Abstract
Background
It has been suggested that low serum zinc levels may be associated with suboptimal outcomes of pregnancy such as prolonged labour, atonic postpartum haemorrhage, pregnancy‐induced hypertension, preterm labour and post‐term pregnancies, although many of these associations have not yet been established.
Objectives
To assess the effects of zinc supplementation in pregnancy on maternal, fetal, neonatal and infant outcomes.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 October 2014) and reference lists of retrieved studies.
Selection criteria
Randomised trials of zinc supplementation in pregnancy. We excluded quasi‐randomised controlled trials.
Data collection and analysis
Three review authors applied the study selection criteria, assessed trial quality and extracted data. When necessary, we contacted study authors for additional information. The quality of the evidence was assessed using GRADE.
Main results
We included 21 randomised controlled trials (RCTs) reported in 54 papers involving over 17,000 women and their babies. One trial did not contribute data. Trials were generally at low risk of bias. Zinc supplementation resulted in a small reduction in preterm birth (risk ratio (RR) 0.86, 95% confidence interval (CI) 0.76 to 0.97 in 16 RCTs; 16 trials of 7637 women). This was not accompanied by a similar reduction in numbers of babies with low birthweight (RR 0.93, 95% CI 0.78 to 1.12; 14 trials of 5643 women). No clear differences were seen between the zinc and no zinc groups for any of the other primary maternal or neonatal outcomes, except for induction of labour in a single trial. No differing patterns were evident in the subgroups of women with low versus normal zinc and nutrition levels or in women who complied with their treatment versus those who did not. The GRADE quality of the evidence was moderate for preterm birth, small‐for‐gestational age, and low birthweight, and low for stillbirth or neonatal death and birthweight.
Authors' conclusions
The evidence for a 14% relative reduction in preterm birth for zinc compared with placebo was primarily represented by trials involving women of low income and this has some relevance in areas of high perinatal mortality. There was no convincing evidence that zinc supplementation during pregnancy results in other useful and important benefits. Since the preterm association could well reflect poor nutrition, studies to address ways of improving the overall nutritional status of populations in impoverished areas, rather than focusing on micronutrient and or zinc supplementation in isolation, should be an urgent priority.
Plain language summary
Zinc supplementation for improving pregnancy and infant outcome
Taking zinc during pregnancy helps to slightly reduce preterm births, but does not prevent other problems such as low birthweight babies.
Many women of childbearing age may have mild to moderate zinc deficiency. Low zinc concentrations may cause preterm birth or they may even prolong labour. It is also possible that zinc deficiency may affect infant growth as well. This review of 21 randomised controlled trials, involving over 17,000 women and their babies, found that although zinc supplementation has a small effect on reducing preterm births, it does not help to prevent low birthweight babies compared with not giving zinc supplements before 27 weeks of pregnancy. One trial did not contribute data. The overall risk of bias was unclear in half of the studies. No clear differences were seen for development of pregnancy hypertension or pre‐eclampsia. The 14% relative reduction in preterm birth for zinc compared with placebo was primarily represented by trials of women with low incomes. In some trials all women were also given iron, folate or vitamins or combinations of these. UNICEF is already promoting antenatal use of multiple‐micronutrient supplementation, including zinc, to all pregnant women in developing countries. Finding ways to improve women's overall nutritional status, particularly in low‐income areas, will do more to improve the health of mothers and babies than supplementing pregnant women with zinc alone. In low‐ to middle‐ income countries, addressing anaemia and infections, such as malaria and hookworm, is also necessary.
Summary of findings
Summary of findings for the main comparison. Zinc supplementation versus no zinc (with or without placebo) for improving pregnancy and infant outcome.
| Zinc supplementation versus no zinc (with or without placebo) for improving pregnancy and infant outcome | ||||||
| Population: Normal pregnant women with no systemic illness Settings: Bangladesh, Chile, China, Denmark, Egypt, Ghana, Indonesia, Iran, Nepal, Pakistan, Peru, South Africa, UK, USA Intervention: Zinc supplementation versus no zinc (with or without placebo) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Zinc supplementation versus no zinc (with or without placebo) | |||||
| Preterm birth | Study population | RR 0.86 (0.76 to 0.97) | 7637 (16 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 129 per 1000 | 111 per 1000 (98 to 125) | |||||
| Moderate | ||||||
| 100 per 1000 | 86 per 1000 (76 to 97) | |||||
| Stillbirth or neonatal death | Study population | RR 1.12 (0.86 to 1.46) | 5100 (8 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 40 per 1000 | 45 per 1000 (34 to 58) | |||||
| Moderate | ||||||
| 25 per 1000 | 28 per 1000 (22 to 37) | |||||
| Birthweight | The mean birthweight in the intervention groups was 0.9lower (22.2 lower to 24.0 higher) | 6757 (17 studies) | ⊕⊕⊝⊝ low1,2 | |||
| Small‐for‐gestational age | Study population | RR 1.02 (0.94 to 1.11) | 4252 (8 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 265 per 1000 | 270 per 1000 (249 to 294) | |||||
| Moderate | ||||||
| 108 per 1000 | 110 per 1000 (102 to 120) | |||||
| Low birthweight | Study population | RR 0.93 (0.78 to 1.12) | 5643 (14 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 196 per 1000 | 182 per 1000 (153 to 219) | |||||
| Moderate | ||||||
| 119 per 1000 | 111 per 1000 (93 to 133) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Most studies contributing data had design limitations. 2 Wide confidence interval crossing the line of no effect.
Background
Description of the condition
The overall nutritional status of the mother during pregnancy is a significant contributor to both maternal and perinatal mortality and morbidity (Koblinsky 1995). This is likely to be even more crucial in developing countries where anaemia and infections, such as malaria and hookworm, compound the issue even further.
Description of the intervention
Zinc is known to play an important role in many biological functions, including protein synthesis and nucleic acid metabolism (Valee 1993). Although severe zinc deficiency is now considered rare, mild to moderate deficiency may be relatively common throughout the world (Sanstead 1991). In a review of literature published between 1970 and 1991, Parr 1996 noted that, on average, pregnant and lactating women worldwide consumed 9.6 mg zinc per day, well below the recommended 15 mg daily, during the last two trimesters of pregnancy (Sanstead 1996; WHO 1996). In animal studies, zinc deficiency during the early stages of pregnancy is associated with reduced fertility (Apgar 1970), fetal neurological malformations and growth retardation (McKenzie 1975), and deficiency in later stages of pregnancy negatively affects neuronal growth and may also be associated with impaired brain function and behavioural abnormalities (Golub 1995).
How the intervention might work
In humans, pregnant women with acrodermatitis enteropathica (an inherited defect in zinc absorption from the bowel) show association with increased risk of congenital malformations and pregnancy losses (Verburg 1974). Numerous reports have noted low serum zinc levels to be linked with abnormalities of labour such as prolonged labour and atonic postpartum haemorrhage (Prema 1980), pregnancy‐induced hypertension (Jameson 1976; Jameson 1993), preterm labour (Jones 1981) and post‐term pregnancies (Simmer 1985). Others (Cherry 1981; Chesters 1982) have failed to show any such association.
Some researchers have also reported an association between low zinc and small‐for‐gestational age babies, and poor perinatal outcome (Kiilholma 1984a; Kiilholma 1984b). Kirksey 1994 reported low maternal serum zinc levels during pregnancy to be associated with an increased risk of low birthweight and preterm birth. Low birthweight babies have higher rates of morbidity and mortality due to infectious disease and impaired immunity and, thus, it is possible that zinc deficiency may affect infant growth and well being too.
Why it is important to do this review
Studies of the effects of zinc supplementation have differed in their findings. These inconsistencies in study findings could be due to lack of consensus on accurate assessment of zinc status (Aggett 1991) and to differences in the populations studied. Randomised controlled trials of zinc supplementation in pregnancy would help to address the association, if any, between zinc deficiency and pregnancy outcome and neonatal and infant health and well being.
The fetal nervous system also develops progressively during pregnancy influencing motor and autonomic functions. Change in the pattern of fetal heart rate and movements monitored electronically have been related to fetal neuro behavioural development (DiPietro 1996) and atypical neurodevelopment has been shown in fetuses that exhibit other indicators of neurologic compromise (Hepper 1995). In a publication from Egypt, Kirskey 1991 also reported a positive association between maternal zinc status during the second trimester of pregnancy and newborn behaviour.
It is plausible that the effect of zinc supplementation would vary among different population groups depending on their nutritional status, with any effect likely to be more apparent in women from the developing world. Currently, UNICEF is already promoting antenatal use of multiple‐micronutrient supplementation, including zinc, to all pregnant women in developing countries (Nepal 2003).
The aim of this review is to systematically review all randomised controlled trials of zinc supplementation in pregnancy and to evaluate the role of zinc as it relates to pregnancy, labour and birth as well as to maternal and infant health and well being.
Objectives
To compare the effects on maternal, fetal, neonatal and infant outcomes in healthy pregnant women receiving zinc supplementation, no zinc supplementation, or placebo.
To assess the above outcomes in a subgroup analysis reviewing studies performed in women who are, or are likely to be, zinc deficient.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials of zinc supplementation versus no zinc supplementation or placebo administration during pregnancy, earlier than 27 weeks' gestation. Quasi‐randomised controlled trials have been excluded. We intended to include studies presented only as abstracts, if they provided enough information or, if necessary, by contacting authors to analyse them against criteria; we did not find such studies.
Types of participants
Normal pregnant women with no systemic illness. Women may have had normal zinc levels or they may have been, or likely to have been, zinc deficient.
Types of interventions
Routine zinc supplementation versus no zinc supplementation, or placebo.
Types of outcome measures
We have included outcomes related to clinical complications of pregnancy on maternal, fetal, neonatal and infant outcomes. We have not included data related to biochemical outcomes or studies reporting only biochemical outcomes.
Primary outcomes
Maternal and pregnancy outcomes
Preterm labour or birth (less than 37 weeks), or both
Neonatal outcomes
Stillbirth or neonatal death Birthweight Small‐for‐gestational age (birthweight less than 10th centile for gestational age) Low birthweight (less than 2.5 kg)
Secondary outcomes
Maternal and pregnancy outcomes
Antepartum haemorrhage Pregnancy‐induced hypertension Prelabour rupture of membranes Post‐term pregnancy Induction of labour Any maternal infection Meconium in liquor Caesarean section Instrumental vaginal birth Retained placenta Postpartum haemorrhage Smell dysfunction Taste dysfunction
Fetal neurodevelopmental assessment
Baseline fetal heart rate Baseline variability Number of accelerations Number of fetal movements Fetal activity level (minutes) Movement amplitude
Neonatal outcomes
Gestational age at birth High birthweight (more than 4.5 kg) Apgar score of less than five at five minutes Head circumference Hypoxia Neonatal sepsis Neonatal jaundice Respiratory distress syndrome Neonatal intraventricular haemorrhage Necrotising enterocolitis Neonatal length of hospital stay Congenital malformation (non‐prespecified outcome)
Infant/child outcomes
Episodes of disease Weight for age Z‐score Weight for height Z‐score Mid‐upper arm circumference Mental development index Psychomotor development index Other measures of infant or child development
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register by contacting the Trials Search Co‐ordinator (31 October 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (OVID);
weekly searches of Embase (OVID);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the references lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, see Mori 2012.
For this update, the following methods, which are based on a standard template used by the Cochrane Pregnancy and Childbirth Group, were used to assess the eight new reports that were identified as a result of the updated search.
Selection of studies
Two review authors Erika Ota (EO), and Celine Miyazaki (CM) independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreements through discussion.
Data extraction and management
We designed a form to extract data. For eligible studies, EO and CM extracted the data using the agreed form. We planned to resolve any discrepancies through discussion or, if required, we would have consulted Rintaro Mori (RM). We entered data into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
EO and CM independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion. PM and RM independently re‐assessed risk of bias using the updated format newly required for all the studies already included in the previous version due to changes in methods (Higgins 2011).
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
For this update the quality of the evidence was assessed using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following primary outcomes for the main comparisons.
Preterm labour or birth (less than 37 weeks), or both.
Stillbirth or neonatal death.
Birthweight.
Small‐for‐gestational age (birthweight less than 10th centile for gestational age).
Low birthweight (less than 2.5 kg).
The GRADEprofiler (GRADE 2014) was used to import data from Review Manager 5.3 (RevMan 2014) in order to create 'Summary of findings' tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. If necessary, we planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We planned to include cluster‐randomised trials in the analyses along with individually‐randomised trials. We would have adjusted their sample sizes or standard errors using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. Had we used ICCs from other sources, we would have reported this and conducted sensitivity analyses to investigate the effect of variation in the ICC. We included one cluster‐randomised trial (Nepal 2003) ‐ analyses adjusted for clustering were presented in study reports and so we did not need to perform the above additional calculations for these study results
We synthesised the relevant information from Nepal 2003 and the individually‐randomised trials. We considered it reasonable to combine the results from both as there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely.
If necessary, we would have acknowledged heterogeneity in the randomisation unit and performed a subgroup analysis to investigate the effects of the randomisation unit.
Cross‐over trials were not considered eligible for this review.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they had been allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
When there were 10 or more studies in a meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually, and used formal tests for funnel plot asymmetry. We performed exploratory analyses to investigate any asymmetry we detected.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary when an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful we did not combine trials.
Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
When we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and when it was, used random‐effects analysis to produce it.
We carried out the following subgroup analysis by incorporating zinc status as subgroups as part of the primary comparison.
Risk of populations (population with no or low risk of zinc deficiency versus population with assumed risk of zinc deficiency).
Study settings (studies conducted in high‐income settings versus low‐income settings).
The primary outcomes were used in the subgroup analysis.
We assessed differences between subgroups by interaction tests. For random‐effects and fixed‐effect meta‐analyses using methods other than inverse variance, we assessed differences between subgroups by interaction tests.
Sensitivity analysis
We carried out sensitivity analysis to explore the effects of adequate allocation concealment, but found that restricting to only trials with adequate allocation concealment made very little difference to the results for the primary outcomes.
Results
Description of studies
Results of the search
In this update, we found an additional seven reports. We added two reports for one new randomised controlled trial (Egypt 2014) to make a total of 21 included trials. We excluded one new trial (Naher 2012) and added two new reports each for the included studies Indonesia 1999 (Prawirohartono 2011; Prawirohartono 2013), and Ghana 2009 (Saaka 2009; Saaka 2012).
Included studies
We included 21 RCTs involving over 17,000 women and their babies. See table of Characteristics of included studies for details.
Participants and settings
Eighteen studies included women from low‐ and middle‐income settings. One of the four studies in the higher‐income or mixed‐income settings only recruited women at risk of giving birth to small‐for‐gestational age babies (UK 1991a).
Baseline zinc concentrations and nutritional status
Women in most of the studies had, or were likely to have low zinc concentrations and low nutritional status. It is difficult to assess zinc status and most studies have assumed that pregnant women from low‐income groups would be low in zinc as part of their overall poor nutritional status. Where studied, the improvement in serum zinc concentrations in the supplemented group supports this assumption (Bangladesh 2000; Peru 1999). The only studies likely to have included women with normal zinc concentrations were UK 1989; UK 1991a; UK 1991b.
Dosage of zinc supplementation
The dose of daily zinc supplementation ranged from 5 mg (China 2001) to 44 mg zinc per day (Denmark 1996). Some women in S Africa 1985 had doses of up to 90 mg zinc per day.
Duration of supplementation
Women were supplemented from before conception in Nepal 2003 with the shortest duration being from 26 completed weeks' gestation in some women in USA 1983; and USA 1985.
Types of interventions
Most trials (15/21) compared zinc with placebo (Bangladesh 2000; China 2001; Chile 2001; Denmark 1996; Egypt 2014; Ghana 2009; Iran 2010; Pakistan 2005; S Africa 1985; UK 1989; UK 1991a; USA 1983; USA 1985; USA 1989; USA 1995). Two trials (Peru 1999; Peru 2004) compared zinc with non‐zinc supplement (iron plus folate). In some trials (seeCharacteristics of included studies table), all women were also given iron, folate or vitamins or combinations of these. Four trials (Egypt 2014; Indonesia 1999; Indonesia 2001; Nepal 2003) had more than two arms, so these trials were analysed to compare women who received zinc with women who did not.
Nepal 2003 was a cluster‐RCT ‐ analyses adjusted for clustering were presented in study reports and so we did not need to perform additional calculations for these study results.
Adherence to treatment
Two studies (Chile 2001; Denmark 1996) excluded women who did not comply with their treatment (85% and 60% compliance respectively) and the other 19 studies included or probably included women in the analysis who did not comply. Of the latter group, two studies (UK 1991a; USA 1983) presented at least some results separately for those women who complied and those who did not comply. Adherence was generally reported to be over 70%, except for Pakistan 2005; UK 1989; UK 1991a, where it was 50% to nearly 70%.
Excluded studies
We excluded 16 studies. See table of Characteristics of excluded studies for details.
Risk of bias in included studies
Risk of bias for included studies is summarised in Figure 1 and Figure 2.
1.
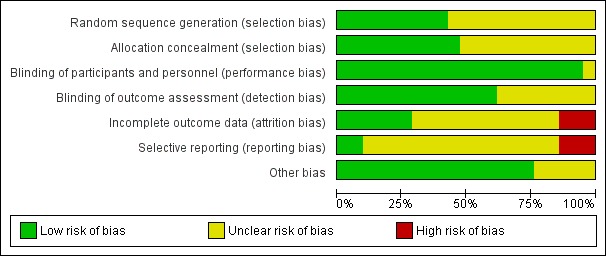
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
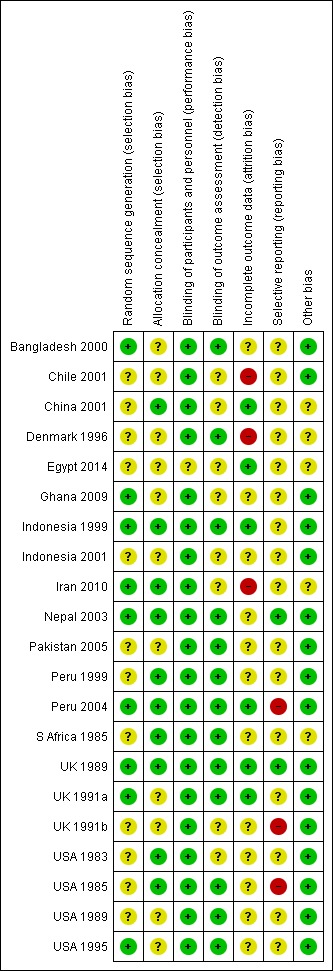
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Allocation concealment was considered adequate in 10 trials (China 2001; Indonesia 1999; Iran 2010; Nepal 2003; Peru 1999;Peru 2004; S Africa 1985;UK 1989;USA 1985; USA 1983). Allocation concealment was rated as unclear in 11 trials: Bangladesh 2000; Chile 2001; Denmark 1996; Egypt 2014; Ghana 2009; Indonesia 2001; Pakistan 2005; UK 1991a; UK 1991b; USA 1989; USA 1995 (method not described or not clearly described); and in Indonesia 2001 there was third party randomisation but no details of how allocations were concealed.
Blinding
All trials stated that both investigators and mothers were blinded or that the trial was double‐blinded.
Blinding of outcome assessors was not well described but was likely to have happened in most trials (at least for short‐term outcomes) as the majority were placebo‐controlled.
Incomplete outcome data
Losses to follow‐up ranged from 1% in UK 1989 to 40% in Denmark 1996. Attrition bias was judged to be at high risk in only three trials (Chile 2001; Denmark 1996; Iran 2010).
Selective reporting
Selective reporting bias was mostly rated as unclear, with three RCTs judged to be at high risk due to expected outcomes not being reported, or reported incompletely.
Other potential sources of bias
Other sources of bias were not generally evident although several trials reported some baseline imbalances and several had restricted analyses.
Effects of interventions
See: Table 1
We included 21 RCTs involving over 17,000 women and their babies. Egypt 2014 did not report any of our primary or secondary outcomes, thus we were unable to include any data from this trial in the analyses.
Primary outcomes:
There was a 14% reduction in preterm birth in zinc groups compared with no zinc groups (risk ratio (RR) 0.86, 95% confidence interval (CI) 0.76 to 0.97; 16 RCTs, 7637 women; moderate quality evidence, Analysis 1.1).
