Abstract
The pressure on healthcare budgets including laboratory medicine is relentless and the focus on activities and costs remains the dominant funding model of laboratory medicine everywhere. The limitations of this model are well documented and for a decade or more laboratory professions worldwide have started looking at alternative models where the value of laboratory medicine and its impact on patient outcomes are the predominant driving force. There are multiple ways to determine the value of a medical test, particularly if one takes into consideration its impact upon the complete clinical pathway. Thus various approaches to value determination are being explored by a number of international organisations. These organisations will be reviewed below, including one which uses the concept of a value proposition that describes in detail how a test should be implemented by measuring its clinical, operational and economic impact. All approaches for determination of value require professional leadership. There is a need for research of varying types including that related to translating global evidence into local practice, a key challenge facing laboratory medicine and healthcare generally. Another challenge is to think and act beyond the silo of the laboratory to achieve greater collaboration with those colleagues more directly involved in patient care.
Introduction
For more than a decade there has been an ongoing debate and focus on delivering greater value in healthcare.1 This has been driven primarily by the need to restrain ongoing increases in healthcare budgets but also by factors such as the growing recognition of what patients desire and need – so-called patient-centred healthcare.2 These demands also apply to laboratory medicine and there is a considerable literature on what value-based laboratory medicine is or should be, and how it might be achieved.
It might be argued that the debate on value started in laboratory medicine even before the debate in healthcare more widely. Advances in analytical and information technology have led to significant improvements in the productivity of laboratories over several decades. While this has undoubtedly contributed to improvements in patient care, it has also led to a focus on the analytical activities and costs within the laboratory itself rather than a broader consideration of the impact of testing on patient care.3 While there are obvious and well-known difficulties in identifying and quantifying medical tests as an intervention, the so called commoditisation of laboratory and medical testing in general has been lamented by many in the profession and these concerns have appeared in the literature for several decades.4
In recent years the profession has made the claim that laboratory medicine contributes to 70% of clinical decisions. The provenance and accuracy of this claim is unknown but however value is defined, it is clear that laboratory medicine forms a part of many clinical decisions. Furthermore, the various aspects of laboratory testing which contribute value were comprehensively summarised in a previous David Curnow Plenary Lecture delivered by Ken Sikaris in 2016 and subsequently published.5 The review by Sikaris identified how the many activities of what might collectively be called best practice laboratory medicine, contribute to the overall value of testing with particular emphasis on the importance of the requesting and reporting processes to the overall value.5
Despite all of the above we continue to lack the systematic or hard evidence of value that can be attributed to testing, a point highlighted by Hallworth et al in the report from the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Task Force on the Impact of Laboratory Medicine on Clinical Management and Outcomes.6 As part of its investigations and recommendations, the task force identified work in five specific areas that could maximise the value of laboratory medicine. Some of these areas will be touched upon later in this review.
Value can be defined in many ways and will depend upon the perspective of the various healthcare stakeholders including the patient. Most perspectives of value involve making some form of economic analysis and such analyses are central to some of the concepts and initiatives that will be discussed below in relation to laboratory medicine. However, while they will be referred to in relation to aspects such as evidence and the implementation of tests, the tools of economic analyses will not be the focus of this review.
The review will firstly provide an overview of some specific international initiatives which have been described in the literature and are broadly aimed at increasing the value of laboratory medicine. One of these, the IFCC and World Association of Societies of Pathology and Laboratory Medicine (WASPaLM) Committee for the Value Proposition in Laboratory Medicine, will be described in some detail. This will be followed by an overview of value-based initiatives which are taking place in Australia. The review will conclude with some of the challenges associated with identifying and improving the value that laboratory medicine brings to patient care.
