Abstract
N-acetyl-L-cysteine (NAC) commonly used as an antidote in acetaminophen poisoning has shown promise in the treatment of neurological disorders such as cerebral palsy (CP). However, NAC suffers from drawbacks such as poor oral bioavailability and suboptimal blood-brain-barrier (BBB) permeability limiting its clinical success. It was previously demonstrated that intravenous administration of dendrimer-NAC (D-NAC) conjugates have shown significant promise in the targeted treatment of neuroinflammation, in multiple preclinical models. Development of an oral formulation of D-NAC may open new administrative routes for this compound. Here, we report the gastrointestinal stability, in vitro transepithelial permeability, and in vivo oral absorption and pharmacokinetics in rats of a pediatric formulation of D-NAC containing Capmul MCM (glycerol monocaprylate) as a penetration enhancer. D-NAC was stable for 6 hours in all five simulated gastrointestinal fluids with no signs of chemical degradation. The apparent permeability (Papp) of D-NAC increased 9-fold in the formulation containing Capmul. The area under the curve [AUC]o−∞ of D-NAC with Capmul increased by 47% when compared to D-NAC alone. These results indicate that an oral pediatric formulation containing D-NAC and Capmul can be an effective option for the treatment of neuroinflammation.
Keywords: Neuroinflammation, PAMAM Dendrimers, Permeability, Pediatric formulations, Oral bioavailability, Pharmacokinetics
Graphical Abstract
Introduction:
N-acetyl-L-cysteine (NAC), an antioxidant and anti-inflammatory drug commonly used as an antidote to acetaminophen overdose, is beneficial in the treatment of neurological disorders (Deepmala et al., 2015). Despite its success in neurologic disorders, the ability of NAC to efficiently cross the BBB is limited. NAC has shown differential ability to cross BBB which is dependent on its dose and administration (Bavarsad Shahripour et al., 2014). NAC has poor oral bioavailability (~10 %) and requires very high doses to elicit therapeutic activity (Dekhuijzen, 2004). For example, NAC was administered orally at 8000 mg/day dose for the treatment of Glutathione (GSH) deficiency (Atkuri et al., 2007). Therefore, an oral formulation of NAC which can improve its bioavailability and BBB permeability will be of high value for the clinical applications of NAC in neurologic disorders such as cerebral palsy (CP) by reducing the neuroinflammation. Neuroinflammation is a biochemical and cellular response to brain injury involving the activation of glia, release of inflammatory mediators, and generation of reactive oxygen and nitrogen species (Bazan, 2013). Chronic neuroinflammation is a leading cause in the pathogenesis of many brain disorders.
Poly(amido amine) (PAMAM ) dendrimers are branched biomimetic spherical and nanostructured polymers (~5–10 nm) investigated in cancer therapy, inflammation, and targeted delivery applications (Yellepeddi et al., 2009). Previous research in our laboratories (Pisal et al., 2008; Thiagarajan et al., 2013b) as well as others (Najlah et al., 2007) have reported that PAMAM dendrimers are transported across the intestinal epithelial barrier of the gut. PAMAM dendrimers were demonstrated to follow a combination of transcellular and paracellular routes for their transport across the intestinal epithelial barrier (El-Sayed et al., 2002; El-Sayed et al., 2003; Tajarobi et al., 2001). Therefore, PAMAM dendrimers can be effective vehicles for developing oral pediatric formulations for pediatric brain injury.
It has been shown that PAMAM dendrimers with hydroxyl surface groups localize in activated microglia and astrocytes in the presence of neuroinflammation when administered intravenously and intrathecally (Dai et al., 2010). Such selective, intrinsic localization of the hydroxyl PAMAM dendrimers in diffuse neuroinflammatory cells, from systemic administration, has been shown in mouse, rat, rabbit, canine, and primate models (Dai et al., 2010; Guo et al., 2016; Kambhampati et al., 2015; Nance et al., 2016).These results indicate that PAMAM dendrimers can be used for targeted therapy in neuroinflammatory conditions such as CP (Dai et al., 2010). The ability of PAMAM dendrimers to localize in activated microglia and astrocytes when administered intravenously to newborn rabbits with CP was further investigated (Kannan et al., 2012).
