Abstract
Background:
Alcohol use disorder is a significant societal and medical burden that is associated with both organ pathology and addiction. Excessive alcohol use results in neuroinflammation characterized by activation of the inflammasome, a multiprotein complex, and IL-1β increase in the brain. Recent studies suggest that inflammation could contribute to alcohol addiction. Here, we targeted components of the NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome cascade, which senses and responds to immunologic stimuli, to determine if NLRP3 inhibition modulates alcohol consumption.
Methods:
C57BL/6J male and female mice were provided a two-bottle choice of alcohol at increasing concentrations (3, 6, 9 and 12%, four days each) or water and some were treated with daily injections of an NLRP3 inhibitor (MCC950), a caspase-1 inhibitor (VX765), IL-1 receptor antagonist (IL-1ra; anakinra), or vehicle injection.
Results:
Treatment with VX765, MCC950 and IL-1ra significantly reduced alcohol consumption and preference in female mice (p<0.05). Treatment with MCC950 and IL-1ra reduced alcohol consumption, while IL-1ra reduced alcohol preference in male mice (p<0.05). VX765 did not affect alcohol consumption or preference in male mice.
Conclusions:
These findings highlight gender differences in alcohol preference and demonstrate that inhibition of different steps in inflammasome signaling can reduce alcohol consumption in females. Inhibition of NLRP3 inflammasome activation and the inflammasome-IL-1β cascade opens novel insights into the development of new therapies to address alcohol use disorder in an era of targeted and precision medicine.
Keywords: alcohol, anakinra, NLPR3, caspase-1, interleukin-1 beta
Introduction
Alcohol consumption is prevalent in our society with a quarter of American adults consuming four to five drinks in one sitting, qualifying for binge drinking behavior (National Institute on Alcohol Abuse and Alcoholism, 2017). In the United States, 15 million adults and more than 500,000 adolescents have alcohol use disorder (AUD), which causes the death of 88,000 individuals per year (National Institute on Alcohol Abuse and Alcoholism, 2017). AUD is the third leading preventable cause of death in the US, behind tobacco and poor diet/physical activity (National Institute on Alcohol Abuse and Alcoholism, 2017). Current therapies to treat AUD vary in their therapeutic mechanism of action and include naltrexone (an opioid antagonist), acamprosate (a modulator of neurotransmitter signaling) and disulfiram (an inhibitor of acetaldehyde dehydrogenase)(Peng et al., 2017). However, millions of patients continue to suffer with AUD. Methods to reduce alcohol consumption, including pharmacologic therapies to affect patient behavior, can have a significant impact on relieving a preventable, but often untreated, health burden.
Recent gene expression studies of AUD and the mechanisms involved in disease progression have highlighted the role of immune pathways, suggesting a new avenue of possible candidates for targeted interventions (Liu et al., 2006). Studies in animal models showed that manipulation of various immune pathways could reduce alcohol consumption in mice (Blednov et al., 2012). The reverse has also been demonstrated where inducing systemic inflammation, via peripheral injection of the bacterial wall component lipopolysaccharide (LPS) or with the TLR3 ligand poly(I:C), increased alcohol consumption in mice (Blednov et al., 2011, Warden et al., 2019, Randall et al., 2019). Interestingly, studies in human patients correlate alcohol craving severity with increased markers of systemic inflammation, including cytokines (Leclercq et al., 2012).
We and others have previously identified interleukin-1β (IL-1β) as an important cytokine that is increased in the brain following chronic alcohol feeding in mice (Lippai et al., 2013a, Coleman et al., 2017, Pascual et al., 2017b, Asatryan et al., 2015) and treatment with IL-1 receptor antagonist (IL-1ra) ameliorated alcohol-induced neuroinflammation (Lippai et al., 2013b) as well as hallmarks of alcoholic liver disease (Petrasek et al., 2012). IL-1β production is a result of activation of the intracellular NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome complex (Martinon et al., 2002). The NLRP3 inflammasome is a multiprotein complex including NLRP3 as the sensor, apoptosis-associated speck-like protein (ASC) as the adaptor and caspase-1 (Casp1) as the enzymatic effector. Upon inflammasome activation, Casp1 becomes active and cleaves pro-IL-1β into its active proinflammatory form, IL-1β (Ogura et al., 2006, Szabo and Petrasek, 2015). Preclinical studies have revealed signaling through the NLRP3 inflammasome as a key component in alcohol-induced liver pathology in chronic alcohol mouse models (Iracheta-Vellve et al., 2017, Iracheta-Vellve et al., 2015). We have also shown that mice lacking functional ASC, Casp1 and IL-1 receptor are protected from alcoholic liver disease (Petrasek et al., 2012, Petrasek et al., 2013).
Based on evidence that immune signaling can impact alcohol consumption and the importance of NLRP3 inflammasome in alcohol-induced pathology, we hypothesized that pharmacological targeting of the inflammasome could be an effective approach to modulate AUD. Multiple agents exist for targeting of NLRP3 inflammasome components (Baldwin et al., 2016). MCC950 is a small molecule inhibitor that specifically targets and inhibits the inflammasome sensor NLRP3(Coll et al., 2015). VX765 inhibits the effector protein of the inflammasome, Casp1, blocking its ability to enzymatically activate its cleavage targets, including pro-IL-1β and IL-18 (Wannamaker et al., 2007, Stutz et al., 2009). Anakinra is a recombinant IL-1 receptor antagonist (IL-1ra) capable of binding the IL-1 receptor (IL-1r) without inducing downstream signaling. Thus, IL-1ra acts as a competitive inhibitor of the endogenous IL-1r, thereby blocking the ability of IL-1β, IL-1α and/or IL-1r to carry out inflammation signaling and communication (Bresnihan et al., 1998, Dinarello, 2000).
In this study, we aimed to test the novel hypothesis that inflammasome signaling is an important driver of alcohol consumption. Using the two-bottle choice paradigm with male and female mice, we describe the effects of three inflammasome cascade inhibitors (MCC950, VX765 and anakinra) on alcohol consumption. These data will provide critical insight not only into the role of the inflammasome and inflammation in the behavior associated with alcohol consumption but may also offer rationale for the use of inflammasome inhibitors as adjuvants in clinical alcohol cessation therapy.
Material and Methods
Mice
All animals were cared for in strict accordance with the approved Institutional Animal Care and Use Committee protocol specific to the procedures described in this study at the University of Massachusetts Medical School (Protocol #A-1154–14; G.S.). Wild-type C57BL/6J 6- to 8-weeks-old male and female mice were purchased from Jackson Laboratories and were cohoused in the University of Massachusetts Medical School Animal Medicine Facility for one week prior to the start of the experiment at which time they were singly housed. Mice were maintained on a 12h light/dark cycle with lights on at 7:00 AM. Inhibitor injections and feeder changes occurred each day between 1:00 and 3:00 PM to maintain consistency in mouse handling, feeder replenishment and dosing of inhibitors and to ensure mice had time to resume normal behavior and recover from any stress of handling well before the initial hours of lights off, when alcohol consumption in mice peaks.
