Abstract
Background
Secondary ischaemia is a frequent complication after aneurysmal subarachnoid haemorrhage (SAH), and responsible for a substantial proportion of patients with poor outcome after SAH. The cause of secondary ischaemia is unknown, but hypovolaemia and fluid restriction are important risk factors. Therefore, volume expansion therapy (hypervolaemia) is frequently used in patients with SAH to prevent or treat secondary ischaemia.
Objectives
To determine the effectiveness of volume expansion therapy for improving outcome in patients with aneurysmal SAH.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched September 2003). In addition we searched MEDLINE (1966 to January 2004) and EMBASE (1980 to January 2004) and contacted trialists to identify further published and unpublished studies.
Selection criteria
All randomised controlled trials of volume expansion therapy in patients with aneurysmal SAH. We also sought controlled trials based on consecutive groups of patients quasi‐randomly allocated to treatment or control group and included these in the analysis if the two groups were well comparable with regard to major prognostic factors.
Data collection and analysis
Two reviewers independently extracted the data and assessed trial quality. Trialists were contacted to obtain missing information.
Main results
We identified three trials. One truly randomised trial and one quasi‐randomised trial with comparable baseline characteristics for both groups were included in the analyses. Volume expansion therapy did not improve outcome (Relative Risk (RR) 1.0; 95% Confidence Interval (CI) 0.5 to 2.2), nor the occurrence of secondary ischaemia (RR 1.1; 95% CI 0.5 to 2.2). Hypervolaemia tended to increase the rate of complications (RR 1.8; 95% CI 0.9 to 3.7) In another quasi‐randomised trial, outcome assessment was done only at the day of operation (7 to 10 days after SAH). In the period before operation, treatment resulted in a reduction of secondary ischaemia (RR 0.33; 95% CI 0.11 to 0.99) and case fatality (RR 0.20; 95% CI 0.07 to 1.2).
Authors' conclusions
The effects of volume expansion therapy have been studied properly in only two trials of patients with aneurysmal SAH, with very small numbers. At present, there is no sound evidence for the use of volume expansion therapy in patients with aneurysmal SAH.
Plain language summary
Circulatory volume expansion therapy for aneurysmal subarachnoid haemorrhage
There is no evidence that administration of large volume of fluids is beneficial in patients with subarachnoid haemorrhage. Subarachnoid haemorrhage is a subset of stroke that occurs frequently in relatively young persons (mostly 40 to 60 years of age). Secondary ischaemia is an important contributor to poor outcome after a subarachnoid haemorrhage (half the patients die within a month after the haemorrhage). This type of ischaemia occurs 4 to 10 days (hence: secondary) after the haemorrhage, possibly due to fluid loss through increased urinary production. This review shows that there is no evidence to support giving additional fluids to not only compensate for the loss of fluid but also to increase the amount of fluid in the body.
Background
Subarachnoid haemorrhage (SAH) has an incidence of 6 per 100 000 person years (Linn 1996) and half the patients are younger than 55 years of age (ACROSS 2000). Poor outcome (death or dependence) from aneurysmal SAH occurs in approximately 70% of patients (Hop 1997) and is attributed to secondary ischaemia in approximately 30% of all patients with poor outcome (Kassell 1990). Secondary ischaemia occurs most often between 4 and 10 days after the haemorrhage (Brilstra 2000). The cause of secondary ischaemia is unknown, but hypovolaemia (Maroon 1979; Solomon 1984) and fluid restriction (Wijdicks 1985) are important risk factors for the development of secondary ischaemia. Therefore, hypervolaemia is frequently the goal of management in patients with SAH to prevent or treat episodes of secondary ischaemia. However, besides the potentially beneficial effect on secondary ischaemia, volume expansion therapy also carries disadvantages and risks, such as heart failure, or increased risk of re‐bleeding if applied before occlusion of the aneurysm.
Our aim is to review the evidence on effectiveness of volume expansion therapy in patients with aneurysmal SAH.
Objectives
Primary
To determine the effects of volume expansion therapy on poor outcome (death or dependence) or case fatality in patients with aneurysmal SAH.
Secondary
(1) To determine the effects of volume expansion therapy on the rate of secondary ischaemia in patients with aneurysmal SAH. (2) To determine the effects of volume expansion therapy on the rate of rebleeding in patients with aneurysmal SAH. (3) To study the complications of volume expansion therapy in patients with aneurysmal SAH.
Methods
Criteria for considering studies for this review
Types of studies
We sought to identify all randomised controlled trials of volume expansion therapy in patients with aneurysmal SAH. As we assumed that we would not find many randomised clinical trials, we also searched for controlled trials based on consecutive groups of patients quasi‐randomly allocated to treatment or control regimen (e.g. by date of birth) and included these in the analysis if the two groups were well comparable with regard to major prognostic factors. Uncontrolled studies, controlled trials with non‐consecutive patients where the method of allocation was prone to bias (e.g. allocation by day of the week) were excluded to prevent sampling bias and biased treatment allocation.
