Abstract
Background
Preterm infants are at risk of periventricular haemorrhage (PVH). This can be a sign of brain damage that might lead to neurodevelopmental abnormalities, including cerebral palsy. It has been suggested that vitamin K might improve coagulation in preterm infants and thereby decrease the risk of PVH.
Objectives
To assess the effects of vitamin K administered to women at risk of imminent very preterm birth to prevent PVH and associated neurological injury in the infant.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (20 December 2010).
Selection criteria
Randomised or quasi‐randomised trials of vitamin K administered parenterally or orally to women at risk of imminent preterm birth. The primary outcomes were neonatal mortality, neonatal neurological morbidity, as measured by the presence of PVH on ultrasound during the first week of life, and long‐term neurodevelopment. Secondary outcomes included other neonatal morbidity and any maternal side effects.
Data collection and analysis
Two review authors independently assessed eligibility, trial quality and extracted data.
Main results
Eight trials were included but only seven (843 women) contributed data to the results. The trials were of variable quality. Antenatal vitamin K was associated with a non‐significant reduction in all grades of PVH (risk ratio (RR) 0.76; 95% confidence interval (CI) 0.54 to 1.06) and a significant reduction in severe PVH (grades 3 and 4) (RR 0.58; 95% CI 0.37 to 0.91) for babies receiving prenatal vitamin K compared with control babies. When the two quasi‐randomised trials were excluded, antenatal vitamin K was associated with a non‐significant reduction in all grades of PVH (RR 0.87; 95% CI 0.60 to 1.26) and a non‐significant reduction in severe PVH (RR 0.82; 95% CI 0.49 to 1.36).
Treatment with vitamin K resulted in a significant reduction in the Bayley Mental Development Index at two years of age (mean difference (MD) ‐9.00; 95% CI ‐16.66 to ‐1.34, one trial, 121 children); however, these results are derived from one trial with many participants lost to follow up. No difference was found in the incidence of other neurodevelopmental abnormalities at paediatric follow up at 18 to 24 months or seven years of age between children born to mothers given vitamin K and children not so exposed.
Authors' conclusions
Vitamin K administered to women prior to very preterm birth has not been shown to significantly prevent PVH in preterm infants or improve neurodevelopmental outcomes in childhood.
Plain language summary
Vitamin K prior to preterm birth for preventing neonatal periventricular haemorrhage
Vitamin K given to women before a very preterm birth does not decrease the risk of bleeding in the brain and associated neurological injury in babies born very preterm.
Babies born very early (before 34 weeks) are at risk of bleeding in the brain (periventricular haemorrhage). This can be a cause of brain damage that might lead to neurological disabilities including cerebral palsy. Preterm infants have reduced levels of clotting factors, some of which require vitamin K for activation. Vitamin K may therefore help the blood to clot in preterm babies and so decrease this risk of haemorrhage. The review of trials did not find vitamin K, given as an injection to women immediately prior to a very preterm birth, decreased the risk of periventricular haemorrhage in their babies. There were too few data on children at follow up to assess the effects of vitamin K given immediately before very preterm birth on child development. Eight trials were included but only seven contributed data to the results. These seven trials involved 843 women. The trials were of variable quality and only two trials used a placebo.
In four trials that did not use placebo, more women were treated with vitamin K also received corticosteroids before giving birth compared to those not receiving vitamin K. Prenatal corticosteroids are known to reduce the rate of haemorrhage. Women receiving vitamin K were also more likely to be treated with phenobarbital in one trial.
Women given vitamin K reported a rash in two trials.
Background
Description of the condition
Preterm infants born before 34 weeks' gestation, and especially if born before 30 weeks, are at significant risk of developing brain injury in association with periventricular haemorrhage (PVH). Such lesions are associated with later neurodevelopmental abnormalities, including cerebral palsy.
The cause of haemorrhage is uncertain, although it has been hypothesised that brain ischaemia followed by bleeding, usually arising in the subependymal germinal matrix, is the most likely explanation. Fluctuations in blood pressure and cerebral perfusion around birth and during early postnatal adaptation might also be involved. Coagulation disorders have been described in infants with PVH. Preterm infants in general have reduced levels of clotting factors, some of which require vitamin K for activation.
Most PVHs are manifest on head ultrasound within 72 hours of birth although the severity may change after this time (Vohr 2000).
Haemorrhages are usually graded for severity: grade 1 ‐ confined to the germinal matrix; grade 2 ‐ in the lateral ventricle; grade 3 ‐ distending the ventricle; grade 4 ‐ intracerebral.
The latter two grades are strongly associated with long‐term neurological sequelae.
Description of the intervention
Vitamin K given intramuscularly, intravenously or orally in the antenatal period.
How the intervention might work
It has been postulated that vitamin K might improve the coagulation status of preterm infants and reduce the frequency of PVH. As many haemorrhages are thought to originate close to the time of birth, prophylactic antenatal rather than postnatal treatment may be preferable. Maternally administered vitamin K only crosses the placenta in small amounts with a maternal‐fetal gradient of 30:1 (Shearer 1992).
For further background and discussion regarding prevention and treatment of PVH, seeHorbar 1992.
Why it is important to do this review
PVH has severe effects on the infant's brain and subsequent development. There is a need to identify the most safe and efficacious method to prevent PVH in preterm infants to improve mortality and neurodevelopmental outcomes.
Objectives
To assess the benefits and harms of vitamin K administered to women at risk of imminent very preterm birth with the primary aims of preventing neonatal mortality, PVH and the associated neurological injury in the infant.
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished and ongoing randomised or quasi‐randomised trials with reported data which compared outcomes for women at risk of preterm birth who were given vitamin K therapy, placebo or no treatment.
Types of participants
Women at risk of imminent very preterm birth.
Types of interventions
Vitamin K administered parenterally or orally to the mother prior to preterm birth (before 37 weeks' gestation).
Types of outcome measures
We only considered clinical outcome measures.
Primary outcomes
Neonatal mortality;
neonatal neurological morbidity as measured by the presence of periventricular haemorrhage on ultrasound during the first week of life;
long‐term neurodevelopment.
Secondary outcomes
Any maternal side effects;
other neonatal morbidity.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (20 December 2010).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We also searched the citation lists of relevant publications, review articles and included studies.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third person. We assessed included studies for quality and methodological details without consideration of the results.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors independently extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third person. There was no blinding of authorship. We entered data into Review Manager software (RevMan 2008) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the methods used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should have produced comparable groups.
We assessed the methods as:
adequate (any truly random process, e.g. random number table; computer random number generator);
inadequate (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence in sufficient detail and determined whether intervention allocation could have been foreseen in advance of, or during recruitment or changed after assignment.
We assessed the methods as:
adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear.
(3) Blinding (checking for possible performance bias)
We described for each included study all the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel;
adequate inadequate or unclear for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
adequate (e.g. where there were no missing data or where reasons for missing data are balanced across groups);
inadequate (e.g. where missing data are likely to be related to outcomes or are not balanced across groups);
unclear (e.g. where there is insufficient reporting of attrition or exclusions to permit a judgement to me made).
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
adequate (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
inadequate (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear.
(6) Other sources of bias
We described for each included study any important concerns we had about other possible sources of bias. For example, was there a potential source of bias related to the specific study design? Was the trial stopped early due to some data‐dependent process? Was there extreme baseline imbalance? Has the study been claimed to be fraudulent?
We assessed whether each study was free of other problems that could put it at risk of bias:
yes;
no;
unclear.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2009). With reference to (1) and (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
We carried out statistical analysis using the Review Manager software (RevMan 2008). We used fixed‐effect meta‐analysis for combining data in the absence of significant heterogeneity if trials were sufficiently similar. If we found significant heterogeneity, we explored this by sensitivity analysis followed by random‐effects if required.
Dichotomous data
For dichotomous data, we presented results as summary risk ratios with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measured the same outcome, but used different methods. If there was evidence of skewness, this was reported.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Where data were not reported for some outcomes or groups, we attempted to contact the study authors for further information.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the I² statistic. We regarded heterogeneity as substantial if I² was greater than 50%.
Assessment of reporting biases
Where we suspected reporting bias (see 'Selective reporting bias' above), we attempted to contact study authors asking them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Where we suspected publication bias (e.g. where only statistically significant results are reported), this was explored using funnel plots (Higgins 2009). We involved the project statistician in the interpretation of such analysis.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2008). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials' populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful we did not combine trials.
If we used random‐effects analyses, the results were presented as the average treatment effect with its 95% confidence interval, and the estimates of I².
If appropriate, we synthesised data from studies where results are expressed as dichotomous and continuous data. We involved the project statistician before attempting to synthesise different measures of treatment effect.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses based on gestational age or birthweight strata, or both, but they were not performed because of insufficient data.
We conducted the following planned subgroup analyses classifying whole trials by interaction tests as described by Deeks 2001:
gestational age;
birthweight;
gestational age and birthweight.
We were unable to perform the planned subgroup analyses due to insufficient data.
