Abstract
Background
Ischemia-reperfusion injury is caused by a blood reperfusion injury in ischemic brain tissue, and usually occurs in the treatment stage of ischemic disease, which can aggravate brain tissue injury. MiR-122 is closely related to ischemia-reperfusion injury in the myocardium, kidney, and liver; however, the role in cerebral ischemia-reperfusion injury has not been established.
Material/Methods
In this study, cerebral ischemia-reperfusion injury was established in a rat model, and the control group was a sham-operated group. After ischemia-reperfusion injury for 6, 12, and 24 hours, brain tissue specimens were collected and the expression of miR-122 and DJ-1 were determined using quantitative real-time polymerase chain reaction. Flow cytometry was used to determine the reactive oxygen species (ROS) content. The modified Neurological Severity Score (mNSS) scale was used to evaluate the sensory and motor function defects of the rats. The malondialdehyde (MDA), superoxide dismutase (SOD), and enzyme activity were determined. The rats in the cerebral ischemia-reperfusion injury model were divided into 2 groups (antagomir-NC group and antagomir miR-122 group). Brain neuron RN-c cells were divided into the following 4 groups: antagomir-NC, antagomir miR-122, pIRES2-blank, and pIRES2-DJ-1. Seventy-two hours after transfection, ischemia-reperfusion treatment was carried out and conventional cultured RN-c cells were used as the control group. Flow cytometry was used to detect apoptosis and western blot was used to detect the expression of DJ-1, PTEN, AKT, and p-AKT.
Results
The expression of miR-122 increased significantly in the process of ischemia-reperfusion damage after cerebral infarction, while the expression of DJ-1 decreased significantly. Downregulation of miR-122 significantly increased the expression of DJ-1, enhanced the activity of the PTEN/PI3K/AKT pathway, reduced cell apoptosis, and alleviated cerebral ischemia-reperfusion injury.
Conclusions
Inhibition of miR-122 can decrease cerebral ischemia-reperfusion injury by upregulating DJ-1-PTEN/PI3K/AKT pathway.
MeSH Keywords: Apoptosis, Cerebral Infarction, Reperfusion Injury
Background
Acute cerebral infarction is a common ischemic cerebrovascular disease and is one of the important causes of adult disability [1,2]. Ischemia-reperfusion injury is caused by a blood reperfusion injury in ischemic brain tissue, and usually occurs in the treatment stage of ischemic disease [3–5], which can aggravate the brain tissue injury.
Phosphonosinol-3 kinase (pPI3K)/protein kinase B (AKT/PKB) is a signaling pathway that exists widely in many tissues and cells and participates in the regulation of many important biological processes such as cell growth, survival, apoptosis, and other important biological processes. Phosphatase and tensin homologue deleted on chromosome 10 (PTEN) is a tumor suppressor gene that inhibits conduction of the PI3K/AKT signaling pathway via negative feedback and participates in regulation of ischemia-reperfusion injury [6–8]. PTEN can regulate the activity of the PI3K/AKT signaling pathway by negative feedback and is also involved in the regulation of ischemia-reperfusion damage Some studies have shown that miRNA and the PI3K/AKT signaling pathway play important roles in cerebral ischemia-reperfusion injury [9,10]. By inhibiting the expression and function of PTEN, DJ-1 enhances the transduction activity of the PI3K/AKT signaling pathway and plays a role antagonizing cell apoptosis, promoting cell survival, and proliferation [11]. Many studies have shown that DJ-1/PARK7 plays a very important role in ischemia-reperfusion injury [12]. The expression of DJ-1 in ischemia-reperfusion injury is reported to significantly decreased, while upregulating DJ-1 expression could alleviate the damage of ischemia-reperfusion injury and alleviate the apoptosis of neurons, thus having a neuroprotective effect [13–15].
MicroRNA (miRNA) is an endogenous small non-coding RNA with 22~25 nucleotides in eukaryotes. MiRNA is combined with the 3′-terminal non-translation region (3′-UTR) of mRNA of the target gene to degrade mRNA or inhibit mRNA translation, and regulate the expression of the target gene to participate in cell survival and proliferation, and regulation of various biological processes such as apoptosis, oxidative stress, and other biological processes. Moreover, miRNA plays a crucial role in the regulation of ischemia-reperfusion injury [16–18]. MiR-122 is one of the most studied miRNAs, and it is closely related to ischemia-reperfusion injury in various tissues and organs [19–21], but has not been reported in cerebral ischemia-reperfusion injury. Bioinformatics analysis has shown that there is a target complementary binding site between miR-122 and 3′-UTR of DJ-1 mRNA, indicating a target regulatory relationship. This study investigated whether or not miR-122 plays a role in regulating the DJ-1-PTEN/PI3K/AKT signaling pathway and cerebral ischemia-reperfusion injury.
Material and Methods
Materials
Healthy adult male Sprague Dawley rats (6~8 weeks; 220~240 g) were purchased from Beijing Wei Tong Li Hua Experimental Animal Technology Co., Ltd. (Beijing, China), the study was approved by the Ethics Committee of Liaocheng People’s Hospital. Rat cortical neurons (RN-c cells; #TC-axbz-207) were purchased from Shanghai Xinyu Biological Science and Technology Co., Ltd. (Shanghai, China). Dulbecco’s Modified Eagle Medium (DMEM) culture solution was purchased from Dalian Meilun Biotechnology Co., Ltd. (Shanghai China).
Establishment of cerebral artery occlusion model
The rats were given anesthesia by intraperitoneal injection of 10% chloral hydrate. After anesthesia was established, the neck skin was disinfected and cut at the midline. The right cervical artery, the external carotid artery, and the internal carotid artery were separated and exposed. The common and external carotid arteries were ligated through the line, and a reservation line was placed near the common carotid artery. The fork of the internal and external carotid arteries was clipped, and a small mouth was cut at the terminus of the internal carotid artery and inserted into the bolt line from the small mouth to the internal carotid artery slowly. The insertion depth was 18 mm, indicating that the embolus had caused a right middle cerebral artery embolism. After 2 hours, the embolus was slowly withdrawn. The blood supply of the middle cerebral artery was restored, and reperfusion injury was established as the rat brain ischemia-reperfusion damage model (I-R group). The incision was sutured, the skin was disinfected, and antibiotic prophylaxis was administered. The right common, internal, and external carotid arteries were exposed in rats in the sham operation group (sham group), but an embolus was not inserted. The 2 groups of rats were sacrificed at 6 hours, 12 hours, and 24 hours after the operation, and the brain tissues were collected. TTC (triphenyl tetrazolium chloride) staining was performed on brain tissue sections. The expression of related genes and proteins were determined, and malondialdehyde (MDA), superoxide dismutase (SOD), and catalase (CAT) levels were measured. There were 18 rats in each group for this experiment.
The brain tissues that were collected 24 hours after the operation were digested for 30 minutes with 0.1% collagenase. The tissue fragments were removed, and the cell suspension was removed after centrifugation. The cell suspension was centrifuged, and the cells were deposited. Serum-free 1640 medium was diluted by DCFH-DA (1: 1000 ratio). The cell precipitation was suspended in the diluted DCFH-DA, incubated for 30 minutes at 37oC, then washed with phosphate-buffered saline (PBS). After 500 μL PBS suspension, the reactive oxygen species (ROS) content in cells was determined using a Beckman Kurt FC500MCL flow cytometry; there were 6 rats in each group for this experiment.
