Abstract
Background
Through observing the changes of indexes of the intestinal mucosal barrier and intestinal flora in rats, we explored the mechanism by which Hetiao Jianpi Decoction (HTJPD) treats antibiotic-associated diarrhea (AAD) by repairing intestinal mucosal injury and regulating intestinal flora.
Material/Methods
Samples of colon tissues were collected for HE staining. Enzyme-linked immunosorbent assay (ELISA) was used to assess levels of diamine oxidase (DAO) and D-lactic acid in rat plasma and the expression of secretory immunoglobulin A (SIgA) in colon tissue. We assessed the abundance of intestinal contents by high-throughput sequencing of the 16S rRNA gene.
Results
Compared with the Model group, the muscle layer and intestinal mucosal edema were improved, and the continuity was restored; the levels of DAO and D-lactic acid in plasma decreased, and the SIgA level were increased in the HTJPD group. The structure of the intestinal flora changed, as indicated by increased levels of certain beneficial bacteria (Verrucomicrobia, Actinobacteria, CF231, and Akkermansia), decreased levels of pathogenic bacteria (Spirochaetes and Treponema), and increased species diversity.
Conclusions
By improving the permeability and immune function of the intestinal mucosa, Hetiao Jianpi decoction prevented the occurrence of AAD by repairing the intestinal mucosal damage and regulating the structure and diversity of intestinal flora.
MeSH Keywords: Diarrhea; Intestinal Mucosa; Medicine, Chinese Traditional
Background
According to a recent report titled “Antibacterial Drug Management and Bacterial Resistance in China” (2018) issued by the State Health Commission, the utilization rate of antibiotics in hospital inpatients reached 36.9% in 2017 [1]. Clinical complications due to misuse of antibiotics have increased significantly, among which, AAD is a relatively common type. It was found that the occurrence of AAD is closely related to imbalances in intestinal flora [2]. When the intestinal microbiota balance is upset, disease can result; therefore, maintaining a stable microbial environment is crucial to human health [3–6]. The intestinal mucosa is an important part of the human body’s contact with the external environment, and it directly participates in exchange of materials. The integrity of the intestinal mucosal barrier determines the health of the intestinal tract [7]. As an important biological barrier, the intestinal flora is continuously and relatively fixedly distributed in the intestinal mucosa and is involved in immune regulation, metabolism of nutrients and their decomposition products, and metabolism and regulation of drugs and toxins [8]. After treatment with antibiotics for various reasons, the equilibrium of the intestinal flora is upset; the growth of beneficial bacteria in the intestine is inhibited, and pathogenic bacteria multiply, resulting in dysbacteriosis [9]. Intestinal flora imbalance can affect intestinal mucosal immune function and intestinal mucosal structure integrity, resulting in weakened intestinal mucosal barrier function, as well as colonization and multiplication of foreign or pathogenic bacteria in the intestine, which then became the dominant flora, resulting in diarrhea or enteritis. Although modern treatments are somewhat effective, they cannot repair damaged mucosal barriers or regulate the intestinal flora.
According to reports [10–12], traditional Chinese medicine is effective in the treatment of various symptoms of diarrhea and intestinal microbial disorders, in addition to being safe, lacking adverse effects, and having clear efficacy. The application of HTJPD in the present study used the classic prescription of Shenlin Baizhu San in the treatment of spleen deficiency and diarrhea [13,14] and focused on improving body function, reconciling the viscera and blood, regulating respiration, balancing the yin and yang, and thus restoring homeostasis. Our results provide a theoretical basis for use of traditional Chinese medicine in multi-targeted treatment of AAD, and provide new ideas for clinical treatment of the disease.
Material and Methods
Experimental animals
SPF Wistar rats (n=30; 200±20 g; male) were purchased from Chongqing Shiyi Biotechnology Co. (Chongqing, China; license no. SCXK-Liao-2015-0001). The rats were raised in the Laboratory Animal Center of Yunnan University of Chinese Medicine in an environment with 20±4°C average temperature, 50–80% relative humidity, and free access to food and water. The experiments were approved by the Laboratory Animal Welfare and Ethics Committee of Yunnan University of Chinese Medicine (Yunnan, China). All animal care and experimental procedures were conducted according to the Chinese Laboratory Animals’ Welfare and Ethics guidelines [15].
Animal grouping
The 30 rats were randomly divided into 5 groups with 6 rats in each group: a Control group (Control), a Model group (Model), a low-dose Hetiao Jianpi Decoction group (HTJPD-L), a moderate-dose group (HTJPD-M), and a high-dose group (HTJPD-H).
Establishment of AAD Model
The AAD rat model was established according to the method described by Biqiang Sun and others [16–18]. After 7 days of adaptation to the environment, the remaining 24 rats were gavaged with 2 linked antibiotics: cefotaxime sodium (purchased from Zhejiang Xianyi Pharmaceutical Co. (production batch no. SH6433) and lincomycin hydrochloride (purchased from Jiangsu Yabang Johnson Pharmaceutical Co. (production batch no. SL8890) at 1.82 mg/d administered intragastrically for 3 days, one in the morning and the other in the afternoon. The interval between the 2 treatments was 6 hours, causing an intestinal flora imbalance in the rats. Subsequently, we administered rhubarb decoction at 13.3 g/(kg/d) per day [19] continuously for 7 days, resulting in the model of spleen deficiency and diarrhea in rats. The 6 rats in the Control group were given normal saline.
