the review.
Declarations of interest
Chris Del Mar (CDM) declares no conflicts of interests in the current work. Maroeska M. Rovers (MMR) has participated in workshops and educational activities on otitis media organised by GlaxoSmithKline and received a grant from GlaxoSmithKline for a study on the microbiology of otitis media in 2009. Roderick P. Venekamp (RPV) is an Editor of the Cochrane Acute Respiratory Infections Group. Sharon L Sanders (SLS) declares no conflicts of interests in the current work. Paul P Glasziou (PPG) is co‐investigator on NHMRC funded grant Antibiotic Resistance.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Appelman 1991 {published data only}
- Appelman CL, Claessen JQ, Touw Otten FW, Hordijk GJ, Melker RA. Co‐amoxiclav in recurrent acute otitis media: placebo controlled study. BMJ 1991;303:1450‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelman CL, Claessen JQ, Touw Otten FW, Hordijk GJ, Melker RA. Severity of inflammation of tympanic membrane as predictor of clinical course of recurrent acute otitis media. BMJ 1993;306:895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claessen JQ, Appelman CL, Touw Otten FW, Melker RA, Hordijk GJ. Persistence of middle ear dysfunction after recurrent acute otitis media. Clinical Otolaryngology 1994;19:35‐40. [DOI] [PubMed] [Google Scholar]
Burke 1991 {published data only}
- Burke P, Bain J, Robinson D, Dunleavey J. Acute red ear in children: controlled trial of non‐antibiotic treatment in general practice. BMJ 1991;303:558‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Damoiseaux 2000 {published data only}
- Bezáková N, Damoiseaux RA, Hoes AW, Schilder AG, Rovers MM. Recurrence up to 3.5 years after antibiotic treatment of acute otitis media in very young Dutch children: survey of trial participants. BMJ 2009;338:b2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux RAMJ, Balen FAM, Hoes AW, Verheij TJM, Melker RA. Primary care based randomised, double blind trial of amoxicillin versus placebo for acute otitis media in children aged under 2 years. BMJ 2000;320:350‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halsted 1968 {published data only}
- Halsted C, Lepow ML, Balassanian N, Emmerich J, Wolinsky E. Otitis media: clinical observation, microbiology and evaluation of therapy. American Journal of Diseases of Children 1968;115(5):542‐51. [DOI] [PubMed] [Google Scholar]
Hoberman 2011 {published data only}
- Hoberman A, Paradise JL, Rockette HE, Shaikh N, Wald ER, Kearney DH, et al. Treatment of acute otitis media in children under 2 years of age. New England Journal of Medicine 2011;364(2):105‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradise JL, Hoberman A, Rockette HE, Shaikh N. Treating acute otitis media in young children: what constitutes success?. Pediatric Infectious Disease Journal 2013;32(7):745‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howie 1972 {published data only}
- Howie VM, Ploussard JH. Efficacy of fixed combination antibiotics versus separate components in otitis media: effectiveness of erythromycin estolate, triple sulfonamide, ampicillin, erythromycin estolate‐triple sulfonamide, and placebo in 280 patients with acute otitis media under two and one‐half years of age. Clinical Pediatrics 1972;11(4):205‐14. [DOI] [PubMed] [Google Scholar]
Kaleida 1991 {published data only}
- Kaleida PH, Casselhrant ML, Rockette HE, Paradise JL, Bluestone CD, Blatter MM, et al. Amoxicillin or myringotomy or both for acute otitis media: results of a randomized clinical trial. Paediatrics 1991;87(4):466‐74. [PubMed] [Google Scholar]
Laxdal 1970 {published data only}
- Laxdal OE, Merida J, Jones RHT. Treatment of acute otitis media: a controlled study of 142 children. Canadian Medical Association Journal 1970;102(3):263‐8. [PMC free article] [PubMed] [Google Scholar]
Le Saux 2005 {published data only}
- Saux N, Gaboury I, Baird M, Klassen TP, MacCormick J, Blanchard C, et al. A randomized, double‐blind, placebo‐controlled noninferiority trial of amoxicillin for clinically diagnosed acute otitis media in children 6 months to 5 years of age. Canadian Medical Association Journal 2005;172(3):335‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Little 2001 {published data only}
- Little P, Gould C, Williamson I, Moore M, Warner G, Dunleavey J. Pragmatic randomised controlled trial of two prescribing strategies for childhood acute otitis media. BMJ 2001;322:336‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little P, Moore M, Warner G, Dunleavy J, Williamson I. Longer term outcomes from a randomised trial of prescribing strategies in otitis media. British Journal of General Practitioners 2006;56(524):176‐82. [PMC free article] [PubMed] [Google Scholar]
