Abstract
Background
It has been estimated that arthritis occurs in 5‐7% of those with psoriasis. Relatively few clinical trials of treatment are available for psoriatic arthritis and data presentation in these trials is far from uniform making comparison difficult.
Objectives
To assess the effects of sulfasalazine, auranofin, etretinate, fumaric acid, IMI gold, azathioprine, efamol marine and methotrexate, in psoriatic arthritis.
Search methods
We searched MEDLINE up to February 2000, and Excerpta Medica (June 1974‐95). Search terms were psoriasis, arthritis, therapy and/or controlled trial. This was supplemented by manually searching bibliographies of previously published reviews, conference proceedings, contacting drug companies and referring to the Cochrane Clinical Trials Register. All languages were included in the initial search.
Selection criteria
All randomized trials comparing sulfasalazine, auranofin, etretinate, fumaric acid, IMI gold, azathioprine, and methotrexate, in psoriatic arthritis.
Following a published a priori protocol, the main outcome measures included individual component variables derived from Outcome Measures in Rheumatology Clinical Trials (OMERACT). These include acute phase reactants, disability, pain, patient global assessment, physician global assessment, swollen joint count, tender joint count and radiographic changes of joints in any trial of one year or longer [Tugwell 1993], and the change in pooled disease index (DI).
Only English trials were included in the review.
Data collection and analysis
Data were independently extracted from the published reports by two of the reviewers (MC, GJ). An independent blinded quality assessment was also performed.
Main results
Twenty randomized trials were identified of which thirteen were included in the quantitative analysis with data from 1022 subjects. Although all agents were better than placebo, parenteral high dose methotrexate (not included), sulfasalazine, azathioprine and etretinate were the agents that achieved statistical significance in a global index of disease activity (although it should be noted that only one component variable was available for azathioprine and only one trial was available for etretinate suggesting some caution is necessary in interpreting these results). Analysis of response in individual disease activity markers was more variable with considerable differences between different medications and responses. In all trials the placebo group improved over baseline (pooled improvement 0.39 DI units, 95% CI 0.26‐0.54). There was insufficient data to examine toxicity.
Authors' conclusions
Parenteral high dose methotrexate and sulfasalazine are the only two agents with well demonstrated published efficacy in psoriatic arthritis. The magnitude of the effect seen with azathioprine, etretinate, oral low dose methotrexate and perhaps colchicine suggests that they may be effective but that further multicentre clinical trials are required to establish their efficacy. Furthermore, the magnitude of the improvement observed in the placebo group strongly suggests that uncontrolled trials should not be used to guide management decisions in this condition.
Plain language summary
Interventions for treating psoriatic arthritis
It has been estimated that arthritis occurs in 5‐7 % of those with psoriasis, which can cause substantial disability in some patients.
The objective was to assess the benefits of the treatment [sulfasalazine, auranofin, etretinate, fumaric acid, IMI gold, azathioprine, methotrexate] for psoriatic arthritis and to assess the side effects. Parenteral methotrexate and sulfasalazine resulted in important benefit in over half the patients for psoriatic arthritis in these studies. There was insufficient data to evaluate other therapies and to examine toxicity. Further multicentre trials are required to establish the efficacy of azathioprine, oral methotrexate, etretinate, and colchicine.
Background
It has been estimated that arthritis occurs in 5‐7% of those with psoriasis (Wright 1976). However, this figure becomes much higher in those who are hospitalized with psoriasis (Leonard 1978). Certainly, psoriatic arthritis is commonly encountered by rheumatologists and can, on occasion, be difficult to manage. Its overall natural history appears somewhat better than rheumatoid arthritis (Roberts 1976) although not all agree with this optimism (Gladman 1990). Relatively few clinical trials of treatment are available for this condition and data presentation in these trials is far from uniform making comparison difficult. Often management is extrapolated from trials in rheumatoid arthritis but there is some evidence in the literature to suggest that this may not be appropriate (Dorwart 1978).
Objectives
In this study we used the technique of meta‐analysis to attempt to answer the following question: How do various treatments compare in terms of both efficacy and toxicity in the treatment of psoriatic arthritis?
Methods
Criteria for considering studies for this review
Types of studies
Following a published a priori protocol the following criteria was considered.
For inclusion in the meta‐analysis, studies had to have at least two treatment groups, and the allocation to these must have been by formal randomization. Only articles published in English were included due to the necessity of full text evaluation for quality assessment.
Types of participants
Trials were included of patients aged 20 years and over, with a clinical diagnosis of psoriatic arthritis.
Types of interventions
All conservative therapeutic agents were eligible for inclusion in this review. Comparative trials without a placebo arm were not included. Trials which were placebo‐controlled and which also involved the comparison of two different agents were included.
Types of outcome measures
The main outcome measures included individual component variables derived from Outcome Measures in Rheumatology Clinical Trials (OMERACT). These include acute phase reactants, disability, pain, patient global assessment, physician global assessment, swollen joint count, tender joint count and radiographic changes of joints in any trial of 1 year or longer (Tugwell 1993), and the change in pooled disease index.
Search methods for identification of studies
A computerized literature search was conducted in May 1994 and updated regularly until February 2000. Data bases used were MEDLINE (1966‐2000) and Excerpta Medica (June 1974‐95). Search terms were psoriasis, arthritis, therapy and/or controlled trial. The search strategy was supplemented by manually searching bibliographies of previously published reviews and papers as well as the conference proceedings of the American College Rheumatology Association and the Cochrane Clinical Trials Register. There was no language restriction in the initial search. We also contacted drug companies who might be involved in clinical trials.
