Abstract
Morbidity and mortality attributed to type 2 diabetes have exponentially increased in the US. At exceptionally high risk is a subpopulation of persons with type 2 diabetes who smoke, which are shown to have decreased success rates of smoking cessation than euglycemic smokers. Preclinical research in our laboratory has shown that the rewarding effects of nicotine are enhanced in the streptozotocin and high-fat diet rodent model of diabetes. It is presently unclear whether this enhancement of nicotine reward can be demonstrated in other insulin resistant rat models. This study aimed to determine if a similar increase in nicotine reward is found in Goto-Kakizaki (GK) rats, a model of the spontaneous formation of insulin resistance in an inbred sub-strain of Wistar rat. Nicotine conditioned place preference (CPP) was examined in Sprague-Dawley (SD), Wistar, and GK rats. A robust nicotine CPP was found in SD and Wistar rats, but nicotine CPP was not detected in GK rats. Locomotor activity was also evaluated in all three strains, and GK rats demonstrated significantly less activity as compared to SD and Wistar rats. To further assess reward behavior in GK rats, consumption of saccharin solution was measured over a 48-hour period. GK rats showed a significant increase in saccharin intake compared to SD rats. These findings suggest that GK rats experience an enhanced hedonic processing as compared to SD rats. The lack of nicotine CPP in GK rats may be due to deficits in learning and memory, thus hindering their ability to acquire or express a place preference.
Keywords: nicotine reward, diabetes, Goto-Kakizaki, place preference
1. Introduction
The incidence of diabetes increases every year and currently sits at 9.4% of the US population, translating to 30 million Americans with diabetes [1]. Persons with diabetes have a higher propensity for numerous health conditions, including cardiovascular and metabolic diseases. Individuals who have diabetes and smoke are twice as likely to experience mortality and adverse health outcomes than those who do not smoke [2,3]. Diabetic smokers are strongly encouraged to undergo smoking cessation therapy, yet their success rate is much lower, and they experience higher rates of depression and negative affective states during nicotine abstinence as compared to euglycemic smokers [4–6]. It is unclear whether diabetic smokers have altered nicotine reward circuitry as compared to non-diabetic smokers. Although preclinical studies indicate that the rewarding effects of nicotine, and nicotine withdrawal symptoms are enhanced in rodent models of type 1 and type 2 diabetes [7–10].
A major hurdle in studying diabetes is our lack of understanding of the underlying etiology of the disease, which has resulted in numerous animal models that have been developed to study the disease state and associated complications. In general, three avenues are employed to evaluate diabetes in animal models: 1- using a toxin, such as streptozotocin (STZ) that destroys insulin-producing beta cells in the pancreas, resulting in hypoinsulinemia and hyperglycemia that resemble type 1 and advanced stages of type 2 diabetes; 2-using a chronic high fat diet (HFD) regimen leading to obesity and insulin resistance that resemble the pathogenesis of type 2 diabetes; and 3- using transgenic or inbred strains of rodents that develop spontaneous insulin resistance and hyperglycemia resembling type 2 diabetes. Previous studies by our group made use of the STZ and HFD models of diabetes to demonstrate that the rewarding effects of nicotine are enhanced in rodent models of diabetes and that the enhancement in nicotine reward is reduced upon insulin replacement or blood glucose normalization [7,11]. The present work expands previous studies by assessing the rewarding effects of nicotine in the Goto-Kakizaki (GK) rat, a Wistar rat sub-strain that was produced by selective inbreeding of Wistar rats using glucose tolerance as the selection criteria, resulting in rats that spontaneously develop insulin resistance similar to that in type 2 diabetes [12]. The GK rat has been widely used in research exploring type 2 diabetes phenotype. In continuation of our previous work, this study aimed to discern whether a spontaneous animal model of type 2 diabetes exhibits an enhancement in nicotine reward. As a control procedure, the present study also compared hedonic reward processing by comparing saccharin intake in rats.
2. Methods
2.1. Subjects
Sixty-day-old adult male Sprague-Dawley (SD; Envigo, Indianapolis, IN), Wistar and Goto-Kakizaki (Charles River, Wilmington, MA) rats weighing 250–300 g were pair-housed (according to strain) in standard cages and placed on a 12-h light/dark cycle (lights on at 6 a.m.) with unrestricted access to food and water. SD and Wistar rats were both selected as controls. All animals had blood glucose levels (BGLs) measured using an AlphaTRAK glucometer (Abbott Laboratories, Chicago, IL) before the start of the behavioral experiments. All GK animals were evaluated and confirmed as hyperglycemic according to their blood glucose measurements (indicated by a baseline fasting BGL >150 mg/dL) before the start of the behavioral evaluation. All experiments took place between 9 a.m. and 2 p.m. Animal care and use were in accordance with the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication 85–23, Bethesda, MD) and approved by the Institutional Animal Care and Use Committee of Western University of Health Sciences.
