Streptococcus mutans is a pathogenic bacterium that is the primary etiologic agent of dental caries, a disease that affects billions yearly. Rhamnose biosynthesis is conserved not only in streptococcal species but in other Gram-positive, as well as Gram-negative, organisms. This study highlights the importance of rhamnose biosynthesis in RGP production for protection of the organism against acid and oxidative stresses, the two major stressors that the organism encounters in the oral cavity. Loss of RGP also severely impacts biofilm formation, the first step in the onset of dental caries. The high conservation of the rhamnose synthesis enzymes, as well as their importance in S. mutans and other organisms, makes them favorable antibiotic targets for the treatment of disease.
KEYWORDS: Streptococcus mutans, rhamnose, RGP, cell wall, acid tolerance, oxidative stress
ABSTRACT
The rhamnose-glucose cell wall polysaccharide (RGP) of Streptococcus mutans plays a significant role in cell division, virulence, and stress protection. Prior studies examined function of the RGP using strains carrying deletions in the machinery involved in RGP assembly. In this study, we explored loss of the substrate for RGP, l-rhamnose, via deletion of rmlD (encoding the protein responsible for the terminal step in l-rhamnose biosynthesis). We demonstrate that loss of rhamnose biosynthesis causes a phenotype similar to strains with disrupted RGP assembly (ΔrgpG and ΔrgpF strains). Deletion of rmlD not only caused a severe growth defect under nonstress growth conditions but also elevated susceptibility of the strain to acid and oxidative stress, common conditions found in the oral cavity. A genetic complement of the ΔrmlD strain completely restored wild-type levels of growth, whereas addition of exogenous rhamnose did not. The loss of rhamnose production also significantly disrupted biofilm formation, an important aspect of S. mutans growth in the oral cavity. Further, we demonstrate that loss of either rmlD or rgpG results in ablation of rhamnose content in the S. mutans cell wall. Taken together, these results highlight the importance of rhamnose production in both the fitness and the ability of S. mutans to overcome environmental stresses.
IMPORTANCE Streptococcus mutans is a pathogenic bacterium that is the primary etiologic agent of dental caries, a disease that affects billions yearly. Rhamnose biosynthesis is conserved not only in streptococcal species but in other Gram-positive, as well as Gram-negative, organisms. This study highlights the importance of rhamnose biosynthesis in RGP production for protection of the organism against acid and oxidative stresses, the two major stressors that the organism encounters in the oral cavity. Loss of RGP also severely impacts biofilm formation, the first step in the onset of dental caries. The high conservation of the rhamnose synthesis enzymes, as well as their importance in S. mutans and other organisms, makes them favorable antibiotic targets for the treatment of disease.
INTRODUCTION
The cell wall of Streptococcus mutans is decorated with a polysaccharide termed the rhamnose-glucose polysaccharide (RGP) (1). The function of these structures in S. mutans has been recently studied, demonstrating that the RGP plays a role in virulence, stress protection, cell morphology, and cell division (2–6). Unlike those of other model Gram-positive organisms, such as Staphylococcus aureus and Bacillus subtilis, the cell wall of S. mutans lacks wall teichoic acid (WTA) structures. WTAs are found in many Gram-positive organisms, and their importance to those organisms has been studied extensively (7–10). Like the RGP in S. mutans, WTAs play a role in proper cell division, biofilm formation, and stress tolerance (11–13). Previous studies have highlighted the homology between the S. mutans RGP and the WTAs of other Gram-positive bacteria (2–4).
Assembly of the RGP structure begins with the addition of an N-acetylglucosamine (GlcNAc) subunit to an undecaprenol lipid carrier by RgpG (14); for WTA assembly, a similar mechanism is catalyzed by TagO (15). After addition of the GlcNAc base, production of the polyrhamnose backbone can begin. RgpA is recognized as the enzyme that adds the first rhamnose to the GlcNAc. From this point, the rhamnosyltransferases RgpB and RgpF add rhamnose monomers to the growing RGP chain. RgpB adds the second rhamnose, while RgpF adds the third rhamnose, with the two enzymes alternating to elongate the chain (16), whereas in WTA formation the backbone is made of either glycerol-3-phosphate or ribitol-5-phosphate, depending on the organism (17). Three different glucosyltransferases (RgpE, RgpH, and RgpI) add α1,2-linked glucose side chains to the rhamnose subunits (18). Recently, the membrane-bound protein encoded by the gene previously annotated as SMU.831 was shown to add a glycerol phosphate to the RGP structure (3). RgpC and RgpD share homology with ATP-binding cassette (ABC) transporters for polysaccharides in other organisms and have been shown to be essential in S. mutans, required to transport the RGP from the cytosol to the exterior of the cell (16, 19). After the polysaccharide is exported, the LytR/CpsA/Psr family of proteins cross-links the RGP structure to the peptidoglycan. The phosphodiester bond between the GlcNAc and the lipid carrier is initially hydrolyzed, and a phosphodiester linkage is produced between the GlcNAc of the RGP and the peptidoglycan (20).
The deoxy-sugar rhamnose is produced by many plants and bacteria; however, it is absent in mammals. In Gram-negative bacteria, rhamnose is found in the lipopolysaccharide (21), while it is essential in Mycobacterium spp. as part of the glycopeptidolipid (22). Streptococcal species produce the enantiomer form, l-rhamnose, via the same four biosynthetic steps carried out by the RmlA, RmlB, RmlC, and RmlD enzymes from the precursor glucose-1-phosphate (23). As the final enzyme in the rhamnose production pathway, RmlD catalyzes the final reduction to dTDP–l-rhamnose (24). In group A streptococci (GAS), the RmlD enzyme is essential for growth. Deletion of rmlD in S. mutans Xc has been shown to cause defects in cell morphology and growth, similar to a deletion of rgpG (4, 25, 26).
In S. mutans, rmlA, rmlB, and rmlC are in the same genetic locus, while rmlD is positioned immediately preceding the locus containing genes responsible for formation of the RGP polymer (27) and is cotranscribed with the downstream locus (28). With the exception of the enzyme encoded by rgpG, which is located over 50 kb upstream of rmlD, all enzymes known to be involved in the production of the RGP structure are encoded by genes in the rgp operon. While S. mutans can produce rhamnose, it has no known mechanism for its catabolism. The only known use for rhamnose in S. mutans is in production of the rhamnose-glucose polysaccharide.
Functionally, deletion of rgpG or rmlD results in loss of the RGP structure. In the absence of rgpG, S. mutans loses the ability to transfer the GlcNac to an undecaprenol carrier. Deletion of rmlD results in the loss of dTDP-rhamnose production and, thereby, elimination of the major substrate of the RGP. In the present study, we characterized the rmlD mutant strain, with respect to strains carrying deletions in rgp genes, and demonstrate that the phenotypes and increased susceptibility to stress observed in strains that lack the RGP, or contain a defective RGP, are due to the loss of rhamnose in the cell (and, therefore, the RGP) and not to any consequences of excess rhamnose in the cells.
RESULTS
Loss of rgpG or rmlD eliminates cell wall rhamnose content.
We anticipated that the phenotypic similarities that result from deletion of either rgpG or rmlD are caused by similar outcomes of these mutant strains, a lack of cell wall-associated RGP structures. To confirm this, we extracted the cell walls of S. mutans strains tested in this study, hydrolyzed the polysaccharides into monosaccharides, and assayed for the amount of rhamnose present in each sample. Loss of both rgpG and rmlD resulted in cell walls devoid of any detectable rhamnose content, while cell walls of the rmlD+ complement strain were restored to levels comparable to those of the UA159 parent strain (Fig. 1). These data demonstrate that both rgpG and rmlD are critically required for the formation of RGP structures, albeit via independent mechanisms.
FIG 1.