Systemic treatment with D-NAC in neonatal CP rabbit kits suppressed neuroinflammation and led to significant improvement in motor function (Kannan et al., 2012). The success of D-NAC based therapies in multiple brain injury models (Kannan et al., 2012; Mishra et al., 2014; Nance et al., 2017; Nance et al., 2015; Nance et al., 2016) suggests a new therapeutic opportunity for treatment of pediatric brain injury, after birth in humans.
In this short communication, we report development, characterization, in vitro permeability, and in vivo absorption of the pediatric oral formulation of D-NAC for the treatment of neuroinflammation. Since NAC dosage regimen usually requires administration of high dose of NAC for prolonged periods of time (Grandjean et al., 2000), we hypothesize that an oral formulation of D-NAC will enhance patient compliance with respect to neonates and significantly ease the mode of administration and enable broader adaptation of therapy in other chronic brain disorders. The formulation contains Capmul MCM (glyceryl caprylate) as penetration enhancer. Capmul MCM is recognized as Generally Regarded as Safe (GRAS) by FDA (Abitec, 2018). Capmul MCM was reported to enhance penetration of hydrophilic markers such as mannitol and poly(ethylene glycol) (Ghandehari et al., 1997) and drugs such as Zanamavir (Holmes et al., 2013). Therefore, incorporating Capmul MCM can have a synergistic effect of increasing the permeability of D-NAC significantly thereby increasing the oral bioavailability of NAC for treating CNS related diseases.
D-NAC and FITC labeled D-NAC (FITC-D-NAC) were synthesized, purified, and characterized by previously established protocols (Burd et al., 2014; Hubbard et al., 2014; Kannan et al., 2012; Nemeth et al., 2017).The D-NAC purity was analyzed using HPLC (~97%), and from 1H NMR spectrum it was estimated to be ~18 NAC molecules attached to each dendrimer (16% loading). The particle size and Zeta potential of D-NAC measured by Dynamic Light Scattering (DLS) were 5.3 ± 1.8 nm and +6.8 ± 0.3 mV respectively. For stability studies, two United States Pharmacopeia (USP) [simulated gastric fluid (USPSGF), pH 1.2 and simulated intestinal fluid (USPSIF), pH 6.8] and three Biorelevant media [fasted state simulated gastric fluid (FaSSGF), pH 1.6, fasted state simulated intestinal fluid (FaSSIF), pH 6.5 and fed state simulated intestinal fluid (FeSSIF), pH 5.0] were used. D-NAC stability was quantified using HPLC. The in vitro permeability of D-NAC was evaluated using a Caco-2 cell monolayer model and included 5 μL of 14C - mannitol as an internal control. For in vivo pharmacokinetics experiments FITC-D-NAC was quantified using fluorescence spectrophotometer (λexc/ λem - 495/525 nm) (Synergy®, BioTek, VT, USA). Furthermore, the stability of FITC-D-NAC in rat serum was assessed for 24 hours using HPLC to confirm the integrity of FITC conjugation to D-NAC. The stability studies showed that after 24 hours, 89.8 ± 2.2 % of FITC-D-NAC was recovered from the rat serum.
The single-dose oral pharmacokinetics of FITC-D-NAC was performed in jugular vein catheterized (JVC) male Sprague-Dawley rats (Charles River Laboratories, MA, USA). On the day of the experiment, 50 mg/kg of FITC-D-NAC and FITC-D-NAC+2% Capmul MCM prepared in 1 mL deionized (DI) water were administered to rats by oral gavage. At time points 0, 0.25, 0.5,1,2,4,6,8, and 24 hours, 500 μL of blood was collected and serum was separated. FITC-D-NAC was extracted from serum and quantified using fluorescence spectroscopy. The pharmacokinetic parameters such as maximum serum concentration (Cmax), time to reach maximum serum concentration (Tmax), and area under the curve (AUC)0−∞ for FITC-D-NAC were calculated by non-compartmental analysis using Kinetica® Version 5.1, Thermo Fisher Scientific, USA. The statistical analysis of data included t-test and one-way analysis of variance (ANOVA). The a priori level of significance was set to 0.05. The statistical analyses were performed using GraphPad® Prism 6 (GraphPad Inc., La Jolla, CA, USA).