Types of participants
Patients of any age and either gender with aneurysmal or presumed aneurysmal SAH documented by CT scan or cerebrospinal fluid examination who entered the trial within the first two weeks after the SAH. This long period was chosen to allow inclusion of trials that started the intervention only after the onset of symptoms from secondary ischaemia.
Types of interventions
We included treatment with any volume expansion therapy fluids such as 5% human albumin, high sodium crystalloid, colloid, blood plasma, whole blood, or any combination of these. Studies were included regardless of whether the therapy was instituted before or after treatment of the aneurysm.
Types of outcome measures
To provide an intention‐to‐treat analysis, we aimed to extract from each trial the number of patients who were originally allocated to each treatment group. The main outcome measures to be extracted from each treatment group during the scheduled treatment or follow‐up period were the following: (1) poor outcome (the composite endpoint of death, vegetative state and severe disability); (2) death; (3) the occurrence of secondary ischaemia; (4) the occurrence of rebleeding; (5) complications of the treatment (such as pulmonary oedema, cardiac failure). These outcome events are analysed separately for trials where treatment was initiated before or only after occlusion of the aneurysm. Because most studies provide an unclear definition of secondary ischaemia, or no definition at all (van Gijn 1994), we used the number of patients with secondary ischaemia given by the authors, without adjusting these numbers to a predefined definition of secondary ischaemia.
Search methods for identification of studies
See: 'Specialized register' section in Cochrane Stroke Group
We identified relevant trials in the Cochrane Stroke Group trials register (last searched by the Review Group Co‐ordinator in September 2003). In addition, we searched MEDLINE (1966 to January 2004) (Appendix 1) and EMBASE (1980 to January 2004) (Appendix 2). We also contacted trialists of the identified trials for any published or unpublished studies they might be aware of.
Data collection and analysis
Two reviewers independently selected trials to be included in the review and extracted details of randomisation methods, blinding of outcome assessments, whether intention‐to‐treat analysis was possible from the published data, whether treatment groups were comparable with regard to major prognostic factors for poor outcome (clinical condition on admission, amount of extravasated blood, and age), the number of patients who were excluded or lost to follow‐up, definition of outcome events, and criteria for inclusion and exclusion criteria. Dose and timing of the fluid administration, duration of follow‐up, the numbers of deaths, numbers of patients with poor outcome (dependence or death), and complications of treatment were also recorded. In case of disagreement between the two data‐extractors, a consensus meeting was held together with one of the other reviewers. If patients were excluded or lost to follow‐up after randomisation or any of the above data not available from the publications, further information was sought by correspondence with the trialists to allow an intention‐to‐treat analysis. If the data about patients who were excluded or lost to follow‐up remained unavailable, a worst case scenario analysis for clinical outcome measures of interest was provided to ensure significance of the results (in this analysis it was assumed that those patients who were lost to follow‐up in the treatment group had the worst outcome while those patients who were lost to follow‐up in the control group had the best outcome).
Data analysis
We planned the following analyses. The primary analyses are based on the intention‐to‐treat results (if available) of the individual trials, for 'poor outcome' (death or dependence), for case fatality, and for the occurrence of specific events (secondary ischaemia and rebleeding). An estimate of the treatment effect across trials (Relative Risk (RR) with a 95% confidence interval (CI)) is calculated with standard methods. The statistical validity of aggregating the trials is assessed with a chi ‐squared test statistic for heterogeneity by using a standard fixed‐effect method of overview analysis (p > 0.05 suggested that the assumption of homogeneity was not violated).
If primary analyses suggest a beneficial effect but follow up was not complete, a worst case scenario analyses is performed. If the effects of primary and worst‐case meta‐analyses are in the same direction and magnitude, a definitive conclusion about the treatment effectiveness is made; otherwise no definitive conclusion is made. Worst case scenario analyses are also performed if efficacy is found for the prevention of secondary ischaemia.
Subgroup analyses include: (1) the comparison of the efficacy of preoperative volume expansion therapy with post operative volume expansion therapy; (2) the comparison of the efficacy of volume expansion therapy with crystalloid versus colloid; and (3) the comparison of the efficacy of varying degrees of aggressiveness of volume expansion as measured by haematocrit level or the central venous pressure (CVP).
Results
Description of studies
We identified three trials. One trial, performed in the Columbia Presbyterian Medical Center, New York, USA, was truly randomised (CPMC), two others were quasi‐randomised but had comparable baseline criteria in both groups (Egge 2001; Rosenwasser 1983).