Model success evaluation indicators
According to “Lactobacillus reuteri DSM 17938 in the prevention of antibiotic-associated diarrhea in children” edited by Kołodziej [20], the success of establishing the AAD model was assessed by observation of general behavioral changes (such as behavioral status and fecal morphology). Based on the results of this experiment, the Model group rats became lethargic, with hunched posture and fecal staining of the perianal area, and the feces were pale yellow and wet or loose.
Preparation of Hetiao Jianpi decoction
The decoction was composed of lotus seed (20 g), common yam rhizome (15 g), Codonopsis (15 g), Gordon Euryale seed (10 g), and bitter orange (9 g) (all purchased from Yunnan Hongxiang Yixintang Pharmaceutical). The total amount was 69 g. According to the “Chinese medicine pharmacology experimental methodology” edited by Yikui Li [21], the Chinese medicine decoction pieces were soaked for 1–2 hours after adding 5–8 times the amount of the medicine and then filtered after boiling for 30 minutes. The dregs were diluted 3- to 6-fold with water, then boiled for a further 15–20 minutes, and then filtered. The 2 decoctions were combined and further decocted to make an extract. After preparation, it was stored at 4°C until use. The daily human therapeutic dose was converted into the dose based on the rat body surface area, and the rat conversion factor was 0.018 [21]. We used the human daily treatment dose (69 g) as the medium dose, so the HTJPD-M rats received the equivalent dose (1.24 g), and the ratio of HTJPD-L (0.62 g), HTJPD-M (1.24 g), and HTJPD-H (2.48 g) was 1/2: 1: 2.
Administration of AAD model
After modeling, rats were randomly divided into 4 groups: the Model group, the HTJPD-L group, the HTJPD-M group, and the HTJPD-H group, with 6 rats in each group. The intragastric administration was started on the 11th day after the start of modeling [16–18]. The HTJPD-L (0.62 g/d), HTJPD-M (1.24 g/d), and HTJPD-H (2.48g/d) groups were gavaged for 7 consecutive days. The Control and Model groups were orally gavaged with equal volumes of normal saline (once a day) for 7 consecutive days. All rats were sacrificed on the 24th day of the experiment, and the samples were taken under aseptic conditions.
Observing the pathological changes of colon tissue
The fixed 1-cm colon tissue was paraffin-embedded according to the standard procedure of dehydration, transparency, and wax immersion. It was then cut into 5-μm-thick tissue pieces for HE staining. The field of view was selected under a high-power microscope (100×, 400×). The pathological changes, such as epithelial edema, muscle layer damage, number of goblet cells, and intestinal mucosal continuity, were observed.
Detecting the levels of DAO and D-lactic acid in plasma of rats
After the rats were sacrificed, 5 ml of blood was taken from the abdominal aorta and placed in heparin anticoagulation tubes for centrifuging at 4°C for 20 min (3000 rpm). The upper-layer serum was taken and frozen at −80°C. The method and procedure were completed according to the kit operating instructions.
Detecting the SIgA in intestinal mucosa of rats
Colon tissue of rats, taken from 5 cm below the pylorus to about 20 cm in the ileocecal area, was placed in a sterile centrifuge tube and stored at −80°C. The method and procedure were completed according to the kit operating instructions.
Detecting the bacterial abundance of rat intestinal contents
The determination of bacterial abundance of intestinal contents was performed by Shenzhen Microscience and Technology Co., using high-throughput sequencing of the 16S rRNA gene. By using the E.Z.N.A.® soil DNA Kit, OmegaV3-V4 hypervariable regions of the bacterial 16S rRNA gene were amplified with primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) using the thermocycler PCR system (GeneAmp 9700, ABI, USA). The resulting PCR products were further purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA)and quantified using Quant Fluor™-ST (Promega, USA) according to the manufacturer’s protocol. We then performed sequencing using the Illumina MiSeq platform.
Statistical analysis
We used GraphPad Prism 8.0 statistical software, and the data are expressed as (x±s). Measurement data were tested for homogeneity of variance using the t test to assess differences between 2 groups and using one-way ANOVA to assess differences among multiple groups. P<0.05 was considered statistically significant.
Results
Behavioral changes
Rats in the Control group continued to eat normally, maintained normal weight and mental state, and had brown stools and shiny coats. Compared with the Control group, during the model establishment, the rats in the Model group lost significant weight loss and appeared dispirited and lethargic; they had darker hair and their perianal area became dirty, and their stools became pale yellow, wet, or unformed. Compared with the Model group, rats in the HTJPD groups had increased weight and better mental state and were more active, with glossy coats and normal feces.
Pathological section of colon tissue
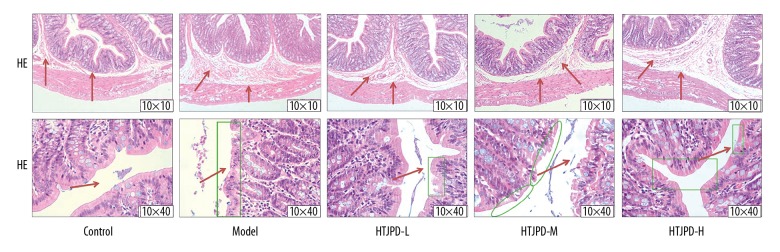
Compared with Control group, the muscle tissues of Model rats became thinner, there was diffuse edema of the intestinal mucosa, and its integrity was impaired. Compared with Model group rats, the muscle tissues and mucosal edema of the colon tissue in the HTJPD groups were significantly improved and the intestinal mucosal integrity was restored (Figure 1).