International Value-Based Initiatives in Laboratory Medicine
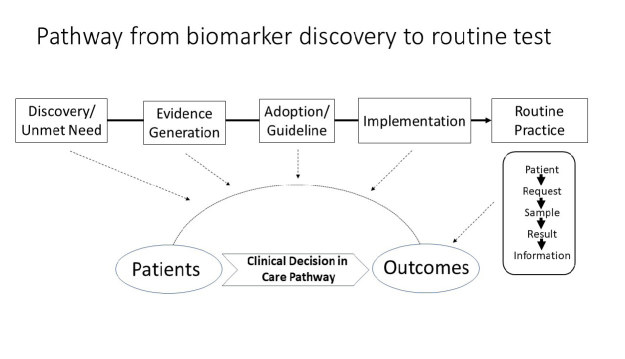
The pathway by which a newly discovered biomarker is developed, evaluated and implemented as a routine test can be illustrated in the diagram shown as Figure 1. Although the process is in reality much more complex than shown here, it can be considered as a number of discrete stages such as evidence generation, adoption and implementation, all of which involve testing the biomarker in the clinical pathway until there is sufficient evidence to justify its use in routine testing (shown on the right-hand-side of Figure 1). The value-based initiatives discussed below address various parts of this overall process, including two that operate in relation to routine testing and two at earlier processes in this pathway.
Figure 1.
Complete process from biomarker discovery through test evaluation to adoption and implementation of routine testing.
While all of these initiatives can be seen as focusing on the value of individual tests, it is important to emphasise that it is about the value of such tests for individual patients and not the test in isolation. In other words, we must always consider the clinical circumstances in which the test has been used in order to determine its value.
Diagnostic Harm and The Testing Process
This initiative applies to routine testing and has been led primarily by Paul Epner through the Society to Improve Diagnosis in Medicine.7 The core concept is to demonstrate the value of laboratory medicine by showing the harms that can occur to patients from diagnostic errors which may occur with sub-optimal laboratory testing. In advocating for this approach Epner argues that healthcare providers and funders take the quality of laboratory medicine for granted and that only by demonstrating the risks associated with poor-quality testing, can the true value of high-quality testing be demonstrated.8 Accordingly, Epner and colleagues have developed a taxonomy of the causes of diagnostic error:
an inappropriate test is ordered
an appropriate test is not ordered
an appropriate test result is misapplied
an appropriate test is ordered but a delay occurs somewhere in the total testing process
the result of an appropriately ordered test is inaccurate.
Using this taxonomy to identify and classify errors, Epner proposes that the value of laboratory medicine (V) can be described as the ‘aggregate delivered benefits (B) offset by both the harms delivered (H) and the missed opportunities (MO)’; so in summary: V = (B – H – MO).9 Epner subsequently reported on the development of a framework using the above concepts which would define the structures and processes that are required for a high value laboratory service but following this publication, further details of this framework have yet to appear.
Clinical Lab 2.0
A second initiative based on routine testing is called the Clinical Lab 2.0 or the Santa Fe Project.10 The driving force behind this project has been major changes to US healthcare facilitating adoption of a more value-based reimbursement system. In response, regional laboratory networks have collaborated to find new value-based practices by moving away from the transactional model of just providing individual results, and towards providing additional information from data analysis of the large numbers of results that are produced on a daily basis. This initiative principally operates through a yearly conference which includes presentations and workshops and provides delegates with updates and education on this new value-based approach.10
One of the project’s specific initiatives is to encourage laboratories to harness the power of longitudinal data, both in a single patient where it can provide additional clinical insights and in multiple patients where the data can be used as tool in population health management. This data analysis approach has been applied to clinical problems where gaps in care have been previously demonstrated, such as the identification and tracking of the well-being of pregnant women in the Medicaid population and the identification of acute kidney injury (AKI) during hospital admission. Publications describing this work also cite the challenges associated with conducting this value-based analysis, including the key requirement of laboratory information systems and other IT infrastructure that are fit for purpose, and the need for laboratory professionals to have data analysis skills.11,12 These challenges are common to the whole area of value-based laboratory medicine and will be discussed further in the concluding section of this review.
EFLM Test Evaluation Working Group
The Test Evaluation Group of the European Federation of Laboratory Medicine (EFLM) came into existence in 2012 and was developed to improve the overall efficiency of bringing newly discovered biomarkers to commercial reality and everyday use in routine testing. This test evaluation process is currently poorly performed with reports of wasted expenditure extending to billions of dollars.13 The working group aims to provide practical tools to help all groups involved with test evaluation including researchers and laboratory professionals. One of these tools is a checklist to determine the unmet clinical need of a proposed new test. This is a critical step early in the test evaluation process, and when performed correctly, will help correctly identify which biomarkers should be further evaluated and which should be rejected.14
Other important activities of this working group include identifying that the test evaluation process is not a simple linear sequence but is in fact a more complex, interactive and iterative process where the analytical and clinical performance requirements for a test are driven by the clinical pathway in which the test is intended to be used.15 The original scoping paper of the working group also includes a useful glossary of terms which should be disseminated and used more widely in an effort to curb the multiplicity and confusion of terms used by the various professional groups in this area.
