Background:
Little is known about the efficacy of newer skin substitute scaffolds to reconstruct complex lower extremity wounds. The investigators present a multihospital experience of reconstructive surgeons utilizing collagen-GAG bilayer wound matrix in lower extremity soft-tissue reconstruction with the goals to (1) characterize a suitable patient population, (2) categorize failures to optimize patient selection, and (3) determine wound factors affecting success.
Methods:
Subjects underwent collagen-GAG–based lower extremity wound reconstruction from May of 2010 to June of 2017. The primary outcome variable was 180-day graft success, defined as eventual split-thickness skin grafting after bilayer wound matrix application; failure was defined as inadequate wound bed for split-thickness skin grafting, requirement for vascularized tissue transfer, or eventual amputation. Eligible subjects had at least one lower extremity wound and were at least 18 years old. Exclusion criteria included third-degree burn wounds or failure to follow up for at least 60 days postoperatively. Predictor variables included demographics, medical comorbidities, perioperative characteristics, postoperative complications, and cost-related data for each hospitalization.
Results:
There were 147 subjects with 191 wounds. Mean patient age was 60.1 years (range, 21.0 to 95.6 years), and mean body mass index was 30.5 kg/m2 (range, 14.4 to 64.7 kg/m2). Average wound size was 73.1 ± 137.7 cm2, with 49.0 percent of subjects receiving adjunct postoperative negative-pressure wound therapy. Seventy percent of wounds were successfully healed at 180 days. Most were localized between the knee and ankle (50.8 percent) or foot (46.1 percent). Tendon exposure (p < 0.05), bone exposure (p < 0.01), and bone excision (p < 0.04) were associated with reconstructive failure.
Conclusions:
The authors present the largest reported multihospital, multidisciplinary experience with collagen-GAG wound matrix for lower extremity reconstruction. Tendon and/or bone exposure and socioeconomic factors were associated with failure.
CLINICAL QUESTION/LEVEL OF EVIDENCE:
Risk, III.
Lower extremity wounds represent a heterogeneous and often complex clinical situation associated with high rates of failure and morbidity. These high failure and morbidity rates are due to the paucity of soft tissue in between the skin and bones of the lower extremity, which leads to frequent exposure and subsequent higher rates of osteomyelitis and limb amputation after wound development. The presence of common comorbidities, such as diabetes mellitus, peripheral arterial disease, bacterial colonization, impaired tissue perfusion, and venous insufficiency, further complicate healing. The economic burden associated with diabetic complications in the United States, for example, was $116 billion in 2007, with 33 percent of this cost attributed to neuropathic foot ulcers.1 Following limb amputation, the mortality rates for patients with and without diabetes are estimated to be 61 percent and 54.3 percent approximately 5.2 and 5.3 years after amputation, respectively.2,3 These numbers are predicted to rise proportionately with the increasing incidence of diabetes and obesity. Given this risk, extremity salvage has a tremendous impact on overall survival, quality of life, and function.
Wound management has continued to evolve rapidly over the last several decades, with the ultimate goal of functional restoration, where function is defined as a weight-bearing limb with sufficient soft-tissue coverage to protect underlying bone.4 With the advancements in microvascular techniques made during the 1980s, fasciocutaneous flaps supplemented muscle flaps in the reconstruction of complex, large lower extremity wounds, while smaller defects continued to be reconstructed with local tissues that offered better color match, pliability, and less donor-site morbidity.5–7 Yet these autologous tissue flaps remain somewhat limited in their ability to treat lower extremity wounds of varying size, location, and depth. Christy et al.8 described limitations of successful lower extremity reconstruction using free fasciocutaneous flaps, including subjects with a history of tobacco use and risk factors for atherosclerosis. Postoperative complications included hematoma, infection, thromboembolism, flap loss, and progression to amputation. In a systemic review by Bekara et al.,9 risk factors associated with propeller flap failure in lower extremity reconstruction included age greater than 60 years, diabetes, and arteriopathy, while Nelson et al.5 found partial and complete flap necrosis rates of 11.6 and 5.5 percent, respectively. Moreover, defects of increasing size consistently required donor-site skin grafting. The procedures utilized for lower extremity wound reconstruction, such as primary closure, skin grafting, and local, pedicled, or free tissue flaps, have been widely used by surgeons, but little is known about the efficacy of newer skin substitute allografts and xenograft scaffolds to reconstruct these complex wounds. The decision-making process of which procedure or product to use is patient-specific and multifactorial, based on patient-specific end goals, comorbidities, social and psychological factors, wound etiology, and socioeconomic factors related to health literacy.
The ultimate goal in lower extremity reconstruction remains consistent—the restoration or maintenance of function, where function is defined as a stable limb that allows for weight bearing with sufficient soft-tissue coverage to protect the underlying bone.4 Introduced in the 2000s, acellular dermal matrix substitute grafts provide even more options for reconstruction. Products such as Integra bilayer wound matrix (Integra Lifesciences, Plainsboro, N.J.) have achieved satisfactory outcomes for this purpose, which “challenge the current gold-standard treatment” of lower extremity defects.10 Serving as a dermal equivalent not reliant on immediate imbibition or inosculation for successful take, the scaffold can vascularize within 2 to 4 weeks even over poorly perfused structures, after which the outer silicone layer is subsequently replaced with a thin split-thickness skin graft.11 When compared to autologous tissues, these xenograft-based reconstructions avoid donor-site morbidity, offer ease of use and immediate wound coverage, provide a treatment modality in hospital centers that lack microvascular-trained surgeons to harvest pedicled or free tissue flaps, and serve as an alternative for patients with comorbidities and donor sites precluding sophisticated autologous tissue reconstruction (i.e., peripheral arteriopathy, excessive adiposity).