Antagomir miR-122 treatment of I-R model rats
The rats in the I-R damage model were randomly divided into 2 groups. antagomir-NC group was given 50 nmol of antagomir-NC by intravenous injection before surgery, and the antagomir miR-122 group was given 50 nmol of antagomir miR-122 at 24 hours before surgery; there were 5 rats in each group for this experiment. Brain tissue was collected 24 hours after surgery from rats in each group, and the expression of protein was determined by western blot. The activity of caspase-3 was detected using a spectrophotometric method, the content of ROS was determined by flow cytometry, and the content of MDA and the activity of SOD and CAT were determined. There were 30 rats in each group for this experiment.
Behavioral score
Twenty-four hours before and after the surgery, the improved modified Neurological Severity Score (mNSS) was administered. The mNSS has a total score of 18 points and is divided into 4 parts as follows: motion, sensation, balance, and reflex. The score of normal rats was 0. The higher the score, the more severe the symptoms of neural power deficiency. There were 24 rats in each group for this experiment.
TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) staining to detect cell apoptosis
Three rat brains were removed in each group, and serial coronal sections were cut on a cryostat and mounted on coverslips. The tissue sections were dewaxed before being dehydrated with ethanol. Sections were permeabilized with proteinase K solution for 20 minutes. The TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) reaction solution and compound solution were added and staining with DAB (3,3-diaminobenzidine) was done. Finally, the tissue sections were stained with hematoxylin and observed after sealing. Five fields were randomly selected, and the cells with brown color were considered apoptotic positive cells. Brown positive cells were detected in each field, and the number was calculated and divided by the total number of cells; the average was the cell apoptosis rate.
Detection of caspase-3 enzyme activity in brain tissues
According to the kit instructions, the steps were as follows: the standard products of peptide nucleic acids (pNA) standard products were diluted by concentration gradient; the standard products with concentration of 200 μM, 100 μM, 50 μM, 25 μM, 12.5 μM, 6.25 μM, and 0 μM were prepared; the absorbance value at 405 nm was A405; and a standard curve was made. Caspase lysis buffer was used to cleave the brain tissue in an ice bath, tissue was homogenized with a glass homogenizer, transferred to a 1.5-mL centrifuge tube, cleaved with caspase lysis buffer for 5~10 minutes on the ice, centrifuged at 12 000g for 10~15 minutes at 4°C, and the supernatant was transferred to a new 1.5-mL centrifuge tube. After adding 65 μL of assay buffer, 25 μL of lysate supernatant and 10 μL of Ac-DEVD-pNA (2 mM) were added into the 96-well plate, incubated 60~120 minutes at 37°C, and the A405 was detected immediately by the color change without magnification, and the relative enzyme activity units were calculated as follows: experimental group A405/control group A405×100%.
Detection of MDA and the antioxidant index
MDA is the final product of lipid peroxidation, and the level of lipid peroxidation was evaluated by measuring the content of MDA. The content of MDA in the cerebrospinal fluid of rats was determined in strict accordance with the instructions of the MDA detection kit. The levels of SOD and CAT in cerebrospinal fluid were determined by the kit, and the oxidative stress status was evaluated.
Ischemia-reperfusion injury treatment of RN-c cells
Ischemia-reperfusion injury treatment was as follows: DMEM culture medium with a low glucose serum-free model was used to simulate the ischemic environment in vivo. The hypoxic environment was simulated in the incubator with a volume fraction of 5% CO2 and 95% N2. After 12 hours, the normal serum medium was replaced, cultured in a 5% CO2 and 95% air incubator for 12 hours, and the related indices were determined.
RN-c cells in the logarithmic phase were divided into the following 4 groups: antagomir-NC transfection, antagomir miR-122 transfection, pIRES2-blank transfection, and pIRES2–DJ-1 transfection. Seventy-two hours after transfection, ischemia-reperfusion injury treatment was carried out according to the aforementioned methods; the conventional cultured RN-c cells were used as a control. Cells were used to determine gene and protein expression and apoptosis.
Flow cytometry to detect cell apoptosis
The cell suspension was digested in trypsin, the cells were washed in pre-cooled PBS, and centrifuged at 300 g for 5 minutes. Binding buffer (500 μL) was added to the suspension of cells, then 5 μL of Annexin V-FITC was added while avoiding light and incubated for 15 minutes at room temperature before adding 5 μL of propidium iodide (PI) stain, and immediately using a FC500MCL flow cytometer for detection.
Detection of intracellular ROS by flow cytometry
The cells were collected by trypsin digestion. A 0.1% DCFH-DA probe was diluted with 500 μL of serum-free medium and incubated with the cell preparation at 37°C for 20 minutes. The cell preparation and ROS probe were evenly and fully exposed 3~5 times, the serum-free culture medium was used to wash the cells, and DCFH-DA was removed from the cells. FC500MCL flow cytometry was used for detection.
Luciferase reporter gene experiment
The RNA of HEK293T cells was used as a template to amplify the fragment of the target binding site in the 3′-UTR of DJ-1 mRNA or its mutant fragment. After polymerase chain reaction (PCR) amplification, PCR products and pMIR empty vector plasmid were cut by Sac I and Hind III double enzymes. The enzyme digestion conditions were 37°C for 4 hours. A 1.5% agarose gel electrophoresis was used to extract and purify the product. The purified PCR product was incubated with the carrier at 16°C, overnight. The linked products were converted to DH5α receptive cells and incubated at 37°C for overnight in medium containing penicillin, and single positive clones were selected. After shaking in the Escherichia coli culture solution overnight at 37°C, the plasmid was extracted and sequenced, and identified as pMIR-DJ-1–WT and pMIR-DJ-1–MUT.
HEK-293T cells (1×105) were inoculated in 24-well plates. After 24 hours, 100 ng of pMIR-DJ-1-WT (or pMIR-DJ-1-MUT) and 50 nmol miR-122 mimic (or miR-NC) were transfected to HEK293T cells by Lipo 2000 and incubation continued for 48 hours. A Dual-Glo Luciferase Assay System kit was used to detect the activity of double luciferase. The experimental procedure was as follows: 100 μL of Passive Lysis Buffer was added to the cell lysate per well in 24-well plates; the plates were slowly shaken for 15 minutes at room temperature; 20 μL of cell lysate was added to 100 μL of luciferase detection reagent II (LAR II) and mixed; fluoreli luminescence detected fluoro activity; then, the luciferase activity of sea kidney was detected with 100 μL of sea kidney fluorescein reagent; and firefly luciferase activity/sea kidney luciferase activity as a relative activity value was analyzed statistically.
Quantitative real-time PCR
Tissues (50 mg) or cells (1×107) were trypsinized and lysed with 1 mL TRIzol. After lysis, 200 μL of chloroform was added to the cleavage product, which was placed at room temperature after 20 minutes. RNA was transferred to the new Eppendorf (EP) tube, and 1 mL of isopropanol was added to the RNA and centrifuged at 10 000 g for 10 minutes. One milliliter of 75% ethanol was used to wash RNA, then centrifuged at 10 000g for 10 minutes, and dissolved in 50 μL of DEPC water to obtain RNA. A PrimeScript RT Reagent Kit (R&D Systems, MN, USA) was used to generate RNA by reverse transcriptase cDNA. The PCR system (10 μL) included 2.0 μL of PrimeScript Buffer, 0.5 μL of PrimeScript RT Enzyme, 0.5 μL of Oligo dT Primer (50 μM), 0.5 μL of random 6 mers (100 μM), 500 ng of RNA, and ddH2O to a 10-μL volume. The reverse transcription conditions were as follows: initial denaturation at 95°C for 5 minutes; and 40 cycles of 95°C for 15 second and 60°C for 1 minute. The data were preserved.