1.1. Analysis.
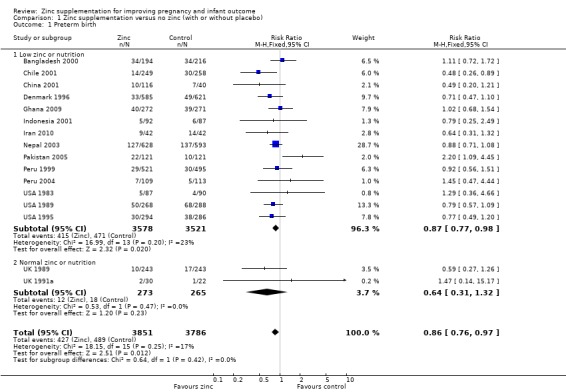
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 1 Preterm birth.
No significant differences between zinc and no zinc were seen for stillbirth or neonatal death: (RR 1.57 95% CI 0.83 to 2.98; four RCTs of 1364 women; low zinc or RR 0.93 95% CI 0.24 to 3.65; three RCTs of 683 women; normal zinc; low quality evidence, (Analysis 1.2).
1.2. Analysis.
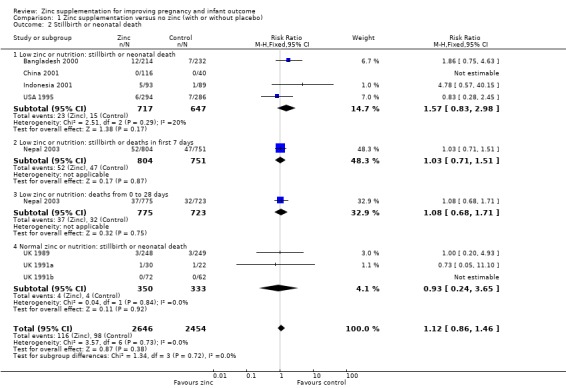
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 2 Stillbirth or neonatal death.
There was no significant difference in birthweight between zinc and no‐zinc groups (mean difference (MD) 0.90; 95% CI ‐22.23 to 24.02; 17 RCTs, 6757 babies; low quality evidence, Analysis 1.3); small‐for‐gestational age (RR 1.02 95% CI 0.94 to 1.11; eight RCTs, 4252 babies Analysis 1.4; moderate quality evidence) or low birthweight (RR 0.93, 95% CI 0.78 to 1.12; 14 RCTs, 5643 babies; moderate quality evidence, Analysis 1.5).
1.3. Analysis.
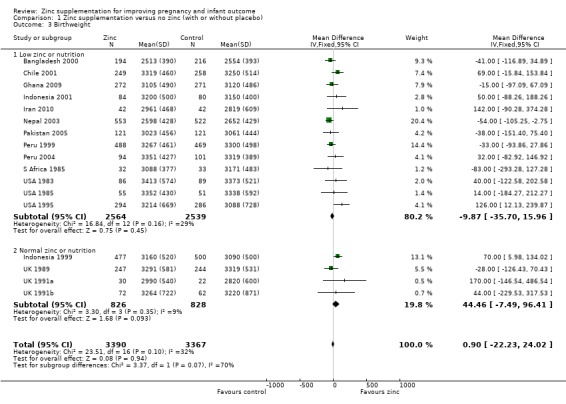
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 3 Birthweight.
1.4. Analysis.
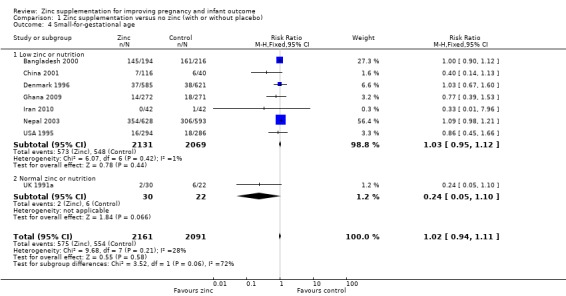
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 4 Small‐for‐gestational age.
1.5. Analysis.
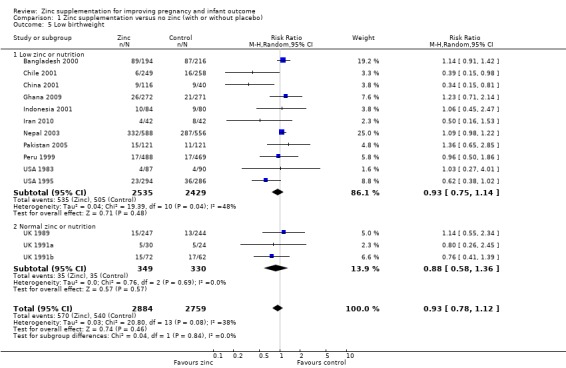
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 5 Low birthweight.
Secondary outcomes
Maternal outcomes
No significant difference was seen for pregnancy hypertension or pre‐eclampsia (RR 0.83, 95% CI 0.64 to 1.08; seven RCTs, 2975 women; Analysis 1.7) or prelabour rupture of membranes (Analysis 1.8), antepartum haemorrhage (Analysis 1.6), post‐term birth (Analysis 1.9), retention of placenta (Analysis 1.15), meconium in liquor (Analysis 1.12), instrumental vaginal birth (Analysis 1.14) and smell dysfunction or taste dysfunction (Analysis 1.17; Analysis 1.18), but these outcomes were measured in only one or two trials. In one trial of women at risk for small‐for‐gestational age babies (UK 1991a), significantly fewer women in the zinc group than in the no‐zinc group were induced (RR 0.27, 95% CI 0.10 to 0.73, 52 women; Analysis 1.10).
1.7. Analysis.
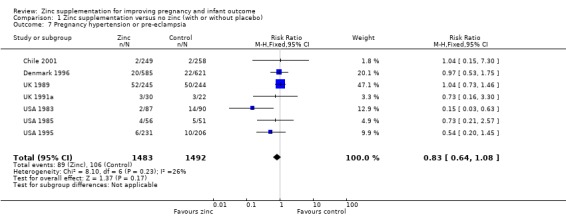
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 7 Pregnancy hypertension or pre‐eclampsia.
1.8. Analysis.
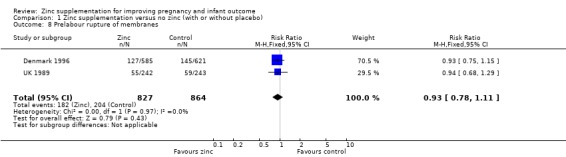
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 8 Prelabour rupture of membranes.
1.6. Analysis.
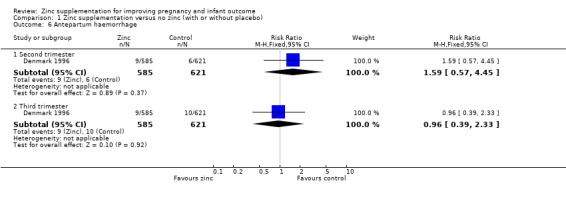
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 6 Antepartum haemorrhage.
1.9. Analysis.
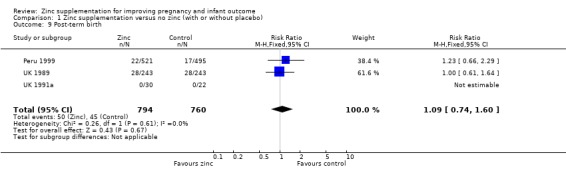
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 9 Post‐term birth.
1.15. Analysis.
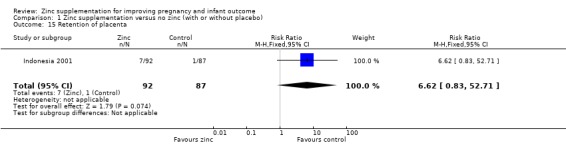
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 15 Retention of placenta.
1.12. Analysis.
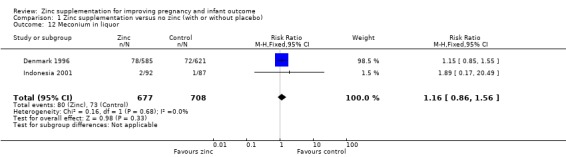
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 12 Meconium in liquor.
1.14. Analysis.
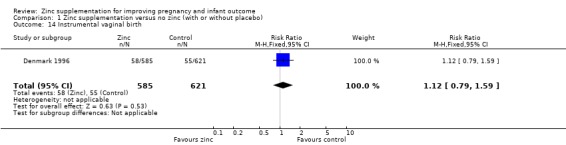
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 14 Instrumental vaginal birth.
1.17. Analysis.
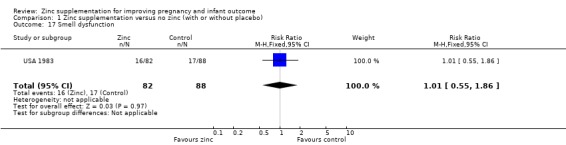
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 17 Smell dysfunction.
1.18. Analysis.
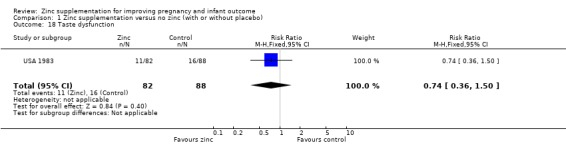
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 18 Taste dysfunction.
1.10. Analysis.
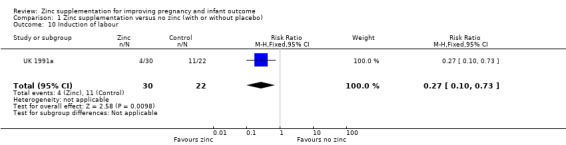
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 10 Induction of labour.
No significant differences were seen for postpartum haemorrhage (Analysis 1.16) or maternal infections (Analysis 1.11) (three trials each) or gestational age at birth (Analysis 1.25) (seven trials) or caesarean section (Analysis 1.13; random‐effects) (six trials). The heterogeneity in caesarean section seemed to be contributed to by the income settings of the countries, as trials in high‐income settings tend to favour zinc supplement, while trials in low‐income settings tend to favour the controls.
1.16. Analysis.
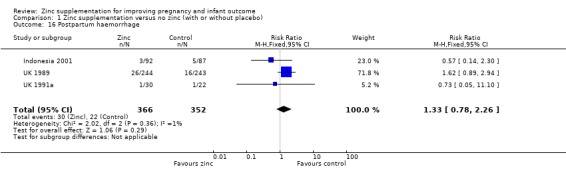
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 16 Postpartum haemorrhage.
1.11. Analysis.
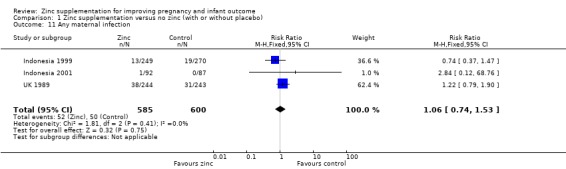
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 11 Any maternal infection.
1.25. Analysis.
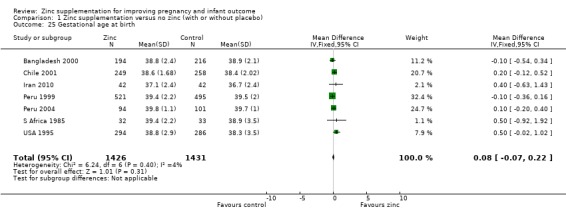
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 25 Gestational age at birth.
1.13. Analysis.
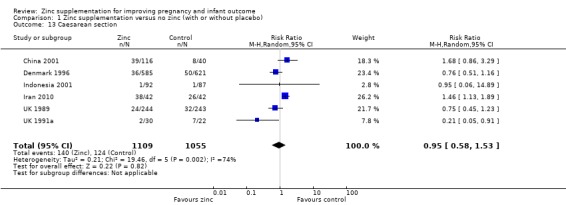
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 13 Caesarean section.
Birthweight and associated outcomes
No differences between the zinc and no zinc groups were seen for high birthweight (Analysis 1.26) (five RCTs), head circumference (Analysis 1.28) (seven RCTs) or mid‐upper arm circumference (Analysis 1.44) (three RCTs). A high level of heterogeneity was apparent in the results for head circumference (I² = 45%). A random‐effects model did not change the conclusion of no significant difference between the zinc and no‐zinc groups.
1.26. Analysis.
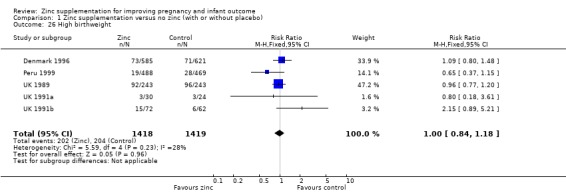
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 26 High birthweight.
1.28. Analysis.
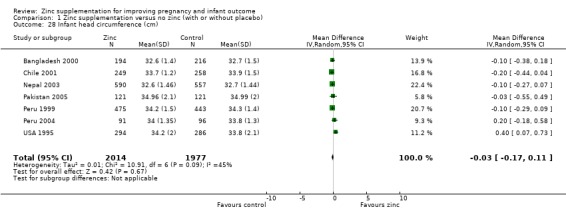
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 28 Infant head circumference (cm).
1.44. Analysis.
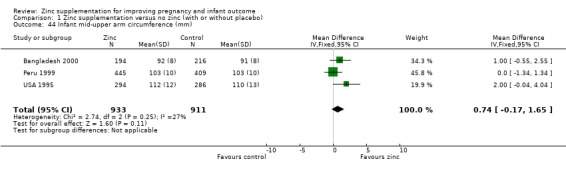
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 44 Infant mid‐upper arm circumference (mm).
Other neonatal outcomes
No significant differences were seen for congenital malformations (Analysis 1.36) (six RCTs).
1.36. Analysis.
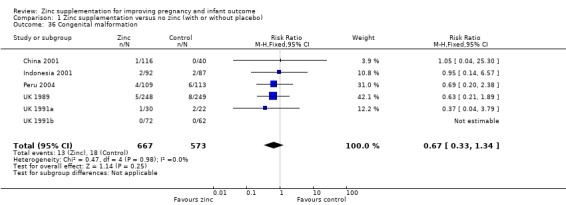
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 36 Congenital malformation.
There were no significant differences between the zinc and no‐zinc groups for the following outcomes: Apgar scores less than five at five minutes, neonatal hypoxia, jaundice, fever, infant umbilical infection, neonatal sepsis, respiratory distress syndrome, neonatal intraventricular haemorrhage, necrotising enterocolitis, and neonatal hospital stay. Each of these outcomes was only available from one or two RCTs.
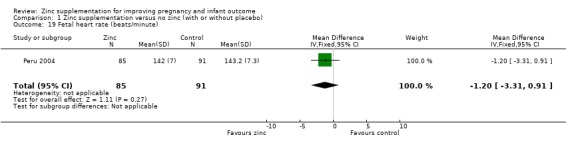
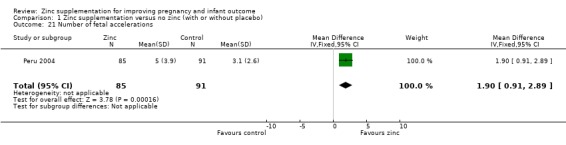
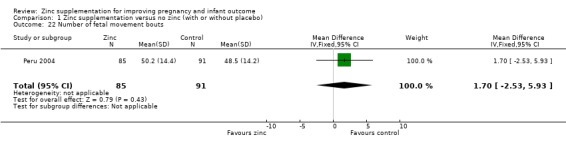
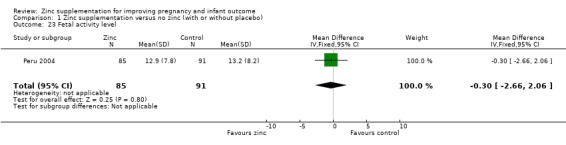
In one RCT of 176 babies (Peru 2004), four measures of fetal heart rate (fetal heart rate, number of fetal movement bouts, fetal activity level, and fetal movement amplitude) showed no evidence of differences between the zinc and no‐zinc groups, while fetal heart rate variability and number of fetal accelerations were significantly higher in the zinc groups.
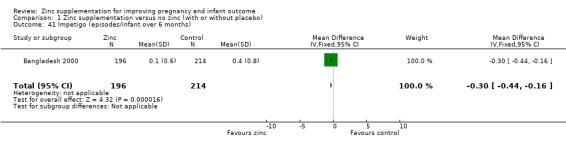
In one RCT of 410 infants (Bangladesh 2000), the zinc group (196 infants) had significantly fewer episodes per infant of acute diarrhoea over six months (MD ‐0.40 episodes, 95% CI ‐0.79 to ‐0.01; Analysis 1.37), and significantly fewer episodes per infant of impetigo. No significant differences were seen for episodes of persistent diarrhoea, dysentery, cough, and acute lower respiratory infection) over the same period.
1.37. Analysis.
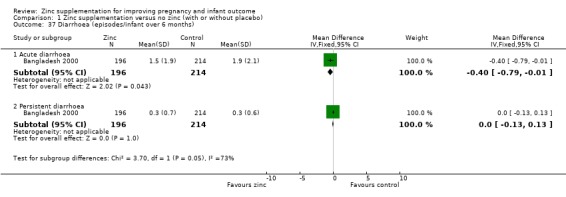
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 37 Diarrhoea (episodes/infant over 6 months).
Results of infant weight‐for‐age (Z‐score) showed no evidence of difference at six months for the zinc and no‐zinc groups in two RCTs (304 infants), but by 13 months, the no‐zinc group showed significantly higher scores (in one RCT of 168 infants, Bangladesh 2000) (Analysis 1.42). No evidence of difference was seen for weight‐for‐height at six months in one RCT of 136 infants (Indonesia 2001) (Analysis 1.43).
1.42. Analysis.
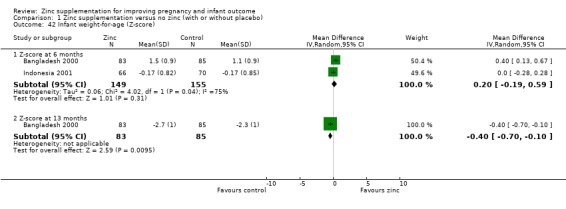
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 42 Infant weight‐for‐age (Z‐score).
1.43. Analysis.
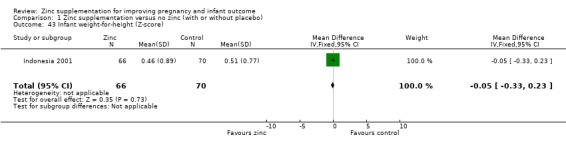
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 43 Infant weight‐for‐height (Z‐score).
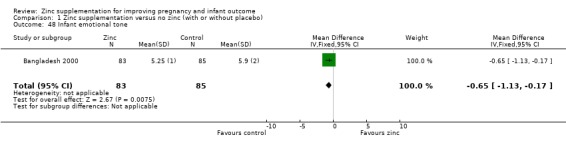
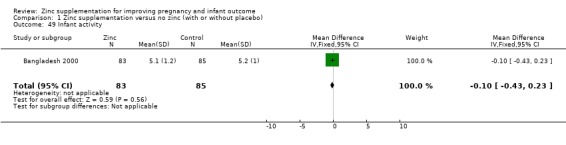
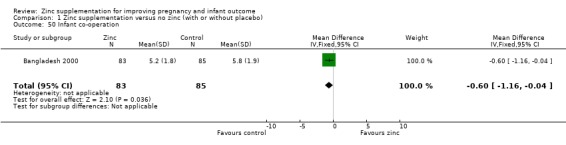
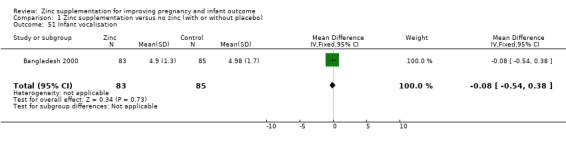
Infant/child development
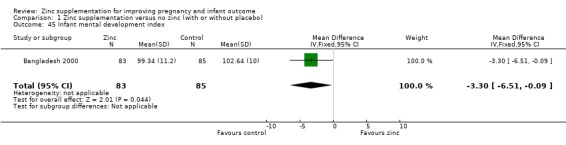
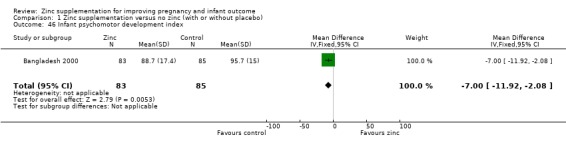
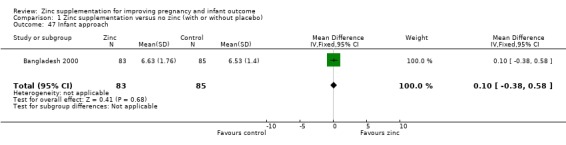
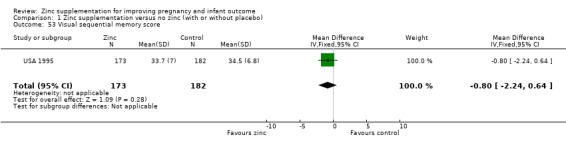
Three RCTs (Bangladesh 2000; Peru 2004; USA 1995) measured child development outcomes. A subset of 168 infants from Bangladesh 2000 assessed at 13 months found that the zinc group had significantly worse mental development, psychomotor development index scores, emotional tone and co‐operation than the no‐zinc group, with infant approach, activity, and vocalisation showing no significant differences. The US RCT (USA 1995) followed up 355 infants at five years, finding no evidence of differences between zinc and no‐zinc groups for differential abilities, visual or auditory sequential memory scores, Knox cube, gross motor scale and grooved pegboard scores. The trial in Peru (Peru 2004) reported intelligence quotient of infants at 54 months, which showed no evidence of difference.