We present a multihospital experience of multidisciplinary reconstructive surgeons utilizing this collagen-GAG wound matrix in complex lower extremity soft-tissue reconstruction with the goals to (1) characterize a suitable patient population, (2) categorize failures to optimize patient selection, and (3) determine wound factors affecting success. We hypothesized that collagen-GAG bilayer wound matrices can be utilized successfully in the reconstruction of complex lower extremity wounds of varying location, size, and depth.
METHODS
Study Design and Sample Selection
This investigator-designed, retrospective case-control study of patients undergoing lower extremity wound reconstruction with collagen-GAG wound matrix (Integra bilayer wound matrix) within the University of Pennsylvania Health Systems from May of 2010 to June of 2017 was implemented after institutional review board approval was obtained. The patient population was derived from a free-text search engine (PennSeek; Penn Medicine, Philadelphia, Pa.) of operative notes using Boolean search terms “Integra AND lower extremity wound.” Subjects eligible for study inclusion presented to plastic, orthopedic, or podiatric surgery with at least one lower extremity wound and were at least 18 years of age. Subjects were excluded from the study if they presented with a third-degree burn wound or failed to follow up for at least 60 days postoperatively. The primary outcome variable was 180-day split-thickness skin graft success. Two cohorts were identified: those whose reconstruction succeeded and those for whom it failed. Successful reconstruction was defined as the ability to successfully stage a split-thickness skin graft onto the given wound bed, and failure was defined as an inadequate wound bed for split-thickness skin grafting, requirement for vascularized tissue transfer, such as local or free flap, or eventual amputation within the 180-day postoperative time period.
Study Variables
Graft success at 180 days served as the binary outcome variable of interest. Additional secondary outcomes included graft success at the 60- and 120-day intervals and postoperative complications, including dehiscence, infection, and amputation. Predictor variables included demographic information (i.e., age, gender, race, body mass index, smoking history, corticosteroid use, medical comorbidities, and ambulatory status) and perioperative characteristics (i.e., wound location, wound type, exposed deep structures, necrotizing soft-tissue infection, length of procedure, length of stay, wound size, wound age, and total cost).
The investigators derived cost-related data for each patient’s hospital course and subsequent wound-related admissions or reoperations from the Department of Finance at the Hospital of the University of Pennsylvania. Total costs did not include professional service fees and only reflected costs to the University of Pennsylvania Health System.
Data Analyses
Data were analyzed as two cohorts, divided by success or failure of the collagen-GAG wound matrix reconstruction. Categorical variables were analyzed using Pearson chi-square or Fisher’s exact tests, while continuous variables were examined with Wilcoxon rank sum or Mann-Whitney tests. Preoperative and intraoperative variables with a p < 0.10 on univariate analysis were included in a multivariate logistic regression analysis as independent variables, with bilayer wound matrix failure as the dependent variable. All tests were two-tailed, with statistical significance defined as p < 0.05. Analyses were performed using STATA IC 11.0 (StataCorp, College Station, Texas).
RESULTS
Patient Characteristics
A total of 147 subjects with 191 lower extremity wounds underwent reconstruction using collagen-GAG wound matrices (Table 1). Subjects presented for reconstruction to orthopedic (23.1 percent), plastic (35.6 percent), podiatric (38.9 percent), and vascular (2.4 percent) surgeons. There was no significant difference in reconstructive success based on surgeon subspecialty (p < 0.60) (Table 2). Average age at reconstruction was 60.1 years, and an average body mass index was 30.5 kg/m2. Male patients and Caucasian patients comprised 59.2 percent and 55.1 percent, respectively, of the study subjects. The most prevalent comorbidities included hypertension (76.9 percent), diabetes (52.4 percent), and peripheral vascular disease (44.9 percent). Wound types were cellulitic (7.2 percent), diabetic (21.6 percent), pressure (8.7 percent), surgical site (30.3 percent), traumatic (11.5 percent), and vascular (20.7 percent). Fifty-seven wounds (30.0 percent) at the 180-day timepoint experienced failed reconstruction using collagen-GAG bilayer wound matrix. There were no significant differences in demographics, ambulatory status, or comorbidities, including cancer, chronic obstructive pulmonary disease, corticosteroid use, smoking status, diabetes, hypertension, or peripheral vascular disease, when comparing subjects who underwent successful reconstruction with those whose reconstruction failed.
Table 1.
