Summary
Objective
To estimate the direct and indirect costs of diabetes mellitus care at five public health facilities in Kenya.
Methods
We conducted a cross‐sectional study in two counties where diabetes patients aged 18 years and above were interviewed. Data on care‐seeking costs were obtained from 163 patients seeking diabetes care at five public facilities using the cost‐of‐illness approach. Medicines and user charges were classified as direct health care costs while expenses on transport, food, and accommodation were classified as direct non–health care costs. Productivity losses due to diabetes were classified as indirect costs. We computed annual direct and indirect costs borne by these patients.
Results
More than half (57.7%) of sampled patients had hypertension comorbidity. Overall, the mean annual direct patient cost was KES 53 907 (95% CI, 43 625.4‐64 188.6) (US$ 528.5 [95% CI, 427.7‐629.3]). Medicines accounted for 52.4%, transport 22.6%, user charges 17.5%, and food 7.5% of total direct costs. Overall mean annual indirect cost was KES 23 174 (95% CI, 20 910‐25 438.8) (US$ 227.2 [95% CI, 205‐249.4]). Patients reporting hypertension comorbidity incurred higher costs compared with diabetes‐only patients. The incidence of catastrophic costs was 63.1% (95% CI, 55.7‐70.7) and increased to 75.4% (95% CI, 68.3‐82.1) when transport costs were included.
Conclusion
There are substantial direct and indirect costs borne by diabetic patients in seeking care from public facilities in Kenya. High incidence of catastrophic costs suggests diabetes services are unaffordable to majority of diabetic patients and illustrate the urgent need to improve financial risk protection to ensure access to care.
Keywords: catastrophe, diabetes mellitus, Kenya, out‐of‐pocket costs, productivity losses
1. BACKGROUND
Diabetes mellitus (DM) is a chronic, incurable, and potentially disabling disease that presents a substantial public health challenge worldwide.1 Evidence suggests that the unprecedented increase in DM burden has major clinical, economic, and social implications particularly in low‐ and middle‐income countries (LMICs).2, 3, 4, 5, 6, 7 In addition to reducing well‐being, the chronic nature and complications associated with DM may lead to substantial costs of medical care and productivity losses to patients and their households. Unfortunately, progress in attainment of Sustainable Development Goal 3.4 that aims to reduce premature mortality from noncommunicable diseases (NCDs) by one‐third by 2030 continues to be constrained by, inter alia, weak health systems in most LMICs.1
The Kenyan health system is devolved, with the National Ministry of Health (MOH) having policy and regulatory roles while the 47 county health systems have service provision roles.8 Service delivery is pluralistic and is characterized by a mix of public, private for‐profit, and private not‐for‐profit providers.9, 10 Kenya's public health care delivery system is organized into six levels. Level 1 is composed of community health services that include all community‐based demand creation activities that are guided by the MOH community strategy. Levels 2 and 3 refer to dispensaries and health centers, respectively, which offer outpatient primary health care services. Level 4 represents subcounty hospitals that are first referral hospitals while level 5 represents county referral hospitals that provide secondary care. Level 6 represents national tertiary referral hospitals. Diabetes care is typically offered through dedicated specialized clinics located in public levels 4 to 6 hospitals.11, 12, 13, 14 In some areas, however, patients can access medication from health centres and dispensaries, but this is the exception rather than the norm. Each of these levels are expected to provide some aspects of preventive, promotive, curative, and rehabilitative services as outlined in the Kenya Essential Package for Health,15 which includes interventions and services targeted at DM. Private providers mimic this classification though most are stand‐alone units with weak referral mechanisms. Kenya's health system is financed by (a) tax revenues collected by the government (national and county), (b) donor funding, (c) household contributions to the National Hospital Insurance Fund (NHIF), (d) household contributions to private health insurance companies, and (d) out‐of‐pocket (OOP) payments at points of care.
The first nationally representative survey of 2015 found an age‐standardized DM prevalence of 2.4% with 3.1% of Kenya's population having impaired fasting glycemia.12, 16 The increasing prevalence and the chronic nature of DM makes it a costly disease both to Kenya's health system and the affected households as it has been shown that persons with diabetes incur up to three times higher medical costs compared with nondiabetics.17, 18, 19 In addition, delay in diagnosis, poor quality of care or the lack thereof, presence and severity of complications, and comorbid conditions are the most important factors related to DM care costs.20, 21
Evidence of patient costs associated with DM care is needed to assess the economic impact of DM to households, the extent to which DM patients and households are protected from financial hardship due to health care use and to design effective financial risk protection mechanisms for this group of patients.6, 21, 22 We therefore conducted this study to document the patient costs of DM at primary care level in Kenya.
2. METHODS
2.1. Study setting
The study was conducted from June to December 2017 in two sites in Kenya (Kilifi and Bungoma County) purposively selected to reflect a diverse set of demographic, socio‐economic, and geographical settings. Kilifi is located on the coast of Kenya, and a high burden of stroke and heart failure has been described in this area.23 The population in Kilifi has been well characterized by data from the health and demographic surveillance system run by the KEMRI Wellcome Trust Research Programme.24 The Webuye Health and Demographic Surveillance System run by Moi University is located in Bungoma County in the western region of Kenya.25 Multiple cardiovascular risk factors have been identified in this area.26 Table 1 outlines the study site characteristics.
Table 1.
Selected study site indicators in 2017
| County | Estimated Population | No. of Public Hospitals27 | No. of Health Centers and Dispensaries27 | No. of OPD Patients | No. of Admissions | No. of Diabetes Cases | No. of Hypertension Cases |
|---|---|---|---|---|---|---|---|
| Bungoma | 1 759 49928 | 9 | 125 | 1 215 525 | 70 665 | 3038 | 15 908 |
| Kilifi | 1 466 85629 | 5 | 123 | 1 243 315 | 28 746 | 4663 | 26 458 |
(all data except where otherwise indicated30).
Six public health care facilities were purposively selected in consultation with county health officials in respective counties to generate a sample of facilities with different workloads, plus the location of the clinics relative to the communities served. However, due to the 150‐day nation‐wide nurses' strike at the time of data collection,27 data were collected from five facilities unlike the anticipated six facilities in the two counties. In Kilifi, a public hospital and a health center that provided DM treatment were selected, while in Bungoma, three public hospitals were sampled. For this descriptive analysis, data from all the facilities were pooled.
2.2. Sample size and sampling
The target enrolment was 282 patients for a sample size sufficient to obtain an estimate of DM patient costs based on the formulae by Kirkwood28:
where Z α/2 is the critical value of the normal distribution at α/2 (for a confidence level of 95%, α is .05 and the critical value is 1.96), Z β is the critical value of the normal distribution at β (for a power of 80%, β is 0.2 and the critical value is 0.84), P is the expected true proportion of DM in the population in Kenya of 10% (0.10), and e is the desired standard size of standard error around the estimated proportion of 5% (±0.05).
Every DM patient receiving treatment and available at participating facilities during data collection was approached to participate in the study. Patients were eligible if they self‐reported DM diagnosis, had received treatment for a minimum of 6 months after diagnosis and were more than 18 years of age. Consenting patients were selected based on meeting the eligibility criteria and the order of arrival at the clinic. Respondents were asked to report on their health service use, associated costs, income, and coping mechanisms if they undertook any of the following to meet DM care costs: borrowing (having taken a loan), selling household items or assets (eg, livestock), and use of savings.
2.3. Measuring patient costs
The cost‐of‐illness approach was used to document patient costs.29 Interviews were conducted using a structured questionnaire. Three trained interviewers collected the data in the two study sites following a pilot from a facility in Kilifi that was not selected in the main study. Interviews were conducted primarily in Kiswahili, with local languages (Kigiriama and Kibukusu in Kilifi and Bungoma, respectively) used to clarify questions where necessary. Respondents were asked about costs incurred for different care‐seeking episodes described in Table 2.
Table 2.
Care‐seeking episodes included in patient cost estimates
| Care‐seeking Episode | Description | Recall Period |
|---|---|---|
| Sick visit | Cost of current care seeking (during interview), and any out‐patient visit when the patient was ill due to DM outside the scheduled clinic appointments | 1 month |
| Inpatient visit | Cost of admission due to DM | 12 months |
| Drug collection visit | Cost of regular medicines prescribed to the patient to manage DM | Frequency of drug collection, ie, monthly/quarterly |
| Laboratory/diagnostic visits | Cost of routine laboratory/diagnostic services done at a health facility | Frequency of lab/diagnostic services, ie, monthly/quarterly |
| Scheduled clinic check‐up visits | Costs due to regular clinic appointments | Frequency of clinic appointments, ie, monthly/quarterly |
To annuitize sick visit costs, we summed up costs incurred during current care visit and any reported outpatient visit costs that occurred due to DM in the last 4 weeks prior to the study then multiplied by 13 (assuming there are 52 weeks in a year). On the other hand, to annuitize costs in other care‐seeking episodes described in Table 2, reported costs were multiplied by the frequency of visits, i.e., weekly, monthly, or quarterly for each episode. Furthermore, any inpatient admission costs in the last 1 year were also collected. Overall DM care costs for all care‐seeking episodes were calculated by summing up the annual costs in each care‐seeking episode. Costs incurred by patients in the overall sample, costs incurred by diabetics without comorbidities (hereafter called diabetes‐only patients), and costs incurred by diabetics with comorbidities (hereafter called comorbid patients), i.e., patients who reported both DM and hypertension diagnosis, were analysed and reported separately.