2.2. CPP Apparatus
A detailed description of the CPP apparatus has been previously provided [13,14]. In brief, the CPP apparatus was a truncated T-maze consisting of two adjacent conditioning chambers, one chamber having black and white vertical striped walls and a wire mesh floor and the other chamber having black and white horizontal striped walls with a metal rod floor. A start box was located on the side of the apparatus and had gray walls and a smooth gray floor.
2.3. CPP Procedure
All rats were handled for at least three days before the start of the CPP experiment. An 8-day CPP paradigm was used, consisting of a preconditioning day, six conditioning days, with one pairing session per day counterbalanced and alternating between saline and drug/saline (i.e., three drug pairings and three saline-vehicle pairings), and a postconditioning test day. The study had two groups of rats. Those that were conditioned with nicotine in alternating administration patters as noted above, and control rats that were only conditioned with saline (non-nicotine controls). On the preconditioning day, rats were placed in the start box for 5 min and then permitted to enter the conditioning chambers once the start box door was opened. Upon exiting the start box, the door was closed at which time rats were given 15 min to travel and explore the two conditioning chambers. Time spent in each chamber was recorded and quantified using EthoVision XT video tracking software (Noldus Information Technology, Leesburg, VA, USA). On conditioning days, rats were injected subcutaneously with either saline (1ml/kg) or nicotine (0.2 mg/kg) and placed either in the horizontally or vertically striped conditioning chambers for 30 min. We used a biased procedure, where nicotine was paired with the initially non-preferred chamber of the conditioning apparatus. On the post-conditioning test day, rats were placed in the start box for 5 min and then permitted to enter the conditioning chambers. The amount of time spent in the drug-paired or saline-paired chamber was measured for 15 min. The difference in time spent in the drug-paired chamber between the postconditioning and preconditioning days are reported. CPP was defined as a greater amount of time spent in the drug-paired side between rats conditioned with nicotine versus rats conditioned with saline.
2.4. Locomotor Activity
Locomotor activity (distance traveled in cm) was assessed inside the CPP apparatus on the preconditioning and postconditioning test days using the EthoVision Software.
2.5. Saccharin Consumption
Two weeks after the conclusion of the CPP experiment, the previously saline treated SD and GK rats were acclimated to dual water bottles placed in their home cage for three days. Rats were then exposed to 0.1% saccharin solution placed in pre-weighed water bottles positioned next to an identical bottle filled with water. Weights of bottles were recorded after 24- and 48-hr exposures. Twenty-four hours after the start of the measurement, bottle positions were switched to control for potential side preference. Consumption measured at 24 and 48 hrs were averaged and presented. Wistar rats were not included in this experiment as Wistar and SD rats consume similar levels of saccharin [15], although Wistar rats are also an appropriate control group for GK rats in this study.
2.6. Drugs
Nicotine tartrate salt was purchased from Sigma-Aldrich (St. Louis, MO, USA). Saccharin was purchased from (Spectrum Chemicals, New Brunswick, NJ, USA). The nicotine dose is reported in free-base form. Nicotine was dissolved in saline and physiologically pH (7.0–7.4) and saccharin was dissolved in water.
2.7. Statistical Analysis
CPP data were analyzed using a 2-way ANOVA with Drug (saline or nicotine) and Rat Strain (SD, Wistar, and GK) as between-subject factors. Locomotor activity data were analyzed using a 1-way ANOVA with Strain as the factor. Saccharin and water solution consumption was analyzed using a 2-way ANOVA with Strain (SD and GK) as a between subjects factor and Solution (Saccharin, Water and Total) as a within-subjects factor. For the CPP data, pairwise comparisons were made using a Bonferroni test with an alpha level set at 0.01 probability level (p<0.01). The choice of a more conservative pairwise comparison in the CPP data was due to the lack of a significant 2-way interaction, in light of planned comparisons that were initially intended to examine nicotine vs saline effects within strains. Post-hoc comparisons for saccharin and water solution consumption data, as well as locomotor activity data were made using Tukey’s test with the alpha level set at the 0.05 probably level (p< 0.05). The same animals were evaluated for the CPP, locomotor activity and saccharin consumption tests. In saline treated SD, Wistar and GK rats group sample sizes were n= 6, 7, and 5, respectively. In nicotine treated SD, Wistar and GK rats group sample sizes were n= 6, 5, and 7, respectively.