Western blot analysis
Tissues (50 mg) or cells (1×106) were trypsinized and lysed in 100 μL of RIPA (radioimmunoprecipitation assay), then 100 mu LRIPA lysate was added to every 50 mg or 1×106 cells. After lysis for 50 minutes on ice, the mixture was centrifuged at 10 000 g and 4°C for 15 minutes. The supernatant was transferred to a new EP tube, and 40 μg was separated on 10% sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gel electrophoresis (50V for 4 hours), transferred to Polyvinylidene difluoride (PVDF) membranes (300 mA for 90 minutes), and blocked with 5% non-fat milk at room temperature for 60 minutes. Then, the membranes were incubated with primary antibodies (DJ-1, PTEN, AKT, p-AKT, and beta -actin [1: 1000, 1: 1000, 1: 1000, 1: 500, and 1: 10000, respectively]) at 4°C overnight. After rinsing 3 times with TBST (Tris buffered saline with Tween 2) buffer, the membranes were incubated with secondary antibody at a 1: 15 000 dilution at room temperature for 60 minutes. After rinsing 3 times with TBST buffer, the membrane was developed with an enhanced chemiluminescence detection kit (ECL).
Statistical analysis
The statistical analysis of data was carried out with SPSS 18.0, and the measurement data are expressed as the mean ± standard deviation. Student’s t-test was used to compare the difference between 2 groups. The comparison of the data between the multiple groups adopted one-way analysis of variance (ANOVA), then the 2 groups were compared using the Bonferroni method. A value of P<0.05 was considered statistically significant.
Results
Rats in the I-R group showed more area of cerebral infarction
Before establishing the model, the mNSS scores of the rats in the 2 groups were 0. After the operation, the rats in the sham group had no symptoms of a nerve function defect, and the mNSS scores were 0, while the rats in the I-R group had different symptoms of nerve function defect 24 hours after reperfusion, including hemiplegia, instability upon standing, and falling or circling. When the rat’s tail was lifted, the contralateral forelimb of the rat was flexed and the head was raised to the affected side, and the mNSS score of 12.56±3.28 indicated the success of the model.
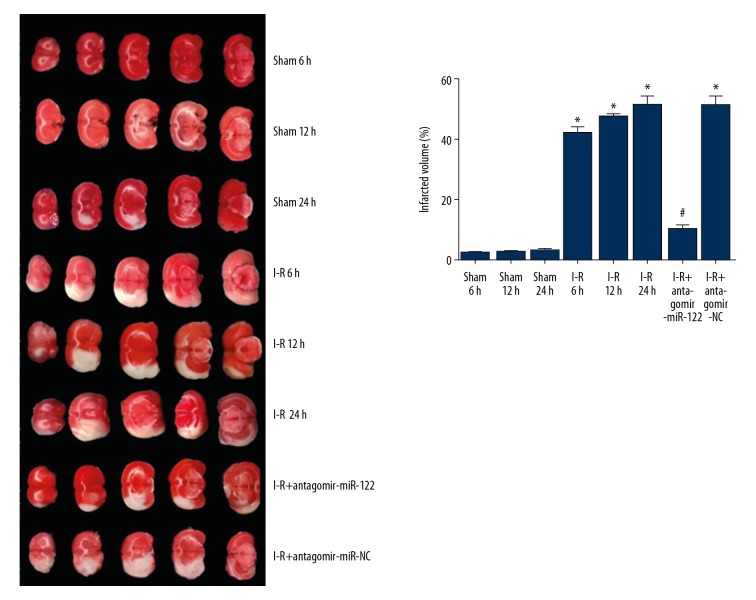
TTC staining showed that no obvious cerebral infarction in sham group, the cerebral infarction area of rats in the I-R group was significantly larger than that in the sham group. Compared with the antagomir-NC group, the cerebral infarction area of the antagomir mir-122 injection group was significantly reduced, and there was no significant difference between the 2 groups (Figure 1).
Figure 1.
Cerebral tissue TTC staining in each group showed infarct area. * P<0.05 compared with the sham group; # P<0.05 compared with the I-R group. Each experiment was repeated 3 times. TTC – triphenyl tetrazolium chloride; I-R group – ischemia-reperfusion damage model.
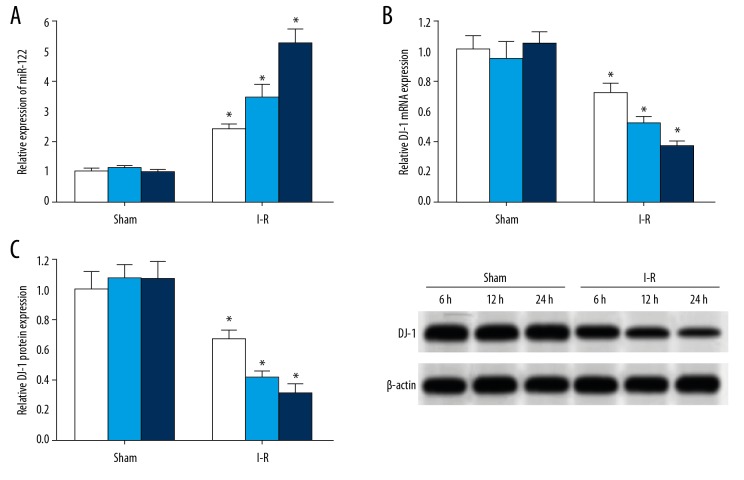
Expression of miR-122 in brain tissues of rats in the I-R group increased significantly, and the expression of DJ-1 decreased significantly
The qRT-PCR showed that the expression of miR-122 in the brain tissue of rats in the I-R group was significantly higher than the sham group. Moreover, the expression of miR-122 increased with the prolongation of ischemia-reperfusion injury, while the expression of DJ-1 mRNA decreased significantly, and the amount of DJ-1 mRNA was also lower in rats with a prolonged ischemia-reperfusion injury (Figure 2A, 2B). Similarly, western blot analysis showed that the expression of DJ-1 protein in 6-hour, 12-hour, and 24-hour brain tissue samples of the rats in the I-R group was significantly higher than the sham group, and the longer the ischemia-reperfusion injury time, the lower the expression of DJ-1 protein (Figure 2C).
Figure 2.
There is a target regulatory relationship between miR-122 and DJ-1. (A) Schematic diagram of interaction sites between miR-122 and the 3′-UTR of DJ-1 mRNA. (B) There was a target complementary binding site between miR-122 and the 3′-UTR of DJ-1 mRNA. (C) Double luciferase gene report. White represents miR-NC and light blue represents miR-122 mimics. * P<0.05 compared with the miR-NC group (white bar is miR-NC and dark blue bar is miR-122 mimic). Each experiment was repeated 3 times. The results of the double luciferase gene report showed that the transfection of miR-122 mimic significantly reduced the relative luciferase activity in the HEK293T cells transfected by pMIR-DJ-1-WT, but there was no significant effect on the relative luciferase activity in the HEK293T cells transfected by pMIR-DJ-1-MUT, indicating that there was a targeting regulation between miR-122 and DJ-1.