One trial of peri‐operative haemodilution was not included because most of the patients were operated upon later than 14 days after onset of the SAH. Moreover, comparison was by two cohorts, and not by random allocation.
In the truly randomised trial, patients undergoing aneurysmal clipping on or before the sixth day after the SAH were randomised after operation to either hypervolaemic treatment or normovolaemic treatment (CPMC). Patients assigned to the hypervolaemia treatment received 5% albumin solution (250 ml/2 hrs) if pulmonary artery diastolic pressure (PADP) fell below 14 mm Hg or CVP fell below 8 mm Hg. Patients in the control group received similar albumin infusions if PADP fell below 7 mm Hg or CVP below 5 mm Hg. In both groups, patients who developed secondary ischaemia were switched to a hypertensive‐hypervolaemic protocol. Clinical outcome was assessed according to the Glasgow Outcome Scale at 14 days and three months after SAH. Symptomatic vasospasm was defined as a focal neurological deficit or deterioration in the level of consciousness, with either confirmation of infarction in CT scans or exclusion of other possible causes.
One of the quasi‐randomised studies, performed in one centre in Norway, included 16 patients in the intervention arm and 16 in the control arm (Egge 2001). Patients were eligible if they were in a fair or good clinical condition before operation. The study treatment was begun after operative clipping of aneurysm. The intervention strategy consisted of triple‐H fluid management therapy for 12 days, aiming for central venous pressure between 8 and 12 mm Hg, haematocrit between 0.30 and 0.35 and postoperative mean arterial pressure greater than 20 mm Hg higher than pre‐operatively. Baseline fluid management consisted of 2000 ml of 5% dextrose and 2000 ml of 0.9% saline and 1000 to 1500 ml colloids. The control strategy consisted of normovolaemic fluid therapy aiming for neutral fluid balance. Baseline fluid management consisted of 1000 ml of 5% dextrose and 1000 ml of 0.9% saline, without administering albumen or colloids. Clinical outcome assessment was done at 14 days and after one year.
The other quasi randomised trial, performed in one centre in Philadelphia, included 30 hypertensive patients (no definition given for hypertension) with angiographically documented aneurysmal SAH (Rosenwasser 1983). This trial was the only one where study treatment was installed before occlusion of the aneurysm. The study population was a consecutive series of patients who were not moribund (Hunt and Hess grade I through IV) on admission. Every other patient was given study treatment (hypervolaemia); the other patients served as controls (normovolaemia) (Rosenwasser 1996). There is no information regarding baseline‐characteristics for the treatment and control groups. The hypervolaemic treatment with 5% albumin and crystalloid aimed to obtain a haematocrit level of 0.45 and a pulmonary wedge pressure at 12 to 15 mm Hg. All patients received antihypertensive treatment to maintain the systolic blood pressure around 120 mm Hg, but if clinical signs of secondary ischaemia developed, hypertension was induced in the treatment group patients. Aneurysm clipping was scheduled for 7 to 10 days after SAH. Outcome assessment was done at the day of operation; no data were collected for the period after operation, and information on adverse effects of the treatment is not available; therefore, this trial is not included in the analysis.
Risk of bias in included studies
In the New York trial (CPMC), patients were stratified according to the number of days lapsed since the onset of SAH and the postoperative clinical condition. Randomisation was done postoperatively by means of an on‐site computer system (Klebanoff 1996) to either hypervolaemic treatment or normovolaemic treatment. Clinical outcome and the occurrence of symptomatic vasospasm were assessed in an unblinded fashion. Clinical outcome was assessed according to an intention‐to‐treat analysis.
In the Norwegian trial (Egge 2001), patients were stratified according to the amount of extravasated blood on CT (assessed by means of Fisher's grading system). The first included patient per Fisher category was randomly (method unknown) entered into one of the two treatment strategies. Thereafter, consecutive patients were entered alternately into the groups. No definition is provided for 'clinical vasospasm'. Clinical outcome by means of the Glasgow Outcome Scale was assessed at 12 months, in an unblinded fashion.
In the trial from Philadelphia (Rosenwasser 1983), patients were allocated alternately between study‐ and control treatment (Rosenwasser 1996). Outcome assessment was on the day of operation (day 7 to 10 after the SAH), and unblinded. No further follow up data were collected.
Effects of interventions
One truly randomised trial and one quasi‐randomised trial with comparable baseline‐characteristics for both groups were included in the analyses. Volume expansion therapy did not improve outcome (RR 1.0; 95% CI 0.5 to 2.2), nor the occurrence of secondary ischaemia (RR 1.1; 95% CI 0.5 to 2.2). Hypervolaemia tended to increase the rate of complications (RR 1.8; 95% CI 0.9 to 3.7).