Sensitivity analysis
If we identified high levels of heterogeneity among the trials (I² exceeding 50%), we explored it by prespecified subgroup analysis and performed sensitivity analysis. A random‐effects meta‐analysis was used as an overall summary if this was considered appropriate. This involved analysis based on the rating of selection bias and attrition bias. This was done by excluding trials given an inadequate rating in the quality assessment for allocation of concealment and then inadequate (20% or more or unclear) for exclusions. Post hoc sensitivity analysis was done by removing the trial with dual intervention (Thorp 1994) in which both phenobarbital and vitamin K were compared with placebo control.
Results
Description of studies
Results of the search
Nine trials of prenatal vitamin K prior to preterm birth for the prevention of periventricular haemorrhage (PVH) were identified of which seven provided clinical outcomes and met the inclusion criteria.
Included studies
Eight trials were included but only seven trials contributed data to the results. Eight hundred and forty‐three women were recruited into these seven included trials. The details of each of these seven studies are given in the table of Characteristics of included studies. The Kazzi 1989 study used 10 mg of vitamin K intramuscularly repeated after four days then 20 mg of oral vitamin K daily. The Liu 2006 study used 10 mg of intravenous or intramuscular vitamin K daily for two to seven days. Ten milligrams of vitamin K intramuscularly every five days was the treatment in the Morales 1988 study. The Pathak 1990 study used 10 mg of intramuscular vitamin K between four and 96 hours before delivery. The Pomerance 1987 study used 10 mg of intramuscular vitamin K four hours before delivery. The treatment was repeated after five days if the women had not given birth. One study (Thorp 1994) used both vitamin K and phenobarbital as the treatment. Ten milligrams of intramuscular vitamin K was given, repeated in four days then 20 mg of oral vitamin K was given daily for eight days or until delivery. One study (Yang 1989) used a lower dose of vitamin K compared to the other trials.
Excluded studies
One trial was excluded (Anai 1993) because it did not specify that women in the trial were at imminent risk of preterm birth.
Risk of bias in included studies
Allocation
All except two of the trials (Liu 2006; Pomerance 1987) used formal randomisation although allocation concealment was unclear for Dickson 1994, Kazzi 1989, Morales 1988, Yang 1989 and Pathak 1990. Allocation was not concealed in the Pomerance 1987 trial as they used the last digit of the medical record number. In this trial, imbalance in prenatal corticosteroid use favouring the treatment group (50% compared with the control group 33%), as well as imbalances in neonatal factors, unlikely to be related to vitamin K treatment, suggest bias in treatment allocation. Allocation was not concealed in the Liu 2006 trial because participants were randomised according to their inpatient sequence.
Blinding
A placebo was used in three trials (Dickson 1994; Pathak 1990;Thorp 1994). The assessment of the primary outcome of PVH was blinded in all trials reporting this outcome except Pathak 1990 and Liu 2006.
Incomplete outcome data
The rates of exclusion of women after randomisation prior to hospital discharge varied being high (Kazzi 1989, 20%), moderate (Dickson 1994, 16% of the control group and none in the treatment group; Thorp 1994, 10%; Yang 1989, 7%), or not stated (Liu 2006; Morales 1988; Pathak 1990; Pomerance 1987). The main reason for exclusion was continuation of the pregnancy beyond 34 weeks or birth of an infant of more than 1500 g, or both.
In the one trial that reported follow up at two years (Thorp 1994), the rate of exclusion was high at 71%, although at the seven‐year follow up the rate of loss to follow up was lower at 28%.
Selective reporting
Six trials were free of selective reporting bias (Kazzi 1989; Liu 2006; Morales 1988; Pomerance 1987; Thorp 1994; Yang 1989). It was unclear in the Pathak 1990 study if all outcomes were reported. In Dickson 1994, only biochemical outcomes were reported.
Other potential sources of bias
The trials without a placebo had a higher rate of prenatal corticosteroid use in the vitamin K treated group. For Kazzi 1989 this was 5% versus 0%, and for Yang 1989 43% versus 17%. Similarly in the Morales 1988 trial, there was a significantly higher rate of prenatal corticosteroid use (74% versus 57%) and a higher rate of use of prenatal phenobarbital (39% versus 22%) in the treatment group compared with the control group. The rate of corticosteroid use was not recorded for Pathak 1990 or Liu 2006, although 44 of 84 (52%) of women in the Liu 2006 trial who were allocated to vitamin K, received dexamethasone as part of their treatment. The rate of PVH was much higher in the control groups in the Liu 2006 trial (66%) compared to the rate of PVH in the control groups of other trials.
SeeFigure 1 for review authors' judgements about each methodological quality item for each included study and Figure 2 for review authors' judgements about each methodological quality item presented as percentages across all included studies.
1.
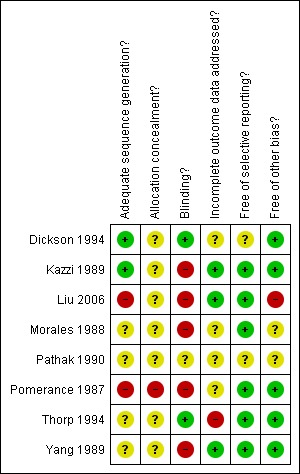
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
2.
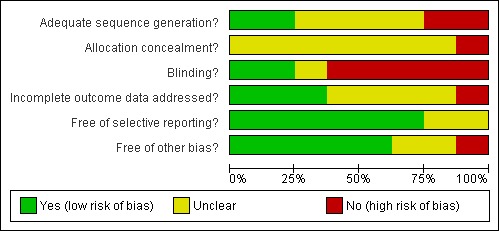
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Effects of interventions
1. Analyses of all trials
Primary outcomes
There was no significant difference in the risk of perinatal mortality (stillbirths, deaths prior to discharge and deaths postdischarge) between infants of mothers in the vitamin K group compared with the control group. Overall there was a non‐significant trend to a reduction in the rate of all grades of PVH (risk ratio (RR) 0.76; 95% confidence interval (CI) 0.54 to 1.06, seven trials, 851 infants) and a significant reduction in severe PVH (grades 3 and 4) (RR 0.58; 95% CI 0.37 to 0.91, seven trials, 851 infants) for infants receiving prenatal vitamin K compared with control babies.
Only one trial (Thorp 1994) reported on neurodevelopmental outcomes at paediatric follow up; however, this trial used a dual intervention of vitamin K and phenobarbital. For the Thorp 1994 trial, losses to follow up at two years were 71% and results should be interpreted cautiously. There was a discrepancy between the two published reports on childhood follow up at two years (Thorp 1997; Thorp 1999). In Thorp 1999, at two years of age, children in the phenobarbital group had significantly lower Bayley Mental Developmental Index (MDI) scores than children in the control group (mean MDI score 104, standard deviation 21 compared with mean MDI score 113, standard deviation 22). For the Thorp 1994 trial, losses to follow up at seven years were 28% and no significant difference was found between children in the treatment and control groups in developmental outcomes at seven years of age (Thorp 2003).
Secondary outcomes
One woman given vitamin K was reported as having a pruritic rash at the injection site in the Pomerance 1987 trial, and one woman in the vitamin K group developed a rash in the Thorp 1994 trial. Maternal side effects were not seen in three trials (Kazzi 1989; Pathak 1990; Yang 1989) and were not reported in two trials (Liu 2006; Morales 1988).
There were no significant differences in the risk of respiratory distress syndrome, patent ductus arteriosus, use of mechanical ventilation, pulmonary air lea or a low Apgar score at five minutes.
2. Analyses with the exclusion of trials with inadequate allocation of treatment ( Liu 2006; Pomerance 1987)
This analysis included five trials (Kazzi 1989; Morales 1988; Pathak 1990; Thorp 1994; Yang 1989) with a total of 639 women.
Primary outcomes
There were no differences in the risk of perinatal death between the vitamin K and control groups. No significant difference was seen in the risk of having any grade of PVH or in severe PVH between babies receiving prenatal vitamin K and control babies.
As above, the one trial (Thorp 1994) which reported on neurodevelopment at two years of age showed significantly lower Bayley MDI scores from children in the vitamin K and phenobarbital group compared with the control group at two‐year paediatric follow up (Thorp 1997; Thorp 1999). For the Thorp 1994 trial, at seven years of age no differences were found between children in the treatment and control groups (Thorp 2003).
Secondary outcomes
Similar to the overall analysis, there was no significant increase in the risk of maternal side effects or neonatal morbidity between the vitamin K and the control groups.
3. Pre‐specified analyses based on high‐quality trials
This analysis excluded trials with inadequate allocation of treatment (Liu 2006; Pomerance 1987) and trials with 20% or more exclusions after randomisation or unclear for exclusions (Kazzi 1989; Morales 1988; Pathak 1990; Yang 1989). Only one trial fulfilled these criteria (Thorp 1994). However, this trial had exclusions of over 71% for their two‐year childhood follow up and 28% for their seven‐year follow up and so the data for these assessments have not been included in this analysis.
Primary outcomes
No significant difference was seen in the risk of perinatal death. Similarly, there were no differences in the risk of having any grade of PVH, or in severe PVH, between babies who received prenatal vitamin K and control babies.