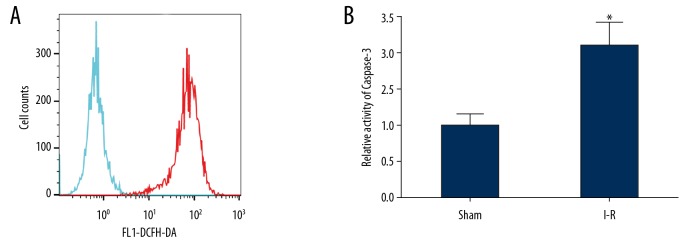
The oxidative stress of brain tissue and the activity of caspase-3 increased in the rats in the I-R group
The flow cytometry results showed that compared with the sham group, the ROS content in brain tissue of the rats in the I-R group increased significantly (Figure 3A). The level of lipid peroxidation testing showed that compared with the sham group, the content of MDA in the brain tissues of the rats in the I-R group increased significantly (P<0.001). The test results of the antioxidant index showed that the content of the antioxidant enzymes, SOD, and CAT in the brain tissues of the rats in the I-R injury model were significantly lower than the sham group (P<0.001; Table 1). The results of spectrophotometry showed that compared with the sham group, the activity of caspase-3 in the brain tissues of rats in the I-R group increased significantly (Figure 3B).
Figure 3.
The oxidative stress in the brain tissues of rats in the I-R group was apparent, and the activity of caspase-3 was significantly increased. (A) Flow cytometry was used to detect ROS content in the 2 groups of rats. (Green line is sham group and red line is I-R group) (B) The caspase-3 enzyme activity in the 2 groups of brain tissues was detected by spectrophotometry. * P<0.05 compared with the sham group. Each experiment was repeated 3 times. Compared with the sham group, the ROS content in brain tissue of the I-R group increased significantly (A). The results of spectrophotometry showed that compared with the sham group, the activity of caspase-3 in the brain tissues of I-R group increased significantly (B). I-R group – ischemia-reperfusion damage model; ROS – reactive oxygen species.
Table 1.
Detection results of MDA and antioxidant capacity in rats brain tissues in the 2 groups.
| Index | Sham group (n=5) | I-R group (n=5) | t Test | P |
|---|---|---|---|---|
| MDA (nmol/mg protein) | 18.16±3.57 | 42.81±5.66 | 8.237 | <0.001 |
| SOD (U/mg protein) | 78.63±8.15 | 41.29±4.93 | 8.766 | <0.001 |
| CAT (U/g protein) | 26.59±3.46 | 12.61±2.57 | 7.253 | <0.001 |
MDA – malondialdehyde.
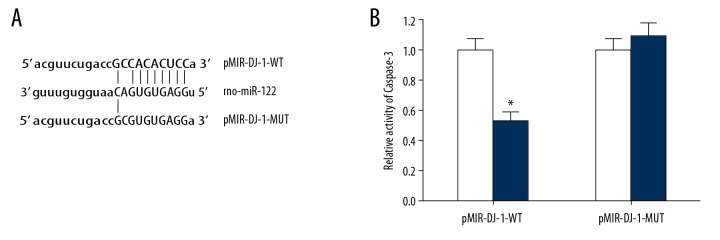
MiR-122 targeting 3′-UTR region of DJ-1mRNA
The online prediction results of microRNA.org showed that there was a target complementary binding site between miR-122 and the 3′-UTR of DJ-1 mRNA (Figure 4A). The results of the double luciferase gene report showed that the transfection of miR-122 mimic significantly reduced the relative luciferase activity in the HEK293T cells transfected by pMIR-DJ-1-WT, but there was no significant effect on the relative luciferase activity in the HEK293T cells transfected by pMIR-DJ-1-MUT (Figure 4B), indicating that there was a targeting regulation between miR-122 and DJ-1.
Figure 4.
There is a target regulatory relationship between miR-122 and DJ-1. (A) Schematic diagram of interaction sites between miR-122 and the 3′-UTR of DJ-1 mRNA. (B) There was a target complementary binding site between miR-122 and the 3′-UTR of DJ-1 mRNA. Double luciferase gene report. White represents miR-NC and deep blue represents miR-122 mimics. * P<0.05 compared with the miR-NC group (white bar is miR-NC and deep blue bar is miR-122mimic) Each experiment was repeated three times. The results of the double luciferase gene report showed that the transfection of miR-122 mimic significantly reduced the relative luciferase activity in the HEK293T cells transfected by pMIR-DJ-1-WT, but there was no significant effect on the relative luciferase activity in the HEK293T cells transfected by pMIR-DJ-1-MUT, indicating that there was a targeting regulation between miR-122 and DJ-1.
MiR-122 significantly reduced DJ-1 expression and alleviated I-R-induced brain injury
The results of oxidation and antioxidant indicators showed that compared with the antagomir-NC group, the content of MDA in the brain tissues of the antagomir miR-122 group decreased significantly, while the activity of SOD and CAT increased significantly (Table 2). The results showed that injection of antagomir miR-122 significantly reduced the neurologic deficits and reduced the mNSS score in rats (Table 2).
Table 2.
Detection of 24-hour MDA, SOD, CAT, mNSS after ischemia-reperfusion injury in rats.
| Grouping | MDA (nmol/mg protein) | SOD (U/mg protein) | CAT (U/g protein) | mNSS |
|---|---|---|---|---|
| Antagomir-NC | 39.57±4.86 | 45.13±6.28 | 15.79±3.21 | 11.92±3.51 |
| Antagomir miR-122 | 28.77±3.96* | 61.39±7.83* | 21.86±3.78* | 7.53±2.16* |
P<0.05 compared with the antagomir-NC group.
MDA – malondialdehyde; SOD – superoxide dismutase; CAT – catalase; mNSS – modified Neurological Severity Score.
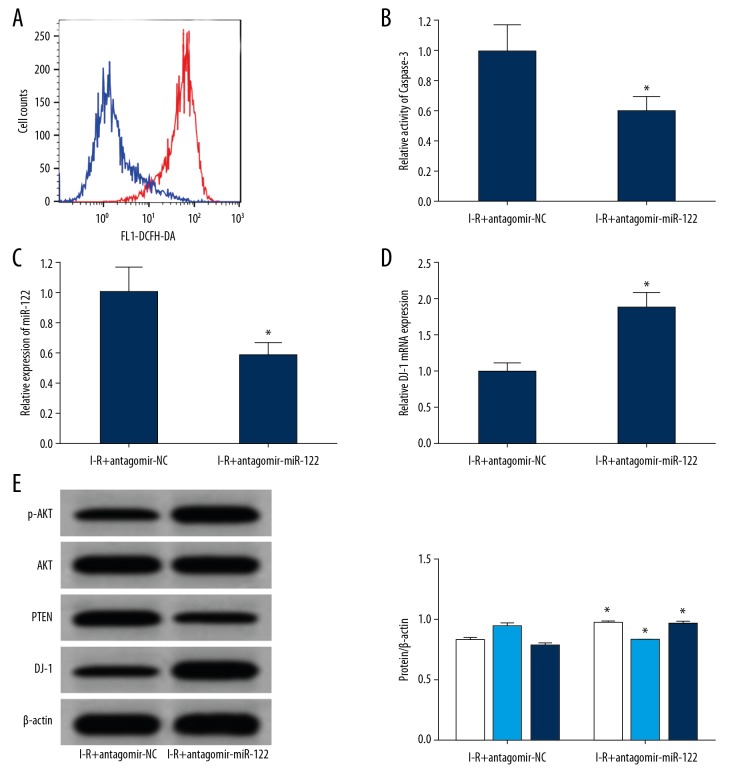
The results of flow testing showed that compared with the antagomir-NC group, the content of ROS in the brain tissues of the antagomir miR-122 group was significantly decreased (Figure 5A), and the activity of caspase-3 in the brain tissues of the rats decreased significantly (Figure 5B). The results of qRT-PCR showed that the expression of miR-122 in the brain tissues of the antagomir miR-122 group was significantly lower than the antagomir-NC group (Figure 5C), while the expression of DJ-1 mRNA increased significantly (Figure 5D). The results of western blot analysis showed that the expression of DJ-1 and p-AKT in the brain tissues of the antagomir miR-122 group was significantly higher than the antagomir-NC group, while the expression of PTEN protein decreased significantly (Figure 5E).