In the Philadelphia trial (Rosenwasser 1983), treatment resulted in a reduction of secondary ischaemia (RR 0.33; 95% CI 0.11 to 0.99) and case fatality (RR 0.20; 95% CI 0.07 to 1.2) in the pre‐operative period. No rebleeding occurred in either group of patients.
As only two trials with small numbers of patients were included in the analyses, the planned subgroup analyses were not performed
Discussion
At present there are insufficient data on the effect of volume expansion on clinical outcome and secondary ischaemia in patients with aneurysmal subarachnoid haemorrhage. In two trials on post‐operative hypervolaemia no beneficial effect was found, but complications occurred more often in the intervention strategy. The intervention in one of the included studies consisted of a combination of hypervolaemia, haemodilution, and hypertension. The effect of hypervolaemia per se could not be assessed from this particular trial.
In the two trials of post‐operative treatment included in the analyses not even a trend for effect was found on clinical outcome measurements or physiological parameters (CPMC; Egge 2001). This seems to contrast with the finding in the Philadelphia trial that volume expansion tended to reduce the frequency of preoperative secondary ischaemia (Rosenwasser 1983). However, all studies included only small numbers of patients; moreover there are differences in the treatment strategies. Whereas in the trials included in the analyses the hypervolaemic treatment was initiated only after aneurysm clipping and was targeted to maintain a haematocrit below 0.35 (CPMC; Egge 2001), in the Philadelphia trial treatment was started preoperatively (on admission) and was targeted to obtain a haematocrit of 0.45 (Rosenwasser 1983). Because initiation of hypervolaemia might increase the risk of rebleeding, it is important to discriminate between trials initiating hypervolaemia before aneurysm occlusion and those initiating hypervolaemia only after aneurysm occlusion. Unfortunately, there are currently no relevant outcome data for hypervolaemia initiated before aneurysm occlusion.
In May 2003, a systematic review of treatment with hypertension, hypervolaemia and haemodilution was published (Treggiari 2003). The review was done before the Norwegian trial (Egge 2001) had been published; therefore that trial could not be included. Four trials were included; in addition to the trials from New York (CPMC) and Philadelphia (Rosenwasser 1983) that were included in our review, two other trials were included. One of these trials used historical controls (Vermeij 1998), and in the other trial hypervolaemia was the control strategy whereas antiplatelet therapy was under study (Yano 1993). Results from this meta‐analysis including potentially biased studies suggested that triple‐H therapy reduces the case fatality rate. Nevertheless, the other reviewers concluded that the paucity of information and important limitations in the design of the studies included in their review precluded evaluation of the efficacy of this therapy and formulation of any of the recommendations regarding its use. This conclusion is in keeping with the conclusion of our review.
Authors' conclusions
Implications for practice.
There is at present no convincing evidence to support the routine use of volume expansion therapy in patients with aneurysmal subarachnoid haemorrhage, either before or after aneurysm surgery.
Implications for research.
Volume expansion is often used in the treatment of patients with aneurysmal subarachnoid haemorrhage but there is no good evidence to support this therapy, so randomised trials are urgently needed. These trials should have a well defined method of volume expansion (type of volume expander, target values for physiological variables, concurrent use of induced hypertension), a well defined study population (pre‐operative period or post‐operative period) and a well defined objective (treatment is given in all patients to prevent delayed cerebral ischaemia, or treatment is given only in patients who have clinical features of delayed cerebral ischaemia). Since volume expansion might increase the risk of rebleeding in patients with untreated aneurysms, the trials should initially be confined to patients with occluded aneurysms. If the aforementioned trials prove that the treatment is effective then, further trials should include patients with still untreated aneurysms.
What's new
| Date | Event | Description |
|---|---|---|
| 30 July 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 1997 Review first published: Issue 1, 2000
| Date | Event | Description |
|---|---|---|
| 5 January 2004 | New search has been performed | In the previous version, trials were included if study medication was begun within three days after onset of subarachnoid haemorrhage. In this updated version we have allowed trials that started study medication within two weeks after onset. This change was made to include studies that use hypervolaemia as a treatment if secondary ischaemia develops and not only as a preventive measure. Since the last publication of the review, one new trial has been identified and found eligible, and for one other trial clinical data have become available. Therefore, we now have two trials included in the analyses. Nevertheless, the amount of data is still sparse because both trials included only small numbers of patients. One new review on hypervolaemia has been identified and is discussed in this updated version. |
Acknowledgements
We would like to thank Drs. RH Rosenwasser and Mitosek‐Sabbo for providing us with extra data from their trials.