McCormick 2005 {published data only}
- McCormick DP, Chonmaitree T, Pittman C, Saeed K, Friedman NR, Uchida T, et al. Nonsevere acute otitis media: a clinical trial comparing outcomes of watchful waiting versus immediate antibiotic treatment. Pediatrics 2005;115(6):1455‐65. [DOI] [PubMed] [Google Scholar]
Mygind 1981 {published data only}
- Mygind N, Meistrup‐Larsen K‐I, Thomsen J, Thomsen VF, Josefsson K, Sorensen H. Penicillin in acute otitis media: a double‐blind placebo‐controlled trial. Clinical Otolaryngology 1981;6:5‐13. [DOI] [PubMed] [Google Scholar]
- Thomsen J, Meistrup‐Larsen KI, Sorensen H, Larsen PK, Mygind N. Penicillin and acute otitis: short and long term results. Annals of Otology, Rhinology and Laryngology, Supplement 1980;89:271‐4. [DOI] [PubMed] [Google Scholar]
Neumark 2007 {published data only}
- Neumark T, Molstad S, Rosen C, Persson L, Torngren A, Brudin L, et al. Evaluation of phenoxymethylpenicillin treatment of acute otitis media in children aged 2‐16. Scandinavian Journal of Primary Health Care 2007;25:166‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spiro 2006 {published data only}
- Spiro DM, Tay K, Arnold DH, Dziura J, Baker M, Shapiro ED. Wait‐and‐see prescription for the treatment of acute otitis media. JAMA 2006;296(10):1235‐41. [DOI] [PubMed] [Google Scholar]
Tähtinen 2011 {published data only}
- Tähtinen PA, Laine MK, Huovinen P, Jalava J, Ruuskanen O, Ruohola A. A placebo‐controlled trial of antimicrobial treatment for acute otitis media. New England Journal of Medicine 2011;364(2):116‐26. [DOI] [PubMed] [Google Scholar]
Tapiainen 2014 {published data only}
- Tapiainen T, Kujala T, Renko M, Koivunen P, Kontiokari T, Kristo A, et al. Effect of antimicrobial treatment of acute otitis media on the daily disappearance of middle ear effusion: a placebo‐controlled trial. JAMA Pediatrics 2014;168(7):635‐41. [DOI] [PubMed] [Google Scholar]
Thalin 1985 {published data only}
- Thalin A, Densert O, Larsson A, Lyden E, Ripa T. Is penicillin necessary in the treatment of acute otitis media?. Proceedings of the International Conference on Acute and Secretory Otitis Media 1985, Jerusalem. Amsterdam: Kugler Publications, 1985:441‐6.
van Buchem 1981a {published data only}
- Buchem FL, Dunk JHM, van't Hof MA. Therapy of acute otitis media: myringotomy, antibiotics or neither? A double‐blind study in children. Lancet 1981;2:883‐7. [DOI] [PubMed] [Google Scholar]
van Buchem 1981b {published data only}
- Buchem FL, Dunk JHM, van't Hof MA. Therapy of acute otitis media: myringotomy, antibiotics or neither? A double‐blind study in children. Lancet 1981;2:883‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arguedas 2011 {published data only}
- Arguedas A, Soley C, Kamicker BJ, Jorgensen DM. Single‐dose extended‐release azithromycin versus a 10‐day regimen of amoxicillin/clavulanate for the treatment of children with acute otitis media. International Journal of Infectious Diseases 2011;15(4):e240‐8. [DOI] [PubMed] [Google Scholar]
Casey 2012 {published data only}
- Casey JR, Block SL, Hedrick J, Almudevar A, Pichichero ME. Comparison of amoxicillin/clavulanic acid high dose with cefdinir in the treatment of acute otitis media. Drugs 2012;72:1991‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaput 1982 {published data only}
- Chaput de Saintonge DM, Levine DF, Temple Savage IT, Burgess GW, Sharp J, Mayhew SR, et al. Trial of three‐day and ten‐day courses of amoxycillin in otitis media. British Medical Journal Clinical Research Edition 1982;284(6322):1078‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Engelhard 1989 {published data only}
- Engelhard D, Strauss N, Jorczak‐Sarni L, Cohen D, Sacjs TG, Shapiro M. Randomised study of myringotomy, amoxycillin/clavulanate, or both for acute otitis media in infants. Lancet 1989;2(8655):141‐3. [DOI] [PubMed] [Google Scholar]
Liu 2011 {published data only}
- Liu P, Fang AF, LaBadie RR, Crownover PH, Arguedas AG. Comparison of azithromycin pharmacokinetics following single oral doses of extended‐release and immediate‐release formulations in children with acute otitis media. Antimicrobial Agents and Chemotherapy 2011;55(11):5022‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ostfeld 1987 {published data only}
- Ostfeld E, Segal J, Kaufstein M, Gelernter I. Management of acute otitis media without primary administration of systemic antimicrobial agents. Recent advances in otitis media. Proceedings of the Fourth International Symposium. Toronto: BC Decker, 1987:235‐9.