Figure 5.
MiR-122 significantly reduced DJ-1 expression and alleviates ischemia-reperfusion induced brain injury. (A) Flow cytometry detection of ROS content in 2 groups of rats (the bnlue line is I-R+antagomir NC and the red line is I-R+antagomir miR-122) (B) Spectrophotometry detection the caspase-3 enzyme activity in the 2 groups of brain tissues. (C) qRT-PCR detected the expression of miR-122 in rat brains. (D) qRT-PCR detected the expression of DJ-1 mRNA in rat brains. (E) Western blot detected protein expression in brain tissues (the white represents p-Akt/Akt, the light blue line represents PTEN, and the dark blue line represents DJ-1). * P<0.05 compared with the agomir control group. Each experiment was repeated 3 times. Flow testing showed that compared with the antagomir-NC group, the content of ROS in the brain tissues of the antagomir miR-122 group was significantly decreased (A), and the activity of caspase-3 in the brain tissues of the rats decreased significantly (B). The results of qRT-PCR showed that the expression of miR-122 in the brain tissues of the antagomir miR-122 group was significantly lower than the antagomir-NC group (C), while the expression of DJ-1 mRNA increased significantly (D). The results of western blot analysis showed that the expression of DJ-1 and p-AKT in the brain tissues of the antagomir miR-122 group was significantly higher than the antagomir-NC group, while the expression of PTEN protein decreased significantly (E). I-R group – ischemia-reperfusion damage model; ROS – reactive oxygen species; qRT-PCR – quantitative real-time polymerase chain reaction.
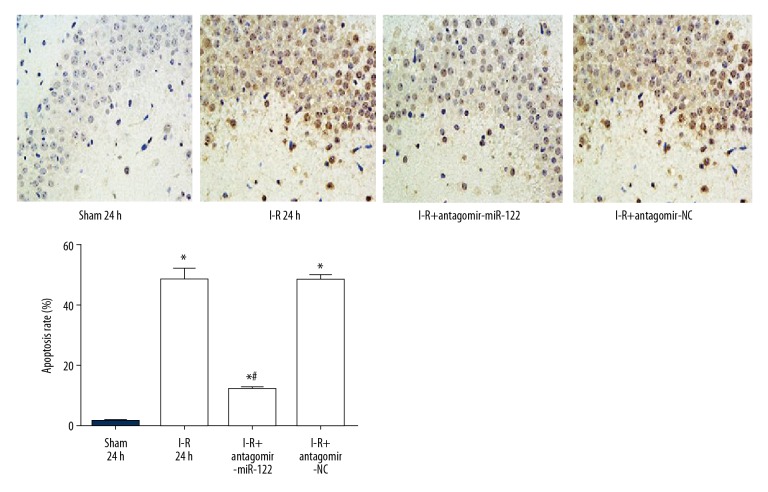
Compared to the I-R group and antagomir-NC groups, TUNEL staining showed that the apoptosis rate of brain tissue neurons declined significantly more in the antagomir mir-122 injection group (Figure 6).
Figure 6.
MiR-122 significantly reduced neuron apoptosis and alleviates ischemia-reperfusion induced brain injury. * P<0.05 compared with the sham group; # P<0.05 compared with the I-R group. Each experiment was repeated 3 times.
Downregulation of miR-122 or an increase in DJ-1 attenuated ischemia-reperfusion injury-induced apoptosis in RN-c cells
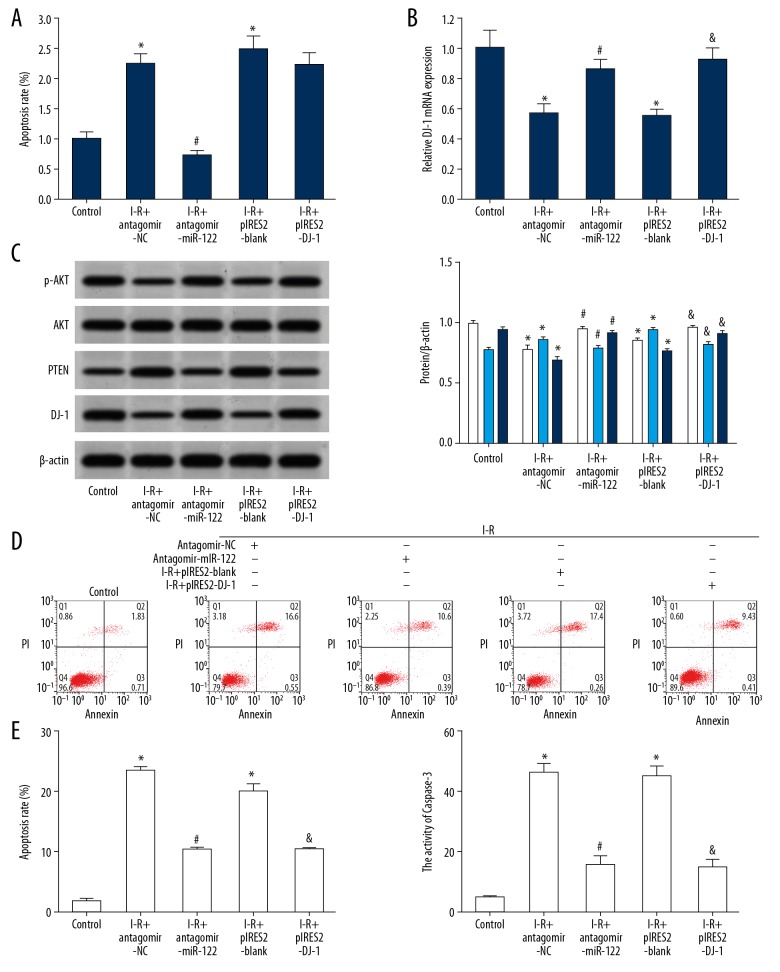
The results of qRT-PCR showed that compared with the control group, ischemia-reperfusion injury treatment significantly increased the expression of miR-122 in RN-c cells (Figure 7A), and the expression of DJ-1 mRNA was decreased significantly (Figure 7B). Western blot analysis showed that compared with the control group, ischemia-reperfusion injury treatment reduced the expression of DJ-1 protein in RN-c cells (Figure 7C). Flow cytometry results showed that compared with the control group, ischemia-reperfusion injury treatment significantly increased apoptosis of RN-c cells (Figure 7D). Compared with the control group, the caspase-3 activity of ischemia-reperfusion injury treatment significantly increased in RN-c cells (Figure 7E). The expression of DJ-1 in RN-c cells was obviously upregulated by transfection of antagomir miR-122 and pIRES2-DJ-1, which significantly reduced the expression of PTEN protein, increased the expression of p-AKT protein, reduced apoptosis of the cells, and weakened the cell damage induced by ischemia-reperfusion injury.