For each of the care‐seeking episode, two broad cost categories were estimated: direct OOP costs and indirect (productivity losses) costs for both patients and their caregivers. Direct health care costs included any charges levied for medicines and user fees, i.e., registration, consultation, and laboratory services. Direct non–health care costs included transport costs to and from a health provider and any costs associated with food or accommodation while seeking care. For this analysis, OOP costs were defined as the sum of direct health care and direct non–health care costs. The analysis was restricted to patients who reported any OOP costs for each care‐seeking episode.
Indirect costs were estimated based on the total hours lost while seeking care as well as the cost of illness‐related to lost productivity for both patients and their caregivers, assuming that these hours would have been used for productive activity in the absence of DM.30 Income lost due to DM illness was therefore estimated by multiplying the estimated number of lost production hours due to DM by the official minimum wage; by the official minimum wage of Kenya shillings (KES) 8568 (US$ 84/month) in the agricultural sector in 2017 (given the main economic activities in our study sites).31 We assumed an average workday of 8 h/day and 22 working days/month. Caregivers' lost productivity was also estimated by multiplying the total number of hours spent caring for the patient by the official minimum wage rate.
Income was estimated by asking detailed questions about income categories, including patient income, income for household members, welfare payments, and government assistance. As a measure of financial risk protection, we compared total direct costs incurred in the overall sample and by socio‐economic status, against annual household income and total direct costs excluding transportation costs and defined costs as catastrophic if they exceeded 10% of annual household income.32
2.4. Data management and analysis
Data were double entered to enhance data quality. The two data sets were compared to eliminate data entry errors. Consistency and range checks were used to ensure the completeness of data. Data were analysed using STATA 14.0 (STATA, StataCorp, Texas). Frequency counts and percentages were used to describe patient demographic and socio‐economic variables. Due to skewed nature of costs data, mean and median values were reported for all cost estimates as a measure of central tendency and 95% confidence intervals (CI) and interquartile range (IQR) were reported. Cost results are presented in KES and US Dollars (US$). Cost in KES was converted to US$ using the following exchange rate: US$ 1 = KES 102 (average exchange rate, January‐December 2017).33
2.5. Ethical approval
Ethical approval for the study was obtained from the Scientific Ethics Review Unit (SERU) of KEMRI (Ref: KEMRI/SERU/CGMR‐C/041/3270) and from the County Department of Health in both study sites. Written consent was obtained from each participant. Facility managers were informed about the study and granted permission to access clinics and patients.
3. RESULTS
3.1. Participants characteristics and health services utilization
Overall, 163 patients were interviewed: 92 (56.4%) from Bungoma and 71 (43.6%) from Kilifi. The mean age in the sample was 58.9 years, and 58.9% of the respondents were female. More than half of the patients reported hypertension comorbidity (57.7%), were not enrolled to any health insurance scheme (68.1%), and slightly under half were diagnosed with either DM or hypertension (HTN) more than 5 years ago (47.9%) (Table 3). The median travel time to a health facility was 30 minutes (IQR, 18‐60). Forty‐five patients (27.6%) reported that the facility where they sought care was not the nearest to them; of these, 48.9% (95% CI, 34.2‐63.7) reported lack of resources such as medicines and/or diagnostic equipment, 37.8% (95% CI, 24.5‐53.2) reported referral, and 13.3% (95% CI, 5.9‐27.3) reported other reasons (eg, time consuming to wait) for not visiting the nearest facility.
Table 3.
Patient characteristics
| Characteristic | Observations (n = 163) | Proportion (95% CI) |
|---|---|---|
| Mean age in years | 163 | 58.9 (56.8‐61.0) |
| Gender | ||
| Male | 67 | 41.1% (33.7‐48.9) |
| Female | 96 | 58.9% (51.1‐66.2) |
| Illness condition | ||
| Diabetes mellitus | 69 | 42.3% (34.9‐50.1) |
| Diabetes mellitus & Hypertension | 94 | 57.7% (49.9‐65.1) |
| Enrolled to a health insurance scheme | ||
| Yes | 52 | 31.9% (25.1‐39.5) |
| No | 111 | 68.1% (60.5‐74.9) |
| Employment status | ||
| Formal employment | 32 | 19.6 (14.2‐26.5) |
| Informal/unemployed | 131 | 80.4 (73.5‐85.8) |
| Reason for not working | ||
| Related to DM/DM & HTN comorbidity | 48 | 29.4% (22.9‐37.0) |
| Not related to DM/DM & HTN comorbidity | 115 | 70.6% (63.0‐77.1) |
| Highest level of education | ||
| None | 27 | 16.6% (11.6‐23.2) |
| Primary | 66 | 40.5% (33.2‐48.3) |
| Secondary | 47 | 28.8% (22.3‐36.3) |
| Graduate/certificate | 23 | 14.1% (9.5‐20.4) |
| Where diagnosed | ||
| Public facility | 132 | 81.0% (74.1‐86.3) |
| Mission facility | 7 | 4.3% (2.0‐8.7) |
| Private facility | 24 | 14.7% (10.0‐21.1) |
| Illness duration | ||
| 6 mo to <1 y | 16 | 9.8% (6.1‐15.5) |
| 1‐5 y | 69 | 42.3% (34.9‐50.1) |
| ≥5 y | 78 | 47.9% (40.2‐55.6) |
3.2. Patient costs associated with health care use
3.2.1. Sick visit costs
In the overall sample, 36% (n = 59) of patients reported a sick visit, i.e., sought care out of their scheduled clinic appointments a month before the survey. Similarly, 29% (n = 20) of diabetes‐only patients and 41% (n = 39) of comorbid patients reported a sick visit. Mean annual costs for medicines attracted the highest direct cost in the overall sample—KES 15 340.8 (95% CI, 6120‐24 561.6) (US$ 150.4 [95% CI, 60‐240.8]) mainly driven by the contribution of medicine costs for comorbid patients—KES 17 880 (95% CI, 4641.7‐31 118.3) (US$ 175.3 [95% CI, 45.5‐305.1]). However, among diabetes‐only patients, user charges attracted the highest mean annual cost—KES 12 733.5 (95% CI, 1825‐27 292) (US$ 124.8 [95% CI, 17.9‐267.6]) (Table 5). Transport costs contributed 25.8% of total costs in the overall sample, 26.3% in the diabetes‐only group, and 25.5% in the comorbid group (Tables 4, 5, 6).
Table 5.