Two-bottle choice test
A continuous access (24h access) two-bottle choice between alcohol or drinking water was used to measure alcohol consumption as previously described (Blednov et al., 2005). Briefly, we constructed glass graduated feeder tubes as described (Monell Mouse Taste Phenotyping Project, 1999). Feeders were placed in the cages 2.5 cm from the left wall of the cage or 1 cm from the right pellet food divider with 5 cm between the two bottle feeders. Chow diet was provided ad libitum throughout the experiment. Singly housed mice received one-day acclimation to the new feeders containing water only. Thereafter, one feeder contained alcohol and the other normal drinking water. Mice consecutively received four days each of 3, 6, 9 and 12% (v/v) alcohol in drinking water. Alcohol was made fresh daily to avoid concentration fluctuations due to evaporation. The side of the alcohol and water feeders was altered each day to control for a side preference bias. Alcohol and water volume consumed was measured daily and a control cage without mice was included on each mouse rack to account for spillage of liquid throughout the 24h period. Alcohol consumption was calculated by converting the volume consumed (corrected for the spilled volume from the control cage) to g of alcohol and normalizing to the kg body weight of each mouse. Preference for alcohol was calculated by dividing the volume of alcohol consumed by the total liquid intake for that 24h period (water plus alcohol volume consumed). Total fluid intake was calculated by adding the amount of water and ethanol in g consumed per day and normalizing to the kg body weight of each mouse (this provides the ability to observe variations caused by changes in alcohol consumption as the volume of water consumed daily was much larger than the volume of alcohol solution consumed each day and thus would mask ability to detect changes in total fluid intake). In the event of leakage from a feeder or if cage bedding was stuffed into the feeder by a mouse, data from that cage for that day was excluded from analysis.
Non-Alcohol Tastants
After receiving 12% alcohol, alcohol exposure and inhibitor injections were ceased and mice were allowed to rest for four weeks. Following this rest phase, all inhibitors were restarted and some mice received saccharin (n=3; 0.033% and 0.066% w/v consecutively for four days at each concentration; Sigma, St. Louis, MO) while others received quinine (n=3; 0.03mM and 0.06mM consecutively for four days at each concentration; Sigma, St. Louis, MO) similar to previous studies (Blednov et al., 2011, Blednov et al., 2012). The side of the tastant and water feeders was changed each day to control for a side preference bias. The number of mice included in each treatment group was limited by the amount of treatment compound available for study and therefore the data are provided here to inform the alcohol data without significant conclusions drawn from the non-alcohol tastant data.
Inflammasome Inhibitor Administration
Inhibitors of various inflammasome-related molecules were resuspended, aliquoted and frozen until use. MCC950 (Cayman Chemicals, Ann Arbor, MI) was resuspended with DMSO to a stock concentration of 50mg/mL and diluted to a working concentration of 0.25 mg/mL in 0.5% DMSO in 0.9% saline. VX765 (ApexBio, Houston, TX) was resuspended with DMSO to a stock concentration of 100 mg/mL and diluted to a working concentration of 1 mg/mL in 1% DMSO in 0.9% saline. Anakinra (Kineret™) was purchased in solution at a concentration of 100 mg/0.67 mL and diluted in saline to a working concentration of 2.5 mg/mL. Inhibitors were given by intraperitoneal injection of the following doses: MCC950 5mg/kg body weight; VX765 20mg/kg body weight; anakinra 25mg/kg body weight. 1% DMSO in 0.9% saline was used as a vehicle control injection for MCC950 and VX765 and 0.9% saline was used as a vehicle control for anakinra. Some mice did not receive any injection.
Statistical Analysis
Group sizes were chosen to provide 80% power to detect an effect size of 33%. Statistical analysis was carried out using GraphPad Prism Version 7.0. To test differences between treatment groups, two-way analysis of variance (ANOVA) with post-hoc Bonferroni corrections or student T test and p < 0.05 was considered statistically significant.
Results
NLRP3 inflammasome inhibition with MCC950 reduces alcohol consumption and preference in female mice
Previously, we have shown the importance of the NLRP3 inflammasome and its role in alcoholic liver disease pathology (Iracheta-Vellve et al., 2015, Petrasek et al., 2013) as well as in neuroinflammation (Lippai et al., 2013b). Here, we hypothesize that signaling through the inflammasome may contribute to alcohol consumption. We selected inhibitors of the NLRP3 inflammasome signaling cascade based on their prior use and efficacy in inhibiting inflammation and other aspects of alcohol-induced pathology. These include the NLRP3 inhibitor MCC950 which reduces inflammation in infection models at 5mg/kg (Tate et al., 2016); VX765, an inhibitor of caspase-1 that has effectively reduced neuroinflammation at doses comparable to our study (10–25mg/kg) (Flores et al., 2018); and anakinra (IL-1ra) which we have previously shown inhibits alcohol-induced disease at 25mg/kg (Lippai et al., 2013b, Petrasek et al., 2012). Dose selection was also informed by reductions in alcohol-induced inflammation in preliminary studies (unpublished data). Statistical analyses for consumption, preference and total fluid intake are provided in Supplemental Table 1.
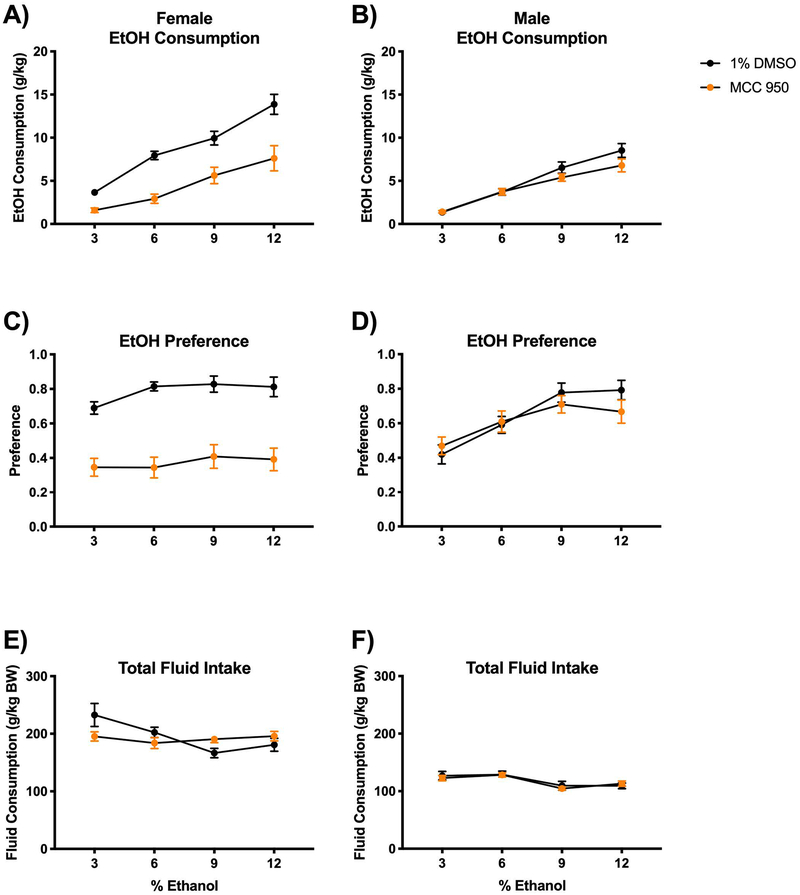
We treated mice with daily i.p. injections of the NLRP3 inhibitor, MCC950. Control mice were injected daily with an equal volume of the vehicle (1% DMSO). We found that female mice treated with MCC950 consistently consumed less alcohol per kg body weight (Fig 1A) and had significantly lower alcohol preference (Fig 1C) compared to vehicle-treated controls (consumption interaction F(3,158)=2.422; P=0.0680 and treatment groups F(1,158)=62.64; P<0.0001; preference interaction F(3,158)=0.4829; P=0.6947 and treatment groups F(1,158) = 117.0; P<0.0001). MCC950-treated male mice consumed less alcohol per kg body weight (consumption interaction F(3,162)=1.536; P=0.2073 and treatment groups F(1,162)=4.048; P<0.05), however they were unaffected by MCC950 treatment in alcohol preference (consumption interaction F(3,162)=0.9879; P=0.4000 and treatment groups F(1,162)=0.6225; P=0.4313) (Fig 1B, D). Total fluid was also measured as grams of ethanol and water consumed and normalized to body weight and unchanged in female and male mice (female interaction F(3,158)=3.833; P=0.0110 and treatment groups F(1,158)=0.3225; P=0.5709; male interaction F(3,162)=0.2753; P=0.8432 and treatment groups F(1,162)=0.1600; P=0.6897) (Fig 1E, F). Weight change following alcohol self-administration was not significantly affected by MCC950 treatment (Supplemental Table 2).