3. Results
The CPP results are shown in Figure 1. The statistical analysis did not detect a significant Strain x Drug interaction F (2, 30) = 0.65, p = 0.52. Although, significant Drug and Strain main effects were detected: Drug F (1,30) = 29.38, p < 0.0001; Strain F (2,30) = 3.65, p < 0.05. Planned comparisons were made to examine differences between nicotine vs saline within strains. Nicotine treatment produced an increase in the time spent in the drug-paired side compared to saline controls in SD and Wistar rats, indicative of nicotine CPP. Nicotine treatment did not produce a CPP in GK rats. The locomotor activity data are shown in Figure 2. The analysis revealed that GK rats had lower locomotor activity counts as compared to SD and Wistar rats during the preconditioning F (2, 33) = 29.93, p < 0.0001, and postconditioning test sessions F (2,37) = 15.78, p < 0.0001, in the CPP apparatus. Saccharin solution, water and total fluid consumption is shown in Figure 3. The statistical analysis detected a significant Strain by Solution interaction F (2, 18) = 16.56. GK rats consumed less water and more saccharin solution as compared to SD rats over a 48-hr period. Total fluid consumption was similar between SD and GK rats.
Figure 1.
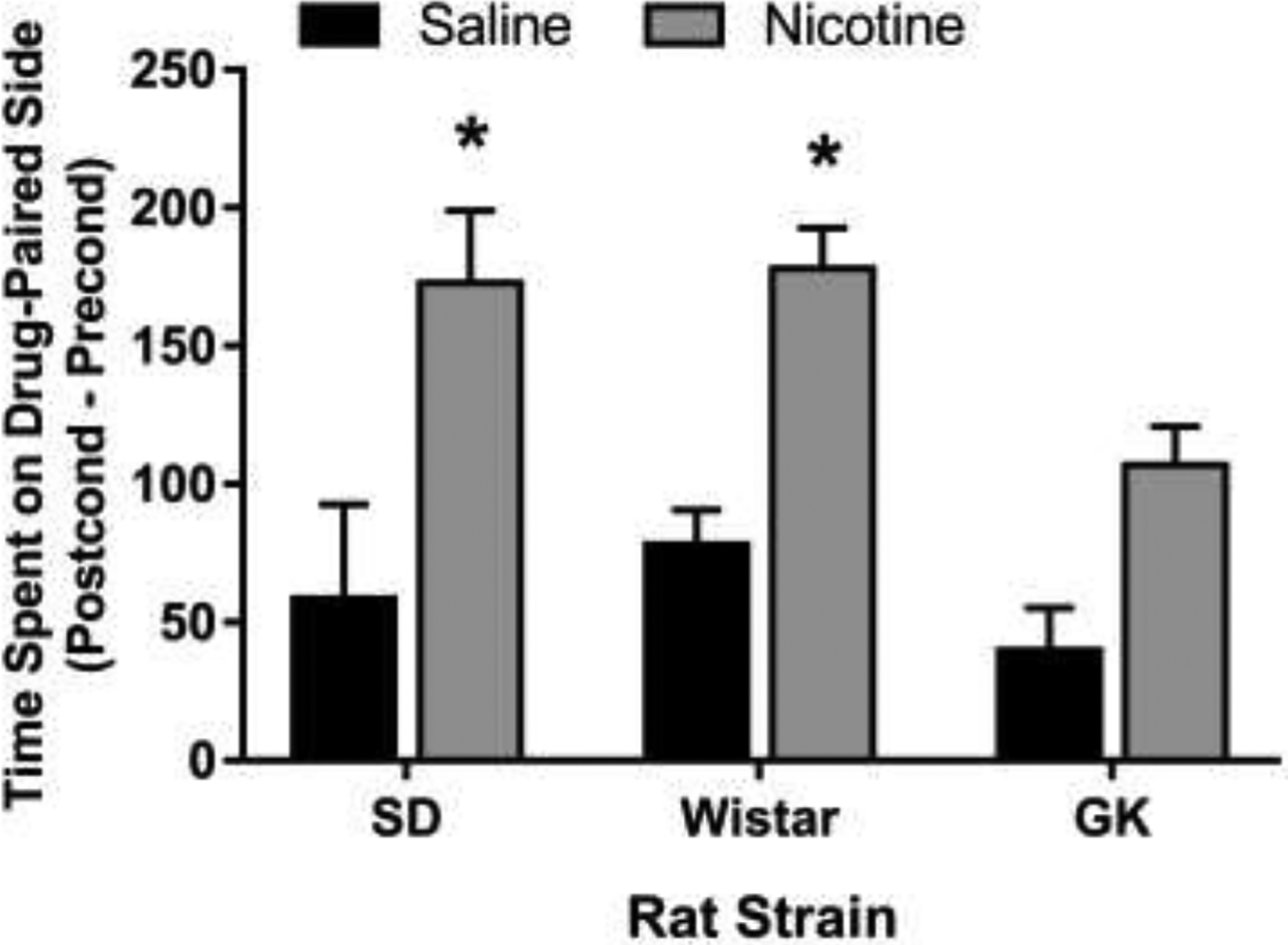
Nicotine CPP in SD, Wistar, and GK rats. Nicotine treated SD and Wistar rats displayed a significant increase in the time spent (s) on the drug-paired side as compared to saline-treated rats. GK rats did not exhibit a nicotine CPP. Data are presented as means (±S.E.M.). n = 5–7 rats per group. Asterisks represent a significant difference from the saline group within strain (p < 0.01).