Figure 1.
Pathological section of colon tissue in rats. Observation of colonic muscle layer tissue and intestinal mucosa edema in rats by HE staining (10×10). Observation of intestinal mucosal continuity in the colonic tissues of rats by HE staining (10×40).
Changes in plasma DAO content after HTJPD treatment
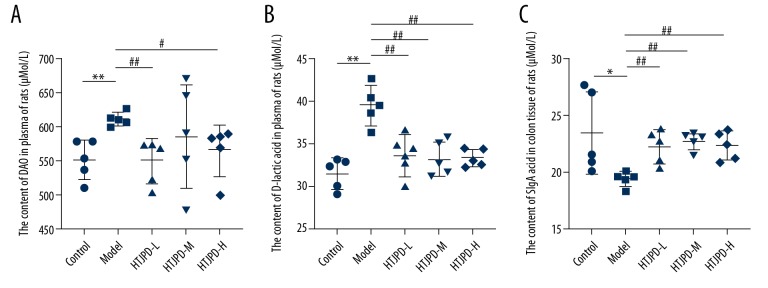
The level of DAO in the plasma was significantly higher in the Model group than in the Control group (P<0.01). Compared with the Model group, the expression of DAO in the HTJPD-L group was significantly lower (P<0.01). The HTJPD-M group showed a decreasing trend (P>0.05), and the level in the HTJPD-H group was decreased (P<0.05) (Figure 2A).
Figure 2.
ELISA. (A) Changes in plasma DAO level in rats. (B) Changes in plasma D-lactic acid level in rats. (C) Changes in SIgA content in rat intestinal mucosa. Data (n=5) are presented as mean±SD. * P<0.05 and ** P<0.01 versus Control group; # P<0.05 and ## P<0.01 versus Model group.
Changes of plasma D-lactic acid content after HTJPD treatment
Compared with the Control group, the expression of D-lactic acid in the plasma of Model group rats was significantly increased (P<0.01). However, we observed that after administration of HTJPD, the expression of D-lactic was decreased (P<0.05) (Figure 2B).
Changes of SIgA levels in rat intestinal mucosa after HTJPD treatment
Compared with the Control group, the expression of SIgA in intestinal mucosa of the Model group was significantly decreased (P<0.01). After administration of HTJPD, there was higher expression of SIgA in the HTJPD-L group and HTJPD-M group than in the Model group rats (P<0.05), but rats in the HTJPD-H group showed an increasing trend (P>0.05) (Figure 2C).
Overall structural modulation of gut microbiome after HTJPD treatment
To examine the effect of HTJPD on the gut microbiome structure in the AAD rat Model, an Illumina MiSeq platform was used to generate 1 010 340 high-quality sequences from 25 fecal samples.
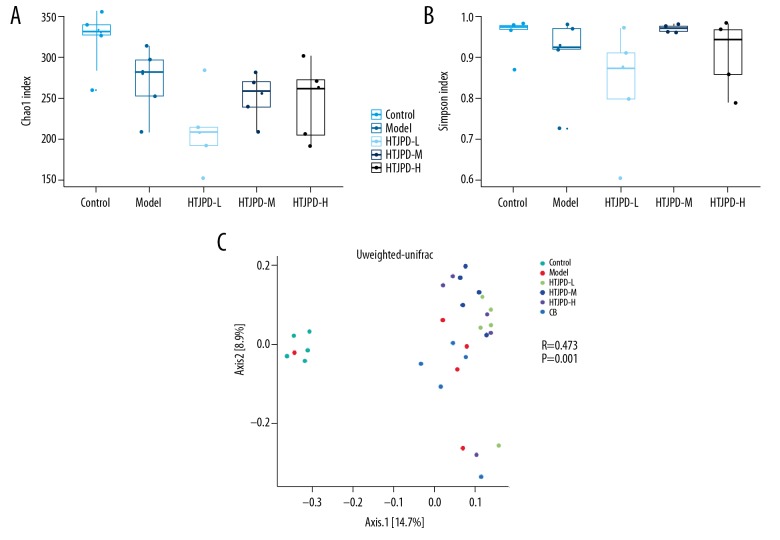
The Chao 1 index was used to estimate the total number of OTUs in a sample; the higher the index, the more complex the abundance of the sample. The Simpson index was used to assess the species diversity of a sample; the higher the index, the more complex the sample diversity. All 5 groups in this study showed differences in microbial diversity. The diversity of the intestinal flora of the Model group showed a downward trend. After HTJPD treatment, there were various degrees of change in different dose groups. Among them, the diversity of HTJPD-M increased significantly, while the species richness was still lower than in the Model group (Figure 3A, 3B).
Figure 3.
Overall structural modulation of gut microbiome after HTJPD treatment (n=5). (A) Chao 1 index was used to estimate the total number of OTUs in a sample; the higher the index, the more complex the abundance of the sample. (B) Simpson index was used to evaluate the species diversity of a sample; the higher the index, the more complex the diversity of the sample (P>0.05). (C) Unweighted-Unifrac-PCoA. Observation of the differences among groups of intestinal content bacteria in rats and evaluation of differences in microbial community structure among different samples.