The cell walls of the ΔrmlD and ΔrgpG strains are devoid of l-rhamnose content. Cell walls were isolated from the S. mutans UA159, ΔrmlD, and ΔrgpG strains and hydrolyzed with trifluoroacetic acid to release monosaccharides. Samples were normalized to 10 mg ml−1. Distilled water was used as a negative control (NC) to determine background. Rhamnose content was measured using a Megazyme l-rhamnose assay kit. Data were derived from three independent experiments and represented as the micrograms of rhamnose per milliliter of reaction mixture. **, P < 0.01 in a pairwise comparison to UA159.
S. mutans strains devoid of RGP structure display severe growth defects.
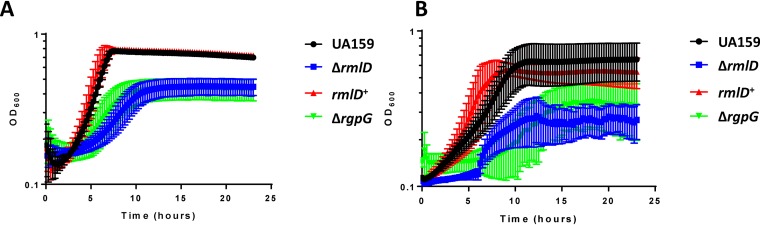
The van Sorge group had described a ΔrmlD strain in the S. mutans Xc background that grew poorly (25). Sequence analysis of the rmlD region from both S. mutans UA159 and Xc indicated no differences between them; therefore, we deleted rmlD in the UA159 background using the ΔrmlD construct from the Xc strain (25). Growth of the two rmlD deletion strains was not significantly different (P > 0.05) (data not shown). Previously, we reported that the ΔrgpG strain exhibited severe growth defects (4). Given this observation and the results from the rhamnose content assay that revealed neither the ΔrgpG nor the ΔrmlD strain contained rhamnose in their cell wall fraction, we set out to examine the growth rate of the UA159 ΔrmlD strain with respect to the ΔrgpG strain. Cultures of both the ΔrgpG (26) and ΔrmlD derivatives of UA159 were flocculated at the bottom of the growth vessel and grew significantly worse than the wild-type in microaerobic conditions (under an oil overlay) (Fig. 2A), both with doubling times of approximately 500 min (Table 1). The differences in generation time were not found to be significant (P > 0.05). Complementation of the rmlD gene (rmlD+) restored the growth rate of the organism to a value similar to that of the wild-type strain (UA159) and also resulted in turbid growth, as observed with the parent strain. When the test strains were assayed for growth without an oil overlay (i.e., in conditions of atmospheric oxygen), the maximum optical density (OD) was decreased for all strains (Fig. 2B), with a concomitant increase in doubling time (Table 1). These results support the idea that loss of the RGP structure, via deletion of rmlD or rgpG, causes severe defects in growth.
FIG 2.
Loss of the RGP structure results in a severe growth defect in S. mutans. Strains of S. mutans UA159 deficient in the RGP structure were grown in BHI medium with (A) or without (B) an oil overlay. Optical density readings were taken at 600 nm as a measure of growth rate using a Bioscreen C plate reader (±standard deviation). The doubling times of each strain can be found in Table 1. Three independent experiments were performed.
TABLE 1.
Doubling times of S. mutans UA159, ΔrmlD, ΔrgpG, and rmlD+ strains
| Strain or genotype | Growth condition | Doubling time (min) | SDa | Significant difference from UA159 BHI (oil)a |
|---|---|---|---|---|
| UA159 | BHI (oil) | 107.09 | 4.70 | N/A |
| rmlD+ | BHI (oil) | 102.61 | 5.61 | NS |
| ΔrmlD | BHI (oil) | 518.91 | 34.03 | ** |
| ΔrgpG | BHI (oil) | 472.85 | 67.94 | ** |
| UA159 | BHI (no oil) | 365.32 | 23.96 | ** |
| rmlD+ | BHI (no oil) | 297.27 | 98.46 | NS |
| ΔrmlD | BHI (no oil) | 872.87 | 391.37 | NS |
| ΔrgpG | BHI (no oil) | 744.71 | 220.64 | * |
| UA159 | BHI + H2O2 | 187.54 | 20.53 | * |
| rmlD+ | BHI + H2O2 | 159.82 | 2.01 | ** |
| ΔrmlD | BHI + H2O2 | No growth | N/A | N/A |
| UA159 | BHI (pH 5.4) | 181.36 | 5.87 | *** |
| rmlD+ | BHI (pH 5.4) | 182.53 | 3.46 | *** |
| ΔrmlD | BHI (pH 5.4) | 2,454.54 | 232.60 | ** |
N/A, not available; NS, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The ΔrmlD strain exhibits hypersensitivity to acid and oxidative stress.
As an acidogenic bacterium, the ability of S. mutans to adapt and grow in a low pH environment is an important aspect of its virulence. Deletion of rgp genes has previously been shown to increase the organism’s susceptibility to acid (2, 26). When the ΔrmlD strain was grown in medium that was buffered to a pH of 5.4, it grew significantly more poorly than in brain heart infusion (BHI) medium alone (Fig. 3A), as evidenced by an approximately 5-fold increase in doubling time (Table 1). The acid sensitivity of the ΔrmlD strain was further demonstrated in a spot plating assay as described in Materials and Methods. Outgrowth of the ΔrmlD strain was substantially arrested when it was plated so as to be adjacent to the acidogenic parent strain, UA159 (Fig. 3B), or the rmlD complement strain (rmlD+) (see Fig. S1 in the supplemental material), whereas growth of the rmlD+ strain was not affected in either experiment (Fig. 3B; see also Fig. S1). These strains were also plated on agar medium buffered to pH 7 to abrogate the effects of acidic end products generated by S. mutans, and the results showed that both the rmlD+ and the ΔrmlD strains had no defect in growth (Fig. 3B, right), again illustrating that loss of rmlD affects the organism’s acid sensitivity.
FIG 3.
The ΔrmlD strain is susceptible to acid stress. (A) S. mutans UA159, ΔrmlD, and rmlD+ strains were grown in BHI medium titrated to pH 5.4 with an oil overlay. Optical density readings were taken at 600 nm as a measure of growth rate using a Bioscreen C plate reader (±standard deviation). The doubling times of each strain can be found in Table 1. (B) Competition spot assays were used to determine the fitness of the ΔrmlD and rmlD+ strains against acid (S. mutans UA159). A primary spot, S. mutans UA159, was allowed to grow for 24 h before secondary spots were placed adjacently. (Top) BHI agar medium; (bottom) BHI agar medium buffered to pH 7 was used as a control. Representative images from three independent experiments are shown.
Similarly, growth of the ΔrmlD strain was halted when exposed to oxidative stress in the form of a sublethal dose of H2O2. While both the UA159 and rmlD+ strains had greatly extended lag phases (Fig. 4A) and significantly increased generation times compared to growth in BHI medium alone (Table 1), the ΔrmlD strain did not grow at all and exhibited no change in optical density at 600 nm (OD600) over the 20-h experimental time period (Fig. 4A). This effect on the growth rate of the ΔrmlD strain was even more drastic than what was observed in acidic conditions (Fig. 3A; Table 1). Again, we employed a spot competition assay, this time using peroxigenic streptococci, either Streptococcus gordonii or Streptococcus sanguinis, as the primary colonizer, as these bacteria have previously been shown to produce millimolar levels of H2O2 (29). Similar to observations from the response to acidic stress (Fig. 3B), the ΔrmlD strain displayed elevated susceptibility to oxidative stress compared to that of the parent strain or rmlD+ (Fig. 4B). When catalase was added to the cultures of S. sanguinis and S. gordonii to ablate the effects of H2O2, the growth defect observed with the ΔrmlD strain was eliminated (Fig. 4C), further proving that it was indeed the production of H2O2 that limited growth.