Figure 7.
Downregulation of miR-122 or an increase in DJ-1 attenuated ischemia-reperfusion induced apoptosis in RN-c cells. (A) qRT-PCR) detected the expression of miR-122; (B) qRT-PCR detected the expression of DJ-1 mRNA; (C) Western blot detected protein expression; (White represents p-Akt/Akt, light grey represents PTEN, and dark grey represents DJ-1.) (D) Flow cytometry detected cell apoptosis. (E) The activity of caspase-3 was detected. * P<0.05 compared with the control group. # P<0.05, I-R+antagomir miR-122 compared with the I-R+antagomir-NC; & P<0.05, I-R+pIRES2–DJ-1 compared with the I-R+pIRES2-blank. Each experiment was repeated 3 times. The results of qRT-PCR showed that compared with the control group, I ischemia-reperfusion treatment significantly increased the expression of miR-122 in RN-c cells (A), and the expression of DJ-1 mRNA was decreased significantly (B). Western blot analysis showed that compared with the control group, I ischemia-reperfusion treatment reduced the expression of DJ-1 protein in RN-c cells (C). Flow cytometry results showed that compared with the control group, ischemia-reperfusion treatment significantly increased apoptosis of RN-c cells (D). Compared with the control group, the caspase-3 activity of ischemia-reperfusion treatment significantly increased in RN-c cells (E). qRT-PCR – quantitative real-time polymerase chain reaction.
Discussion
Acute cerebral infarction has high mortality rate, morbidity, and disability rate. Acute cerebral infarction seriously threatens the life, health, and quality of life of patients, and also can cause serious economic burden to the family and society [1,2]. Ischemia-reperfusion injury is the injury of tissues and organs caused by ischemia, hypoxia, and oxygen recovery after a period of ischemia and hypoxia. Ischemia-reperfusion injury can cause damage of tissue and organ function, and even cause irreversible structural changes. The pathogenesis of ischemia-reperfusion injury is extremely complex and is not fully understood. Many mechanisms, such as inflammatory reactions, apoptosis and necrosis, oxidative stress, and autophagy, are involved in the occurrence of ischemia-reperfusion injury [22–26].
Many studies have shown that PI3K/AKT signaling pathway plays an important role in reducing oxidative damage, antagonizing cell apoptosis, promoting cell survival, and alleviating ischemia-reperfusion damage [27–29]. PTEN is the only tumor suppressor gene that has been reported to have the double activity of protein esterase and phosphatase. PTEN can regulate the activity of the PI3K/AKT signaling pathway by negative feedback and is also involved in the regulation of ischemia-reperfusion damage [7,8]. The DJ-1/PARK7 gene (Parkinson gene 7) is located on human chromosome 1p36.2–36.3 and the entire length of the gene is approximately 24 kb, which encodes a protein composed of 189 amino acids and a molecular weight of 21kD [11]. DJ-1 is a negative regulator of PTEN, which plays an important role in inhibiting PTEN expression and function and enhancing the transduction activity of the PI3K/AKT signaling pathway. MiR-122 is a widely studied miRNA, which is closely related to ischemia-reperfusion injury of various tissues and organs, such as heart [19], lung [20], and liver [21]; however, a study involving ischemia-reperfusion injury in brain tissues has not been reported. Bioinformatics analysis has shown that there is a target complementary binding site between miR-122 and the 3′-UTR of DJ-1 mRNA, indicating a target regulatory relationship. This study was conducted to investigate whether or not miR-122 plays a role in regulating the DJ-1-PTEN/PI3K/AKT signaling pathway and cerebral ischemia-reperfusion injury.
The results of the double luciferase gene report showed that the transfection of miR-122 mimic significantly reduced relative luciferase activity in the HEK293T cells transfected by pMIR-DJ-1-WT, but had no obvious effect on the relative luciferase activity in the HEK293T cells transfected by pMIR-DJ-1-MUT, indicating that there is a targeting regulation relationship between miR-122 and DJ-1. The results of brain tissue detection in the rat model showed that after ischemia-reperfusion injury, the expression of miR-122 in the brain tissue of rats increased, while the expression of DJ-1 gradually decreased, suggesting that the increase in miR-122 expression may play a role in reducing the expression of DJ-1 and regulating the process of cerebral ischemia-reperfusion injury. Increased expression of miR-122 was associated with brain tissue ischemia-reperfusion injury. In the study of the relationship between miR-122 and ischemia-reperfusion damage, Akbari et al. [30] showed that the expression of miR-122 in the serum of rats in the I-R model group was up to 50 times higher than the sham group. Farid et al. [31] showed that the expression of miR-122 in the serum of patients with ischemia-reperfusion injury caused by liver transplantation was 10~100 times higher than the control group. Moreover, the more serious the liver injury, the higher the expression of miR-122. The results of Van Caster et al. [21] showed that the expression of miR-122 in the serum of the liver rat model was significantly higher than the sham operation group (up to 100 times higher than the sham group), and miR-122 expression was positively correlated with the liver function index (AST), after the 4 hours of the liver ischemia-reperfusion damage model was made. Leelahavanichkul et al. [32] also observed that the expression of miR-122 increased significantly during the process of liver ischemia-reperfusion injury. The results of this study showed that the abnormal increase in miR-122 expression may be involved in cerebral ischemia-reperfusion injury, similar to the results of other studies [17,30].
The results of Akbaris et al. [30] showed that the pre-treatment of saffron (Crocin) significantly reduced the expression of miR-122 in the serum of liver ischemia-reperfusion injury model rats enhanced the antioxidant capacity of liver tissues, reduced the liver ischemia-reperfusion injury and improved liver function. The results suggest that inhibition of miR-122 expression may be a target molecule for reducing ischemia-reperfusion injury in the liver. So, can inhibition of miR-122 expression also play a role in alleviating ischemia-reperfusion damage in brain tissue? Therefore, the aim of this study was to inject antagomir miR-122 into rats before the I-R model was established, so as to achieve the goal of downregulating the expression of miR-122 after ischemia-reperfusion injury. The results showed that after the injection of antagomir miR-122, the expression of DJ-1 in the brain tissue of rats in the I-R group increased, the expression of PTEN was reduced, the activity of PI3K/AKT pathway was enhanced, the content of ROS and MDA in the brain tissue was reduced and the activity of antioxidase was increased, and the symptoms of the function defect was improved. The results of this study show that the increase in miR-122 expression can play a role in reducing the expression of DJ-1, inhibiting the activity of PI3K/AKT pathway and enhancing the ischemia-reperfusion damage in brain tissue, and miR-122 is a therapeutic target for ischemia-reperfusion injury in brain tissue, which is in agreement with the results of another study [30]. In addition, the results of Mard et al. [33] showed that crocin and zinc sulfate inhibit the abnormal expression of miR-122 during the liver ischemia-reperfusion injury, enhance the antioxidant capacity, reduce the liver damage and lesions, and protect the liver ischemia-reperfusion injury. Xiao et al. [30] showed that low temperature pre-treatment can downregulate the expression of miR-122, activate the IGF-1R/AKT pathway, inhibit FOXO3a activity and caspase-3 expression, reduce ischemia-reperfusion injury in liver cells, while increased the expression of miR-122 can attenuate the protective effect of low temperature pretreatment on liver ischemia-reperfusion injury. and increase miR-122 expression. The protective effect of low temperature pre-treatment on liver ischemia-reperfusion injury can be attenuated. The results of this study are also supported by research results of other studies [33,34].