The current focus of the working group is to contribute to the process of introducing the new European IVD Directive for medical tests. When finalised, the directive will require a more extensive clinical evaluation of tests prior to their introduction into routine testing. A full list of the working group’s terms of reference and achievements to date can be found on the EFLM website.16
IFCC-WASPaLM Committee for the Value Proposition in Laboratory Medicine
This value-based initiative also focuses on the test development pathway prior to routine testing, but on different parts to the EFLM initiative, namely the processes of adoption and implementation (Figure 1). Technology adoption is poorly implemented in healthcare which results in wasted money, even when there is good clinical and economic evidence for a test.17 Poor translation into effective local practice is manifested in a number of ways including variability in testing across healthcare systems in the United Kingdowm and in Australia.18,19
To improve the process of test adoption and implementation, Price et al have proposed using a concept from the business world called a value proposition. The term is utilised by businesses to demonstrate to prospective customers in a detailed and explicit way, the full range of benefits, costs and value that an organisation can deliver through its product or service. Translating this concept to a medical test, the value proposition can be defined as: ‘The provision of information to enable clinicians and other stakeholders to make better decisions about the care of individual patients’.20 This information can be presented as a series of questions, the answers to which describe in detail all the relevant aspects of the test that will enable it to be adopted and implemented effectively into local practice. This information is known as the Value Proposition Framework and is shown in the Box.21
Box.
Complete framework for the description of a value proposition for a medical test (ref. 21).
The unmet clinical need: this represents a definition of the problem and is complemented by the impact on clinical, operational and economic outcomes.
Patient population that will benefit: this will include gender, age and setting in which problem arises, including in the home, primary care, paramedical vehicle, emergency department and other hospital settings, e.g. intensive care.
Identity of the test and its properties: this will include the name of the test and a statement of the basic pathology with which it is associated, reference intervals or clinical decision cut-off values, biological variation and expected analytical performance.
Test intervention purpose: screening, diagnosis, prognosis, risk stratification and/or monitoring.
Expected outcomes: clinical, process and/or resource utilisation.
Location where test is performed: laboratory and/or point of care setting, e.g. home, primary care, ambulatory hospital clinic, paramedical vehicle, hospital department.
Quality of evidence available: results from formal trials, observational studies, systematic review and meta-analysis.
Part(s) of the care pathway in which the test will be used: linked with test purpose.
Stakeholders involved in delivering and receiving the care identified in the care pathway; the potential beneficiaries.
Benefits to each stakeholder in relation to the outcomes identified above: again clinical, operational and/or economic benefits linked with the stated outcome measures.
Potential limitations and risks that might be associated with introduction of the test, and a proposed mitigation strategy: this could be relevant to all the beneficiary stakeholders and may cover clinical, operational and economic outcomes.
Resource/activity contributed by each of the service lines involved in the care pathway with and without the test intervention.
Statement of the reimbursement received for delivering the care pathway with and without (before and after) the test intervention.
A proposed implementation plan including the metrics for monitoring appropriate adoption.
The IFCC’s Committee for the Value Proposition in Laboratory Medicine (C-VPLM) was set up to apply this value proposition concept to a number of different tests and then to ask laboratories to evaluate whether the concept helps with better test implementation. Another goal of the Committee is to develop some practical tools laboratory professionals can use as part of better test implementation including economic modelling.
To date the Committee has published the application of the value proposition framework to a number of different tests where information and evidence has been provided to answer all the questions in the framework.22–25 In effect, information to complete questions 1–7 of the framework are essentially the principles of evidence based medicine while steps 8–14 are primarily focused on how the test will be implemented on a local basis. With further development and wider application, it is likely that the framework will not need to be so detailed and could be reduced to the key components which contribute to better test adoption and implementation, which may vary with the particular test.