Rudberg 1954 {published data only}
- Rudberg RD. Acute otitis media: comparative therapeutic results of sulphonamide and penicillin administered in various forms. Acta Oto‐Laryngologica 1954;113(Suppl):1‐79. [PubMed] [Google Scholar]
Ruohola 2003 {published data only}
- Ruohola A, Heikkinen T, Meurman O, Puhakka T, Lindblad N, Ruuskanen O. Antibiotic treatment of acute otorrhea through tympanostomy tube: randomized double‐blind placebo‐controlled study with daily follow‐up. Pediatrics 2003;111(5):1061‐7. [DOI] [PubMed] [Google Scholar]
Sarrell 2003 {published data only}
- Sarrell EM, Cohen HA, Kahan E. Naturopathic treatment for ear pain in children. Pediatrics 2003;111(5):574‐9. [DOI] [PubMed] [Google Scholar]
Tähtinen 2012 {published data only}
- Tähtinen PA, Laine MK, Ruuskanen O, Ruohola A. Delayed versus immediate antimicrobial treatment for acute otitis media. Pediatric Infectious Disease Journal 2012;31(12):1227‐32. [DOI] [PubMed] [Google Scholar]
van Buchem 1985 {published data only}
- Buchem F, Peeters M, Van' t Hof M. Acute otitis media: a new treatment strategy. BMJ 1985;290:1033‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12608000424303 {published data only}
- ACTRN12608000424303. Antibiotics for asymptomatic acute otitis media. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12608000424303 (accessed 26 November 2014).
Additional references
AAP 2013
- American Academy of Pediatrics, American Academy of Family Physicians. Clinical practice guidelines: diagnosis and management of acute otitis media. Pediatrics 2013;131(3):e964‐99. [DOI] [PubMed] [Google Scholar]
Akkerman 2005
- Akkerman AE, Kuyvenhoven MM, Wouden JC, Verhij TJM. Analysis of under‐and overprescribing of antibiotics in acute otitis media in general practice. Journal of Antimicrobial Chemotherapy 2005;56:569‐74. [DOI] [PubMed] [Google Scholar]
Arnold 2005
- Arnold SR, Straus SE. Interventions to improve antibiotic prescribing in ambulatory care. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003539] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arola 1990
- Arola M, Ruuskanen O, Ziegler T, Mertsola J, Näntö‐Salonen K, Putto‐Laurila A, et al. Clinical role of respiratory virus infection in acute otitis media. Pediatrics 1990;86:848‐55. [PubMed] [Google Scholar]
Barkai 2009
- Barkai G, Leibovitz E, Givon‐Lavi N, Dagan R. Potential contribution by nontypable Haemophilus influenzae in protracted and recurrent acute otitis media. Pediatric Infectious Disease Journal 2009;28:466‐71. [DOI] [PubMed] [Google Scholar]
Berman 1995
- Berman S. Otitis media in developing countries. Pediatrics 1995;96:126‐31. [PubMed] [Google Scholar]
Casey 2013
- Casey JR, Kaur R, Friedel VC, Pichichero ME. Acute otitis media otopathogens during 2008 to 2010 in Rochester, New York. Pediatic Infectious Disease Journal 2013;32(8):805‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cates 1999
- Cates C. An evidence based approach to reducing antibiotic use in children with acute otitis media: controlled before and after the study. BMJ 1999;318:715‐6. [The handout is available at http://www.cates.cwc.net/] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chao 2008
- Chao JH, Kunkov S, Reyes LB, Lichten S, Crain EF. Comparison of two approaches to observation therapy for acute otitis media in the emergency department. Pediatrics 2008;121:31352‐e6. [DOI] [PubMed] [Google Scholar]
Chonmaitree 1992
- Chonmaitree T, Owen MJ, Patel JA, Hedgpeth D, Horlick D, Howie VM. Effect of viral respiratory tract infection on outcome of acute otitis media. Journal of Pediatrics 1992;120:856‐62. [DOI] [PubMed] [Google Scholar]
Coker 2010
- Coker TR, Chan LS, Newberry SJ, Limbos MA, Suttorp MJ, Shekelle PG, et al. Diagnosis, microbial epidemiology, and antibiotic treatment of acute otitis media in children: a systematic review. JAMA 2010;304(19):2161‐9. [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309(6964):1286‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Froom 2001
- Froom J, Culpepper L, Green LA, Melker RA, Grob P, Heeren T, et al. A cross‐national study of acute otitis media: risk factors, severity, and treatment at initial visit. Report from the International Primary Care Network (IPCN) and the Ambulatory Sentinel Practice Network (ASPN). Journal of the American Board of Family Practice 2001;14:406‐17. [PubMed] [Google Scholar]