Secondary outcomes
There was no significant increase in the risk of any maternal side effects with prenatal vitamin K treatment. No significant difference was seen in the risk of neonatal morbidities between babies of mothers who were treated with vitamin K or placebo.
Subgroup analyses planned, after exclusion of the trial with combined therapy of vitamin K and phenobarbital (Thorp 1994) and poorer quality trials, left no trials for consideration. Subgroup analyses based on gestational age or birthweight strata, or both, were not possible with the limited data reported.
Discussion
Summary of main results
The benefit of vitamin K in preventing severe periventricular haemorrhage (PVH) and the trend to an apparent benefit of prenatal vitamin K in preventing all grades of PVH, shown in the overall analysis, is unlikely to be a true effect of the drug. This is suggested by the change in treatment effect when only higher quality trials are considered. There was an imbalance in the use of prenatal corticosteroids, which is known to reduce rates of PVH, in all four trials that were not placebo blinded. The effect of prenatal vitamin K on long‐term neurodevelopment is uncertain. No differences were seen in the one trial (Thorp 1994) that reported follow up after hospital discharge of the children, although the rates of exclusion were high (71% at two years and 28% at seven years).
The apparent lack of effect of vitamin K could be explained by poor placental transfer of vitamin K and, therefore, poor effect on fetal coagulation factors.
Overall completeness and applicability of evidence
No data are available at present on the effects of vitamin K when given to women prior to preterm birth at different gestations. It is not recommended that vitamin K be used in routine practice due to the poor quality of the current evidence and the questionable efficacy of vitamin K in preventing PVH in the high‐quality trial. Also, only four of the eight included trials reported on maternal side effects. This must be assessed more completely before vitamin K is accepted into routine practice.
Quality of the evidence
There is a paucity of high‐quality evidence in assessing the efficacy of vitamin K to reduce PVH in preterm infants. Only one trial in this review (Thorp 1994) had adequate sequence generation, allocation concealment and less than 20% post‐randomisation exclusions.
Potential biases in the review process
The largest trial (Thorp 1994), which was also the best quality trial, had phenobarbital as a co‐treatment with vitamin K. Evidence from the systematic review of trials evaluating prenatal phenobarbital prior to preterm birth (Crowther 2010a) suggests that phenobarbital does not influence the rate of PVH.
Authors' conclusions
Implications for practice.
Overall this analysis suggests that vitamin K administration to the mother prior to imminent very preterm birth does not prevent intraventricular haemorrhage.
Implications for research.
Further trials cannot be considered a priority. However, if future trials are planned, they should be of high quality and should examine the effects of vitamin K administration at gestational ages with a high risk of periventricular haemorrhage, stratify for gestational age and ensure minimal exclusions after randomisation. Neurodevelopmental status at follow up should be measured as the most important outcome.
What's new
| Date | Event | Description |
|---|---|---|
| 20 December 2010 | New search has been performed | Search updated. Second reference for Liu 2006 trial added. |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 3, 1997
| Date | Event | Description |
|---|---|---|
| 11 November 2009 | New citation required but conclusions have not changed | New author helped prepare the last update. |
| 14 April 2008 | Amended | Converted to new review format. |
| 31 March 2008 | New search has been performed | Search updated. Two new trials identified (Liu 2006; Pathak 1990) and Thorp 2003 study found which is a follow‐up report on the Thorp 1994 trial ‐ this reports developmental outcomes in children at age seven. |
| 30 September 2000 | New search has been performed | Search updated. One new reference found. No new trials identified. |
Acknowledgements
Professor Roger Soll compiled an earlier version of this review in 1991.
Special thanks to Lynn Hampson who performed the literature search for this update and to Philippa Middleton who commented on all drafts of this update.
We acknowledge the significant contributions of Professor David Henderson‐Smart to the development of the original protocol, identification and selection of studies for inclusion, data extraction and preparation of the text of the initial review and previous updates.
Data and analyses
Comparison 1. Vitamin K versus control ‐ all studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal mortality | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Stillbirths | 1 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.18, 21.06] |
| 1.2 Death of live born infants prior to discharge | 4 | 578 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.68, 1.94] |
| 1.3 Infant deaths post‐discharge | 1 | 375 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.96 [0.36, 133.88] |
| 2 All periventricular haemorrhage (PVH) | 7 | 851 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.54, 1.06] |
| 3 Severe (grades 3 and 4) PVH | 7 | 851 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.37, 0.91] |
| 4 Bayley Mental Developmental Index at 2 years | 1 | 121 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐16.66, ‐1.34] |
| 5 Bayley Psychomotor Developmental Index at 2 years | 1 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐11.39, 1.39] |
| 6 Cerebral palsy at 7 year follow up | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.33, 1.76] |
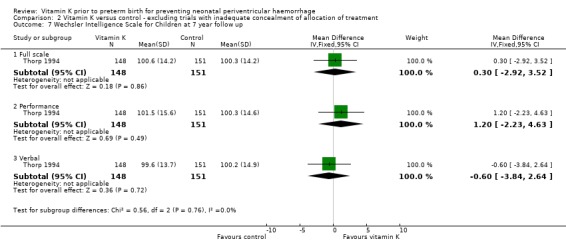
| 7 Wechsler Intelligence Scale for Children at 7 year follow up | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Full scale | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐2.92, 3.52] |
| 7.2 Performance | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐2.23, 4.63] |
| 7.3 Verbal | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐3.84, 2.64] |
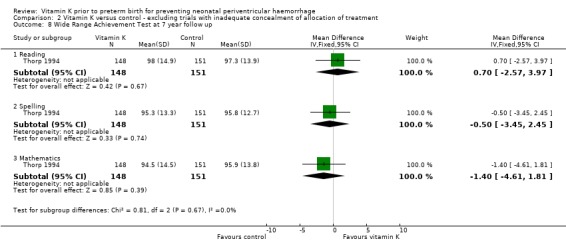
| 8 Wide Range Achievement Test at 7 year follow up | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Reading | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐2.57, 3.97] |
| 8.2 Spelling | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐3.45, 2.45] |
| 8.3 Mathematics | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐4.61, 1.81] |
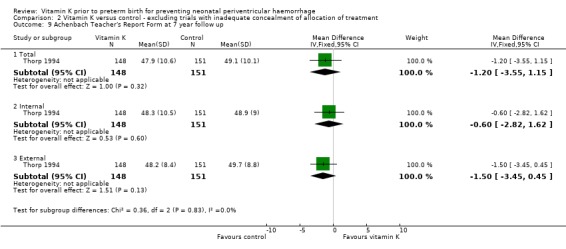
| 9 Achenbach Teacher's Report Form at 7 year follow up | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Total | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐3.55, 1.15] |
| 9.2 Internal | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.82, 1.62] |
| 9.3 External | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐3.45, 0.45] |
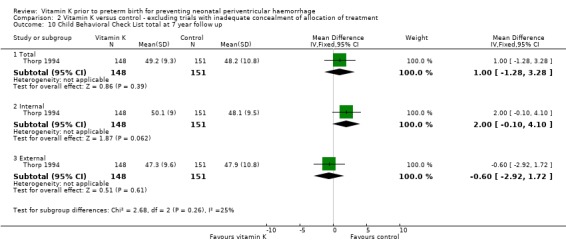
| 10 Child Behavioral Check List total at 7 year follow up | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Total | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐1.28, 3.28] |
| 10.2 Internal | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐0.10, 4.10] |
| 10.3 External | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.92, 1.72] |
| 11 Maternal side effects (any) | 4 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.78 [0.41, 35.07] |
| 12 Respiratory distress syndrome | 4 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.73, 1.26] |
| 13 Patent ductus arteriosus | 3 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.57, 1.63] |
| 14 Use of mechanical ventilation | 5 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.84, 1.10] |
| 15 Pulmonary air leak | 2 | 475 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.59, 5.10] |
| 16 Low Apgar score at 5 minutes | 3 | 646 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.69, 1.62] |
1.1. Analysis.
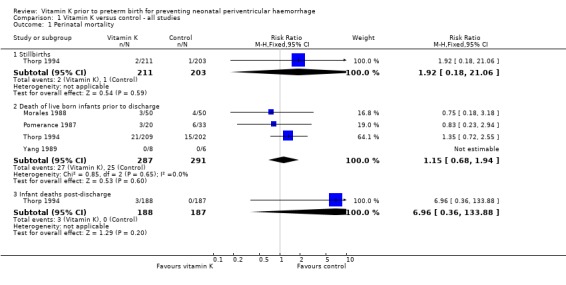
Comparison 1 Vitamin K versus control ‐ all studies, Outcome 1 Perinatal mortality.
1.2. Analysis.
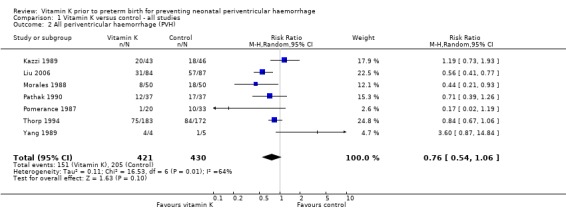
Comparison 1 Vitamin K versus control ‐ all studies, Outcome 2 All periventricular haemorrhage (PVH).