Demographic Information Stratified by 180-Day Success and Failure
| Success | Failure | p | |
|---|---|---|---|
| No. of patients (%) | 103 (70.1) | 44 (29.9) | |
| No. of wounds (%) | 134 (70.2) | 57 (29.8) | |
| Median age (IQR), yr | 60.6 (51.6 75.0) | 58.8 (45.4–74.7) | 0.60 |
| Gender, no. (%) | 0.28 | ||
| Female | 45 (43.7) | 15 (34.1) | |
| Male | 58 (56.3) | 29 (65.9) | |
| Race, no. (%) | 0.001* | ||
| African American | 32 (31.1) | 27 (61.4) | |
| Caucasian | 64 (62.1) | 17 (38.6) | |
| Other/unknown | 7 (6.8) | 0 (0.0) | |
| Median BMI (IQR), kg/m2 | 28.8 (24.0–33.5) | 29.8 (23.5–33.5) | 0.90 |
| DM, no. (%) | 50 (48.5) | 27 (61.4) | |
| Smoking, no. (%) | 0.62 | ||
| Never smoked | 56 (54.4) | 22 (50.0) | |
| Former smoker | 29 (28.2) | 16 (36.4) | |
| Current smoker | 17 (16.5) | 6 (13.6) | |
| COPD, no. (%) | 14 (13.6) | 5 (11.4) | 0.71 |
| PVD, no. (%) | 42 (40.8) | 24 (54.5) | 0.12 |
| HTN, no. (%) | 76 (73.8) | 37 (84.1) | 0.18 |
| Cancer history, no. (%) | 21 (20.4) | 6 (13.6) | 0.49 |
| Corticosteroid use, no. (%) | 17 (16.5) | 2 (4.5) | 0.06 |
| Ambulatory, no. (%) | 99 (96.1) | 42 (95.5) | 1 |
IQR, interquartile range; no., number of subjects; BMI, body mass index; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; PVD, peripheral vascular disease; HTN, hypertension.
*p < 0.05.
Table 2.
Perioperative Characteristics and Costs Stratified by 180-Day Success and Failure
| Success | Failure | p | |
|---|---|---|---|
| Surgical subspecialty, no. (%) | 0.60 | ||
| Orthopedic | 33 (24.6) | 11 (19.3) | |
| Plastic | 49 (36.6) | 18 (31.6) | |
| Podiatric | 49 (36.6) | 26 (45.6) | |
| Vascular | 3 (2.2) | 2 (3.5) | |
| Wound location, no. (%) | 0.30 | ||
| Hip | 1 (0.7) | 1 (1.8) | |
| Thigh | 3 (2.2) | 1 (1.8) | |
| Knee/ankle | 73 (54.5) | 24 (42.1) | |
| Foot | 57 (42.5) | 31 (54.4) | |
| Wound type, no. (%) | 0.11 | ||
| Cellulitic | 8 (6.0) | 6 (10.5) | |
| Diabetic | 24 (17.9) | 19 (33.3) | |
| Pressure | 12 (9.0) | 5 (8.8) | |
| Surgical site | 47 (35.1) | 13 (22.8) | |
| Traumatic | 16 (11.9) | 3 (5.3) | |
| Vascular | 27 (20.1) | 11 (19.3) | |
| Tendon exposure, no. (%) | 25 (18.7) | 18 (31.6) | 0.05* |
| Tendon excision, no. (%) | 20 (14.9) | 10 (17.5) | 0.65 |
| Bone exposure, no. (%) | 28 (20.9) | 22 (38.6) | 0.01* |
| Bone excision, no. (%) | 15 (11.2) | 13 (22.8) | 0.04* |
| Preoperative wound colonization, no. (%) | 32 (23.9) | 21 (36.8) | 0.07 |
| Necrotizing fasciitis, no. (%) | 10 (7.5) | 0 (0.0) | 0.04* |
| Edema, no. (%) | 9 (6.7) | 6 (10.5) | 0.37 |
| Median length of procedure, min (IQR) | 28.5 (24.1–33.5) | 43.0 (20.0–82.0) | 0.25 |
| Median total LOS, days (IQR) | 5.5 (0.0–14.0) | 18.0 (11.0–27.0) | <0.01* |
| Median wound area, cm2 (IQR) | 25.0 (9.0–68.0) | 28.0 (12.0–63.0) | 0.98 |
| Median wound age, days (IQR) | 45.1 (10.3–169.4) | 173.7 (35.8–760.0) | <0.01* |
| Postoperative wound VAC, no. (%) | 61 (45.5) | 29 (50.9) | 0.50 |
| Median length of wound VAC, days (IQR) | 6.0 (5.0–20.0) | 6.5 (5.0–10.0) | 0.77 |
| Median direct cost, $ (IQR) | 16,714.29 (8,662.52–30,573.00) |
39,221.35 (27,860.71–58,448.63) |
<0.01* |
| Median total cost, $ (IQR) | 24,510.75 (12,520.67–46,015.13) |
59,680.11 (41,987.46–89,542.26) |
<0.01* |
| Median total charges, $ (IQR) | 112,441.20 (68,404.57–207,196.30) |
269,528.20 (17,9242.50--413,428.30) | <0.01* |
| Median income by zip code, $ (IQR) | 78,767.00 (49,509–107,341) |
64,317.00 (44,288–82,959) |
<0.01* |
| Insurance type, no. (%) | 0.02* | ||
| Commercial | 45 (35.2) | 15 (19.7) | |
| Government | 83 (64.8) | 61 (80.3) |
No., number of wounds; IQR, interquartile range; LOS, length of stay; VAC, vacuum-assisted closure.