Figure 1. NLRP3 inflammasome inhibitor MCC950 reduces alcohol preference in female mice and consumption in both female and male mice.
Mice were housed singly and received daily intraperitoneal injection of either the NLRP3 inflammasome inhibitor MCC950 (5mg/kg BW; diluted in 0.5% DMSO) or vehicle injection (1% DMSO in 0.9% saline). Two-bottle choice of water or alcohol in drinking water at increasing concentrations (3%, 6%, 9% and 12%) for four days each was provided ad libitum. Consumption was measured and mice were provided fresh water and alcohol daily. A-B) Dose of alcohol consumed at each concentration was normalized to mouse body weight for male and female mice. C-D) Alcohol preference was determined as a ratio of the volume of alcohol consumed to the total liquid volume consumed per day in male and female mice. E-F) Total fluid intake (water plus alcohol) was measured and normalized to body weight in male and female mice. n=6 mice/group. Two-way ANOVA with Bonferroni correction results are described in the text and Supplemental Table 1; error bars depict mean ± SEM.
VX765, a Caspase-1 inhibitor, mildly attenuates alcohol consumption and preference in female mice
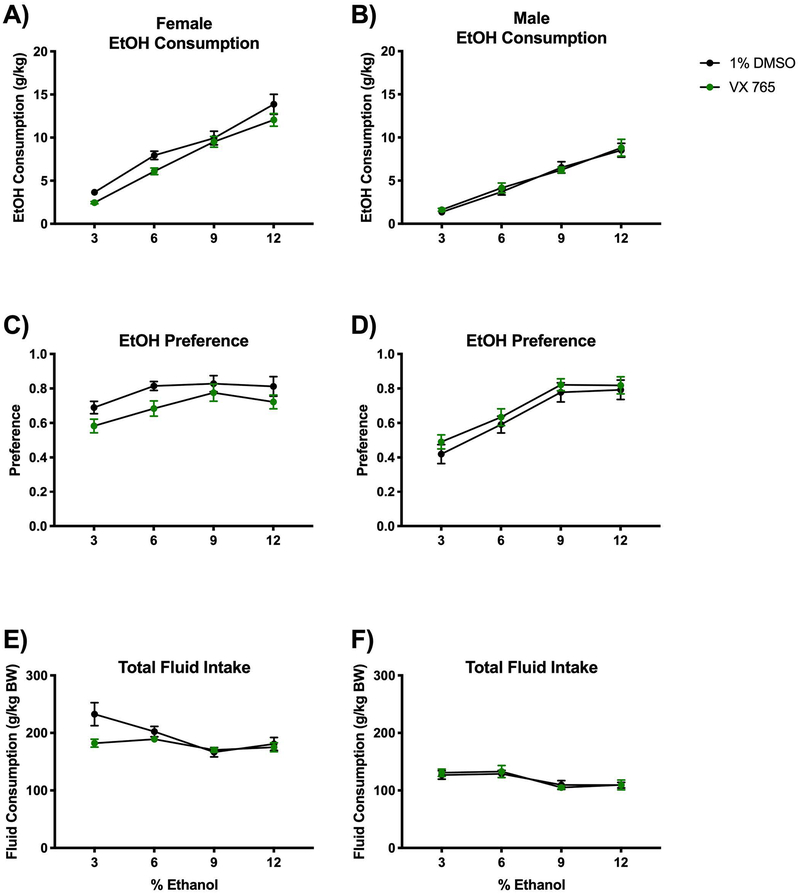
To target the catalytic component of the NLRP3 inflammasome, Caspase-1, we used the inhibitor VX765 and compared with vehicle-treated (1% DMSO) mice. Daily VX765 treatment reduced alcohol consumption and alcohol preference in female mice (consumption interaction F(3,160)=0.6647; P=0.5749 and treatment groups F(1,160=10.00; P=0.0019; preference interaction F(3,160)=0.3174; P=0.8128 and treatment groups F(1,160)=9.525; P=0.0024) (Fig 2A, C). Alcohol consumption and preference were unaffected in male mice treated with the Casp1 inhibitor, VX765 (consumption interaction F(3,160)=0.1883; P=0.9043 and treatment groups F(1,160)=0.2050; P=0.6513; preference interaction F(3,160)=0.06727; P=0.9772 and treatment groups F(1,160)=1.749; P=0.1879) (Fig 2B, D). Total fluid was also measured as grams of ethanol and water consumed and normalized to body weight and VX765 modestly reduced total fluid intake in female but not male mice (female interaction F(3,160)=3.162; P=0.0262 and treatment groups F(1,160)=5.737; P=0.0178; male interaction F(3,160)=0.1572; P=0.9249 and treatment groups F(1,160)=0.03091; P=0.8607) (Fig 2E, F). Weight change following alcohol self-administration was not significantly affected by VX765 treatment in female mice, although VX765-treated males gained less than vehicle controls (mean 2.4±0.74 vs. 3.7±1.05 g, P=0.035; Supplemental Table 2).
Figure 2. Caspase-1 inhibition with VX765 mildly reduces alcohol consumption.
Mice were housed singly and received daily treatment with either a Caspase-1 inhibitor VX765 (20mg/kg BW; diluted in 1% DMSO) or vehicle injection (1% DMSO in 0.9% saline). Two-bottle choice of water or alcohol in drinking water at various concentrations (3%, 6%, 9% and 12%) for four days each was provided ad libitum. Consumption was measured and mice were provided fresh water and alcohol daily. A-B) Dose of alcohol consumed at each concentration was normalized to mouse body weight for male and female mice. C-D) Alcohol preference was determined as a ratio of the volume of alcohol consumed to the total liquid volume consumed per day in male and female mice. E-F) Total fluid intake (water plus alcohol) was measured and normalized to body weight in male and female mice. NB: Saline/DMSO data replotted from Figure 2. n=6 mice/group. Two-way ANOVA with Bonferroni correction results are described in the text and Supplemental Table 1; error bars depict mean ± SEM.