Data collection and analysis
Data Extraction Data were independently extracted from the published reports by two of the authors (MC, GJ). Updated reports were done by one author only (GJ). Disagreements were resolved by discussion. For each trial we documented the following: the number of subjects, the outcome measures used, the type of treatment, the dose regimen used, the nature of the control group, the method of allocation, the extent of blinding, the duration of the treatment, the method of analysis and the adverse affects.
In trials where the results were not expressed in a form that allowed extraction of all the necessary data, we approached the investigators by letter for more information. Nine investigators were approached, eight responded and six provided data. This procedure was not followed for updates.
Outcome Measures The trials reported a wide variety of outcome measures including joint index, pain score, global assessments and blood parameters. No consensus exists on the appropriate endpoints to be reported in psoriatic arthritis clinical trials however a core set of outcome measures has been developed to measure disease activity for rheumatoid arthritis clinical trials at the OMERACT (Outcome Measures in Rheumatology). These measures (acute phase reactants, disability, pain, patient global assessment, physician global assessment, swollen joint count, tender joint count and radiographic changes of joints in any trial of 1 year or longer) known as the core set (Tugwell 1993) have been selected on the basis of validity and we accepted any or all of these measures for inclusion in the pooled disease index.
We chose to look at change in individual disease activity measures where available for each of the agents. Some modification was necessary for comparison and pooling purposes:
a. Pain scales were all converted to the same scale as a 100mm visual analogue scale for both continuous and categorical scales. b. Joint activity scores were all converted to the same scale as a Ritchie Index (the most common scale used) where possible. Other component variables did not require any modification.
We also examined a summary measure of treatment effect: the pooled disease index (Goldsmith 1993). This index weights each component variable equally allowing them to be combined. We modified this to allow comparison between agents by dividing by the number of component variables (which varied between studies) so that the index generated has a mean of 0 and a standard deviation (SD) of 1. Thus, unlike the normal disease index, a change in Disease Index (DI) of 1 unit approximates a 1 SD change in each of the component variables (if the component variables are highly correlated).
Data Analysis As RevMan allows for only one entry for placebo and treatment arms and data was available for both baseline and follow‐up periods, we calculated N using the formula for the harmonic mean N = 2N1N2/(N1 + N2) We calculated the SD by using a weighted formula of individual SDs SD squared = [SD1 squared (N1‐1) + SD2 squared (N2 ‐1)]/ (N1+N2‐2) where N1 and N2 are the baseline and follow‐up numbers and SD1 and SD2 are the baseline and follow‐up standard deviations.
Where there was only one trial looking at an agent, results were generated as both disease index and component value listed with 95% confidence intervals (CI). The variance of the change in the disease index was calculated by assuming that baseline and follow‐up measurements for both the treatment and placebo arms were unpaired. This was necessary as data were only available as group means and standard deviation at baseline and follow‐up rather than individual data. Also, because of dropouts patient numbers were often not the same in the two groups. This represents a conservative approach, in terms of type l error, to estimation of the variance. In crossover trials the treatment‐placebo difference was a paired comparison rather than a comparison of two independent groups. An additional assumption was that a disease index has an SD of one unit (from the standardised normal distribution).
When there was more than one trial looking at the same agent a pooled DI (and pooled component variable where available) were calculated by the weighted average of the individual disease indices (or component variable) with the weighting inversely proportional to the variance of each individual disease index (or component variable) again listed with 95% CIs. Thus, a study with a larger sample size will have a disproportionately higher weighting than a smaller study. We also adapted the conventional chi‐squared test for homogeneity which was applied where there was more than one trial for an individual agent as well as for placebo versus baseline. This test is to check the assumption that all trials are samples from the same population of studies (the central limit theorem) and thus their 95% confidence limits should all overlap.
Results
Description of studies
See characteristics of included studies
Risk of bias in included studies
Each of the trials included in the meta‐analysis was assessed for quality by using a modified version of previously published work (Wilson 1992) as well as the Allocation Concealment (Schulz 1995). For this purpose "quality" is defined in terms of the measures taken by the investigators to minimize bias in each trial. The trials were scored independently of the method of data extraction and were rated in the range 0‐3 on each of the following features: a) degree to which randomization was truly blind b) inclusion of data from subjects who subsequently withdrew from the study (intention to treat) c) degree to which assessors of outcome were blind to treatment allocation d) whether subjects were assessed to determine if they had accurately guessed their treatment status e) whether an appropriate range of clinical outcome measures had been used Scores for the individual quality items were summed for the purposes of analysis. The total possible score was 15 points.
Effects of interventions
A total of twenty randomized controlled trials were identified [see references]. Complete or near complete data were available on thirteen trials which included 1022 subjects as randomized. There was inadequate data to consider toxicity. Randomized controlled trials were not available for antimalarials, cyclosporin or alkylating agents.
Data necessary for pooling could not be extracted from papers by Black (high dose parenteral methotrexate) (Black 1964) , Price (D‐Penicillamine) (Price 1986), Seideman (colchicine) (Seideman 1987), Caperton (Ceftriaxone) (Caperton 1990), Fierlbeck (Interferon) (Fierlbeck 1990) and efamol marine [Veale]. A further trial of gold versus auranofin was not included as there was no placebo comparison (Bruckle 1994). Of the included trials the effect of auranofin was each examined by two trials; colchicine, etretinate, fumaric acid, IM Gold, azathioprine and oral methotrexate by one trial and sulfasalazine by six trials. The quality scores varied widely There was a weak inverse correlation between trial quality and magnitude of improvement (Spearmans rho=‐0.3, p=0.20). However trial quality was strongly associated with year of publication (Spearmans rho=0.65, p=0.01).