*p < 0.05.
Operative Characteristics
The treated wounds were located between the knee and ankle (50.8 percent), foot (46.1 percent), thigh (2.1 percent), and hip (1.0 percent). Average wound size was 73.1 ± 137.7 cm2, with 49.0 percent of subjects receiving adjunct negative-pressure wound therapy for a mean period of 13.9 ± 18.3 days. Exposed bone, exposed tendon, preoperative infection, and necrotizing fasciitis were observed in 26.2 percent, 22.5 percent, 27.7 percent, and 5.2 percent of wounds, respectively (Table 2). In univariate regression analysis, tendon exposure (p < 0.05) and bone exposure (p < 0.01) were associated with reconstructive failure when using collagen-GAG matrix. Wound area treated (70.6 cm2 versus 58.6 cm2 for success versus failure, respectively) did not differ significantly between groups; however, wound age differed significantly (success, 203.1 days versus failure, 814.2 days) (Table 2). Preoperative wound colonization, which was determined by wound biopsy, was not significantly associated with reconstructive failure (p < 0.07), while chronic wound colonization was measured in a subset of patients (28.9 percent) in this retrospective study. Figure 1 highlights successful collagen-GAG matrix–based reconstruction of a 7-day-old complex degloving injury of the lower extremity measuring 1450 cm2 in an 86-year-old diabetic Caucasian man.
Fig. 1.
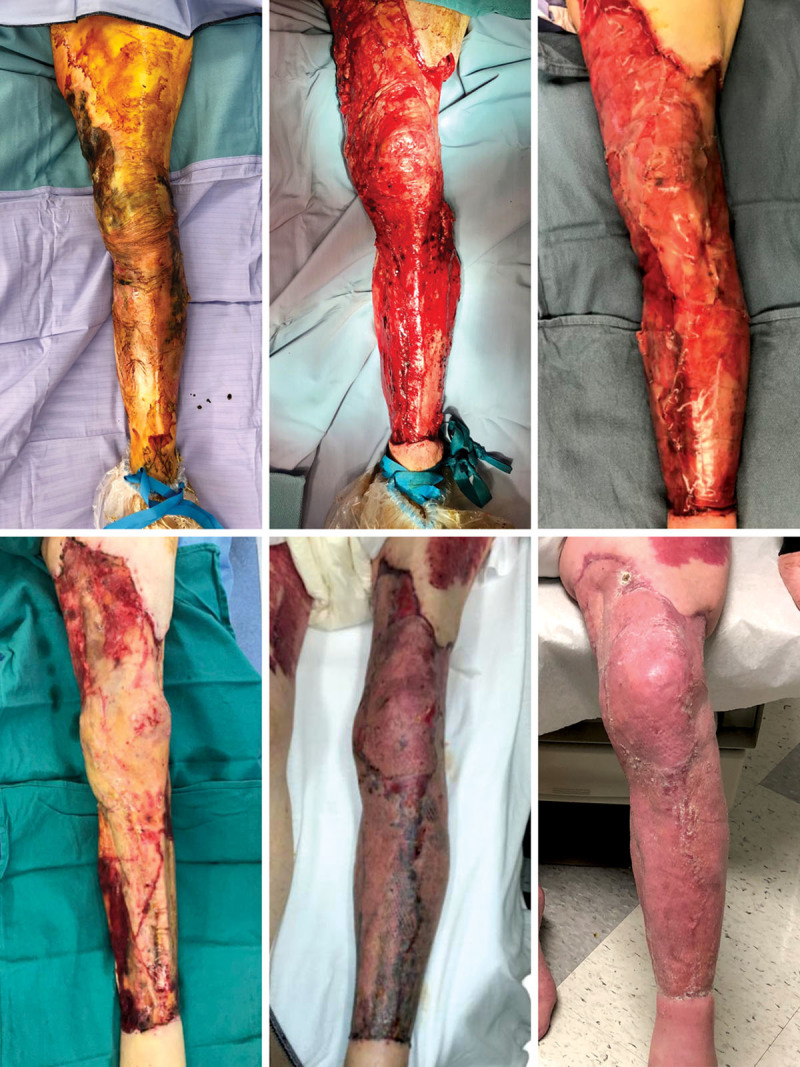
Lower extremity salvage using bilayer wound matrix following extensive debridement of degloving wound. (Above, left) An 86-year-old male subject presented 7 days after experiencing an extensive degloving injury from midthigh to proximal ankle. (Above, center) Exposed tendon and tibia with intact peritenon and periosteum. (Above, right) Application of Integra bilayer wound matrix measuring 1450 cm2. (Below, left) Removal of silicone layer 3 weeks after application revealing well-granulated tissue bed. (Below, center) Application of meshed split-thickness skin graft. (Below, right) Long-term result with 100 percent graft take and successful limb salvage. These photographs were obtained with written permission.