Treatment with anakinra reduces alcohol consumption and preference in both female and male mice
Secretion of the effector molecules IL-1β and IL-18 is the ultimate step of inflammasome activation. We have previously highlighted the important role of IL-1β in alcohol-induced liver disease (Petrasek et al., 2012, Iracheta-Vellve et al., 2017) and neuroinflammation (Lippai et al., 2013b). Anakinra (recombinant IL-1ra) is currently in clinical trials for numerous diseases including severe acute alcoholic hepatitis (). We found that female mice treated with anakinra consumed less alcohol and had lower alcohol preference than vehicle-treated (saline) animals (consumption interaction F(3,157)=0.2019; P=0.8950 and treatment groups F(1,157)=4.114; P=0.0442; preference interaction F(3,157)=1.174; P=0.3217 and treatment groups F(1,157)=14.73; P=0.0002) (Fig 3A, C). Male mice also showed a reduction in alcohol consumption and preference (consumption interaction F(3,167)=0.1909; P=0.9025 and treatment groups F(1,167)=39.83; P<0.0001; preference interaction F(3,167)=2.707; P=0.0470 and treatment groups F(1,167)=13.12; P=0.0004) (Fig 3B, D). These results indicate that inhibition of IL-1 receptor signaling with the IL-1ra, anakinra, can attenuate alcohol consumption in both genders in mice. Total fluid was unchanged in female and decreased in male mice (female interaction F(3,157)=0.5514; P=0.6479 and treatment groups F(1,157)=1.350; P=0.2471; male interaction F(3,167)=0.7967; P=0.4973 and treatment groups F(1,167)=42.26; P<0.0001) (Fig 3E, F). Weight change following alcohol self-administration was not significantly affected by anakinra treatment (Supplemental Table 2). Complete statistical two-way ANOVA analysis results of conditions presented in Fig 1–3 is provided in Supplemental Table 1.
Figure 3. Recombinant IL-1 receptor antagonist, anakinra, reduces alcohol consumption in both female and male mice.
Mice were housed singly and received daily treatment with either a recombinant IL-1 receptor antagonist anakinra (25mg/kg BW) or vehicle injection (0.9% saline). Two-bottle choice of water or alcohol in drinking water at various concentrations (3%, 6%, 9% and 12%) for four days each was provided ad libitum. Consumption was measured and mice were provided fresh water and alcohol daily. A-B) Dose of alcohol consumed at each concentration was normalized to mouse body weight for male and female mice. C-D) Alcohol preference was determined as a ratio of the volume of alcohol consumed to the total liquid volume consumed per day in male and female mice. E-F) Total fluid intake (water plus alcohol) and normalized to body weight in male and female mice. NB: Saline/DMSO data replotted from Figure 2. n=6 mice/group. Two-way ANOVA with Bonferroni correction results are described in the text and Supplemental Table 1; error bars depict mean ± SEM.
Consumption of sweet and bitter tastants is mildly affected by inflammasome cascade inhibitor treatment
In order to test if inhibitor treatment had an effect on consumption based on taste or other factors, we provided two alternate non-alcoholic tastants in a follow up experiment. At the conclusion of the last 12% alcohol exposure, mice were rested for 30 days then some mice were then provided choice of either water versus sweet tastant (saccharin) or water versus bitter tastant (quinine). Mice that had previously received daily inhibitor or vehicle injections again received the same treatments. The number included in each treatment group was limited by the amount of treatment compound and therefore the data are provided here to inform the alcohol data, without significant conclusions drawn from the non-alcohol tastant data.
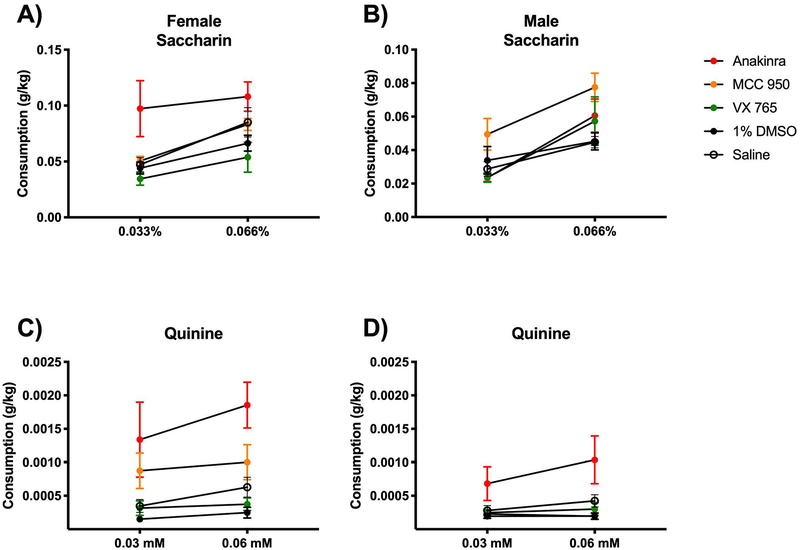
Although inhibitor treatments reduced alcohol consumption and preference, particularly in female mice, treatments with MCC950 and VX765 did not tend to reduce consumption of saccharin (Fig 4A–B) or quinine (Fig 4C–D) in either female or male mice compared with 1% DMSO vehicle treatment. Rather, anakinra tended to increase 0.033% saccharin consumption in female mice compared to control saline-treated mice. Anakinra also tended to increase 0.06mM quinine consumption in male and female mice compared to saline vehicle controls. These results suggest that while some inhibitors were effective at reducing alcohol consumption, they also have nonspecific effects leading to an increase in other tastants.
Figure 4. Inhibitors did not reduce consumption of non-alcoholic tastants including saccharin (sweet) or quinine (bitter).
A-B) Female and male mice were provided two-bottle choice between drinking water and saccharin in drinking water at two doses (0.033 and 0.066%) for four days each. C-D) A different cohort of female and male mice were provided two-bottle choice between drinking water and quinine in drinking water at two doses (0.03 and 0.06mM) for four days each. In all cases (A-D), some mice received daily treatment with anakinra (25mg/kg BW), MCC950 (5mg/kg BW), VX765 (20mg/kg BW) or vehicle injection (1% DMSO in 0.9% saline). n=3 mice/group. Error bars depict mean ± SEM.
Daily injection and handling modestly effects alcohol consumption
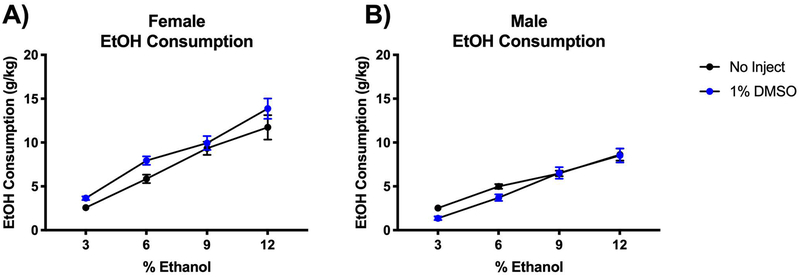
To investigate whether the stress of daily handling and injection impacted alcohol consumption, we compared mice injected daily with the vehicle to mice that were not injected and not regularly handled. Daily injection of vehicle modestly increased alcohol consumption in female mice (F(1,154) = 8.79; P=0.0035) (Fig 5A). Male mice alcohol consumption was not affected by daily injection (F(1,161) = 3.640; P=0.0582) (Fig 5B). This important observation suggests that, particularly in female mice who were most impacted by inflammasome inhibition, daily handling and injecting did not in and of itself reduce alcohol consumption.
Figure 5. Daily injection had modest effect on male and female mice alcohol consumption.
Two-bottle choice of water or alcohol in drinking water at various concentrations (3%, 6%, 9% and 12%) for four days each was provided ad libitum. Consumption was measured and mice were provided fresh water and alcohol daily. Some mice were handled daily and received injection of vehicle (1% DMSO in 0.9% saline) while other mice were untreated. A-B) Dose of alcohol consumed at each concentration was normalized to mouse body weight for female and male mice. n=6 mice/group. Two-way ANOVA with Bonferroni correction results are described in the text; error bars depict mean ± SEM.