The compendial media USPSGF and USPSIF do not mimic the highly variable and dynamic environment of the human gut accurately enough. These media are just aqueous buffers that can reflect pH conditions in the gastrointestinal tract but not key factors such as osmolality, ionic strength, viscosity, and surface tension. To address these limitations, biorelevant dissolution media have been developed to represent conditions in the stomach and intestines more accurately (Mann et al., 2017; Wagner et al., 2012). These ‘biologically relevant’ media take into consideration important properties such as pH, osmolality, the presence of bile, buffer capacity, and surface tension of the gastrointestinal fluids. They also contain bile salts and phospholipids, physiologically relevant surfactants absent from compendial gastrointestinal media.
Furthermore, it is more important to evaluate the stability of D-NAC in biorelevant media as the constituents of gastrointestinal fluids can influence the chemical structure of D-NAC. Table 1 provides the data on stability of D-NAC in both USP and biorelevant gastrointestinal media. The results have shown that for the entire period of study (6 hours) in all media tested, D-NAC stability was >90% indicating that D-NAC is stable in the gastrointestinal environment.
Table 1.
Stability of D-NAC in various simulated gastric and intestinal fluids
| Simulated Gastric Fluids | Simulated Intestinal Fluids | ||||
|---|---|---|---|---|---|
| Time (hrs.) | USPGF | FaSSGF | USPSIF | FaSSIF | FeSSIF |
| 0 | 100±0.9 | 100±6.8 | 100±1.7 | 100±3 | 100±1.5 |
| 2 | 126.3±3.4 | 104.1±4.3 | 105±0.6 | 109.4±1.4 | 93.4±0.4 |
| 4 | 119±2.7 | 112.9±8.7 | 113.3±1.2 | 113.9±1.4 | 88±1.2 |
| 6 | 112±3.2 | 108±3.2 | 102.1±2.2 | 106.2±3.4 | 95.8±1.3 |
The data is presented as % Assay ± SD of D-NAC at 100 μg/m concentration
USPGF-United States Pharmacopeia Simulated Gastric Fluid, FaSSGF-Fasted State Simulated Gastric Fluid from Biorelevant.com;
USPSIF- United States Pharmacopeia Simulated Gastric Fluid, FaSSIF- Fasted State Simulated Intestinal Fluid from Biorelevant.com, FeSSIF-Fed State Simulated Intestinal Fluid from Biorelevant.com
Figure 1 shows the permeability of FITC-D-NAC conjugates across Caco-2 cells in the presence of various concentrations of the penetration enhancer Capmul. The literature reported concentrations of Capmul MCM for enhancing oral permeability typically ranged from 0.25% to 10 % (Hintzen et al., 2014; Holmes et al., 2013). In the current study, the concentrations of Capmul MCM used were 0.5% to 2% representing a range within the reported concentrations. For both permeability and in vivo absorption studies, FITC-D-NAC was quantified using fluorescence spectroscopy as it was reported to be the most sensitive analytical method to quantify D-NAC in a biological specimen (Lesniak et al., 2013). The 14C-mannitol Papp values (monolayers with 14C-mannitol Papp< 2 × 10−6 cm/s were considered confluent and intact) and the transepithelial electrical resistance (TEER) values showed that the Caco-2 monolayer was intact during the transport studies. Both D-NAC and Capmul were not toxic to Caco-2 cells at the concentrations tested in the permeability studies (data not shown). Capmul showed a concentration-dependent increase in transepithelial permeability of D-NAC (Figure 1). Specifically, D-NAC oral formulation with 2% Capmul showed a 9-fold increase in permeability when compared to the formulation containing D-NAC. Our results are consistent with previous reports wherein, Capmul significantly increased the transepithelial permeability of hydrophilic macromolecules (Ghandehari et al., 1997) (Yeh, 1995).
Figure 1.
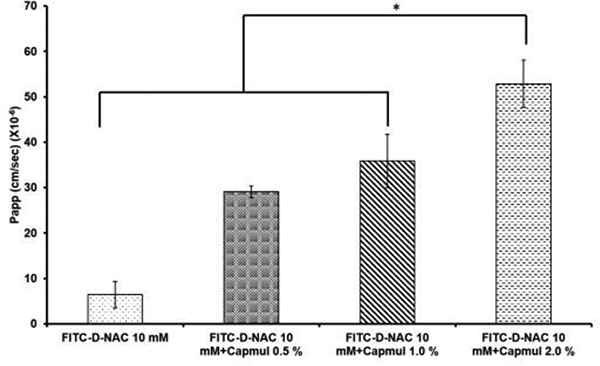
Caco-2 permeability of FITC-D-NAC conjugates containing various concentrations of Capmul. The FITC-D-NAC was at 10 mM concentration, and Capmul was added as % v/v. Data are represented as mean ± SD of apparent permeability (Papp) values from six replicates (n=6) of each sample.