Mean and median annual cost for diabetes‐only patients at five public facilities in Kenya (2017 US$)
| Care‐seeking Episode | Cost Category | n | Mean KES (95% CI) | Median KES (IQR) | Mean US$ (95% CI) | Median US$ (IQR) | As a % of Total Direct Costs |
|---|---|---|---|---|---|---|---|
| Sick visit | Direct health care costs | ||||||
| User charges | 20 | 12 733.5 (1825.0‐27 292.0) | 3055.0 (845.0‐6110.0) | 124.8 (17.9‐267.6) | 30.0 (8.3‐59.9) | 40.6 | |
| Medicines | 20 | 10 387 (488.6‐20 305.4) | 2340 (520‐9425) | 101.8 (4.8‐199.1) | 22.9 (5.1‐92.4) | 33.1 | |
| Direct non–health care costs | |||||||
| Transport | 20 | 8268 (3090.8‐13 445.2) | 3120 (1560‐9100) | 81.1 (30.3‐131.8) | 30.6 (15.3‐89.2) | 26.3 | |
| Food | 20 | 0 | 0 | 0 | 0 | 0.0 | |
| Subtotal (direct costs) | 20 | 31 388.5 (7168.3‐55 608.7) | 10 335 (6630‐33 605) | 307.7 (70.3‐545.2) | 101.3 (65.0‐329.5) | ||
| Indirect costs | 20 | 7360.5 (4925.0‐9796.1) | 4545.6 (3860.0‐10 713.2) | 72.2 (48.3‐96.0) | 44.6 (37.8‐105.0) | ||
| Direct + Indirect costs | 20 | 38 749.0 (14 080.6‐63 417.5) | 18 804.3 (11 197.4‐42 818.0) | 379.9 (138.0‐621.7) | 184.4 (109.8‐419.8) | ||
| In‐patient admission | Direct health care costs | ||||||
| User charges | 7 | 18 568.6 (11 234.9‐48 372.1) | 4700 (3150‐16 000) | 182.0 (110.1‐474.2) | 46.1 (30.9‐156.9) | 76.8 | |
| Medicines | 5 | 3372 (1624.9‐8368.9) | 1200 (1110‐4350) | 33.1 (15.9‐82.0) | 11.8 (10.9‐42.6) | 10.0 | |
| Direct non–health care costs | |||||||
| Transport | 15 | 910.7 (225.3‐1596.1) | 600 (200‐1080) | 8.9 (2.2‐15.6) | 5.9 (2.0‐10.6) | 8.1 | |
| Food | 6 | 1441.7 (265.4‐2618.0) | 850 (750‐2500) | 14.1 (2.6‐25.7) | 8.3 (7.4‐24.5) | 5.1 | |
| Subtotal (direct costs) | 15 | 11 276.7 (3958.3‐26 511.7) | 1860 (400‐7130) | 110.6 (38.8‐259.9) | 18.2 (3.9‐69.9) | ||
| Indirect costs | 15 | 5060.3 (2042.4‐8078.2) | 3101.0 (1611.6‐6006.7) | 49.6 (20.0‐79.2) | 30.4 (15.8‐58.9) | ||
| Direct + Indirect costs | 15 | 16 337.0 (1514.0‐34 187.9) | 5968.5 (2567.1‐14 181.8) | 160.2 (14.8‐335.2) | 58.5 (25.2‐139.0) | ||
| Medicine collection | Direct health care costs | ||||||
| Medicines | 57 | 9230.5 (6253.9‐12 207.1) | 5040 (2400‐10 680) | 90.5 (61.3‐119.7) | 49.4 (23.5‐104.7) | 84.7 | |
| Direct non–health care costs | |||||||
| Transport | 8 | 10 950 (581.6‐22 481.6) | 6000 (2400‐13 800) | 107.4 (5.7‐220.4) | 58.8 (23.5‐135.3) | 14.1 | |
| Food | 1 | 7200 | 7200 | 70.6 | 70.6 | 1.2 | |
| Subtotal (direct costs) | 57 | 10 893.7 (6884.9‐14 902.5) | 6000 (3360‐12 000) | 106.8 (67.5‐146.1) | 58.8 (32.9‐117.6) | ||
| Indirect costs | 69 | 7771.4 (4800.1‐10 742.7) | 4688.2 (3223.1‐7325.3) | 76.2 (47.1‐105.3) | 46.0 (31.6‐71.8) | ||
| Direct + Indirect costs | 69 | 16 770.6 (11 672.9‐21 868.2) | 10 395.2 (5225.2‐16 739.7) | 164.4 (114.4‐214.4) | 101.9 (51.2‐164.1) | ||
| Diagnostic visit | Direct health care costs | ||||||
| Test cost | 47 | 7598.3 (201.4‐15 398.0) | 1800 (1800‐3600) | 74.5 (2.0‐151.0) | 17.6 (17.6‐35.3) | 90.6 | |
| Direct non–health care costs | |||||||
| Transport | 7 | 4971.4 (2075.4‐7867.5) | 4800 (2400‐7200) | 48.7 (20.3‐77.1) | 47.1 (23.5‐70.6) | 8.8 | |
| Food | 1 | 2400 | 2400 | 23.5 | 23.5 | 0.6 | |
| Subtotal (direct costs) | 50 | 7886.4 (572.7‐15 200.1) | 1800 (1800‐3600) | 77.3 (5.6‐149.0) | 17.6 (17.6‐35.3) | ||
| Indirect costs | 68 | 4423.4 (3644.7‐5202.1) | 3850.2 (2607.8‐4887.4) | 43.4 (35.7‐51.0) | 37.7 (25.6‐47.9) | ||
| Direct + Indirect costs | 68 | 10 222.2 (4827.4‐15 616.9) | 5682.4 (3809.1‐9273.8) | 100.2 (47.3‐153.1) | 55.7 (37.3‐90.9) | ||
| Scheduled clinics | Direct health care costs | ||||||
| User charges | 55 | 6362.2 (1953.2‐10 771.1) | 3000 (2400‐4080) | 62.4 (19.1‐105.6) | 29.4 (23.5‐40.0) | 38.6 | |
| Medicines | 50 | 6289.2 (4546.0‐8032.4) | 4350 (2400‐9000) | 61.7 (44.6‐78.7) | 42.6 (23.5‐88.2) | 34.7 | |
| Direct non–health care costs | 69 | 13 148.7 (9166.8‐17 130.6) | 9000 (4080‐17 640) | 128.9 (89.9‐167.9) | 88.2 (40‐172.9) | ||
| Transport | 62 | 3878.7 (2987.6‐4769.8) | 2400 (1200‐4800) | 38.0 (29.3‐46.8) | 23.5 (11.8‐47.1) | 26.5 | |
| Food | 1 | 2400 | 2400 | 23.5 | 23.5 | 0.3 | |
| Subtotal (direct costs) | 69 | 13 148.7 (9166.8‐17 130.6) | 9000 (4080‐17 640) | 128.9 (89.9‐167.9) | 88.2 (40‐172.9) | ||
| Indirect costs | 69 | 4071.1 (3542.5‐4599.6) | 3516.1 (2344.1‐5086.7) | 39.9 (34.7‐45.1) | 34.5 (23.0‐49.9) | ||
| Direct + Indirect costs | 69 | 17 219.8 (13 072.6‐21 366.9) | 12 792.3 (6876.1‐21 156.1) | 168.8 (128.2‐209.5) | 125.4 (67.4‐207.4) | ||
| Overall costs | Direct health care costs | ||||||
| User charges | 58 | 14 839.1 (4730.7‐24 947.6) | 6250 (5000‐7880) | 145.5 (46.4‐244.6) | 61.3 (49.0‐77.3) | 30.3 | |
| Medicines | 57 | 22 275.3 (16 647.7‐27 902.8) | 18 400 (7400‐30 500) | 218.4 (163.2‐273.6) | 180.4 (75.5‐299.0) | 44.0 | |
| Direct non–health care costs | |||||||
| Transport | 63 | 11 218.4 (7919.4‐14 517.5) | 6300 (3360‐14 640) | 110.0 (77.6‐142.3) | 61.8 (32.9‐143.5) | 24.7 | |
| Food | 9 | 3016.7 (609.4‐5423.9) | 2400 (800‐3200) | 29.6 (6.0‐53.2) | 23.5 (7.8‐31.4) | 1.0 | |
| Subtotal (direct costs) | 69 | 46 754.4 (31 008.3‐62 500.4) | 31 500 (13 880‐50 840) | 458.4 (304.0‐612.7) | 308.8 (136.1‐498.4) | ||
| Indirect costs | 64 | 21 861.1 (18 070.1‐25 652.1) | 17 483.0 (13 136.7‐26 187.8) | 214.3 (177.2‐251.5) | 171.4 (128.8‐256.7) | ||
| Direct + Indirect costs | 69 | 68 615.5 (51472.6‐85 758.3) | 52 372.5 (28 458.9‐75 288.0) | 672.7 (504.6‐840.8) | 513.5 (279.0‐738.1) | ||
Table 4.