Subgroup analyses
No differing patterns were clearly evident in the subgroups of women with low versus normal zinc concentrations and nutrition status (with the possible exception for small‐for‐gestational age where women with normal zinc concentrations may show more benefit for this outcome), or in women who adhered to their treatment versus those who did not (latter subgroup analysis not presented in the graphs), though the interaction test showed borderline P value (P = 0.06).
Reporting bias
There are three outcomes whose meta‐analyses included more than 10 studies (Figure 3; Figure 4; Figure 5). Although there was no evidence of reporting bias in preterm birth and birthweight, the distribution of the results on low birthweight were skewed. This means there is a possibility of reporting bias and warrants careful interpretation of the results. The result on effectiveness by zinc could have been overestimated.
3.
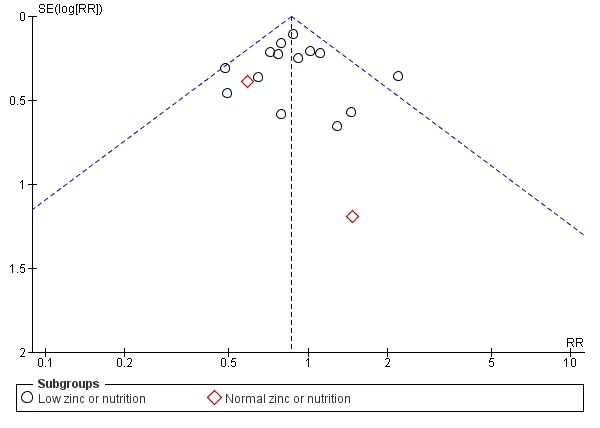
Funnel plot of comparison: 1 Zinc supplementation versus no zinc (with or without placebo), outcome: 1.1 Preterm birth.
4.
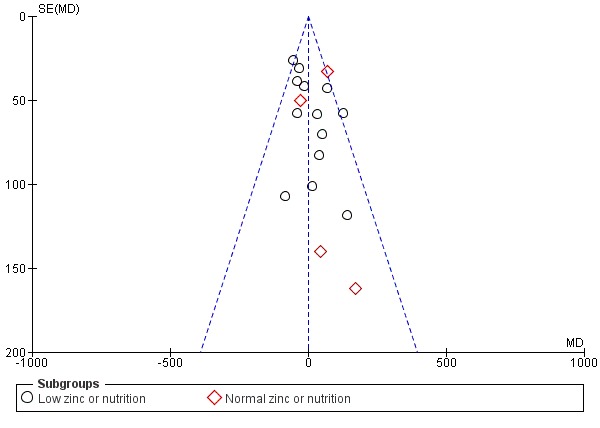
Funnel plot of comparison: 1 Zinc supplementation versus no zinc (with or without placebo), outcome: 1.3 Birthweight.
5.
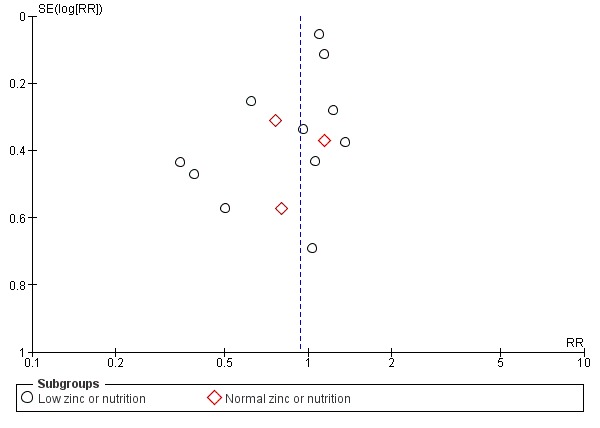
Funnel plot of comparison: 1 Zinc supplementation versus no zinc (with or without placebo), outcome: 1.5 Low birthweight.
Discussion
Summary of main results
Many studies have demonstrated some positive response on biochemical parameters such as serum zinc status of mother or baby, or both, with supplementation (Bangladesh 2000; Peru 1999) as have studies of iron supplementation in pregnancy (Pena‐Rosas 2006). It is now crucial to focus on the impact of any intervention on outcomes that are of clinical significance and particularly those that may be related to maternal, fetal, neonatal and infant mortality and morbidity. This is relevant because of the limited resources, both financial and human, currently available worldwide but in particular to the developing countries where such morbidity and mortality is high.
This review of 21 randomised controlled trials, including over 17,000 women and their babies, has not provided compelling evidence for routine zinc supplementation during pregnancy, although the finding of a reduction in preterm births warrants further investigation, as does the suggestion of reporting bias from the funnel plot on small‐for‐gestational age. Subgroup analysis of the 17 studies involving women who are or are likely to be zinc deficient, such as populations from developing countries or from low socioeconomic groups from western countries, also did not make a case for zinc supplementation in those groups of women. This is consistent with a review of maternal zinc supplementation in developing countries (Osendarp 2003).
Overall completeness and applicability of evidence
The small but significant reduction in preterm birth in the zinc group deserves further attention; is it possible that improving nutrition would cause an even greater reduction? The Cochrane review on micronutrient supplementation did not show any significance in reduction of preterm birth (Haider 2012). Although dosage of zinc may play a role, no dose‐response pattern was evident in this review (with the possible exception of pre‐eclampsia). It is possible that zinc used in conjunction with iron may dilute the effect of supplementation. The intrauterine growth effect seen in UK 1991a, where women were selected on the basis of being at risk for giving birth to a small‐for‐gestational age baby, has not been replicated. In the Bangladesh 2000 study, where the incidence of small‐for‐gestational age was 75% and low birthweight was 43%, supplementation with 30 mg zinc daily did not improve pregnancy outcomes. This is most likely due to the presence of other concurrent nutrient deficiencies. Peru 1999, Bangladesh 2000 and USA 1995 studies attempted to assess the neurodevelopmental effect of zinc supplementation on infants. The inconsistencies in their results probably reflect the dependence of such outcomes on many variables.
Quality of the evidence
The overall risk of bias was unclear in the half of the studies. We assessed the quality of the evidence using GRADE comparing the effects of zinc supplementation versus placebo/no intervention during pregnancy (Table 1). The GRADE quality of the evidence was moderate for preterm birth, small‐for‐gestational age, and low birthweight, downgraded by one level due to the fact that most studies had design limitations. Stillbirth or neonatal death, and birthweight were considered to be low quality of evidence, downgraded by two levels because of the design limitations and wide 95% CIs crossing the line of no effect.
Potential biases in the review process
We followed the Cochrane Pregnancy and Childbirth Group search strategies and review process to reduce potential biases.
Agreements and disagreements with other studies or reviews
Zinc is likely to be only one micronutrient in the overall picture of maternal nutrition prior to and during the course of pregnancy. Although the Cochrane review on micronutrient supplementation concludes that there is a reduction for low birthweight and small‐for‐gestational age with multiple‐micronutrient supplements compared with iron folic acid supplementation, but there is no added benefit for preterm birth (Haider 2012). In order to make any significant impact on morbidity and mortality, we really need to address the underlying problem of poor nutrition, due to low socioeconomic status (Peru 1999). Villar and colleagues (Villar 2003) indicated that while zinc supplementation may be promising, they go on to say that "it is unlikely that any specific nutrient on its own ... will prevent .... preterm delivery or death during pregnancy".
Although improving birthweight, particularly in women from low‐income countries is desirable, data from Nepal 2003 imply a degree of caution. In the overall Nepal 2003 study, multiple‐micronutrient supplementation (but not other combinations of micronutrients) compared with controls was associated with more babies with a birthweight greater than 3.3 kg; and this high birthweight was associated with an increased risk of symptoms of birth asphyxia (risk ratio 1.49, 95% confidence interval 1.04 to 2.13).
Despite uncertainty about the effects of maternal zinc supplementation, many pharmaceutical companies have added zinc to their multivitamin preparations. Lack of any significant benefit from zinc supplementation of mothers suggests that we should now not waste valuable resources looking at zinc in isolation. In addition, infant micronutrient supplementation (including zinc) may be more effective than maternal supplementation (Lassi 2010; Shrimpton 2005). Any future research aimed at improving outcomes related to maternal nutrition should address ways of modifying the overall nutritional status of pregnant women particularly in developing countries. This may not come from the scientific but from the political community where more resources need to be put into improving the overall socioeconomic status of impoverished populations and also to improve the status of the women in such populations. Future research should also address other interventions such as work reduction in populations of pregnant women at high risk of nutritional deficiency.
Authors' conclusions
Implications for practice.
The 14% relative reduction in preterm birth for zinc compared with placebo was primarily in studies of women of low income and this has some relevance in areas of high perinatal mortality. Some trials showed inconsistent findings, but overall, there is not enough evidence to show that routine zinc supplementation in women results in other clinically relevant outcomes.
Implications for research.
There appeared to be inconsistency between trials regarding some pregnancy outcomes. The reduction in preterm birth needs further assessment probably in association with protein‐calorie nutrition. Future research aimed at improving outcomes related to maternal nutrition should address ways of modifying the overall nutritional status of pregnant women particularly in low‐income regions, but avoid looking at zinc in isolation. Future research should also address other interventions such as work reduction in populations of pregnant women at high risk of nutritional deficiency.
What's new
| Date | Event | Description |
|---|---|---|
| 15 September 2015 | Amended | Added additional information to Characteristics of included studies and Characteristics of excluded studies tables. |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 3, 1997
| Date | Event | Description |
|---|---|---|
| 31 October 2014 | New search has been performed | Search updated. Seven new reports identified from the updated search: two reports of one new trial included (Egypt 2014); one new trial excluded (Naher 2012) and four new reports of existing trials added. Methods have been updated. A 'Summary of findings' table incorporated. |
| 31 October 2014 | New citation required but conclusions have not changed | The inclusion of one new trial (Egypt 2014) did not change the conclusions. |
| 9 November 2011 | New search has been performed | Search updated. Three new trials included (China 2001; Ghana 2009; Iran 2010) and four new trials excluded (Mahmoudian 2005; Van Vliet 2001; Villamor 2006; Yalda 2010). |
| 9 November 2011 | New citation required but conclusions have not changed | New authors helped to update this review. |
| 1 July 2011 | Amended | Search updated. Thirteen trial reports added to Studies awaiting classification. |
| 6 November 2008 | Amended | Converted to new review format. |
| 20 December 2006 | New search has been performed | Search updated. Nine new studies have been added to the original seven included studies, plus one previously excluded study (USA 1985) has now been included, making a total of 17 studies included in the 2006 update. A total of 11 studies have been excluded in this update and two studies have been placed in Studies awaiting classification. The Background and Methods sections have been expanded in this update, and additional outcomes have been added. The title has been changed from 'Zinc supplementation in pregnancy' to 'Zinc supplementation for improving pregnancy and infant outcome'. The conclusions regarding the effect of zinc supplementation on reducing preterm birth have been slightly strengthened. |
Acknowledgements
S Osendarp for providing information about unpublished trials. We also thank Sally J. Reynolds and Becky Ann Davie for their support for the 'Risk of bias' assessment in this update.
Data and analyses
Comparison 1. Zinc supplementation versus no zinc (with or without placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth | 16 | 7637 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.76, 0.97] |
| 1.1 Low zinc or nutrition | 14 | 7099 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.77, 0.98] |
| 1.2 Normal zinc or nutrition | 2 | 538 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.31, 1.32] |
| 2 Stillbirth or neonatal death | 8 | 5100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.86, 1.46] |
| 2.1 Low zinc or nutrition: stillbirth or neonatal death | 4 | 1364 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.83, 2.98] |
| 2.2 Low zinc or nutrition: stillbirth or deaths in first 7 days | 1 | 1555 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.71, 1.51] |
| 2.3 Low zinc or nutrition: deaths from 0 to 28 days | 1 | 1498 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.68, 1.71] |
| 2.4 Normal zinc or nutrition: stillbirth or neonatal death | 3 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.24, 3.65] |
| 3 Birthweight | 17 | 6757 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐22.23, 24.02] |
| 3.1 Low zinc or nutrition | 13 | 5103 | Mean Difference (IV, Fixed, 95% CI) | ‐9.87 [‐35.70, 15.96] |
| 3.2 Normal zinc or nutrition | 4 | 1654 | Mean Difference (IV, Fixed, 95% CI) | 44.46 [‐7.49, 96.41] |
| 4 Small‐for‐gestational age | 8 | 4252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.94, 1.11] |
| 4.1 Low zinc or nutrition | 7 | 4200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.95, 1.12] |
| 4.2 Normal zinc or nutrition | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.05, 1.10] |
| 5 Low birthweight | 14 | 5643 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.78, 1.12] |
| 5.1 Low zinc or nutrition | 11 | 4964 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.14] |
| 5.2 Normal zinc or nutrition | 3 | 679 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.58, 1.36] |
| 6 Antepartum haemorrhage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Second trimester | 1 | 1206 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.57, 4.45] |
| 6.2 Third trimester | 1 | 1206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.39, 2.33] |
| 7 Pregnancy hypertension or pre‐eclampsia | 7 | 2975 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.64, 1.08] |
| 8 Prelabour rupture of membranes | 2 | 1691 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.78, 1.11] |
| 9 Post‐term birth | 3 | 1554 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.74, 1.60] |
| 10 Induction of labour | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.10, 0.73] |
| 11 Any maternal infection | 3 | 1185 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.74, 1.53] |
| 12 Meconium in liquor | 2 | 1385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.86, 1.56] |
| 13 Caesarean section | 6 | 2164 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.58, 1.53] |
| 14 Instrumental vaginal birth | 1 | 1206 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.79, 1.59] |
| 15 Retention of placenta | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.62 [0.83, 52.71] |
| 16 Postpartum haemorrhage | 3 | 718 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.78, 2.26] |
| 17 Smell dysfunction | 1 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.55, 1.86] |
| 18 Taste dysfunction | 1 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.36, 1.50] |
| 19 Fetal heart rate (beats/minute) | 1 | 176 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐3.31, 0.91] |
| 20 Fetal heart rate variability (beats/minute) | 1 | 176 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [0.04, 1.16] |
| 21 Number of fetal accelerations | 1 | 176 | Mean Difference (IV, Fixed, 95% CI) | 1.9 [0.91, 2.89] |
| 22 Number of fetal movement bouts | 1 | 176 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐2.53, 5.93] |
| 23 Fetal activity level | 1 | 176 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐2.66, 2.06] |
| 24 Fetal movement amplitude | 1 | 176 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.79, 1.19] |
| 25 Gestational age at birth | 7 | 2857 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.07, 0.22] |
| 26 High birthweight | 5 | 2837 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.84, 1.18] |
| 27 Five‐minute Apgar score less than 5 | 2 | 1692 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.26, 4.03] |
| 28 Infant head circumference (cm) | 7 | 3991 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.17, 0.11] |
| 29 Blue or floppy (neonatal hypoxia) | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.67 [0.70, 46.18] |
| 30 Neonatal sepsis | 2 | 736 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.03, 1.01] |
| 31 Neonatal jaundice | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.20, 4.56] |
| 32 Respiratory distress syndrome | 2 | 1136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.40, 1.14] |
| 33 Neonatal intraventricular haemorrhage | 1 | 580 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.14, 6.86] |
| 34 Necrotising enterocolitis | 1 | 580 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.18, 21.34] |
| 35 Neonatal hospital stay | 1 | 580 | Mean Difference (IV, Fixed, 95% CI) | ‐1.1 [‐2.39, 0.19] |
| 36 Congenital malformation | 6 | 1240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.33, 1.34] |
| 37 Diarrhoea (episodes/infant over 6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 37.1 Acute diarrhoea | 1 | 410 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.79, ‐0.01] |
| 37.2 Persistent diarrhoea | 1 | 410 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.13, 0.13] |
| 38 Dysentery (episodes/infant over 6 months) | 1 | 410 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.12, 0.00] |
| 39 Cough (episodes/infant over 6 months) | 1 | 410 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.56, 0.16] |
| 40 Acute lower respiratory infection (episodes/infant over 6 months) | 1 | 410 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.34, 0.14] |
| 41 Impetigo (episodes/infant over 6 months) | 1 | 410 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.44, ‐0.16] |
| 42 Infant weight‐for‐age (Z‐score) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 42.1 Z‐score at 6 months | 2 | 304 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.19, 0.59] |
| 42.2 Z‐score at 13 months | 1 | 168 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.70, ‐0.10] |
| 43 Infant weight‐for‐height (Z‐score) | 1 | 136 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.33, 0.23] |
| 44 Infant mid‐upper arm circumference (mm) | 3 | 1844 | Mean Difference (IV, Fixed, 95% CI) | 0.74 [‐0.17, 1.65] |
| 45 Infant mental development index | 1 | 168 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐6.51, ‐0.09] |
| 46 Infant psychomotor development index | 1 | 168 | Mean Difference (IV, Fixed, 95% CI) | ‐7.0 [‐11.92, ‐2.08] |
| 47 Infant approach | 1 | 168 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.38, 0.58] |
| 48 Infant emotional tone | 1 | 168 | Mean Difference (IV, Fixed, 95% CI) | ‐0.65 [‐1.13, ‐0.17] |
| 49 Infant activity | 1 | 168 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.43, 0.23] |
| 50 Infant co‐operation | 1 | 168 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.16, ‐0.04] |
| 51 Infant vocalisation | 1 | 168 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.54, 0.38] |
| 52 Differential abilities score at 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 52.1 Non‐verbal ability | 1 | 355 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐5.70, 0.90] |
| 52.2 Verbal ability | 1 | 355 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐2.56, 1.96] |
| 52.3 General conceptual ability, IQ | 1 | 355 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐3.74, 1.54] |
| 53 Visual sequential memory score | 1 | 355 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐2.24, 0.64] |
| 54 Auditory sequential memory score | 1 | 355 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.65, 1.85] |
| 55 Knox cube score | 1 | 355 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.19, 0.39] |
| 56 Gross motor scale score | 1 | 355 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐4.79, 0.79] |
| 57 Grooved pegboard score | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 57.1 Dominant hand | 1 | 355 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [‐1.26, 6.26] |
| 57.2 Non‐dominant hand | 1 | 355 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐2.71, 5.11] |
| 58 Intelligence quotient of infants at 54 months | 1 | 181 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐3.33, 2.53] |
1.19. Analysis.
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 19 Fetal heart rate (beats/minute).
1.20. Analysis.
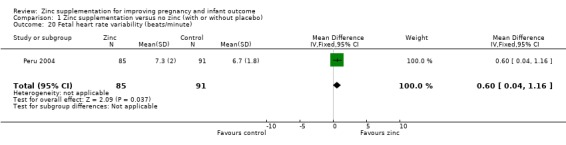
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 20 Fetal heart rate variability (beats/minute).
1.21. Analysis.
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 21 Number of fetal accelerations.
1.22. Analysis.
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 22 Number of fetal movement bouts.
1.23. Analysis.
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 23 Fetal activity level.