1.3. Analysis.
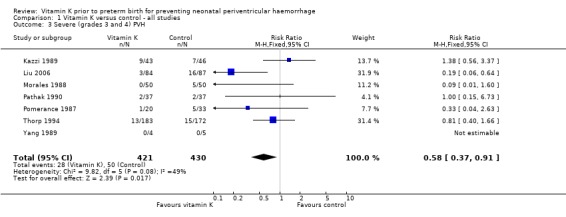
Comparison 1 Vitamin K versus control ‐ all studies, Outcome 3 Severe (grades 3 and 4) PVH.
1.4. Analysis.
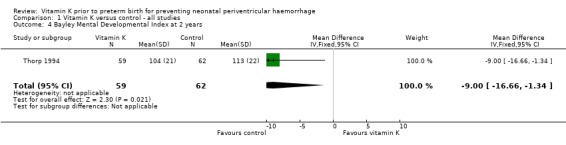
Comparison 1 Vitamin K versus control ‐ all studies, Outcome 4 Bayley Mental Developmental Index at 2 years.
1.5. Analysis.
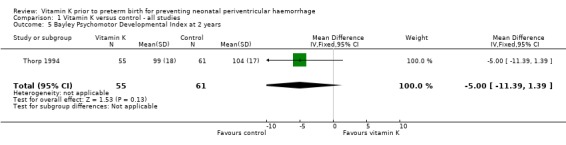
Comparison 1 Vitamin K versus control ‐ all studies, Outcome 5 Bayley Psychomotor Developmental Index at 2 years.
1.6. Analysis.
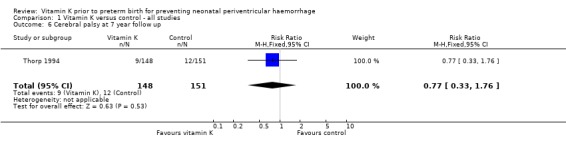
Comparison 1 Vitamin K versus control ‐ all studies, Outcome 6 Cerebral palsy at 7 year follow up.
1.7. Analysis.
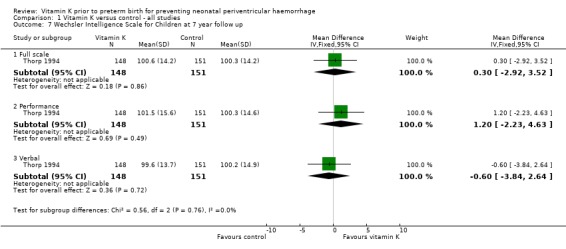
Comparison 1 Vitamin K versus control ‐ all studies, Outcome 7 Wechsler Intelligence Scale for Children at 7 year follow up.
1.8. Analysis.
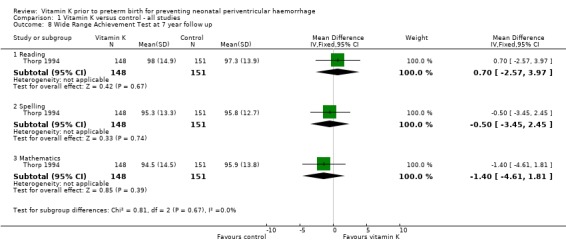
Comparison 1 Vitamin K versus control ‐ all studies, Outcome 8 Wide Range Achievement Test at 7 year follow up.
1.9. Analysis.
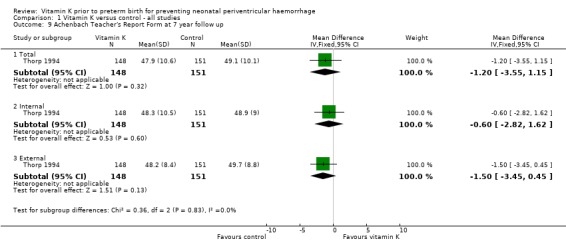
Comparison 1 Vitamin K versus control ‐ all studies, Outcome 9 Achenbach Teacher's Report Form at 7 year follow up.
1.10. Analysis.
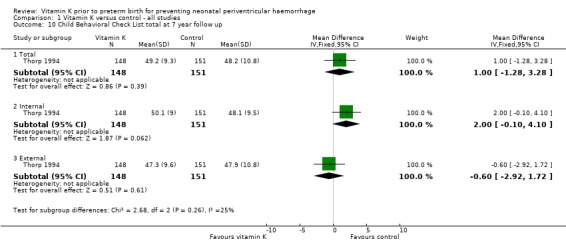
Comparison 1 Vitamin K versus control ‐ all studies, Outcome 10 Child Behavioral Check List total at 7 year follow up.
1.11. Analysis.
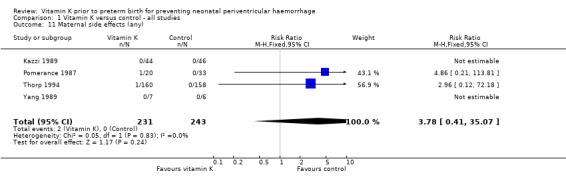
Comparison 1 Vitamin K versus control ‐ all studies, Outcome 11 Maternal side effects (any).
1.12. Analysis.
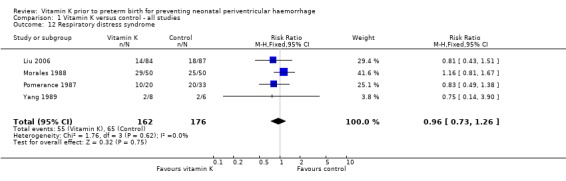
Comparison 1 Vitamin K versus control ‐ all studies, Outcome 12 Respiratory distress syndrome.
1.13. Analysis.
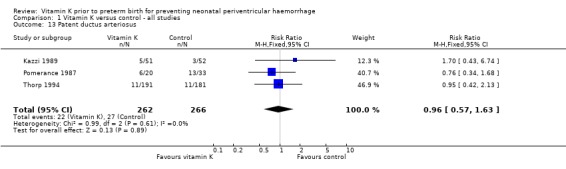
Comparison 1 Vitamin K versus control ‐ all studies, Outcome 13 Patent ductus arteriosus.
1.14. Analysis.
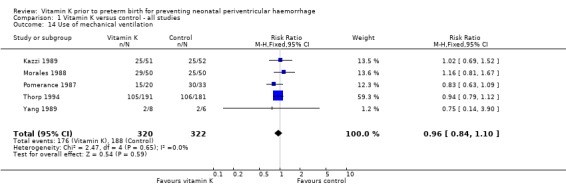
Comparison 1 Vitamin K versus control ‐ all studies, Outcome 14 Use of mechanical ventilation.
1.15. Analysis.
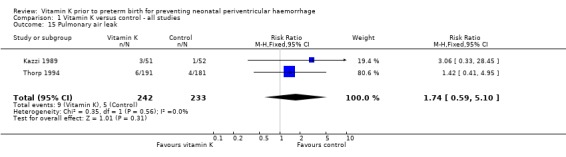
Comparison 1 Vitamin K versus control ‐ all studies, Outcome 15 Pulmonary air leak.
1.16. Analysis.
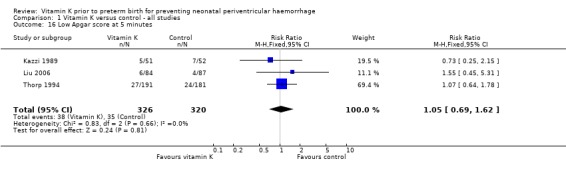
Comparison 1 Vitamin K versus control ‐ all studies, Outcome 16 Low Apgar score at 5 minutes.
Comparison 2. Vitamin K versus control ‐ excluding trials with inadequate concealment of allocation of treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal mortality | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Stillbirths | 1 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.18, 21.06] |
| 1.2 Death of live born infants prior to discharge | 3 | 525 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.69, 2.19] |
| 1.3 Infant deaths post‐discharge | 1 | 375 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.96 [0.36, 133.88] |
| 2 All periventricular haemorrhage (PVH) | 5 | 627 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.60, 1.26] |
| 3 Severe (grades 3 and 4) PVH | 5 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.49, 1.36] |
| 4 Bayley Mental Developmental Index at 2 years | 1 | 121 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐16.66, ‐1.34] |
| 5 Bayley Psychomotor Developmental Index at 2 years | 1 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐11.39, 1.39] |
| 6 Cerebral palsy at 7 year follow up | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.33, 1.76] |
| 7 Wechsler Intelligence Scale for Children at 7 year follow up | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Full scale | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐2.92, 3.52] |
| 7.2 Performance | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐2.23, 4.63] |
| 7.3 Verbal | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐3.84, 2.64] |
| 8 Wide Range Achievement Test at 7 year follow up | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Reading | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐2.57, 3.97] |
| 8.2 Spelling | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐3.45, 2.45] |
| 8.3 Mathematics | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐4.61, 1.81] |
| 9 Achenbach Teacher's Report Form at 7 year follow up | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Total | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐3.55, 1.15] |
| 9.2 Internal | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.82, 1.62] |
| 9.3 External | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐3.45, 0.45] |
| 10 Child Behavioral Check List total at 7 year follow up | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Total | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐1.28, 3.28] |
| 10.2 Internal | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐0.10, 4.10] |
| 10.3 External | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.92, 1.72] |
| 11 Maternal side effect (any) | 3 | 421 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.96 [0.12, 72.18] |
| 12 Respiratory distress syndrome | 2 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.79, 1.61] |
| 13 Patent ductus arteriosus | 2 | 475 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.55, 2.21] |
| 14 Pulmonary air leak | 2 | 475 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.59, 5.10] |
| 15 Use of mechanical ventilation | 4 | 589 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.85, 1.14] |
| 16 Low Apgar score at 5 minutes | 2 | 475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.63, 1.57] |
2.1. Analysis.
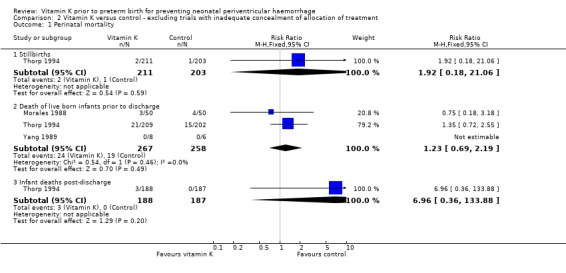
Comparison 2 Vitamin K versus control ‐ excluding trials with inadequate concealment of allocation of treatment, Outcome 1 Perinatal mortality.