FIG 4.
(A) The ΔrmlD strain displays an increased susceptibility to hydrogen peroxide. S. mutans UA159, ΔrmlD, and rmlD+ strains were grown in BHI medium containing 0.5 mM H2O2 with an oil overlay. Optical density readings were taken at 600 nm as a measure of growth rate using a Bioscreen C plate reader. The doubling times of each strain can be found in Table 1. (B) Competition spot assays were used to determine the fitness of S. mutans strains against hydrogen peroxide-producing streptococci (S. gordonii [left] and S. sanguinis [right]). A primary spot was allowed to grow for 24 h before secondary spots were placed adjacently. Representative images from three independent experiments are shown. (C) Competition spot assays were used to determine the fitness of S. mutans strains against hydrogen peroxide-producing streptococci (S. gordonii [left] and S. sanguinis [right]). Catalase (8 μg) was added to the primary cultures prior to spotting the culture on the agar plate. The primary spot was allowed to grow for 24 h before secondary spots were placed adjacently. Representative images from three independent experiments are shown.
Loss of rmlD leads to an elevated minimum glycolytic pH.
S. mutans thrives in the oral cavity not only due to its ability to produce acid but also because it can survive in the low pH microenvironment that it creates. Since we observed that the ΔrmlD strain exhibited an increased sensitivity to acidic stress, we hypothesized that it might be less adept at acid production. Measurement of the minimum glycolytic pH examines the amount of acid produced by S. mutans via the fermentation of glucose to lactic acid through glycolysis; thus, alterations in the minimum pH would indicate an altered glycolytic output. In fact, deletion of rmlD resulted in a significant increase in the minimum glycolytic pH (0.15 pH unit) compared to that of UA159 (Fig. 5). Further, these results are consistent with observations for the ΔrgpG strain (26). Interestingly, complementation of rmlD (rmlD+) resulted in a minimum glycolytic pH that was 0.1 pH unit lower than that of UA159. These results indicate that the loss of rmlD, or the RGP structure, affects glycolytic activity and acid production.
FIG 5.
The ΔrmlD strain exhibits a reduction in glycolytic activity. The minimum glycolytic pH values of S. mutans UA159, ΔrmlD, and rmlD+ strains were determined after excess glucose was added to cell suspensions as described in Materials and Methods. The minimum pH at which glycolysis could be performed was recorded from three independent experiments. ***, P < 0.001.
Defects in the RGP cause impairment in biofilm formation.
The ability of S. mutans to form biofilms is another important virulence attribute in the formation of dental caries. Previously, alterations in the RGP have been shown to significantly alter the ability of the deletion strains to grow robust biofilms (2, 30). To test the ability of the ΔrmlD strain to form biofilms, the organism was grown in the presence of either glucose or sucrose, two preferred sugars for biofilm formation. When the ΔrmlD strain was grown with glucose as the sole carbon source (tryptone-yeast extract-glucose [TYG]), very little biofilm accumulation was observed (a 98% reduction compared to that of UA159) (Fig. 6). In the presence of sucrose (tryptone-yeast extract-sucrose [TYS]), an 84% reduction in biofilm production of the ΔrmlD strain was measured compared to that of UA159 (Fig. 6). In the rmlD+ strain, where rhamnose biosynthesis was restored, biofilm accumulation returned to wild-type levels in the presence of either carbon source (Fig. 6). Altogether, these results demonstrate that loss of rmlD and of concomitant rhamnose production severely impacts biofilm formation.
FIG 6.
Biofilm formation is impaired in the ΔrmlD strain. S. mutans UA159, ΔrmlD, and rmlD+ strains were grown in 96-well microtiter plates in TY medium containing either glucose or sucrose as the sole carbon source. Biofilms were allowed to form for 24 h before being measured via crystal violet staining. Data were obtained from three independent cultures and represented as the optical density at 575 nm. **, P < 0.01.
Addition of exogenous rhamnose does not restore the growth of the ΔrmlD strain.
Since rmlD encodes the final enzymatic step in rhamnose biosynthesis, we next tested whether addition of exogenous rhamnose to the culture medium for the ΔrmlD strain would complement the loss of native rhamnose. S. mutans UA159 and ΔrmlD strains were grown under four different conditions as follows: tryptone-yeast extract (TY) broth (no carbon source), TY with 1% (vol/vol) glucose (TYG), TY with 1% (vol/vol) rhamnose (TYR), and TY with 1% (vol/vol) glucose plus 1% (vol/vol) rhamnose (TYGR) (Fig. 7). As expected, neither strain grew in the TY medium, as it lacked a metabolizable carbon source. Also, as expected, growth was observed for both strains in the TYG medium. When rhamnose was the only carbon source, there was no growth of any of the test strains, similar to the results seen with the TY medium alone, indicating that rhamnose is not a viable carbon source for S. mutans. While both strains grew in the TYGR medium, there was no significant difference between TYG and TYGR, indicating that addition of exogenous rhamnose does not restore growth in a mutant that lacks the ability to produce rhamnose.
FIG 7.
Exogenous rhamnose does not restore growth of a ΔrmlD mutant strain. S. mutans UA159 and ΔrmlD strains were grown in tryptone-yeast extract (TY) medium lacking a carbon source, containing glucose as the sole carbon source (TYG), with rhamnose as the sole carbon source (TYR), or with equal quantities of glucose and rhamnose (TYGR). OD600 readings were taken at 7 h and 24 h postinoculation. Three independent experiments were performed. Pairwise comparison using Student’s t test was used to determine statistical significance (P < 0.01) between cultures of the UA159 and ΔrmlD strains at 7 h in TYG (*), at 24 h in TYG (#), at 7 h in TYGR (†), and at 24 h in TYGR (^).
DISCUSSION
Previous studies have examined the role of rgp gene deletion on the overall fitness of the organism (4, 30); however, those deletion strains still encoded the mechanism for rhamnose production. No enzymes have been identified with the ability to catabolize rhamnose in S. mutans, and, in fact, the organism cannot grow with rhamnose as the sole carbon source (Fig. 7), suggesting that rhamnose is only utilized by S. mutans for incorporation into the RGP. The accumulation of the nucleotide sugar within the cells could have a negative impact on fitness of rgp deletion strains, as its biosynthesis diverts glucose from energy-producing pathways. By studying a ΔrmlD strain, we can eliminate the effects of rhamnose buildup by removing rhamnose production, suggesting that any fitness defects in the ΔrmlD strain are due to lack of RGP and not due to a stockpile of unusable end products. As a comparator for the work in this study and as a model organism for RGP deficiency, we utilized the ΔrgpG strain, which we have previously reported (4).
It has been shown that an S. mutans Xc derivative lacking rmlD does not contain rhamnose in its cell wall (25). In this study, we show that an S. mutans UA159 ΔrmlD strain is also devoid of cell wall rhamnose and, further, that the RGP-deficient ΔrgpG strain is likewise lacking the nucleotide sugar in the cell wall (Fig. 1). It is important to note that the ΔrgpG strain possesses an intact rhamnose production pathway and that rmlD copy number in this strain is not altered compared to that of the parent strain (see Fig. S2 in the supplemental material). We measured rhamnose content from cell-free lysates of the ΔrgpG strain to determine if they were comprised of an elevated quantity of rhamnose compared to that of the parent strain or the ΔrmlD strain, though no intracellular rhamnose could be detected (data not shown). There are several possibilities to explain this finding. The amount of rhamnose may be too dilute in whole-cell lysates to be detectable by the assay method, or, more likely, the dTDP form of the sugar may be interfering with the assay. Therefore, it is difficult to say whether there is a buildup of rhamnose in the ΔrgpG strain, though the fact that the growth phenotypes of the ΔrmlD and ΔrgpG strains are similar indicates that this is not the cause of the growth defects observed (Fig. 2 and Table 1).