In the process of cerebral ischemia-reperfusion injury, in addition to the abnormal increase of miR-122 expression, the decrease in DJ-1 expression is also an important molecular event. In the study of the relationship between DJ-1 and cerebral ischemia-reperfusion injury, the results of Ruan et al. [35] showed that compared with the sham group, the ischemia-reperfusion damage of the brain tissues of the MCAO model group was apparent, the neurologic function score was decreased, and the expression of DJ-1 in the brain tissue of the mice with ischemia-reperfusion damage was significantly reduced. Yang et al. [36] showed that the expression of DJ-1 in brain tissues of ischemia-reperfusion injury mice was significantly reduced. Moreover, Yanagida et al. [37] showed that the expression of DJ-1 increased the sensitivity of nerve cells to oxidative stress, and the increased expression of DJ-1 reduced the sensitivity of nerve cells to oxidative stress and increased cell vitality. In this study, we observed that the expression of DJ-1 in brain tissue was significantly reduced during ischemia-reperfusion injury, which is similar to the observed phenomena and aforementioned results. To further investigate whether or not the decrease in DJ-1 expression is a promoter of ischemia-reperfusion damage in brain neurons, DJ-1 was overexpressed in neuron cells cultured in vitro and observed whether or not the ischemia-reperfusion damage of neuron cells was reduced. The results showed that over-expression of DJ-1 in RN-c cells significantly reduced ischemia-reperfusion injury-induced apoptosis. Tajiri et al. [38] showed that treatment with cyclosporine A (CSA) increased the expression of DJ-1 in cells, protected the mitochondrial function of rat neural cells and reduced reperfusion injury. Yang et al. [36] showed that treatment with sodium phenylbutyrate (SPB), an agonist, increased the expression of DJ-1 in cultured neurons, increased cell viability, reduced apoptosis, and reduced the neuronal damage induced by ischemia-reperfusion injury. Ruan et al. [35] showed that treatment with tetrandrine significantly increased the expression of DJ-1 in the brain tissues of mice with ischemia-reperfusion damage, reduced the brain ischemia-reperfusion damage, and improved nerve function. These studies have confirmed that the decreased DJ-1 expression is the promoter of cerebral ischemia-reperfusion injury, and the expression of DJ-1 plays a role in protecting the brain cells and may be a protective target molecule for the ischemia-reperfusion damage of brain nerve cells.
At present, the relationship between miR-122 and ischemia-reperfusion injury is mostly concentrated in the liver. This study showed that increased expression of miR-122 may be a contributing factor to ischemia-reperfusion injury after cerebral infarction. However, some studies reported that serum miR-122 level decreased in patients with cerebral infarction [38]. The expression of miR-122 decreased in rats with ischemia-reperfusion injury [39]. While the expression of miR-122 in hepatic and cardiac ischemia-reperfusion injury was abnormally increased [21,30–32]. Therefore, whether miR-122 has bidirectional regulation in ischemia-reperfusion injury needs further research. This study combined the relationship between miR-122 and target DJ-1. While miR-122 is elevated, the expression of DJ-1 is decreased. By reducing the expression of miR-122, it can increase the expression of DJ-1, thereby increasing the protective effect of brain tissue ischemia-reperfusion injury. It is revealed that inhibition of miR-122 can attenuate cerebral ischemia-reperfusion injury by upregulating DJ-1-PTEN/PI3K/AKT pathway, which is the innovation of this research.
Conclusions
The expression of miR-122 increased significantly in the process of ischemia-reperfusion damage after cerebral infarction, while the expression of DJ-1 decreased significantly. Downregulation of miR-122 significantly increased the expression of DJ-1, enhanced the activity of the PTEN/PI3K/AKT pathway, reduced cell apoptosis, and alleviated cerebral ischemia-reperfusion injury. Inhibition of miR-122 can decrease cerebral ischemia-reperfusion injury by upregulating DJ-1-PTEN/PI3K/AKT.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Hsu CC, Kwan GNC, Hapugoda S, et al. Susceptibility weighted imaging in acute cerebral ischemia: Review of emerging technical concepts and clinical applications. Neuroradiol J. 2017;30(2):109–19. doi: 10.1177/1971400917690166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo WL, Mao YR, Li L, et al. Prospective clinical study of rehabilitation interventions with multisensory interactive training in patients with cerebral infarction: Study protocol for a randomised controlled trial. Trials. 2017;18(1):173. doi: 10.1186/s13063-017-1874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obadia N, Lessa MA, Daliry A, et al. Cerebral microvascular dysfunction in metabolic syndrome is exacerbated by ischemia-reperfusion injury. BMC Neurosci. 2017;18(1):67. doi: 10.1186/s12868-017-0384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuluaga M, Gueguen V, Letourneur D, Pavon-Djavid G. Astaxanthin-antioxidant impact on excessive reactive oxygen species generation induced by ischemia and reperfusion injury. Chem Biol Interact. 2018;279:145–58. doi: 10.1016/j.cbi.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Yu W, Gao D, Jin W, et al. Propofol prevents oxidative stress by decreasing the ischemic accumulation of succinate in focal cerebral ischemia-reperfusion injury. Neurochem Res. 2018;43(2):420–29. doi: 10.1007/s11064-017-2437-z. [DOI] [PubMed] [Google Scholar]
- 6.Oudit GY, Sun H, Kerfant BG, et al. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37(2):449–71. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Ke ZP, Xu P, Shi Y, Gao AM. MicroRNA-93 inhibits ischemia-reperfusion induced cardiomyocyte apoptosis by targeting PTEN. Oncotarget. 2016;7(20):28796–805. doi: 10.18632/oncotarget.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li CM, Shen SW, Wang T, Zhang XH. Myocardial ischemic post-conditioning attenuates ischemia reperfusion injury via PTEN/Akt signal pathway. Int J Clin Exp Med. 2015;8(9):15801–7. [PMC free article] [PubMed] [Google Scholar]
- 9.Muniz LRF, Faria MHG, Vasconcelos PRLD. Metabolic evaluation of cerebral ischemia and reperfusion injuries after bilateral carotid artery occlusion: Experimental study in rats. Acta Cirurgica Brasileira. 2004;19(5):529–34. [Google Scholar]
- 10.Li J, Wang Z, Sun Q, Du Y. Expression of Tau protein in rats with cognitive dysfunction induced by cerebral hypoperfusion. Int J Clin Exp Med. 2015;8(10):19682–88. [PMC free article] [PubMed] [Google Scholar]
- 11.Kim RH, Peters M, Jang Y, et al. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005;7(3):263–73. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Kitamura Y, Yanagida T, Takata K, et al. Neuroprotective effect of DJ-1 (park7) on focal cerebral ischemia and reperfusion in rats. Journal of Physiological Sciences. 2009;59:344. [Google Scholar]