2.2. Analysis.
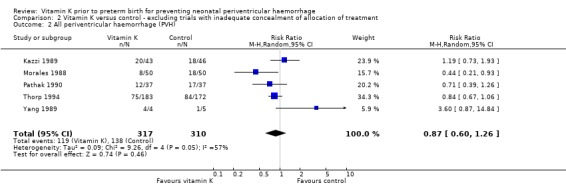
Comparison 2 Vitamin K versus control ‐ excluding trials with inadequate concealment of allocation of treatment, Outcome 2 All periventricular haemorrhage (PVH).
2.3. Analysis.
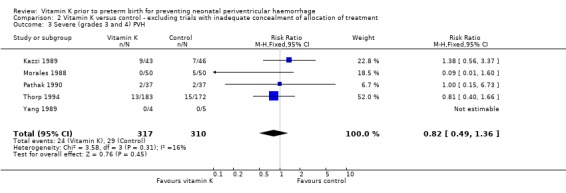
Comparison 2 Vitamin K versus control ‐ excluding trials with inadequate concealment of allocation of treatment, Outcome 3 Severe (grades 3 and 4) PVH.
2.4. Analysis.
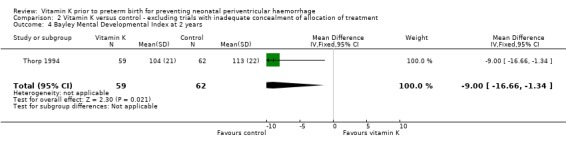
Comparison 2 Vitamin K versus control ‐ excluding trials with inadequate concealment of allocation of treatment, Outcome 4 Bayley Mental Developmental Index at 2 years.
2.5. Analysis.
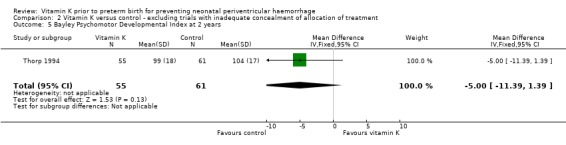
Comparison 2 Vitamin K versus control ‐ excluding trials with inadequate concealment of allocation of treatment, Outcome 5 Bayley Psychomotor Developmental Index at 2 years.
2.6. Analysis.
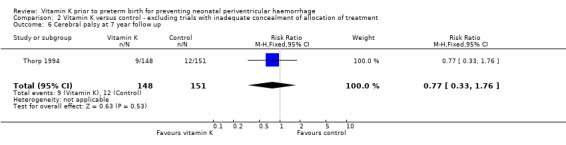
Comparison 2 Vitamin K versus control ‐ excluding trials with inadequate concealment of allocation of treatment, Outcome 6 Cerebral palsy at 7 year follow up.
2.7. Analysis.
Comparison 2 Vitamin K versus control ‐ excluding trials with inadequate concealment of allocation of treatment, Outcome 7 Wechsler Intelligence Scale for Children at 7 year follow up.
2.8. Analysis.
Comparison 2 Vitamin K versus control ‐ excluding trials with inadequate concealment of allocation of treatment, Outcome 8 Wide Range Achievement Test at 7 year follow up.
2.9. Analysis.
Comparison 2 Vitamin K versus control ‐ excluding trials with inadequate concealment of allocation of treatment, Outcome 9 Achenbach Teacher's Report Form at 7 year follow up.
2.10. Analysis.
Comparison 2 Vitamin K versus control ‐ excluding trials with inadequate concealment of allocation of treatment, Outcome 10 Child Behavioral Check List total at 7 year follow up.
2.11. Analysis.
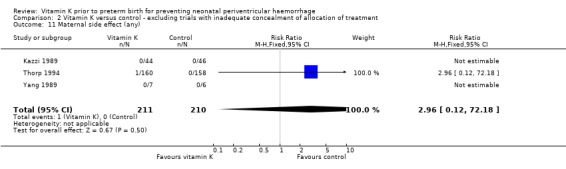
Comparison 2 Vitamin K versus control ‐ excluding trials with inadequate concealment of allocation of treatment, Outcome 11 Maternal side effect (any).
2.12. Analysis.
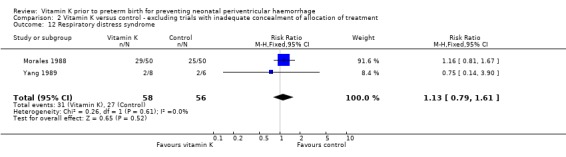
Comparison 2 Vitamin K versus control ‐ excluding trials with inadequate concealment of allocation of treatment, Outcome 12 Respiratory distress syndrome.
2.13. Analysis.
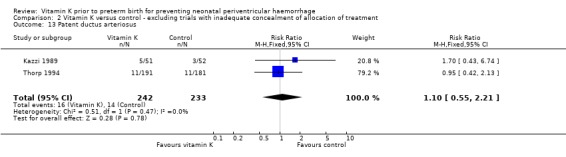
Comparison 2 Vitamin K versus control ‐ excluding trials with inadequate concealment of allocation of treatment, Outcome 13 Patent ductus arteriosus.
2.14. Analysis.
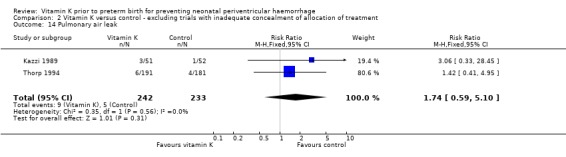
Comparison 2 Vitamin K versus control ‐ excluding trials with inadequate concealment of allocation of treatment, Outcome 14 Pulmonary air leak.
2.15. Analysis.
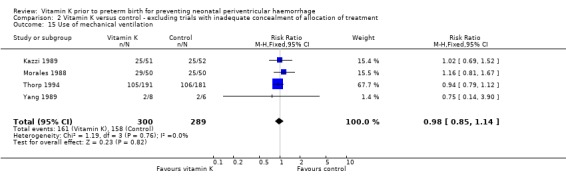
Comparison 2 Vitamin K versus control ‐ excluding trials with inadequate concealment of allocation of treatment, Outcome 15 Use of mechanical ventilation.
2.16. Analysis.
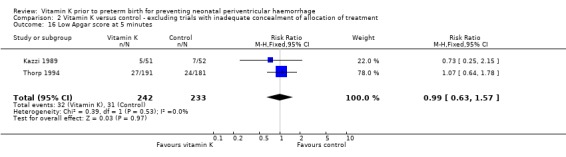
Comparison 2 Vitamin K versus control ‐ excluding trials with inadequate concealment of allocation of treatment, Outcome 16 Low Apgar score at 5 minutes.
Comparison 3. Vitamin K versus control ‐ excluding trials with inadequate randomisation and > 20% or unclear for exclusions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Stillbirths | 1 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.18, 21.06] |
| 1.2 Death of live born infants prior to discharge | 1 | 411 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.72, 2.55] |
| 1.3 Infant deaths post‐discharge | 1 | 375 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.96 [0.36, 133.88] |
| 2 All periventricular haemorrhage (PVH) | 1 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.67, 1.06] |
| 3 Severe (grades 3 and 4) PVH | 1 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.40, 1.66] |
| 4 Maternal side effect (any) | 1 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.96 [0.12, 72.18] |
| 5 Patent ductus arteriosus | 1 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.42, 2.13] |
| 6 Pulmonary air leak | 1 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.41, 4.95] |
| 7 Use of mechanical ventilation | 1 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.79, 1.12] |
| 8 Low Apgar score at 5 minutes | 1 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.64, 1.78] |
3.1. Analysis.
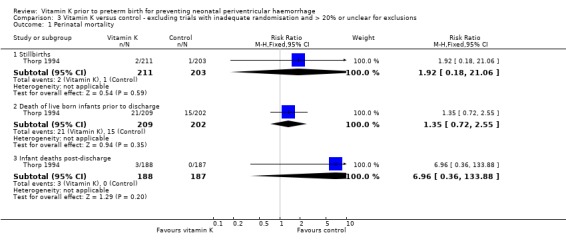
Comparison 3 Vitamin K versus control ‐ excluding trials with inadequate randomisation and > 20% or unclear for exclusions, Outcome 1 Perinatal mortality.