Appendices
Appendix 1. MEDLINE search strategy
MEDLINE (Ovid)
1. Subarachnoid Hemorrhage/ 2. intracranial hemorrhages/ or cerebral hemorrhage/ or vasospasm, intracranial/ 3. Intracranial Aneurysm/ 4. Rupture, Spontaneous/ 5. 3 and 4 6. Aneurysm, Ruptured/ 7. exp brain/ 8. 6 and 7 9. ((subarachnoid or arachnoid) adj6 (haemorrhage$ or hemorrhage$ or bleed$ or blood$)).tw. 10. Vasospasm, Intracranial/ 11. ((cerebral or intracranial or cerebrovascular) adj6 (vasospasm or spasm)).tw. 12. sah.tw. 13. 1 or 2 or 5 or 8 or 9 or 10 or 11 or 12 14. exp Plasma Substitutes/ 15. Blood Substitutes/ 16. blood/ or plasma/ 17. Blood Transfusion/ 18. Fluid Therapy/ 19. Rehydration Solutions/ 20. Hemodilution/ 21. exp Albumins/ 22. Sodium Chloride/ 23. exp Colloids/ 24. Gelatin/ 25. exp Dextrans/ 26. Hetastarch/ 27. (volume adj (expand$ or expans$ or replace$)).tw. 28. plasma substitute$.tw. 29. (hydration or rehydration or fluid therapy).tw. 30. (hypervolaem$ or hypervolem$ or hemodilution or haemodilution).tw. 31. (albumin$ or crystalloid or colloid$ or gelatin or gelafusal or dextran$ or hetastarch or hydroxyethyl starch or pentastarch or HES or saline or blood plasma or fresh frozen plasma).tw. 32. plasma volume/de or blood volume/de 33. or/14‐32 34. 13 and 33 35. limit 34 to human
Appendix 2. EMBASE search strategy
EMBASE (Ovid)
1. subarachnoid hemorrhage/ 2. brain hemorrhage/ or brain vasospasm/ or intracranial aneurysm/ or brain artery aneurysm/ 3. brain artery aneurysm rupture/ 4. ((subarachnoid or arachnoid) adj6 (haemorrhage$ or hemorrhage$ or bleed$ or blood$)).tw. 5. ((cerebral or intracranial or cerebrovascular) adj6 (vasospasm or spasm)).tw. 6. sah.tw. 7. or/1‐6 8. exp plasma substitute/ or exp blood substitution/ or exp blood transfusion/ 9. exp fluid therapy/ or hemodilution/ or hypervolemia/ 10. albumin/ or sodium chloride/ or exp colloid/ or crystalloid/ or gelatin/ or dextran/ or hetastarch/ 11. ((volume or plasma) adj (expand$ or expans$ or replace$ or substitut$ or therap$)).tw. 12. (hydration or rehydration or fluid therapy).tw. 13. (hypervolaem$ or hypervolem$ or hemodilution or haemodilution).tw. 14. (albumin$ or crystalloid or colloid$ or gelatin or gelafusal or dextran$ or hetastarch or hydroxyethyl starch or pentastarch or HES or saline or blood plasma or fresh frozen plasma).tw. 15. or/8‐14 16. 7 and 15 17. limit 16 to human
Data and analyses
Comparison 1. Poor outcome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 treatment started after occlusion of the aneurysm | 2 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.45, 2.22] |
1.1. Analysis.
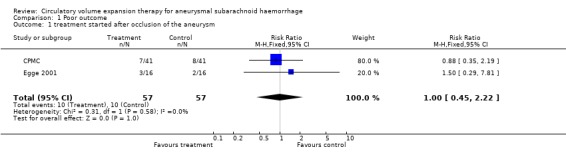
Comparison 1 Poor outcome, Outcome 1 treatment started after occlusion of the aneurysm.
Comparison 2. Case fatality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 treatment started after occlusion of the aneurysm | 2 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.18, 3.20] |
2.1. Analysis.
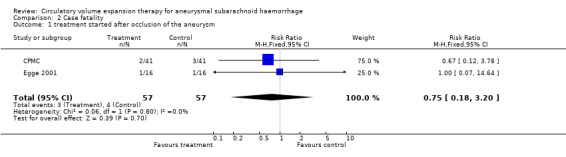
Comparison 2 Case fatality, Outcome 1 treatment started after occlusion of the aneurysm.
Comparison 3. Secondary ischaemia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 clinical signs of secondary ischaemia | 2 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.54, 2.16] |
| 1.1 treatment started before occlusion of the aneurysm | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 treatment started after occlusion of the aneurysm | 2 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.54, 2.16] |
| 2 infarct on CT/MR | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.55, 5.53] |
| 2.1 treatment started before occlusion of the aneurysm | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 treatment started after occlusion of the aneurysm | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.55, 5.53] |
3.1. Analysis.
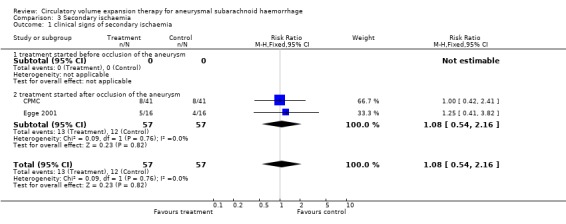
Comparison 3 Secondary ischaemia, Outcome 1 clinical signs of secondary ischaemia.