Three of the key components will be highlighted here. First is that of the clinical pathway and how the new test will change that pathway (Question 8 of the framework). For example, in the case of NT-proBNP the evidence and associated guidelines indicate that if the test result is lower than a specified cut-off then heart failure can be ruled out and other causes of the patient’s symptoms need to be investigated. Thus, measures should be included in the implementation plan which will monitor whether the newly changed pathway is being adhered to in accordance with the guideline.23
The second critical component of the framework is consideration of all the stakeholders who will be involved in the pathway and the benefits to these stakeholders. To use the example of high-sensitive troponin, the application of the test has the potential to bring benefits to the patient, ED physician and healthcare provider through faster discharge of some patients but there is also the possibility that another key stakeholder, the cardiologist, may receive more referrals and this may be perceived as a disbenefit which may affect the overall benefits of the test. The balance of these benefits and disbenefits must be considered and will determine whether the test is successfully implemented.22
The third component to consider is the Implementation Plan and the metrics within that plan. This is related to the previous critical components discussed above where there needs to be a monitoring process in place to assess adherence to the clinical pathway and to the impact on stakeholders. There are likely to be other additional measures of whether the test is being effective which need to be incorporated into the plan. A review of the literature on the use of point-of-care testing for glycated haemoglobin revealed a wide range of clinical, operational and economic measures across all the reviewed papers. While the change in HbA1c level featured in nearly all the papers, only very few contained measures to assess the economic benefits and were thus unable to show that the testing was cost effective.24
To summarise this section of the review, there are a number of specific initiatives being conducted in various countries to more strongly identify value in laboratory medicine. There are no doubt others which have not been mentioned here, and while there will be some overlap with all these different approaches, a plurality of approach is probably beneficial. As mentioned previously, value can be defined in many different ways and therefore multiple approaches to identify and quantify value will be required. It is important to emphasise that these initiatives supplement and do not replace existing processes conducted as part of good laboratory practice and contribute to value, albeit in a general and largely unquantifiable way, as summarised by Sikaris and others in the literature.5 The challenge for all such new value-based initiatives is to go beyond what might be called the academic and theoretical, and to get them evaluated, possibly modified based on practical experience and implemented to see whether they improve the potential value of testing.
Value-Based Pathology Initiatives in Australia
The concerns expressed in the introduction regarding the commoditisation of laboratory testing worldwide are also relevant to Australia. The need for a more value-based service formed the basis of an opinion piece published in 2015 by members of the profession and IVD industry.4 Unrelated to this publication, but at about the same time, the Australian Government announced a major review of the fee schedule which determines which services are reimbursed, including pathology tests.26
In response to these events, the Australasian Association of Clinical Biochemists (AACB), with support from the Federal Government, organised a one day workshop to review the various value-based initiatives taking place around the world, including those mentioned above, and to determine how the pathology profession should respond in order to achieve a more value-orientated practice in Australia. The Value of Pathology Workshop included a broad range of healthcare professionals who had some interaction with pathology practice. Following presentations, the facilitated audience discussion generated 10 initiatives from which two were selected to be developed and are discussed further below. The presentations and a summary report from the workshop can be found on the AACB website.19
Value of Pathology Initiative - Centre of Excellence for Research into the Value of Pathology
Within the Australian pathology system there is data on test usage but its validity is often disputed. The nature of the reimbursement system also prevents easy access to accurate data. Furthermore, there is very little information on how tests are used and the outcomes they generate. Such information might be gained from clinical audit processes but these are rarely conducted by Australian laboratories. Thus, a clear outcome from the workshop was a need for greater knowledge and insight into the impact and value of testing. It was recommended that a dedicated research centre should be set up for this purpose. The high level aims of such a centre would be:
a better understanding of the relationship between pathology data and patient outcomes; and
development of relevant research capacity and skills in laboratory professionals.