Using the Unifrac distance method, we calculated the distance between samples and compared the differences in species diversity between samples and groups. The Unweighted Unifrac distance was used to assess microbial community differences between different samples, showing that the microbial community of the Model group was much less diverse than that of the Control group and HTJPD groups. The Anosim method was used in connection with the grouping information to assess differences in microbial composition among the various sample groups. The results showed that the differences in intestinal content flora in each group were greater than that the intra-group differences (P<0.05) (Figure 3C).
Key phylotypes of gut microbiome were altered by HTJPD treatment
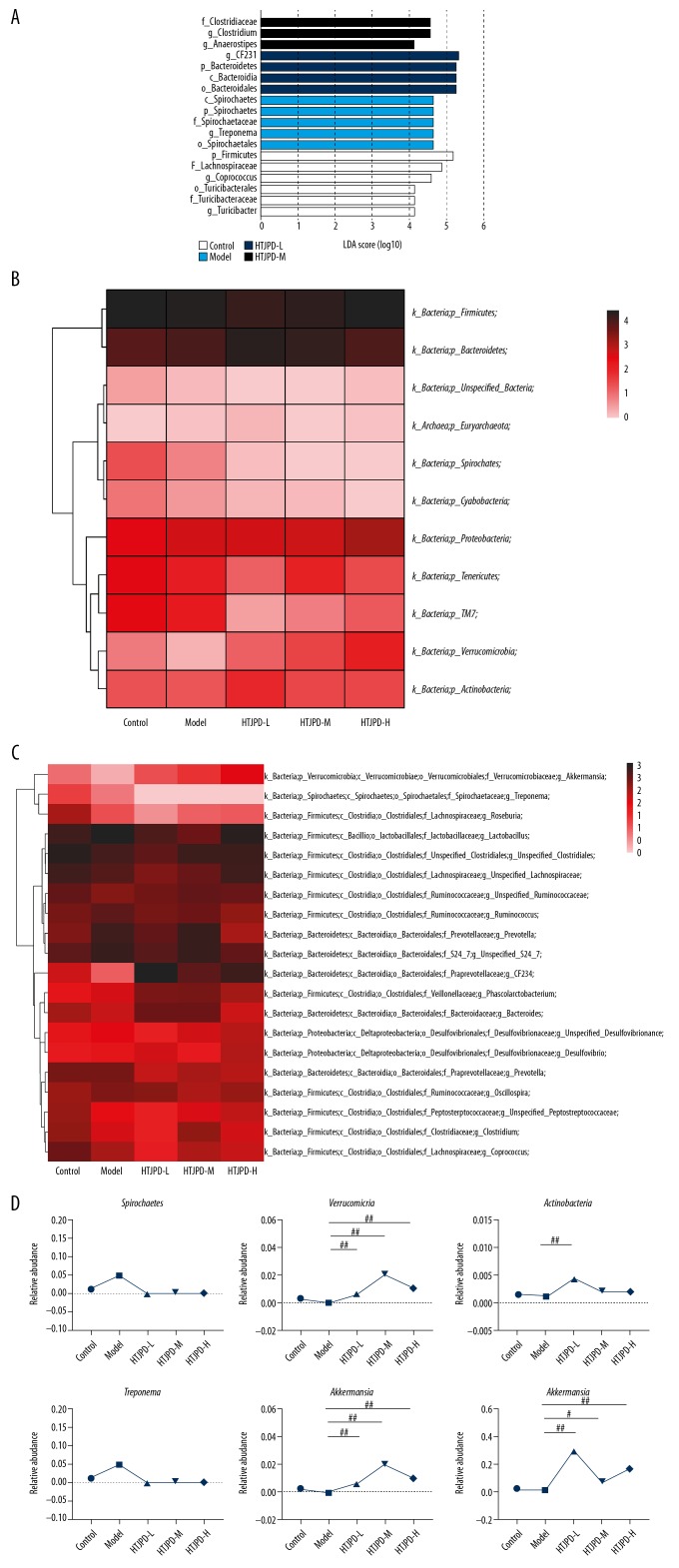
The LDA value distribution revealed that there were obvious differences in leading microbiota among the 4 groups (Figure 4A). We found 6, 5, 4, and 3 dominant taxa in the Control, Model, HTJPD-L, and HTJPD-H groups, respectively. The predominant microbiota in the Control group were Firmicutes, Lachnospiraceae, and Coprococcus. The predominant gut microbiota in the Model group were Spirochaetes, Spirochaetaceae, and Treponema. The predominant gut microbiota in the HTJPD-L group were CF231, Bacteroidetes, and Bacteroidales. The predominant gut microbiota in the HTJPD-M group were Clostridiaceae, Clostridium, and Anaerostipes. LDA scores further showed the important microbiota in the 4 groups.
Figure 4.
Key phylotypes of gut microbiome modulated by HTJPD treatment in rats with AAD (n=5). (A) Histogram of LDA value. Each lateral column represented a species, and the length of the column corresponds to the LDA value. (B, C) Heatmap of the most abundant OTUs classified by phylum and genus, reflecting the abundance and species clustering and sample clustering information of different species in the sample by using a color gradient. (D) Relative abundances within Spirochaetes, Verrucomicrobia, and Actinobacteria. Relative abundances within Treponema, CF231, and Akkermansi. # P<0.05 and ## P<0.01 compared with the Model group.