Outcomes and Costs
Overall, 70 percent of the treated wounds were healed successfully. Bilayer wound matrix failure rates at the 60-day, 120-day, and 180-day intervals were 18.6 percent, 24.6 percent, and 30.0 percent, respectively (Table 3). There was a significant difference in bilayer wound matrix failure and wound type at the 60-day timepoint (p < 0.035), with cellulitic (40.0 percent) and diabetic (28.9 percent) wounds comprising the majority of early failures (Table 4). There were significant intergroup differences based on wound type at the 60-, 120-, and 180-day timepoints (p < 0.001) (Fig. 2).
Table 3.
Failure Rates and Amputation Rates*
| No. (%) | p | |
|---|---|---|
| 60-Day failure | 38 (18.6) | – |
| 120-Day failure | 52 (26.4) | – |
| 180-Day failure | 57 (30.0) | – |
| Amputation rates stratified by wound type | 0.50 | |
| Cellulitic | 2 (13.3) | |
| Diabetic | 11 (24.4) | |
| Pressure | 2 (11.1) | |
| Surgical site | 9 (14.8) | |
| Traumatic | 4 (17.4) | |
| Vascular | 4 (9.3) |
No., number of wounds.
*The 60-day follow-up rate was 98.6 percent, the 120-day follow-up rate was 96.1 percent, and the 180-day follow-up rate was 96.4 percent.
Table 4.
Failure Rates Stratified by Wound Type*
| Timepoint | Wound Type | p | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cellulitic | Diabetic | Pressure | Surgical Site | Traumatic | Vascular | ||||||||
| Success | Failure | Success | Failure | Success | Failure | Success | Failure | Success | Failure | Success | Failure | ||
| Day 60 | 9 (60.0) | 6 (40.0) | 32 (71.1) | 13 (28.9) | 14 (77.8) | 4 (22.2) | 54 (87.1) | 8 (12.9) | 20 (87.0) | 3 (13.0) | 38 (90.5) | 4 (9.5) | 0.038† |
| Day 120 | 8 (57.1) | 6 (42.9) | 27 (61.4) | 17 (38.6) | 13 (72.2) | 5 (27.8) | 51 (82.3) | 11 (17.7) | 16 (84.2) | 3 (15.8) | 30 (75.0) | 10 (25.0) | 0.12 |
| Day 180 | 8 (57.1) | 6 (42.9) | 24 (55.8) | 19 (44.2) | 12 (70.6) | 5 (29.4) | 47 (78.3) | 13 (21.7) | 16 (84.2) | 3 (15.8) | 27 (71.1) | 11 (29.0) | 0.11 |
*There was a significant difference in failure rates at the early 60-day timepoint when stratifying subjects by wound type (p < 0.038). This difference did not persist at the later timepoints.
†p < 0.05.
Fig. 2.
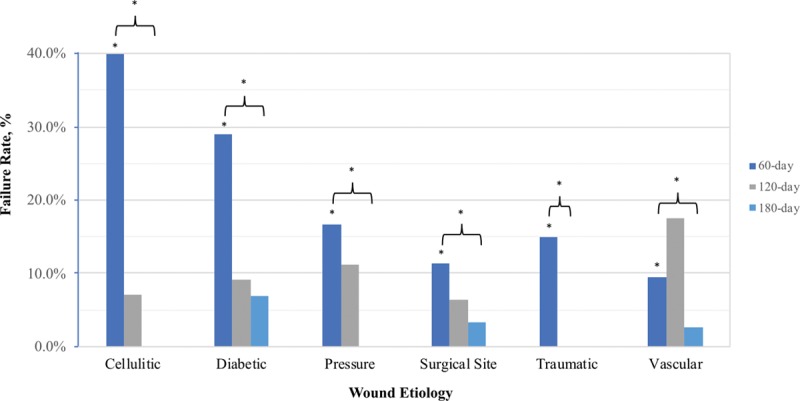
Bilayer wound matrix failure rates stratified by wound type at varying timepoints. There was a significant difference between failure and wound type at the 60-day timepoint (p < 0.035), with cellulitic (40.0 percent) and diabetic (28.9 percent) wounds comprising the majority of early failures. There were significant intergroup differences based on wound type at the various timepoints (brackets). *p < 0.05.
Wound complications were significantly increased in subjects whose reconstruction failed, including dehiscence (success, 2.2 percent; failure, 21.1 percent), need for additional debridement (success, 29.9 percent; failure, 38.6 percent), partial necrosis (success, 6.0 percent; failure, 28.1 percent), and full-thickness necrosis (success, 4.5 percent; failure, 43.9 percent) (Table 5). No patients experienced postreconstruction sepsis. Amputations occurred in 27 subjects with failed wound reconstruction, comprising 47.4 percent of all failures. Amputation type was dependent on wound location, as the majority of amputations occurred below the knee (n = 15, 26.3 percent) and the majority of wounds were located from the foot just distal to the knee (n = 97, 50.8 percent). There were no significant differences noted in amputation rates when stratifying by wound type (p < 0.54), although diabetic (34.4 percent) and surgical site (28.1 percent) wounds comprised the majority of wounds proceeding to amputation (Tables 3 and 5).
Table 5.