There were considerable differences in the inclusion and exclusion criteria of the individual trials. For example one of the trials looking at the efficacy of gold and auranofin excluded patients who had previously received gold or other suppressive anti‐rheumatic therapy (Palit 1990) while the other accepted patients who had had prior gold (Carette 1989). Wash out periods between 2 and 3 months were required by five trials (Willkens 1984, Hopkins 1985, Carette 1989, Fraser 1993, Dougados 1995). Similarly trials varied according to whether they permitted the use of prednisone during the trial (see references) and one trial permitted patients to remain on methotrexate (McKendry 1993).
EFFECTS ON OUTCOME The following table shows the results for individual component variables from the eleven studies included in the quantitative analysis
1. Component variables achieving statistical significance with different agents.
| Variables | Agent |
| Pain | Salazopyrin, Auranofin |
| ESR | Salazopyrin, Etretinate, Fumaric Acid |
| Tender Joint Count | IMI Gold, Azathioprine |
| Swollen Joint Count | Auranofin |
| Patient Global Assessment | Salazopyrin, Methotrexate |
| Physician Global Assessment | Salazopyrin, Methotrexate |
sulfasalazine achieved the largest number of statistically significant results reflecting both the fact that it was the agent studied in the most subjects as well as being efficacious.
The disease index was statistically significantly improved relative to placebo in one study of sulfasalazine (Dougados 1995), azathioprine (Levy 1972) and etretinate (Hopkins 1985) and confidence limits just overlapped zero in a further trial of low dose methotrexate (Willkens 1984). When the pooled indices for individual agents were examined sulfasalazine (improvement in disease index 0.38 units (95% CI 0.21‐0.54) and azathioprine (improvement in disease index 2.20 units (95% CI 1.06‐3.33) and etretinate (improvement in disease index 0.84 units (95% CI 0.08‐1.59) were statistically better than placebo. However, only one component variable (Ritchie score) was available for azathioprine and there was only one small trial of etretinate with a high dropout rate in the placebo arm suggesting some caution is necessary in interpreting both of these results. Data for high dose parenteral methotrexate were unavailable for comparison purposes but it was clearly much better than placebo (p<0.001). Combinable data were not available for one trial of colchicine (Seideman 1987) but an estimate was possible and showed a large improvement in disease index (3.66 units 95% CI 2.84‐4.47). If this was combined with the included trial a pooled estimate of 1.70 units improvement was obtained (95% CI ‐2.20‐5.60) but there was marked heterogeneity (p<0.0001) which indicates that marked caution should be used in the interpretation of this result. All other pooled indices were homogeneous.
In all the trials the placebo group improved over baseline (pooled mean improvement 0.39 DI units, 95% CI 0.26‐0.52). This improvement was also homogeneous (p=0.18).
Table 2 shows a comparison between previously published effect sizes in rheumatoid arthritis (Felson 1990) and psoriatic arthritis. The outstanding difference is the placebo group improves three times as much as the placebo rheumatoid group (p<0.05). Gold compounds appear less efficacious in psoriatic arthritis but these differences were not statistically significant.
2. Psoriatic arthritis versus Rheumatoid arthritis.
| placebo vs baseline | 0.39 (+0.26‐+0.52) 0.15 (+0.11‐+0.19) |
| treatment vs placebo | |
| Auranofin | 0.12(‐0.13‐+0.38) 0.30 (+0.06‐+0.54) |
| IM Gold | 0.23 (‐0.13‐+0.38) 0.56 (+0.11‐+1.01 |
| Methotrexate | 0.65 (‐0.00‐+1.30) 0.58 (+0.10‐+1.06) |
| Salazopyrin | 0.38 (+0.21‐+0.54) 0.60 (+0.18‐+1.02) |
#all results are listed with 95% confidence intervals. * extracted from (Felson 1990).
Discussion
The study provides the first comprehensive overview of randomized controlled trials of psoriatic arthritis. It demonstrates published efficacy for sulfasalazine and parenteral high dose methotrexate and suggestive evidence for azathioprine, etretinate and low dose methotrexate. Importantly, it shows differences between rheumatoid arthritis and psoriatic arthritis not only in response to treatment but also in the magnitude of improvement observed in the placebo arm. This latter finding, in particular, has significant implications for the interpretation of uncontrolled trials in this condition. There are a number of potential limitations in this review stemming from the methodological shortcomings in the primary trials. The trial sizes are generally small with insufficient statistical power increasing the likelihood of type II errors. This could affect the results with low dose methotrexate and perhaps colchicine. Secondly, subgroup analyses of results were not possible with this meta analysis as the patient groups had been poorly characterized. There is some limited support in the literature for the hypothesis that different subtypes respond differently to treatment [Price 1984]. This may indicate that bias could be introduced by maldistribution of arthritis subtypes between treatment and placebo arms. Thirdly these studies are of short duration with a mean duration of six months and these results cannot be extrapolated beyond this period. Finally, it would be possible to criticize the outcome measure we chose for this review. In this overview, we limited the component variables to those described at OMERACT 1 (Tugwell 1993). We were careful to equally weigh all variables and ensure that the final disease index had a variance of 1 for each agent to allow direct comparison. Ideally each disease index should have had the same number of component variables but that was not possible in this case. This would not bias our findings unless one component variable was much more sensitive to change than others which, intuitively, appears unlikely in this case as all the variables are correlated with each other. This may not apply in the case of azathioprine where only one variable was available for pooling with a highly significant result that was an outlier in terms of effect size (in comparison to other agents) and may well have been diluted with further variables or an increase in sample size. We would still contend, however, that this measure is among the best available and has been well validated and widely accepted in rheumatoid arthritis.