3.2. Analysis.
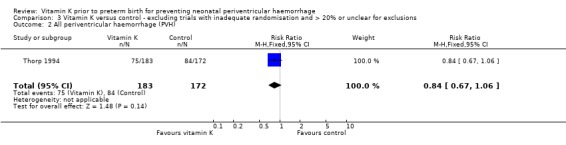
Comparison 3 Vitamin K versus control ‐ excluding trials with inadequate randomisation and > 20% or unclear for exclusions, Outcome 2 All periventricular haemorrhage (PVH).
3.3. Analysis.
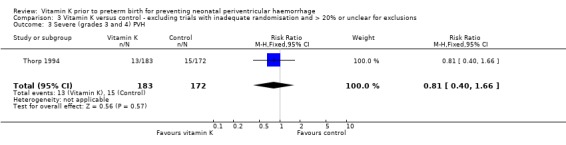
Comparison 3 Vitamin K versus control ‐ excluding trials with inadequate randomisation and > 20% or unclear for exclusions, Outcome 3 Severe (grades 3 and 4) PVH.
3.4. Analysis.
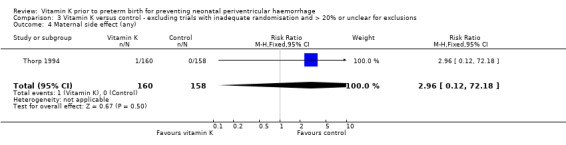
Comparison 3 Vitamin K versus control ‐ excluding trials with inadequate randomisation and > 20% or unclear for exclusions, Outcome 4 Maternal side effect (any).
3.5. Analysis.
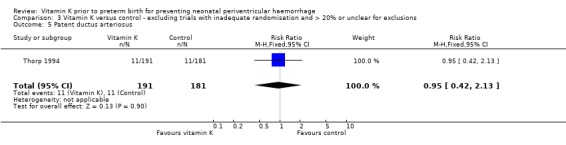
Comparison 3 Vitamin K versus control ‐ excluding trials with inadequate randomisation and > 20% or unclear for exclusions, Outcome 5 Patent ductus arteriosus.
3.6. Analysis.
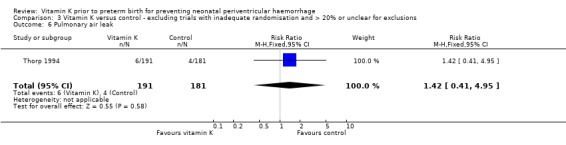
Comparison 3 Vitamin K versus control ‐ excluding trials with inadequate randomisation and > 20% or unclear for exclusions, Outcome 6 Pulmonary air leak.
3.7. Analysis.
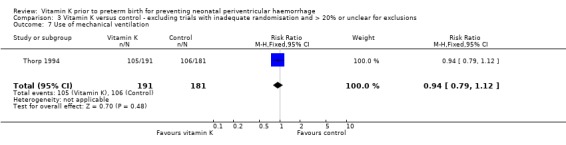
Comparison 3 Vitamin K versus control ‐ excluding trials with inadequate randomisation and > 20% or unclear for exclusions, Outcome 7 Use of mechanical ventilation.
3.8. Analysis.
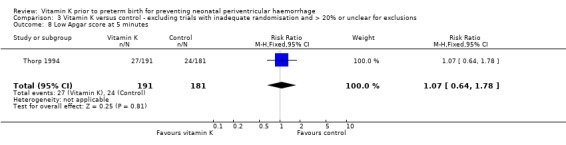
Comparison 3 Vitamin K versus control ‐ excluding trials with inadequate randomisation and > 20% or unclear for exclusions, Outcome 8 Low Apgar score at 5 minutes.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dickson 1994.
| Methods | Prospective randomised trial. | |
| Participants | 36 mothers in preterm labour admitted to hospital. | |
| Interventions | 17 women allocated to intramuscular vitamin K1, 19 allocated to placebo. | |
| Outcomes | Prothrombin time, activated partial thromboplastin time, factor II and protein C activity in cord blood samples | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "random numbers table". |
| Allocation concealment? | Unclear risk | Not reported, but likely to have been pharmacy controlled. |
| Blinding? All outcomes | Low risk | Placebo was used. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Threre of the 19 women (16%) allocated to the placebo control group were subsequently excluded either because a delay in giving birth or missing data. |
| Free of selective reporting? | Unclear risk | No clinical or health outcomes reported. |
| Free of other bias? | Low risk | No apparent evidence of other bias. |
Kazzi 1989.
| Methods | Prospective randomised clinical trial. | |
| Participants | 112 women in preterm labour or with prelabour preterm rupture of the membranes < 35 weeks. Ineligible if known congenital abnormalities, maternal bleeding diathesis, severe pre‐eclampsia, or fetal distress needing delivery. | |
| Interventions | 10 mg vitamin K intramuscularly repeated after 4 days if undelivered. If undelivered after a further 4 days oral vitamin K (20 mg) daily until end of 34th week (n = 56). The control group (n = 56) was given no treatment. | |
| Outcomes | IVH assessed by a radiologist blinded to treatment allocation on day 1, 3, and 14 after delivery. Other neonatal morbidity, patent ductus arteriosus, apnoea. Cord blood clotting profiles for a partial thromboplastin time, factor II, VII, X activity and antigen levels of factor II and X. | |
| Notes | Sample size. 50% reduction in PVH (from 40% to 20%) needed 46 babies in each group. Patent ductus arteriosus reported as seen on echocardiogram, uncertain as to whether required treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The cards were pre‐coded from a table of random numbers. |
| Allocation concealment? | Unclear risk | Women randomised to a treatment or control group by consecutively drawing cards. |
| Blinding? All outcomes | High risk | No placebo used, but assessment of the primary outcome of IVH was blinded. |
| Incomplete outcome data addressed? All outcomes | Low risk | 22/112 (19.6%) women lost to follow up due to continuation of pregnancy beyond 34 weeks. |
| Free of selective reporting? | Low risk | No indication of selective reporting. |
| Free of other bias? | Low risk | No other bias evident. |
Liu 2006.
| Methods | Randomised clinical trial. | |
| Participants | 161 women at high risk of preterm birth at less than 35 weeks admitted to the Beijing Obstetrics and Gynecology Hospital. | |
| Interventions | Antenatal intramuscular or intravenous injection of vitamin K1 10 mg per day for 2 to 7 days (n = 79). The control group (n = 82) received no treatment. | |
| Outcomes | Peri/intraventricular haemorrhage (PIVH) assessed by bedside cranial sonography on the 1st, 3rd and 7th day after birth. The ultrasound was performed by the same sonographer each time. Other variables analysed: maternal age, prenatal complications, mode of delivery, gestational age, birthweight, head circumference, respiratory distress syndrome, neonatal surfactant treatment, ventilation. | |
| Notes | PIVH diagnosed in 42.5% of neonates in vitamin K1 group, compared with 65.2% in control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Randomly assigned by inpatient sequence. |
| Allocation concealment? | Unclear risk | Not reported. |
| Blinding? All outcomes | High risk | Blinding of outcome assessment not reported, placebo not used. |
| Incomplete outcome data addressed? All outcomes | Low risk | No losses to follow up evident. |
| Free of selective reporting? | Low risk | No indication of selective reporting. |
| Free of other bias? | High risk | 44/84 (52%) women receiving vitamin K also received dexamethasone as a part of their treatment. Higher rate of PVH in the control group (66%) compared to the control groups in other trials (49% PVH in the Thorp 1994 trial, 39% PVH in the Kazzi 1989 trial). Women were excluded if the time between dexamethasone administration and delivery was less than 24 hours (applies to women who received dexamethasone and vitamin K). |
Morales 1988.
| Methods | Randomised clinical trial. All women received betamethasone. |
|
| Participants | 92 women at less than 33 weeks' gestation. Not eligible if intrauterine growth restriction, known congenital abnormalities, or maternal bleeding disorder. | |
| Interventions | 10 mg vitamin K intramuscularly every 5 days until delivery (n = 46). Control group (n = 46) given no treatment. | |
| Outcomes | IVH assessed by cranial ultrasound 24 and 72 hours after birth reviewed by a radiologist blinded to treatment. Other neonatal morbidity including respiratory distress syndrome. Cord blood clotting profiles. | |
| Notes | No sample size estimation reported. All babies with any respiratory distress syndrome received mechanical ventilation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported by authors. |
| Allocation concealment? | Unclear risk | Allocation by sealed envelopes. Not reported if the envelopes were sequentially numbered or opaque. |
| Blinding? All outcomes | High risk | No placebo used, however, assessment of the primary outcome was blinded. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Losses to follow up not reported. |
| Free of selective reporting? | Low risk | No indication of selective reporting. |
| Free of other bias? | Unclear risk | Unclear. |
Pathak 1990.