The growth defect of the ΔrmlD strain was not surprising given previous reports involving strains defective in RGP assembly (2, 25, 30). The generation time for the ΔrmlD strain was significantly increased compared to that of the parent strain when grown in microaerobic conditions (Fig. 2A), under atmospheric levels of oxygen (Fig. 2B), under acidic conditions (Fig. 3), and in the presence of sublethal quantities of H2O2 (Fig. 4A). These observations are mirrored in the ΔrgpG strain grown under the same conditions (Fig. 2; Table 1) (4). The increased sensitivity of the ΔrmlD strain to environmental stresses was further demonstrated in spot competition assays where the mutant displayed reduced competitive fitness when grown adjacently to both acid-producing S. mutans and peroxigenic streptococci (Fig. 3B and 4B). Taken together, these results highlight the integral role that RGP, and rhamnose, play in protecting the organism against the acidic environment it creates as well as acid and H2O2 produced by competing commensal bacteria.
Importantly, we demonstrate that loss of rhamnose production, a nucleotide sugar not typically associated with the S. mutans oxidative stress response, leads to severe defects in growth rate and fitness. Classical oxidative stress responses in S. mutans, such as NADH oxidase (31), superoxide dismutase (32), and Dpr (33), are all present in the ΔrmlD strain, leading us to the hypothesis that the RGP might be acting as an outer defense against oxidative stress through a mechanism that has yet to be elucidated. The recent finding that SMU.831 adds a glycerol phosphate to the RGP to confer a negative charge to the polysaccharide (3) hints at a role for the RGP in sequestering positively charged ions such as iron ions, which participate in the Fenton reaction (34) that mediates oxidative stress (35). Analysis of the polysaccharides of fungi have correlated elevated ratios of rhamnose in cell wall polysaccharides with protection against oxidative stress in the form of an enhanced antioxidant ability, greater reducing power, and the capacity to chelate metals (36). While these effects have not yet been examined for S. mutans cell wall polysaccharides, the rhamnose in the RGP may indicate an ability of the structure to play some protective role for the organism. In fact, the addition of d-alanine to WTAs has been shown to protect S. aureus from oxygen-mediated killing by neutrophils (7). While the RGP does not contain d-alanylation in the structure, it is possible that the rhamnose itself is protecting the cells from oxidative stress (36), acting as an antioxidant against reactive oxygen species, scavenging harmful free radicals, or chelating metal ions such as iron ions.
The loss of the RGP, via deletion of either rmlD or rgpG, affected the metabolic potential of S. mutans, evidenced by the elevated minimum glycolytic pH in the ΔrgpG (26) and ΔrmlD (Fig. 5) (∼0.15 unit increase in pH) strains compared to that of the UA159 parent strain. The rhamnose biosynthesis pathway and glycolysis (via conversion to glucose-6-phosphate) use the same substrate, glucose-1-phosphate, such that any substrate shifted to one pathway is therefore shuttled away from the other. Previous studies have shown that RGP disruption alters growth rate (2, 25) and that one of the causes of this is the mislocalization of cell wall production machinery (4). The decreased metabolic potential of the ΔrmlD strain likely also has an effect on growth rate, as a defect in metabolism will alter the ability of the cells to grow. Loss of rgpG had a similar effect on metabolic potential (26), indicating that it is the loss of RGP production that alters metabolism and not necessarily the loss of rhamnose production.
While S. mutans has the ability to import many different sugars, rhamnose specifically has not been reported among them (37–39). Further, the rhaEWRBMA rhamnose catabolism operon is lacking in the genome of S. mutans and other closely related streptococcal species (27). In contrast, this genetic locus has been well characterized in the Gram-positive organism Bacillus subtilis, where rhamnose degradation is tightly linked to metabolism (40). Unlike streptococci that incorporate rhamnose into their cell wall polysaccharides, the WTAs of B. subtilis are devoid of any rhamnose, nor does it produce any other known structures into which rhamnose is incorporated. As a ubiquitous soil-inhabiting organism, the environmental niche of B. subtilis is rich with plants that naturally produce rhamnose, making it a viable carbon source for the bacterium (41). Also important to note is that dTDP, added by rmlA, has been shown to be required for addition of rhamnose to the cell wall polysaccharide (42).
With rhamnose production being a dead-end pathway in S. mutans (i.e., rhamnose cannot be converted back to glucose), it could be predicted that loss of the ability to utilize rhamnose would slow or stop rhamnose production. A non-statistically significant decrease in rmlD expression, compared to that of UA159, was measured in the ΔrgpG strain (see Fig. S2). Unexpectedly, the only rgp deletion strain tested that exhibited a significant decrease in rmlD expression (excluding the ΔrmlD strain) was the ΔrgpF strain (Fig. S2). RgpF, which adds rhamnose to the RGP, is encoded downstream of the rmlD gene in the RGP operon (16). Interestingly, deletion of rgpE, which is adjacent to rgpF in the RGP operon, had no effect on rmlD expression (Fig. S2), perhaps indicating that the addition of rhamnose to the structure is a regulated event. As rgpG is not located within the RGP operon, it might not be subject to the same effect on regulation as the remaining rgp genes, as evidenced by the reverse transcription-quantitative PCR (qRT-PCR) data. The qRT-PCR results highlight, however, that disruption of the RGP via rgpG deletion has no significant effect on rmlD expression.
The rmlD gene resides in an interesting location in the S. mutans genome as the first gene in the 10-gene rgp operon (27). The location of rmlD is conserved in other streptococcal species as well, including Streptococcus pyogenes and Streptococcus agalactiae (43, 44). As the first gene in an operon that is cotranscribed (28), regulation of the rmlD promoter can have significant effects on the expression of downstream genes in the operon. While the regulation of the operon is currently not well understood, some of our evidence hints at high levels of regulation (see Fig. S2; additional data not shown). Current studies are focused on elucidating on the role that rmlD regulation has on expression of the downstream rgp genes.
Complementation of the ΔrmlD strain proved to be difficult as was previously reported for the rgpG deletion (4). The ΔrmlD strain was recalcitrant to all complementation attempts, including the addition of competence stimulating peptide (CSP), a peptide that stimulates transcription of com genes involved in genetic competence in streptococcal species (45). Instead, an alternative approach was undertaken, similar to that used to create the rgpG+ strain (4). A second copy of the rmlD gene was inserted into the genome of UA159, creating a strain of S. mutans with two functional copies of rmlD. This strain was then deleted for rmlD in the native locus. Unlike the complement of the ΔrgpG strain (4), growth phenotypes returned to wild-type levels in the rmlD+ strain (Fig. 2 and Table 1) as well as the strain’s ability to combat environmental stress (Fig. 3 and 4). By using qRT-PCR, we were also able to show that expression of rmlD is restored to wild-type levels (see Fig. S2).