1.24. Analysis.
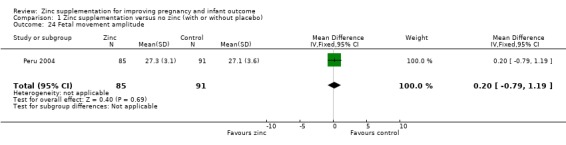
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 24 Fetal movement amplitude.
1.27. Analysis.
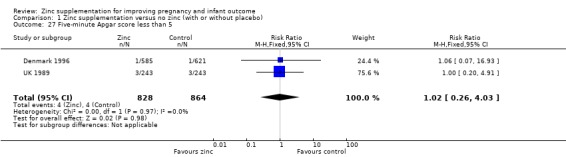
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 27 Five‐minute Apgar score less than 5.
1.29. Analysis.
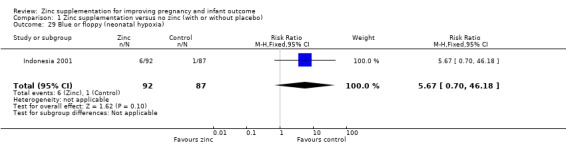
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 29 Blue or floppy (neonatal hypoxia).
1.30. Analysis.
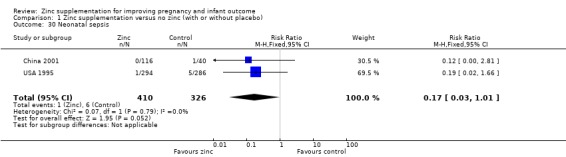
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 30 Neonatal sepsis.
1.31. Analysis.
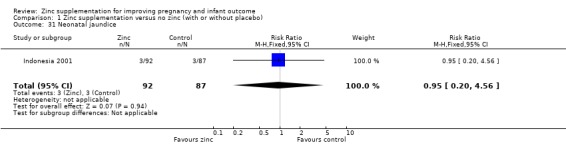
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 31 Neonatal jaundice.
1.32. Analysis.
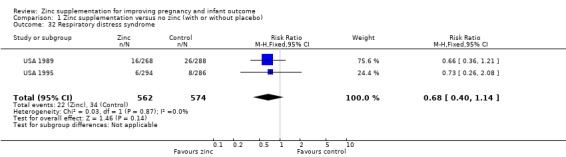
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 32 Respiratory distress syndrome.
1.33. Analysis.
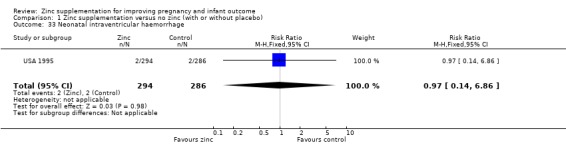
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 33 Neonatal intraventricular haemorrhage.
1.34. Analysis.
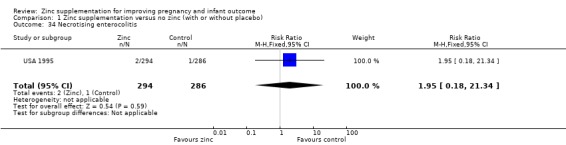
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 34 Necrotising enterocolitis.
1.35. Analysis.
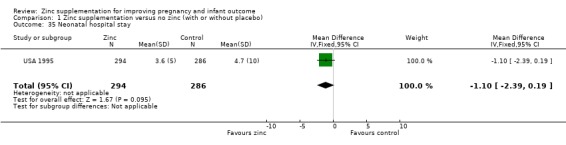
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 35 Neonatal hospital stay.
1.38. Analysis.
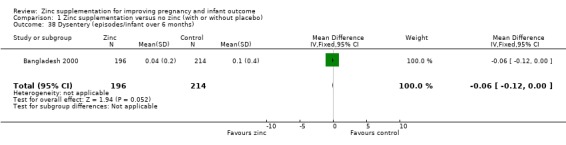
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 38 Dysentery (episodes/infant over 6 months).
1.39. Analysis.
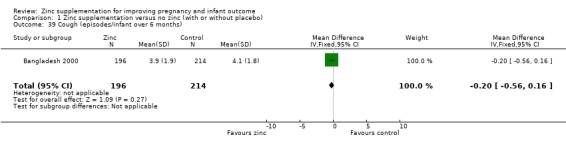
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 39 Cough (episodes/infant over 6 months).
1.40. Analysis.
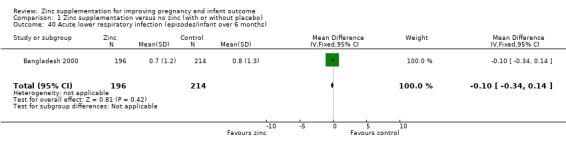
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 40 Acute lower respiratory infection (episodes/infant over 6 months).
1.41. Analysis.
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 41 Impetigo (episodes/infant over 6 months).
1.45. Analysis.
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 45 Infant mental development index.
1.46. Analysis.
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 46 Infant psychomotor development index.
1.47. Analysis.
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 47 Infant approach.
1.48. Analysis.
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 48 Infant emotional tone.
1.49. Analysis.
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 49 Infant activity.
1.50. Analysis.
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 50 Infant co‐operation.
1.51. Analysis.
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 51 Infant vocalisation.
1.52. Analysis.
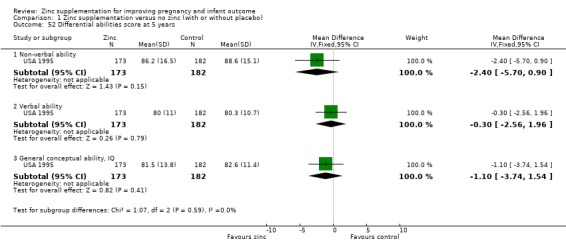
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 52 Differential abilities score at 5 years.
1.53. Analysis.
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 53 Visual sequential memory score.
1.54. Analysis.
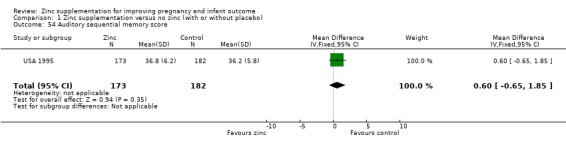
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 54 Auditory sequential memory score.
1.55. Analysis.
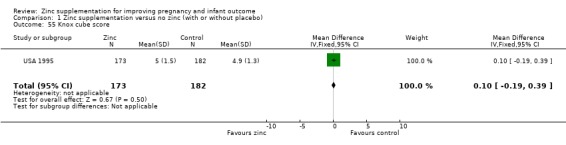
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 55 Knox cube score.
1.56. Analysis.
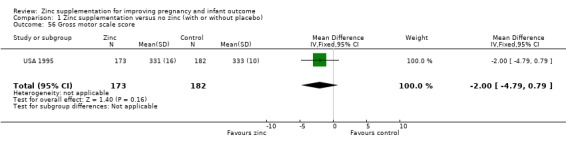
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 56 Gross motor scale score.
1.57. Analysis.
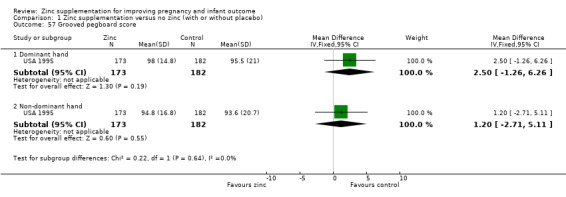
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 57 Grooved pegboard score.
1.58. Analysis.
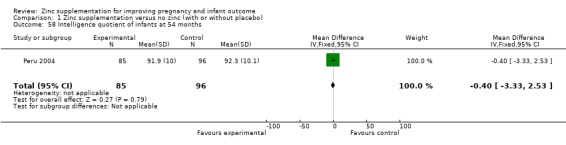
Comparison 1 Zinc supplementation versus no zinc (with or without placebo), Outcome 58 Intelligence quotient of infants at 54 months.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bangladesh 2000.
| Methods | A double‐blind, randomised, placebo‐controlled trial. | |
| Participants | 559 pregnant women between 12 and 16 weeks' gestation, from Dhaka city slums. The 446 women who completed follow‐up had a mean baseline serum zinc level of 15.3 [SD 4.3] µmol/L (similar to those lost to follow‐up). Energy intakes were low at 4 months' gestation (median 6065 kJ/day). A total of 559 pregnant women from selected areas of Dhaka city slums were identified between 12 and 16 weeks of gestation through an established pregnancy identification system between March and June 1996. Women were included if they remain at or near their residences in Dhaka for the delivery without established medical risk for reduced or excessive birthweight (e.g. hypertension, renal disease, or diabetes). The 446 women who completed follow‐up had a mean baseline serum zinc level of 15.3 [SD 4.3] µmol/L (similar to those lost to follow‐up). Energy intakes were low at 4 months' gestation (median 6065 kJ/day). | |
| Interventions | Zinc was given twice the recommended daily intake during the last 2 trimesters of pregnancy. The zinc tablets contained (31.0 mg Zn/tablet; range: 28.6–32.6) and placebo tablets contained (0.0 mg Zn/tablet; range: 0.0–0.1) were was verified and confirmed by 2 independent laboratories. The placebo was a cellulose tablet indistinguishable from the zinc supplement in both appearance and taste. Health workers provided a 1‐week supply of zinc or placebo tablets (ACME Ltd, Dhaka) to the houses of the women weekly and instructed the women to consume 1 tablet daily between meals and not together with other vitamin or mineral supplements. Compliance was assessed by counting the remaining tablets in each strip at the next visit. Unannounced compliance checks between regular visits were performed monthly in subsamples of 10% of the study participant. Zinc: 30 mg elemental zinc/day (n = 269 [214]) . Placebo: n = 290 [232]). |
|
| Outcomes |
Maternal outcomes Serum zinc concentrations at 7 months' gestation; haemoglobin concentrations at 7 months' gestation; blood pressure at 7 months' gestation; preterm birth and gestational age; stillbirth. Neonatal outcomes Birthweight; low birthweight, < 2500 g, < 2000 g, < 1500 g; gestational age (weeks); prematurity,< 37 weeks, < 32 weeks; small‐for‐gestational age; length (cm), head circumference (cm), chest circumference (cm), and mid‐upper arm circumference (mm). |
|
| Notes | Adherence: percentage of days during follow‐up that a woman reported having consumed a supplement was 86%. Final sample size of 410 infants was sufficient to detect a 110 g difference in birthweight. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random letter assignment." |
| Allocation concealment (selection bias) | Unclear risk | "randomly assigned" ‐ no details given regarding allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both investigators and participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specifically mentioned but assessors were also likely to have been blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 113/559 (20.2%) women were lost to follow‐up before birth; (55 (20.4%) in the zinc group and 58 (20.0%) in the placebo group) ‐ most (60) due to migration out of the area. By 13 months follow‐up, 383 (68.5%) infants remained in the trial, with only 168 of these infants being included in the 13‐month analysis. |
| Selective reporting (reporting bias) | Unclear risk | Some primary outcomes such as mode of birth not reported. |
| Other bias | Low risk | No apparent source of other bias. |
Chile 2001.
| Methods | A double‐blind, randomised, placebo‐controlled trial. | |
| Participants | 804 pregnant adolescents were recruited from 5 Primary Care Centres in southern urban slums in Santiago, Chile. They were selected from their prenatal clinic visits before 20 weeks of gestation and aged < 19 years at the estimated time of delivery. The pregnant adolescents identified with chronic diseases, drug abuse, mental retardation, illiteracy or those with pregnancies due to incest or rape were not considered. Subgroup of 220 randomly selected pregnant adolescents at their 28‐30 weeks of gestation with a low zinc intake (7.4 SD 2.3 mg) at the initial admission were evaluated for dietary nutrient intake. Women showed adequate protein intakes but a relatively low mean energy intake. | |
| Interventions | Zinc‐supplemented group (S) received 20 mg of Zn capsules daily (sulphate), or the placebo group (P) received an equivalent capsule of a placebo containing lactose. The group codes changed twice during the study and were kept by the pharmacist who prepared the capsules until the end of computational analysis for double‐blinding procedure. The individuals who ingested less than 50% of the capsules in any month of the study were excluded. All subjects received 40 mg iron (sulphate) supplements daily. Compliance with zinc intake was evaluated by counting the remaining capsules during the monthly visits. Zinc: 20 mg zinc/day (n = 249). Placebo: (n = 258). All women also received 40 mg iron per day. |
|
| Outcomes |
Maternal outcomes Pre‐eclampsia; plasma zinc; hair zinc; gestational age at birth; preterm birth; maternal oedema; maternal cholestasis. red blood cell membrane alkaline phosphatases; plasma alkaline phosphatases . Neonatal outcomes Low birthweight; birthweight; spontaneous abortions; length at birth; head circumference. |
|
| Notes | Adherence: non‐adherers were excluded from analysis; this included individuals who ingested less than 50% of zinc supplements in any month of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned" ‐ no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | "randomly assigned"; pharmacist kept codes ‐ no further details reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind fashion." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up: 297/804 (37%) ‐ failure to come to visits (137), taking less than 15 zinc capsules in any 1 month (115), spontaneous abortion (12), intervention began after 20 weeks' gestation (10), absence of pregnancy (7), change of address (6), apparent intolerance to zinc or placebo (6), twin pregnancy (4). |
| Selective reporting (reporting bias) | Unclear risk | Not all expected maternal primary outcomes reported, but most primary infant outcomes specified in this review were reported. |
| Other bias | Low risk | No apparent risk of other bias. |
China 2001.
| Methods | A double‐blind, randomised, placebo‐controlled trial. A 4‐arm trial. | |
| Participants | 146 pregnant women, less than 12 weeks' gestation, who were living in southwest Shanghai, Maqiao countryside and was attending the prenatal clinic at Maqiao Primary Health Care Center were selected for the study. The people living in this area were uneducated with nutritional knowledge, and took cereal‐based diet with a little even no milk or milk products; therefore, they were supposed to have mild to moderate zinc deficiency according to Chinese recommended dietary allowance. The zinc content of drinking water in this area was considered negligible and no women received folic acid, iron supplementation and any commercial nutrition products during this trial study. | |
| Interventions | For the zinc treatment groups, zinc lactate in capsule were given daily. Group A (GpA, 5 mg/day of zinc (n = 27)); Group B (GpB 10 mg/day of zinc (n = 40)); Group C (GpC, 30 mg/day of zinc (n = 39)). Group D was given placebo where the capsule was of maize starch. (GpD, 0 mg/day of zinc (n = 40)). All capsules were prepared by Laboratory, Second Military Medical University with indistinguishable appearance. Women were instructed to take a single capsule per day 1 hour before or 3 hours after the evening meal. The content of the capsules and the code of the capsule bottles were not known by the investigator or the pregnant women. Only 156 women were followed up under antenatal care. | |
| Outcomes |
Maternal outcomes
Caesarean section;
weight gains;
gestational age;
intrauterine growth restriction;
duration of labour;
oxygen demand;
forceps. Neonatal outcomes Small‐for‐gestational age; neonatal sepsis; low birthweight; congenital malformations; stillbirth; preterm birth. Apgar score; chest, neck, head circumference; crown‐heel length; ponderal index. |
|
| Notes | For the purposes of this review, Group A, B and C were combined as an intervention group and Group D served as a control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description other than the allocation was made randomly. |
| Allocation concealment (selection bias) | Low risk | All capsules were prepared by pharmacy and allocation was concealed for both investigators and women. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All capsules were prepared by pharmacy and both investigators and enrolled pregnant women were concealed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop outs for maternal and neonatal clinical outcomes reported. |
| Selective reporting (reporting bias) | Unclear risk | There is no information on protocol published prior to this trial and no information to make appropriate judgements on this. |
| Other bias | Unclear risk | It was reported that obstetric and physical background data between the groups were not significantly different, though actual data were not reported. |
Denmark 1996.
| Methods | A double‐blinded, randomised placebo‐controlled trial. | |
| Participants | Normal healthy middle‐class women were (at least 18 years old) less than 20 weeks pregnant confirmed by scan for their first visit and booked for delivery at Kolding hospital and Horsens hospital, Denmark. Any known intolerance towards zinc, diabetes mellitus, thyrotoxicosis or earlier rhesus immunization were excluded from the trials. The women thought likely to be zinc deficient by the previous study project 'Pregnancy, environment and way of life' in Denmark. | |
| Interventions | Women received 2 tablets of Zinclet@ (44 mg elemental zinc in total) or 2 placebo tablets containing inert substances. They were indistinguishable in appearance and taste. The tablets were prepared by the Gunnar Kjems Aps company. Women were advised to take 2 tablets daily after breakfast and to avoid taking tablets possibly containing iron together with those of the study, as iron reduces zinc uptake. Women were excluded later, if there were any side‐effects caused by the tablets, if they wanted to stop or if she had not taken the tablets for 14 days in all. Zinc: 2 tablets with 44 mg elemental zinc (n = 1000). No zinc: 2 placebo tablets indistinguishable from active tablets (n = 1000). |
|
| Outcomes |
Maternal
outcomes Prelabour rupture of membranes; preterm labour; pre‐eclampsia; antepartum haemorrhage; caesarean section. Neonatal outcomes Low 5‐minute Apgar score; large‐for‐gestational age; small‐for‐gestational age; birthweight (not able to be used in graphs since no SDs provided). |
|
| Notes | Adherence: non‐adherers were excluded from the final analysis; reasons included side‐effects from tablets, if a woman wished to stop or if a woman had not taken the tablets for 14 days in all. The authors noted that women did not differ in basic characteristics. There were however, significantly more smokers in the non‐adherers group and thus the numbers in the final analysis related to labour and birth have also excluded smokers. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was performed in successive groups of 10 active and 10 placebos; no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators and mothers were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported, but probably done as paper reports that the code was not broken until the end of the study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 794/2000 (39.7%); 415 in zinc group and 379 in placebo group. |
| Selective reporting (reporting bias) | Unclear risk | Not all expected maternal primary outcomes reported, but most primary infant outcomes specified in this review were reported. |
| Other bias | Unclear risk | Analyses relating to labour and birth excluded smokers. |
Egypt 2014.
| Methods | A double‐blind, randomised, placebo‐controlled trial. A 3‐arm parallel group trial. | |
| Participants | 1055 healthy pregnant women from low‐ and middle‐income pregnant populations attending 2 antenatal care centres were screened for low level of zinc serum. Of these women, 675 were with low zinc serum level and were eligible for the trial in Alexandria, Egypt. The age range between 20 and 45 years, with gestational age below 16 weeks with normal course of pregnancy were included for the trial. Women identified through interviews to be on any other form of zinc supplements at any dosage, or risk of having reduced or excessive birthweight of infants (e.g. diabetes, hypertension, renal and heart disease) were excluded. Zinc supplements were provided from 16 weeks until delivery and a subgroup of 100 women were monitored for their dietary intake. Of the 675 women, 597 of women completed the study. | |
| Interventions | The control group (group 1) received placebo, the zinc group (group 2) received a daily supplement of 30 mg of zinc sulphate, and the zinc plus multivitamins group (group 3) received 30 mg zinc sulphate with added multivitamins. Placebo: (n = 199/223 (89%)). Zinc supplement (30 mg daily): (n = 198/225 (88%)). Zinc plus multivitamins (30 mg daily): (n = 200/227 (88%)). | |
| Outcomes |
Maternal outcomes
Zinc serum level;
haemoglobin level. Subsample of women (n = 100) Intake of macronutrients; intake of micronutrients; zinc absorption with dietary food enhancers; zinc absorption with dietary food inhibitors. |
|
| Notes | The mean maternal dietary intake of zinc was 7 mg/day in the 3 groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomized trial.” “participants were randomly assigned to one of the three parallel groups in a 1:1:1 ratio.” Insufficient information on how the random sequence was generated to make a judgement. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “double‐blinded” without further information, so insufficient information to make a judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | “a structured interview was administered to mothers to collect the following data.” Insufficient information on whether the interviewer was blinded to make a judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No description of the women who dropped out from study, although drop out rates is in balance (intervention 88% versus placebo 89%). |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available so insufficient information to make a judgement. |
| Other bias | Unclear risk | The report appears to be free of other sources of bias. |
Ghana 2009.