| Methods | Randomised double blind trial. | |
| Participants | 67 mothers, 74 infants at less than 34 weeks' gestation. | |
| Interventions | Antenatal intramuscular injection of vitamin K1 10 mg given to women at less than 34 weeks' gestation between 4 and 96 hours before delivery. | |
| Outcomes | Primary: IVH (grades I‐IV). Secondary: mode of delivery, severity of respiratory illness, survival and incidence of patent ductus arteriosus. | |
| Notes | Vitamin K1 and control groups were similar with respect to birthweight and gestational age. No adverse effects due to vitamin K in mothers or infants. Losses to follow up were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported. |
| Allocation concealment? | Unclear risk | Not reported. |
| Blinding? All outcomes | Unclear risk | Placebo used. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Unclear. |
| Free of selective reporting? | Unclear risk | Unclear. |
| Free of other bias? | Unclear risk | Unclear. |
Pomerance 1987.
| Methods | Controlled clinical trial. | |
| Participants | 43 women in threatened preterm labour between 24‐34 weeks' gestation. Babies weighing < 1500 g at birth and born < 34 weeks' gestation. | |
| Interventions | 10 mg vitamin K intramuscularly 4 hours before delivery. Repeated after 5 days if not delivered and when in active labour. | |
| Outcomes | IVH assessed by cranial ultrasound on day 2 and day 4 after birth by a radiologist blinded to treatment allocation. Maternal problems at site of injection. Cord blood clotting profiles. | |
| Notes | Sample size calculated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | No sequence generation, allocated women based on odd and even hospital record numbers. |
| Allocation concealment? | High risk | No concealment due to usage of hospital record numbers. |
| Blinding? All outcomes | High risk | No placebo was used however assessment of the primary outcome of IVH was blinded. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | One infant in the treatment group did not have prothrombin time or PTT measured. |
| Free of selective reporting? | Low risk | No indication of selective reporting. |
| Free of other bias? | Low risk | |
Thorp 1994.
| Methods | Randomised controlled trial stratified by gestational age. | |
| Participants | 353 women (414 fetuses) at 24‐34 weeks' gestation at risk of spontaneous or indicated preterm birth.All received betamethasone and had ampicillin until known culture negative for GBS. | |
| Interventions | Vitamin K 10 mg intramuscularly. Repeated in 4 days then 20 mg per day orally after 8 days. Phenobarbital 780 mg intravenously over 90 minutes or 720 mg orally in 3 doses over 8 hours, then 60 mg/6 hours intravenously or oral (n = 177). Versus identical appearing placebo (n = 176). | |
| Outcomes | Primary outcome, PVH; other, neonatal mortality and morbidity PVH assessed by cranial ultrasound on day 2‐3 and day 7 by a radiologist blinded to treatment group. Secondary outcome: infant neurodevelopment. Developmental outcomes at 2 and 7 year follow up. | |
| Notes | Power calculation based on reduction of all PVH 50% to 25% and severe PVH 20% to 5%, needed 200. Interim analysis expanded the sample size because the frequency of severe IVH was substantially lower in the study compared to previous studies. Stopped trial early because baseline risk lower and no difference found. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Block randomisation. |
| Allocation concealment? | Unclear risk | Randomisation completed by pharmacist. |
| Blinding? All outcomes | Low risk | Placebo identical to treatment. Phenobarbital levels not followed in treatment group. Blinded assessment of primary outcome. |
| Incomplete outcome data addressed? All outcomes | High risk | 22/203 (11%) babies from the placebo group and 20/211 (9%) babies from the treatment group were lost to follow up initially because they were delivered at or after 34 weeks' gestation (in total 42/414 (10%) infants were excluded). 141/203 (69%) children from the placebo group and 152/211 (72%) children from the treatment group were excluded at 2 years (in total 293/414 (71%) infants randomised were excluded). Reasons for exclusion were antenatal and postnatal demise and mothers declining to be involved in follow up. 52/203 (26%) children in the placebo group and 63/211 (30%) children in the treatment group were lost to follow up at 7 years (in total 115/414 (28%) children randomised were excluded). Reasons for exclusion included antenatal and postnatal demise, and death since discharge. |
| Free of selective reporting? | Low risk | No indication of selective reporting. |
| Free of other bias? | Low risk | All patients received betamethasone and ampicillin. |
Yang 1989.
| Methods | Randomised clinical trial. | |
| Participants | 15 women in labour at 34 weeks or less. | |
| Interventions | 5 mg vitamin K at least 30 minutes and ideally 4 hours prior to delivery (n = 7). Control group received no treatment (n = 6). | |
| Outcomes | IVH assessed by cranial ultrasound on day 3 by a radiologist. Cord blood clotting profiles. | |
| Notes | No sample size calculation reported. Information given on respiratory distress syndrome in babies only if required ventilation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported. |
| Allocation concealment? | Unclear risk | Not reported. |
| Blinding? All outcomes | High risk | No placebo used however assessment of the primary outcome of IVH was blinded. |
| Incomplete outcome data addressed? All outcomes | Low risk | Losses to follow up 7% (1/15) due to the mother not receiving the vitamin K. |
| Free of selective reporting? | Low risk | No indication of selective reporting. |
| Free of other bias? | Low risk | |
GBS: group B streptococci IVH: intraventricular haemorrhage PIVH: periventricular intraventricular haemorrhage PVH: periventricular haemorrhage
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anai 1993 | This study did not specify if women were at risk of imminent preterm birth. |
Characteristics of studies awaiting assessment [ordered by study ID]
Liu 2005.
| Methods | Randomised clinical trial. |
| Participants | Pregnant women in preterm labour at less than 35 weeks' gestation. |
| Interventions | IM or IV vitamin K 10 mg per day for 2 to 7 days (44 infants), or no vitamin K (133 infants). Dexamethasone was given to both groups as per routine practice. |
| Outcomes | Vitamin K dependent coagulation factors in umbilical blood samples, PIVH. |
| Notes | Incidence of PIVH was 31.8% in the vitamin K group and 52.6% in the control group (P = 0.017). Incidence of severe PIVH was 2.3% in the vitamin K group and 12.0% in the control group (P = 0.057). |
Contributions of authors
CA Crowther (CAC) contributed to the development of the protocol, identification and selection of studies for inclusion, data extraction and preparation of the text of the initial review and previous updates.
For this update CAC and DD Crosby (DDC) assessed the new studies for inclusion and performed the data extraction. DDC drafted the changes to the text and prepared subsequent drafts. CAC contributed to the drafts and final version.
Sources of support
Internal sources
Discipline of Obstetrics and Gynaecology, The University of Adelaide, Australia.
NSW Centre for Perinatal Health Services Research, University of Sydney, Australia.