The relative abundance of the intestinal flora is presented in stacked histograms, which were used to identify the marked differences at the phylum and genus levels among the 4 groups (Figure 4B, 4C). At the species and phylum levels, the intestinal contents were dominated by Firmicutes (42.785%~71.481%) and Bacteroidetes (19.489~54.328%), followed by Proteobacteria (1.100~5.413%) and Spirochaetes (0.000~5.023%). At the genus level, the intestinal contents of rats were dominated by Lactobacillus (5.820~26.806%) and unspecified Clostridiales (6.566~15.702%), followed by CF231 (0.868~30.121%), unspecified S24–7 (4.418~12.906%), and Prevotella (1.419~9.820%). The relative abundance of Spirochaetes was 1.35% in Control group rats, 5.02% in Model group rats, and 0.00% in rats from the HTJPD-L, HTJPD-M, and HTJPD-H groups. Approximately 0.24% of sequences in rats from the Control group were classified into the phylum Verrucomicrobia, whereas only 0.01% of sequences in rats from the Model group were so classified. After HTJPD treatment, however, 0.64%, 2.05%, and 1.08% of sequences were classified into the HTJPD-L, HTJPD-M, HTJPD-H groups, respectively. The relative abundances of Actinobacteria were 0.16%, 0.12%, 0.44%, 0.19%, and 0.19% in the Control, Model, HTJPD-L, HTJPD-M, and HTJPD-H groups, respectively. At the genus level, the results showed that relative abundances of CF231 (0.87%) and Akkermansi (0.01%) were significantly decreased in the Model group compared with those in the Control group (1.15% and 0.24%, respectively). Moreover, HTJPD treatment significantly increased the abundances of CF231 (30.12%, 7.00%, and 17.23%) and Akkermansi (0.64%, 2.05%, 1.08%). In the Model group, there was an increase in Treponema (5.02%) compared with rats from the Control group. After HTJPD treatment, the abundances of Treponema decreased (0.00%). Taken collectively, these data reveal that the gut microbiota was altered by antibiotic treatment, and HTJPD reversed the adverse effects in AAD rats at the phylum and genus levels (Figure 4D).
Discussion
The pathogenesis of AAD is complicated, often involving disordered intestinal flora, antibiotics interfering with carbohydrates and bile acid metabolism, and the direct effects of antibiotics [22]. Studies [23,24] have shown that many long-term applications of antibacterial drugs can cause intestinal flora imbalance and damage intestinal mucosal structure via toxic adverse the effects of drugs, as well as reducing the intestinal mucosa barrier function, leading to intestinal dysfunction and causing AAD. DAO is one of the marker enzymes widely present in mammalian intestinal mucosal epithelial villus cells, and DAO levels can objectively reflect the degree of intestinal mucosal damage [25,26]. D-lactic acid is a unique metabolic end-product of intestinal bacteria, and its levels can reflect the degree of intestinal mucosal damage and permeability changes [27,28]. Some scholars [29] found that the serum DAO levels of patients with spleen and stomach deficiency syndrome were significantly associated with diarrhea. In the present study, the levels of DAO (P<0.05) and D-lactic acid (P>0.05) in the Model group were significantly increased, suggesting that AAD rats had various degrees of intestinal mucosal structure damage during the modeling process, and their barrier permeability changed. As the main component and antibody of the mucosal immune system, SIgA can reflect the mucosal immunity of the body [30]. Yang [31] found that, after gavaging with rifampicin and rhubarb, rats with damaged duodenal mucosal cells had decreased levels of intestinal mucosal SIgA. We found that the level of SIgA in intestinal mucosa of AAD rats was significantly decreased (P<0.01), suggesting that the intestinal mucosal immune function of AAD rats was disordered. The intestinal tracts of mammals contain many species of microorganisms that have an important influence on maintaining the homeostasis of the intestinal environment and host health. The diversity and richness of the human intestinal microbiota are influenced by many factors, including drugs (antibiotics), diet, and environmental factors [32–34]. Studies [35,36] have found that, after antibiotic treatment, the diversity of intestinal flora was significantly decreased, and many clinical complications occurred in patients. AAD is one of the main complications in the course of treatment, and its occurrence and development are closely related to intestinal microflora disorders and changes in intestinal structure [37–39]. In the present study, high-throughput sequencing of the 16S rRNA gene was used to detect changes in diversity of the intestinal microbiota in rats. The Simpson index and Shannon index indicated that the biological diversity of colon microflora in AAD rats was significantly decreased. The structure and abundance of the intestinal flora were more different among the groups than among the samples in each group (P<0.05). In summary, the results showed that overuse of antibiotics decreased the diversity of intestinal flora. Consistent with the results of Bezirtzogloue [40], Quwei [41], and Kaili [42], we found that the development of AAD was accompanied by disordered intestinal flora. We also found that repairing mucosal damage and regulating intestinal flora balance were particularly important for the treatment of AAD.