| Methods | A double‐blind, RCT. | |
| Participants | 400 pregnant women in Ghana earlier than 16 weeks of gestation who presented themselves for antenatal care and have been screened for their gestational age in the Wa Regional Hospital of the Upper West Region in Ghana. Women who were receiving zinc supplements at any dosage level or were severely anaemic (that is, Hb less than 7.0 g/dL) were excluded. The iron‐zinc and iron‐only supplements were pre‐coded and supplied by Nutricaps pharmaceutical company in the United States of America. The supplements (in the form of capsules) were of the same shape, colour and taste and packaging. The women were advised to take the supplements at least 2 hours before or after meals, and at night, just before going to bed. Compliance was monitored by interviewing all participants after having being enrolled for 4 weeks using structured questionnaire to check the frequency and dosage of supplement intake. A subsample of 213 women at recruitment were assessed for serum ferritin but only 173 were repeated at 34‐36 weeks' gestation. N = 299 for intervention and n = 301 for control allocated. 27 out of 299 of the intervention group and 30 out of 301 of the control group were lost to follow‐up and excluded from the analysis. | |
| Interventions | Women received a combined supplement of 40 mg zinc as zinc gluconate and 40 mg iron as ferrous sulphate and women in the control group received 40 mg elemental iron as ferrous sulphate without zinc content. Both groups received malaria chemoprophylaxis in the form of sulphadoxine pyrimethamine, and 400 μg folic acid. The supplements were taken every other day from enrolment until delivery. Intervention group: 40 mg zinc plus 40 mg iron (n = 299). Control group: 40 mg iron only (n = 301). | |
| Outcomes | Intrauterine growth restriction/small‐for‐gestational age; low birthweight; preterm birth; birthweight; haemoglobin concentration; serum ferritin concentration; plasma zinc concentration. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By computer‐generated random number. |
| Allocation concealment (selection bias) | Unclear risk | Opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The capsules for both intervention and placebo were the same. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 27 out of 299 of the intervention group and 30 out of 301 of the control group were lost to follow‐up and excluded from the analysis. |
| Selective reporting (reporting bias) | Unclear risk | It was not clear if a protocol of this trial had been published prior to the study; no maternal outcomes reported. |
| Other bias | Low risk | Baseline characteristics were compared, with no significant difference seen between groups. |
Indonesia 1999.
| Methods | A double‐blinded, randomised placebo‐controlled trial. A factorial design 4‐arm (Zibuvita study) trial and then included a follow‐up study of the infant (the Zinak and Pronak study). | |
| Participants | 5736 women who live in Purworejo district of central Java were identified as pregnant from the Indonesian Ministry of Health surveillance. Of these pregnant women, 2173 women at a gestational age of less than (120 days) 17 weeks were eligible for the trial study. After losses to follow‐up, only 2098 delivery and 1956 neonates were analysed in the Zinak and Pronak study. The follow‐up of the children were from birth up to 2 years of age. | |
| Interventions | Women were randomly allocated to 3 treatment groups and placebo group. The treatment groups were given micronutrient capsules from the date of inclusion in the study until delivery. The capsule contained either 2400 RE of vitamin A (as retinyl palmitate) or 20 mg of ZnSO4, or the same dose of both vitamin A and ZnSO4, or placebo. All capsules also contained 2 mg of DL‐a‐tocopherol as an antioxidant and 350 mg of soya bean oil, 20 mg of beeswax and 8 mg of lecithin as capsule filler. The supplements were manufactured by Tishcon Corp. (Westbury, NY, USA) and they were packaged in plastic strips in identical, opaque pink capsules that was sufficient supplements for 2 weeks or 1 month. Fieldworkers distributed capsules and monitored compliance at the home of the women by counting the unused capsule. Vitamin A (2400RE): n = 484/527 (91.8%). Zinc (20 mg): n = 477/531 (89.8%). Vitamin A (2400RE) + zinc (20 mg): n = 495/543 (91.2%). Placebo: n = 500/523 (95.6%). | |
| Outcomes |
Maternal outcome
Pregnancy weight. Neonatal outcomes Birthweight; low birthweight; stillbirth/neonatal death; blue/floppy (neonatal hypoxia); fever/not drinking; umbilical infection; 6‐month Z‐scores; 6‐month haemoglobin, plasma retinol, plasma zinc; birth size (weight and length); small‐for‐gestational age. |
|
| Notes | Adherence: mean adherence ranged from 71% to 73% across the 4 arms of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pseudo‐random number generator in blocks of 12. |
| Allocation concealment (selection bias) | Low risk | Treatment allocation sequence was prepared and held at a remote site. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All investigators, field and laboratory staff and participants were blinded to the treatment code. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but likely to have been done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 519 of the 1008 women had pregnancies ending between 1 April and 31 October 1997; data available for 503/519 (97%) of these women. |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available and there was not enough information to make this judgement. |
| Other bias | Low risk | No apparent risk of other bias. |
Indonesia 2001.
| Methods | A double‐blinded, RCT. A follow‐up study of the infants with a factorial design. A 4‐arm trial. | |
| Participants | 230 pregnant women were recruited before 20 weeks of gestational age from 13 adjacent villages in Bogor District, Indonesia. Of 230 women, 179 women remain until delivery and only and only 170 women were enrolled for follow up of infant and mother until 6 months postpartum study. Each woman was supplemented daily during pregnancy until delivery. Exclusion criteria at enrolment were twin pregnancy and congenital abnormalities. Women had mean plasma zinc concentrations of about 11 µmol/L. | |
| Interventions | All women received iron and folic acid (30 mg iron as ferrous fumarate/d and 0.4 mg pteroylglutamic acid/d). In addition, 1 group of women received ß‐carotene (4.5 mg as water‐soluble granulate/d; ß‐carotene group), 1 group received zinc (30 mg zinc as sulphate/day; zinc group), 1 group received ß‐carotene plus zinc (4.5 mg ß‐carotene and 30 mg zinc/d; ß‐carotene + zinc group), and 1 group received only iron and folic acid (control group). Capsules were prepared by the pharmacy of the Gelderse Vallei Hospital (Ede, Netherlands) and given a letter code and the micronutrients were indistinguishable from each other. Compliance, expressed as a proportion of the intended supplements consumed during pregnancy, did not differ among the groups with a mean compliance of > 80% in all groups, and 90% of the women taking > 50% of the intended dose. Iron + folate acid: (n = 41). Iron + folate acid + β‐carotene: (n = 43). Iron + folate acid + zinc: (n = 44). Iron + folate acid + β‐carotene + zinc (n = 42). | |
| Outcomes |
Maternal outcomes Preterm birth; caesarean section; prolonged labour; retention of placenta; postpartum haemorrhage; infection; 6‐month serum zinc. Neonatal outcomes Birthweight; low birthweight; congenital malformation; stillbirth/neonatal death; blue/floppy (neonatal hypoxia); jaundice; fever/not drinking; umbilical infection; 6‐month Z‐scores; 6‐month haemoglobin; plasma retinol; plasma zinc. |
|
| Notes | Adherence: mean adherence was over 80%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Supplements were prepared by a third party (hospital pharmacy in the Netherlands), but no detail given of how the contents of the bottles were concealed from the investigators or the participants. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as being "double‐blind"; probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up: 50/229 (22%) women before giving birth; 136 newborns completed follow‐up at 6 months. |
| Selective reporting (reporting bias) | Unclear risk | Not all expected maternal primary outcomes reported, but most primary infant outcomes specified in the review were reported. |
| Other bias | Low risk | No apparent risk of other bias. |
Iran 2010.
| Methods | A double‐blind, randomised, placebo‐controlled trial. | |
| Participants | 110 healthy pregnant women with a previous preterm delivery who were receiving prenatal care from obstetrics and gynaecology outpatient clinics of Isfahan University of Medical Sciences were recruited for the trial. The healthy pregnant women were 18–35 years, at 12–16 weeks' gestational age at delivery, height > 150 cm, weight > 45 kg, non‐smoker, no complicated pregnancy, but with history of preterm delivery, carrying a singleton fetus, living in Isfahan and willingness to continue current medications for the duration of the study. | |
| Interventions | The treatment group received (50 mg/day Zn as Zn sulphate) produced by a local pharmaceutical company, Alhavi Pharmaceutical Laboratory, Tehran, Iran, from the day of reporting (12–16th weeks of gestation) until delivery, and the control group received placebo. Both groups administered capsules orally before meals once a day. The capsules were distributed monthly during prenatal visit. Compliance with study treatment was established by asking the women about missed doses and by counting unused sachets. The doses used are safe during pregnancy. Intervention: 50 mg/day Zn as Zn sulphate (n = 42). Placebo: (n = 42). | |
| Outcomes |
Maternal outcomes
Caesarean section;
pre‐eclampsia;
intrauterine growth restriction. Neonatal outcomes Small‐for‐gestational age; gestational age at birth; preterm birth; low birthweight. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomised according to a pre‐existing list produced by a computer program. |
| Allocation concealment (selection bias) | Low risk | Both woman and physician who assessed the outcome were not aware of treatment type that the woman was receiving. The masking of the active and placebo treatments was preserved by creating treatments that looked identical. The hospital pharmacist was informed of all randomisation assignments and was responsible for labelling the study drug and maintaining a master list linking the women and their treatment assignments. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | As above. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 42 out of allocated 55 women in the intervention group and 42 out of 55 women in the control group were analysed (26% lost to follow‐up in each group). |
| Selective reporting (reporting bias) | Unclear risk | Not enough information to make this judgement. No information on if the protocol had been published prior to the trial. |
| Other bias | Unclear risk | No significant baseline differences except for higher haemoglobin concentrations in the zinc group (MD 0.5 g/dL). |
Nepal 2003.
| Methods | A double‐blind, cluster‐randomised, controlled trial (also factorial design). It was an 1‐5 treatment arms intervention. | |
| Participants | 4926 pregnant women and 4130 liveborn infants in a rural plains district of Sarlahi, community in Nepal, which had 426 sectors (communities of about 100‐150 households) ‐ only 2 of the 5 arms (total of 1659 infants) used in this review. This is the same area of Nepal in which we previously recorded evidence of vitamin A, iron, and zinc deficiency among pregnant women. Women who were currently pregnant, breastfeeding a baby less than 9 months old, menopausal, sterilised or widowed were excluded. Supplementation commenced before conception. | |
| Interventions | The sectors were randomly assigned to 1 of 5 treatment arms. The control group was vitamin A (1000 µg retinol equivalents). FA group, vitamin A + folic acid (400 µg). FAFe group, vitamin A + folic acid + iron (60 mg). FAFeZn group, vitamin A + folic acid + iron + zinc (30 mg). MN group, vitamin A + folic acid + iron+ zinc + other micronutrients (10 µg vitamin D, 10 mg vitamin E, 1.6 mg thiamine, 1.8 mg riboflavin, 20 mg niacin, 2.2 mg vitamin B‐6, 2.6 µg vitamin B‐12, 100 mg vitamin C, 65 µg vitamin K, 2.0 mg Cu, 100 mg Mg). The supplements were provided from UNICEF, identical in shape, size, and colour, arrived in Nepal in opaque, sealed, and labelled bottles coded 1–5. The code allocation was kept locked at the Johns Hopkins University, Baltimore. The investigators, field staff, and participants were blinded to the codes throughout the study. Zinc: zinc + iron + folate (n = 858). No zinc: iron + folate (n = 801). | |
| Outcomes |
Maternal
outcome Preterm birth. Neonatal outcomes Stillbirth or neonatal death; birthweight; chest circumference; head circumference; length; low birthweight; small‐for‐gestational age. |
|
| Notes | Adherence: mean adherence was 88%. RRs adjusted for the cluster‐design effects were presented for each of the 5 arms of the RCT. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised sectors by "drawing numbered identical chips from a hat" (in blocks of 5 within each community). |
| Allocation concealment (selection bias) | Low risk | Supplements were of identical shape, size and colour and arrived in Nepal in opaque, sealed and labelled bottles coded 1‐5. The code allocation was kept locked at the Johns Hopkins University, Baltimore. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, investigators, field staff and statisticians were all blinded to the codes throughout the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators, field staff and statisticians were all blinded to the codes throughout the study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 155/827 (19%) of infants in the zinc group and 167/872 (19%) in the non‐zinc group were lost to follow‐up or excluded from analysis (infant died, mother refused, home was inaccessible, birthweight was measured more than 72 hours after birth or missing data). |
| Selective reporting (reporting bias) | Low risk | Most expected outcomes were reported with some exceptions such as mode of birth and postpartum haemorrhage. |
| Other bias | Low risk | No apparent evidence of other sources of bias apart from a small imbalance between groups in maternal weight (which was adjusted for in the analyses). |
Pakistan 2005.
| Methods | A double‐blind, RCT. | |
| Participants | By simple random sampling, 250 women from 2 urban hospitals and 1 rural community in Pakistan at 10‐16 weeks' gestation were recruited. 242 women completed the study. The mean (SD) age of the women was 25.7 (4.8) years (range 16–4). Women with known systemic disease were excluded. Serum zinc at enrolment was mean 71.51 µg/dL (SD 21) in the zinc group and 74.09 (SD 23.2) in the placebo group. | |
| Interventions | The supplement was a 20 mg of zinc sulphate powder capsule filled with glucose and a similar capsule as placebo. The supplement were given to the women from the time of booking to the end of their gestational week. In addition, routine supplements of folic acid and iron given. The dietary zinc intake was taken into account by a food diary and various food items were assigned a score. Women were followed up at monthly intervals by trained staff. Compliance was ensured by health visitors and pills were counted out every month before new supply were issued as to double check the consumption of the medicine. Zinc: 20 mg elemental zinc (zinc sulphate powder capsule) (n = 121). Placebo: (n = 121) (capsule); in addition, all women had routine supplements of folic acid and iron. | |
| Outcomes | Maternal outcome Preterm birth. Neonatal outcomes Occipitofrontal circumference; low birthweight; abortion/intrauterine death; birthweight; length. | |
| Notes | Adherence: about 65% of women had good adherence, which was similar in both groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "simple random sampling with preassigned code." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women and health workers were blinded to content of medication. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but likely to have been done. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up: 15% (actual figures not given, but paper notes that losses were non‐differential). |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available. Not enough information to make this judgement. |
| Other bias | Low risk | No apparent risk of other bias. |
Peru 1999.
| Methods | A double‐blind, RCT. | |
| Participants | 1295 women with a low‐risk pregnancy (uncomplicated and eligible for vaginal delivery), at the Hospital Materno Infantil in Lima, Peru and low zinc intake who were carrying a singleton fetus, and had lived in coastal Peru for ≥ 6 months before pregnancy were recruited for the study. These women indicated with low zinc intake living in this region and at 10 to 24 weeks' gestation. The study protocol was approved by the institutional review boards of the Instituto de Investigación Nutricional (IIN) and The Johns Hopkins School of Hygiene and Public Health. | |
| Interventions | 1 group received a daily supplements containing 60 mg Fe (ferrous sulphate) and 250 µg folate with 15 mg of Zn (zinc sulphate) and the other received the same Fe supplement but without an additional 15 mg Zn (zinc sulphate). Women were asked to take 1 pill daily midmorning with a vitamin C–containing drink or water according to the Peruvian guideline. Supplementation began at gestation week 10–24 and continued until 4 weeks postpartum. The supplements were produced by a local pharmaceutical company (Instituto Quimioterápico, SA, Lima, Peru) in coded blister packages. To verify the formulation of the supplements and the integrity of the coding scheme, samples of each supplement type were analysed by the IIN laboratory 2 times during the study.
Zinc: 15 mg zinc plus 60 mg iron plus 250 µg folate (n = 521).
Non‐zinc: 60 mg iron plus 250 µg folate (n = 495). |
|
| Outcomes | Maternal outcomes Duration of pregnancy; preterm birth (< 37 weeks); very preterm birth (< 33 weeks); post‐term birth (> 42 completed weeks); serum and urinary zinc concentrations; haemoglobin; serum ferritin; fetal heart rate and movement measures. Neonatal outcomes Birthweight; low birthweight; high birthweight; cord vein zinc; cord vein haemoglobin; cord vein serum ferritin; crown‐heel length; head circumference; chest, calf and mid‐upper arm circumference; biceps, subcapsular and calf skinfold thicknesses. | |
| Notes | Adherence: mean of about 85% of capsules consumed, which was similar across the groups. Adjustments for baseline differences in maternal age and in‐home electricity were made by multiple regression. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported. |
| Allocation concealment (selection bias) | Low risk | Coded blister packages were prepared by a local pharmaceutical company, and allocation was thus concealed by use of this third party. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators, other health personnel and women were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but likely to have been done due to use of placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 21.5% (279/1295) women lost to follow‐up by time of giving birth ‐ 18 (1%) were found to live in another community and therefore not eligible to participate; 92 (7%) declined to participate; 71 (5%) moved out of the study area; 30 (2%) miscarried; 58 (4%) left the study for other reasons; 10 (1%) were subsequently found to have twin pregnancies or to have developed pregnancy complications. |
| Selective reporting (reporting bias) | Unclear risk | No information on if the protocol had been published prior to the trial. |
| Other bias | Low risk | No apparent risk of other bias. |
Peru 2004.
| Methods | A double‐blind, RCT. | |
| Participants | 242 (low‐income) Peruvian pregnant women at 10 to 16 weeks’ gestation receiving prenatal care at the Hospital Materno Infantil San Jose in Lima, Peru were recruited. The maternal dietary zinc intake is approximately 8 mg/d,12 an intake much lower than recommended intakes at that time of 15 mg/d (US Recommended Dietary Allowance) in this region. Women who were with singleton pregnancy, and had lived in coastal Peru for at least 6 months before becoming pregnant were included in the study. Exclusions was made according to a protocol for fetal neurobehavioural assessment. | |
| Interventions | Women were randomly assigned to receive a daily supplement containing 60 mg iron (ferrous sulphate) and 250 mg folic acid, with 25 mg zinc (zinc sulphate) or the same supplement but without 25 mg zinc (zinc sulphate). The supplements were manufactured in Lima, had the same appearance and taste, and both study personnel and study subjects were masked to treatment assignment. The supplements were distributed in blister packs at monthly intervals, beginning at entry into the study at 10 to 16 weeks’ gestation and continuing until 1 month postpartum. Adherence with supplementation was checked biweekly by health workers who visited the women in their homes and observed the number of tablets remaining in each blister pack. The level of adherence was calculated as the percentage of tablets taken over the number of days in the study. They used a standard questionnaire with specific questions regarding potential benefits or side effects of supplement consumption. Zinc: zinc + iron + folate (n = 109 [94]). No zinc: iron + folate (n = 113 [101]). | |
| Outcomes |
Maternal outcomes
Preterm birth with complications;
gestational age at birth.