Overall mean and median annual costs of diabetes care at five public facilities in Kenya (2017 US$)
| Care‐seeking Episode | Cost Category | n | Mean KES (95% CI) | Median KES (IQR) | Mean US$ (95% CI) | Median US$ (IQR) | As a % of Total Direct Costs |
|---|---|---|---|---|---|---|---|
| Sick visit | Direct health care costs | ||||||
| User charges | 59 | 7435.8 (2611.2‐12 260.4) | 4549.2 (1173.0‐6497.4) | 72.9 (25.6‐120.2) | 44.6 (11.5‐63.7) | 23.9 | |
| Medicines | 59 | 15 340.8 (6120‐24 561.6) | 5202.0 (1815.6‐12 872.4) | 150.4 (60.0‐240.8) | 51.0 (17.8‐126.2) | 49.3 | |
| Direct non–health care costs | |||||||
| Transport | 59 | 8037.6 (4845‐11 220) | 5202 (1815.6‐9098.4) | 78.8 (47.5‐110.0) | 51.0 (17.8‐89.2) | 25.8 | |
| Food | 59 | 326.4 (10.2‐632.4) | 0 | 3.2 (0.1‐6.2) | 0 | 1.0 | |
| Subtotal (direct costs) | 59 | 31 130.4 (18 961.8‐43 299.0) | 17 156.4 (8578.2‐32 762.4) | 305.2 (185.9‐424.5) | 168.2 (84.1‐321.2) | ||
| Indirect costs | 59 | 7466.4 (6262.8‐8659.8) | 6344.4 (3906.6‐8884.2) | 73.2 (61.4‐84.9) | 62.2 (38.3‐87.1) | ||
| Direct + Indirect costs | 59 | 38 596.8 (25 928.4‐51 265.2) | 24 388.2 (12 923.4‐41 524.2) | 378.4 (254.2‐502.6) | 239.1 (126.7‐407.1) | ||
| In‐patient admission | Direct health care costs | ||||||
| User charges | 16 | 16 717.8 (3264.0‐30 161.4) | 7170.6 (2203.2‐15 330.6) | 163.9 (32.0‐295.7) | 70.3 (21.6‐150.3) | 64.5 | |
| Medicines | 11 | 4008.6 (1366.8‐6660.6) | 3468 (683.4‐8496.6) | 39.3 (13.4‐65.3) | 34.0 (6.7‐83.3) | 10.6 | |
| Direct non–health care costs | |||||||
| Transport | 36 | 1917.6 (703.8‐3131.4) | 938.4 (418.2‐1734.0) | 18.8 (6.9‐30.7) | 9.2 (4.1‐17.0) | 16.6 | |
| Food | 17 | 2019.6 (204.0‐3825.0) | 795.6 (499.8‐1999.2) | 19.8 (2.0‐37.5) | 7.8 (4.9‐19.6) | 8.3 | |
| Subtotal (direct costs) | 36 | 11 526.0 (3825‐19 216.8) | 2478.6 (765.0‐14 932.8) | 113.0 (37.5‐188.4) | 24.3 (7.5‐146.4) | ||
| Indirect costs | 37 | 4804.2 (3376.2‐6242.4) | 3417.0 (1662.6‐6058.8) | 47.1 (33.1‐61.2) | 33.5 (16.3‐59.4) | ||
| Direct + Indirect costs | 37 | 16 014.0 (7548.0‐24 490.2) | 6507.6 (3498.6‐20 094.0) | 157.0 (74.0‐240.1) | 63.8 (34.3‐197.0) | ||
| Medicine collection | Direct health care costs | ||||||
| Medicines | 135 | 13 300.8 (9608.4‐16 983.0) | 5997.6 (3243.6‐13 198.8) | 130.4 (94.2‐166.5) | 58.8 (31.8‐129.4) | 87.3 | |
| Direct non–health care costs | |||||||
| Transport | 22 | 9690.0 (5385.6‐13 984.2) | 7201.2 (2397.0‐14 402.4) | 95.0 (52.8‐137.1) | 70.6 (23.5‐141.2) | 10.4 | |
| Food | 8 | 5936.4 (4304.4‐7568.4) | 7201.2 (4845.0‐7201.2) | 58.2 (42.2‐74.2) | 70.6 (47.5‐70.6) | 2.3 | |
| Subtotal (direct costs) | 136 | 15 116.4 (11 189.4‐19 043.4) | 8404.8 (3600.6‐15 595.8) | 148.2 (109.7‐186.7) | 82.4 (35.3‐152.9) | ||
| Indirect costs | 163 | 8017.2 (6446.4‐9598.2) | 5273.4 (3419.2‐9373.8) | 78.6 (63.2‐94.1) | 51.7 (33.5‐91.9) | ||
| Direct + Indirect costs | 163 | 20 634.6 (16 503.6‐24 765.6) | 12 739.8 (5803.8‐23 133.6) | 202.3 (161.8‐242.8) | 124.9 (56.9‐226.8) | ||
| Diagnostic visit | Direct health care costs | ||||||
| Test cost | 118 | 5222.4 (2029.8‐8404.8) | 1795.2 (1795.2‐3600.6) | 51.2 (19.9‐82.4) | 17.6 (17.6‐35.3) | 80.8 | |
| Direct non–health care costs | |||||||
| Transport | 21 | 5844.6 (4192.2‐7486.8) | 4804.2 (2397.0‐9598.2) | 57.3 (41.1‐73.4) | 47.1 (23.5‐94.1) | 16.1 | |
| Food | 7 | 3396.6 (1601.4‐5181.6) | 3600.6 (2397.0‐3600.6) | 33.3 (15.7‐50.8) | 35.3 (23.5‐35.3) | 3.1 | |
| Subtotal (direct costs) | 122 | 6252.6 (3111.0‐9384.0) | 1795.2 (1795.2‐3600.6) | 61.3 (30.5‐92.0) | 17.6 (17.6‐35.3) | ||
| Indirect costs | 161 | 4885.8 (4222.8‐5548.8) | 3886.2 (2641.8‐5865.0) | 47.9 (41.4‐54.4) | 38.1 (25.9‐57.5) | ||
| Direct + Indirect costs | 161 | 9618.6 (7068.6‐12 168.6) | 5661.0 (3825.0‐9455.4) | 94.3 (69.3‐119.3) | 55.5 (37.5‐92.7) | ||
| Scheduled clinics | Direct health care costs | ||||||
| User charges | 128 | 4610.4 (2723.4‐6507.6) | 2998.8 (2397.0‐4141.2) | 45.2 (26.7‐63.8) | 29.4 (23.5‐40.6) | 24.1 | |
| Medicines | 122 | 10 159.2 (7170.6‐13 147.8) | 5967.0 (2397.0‐11 995.2) | 99.6 (70.3‐128.9) | 58.5 (23.5‐117.6) | 50.5 | |
| Direct non–health care costs | |||||||
| Transport | 144 | 3957.6 (3325.2‐4579.8) | 2397.0 (1203.6‐4804.2) | 38.8 (32.6‐44.9) | 23.5 (11.8‐47.1) | 23.2 | |
| Food | 11 | 4834.8 (397.8‐9271.8) | 2397.0 (1795.2‐4804.2) | 47.4 (3.9‐90.9) | 23.5 (17.6‐47.1) | 2.2 | |
| Subtotal (direct costs) | 163 | 15 045.0 (12 056.4‐18 023.4) | 9843.0 (4804.2‐17 401.2) | 147.5 (118.2‐176.7) | 96.5 (47.1‐170.6) | ||
| Indirect costs | 163 | 4396.2 (3957.6‐4824.6) | 3519.0 (2611.2‐5865.0) | 43.1 (38.8‐47.3) | 34.5 (25.6‐57.5) | ||
| Direct + Indirect costs | 163 | 19 441.2 (16 207.8‐22 664.4) | 14 259.6 (7476.6‐23 878.2) | 190.6 (158.9‐222.2) | 139.8 (73.3‐234.1) | ||
| Overall costs | Direct health care costs | ||||||
| User charges | 135 | 11 413.8 (6987.0‐15 840.6) | 6293.4 (5467.2‐9251.4) | 111.9 (68.5‐155.3) | 61.7 (53.6‐90.7) | 17.5 | |
| Medicines | 138 | 33 374.4 (25 092.0‐41 656.8) | 19 818.6 (8404.8‐38 403.0) | 327.2 (246.0‐408.4) | 194.3 (82.4‐376.5) | 52.4 | |
| Direct non–health care costs | |||||||
| Transport | 147 | 12 168.6 (9894.0‐14 433.0) | 7599.0 (3702.6‐14 800.2) | 119.3 (97.0‐141.5) | 74.5 (36.3‐145.1) | 22.6 | |
| Food | 31 | 6232.2 (2743.8‐9720.6) | 2397.0 (7956.0‐6497.4) | 61.1 (26.9‐95.3) | 23.5 (78.0‐63.7) | 7.5 | |
| Subtotal (direct costs) | 163 | 53 907.0 (43 625.4‐64 188.6) | 35 802.0 (16 462.8‐59 884.2) | 528.5 (427.7‐629.3) | 351.0 (161.4‐587.1) | ||
| Indirect costs | 163 | 23 174.4 (20 910.0‐25 438.8) | 19 216.8 (13 963.8‐28 417.2) | 227.2 (205.0‐249.4) | 188.4 (136.9‐278.6) | ||
| Direct + Indirect costs | 163 | 77 081.4 (65 820.6‐88 342.2) | 58 415.4 (33 966.0‐90 922.8) | 755.7 (645.3‐866.1) | 572.7 (333.0‐891.4) |
Table 6.