Based on Caco-2 permeability data, the D-NAC formulation containing 2% Capmul was chosen for in vivo oral absorption studies. The D-NAC was administered at 50 mg/kg dose as it was previously reported that D-NAC was effective at 10 mg/kg dose when administered intravenously (Kannan et al., 2012). D-NAC was synthesized using hydroxyl-terminated generation 4 PAMAM (G4-OH) dendrimers, reported to be tolerated at doses up to 500 mg/kg when administered orally (Thiagarajan et al., 2013a). The LD50 of NAC and Capmul in rats following oral administration is 3,000 mg/kg (Booth, 1982) and 5,000 mg/kg (Abitec, 2016) respectively. Furthermore, there was no reduction in weight, and there were no signs of toxicity in rats that were administered with the oral formulation. As expected, the oral formulation of D-NAC containing 2% Capmul significantly increased the oral absorption of D-NAC (Figure 2). The pharmacokinetic parameters related to oral bioavailability, Cmax, and [AUC]0−∞, were increased by ~ 34% (p < 0.05) and ~ 47% (p < 0.05) respectively indicating that Capmul significantly improved the oral bioavailability of D-NAC. Interestingly, the time to reach maximum concentration (Tmax) for D-NAC in the presence of Capmul was lower by 2 hours when compared to D-NAC alone. This may be due to rapid absorption of D-NAC by Capmul induced de-epithelialization of the gastrointestinal tissue. However, future studies involving toxicity and mechanism of gut translocation of D-NAC as well as efficacy in a CP model needs to be performed.
Figure 2.
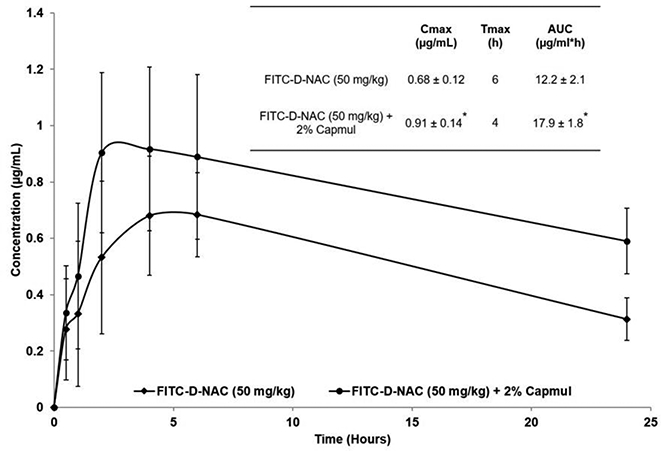
In vivo oral absorption and pharmacokinetics of FITC-D-NAC with Capmul in rats. D-NAC-FITC (50 mg/kg) formulations with Capmul (2%) were administered to rats by oral gavage. The plasma concentrations of FITC-D-NAC were analyzed using fluorescence spectroscopy. The data represent the mean ± SD plasma concentration of FITC-D-NAC obtained from five rats (n=5). The embedded table in the figure provided values of pharmacokinetic parameters representing oral bioavailability. *p < 0.05 vs. FITC-D-NAC. The pharmacokinetic parameters were calculated by noncompartmental analysis using Kinetica® Version 5.1 software.
In conclusion, we demonstrated that D-NAC is stable in simulated gastrointestinal fluids and its oral absorption can be enhanced in the presence of penetration enhancer Capmul. Therefore, a pediatric oral formulation of D-NAC containing Capmul can be beneficial for the treatment of neuroinflammation.