Neonatal and infant outcomes
Fetal heart rate measures;
birthweight;
length;
biparietal diameter;
abdominal circumference;
femur diaphysis length; infant feeding; infant growth; child development at 54 months; dietary and nutritional status at 54 months; mean arterial pressure at 54 months; BMI at 54 months; haemoglobin concentration at 54 months; plasma zinc concentration at 54 months; C‐reactive protein concentration at 54 months; Home Observation for the Measurement of the Environment (HOME) Scale assessment at 54 months; heart rate measures at 54 months. |
|
| Notes | Adherence: mean adherence rate was 87% (86% in the zinc group and 88% in the no zinc group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomly assigned in blocks of 2 using computer‐generated lists from Johns Hopkins and sent to Peru. |
| Allocation concealment (selection bias) | Low risk | The randomisation code was made by the pharmaceutical company and maintained in a sealed and secured envelope in Lima; supplements had the same appearance and taste. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both study personnel and participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specifically stated, but we have assumed that outcome assessors were blinded and remained blinded for the longer‐term analyses. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 222/242 (90.1%) women completed the protocol and 195 (80.6%) were included in the analysis of birth outcomes ‐ 94 (78%) zinc and 101 (84%) no zinc. The 47 lost were made up of 20 change of address, declining to continue in the study, or travel and 27 exclusions for significant obstetric or medical complications. At 54‐month follow‐up, there were 205 eligible children (includes children of 10 mothers excluded from the initial analysis), and evaluations were completed for 184 (90%) of these children (86 (87%) from the zinc group and 98 (92%) from the non‐zinc group. |
| Selective reporting (reporting bias) | High risk | A number of birth outcomes such as postpartum haemorrhage, stillbirth or neonatal death, low birthweight or Apgar scores were not reported; and preterm birth was only reported as preterm birth with complications which were treated as study exclusions. |
| Other bias | Low risk | No apparent source of other bias although the study was designed to primarily assess neonatal and infant outcomes (see selective reporting above). |
S Africa 1985.
| Methods | A double‐blind, randomised, placebo‐controlled. It was a 4‐arm trial. | |
| Participants | 127 black women before 20th week of pregnancy for antenatal medical care with expectation of spontaneous vaginal delivery and a willingness to attend the clinic at Kwa‐Mashu Polyclinc near Durban, South Africa, daily until delivery to eat dietary supplements under supervision were selected for the study. Free transportation was provided daily to the clinic. At entry, each woman had a detailed medical and socioeconomic history, physical examination, assessment of the week of pregnancy by date of LMP and ultrasonic measurements. Serum levels of proteins, cholesterol, triglyceride, carotene, vitamin A, vitamin C, phosphorus, alkaline phosphatase, calcium and magnesium were also measured. An experienced dietitian calculated dietary intake before starting the supplements from a 24‐hour, quantitative, dietary recall history. The recorded diets were deficient in energy, protein, the B vitamins, calcium and iron among these women. Women in the zinc group in this study had a significantly lower mean weight than the women in the placebo group. | |
| Interventions | Women were randomly assigned to 1 of 4 groups. Before supplementation the women in 3 of the 4 groups had similar body weights. Primigravidas were equally distributed by chance among the 4 groups. Group 1 received placebo pills and group 2, 30‐90 mg zinc gluconate daily. Groups 3 and 4 were given food supplements from the 20th week of pregnancy to delivery, Monday through Friday. These supplements were designed to correct dietary deficiencies detected in the dietary recall histories, particularly deficiencies in energy and protein. Group 3 women received a high bulk supplement, a mixture of beans and maize in a 1.2 : 1 ratio as mush with added vitamins. Group 4 women received a low bulk supplement, a porridge containing 100 g dry skimmed milk, maize flour, vitamins and minerals. It differed from the group 3 supplement in its 36 g of animal protein and in its higher levels of several vitamins and calcium. Group 1: placebo (n = 33). Group 2: zinc: zinc gluconate 30‐90 mg daily (n = 32). Group 3: high food supplement (n = 31). Group 4: low food supplement (n = 31). | |
| Outcomes |
Maternal outcomes
Levels of constituents of serum samples: Albumin, cholesterol, triacylglycerol, carotene, vitamin A, vitamin C, phosphorus, alkaline phosphatase, calcium, magnesium. Neonatal outcomes Gestational age at birth; birthweight. |
|
| Notes | Adherence: figures for adherence were not given, but the authors commented that it was high, due to free transportation to the clinic where the supplements or placebo were consumed under supervision. Groups given dietary supplements are not included in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported. |
| Allocation concealment (selection bias) | Low risk | Randomisation by numbered packets prepared at the pharmacy, code held by pharmacy until the end of the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding by use of placebo until end of study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but likely to have been blinded due to use of placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up: 10% (exact figures not given) of women before giving birth, principally due to moving out of the area. |
| Selective reporting (reporting bias) | Unclear risk | Not enough information to make this judgement. No information on if the protocol had been published prior to the trial. |
| Other bias | Unclear risk | No apparent risk of other bias. |
UK 1989.
| Methods | A double‐blind, randomised, placebo‐controlled trial. | |
| Participants | 500 women who were less than 20 weeks pregnant at the first visit booking for delivery at Southmead Hospital, Bristol were recruited for study. An ultrasound scan was done in 95% of cases. At the end of the visit potential volunteers were seen by the research midwife, who explained the study fully. Median zinc concentrations at enrolment were 1.192 µmol/10 x 10 cells in the zinc group and 1.147 in the placebo group. 494 women remained to complete the study. | |
| Interventions | Women were randomly allocated to receive a capsule containing 20 mg elemental zinc (66 mg zinc sulphate) oral capsule containing inert substances (sucrose, maize starch, purified talc, kaolin, gelatin) but which was indistinguishable in appearance and taste from the one containing zinc. The capsules were prepared by Smith Kline and French Ltd. The mothers were advised to take 1 capsule daily after breakfast. Serum haemoglobin and ferritin concentrations were measured in all women at the first visit. Iron and folate supplementation was advised only if the haemoglobin concentration was less than 100 g/l or the serum ferritin concentration was less than 10 µg/l. Supplementation capsule supply were provided enough to last until the next visit. The research midwives visited the women at the 28‐32 weeks and again on the day of delivery. During the study and after delivery clinical details were recorded by the research midwife by interview as well as from the case records. Compliance was assessed by the regularity with which the study capsules were taken. Those who took the supplement daily or on most days were grouped as compliers, and the rest were regarded as non‐compliers. Zinc: 20 mg elemental zinc (n = 246). No zinc: placebo (n = 248). | |
| Outcomes |
Maternal outcomes Preterm delivery (< 37 weeks); post‐term delivery (> 42 weeks); prelabour rupture of membranes; pregnancy hypertension; any maternal infection ‐ (pre or postdelivery); caesarean section; postpartum haemorrhage; congenital malformations; Neonatal outcomes Low birthweight (< 2500 g); birthweight > 3500 g; small‐for‐gestational age (< 10th centile); Apgar score at 1 minute < 6; Apgar score at 5 minutes < 8; stillbirth/neonatal death. |
|
| Notes | Adherence: adherence levels were not reported, but non‐adherers were included in study results. At 28 to 32 weeks' gestation, just over half the women claimed to be taking the supplement every day, and nearly two‐thirds were doing so by the time of giving birth. Although results were not presented separately for adherers and non‐adherers, the authors state that no significant differences between them were found, apart from a significantly lower risk of postpartum infection among the adherers. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation tables. |
| Allocation concealment (selection bias) | Low risk | Bottles prepared by drug company and labelled A/B. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of placebo; code not broken until the end of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but likely to have been done due to use of placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up: 6/500 (1%) ‐ 4 women moved and 2 miscarried. |
| Selective reporting (reporting bias) | Low risk | Most of the outcomes specified in the review were reported. |
| Other bias | Low risk | No apparent risk of other bias. |
UK 1991a.
| Methods | A double‐blind, randomised, placebo‐controlled trial. It was a 2‐arm trial. | |
| Participants | 56 pregnant women between 15‐25 weeks of pregnancy were selected at St Thomas' Hospital, London, UK. To fulfil the criteria for eligibility, women with low maternal pre‐pregnancy weight (less than 95% ideal body weight) were selected. For this study, Asian women and primigravidae who smoked more than 5 cigarettes per day with previous small‐for‐gestational‐age baby were set as criteria. For the zinc supplement group, women with previous small‐for‐gestational‐age baby, Asian, with low pre‐pregnancy weight and primigravidae who smoked were included. The placebo group were women with low pre‐pregnancy weight, previous small‐for‐gestational‐age baby, Asian, primigravidae who smoked. Social class was allocated from the classification of the Office of Population Censuses and Surveys (1980); classes 4‐7 were grouped as lower socio‐economic. | |
| Interventions | A coded placebo and non‐placebo tablet were prepared by Thames Laboratories Ltd, UK. The effervescent Zn table were 22.5 mg. Zinc: 22.5 mg elemental zinc (n = 30). Placebo: (n = 26). | |
| Outcomes |
Maternal outcomes Pregnancy hypertension; preterm delivery; post‐term labour; induction of labour; caesarean section; Neonatal outcomes Small‐for‐gestational age; low birthweight; birthweight > 3500 g; congenital malformations; stillbirth/neonatal death. |
|
| Notes | Adherence was 43% in the zinc group and 67% in the placebo group ‐ outcomes were presented separately for adherers and non‐adherers. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table, no mention of how the numbers were generated but probably adequately done. |
| Allocation concealment (selection bias) | Unclear risk | "coded placebo or non‐placebo tablet or 22.5 mg effervescent zinc...was randomly prescribed." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blind, "all clinical decisions were made by staff in the labour and delivery wards who were unaware of the trial details". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported, but probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/60 (7%); 2 women moved home, 1 termination of pregnancy, 1 miscarriage (all in the placebo group). |
| Selective reporting (reporting bias) | Unclear risk | Trial did not report all of the primary outcomes expected or specified for this review. |
| Other bias | Low risk | No apparent source of other bias. |
UK 1991b.
| Methods | A double‐blind, RCT. | |
| Participants | 152 women resident in Scunthorpe Health District who booked for care before the 18th week of gestation, and who were booked for delivery at either Scunthorpe Maternity Home or at Scunthorpe General Hospital, were recruited for the study. 134 women completed the trial. | |
| Interventions | Women were randomly assigned to 2 groups, X and Y. The the supplements were prepared by Smith Kline and French. The spansules contained 150 mg of FeSO4 and 0‐5 mg of folic acid for Group X. For Group Y, 62 mg of zinc sulphide was added to the 150 mg of FeSO4 and 0‐5 mg of folic acid . Participants were asked to take 1 spansule per day. Haematological tests were undertaken on entering the study and at the 28th week of gestation. Group X: iron + folic acid (n = 62). Group Y: iron + folic and zinc: (n = 72). | |
| Outcomes | Low birthweight < 2500 g;
birthweight > 3500 g; congenital malformations; stillbirth/neonatal death. |
|
| Notes | Adherence was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double blind." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up: 18/152 (12%) due to GI effects, aborted or woman moved, leaving 72 in the zinc group and 62 in the control group. |
| Selective reporting (reporting bias) | High risk | No maternal outcomes reported. |
| Other bias | Low risk | No apparent risk of other bias. |
USA 1983.
| Methods | A double‐blind, RCT. | |
| Participants | 213 Hispanic women of Mexican descent 17 years of age or older who were not over 27 weeks of gestational age. They were without diabetes or heart, renal or thyroid disease were selected for study. Women specifically selected on the basis of being at high risk for low zinc status ‐ at baseline, 81% of women had recalled dietary intakes providing < 2/3 RDA. All participants were agreed to take the test vitamin and mineral supplement as prescribed, return to the clinic for 2 interviews with the research nutritionist, allow 3 blood and 2 hair samples to be taken, and permit the nutritionist to obtain information from their clinic, delivery, and infant records. | |
| Interventions | Women were randomly assigned into 2 groups. The treatment group received a daily vitamin and mineral supplement as a single capsule providing about 20 mg of zinc as zinc acetate. The control group received a similar supplement without zinc. The capsules were indistinguishable in appearance. The capsule bottles were labelled A or B (Balancel Forte, Meyer Laboratories, Ft Lauderdale, FL). Meyer Laboratory provided the supervisor of the clinic pharmacy with the information necessary to identify the capsules containing zinc. The women were instructed to take the capsule with their evening meal to reduce the possible transitory effect on zinc concentrations in serum samples which were collected in the morning. All supplements were formulated to provide 8000 IU vitamin A, 400 IU vitamin D, 30 IU vitamin E, 2 mg thiamin mononitrate, 2 mg riboflavin, 20 mg niacinamide, 5 mg pyridoxine HCI, 1 mg folic acid, 10 µg vitamin B12 (cyanocobalamin), 10 mg D‐calcium pantothenate, 60 mg vitamin C, 100 mg calcium (as carbon ate), 20 mg iron (as ferrous fumarate), 50 mg of magnesium (as oxide), 1 mg of manganese (as sulphate), and 150 µg iodine (as potassium iodide) per day. Zinc: 20 mg elemental zinc plus vitamins (n = 107). Placebo with vitamins: (n = 106). | |
| Outcomes | Pregnancy hypertension; low serum zinc before. birth (< 53.3 µg/dL; low hair zinc; smell dysfunction; taste dysfunction; preterm birth; low birthweight. | |
| Notes | Adherence: defined as a woman who was in the study long enough to take supplements for more than 60 days and who returned to the pharmacy for 1 or more refills of 60 capsules. According to this definition, 82% overall (90% (81/90) in the control group and 75% (65/87) in the zinc group) were adherent in those 177 women who were not lost to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported. |
| Allocation concealment (selection bias) | Low risk | "randomly assigned" ‐ not definitively stated but likely to have been third party randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind"; "capsules were indistinguishable." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported but stated that "code was not broken until the study was completed". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 36/213 (16.9%) lost to follow‐up (3 spontaneous abortions < 20 weeks, 2 sets of twins, 31 records that could not be located). The breakdown was 20/107 (18.7%) lost from the zinc group and 16/106 (15.1%) from the placebo group. Breakdown of reasons was not reported except for spontaneous abortions ‐ 1 in the zinc group and 2 in the control group. |
| Selective reporting (reporting bias) | Unclear risk | A number of primary maternal, pregnancy and neonatal outcomes were not reported (e.g. caesarean section, postpartum haemorrhage, perinatal death). |
| Other bias | Low risk | No apparent source of other bias. |
USA 1985.
| Methods | A double‐blind, RCT. | |
| Participants | 138 Hispanic teenagers who were under 17 years of age and were not over 27 weeks' gestation and who were attending prenatal clinic at Los Angeles were recruited for the study. The mean dietary zinc intakes among these women were about 50% of the RDA. according to LMP. These teenager did not have diabetes, heart, renal or thyroid disease. All participants were agreed to take the test vitamin and mineral supplement as prescribed, return to the clinic for 2 interviews with the research nutritionist, allow 3 blood and 2 hair samples to be taken, and permit the nutritionist to obtaining the study provided by the staff from their clinic, delivery, and infant records. | |
| Interventions | The teenagers were randomly assigned to control group and treatment group. For treatment group, they received daily vitamin and mineral supplement in a single capsule providing (20 mg) of zinc. The control group received a capsule that did not contain zinc. The supplements composed of 8000 IU vitamin A, 400 IU vitamin D, 30 IU vitamin E, 2 mg thiamin mononitrate, 2 mg riboflavin, 20 mg niacinamide, 5 mg pyridoxine HCl, 1 mg folic acid, 10 µg vitamin B12 (cyanocobalamin), 10 mg pantothenic acid, 60 mg vitamin C, 100 mg calcium (as carbonate), 20 mg iron (as ferrous fumarate), 50 mg magnesium (as oxide), 1 mg manganese (as sulphate) and 150 µg iodine (as potassium iodide). The capsules between the 2 groups were identical in taste and appearance by (Pharmavite Pharmaceuticals Coporation, Pacoima, CA). The teenagers were instructed to take the capsule with their evening meal rather than at breakfast. In addition, 108 mg iron/day was prescribed routinely at 20 weeks' gestation. Zinc with vitamin and mineral group: (n = 70). Vitamin and mineral group: (n = 68). | |
| Outcomes | Infant weight;
placental weight; pregnancy‐induced hypertension; meconium‐stained amniotic fluid; birthweight > 2500 g; Apgar scores; preterm birth; fetal death; plasma zinc; haemoglobin; haematocrit; ferritin levels; folacin levels. |
|
| Notes | Adherence: defined as those in study long enough to take supplements for more than 60 days and who then returned to the pharmacy for 1 or more refills of 60 capsules = 93% of teenagers who returned for a final interview. No significant difference in adherence rates between the groups, so results were not presented separately for adherers and non‐adherers. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned" ‐ not further described. |
| Allocation concealment (selection bias) | Low risk | Third party (dispensed by clinic pharmacy). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Capsules were identical in composition and indistinguishable in taste and appearance, and the code was not broken until the end of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but likely to have been blinded due to the use of a placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Birthweight data not available for 31/138 (22%); due to 2 spontaneous abortions and 29 records that could not be located. |
| Selective reporting (reporting bias) | High risk | Data for outcomes such as perinatal death and preterm birth were collected but not fully reported (only that no significant differences were found). |
| Other bias | Low risk | No apparent source of other bias. |
USA 1989.
| Methods | A double‐blind, randomised, placebo‐controlled trial. It was a 2‐arm trial for women in the groups of low weight, normal weight and high weight. | |
| Participants | The pregnant adolescent woman who were at risk for zinc deficiency and enrolled in the prenatal clinic of Charity Hospsital of New Orleans, a large urban state‐supported hospital serving area women without access to private maternity care, were considered for the trial. At the first clinic visit, the pregnant adolescent woman attended the a nutrition lecture presented by nurse and data of their characteristics and background information were collected. In the second visit, 652 low‐income pregnant adolescent women who were at less than 25 weeks' gestation (average age 17.6 years; range 13.5 to 19.6) were recruited for the trial. Women were grouped by their weight percentile, and treatment group. Total of 556 completed the study. | |
| Interventions | Women were randomly assigned to receiving tablets of 30 mg Zn as gluconate the Z group or the placebo (P) group containing cellulose. A sample of blood was taken for chemical analysis and the previous 24‐hour dietary assessments were repeated 8‐10 weeks after enrolment. The course of the pregnancy was documented by the physician at each prenatal visit. Compliance with the treatment regimen was assessed bu a tablet count at each clinic visit and questioning after delivery when details of labour and delivery events were collected. Zinc (30 mg): (n = 268). Placebo: (n = 288). | |
| Outcomes |
Maternal
outcomes Preterm birth; weight. Neonatal outcomes Birthweight; respiratory assistance. |
|
| Notes | Reported compliance was good ‐ 87% consumed 6 or 7 tablets per week. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned." |
| Allocation concealment (selection bias) | Unclear risk | "randomly assigned." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind"; "identical‐appearing tablets". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Neither the subjects nor the investigators were informed of tablet identity until after completion of the data collection." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up: 10.9% (71/652) at entry and 14.7% (96/652) [cumulative] at birth. Breakdown of losses by group was not reported, nor were reasons for losses. |
| Selective reporting (reporting bias) | Unclear risk | A number of primary maternal, pregnancy and neonatal outcomes were not reported (e.g. caesarean, postpartum haemorrhage, perinatal death). |
| Other bias | Low risk | No apparent source of other bias. |
USA 1995.