Gillies 2014
- Gillies M, Ranakusuma A, Hoffmann T, Thorning S, McGuire T, Glasziou P, et al. Common harms from amoxicillin: a systematic review and meta‐analysis of randomized placebo‐controlled trials for any indication. Canadian Medical Association Journal 2014 Nov 17 [Epub ahead of print]:.. [DOI: 10.1503/cmaj.140848] [DOI] [PMC free article] [PubMed]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Klein 1989
- Klein JO. Epidemiology of otitis media. Pediatic Infectious Disease Journal 1989;8(Suppl 1):89. [DOI] [PubMed] [Google Scholar]
Koopman 2008
- Koopman L, Hoes AW, Glasziou PP, Appelman CL, Burke P, McCormick DP, et al. Antibiotic therapy to prevent the development of asymptomatic middle ear effusion in children with acute otitis media. Archives of Otolaryngology ‐ Head and Neck Surgery 2008;134(2):128‐32. [DOI] [PubMed] [Google Scholar]
Kozyrskyj 2010
- Kozyrskyj A, Klassen TP, Moffatt M, Harvey K. Short‐course antibiotics for acute otitis media. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD001095] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Little 2006
- Little P, Moore M, Warner G, Dunleavy J, Williamson I. Longer term outcomes from a randomised trial of prescribing strategies in otitis media. British Journal of General Practice 2006;56:176‐82. [PMC free article] [PubMed] [Google Scholar]
McCormick 2007
- McCormick DP, Chandler SM, Chonmaitree T. Laterality of acute otitis media: different clinical and microbiologic characteristics. Pediatric Infectious Disease Journal 2007;26:583‐8. [DOI] [PubMed] [Google Scholar]
Rosenfeld 1994
- Rosenfeld RM, Vertrees JE, Carr J, Cipolle RJ, Uden DL, Giebink GS, et al. Clinical efficacy of antimicrobial drugs for acute otitis media: meta‐analysis of 5400 children from thirty‐three randomized trials. Journal of Pediatrics 1994;124:355‐67. [DOI] [PubMed] [Google Scholar]
Rothman 2003
- Rothman R, Owens T, Simel D. Does this child have acute otitis media?. JAMA 2003;290(12):1633‐40. [DOI] [PubMed] [Google Scholar]
Rovers 2006
- Rovers MM, Glasziou P, Appelman CL, Burke P, McCormick DP, Damoiseaux RA, et al. Antibiotics for acute otitis media: a meta‐analysis with individual patient data. Lancet 2006;368:1429‐35. [DOI] [PubMed] [Google Scholar]
Spurling 2013
- Spurling GKP, Mar CB, Dooley L, Foxlee R, Farley R. Delayed antibiotics for respiratory infections. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD004417.pub4] [DOI] [PubMed] [Google Scholar]
Stool 1989
- Stool SE, Field MJ. The impact of otitis media. Pediatic Infectious Disease Journal 1989;8(Suppl 1):11‐4. [PubMed] [Google Scholar]
Teele 1989
- Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during first seven years of life in children in greater Boston: a prospective cohort study. Journal of Infectious Diseases 1989;160:83‐94. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Glasziou 1995
- Glasziou PP, Hayem M, Mar CB. A meta‐analysis of treatments for acute otitis media: antibiotic vs placebo, short vs long antibiotic course, and myringotomy. Cochrane Database of Systematic Reviews 1995, Issue 1. [DOI: 10.1002/14651858.CD000219] [DOI] [Google Scholar]
Glasziou 1997
- Glasziou PP, Hayem M, Mar CB. Treatments for acute otitis media in children: antibiotic versus placebo. Cochrane Database of Systematic Reviews 1997, Issue 1. [DOI: 10.1002/14651858.CD000219] [DOI] [PubMed] [Google Scholar]
Glasziou 1999
- Glasziou PP, Hayem M, Mar CB. Antibiotics versus placebo for acute otitis media in children. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD000219.pub2] [DOI] [Google Scholar]
Glasziou 2005
- Glasziou PP, Mar CB, Sanders SL, Hayem M. Antibiotics for acute otitis media in children. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD000219.pub2] [DOI] [PubMed] [Google Scholar]
Sanders 2009
- Sanders SL, Glasziou PP, Mar CB, Rovers MM. Antibiotics for acute otitis media in children. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD000219.pub2] [DOI] [PubMed] [Google Scholar]
Venekamp 2013
- Venekamp RP, Sanders S, Glasziou PP, Mar CB, Rovers MM. Antibiotics for acute otitis media in children. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD000219.pub3] [DOI] [PubMed] [Google Scholar]