The correlation between defects in competence and alteration of the RGP structure is not known at this time, but there are some hypotheses that could be posited. As previously demonstrated, the loss of RGP alters proper localization of GbpB (4). The inability of the cells to divide yields large clusters of cells, which may prevent DNA uptake. The loss of the RGP might also alter the translocation of the transformasome machinery in S. mutans. A type 4-like pilus is known to be required for transformation in streptococcal species, including S. mutans (46). Finally, it is possible that DNA binds to or “sticks” to the RGP structure itself as has been recently shown in B. subtilis, where DNA binds to the WTA during competence (47). Ablation of WTAs from B. subtilis cell walls via addition of tunicamycin rendered the cells incompetent, similar to our observations with the ΔrmlD and ΔrgpG strains. Further, the Carballido-Lopez group noted that modifications by the sugar transferase encoded by tuaH during competence allowed DNA binding to the WTA (47). While the identity of the sugar being transferred is not currently known, it is reminiscent of the activity of the glucosyltransferases RgpE and RgpH in S. mutans. Studies of RgpI have shown that it plays a role in comX-inducing peptide (XIP)-mediated activation of the alternative sigma factor comX (48). ComX, a protein that guides RNA polymerase to the promoters of late competence genes (49) and facilitates their transcription, is not activated in the absence of rgpI, rendering those strains nontransformable in defined medium containing XIP (48). The genes encoding the transformasome are part of the ComX regulon (46), and it is possible that the RGP structure is required to properly localize the transformasome on the outside of the cell. These findings, in combination with those observed in B. subtilis, highlight the importance of the sugar side chain on cell wall polysaccharides for competence. Further work will need to be done to determine the mechanism that the side chain uses to mediate competence, whether it involves coordinating proper localization of transformasome machinery or glucose-mediated DNA attachment to the RGP. With the recent discovery of the addition of the glycerol phosphate modification to the RGP (3), additional unknown modifications cannot be ruled out as well.
In conclusion, we have shown the importance of l-rhamnose synthesis in S. mutans. Loss of rhamnose production, via deletion of rmlD, had drastic effects on the ability of the cells to grow and respond to stresses encountered in the microbial battlefield of the oral cavity. These results highlight the notion that it is not merely the disruption of rhamnose biosynthesis, in particular, that causes the phenotypes described in this study but, rather, the effect that the lack of available substrate, rhamnose, has on RGP assembly. We have shown that a complete loss of the RGP structure has significant consequences for the organism in nearly all aspects required for pathogenesis in the oral cavity and potential to cause disease. The rhamnose biosynthesis pathway is an attractive antimicrobial target (50) and an alternative to targeting the machinery involved in cell wall polysaccharide synthesis. Rhamnose biosynthesis, as a target, might also be extended to life-threatening streptococcal species.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All mutant strains used in this study are derivatives of Streptococcus mutans strain UA159, designated as wild-type in all experiments conducted (27). The ΔrgpF strain was created by a single gene deletion and replacement with an erythromycin resistance cassette as previously described (2, 26). The ΔrmlD strain was created using an amplicon derived from an ΔrmlD strain created in the S. mutans Xc background (generous gift from Nina van Sorge, University Medical Center, Utrecht, Netherlands) (25). A forward primer 700 bp upstream of the rmlD gene (rmlDupF) and a reverse primer 674 bp downstream from rmlD (rmlDdownR) were used to PCR amplify the region surrounding the deleted gene, and the amplicon was gel isolated and used to transform UA159 (see Table S1 in the supplemental material) (25). Transformants were selected on BHI agar medium containing erythromycin (5 μg ml−1) and confirmed via colony PCR using the previously indicated forward primer and a reverse primer that binds in the Ermr cassette (Ermr Rev) (Table S1). Colonies that were positive for the amplicon were sequence verified to ensure proper orientation of the erythromycin cassette.
The first attempt at construction of a complement of the ΔrmlD strain (rmlD+ strain) involved introducing a copy of the rmlD gene into the gtfA locus, as we have reported previously (51–53). Primers were designed to amplify the region containing rmlD (SMU.824) and the intergenic region preceding it (rmlDCompF and rmlDCompR) (see Table S1). These primers contained BglII restriction sites and overhangs complementary to the cloning vector pSUGK-Bgl (53). After PCR amplification, the amplicon was gel isolated, the vector and insert were digested with BglII (New England BioLabs, Ipswich, MA), and the fragments were combined in a ligation-independent fashion using the In-Fusion HD cloning kit (Clontech Laboratories, Inc., Mountain View, CA), according to the manufacturer’s instructions. This cloning reaction was then transformed into Escherichia coli Stellar competent cells (Clontech Laboratories), and transformants were selected on LB agar containing 50 μg ml−1 kanamycin. Colonies were verified by colony PCR using the primer pair gtfAseqkan and rmlDCompR (Table S1). Colonies that were positive for the amplicon were sequence verified. The resulting plasmid, pSUGKrmlD, was isolated and used to transform the S. mutans UA159 ΔrmlD strain. Attempts at recovering viable colonies from this transformation were unsuccessful (further commentary in Discussion), so we used an alternate approach that had previously been effective (4). The plasmid pSUGKrmlD was used to transform UA159, and colonies were selected on BHI agar medium containing 1 mg ml−1 kanamycin. This strain (UA159/rmlD+) was then transformed with the rmlD deletion construct mentioned above, and transformants were selected on BHI agar medium containing 5 μg ml−1 erythromycin. Colonies were confirmed by PCR with the same primer pair used to screen E. coli colonies (gtfAseqkan and rmlDCompR), and clones were sequence verified. A correct construction was named the rmlD+ strain, the rmlD complement strain.
Bacteria were grown in brain heart infusion (BHI) medium (BD Difco, Franklin Lakes, NJ), unless stated otherwise, in which case the strains were grown in tryptone-yeast extract (TY) broth (3% tryptone, 0.1% yeast extract, 0.5% KOH, and 1 mM H3PO4). All E. coli cultures were grown in Luria broth (LB) (1% tryptone, 0.5% yeast extract, and 1% NaCl).
Bacterial growth curves.
Growth rates of all strains were measured using a Bioscreen C plate reader (Growth Curves USA, Piscataway, NJ). Three independent overnight cultures of the UA159, ΔrmlD, and rmlD+ strains were diluted 1:10 into sterile BHI and incubated at 37°C in a 5% (vol/vol) CO2–95% air atmosphere until the cultures reached mid-log phase growth. At this point, a 10-μl aliquot of culture was diluted into 300 μl of fresh medium in a microtiter plate. All cultures were either grown in BHI medium, BHI titrated to pH 5.4, or BHI supplemented with 0.5 mM H2O2. Each well was given a mineral oil overlay (50 μl) to create a microaerobic environment and limit atmospheric oxygen, except where noted. The growth curves were performed at 37°C and readings (OD600) were taken every 15 min for 24 h after inoculation. Prior to each reading, 10 s of shaking at medium amplitude was applied to the microtiter plate to mix culture wells. The generation times for each strain were calculated using the formula 0.3/[(N − N0)/(T − T0)], where N represents the mean OD600 value at the end of the exponential growth phase and N0 represents the mean OD600 at the beginning of the exponential growth phase. T and T0 indicate the times (in minutes) that correspond to the OD600 values for N and N0, respectively.
Competition spot assays.
The ability of S. mutans strains to withstand acid and H2O2 was tested in competition spot assays (54). Primary spots were inoculated on BHI agar medium and allowed to grow for 24 h. S. mutans strains UA159 and rmlD+ were used as the primary spots for the assays examining acid stress, while Streptococcus gordonii DL1 and Streptococcus sanguinis 10904 were used as primary spots for the assays examining oxidative stress. Prior to spotting, overnight cultures of each strain were diluted 1:10 in fresh BHI medium and allowed to grow to mid-log phase (OD600, ∼0.5), and then 10 μl of the culture was plated, in triplicate, on BHI agar medium and allowed to grow for 24 h at 37°C in a 5% (vol/vol) CO2–95% air atmosphere. As a control, catalase (8 μg) (Sigma Chemical Company, St. Louis, MO) was added to cultures of the primary spots to ablate H2O2 diffusion into the medium prior to spotting on the plate. After 24 h, 10 μl of a mid-log phase culture of the secondary strain was spotted directly adjacently to the primary spot and allowed to grow for another 24 h. Assays measuring response to acidic stress were plated on agar medium that was either buffered to pH 7 with phosphate buffer or unbuffered. The experiments were performed three times with three independent cultures of all strains tested. A representative image from each competition assay is shown.
Minimum glycolytic pH determination.