Mean and median annual cost for comorbid (diabetes mellitus and hypertension) patients at five public facilities in Kenya (2017 US$)
| Care‐seeking Episode | Cost Category | n | Mean KES (95% CI) | Median KES (IQR) | Mean US$ (95% CI) | Median US$ (IQR) | As a % of Total Direct Costs |
|---|---|---|---|---|---|---|---|
| Sick visit | Direct health care costs | ||||||
| User charges | 39 | 4720 (3405.9‐6034.1) | 5200 (1300‐6500) | 46.3 (33.4‐59.2) | 51.0 (12.7‐63.7) | 15.2 | |
| Medicines | 39 | 17 880 (4641.7‐31 118.3) | 7670 (3120‐15 600) | 175.3 (45.5‐305.1) | 75.2 (30.6‐152.9) | 57.7 | |
| Direct non–health care costs | |||||||
| Transport | 39 | 7913.3 (3726.5‐12 100.2) | 5200 (2600‐9100) | 77.6 (36.5‐118.6) | 51.0 (25.5‐89.2) | 25.5 | |
| Food | 39 | 486.7 (22.5‐950.8) | 0 | 4.8 (0.2‐9.3) | 0 | 1.6 | |
| Subtotal (direct costs) | 39 | 31 000 (16 546.6‐45 453.4) | 20 800 (10 660‐32 760) | 303.9 (162.2‐445.6) | 203.9 (104.5‐321.2) | ||
| Indirect costs | 39 | 7515.4 (6110.2‐8920.6) | 6856.5 (4063.1‐8888.0) | 73.7 (59.9‐87.5) | 67.2 (39.8‐87.1) | ||
| Direct + Indirect costs | 39 | 38 515.4 (23 247.1‐53 783.7) | 29 688.0 (15 563.4‐41 037.2) | 377.6 (227.9‐527.3) | 291.1 (152.6‐402.3) | ||
| In‐patient admission | Direct health care costs | ||||||
| User charges | 9 | 15 273.9 (287.2‐30 834.9) | 9990 (1000‐14 660) | 149.7 (2.8‐302.3) | 97.9 (9.8‐143.7) | 56.0 | |
| Medicines | 6 | 4545.8 (166.7‐8924.9) | 3598.5 (680‐8500) | 44.6 (1.6‐87.5) | 35.3 (6.7‐83.3) | 11.1 | |
| Direct non–health care costs | |||||||
| Transport | 21 | 2633.3 (592.3‐4674.4) | 1200 (800‐2400) | 25.8 (5.8‐45.8) | 11.8 (7.8‐23.5) | 22.5 | |
| Food | 11 | 2331.8 (589.9‐5253.5) | 600 (300‐2000) | 22.9 (5.8‐51.5) | 5.9 (2.9‐19.6) | 10.4 | |
| Subtotal (direct costs) | 21 | 11 699.5 (2885.6‐20 513.4) | 2750 (1180‐15 960) | 114.7 (28.3‐201.1) | 27.0 (11.6‐156.5) | ||
| Indirect costs | 22 | 4633.5 (3108.5‐6158.5) | 4248.7 (1660.4‐6187.4) | 45.4 (30.5‐60.4) | 41.7 (16.3‐60.7) | ||
| Direct + Indirect costs | 22 | 15 801.2 (6790.6‐24 811.9) | 7149.1 (4060.4‐20 634.9) | 154.9 (66.6‐243.3) | 70.1 (39.8‐202.3) | ||
| Medicine collection | Direct health care costs | ||||||
| Medicines | 78 | 16 269.2 (10 291.7‐22 246.8) | 7200 (3600‐15 000) | 159.5 (100.9‐218.1) | 70.6 (35.3‐147.1) | 88.4 | |
| Direct non–health care costs | |||||||
| Transport | 14 | 8965.7 (4969.6‐12 961.8) | 7200 (4800‐14 400) | 87.9 (48.7‐127.1) | 70.6 (47.1‐141.2) | 8.7 | |
| Food | 7 | 5760 (3876.6‐7643.4) | 7200 (4800‐7200) | 56.5 (38.0‐74.9) | 70.6 (47.1 (70.6) | 2.8 | |
| Subtotal (direct costs) | 79 | 18 162.5 (12 069.3‐24 255.8) | 9120 (4320‐18 000) | 178.1 (118.3‐237.8) | 89.4 (42.4‐176.5) | ||
| Indirect costs | 94 | 8205.7 (6515.2‐9896.2) | 5860.2 (3809.1‐9962.4) | 80.4 (63.9‐97.0) | 57.5 (37.3‐97.7) | ||
| Direct + Indirect costs | 94 | 23 470.0 (17 351.3‐29 588.6) | 14 522.6 (6022.2‐25 785.0) | 230.1 (170.1‐290.1) | 142.4 (59.0‐252.8) | ||
| Diagnostic visit | Direct health care costs | ||||||
| Test cost | 71 | 3643.9 (2132.2‐5155.7) | 1800 (1800‐3600) | 35.7 (20.9‐50.5) | 17.6 (17.6‐35.3) | 70.3 | |
| Direct non–health care costs | |||||||
| Transport | 14 | 6274.3 (4030.7‐8517.8) | 7200 (2400‐9600) | 61.5 (39.5‐83.5) | 70.6 (23.5‐94.1) | 23.9 | |
| Food | 6 | 3560 (1390.9‐5729.1) | 3600 (2400‐3600) | 34.9 (13.6‐56.2) | 35.3 (23.5‐35.3) | 5.8 | |
| Subtotal (direct costs) | 72 | 5110.0 (3282.5‐6937.4) | 1800 (2400‐4080) | 50.1 (32.2‐68.0) | 17.6 (23.5‐40.0) | ||
| Indirect costs | 93 | 5220.8 (4221.9‐6219.6) | 4102.2 (2695.7‐6153.2) | 51.2 (41.4‐61.0) | 40.2 (26.4‐60.3) | ||
| Direct + Indirect costs | 93 | 9176.9 (7078.3‐11 275.4) | 5609.1 (4102.2‐10 060.2) | 90.0 (69.4‐110.5) | 55.0 (40.2‐98.6) | ||
| Scheduled clinics | Direct health care costs | ||||||
| User charges | 73 | 3292.6 (2909.4‐3675.7) | 3000 (2640‐4200) | 32.3 (28.5‐36.0) | 29.4 (25.9‐41.2) | 15.6 | |
| Medicines | 72 | 12 839.2 (7966.7‐17 711.6) | 7200 (3300‐13 200) | 125.9 (78.1‐173.6) | 70.6 (32.4‐129.4) | 59.8 | |
| Direct non–health care costs | 94 | 16 432.2 (12 120.0‐20 744.5) | 10 560 (5640‐17 400) | 161.1 (118.8‐203.4) | 103.5 (55.3‐170.6) | ||
| Transport | 82 | 4013.3 (3125.5‐4901.0) | 2400 (1200‐4800) | 39.3 (30.6‐48.0) | 23.5 (11.8‐47.1) | 21.3 | |
| Food | 10 | 5076 (134.9‐10 017.1) | 2640 (1800‐4800) | 49.8 (1.3‐98.2) | 25.9 (17.6 (47.1) | 3.3 | |
| Subtotal (direct costs) | 94 | 16 432.2 (12 120‐20 744.5) | 10 560 (5640‐17 400) | 161.1 (118.8‐203.4) | 103.5 (55.3‐170.6) | ||
| Indirect costs | 94 | 4632.5 (3985.5‐5279.4) | 3516.1 (2695.7‐5860.2) | 45.4 (39.1‐51.8) | 34.5 (26.4‐57.4) | ||
| Direct + Indirect costs | 94 | 21 064.7 (16 336.1‐25 793.2) | 15 255.1 (8769.4‐24 164.3) | 206.5 (160.2‐252.9) | 149.6 (86.0‐236.9) | ||
| Overall costs | Direct health care costs | ||||||
| User charges | 77 | 8837.2 (6958.2‐10 716.1) | 7500 (5500‐9400) | 86.6 (68.2‐105.1) | 73.5 (53.9‐92.2) | 12.9 | |
| Medicines | 81 | 41 187.2 (27 764.2‐54 610.1) | 21 900 (10 300‐42 700) | 403.8 (272.2‐535.4) | 214.7 (101.0‐418.6) | 63.3 | |
| Direct non–health care costs | |||||||
| Transport | 84 | 12 873.2 (9710.5‐16 035.9) | 8340 (3800‐15 300) | 126.2 (95.2‐157.2) | 81.8 (37.3‐150) | 20.5 | |
| Food | 24 | 7438.3 (2701.2‐12 175.4) | 2300 (725‐10 100) | 72.9 (26.5‐119.4) | 22.5 (7.1‐99.0) | 3.3 | |
| Subtotal (direct costs) | 94 | 59 161.8 (45 440.9‐72 882.8) | 42 750 (17 162‐65 550) | 580.0 (445.5‐714.5) | 419.1 (168.3‐642.6) | ||
| Indirect costs | 94 | 24 137.9 (21 314.9‐26 960.9) | 20 571.8 (15 090.0‐29 984.8) | 236.6 (209.0‐264.3) | 201.2 (147.9‐294.0) | ||
| Direct + Indirect costs | 94 | 83 299.8 (68 223.5‐98 376.0) | 63 970.6 (37 833.6‐100 271.8) | 816.7 (668.9‐964.5) | 627.2 (370.9‐983.1) | ||
3.2.2. Inpatient costs
Twenty‐two percent (n = 37) of the patients in the study reported an inpatient admission in the past year with each admission lasting a mean of 1.6 days (95% CI, 1.3‐1.9). User charges constituted the highest direct costs attracting an estimated KES 16 718.8 (95% CI, 3264‐30 161.4) (US$ 163.9 [95% CI, 32‐295.7]), KES 18 568.6 (95% CI, 11 234.9‐48 372.1) (US$ 182.0 [95% CI, 110.1‐474.2]), and KES 15 273.9 (95% CI, 287.2‐30 834.9) (US$ 149.7 [95% CI, 2.8‐302.3]) mean annual costs in the overall sample, diabetes‐only patients, and comorbid patients, respectively. Additionally, direct costs were higher than indirect costs in all the three groups (Tables 4, 5, 6).