Discussion
The molecular regulatory pathways that promote alcohol consumption and preference are only partially understood. While most studies focused on neuromodulators and neuronal functions, recent evidence suggests a potential role for inflammatory mediators in alcohol addiction (Blednov et al., 2011, Blednov et al., 2012). Based on previous work revealing the importance of the NLRP3 inflammasome in alcohol-related pathology as well as early evidence that inhibiting immune signaling can affect alcohol consumption, in this study we hypothesized that inhibiting the inflammasome would reduce alcohol consumption in mice. We found that female, but not male, mice reduce their alcohol consumption and preference when treated with the NLRP3 inflammasome inhibitor MCC950, the caspase-1 inhibitor VX765, or with IL-1ra, anakinra, even in a model that does not significantly elevate neuroinflammatory cytokines, including IL-1β. This suggests that targeting inflammasome signaling impacts alcohol consumption behavior. These findings emphasize the utility of the two-bottle test for behavioral assessments and provide novel insights into the role of the inflammasome pathway in alcohol use disorder and addiction.
Multiple alcohol consumption models exist for rodent experiments and each provides utility depending on the questions being tested. For example, the chronic 5% alcohol in Lieber-DeCarli liquid diet model induces liver pathology including steatosis, hepatocyte damage and mild inflammation (Wilkin et al., 2016, Bala et al., 2017, Bukong et al., 2016) as well as extrahepatic pathology including neuroinflammation(Lippai et al., 2013a). Our previous research showed that mice deficient in either ASC, Casp1 or IL-1ra were protected from liver pathology in the chronic alcohol feeding model (Petrasek et al., 2012). We found that administration of the IL-1 receptor antagonist, anakinra, in the chronic alcohol feeding model effectively attenuated alcohol-induced liver disease (Petrasek et al., 2012) and neuroinflammation in mice (Lippai et al., 2013b). Recently, we have also shown that an acute-on-chronic alcohol model (daily alcohol consumption plus an acute alcohol binge) induces neuroinflammation, including microglial activation and proinflammatory cytokine expression, while also altering the expression of inflammasome components (such as NLRP3 and ASC) in the CNS(Lowe et al., 2018). Here, we chose to use pharmacologic inhibitors with therapeutic potential to show that targeting this same pathway significantly reduces alcohol consumption in female mice. Importantly, our study reveals that targeting the inflammasome can reduce the development of alcohol self-administration. Future behavioral assays using mice deficient in inflammasome components, and particularly cell-specific deletion of inflammasome component signaling within the CNS and periphery, may yield supporting evidence for targeting this pathway in human patients with AUD and provide insight into the critical cellular sources of inflammasome signaling that contribute to alcohol drinking behavior.
Our findings are consistent with other studies that have used genetic and pharmacologic approaches to influence addiction behavior (Blednov et al., 2005, Blednov et al., 2012, Harris et al., 2017, Mayfield et al., 2016) and establish the inflammasome as a novel target for further research and potential AUD treatment. In order to induce systemic inflammation and liver disease, chronic alcohol or high-dose binges, not included in our present study, are often required (Ambade et al., 2018, Posteraro et al., 2018). However, we did see a significant effect of inflammasome inhibitors on alcohol consumption despite this relatively low alcohol exposure paradigm. This could suggest that signaling through the inflammasome at levels below our detection ability or within a very small population of cells is important for alcohol-seeking behavior. It would be these small populations or subtle signaling changes that are targeted by inhibitors in our present study leading to behavioral changes. Longer-term models of two-bottle choice offer intermittent alcohol with alcohol-free days in between. Studies after thirty days of alcohol (sixty days total) have revealed significant changes in microglia and astrocyte gene expression with significantly upregulated microglial immune signaling (McCarthy et al., 2018, Erickson et al., 2018) and may be an interesting alternative model to investigate with inflammasome inhibition in order to achieve more significant inflammation. Indeed, these models may offer avenues to study expression of inflammasome components and other pro-inflammatory biochemical signals in the setting of alcohol and inhibitor treatments, measurements we were unable to assess in this current self-administration paradigm. Although in our present study we did not note any sensitization or tolerance developed to inflammasome inhibitors across time during treatment (for example, during the four days at each alcohol concentration), a longer-term study would afford more time to observe possible effects such as drug tolerance on alcohol self-administration.
The experimental design of our study focuses on disrupting the development of alcohol self-administration. This represents an important limitation of our study in that we did not test the efficacy of inflammasome inhibition on already established alcohol self-administration (i.e., initiating inhibitor treatment after mice were already exposed to alcohol drinking). This will be an important follow up study to evaluate inflammasome inhibition as a useful approach in human alcohol behavior. In our study, it is also important to note the limitation due to use of DMSO as a vehicle for treatment administration. DMSO has documented effects on inflammation that may influence our target pathways (the inflammasome cascade) and downstream pro-inflammatory signaling (Verheijen et al., 2019). Additionally, we used 0.5% and 1% DMSO to optimally dilute MCC950 and VX765, respectively, and 1% DMSO was used as a common vehicle control for these groups. Given the effect of DMSO on inflammation, these differences in concentration are important to note.
Here we describe a reduction in alcohol consumption and preference in female mice treated with various inflammasome inhibitors. In addition to the effect on alcohol consumption and preference, we also noted an effect on the total fluid intake of alcohol and water by those same groups of female mice, namely those treated with MCC950 and anakinra. In those groups, total fluid consumption was moderately increased, while both voluntary alcohol consumption and preference for alcohol was decreased. Similar effects have been observed in previous studies. For example, Blednov et al. described effects on tastant and total fluid consumption in multiple groups (Blednov et al., 2005). To extrapolate these data clinically, an off-target effect such as increased water intake is of much less significance than an impact of decreasing alcohol consumption and seeking. Interestingly, anakinra also tended to increase the consumption of non-alcohol tastants saccharin and quinine. These are important off-target (i.e., non-alcohol) effects that warrant monitoring if inflammasome inhibitors are to be used experimentally or clinically in the future to alter alcohol consumption.
Interestingly, our previous studies focused on female mice in order to study liver disease because females have been shown to have greater sensitivity to alcohol and develop more severe alcohol-induced liver pathology than males (Iimuro et al., 1997, Frezza et al., 1990, Ikejima et al., 1998). Sex differences related to alcohol-induced pathology have been previously demonstrated and evidence suggests that males and females respond differently to alcohol exposure (Pascual et al., 2017a, Iimuro et al., 1997, Ikejima et al., 1998, Alfonso-Loeches et al., 2013, Yardley et al., 2012). Indeed, women have a stronger immune response and more prominent organ damage than males after alcohol consumption (Pascual et al., 2017a, Iimuro et al., 1997, Ikejima et al., 1998). Neurotoxicity and immune activation is greater in female mice as well (Alfonso-Loeches et al., 2013). Interestingly, IL-1ra knockout mice exhibit reduced alcohol preference in female and male mice, with female mice showing a stronger alcohol-aversive behavior (Blednov et al., 2012). Given these sex differences, it is not surprising to observe differential behavior in male and female animals when treated with immune inhibitors. Knowledge of these differences may be critical to development of future therapeutics for alcohol use disorder and may be important in developing more personalized medicine approaches to treating addiction and disease.
While the gut-liver axis is well established in alcoholic liver disease and alcohol-related liver inflammation as a result of alcohol-induced gut leakiness (Szabo, 2015), communication between the intestine, liver and brain, which may influence alcohol consumption, continues to be an active area of research (Leclercq et al., 2017, Starkel et al., 2018). We have recently demonstrated that by reducing the intestinal microbiome using broad-spectrum antibiotics in mice can significantly protect from inflammation and immune cell activation in both the liver and in the CNS (Lowe et al., 2017, Lowe et al., 2018). Alcohol consumption in the chronic Lieber-DeCarli 5% alcohol feeding model is often unaffected by genotype or treatment, likely due to the fact that this model delivers alcohol along with a liquid food diet so all calorie intake includes alcohol consumption. For this reason, the two-bottle choice provides an important model to measure behavioral changes that cannot be adequately assessed in other models of AUD.