At this point in time, some two years after the workshop, it is unclear whether such a research centre will be established but the need for it remains and the type of research which might be performed in such a centre is illustrated by a recent albeit rare audit into vitamin D testing. A report published by an endocrinologist and public health physician in 2013 identified what they believed to be excessive requesting for 25-hydroxy vitamin D (25-OHD).27 This led to the Australian government, which reimburses pathology testing in the primary care sector, restricting the indications available for 25-OHD testing. A year or so after their introduction, the same authors published data showing that the changes had led to a reduction in testing and accordingly claimed the intervention was successful.28
A more recent audit of testing conducted by a large private pathology laboratory in Queensland over a longer period of time shows that 25-OHD testing is now back to the same levels as before the intervention, as shown in Figure 2. A similar finding has been shown from another research study conducted in a different group of general practitioners to the Queensland study.29 Furthermore, the data from the Queensland audit suggests that following the intervention less people with vitamin D deficiency are being detected than before the changes in guidelines. The reasons for this are unclear and it might relate to more people taking supplementation since the intervention but this is believed to be unlikely in this testing population. Leaving this issue aside, the overall conclusion from this audit is that the intervention to reduce testing has been a failure and this is perhaps not surprising given there is ample evidence in the literature of similarly failed interventions in the test demand management area.30
Figure 2.
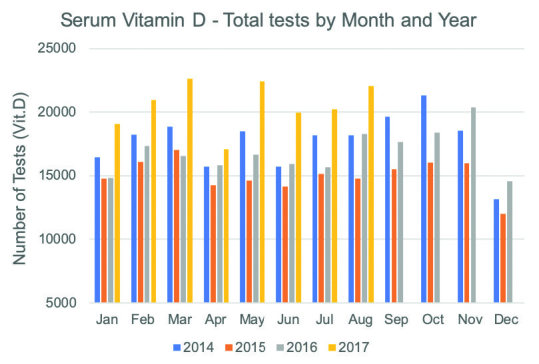
Vitamin D test requests at an Australian pathology practice by month from 2014–2017.
The broader lessons from this example are, first, to show the value of audit-like processes and the need for more of them. Second, they should ideally be conducted by the profession as part of our responsibility to highlight where our testing is failing, whether it is over or under testing, and where it is failing to identify the correct patients, as suggested in the study above. Finally, designing better interventions to optimise test requesting and conducting follow-up studies to measure their effectiveness would appear to be one of the core activities that could be conducted in the putative Centre of Excellence for Research into the Value of Pathology.
Value of Pathology Initiative – Improved Clinical Decision Support for Test Requesters
Improving the value of laboratory testing clearly starts with requesting the correct test. Thus, the second initiative to come out of the Value of Pathology Workshop was a commitment to provide better clinical decision support (CDS) to requesters and particularly to general practitioners. Developing such software has also been a key priority of the Diagnostic Medicine Clinical Committee of the Medicare Benefits Schedule Review mentioned previously. One of the activities within the committee was to commission a literature survey of interventions designed to improve the appropriateness of requesting and of nine possible mechanisms identified, the use of CDS was judged to have the most supportive evidence.26
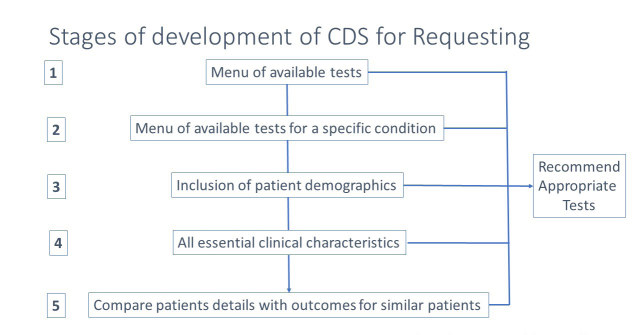
While CDS systems have been available for several decades, until recently they have been quite rudimentary. The steps in the development of CDS systems are shown in Figure 3. Starting with a list of tests, the first development stage is to link these to conditions. The next stage is to include patient demographics and then to enhance these with the essential characteristics of the individual patient. Lastly, an ideal system might compare the individual patient details to outcomes in similar patients. Software is now available in Australia that lies between stages three or four of CDS development as shown in Figure 3. The Diagnostic Medicine Clinical Committee would like to make such software available to general practitioners as soon as is feasible.
Figure 3.
Process of development of Clinical Decision Support (CDS) software for test requesters. (Supplied courtesy of Ken Sikaris, Sonic Healthcare, Melbourne, Australia.)