Figure 2.
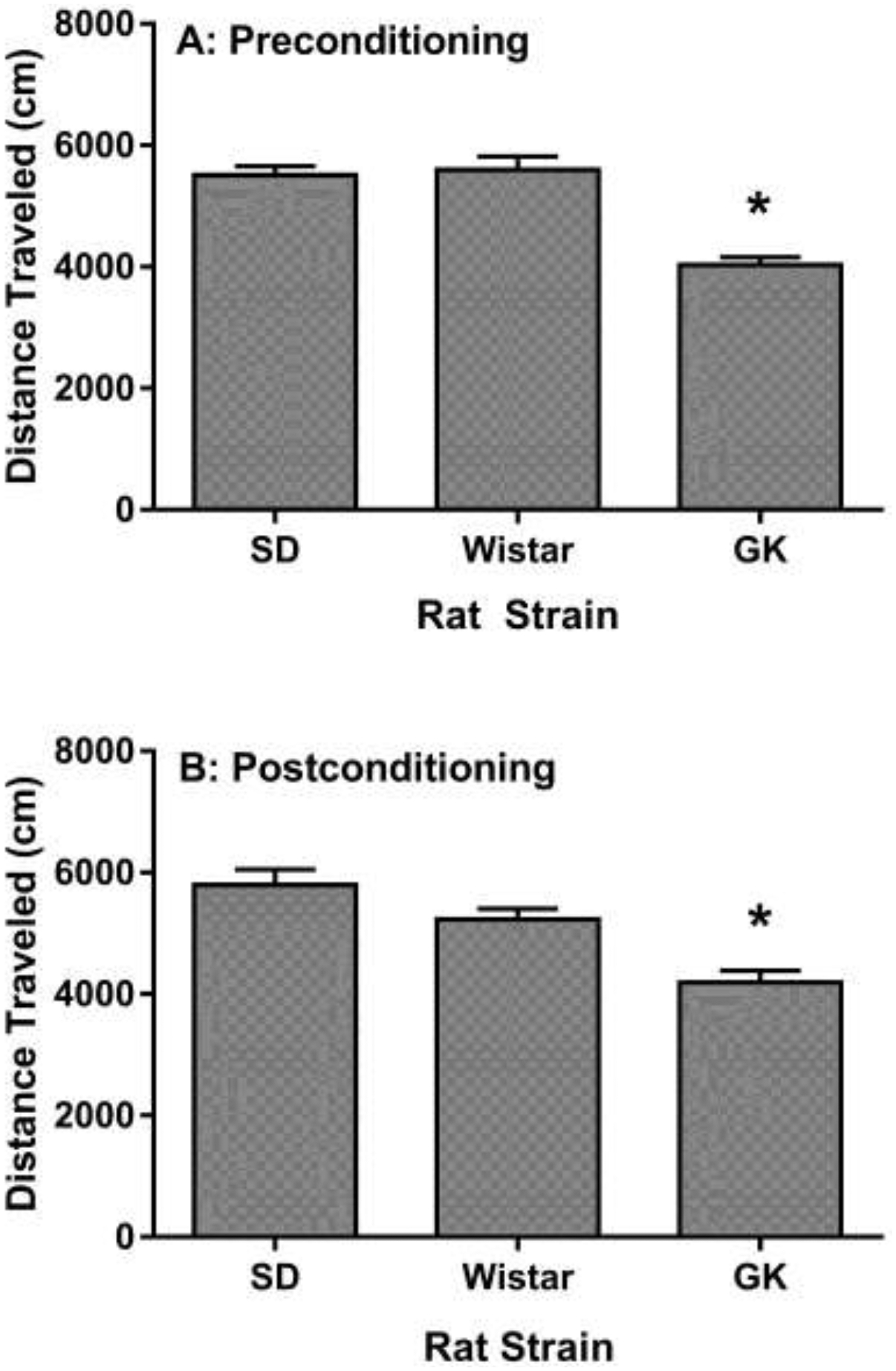
Locomotor activity of rats measured on A) Preconditioning and B) Postconditioning days of the CPP paradigm. GK rats exhibited lower locomotor counts on both days as compared to SD and Wistar rats. Data presented as mean (± S.E.M.) of distance traveled in centimeters. n = 12 rats per group. Asterisks represent a significant difference from the SD and Wistar rat strains (p < 0.05).
Figure 3.
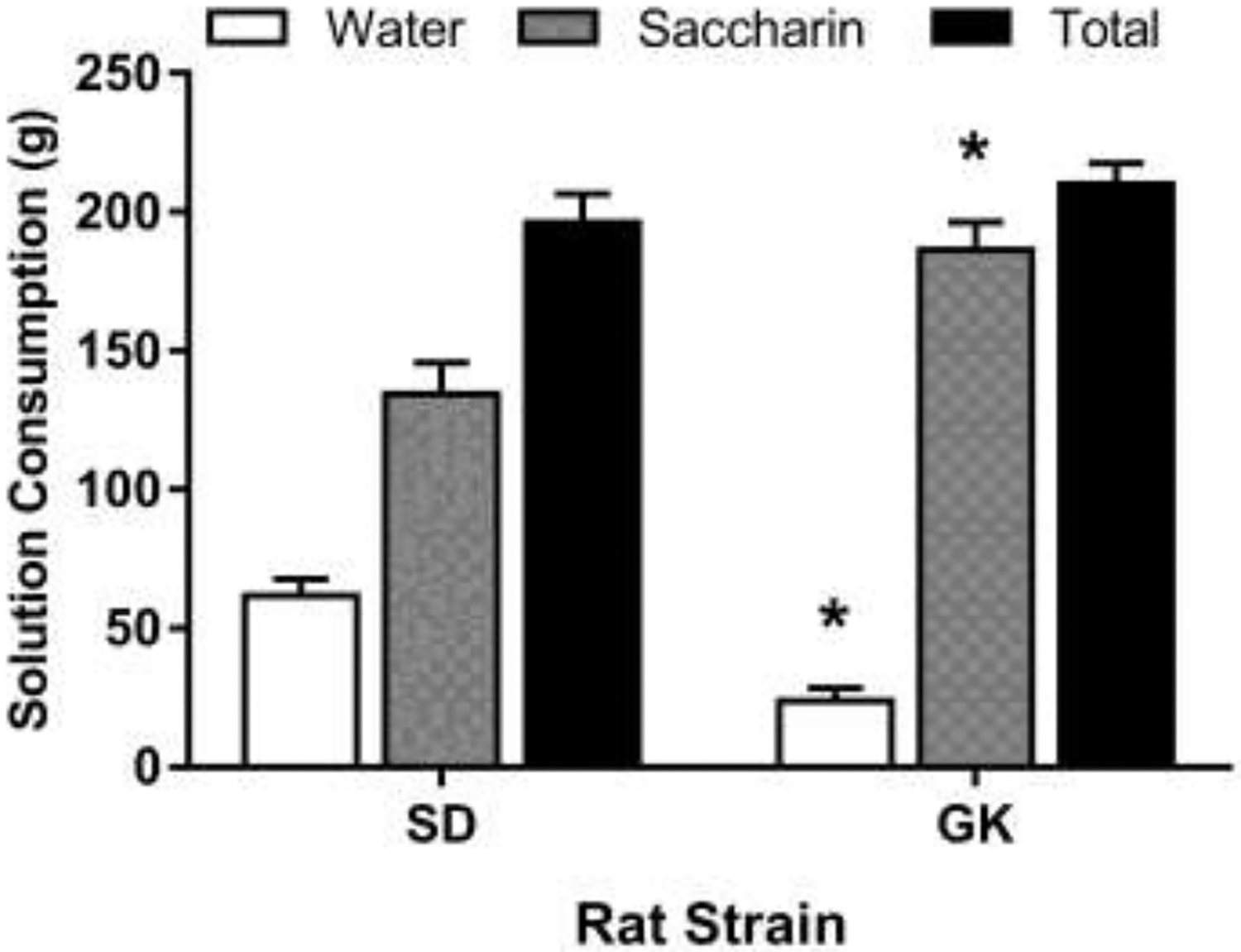
Consumption of saccharin solution (0.1%) in SD and GK rats. Consumption was measured for 48hrs. GK rats consumed more saccharin solution than SD rats. Data presented as mean grams (± S.E.M.) of saccharin solution consumed. n = 5–6 rats per group. Asterisks represent a significant difference from the SD rats (p < 0.05).