3.2. Analysis.
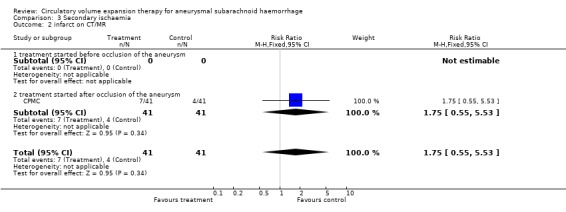
Comparison 3 Secondary ischaemia, Outcome 2 infarct on CT/MR.
Comparison 4. Rebleeding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 treatment started after occlusion of the aneurysm | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.
Comparison 4 Rebleeding, Outcome 1 treatment started after occlusion of the aneurysm.
Comparison 5. Complications.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 treatment started after occlusion of the aneurysm | 2 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.85, 3.71] |
5.1. Analysis.
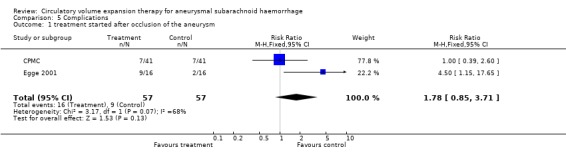
Comparison 5 Complications, Outcome 1 treatment started after occlusion of the aneurysm.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
CPMC.
| Methods | Patients were stratified according to number of days lapsed since the onset of SAH and the postoperative clinical condition (assessed by means of the H&H grading system). Randomisation was done postoperatively by means of an on‐site computer system to either hypervolemic treatment or normovolemic treatment. Clinical outcome and the occurrence of symptomatic vasospasm were assessed in an unblinded fashion. Clinical outcome was assessed according to an intention‐to‐treat analysis. | |
| Participants | Setting: 1 centre (Columbia ‐ Presbyterian Medical Centre). Rx: 42 patients; control: 42 patients. Eligible were patients undergoing aneurysmal clipping on or before the sixth day after onset of SAH. Further inclusion criteria were: (1) H&H grade I through IV after operation; (2) age between 18 and 80 years; (3) no symptomatic vasospasm immediately after operation. Exclusion criteria were: (1) congestive heart failure; (2) pregnancy; and (3) renal insufficiency. | |
| Interventions | All patients received a baseline crystalloid infusion of 80 ml/h 5% dextrose and 0.9% saline. Patients assigned to the hypervolaemia treatment received additional 5% albumin solution (250 ml/2hrs) if PADP fell below 14 mmHg or CVP fell below 8 mmHg. Patients in the control group received similar albumin infusions if PAD fell below 7 mmHg or CVP below 5 mmHg. In both groups, patients who developed secondary ischaemia were switched to a hypertensive‐hypervolemic protocol. | |
| Outcomes | Clinical outcome was assessed according to the Glasgow Outcome Scale at 14 days and 3 months after SAH. Symptomatic vasospasm was defined as a focal neurological deficit or deterioration in the level of consciousness, with either confirmation of infarction in CT scans or exclusion of other possible causes. Other outcome measurements were: cerebral blood volume; cerebral blood flow; blood pressure and volume status. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Egge 2001.
| Methods | Patients were stratified according to amount of extravasated blood on CT (assessed by means of Fisher's grading system). The first included patient per Fisher category was randomly (method unknown) entered into one of the two treatment strategies. Thereafter, consecutive patients were entered alternately into the groups. Outcome assessment was not blinded. | |
| Participants | Location: 1 centre in Norway. Rx: 16 patients; control: 16 patients. Inclusion criteria: (1) operative clipping < 72 hours after SAH; (2) H&H grade I to III; (3) written informed consent. Exclusion criteria: (1) age > 75 years; (2) congestive heart failure; (3) pregnancy; (4) renal insufficiency. Patients were well balanced for major determinants of DCI and clinical outcome. | |
| Interventions | Study treatment was started after operative clipping of aneurysm. Intervention: triple‐H fluid management therapy for 12 days, aiming for CVP 8 to 12 mmHg, hematrocrit between 0.30 and 0.35 and postoperative MAP > 20 mmHg higher than pre‐operative MAP. Baseline fluid management consisted of 2000 ml of 5% dextrose and 2000 ml of 0.9% saline and 1000 to 1500 ml colloids. Control: normovolemic fluid therapy aiming for neutral fluid balance. Baseline fluid management consisted of 1000 ml of 5% dextrose and 1000 ml of 0.9% saline. No albumin or colloids were administered. | |
| Outcomes | (1) MAP and CVP daily; (2) Scandinavian Neurological Stroke Scale assessments; (3) TCD daily; (4) CT on day 8; (5) SPECT on days 4, 8 and 12; (6) GOS at 14 days and at one year; (7) clinical vasospasm (not defined); (8) complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Rosenwasser 1983.