Acknowledgements
This study was partly funded by NIH NICHD [R01HD076901] (RM Kannan) and CP Foundation (RMKannan).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abitec, 2016. Safety Data Sheet, Capmul MCM NF. Abitec, Inc. [Google Scholar]
- Abitec, 2018. Capmul brochure, in: Corporation, A. (Ed.). Abitec. [Google Scholar]
- Atkuri KR, Mantovani JJ, Herzenberg LA, Herzenberg LA, 2007. N-Acetylcysteine--a safe antidote for cysteine/glutathione deficiency. Curr Opin Pharmacol 7, 355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavarsad Shahripour R, Harrigan MR, Alexandrov AV, 2014. -acetylcysteine (NAC) in neurological disorders: mechanisms of action and therapeutic opportunities. Brain Behav 4, 108–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG, 2013. The docosanoid neuroprotectin D1 induces homeostatic regulation of neuroinflammation and cell survival. Prostaglandins Leukot Essent Fatty Acids 88, 127–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth NH, 1982. Veterinary Pharmacology and Therapeutics, 5 ed. Iowa State University Press, Ames, Iowa. [Google Scholar]
- Burd I, Zhang F, Dada T, Mishra MK, Borbiev T, Lesniak WG, Baghlaf H, Kannan S, Kannan RM, 2014. Fetal uptake of intra-amniotically delivered dendrimers in a mouse model of intrauterine inflammation and preterm birth. Nanomedicine 10, 1343–1351. [DOI] [PubMed] [Google Scholar]
- Dai H, Navath RS, Balakrishnan B, Guru BR, Mishra MK, Romero R, Kannan RM, Kannan S, 2010. Intrinsic targeting of inflammatory cells in the brain by polyamidoamine dendrimers upon subarachnoid administration. Nanomedicine (Lond) 5, 1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepmala, Slattery J, Kumar N, Delhey L, Berk M, Dean O, Spielholz C, Frye R, 2015. Clinical trials of N-acetylcysteine in psychiatry and neurology: A systematic review. Neurosci Biobehav Rev 55, 294–321. [DOI] [PubMed] [Google Scholar]
- Dekhuijzen PN, 2004. Antioxidant properties of N-acetylcysteine: their relevance in relation to chronic obstructive pulmonary disease. Eur Respir J 23, 629–636. [DOI] [PubMed] [Google Scholar]
- El-Sayed M, Ginski M, Rhodes C, Ghandehari H, 2002. Transepithelial transport of poly(amidoamine) dendrimers across Caco-2 cell monolayers. J Control Release 81, 355–365. [DOI] [PubMed] [Google Scholar]
- El-Sayed M, Rhodes CA, Ginski M, Ghandehari H, 2003. Transport mechanism(s) of poly (amidoamine) dendrimers across Caco-2 cell monolayers. Int J Pharm 265, 151–157. [DOI] [PubMed] [Google Scholar]
- Ghandehari H, Smith PL, Ellens H, Yeh PY, Kopecek J, 1997. Size-dependent permeability of hydrophilic probes across rabbit colonic epithelium. J Pharmacol Exp Ther 280, 747–753. [PubMed] [Google Scholar]
- Grandjean EM, Berthet P, Ruffmann R, Leuenberger P, 2000. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebo-controlled clinical trials. Clin Ther 22, 209–221. [DOI] [PubMed] [Google Scholar]
- Guo Y, Johnson MA, Mehrabian Z, Mishra MK, Kannan R, Miller NR, Bernstein SL, 2016. Dendrimers Target the Ischemic Lesion in Rodent and Primate Models of Nonarteritic Anterior Ischemic Optic Neuropathy. PLoS One 11, e0154437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintzen F, Perera G, Hauptstein S, Muller C, Laffleur F, Bernkop-Schnurch A, 2014. In vivo evaluation of an oral self-microemulsifying drug delivery system (SMEDDS) for leuprorelin. Int J Pharm 472, 20–26. [DOI] [PubMed] [Google Scholar]
- Holmes EH, Devalapally H, Li L, Perdue ML, Ostrander GK, 2013. Permeability enhancers dramatically increase zanamivir absolute bioavailability in rats: implications for an orally bioavailable influenza treatment. PLoS One 8, e61853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard D, Ghandehari H, Brayden DJ, 2014. Transepithelial transport of PAMAM dendrimers across isolated rat jejunal mucosae in ussing chambers. Biomacromolecules 15, 2889–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambhampati SP, Clunies-Ross AJ, Bhutto I, Mishra MK, Edwards M, McLeod DS, Kannan RM, Lutty G, 2015. Systemic and Intravitreal Delivery of Dendrimers to Activated Microglia/Macrophage in Ischemia/Reperfusion Mouse Retina. Invest Ophthalmol Vis Sci 56, 4413–4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S, Dai H, Navath RS, Balakrishnan B, Jyoti A, Janisse J, Romero R, Kannan RM, 2012. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci Transl Med 4, 130ra146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniak WG, Mishra MK, Jyoti A, Balakrishnan B, Zhang F, Nance E, Romero R, Kannan S, Kannan RM, 2013. Biodistribution of fluorescently labeled PAMAM dendrimers in neonatal rabbits: effect of neuroinflammation. Mol Pharm 10, 4560–4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann J, Dressman J, Rosenblatt K, Ashworth L, Muenster U, Frank K, Hutchins P, Williams J, Klumpp L, Wielockx K, Berben P, Augustijns P, Holm R, Hofmann M, Patel S, Beato S, Ojala K, Tomaszewska I, Bruel JL, Butler J, 2017. Validation of Dissolution Testing with Biorelevant Media: An OrBiTo Study. Mol Pharm 14, 4192–4201. [DOI] [PubMed] [Google Scholar]
- Mishra MK, Beaty CA, Lesniak WG, Kambhampati SP, Zhang F, Wilson MA, Blue ME, Troncoso JC, Kannan S, Johnston MV, Baumgartner WA, Kannan RM, 2014. Dendrimer brain uptake and targeted therapy for brain injury in a large animal model of hypothermic circulatory arrest. ACS Nano 8, 2134–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najlah M, Freeman S, Attwood D, D’Emanuele A, 2007. In vitro evaluation of dendrimer prodrugs for oral drug delivery. Int J Pharm 336, 183–190. [DOI] [PubMed] [Google Scholar]
- Nance E, Kambhampati SP, Smith ES, Zhang Z, Zhang F, Singh S, Johnston MV, Rangaramanujam K, Blue ME, Kannan S, 2017. Dendrimer-mediated delivery of N-acetyl cysteine to microglia in a mouse model of Rett syndrome. J Neuroinflammation 14, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance E, Porambo M, Zhang F, Mishra MK, Buelow M, Getzenberg R, Johnston M, Kannan RM, Fatemi A, Kannan S, 2015. Systemic dendrimer-drug treatment of ischemia-induced neonatal white matter injury. J Control Release 214, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance E, Zhang F, Mishra MK, Zhang Z, Kambhampati SP, Kannan RM, Kannan S, 2016. Nanoscale effects in dendrimer-mediated targeting of neuroinflammation. Biomaterials 101, 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth CL, Drummond GT, Mishra MK, Zhang F, Carr P, Garcia MS, Doman S, Fatemi A, Johnston MV, Kannan RM, Kannan S, Wilson MA, 2017. Uptake of dendrimer-drug by different cell types in the hippocampus after hypoxic-ischemic insult in neonatal mice: Effects of injury, microglial activation and hypothermia. Nanomedicine 13, 2359–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisal DS, Yellepeddi VK, Kumar A, Kaushik RS, Hildreth MB, Guan X, Palakurthi S, 2008. Permeability of surface-modified polyamidoamine (PAMAM) dendrimers across Caco-2 cell monolayers. Int J Pharm 350, 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajarobi F, El-Sayed M, Rege BD, Polli JE, Ghandehari H, 2001. Transport of poly amidoamine dendrimers across Madin-Darby canine kidney cells. Int J Pharm 215, 263–267. [DOI] [PubMed] [Google Scholar]
- Thiagarajan G, Greish K, Ghandehari H, 2013a. Charge affects the oral toxicity of poly(amidoamine) dendrimers. Eur J Pharm Biopharm 84, 330–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan G, Sadekar S, Greish K, Ray A, Ghandehari H, 2013b. Evidence of oral translocation of anionic G6.5 dendrimers in mice. Mol Pharm 10, 988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner C, Jantratid E, Kesisoglou F, Vertzoni M, Reppas C, J BD, 2012. Predicting the oral absorption of a poorly soluble, poorly permeable weak base using biorelevant dissolution and transfer model tests coupled with a physiologically based pharmacokinetic model. Eur J Pharm Biopharm 82, 127–138. [DOI] [PubMed] [Google Scholar]
- Yeh PY, Berenson MM, Samowitz WS, Kopeckova P, Kopecek J, 1995. Site-specific drug delivery and penetration enhancement in the gastrointestinal tract. Journal of controlled release 36, 109–124. [Google Scholar]
- Yellepeddi VK, Kumar A, Palakurthi S, 2009. Surface modified poly(amido)amine dendrimers as diverse nanomolecules for biomedical applications. Expert Opin Drug Deliv 6, 835–850. [DOI] [PubMed] [Google Scholar]