| Methods | A double‐blind, randomised, placebo‐controlled trial. | |
| Participants | 5058 medically indigent African‐American women receiving prenatal care in 4 Jefferson County (Alabama) Health Department clinics considered for selection. The pregnant women who were both nulliparous and multiparous ranged between 13 and 44 years of age were included for the selection criteria. Of these women, 589 at 14‐23 weeks' gestation were selected for the randomisation trial based on a plasma zinc level below the estimated median for gestational age for the population at the time of enrolment in prenatal care. Only 580 women's data completed for analysis due to 9 women had insufficient outcome data. | |
| Interventions | Women were randomly assign to both the zinc supplement and placebo groups. Both group received a daily prenatal multivitamin/mineral tablet not containing zinc but containing folic acid, iron, and other minerals. The tablets were produced by Mission Pharmacal, San Antonio, Texas. Zinc supplement and placebo were prepared in a capsules by Rempak, Carteret, New Jersy and only the zinc supplement contained 25 mg of zinc (zinc sulphate). Women were asked to take 1 of each supplement daily, but the time was not specified. The zinc content of the tablets was verified independently in our laboratory. Prior to this study, pregnant women in this care system received only folic acid and iron supplementation. For each woman, compliance was defined as the percentage of zinc tablets consumed compared with the number of days enrolled in the project prior to delivery. Zinc: 25 mg elemental zinc per day (n = 286). Placebo (n = 294). | |
| Outcomes | Preterm birth;
pregnancy hypertension; low birthweight; small‐for‐gestational age; stillbirth/neonatal death; neonatal sepsis; child mental and psychomotor development at 5 years. |
|
| Notes | Adherence: mean was 78% of days for both groups. Adherence was defined as the percentage of zinc tablets consumed compared with the number of days enrolled in the project prior to birth. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "both caregivers and subjects were blind regarding the content of the supplement." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but likely to have been done due to the use of a placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up: samples unavailable from 24.7% (143/580 women; 63/294 (21.4%) in the zinc group and 80/286 (28%) in the placebo group. At 5 years of age, results were available for 355/580 children (61%). |
| Selective reporting (reporting bias) | Unclear risk | Not enough information to make this judgement. No information on if the protocol had been published prior to the trial. |
| Other bias | Low risk | No apparent source of other bias. |
BMI: body mass index dL: decilitre g: gram GI: gastrointestinal IU: international units kJ: kilojoule L: litre LMP: last menstrual period mg: milligram RCT: randomised controlled trial RDA: recommended daily allowance RR: risk ratio SD: standard deviation µg: micrograms µmol: micromoles
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| An 2001 | 313 healthy pregnant women at their fifth month of gestation enrolled in the hospital for prenatal care in Beijin, China, were given fortified biscuits containing (vitamin D), (vitamin D + calcium), (vitamin D + calcium + iron), (vitamin D + calcium + iron + zinc). Only 1 woman selected from the same hospital (no fortified biscuits given) as control comparison. The study indicated quasi‐randomised study, which there was no randomised sequence generation performed and allocation was by the order of hospital visits. |
| Appelbaum 1979 | The effect of diet supplementation throughout pregnancy on third‐trimester amniotic fluid growth‐supporting activity was studied in 100 African women; 32 were given zinc supplementation, 22 each animal and vegetable supplements, respectively, and 24 served as control subjects. Zinc level was measured among the groups and the inhibitory of zinc was conducted in vitro testing of the amniotic fluid collected. The study design did not indicate randomised controlled trial and the outcome was the zinc level of amniotic fluid only. |
| Christian 2001 | A placebo‐controlled trial in Nepal was conducted on 202 women who reported to be night blind during pregnancy. They were randomly assigned in a double‐blind manner, stratified on vitamin A, ß‐carotene, or placebo receipt, to receive 25 mg Zn or placebo daily for 3 weeks. The participant selection was on women who had night blindness and there was no prespecified outcomes reported related to pregnancy except for vision restoration of pregnant women. |
| Fawzi 2005 | Pregnant women who were HIV‐infected, who resided in Dar es Salaam, Tanzania, at the time of the baseline interview, and who intended to stay in the city until delivery and for were considered. 400 HIV‐infected pregnant women were between 12 and 27 weeks of gestation were randomly assigned to daily oral supplementation with either 25 mg Zn or placebo between recruitment and 6 weeks after delivery. The population selected for the trial were not in healthy state of condition. |
| France 2004 | Healthy pregnant women (n = 100) receiving prenatal care between 12 and 16 weeks of gestation in the Obstetric Departments of Grenoble and Lyon Hospitals in France participated in a double‐blind, randomised, placebo‐controlled trial. The intervention was micronutrients supplement or placebo. The micronutrients contained vitamin C (60 mg), ß‐carotene (4.8 mg), vitamin E (10 mg), thiamin (1.4 mg), riboflavin (1.6 mg), niacin (15 mg), pantothenic acid (6 mg), folic acid (200 mg), cobalamin (1 mg), Zn (15 mg as citrate), Mg (87.5 mg as glycerophosphate), Ca (100 mg as carbonate). Zinc was not given separately as the main intervention. |
| Hambidge 1983 | A longitudinal study (monthly intervals) in 46 pregnant middle‐income women. 10 of the women received a daily supplement of 15 mg Zn and the rest of the women did not receive any zinc supplement. The design was an observational study with no mention of randomisation or allocation to zinc or no‐zinc groups. |
| India 1993 | 90 pregnant women were randomly assigned to control (A) and 120 pregnant women were randomly assigned to zinc treated (B) groups. Group B women were administered a single daily dose of 45 mg zinc as a 200 mg zinc sulphate tablet (Zinfate, Yash Pharma) from the day of reporting till delivery. The control group women were not provided with zinc supplementation. The total number of subjects finally selected in group A served as control was 62, and that in Gp. B, was 106. The study design was a quasi‐randomised study and with large discrepancies in numbers of participants at baseline and follow‐up. |
| Kynast 1986 | A randomly selected study group of 179 pregnant women and a control group of 345 pregnant women were given zinc aspartate. This study investigates the prophylactic effectiveness of zinc replacement in reducing the overall complication rate for both mother and fetus and in particular for large‐for‐date and small‐for‐date infants. The study design was a quasi‐randomised study and allocation was done by alternation. |
| Mahmoudian 2005 | A total of 118 anaemic women were recruited in this randomised controlled trial. Both groups received 100 mg elemental iron daily. The intervention group received an additional dose of 15 mg zinc every day for a period of 12 weeks while the control group received placebo. The participants were all anaemic women and outcomes were haemoglobin concentration only. |
| Makola 2003 | This study was a randomised, placebo‐controlled double‐blind effectiveness trial of a micronutrient‐fortified dietary supplement conducted in pregnant women in Tanzania. Pregnant women who believed that they were between 12 and 34 weeks pregnant were invited to participate in the study. The intervention was micronutrient supplement (orange‐flavoured micronutrient‐fortified powdered beverage mix containing 11 micronutrients, including zinc) in comparison to placebo. Women with gestation greater than 26 weeks were included in the study and zinc was not provided separately as an supplemented intervention. |
| Naher 2012 | A total of 200 pregnant women, age ranging between 18‐40 years and gestational age ranging from 37‐42 weeks were selected for a cross‐sectional study in Bangladesh. Among them, 100 were advised to take 61.8 mg zinc daily and the others did not. The study design was an observational study and not a randomised controlled trial. |
| Nishiyama 1999 | 38 Japanese women at the second trimester of pregnancy had haemoglobin concentrations below 11.0 g/dL and 32 of 38 had normocytic erythrocytes. These women were divided into 3 groups, and they were compared for their haematological status and serum IGF‐I levels before and after iron (Group A) or Zn (Group B) or iron plus Zn (Group C) supplementation. The the women were anaemic and the study design was a clinical controlled trial where women could chose 1 of 3 intervention groups. |
| Nogueira 2003 | 74 low‐income pregnant adolescents in Brazil ranging from 13‐18 years of age received supplementation of (folic acid + iron), (folic acid + zinc sulphate + iron) or only (iron). The pregnant adolescents were divided into 5 groups. The study method was based on longitudinal design and zinc plasma concentration was measured as to support the folic acid metabolism. |
| Van Vliet 2001 | The study was an open, randomised study, using a cross‐over approach for 2 sources of vitamin A (i.e. liver paste and retinyl palmitate containing oil) and a parallel approach for 3 dose levels of vitamin A (i.e. 3.0, 7.5, and 15 mg vitamin A) for women between 19‐47 years of age. Pregnant women were excluded in the study. The intervention did not use zinc supplements or any zinc constituent supplements. |
| Villamor 2006 | Pregnant women with HIV and the HIV status of their babies was assessed at birth and at 6 weeks postpartum at Dar es Salaam Tanzania. Women 12–27 weeks of gestation were randomly assigned to receive a daily oral dose of 25 mg zinc or placebo from the day of the first prenatal visit until 6 weeks postdelivery. All the participants were infected with HIV. |
| Yalda 2010 | Single‐blind randomised clinical control trial conducted in Kurdistan region, Iraq. 100 anaemic pregnant women were selected to receive, first group (A), supplemented daily with 120 mg iron and second group (B) received 120 mg iron + 22.5 mg zinc. The pregnant women were all diagnosed with anaemia and the outcome were to assess the improvement of anaemic condition. |
Characteristics of ongoing studies [ordered by study ID]
Zahiri 2010.
| Trial name or title | Assessment of the effect of zinc supplementation on adverse outcomes of pregnancy. |
| Methods | Randomised controlled trial. |
| Participants | Inclusion criteria: gestational age of 12‐16 weeks based on reliable LMP or first trimester ultrasound, lack of history of high‐risk pregnancy, lack of chronic underlying diseases (such as heart disease, HTN, DM). Exclusion criteria: lack of complete treatment or lack of follow‐up. |
| Interventions | Zinc 30 mg from 12th week of gestation every other day in the intervention group and no zinc is supplemented in the control group. |
| Outcomes | Gestation, birthweight and other pregnancy and neonatal clinical outcomes. |
| Starting date | March 2009. |
| Contact information | Dr Ziba Zahiri (drzibazahiri@gums.ac.ir). |
| Notes |
DM: diabetes mellitus HTN: hypertension LMP: last menstrual period
Differences between protocol and review
We have updated our methods to reflect the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Outcomes have been separated into 'Primary' and 'Secondary' outcomes.
We have added 'congenital malformation' to our secondary outcomes.
GIven the number of trials identified and the standard methods for the Cochrane Pregnancy and Childbirthbirth Group, quasi‐randomised controlled trials have been excluded.
Contributions of authors
E Ota (EO) and C Miyazaki (CM) prepared the first version of this update. R Mori (RM) revised the draft. All the authors (RM, EO, R Tobe‐Gai, P Middleton, CM, Z Bhutta and K Mahomed) read and approved the drafts of the updates. R Mori is the guarantor of the review.
Sources of support
Internal sources
Discipline of Obstetrics and Gynaecology, The University of Adelaide, Australia.
National Center for Child Health and Development, Japan.
External sources
-
Ministry of Health, Labour and Welfare, Japan.
Health Labour Sciences Research Grant (No.13800128)
The Evidence and Programme Guidance, Department of Nutrition for Health and Development, World Health Organization, Switzerland.
Declarations of interest
Kassam Mahomed was principal investigator in a trial included in this review and was not involved in its assessment or data extraction.
Edited (no change to conclusions)
References
References to studies included in this review
Bangladesh 2000 {published data only}
- Darmstadt GL, Osendarp SJM, Ahmed S, Feldman C, Van JMA, Baqui AH, et al. Effect of antenatal zinc supplementation on impetigo in infants in Bangladesh. Pediatric Infectious Disease Journal 2012;31(4):407‐9. [DOI] [PubMed] [Google Scholar]
- Hamadani JD, Fuchs GJ, Osendarp SJM, Huda SN, Grantham‐McGregor SM. Zinc supplementation during pregnancy and effects on mental development and behaviour of infants: a follow‐up study. Lancet 2002;360(9329):290‐4. [DOI] [PubMed] [Google Scholar]
- Osendarp S. Zinc supplementation in Bangladeshi women and infants: effects on pregnancy outcome, infant growth, morbidity and immune response [thesis]. Wageningen: Wageningen University, 2001. [Google Scholar]
- Osendarp SJM, Raaij JMA, Darmstadt GL, Baqui AH, Hautvast JG, Fuchs GJ. Zinc supplementation during pregnancy and effects on growth and morbidity in low birthweight infants: a randomised placebo controlled trial. Lancet 2001;357(9262):1080‐5. [DOI] [PubMed] [Google Scholar]
- Osendarp SJM, Raaij JMA, Arifeen SE, Wahed MA, Baqui AH, Fuchs GJ. A randomized, placebo‐controlled trial of the effect of zinc supplementation during pregnancy outcome in Bangladeshi urban poor. American Journal of Clinical Nutrition 2000;71(1):114‐9. [DOI] [PubMed] [Google Scholar]
Chile 2001 {published data only}
- Castillo‐Duran C, Marin V, Alcazar LS, Iturralde H, Ruz MO. Controlled trial of zinc supplementation in Chilean pregnancy adolescents. Nutrition Research 2001;21:715‐24. [Google Scholar]
China 2001 {published data only}
- Xie L, Chen X, Pan J. The effects of zinc supplementation to Chinese rural pregnant women and their pregnancy outcome. Journal of Shanghai Second Medical University 2001;13(2):119‐24. [Google Scholar]
- Yuan W, Geng G, Chen A, Wu J, Zhang Z, Gao E. Effects of zinc supplementation of rural pregnant women on the growth of offspring in early childhood. Fudan University Journal of Medical Sciences 2004;31(5):496‐501. [Google Scholar]
Denmark 1996 {published data only}
- Jonsson B, Hauge B, Larsen MF, Hald F. Zinc supplementation during pregnancy: a double blind randomised controlled trial. Acta Obstetricia et Gynecologica Scandinavica 1996;75:725‐9. [DOI] [PubMed] [Google Scholar]
Egypt 2014 {published data only}
- Naeem N. Does zinc supplementation improve pregnancy outcome of women in Alexandria, Egypt?. Pan African Clinical Trials Register (accessed 31 May 2013) 2011.
- Naem NE, El‐Sayed NM, Nossier SA, Abu Zeid AA. Zinc status and dietary intake of pregnant women, Alexandria, Egypt. Journal of the Egyptian Public Health Association 2014;89(1):35‐41. [DOI] [PubMed] [Google Scholar]
Ghana 2009 {published data only}
- Saaka M. Combined iron and zinc supplementation improves haematologic status of pregnant women in Upper West Region of Ghana. Ghana Medical Journal 2012;46(4):225‐33. [PMC free article] [PubMed] [Google Scholar]
- Saaka M, Oosthuizen J, Beatty S. Effect of joint iron and zinc supplementation on malarial infection and anaemia. East African Journal of Public Health 2009;6(1):55‐62. [DOI] [PubMed] [Google Scholar]
- Saaka M, Oosthuizen J, Beatty S. Effect of prenatal zinc supplementation on birthweight. Journal of Health, Population & Nutrition 2009;27(5):619‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Indonesia 1999 {published data only}
- Hakimi M, Dibley MJ, Surjono A, Nurdiati D. Impact of vitamin A and zinc supplementation on puerperal sepsis: a randomized controlled trial in rural Indonesia. In: Nurdiati D editor(s). Nutrition and Reproductive Health in Central Java, Indonesia; an Epidemiological Approach [PhD thesis]. Umea, Sweden: Umea University Medical Dissertations, 2001. [Google Scholar]
- Prawirohartono EP, Nystrom L, Ivarsson A, Stenlund H, Lind T. The impact of prenatal vitamin A and zinc supplementation on growth of children up to 2 years of age in rural Java, Indonesia. Public Health Nutrition 2011;14(12):2197‐206. [DOI] [PubMed] [Google Scholar]
- Prawirohartono EP, Nystrom L, Nurdiati DS, Hakimi M, Lind T. The impact of prenatal vitamin A and zinc supplementation on birth size and neonatal survival ‐ a double‐blind, randomized controlled trial in a rural area of Indonesia. International Journal for Vitamin and Nutrition Research 2013;83(1):14‐25. [DOI] [PubMed] [Google Scholar]
Indonesia 2001 {published data only}
- Dijkhuizen MA, Wieringa FT. Vitamin A, iron and zinc deficiency in Indonesia: micronutrient interactions and effects of supplementation [thesis]. Wageningen: Wageningen University, 2001. [Google Scholar]
- Dijkhuizen MA, Wieringa FT, West CE, Muhilal. Zinc plus β‐carotene supplementation of pregnant women is superior to β‐carotene supplementation alone in improving vitamin A status in both mothers and infants. American Journal of Clinical Nutrition 2004;80:1299‐307. [DOI] [PubMed] [Google Scholar]
- Wieringa FT, Dijkhuizen MA, Muhilal, Meer JW. Maternal micronutrient supplementation with zinc and beta‐carotene affects morbidity and immune function of infants during the first 6 months of life. European Journal of Clinical Nutrition 2010;64(10):1072‐9. [DOI] [PubMed] [Google Scholar]
Iran 2010 {published data only}
- Danesh A, Janghorbani M, Mohammadi B. Effects of zinc supplementation during pregnancy on pregnancy outcome in women with history of preterm delivery: a double‐blind randomized, placebo‐controlled trial. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(5):403‐8. [DOI] [PubMed] [Google Scholar]
Nepal 2003 {published data only}
- Christian P, Jiang T, Khatry SK, LeClerq SC, Shrestha SR, West KP Jr. Antenatal supplementation with micronutrients and biochemical indicators of status and subclinical infection in rural Nepal. American Journal of Clinical Nutrition 2006;83(4):788‐94. [DOI] [PubMed] [Google Scholar]
- Christian P, Khatry SK, Katz J, Pradhan EK, LeClerq SC, Ram Shrestha S, et al. Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: double blind randomised community trial. BMJ 2003;326:571‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian P, Shrestha J, LeClerq SC, Khatry SK, Jiang T, Wagner T, et al. Supplementation with micronutrients in addition to iron and folic acid does not further improve the hematologic status of pregnant women in Nepal. Journal of Nutrition 2003;133(11):3492‐8. [DOI] [PubMed] [Google Scholar]
- Christian P, West KP, Khatry SK, Leclerq SC, Pradhan EK, Katz J, et al. Effects of maternal micronutrient supplementation on fetal loss and infant mortality: a cluster‐randomized trial in Nepal. American Journal of Clinical Nutrition 2003;78:1194‐202. [DOI] [PubMed] [Google Scholar]
Pakistan 2005 {published data only}
- Hafeez A, Mahmood G, Hassan M, Batool T, Hayat H, Mazhar F, et al. Serum zinc levels and effects of oral supplementation in pregnant women. JCPSP ‐ Journal of the College of Physicians and Surgeons Pakistan 2005;15(10):612‐5. [PubMed] [Google Scholar]
- Hafeez A, Mehmood G, Mazhar F. Oral zinc supplementation in pregnant women and its effect on birth weight: a randomised controlled trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90:F170‐F171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peru 1999 {published data only}
- Caulfield LE, Donangelo CM, Chen P, Junco J, Merialdi M, Zavaleta N. Red blood cell metallothionein as an indicator of zinc status during pregnancy. Nutrition 2008;24(11‐12):1081‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield LE, Zavaleta N, Figueroa A. Adding zinc to prenatal iron and folate supplements improves maternal and neonatal zinc status in a Peruvian population. American Journal of Clinical Nutrition 1999;69(6):1257‐63. [DOI] [PubMed] [Google Scholar]
- Caulfield LE, Zavaleta N, Figueroa A, Zulema L. Maternal zinc supplementation does not affect size at birth or pregnancy duration in Peru. Journal of Nutrition 1999;129(8):1563‐8. [DOI] [PubMed] [Google Scholar]
- Iannotti LL, Zavaleta N, Leon Z, Huasquiche C, Shankar AH, Caulfield LE. Maternal zinc supplementation reduces diarrheal morbidity in Peruvian infants. Journal of Pediatrics 2010;156(6):960‐4, 964.e1‐ 964.e2. [DOI] [PubMed] [Google Scholar]
- Iannotti LL, Zavaleta N, Leon Z, Shankar AH, Caulfield LE. Maternal zinc supplementation and growth in Peruvian infants. American Journal of Clinical Nutrition 2008;88(1):154‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merialdi M, Caulfield LE, Zavaleta N, Figueroa A, DiPietro JA. Adding zinc to prenatal iron and folate tablets improves fetal neurobehavioral development. American Journal of Obstetrics and Gynecology 1999;180(2 Pt 1):483‐90. [DOI] [PubMed] [Google Scholar]
- O'Brien KO, Zavaleta N, Caulfield LE, Wen J, Abrams SA. Prenatal iron supplements impair zinc absorption in pregnant Peruvian women. Journal of Nutrition 2000;130:2251‐5. [DOI] [PubMed] [Google Scholar]
- O'Brien KO, Zavaleta N, Caulfield LE, Yang D‐X, Abrams SA. Influence of prenatal iron and zinc supplements on supplemental iron absorption, red blood cell incorporation, and iron status in pregnant Peruvian women. American Journal of Clinical Nutrition 1999;69:509‐15. [DOI] [PubMed] [Google Scholar]
- Zavaleta N, Caulfield LE, Garcia T. Changes in iron status during pregnancy in Peruvian women receiving prenatal iron and folic acid supplements with or without zinc. American Journal of Clinical Nutrition 2000;71(4):956‐61. [DOI] [PubMed] [Google Scholar]
Peru 2004 {published data only}
- Caulfield LE, Putnick DL, Zavaleta N, Lazarte F, Albornoz C, Chen P, et al. Maternal gestational zinc supplementation does not influence multiple aspects of child development at 54 mo of age in Peru. American Journal of Clinical Nutrition 2010;92(1):130‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield LE, Zavaleta N, Chen P, Lazarte F, Albornoz C, Putnick DL, et al. Maternal zinc supplementation during pregnancy affects autonomic function of Peruvian children assessed at 54 months of age. Journal of Nutrition 2011;141(2):327‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merialdi M, Caulfield LE, Zavaleta N, Figueroa A, Costigan KA, Dominici F, et al. Randomized controlled trial of prenatal zinc supplementation and fetal bone growth. American Journal of Clinical Nutrition 2004;79:826‐30. [DOI] [PubMed] [Google Scholar]
- Merialdi M, Caulfield LE, Zavaleta N, Figueroa A, Dominici F, DiPietro JA. Randomized controlled trial of prenatal zinc supplementation and the development of fetal heart rate. American Journal of Obstetrics and Gynecology 2004;190:1106‐12. [DOI] [PubMed] [Google Scholar]
S Africa 1985 {published data only}
- Ross SM, Nel E, Naeye RL. Differing effects of low and high bulk maternal dietary supplements during pregnancy. Early Human Development 1985;10:295‐302. [DOI] [PubMed] [Google Scholar]
UK 1989 {published data only}
- James DK, Golding J, Mahomed K, McCabe R. A randomised double blind placebo controlled trial of zinc supplementation in pregnancy. Proceedings of 27th Autumn meeting of British Association of Perinatal Medicine; 1989; UK. 1989.