Complications and Amputation Rates
| Success (n = 134) | Failure (n = 57) | p | |
|---|---|---|---|
| Additional debridement | <0.01* | ||
| 1 | 40 (29.9) | 22 (38.6) | |
| 2+ | 20 (14.9) | 22 (38.6) | |
| Cellulitis/infection | 9 (8.7) | 6 (13.6) | 0.37 |
| Postoperative antibiotic use | 24 (17.9) | 27 (47.4) | <0.01* |
| Dehiscence | 3 (2.2) | 12 (21.1) | <0.01* |
| Necrosis (partial) | 8 (6.0) | 16 (28 .1) | <0.01* |
| Necrosis (full) | 6 (4.5) | 25 (43.9) | <0.01* |
| Sepsis | 0 | 0 | - |
| Amputation | 3 (2.2) | 27 (47.4) | <0.01* |
| TMA | 0 (0.0) | 6 (10.5) | |
| BKA | 2 (1.5) | 15 (26.3) | |
| AKA | 1 (0.7) | 6 (10.5) |
n, number of wounds; AKA, above-knee amputation; BKA, below-knee amputation; TMA, transmetatarsal amputation.
*p < 0.05.
When comparing successfully treated wounds with those that failed, the following factors differed significantly: length of stay: success, 5.5 days; failure, 18.0 days (p < 0.01); direct costs: success, $16,714.29; failure, $39,221.35 (p < 0.01); total costs: success, $24,510.75; failure, $59,680.11 (p < 0.01); total charges: success, $112,441.20; failure, $269,528.20 (p < 0.01); and average income by zip code: success, $78,767.00; failure, $64,317.00 (p < 0.01) (Table 2). Length of procedure (success, 28.5 minutes; failure, 43.0 minutes) did not differ significantly between groups.
Multivariate Regression Analyses
Multivariate risks factors associated with failed reconstruction at the 60-day timepoint included African American race (OR, 3.46), smoking history (OR, 3.04), and wound age (OR, 1.54) (Table 6). Risk factors associated with failed reconstruction at the 120-day timepoint included African American race (OR, 2.33) and wound age (OR, 1.20). Risks factors associated with failed reconstruction at the 180-day timepoint included government insurance (OR, 2.12), African American race (OR, 2.07), tendon exposure (OR, 2.09), and wound age (OR, 1.32).
Table 6.
Multivariate Risk Factors Associated with Failure
| Odds Ratio | 95% CI | p | |
|---|---|---|---|
| 60-Day failure | |||
| African American race | 3.46 | [1.23, 9.70] | 0.02* |
| Former smoker | 3.04 | [1.01, 9.14] | <0.05* |
| Current smoker | 0.21 | [0.04, 1.22] | 0.08 |
| Diabetic ulcer | 1.33 | [0.23, 7.57] | 0.75 |
| Pressure ulcer | 8.28 | [0.85, 81.03] | 0.07 |
| Surgical site wound | 0.87 | [0.16, 4.81] | 0.88 |
| Traumatic wound | 0.40 | [0.06, 2.84] | 0.36 |
| Vascular ulcer | 0.16 | [0.02, 1.38] | 0.10 |
| Wound age | 1.54 | [1.24, 1.91] | <0.01* |
| 120-Day failure | |||
| African American race | 2.33 | [1.07, 5.11] | 0.03* |
| Diabetes mellitus | 1.85 | [0.84, 4.08] | 0.13 |
| Preoperative wound infection | 1.55 | [0.61, 3.91] | 0.36 |
| Bone excision | 2.56 | [0.90, 7.33] | 0.08 |
| Wound age | 1.20 | [1.05, 1.38] | 0.01* |
| 180-Day failure | |||
| Government insurance | 2.12 | [1.15, 3.10] | 0.02* |
| African American race | 2.07 | [1.24, 2.91] | 0.01* |
| Tendon exposure | 2.09 | [1.18, 2.99] | 0.02* |
| Bone exposure | 1.27 | [0.03, 2.50] | 0.68 |
| Preoperative wound infection | 1.24 | [0.22, 2.26] | 0.64 |
| Bone excision | 1.91 | [0.33, 3.47] | 0.26 |
| Wound age | 1.32 | [1.14, 1.51] | <0.01* |
*p < 0.05.
DISCUSSION
Reconstructive principles of the lower extremity relate to size of tissue defect, anatomic location, and depth of injury, with larger, deeper, and distal wounds often requiring complex autologous free flap reconstruction given a dearth of surrounding tissue with which to reconstruct. Bilayer wound matrices such as Integra have gained popularity not only for their ability to efficiently reconstruct wounds of various types and locations but also for their immediate wound coverage, minimal donor-site morbidity, decreased scar burden, and shorter operative times.12 Burke et al.13 initially described the use of Integra bilayer wound matrix in 1981 for the reconstruction of burn wounds; this indication has since been broadened to encompass a variety of wound types. While select literature exists highlighting the use of collagen-GAG matrices in complex lower extremity soft -tissue reconstruction,14 there is a paucity of data to characterize a suitable patient population, categorize failures, and describe successful wound and anatomic factors. In order to critically evaluate the ability of these dermal regenerative matrices to reconstruct complex lower extremity soft-tissue wounds, we describe our experience with Integra bilayer wound matrix in 191 wounds.