The use of one outcome measure gives a global effect estimate and minimizes the risk of type I error by eradicating the need for multiple comparisons with the different possible outcome measures. The results obtained from the individual component variable analysis reflect the greater variation that would be expected when compared to a global or summary effect estimate. However, in general, component results were in the same direction as the global estimate
With regard to the trials with incomplete data only the trial of high dose methotrexate showed a marked improvement in the treatment group as compared to the placebo group (Black 1964). However, as was shown in this trial, this dose would be too toxic for use in current practice. The other studies showed effect sizes that appeared similar in magnitude to Auranofin and IM Gold (Price 1986, Caperton 1990, Fierlbeck 1990). The uniform improvement seen in the placebo arm indicates that uncontrolled trials should not be used to guide management decisions in psoriatic arthritis. The improvement may be due to a number of possible factors but it seems most likely that it reflects the natural history of this condition. The interpretation of uncontrolled studies in this condition will be extremely difficult as virtually all would be expected to show a positive effect which may be erroneously attributed to the treatment. Our calculations suggest that further trials will require 85 subjects per treatment arm to determine a 0.5 unit improvement in disease index for treatment over placebo (with 90% power and 5% significance level). Obviously, inter‐drug comparisons will require much larger sample sizes. This indicates that future therapeutic trials for this condition will need to be multicentre in design.
Authors' conclusions
Implications for practice.
The results of uncontrolled trials are likely to be misleading in psoriatic arthritis. At present, parenteral high dose methotrexate, sulfasalazine and possibly azathioprine and etretinate are the only agents which are statistically better than placebo for the treatment of this condition. However, the magnitude of the effect seen with low dose methotrexate, and perhaps colchicine suggests that they may also be effective.
Implications for research.
Further multicentre clinical trials are required to establish the efficacy of azathioprine, etretinate, low dose oral methotrexate and colchicine.
What's new
| Date | Event | Description |
|---|---|---|
| 29 September 2008 | Amended | Converted to new review format. CMSG ID: A011‐R |
Acknowledgements
The help of Beverley Shea in the original preparation of this review is gratefully acknowledged.
Data and analyses
Comparison 1. Treatment versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pooled disease index | 13 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Salazopyrin | 6 | 564 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [0.21, 0.54] |
| 1.2 Etretinate | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.84 [0.09, 1.59] |
| 1.3 IMI Gold | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.40, 0.86] |
| 1.4 Fumaric acid | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [‐0.36, 1.18] |
| 1.5 Azathioprine | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 2.2 [1.07, 3.33] |
| 1.6 Colchicine | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.85, 0.25] |
| 1.7 Methotrexate | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.65 [‐0.00, 1.30] |
| 1.8 Auranofin | 2 | 230 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.13, 0.39] |
| 2 Pain (VAS) | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Salazopyrin | 4 | 320 | Mean Difference (IV, Fixed, 95% CI) | ‐9.50 [‐15.04, ‐3.96] |
| 2.2 Etretinate | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐5.6 [‐23.15, 11.95] |
| 2.3 IMI Gold | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 5.30 [‐9.17, 19.77] |
| 2.4 Fumaric acid | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐16.64 [‐40.61, 7.33] |
| 2.5 Colchicine | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 14.3 [‐2.31, 30.91] |
| 2.6 Auranofin | 2 | 230 | Mean Difference (IV, Fixed, 95% CI) | ‐2.99 [‐3.23, ‐2.76] |
| 3 ESR (mm/hr) | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Salazopyrin | 4 | 405 | Mean Difference (IV, Fixed, 95% CI) | ‐7.52 [‐10.74, ‐4.29] |
| 3.2 Etretinate | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐9.00 [‐15.23, ‐6.77] |
| 3.3 IMI gold | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐7.10 [‐22.07, 7.87] |
| 3.4 Fumaric acid | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐15.0 [‐27.72, ‐2.28] |
| 3.5 Auranofin | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐13.04, 13.24] |
| 4 Tender joint score (Ritchie Index) | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Salazopyrin | 3 | 361 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐3.09, 0.68] |
| 4.2 Etretinate | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐6.66, 7.26] |
| 4.3 IMI Gold | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐6.60 [‐11.92, ‐1.28] |
| 4.4 Azathioprine | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐12.0 [‐17.56, ‐6.44] |
| 4.5 Auranofin | 2 | 230 | Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐1.81, 0.47] |
| 4.6 Methotrexate | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 1.01 [‐9.47, 11.49] |
| 5 Swollen joint score | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Colchicine | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐2.25, 2.05] |
| 5.2 Fumaric acid | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐3.51, 3.51] |
| 5.3 Auranofin | 1 | 188 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.74, ‐0.06] |
| 5.4 Methotrexate | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐7.32, 6.92] |
| 5.5 Salazopyrine | 2 | 244 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐3.09, 2.79] |
| 6 Patient global assessment | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Salazopyrin | 2 | 159 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐0.79, ‐0.31] |
| 6.2 Methotrexate | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐0.74, ‐0.08] |
| 6.3 Fumaric acid | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.23, 0.43] |
| 7 Physician global assessment | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Salazopyrin | 2 | 159 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.48, ‐0.05] |
| 7.2 Methotrexate | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐0.88 [‐1.22, ‐0.54] |