In parallel to the roll-out of the CDS software, there needs to be development and maintenance of the information base that supports the software, referred to as Appropriate Use Criteria.31 These are the guidelines and other evidence that informs the choice of test in the requesting process. Clearly the various professional bodies and medical specialities will have input into such an information base but a key issue yet to be decided is who or which organisation will coordinate all of these activities. It would seem desirable that the organisation(s) should be part of, or at least have strong links to, the pathology profession.
Challenges to Value-based Pathology Initiatives
This review concludes with a consideration of the challenges to the more widespread adoption of some of the value-based pathology practices that have been described. Without an understanding of these barriers, strategies to overcome them will be difficult and a more value-based pathology practice will remain a theoretical academic exercise.
The first challenge is a lack of resources and skills within the laboratory profession. The achievement of a highly productive laboratory testing model with a focus on costs has meant that there are few spare resources. Increased productivity is only followed by yet more demands for higher productivity and reductions in cost. It is difficult to see how to break this cycle but one would like to envisage in the future a dedicated position(s), at least in the larger laboratories, which is devoted to functions such as audit and outcomes monitoring.
There is also a deficiency of skills. As mentioned earlier, this review has not focused on the tools of economic analysis but such tools are a part of demonstrating value and very few laboratory professionals currently have experience in this area. A review of the literature dealing with the economic analysis of laboratory testing shows that few of the authors come from a laboratory background. This is not to say that laboratory professionals need to become experienced health economists. Useful skills would include awareness of the need and capability to make a business case for introduction of new tests and an understanding of how to apply tools such as economic modelling. One of the key tasks of the IFCC Value Proposition Committee is to provide education in this area and papers are starting to appear in the literature which show the use of economic tools to assist with the implementation of tests.32
A second key challenge is to achieve collaboration with colleagues outside of the laboratory, particularly clinicians. Like many other healthcare entities, the laboratory is a classic isolated silo and this barrier is further exaggerated by the fact that it provides the services, while the benefits of those services are delivered to other departments or silos. Hence the need in the value proposition of a test to quantify the benefits and disbenefits across all the stakeholders (or silos). However, this is not easy to achieve and is a challenge affecting all healthcare providers which often contributes to technology failure.33 The specific need to get more involvement from clinicians in the implementation of tests is especially difficult and solutions to this challenge are not obvious.
The need to involve clinicians extends from the fact that measurement of patient outcomes is a key requirement of a more value-based service. Outcomes measurement is traditionally seen as difficult when it includes the traditional measures of morbidity and mortality. There are other short-term, more operational measures which have economic consequences and which can show the value of testing as has been demonstrated in the value proposition papers mentioned previously for troponin, NT-proBNP and HbA1c.22–25 Unfortunately, while such measures may be easier to measure in theory, practical experience shows difficulties still exist, in part due to inflexible health information systems that cannot be adapted to automatically collect the requisite data. Changes in this area are crucial to demonstrating value-based practice.
Finally, the last of the challenges is essentially that of funding. In Australia at least, the measurement of outcomes is seen as research rather than an integral part of the laboratory or clinical service. Even if adopted under the research agenda there is little if any money available for what might be called translational research, namely ensuring that global evidence is translated into effective local practice which would be a core activity of the putative Centre of Excellence for Research into the Value of Pathology as discussed earlier.
Another way to achieve a program of translational research in this area may be via the proposed roll out of clinical decision support for test requesting by general practitioners. As discussed above, a key component of this initiative will be an organisation that is responsible for the Approved Criteria for Use which will support the CDS. That support will ideally include information about the outcomes of testing in order to ensure that the CDS software is kept up to date and accurately reflects current practice. While this might require significant resources, there would appear to be a good business case for such spending given that healthcare funders, including governments, often claim there are even larger sums of money spent on unnecessary laboratory testing. Such unnecessary testing should be much diminished through the use of CDS software supported by audit and outcomes research thereby delivering enhanced value to the funder and the community at large.
Acknowledgements
This review is dedicated to the memory of Howard Morris who would have been the 2019 David Curnow Plenary Lecturer but for his tragic and untimely death in April of 2019. Amongst his many distinguished scientific activities over the course of his career, Howard established the IFCC-WASPaLM Committee for the Value Proposition in Laboratory Medicine, the work of which forms part of this review. Other colleagues to acknowledge in contributing to and supporting the work described above are Helen Martin, Chris Price, Ken Sikaris and my fellow members from the IFCC-WASPaLM Value Proposition in Laboratory Medicine Committee.