3.2.3. Medicine collection costs
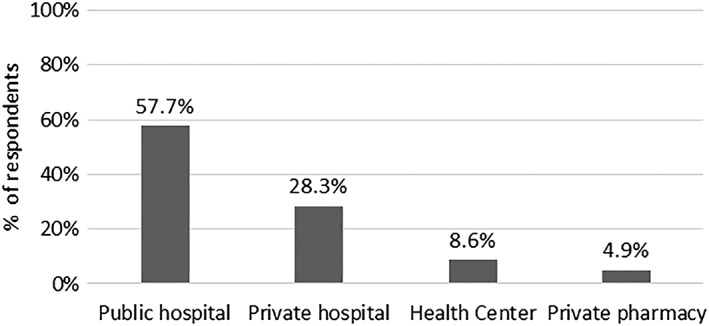
The median number of routine medicines prescribed to diabetes‐only patients was 2 (IQR 1‐2) and 3 (IQR, 3‐4) for comorbid patients. The most expensive antidiabetic regimen was metformin and insulin (mean annual cost KES 15 636.6 (95% CI, 2723.4‐28 539.6) (US$ 153.3 [95% CI, 26.7‐279.8]) while metformin, glibenclamide, and enalapril combinations were the most expensive medicines prescribed to comorbid patients—mean annual cost KES 18 635.4 (95% CI, 3131.4‐40 402.2) (US$ 182.7 [95% CI, 30.7‐396.1]) (Table 7). In addition, more than half (57.7%) of sampled patients reported obtaining their routine medicines from a public hospital (Figure 1). Medicines accounted for 87.3% of total OOP costs during medicine collection visits while transport and food accounted for 10.4% and 2.3% in the overall sample, respectively (Table 4).
Table 7.
Medicines costs
| Drug Name | n | Mean KES (95% CI) | Median KES (IQR) | Mean US$ (95% CI) | Median US$ (IQR) |
|---|---|---|---|---|---|
| Metformin | 16 | 8384.4 (2509.2‐14 259.6) | 3172.2 (897.6‐13 198.8) | 82.2 (24.6‐139.8) | 31.1 (8.8‐129.4) |
| Metformin + insulin | 15 | 15 636.6 (2723.4‐28 539.6) | 10 404.0 (4804.2‐15 004.2) | 153.3 (26.7‐279.8) | 102.0 (47.1‐147.1) |
| Metformin + glibenclamide | 63 | 12 678.6 (6201.6‐19 155.6) | 3835.2 (1438.2‐12 484.8) | 124.3 (60.8‐187.8) | 37.6 (14.1‐122.4) |
| Metformin + glibenclamide + nifedipine | 18 | 17 554.2 (336.6‐34 771.8) | 8160.0 (0.0‐14 881.8) | 172.0 (3.3‐340.9) | 80.0 (0.0‐145.9) |
| Metformin + glibenclamide + enalapril | 15 | 18 635.4 (3131.4‐40 402.2) | 3600.6 (0.0‐16 320.0) | 182.7 (30.7‐396.1) | 35.3 (0.0‐160.0) |
| Metformin + glibenclamide + hydrochlorothiazide | 21 | 13 935.6 (1122‐26 270) | 5395.8 (1438.2‐13 198.8) | 136.6 (11.0‐257.5) | 52.9 (14.1‐90.0) |
| Metformin + nifedipine + hydrochlorothiazide | 11 | 16 218.0 (1530.0‐30 895.8) | 5457.0 (1438.2‐13 198.8) | 159.0 (15.0‐302.9) | 53.5 (14.1‐129.4) |
| Insulin + other antihypertensivesa | 3 | 9557.4 (1315.8‐20 430.6) | 8404.8 (5875.2‐14 402.4) | 93.7 (12.9‐200.3) | 82.4 (57.6‐141.2) |
Atenolol + losartan + amlodipine.
Figure 1.
Source of medicines
3.2.4. Diagnostic/laboratory test costs
The main routine diagnostic tests reported by diabetes‐only patients were fasting blood sugar (FBS) and weight—mean annual cost KES 1499.4 (95% CI, 1030.2‐4029) (US$ 14.7 [95% CI, 10.1‐39.5]) while comorbid patients reported FBS and blood pressure, mean annual cost KES 4518.6 (95% CI, 173.4‐8853.6) (US$ 44.3 [95% CI, 1.7‐86.8]), and echocardiogram and kidney function tests, mean annual cost KES 3335.4 (95% CI, 1081.2‐5589.6) (US$ 32.7 [95% CI, 10.6‐54.8]). The mean annual direct costs for diagnostic/laboratory tests were generally higher for diabetes‐only patients (Table 5) compared with comorbid patients (Table 6). Moreover, 18% (n = 29) of the patients reported incurring costs to purchase of either glucometers or blood pressure monitoring machines, incurring a mean cost of KES 3542.9 (95% CI, 2724.1‐4361.6) (US$ 34.7 [95% CI, 26.7‐42.8]) with additional mean annual cost of KES 16 885.7 (95% CI, 12 377.3‐21 394.1) (US$ 165.5 [95% CI, 121.3‐209.7]) on consumables like glucose strips and lancets.
3.2.5. Scheduled clinic appointment costs
Majority of patients (76.7%, n = 125) attended scheduled clinic visits monthly. Medicines attracted the highest cost in the overall sample, mean annual cost KES 10 159.2 (95% CI, 7170.6‐13 147.8) (US$ 99.6 [95% CI, 70.3‐128.9]), and in the comorbid patients, mean annual cost KES 12 839.2 (95% CI, 7966.7‐17 711.6) (US$ 125.9 [95% CI, 78.1‐173.6]). However, user charges attracted the highest cost, mean annual cost KES 6362.2 (95% CI, 1953.2‐10 771.1) (US$ 62.4 [95% CI, 19.1‐105.6]) in the diabetes‐only group during scheduled clinic appointments. In addition, transport costs accounted for 23.2%, 26.5%, and 21.3% of total direct OOP costs in the overall sample, diabetes‐only, and comorbid patients, respectively (Tables 4, 5, 6).
3.2.6. Overall care‐seeking cost
When costs from all care‐seeking episodes were combined, the average direct annual costs—KES 53 907 (95% CI, 43 625.4‐64 188.6) (US$ 528.5 [95% CI, 427.7‐629.3])—was higher than the average indirect annual costs—KES 23 174.4 (95% CI, 20 910.0‐25 438.8) (US$ 227.2 [95% CI, 205.0‐249.4])—in the overall sample. Of note, medicine costs in the overall sample, in the diabetes‐only patients, and in the comorbid patients attracted the highest costs. This was closely followed by transport costs in the overall sample and in the comorbid patients while user charges were the second highest cost category after medicines in the diabetes‐only group (Tables 4, 5, 6).
3.3. Impact on household income and coping strategies
Costs for DM care services was catastrophic to three quarters (n = 123), 75.5% (95% CI, 68.3‐82.1) of patients at the 10% annual household income threshold. Moreover, comorbid patients (n = 94) realized higher catastrophic costs, 79.8% (95% CI, 71.5‐88.1), compared with diabetes‐only patients (n = 69), 69.6% (95% CI, 58.4‐80.7). Alternatively, when transport costs were excluded, (n = 103) 63.2% (95% CI, 55.7‐70.7) of patients incurred catastrophic costs. Among patients experiencing catastrophe, patients in the lowest wealth quintile incurred higher direct costs with few resources to meet the health care costs (Figure 2). Nonetheless, Figure 3 shows a decreasing proportion of patients experiencing catastrophic costs if the 10% annual income threshold was increased. Patients had to borrow (23.3%) from friends/family, sell an asset (29.9%), and use savings (36.8%) to pay for DM care costs.