Traditional Chinese medicine classifies AAD into the category of “diarrhea”. Excessive and long-term use of antibiotics can easily deplete the qi of the spleen and stomach, causing spleen disease and endogenous dampness, leading to diarrhea [43]. Combined with the experimental results, it was found that the muscle layer and mucosal edema of the colon tissue were obviously improved, and the intestinal mucosal integrity was restored after treating with HTJPD. The levels of DAO and D-lactic acid in the plasma of rats were decreased, and the expression level of SIgA in the intestinal mucosa was increased, especially in the HTJPD-M group. After treatment with HTJPD, the phylum abundance of Verrucomicrobia and Actinobacteria was increased compared to rats in the Model group, but the phylum abundance of Spirochaetes decreased. HTJPD increased the genus abundance of CF231 and Akkermansia, but decreased the abundance of Treponema compared to rats from the Model group. Among these, Verrucomicrobia and some members of the genus Actinobacteria have recently been proposed as indicators of a healthy gut because of its immunostimulant properties and the ability to improve intestinal barrier function [44–46]. Akkermansia is the sole intestinal representative of the Verrucomicrobia in human stools [47]; it is closely related to host intestinal barrier function, nutrient metabolism, immunity, and disease. Akkermansia can specifically degrade mucins and oligosaccharides, producing short-chain fatty acids and propionic acids, respectively, which provides energy for the host and promotes colonization [48]. Spirochaetes plays an important role in some intestinal diseases [49, 50], and it was reported [51] that intestinal spirochetes appear to play a pro-inflammatory role in the etiopathogenesis of colorectal inflammation, showing an interaction with and effect on colonic mucosal immunity. Treponema belongs to the diverse bacterial phylum Spirochaetes, the members of which are distantly related to other bacteria, both Gram-negative and Gram-positive types [52]. Treponema had diacylglycerol-containing glycolipids that resemble lipoteichoic acids of Gram-positive bacteria, which play a role in interaction with animal host receptors [53]. Our data revealed that HTJPD not only promoted the production of probiotics, but also regulated the abundance of gut flora and restored the balance of flora. This was consistent with previous studies [54,55] that suggested that traditional Chinese medicine and intestinal microecosystem balance can make important contributions in the treatment of this disease.
Conclusions
With the widespread overuse of antibiotics in recent years, the incidence of AAD is increasing. When the antibiotics are stopped, the symptoms will be relieved to varying degrees. The patients may be mistakenly perceived to be “healing”, but the hidden intestinal damage persists. The symptoms are subtile and slow to appear, so they are easily missed. Once AAD occurs, the intestinal injury is difficult to reverse and quick recovery is unlikely. Although modern medical treatments and drugs have certain effects, patients often have long-term adverse effects, and the disease is only partially resolved. Therefore, we performed the present study based on the concept that the intestinal flora diversity is essential in AAD, using the theory of traditional Chinese medicine treatment as a guide to restore the balance of intestinal flora, in order to treat or prevent long-term intestinal damage. Our results may provide a theoretical basis for use of traditional Chinese medicine in multi-targeted treatment of AAD, and provide new ideas for clinical treatment of the disease.
Acknowledgements
We thank all the scholars who provided relevant guidance for the study.
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China (No. 81760819) and a grant from the Provincial Innovation Team of Yunnan University of Chinese Medicine for Traditional Chinese Medicine to Regulate Human Microecology (No. 2018HC011)
Conflicts of interest
None.
References
- 1.Zhong NS. The present situation of antibacterial drug management and bacterial resistance in China (2018) Bei Jing: State Health and Health Commission; 2018. [Google Scholar]
- 2.Gillespie D, Hood K, Bayer A, et al. Antibiotic prescribing and associated diarrhoea: A prospective cohort study of care home residents. Age Ageing. 2015;44(5):853–60. doi: 10.1093/ageing/afv072. [DOI] [PubMed] [Google Scholar]
- 3.Dao Maria C, Clément K. Gut microbiota and obesity: Concepts relevant to clinical care. Eur J Intern Med. 2018;48:18–24. doi: 10.1016/j.ejim.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Boulangé CL, Neves AL, Chilloux J, et al. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8(1):42. doi: 10.1186/s13073-016-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheehan D, Shanahan F. The gut microbiota in inflammatory bowel disease. Gastroenterol Clin North Am. 2017;46(1):143–54. doi: 10.1016/j.gtc.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Kosiewicz MM, Zirnheld AL, Alard P. Gut microbiota, immunity, and disease: A complex relationship. Front Microbiol. 2011;2:180. doi: 10.3389/fmicb.2011.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Zhou X. [Intestinal flora, intestinal mucosal immunity and the related intestinal diseases: Research progress]. Chinese Journal of Microecology. 2017;29(4):494–97. [in Chinese] [Google Scholar]
- 8.Wang W, Cui L. [Advances in intestinal microecology and its therapeutic effect on diarrhea]. Academic Journal of Chinese PLA Medical School. 2016;37(7):813–15. [in Chinese] [Google Scholar]
- 9.Jing S. [Pathogenesis and diagnosis and treatment of antibiotic-associated diarrhea]. The Journal of Medical Theory and Practice. 2013;26(23):3112–14. [in Chinese] [Google Scholar]
- 10.Wang X, Li X, Peng Y. Impact of Qi-invigorating traditional Chinese medicines on intestinal flora: A basis for rational choice of prebiotics. Chin J Nat Med. 2017;15(4):241–54. doi: 10.1016/S1875-5364(17)30041-9. [DOI] [PubMed] [Google Scholar]
- 11.Li S, Qi Y, Chen L, et al. Effects of Panax ginseng polysaccharides on the gut microbiota in mice with antibiotic-associated diarrhea. Int J Biol Macromol. 2019;124:931–37. doi: 10.1016/j.ijbiomac.2018.11.271. [DOI] [PubMed] [Google Scholar]
- 12.Xing B, Chen H, Shen W. [Application discussion of edible source of Chinese materia medica used in malignant tumor prevention]. Journal of Sichuan of Traditional Chinese Medicine. 2018;36(2):46–48. [in Chinese] [Google Scholar]
- 13.Shi Y, Yu H. [The local immune regulation of shenling-bai-zhu powder in traditional Chinese medicine spleen deficiency diarrhea model]. Immunological Journal. 2018;34(6):519–23. [in Chinese] [Google Scholar]
- 14.Gu R, Shu Q. [Research progress on pharmacology of shenlingbaizhu powder based on intestinal microecology]. Lishizhen Medicine and Materia Medica Research. 2018;29(3):674–76. [in Chinese] [Google Scholar]
- 15.Laboratory Animals’ Welfare and Ethics guidelines (GB/T 35892-2018) http://www.gb688.cn/bzgk/gb/newGbInfo?hcno=9BA619057D5C13103622A10FF4BA5D14.