Footnotes
Competing Interests: None declared.
References
- 1.Porter ME. What is value in health care? N Engl J Med. 2010;363:2477–81. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 2.Roland M, Sibbald B, McDonald R. Care closer to home. Moving care. Health Serv J. 2007;117(Suppl):6–8. [PubMed] [Google Scholar]
- 3.Lundberg GD. The need for an outcomes research agenda for clinical laboratory testing. JAMA. 1998;280:565–6. doi: 10.1001/jama.280.6.565. [DOI] [PubMed] [Google Scholar]
- 4.St John A, Edwards G, Fisher S, Badrick T, Callahan J. Crothers J. A call for a value based approach to laboratory medicine funding. Clin Biochem. 2015;48:823–6. doi: 10.1016/j.clinbiochem.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Sikaris KA. Enhancing the clinical value of medical laboratory testing. Clin Biochem Rev. 2017;38:107–14. [PMC free article] [PubMed] [Google Scholar]
- 6.Hallworth MJ, Epner PL, Ebert C, Fantz CR, Faye SA, Higgins TN, et al. IFCC Task Force on the Impact of Laboratory Medicine on Clinical Management and Outcomes. Current evidence and future perspectives on the effective practice of patient-centered laboratory medicine. Clin Chem. 2015;61:589–99. doi: 10.1373/clinchem.2014.232629. [DOI] [PubMed] [Google Scholar]
- 7.Society to Improve Diagnosis in Medicine. [Accessed 6 November 2019]. https://www.improvediagnosis.org/about/
- 8.Epner PL, Gans JE, Graber ML. When diagnostic testing leads to harm: a new outcomes-based approach for laboratory medicine. BMJ Qual Saf. 2013;22(Suppl 2):ii6–ii10. doi: 10.1136/bmjqs-2012-001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epner PL. Appraising laboratory quality and value: What’s missing? Clin Biochem. 2017;50:622–4. doi: 10.1016/j.clinbiochem.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Clinical Lab 2.0. [Accessed 6 November 2019]. https://www.cl2lab.org/
- 11.Swanson K, Dodd MR, VanNess R, Crossey M. Improving the Delivery of Healthcare through Clinical Diagnostic Insights: A Valuation of Laboratory Medicine through “Clinical Lab 2.0”. J Appl Lab Med. 2018;3:487–97. doi: 10.1373/jalm.2017.025379. [DOI] [PubMed] [Google Scholar]
- 12.Crawford JM, Shotorbani K, Sharma G, Crossey M, Kothari T, Lorey TS, et al. Improving American healthcare through “Clinical Lab 2.0”: A Project Santa Fe report. Acad Pathol. 2017 doi: 10.1177/2374289517701067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macleod MR, Michie S, Roberts I, Dirnagl U, Chalmers I, Ioannidis JPA, et al. Biomedical research: increasing value, reducing waste. Lancet. 2014;383:101–4. doi: 10.1016/S0140-6736(13)62329-6. [DOI] [PubMed] [Google Scholar]
- 14.Monaghan PJ, Lord SJ, St John A, Sandberg S, Cobbaert CM, Lennartz L, et al. Test Evaluation Working Group of the European Federation of Clinical Chemistry and Laboratory Medicine. Biomarker development targeting unmet clinical needs. Clin Chim Acta. 2016;460:211–9. doi: 10.1016/j.cca.2016.06.037. [DOI] [PubMed] [Google Scholar]
- 15.Horvath AR, Lord SJ, St John A, Sandberg S, Cobbaert CM, Lorenz S, et al. Test Evaluation Working Group of the European Federation of Clinical Chemistry Laboratory Medicine. From biomarkers to medical tests: the changing landscape of test evaluation. Clin Chim Acta. 2014;427:49–57. doi: 10.1016/j.cca.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 16.European Federation of Clinical Chemistry and Laboratory Medicine. [Accessed 6 November 2019]. https://www.eflm.eu/site/page/a/1158.