1.1. Analysis.
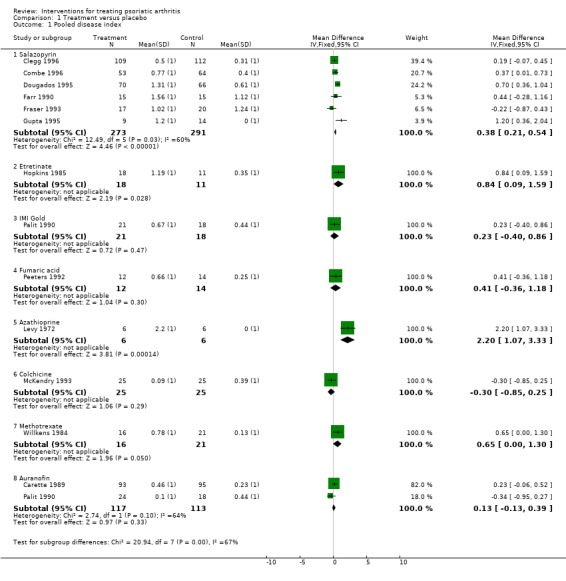
Comparison 1 Treatment versus placebo, Outcome 1 Pooled disease index.
1.2. Analysis.
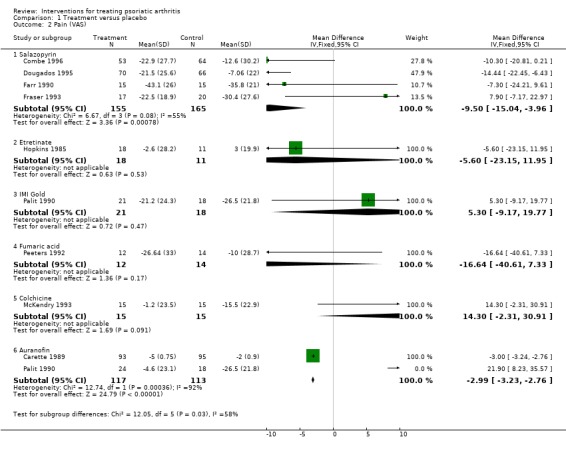
Comparison 1 Treatment versus placebo, Outcome 2 Pain (VAS).
1.3. Analysis.
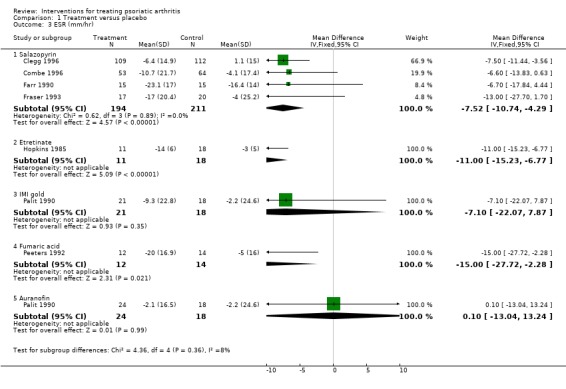
Comparison 1 Treatment versus placebo, Outcome 3 ESR (mm/hr).
1.4. Analysis.
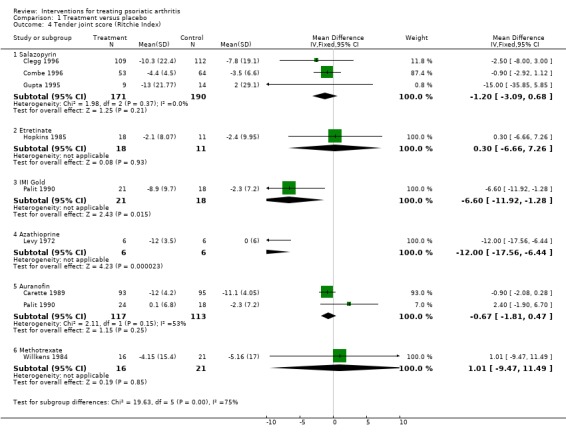
Comparison 1 Treatment versus placebo, Outcome 4 Tender joint score (Ritchie Index).
1.5. Analysis.
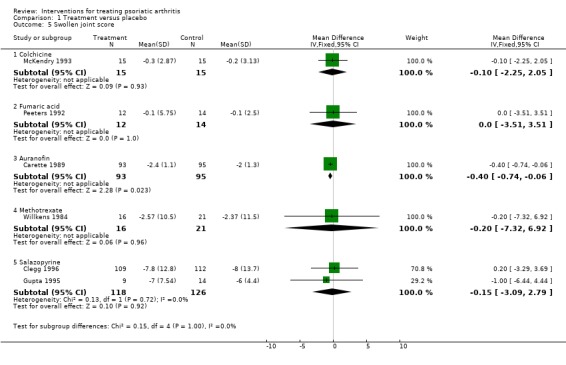
Comparison 1 Treatment versus placebo, Outcome 5 Swollen joint score.
1.6. Analysis.
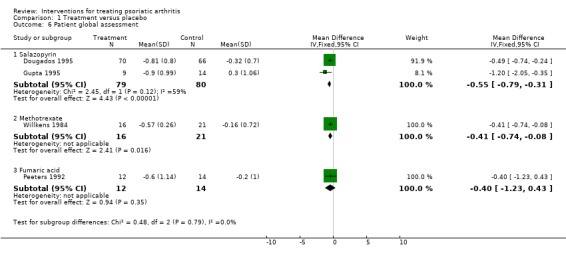
Comparison 1 Treatment versus placebo, Outcome 6 Patient global assessment.
1.7. Analysis.
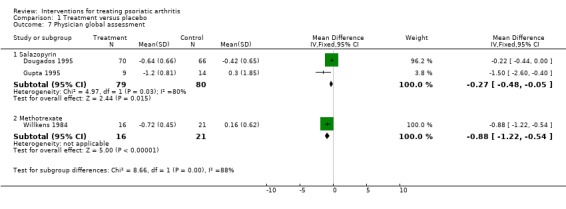
Comparison 1 Treatment versus placebo, Outcome 7 Physician global assessment.