Defining an Ideal Patient Population
Age, body mass index, and comorbid conditions, such as hypertension, diabetes, peripheral vascular disease, tobacco use, and chronic obstructive pulmonary disease, were not associated with reconstructive failure. Not surprisingly, the use of corticosteroids trended toward an unfavorable outcome. Ambulatory status had no bearing on ultimate graft success or failure. Wound type, importantly, had no bearing on ultimate graft success or failure, highlighting an advantage of collagen-GAG wound matrix reconstruction particularly in poorly vascularized wounds beds that require temporization with a neovascularized dermis before skin grafting.11,15 Lee et al.11 commented further on the ability of collagen-GAG matrices such as Integra to regenerate dermis-equivalent tissue suitable for skin grafting over chronic wounds and inherently dysvascular structures.
Categorizing Graft Failures
Patient factors associated with reconstructive failure included tendon exposure, bone exposure, and bone excision. Kim et al.14 described their treatment protocols related to the use of Integra in the setting of exposed tendon and bone. In their study, successful reconstruction of exposed tendon with bilayer wound matrix required the presence of surrounding granulation tissue or peritenon and small defects (<1 cm width of exposed tendon). Negative-pressure therapies can be utilized as temporization measures when no granulation tissue is present, while tissue flaps may also be considered if single-stage reconstruction is favored. Bone exposure greater than 0.5 cm is often associated with collagen-GAG wound matrix failure, which was further substantiated in our patient population.14 Kim et al.14 advocate for alternate reconstruction options for the soft-tissue coverage of the calcaneus, as skin graft bridged with Integra lacks sufficient bulk to bear weight. While collagen-GAG matrices have merit in select cases, our data suggest that complex lower extremity wounds involving extensive tendon and/or bone exposure should proceed directly to sophisticated reconstruction involving propeller flaps or free tissue transfer.
Approximately 19 percent, 26 percent, and 30 percent of total reconstructed wounds failed at the 60-, 120-, and 180-day timepoints, respectively. When stratified by wound type, there was a significant difference in early failure rates at the 60-day timepoint, with cellulitic and diabetic wounds failing early. Surprisingly, bilayer wound matrix–based reconstruction of vascular wounds continued to fail after 60 days (9.5 percent), with 25.0 percent and 29.0 percent of wounds failing by 120 and 180 days, respectively. The incidence of a postoperative complication, such as additional debridement, dehiscence, and necrosis, was ultimately associated with failure within 180 days, further highlighting the importance of an algorithmic approach to lower extremity bilayer wound reconstruction, which we have adopted from Kim et al.’s previous description.14 These wound factors leading to failure highlight the critical and important need for preoperative wound bed optimization before collagen-GAG bilayer wound matrix application.
Multivariate regression analysis identified factors associated with graft failure at early, intermediate, and long-term timepoints. The inclusion of income by zip code (in univariate regression analysis) and race and insurance type (in multivariate regression analysis) as significant factors associated with graft failure likely relates to socioeconomic and health literacy confounding variables not identified in this retrospective review, but highlight the importance of adequate patient education during complex reconstructive endeavors in general.
Wound Characteristics and Anatomic Locations Affecting Success
The major advantage of collagen-GAG matrix relates to its ability to either temporize or reconstruct wounds that are inherently high risk for single-stage skin grafting, regardless of wound size or anatomic location—two factors that historically require complex autologous free tissue transfer in the lower extremity. In our experience, wound size at time of reconstruction and anatomic location were not associated with bilayer wound matrix failure.
Cost-Related Outcomes
At a cost of approximately $2,000 for an 8- × 10-inch graft, use of Integra must be carefully considered in the select patient populations who are predicted to undergo successful bilayer wound matrix–based reconstruction.11 Failure of bilayer wound matrices not only doubled the mean length of stay (10.1 days versus 22.4 days) but also was unsurprisingly associated with significantly greater direct costs, total costs, and total hospital charges. These cost-related data emphasize the importance of careful patient selection, an algorithmic treatment approach, and identifying factors associated with early reconstructive failure, so that alternative reconstructive options, such as local, pedicled, or free tissue flaps, may be appropriately considered.
Limitations
There are, however, clear limitations to this study and several key criticisms are worth exploring. First, these data were collected in a retrospective manner and are therefore subject to unidentified heterogeneity between the cohorts. While we compared subjects who underwent successful reconstruction with those whose reconstruction failed using collagen-GAG bilayer wound matrices, a more comprehensive comparison would include subjects undergoing autologous tissue transfer, which we are currently pursuing. Lastly, patient comorbidities, including diabetes, smoking, and peripheral vascular disease, were not found to be significant contributing factors for successful incorporation of collagen-GAG matrices. Nevertheless, these patient factors undeniably lead to suboptimal and/or delayed wound bed preparation, which should be considered as a morbidity in itself. The racial and socioeconomic factors (i.e., race, insurance type, and income by zip code) significantly associated with reconstructive failure may represent limitations inherent to the study’s retrospective nature, although prior prospective studies have corroborated these results in lower extremity salvage.16 While these are irrational patient characteristics associated with failure, there are healthcare literacy factors driving these associations that remain unidentified in this retrospective study design.