4. Discussion
Preclinical studies have demonstrated that nicotine reward and withdrawal are enhanced in rodent models of diabetes. Animals rendered diabetic via an STZ administration or HFD regimen experience an increase in the rewarding effects of nicotine in the CPP and the intravenous self-administration model of drug reward [7,8,10]. Moreover, STZ treated rats also demonstrate augmented nicotine withdrawal symptoms [9]. The goal of the present study was to determine whether our previous findings would be replicated in a spontaneous model of type 2 diabetes, namely in GK rats. The results revealed that GK rats did not show notable CPP with a nicotine dose that produced a robust CPP in both SD and Wistar rat strains. Furthermore, GK rats displayed lower locomotor activity counts during preconditioning and postconditioning test sessions in the CPP apparatus, indicating an overall reduction in motor activity, consistent with a previous report [16]. Although GK rats had less locomotor activity than SD and Wistar rats, their average distance travelled was approximately 4000 cm, which is notable considering the small size of the CPP chambers. Thus, it is unlikely that a lack of nicotine CPP can be attributed to depressed locomotion or immobility.
Due to the lack of nicotine CPP in GK rats, an additional test compared general hedonic effects using a two-bottle choice task for water or saccharin solution consumed for 48-hrs. The finding that GK rats consumed significantly more saccharin solution than their SD counterparts indicates that GK rats are capable of experiencing rewarding effects. Therefore, the lack of nicotine CPP in GK rats is either due to an inability to experience nicotine reward or due to a learning deficit. A lack of nicotine CPP has been reported in other rat strains. For instance, Wistar Kyoto and Fischer 344 rats did not develop nicotine CPP, neither at low nicotine doses, nor when doses are increased [17–19]. Moreover, a nicotine conditioned place aversion was detected in Fischer rats when the number of nicotine pairings were increased from 5 to 10 [20]. It is noteworthy that Wistar Kyoto and Fischer strains develop a CPP to other drugs such as cocaine [21,22] demonstrating their ability to make associations in a spatial memory task, and suggesting that these strains do not find nicotine to be rewarding. Similarly, GK rats may not find nicotine to be rewarding, which might explain the lack of CPP. However, further studies will be needed to make this determination.
GK rats experience hedonic reward as determined by their higher saccharin consumption as compared to SD rats. It is plausible that a lack of nicotine CPP in GK rats is due to difficulties in making strong associations between the effects of nicotine and the spatial/contextual cues in the CPP chamber, rendering them unable to develop a place preference. Previous reports have demonstrated deficits in spatial learning in GK rats using the morris water maze [23], and the Y-maze [24]. GK rats also display delayed acquisition to lever press for a food reward, and have higher frequency of incorrect responses than controls [16]. Cognitive impairments in GK rats could also be attributed to impaired hippocampal neurogenesis [25], and/or defective differentiation, survivability, and vascularization of the dentate gyrus and its progenitor cells [26]. When considering the behavioral and histological findings together, it is more likely that GK rats do not develop nicotine CPP due to a learning deficit.