Comparison 2. Placebo follow‐up versus baseline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pooled disease index | Other data | No numeric data |
2.1. Analysis.
Comparison 2 Placebo follow‐up versus baseline, Outcome 1 Pooled disease index.
| Pooled disease index | |
|---|---|
| Study | |
| Carette 1989 | Improvement 0.44 (95%CI ‐0.22 ‐ 1.10) |
| Clegg 1996 | Improvement 0.31 (95% CI 0.12‐0.50 ) |
| Combe 1996 | Improvement 0.40 (95%CI 0.04 ‐ 0.76) |
| Dougados 1995 | Improvement 0.61 (95%CI 0.27 ‐ 0.95 ) |
| Farr 1990 | Improvement 1.12 (95%CI 0.38 ‐ 1.86) |
| Fraser 1993 | Improvement 1.24 (95%CI 0.44 ‐ 2.04 |
| Gupta 1995 | Improvement 0.00 (95% CI ‐0.52‐ 0.52 ) |
| Hopkins 1985 | Improvement 0.35 (95%CI ‐0.49 ‐ 1.19) |
| Levy 1972 | Improvement ‐0.09 (95%CI ‐1.25‐ 1.07) |
| McKendry 1993 | Improvement 0.39 (95%CI ‐0.35‐ 1.13) |
| Palit 1990 | Improvement 0.44 (95%CI ‐0.22 ‐ 1.10) |
| Peeters 1992 | Improvement 0.25 (95%CI ‐0.51 ‐ 1.01) |
| Willkens 1984 | Improvement 0.13 (95%CI ‐0.49‐ 0.75) |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Carette 1989.
| Methods | Random allocation (computer generated random numbers block design) Method of concealment not stated | |
| Participants | Psoriasis, arthritis, partial response to NSAIDs, >3 joints, could be seropositive Multicentre design (USA and Canada) N=238 Mean age 44, median duration of arthritis 5 years | |
| Interventions | Auranofin 3mg/day versus identical placebo | |
| Outcomes | All Omeract 1 outcomes 24 weeks duration | |
| Notes | Only three outcomes could be included even after communication with authors due to nature of outcomes (some categorical which meant means and SDs could not be calculated) 21% withdrew | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Clegg 1996.
| Methods | Random allocation (exact method and concealment not stated) | |
| Participants | Psoriasis, active arthritis, failed NSAIDs, steroids not allowed, N=221 | |
| Interventions | Salazopyrin 2g/ day versus identical placebo | |
| Outcomes | All Omeract 1 outcomes , 36 weeks duration | |
| Notes | Only three outcomes could be included due to method of reporting. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Combe 1996.
| Methods | Random allocation (exact method and concealment not stated) | |
| Participants | Psoriatic arthritis (definition not stated) N=120 | |
| Interventions | Salazopyrin 2g/day versus placebo | |
| Outcomes | Pain, patient global assessment, morning stiffness, Ritchie index, ESR, CRP 24 weeks duration | |
| Notes | Intention‐to‐treat analysis 2.5% dropout rate 3 variables available for inclusion after discussion with authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Dougados 1995.
| Methods | Random allocation (computer generated numbers) metrologist blinded to treatment allocation | |
| Participants | Multicentre spondyloarthropathy trial N=136 (psoriatic arthritis only) | |
| Interventions | Salazopyrin 2g/day versus identical placebo | |
| Outcomes | All Omeract 1 outcomes Duration 24 weeks | |
| Notes | 3 variables included in analysis after discussion with authors Intention‐to‐treat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Farr 1990.
| Methods | Random allocation (exact method and concealment not stated) | |
| Participants | Seronegative arthritis associated with psoriasis unresponsive to NSAIDs alone Rheumatology research clinic in Birmingham, UK N=30 mean age 46, equal male female ratio, mean duration of arthritis 7 years | |
| Interventions | Salazopyrin 2g/day versus placebo intra‐articular steroids only permitted duration 24 weeks | |
| Outcomes | Multiple measures of disease activity (EMS, painful joint score, pain score (VAS), global assessment (subjective), grip strength, articular index, laboratory measures) | |
| Notes | Data for pain, articular index and ESR only were available for inclusion after communication with authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Fraser 1993.
| Methods | Random allocation (method not stated) Metrologist blind to treatment allocation | |
| Participants | Psoriasis, seronegative asymmetric polyarthritis, no spinal involvement Hospital and university clinics, Leeds and Glasgow, UK N=39 | |
| Interventions | Salazopyrin 2g/day versus identical placebo | |
| Outcomes | Pain (VAS), Morning stiffness, Ritchie index, grip strength, laboratory measures duration 24 weeks | |
| Notes | 7% dropout rate 3 variables available for inclusion in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Gupta 1995.
| Methods | Random allocation (method not stated) | |
| Participants | Active psoriatic arthritis, NSAIDS permitted, steroids not permitted. N=24 | |
| Interventions | Salazopyrin 3g/day versus placebo | |
| Outcomes | All Omeract 1 measures | |
| Notes | Small sample size | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Hopkins 1985.
| Methods | Random allocation (method and concealment not stated) | |
| Participants | Psoriasis, arthritis >3 joints, seronegative Setting: hospital clinics, UK N=40 | |
| Interventions | Etretinate 0.5 mg/kg/day plus placebo versus ibuprofen 400mg tds plus placebo | |
| Outcomes | Articular index, grip strength, joint size, laboratory tests Duration 24 weeks | |
| Notes | Substantial loss to follow‐up in ibuprofen group (8/20 completed second assessment) Data on three outcome variables included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Levy 1972.