- 16.Sun B, Zhou Y, Liu W, Wu C. [Effect of different formulated Qiwei Baizhu San on mRNA expression of IL-4, IL-10, and IFN-α in diarrhea rat with intestinal flora alteration]. Chinese Journal of Experimental Traditional Medical Formulae. 2016;22(6):84–88. [in Chinese] [Google Scholar]
- 17.Sun B, Wu C, Zhou Y, et al. [Effects of different formulations of Qiwei Baizhu Powder on the ultrastructure of small intestinal mucosa and sIgA in rat with intestinal dysbacteriosis]. Chinese Journal of Experimental Traditional Medical Formulae. 2016;28(2):128–37. [in Chinese] [Google Scholar]
- 18.Sun B, Zhou Y, Liu W, Wu C. Effects of different formulated Qiwei Baizhu powder on intestinal flora and tight junction protein in diarrhea rat. Chinese Journal of Experimental Traditional Medical Formulae. 2016;26(12):2835–37. [in Chinese] [Google Scholar]
- 19.Liu E. Human animal disease Model. Bei Jing: People’s Medical Publishing House; 2014. pp. 1–351. [Google Scholar]
- 20.Kołodziej M, Szajewska H. Lactobacillus reuteri DSM 17938 in the prevention of antibiotic-associated diarrhea in children: A randomized clinical trial. Clin Microbiol Infect. 2019;25(6):699–704. doi: 10.1016/j.cmi.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Li Y. Experimental methodology of traditional Chinese medicine pharmacology. Shang Hai: Shanghai science and technology press; 2016. p. 119. [Google Scholar]
- 22.Zhang W. Animal model of antibiotic-associated diarrhea. Southern Medical University. :2015. [Google Scholar]
- 23.Li J, Wang C, Li Y, et al. [Effect of Shenling Baizhu particles on intestinal epithelial light junctions in rats with functional diarrhea]. Chinese Journal of Experimental Traditional Medical Formulae. 2016;22(12):102–7. [in Chinese] [Google Scholar]
- 24.Ma X, FengyunWang F, Fu J, Tang X. [Research situation and thoughts of mechanism of Traditional Chinese Medicine spleen prescription based on intestinal flora]. Chinese Journal of Experimental Traditional Medical Formulae. 2017;23(5):210–15. [in Chinese] [Google Scholar]
- 25.Ning J, Zhang Y, Yu M, et al. Emodin alleviates intestinal mucosal injury in rats with severe acute pancreatitis via the caspase-1 inhibition. Hepatobiliary Pancreat Dis Int. 2017;16(4):431–36. doi: 10.1016/S1499-3872(17)60041-9. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Yang Y, Chen X, et al. The influence of enteral nutrition therapy for inflammatory bowel disease on occludin, DAO, LPS and sIgA levels. J Clin Exp Med. 2017;16(20):2000–4. [Google Scholar]
- 27.Yan M, Shen M, Cui Y, et al. [Treatment of intestinal barrier function in patients with diarrhea predominant irritable bowel syndrome]. Laboratory Medicine and Clinic. 2019;16(01):27–30. [in Chinese] [Google Scholar]
- 28.Huang J, Nong H, Pei X, et al. [Mechanism underlying effect of Sijunzi decoction on intestinal mucosal barrier of mice with ulcerative colitis]. World Chinese Journal of Digestology. 2015;23(27):4326–34. [in Chinese] [Google Scholar]
- 29.Qiu R, Zhang X, Liu J. [Effect of Shenling Baizhu powder on functional diarrhea (spleen-stomach deficiency) and influence on DAO, TNF-α and other inflammatory factors]. Chinese Archives of Traditional Chinese Medicine. 2018;36(08):1957–59. [in Chinese] [Google Scholar]
- 30.Li J, Wang C, Li Y, et al. [Study on the relationship between functional diarrhea and spleen deficiency syndrome and intestinal mucosal barrier]. Chinese Journal of Integrated Traditional and Western Medicine on Digestion. 2015;23(04):299–302. [in Chinese] [Google Scholar]
- 31.Yang L, Li Y, Zhou Y, et al. [Effects of Sijunzi decoction on SIgA and gut flora in rats with spleen-deficiency diarrhea]. Shanghai Journal of Traditional Chinese Medicine. 2011;45(12):85–87. [in Chinese] [Google Scholar]
- 32.Zmora N, Suez J, Elinav E. You are what you eat: Diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16(1):35–56. doi: 10.1038/s41575-018-0061-2. [DOI] [PubMed] [Google Scholar]
- 33.Suez J, Zmora N, Zilberman-Schapira G, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174(6):1406–23. doi: 10.1016/j.cell.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 34.Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555:623–28. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dethlefsen L, Huse S, Sogin ML, et al. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6(11):280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heinsen F-A, Knecht H, Neulinger SC, et al. Dynamic changes of the luminal and mucosa-associated gut microbiota during and after antibiotic therapy with paromomycin. Gut Microbes. 2015;6(4):243–54. doi: 10.1080/19490976.2015.1062959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lange K, Buerger M, Stallmach A, et al. Effects of antibiotics on gut microbiota. Dig Dis (Basel, Switzerland) 2016;34(3):260–68. doi: 10.1159/000443360. [DOI] [PubMed] [Google Scholar]