The CPP paradigm is a learning and memory task based on classical conditioning principles that require the formation of an association between a stimulus and environmental cues to produce a response indicative of a preference for a chamber. Thus, deficits in learning and memory, as seen in GK rats, limit the acquisition and expression of a CPP. The present findings were limited by the lack of a complete nicotine dose-response consideration, and a less than ideal sample size due to limited availability of GK rats, which together may have contributed to limited statistical findings. With these limitations considered, the findings suggest that GK rats exhibit an intact hedonic reward system, although they are unable to demonstrate rewarding effects in learning and memory based behavioral tasks. As such, while useful for studying the etiology and treatment of type 2 diabetes, the GK rat is a poor animal model to study reward-based behaviors that rely on learning and memory.
Highlights.
Nicotine place preference was not detected in Goto-Kakizaki (GK) rats
GK rats displayed lower locomotor activity as compared to control rats
Saccharin consumption was higher in GK rats as compared to control rats
Acknowledgements
This work was supported by National Institutes of Health grant R15DA040130 to AN and funds provided by Western University of Health Sciences
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].C. for D.C. and Prevention, National diabetes statistics report, 2017, Atlanta, GA: Centers Dis. Control Prev. US Dept Heal. Hum. Serv. (2017). [Google Scholar]
- [2].Scemama O, Hamo-Tchatchouang E, Le Faou AL, Altman JJ, Difficulties of smoking cessation in diabetic inpatients benefiting from a systematic consultation to help them to give up smoking, Diabetes Metab. 32 (2006) 435–441. doi:MDOI-DM-11-2006-32-5-1262-3636-101019-200519824 [pii]. [DOI] [PubMed] [Google Scholar]
- [3].Tonstad S, Cigarette smoking, smoking cessation, and diabetes, Diabetes Res Clin Pr. 85 (2009) 4–13. 10.1016/j.diabres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- [4].Gill GV, Morgan C, MacFarlane IA, Awareness and use of smoking cessation treatments among diabetic patients, Diabet Med. 22 (2005) 658–660. 10.1111/j.1464-5491.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- [5].Haire-Joshu D, Heady S, Thomas L, Schechtman K, Fisher EB, Depressive symptomatology and smoking among persons with diabetes., Res. Nurs. Health 17 (1994) 273–282. [DOI] [PubMed] [Google Scholar]
- [6].Spangler JG, Summerso JH, Bell R. a, Konen JC, Smoking status and psychosocial variables in type 1 diabetes mellitus., Addict. Behav 26 (2001) 21–29. [DOI] [PubMed] [Google Scholar]
- [7].Íbias J, O’Dell LE, Nazarian A, Insulin dependent and independent normalization of blood glucose levels reduces the enhanced rewarding effects of nicotine in a rodent model of diabetes, Behav. Brain Res 351 (2018). doi: 10.1016/j.bbr.2018.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].O’Dell LE, Natividad L. a., Pipkin J. a., Roman F, Torres I, Jurado J, Torres OV, Friedman TC, Tenayuca JM, Nazarian A, Enhanced nicotine self-administration and suppressed dopaminergic systems in a rat model of diabetes, Addict. Biol 19 (2014) 1006–1019. doi: 10.1111/adb.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pipkin JA, Cruz B, Flores RJ, Hinojosa CA, Carcoba LM, Ibarra M, Francis W, Nazarian A, O’Dell LE, Both nicotine reward and withdrawal are enhanced in a rodent model of diabetes., Psychopharmacology (Berl). 234 (2017) 1615–1622. doi: 10.1007/s00213-017-4592-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Richardson JR, a Pipkin J, O’Dell LE, Nazarian A, Insulin resistant rats display enhanced rewarding effects of nicotine., Drug Alcohol Depend. 140 (2014) 205–7. doi: 10.1016/j.drugalcdep.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cruz B, Flores RJ, Uribe KP, Espinoza EJ, Spencer CT, Serafine KM, Nazarian A, O’Dell LE, Insulin modulates the strong reinforcing effects of nicotine and changes in insulin biomarkers in a rodent model of diabetes, Neuropsychopharmacology. 44 (2019) 1141–1151. doi: 10.1038/s41386-018-0306-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Goto Y, Kakizaki M, Masaki N, Production of spontaneous diabetic rats by repetition of selective breeding., Tohoku J. Exp. Med 119 (1976) 85–90. doi: 10.1620/tjem.119.85. [DOI] [PubMed] [Google Scholar]