Targeting inflammatory pathways has previously been shown to modulate alcohol consumption. Blednov et al. showed that alcohol consumption and preference were reduced in multiple drinking models in mice lacking functional inflammation-related genes such as CD14 and IL-6 (Blednov et al., 2012). Harris et al. (Harris et al., 2017) recently showed that TLR4 manipulation in mice and rats had only modest effects on alcohol consumption, however, female TLR4 knockout rats had reduced alcohol preference compared to wild-type animals. These observations are consistent with our finding that inhibition of the inflammatory cytokine circuit attenuates alcohol consumption. It is interesting that both IL-1ra knockout mice (Blednov et al., 2012) and mice treated with anakinra in this present study (recombinant IL-1ra) and elsewhere (Marshall et al., 2016) would exhibit similar alcohol-aversive behavior. It is possible that the total body knockout of IL-1ra, which would be knocked out throughout the animal’s lifetime and development, compared with the temporally-limited i.p. dosing of anakinra could induce different behavioral outcomes. It may also be the case that systemic administration of concentrated anakinra, which has been shown to cross the blood-brain barrier, has an effect different from that of knocking out physiologically normal IL-1ra expression (Shavit et al., 2005). Indeed, anakinra has previously been shown to be effective at reducing alcohol-induced sedation and improving recovery from alcohol-induced motor impairment in mice (Wu et al., 2011) and specific basolateral amygdala infusion of IL-1ra into male mice reduces alcohol binge drinking (Marshall et al., 2016). These results are somewhat confounding as a reduction in sedation and motor dysfunction would seem to allow mice more access and ability to consume alcohol, however our study and others (Marshall et al., 2016) suggests IL-1ra treatment disrupts this behavior despite evidence in the literature of improved motor function (Wu et al., 2011). Indeed, multiple follow up behavioral studies using inflammasome inhibitors in the setting of alcohol exposure are warranted including locomotor function (rotarod, cage hang, etc.), memory testing (memory mazes, novel object, etc.), effect on reducing self-administration after dependence has been established, other addiction studies and more. Assessing the effects of both IL-1ra and other inflammasome inhibitors, such as MCC950 and VX765, will be critical to fully understand how manipulating various immune signals can alter alcohol-related behavior and function.
The link between immune signaling and a behavioral response to alcohol exposure was highlighted in a study by Blednov et al.(Blednov et al., 2011) LPS, a potent TLR4 ligand naturally found on the surface of gram-negative bacteria, was injected in mice who were then provided access to alcohol. LPS injection produced a prolonged alcohol preference and mice deficient in CD14, an important component of the TLR4 receptor complex that recognizes LPS, were protected from this preference induction. In the same study, investigators examined firing rates of dopaminergic neurons in the ventral tegmental region of the brain, a key area in the reward pathway. LPS injection reduced firing rates in these neurons which could lead to an increased threshold for a rewarding dopamine response. These experiments tie together TLR4 immune signaling, neurochemical reward pathways and alcohol consumption (Blednov et al., 2011).
Our present study adds insight into a parallel pathway, the NLRP3 inflammasome, that also influences alcohol preference. Inhibition of NLRP3 inflammasome activation prevents production of IL-1β that is a central amplifier of pro-inflammatory responses and cytokine production (Tilg et al., 2016). Unlike other pro-inflammatory cytokines (such as TNFα, IL-6 or CCL2) that are induced by a single danger signal through NF-kB activation, IL-1β production requires a secondary danger signal and inflammasome activation (Szabo and Petrasek, 2015). This unique regulatory feature of IL-1 highlights the importance of inflammasome activation in alcohol consumption and preference. Indeed, a recent study showed that blockade of IL-1β signaling in the ventral tegmental area (VTA), a critical area in the mesolimbic reward circuitry, reduced dopamine release in response to cocaine exposure (Northcutt et al., 2015), which may contribute to addiction and seeking more reward from a given drug. While the exact signaling mechanism involved has not been fully described, these data highlight an important role for IL-1β and immune signaling in addiction.
Our observations further highlight the potential significance of inflammation pathways in alcohol addiction and provide evidence for new therapeutic interventions to attenuate alcohol preference. It remains to be evaluated whether these gender differences and/or inflammasome pathways play a role in reducing alcohol consumption in humans. The need for more effective therapies to both curb alcohol consumption and treat alcoholic liver disease remains high. Medications that can both reduce consumption and alleviate some of the organ pathology associated with chronic intake would be a significant advancement for the field of alcohol treatment. Targeting the inflammasome may serve this purpose and further research will be needed to show the efficacy of blocking organ disease and reducing consumption.
Conclusion
Here, we show that blocking the NLRP3 inflammasome at multiple points reduces alcohol consumption and preference in female but not in male mice. Our novel findings add new insights into the role of the inflammasome and offer promising new targets for treating alcohol use disorder.
Supplementary Material
Acknowledgements
Authors thank Dr. Liu of the University of Massachusetts Morphology Core for expert histology preparation and staining. The authors are grateful for the assistance of Candice Dufour with the preparation and submission of the manuscript.
Funding
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award numbers 5R01AA017729-05 (to G.S.) and F30AA024680 (to P.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- AUD
alcohol use disorder
- LPS
lipopolysaccharide
- IL-1β
interleukin-1β
- IL-1ra
IL-1 receptor antagonist
- NLRP3
NOD-like receptor family pyrin domain containing 3
- ASC
apoptosis-associated speck-like protein
- Casp1
caspase-1
- IL-1ra
IL-1 receptor antagonist
- IL-1r
IL-1 receptor
- 2-BC
two-bottle choice
- VTA
ventral tegmental area
Footnotes
Data Availability: All data generated or analyzed during this study are included in this published article.
Declaration of interest: The authors declare that they have no competing interests
Ethical Approval and Consent to participate
All animals were cared for in strict accordance with the approved Institutional Animal Care and Use Committee protocol specific to the procedures described in this study at the University of Massachusetts Medical School (Protocol #A-1154-14; G.S.)