Minimum glycolytic pH of S. mutans strains tested was measured using established protocols (51, 55). Briefly, three independent cultures of the S. mutans UA159, ΔrmlD, and rmlD+ strains were grown overnight and harvested. Cell pellets were washed twice in a solution of 50 mM KCl and 1 mM MgCl2. The cells were then resuspended to a final concentration of 10 mg cell dry weight ml−1. Five milliliters of the resuspended cells was titrated to a pH of 7.2 using 0.5 M KOH with continuous stirring. Then, 100 μl 50% (wt/vol) glucose was added, and the cell suspension was incubated at 37°C in a 5% (vol/vol) CO2–95% air atmosphere for 90 min, after which a final pH reading was recorded.
Biofilm formation assay.
Biofilm formation was assayed as previously described (26). Briefly, 200 μl of either 0.5× tryptone-yeast extract plus glucose (TYG) broth or 0.5× tryptone-yeast extract plus sucrose (TYS) broth was placed into the wells of a flat-bottomed 96-well microtiter plate. Six technical replicates of each strain, the S. mutans UA159, ΔrmlD, or rmlD+ strain, were used per plate for both the TYG and the TYS media. The wells were inoculated with a 5-μl aliquot of an overnight culture, and the microtiter plate was incubated at 37°C in a 5% (vol/vol) CO2–95% air atmosphere for 24 h. The medium was carefully removed, the plate was washed twice with water to remove remaining medium, and the plate was allowed to dry overnight. The biofilm was then stained with 50 μl 0.1% crystal violet for 15 min at room temperature. Unbound crystal violet was removed by rinsing the plate twice in water. After rinsing, 200 μl of 500 mM acetic acid was added to each well, and the plate was incubated for 1 h at room temperature to extract the bound crystal violet. An aliquot of the acetic acid/dye mix (100 μl) was then transferred from all wells to a clean microtiter plate, with the remaining 100 μl discarded. A fresh aliquot (200 μl) of 500 mM acetic acid was added to the plate for a second dye extraction and incubated for 15 min. A 100-μl aliquot was removed and combined with the first extraction in the second microtiter plate. Absorbance at OD575 was read using a Benchmark Plus microplate reader (Bio-Rad, Hercules, CA), representing the acetic acid-extracted crystal violet bound to the biofilms. Three independent cultures were used to determine significance and standard deviation by Student’s t test.
Chemical complementation of a rhamnose-deficient mutant.
To determine whether exogenous rhamnose could restore the growth of a ΔrmlD strain, we grew the strain in the presence or absence of glucose and/or rhamnose. Four different growth conditions were used as follows: tryptone-yeast extract (TY) alone, TY plus 1% (vol/vol) glucose, TY plus 1% (vol/vol) rhamnose, and TY plus 1% (vol/vol) glucose plus 1% (vol/vol) rhamnose. Overnight cultures of the UA159 and ΔrmlD strains (grown in TYG) were diluted 1:10 in fresh medium and incubated at 37°C in a 5% (vol/vol) CO2–95% air atmosphere. Absorbance at 600 nm was recorded 7 h and 24 h postinoculation. The experiment was performed with three independent cultures.
RGP isolation and l-rhamnose measurement.
Isolation of the S. mutans RGP was performed as described previously, with minor modifications (25). Three independent overnight cultures of the S. mutans UA159, ΔrgpG, ΔrmlD, and rmlD+ strains were grown overnight, harvested, washed twice with ice-cold citrate buffer (0.1 M citric acid, pH 4.6), and then resuspended with 25 ml citrate buffer per 5 g cell wet weight. The cells were mechanically lysed with 0.1-mm glass beads in a Bead Ruptor 12 (Omni International, Kennesaw, GA) five times in 1-min intervals. Cell lysates were transferred to 50-ml conical tubes, and the beads were allowed to settle. The supernatant was transferred to a fresh tube. Residual lysate was removed from the beads by washing with 5 ml of citrate buffer at 1,000 × g for 5 min. The supernatants were removed, combined, and pelleted at 15,000 × g for 30 min. The white pellets were resuspended in 10 ml lysis buffer (0.1 M sodium acetate, pH 4.6, with 4% sodium dodecyl sulfate), boiled for 1 h, and allowed to cool at room temperature overnight. After cooling, the boiled supernatants were centrifuged at 35,000 × g for 30 min at room temperature. The pellet was washed five times in distilled water (dH2O) and resuspended in 10 ml dH2O. Samples were treated with 10 μg ml−1 DNase and 10 μg ml−1 RNase (Sigma Chemical Company, St. Louis, MO) for 2 h at 37°C. After DNase/RNase treatment, the samples were treated with 100 μg ml−1 Pronase E overnight at 37°C. The cell suspension was centrifuged at 35,000 × g for 30 min and washed three times with dH2O. After the final wash, the supernatant was discarded, and the samples frozen at −80°C and then lyophilized. A portion of the lyophilized samples (10 mg) was resuspended in 5 ml 4 M trifluoroacetic acid (TFA) (Sigma Chemical Company, St. Louis, MO) and boiled for 2 h to hydrolyze polysaccharides into monosaccharides for further analysis. The cooled solution was frozen at −80°C overnight before being lyophilized again, leaving behind a brown pellet.
The monosaccharides isolated from each strain were diluted to 10 mg ml−1 for normalization. The RGP structures were assayed to determine rhamnose content across test strains using an l-rhamnose assay kit (Megazyme, Ireland) per the manufacturer’s instructions. Results are represented as micrograms of l-rhamnose per milliliter of reaction mixture.
Quantitative real-time PCR.
RNA was extracted from three independent cultures of the S. mutans UA159, ΔrmlD, or rmlD+ strain grown to mid-log phase as previously described (56). The high-capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA) was used to produce cDNA from RNA with random primers. The primer pair rmlDqPCRFor and rmlDqPCRRev (see Table S1) was used with Power SYBR green master mix (Applied Biosystems), and the reactions were performed in a StepOnePlus real-time PCR system (Applied Biosystems). The mRNA copy number was measured based on a standard curve of PCR products for the rmlD fragment.
Statistics.
Statistical significance was determined by pairwise comparison using Student’s t test.
Supplementary Material
ACKNOWLEDGMENTS
We graciously thank Nina van Sorge (University Medical Center Utrecht, The Netherlands) for the gift of the S. mutans Xc-derived ΔrmlD strain. We also thank Martin Pavelka (University of Rochester Medical Center, Rochester, NY) for his assistance in adapting the RGP isolation protocol and Elizabeth Lindsay for helpful discussions.