- 13.Kwon WY, Suh GJ, Kim KS, et al. Niacin and selenium attenuate brain injury after cardiac arrest in rats by up-regulating DJ-1-Akt signaling. Crit Care Med. 2018;46(8):E788–96. doi: 10.1097/CCM.0000000000003198. [DOI] [PubMed] [Google Scholar]
- 14.Yang RX, Lei J, Wang BD, et al. Pretreatment with sodium phenylbutyrate alleviates cerebral ischemia/reperfusion injury by upregulating DJ-1 protein. Front Neurol. 2017;8:256. doi: 10.3389/fneur.2017.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdel-Aleem GA, Khaleel EF, Mostafa DG, Elberier LK. Neuroprotective effect of resveratrol against brain ischemia reperfusion injury in rats entails reduction of DJ-1 protein expression and activation of PI3K/Akt/GSK3b survival pathway. Arch Physiol Biochem. 2016;122(4):200–13. doi: 10.1080/13813455.2016.1182190. [DOI] [PubMed] [Google Scholar]
- 16.Meng ZY, Kang HL, Duan W, et al. MicroRNA-210 promotes accumulation of neural precursor cells around ischemic foci after cerebral ischemia by regulating the SOCS1-STAT3-VEGF-C pathway. J Am Heart Assoc. 2018;7(5) doi: 10.1161/JAHA.116.005052. pii: e005052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C, Zhang C, Yang J, et al. Screening circular RNA expression patterns following focal cerebral ischemia in mice. Oncotarget. 2017;8(49):86535–47. doi: 10.18632/oncotarget.21238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta SL, Pandi G, Vemuganti R. Circular RNA expression profiles alter significantly in mouse brain after transient focal ischemia. Stroke. 2017;48(9):2541–48. doi: 10.1161/STROKEAHA.117.017469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersson P, Gidlof O, Braun OO, et al. Plasma levels of liver-specific miR-122 is massively increased in a porcine cardiogenic shock model and attenuated by hypothermia. Shock. 2012;37(2):234–38. doi: 10.1097/SHK.0b013e31823f1811. [DOI] [PubMed] [Google Scholar]
- 20.Rancan L, Simon C, Marchal-Duval E, et al. Lidocaine administration controls microRNAs alterations observed after lung ischemia-reperfusion injury. Anesth Analg. 2016;123(6):1437–47. doi: 10.1213/ANE.0000000000001633. [DOI] [PubMed] [Google Scholar]
- 21.Van Caster P, Brandenburger T, Strahl T, et al. Circulating microRNA-122, −21 and −223 as potential markers of liver injury following warm ischaemia and reperfusion in rats. Mol Med Rep. 2015;12(2):3146–315. doi: 10.3892/mmr.2015.3742. [DOI] [PubMed] [Google Scholar]
- 22.Sadatomo A, Inoue Y, Ito H, et al. Interaction of neutrophils with macrophages promotes IL-1beta maturation and contributes to hepatic ischemia-reperfusion injury. J Immunol. 2017;199(9):3306–15. doi: 10.4049/jimmunol.1700717. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Jiang S, Wang H, et al. SIRT6 protects against hepatic ischemia/reperfusion injury by inhibiting apoptosis and autophagy related cell death. Free Radical Biol Med. 2017;115:18–30. doi: 10.1016/j.freeradbiomed.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Wang K, Liu F, Liu CY, et al. The long noncoding RNA NRF regulates programmed necrosis and myocardial injury during ischemia and reperfusion by targeting miR-873. Cell Death Differ. 2016;23(8):1394–405. doi: 10.1038/cdd.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diao H, Liu B, Shi Y, et al. MicroRNA-210 alleviates oxidative stress-associated cardiomyocyte apoptosis by regulating BNIP3. Biosci Biotechnol Biochem. 2017;81(9):1712–20. doi: 10.1080/09168451.2017.1343118. [DOI] [PubMed] [Google Scholar]
- 26.Han YF, Zhao YB, Li J, et al. Stat3-Atg5 signal axis inducing autophagy to alleviate hepatic ischemia-reperfusion injury. J Cell Biochem. 2017;119(4):3440–50. doi: 10.1002/jcb.26516. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Tang L, Wang Y, et al. Exendin-4 protects HUVECs from t-BHP-induced apoptosis via PI3K/Akt-Bcl-2-caspase-3 signaling. Endocr Res. 2016;41(3):229–35. doi: 10.3109/07435800.2015.1110162. [DOI] [PubMed] [Google Scholar]
- 28.Fan Y, Yang F, Cao X, et al. Gab1 regulates SDF-1-induced progression via inhibition of apoptosis pathway induced by PI3K/AKT/Bcl-2/BAX pathway in human chondrosarcoma. Tumour Biol. 2016;37(1):1141–49. doi: 10.1007/s13277-015-3815-2. [DOI] [PubMed] [Google Scholar]
- 29.Hu L, Sun Y, Hu J. Catalpol inhibits apoptosis in hydrogen peroxide-induced endothelium by activating the PI3K/Akt signaling pathway and modulating expression of Bcl-2 and Bax. Eur J Pharmacol. 2010;628(1–3):155–63. doi: 10.1016/j.ejphar.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 30.Akbari G, Mard SA, Dianat M, Mansouri E. The hepatoprotective and microRNAs downregulatory effects of crocin following hepatic ischemia-reperfusion injury in rats. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/1702967. 1702967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farid WR, Pan Q, van der Meer AJ, et al. Hepatocyte-derived microRNAs as serum biomarkers of hepatic injury and rejection after liver transplantation. Liver Transpl. 2012;18(3):290–97. doi: 10.1002/lt.22438. [DOI] [PubMed] [Google Scholar]
- 32.Leelahavanichkul A, Somparn P, Panich T, et al. Serum miRNA-122 in acute liver injury induced by kidney injury and sepsis in CD-1 mouse models. Hepatol Res. 2015;45(13):1341–52. doi: 10.1111/hepr.12501. [DOI] [PubMed] [Google Scholar]
- 33.Mard SA, Akbari G, Dianat M, Mansouri E. Protective effects of crocin and zinc sulfate on hepatic ischemia-reperfusion injury in rats: A comparative experimental model study. Biomed Pharmacother. 2017;96:48–55. doi: 10.1016/j.biopha.2017.09.123. [DOI] [PubMed] [Google Scholar]
- 34.Xiao Q, Ye QF, Wang W, et al. Mild hypothermia pretreatment protects hepatocytes against ischemia reperfusion injury via down-regulating miR-122 and IGF-1R/AKT pathway. Cryobiology. 2017;75:100–5. doi: 10.1016/j.cryobiol.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Ruan L, Huang HS, Jin WX, et al. Tetrandrine attenuated cerebral ischemia/reperfusion injury and induced differential proteomic changes in a MCAO mice model using 2-D DIGE. Neurochem Res. 2013;38(9):1871–79. doi: 10.1007/s11064-013-1093-1. [DOI] [PubMed] [Google Scholar]
- 36.Yanagida T, Tsushima J, Kitamura Y, et al. Oxidative stress induction of DJ-1 protein in reactive astrocytes scavenges free radicals and reduces cell injury. Oxid Med Cell Longev. 2009;2(1):36–42. doi: 10.4161/oxim.2.1.7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tajiri N, Borlongan CV, Kaneko Y. Cyclosporine a treatment abrogates ischemia-induced neuronal cell death by preserving mitochondrial integrity through upregulation of the Parkinson’s disease-associated protein DJ-1. CNS Neurosci Ther. 2016;22(7):602–10. doi: 10.1111/cns.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jickling GC, Ander BP, Zhan X, et al. MicroRNA expression in peripheral blood cells following acute ischemic stroke and their predicted gene targets. PLoS One. 2014;9(6):e99283. doi: 10.1371/journal.pone.0099283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu DZ, Jickling GC, Ander BP, et al. Elevating microRNA-122 in blood improves outcomes after temporary middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2016;36(8):1374–83. doi: 10.1177/0271678X15610786. [DOI] [PMC free article] [PubMed] [Google Scholar]