| Methods | Allocation: every other patient was given study treatment according to order of admission. Treatment and outcome assessment were not blinded. | |
| Participants | Location: Temple University Heath Sciences Center, Philadelphia, Pennsylvania. Rx: 15 patients; control: 15 patients. Inclusion criteria: hypertensive (not specified) patients with SAH and angiographically confirmed aneurysm. Exclusion criterion: H&H grade V (moribund patients). | |
| Interventions | Study treatment: hypervolaemia induced with packed red cells (target: hematocrit 0.45), 5% albumin and crystalloid (PCWP target 12 to 15 mmHg). Both study and control patients were treated with antihypertensive medications (target: systolic blood pressure 120 mmHg) | |
| Outcomes | Pre operative death and pre operative "clinical signs of vasospasm" (not defined) follow up until operation (performed 7 to 10 days after SAH). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
CT: computerised axial tomography CVP: central venous pressure DCI: delayed cerebral ischaemia GOS: Glasgow Outcome Scale H&H: Hunt and Hess grading system for patients with SAH IV: Intravenous MAP: mean arterial pressure PADP: pulmonary artery diastolic pressure PCWP: pulmonary capillary wedge pressure RCT: randomised controlled trial Rx: treated group patients (volume expansion therapy) SAH: subarachnoid haemorrhage SPECT: single photon emission computed tomography TC: trans cranial doppler
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Mitosek‐Sabbo | In an email of 26 September 2003, Dr Mitosek‐Sabbo stated this was a randomised study. Methods: 80 consecutive patients; first 50 underwent peri‐operative hemodilution, the remaining 30 constituted a control group. Intervention: peri‐operative hemodilution by extracting blood and replacement by colloids aiming at a hematocrit of 0.3. Blood retransfused after operation. Patients: WFNS I, II or III before surgery. Only 17 of 50 treatment patients and 12 of 30 controls were operated within 14 days; the remaining patients were operated more than 14 days after the SAH. Main reasons for exclusion: probably not randomised, and most patients treated more than 14 days after SAH. |
SAH: subarachnoid haemorrhage WFNS: World Federation of Neurological Surgeons (grading scale for subarachnoid haemorrhage)
Contributions of authors
VL Feigin: participated in developing the protocol; retrieving papers; data extraction; appraising the quality of studies; data management; data analysis; data interpretation; and writing the review.
GJE Rinkel: participated in writing the grant application; developing the protocol; data extraction; appraising the quality of studies; data analysis; data interpretation; writing the review; and entering the review into RevMan. Prof Rinkel is the guarantor for this review.
A Algra: participated in writing the grant application; developing the protocol; appraising the quality of studies; data analysis; data interpretation; and writing the review.
J van Gijn: participated in writing the grant application; developing the protocol; appraising the quality of studies; data interpretation; writing the review; and entering the review into RevMan.
The updates in November 2001 and November 2003 were prepared by GJE Rinkel.
Sources of support
Internal sources
University Medical Centre, Utrecht, Netherlands.
External sources
The Netherlands Heart Foundation, Netherlands.
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
CPMC {published data only}
- Klebanoff L, Fink ME, Lennihan L, Solomon RA, Mayer SA, Beckford AR, et al. Management of cerebral vasospasm in the 1990s. Clinical Neuropharmacology 1995;18(2):127‐37. [DOI] [PubMed] [Google Scholar]
- Lennihan L, Mayer SA, Fink ME, Beckford A, Paik MC, Zhang H, et al. Effect of hypervolemic therapy on cerebral blood flow after subarachnoid hemorrhage: a randomized controlled trial. Stroke 2000;31:383‐91. [DOI] [PubMed] [Google Scholar]
- Lennihan L, Solomon RA, Beckford AR, Fink ME, Paik M, et al. Comparison of effects of hypervolemia and normovolemia on cerebral blood flow after subarachnoid hemorrhage. Stroke 1997;28:249. [DOI] [PubMed] [Google Scholar]