- Mahomed K, James DK, Golding J, McCabe R. Failure to taste zinc sulphate solution does not predict zinc deficiency in pregnancy. European Journal of Obstetrics & Gynecology and Reproductive Biology 1993;48:169‐75. [DOI] [PubMed] [Google Scholar]
- Mahomed K, James DK, Golding J, McCabe R. Zinc supplementation during pregnancy: a double blind randomised controlled trial. BMJ 1989;299:826‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
UK 1991a {published data only}
- Simmer K, Lort‐Phillips L, James C, Thompson RPH. A double blind trial of zinc supplementation in pregnancy. European Journal of Clinical Nutrition 1991;45:139‐44. [PubMed] [Google Scholar]
UK 1991b {published data only}
- Robertson JS, Heywood B, Atkinson SM. Zinc supplementation during pregnancy. Journal of Public Health Medicine 1991;13:227‐9. [DOI] [PubMed] [Google Scholar]
USA 1983 {published data only}
- Hunt IF, Murphy NJ, Cleaver AE, Faraji B, Swendseid ME, Coulson AH, et al. Zinc supplementation during pregnancy: effects on selected blood constituents and on progress and outcome of pregnancy in low‐income women of Mexican descent. American Journal of Clinical Nutrition 1984;40:508‐21. [DOI] [PubMed] [Google Scholar]
- Hunt IF, Murphy NJ, Cleaver AE, Faraji B, Swendseid ME, Coulson AM, et al. Zinc supplementation during pregnancy: zinc concentration of serum and hair from low‐income women of Mexican descent. American Journal of Clinical Nutrition 1983;37:572‐82. [DOI] [PubMed] [Google Scholar]
USA 1985 {published data only}
- Hunt IF, Murphy NJ, Cleaver AE, Faraji B, Swendseid ME, Browdy BL, et al. Zinc supplementation during pregnancy in low income teenagers of Mexican descent: effects on selected blood constituents and on progress and outcome of pregnancy. American Journal of Clinical Nutrition 1985;42:815‐28. [DOI] [PubMed] [Google Scholar]
USA 1989 {published data only}
- Cherry FF, Sandstead HH, Rojas P, Johnson LK, Batson HK, Wang XB. Adolescent pregnancy: associations among body weight, zinc nutriture, and pregnancy outcome. American Journal of Clinical Nutrition 1989;50:945‐54. [DOI] [PubMed] [Google Scholar]
USA 1995 {published data only}
- Goldenberg R, Tamura T, Neggers Y, Copper R, Johnston K, DuBard M, et al. Maternal zinc supplementation increases birthweight and head circumference. American Journal of Obstetrics and Gynecology 1995;172(1 Pt 2):368. [Google Scholar]
- Goldenberg RL, Tamura T, Neggers Y, Cooper RL, Johnston KE, DuBard MB, et al. The effect of zinc supplementation on pregnancy outcome. JAMA 1995;274:463‐8. [DOI] [PubMed] [Google Scholar]
- Hogg B, Tamura T, Johnston K, DuBard M, Goldenberg RL. Homocysteine levels in pregnancy induced hypertension (PIH), preeclampsia (PE) and intrauterine growth retardation (IUGR) [abstract]. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S90. [DOI] [PubMed] [Google Scholar]
- Hogg BB, Tamura T, Johnston KE, DuBard MB, Goldenberg RL. Second‐trimester plasma homocysteine levels and pregnancy‐induced hypertension, preeclampsia, and intrauterine growth restriction. American Journal of Obstetrics and Gynecology 2000;183(4):805‐9. [DOI] [PubMed] [Google Scholar]
- Neggers YH, Goldenberg RL, Tamura T, Johnston KE, Copper RL, DuBard M. Plasma and erythrocyte zinc concentrations and their relationship to dietary zinc intake and zinc supplementation during pregnancy in low‐income African‐American women. Journal of the American Dietetic Association 1997;97:1269‐74. [DOI] [PubMed] [Google Scholar]
- Tamura T, Goldenberg RL, Ramey SL, Nelson KG, Chapman VR. Effect of zinc supplementation of pregnant women on the mental and psychomotor development of their children at 5 y of age. American Journal of Clinical Nutrition 2003;77(6):1512‐6. [DOI] [PubMed] [Google Scholar]
- Tamura T, Goldenberg RN, Johnston KE, DuBard MB. Effect of smoking on plasma ferritin concentrations in pregnant women. Clinical Chemistry 1995;41(8):1190‐1. [PubMed] [Google Scholar]
- Tamura T, Olin KL, Goldenberg RL, Johnston KE, Dubard MB, Keen CL. Plasma extracellular superoxide dismutase activity in healthy pregnant women is not influenced by zinc supplementation. Biological Trace Element Research 2001;80(2):107‐13. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
An 2001 {published data only}
- An H, Yin S, Xu Q. Effects of supplementing calcium, iron and zinc on the fetus development and growth during pregnancy [Chinese]. Chung‐Hua Yu Fang i Hsueh Tsa Chih [Chinese Journal of Preventive Medicine] 2001;35(6):370‐3. [PubMed] [Google Scholar]
Appelbaum 1979 {published data only}
- Appelbaum PC, Ross SM, Dhupelia I, Naeye RL. The effect of diet supplementation and addition of zinc in vitro on the growth‐supporting property of amniotic fluid in African women. American Journal of Obstetrics and Gynecology 1979;135:82‐4. [PubMed] [Google Scholar]
Christian 2001 {published data only}
- Christian P, Khatry SK, LeClerq SC, Shrestha SR, Kimbrough‐Pradhan E, West KP Jr. Iron and zinc interactions among pregnant Nepali women. Nutrition Research 2001;21(1‐2):141‐8. [Google Scholar]
- Christian P, Khatry SK, Yamini S, Stallings R, LeClerq SC, Shrestha SR, et al. Zinc supplementation might potentiate the effect of vitamin A in restoring night vision in pregnant Nepalese women. American Journal of Clinical Nutrition 2001;73(6):1045‐51. [DOI] [PubMed] [Google Scholar]
Fawzi 2005 {published data only}
- Fawzi WW, Villamor E, Msamanga GI, Antelman G, Aboud S, Urassa W, et al. Trial of zinc supplements in relation to pregnancy outcomes, hematologic indicators, and T cell counts among HIV‐1‐infected women in Tanzania. American Journal of Clinical Nutrition 2005;81(1):161‐7. [DOI] [PubMed] [Google Scholar]
France 2004 {published data only}
- Hininger I, Favier M, Arnaud J, Faure H, Thoulon JM, Hariveau E, et al. Effects of a combined micronutrient supplementation on maternal biological status and newborn anthropometrics measurements: a randomized double‐blind, placebo‐controlled trial in apparently healthy pregnant women. European Journal of Clinical Nutrition 2004;58:52‐9. [DOI] [PubMed] [Google Scholar]
Hambidge 1983 {published data only}
- Hambidge KM, Krebs NF, Jacobs MA, Favier A, Guyette L, Ikle DN. Zinc nutritional status during pregnancy: a longitudinal study. American Journal of Clinical Nutrition 1983;37:429‐42. [DOI] [PubMed] [Google Scholar]
India 1993 {published data only}
- Garg HK, Singal KC, Arshad Z. Zinc taste test in pregnant women and its correlation with serum zinc level. Indian Journal of Physiology and Pharmacology 1993;37(4):318‐22. [PubMed] [Google Scholar]
- Garg HK, Singhal KC, Arshad Z. A study of the effect of oral zinc supplementation during pregnancy on pregnancy outcome. Indian Journal of Physiology and Pharmacology 1993;37(4):276‐84. [PubMed] [Google Scholar]
- Garg HK, Singhal KC, Arshad Z. Effect of oral zinc supplementation on copper and haemoglobin levels in pregnant women. Indian Journal of Physiology and Pharmacology 1994;38:272‐6. [PubMed] [Google Scholar]
Kynast 1986 {published data only}
- Kynast G, Saling E. Effect of oral zinc application during pregnancy. Gynecologic and Obstetric Investigation 1986;21:117‐23. [DOI] [PubMed] [Google Scholar]
Mahmoudian 2005 {published data only}
- Mahmoudian A, Khademloo MD. The effect of simultaneous administration of zinc sulfate and ferrous sulfate in the treatment of anemic pregnant women. Journal of Research in Medical Sciences 2005;10(4):205‐9. [Google Scholar]
Makola 2003 {published data only}
- Makola D, Ash DM, Tatala SR, Latham MC, Ndossi G, Mehanso H. A micronutrient‐fortified beverage prevents iron deficiency, reduces anemia and improves the hemoglobin concentration of pregnant Tanzanian women. Journal of Nutrition 2003;133:1339‐46. [DOI] [PubMed] [Google Scholar]
Naher 2012 {published data only}
- Naher K, Nahar K, Aziz MA, Hossain A, Rahman R, Yasmin N. Maternal serum zinc level and its relation with neonatal birth weight. Mymensingh Medical Journal : MMJ 2012;21(4):588‐93. [PubMed] [Google Scholar]
Nishiyama 1999 {published data only}
- Nishiyama S, Kiwaki K, Miyazaki Y, Hasuda T. Zinc and IGF‐I concentrations in pregnant women with anemia before and after supplementation with iron and/or zinc. Journal of the American College of Nutrition 1999;18(3):261‐7. [DOI] [PubMed] [Google Scholar]
Nogueira 2003 {published data only}
- Nogueira NDN, Macedo ADS, Parente JV, Cozzolino SMF. Nutritional profile of newborns of adolescent mothers supplemented with iron, in different concentrations, zinc and pholic acid. Revista de Nutricao 2002;15:193‐200. [Google Scholar]
- Nogueira Ndo N, Parente JV, Cozzolino SM. Changes in plasma zinc and folic acid concentrations in pregnant adolescents submitted to different supplementation regimens. Cadernos de Saude Publica 2003;19(1):155‐60. [DOI] [PubMed] [Google Scholar]
Van Vliet 2001 {published data only}
- Vliet T, Boelsma E, Vries AJ, Berg H. Retinoic acid metabolites in plasma are higher after intake of liver paste compared with a vitamin a supplement in women. Journal of Nutrition 2001;131(12):3197‐203. [DOI] [PubMed] [Google Scholar]
Villamor 2006 {published data only}
- Villamor E, Aboud S, Koulinska IN, Kupka R, Urassa W, Chaplin B, et al. Zinc supplementation to HIV‐1‐infected pregnant women: effects on maternal anthropometry, viral load, and early mother‐to‐child transmission. European Journal of Clinical Nutrition 2006;60(7):862‐9. [DOI] [PubMed] [Google Scholar]
Yalda 2010 {published data only}
- Yalda MA, Ibrahiem AA. The effect of combined supplementation of iron and zinc versus iron alone on anemic pregnant patients in Dohuk. Jordan Medical Journal 2010;44(1):9‐16. [Google Scholar]
References to ongoing studies
Zahiri 2010 {published data only}
- Zahiri Z. Assessment the effect of zinc supplementation on adverse outcomes of pregnancy, a randomized controlled clinical trial. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 December 2010).
Additional references
Aggett 1991
- Aggett PJ. The assessment of zinc status: a personal view; workshop on assessment of zinc status. Proceedings of the Nutrition Society 1991;50:9‐17. [DOI] [PubMed] [Google Scholar]
Apgar 1970
- Apgar J. Effect of zinc deficiency in maintenance of pregnancy in the rat. Journal of Nutrition 1970;100:470. [DOI] [PubMed] [Google Scholar]
Cherry 1981
- Cherry FF, Bennett EA, Bazzono GS, Johnson LK, Fosmire GJ, Batson HK. Plasma zinc in hypertension/toxaemia and other reproductive variables in adolescent pregnancy. American Journal of Clinical Nutrition 1981;34:194‐201. [DOI] [PubMed] [Google Scholar]
Chesters 1982
- Chesters JK. Metabolism and biochemistry of zinc. In: Prasad AS editor(s). Clinical Biochemistry and Nutritional Aspects of Trace Elements. New York: Alan R Liss, 1982. [Google Scholar]
DiPietro 1996
- DiPietro JA, Hodgson DM, Costigan KA, Johnson TRB. Fetal neurobehavioral development. Child Development 1996;67:2553‐7. [PubMed] [Google Scholar]
Golub 1995
- Golub MS, Keen CL, Gershwin ME, Hendrickx AG. Developmental zinc deficiency and behaviour. Journal of Nutrition 1995;125 Suppl:2263S‐2271S. [DOI] [PubMed] [Google Scholar]
GRADE 2014 [Computer program]
- McMaster University. GRADEpro [Computer program on www.gradepro.org]. Version 2014. McMaster University, 2014.
Haider 2012
- Haider BA, Bhutta ZA. Multiple‐micronutrient supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD004905.pub3] [DOI] [PubMed] [Google Scholar]
Hepper 1995
- Hepper PG. The behavior of the fetus as an indicator of neural functioning. In: Lecaneut J, Fifer W, Krasnegor N, Smotherman W editor(s). Fetal Development: a Psychological Perspective. Hillsdale (NJ): Lawrence Erlbaum Associates, 1995:405‐17. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jameson 1976
- Jameson S. Effect of zinc deficiency in human reproduction. Acta Medica Scandinavica Supplement 1976;593:3. [PubMed] [Google Scholar]
Jameson 1993
- Jameson S. Zinc status in pregnancy: the effect of zinc therapy on perinatal mortality, prematurity and placental ablation. Annals of the New York Academy of Science 1993;678:178‐92. [DOI] [PubMed] [Google Scholar]
Jones 1981
- Jones RB, Keeling PWN, Hilton PJ, Thompson RP. The relationship between leucocyte and muscle zinc in health and disease. Clinical Sciences 1981;60:237‐9. [DOI] [PubMed] [Google Scholar]
Kiilholma 1984a
- Kiilholma P, Gronroos M, Erkkola P, Pakarinen P, Nanto V. The role of calcium, iron, copper and zinc in preterm delivery and premature rupture of membranes. Gynecologic and Obstetric Investigation 1984;17:194‐201. [DOI] [PubMed] [Google Scholar]
Kiilholma 1984b
- Kiilholma P, Erkkola P, Pakarinen P, Gronroos M. Trace metals in post date pregnancy. Gynecologic and Obstetric Investigation 1984;18:45‐6. [DOI] [PubMed] [Google Scholar]
Kirksey 1994
- Kirksey A, Wachs TD, Yunis F, Srinath U, Rahmanifar A, McCabe GP, et al. Relation of maternal zinc nutriture to pregnancy outcome and infant development in an Egyptian village. American Journal of Clinical Nutrition 1994;60:782‐92. [DOI] [PubMed] [Google Scholar]
Kirskey 1991
- Kirksey A, Rahmanifar A, Wachs TD, McCabe GP, Bessily NS, Bishry Z, et al. Determinants of pregnancy outcome and newborn behaviour in a semirural Egyptian population. American Journal of Clinical Nutrition 1991;54:657‐67. [DOI] [PubMed] [Google Scholar]
Koblinsky 1995
- Koblinsky MA. Beyond maternal mortality ‐ magnitude, interrelationship, and consequences of women's health, pregnancy related complications and nutritional status on pregnancy outcomes. International Journal of Gynecology & Obstetrics 1995;48:S21‐S32. [DOI] [PubMed] [Google Scholar]
Lassi 2010
- Lassi ZS, Haider BA, Bhutta ZA. Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD005978.pub2] [DOI] [PubMed] [Google Scholar]
McKenzie 1975
- McKenzie JM, Forsmire CJ. Zinc deficiency during the latter third of pregnancy: effect on fetal rat brain, liver and placenta. Journal of Nutrition 1975;105:1466‐73. [DOI] [PubMed] [Google Scholar]
Osendarp 2003
- Osendarp SJM, West CE, Black RE, on behalf of the Maternal Zinc Supplementation Study Group. The need for maternal zinc supplementation in developing countries: an unresolved issue. Journal of Nutrition 2003;133:817S‐827S. [DOI] [PubMed] [Google Scholar]
Parr 1996
- Parr RM. Assessment of dietary intakes. Trace Elements in Human Nutrition and Health. Geneva: World Health Organization, 1996:265‐88. [Google Scholar]
Pena‐Rosas 2006
- Pena‐Rosas JP, Viteri FE. Effects of routine oral iron supplementation with or without folic acid for women during pregnancy. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004736.pub2] [DOI] [PubMed] [Google Scholar]
Prawirohartono 2011
- Prawirohartono EP, Nystrom L, Ivarsson A, Stenlund H, Lind T. The impact of prenatal vitamin A and zinc supplementation on growth of children up to 2 years of age in rural Java, Indonesia. Public Health Nutrition 2011;14(12):2197‐206. [DOI] [PubMed] [Google Scholar]
Prawirohartono 2013
- Prawirohartono EP, Nystrom L, Nurdiati DS, Hakimi M, Lind T. The impact of prenatal vitamin A and zinc supplementation on birth size and neonatal survival ‐ a double‐blind, randomized controlled trial in a rural area of Indonesia. International Journal for Vitamin and Nutrition Research 2013;83(1):14‐25. [DOI] [PubMed] [Google Scholar]
Prema 1980
- Prema K, Ramalagshmi BA, Neelakumari S. Serum copper and zinc in pregnancy. Indian Medical Research 1980;71:547‐53. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saaka 2009
- Saaka M, Oosthuizen J, Beatty S. Effect of joint iron and zinc supplementation on malarial infection and anaemia. East African Journal of Public Health 2009;6(1):55‐62. [DOI] [PubMed] [Google Scholar]
Saaka 2012
- Saaka M. Combined iron and zinc supplementation improves haematologic status of pregnant women in Upper West Region of Ghana. Ghana Medical Journal 2012;46(4):225‐33. [PMC free article] [PubMed] [Google Scholar]
Sanstead 1991
- Sandstead HH. Zinc deficiency: a public health problem?. American Journal of Diseases of Children 1991;145:853‐9. [DOI] [PubMed] [Google Scholar]
Sanstead 1996
- Sandstead HH, Smith JC. Deliberations and evaluations of approaches, endpoints and paradigms for determining zinc dietary recommendations. Journal of Nutrition 1996;126:2410S‐2418S. [DOI] [PubMed] [Google Scholar]
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [PUBMED: 19839216] [DOI] [PubMed] [Google Scholar]
Shrimpton 2005
- Shrimpton R, Gross R, Darnton‐Hill I, Young M. Zinc deficiency: what are the most appropriate interventions?. BMJ 2005;330:347‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simmer 1985
- Simmer K, Thompson RPH. Maternal zinc and intrauterine growth retardation. Clinical Sciences 1985;68:395‐9. [DOI] [PubMed] [Google Scholar]
Valee 1993
- Valee BL, Flachuk KH. The biochemical basis of zinc physiology. Physiology Review 1993;73:79‐118. [DOI] [PubMed] [Google Scholar]
Verburg 1974
- Verburg DI, Burd LJ, Hoxtill EO, Merrill LK. Acrodermatitis enteropathica and pregnancy. Obstetrics & Gynecology 1974;593:233. [PubMed] [Google Scholar]
Villar 2003
- Villar J, Merialdi M, Gulmezoglu AM, Abalos E, Carroli G, Kulier R, et al. Nutritional interventions during pregnancy for the prevention or treatment of maternal morbidity and preterm delivery: an overview of randomized controlled trials. Journal of Nutrition 2003;133:1606S‐1625S. [DOI] [PubMed] [Google Scholar]
WHO 1996
- World Health Organization Committee. Zinc. Trace Elements in Human Nutrition and Health. Geneva: WHO, 1996:72‐104. [Google Scholar]
References to other published versions of this review
Mahomed 1995
- Mahomed K. Routine zinc supplementation in pregnancy. [revised 28 April 1993]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM] The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Mahomed 1997
- Mahomed K. Zinc supplementation in pregnancy. Cochrane Database of Systematic Reviews 1997, Issue 3. [DOI: 10.1002/14651858.CD000230] [DOI] [PubMed] [Google Scholar]
Mahomed 2006
- Mahomed K. Zinc supplementation in pregnancy. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD000230.pub2] [DOI] [PubMed] [Google Scholar]
Mahomed 2007
- Mahomed K, Bhutta ZA, Middleton P. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD000230.pub3] [DOI] [PubMed] [Google Scholar]
Mori 2012
- Mori R, Ota E, Middleton P, Tobe‐Gai R, Mahomed K, Bhutta ZA. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD000230.pub4] [DOI] [PubMed] [Google Scholar]