Figure 2.
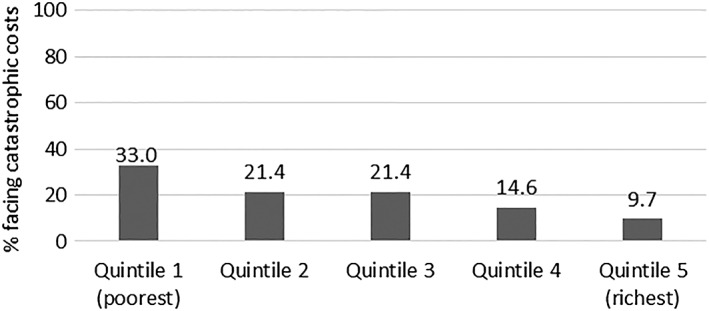
Relationship between catastrophic costs and socio‐economic status
Figure 3.
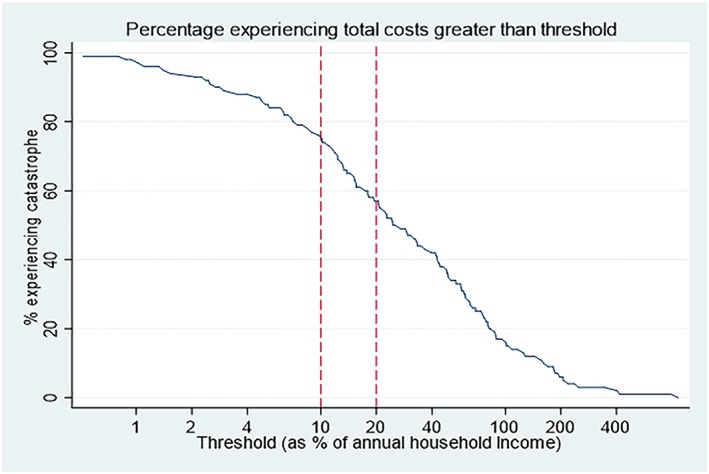
Percentage experiencing total costs greater than threshold [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.4. Productivity and social impact of diabetes mellitus
Patients were asked to report any work days missed due to DM illness in the last 3 months before the survey. Of the 163 patients, 33% reported to miss a median of 21 (IQR, 7‐60) working days. Forty‐five percent of the respondents reported disrupted social life due to DM, affecting sexual life (n = 11), job loss (n = 48), divorce (n = 4), and separation from spouse (n = 6).
4. DISCUSSION
This study has estimated patient costs for adults with DM that sought care at five public health care facilities in two counties in Kenya. The study's main finding is that patient cost for DM are driven by medicine expenses. Similar findings have been reported by recent studies in Kenya and South Africa.19, 34 Costs due to medicine have been shown to reduce adherence to medication and demand for health services by patients with NCDs.35 Past studies conducted in LMICs have however shown that social health insurance schemes do not comprehensively cover the costs for medicines36 and that OOP costs, which are majorly contributed by medicines, are a hindrance to attainment of universal health coverage in many low resource settings.37, 38 Indeed, any reductions or removal of medicine costs is likely to increase access to DM health care services, but additional resources will be required to cover any concomitant increase in service utilization.
The incidence of catastrophic costs documented in this study is arguably high and suggests that DM care in the sampled health facilities is unaffordable to majority of patients, especially those in the lowest wealth quintile whose capacity to pay is limited compared with those in higher socio‐economic group. This is a concern given the high poverty rates in Kenya (36.1%) and that only 19% of Kenya's population have a form of health insurance.39, 40 Furthermore, a past study has shown that families with a member with an NCD incurs three times higher costs compared with families without a member with an NCD.17 Our study has also shown that DM patients reporting hypertension comorbidity incur higher costs overall compared with diabetes‐only patients. This places additional financial burden on families of these patients, similar to findings of previous studies.18, 41
Our results indicate that transport cost offers an access barrier to DM patients given that it takes a significant proportion of total direct costs in all care‐seeking episodes. In part, the high transport costs reported in this study can be attributed to poor quality of care in public health care facilities. For instance, 48.9% of the patients reported lack of medicines and diagnostic facilities as a reason for not visiting nearest facilities. This phenomenon has been observed from studies in Uganda and Zambia.42, 43 Prior studies conducted in Kenya highlight that transport costs are a key access barrier especially to poor patients.38, 44 Moreover, 36% of patients in the overall sample reported a sick visit outside of scheduled clinic appointments incurring an annual mean cost of KES 38 597 (US$ 378.4). Failure in health‐care delivery has been shown to increase the risk of catastrophe, exacerbate socio‐economic iniquities, and reduce the probability of comprehensive treatment.45 These findings therefore reiterate the need for policymakers to develop mechanisms to improve quality of care for diabetic patients in public health facilities since this has serious cost implications on patients. Additionally, since more than three quarters (76.7%) of the patients reported attending their routine scheduled clinics monthly, introducing mechanisms to minimize facility visits, for example, by enhancing and supporting self‐care by patients is likely to reduce transport costs.
The indirect costs due DM care in this study are noteworthy. For instance, of the 163 patients enrolled in the study, 48 (29.5%) had to stop working because of DM and 54 (33%) reported a median loss of 21 working days over the past 90‐day period, which is equal to 1 month's wage in the informal sector in Kenya. Similarly, the overall mean indirect costs in all care‐seeking episodes was KES 23 174 (US$ 227.2) and were primarily contributed by long waiting times at health facilities. It has been shown that long‐waiting times while receiving care reduces demand for chronic care services.46 To achieve optimal efficiency and increase demand for service delivery for patients with chronic illnesses like diabetes, there is urgent need to redesign health service delivery for these patients with a view to making care more patient‐centred to meet the unique needs of these patients.
5. LIMITATIONS
Our study had some limitations. Due to the recall periods, results may be subject to recall bias. Our study only focused on those who utilized health services, thus excluding those with undiagnosed diabetes who may still incur costs due to symptoms associated with diabetes. Also, costs reported in our study could be potentially overestimated since patients were selected purposefully. Consequently, our findings are not nationally representative. Also, our study relied on the diagnosis reported by patients, hence could not distinguish between type 1 and type 2 diabetes. Use of an official minimum wage to estimate productivity losses for all patients could have potentially overestimated indirect costs among patients who were unemployed prior to their illness or underestimated indirect costs among those who were employed.30 These limitations notwithstanding, the findings presented are potentially useful as inputs in costing and/or cost‐effectiveness models that require patient cost and suggest there are significant OOP costs associated with DM management in public facilities in Kenya, which offer a barrier of access to care.
AUTHOR CONTRIBUTIONS
Edwine Barasa, Anthony Etyang, Kenneth Munge, Jane Mbui, Zipporah Bukania, Fredrick Kirui, and Andrew Obala conceived the study. Robinson Oyando, Martin Njoroge, Antipa Sigilai, Kenneth Munge, Peter Nguhiu, and Edwine Barasa contributed to the development of data collection tools. Robinson Oyando collected the data. Robinson Oyando and Edwine Barasa analysed the data. Robinson Oyando developed the first draft of the manuscript. All authors contributed to writing subsequent versions of the manuscript.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
FUNDING SOURCE
This work is funded by a KEMRI internal research grant (IRG‐04) awarded to Anthony Etyang. Additional funds from a Wellcome Trust research training fellowship (#107527) awarded to Edwine Barasa, a Wellcome Trust International Master's Fellowship (#214622) awarded to Robinson Oyando, and a Wellcome Trust core grant (#092654) awarded to the KEMRI‐Wellcome Trust research program supported this work.
ACKNOWLEDGEMENTS
We would like to thank the participants who willingly enrolled in the study as well as county and facility managers who gave permission for the study to be conducted. The field team are sincerely appreciated for their tireless efforts in ensuring the success of this project.