References
- ALFONSO-LOECHES S, PASCUAL M & GUERRI C 2013. Gender differences in alcohol-induced neurotoxicity and brain damage. Toxicology, 311, 27–34. [DOI] [PubMed] [Google Scholar]
- AMBADE A, LOWE P, KODYS K, CATALANO D, GYONGYOSI B, CHO Y, IRACHETA VELLVE A, ADEJUMO A, SAHA B, CALENDA C, MEHTA J, LEFEBVRE E, VIG P & SZABO G 2018. Pharmacological inhibition of CCR2/5 signaling prevents and reverses alcohol-induced liver damage, steatosis and inflammation in mice. Hepatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASATRYAN L, KHOJA S, RODGERS KE, ALKANA RL, TSUKAMOTO H & DAVIES DL 2015. Chronic ethanol exposure combined with high fat diet up-regulates P2X7 receptors that parallels neuroinflammation and neuronal loss in C57BL/6J mice. J Neuroimmunol, 285, 169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALA S, CSAK T, KODYS K, CATALANO D, AMBADE A, FURI I, LOWE P, CHO Y, IRACHETA-VELLVE A & SZABO G 2017. Alcohol-induced miR-155 and HDAC11 inhibit negative regulators of the TLR4 pathway and lead to increased LPS responsiveness of Kupffer cells in alcoholic liver disease. J Leukoc Biol, 102, 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALDWIN AG, BROUGH D & FREEMAN S 2016. Inhibiting the Inflammasome: A Chemical Perspective. J Med Chem, 59, 1691–710. [DOI] [PubMed] [Google Scholar]
- BLEDNOV YA, BENAVIDEZ JM, GEIL C, PERRA S, MORIKAWA H & HARRIS RA 2011. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun, 25 Suppl 1, S92–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLEDNOV YA, BERGESON SE, WALKER D, FERREIRA VM, KUZIEL WA & HARRIS RA 2005. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behav Brain Res, 165, 110–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLEDNOV YA, PONOMAREV I, GEIL C, BERGESON S, KOOB GF & HARRIS RA 2012. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol, 17, 108–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRESNIHAN B, ALVARO-GRACIA JM, COBBY M, DOHERTY M, DOMLJAN Z, EMERY P, NUKI G, PAVELKA K, RAU R, ROZMAN B, WATT I, WILLIAMS B, AITCHISON R, MCCABE D & MUSIKIC P 1998. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. Arthritis Rheum, 41, 2196–204. [DOI] [PubMed] [Google Scholar]
- BUKONG TN, IRACHETA-VELLVE A, SAHA B, AMBADE A, SATISHCHANDRAN A, GYONGYOSI B, LOWE P, CATALANO D, KODYS K & SZABO G 2016. Inhibition of spleen tyrosine kinase activation ameliorates inflammation, cell death, and steatosis in alcoholic liver disease. Hepatology, 64, 1057–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLEMAN LG JR., ZOU J, QIN L & CREWS FT 2017. HMGB1/IL-1beta complexes regulate neuroimmune responses in alcoholism. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLL RC, ROBERTSON AA, CHAE JJ, HIGGINS SC, MUNOZ-PLANILLO R, INSERRA MC, VETTER I, DUNGAN LS, MONKS BG, STUTZ A, CROKER DE, BUTLER MS, HANEKLAUS M, SUTTON CE, NUNEZ G, LATZ E, KASTNER DL, MILLS KH, MASTERS SL, SCHRODER K, COOPER MA & O’NEILL LA 2015. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med, 21, 248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINARELLO CA 2000. The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med, 343, 732–4. [DOI] [PubMed] [Google Scholar]
- ERICKSON EK, FARRIS SP, BLEDNOV YA, MAYFIELD RD & HARRIS RA 2018. Astrocyte-specific transcriptome responses to chronic ethanol consumption. Pharmacogenomics J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLORES J, NOEL A, FOVEAU B, LYNHAM J, LECRUX C & LEBLANC AC 2018. Caspase-1 inhibition alleviates cognitive impairment and neuropathology in an Alzheimer’s disease mouse model. Nat Commun, 9, 3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREZZA M, DI PADOVA C, POZZATO G, TERPIN M, BARAONA E & LIEBER CS 1990. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med, 322, 95–9. [DOI] [PubMed] [Google Scholar]
- HARRIS RA, BAJO M, BELL RL, BLEDNOV YA, VARODAYAN FP, TRUITT JM, DE GUGLIELMO G, LASEK AW, LOGRIP ML, VENDRUSCOLO LF, ROBERTS AJ, ROBERTS E, GEORGE O, MAYFIELD J, BILLIAR TR, HACKAM DJ, MAYFIELD RD, KOOB GF, ROBERTO M & HOMANICS GE 2017. Genetic and Pharmacologic Manipulation of TLR4 Has Minimal Impact on Ethanol Consumption in Rodents. J Neurosci, 37, 1139–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IIMURO Y, FRANKENBERG MV, ARTEEL GE, BRADFORD BU, WALL CA & THURMAN RG 1997. Female rats exhibit greater susceptibility to early alcohol-induced liver injury than males. Am J Physiol, 272, G1186–94. [DOI] [PubMed] [Google Scholar]
- IKEJIMA K, ENOMOTO N, IIMURO Y, IKEJIMA A, FANG D, XU J, FORMAN DT, BRENNER DA & THURMAN RG 1998. Estrogen increases sensitivity of hepatic Kupffer cells to endotoxin. Am J Physiol, 274, G669–76. [DOI] [PubMed] [Google Scholar]
- IRACHETA-VELLVE A, PETRASEK J, GYOGYOSI B, BALA S, CSAK T, KODYS K & SZABO G 2017. Interleukin-1 inhibition facilitates recovery from liver injury and promotes regeneration of hepatocytes in alcoholic hepatitis in mice. Liver Int, 37, 968–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRACHETA-VELLVE A, PETRASEK J, SATISHCHANDRAN A, GYONGYOSI B, SAHA B, KODYS K, FITZGERALD KA, KURT-JONES EA & SZABO G 2015. Inhibition of sterile danger signals, uric acid and ATP, prevents inflammasome activation and protects from alcoholic steatohepatitis in mice. J Hepatol, 63, 1147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LECLERCQ S, CANI PD, NEYRINCK AM, STARKEL P, JAMAR F, MIKOLAJCZAK M, DELZENNE NM & DE TIMARY P 2012. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav Immun, 26, 911–8. [DOI] [PubMed] [Google Scholar]
- LECLERCQ S, DE TIMARY P, DELZENNE NM & STARKEL P 2017. The link between inflammation, bugs, the intestine and the brain in alcohol dependence. Transl Psychiatry, 7, e1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIPPAI D, BALA S, CSAK T, KURT-JONES EA & SZABO G 2013a. Chronic alcohol-induced microRNA-155 contributes to neuroinflammation in a TLR4-dependent manner in mice. PLoS One, 8, e70945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIPPAI D, BALA S, PETRASEK J, CSAK T, LEVIN I, KURT-JONES EA & SZABO G 2013b. Alcohol-induced IL-1beta in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J Leukoc Biol, 94, 171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU J, LEWOHL JM, HARRIS RA, IYER VR, DODD PR, RANDALL PK & MAYFIELD RD 2006. Patterns of gene expression in the frontal cortex discriminate alcoholic from nonalcoholic individuals. Neuropsychopharmacology, 31, 1574–82. [DOI] [PubMed] [Google Scholar]
- LOWE PP, GYONGYOSI B, SATISHCHANDRAN A, IRACHETA-VELLVE A, AMBADE A, KODYS K, CATALANO D, WARD DV & SZABO G 2017. Alcohol-related changes in the intestinal microbiome influence neutrophil infiltration, inflammation and steatosis in early alcoholic hepatitis in mice. PLoS One, 12, e0174544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWE PP, GYONGYOSI B, SATISHCHANDRAN A, IRACHETA-VELLVE A, CHO Y, AMBADE A & SZABO G 2018. Reduced gut microbiome protects from alcohol-induced neuroinflammation and alters intestinal and brain inflammasome expression. J Neuroinflammation, 15, 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSHALL SA, CASACHAHUA JD, RINKER JA, BLOSE AK, LYSLE DT & THIELE TE 2016. IL-1 receptor signaling in the basolateral amygdala modulates binge-like ethanol consumption in male C57BL/6J mice. Brain Behav Immun, 51, 258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTINON F, BURNS K & TSCHOPP J 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell, 10, 417–26. [DOI] [PubMed] [Google Scholar]
- MAYFIELD J, ARENDS MA, HARRIS RA & BLEDNOV YA 2016. Genes and Alcohol Consumption: Studies with Mutant Mice. Int Rev Neurobiol, 126, 293–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCARTHY GM, FARRIS SP, BLEDNOV YA, HARRIS RA & MAYFIELD RD 2018. Microglial-specific transcriptome changes following chronic alcohol consumption. Neuropharmacology, 128, 416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONELL MOUSE TASTE PHENOTYPING PROJECT. 1999. Construction of graduated drinking tubes [Online]. Monell Chemical Senses Center. Available: http://www.monell.org/MMTPP/Drinkingtubes.htm [Accessed 6 Jun 2018].