- 38.Pajau V, Ward T, Gerber JS, et al. Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe. 2015;17(5):553–64. doi: 10.1016/j.chom.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas S, Izard J, Walsh E, et al. The host microbiome regulates and maintains human health: A primer and perspective for non-microbiologists. Cancer Res. 2017;77(8):1783–812. doi: 10.1158/0008-5472.CAN-16-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bezirtzoglou E, Tsiotsias A, Welling GW. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH) Anaerobe. 2011;17(6):478–82. doi: 10.1016/j.anaerobe.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Qu W, Zhang Z, Ma J, et al. [Effect of probiotics on gut microbiota in rat evaluated by high-throughput sequencing]. Food Science. 2017;38(1):214–19. [in Chinese] [Google Scholar]
- 42.Zang K, Jiang Y, Sun Y, et al. Relationship between microecologics and the expression of short chain fatty acids synthesis genes in key bacterial genera in the regulation of intestinal flora structure in populations with constipation and diarrhea. Food Science. 2018;39(5):155–65. [in Chinese] [Google Scholar]
- 43.Mu X, Du J. [Research progress on prevention and treatment of antibiotic associated Diarrhea with traditional Chinese Medicine]. J Diseases Monitor and Control Sept. 2016;10(9):732–33. [in Chinese] [Google Scholar]
- 44.Xie P, Ru W, Liang H, et al. Enterococcus faecium NCIMB 10415 administration improves the intestinal health and immunity in neonatal piglets infected by enterotoxigenic Escherichia coli K88. J Anim Sci Biotechnol. 2019;10:72. doi: 10.1186/s40104-019-0376-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujio-Vejar S, Vasquez Y, Morales P, et al. The gut microbiota of healthy Chilean subjects reveals a high abundance of the phylum verrucomicrobia. Front Microbiol. 2017;8:1221. doi: 10.3389/fmicb.2017.01221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu H, Yawei L, Yanfang G, et al. Diversity of intestinal bacterial lactase gene in antibiotics-induced diarrhea mice treated with Chinese herbs compound Qi Wei Bai Zhu San. 3 Biotech. 2018;8(1):1–8. doi: 10.1007/s13205-017-1024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Vos Willem M. Microbe profile: Akkermansia muciniphila: A conserved intestinal symbiont that acts as the gatekeeper of our mucosa. Microbiology (Reading, England) 2017;163(5):646–48. doi: 10.1099/mic.0.000444. [DOI] [PubMed] [Google Scholar]
- 48.Feng Z, Bao X, Ying Y. The interaction of colonization of Akkermansia muciniphila gastrointestinal tract and its host. Sci Agric Sin. 2016;49(8):1577–84. [Google Scholar]
- 49.Andoh A, Fujiyama Y. Therapeutic approaches targeting intestinal microflora in inflammatory bowel disease. W J Gastroenterol. 2006;28:4452–60. doi: 10.3748/wjg.v12.i28.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iwamoto J, Ogata S, Honda A, et al. Human intestinal spirochaetosis in two ulcerative colitis patients. Intern Med. 2014;53:2067–71. doi: 10.2169/internalmedicine.53.2386. [DOI] [PubMed] [Google Scholar]
- 51.Shin N, Masaaki H, Sho O, et al. Human intestinal spirochetosis mimicking ulcerative colitis. Clin J Gastroenterol. 2018;11(2):145–49. doi: 10.1007/s12328-017-0807-3. [DOI] [PubMed] [Google Scholar]
- 52.Buyuktimkin B, Zafar H, Saier MH. Comparative genomics of the transportome of Ten Treponema species. Microb Pathog. 2019;132:87–99. doi: 10.1016/j.micpath.2019.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schröder NW, Eckert J, Stübs G, Schumann RR. Immune responses induced by spirochetal outer membrane lipoproteins and glycolipids. Immunobiology. 2007;213(3):329–40. doi: 10.1016/j.imbio.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 54.Lv W, Liu C, Ye C, et al. Structural modulation of gut microbiota during alleviation of antibiotic-associated diarrhea with herbal formula. Int J Biol Macromol. 2017;105:1622–29. doi: 10.1016/j.ijbiomac.2017.02.060. [DOI] [PubMed] [Google Scholar]
- 55.Jun X, Hu-Biao C, Song-Lin L. Understanding the molecular mechanisms of the interplay between herbal medicines and gut microbiota. Med Res Rev. 2017;379(2):1140–85. doi: 10.1002/med.21431. [DOI] [PubMed] [Google Scholar]