This study was supported by NIH/NIDCR DE-13683 and DE-17425 (to R.G.Q.) and the NIH/NIDCR Training Program in Oral Sciences DE021985 (to A.P.B. and C.J.K.).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Van de Rijn I, Bleiweis AS. 1973. Antigens of Streptococcus mutans. I. Characterization of a serotype-specific determinant from Streptococcus mutans. Infect Immun 7:795–804. doi: 10.1128/IAI.7.5.795-804.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovacs CJ, Faustoferri RC, Quivey RG Jr. 2017. RgpF is required for maintenance of stress tolerance and virulence in Streptococcus mutans. J Bacteriol 199:e00497-17. doi: 10.1128/JB.00497-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edgar RJ, van Hensbergen VP, Ruda A, Turner AG, Deng P, Le Breton Y, El-Sayed NM, Belew AT, McIver KS, McEwan AG, Morris AJ, Lambeau G, Walker MJ, Rush JS, Korotkov KV, Widmalm G, van Sorge NM, Korotkova N. 2019. Discovery of glycerol phosphate modification on streptococcal rhamnose polysaccharides. Nat Chem Biol 15:463–471. doi: 10.1038/s41589-019-0251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovacs CJ, Faustoferri RC, Bischer AP, Quivey RG Jr. 2019. Streptococcus mutans requires mature rhamnose-glucose polysaccharides for proper pathophysiology, morphogenesis and cellular division. Mol Microbiol 112:944–959. doi: 10.1111/mmi.14330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rainey K, Michalek SM, Wen ZT, Wu H. 2019. Glycosyltransferase-mediated biofilm matrix dynamics and virulence of Streptococcus mutans. Appl Environ Microbiol 85:e02247-18. doi: 10.1128/AEM.02247-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rainey K, Wilson L, Barnes S, Wu H. 2019. Quantitative proteomics uncovers the interaction between a virulence factor and mutanobactin synthetases in Streptococcus mutans. mSphere 4:e00429-19. doi: 10.1128/mSphere.00429-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins LV, Kristian SA, Weidenmaier C, Faigle M, Van Kessel KP, Van Strijp JA, Gotz F, Neumeister B, Peschel A. 2002. Staphylococcus aureus strains lacking D-alanine modifications of teichoic acids are highly susceptible to human neutrophil killing and are virulence attenuated in mice. J Infect Dis 186:214–219. doi: 10.1086/341454. [DOI] [PubMed] [Google Scholar]
- 8.Campbell J, Singh AK, Santa Maria JP Jr, Kim Y, Brown S, Swoboda JG, Mylonakis E, Wilkinson BJ, Walker S. 2011. Synthetic lethal compound combinations reveal a fundamental connection between wall teichoic acid and peptidoglycan biosyntheses in Staphylococcus aureus. ACS Chem Biol 6:106–116. doi: 10.1021/cb100269f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhavsar AP, Beveridge TJ, Brown ED. 2001. Precise deletion of tagD and controlled depletion of its product, glycerol 3-phosphate cytidylyltransferase, leads to irregular morphology and lysis of Bacillus subtilis grown at physiological temperature. J Bacteriol 183:6688–6693. doi: 10.1128/JB.183.22.6688-6693.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kern T, Giffard M, Hediger S, Amoroso A, Giustini C, Bui NK, Joris B, Bougault C, Vollmer W, Simorre JP. 2010. Dynamics characterization of fully hydrated bacterial cell walls by solid-state NMR: evidence for cooperative binding of metal ions. J Am Chem Soc 132:10911–10919. doi: 10.1021/ja104533w. [DOI] [PubMed] [Google Scholar]
- 11.Vergara-Irigaray M, Maira-Litrán T, Merino N, Pier GB, Penadés JR, Lasa I. 2008. Wall teichoic acids are dispensable for anchoring the PNAG exopolysaccharide to the Staphylococcus aureus cell surface. Microbiology 154:865–877. doi: 10.1099/mic.0.2007/013292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, Gotz F. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem 274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 13.Schlag M, Biswas R, Krismer B, Kohler T, Zoll S, Yu W, Schwarz H, Peschel A, Gotz F. 2010. Role of staphylococcal wall teichoic acid in targeting the major autolysin Atl. Mol Microbiol 75:864–873. doi: 10.1111/j.1365-2958.2009.07007.x. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita Y, Shibata Y, Nakano Y, Tsuda H, Kido N, Ohta M, Koga T. 1999. A novel gene required for rhamnose-glucose polysaccharide synthesis in Streptococcus mutans. J Bacteriol 181:6556–6559. doi: 10.1128/JB.181.20.6556-6559.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holland LM, Conlon B, O'Gara JP. 2011. Mutation of tagO reveals an essential role for wall teichoic acids in Staphylococcus epidermidis biofilm development. Microbiology 157:408–418. doi: 10.1099/mic.0.042234-0. [DOI] [PubMed] [Google Scholar]
- 16.Shibata Y, Yamashita Y, Ozaki K, Nakano Y, Koga T. 2002. Expression and characterization of streptococcal rgp genes required for rhamnan synthesis in Escherichia coli. Infect Immun 70:2891–2898. doi: 10.1128/iai.70.6.2891-2898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward JB. 1981. Teichoic and teichuronic acids: biosynthesis, assembly, and location. Microbiol Rev 45:211–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozaki K, Shibata Y, Yamashita Y, Nakano Y, Tsuda H, Koga T. 2002. A novel mechanism for glucose side-chain formation in rhamnose-glucose polysaccharide synthesis. FEBS Lett 532:159–163. doi: 10.1016/s0014-5793(02)03661-x. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita Y, Tsukioka Y, Tomihisa K, Nakano Y, Koga T. 1998. Genes involved in cell wall localization and side chain formation of rhamnose-glucose polysaccharide in Streptococcus mutans. J Bacteriol 180:5803–5807. doi: 10.1128/JB.180.21.5803-5807.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eberhardt A, Hoyland CN, Vollmer D, Bisle S, Cleverley RM, Johnsborg O, Havarstein LS, Lewis RJ, Vollmer W. 2012. Attachment of capsular polysaccharide to the cell wall in Streptococcus pneumoniae. Microb Drug Resist 18:240–255. doi: 10.1089/mdr.2011.0232. [DOI] [PubMed] [Google Scholar]
- 21.West NP, Sansonetti P, Mounier J, Exley RM, Parsot C, Guadagnini S, Prevost MC, Prochnicka-Chalufour A, Delepierre M, Tanguy M, Tang CM. 2005. Optimization of virulence functions through glucosylation of Shigella LPS. Science 307:1313–1317. doi: 10.1126/science.1108472. [DOI] [PubMed] [Google Scholar]
- 22.Brennan PJ, Nikaido H. 1995. The envelope of mycobacteria. Annu Rev Biochem 64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 23.Tsukioka Y, Yamashita Y, Nakano Y, Oho T, Koga T. 1997. Identification of a fourth gene involved in dTDP-rhamnose synthesis in Streptococcus mutans. J Bacteriol 179:4411–4414. doi: 10.1128/jb.179.13.4411-4414.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blankenfeldt W, Kerr ID, Giraud MF, McMiken HJ, Leonard G, Whitfield C, Messner P, Graninger M, Naismith JH. 2002. Variation on a theme of SDR. dTDP-6-deoxy-L- lyxo-4-hexulose reductase (RmlD) shows a new Mg2+-dependent dimerization mode. Structure 10:773–786. doi: 10.1016/S0969-2126(02)00770-0. [DOI] [PubMed] [Google Scholar]
- 25.van der Beek SL, Le Breton Y, Ferenbach AT, Chapman RN, van Aalten DM, Navratilova I, Boons GJ, McIver KS, van Sorge NM, Dorfmueller HC. 2015. GacA is essential for group A Streptococcus and defines a new class of monomeric dTDP-4-dehydrorhamnose reductases (RmlD). Mol Microbiol 98:946–962. doi: 10.1111/mmi.13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quivey RG Jr, Grayhack EJ, Faustoferri RC, Hubbard CJ, Baldeck JD, Wolf AS, MacGilvray ME, Rosalen PL, Scott-Anne K, Santiago B, Gopal S, Payne J, Marquis RE. 2015. Functional profiling in Streptococcus mutans: construction and examination of a genomic collection of gene deletion mutants. Mol Oral Microbiol 30:474–495. doi: 10.1111/omi.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ajdic D, McShan WM, McLaughlin RE, Savic G, Chang J, Carson MB, Primeaux C, Tian R, Kenton S, Jia H, Lin S, Qian Y, Li S, Zhu H, Najar F, Lai H, White J, Roe BA, Ferretti JJ. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc Natl Acad Sci U S A 99:14434–14439. doi: 10.1073/pnas.172501299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovacs CJ. 2018. Defining functional roles for the rhamnose-glucose polysaccharides of Streptococcus mutans. PhD dissertation. University of Rochester, Rochester, New York. [Google Scholar]