- 17.Robert G, Greenhalgh T, MacFarlane F, Peacock R. Adopting and assimilating new non-pharmaceutical technologies into health care: a systematic review. J Health Serv Res Policy. 2010;15:243–50. doi: 10.1258/jhsrp.2010.009137. [DOI] [PubMed] [Google Scholar]
- 18.The NHS Atlas of Variation in Diagnostic Services. [Accessed 6 November 2019]. https://ukgtn.nhs.uk/fileadmin/uploads/ukgtn/Documents/Resources/Library/Reports_Guidelines/Right_Care_Diagnostics_Atlas_2013.pdf.
- 19.AACB Value of Pathology Workshop Melbourne, May 26, 2017- Australasian Association of Clinical Biochemists. [Accessed 6 November 2019]. https://www.aacb.asn.au/professionaldevelopment/value-of-pathology-workshop.
- 20.Price CP, St John A. Anatomy of a value proposition for laboratory medicine. Clin Chim Acta. 2014;436:104–11. doi: 10.1016/j.cca.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Price CP, St John A, Christenson R, Scharnhorst V, Oellerich M, Jones P, et al. Leveraging the real value of laboratory medicine with the value proposition. Clin Chim Acta. 2016;462:183–6. doi: 10.1016/j.cca.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 22.St John A, Cullen L, Jülicher P, Price CP. Developing a value proposition for high-sensitivity troponin testing. Clin Chim Acta. 2018;477:154–9. doi: 10.1016/j.cca.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 23.O’Kane M, Porter D, McCann M, Jülicher P, Christenson R, Oellerich M, et al. A value proposition for natriuretic peptide measurement in the assessment of patients with suspected acute heart failure. Clin Chim Acta. 2020;500:98–103. doi: 10.1016/j.cca.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 24.Price CP, St John A. The value proposition for point-of-care testing in healthcare: HbA1c for monitoring in diabetes management as an exemplar. Scand J Clin Lab Invest. 2019;79:298–304. doi: 10.1080/00365513.2019.1614211. [DOI] [PubMed] [Google Scholar]
- 25.Oellerich M, Christenson RH, Beck J, Walson PD. Plasma EGFR mutation testing in non-small cell lung cancer: A value proposition. Clin Chim Acta. 2019;495:481–6. doi: 10.1016/j.cca.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 26.Australian Government Department of Health. Medicare Benefits Schedule Review. [Accessed 6 November 2019]. https://www1.health.gov.au/internet/main/publishing.nsf/Content/MBSR-about.
- 27.Bilinski KL, Boyages SC. The rising cost of vitamin D testing in Australia: time to establish guidelines for testing. Med J Aust. 2012;197:90. doi: 10.5694/mja12.10561. [DOI] [PubMed] [Google Scholar]
- 28.Boyages SC. Vitamin D testing: new targeted guidelines stem the overtesting tide. Med J Aust. 2016;204:18. doi: 10.5694/mja15.00497. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Chica D, Stocks N. Changes to the frequency and appropriateness of vitamin D testing after the introduction of new Medicare criteria for rebates in Australian general practice: evidence from 1.5 million patients in the NPS MedicineInsight database. BMJ Open. 2019;9:e024797. doi: 10.1136/bmjopen-2018-024797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baird GS, Jackson BR, Dickerson J, Sikaris K, Croal B, Laposata M. What’s New in Laboratory Test Utilization Management? Clin Chem. 2018;64:994–1000. doi: 10.1373/clinchem.2017.280214. [DOI] [PubMed] [Google Scholar]
- 31.Medicare Benefits Schedule Review Taskforce: Report from the Diagnostic Medicine Clinical Committee. [Accessed 6 November 2019]. pp. 25–30. https://www1.health.gov.au/internet/main/publishing.nsf/Content/mbs-review-2018-taskforce-reports-cp/$File/Diagnostic-Medicine-Clinical-Committee-Final%20Report.pdf.
- 32.Price CP, Wolstenholme J, McGinley P, St John A. Translational health economics: The key to accountable adoption of in vitro diagnostic technologies. Health Serv Manage Res. 2018;31:43–50. doi: 10.1177/0951484817736727. [DOI] [PubMed] [Google Scholar]
- 33.Falling short: Why the NHS is still struggling to make the most of new innovations. [Accessed 6 November 2019]. https://www.nuffieldtrust.org.uk/research/falling-short-why-the-nhs-is-still-struggling-to-make-the-most-of-new-innovations.