| Methods | Random allocation (method and concealment not stated) Crossover design | |
| Participants | Psoriatic arthritis USA N=6 | |
| Interventions | Azathioprine 3mg/kg/day versus placebo | |
| Outcomes | Tender joint score, Morning stiffness duration 26 weeks | |
| Notes | Small study only reported in abstract form Only one variables included in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
McKendry 1993.
| Methods | Random allocation by computer code (exact method of concealment not stated) Crossover design (1 week washout period) | |
| Participants | Arthritis and psoriasis, age >18, >3 joints, seronegative, methotrexate and oral steroids permitted University of Ottawa rheumatic disease unit N=25 | |
| Interventions | Colchicine 0.6‐1.8 mg/day versus placebo | |
| Outcomes | Pain, morning stiffness, grip strength, Lansbury joint count, swollen joints, joint circumference, laboratory measures, global assessment Duration 23 weeks | |
| Notes | Data only adequate for inclusion of 2 variables No loss to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Palit 1990.
| Methods | Random allocation (method not stated) Metrologist blinded to treatment | |
| Participants | Active symptomatic psoriatic arthritis >1 year duration Steroids not permitted N=82 | |
| Interventions | Auranofin 3mg bd versus IM gold thiomalate versus placebo tablet only | |
| Outcomes | Ritchie index, pain (VAS), grip strength, ESR 24 weeks duration | |
| Notes | 3 variables included after discussion with authors 38% dropout rate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Peeters 1992.
| Methods | Random allocation (exact method and concealment not stated) | |
| Participants | Psoriatic arthritis Mean age 40, mean duration 6 years N=27 | |
| Interventions | Fumaric acid 120mg/day versus placebo tablet | |
| Outcomes | Morning stiffness, pain score, global assessment, grip strength, joint index, laboratory measures 16 weeks duration | |
| Notes | Four variables included in analysis 7% dropouts. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Willkens 1984.
| Methods | Random allocation (exact method and concealment not stated) | |
| Participants | Psoriatic arthritis (DIP involvement/seronegative polyarthritis with psoriasis/arthrosis mutilans with psoriasis) Multicentre recruitment 16 treatment/21 placebo Corticosteroids not permitted | |
| Interventions | Oral methotrexate 7.5‐15 mg/wk versus identical placebo | |
| Outcomes | Tender joint count, joint swelling, physician assessment, grip strength, laboratory measures duration 12 weeks | |
| Notes | 4 variables were included after discussion with the authors 11% dropout rate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Black 1964 | Data not available to allow comparison with other studies. |
| Bruckle 1994 | No placebo arm. |
| Caperton 1990 | Different study group (Lyme positive subjects with psoriatic arthritis). Authors not contacted. |
| Fierlbeck 1990 | Data not available to allow comparison with included studies. Authors contacted but failed to respond. |
| Price 1986 | Data not available to allow comparison with included studies. Authors were contacted but data was lost. |
| Seideman 1987 | Data not available in format to include in this overview |
| Veale 1994 |
Contributions of authors
G Jones was responsible for the overall review. M Crotty assisted with the data abstraction and quality assessment. P Brooks provided comments on the initial preparation of the review and provided editorial support.
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Carette 1989 {published data only}
- Carette S, Calin A, McCafferty JP, Wallin BA and the Auranofin Cooperating Group. A double‐blind placebo controlled trial of auranofin in psoriatic arthritis. Arthritis and Rheumatism 1989;32:158‐65. [DOI] [PubMed] [Google Scholar]
Clegg 1996 {published data only}
- Clegg DO, Reda DJ, Mejias E, et al. Comparison of sulphasalzine and placebo for the treatment of psoriatic arthritis and cutaneous psoriasis. Arthritis and Rheumatism 1996;39:2013‐20. [DOI] [PubMed] [Google Scholar]
Combe 1996 {published and unpublished data}
- Combe B, Goupille P, Kuntz JL, et al. Sulphasalazine in psoriatic arthritis: a randomised multicentre placebo‐controlled study. British Journal of Rheumatology 1996;35:664‐8. [DOI] [PubMed] [Google Scholar]
Dougados 1995 {published and unpublished data}
- Dougados M, Linden S, Leirisalo‐Repo M. Sulphasalazine in the treatment of spondyloarthropathy: a randomised multicentre double‐blind placebo controlled study. Arthritis and Rheumatism 1995;38:618‐27. [DOI] [PubMed] [Google Scholar]
Farr 1990 {published and unpublished data}
- Farr M, Kitas GD, Waterhouse L, et al. Suplhasalazine in psoriatic arthritis: a double‐blind placebo‐controlled study. British Journal of Rheumatology 1990;29:46‐9. [DOI] [PubMed] [Google Scholar]
Fraser 1993 {published and unpublished data}
- Fraser SM, Hopkins R, Hunter JA, Neumann V, Capell HA, Bird HA. Sulphasalazine in the management of psoriatic arthritis. British Journal of Rheumatology 1993;32:923‐5. [DOI] [PubMed] [Google Scholar]
Gupta 1995 {published data only}