- [13].Nazarian A, Are D, Tenayuca JM, Acetaminophen modulation of hydrocodone reward in rats., Pharmacol. Biochem. Behav 99 (2011) 307–10. doi: 10.1016/j.pbb.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tenayuca JM, Nazarian A, Hydrocodone and morphine possess similar rewarding effects and reduce ERK and CREB phosphorylation in the nucleus accumbens., Synapse. 66 (2012) 918–22. doi: 10.1002/syn.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Malkesman O, Braw Y, Zagoory-Sharon O, Golan O, Lavi-Avnon Y, Schroeder M, Overstreet DH, Yadid G, Weller A, Reward and anxiety in genetic animal models of childhood depression., Behav. Brain Res 164 (2005) 1–10. doi: 10.1016/j.bbr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- [16].Moreira T, Malec E, Östenson CG, Efendic S, Liljequist S, Diabetic type II Goto-Kakizaki rats show progressively decreasing exploratory activity and learning impairments in fixed and progressive ratios of a lever-press task, Behav. Brain Res 180 (2007) 28–41. doi: 10.1016/j.bbr.2007.02.034. [DOI] [PubMed] [Google Scholar]
- [17].Philibin SD, Vann RE, Varvel SA, Covington HE, Rosecrans JA, James JR, Robinson SE, Differential behavioral responses to nicotine in Lewis and Fischer-344 rats, Pharmacol. Biochem. Behav 80 (2005) 87–92. doi: 10.1016/j.pbb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- [18].Rauhut AS, Zentner IJ, Mardekian SK, Tanenbaum JB, Wistar Kyoto and Wistar rats differ in the affective and locomotor effects of nicotine., Physiol. Behav 93 (2008) 177–88. doi: 10.1016/j.physbeh.2007.08.011. [DOI] [PubMed] [Google Scholar]
- [19].Watterson E, Daniels CW, Watterson LR, Mazur GJ, Brackney RJ, Olive MF, Sanabria F, Nicotine-induced place conditioning and locomotor activity in an adolescent animal model of attention deficit/hyperactivity disorder (ADHD)., Behav. Brain Res 291 (2015) 184–188. doi: 10.1016/j.bbr.2015.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Horan B, Smith M, Gardner EL, Lepore M, Ashby CR, (−)-Nicotine produces conditioned place preference in Lewis, but not Fischer 344 rats., Synapse. 26 (1997) 93–4. doi:. [DOI] [PubMed] [Google Scholar]
- [21].Nazarian A, Russo SJ, Festa ED, Kraish M, Quinones-Jenab V, The role of D1 and D2 receptors in the cocaine conditioned place preference of male and female rats., Brain Res. Bull 63 (2004) 295–299. doi: 10.1016/j.brainresbull.2004.03.004. [DOI] [PubMed] [Google Scholar]
- [22].Dennis TS, Beck KD, Bobzean SAM, Dougall AL, Perrotti LI, Assessing learned associations between conditioned cocaine reward and environmental stimuli in the Wistar Kyoto rat., Pharmacol. Biochem. Behav 103 (2012) 76–82. doi: 10.1016/j.pbb.2012.08.005. [DOI] [PubMed] [Google Scholar]
- [23].Tian Z, Ren N, Wang J, Zhang D, Zhou Y, Ginsenoside Ameliorates Cognitive Dysfunction in Type 2 Diabetic Goto-Kakizaki Rats., Med. Sci. Monit 24 (2018) 3922–3928. doi: 10.12659/MSM.907417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Duarte JMN, Skoug C, Silva HB, Carvalho RA, Gruetter R, Cunha RA, Impact of Caffeine Consumption on Type 2 Diabetes-Induced Spatial Memory Impairment and Neurochemical Alterations in the Hippocampus., Front. Neurosci 12 (2018) 1015. doi: 10.3389/fnins.2018.01015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lang BT, Yan Y, Dempsey RJ, Vemuganti R, Impaired neurogenesis in adult type-2 diabetic rats, Brain Res. 1258 (2009) 25–33. doi: 10.1016/j.brainres.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Beauquis J, Homo-Delarche F, Giroix MH, Ehses J, Coulaud J, Roig P, Portha B, De Nicola AF, Saravia F, Hippocampal neurovascular and hypothalamic-pituitary-adrenal axis alterations in spontaneously type 2 diabetic GK rats, Exp. Neurol 222 (2010) 125–134. doi: 10.1016/j.expneurol.2009.12.022. [DOI] [PubMed] [Google Scholar]