External sources
Australian Government. Department of Health and Ageing, Australia.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Dickson 1994 {published data only}
- Dickson R, Stubbs T, Lazarchick J. Antenatal vitamin K therapy of the low‐birth‐weight infant. American Journal of Obstetrics and Gynecology 1994;170:85‐9. [DOI] [PubMed] [Google Scholar]
Kazzi 1989 {published data only}
- Kazzi N, Ilagan N, Liang K, Kazzi G, Grietsell L, Brans Y. Placental transfer of vitamin K1 in preterm pregnancy. Obstetrics and Gynecology 1990;75:334‐7. [PubMed] [Google Scholar]
- Kazzi N, Ilagan N, Liang K, Kazzi G, Poland R. Effects of antenatal vitamin K on cord blood coagulation profile of preterm infants. Pediatric Research 1988;23:412A, Abstract no: 1267. [Google Scholar]
- Kazzi N, Ilagan N, Liang K, Kazzi G, Poland R. Effects of antenatal vitamin K on the incidence of IVH and mortality in preterm infants. Pediatric Research 1988;23:412A, Abstract no: 1266. [Google Scholar]
- Kazzi N, Ilagan N, Liang K, Kazzi G, Poland R, Grietsell L, et al. Maternal administration of vitamin K does not improve the coagulation profile of preterm infants. Pediatrics 1989;84:1045‐50. [PubMed] [Google Scholar]
- Kazzi NJ, Ilagan NB, Liang KC, Kazzi GM, Poland RL. Does vitamin K cross the placenta in pregnancy before term? [abstract]. Pediatric Research 1988;23:412A. [Google Scholar]
Liu 2006 {published data only}
- Liu J, Wang Q, Gao F, He JW, Zhao JH. Maternal antenatal administration of vitamin K1 results in increasing the activities of vitamin K‐dependent coagulation factors in umbilical blood and in decreasing the incidence rate of periventricular‐intraventricular hemorrhage in premature infants. Journal of Perinatal Medicine 2006;34(2):173‐6. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang Q, Zhao JH, Chen YH, Qin GL. The combined antenatal corticosteroids and vitamin K therapy for preventing periventricular‐intraventricular hemorrhage in premature newborns less than 35 weeks gestation. Journal of Tropical Pediatrics 2006;52(5):355‐9. [DOI] [PubMed] [Google Scholar]
Morales 1988 {published data only}
- Morales W, Angel J, O'Brien W, Knuppel R, Marsalisi F. The use of antenatal vitamin K in the prevention of early neonatal intraventricular hemorrhage. American Journal of Obstetrics and Gynecology 1988;159:774‐9. [DOI] [PubMed] [Google Scholar]
Pathak 1990 {published data only}
- Pathak A, Hamm CR, Eyal FG, Walter K, Rijhsinghani A, Bohlman M. Maternal vitamin K administration for prevention of intraventricular hemorrhage in preterm infants [abstract]. Pediatric Research 1990;27:219A. [Google Scholar]
Pomerance 1987 {published data only}
- Pomerance J, Teal J, Gogolok J, Brown S, Stewart M. Maternally administered antenatal vitamin K effect on neonatal prothrombin activity, partial thromboplastin time, and intraventricular hemorrhage. Obstetrics & Gynecology 1987;70:235‐41. [PubMed] [Google Scholar]
Thorp 1994 {published data only}
- Lia JE, Billman D. Antepartum vitamin K and phenobarbital for preventing intraventricular hemorrhage in the premature newborn; a randomized, double‐blind, placebo‐controlled trial [letter]. Obstetrics & Gynecology 1994;83(6):1067‐8. [DOI] [PubMed] [Google Scholar]
- Thorp J, O'Connor M, Jones A, Hoffman E, Belden B. Does perinatal phenobarbital exposure affect developmental outcome at age 2?. American Journal of Perinatology 1999;16:51‐60. [DOI] [PubMed] [Google Scholar]
- Thorp J, Parriot J, Ferrette‐Smith D, Meyer B, Cohen GR, Johnson J. Antepartum vitamin K and phenobarbital for preventing intraventricular hemorrhage in the premature newborn; a randomized, double‐blind, placebo‐controlled trial. Obstetrics & Gynecology 1994;83(1):70‐6. [PubMed] [Google Scholar]
- Thorp J, Yeast J, Cohen G, Poskin M, Peng V, Hoffman E. Does in‐utero phenobarbital lower IQ? Follow up of the intracranial hemorrhage prevention trial. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S117. [Google Scholar]
- Thorp JA. Antepartum vitamin K and phenobarbital for preventing intraventricular hemorrhage in the premature newborn; a randomized, double‐blind, placebo‐controlled trial [In reply]. Obstetrics and Gynecology 1994;83(6):1068‐9. [PubMed] [Google Scholar]
- Thorp JA, Caspers DR, Cohen GR, Zucker ML, Strope BD, McKenzie DR. The effect of combined antenatal vitamin K and phenobarbital therapy on umbilical blood coagulation studies in infants less than 34 weeks' gestation. Obstetrics & Gynecology 1995;86:982‐9. [DOI] [PubMed] [Google Scholar]
- Thorp JA, Etzenhouser J, OConnor M, Jones A, Jones P, Belden B, et al. Effects of phenobarbital and multiple‐dose antenatal/postnatal steroid on developmental outcome at age 7 years [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S87. [Google Scholar]
- Thorp JA, Ferrette‐Smith D, Gaston L, Johnson J, Caspers D, Yeast JD, et al. Antenatal vitamin K (VK) and phenobarbital (PH) for preventing intracrancial hemorrhage (ICH) in the premature newborn: a randomized double blinded placebo controlled trial. American Journal of Obstetrics and Gynecology 1995;172:253. [Google Scholar]
- Thorp JA, Ferrette‐Smith D, Gaston L, Johnson J, Yeast JD, Myer B. Combined antenatal vitamin K and phenobarbital therapy for preventing intracranial hemorrhage in newborns less than 34 weeks' gestation. Obstetrics and Gynecology 1995;86:1‐8. [DOI] [PubMed] [Google Scholar]
- Thorp JA, Gaston L, Ferrette‐Smith D, Caspers D, Wickstrom E, Pal M, et al. Mode of delivery and prediction of severe intracranial hemorrhage (ICH): a randomized double blinded placebo controlled trial. American Journal of Obstetrics and Gynecology 1995;172:289. [Google Scholar]
- Thorp JA, McKenzie DR. Antenatal phenobarbital therapy and neonatal outcome. Pediatrics 1997;99:751‐3. [DOI] [PubMed] [Google Scholar]
- Thorp JA, O'Connor M, Belden B, Etzenhouser J, Hoffman EL, Jones PG. Effects of phenobarbital and multiple‐dose corticosteroids on developmental outcome at age 7 years. Obstetrics & Gynecology 2003;101(2):363‐3. [DOI] [PubMed] [Google Scholar]
- Thorp JA, Parriott J, Ferrette‐Smith D, Holst V, Meyer BA, Cohen GR, et al. Antepartum vitamin K and phenobarbital for preventing intraventricular hemorrhage in the premature newborn: a randomized double blinded placebo controlled trial. American Journal of Obstetrics and Gynecology 1993;168:367. [PubMed] [Google Scholar]
- Thorp JA, Parriott J, Ferrette‐Smith D, Meyer BA, Cohen GR, Johnson J. Antepartum vitamin K and phenobarbital for preventing intraventricular hemorrhage in the premature newborn: a randomized, double blind, placebo‐controlled trial. Obstetrics and Gynecology 1994;83:70‐6. [PubMed] [Google Scholar]
- Thorp JA, Zucker M, Strope B, Claflin K, Callenbach JC, Shaffer S, et al. The effect of antenatal vitamin K (VK) and phenobarbital (PH) on cord blood coagulation studies at birth: a randomized double blinded placebo controlled trial. American Journal of Obstetrics and Gynecology 1995;172:327. [Google Scholar]
Yang 1989 {published data only}
- Yang Y, Simon N, Maertens P, Brigham S, Liu P. Maternal‐fetal transport of vitamin K and its effects on coagulation in premature infants. Journal of Pediatrics 1989;115:1009‐13. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anai 1993 {published data only}
- Anai T, Hirota Y, Yoshimatsu J, Oga M, Miyakawa I. Can prenatal vitamin K (phylloquinone) supplementation replace prophylaxis at birth?. Obstetrics & Gynecology 1993;81:251‐4. [PubMed] [Google Scholar]
References to studies awaiting assessment
Liu 2005 {published data only}
- Liu J, Wang Q, Chen YH, Qin GL, Zhao JH, Zhu LC. Level of vitamin K‐dependent coagulation factors in premature infants and the influence of maternal antenatal administration of vitamin K1 on their activity. Zhonghua Erke Zazhi 2005;43(12):908‐10. [PubMed] [Google Scholar]
Additional references
Crowther 2010a
- Crowther CA, Crosby DD, Henderson‐Smart DJ. Phenobarbital prior to preterm birth for preventing neonatal periventricular haemorrhage. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD000164.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. Egger M, Davey Smith G, Altman DG, editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. London: BMJ Books, 2001. [Google Scholar]
Higgins 2009
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Horbar 1992
- Horbar J. Prevention of periventricular‐intraventricular haemorrhage. In: Sinclair JC, Bracken MB editor(s). Effective care of the newborn infant. Oxford: Oxford University Press, 1992. [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Shearer 1992
- Shearer M. Vitamin K metabolism and nutriture. Blood Reviews 1992;6:92‐104. [DOI] [PubMed] [Google Scholar]
Thorp 1997
- Thorp J, Yeast J, Cohen G, Poskin M, Peng V, Hoffman E. Does in‐utero phenobarbital lower IQ? Follow up of the intracranial hemorrhage prevention trial. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S117. [Google Scholar]
Thorp 1999
- Thorp J, O'Connor M, Jones A, Hoffman E, Belden B. Does perinatal phenobarbital exposure affect developmental outcome at age 2?. American Journal of Perinatology 1999;16:51‐60. [DOI] [PubMed] [Google Scholar]
Thorp 2003
- Thorp JA, O'Connor M, Belden B, Etzenhouser J, Hoffman EL, Jones PG. Effects of phenobarbital and multiple‐dose corticosteroids on developmental outcome at age 7 years. Obstetrics & Gynecology 2003;101(2):363‐3. [DOI] [PubMed] [Google Scholar]
Vohr 2000
- Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Developmental Neonatal Research Network 1993‐1994. Pediatrics 2000;105:1216‐26. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Crowther 1995
- Crowther CA, Alfirevic Z. Vitamin K prior to preterm delivery. [revised 07 April 1994]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.). Pregnancy and Childbirth Module. The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software 1995.
Crowther 1997
- Crowther CA, Henderson‐Smart DJ. Vitamin K prior to preterm birth.. In: Neilson JP, Crowther CA, Hodnett ED, Hofmeyr GJ, Keirse MJNC (eds). Pregnancy and Childbirth Module of The Cochrane Database of Systematic Reviews, [updated 03 June 1997]. Available in The Cochrane Library [database on disk and CDROM]. The Cochrane Collaboration; Issue 3. Oxford: Update Software; 1997. Updated quarterly.
Crowther 1999
Crowther 2001
- Crowther CA, Crosby DD, Henderson‐Smart DJ. Vitamin K prior to preterm birth for preventing neonatal periventricular haemorrhage. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD000229] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crowther 2010b
- Crowther CA, Crosby DD, Henderson‐Smart DJ. Vitamin K prior to preterm birth for preventing neonatal periventricular haemorrhage. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD000229.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