- Gupta AK, Grober JS, Hamilton TA, et al. Sulfasalazine therapy for psoriatic arthritis: a double‐blind, placebo controlled trial. Journal of Rheumatology 1995;22:894‐8. [PubMed] [Google Scholar]
Hopkins 1985 {published data only}
- Hopkins R, Bird HA, Jones H, Hill J, Surrall KE, Astbury, et al. A double‐blind controlled trial of etretinate (Tigason) and ibuprofen in psoriatic arthritis. Annals of the Rheumatic Diseases 1985;44:189‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levy 1972 {published data only}
- Levy J, Paulus HE, Barnett EV, Sokoloff M, Bangert R, Pearson CM. A double‐blind controlled evaluation of azathioprine treatment in rheumatoid arthritis and psoriatic arthritis. Arthritis and Rheumatism 1972;15:116‐7. [Google Scholar]
McKendry 1993 {published and unpublished data}
- McKendry RJR, Kraag G, Seigel S, Awadhi AA. Therapeutic value of colchicine in the treatment of patients with psoriatic arthritis. Annals of the Rheumatic Diseases 1993;52:826‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palit 1990 {published and unpublished data}
- Palit J, Hill J, Capell HA, et al. A multicentre double‐blind comparison of auranofin, intramuscular gold thiomalate and placebo in patients with psoriatic arthritis. British Journal of Rheumatology 1990;29:280‐3. [DOI] [PubMed] [Google Scholar]
Peeters 1992 {published data only}
- Peeters AJ, Dijkmans BA, Scroeff JG. Fumaric acid therapy for psoriatic arthritis. A randomised double‐blind placebo‐controlled study (letter). British Journal of Rheumatology 1992;31(7):502‐4. [DOI] [PubMed] [Google Scholar]
Willkens 1984 {published and unpublished data}
- Willkens RE, Williams J, Ward JR, et al. Randomized, double‐blind, placebo controlled trial of low‐dose pulse methotrexate in psoriatic arthritis. Arthritis and Rheumatism 1984;27(4):376‐81. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Black 1964 {published data only}
- Black RL, O'Brien WM, Scott EJ, et al. Methotrexate therapy in psoriatic arthritis. JAMA 1964;189:743‐7. [PubMed] [Google Scholar]
Bruckle 1994 {published data only}
- Bruckle W, Dexel T, Grasedyck K, Schattenkirchner M. Treatment of psoriatic arthritis with auranofin and gold sodium thiomalate. Clinical Rheumatology 1994;13:209‐16. [DOI] [PubMed] [Google Scholar]
Caperton 1990 {published data only}
- Caperton EM, Heim‐Duthoy KL, Matzke GR. Ceftriaxone therapy of chronic inflammatory arthritis, A double‐blind placebo controlled trial. Archives of Internal Medicine 1990;150:1677‐82. [PubMed] [Google Scholar]
Fierlbeck 1990 {published data only}
- Fierlbeck G, Rassner G. Treatment of psoriasis and psoriatic arthritis with interferon gamma. Journal of Investigative Dermatology 1990;95:138‐41S. [DOI] [PubMed] [Google Scholar]
Price 1986 {published data only}
- Price R, Gibson T. D‐Penicillamine and psoriatic arthropathy. British Journal of Rheumatology 1986;24:228. [DOI] [PubMed] [Google Scholar]
Seideman 1987 {published data only}
- Seideman P, Fjellner B, Johanesson L. Psoriatic arthritis treated with oral colchicine. Journal of Rheumatology 1987;14:777‐9. [PubMed] [Google Scholar]
Veale 1994 {published data only}
- Veale DJ, Torley HI, Richards IM, O'Dowd, A, Fitzsimons C, Belch JJF, Sturrock RD. A double‐blind placebo controlled trial of efamol marine on skin and joint symptoms of psoriatic arthritis. British Journal of Rheumatology 1994;33:954‐8. [DOI] [PubMed] [Google Scholar]
Additional references
Dorwart 1978
- Dorwart BB, Gall EP, Schumacher HR, Krauser RE. Chrysotherapy in psoriatic arthritis: efficacy and toxicity compared to rheumatoid arthritis. Arthritis and Rheumatism 1978;21:513‐5. [DOI] [PubMed] [Google Scholar]
Felson 1990
- Felson DT, Anderson JJ, Meenan RF. The comparative efficacy and toxicity of second‐line drugs in rheumatoid arthritis. Arthritis and Rheumatism 1990;33:1449‐61. [DOI] [PubMed] [Google Scholar]
Gladman 1990
- Gladman DD, Stafford‐Brady F, Chang CH, Lewandowksi K, Russell MC. Longterm study of clinical and radiological progression in psoriatic arthritis. Journal of Rheumatology 1990;17:809‐12. [PubMed] [Google Scholar]
Goldsmith 1993
- Goldsmith CH, Smythe HA, Helewa A. Interpretation and power of a pooled index. Journal of Rheumatology 1993;20:575‐8. [PubMed] [Google Scholar]
Leonard 1978
- Leonard DG, O'Duffy JD, Rogers RS. Prospective analysis of psoriatic arthritis in patients hospitalised for psoriasis. Mayo Clinic Proceedings 1978;53:511‐8. [PubMed] [Google Scholar]
Roberts 1976
- Roberts MCT, Wright V, Hill AGS, Mehra AC. Psoriatic arthritis: a follow‐up study. Annals of the Rheumatic Diseases 1976;35:206‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Tugwell 1993
- Tugwell P, Boers M. OMERACT Conference on outcome measures in rheumatoid arthritis clinical trials: conclusion. Journal of Rheumatology 1993;20:590. [PubMed] [Google Scholar]
Wilson 1992
- Wilson A, Henry DA. Meta‐analysis. Part 2: Assessing the quality of published meta‐analyses. Medical Journal of Australia 1992;156:173‐87. [PubMed] [Google Scholar]
Wright 1976
- Wright V, Moll MH. Seronegative polyarthritis. Amsterdam: North Holland Publishing Company, 1976. [Google Scholar]