- 29.Kreth J, Zhang Y, Herzberg MC. 2008. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J Bacteriol 190:4632–4640. doi: 10.1128/JB.00276-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De A, Liao S, Bitoun JP, Roth R, Beatty WL, Wu H, Wen ZT. 2017. Deficiency of RgpG causes major defects in cell division and biofilm formation, and deficiency of LytR-CpsA-Psr family proteins leads to accumulation of cell wall antigens in culture medium by Streptococcus mutans. Appl Environ Microbiol 83:e00928-17. doi: 10.1128/AEM.00928-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higuchi M, Shimada M, Yamamoto Y, Hayashi T, Koga T, Kamio Y. 1993. Identification of two distinct NADH oxidases corresponding to H2O2-forming oxidase and H2O-forming oxidase induced in Streptococcus mutans. J Gen Microbiol 139:2343–2351. doi: 10.1099/00221287-139-10-2343. [DOI] [PubMed] [Google Scholar]
- 32.Martin ME, Byers BR, Olson MO, Salin ML, Arceneaux JE, Tolbert C. 1986. A Streptococcus mutans superoxide dismutase that is active with either manganese or iron as a cofactor. J Biol Chem 261:9361–9367. [PubMed] [Google Scholar]
- 33.Yamamoto Y, Higuchi M, Poole LB, Kamio Y. 2000. Role of the dpr product in oxygen tolerance in Streptococcus mutans. J Bacteriol 182:3740–3747. doi: 10.1128/jb.182.13.3740-3747.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fenton H. 1894. Oxidation of tartaric acid in presence of iron. J Chem Soc, Trans 65:899–910. doi: 10.1039/CT8946500899. [DOI] [Google Scholar]
- 35.Yamamoto Y, Fukui K, Koujin N, Ohya H, Kimura K, Kamio Y. 2004. Regulation of the intracellular free iron pool by Dpr provides oxygen tolerance to Streptococcus mutans. J Bacteriol 186:5997–6002. doi: 10.1128/JB.186.18.5997-6002.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lo T, Chang C, Chiu K-H, Tsay P-K, Jen J-F. 2011. Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohyd Polym 86:320–327. doi: 10.1016/j.carbpol.2011.04.056. [DOI] [Google Scholar]
- 37.Ajdic D, Pham VT. 2007. Global transcriptional analysis of Streptococcus mutans sugar transporters using microarrays. J Bacteriol 189:5049–5059. doi: 10.1128/JB.00338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith EG, Spatafora GA. 2012. Gene regulation in S. mutans: complex control in a complex environment. J Dent Res 91:133–141. doi: 10.1177/0022034511415415. [DOI] [PubMed] [Google Scholar]
- 39.Zeng L, Xue P, Stanhope MJ, Burne RA. 2013. A galactose-specific sugar: phosphotransferase permease is prevalent in the non-core genome of Streptococcus mutans. Mol Oral Microbiol 28:292–301. doi: 10.1111/omi.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirooka K, Kodoi Y, Satomura T, Fujita Y. 2015. Regulation of the rhaEWRBMA operon involved in L-rhamnose catabolism through two transcriptional factors, RhaR and CcpA, in Bacillus subtilis. J Bacteriol 198:830–845. doi: 10.1128/JB.00856-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Earl AM, Losick R, Kolter R. 2008. Ecology and genomics of Bacillus subtilis. Trends Microbiol 16:269–275. doi: 10.1016/j.tim.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeleznick LD, Boltralik JJ, Barkulis SS, Smith C, Heymann H. 1963. Biosynthesis of streptococcal cell walls: a rhamnose polysaccharide. Science 140:400–401. doi: 10.1126/science.140.3565.400. [DOI] [PubMed] [Google Scholar]
- 43.Mistou MY, Sutcliffe IC, van Sorge NM. 2016. Bacterial glycobiology: rhamnose-containing cell wall polysaccharides in Gram-positive bacteria. FEMS Microbiol Rev 40:464–479. doi: 10.1093/femsre/fuw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zorzoli A, Meyer BH, Adair E, Torgov VI, Veselovsky VV, Danilov LL, Uhrin D, Dorfmueller HC. 2019. Group A, B, C, and G Streptococcus Lancefield antigen biosynthesis is initiated by a conserved alpha-d-GlcNAc-beta-1,4-l-rhamnosyltransferase. J Biol Chem 294:15237–15256. doi: 10.1074/jbc.RA119.009894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li YH, Lau PC, Lee JH, Ellen RP, Cvitkovitch DG. 2001. Natural genetic transformation of Streptococcus mutans growing in biofilms. J Bacteriol 183:897–908. doi: 10.1128/JB.183.3.897-908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaccaria E, Wels M, van Baarlen P, Wells JM. 2016. Temporal regulation of the transformasome and competence development in Streptococcus suis. Front Microbiol 7:1922. doi: 10.3389/fmicb.2016.01922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mirouze N, Ferret C, Cornilleau C, Carballido-López R. 2018. Antibiotic sensitivity reveals that wall teichoic acids mediate DNA binding during competence in Bacillus subtilis. Nat Commun 9:5072. doi: 10.1038/s41467-018-07553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shields RC, O’Brien G, Maricic N, Kesterson A, Grace M, Hagen SJ, Burne RA. 2018. Genome-wide screens reveal new gene products that influence genetic competence in Streptococcus mutans. J Bacteriol 200:e00508-17. doi: 10.1128/JB.00508-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee MS, Morrison DA. 1999. Identification of a new regulator in Streptococcus pneumoniae linking quorum sensing to competence for genetic transformation. J Bacteriol 181:5004–5016. doi: 10.1128/JB.181.16.5004-5016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Beek SL, Zorzoli A, Canak E, Chapman RN, Lucas K, Meyer BH, Evangelopoulos D, de Carvalho LPS, Boons GJ, Dorfmueller HC, van Sorge NM. 2019. Streptococcal dTDP-L-rhamnose biosynthesis enzymes: functional characterization and lead compound identification. Mol Microbiol 111:951–964. doi: 10.1111/mmi.14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santiago B, MacGilvray M, Faustoferri RC, Quivey RG Jr. 2012. The branched-chain amino acid aminotransferase encoded by ilvE is involved in acid tolerance in Streptococcus mutans. J Bacteriol 194:2010–2019. doi: 10.1128/JB.06737-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Faustoferri RC, Hubbard CJ, Santiago B, Buckley AA, Seifert TB, Quivey RG Jr. 2015. Regulation of fatty acid biosynthesis by the global regulator CcpA and the local regulator FabT in Streptococcus mutans. Mol Oral Microbiol 30:128–146. doi: 10.1111/omi.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Derr AM, Faustoferri RC, Betzenhauser MJ, Gonzalez K, Marquis RE, Quivey RG Jr. 2012. Mutation of the NADH oxidase gene (nox) reveals an overlap of the oxygen- and acid-mediated stress responses in Streptococcus mutans. Appl Environ Microbiol 78:1215–1227. doi: 10.1128/AEM.06890-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kreth J, Merritt J, Shi W, Qi F. 2005. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J Bacteriol 187:7193–7203. doi: 10.1128/JB.187.21.7193-7203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belli WA, Marquis RE. 1994. Catabolite modification of acid tolerance of Streptococcus mutans GS-5. Oral Microbiol Immunol 9:29–34. doi: 10.1111/j.1399-302x.1994.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 56.Baker JL, Derr AM, Karuppaiah K, MacGilvray ME, Kajfasz JK, Faustoferri RC, Rivera-Ramos I, Bitoun JP, Lemos JA, Wen ZT, Quivey RG Jr. 2014. Streptococcus mutans NADH oxidase lies at the intersection of overlapping regulons controlled by oxygen and NAD+ levels. J Bacteriol 196:2166–2177. doi: 10.1128/JB.01542-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.