- Lennihan L, Solomon RA, Fink ME, Paik M, Klebanoff L, Beckford AR, et al. Comparison of effects of hypervolemia and normovolemia on clinical outcome and medical complications after subarachnoid hemorrhage. Neurology 1997;3:365. [Google Scholar]
- Lennihan L, Solomon RA, Mayer SA, Prohovnik I, Fink ME, Klebanoff L, et al. Effect of volume therapy on cerebral blood flow after subarachnoid hemorrhage. Neurology 1994;44 (Suppl 2):862S. [Google Scholar]
- Mayer SA, Solomon RA, Fink ME, Lennihan L, Stern L, Beckford AR, et al. Effect of 5% albumin solution on sodium balance and blood volume after subarachnoid hemorrhage. Neurosurgery 1998;42:759‐68. [DOI] [PubMed] [Google Scholar]
Egge 2001 {published data only}
- Egge A, Waterloo K, Sjoholm H, Solberg T, Ingebrigtsen T, Romner B. Prophylactic hyperdynamic postoperative fluid therapy after aneurysmal subarachnoid hemorrhage: a clinical, prospective, randomized, controlled study. Neurosurgery 2001;49(3):593‐606. [DOI] [PubMed] [Google Scholar]
Rosenwasser 1983 {published and unpublished data}
- Rosenwasser RH, Delgago TE, Buchheit WA, Freed MH. Control of hypertension and prophylaxis against vasospasm in cases of subarachnoid hemorrhage. Neurosurgery 1983;12:658‐61. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Mitosek‐Sabbo {published and unpublished data}
- Mitosek‐Sabbo D, Trojanowski T, Czochra M. Peri‐operative hemodilution in intracranial aneurysm surgery. Medical Science Monitor 1998;4(2):292‐6. [Google Scholar]
Additional references
ACROSS 2000
- The ACROSS Study Group. Epidemiology of aneurysmal subarachnoid hemorrhage in Australia and New Zealand. Stroke 2000;31:1843‐50. [DOI] [PubMed] [Google Scholar]
Brilstra 2000
- Brilstra EH, Rinkel GJE, Algra A, Gijn J. Rebleeding, secondary ischemia and timing of operation in patients with subarachnoid hemorrhage. Neurology 2000;55:1656‐60. [DOI] [PubMed] [Google Scholar]
Hop 1997
- Hop JW, Rinkel GJE, Algra A, Gijn J. Case fatality rates and functional outcome after subarachnoid hemorrahge: a systematic review. Stroke 1997;28:660‐4. [DOI] [PubMed] [Google Scholar]
Kassell 1990
- Kassell NF, Torner JC, Haley EC Jr. The International Cooperative Study on the timing of aneurysm surgery. Part 1: Overall management results. Journal of Neurosurgery 1990;73:18‐36. [DOI] [PubMed] [Google Scholar]
Klebanoff 1996
- Klebanoff L. Personal communication 1996.
Linn 1996
- Linn FHH, Rinkel GJE, Algra A, Gijn J. Incidence of subarachnoid hemorrhage: role of region, year and rate of computed tomography: a meta‐analysis. Stroke 1996;27:625‐9. [DOI] [PubMed] [Google Scholar]
Maroon 1979
- Maroon JC, Nelson PB. Hypovolemia in patients with subarachnoid hemorrhage: therapeutic implications. Neurosurgery 1979;4:223‐6. [DOI] [PubMed] [Google Scholar]
Rosenwasser 1996
- Rosenwasser R. Personal communication 1996.
Solomon 1984
- Solomon RA, Post KD, McMurtry JG III. Depression of circulating blood volume in patients after subarachnoid hemorrhage: implications for the treatment of symptomatic vasospasm. Neurosurgery 1984;15:354‐61. [DOI] [PubMed] [Google Scholar]
Treggiari 2003
- Treggiari MM, Walder B, Suter PM, Romand J‐A. Systematic review of the prevention of delayed neurological deficits with hypertension, hypervolemia, and hemodilution therapy following subarachnoid hemorrhage. Journal of Neurosurgery 2003;98(5):978‐84. [DOI] [PubMed] [Google Scholar]
van Gijn 1994
- Gijn J, Bromberg JEC, Lindsay KW, Vermeulen M. Definition of initial grading, specific events, and overall outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke 1994;25(8):1623‐7. [DOI] [PubMed] [Google Scholar]
Vermeij 1998
- Vermeij FH, Hasan D, Bijvoet HW, Avezaat CJ. Impact of medical treatment on the outcome of patients after aneurysmal subarachnoid hemorrhage. Stroke 1998;29:924‐30. [DOI] [PubMed] [Google Scholar]
Wijdicks 1985
- Wijdicks EFM, Vermeulen M, Haaf JA, Bakker WH, Gijn J. Volume depletion and natriuresis in patients with a ruptured intracranial aneurysm. Annals of Neurology 1985;18:211‐6. [DOI] [PubMed] [Google Scholar]
Yano 1993
- Yano K, Kuroda T, Tanabe Y, Yamada H. Preventive therapy against delayed cerebral ischaemia after aneurysmal subarachnoid haemorrhage: trials of thromboxane A2 synthetase inhibitor and hyperdynamic therapy. Acta Neurochirurgica 1993;125:15‐9. [DOI] [PubMed] [Google Scholar]