- NATIONAL INSTITUTE ON ALCOHOL ABUSE AND ALCOHOLISM. 2017. Alcohol Facts and Statistics [Online]. Available: https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/alcohol-facts-and-statistics [Accessed 5 Jun 2018].
- NORTHCUTT AL, HUTCHINSON MR, WANG X, BARATTA MV, HIRANITA T, COCHRAN TA, POMRENZE MB, GALER EL, KOPAJTIC TA, LI CM, AMAT J, LARSON G, COOPER DC, HUANG Y, O’NEILL CE, YIN H, ZAHNISER NR, KATZ JL, RICE KC, MAIER SF, BACHTELL RK & WATKINS LR 2015. DAT isn’t all that: cocaine reward and reinforcement require Toll-like receptor 4 signaling. Mol Psychiatry, 20, 1525–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGURA Y, SUTTERWALA FS & FLAVELL RA 2006. The inflammasome: first line of the immune response to cell stress. Cell, 126, 659–62. [DOI] [PubMed] [Google Scholar]
- PASCUAL M, MONTESINOS J, MARCOS M, TORRES JL, COSTA-ALBA P, GARCIA-GARCIA F, LASO FJ & GUERRI C 2017a. Gender differences in the inflammatory cytokine and chemokine profiles induced by binge ethanol drinking in adolescence. Addict Biol, 22, 1829–1841. [DOI] [PubMed] [Google Scholar]
- PASCUAL M, MONTESINOS J, MONTAGUD-ROMERO S, FORTEZA J, RODRIGUEZ-ARIAS M, MINARRO J & GUERRI C 2017b. TLR4 response mediates ethanol-induced neurodevelopment alterations in a model of fetal alcohol spectrum disorders. J Neuroinflammation, 14, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENG JL, PATEL MP, MCGEE B, LIANG T, CHANDLER K, TAYARACHAKUL S, O’CONNOR S & LIANGPUNSAKUL S 2017. Management of alcohol misuse in patients with liver diseases. J Investig Med, 65, 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETRASEK J, BALA S, CSAK T, LIPPAI D, KODYS K, MENASHY V, BARRIEAU M, MIN SY, KURT-JONES EA & SZABO G 2012. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest, 122, 3476–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETRASEK J, IRACHETA-VELLVE A, CSAK T, SATISHCHANDRAN A, KODYS K, KURT-JONES EA, FITZGERALD KA & SZABO G 2013. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc Natl Acad Sci U S A, 110, 16544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTERARO B, PARONI STERBINI F, PETITO V, ROCCA S, CUBEDDU T, GRAZIANI C, ARENA V, VASSALLO GA, MOSONI C, LOPETUSO L, LORRAI I, MACCIONI P, MASUCCI L, MARTINI C, GASBARRINI A, SANGUINETTI M, COLOMBO G & ADDOLORATO G 2018. Liver Injury, Endotoxemia, and Their Relationship to Intestinal Microbiota Composition in Alcohol-Preferring Rats. Alcohol Clin Exp Res, 42, 2313–2325. [DOI] [PubMed] [Google Scholar]
- RANDALL PA, VETRENO RP, MAKHIJANI VH, CREWS FT & BESHEER J 2019. The Toll-Like Receptor 3 Agonist Poly(I:C) Induces Rapid and Lasting Changes in Gene Expression Related to Glutamatergic Function and Increases Ethanol Self-Administration in Rats. Alcohol Clin Exp Res, 43, 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAVIT Y, WOLF G, GOSHEN I, LIVSHITS D & YIRMIYA R 2005. Interleukin-1 antagonizes morphine analgesia and underlies morphine tolerance. Pain, 115, 50–9. [DOI] [PubMed] [Google Scholar]
- STARKEL P, LECLERCQ S, DE TIMARY P & SCHNABL B 2018. Intestinal dysbiosis and permeability: the yin and yang in alcohol dependence and alcoholic liver disease. Clin Sci (Lond), 132, 199–212. [DOI] [PubMed] [Google Scholar]
- STUTZ A, GOLENBOCK DT & LATZ E 2009. Inflammasomes: too big to miss. J Clin Invest, 119, 3502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABO G 2015. Gut-liver axis in alcoholic liver disease. Gastroenterology, 148, 30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABO G & PETRASEK J 2015. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol, 12, 387–400. [DOI] [PubMed] [Google Scholar]
- TATE MD, ONG JDH, DOWLING JK, MCAULEY JL, ROBERTSON AB, LATZ E, DRUMMOND GR, COOPER MA, HERTZOG PJ & MANSELL A 2016. Reassessing the role of the NLRP3 inflammasome during pathogenic influenza A virus infection via temporal inhibition. Sci Rep, 6, 27912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TILG H, MOSCHEN AR & SZABO G 2016. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology, 64, 955–65. [DOI] [PubMed] [Google Scholar]
- VERHEIJEN M, LIENHARD M, SCHROODERS Y, CLAYTON O, NUDISCHER R, BOERNO S, TIMMERMANN B, SELEVSEK N, SCHLAPBACH R, GMUENDER H, GOTTA S, GERAEDTS J, HERWIG R, KLEINJANS J & CAIMENT F 2019. DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Sci Rep, 9, 4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANNAMAKER W, DAVIES R, NAMCHUK M, POLLARD J, FORD P, KU G, DECKER C, CHARIFSON P, WEBER P, GERMANN UA, KUIDA K & RANDLE JC 2007. (S)-1-((S)-2-{[1-(4-amino-3-chloro-phenyl)-methanoyl]-amino}−3,3-dimethyl-butanoy l)-pyrrolidine-2-carboxylic acid ((2R,3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765), an orally available selective interleukin (IL)-converting enzyme/caspase-1 inhibitor, exhibits potent anti-inflammatory activities by inhibiting the release of IL-1beta and IL-18. J Pharmacol Exp Ther, 321, 509–16. [DOI] [PubMed] [Google Scholar]
- WARDEN AS, AZZAM M, DACOSTA A, MASON S, BLEDNOV YA, MESSING RO, MAYFIELD RD & HARRIS RA 2019. Toll-like receptor 3 dynamics in female C57BL/6J mice: Regulation of alcohol intake. Brain Behav Immun, 77, 66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKIN RJ, LALOR PF, PARKER R & NEWSOME PN 2016. Murine Models of Acute Alcoholic Hepatitis and Their Relevance to Human Disease. Am J Pathol, 186, 748–60. [DOI] [PubMed] [Google Scholar]
- WU Y, LOUSBERG EL, MOLDENHAUER LM, HAYBALL JD, ROBERTSON SA, COLLER JK, WATKINS LR, SOMOGYI AA & HUTCHINSON MR 2011. Attenuation of microglial and IL-1 signaling protects mice from acute alcohol-induced sedation and/or motor impairment. Brain Behav Immun, 25 Suppl 1, S155–64. [DOI] [PubMed] [Google Scholar]
- YARDLEY MM, WYATT L, KHOJA S, ASATRYAN L, RAMAKER MJ, FINN DA, ALKANA RL, HUYNH N, LOUIE SG, PETASIS NA, BORTOLATO M & DAVIES DL 2012. Ivermectin reduces alcohol intake and preference in mice. Neuropharmacology, 63, 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.