CONCLUSIONS
This study represents the largest reported multihospital and multidisciplinary experience with lower extremity reconstruction using collagen-GAG wound matrix (Integra). Seventy percent of wounds treated with bilayer wound matrix were successfully salvaged at the 180-day timepoint. Half of the wounds that failed ultimately progressed to amputation. Wounds that experienced postoperative complications and/or need for additional debridement were associated with significantly higher failure rates. Patient comorbidities, including diabetes, peripheral vascular disease, hypertension, and chronic obstructive pulmonary disease, and wound factors, including location and size, were not associated with reconstructive failure. Patient factors associated with failure included wound age, tendon or bone exposure, and bone excision. These results suggest that collagen-GAG wound matrices in combination with split-thickness skin grafting may be used reliably in the reconstruction of large, superficial lower extremity wounds, but they may not be indicated for the reconstruction of complex, deep wounds with exposed tendon and/or bone. However, collagen-GAG wound matrices may be considered in special indications, such as the multimorbid patient with compromised vascular extremities who is not an ideal candidate for local flaps or free tissue transfer. Future investigations should include direct comparison of these wound matrices to autologous tissue transfer in lower extremity salvage.
ACKNOWLEDGMENT
The authors would like to acknowledge Ari M. Wes, M.D., for his assistance with statistical analysis.
Footnotes
Disclosure: Dr. Fischer has received consultant payments from Becton Dickinson, Integra Life Sciences, Gore, and Allergan. Dr. Kovach has received consultant payments from Becton Dickinson and Integra Life Sciences. This study was supported as an investigator-initiated study through Integra Life Sciences. The remaining authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: The economic case for the limb salvage team. J Vasc Surg. 2010;52(3 Suppl):17S–22S. [DOI] [PubMed] [Google Scholar]
- 2.Tentolouris N, Al-Sabbagh S, Walker MG, Boulton AJ, Jude EB. Mortality in diabetic and nondiabetic patients after amputations performed from 1990 to 1995: A 5-year follow-up study. Diabetes Care 2004;27:1598–1604. [DOI] [PubMed] [Google Scholar]
- 3.Iorio ML, Goldstein J, Adams M, Steinberg J, Attinger C. Functional limb salvage in the diabetic patient: The use of a collagen bilayer matrix and risk factors for amputation. Plast Reconstr Surg. 2011;127:260–267. [DOI] [PubMed] [Google Scholar]
- 4.Jordan DJ, Malahias M, Hindocha S, Juma A. Flap decisions and options in soft tissue coverage of the lower limb. Open Orthop J. 2014;8:423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson JA, Fischer JP, Brazio PS, Kovach SJ, Rosson GD, Rad AN. A review of propeller flaps for distal lower extremity soft tissue reconstruction: Is flap loss too high? Microsurgery 2013;33:578–586. [DOI] [PubMed] [Google Scholar]
- 6.Pontén B. The fasciocutaneous flap: Its use in soft tissue defects of the lower leg. Br J Plast Surg. 1981;34:215–220. [DOI] [PubMed] [Google Scholar]
- 7.Rad AN, Singh NK, Rosson GD. Peroneal artery perforator-based propeller flap reconstruction of the lateral distal lower extremity after tumor extirpation: Case report and literature review. Microsurgery 2008;28:663–670. [DOI] [PubMed] [Google Scholar]
- 8.Christy MR, Lipschitz A, Rodriguez E, Chopra K, Yuan N. Early postoperative outcomes associated with the anterolateral thigh flap in Gustilo IIIB fractures of the lower extremity. Ann Plast Surg. 2014;72:80–83. [DOI] [PubMed] [Google Scholar]
- 9.Bekara F, Herlin C, Mojallal A, et al. A systematic review and meta-analysis of perforator-pedicled propeller flaps in lower extremity defects: Identification of risk factors for complications. Plast Reconstr Surg. 2016;137:314–331. [DOI] [PubMed] [Google Scholar]
- 10.Weigert R, Leclere FM, Delia G, De Luca L, Al Mutairi K, Casoli V. Long-term patient-reported functional and cosmetic outcomes following severe traumatic foot and ankle wound reconstruction with acellular dermal matrix. J Cosmet Laser Ther. 2015;17:321–329. [DOI] [PubMed] [Google Scholar]
- 11.Lee LF, Porch JV, Spenler W, Garner WL. Integra in lower extremity reconstruction after burn injury. Plast Reconstr Surg. 2008;121:1256–1262. [DOI] [PubMed] [Google Scholar]
- 12.Johnson MB, Wong AK. Integra-based reconstruction of large scalp wounds: A case report and systematic review of the literature. Plast Reconstr Surg Glob Open 2016;4:e1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke JF, Yannas IV, Quinby WC, Jr, Bondoc CC, Jung WK. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg. 1981;194:413–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim PJ, Attinger CE, Steinberg JS, Evans KK. Integra bilayer wound matrix application for complex lower extremity soft tissue reconstruction. Surg Technol Int. 2014;24:65–73. [PubMed] [Google Scholar]
- 15.Moiemen NS, Staiano JJ, Ojeh NO, Thway Y, Frame JD. Reconstructive surgery with a dermal regeneration template: Clinical and histologic study. Plast Reconstr Surg. 2001;108:93–103. [DOI] [PubMed] [Google Scholar]
- 16.Bosse MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation after leg-threatening injuries. N Engl J Med. 2002;347:1924–1931. [DOI] [PubMed] [Google Scholar]