Oyando R, Njoroge M, Nguhiu P, et al. Patient costs of diabetes mellitus care in public health care facilities in Kenya. Int J Health Plann Mgmt. 2020;35:290–308. 10.1002/hpm.2905
REFERENCES
- 1. World Health Organization . Global report on diabetes. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 2. Abdulganiyu G, Fola T. What is the cost of illness of type II diabetes mellitus in a developing economy. Int J Pharm Pharm Sci. 2014;6(Suppl 2):927‐931. [Google Scholar]
- 3. Bahia LR, Araujo DV, Schaan BD, et al. The costs of type 2 diabetes mellitus outpatient care in the Brazilian public health system. Value Health. 2011;14(5):S137‐S140. [DOI] [PubMed] [Google Scholar]
- 4. Bhansali A. Cost of diabetes care: prevent diabetes or face catastrophe. JAPI. 2013;61:9. [PubMed] [Google Scholar]
- 5. Ramachandran A, Ramachandran S, Snehalatha C, et al. Increasing expenditure on health care incurred by diabetic subjects in a developing country: a study from India. Diabetes Care. 2007;30(2):252‐256. [DOI] [PubMed] [Google Scholar]
- 6. Barceló A, Aedo C, Rajpathak S, Robles S. The cost of diabetes in Latin America and the Caribbean. Bull World Health Organ. 2003;81(1):19‐27. [PMC free article] [PubMed] [Google Scholar]
- 7. Suhrcke M, Nugent RA, Stuckler D, Rocco L. Chronic disease: an economic perspective. 2006.
- 8. The Constitution of Kenya , 2010. http://www.icla.up.ac.za/images/constitutions/kenya_constitution.pdf (Accessed 31st December 2018)
- 9. Ministry of Health Kenya . In: Ministry of Health , ed. Kenya Health Policy 2014‐2030: towards attaining the highest standard of health. Nairobi, Kenya: Ministry of Health, Republic of Kenya; 2014. [Google Scholar]
- 10. Government of Kenya . Kenya Service Availablity and Readiness Assessment Mapping (SARAM) Report. Nairobi, Kenya: Ministry of Health; 2013. [Google Scholar]
- 11. Shannon GD, Haghparast‐Bidgoli H, Chelagat W, Kibachio J, Skordis‐Worrall J. Innovating to increase access to diabetes care in Kenya: an evaluation of Novo Nordisk's base of the pyramid project. Glob Health Action. 2019;12(1):1605704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ministry of Health . Kenya STEPwise Survey For Non‐Communicable Diseases Risk Factors 2015 Report. Nairobi: Kenya; 2015.
- 13. Ministry of Health . National Clinical Guidelines for Management of Diabetes Mellitus. Nairobi: Kenya; 2010.
- 14. Ministry of Health . Strategy for Community Health 2014‐2019. Nairobi: Kenya; 2014.
- 15. Ministy of Health . The Kenya Essential Package for Health: the health services persons in Kenya are entitled to, for movement towards attainment of the right to health. Nairobi: Ministry of Health, Secretariat HSR; 2015. [Google Scholar]
- 16. Mohamed SF, Mwangi M, Mutua MK, et al. Prevalence and factors associated with pre‐diabetes and diabetes mellitus in Kenya: results from a national survey. BMC Public Health. 2018;18(3):1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chatterjee S, Riewpaiboon A, Piyauthakit P, Riewpaiboon W. Cost of informal care for diabetic patients in Thailand. Prim Care Diabetes. 2011;5(2):109‐115. [DOI] [PubMed] [Google Scholar]
- 18. Rubin RJ, Altman WM, Mendelson DN. Health care expenditures for people with diabetes mellitus, 1992. J Clin Endocrinol Metabol. 1994;78(4):809A‐809F. [DOI] [PubMed] [Google Scholar]
- 19. Subramanian S, Gakunga R, Kibachio J, et al. Cost and affordability of non‐communicable disease screening, diagnosis and treatment in Kenya: patient payments in the private and public sectors. PLoS ONE. 2018;13(1):e0190113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kapur A. Economic analysis of diabetes care. Indian J Med Res. 2007;125(3):473‐482. [PubMed] [Google Scholar]
- 21. Rayappa P, Raju K, Kapur A, Bjork S, Sylvest C, Kumar KD. Economic cost of diabetes care: the Bangalore urban district diabetes study. Int J Diab Dev Countries. 1999;19(3):87‐86. [Google Scholar]
- 22. Elrayah H, Eltom M, Bedri A, Belal A, Rosling H, Östenson C‐G. Economic burden on families of childhood type 1 diabetes in urban Sudan. Diabetes Res Clin Pract. 2005;70(2):159‐165. [DOI] [PubMed] [Google Scholar]
- 23. Etyang AO, Munge K, Bunyasi EW, et al. Burden of disease in adults admitted to hospital in a rural region of coastal Kenya: an analysis of data from linked clinical and demographic surveillance systems. Lancet Glob Health. 2014;2(4):e216‐e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scott JAG, Bauni E, Moisi JC, et al. Profile: the Kilifi health and demographic surveillance system (KHDSS). Int J Epidemiol. 2012;41(3):650‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Simiyu C, Naanyu V, Obala A, et al. Establishing Webuye Health and Demographic Surveillance Site in rural western Kenya: challenges and lessons learned. Paper presented at: Population Association of America Annual Meeting Problem 2013.
- 26. Bloomfield GS, Mwangi A, Chege P, et al. Multiple cardiovascular risk factors in Kenya: evidence from a health and demographic surveillance system using the WHO STEPwise approach to chronic disease risk factor surveillance. Heart. 2013;99(18):1323‐1329. heartjnl‐2013‐303913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Irimu G, Ogero M, Mbevi G, et al. Tackling health professionals' strikes: an essential part of health system strengthening in Kenya. BMJ Glob Health. 2018;3(6):e001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kirkwood BR, Sterne JA. Essential medical statistics. Chichester, UK: John Wiley & Sons; 2010. [Google Scholar]
- 29. Drummond M. Cost‐of‐illness studies. Pharmacoeconomics. 1992;2(1):1‐4. [DOI] [PubMed] [Google Scholar]
- 30. Ramma L, Cox H, Wilkinson L, et al. Patients' costs associated with seeking and accessing treatment for drug‐resistant tuberculosis in South Africa. Int J Tuberc Lung Dis. 2015;19(12):1513‐1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kenya National Bureau of Statistics . Kenya Economic Survey. 2018.
- 32. Wagstaff A, Flores G, Hsu J, et al. Progress on catastrophic health spending in 133 countries: a retrospective observational study. Lancet Glob Health. 2018;6(2):e169‐e179. [DOI] [PubMed] [Google Scholar]
- 33. OANDA . Corporation. Currency converter New York: OANDA Corporation. Available from: https://www1.oanda.com/currency/converter/. [Accessed on 16 May 2018].
- 34. Mutyambizi C, Pavlova M, Hongoro C, Booysen F, Groot W. Incidence, socio‐economic inequalities and determinants of catastrophic health expenditure and impoverishment for diabetes care in South Africa: a study at two public hospitals in Tshwane. Int J Equity Health. 2019;18(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oyando R, Njoroge M, Nguhiu P, et al. Patient costs of hypertension care in public health care facilities in Kenya. Int J Health Plann Manage. 2019;34(2):1166‐1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bredenkamp C, Buisman LR. Financial protection from health spending in the Philippines: policies and progress. Health Policy Plan. 2016;31(7):919‐927. [DOI] [PubMed] [Google Scholar]
- 37. Opwora A, Waweru E, Toda M, et al. Implementation of patient charges at primary care facilities in Kenya: implications of low adherence to user fee policy for users and facility revenue. Health Policy Plan. 2014;30(4):508‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barasa EW, Maina T, Ravishankar N. Assessing the impoverishing effects, and factors associated with the incidence of catastrophic health care payments in Kenya. Int J Equity Health. 2017;16(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. KNBS . Kenya integrated household budget survey 2017.
- 40. Kazungu JS, Barasa EW. Examining levels, distribution and correlates of health insurance coverage in Kenya. Trop Med Int Health. 2017;22(9):1175‐1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mutyambizi C, Pavlova M, Chola L, Hongoro C, Groot W. Cost of diabetes mellitus in Africa: a systematic review of existing literature. Glob Health. 2018;14(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Criel B, Kirunga C, Mugisha F, Macq J, Nabyonga Orem J. Abolition of user fees: the Uganda paradox. Health Policy Plan. 2011;26(suppl_2):ii41‐ii51. [DOI] [PubMed] [Google Scholar]
- 43. Masiye F, Kaonga O, Kirigia JM. Does user fee removal policy provide financial protection from catastrophic health care payments? Evidence from Zambia. PLoS ONE. 2016;11(1):e0146508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kabia E, Mbau R, Oyando R, et al. “We are called the et cetera”: experiences of the poor with health financing reforms that target them in Kenya 2018. (submitted). [DOI] [PMC free article] [PubMed]
- 45. Laokri S, Drabo MK, Weil O, Kafando B, Dembele SM, Dujardin B. Patients are paying too much for tuberculosis: a direct cost‐burden evaluation in Burkina Faso. PLoS ONE. 2013;8(2):e56752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rayner B, Blockman M, Baines D, Trinder Y. A survey of hypertensive practices at two community health centres in Cape Town. S Afr Med J. 2007;97(4):280‐281. [PubMed] [Google Scholar]


